Abstract
BACKGROUND
Wernicke's encephalopathy is a disease caused by thiamine deficiency. The lesions usually involve the periphery of the aqueduct, midbrain, tectum, third ventricle, papillary body, and thalamus. It is very rare to affect the vermis and cerebellar hemispheres.
CASE SUMMARY
We report a 77-year-old female patient admitted to the emergency department of our hospital for 2 d of unconsciousness. Brain magnetic resonance imaging showed increased diffusion weighted imaging signals in the bilateral thalamus, periventricular regions of the third ventricle, corpora quadrigemina, vermis, and cerebellar hemispheres. Wernicke's encephalopathy was considered. She was given thiamine therapy and became conscious after the treatment.
CONCLUSION
Wernicke's encephalopathy may have various imaging manifestations. Clinicians should keep in mind that Wernicke’s encephalopathy may occur in patients who experience prolonged periods of malnutrition.
Keywords: Wernicke's encephalopathy, Atypical radiological features, Vermis, Cerebellar hemispheres, Case report
Core Tip: Wernicke's encephalopathy is a disease caused by thiamine deficiency. Wernicke's encephalopathy may have various imaging manifestations. Typically, the lesions are distributed symmetrically in the thalamus, mammillary bodies, corpora quadrigemina, and periaqueductal areas. Lesions can also be found atypically in the cerebellum, cranial nerve nucleus, red nucleus, caudate nucleus, cerebral cortex, and other atypical areas. We report a female patient with atypical lesions involving the vermis and cerebellar hemispheres. She was given thiamine therapy and became conscious after the treatment.
INTRODUCTION
Wernicke's encephalopathy is a disease caused by thiamine (vitamin B1) deficiency. It was first believed that Wernicke's encephalopathy was caused by the malabsorption of vitamin B1 after chronic alcohol abuse. Alcohol abuse is related to the complications of liver cirrhosis, such as the gastrointestinal tract having a low absorption rate at the mucosal level and the consequent malnourishment. In addition to alcohol abuse, severe vomiting, fasting, gastrointestinal surgery, chronic diarrhea, systematic disease, hemodialysis, hyperthyroidism, anorexia nervosa, and genetic factors[1] may cause insufficient intake of vitamin B1 or vitamin B1 use disorder. Wernicke's encephalopathy may have various imaging manifestations[2]. We report a rare case of Wernicke’s encephalopathy affecting the cerebellum.
CASE PRESENTATION
Chief complaints
A 77-year-old female patient was admitted to the emergency department of our hospital for 2 d of unconsciousness.
History of present illness
Approximately 1 mo prior to this visit, the patient presented with recurrent vomiting and bloody stools. Diarrhea, breathing problems, dizziness, and limb weakness were denied by the patient’s family in the course of the disease.
History of past illness
The patient had hypertension for 8 years. The blood pressure ranged from 120-140/70-100 mmHg after taking amlodipine.
Personal and family history
The patient had a disease-free personal and family history.
Physical examination
On admission, the vital signs were normal. Physical examination showed that the patient was confused. The neurological examination revealed that the diameter of both pupils was 0.3 cm, and the pupillary light reflex was sensitive. She could move her extremities unconsciously, and bilateral pathological signs were negative.
Laboratory examinations
Biochemical tests, thyroid function, autoantibodies, and vasculitis antibodies were normal. The results of blood electrolyte and arterial blood gas analyses were unremarkable. Blood tests showed decreased hemoglobin (93 g/L) and increased D-dimer (1380 µg/L). One tumor index, carcinoembryonic antigen, was increased mildly (10.02 g/mL). Fecal occult blood test result was positive.
