Abstract
BACKGROUND
There are various studies showing the relationship between irritable bowel syndrome (IBS) and diet, and some dietary adjustments are recommended to reduce symptoms. In recent years, there is a growing number of studies that show a 4-8 wk low fermentable oligo, di- and mono-saccharides and polyols (FODMAP) diet has a 50%-80% significant effect on symptoms in IBS patients. There is strong evidence suggesting that changes in fecal microbiota have an impact on IBS pathogenesis. Based on this argument, probiotics have been used in IBS treatment for a long time. As is seen, the FODMAP diet and probiotics are used separately in IBS treatment.
AIM
To evaluate the effectiveness of adding probiotics to a low FODMAP diet to control the symptoms in patients with IBS.
METHODS
The patients who were admitted to the Gastroenterology Clinic of Dokuz Eylul University Hospital and diagnosed with IBS according to Rome IV criteria were enrolled into the study. They were randomized into 2 groups each of which consisted of 50 patients. All patients were referred to a dietitian to receive dietary recommendations for the low FODMAP diet with a daily intake of 9 g. The patients were asked to keep a diary of foods and beverages they consumed. The patients in Group 1 were given supplementary food containing probiotics (2 g) once a day in addition to their low FODMAP diet, while the patients in Group 2 were given a placebo once a day in addition to their low FODMAP diet. Visual analogue scale (VAS), the Bristol Stool Scale and IBS Symptom Severity Scale (IBS-SSS) scores were evaluated before and after the 21 d treatment.
RESULTS
The rate of adherence of 85 patients, who completed the study, to the FODMAP restricted diet was 92%, being 90% in Group 1 and 94% in Group 2. The mean scores of VAS and IBS-SSS of the patients in Group 1 before treatment were 4.6 ± 2.7 and 310.0 ± 78.4, respectively, and these scores decreased to 2.0 ± 1.9 and 172.0 ± 93.0 after treatment (both P < 0.001). The mean VAS and IBS-SSS scores of the patients in Group 2 before treatment were 4.7 ± 2.7 and 317.0 ± 87.5, respectively, and these scores decreased to 1.8 ± 2.0 and 175.0 ± 97.7 after treatment (both P < 0.001). The IBS-SSS score of 37 patients (86.04%) in Group 1 and 36 patients (85.71%) in Group 2 decreased by more than 50 points. Group 1 and Group 2 were similar in terms of differences in VAS and IBS-SSS scores before and after treatment. When changes in stool shape after treatment were compared using the Bristol Stool Scale, both groups showed significant change.
CONCLUSION
This study is the randomized controlled study to examine the efficiency of probiotic supplementation to a low FODMAP diet in all subtypes of IBS. The low FODMAP diet has highly positive effects on symptoms of all subtypes of IBS. It was seen that adding probiotics to a low FODMAP diet does not make an additional contribution to symptom response and adherence to the diet.
Keywords: Irritable bowel syndrome; Probiotics; Low Fermentable Oligo, Di- and Mono-Saccharides and Polyols diet; Visual Analogue Scale; the Bristol Stool Scale; Irritable Bowel Syndrome Symptom Severity Scale
Core Tip: A low fermentable oligo, di- and monosaccharides and polyols diet significantly relieved irritable bowel syndrome symptoms in all irritable bowel syndrome subtypes in the initial phase; however, adding probiotics to the diet did not make an additional contribution to symptom relief. Further longer term research using different probiotics is needed to examine the efficiency of probiotic supplementation to a low fermentable oligo, di- and monosaccharides and polyols diet in irritable bowel syndrome.
INTRODUCTION
Irritable bowel syndrome (IBS) is a common functional disorder characterized by symptoms of chronic abdominal pain, changes in bowel habits and defecation[1]. Although its prevalence varies by country, about one in five people around the world is affected by this disease. IBS is more common among women and people under the age of 50. The course of this disorder is chronic, episodic or relapsing[2]. The pathogenesis of IBS is complex and multifactorial. Visceral hypersensitivity, psychosocial factors, changes in the gut-brain axis and gastrointestinal motility, immune dysregulation and dysbiosis are considered to be the most critical factors that affect etiopathogenesis[3]. IBS causes recurring admission to clinics, redundant medical examinations and unnecessary procedures. Thus, it not only deteriorates the quality of life of patients, but it also increases healthcare costs significantly and causes workforce loss[4].
In addition to medical treatment, the doctor-patient relationship and diet and lifestyle changes play an essential role in the treatment of IBS[5]. Treatment may include various diets, antispasmodics, loperamide, antidepressants, psychological treatments (psychotherapy, hypnosis, etc.), laxatives, biofeedback, prebiotics or probiotics. Although these treatment(s) relieve the symptoms in some patients, some patients do not get better sufficiently, hence requiring other treatment options.
There are various studies showing the relationship between IBS and diet, and some dietary adjustments are recommended to reduce symptoms. One of them is the elimination diet, which is widely used in IBS treatment. In recent years, there is a growing number of studies that show a 4-8 wk low fermentable oligo, di- and monosaccharides and polyols (FODMAP) diet has a 50%-80% significant effect on symptoms in IBS patients[3,6,7]. The term FODMAP refers to all short-chain carbohydrates. These substances are not appropriately absorbed and rapidly fermented by intestinal bacteria. Thus, it is thought that intraluminal fluid content and an increase in colonic hydrogen production increase the symptoms[8]. Excessive consumption of these carbohydrate derivatives (fructose, fructooligosaccharides, sorbitol) may trigger IBS symptoms due to their direct or indirect effects on microbiota, intestinal barrier, immune response and visceral sensitivity[9-11]. Patient’s demographic characteristics, microbiota composition and metabolism as well as IBS subtype and patient’s adherence to diet in particular may affect the effectiveness of a low FODMAP diet[12]. Evidence suggests that microbiota plays a vital role in the pathophysiology of IBS[13]. The studies show that Lactobacillus and Bifidobacterium decrease while proinflammatory bacterial species, Enterobacteriaceae in particular, increase relatively in IBS[12]. It is thought that low FODMAP diet’s reducing colonic motility may harm bacterial flora. Hence, according to a study on the fecal microbiota comparing the patients on a low FODMAP diet and those on a regular diet, it was found that Bifidobacteria spp. rate and concentration decrease in those who followed a low FODMAP diet[14]. Another study showed that the rate of fecal bacteria in those who follow a low FODMAP diet is 47% less than those who follow an Australian-type diet, and there is an increase in the number of harmful bacteria with a decrease in beneficial bacteria[15].
However, there are also some studies showing that although a low FODMAP diet decreases the number of bacteria, it does not affect their diversity[16]. The low FODMAP diet positively affects IBS symptoms, but it may have adverse effects on colon health. The components of the FODMAP diet act as a substrate for bacteria[17]. Butyrate, a short-chain fatty acid formed as a result of bacterial fermentation, is not only an energy source for colon epithelium but also an important regulator and immunomodulator for colonocyte proliferation and apoptosis[18,19]. Recent studies have reported that a low FODMAP diet reduces proinflammatory cytokines such as interleukin 6 and interleukin 8. It also changes the content of fecal bacteria and decreases the amount of fecal butyric acid[12]. Furthermore, there is doubt regarding the long-term sustainability of this diet, its long-term effects on symptoms and possible nutritional deficiencies[3].
Probiotics balance intestinal flora by limiting the colonization of pathogenic bacteria[15]. As mentioned above, there is strong evidence suggesting that changes in fecal microbiota have an impact on IBS pathogenesis. Based on this argument, probiotics have been used in IBS treatment for a long time[20].
