Abstract
BACKGROUND
Tracheobronchial tuberculosis (TBTB) is a common subtype of pulmonary tuberculosis. Concomitant diseases often obscure the diagnosis of senile TBTB.
AIM
To characterize senile patients with TBTB and to identify the potential causes of misdiagnosis.
METHODS
One hundred twenty patients with senile TBTB who were admitted to the Anhui Chest hospital between May 2017 and May 2019 were retrospectively analyzed. Patients were classified as diagnosed group (n = 58) and misdiagnosed group (n = 62). Clinical manifestations, laboratory results, radiographic data, and endoscopic findings were compared between the two groups.
RESULTS
Patients in the misdiagnosed group were most commonly diagnosed as pulmonary tuberculosis (non-TBTB, 29/62, 46.8%), general pneumonia (9/62, 14.5%), chronic obstructive pulmonary disease (8/62, 12.9%), and tracheobronchial carcinoma (7/62, 11.3%). The time elapsed between disease onset and confirmation of diagnosis was significantly longer in the misdiagnosed group [median (first quartile, third quartile): 6.32 (4.94, 16.02) mo vs 3.73 (2.37, 8.52) mo]. The misdiagnosed group had lower proportion of patients who underwent bronchoscopy [33.87% (21/62) vs 87.93% (51/58)], chest computed tomography (CT) scan [69.35% (43/62) vs 98.28% (57/58)], and those who showed CT signs of tuberculosis [27.91% (12/62) vs 50% (29/58)] as compared to that in the diagnosed group (P < 0.05). There were no significant between-group differences with respect to age, gender, occupation, clinical manifestations, or prevalence of comorbid chronic diseases (P > 0.05).
CONCLUSION
Insufficient or inaccurate radiographic or bronchoscopic assessment was the predominant cause of delayed diagnosis of TBTB. Increased implementation and better interpretation of CT scan and early implementation of bronchoscopy can help reduce misdiagnosis of senile TBTB.
Keywords: Senile tracheobronchial tuberculosis, Misdiagnosis, Clinical characteristics, Pulmonary tuberculosis, Tuberculosis
Core Tip: Tracheobronchial tuberculosis (TBTB) is commonly misdiagnosed in clinical practice, especially among senile patients. To identify the determinants of misdiagnosis of TBTB, we systematically compared the clinical features and diagnostic workup between senile patients with TBTB that had been correctly diagnosed and those that had been misdiagnosed. Insufficient or inaccurate radiographic or bronchoscopy assessment was the predominant cause of delayed diagnosis of TBTB. Clinical features like age, gender, occupation, clinical manifestations, or prevalence of comorbid chronic diseases were not related to the misdiagnosis of TBTB.
INTRODUCTION
Pulmonary tuberculosis (PTB) remains a major global public health issue. According to the 2019 global tuberculosis (TB) report by the World Health Organization[1], the estimated global caseload of TB exceeds 1.7 billion; an estimated 10.0 million new cases were diagnosed, and an estimated 1.2 million people died of TB in the year 2018. The TB-related burden varies enormously among countries, and China is among the top 22 countries that are hard-hit by TB. According to the China Health Statistics Yearbook[2], more than 0.83 million new cases of TB were diagnosed in China in 2018, with the estimated morbidity rate in the same year exceeding 60.53/100000. According to an epidemiological survey, the major peak of incidence was in the age-group of 75-80 years[3,4]. Senile patients typically have multiple comorbid conditions and are immunocompromised; therefore, the diagnosis and management of senile PTB is an even more challenging issue.
Tracheobronchial TB (TBTB) is a common subtype of PTB that mainly affects the mucosa, submucosa, smooth muscle, cartilage, and even the outer membranes of the trachea or bronchi[5]. Owing to the airway involvement, patients with TBTB commonly develop obstructive pneumonia and pulmonary atelectasis, resulting in high mortality and lower cure rate as compared to the other subtypes of PTB[5]. Despite the high incidence, early diagnosis of TBTB is a challenge; this is largely attributable to the atypical manifestations and the lack of specific approaches for assessment[6,7]. Moreover, presence of concomitant diseases (especially other respiratory diseases) in senile patients further obscures the diagnosis of senile TBTB[6]. In this study, we sought to characterize the potential causes of missed diagnosis and misdiagnosis of TBTB in senile patients. Insights from our study may help improve the diagnostic workup for TBTB in clinical practice.