Imaging examinations
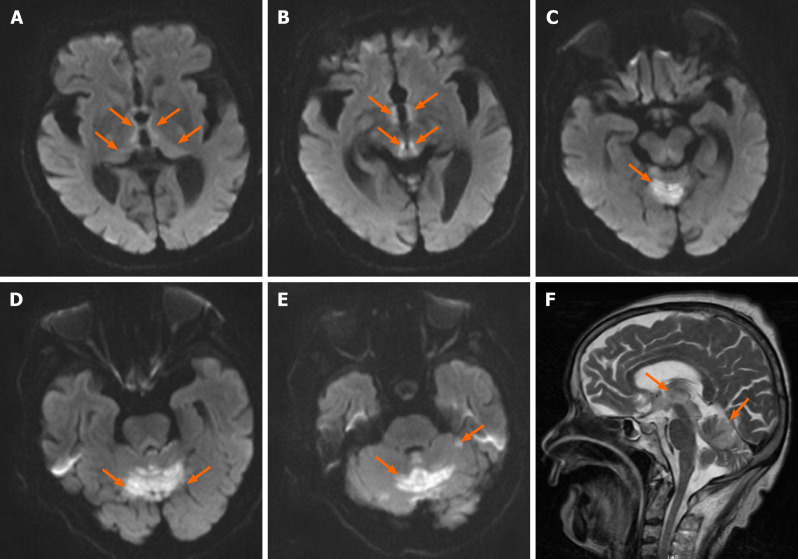
Abdominal contrast-enhanced computed tomography (CT) revealed thickened walls of the lower part of the rectum and distal bowel obstruction. Brain CT findings were unremarkable. Chest CT revealed a pleural effusion on the left. Brain magnetic resonance imaging (MRI) showed extensive abnormal signal lesions not only in the periaqueductal region but also in the cerebellum. Specifically, lesions were located in the bilateral thalamus, the periventricular regions of the third ventricle, the corpora quadrigemina, the vermis, and the cerebellar hemispheres (Figure 1), with increased signal intensity on T1-weighted imaging, T2-weighted imaging, and diffusion weighted imaging (DWI).
Figure 1.
Diffusion-weighted imaging. A-E: Axial diffusion-weighted imaging showing bilateral and symmetric high signals at the level of the periventricular regions of the third ventricle, medial portion of bilateral thalamus, corpora quadrigemina (A and B), vermis, (C) and cerebellar hemispheres (D and E); F: Sagittal T2-weighted imaging lesions.
FINAL DIAGNOSIS
At first, the radiologists indicated that the periaqueductal lesions suggested Wernicke's encephalopathy and that the cerebellar lesions suggested acute infarction. After combining the clinical manifestations and a literature review, all these abnormal signal intensity lesions were considered Wernicke's encephalopathy lesions. First, the lesions in the cerebellum involved both the vermis and the bilateral cerebellar hemispheres, which were basically symmetrical and did not match the distribution of blood vessels. Second, a few papers indeed reported the involvement of the cerebellar hemispheres in Wernicke's encephalopathy.
TREATMENT
The above examinations also showed gastrointestinal bleeding. The patient was highly suspected of having rectal cancer. She was given gastrointestinal decompression and intravenous nutrition. She was immediately given 200 mg vitamin B1 thrice a day intravenously.
OUTCOME AND FOLLOW-UP
Four hours after the injection, the patient became sober. She complained of dizziness and weakness. Physical examination showed nystagmus and bilateral limb ataxia. On the 7th day after administering vitamin B1, the patient underwent colonoscopy, which indicated a rectal tumor. Biopsy pathology confirmed a moderately differentiated adenocarcinoma. Finally, she refused surgical assessment and was discharged on the 14th day after hospitalization with a modified Rankin scale score of 1.