As is seen, the FODMAP diet and probiotics are used separately in IBS treatment. These are preferred treatments today thanks to their relatively high efficiency and low side effects. Combining both may enhance symptom control in patients. However, the negative impact of a low FODMAP diet on intestinal flora and its long-term side effects are still ambiguous. Furthermore, although the rate of adherence to diet is high, there is also a group of patients who cannot tolerate it. We added probiotics to the low FODMAP diet in our study considering that it may affect symptom control in IBS and that it has a potential to prevent possible harmful effects of the low FODMAP diet on the intestinal flora. This randomized double-blind prospective controlled study aimed to find out how probiotic supplementation to a low FODMAP diet affected symptoms in all subtypes of IBS.
MATERIALS AND METHODS
Patients and study design
This randomized double-blind prospective study included 100 patients between the ages of 18 and 65 years, who were admitted to the Gastroenterology Clinic of Faculty of Medicine of Dokuz Eylul University between December 1, 2017 and December 24, 2018, who were newly and/or previously diagnosed with IBS according to Rome IV criteria and whose IBS treatment were not modified in the last 4 wk. For both treatment groups, the mean effect size was determined as 0.5 for the comparison of IBS symptom severity scale (IBS-SSS) and Visual Analog Scale (VAS) scores, taking 50 patients for each group at 80% power and 95% confidence interval. Based on their IBS subtypes, the patients were classified as IBS-D (diarrhea dominant type), IBS-C (constipation dominant type) and IBS-mixed type. This prospective study was designed in compliance with the ethical rules set forth by the Helsinki Declaration, and we took the consent of all patients by asking them to fill in informed consent forms before the study. Patients were randomly assigned to two groups as follows: 21 d low FODMAP diet + probiotic (Group 1) and 21 d low FODMAP diet + placebo (Group 2). We tossed a coin to randomly categorize the patients into two groups, and neither the patient nor the researcher was informed about the treatment administered to the patient. When we ran out of the product in the probiotic or placebo group, the patient was given the product used in the other group.
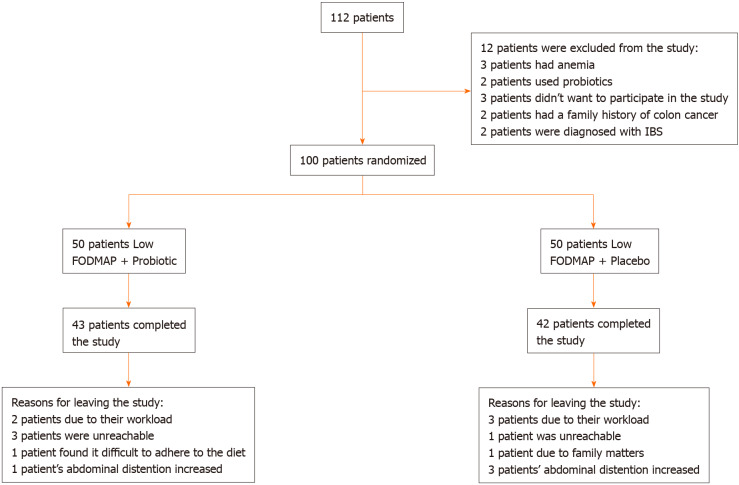
Patients with inflammatory bowel disease, celiac disease, history of bowel operation and diabetes mellitus who were currently using food supplements such as probiotics were excluded from the study. In addition, patients with alarm symptoms such as fever, anemia and weight loss, those who had a family history of colon cancer, patients whose body mass index was 18 and below and pregnant women were excluded from the study. Based on the above-mentioned exclusion criteria, 12 patients were excluded from the study (Figure 1).
Figure 1.
Study flow diagram. IBS: Irritable bowel syndrome; FODMAP: Fermentable oligo, di- and monosaccharides and polyols; IBD: Irritable bowel disease.
The demographic data (age, gender, occupation) of all patients were recorded, and they were asked in detail about their history of chronic diseases (hypertension, cerebrovascular disease, hypothyroidism, coronary artery disease, osteoporosis, asthma, depression, urinary incontinence, neuromuscular disorders, severe osteoarthritis), drugs they use, operations they had (thyroidectomy, tonsillectomy, appendectomy, etc.), dominant symptoms and treatments they received.
This study was conducted with the approval of the Clinical Research Ethics Committee of Dokuz Eylul University (approval no. 2017/01-02) and the Medicines and Medical Devices Agency of the Ministry of Health of Turkey (approval No. 67116).
Dietary advice and probiotic/placebo
An expert dietitian had a face-to-face conversation for at least 45 min with each patient to explain the diet to be followed throughout the study. Furthermore, patients were given the low FODMAP diet therapy (Table 1) and informed about the foods they must avoid (Supplementary material) to ensure that they consume sufficient amounts of calcium and fiber.
Table 1.
Content of low fermentable oligo, di- and monosaccharides and polyols diet recommended for patients
| Energy | 1600-2000 kcal/d |
| Protein | 75 -90 g/d |
| Fat | 75-85 g/d |
| Carbohydrate | 186-240 g/d |
| Starch | 120-25 g/d |
| Sugars | 50 -75 g/d |
| Non-starch polysaccharide | 13–16 g/d |
| Total FODMAPs1 | 9.0-9.6 g/d |
| Fructans | 2.5-3.5 g/d |
| GOS | 0.8-1.4 g/d |
| Lactose | 4.3-4.5 g/d |
| Total fructose | 12.7-5.9 g/d |
| Excess fructose | 1.9-2.0 g/d |
| Sorbitol | 0.3-0.5 g/d |
| Mannitol | 0.1 -0.2 g/d |
Total fermentable oligo, di- and monosaccharides and polyols (FODMAPs) are calculated as the sum of individual carbohydrates including excess fructose (not total fructose). Data are mean total and individual FODMAPs (g/d), energy (kcal/d) and nutrient intake (g/d). FODMAPs: Fermentable oligo, di- and monosaccharides and polyols; GOS: Galacto-oligosaccharides.
Nobel Ilac San. ve Tic. A.S. Istanbul, Turkey® provided probiotic and placebo drugs. As a probiotic, supplementary food containing probiotics (2 g) once a day was prescribed to patients. This product contained Streptococcus thermophilus (5.4x108 cfu), Bifidobacterium lactis (5.4 × 108 cfu), Lactobacillus acidophilus (4.5 × 108 cfu), Lactobacillus plantarum (4 × 108 cfu) and Bifidobacterium breve (4 × 108 cfu). Placebo products were identical capsules without any probiotics. The active and placebo products were similar in appearance, taste and smell and bottled in identically.
All patients were asked to keep a diary of the foods and beverages they consumed. Furthermore, they were called by phone every week to follow up with their adherence to diet in terms of probiotic/placebo compliance. We used the diaries of the patients to evaluate their adherence to the given diet. The percentage of cheatings within a diet during the day was calculated separately, and the daily dietary compliance was averaged. Patients who adhered to their diet for 17 d (> 80%) of the 21 d diet were considered to have adhered to their diet. The symptoms of patients were evaluated with the below-mentioned scale and scoring systems before and after 21 d of a low FODMAP diet + placebo or a low FODMAP diet + probiotic and the resulting data were analyzed. During the study, the patients were not allowed to use drugs that could affect their symptoms (laxatives, antidiarrheal drugs, etc.).