MATERIALS AND METHODS
Study population
Data pertaining to a total of 120 senile patients with TBTB who were hospitalized at the Anhui Chest Hospital between May 2017 and May 2019 were retrospectively analyzed. Patients that met all the following criteria were included: (1) Age ≥ 60 years; met diagnostic criteria for TBTB as described in the Diagnosis and treatment guidelines for TBTB 2012[8]; (2) had a previous diagnosis and treatment experience related to the current disease; and (3) complete medical records pertaining to previous diagnosis and treatment. The study population was classified into two groups: diagnosed group (n = 58, diagnosis of TBTB was confirmed before admission) and misdiagnosed group (n = 62, patients who were misdiagnosed before admission). The study was approved by the ethics committee of the Anhui Chest Hospital. Due to the retrospective nature of this study, the requirement for informed consent was waived.
Data collection
Data pertaining to demographic characteristics including age, gender, occupation, area of residence (rural/urban), and onset time (time elapsed from disease onset to the admission in our hospital) were collected. Medical records, including clinical manifestations (signs and symptoms), presence of concurrent extrapulmonary TB and other underlying diseases, were obtained. Results of radiographic assessment or bronchoscopy performed before admission were also collected.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Science (SPSS 21.0). Normally distributed continuous variables are presented as median ± SD and between-group differences were assessed using the unpaired t test. Non-normally distributed continuous variables are presented as median (interquartile range) [M (QR)], and between-group differences were assessed using the Mann-Whitney U test. Dichotomous or categorical variables are expressed as frequencies (percentage) and between-group differences were assessed using the Chi-squared or Fisher exact test. For all statistical analysis, P values < 0.05 were considered indicative of statistical significance.
RESULTS
TBTB misdiagnosis in senile patients
Among the 120 included patients who were finally confirmed as TBTB, 62 patients (51.7%) were previously incorrectly diagnosed. These patients were most commonly misdiagnosed as pulmonary TB (non TBTB, n = 29, 46.8%), general pneumonia (n = 9, 14.5%), chronic obstructive pulmonary disease (n = 8, 14.5%), tracheobronchial carcinoma (n = 7, 11.3%), chronic bronchitis (n = 5, 8.1%), foreign body in bronchus (n = 2, 3.2%), bronchial asthma (n = 1, 1.6%), and non-tuberculous mycobacterial lung disease (n = 1, 1.6%).
General characteristics of patients in the diagnosed and misdiagnosed groups
We compared the basic characteristics of patients in the two groups to identify potential factors that may contribute to the misdiagnosis of TBTB. We found no significant differences between the two groups with respect to age, gender, occupation, or area of residence (Table 1). The onset time of the disease (time elapsed between disease onset and the present admission) in the misdiagnosed group was significantly longer than that in the diagnosed group (P < 0.001, Table 1); this indicated that misdiagnosis could jeopardize the early diagnosis and treatment of the disease.
Table 1.
Comparison of general characteristics of patients in the two study groups
|
Variables
|
Categories
|
Misdiagnosed group, n = 62
|
Diagnosed group, n = 58
|
χ2
|
P
value
|
| Gender | Male | 24 (38.71) | 27 (46.55) | 0.754 | 0.385 |
| Female | 38 (61.29) | 31 (53.45) | |||
| Age (yr) | 60-70 | 48 (77.42) | 39 (67.24) | 3.283 | 0.194 |
| 71-80 | 10 (16.13) | 17 (29.31) | |||
| > 80 | 4 (6.45) | 2 (3.45) | |||
| Occupation | Farmer | 48 (77.42) | 45 (77.59) | 0.000 | 1.000 |
| Non-farmer | 14 (22.58) | 13 (22.41) | |||
| Resident area | Rural | 41 (66.13) | 38 (65.52) | 0.005 | 0.944 |
| Urban | 21 (33.87) | 20 (34.48) | |||
| Onset time | 6.32 (4.94, 16.02) | 3.73 (2.37, 8.52) | -3.899 | 0.000b |
Data presented as n (%) or as median (first quartile, third quartile).