DISCUSSION
Wernicke’s encephalopathy is an encephalopathy caused by vitamin B1 (thiamine) deficiency first discovered by Carl Wernicke in 1881. It is more commonly observed as a metabolic encephalopathy caused by chronic alcoholism, long-term heavy alcohol abuse, and thiamine deficiency. Alcoholism accounts for about 50% of causes of Wernicke’s encephalopathy[3]. The causes of thiamine deficiency include vomiting in pregnancy, malnutrition, anorexia nervosa, liver disease, total gastrectomy, jejunectomy, gastric cancer, malignant tumors, pernicious anemia, chronic diarrhea, long-term renal dialysis, parenteral nutrition deficiency, etc. Animal experiments have shown that chronic alcoholism can lead to malnutrition, mainly thiamine deficiency, which can then aggravate the chronic alcoholism[4,5]. It has been reported that the proportion of patients with nonethanol poisoning is 39% to 50%[1]. Hyperemesis during pregnancy, acute pancreatitis, and temporary fasting are more common after total parenteral nutrition. Supplementing vitamin B1 as early as possible is the key to the treatment of this disease. Patients diagnosed and treated in time can fully recover.
The classic triad of mental impairment, ataxia, and ocular symptoms occurs in only 8% of patients[6], while some only show one or two of the triad. In addition to these typical clinical manifestations, Wernicke's encephalopathy may also be characterized by many atypical symptoms or signs, leading to clinical misdiagnosis. According to various reports, these atypical manifestations include stupor, hypotension, tachycardia, hypothermia, seizures, hearing disorders, fever, and spastic paralysis[7]. In our case, recurrent vomiting and subsequent ileus decreased the absorption of nutrients. Finally, the patient appeared unconscious due to a long-term lack of absorption of vitamin B1.
Patients with Wernicke's encephalopathy can have pathological lesions in multiple locations, such as the mammillary bodies, brain stem, periventricular regions of the third ventricle, thalamus, hypothalamus, vermis, and vestibule nuclei. Studies suggest that CT is not reliable in the diagnosis of Wernicke's encephalopathy[2]. In the acute stage of Wernicke's encephalopathy, head CT is negative. MRI is the preferred routine imaging method for diagnosis. It has been reported that its sensitivity is 53%, specificity is 93%, and positive predictive value is 89%[8,9]. MRI is currently considered to be the most valuable method for diagnosis. On MRI, the lesions are consistent with the pathological features of the disease, showing long T1 signals, long T2 signals, high fluid-attenuated inversion recovery signals, and high DWI signals in the acute phase. The most sensitive diagnostic modality for Wernicke's encephalopathy is DWI. Sometimes the signal changes in the other phases are not obvious. Typically, the lesions are distributed symmetrically in the thalamus, mammillary bodies, corpora quadrigemina, and periaqueductal areas. Lesions can also be found atypically in the cerebellum, cranial nerve nucleus, red nucleus, caudate nucleus, cerebral cortex, and other atypical areas. Atypical findings are found more frequently in nonalcoholic patients[10]. Cerebellum involvement on imaging is rare, but autopsy studies have demonstrated that the anterior-superior vermis or anterior hemisphere is affected in more than half of patients with Wernicke’s encephalopathy. The involvement of atypical sites is usually seen in association with the involvement of typical sites and is thought to indicate progression of the disease[1]. Lesions may persist or disappear after treatment.
In our case, there were lesions in the vermis and cerebellar hemispheres in addition to typical imaging changes around the aqueduct. It is extremely rare in the published papers[11]. Wernicke's encephalopathy is an emergent event in the neurology department. Nonalcoholic Wernicke's encephalopathy is easily misdiagnosed. Once a diagnosis is suspected, it is advised that vitamin B1 should be administered immediately. The guidelines recommend administration of 200 mg vitamin B1 three times daily, preferably intravenously instead of intramuscularly[6]. It is generally considered that adverse reactions to vitamin B1 are very rare. The effect of vitamin B1 may also partly or completely reverse the damage to nervous tissue[12]. However, delayed diagnosis and treatment can lead to progression of the disease. Ultimately, the patient may develop Korsakov's syndrome and even die. The treatment course of vitamin B1 use is not clear. Our patient’s symptoms improved dramatically after supplementation with vitamin B1, potentially confirming her condition as Wernicke’s encephalopathy.