Evaluation of symptoms by questionnaires
For patients diagnosed with IBS according to Rome IV criteria, we used the following scales for evaluation: VAS to evaluate pain before and after 21 d of a low FODMAP diet + probiotic/placebo, Bristol Stool Scale to evaluate IBS type and any changes in stool and IBS-SSS to evaluate the severity of IBS symptoms.
The following results were accepted as clinical improvement after the evaluation of 21 d treatment: a decrease of at least 50 points in the IBS-SSS score, a decrease of more than 10 mm in pain severity according to VAS and change in stool characterization to Type 3 and Type 4 according to Bristol Stool Chart.
Statistical analysis
The SPSS 22.0 version (SPSS Inc, Chicago, IL, United States) package program was used to perform all statistical analyses. Demographic data, symptoms and IBS subtypes were analyzed descriptively. The Shapiro Wilk Test was used to test the compatibility of the numerical variables to normal distribution. Numerical variables were described with mean and standard deviation while categorical variables were described with frequency and percentage values. The Mann Whitney U Test was used to compare differences between two independent means. The relationship between two independent categorical variables was measured using the χ2 Test. The Wilcoxon Signed-rank Test was used to compare the relationship between two dependent means. The conformity between two dependent categorical variables was evaluated using the Kappa Test. The Mann Whitney U Test was used to determine the difference between two independent medians. The relationship between two independent numerical variables was analyzed using Spearman’s Correlation. The confidence level of the study was 95%, and the P value below 0.05 was considered to be significant.
RESULTS
Subjects
Fifteen patients (15%; 7 from Group 1, 8 from Group 2) out of 100 patients, who were included in the study, left the study during the follow-up period. The patients left the study due to the following reasons: workload, being unreachable by phone, increased bloating, family problems and difficulty in following the diet (Figure 1).
A total of 85 patients completed the study, 43 patients from Group 1 and 42 patients from Group 2. A total of 62 of them (72.9%) were female while 23 of them (27.1%) were male, and the mean age of the patients was 43.6 ± 13.0. Breakdown by gender shows that 33 patients (76.7%) in Group 1 and 29 patients (69.0%) in Group 2 were female. The mean age was 43.6 ± 12.2 in Group 1 and 43.6 ± 14.0 in Group 2, i.e. they were similar.
Some patients had comorbid diseases such as Hashimoto’s thyroiditis, hypertension and allergic asthma. In this study, 69.4% of the patients had a comorbid disease, while 30.6% did not have any comorbidities. A total of 35 (41.2%) patients underwent an abdominal operation, 19 (44.2%) of whom were in the probiotic group, while 16 (38.1%) of them were in the placebo group. The patients in both groups had similar demographic characteristics. Table 2 summarizes demographic data, comorbidities, history of treatments and drug use of all patients.
Table 2.
Patients’ demographic characteristics, comorbidities and treatments they received
|
|
Total, n = 85
|
Group 1, n = 43
|
Group 2, n = 42
|
P
value
|
| Age (mean ± SD) | 43.6 ± 13.0 | 43.6 ± 12.2 | 43.6 ± 14.0 | 0.888 |
| Gender, n (%) | ||||
| Female | 62 (72.9) | 33 (76.7) | 29 (69.0) | 0.425 |
| Male | 23 (27.1) | 10 (23.3) | 13 (31.0) | |
| Employment status, n (%) | ||||
| No | 49 (57.6) | 25 (58.1) | 24 (57.1) | 0.926 |
| Yes | 36 (42.4) | 18 (41.9) | 18 (42.9) | |
| Comorbid diseases | ||||
| Hashimoto’s thyroiditis, n (%) | ||||
| No | 67 (78.8) | 36 (83.7) | 31 (73.8) | 0.263 |
| Yes | 18 (21.2) | 7 (16.3) | 11 (26.2) | |
| Allergic asthma, n (%) | ||||
| No | 77 (90.6) | 36 (83.7) | 41 (97.6) | 0.058 |
| Yes | 8 (9.4) | 7 (16.3) | 1 (2.4) | |
| Hypertension, n (%) | ||||
| No | 72 (84.7) | 38 (88.4) | 34 (81.0) | 0.342 |
| Yes | 13 (15.3) | 5 (11.6) | 8 (19.0) | |
| Gastritis, n (%) | ||||
| No | 77 (90.6) | 37 (86.0) | 40 (95.2) | 0.265 |
| Yes | 8 (9.4) | 6 (14.0) | 2 (4.8) | |
| Hyperlipidemia, n (%) | ||||
| No | 78 (91.8) | 40 (93.0) | 38 (90.5) | 0.713 |
| Yes | 7 (8.2) | 3 (7.0) | 4 (9.5) | |
| Depression, n (%) | ||||
| No | 80 (94.1) | 41 (95.3) | 39 (92.9) | 0.676 |
| Yes | 5 (5.9) | 2 (4.7) | 3 (7.1) | |
| Comorbidity, n (%) | ||||
| No | 26 (30.6) | 12 (27.9) | 14 (33.3) | 0.587 |
| Yes | 59 (69.4) | 31 (72.1) | 28 (66.7) | |
| Abdominal surgery, n (%) | ||||
| No | 50 (58.8) | 24 (55.8) | 26 (61.9) | 0.568 |
| Yes | 35 (41.2) | 19 (44.2) | 16 (38.1) | |
| Drug use | ||||
| Systematic drug use, n (%) | ||||
| No | 28 (32.9) | 14 (32.6) | 14 (33.3) | 0.939 |
| Yes | 57 (67.1) | 29 (67.4) | 28 (66.7) | |
| PPI use, n (%) | ||||
| No | 67 (78.8) | 32 (74.4) | 35 (83.3) | 0.315 |
| Yes | 18 (21.2) | 11 (25.6) | 7 (16.7) | |
| Psychiatric drug use, n (%) | ||||
| No | 71 (83.5) | 34 (79.1) | 37 (88.1) | 0.262 |
| Yes | 14 (16.5) | 9 (20.9) | 5 (11.9) | |
| CCB use, n (%) | ||||
| No | 69 (81.2) | 36 (83.7) | 33 (78.6) | 0.544 |
| Yes | 16 (18.8) | 7 (16.3) | 9 (21.4) | |
| Beta-blocker use, n (%) | ||||
| No | 75 (88.2) | 38 (88.4) | 37 (88.1) | > 0.999 |
| Yes | 10 (11.8) | 5 (11.6) | 5 (11.9) | |
Group 1: Low fermentable oligo, di- and monosaccharides and polyol diet + probiotic; Group 2: Low fermentable oligo, di- and monosaccharides and polyol diet + placebo. PPI: Proton pump inhibitor; CCB: Calcium canal blocker; SD: Standard deviation.
The breakdown of IBS subtypes shows that the most common group is IBS-C, followed by IBS-D. The least common type is mixed IBS (Table 3).
Table 3.
Irritable bowel syndrome sub-type breakdown by groups
|
|
Total, n = 85
|
Group 1, n = 43
|
Group 2, n = 42
|
P
value
|
| IBS subtype, n (%) | ||||
| Constipation | 43 (50.6) | 23 (53.5) | 20 (47.6) | 0.415 |
| Diarrhea | 23 (27.1) | 9 (20.9) | 14 (33.3) | |
| Mixed | 19 (22.4) | 11 (25.6) | 8 (19.0) |
Group 1: Low fermentable oligo, di- and monosaccharides and polyol diet + probiotic; Group 2: Low fermentable oligo, di- and monosaccharides and polyol diet + placebo. IBS: Irritable bowel syndrome.