P < 0.01.
Clinical features of patients in the two groups
We further compared the clinical features of patients in the two groups, including clinical manifestations, presence of extracellular TB, and other concurrent diseases. Cough with expectoration were the most common symptoms in both groups; there was no significant between-group difference in this respect (P > 0.05, Table 2). The two groups were also comparable in terms of the incidence of other less common symptoms including hemoptysis, breathlessness, or fever (P > 0.05, Table 2). The presence of extrapulmonary TB (n = 11, 9.17%) was rare in our study cohort; 3 patients had cervical lymphatic TB, five had laryngeal TB, one had bone TB, and one had tuberculous meningitis. No significant between-group difference was observed with respect to the incidence of extrapulmonary TB (P > 0.05, Table 2). Half of the study population had concurrent chronic diseases (n = 61, 50.8%); of these, 10 patients (8.33%) had more than 3 types of concurrent diseases other than TBTB. We did not observe any significant between-group difference with respect to the prevalence of concurrent chronic diseases (P > 0.05, Table 2).
Table 2.
Comparison of clinical features in the two groups
|
Variables
|
Categories
|
Misdiagnosed group, n = 62
|
Diagnosed group, n = 58
|
χ2
|
P
value
|
| Clinical manifestations, n (%) | Cough | 53 (85.48) | 49 (84.48) | 0.024 | 0.878 |
| Expectoration | 38 (61.29) | 40 (68.97) | 0.776 | 0.378 | |
| Hemoptysis | 11 (17.74) | 14 (24.14) | 0.743 | 0.389 | |
| Breathlessness | 23 (37.10) | 18 (31.03) | 0.49 | 0.484 | |
| Fever (≥ 37.3 °C) | 19 (30.65) | 10 (17.24) | 2.938 | 0.087 | |
| Other systemic symptoms | 14 (22.58) | 11 (18.97) | 0.237 | 0.626 | |
| Extrapulmonary tuberculosis, n (%) | Cervical lymphatic tuberculosis | 2 (3.23) | 1 (1.72) | - | 1.000 |
| Bone tuberculosis | 1 (1.61) | 1 (1.72) | - | 1.000 | |
| Laryngeal tuberculosis | 2 (3.23) | 3 (5.17) | - | 0.672 | |
| Tuberculous meningitis | 1 (1.61) | 0 (0.00) | - | 1.000 | |
| Chronic diseases, n (%) | 1 | 15 (24.19) | 7 (12.07) | 2.942 | 0.086 |
| 2 | 18 (29.03) | 11 (18.97) | 1.657 | 0.198 | |
| ≥ 3 | 7 (11.29) | 3 (5.17) | 1.468 | 0.226 |
Comparison of imaging findings
We presumed that implementation of chest computed tomography (CT) scan and the correct interpretation of CT findings play an important role in the diagnosis of TBTB. We compared the pre-admission CT scan data between the two groups. Almost all the patients (n = 57, 98.28%) in the diagnosed group had undergone chest CT scan prior to admission; however, only 43 out of 62 patients (69.35%) in the misdiagnosed group had undergone CT scan prior to admission (P < 0.001, Table 3). Among the patients for whom prior CT scan data was available, cases with reported signs of TBTB, like segmental atelectasis, airway stricture, or stenosis, were significantly lower in the misdiagnosed group as compared to that in the diagnosed group (P < 0.05, Table 3). A similar finding was observed with respect to the percentage of positive CT results (CT findings indicative of TBTB) (P = 0.021, Table 4).
Table 3.
Comparison of computed tomography findings in the two groups
|
Variables
|
Categories
|
Misdiagnosed group, n = 62
|
Diagnosed group, n = 58
|
χ2
|
P
value
|
| CT scan | Yes | 43 (69.35) | 57 (98.28) | 18.047 | 0.000b |
| No | 19 (30.65) | 1 (1.72) | |||
| CT findings | Segmental atelectasis | 21 (33.87) | 30 (51.72) | 3.909 | 0.048a |
| Airway stricture/stenosis | 15 (24.19) | 23 (39.66) | 3.311 | 0.069 | |
| Mediastinal/hilar lymphadenectasis | 6 (9.68) | 3 (5.17) | - | 0.493 | |
| Others | 1 (1.61) | 1 (1.72) | - | 1.000 |
Data presented as n (%).