CONCLUSION
Our case illustrates that Wernicke's encephalopathy may have various imaging manifestations. It can cause not only structural lesions around the aqueduct but also large-area limited signals on DWI in the cerebellum. It is difficult to diagnose early, especially in patients with atypical syndromes and imaging changes. Careful recording of medical features and analysis of imaging changes are necessary. Clinicians should keep in mind that patients who have difficulty eating due to recurrent vomiting are at high risk of Wernicke's encephalopathy due to malnutrition.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: All authors declare that they have no conflicts of interest to disclose.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: April 22, 2021
First decision: May 24, 2021
Article in press: July 14, 2021
Specialty type: Neuroimaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Naem A S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Zhang YL
Contributor Information
Tian Nie, Department of Neurology, The Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou 310006, Zhejiang Province, China. nietianshn@163.com.
Jian-Li He, Department of Neurology, The Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou 310006, Zhejiang Province, China.
References
- 1.Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6:442–455. doi: 10.1016/S1474-4422(07)70104-7. [DOI] [PubMed] [Google Scholar]
- 2.Ota Y, Capizzano AA, Moritani T, Naganawa S, Kurokawa R, Srinivasan A. Comprehensive review of Wernicke encephalopathy: pathophysiology, clinical symptoms and imaging findings. Jpn J Radiol. 2020;38:809–820. doi: 10.1007/s11604-020-00989-3. [DOI] [PubMed] [Google Scholar]
- 3.Sinha S, Kataria A, Kolla BP, Thusius N, Loukianova LL. Wernicke Encephalopathy-Clinical Pearls. Mayo Clin Proc. 2019;94:1065–1072. doi: 10.1016/j.mayocp.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Zahr NM, Sullivan EV, Rohlfing T, Mayer D, Collins AM, Luong R, Pfefferbaum A. Concomitants of alcoholism: differential effects of thiamine deficiency, liver damage, and food deprivation on the rat brain in vivo. Psychopharmacology (Berl) 2016;233:2675–2686. doi: 10.1007/s00213-016-4313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zahr NM, Pfefferbaum A. Alcohol's Effects on the Brain: Neuroimaging Results in Humans and Animal Models. Alcohol Res. 2017;38:183–206. doi: 10.35946/arcr.v38.2.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galvin R, Bråthen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA EFNS. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17:1408–1418. doi: 10.1111/j.1468-1331.2010.03153.x. [DOI] [PubMed] [Google Scholar]
- 7.Shah IA, Asimi RP, Kawoos Y, Wani M, Saleem T, Baba WN. Nonalcoholic Wernicke's Encephalopathy: A Retrospective Study from a Tertiary Care Center in Northern India. J Neurosci Rural Pract. 2017;8:401–406. doi: 10.4103/jnrp.jnrp_14_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antunez E, Estruch R, Cardenal C, Nicolas JM, Fernandez-Sola J, Urbano-Marquez A. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke's encephalopathy. AJR Am J Roentgenol. 1998;171:1131–1137. doi: 10.2214/ajr.171.4.9763009. [DOI] [PubMed] [Google Scholar]
- 9.Elefante A, Puoti G, Senese R, Coppola C, Russo C, Tortora F, de Divitiis O, Brunetti A. Non-alcoholic acute Wernicke's encephalopathy: role of MRI in non typical cases. Eur J Radiol. 2012;81:4099–4104. doi: 10.1016/j.ejrad.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Yin H, Xu Q, Cao Y, Qi Y, Yu T, Lu W. Nonalcoholic Wernicke's encephalopathy: a retrospective study of 17 cases. J Int Med Res. 2019;47:4886–4894. doi: 10.1177/0300060519870951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuccoli G, Motti L. Atypical Wernicke's encephalopathy showing lesions in the cranial nerve nuclei and cerebellum. J Neuroimaging. 2008;18:194–197. doi: 10.1111/j.1552-6569.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 12.Nishimoto A, Usery J, Winton JC, Twilla J. High-dose Parenteral Thiamine in Treatment of Wernicke's Encephalopathy: Case Series and Review of the Literature. In Vivo. 2017;31:121–124. doi: 10.21873/invivo.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]