Gastrointestinal symptoms response
The VAS score, IBS-SSS total score and IBS-SSS sub-parameters measured before treatment were similar in both groups (Table 4).
Table 4.
Comparison of visual analogue scale and irritable bowel syndrome symptom severity scale scores before and after treatment
|
|
Group 1
|
|
|
Group 2
|
|
|
|
|
Before treatment
|
After treatment
|
P value |
Before treatment
|
After treatment
|
P value | P ’ value | |
|
mean ± SD
|
mean ± SD
|
mean ± SD
|
mean ± SD
|
|
|||
| VAS | 4.6 ± 2.7 | 2.0 ± 1.9 | < 0.001 | 4.7 ± 2.7 | 1.8 ± 2.0 | < 0.001 | 0.754 |
| IBS-SSS | 310.0 ± 78.4 | 172.0 ± 93.0 | < 0.001 | 317.0 ± 87.5 | 175.0 ± 97.7 | < 0.001 | 0.610 |
| Pain severity | 49.4 ± 24.1 | 24.4 ± 20.8 | < 0.001 | 46.4 ± 23.1 | 22.6 ± 19.8 | < 0.001 | 0.616 |
| Abdominal pain severity | 52.9 ± 23.9 | 26.7 ± 21.4 | < 0.001 | 53.6 ± 25.0 | 28.6 ± 23.1 | < 0.001 | 0.843 |
| Abdominal distention severity | 65.1 ± 25.1 | 34.3 ± 25.0 | < 0.001 | 68.5 ± 23.5 | 33.9 ± 24.0 | < 0.001 | 0.660 |
| Satisfaction with intestinal habits | 70.9 ± 24.9 | 41.6 ± 33.3 | < 0.001 | 73.5 ± 27.2 | 43.4 ± 31.6 | < 0.001 | 0.559 |
| IBS quality of life | 74.8 ± 22.1 | 44.6 ± 26.1 | < 0.001 | 75.0 ± 22.4 | 46.5 ± 23.3 | < 0.001 | 0.957 |
P’: Comparison between both groups before treatment. Group 1: Low fermentable oligo, di- and monosaccharides and polyols (FODMAP) diet + probiotic, Group 2: low fermentable oligo, di- and monosaccharides and polyols diet + placebo. VAS: Visual analogue scale; IBS-SSS: Irritable bowel syndrome symptom severity scale; IBS: Irritable bowel syndrome; SD: Standard deviation.
The average VAS score of the patients in Group 1 before treatment was 4.6 ± 2.7 with an average IBS-SSS score of 310.0 ± 78.4. The mean VAS score decreased to 2.0 ± 1.9, and the mean IBS-SSS score decreased to 172.0 ± 93.0. The VAS score, IBS-SSS total score and IBS-SSS sub-parameter scores of Group 1 significantly reduced after treatment (P < 0.001). In Group 1, the IBS-SSS score of 37 (86.04%) patients decreased by more than 50 points (Table 4).
The average VAS score of the patients in Group 2 before treatment was 4.7 ± 2.7 with an average IBS-SSS score of 317.0 ± 87.5. The mean VAS score decreased to 1.8 ± 2.0, and the mean IBS-SSS score decreased to 175.0 ± 97.7. The VAS score, IBS-SSS total score and IBS-SSS sub-parameter scores of Group 2 significantly reduced after treatment (P < 0.001). In Group 2, the IBS-SSS score of 36 (85.71%) patients decreased by more than 50 points (Table 4). In conclusion, abdominal pain and symptoms in the scope of IBS-SSS in both groups decreased significantly after treatment. Furthermore, no side effects were observed in any of the patients that were included in this study.
The results showed that the scores of 38 (79.2%) out of 48 patients who had severe IBS before treatment improved after treatment. In Group 1 and Group 2, the IBS-SSS scores of 20 (80.0%) and 18 (78.3%) patients out of 25 and 23 patients, respectively, with severe IBS decreased after treatment. When we evaluated the changes in the VAS and IBS-SSS scores of the patients before and after the treatment, we observed that the scores decreased significantly in both groups, and the scores decreased similarly in Group 1 and Group 2 (Tables 5 and 6).
Table 5.
Comparison of the change in visual analogue scale and irritable bowel syndrome symptom severity scale scores in both groups before and after treatment
|
Parameters
|
Total, n = 85
|
Group 1, n = 43
|
Group 2, n = 42
|
P
value
|
| VAS [Median (min-max)] | -50.0 [(-100.0)-40.0] | -50.0 [(-100.0)-0.0] | -58.3 [(-100.0)-40.0] | 0.778 |
| IBS-SSS[Median (min-max)] | -47.3 [(-100.0)-30.3)] | -43.5 [(-93.0)-0.0)] | -50.0 [(-100.0)-30.3) | 0.871 |
| Pain severity | -50.0 [(-100.0)-50.0] | -50.0 [(-100.0)-0.0] | -50.0 [(-100.0)-50.0] | 0.799 |
| Abdominal pain severity | -50.0 [(-100.0)-50.0] | -50.0 [(-100.0)-0.0] | -50.0 [(-100.0)-50.0] | 0.857 |
| Abdominal distention severity | -50.0 [(-100.0)-0.0] | -50.0 [(-100.0)-0.0] | -50.0 [(-100.0)-0.0] | 0.561 |
| Satisfaction with intestinal habits | -50.0 [(-100.0)-203.0] | -50.0 [(-100.0)-100.0] | -50.0 [(-100.0)-203.0] | 0.953 |
| IBS quality of life | -50.0 [(-100.0)-51.5] | -50.0 [(-100.0)-0.0] | -50.0 [(-100.0)-51.5] | 0.960 |
Group 1: Low fermentable oligo, di- and monosaccharides and polyols (FODMAP) diet + probiotic, Group 2: low fermentable oligo, di- and monosaccharides and polyols diet + placebo. VAS: Visual analogue scale; IBS-SSS: Irritable bowel syndrome symptom severity scale; IBS: Irritable bowel syndrome.
Table 6.
Irritable bowel syndrome symptom severity scale scores before and after treatment
| After treatment |
Before treatment, n (%)
|
Kappa | P value | |||
|
Mild
|
Moderate
|
Severe
|
||||
| All patients | Asymptomatic | 3 (50.0) | 2 (6.5) | 4 (8.3) | 0.021 | 0.648 |
| Mild | 3 (50.0) | 22 (71.0) | 17 (35.4) | |||
| Moderate | 0 (0) | 6 (19.4) | 17 (35.4) | |||
| Severe | 0 (0) | 1 (3.2) | 10 (20.8) | |||
| Group 1 | Asymptomatic | 2 (66.7) | 1 (6.7) | 2 (8.0) | 0.013 | 0.840 |
| Mild | 1 (33.3) | 11 (73.3) | 9 (36.0) | |||
| Moderate | 0 (0) | 3 (20.0) | 9 (36.0) | |||
| Severe | 0 (0) | 0 (0) | 5 (20.0) | |||
| Group 2 | Asymptomatic | 1 (33.3) | 1 (6.2) | 2 (8.7) | 0.031 | 0.649 |
| Mild | 2 (66.7) | 11 (68.8) | 8 (34.8) | |||
| Moderate | 0 (0) | 3 (18.8) | 8 (34.8) | |||
| Severe | 0 (0) | 1 (6.2) | 5 (21.7) | |||
Group 1: Low fermentable oligo, di- and monosaccharides and polyols (FODMAP) diet + probiotic, Group 2: low fermentable oligo, di- and monosaccharides and polyols diet + placebo.