P < 0.05.
P < 0.01.
Table 4.
Comparison of computed tomography scan interpretation between the two groups
|
CT interpretation
|
||
|
Positive
|
Negative
|
|
| Misdiagnosed group, n = 43 | 12 (27.91) | 31 (72.09) |
| Diagnosed group, n = 57 | 29 (50.00) | 28 (49.12) |
| χ 2 | 5.346 | |
| P value | 0.021a | |
Data presented as n (%).
P < 0.05.
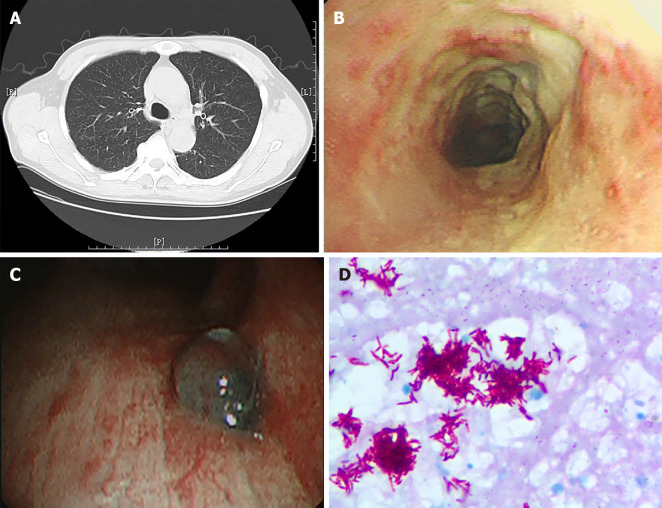
Some patients with TBTB may have completely normal chest CT scan, but can only be diagnosed by bronchoscopy and bronchoscopic biopsy (as shown in Figure 1). Therefore, we also compared the implementation of bronchoscopy prior to admission in the two groups and evaluated the bronchoscopic findings. The percentage of patients who had undergone bronchoscopy prior to admission in the misdiagnosed group (21/62, 33.9%) was significantly lower than that in the diagnosed group (51/58, 87.9%) (P < 0.001, Table 5). Among all patients who had undergone bronchoscopy prior to admission, the endoscopic findings of patients in the two groups were similar in terms of subtype classification (P = 0.369) and range of lesion involvement (P = 0.855) (Table 5).
Figure 1.
Chest computed tomography, bronchoscopy image and pathology image of a patient. A: Chest computed tomography of a patient with tracheobronchial tuberculosis showing no obvious abnormalities; B: Bronchoscopy image showing white caseous necrotic tissue on tracheal wall; C: Bronchoscopy image showing granulomatous proliferation on the wall of lower right trachea; D: Necrotic granulomatosis was detected on the pathology image, accompanied with positive acid fast-staining and detection of mycobacterium tuberculosis complex, × 1000 magnification.
Table 5.
Comparison of endoscopic features and classification of tracheobronchial tuberculosis in the two groups
|
Variables
|
Categories
|
Misdiagnosed group, n = 62
|
Diagnosed group, n = 58
|
χ2
|
P
value
|
| Bronchoscopy before admission | 21 (33.87) | 51 (87.93) | 36.491 | 0.000b | |
| Classification of TBTB | I | 2 (3.23) | 4 (6.9) | - | 0.369 |
| II | 17 (27.42) | 19 (32.76) | |||
| III | 6 (9.68) | 2 (3.45) | |||
| IV | 27 (43.55) | 20 (34.48) | |||
| V | 1 (1.61) | 0 (0) | |||
| VI | 9 (14.52) | 13 (22.41) | |||
| Range of lesion involvement | Trachea | 3 (4.84) | 7 (12.07) | - | 0.855 |
| left principal bronchus | 6 (9.68) | 8 (13.79) | |||
| left upper bronchus | 16 (25.81) | 13 (22.41) | |||
| left lower bronchus | 7 (11.29) | 6 (10.34) | |||
| Right principal bronchus | 15 (24.19) | 12 (20.69) | |||
| Right upper bronchus | 4 (6.45) | 3 (5.17) | |||
| Right middle bronchus | 11 (17.74) | 9 (15.52) |
Data presented as n (%).
P < 0.01.
DISCUSSION
PTB is highly prevalent across the world, especially in developing countries; TBTB accounts for 10%-40% of all cases of PTB[9]. The diagnosis of TBTB is challenging, as the bronchial lesions are usually not detectable on X-ray and the symptoms are non-specific and insidious at onset; this is especially so in senile patients in whom the manifestations can be obscured by co-existing pulmonary diseases[10]. Early diagnosis of senile TBTB is a key imperative owing to the extremely high mortality rate. In the present study, we sought to identify means to improve the diagnostic accuracy in senile patients with TBTB. Our study revealed that senile TBTB is commonly omitted or misdiagnosed as other pulmonary diseases. We found no significant difference between misdiagnosed patients and those who were correctly diagnosed in terms of demographic characteristics, clinical manifestations, or concomitant diseases. However, a significantly lesser percentage of patients received chest CT scan and bronchoscopy in the misdiagnosed group as compared to that in the correctly-diagnosed group. Our findings indicate that the lack of CT scan or bronchoscopy examination is the major factor that hinders the diagnosis of TBTB in senile patients.
TBTB is a specific subtype of PTB in which the tuberculous lesions are primarily located in the wall of airway, and are not necessarily accompanied by involvement of pulmonary parenchyma[9,11]. Pathological changes in the airway wall can lead to airway stenosis in up to 90% of TBTB patients, especially in the late stage of disease; this is a major cause of mortality in these patients[7,12]. Early diagnosis and treatment is the key strategy to stop the progression of airway stenosis. However, misdiagnosis or delayed diagnosis of TBTB is a common phenomenon, resulting in increased likelihood of progression to fibro-stenosis[5]. More than half of all TBTB patients in the present study were initially misdiagnosed, usually as PTB, pneumonia, or chronic obstructive pulmonary disease. Although the diagnosis of PTB would not delay the initiation of antitubercular treatment, TBTB is the most aggressive subtype of PTB, which requires more intensive therapeutic strategy to prevent the occurrence or progression of airway ulceration, necrosis, and fibrostenosis. Therefore, identification of TBTB at an early stage is a key imperative. As expected, misdiagnosis significantly prolonged the disease onset time (time elapsed between disease onset and confirmation of diagnosis) and delayed the start of treatment.
Several factors contribute to the delayed diagnosis of TBTB in clinical practice. Firstly, the typically insidious disease onset and the non-specific clinical presentation tends to hinder the diagnosis, especially in the early stages[9]. A high index of clinical suspicion supported by evidence from radiological and bronchoscopic detection is key for early diagnosis[10]. Secondly, identification of TBTB relies on evidence of pathological changes in airway tissues; however, these changes are usually undetectable on X-ray until the development of severe tracheobronchial stenosis at late stage[13]. Even when the concomitant parenchymal changes are detectable on general radiography, it tends to obscure the presence of airway involvement and lead to misdiagnosis of parenchymal disease[9]. The lack of specific diagnostic approach is the major obstacle for early diagnosis of TBTB. Lastly, senile patients with underlying conditions are most vulnerable to TBTB; these patients tend to have underlying pulmonary diseases, such as chronic obstructive pulmonary disease, parenchymal TB, and pneumonia. These preexisting diseases can also induce respiratory symptoms like cough, hemoptysis, breathlessness, and fever; this tends to obscure the concurrent TBTB[6,7]. All these factors make the early detection of TBTB extremely challenging, especially among senile patients.
In the present study, we first assessed whether the delayed diagnosis of TBTB was due to atypical clinical presentation or the presence of comorbid conditions. Unexpectedly, we did not observe any significant between-group difference in terms of clinical manifestations, concomitant chronic diseases or demographic features. This implies that the insidious manifestations and concomitant diseases were not the major causes of TBTB misdiagnosis in clinical practice.