When changes in stool shape were analyzed with Kappa according to the Bristol Stool Scale after treatment, it was found that there were significant changes in both groups. When the results of all patients were analyzed, stool type after treatment was evaluated as normal (type 3-4) in 19 out of 29 patients (65.5%) with stool type 5-6-7 (diarrhea predominant) and in 18 out of 34 patients (52.9%) with stool type 1-2 (constipation predominant). In Group 1, the stool type was evaluated as normal (type 3-4) after treatment in 9 (75.0%) out of 12 patients who had stool type 5-6-7 (IBS-D) and in 12 (70.6%) out of 17 patients who had stool type 1-2 (IBS-C). In Group 2, the stool type was evaluated as normal (type 3-4) after treatment in 10 (58.8%) out of 17 patients who had stool type 5-6-7 (IBS-D) and in 6 (35.3%) out of 17 patients who had stool type 1-2 (IBS-C) (Table 7). All patients were evaluated, and no severe side effects were observed except for increased bloating in 4 patients.
Table 7.
Comparison of stool changes in groups before and after treatment according to the Bristol stool scale
|
After treatment
|
Before treatment
|
Kappa
|
P
value
|
|||
|
Type 1, 2
|
Type 3, 4
|
Type 5-7
|
||||
| All patients | Type 1, 2 | 12 (35.3) | 2 (9.1) | 2 (6.9) | 0.175 | 0.006 |
| Type 3, 4 | 18 (52.9) | 16 (72.7) | 19 (65.5) | |||
| Type 5-7 | 4 (11.8) | 4 (18.2) | 8 (27.6) | |||
| Group 1 | Type 1, 2 | 4 (23.5) | 2 (14.3) | 1 (8.3) | 0.061 | 0.448 |
| Type 3, 4 | 12 (70.6) | 10 (71.4) | 9 (75.0) | |||
| Type 5-7 | 1 (5.9) | 2 (14.3) | 2 (16.7) | |||
| Group 2 | Type 1, 2 | 8 (47.1) | 0 (0) | 1 (5.9) | 0.26 | 0.005 |
| Type 3, 4 | 6 (35.3) | 6 (75.0) | 10 (58.8) | |||
| Type 5-7 | 3 (17.6) | 2 (25.0) | 6 (35.3) | |||
Group 1: Low fermentable oligo, di- and monosaccharides and polyols (FODMAP) diet + probiotic, Group 2: low fermentable oligo, di- and monosaccharides and polyols diet + placebo.
The rate of adherence of 85 patients, who completed the study, to the FODMAP restricted diet was 92%, being 90% in Group 1 and 94% in Group 2. There was no significant difference between these two groups (P = 0.066). All patients in the study completely adhered to their probiotic and placebo doses. Although there was no statistically significant relationship between the change in scores with increased adherence to the diet in Group 1, the enhanced dietary adherence in Group 2 was found to decrease the VAS scores, IBS-SSS total score, satisfaction with bowel habits and IBS quality of life sub-score significantly (Table 8).
Table 8.
The relationship between adherence to diet and changes in visual analogue scale and irritable bowel syndrome symptom severity scale scores
| Scores |
Group 1; adherence to diet
|
Group 2; adherence to diet
|
||
|
r
|
P
value
|
r
|
P
value
|
|
| VAS | 0.226 | 0.145 | 0.425 | 0.005 |
| IBS-SSS | 0.226 | 0.144 | 0.434 | 0.004 |
| Pain severity | 0.233 | 0.133 | 0.253 | 0.106 |
| Abdominal pain severity | 0.298 | 0.052 | 0.287 | 0.065 |
| Abdominal distention severity | 0.001 | 0.995 | 0.298 | 0.055 |
| Satisfaction with intestinal habits | 0.092 | 0.557 | 0.328 | 0.034 |
| IBS quality of life | 0.264 | 0.088 | 0.390 | 0.011 |
Group 1: Low fermentable oligo, di- and monosaccharides and polyols (FODMAP) diet + probiotic, Group 2: Low fermentable oligo, di- and monosaccharides and polyols diet + placebo. The discrepancy between the initial and final values of the evaluated scores (visual analogue scale, irritable bowel syndrome, etc.) was proportioned to the initial value to create a new variable called “percentage change from baseline” ((last value - initial value)/initial value). The correlation of this variable with dietary compliance in two different groups was investigated. The relationship between the improvement in other scores (numerical) and the diet compliance score (numerical) was investigated. The “r” values we received provide us with knowledge about the relationship’s course and power. VAS: Visual analogue scale; IBS-SSS: Irritable bowel syndrome symptom severity; IBS: Irritable bowel syndrome.
DISCUSSION
IBS is a chronic disease that reduces quality of life, and the desired results are still not achievable with the current treatment options. Therefore, there is an ongoing search for effective and long-term treatments. There are many studies showing that both a low FODMAP diet and probiotics alone have a positive effect on IBS symptoms[21-25]. However, there is a group of patients who cannot adhere to a low FODMAP diet and are therefore not treated successfully. There is also some evidence showing that a low FODMAP diet may have adverse effects on microbiota, and long-term use of this diet may have unintended consequences[26,27]. Adding probiotics to a low FODMAP diet may increase treatment success and have a positive effect on the unintended consequences on microbiota due to this dietary approach. From this point of view, this randomized double-blind prospective controlled study aimed to find out whether adding probiotics to a low FODMAP diet contributes to the success of treatment of all subtypes of IBS.
Visceral hypersensitivity due to luminal distension is an important cause of IBS-related gastrointestinal symptoms. A low FODMAP diet has a positive effect on such symptoms by decreasing fluid content in the intestinal lumen and hence gas production. Many clinical studies showed that a low FODMAP diet significantly relieved symptoms in approximately 50% to 80% of IBS patients[28-30].
Böhn et al[31] from Sweden included 75 patients diagnosed with IBS according to Rome III criteria in their multicenter, double-blind parallel-group study. They included 38 patients on a 4-wk-long FODMAP restricted diet, while 37 patients in the other group were asked to adhere to a different diet that is frequently recommended to patients with IBS. The severity of symptoms was evaluated by using IBS-SSS. The study concluded that there was a decrease in IBS symptoms in both groups (P < 0.0001 in both groups) with similar decrease rates (P = 0.62). In that study, the IBS-SSS score of 19 patients (50%) who followed a low FODMAP diet decreased by 50 points or more compared to their scores before the study. In our study, we also saw that the IBS-SSS score decreased more than 50 points compared to the baseline in 37 (86.04%) patients in Group 1 and in 36 (85.71%) patients in Group 2. In another randomized controlled trial study by Harvie et al[32], a total of 23 patients in the interventional group were asked to follow a low FODMAP diet (10.0 ± 7.9 g/d), while 27 patients in the control group were given a regular diet. It was found that there was a significant difference between changes in FODMAP content and relief of symptoms. Furthermore, a low FODMAP diet was proved to have a positive effect on patient quality of life. In our study, the patients were given 9 g of FODMAP on average per day, and the severity of symptoms decreased significantly. We did not use IBS Quality of Life in our study; still, significant improvement was achieved with a low FODMAP diet in both groups in the parameter that shows the quality of life parameter, which is a subgroup of the IBS-SSS scores (P < 0.01 in both groups).