As mentioned above, the lack of specific diagnostic modalities hinder the early identification of TBTB. The gold standard for diagnosis of PTB is the positive culture of tubercular bacilli from sputum or bronchoalveolar lavage fluid; this is a time-consuming method and is associated with a low sensitivity[14]. The development of TB antibodies/RNA/DNA detection, Interferon-Gamma Release Assays (IGRAs), and Gene Xpert MTB/RIF have helped improve the diagnostic yield; however, the early diagnosis of PTB remains a challenge[15,16]. The diagnosis of TBTB requires evidence of airway involvement in addition to the detection of tubercular bacilli; this makes the diagnosis of TBTB even more challenging. X-ray has a poor sensitivity for the detection of tracheobronchial lesions; however, CT scan (especially 64-slice spiral CT) has been shown to efficiently detect the TBTB lesions[17-19]. CT imaging can clearly delineate the extent of bronchial or parenchymal involvement and determine the stage of disease progression[9]. Bronchoscopic examination is another highly recommended modality for detection of TBTB. Bronchoscopy can detect morphological features (e.g., edematous, fibrostenotic, granular or ulcerative lesions) of the airway lesions, which provides critical information for the staging of TBTB[20]. Moreover, it is a convenient method for obtaining biopsy tissue for pathological examination[21,22]. Specifically, for TBTB progressing to fibro-stenosis stage, when tuberculous bacilli are undetectable, bronchoscopic evidence of chronic fibrosis and granular changes can support the diagnosis of TBTB[20,22]. Given the critical role of CT scan and bronchoscopy in the diagnosis of TBTB, we assessed whether the lack of implementation of these examinations contributed to the delayed diagnosis of TBTB. Our results showed that the percentage of patients who did not receive CT scan or bronchoscopy examination before admission in the misdiagnosed group was significantly greater than that in the correctly-diagnosed group. A large proportion of TBTB patients did not receive CT scan or bronchoscopy during diagnostic workup; this indicates the need for greater awareness among physicians about this disease. Our findings highlight the importance of a high suspicion index for TBTB along with early implementation and accurate interpretation of chest CT scan and bronchoscopy for the diagnosis of TBTB. Detailed medical history and meticulous physical examination also play a role in the early identification of TBTB. For patients with signs or symptoms suggestive of TBTB, CT scan and bronchoscopy should be considered especially in senile patients.
To the best of our knowledge, this is the first study to identify factors associated with misdiagnosis of senile TBTB in contemporary clinical practice. However, some limitations of our study should be considered while interpreting the results. First, the sample size in this study was relatively small; larger studies are required to obtain more definitive evidence. Moreover, most of the medical data analyzed was obtained from other institutions. The analysis did not take into account the level of care and diagnostic competence of these medical institutions. Finally, we only included cases who were admitted at our center; our conclusions need to be verified in a multi-center study.
CONCLUSION
To conclude, our study identified factors that may contribute to the misdiagnosis of TBTB in contemporary clinical practice. Early implementation and accurate interpretation of chest CT scan and bronchoscopy would facilitate early diagnosis and minimize misdiagnosis of TBTB in senile patients.
ARTICLE HIGHLIGHTS
Research background
Tracheobronchial tuberculosis (TBTB) is commonly misdiagnosed in clinical practice, especially among senile patients.
Research motivation
To characterize senile patients with TBTB and to identify the potential causes of misdiagnosis.
Research objectives
One hundred twenty patients with senile TBTB who were admitted to the Anhui Chest hospital between May 2017 and May 2019 were retrospectively analyzed.
Research methods
Patients were classified as diagnosed group (n = 58) and misdiagnosed group (n = 62). Clinical manifestations, laboratory results, radiographic data, and endoscopic findings were compared between the two groups to identify the major factors that contribute to the misdiagnosis or delayed diagnosis of the disease.
Research results
Patients in the misdiagnosed group were most commonly diagnosed as pulmonary tuberculosis (non-TBTB, 29/62, 46.8%), general pneumonia (9/62, 14.5%), chronic obstructive pulmonary disease (8/62, 12.9%), and tracheobronchial carcinoma (7/62, 11.3%). The misdiagnosed group had lower proportion of patients who underwent bronchoscopy [33.87% (21/62) vs 87.93% (51/58)], chest CT scan [69.35% (43/62) vs 98.28% (57/58)], and those who showed CT signs of tuberculosis [27.91% (12/62) vs 50% (29/58)] as compared to that in the diagnosed group (P < 0.05).