If symptoms cannot be relieved with a diet, first of all, the patient’s adherence to diet must be examined. Adherence to a low FODMAP diet was reported to be 76% and 80% in two different studies that administered a diet for 3 wk and 6 wk, respectively[7,24]. According to the study of Shepherd et al[3], the adherence to a low FODMAP diet in the long term (14 mo) was reported to be 77%. In our study, we used a 3 wk long diet, adherence to which was 90% in Group 1 and 94% in Group 2. The fact that there was no difference between two groups with regards to adherence to diet shows that adding probiotics to a low FODMAP diet is not of additional benefit to patients’ adherence to the diet. These high adherence rates may be the result of the success of the diet in symptom control as well as the fact that the patients were adequately informed about their diet by an expert dietician. A dietician and a doctor also closely monitored them during the study. In their study investigating the efficiency of and adherence to a long-term low FODMAP diet in IBS patients, Weynants et al[33] stated that patients who visited a dietician multiple times could choose the right nutritional products easily, and their adherence to the diet was higher.
Dietary approaches for IBS generally aim to diversify patient’s nutritional intake as much as possible while controlling the symptoms at a maximum level with fewer restrictions[34]. While treating IBS, it is recommended to apply a low FODMAP diet in three stages. According to this recommendation, FODMAP is restricted in the initial stage, then FODMAP is reintroduced in the second stage, and the treatment is tailored to the needs of the patient in the last stage. The type of FODMAP containing food that triggers the symptoms and the amount of FODMAP in a diet may vary from person to person. Consequently, in the studies investigating the efficiency of a low FODMAP diet in the initial appointment in IBS, the amounts of FODMAP in diet are variable. Böhn et al[31] significantly controlled the symptoms with an average of 3.8 g of FODMAP per day, while Staudacher et al[28] achieved significant symptom control with a daily intake of 17.7 g of FODMAP. In our study, symptoms were relieved, and quality of life was enhanced in more than 85% of our patients with a daily average of 9 g of FODMAP. Over time, the FODMAP amount in diet can be modified and tailored to the needs of individual patients based on their symptom response and tolerance.
A FODMAP restricted diet may worsen constipation as it has low fiber content, and it decreases the amount of water in the small intestine[30]. However, the studies in which a low FODMAP diet was used reported symptom response in all subgroups of IBS including IBS-C[31,32,35]. In our study, out of all IBS cases, 53.5% of the patients in Group 1 and 47.6% in Group 2 were constipation predominant type, and symptom response was significant in both groups. Furthermore, both groups had a similar response rate. This finding supports the argument that a low FODMAP diet can be applied in all subtypes of IBS[6,7,31]. In the group of patients who took probiotics and had both IBS-D and IBS-C, the appearance of stool turned to be highly normal, although not statistically significant (kappa= 0.061, P = 0.448).
It is not clear how significant the changes are in microbiota after a low FODMAP diet. Our knowledge is restricted to the results of the short-term studies in which a low FODMAP diet was applied, and there is limited data on their long-term consequences. A 4 wk study on how a low FODMAP diet causes changes in microbiota and on the effects of probiotics showed that the proportion of total bacteria (Bifidobacterium species in particular) in the gastrointestinal lumen decreased[28]. This blind study included 104 patients, who were diagnosed with IBS according to Rome III criteria, and they were randomly categorized into four groups of sham diet, a low FODMAP diet, placebo and multistrain probiotic formulation. The amount of Bifidobacterium species in stool samples was significantly lower in patients who followed a low FODMAP diet (8.8 rRNA genes/g) when compared to the patients on sham diet (9.2 rRNA genes/g) (P = 0.008). The amount found in the probiotic group (9.1 rRNA genes/g) was significantly higher than the placebo group (8.8 rRNA genes/g) (P = 0.019), and it was seen that a low FODMAP diet did not affect microbiota diversity in fecal samples. In this study, symptom response rates were higher in the low FODMAP diet group and the sham diet group (57% and 38%, respectively, P = 0.051) according to the intention-to-treat analysis. The total mean IBS-SSS score was significantly lower in the low FODMAP diet group (173 ± 95) compared to the sham diet group (224 ± 89) (P = 0.001). At the same time, there was no difference between the probiotic group (207 ± 98) and the placebo group (192 ± 93), (P = 0.721).
We also found that a low FODMAP diet ensured significant improvement in IBS symptoms in all patients; however, adding probiotics to the diet did not have an additional effect on symptom response. VAS scores before treatment were 4.6 ± 2.7 and 4.7 ± 2.7 for Group 1 and Group 2, respectively, and these scores decreased to 2.0 ± 1.9 and 1.8 ± 2.0 after treatment (P < 0.001 and P < 0.001). Before treatment, the IBS-SSS scores were 310.0 ± 78.4 and 317.0 ± 87.5 for Group 1 and Group 2, respectively, which decreased to 172.0 ± 93.0 and 175.0 ± 97.7 after treatment (P < 0.001 and < 0.001). As is seen, symptoms were relieved significantly in both groups.
The multistrain probiotic preparation that was used in the study of Staudacher et al[28] contained Streptococcus thermophilus DSM 24731, Bifidobacterium breve DSM 24742, Bifidobacterium longum DSM 24736, Bifidobacterium infantis DSM 24737, Lactobacillus acidophilus DSM 24735, Lactobacillus plantarum DSM 24730, Lactobacillus paracasei DSM 24733 and Lactobacillus delbruckeii subsp. bulgaricus DSM 24734, while the probiotic preparation we used in our study contained Streptococcus thermophilus (5.4 × 108 cfu), Bifidobacterium lactis (5.4 × 108 cfu), Lactobacillus acidophilus (4.5 × 108 cfu), Lactobacillus plantarum (4 × 108 cfu) and Bifidobacterium breve (4 × 108 cfu). It was found that the multistrain probiotics used in both studies did not provide an additional positive effect on the symptoms. Staudacher et al[28] did not include IBS-C patients in their study. In our study, around half of the patients in both the probiotics and placebo groups had IBS-C. To the best of our knowledge, no study has been conducted on the effects of adding probiotics to a low FODMAP diet.
In their randomized unblinded controlled study, Pedersen et al[36] compared the efficiency of treatment with a 6 wk low FODMAP diet and Lactobacillus rhamnosus (LGG) in 123 patients with IBS. The study showed that the IBS-SSS score significantly decreased in all three groups (P < 0.001 for low FODMAP, P < 0.01 for LGG and P = 0.03 for the control group) after 6 wk of treatment. The scores decreased more in both low FODMAP and LGG groups compared to the control group. Considering the subtypes of IBS in this study, IBS-SSS score was significantly reduced with a low FODMAP diet and LGG in both IBS-D and IBS-mixed type groups (133 ± 122 vs 68 ± 107, 133 ± 122 vs 34 ± 95 respectively, P < 0.01), while there was no significant decrease in patients with IBS-C. In this study, the difference between results can be explained by the use of LGG and by the fact that the majority of the patients in this study were female patients with IBS-D.
Our study is a randomized controlled study that included all IBS subtypes and examined the effects of probiotic supplementation to a low FODMAP diet on IBS symptoms. Our study’s limitation is that we did not analyze stool microbiota before and after treatment of the patient group that received probiotics or placebo in addition to the FODMAP restricted diet.
CONCLUSION
In conclusion, a low FODMAP diet significantly relieved IBS symptoms in all IBS subtypes in the initial phase; however, adding probiotics to the diet did not make an additional contribution to symptom relief. Further longer term research using different probiotics is needed to examine the efficiency of probiotic supplementation to a low FODMAP diet in IBS.
ARTICLE HIGHLIGHTS
Research background
Irritable bowel syndrome (IBS) has a complicated pathogeny involving many factors. IBS patients apply to clinics again and again and undergo numerous examinations and procedures that may not be necessary at all. Among the tools for treating IBS, various diets, antispasmodics, loperamide, antidepressants, psychological therapies (psychotherapy, hypnosis, etc.), laxatives, biofeedback, prebiotics or probiotics can be used. Even though some or all of these treatments relieve the symptoms in some patients, some of the patients do not recover sufficiently and may need other treatment alternatives. Because the presence of a relationship between IBS and diet has been shown by various studies, some diet modifications are recommended to reduce symptoms. The elimination diet is a widely used treatment of IBS. Recently, a gradually increasing number of studies have shown a 50%-80% significant effect of a 4-8 wk low fermentable oligo, di- and mono-saccharides and polyols (FODMAP) diet on the symptoms of IBS patients. The efficacy of a low FODMAP diet may depend on factors such as the demographic characteristics, microbiota composition and metabolism besides the IBS subtype and particularly dietary adherence of the patients. There is evidence to suggest the vital role of microbiota in pathophysiology of IBS. The balance of the intestinal flora is corrected by probiotics by way of limiting the pathogenic bacteria colonization. Reasoning on this argument, probiotics have been used in treatment of IBS for a long time.
Research motivation
A low FODMAP diet and probiotics are used individually in IBS treatment. Today, they are preferable treatments due to their relatively high efficacy and few side effects. Combination of these two may improve symptom control in patients. However, the negative effects of a low FODMAP diet on intestinal flora and its long-term adverse effects are still inconclusive. Also, even though the dietary adherence rate is high, a group of patients still cannot tolerate it. Having the potential of preventing possible harmful effects of low FODMAP diet on the intestinal flora, adding probiotics into it may affect control of IBS symptoms.
Research objectives
This randomized double-blind prospective controlled study aimed to find out how probiotic supplementation to a low FODMAP diet affects symptoms in all subtypes of IBS.
Research methods
This double-blind randomized prospective study included 100 patients aged 18-65 newly and/or previously diagnosed with IBS as per the Rome IV criteria whose IBS treatment was not changed during the last 4 wk. According to their IBS subtypes, the patients were divided into subgroups categorized as IBS-diarrhea dominant type, IBS-constipation dominant type and IBS-mixed type. Patients were randomly assigned to two groups: 21 d low FODMAP diet + probiotic (Group 1) and 21 d low FODMAP diet + placebo (Group 2). A coin was tossed to categorize the patients into two groups randomly and neither the patient nor the researcher knew about the treatment administered to the patient. Each patient had a face-to-face explanatory conversation lasting at least 45 min with an expert dietitian about the diet to be followed throughout the trial. The provider of the probiotic and placebo drugs was Nobel Ilac San. ve Tic. A.S. Istanbul, Turkey®. Supplementary food containing probiotics (2 g) was prescribed to patients once a day as a probiotic. This product contained Streptococcus thermophilus (5.4 × 108 cfu), Bifidobacterium lactis (5.4 × 108 cfu), Lactobacillus acidophilus (4.5 × 108 cfu), Lactobacillus plantarum (4 × 108 cfu) and Bifidobacterium breve (4 × 108 cfu). Placebos were identical-looking capsules without any probiotics. All the participants were asked to record all the foods and beverages they consumed in a diary. Moreover, by weekly phone calls their dietary adherence in terms of probiotic/placebo compliance was monitored. Before and after applying a 21 d low FODMAP diet + placebo or a low FODMAP diet + probiotic, the symptoms of the patients were evaluated with the reference scale and scoring systems, and the results obtained were analyzed. We used the following scales to evaluate the patients: visual analogue scale (VAS) to evaluate pain before and after a 21 d low FODMAP diet + probiotic/placebo, Bristol Stool Scale to identify IBS type and any changes observed in stool and IBS-Symptom Severity Scale (IBS-SSS) to evaluate the severity of IBS symptoms. In the evaluation made after 21 d treatment, the following outcomes were acknowledged as clinical improvement: a decrease of at least 50 points in the IBS-SSS score, a decrease of more than 10 mm in pain severity according to VAS and change in stool characterization to Type 3 and Type 4 according to the Bristol Stool Chart.
Research results
A total of 85 patients, 43 from Group 1 and 42 from Group 2, completed the study to the end. IBS subtype breakdown showed that the most common subgroup was the constipation-predominant IBS, which was followed by diarrhea-predominant IBS. In both groups, the VAS score, IBS-SSS total score and IBS-SSS sub-parameters measured before treatment were similar. There was a significant decrease in the VAS score, IBS-SSS total score and IBS-SSS sub-parameter scores of Groups 1 and 2 after treatment (P < 0.001). The IBS-SSS score of 37 (86.04%) patients in Group 1 and 36 (85.71%) patients in Group 2 decreased by more than 50 points. In conclusion, in both groups abdominal pain and symptoms within the scope of IBS-SSS decreased significantly after treatment. Moreover, no side effects were observed in any of the patients that were included in this study. Treatment outcomes showed that the scores of 38 (79.2%) out of 48 patients who had severe IBS improved after treatment. Significant changes in stool shape were found in both groups when they were analyzed with Kappa index according to the Bristol Stool Scale after treatment. The average adherence rate of the 85 patients who completed the study to the FODMAP restricted diet was 92%, being 90% in Group 1 and 94% in Group 2. There was no significant difference between these two groups (P = 0.066).
Research conclusions
According to our findings, a low FODMAP diet significantly relieved IBS symptoms in all IBS subtypes at the initial phase; however, adding probiotics to the diet made no additional contributions to symptom relief.
Research perspectives
The limitation of our study was that we did not analyze stool microbiota of the patient group that received probiotics or placebo in addition to the FODMAP restricted diet before and after treatment. Further and longer term research using different probiotics is needed to assess the efficacy of probiotic supplementation to a low FODMAP diet in IBS.
ACKNOWLEDGEMENTS
Our dietitian Ferya Bertan met with patients face-to-face during the study and organized the diet treatment. Unfortunately, we are so sorry she passed away in a traffic accident after the study completion. We dedicate this work to her. We also thank Professor Pembe Keskinoğlu and Tolga Cevizci for their assistance with statistical analysis.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Institutional Ethics committee of Dokuz Eylul University Izmir.
Clinical trial registration statement: This study was conducted with the approval of the Clinical Research Ethics Committee of Dokuz Eylul University (approval No. 2017/01-02) and the Medicines and Medical Devices Agency of the Ministry of Health of Turkey (approval No. 67116).
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare that there are no conflicts of interest.
CONSORT 2010 statement: The authors have read the CONSORT 2010 Statement, and the manuscript was prepared and revised according to the CONSORT 2010 Statement.
Manuscript source: Unsolicited manuscript
Peer-review started: April 29, 2021
First decision: May 26, 2021
Article in press: July 16, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang YJ S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Liu JH
Contributor Information
Beril Turan, Department of Internal Medicine, Dokuz Eylül University, Izmir 35000, Turkey.
Göksel Bengi, Department of Gastroenterology, Dokuz Eylul University Izmir, Izmir 35000, Turkey.
Ruksan Cehreli, Department of Preventive Oncology, Dokuz Eylül University, Institute of Oncology, İzmir 35000, Turkey.