Research conclusions
Insufficient or inaccurate radiographic or bronchoscopic assessment was the major cause of delayed diagnosis of TBTB.
Research perspectives
Increased implementation and better interpretation of CT scan and early implementation of bronchoscopy can help reduce misdiagnosis of senile TBTB.
Footnotes
Institutional review board statement: The study was approved by the ethics committee of the Anhui Chest Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent statement: Due to the retrospective nature of this study, informed consent was waived.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: January 23, 2021
First decision: April 29, 2021
Article in press: July 12, 2021
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barbosa OA, Karmakar S S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li X
Contributor Information
Fei Tang, Department of Geriatric Respiratory and Critical Care, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui Province, China; Department of Interventional Pulmonology and Endoscopic Diagnosis and Treatment Center, Anhui Chest Hospital, Hefei 230022, Anhui Province, China.
Lian-Jun Lin, Department of Respiratory and Critical Care Medicine, Peking University First Hospital, Beijing 100034, China.
Shu-Liang Guo, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China.
Wei Ye, Department of Interventional Pulmonology and Endoscopic Diagnosis and Treatment Center, Anhui Chest Hospital, Hefei 230022, Anhui Province, China.
Xian-Kui Zha, Department of Interventional Pulmonology and Endoscopic Diagnosis and Treatment Center, Anhui Chest Hospital, Hefei 230022, Anhui Province, China.
Yu Cheng, Department of Interventional Pulmonology and Endoscopic Diagnosis and Treatment Center, Anhui Chest Hospital, Hefei 230022, Anhui Province, China.
Ying-Feng Wu, Department of Interventional Pulmonology and Endoscopic Diagnosis and Treatment Center, Anhui Chest Hospital, Hefei 230022, Anhui Province, China.
Yue-Ming Wang, Department of Interventional Pulmonology and Endoscopic Diagnosis and Treatment Center, Anhui Chest Hospital, Hefei 230022, Anhui Province, China.
Xiao-Mei Lyu, Department of Interventional Pulmonology and Endoscopic Diagnosis and Treatment Center, Anhui Chest Hospital, Hefei 230022, Anhui Province, China.
Xiao-Yun Fan, Department of Geriatric Respiratory and Critical Care, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui Province, China. 115367686@qq.com.
Li-Ping Lyu, Department of Interventional Pulmonology and Endoscopic Diagnosis and Treatment Center, Anhui Chest Hospital, Hefei 230022, Anhui Province, China.
Data sharing statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.World Health Organization. Global tuberculosis report 2019. [cited 29 July 2020]. In: World Health Organization. Available from: https://www.who.int/tb/publications/global_report/en/
- 2.China Union Medical University Press. China Health Statistics Yearbook 2018, 2018. [cited 29 July 2020]. In: China Union Medical University Press [Internet]. Available from: http://cdi.cnki.net/Titles/SingleNJ?NJCode=N2019030282 .