Hale Akpınar, Department of Gastroenterology, Dokuz Eylul University Izmir, Izmir 35000, Turkey.
Müjde Soytürk, Department of Gastroenterology, Dokuz Eylul University Izmir, Izmir 35000, Turkey. soyturkmuj@gmail.com.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author at soyturkmuj@gmail.com. Participants gave informed consent for data sharing.
References
- 1.Tanaka Y, Kanazawa M, Fukudo S, Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:131–139. doi: 10.5056/jnm.2011.17.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson WG. Irritable bowel syndrome: a management strategy. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:453–460. doi: 10.1053/bega.1999.0039. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106:1631–1639. doi: 10.1016/j.jada.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Longstreth GF, Wilson A, Knight K, Wong J, Chiou CF, Barghout V, Frech F, Ofman JJ. Irritable bowel syndrome, health care use, and costs: a U.S. managed care perspective. Am J Gastroenterol. 2003;98:600–607. doi: 10.1111/j.1572-0241.2003.07296.x. [DOI] [PubMed] [Google Scholar]
- 5.Locke GR 3rd. The epidemiology of functional gastrointestinal disorders in North America. Gastroenterol Clin North Am. 1996;25:1–19. doi: 10.1016/s0889-8553(05)70362-9. [DOI] [PubMed] [Google Scholar]
- 6.Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) vs standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. 2011;24:487–495. doi: 10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 7.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 8.Barrett JS. Extending our knowledge of fermentable, short-chain carbohydrates for managing gastrointestinal symptoms. Nutr Clin Pract. 2013;28:300–306. doi: 10.1177/0884533613485790. [DOI] [PubMed] [Google Scholar]
- 9.Ravich WJ, Bayless TM, Thomas M. Fructose: incomplete intestinal absorption in humans. Gastroenterology. 1983;84:26–29. [PubMed] [Google Scholar]
- 10.Fernandez-Bañares F, Esteve-Pardo M, Humbert P, de Leon R, Llovet JM, Gassull MA. Role of fructose-sorbitol malabsorption in the irritable bowel syndrome. Gastroenterology. 1991;101:1453–1454. doi: 10.1016/0016-5085(91)90112-x. [DOI] [PubMed] [Google Scholar]
- 11.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang X, Xiong L, Li L, Li M, Chen M. Alterations of gut microbiota in patients with irritable bowel syndrome: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:28–38. doi: 10.1111/jgh.13471. [DOI] [PubMed] [Google Scholar]
- 13.Rodiño-Janeiro BK, Vicario M, Alonso-Cotoner C, Pascua-García R, Santos J. A Review of Microbiota and Irritable Bowel Syndrome: Future in Therapies. Adv Ther. 2018;35:289–310. doi: 10.1007/s12325-018-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142:1510–1518. doi: 10.3945/jn.112.159285. [DOI] [PubMed] [Google Scholar]
- 15.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64:93–100. doi: 10.1136/gutjnl-2014-307264. [DOI] [PubMed] [Google Scholar]
- 16.Harvie RM, Chisholm AW, Bisanz JE, Burton JP, Herbison P, Schultz K, Schultz M. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs. World J Gastroenterol. 2017;23:4632–4643. doi: 10.3748/wjg.v23.i25.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook SI, Sellin JH. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998;12:499–507. doi: 10.1046/j.1365-2036.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 18.Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424–429. [PubMed] [Google Scholar]
- 19.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 20.Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21:3072–3084. doi: 10.3748/wjg.v21.i10.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765–771. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 22.Pérez y López N, Torres-López E, Zamarripa-Dorsey F. Clinical response in Mexican patients with irritable bowel syndrome treated with a low diet low in fermentable carbohydrates (FODMAP) Rev Gastroenterol Mex. 2015;80:180–185. doi: 10.1016/j.rgmx.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 24.de Roest RH, Dobbs BR, Chapman BA, Batman B, O'Brien LA, Leeper JA, Hebblethwaite CR, Gearry RB. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract. 2013;67:895–903. doi: 10.1111/ijcp.12128. [DOI] [PubMed] [Google Scholar]
- 25.Nikfar S, Rahimi R, Rahimi F, Derakhshani S, Abdollahi M. Efficacy of probiotics in irritable bowel syndrome: a meta-analysis of randomized, controlled trials. Dis Colon Rectum. 2008;51:1775–1780. doi: 10.1007/s10350-008-9335-z. [DOI] [PubMed] [Google Scholar]
- 26.Rajilić-Stojanović M, Jonkers DM, Salonen A, Hanevik K, Raes J, Jalanka J, de Vos WM, Manichanh C, Golic N, Enck P, Philippou E, Iraqi FA, Clarke G, Spiller RC, Penders J. Intestinal microbiota and diet in IBS: causes, consequences, or epiphenomena? Am J Gastroenterol. 2015;110:278–287. doi: 10.1038/ajg.2014.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Liang L, Deng H, Guo J, Shu H, Zhang L. Efficacy and Safety of Probiotics in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Front Pharmacol. 2020;11:332. doi: 10.3389/fphar.2020.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staudacher HM, Lomer MCE, Farquharson FM, Louis P, Fava F, Franciosi E, Scholz M, Tuohy KM, Lindsay JO, Irving PM, Whelan K. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology. 2017;153:936–947. doi: 10.1053/j.gastro.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Altobelli E, Del Negro V, Angeletti PM, Latella G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients. 2017;9 doi: 10.3390/nu9090940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whelan K, Martin LD, Staudacher HM, Lomer MCE. The low FODMAP diet in the management of irritable bowel syndrome: an evidence-based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J Hum Nutr Diet. 2018;31:239–255. doi: 10.1111/jhn.12530. [DOI] [PubMed] [Google Scholar]
- 31.Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, Törnblom H, Simrén M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149:1399–1407.e2. doi: 10.1053/j.gastro.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 32.Harvie R, Schultz M, Chisholm A. A reduction in FODMAP intake corralates strongly with a reduction in IBS-symptoms-the FIBS study. J Gastroenterol Hepatol. 2013;28:623–693. [Google Scholar]
- 33.Weynants A, Goossens L, Genetello M, De Looze D, Van Winckel M. The long-term effect and adherence of a low fermentable oligosaccharides disaccharides monosaccharides and polyols (FODMAP) diet in patients with irritable bowel syndrome. J Hum Nutr Diet. 2020;33:159–169. doi: 10.1111/jhn.12706. [DOI] [PubMed] [Google Scholar]
- 34.Halmos EP, Gibson PR. Controversies and reality of the FODMAP diet for patients with irritable bowel syndrome. J Gastroenterol Hepatol. 2019;34:1134–1142. doi: 10.1111/jgh.14650. [DOI] [PubMed] [Google Scholar]
- 35.Gravina AG, Dallio M, Romeo M, Di Somma A, Cotticelli G, Loguercio C, Federico A. Adherence and Effects Derived from FODMAP Diet on Irritable Bowel Syndrome: A Real Life Evaluation of a Large Follow-Up Observation. Nutrients. 2020;12 doi: 10.3390/nu12040928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen N, Andersen NN, Végh Z, Jensen L, Ankersen DV, Felding M, Simonsen MH, Burisch J, Munkholm P. Ehealth: low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J Gastroenterol. 2014;20:16215–16226. doi: 10.3748/wjg.v20.i43.16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author at soyturkmuj@gmail.com. Participants gave informed consent for data sharing.