- 3.Huang L, Cheng S, Chen M, Zhao L. Report on the Fifth National Tuberculosis Epidemiological Sampling Survey in 2010. Zhongguo Fanglao Zazhi . 2012;34:485–508. [Google Scholar]
- 4.Khan MK, Islam MN, Ferdous J, Alam MM. An Overview on Epidemiology of Tuberculosis. Mymensingh Med J. 2019;28:259–266. [PubMed] [Google Scholar]
- 5.Gil Guerra AB, Gómez San Martín E, López Pedreira MR. Tracheobronchial Tuberculosis. Arch Bronconeumol (Engl Ed) 2018;54:41. doi: 10.1016/j.arbres.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Su Z, Cheng Y, Wu Z, Zhang P, Chen W, Zhou Z, Zhong M, Luo W, Guo W, Li S. Incidence and Predictors of Tracheobronchial Tuberculosis in Pulmonary Tuberculosis: A Multicentre, Large-Scale and Prospective Study in Southern China. Respiration. 2019;97:153–159. doi: 10.1159/000492335. [DOI] [PubMed] [Google Scholar]
- 7.Guo X, Wang C, Wang X, Ma J, Xv L, Luan T, Kou C. Characteristics and risk factor analysis of 410 cases of tracheobronchial tuberculosis. Exp Ther Med. 2014;8:781–784. doi: 10.3892/etm.2014.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding WM. Guidelines for the diagnosis and treatment of tracheobronchial tuberculosis. The 2012 Annual Academic Conference and Professional Group Establishment Conference of the Clinical Professional Committee of the Chinese Tuberculosis Association; 2012 August 10; Changchun, Jilin, China. [Google Scholar]
- 9.Pathak V, Shepherd RW, Shojaee S. Tracheobronchial tuberculosis. J Thorac Dis. 2016;8:3818–3825. doi: 10.21037/jtd.2016.12.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siow WT, Lee P. Tracheobronchial tuberculosis: a clinical review. J Thorac Dis. 2017;9:E71–E77. doi: 10.21037/jtd.2017.01.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campos J, Ernst G, Borsini E, Garcia A, Blasco M, Bosio M, Salvado A. Tracheobronchial Tuberculosis Without Lung Involvement. J Clin Med Res. 2015;7:646–648. doi: 10.14740/jocmr2182w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz AC, Carrascosa MF, García-Rivero JL, Rodríguez GB, Hoz MC, Sáenz EC. One not to miss: Tuberculous tracheal stenosis. Respir Med Case Rep. 2020;30:101040. doi: 10.1016/j.rmcr.2020.101040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YB, Nguyen QK, Le HL, Phan TH, Hoeffel CC. [Diagnostic imaging of tracheobronchial tuberculosis. Apropos of a case] Rev Pneumol Clin. 1999;55:223–226. [PubMed] [Google Scholar]
- 14.Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393:1642–1656. doi: 10.1016/S0140-6736(19)30308-3. [DOI] [PubMed] [Google Scholar]
- 15.Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir Med. 2020;8:19. doi: 10.1016/S2213-2600(19)30418-7. [DOI] [PubMed] [Google Scholar]
- 16.Dheda K, Gumbo T, Maartens G, Dooley KE, Murray M, Furin J, Nardell EA, Warren RM Lancet Respiratory Medicine drug-resistant tuberculosis Commission group. The Lancet Respiratory Medicine Commission: 2019 update: epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant and incurable tuberculosis. Lancet Respir Med. 2019;7:820–826. doi: 10.1016/S2213-2600(19)30263-2. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y, Lee KS, Yoon JH, Chung MP, Kim H, Kwon OJ, Rhee CH, Han YC. Tuberculosis of the trachea and main bronchi: CT findings in 17 patients. AJR Am J Roentgenol. 1997;168:1051–1056. doi: 10.2214/ajr.168.4.9124114. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz E, Akkoclu A, Sevinc C. CT and MRI appearance of a fistula between the right and left main bronchus caused by tracheobronchial tuberculosis. Br J Radiol. 2001;74:1056–1058. doi: 10.1259/bjr.74.887.741056. [DOI] [PubMed] [Google Scholar]
- 19.Im JG, Itoh H, Han MC. CT of pulmonary tuberculosis. Semin Ultrasound CT MR. 1995;16:420–434. doi: 10.1016/0887-2171(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 20.Rikimaru T, Tanaka Y, Ichikawa Y, Oizumi K. Endoscopic classification of tracheobronchial tuberculosis with healing processes. Chest. 1994;105:318–319. doi: 10.1378/chest.105.1.318. [DOI] [PubMed] [Google Scholar]
- 21.Sabour S. Letter to the Editor: Diagnostic Value of Virtual Bronchoscopic Navigation in the Bronchial Tuberculosis-Induced Central Airway Stenosis. Infect Dis Ther. 2020;9:403–405. doi: 10.1007/s40121-020-00298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng LP, Gu Y, Gui XW, Fang Y, Wang H, Sha W. Diagnostic Value of Virtual Bronchoscopic Navigation in the Bronchial Tuberculosis Induced Central Airway Stenosis. Infect Dis Ther. 2020;9:165–174. doi: 10.1007/s40121-020-00283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.