Abstract
BACKGROUND
To date, no treatment has proven to be absolutely effective for coronavirus disease 2019 (COVID-19) patients, and further research is necessary. As a traditional antiviral drug, arbidol was widely used in Wuhan at the beginning of the COVID-19 epidemic and is of increasing interest for treating COVID-19 based on in vitro data suggesting activity against severe acute respiratory syndrome (SARS). Although arbidol has been widely used in China to treat COVID-19 patients, clinical trials to date have not clearly substantiated this approach.
AIM
To evaluate the efficacy of arbidol for COVID-19.
METHODS
A retrospective study was conducted on 132 moderate and severe COVID-19 patients admitted to Jinyintan Hospital and Huoshenshan Hospital (officially designated for COVID-19 treatment) from February to March 2020 in Wuhan, China. This study mainly evaluated the efficacy of arbidol in patients with COVID-19 in the early stage of the SARS coronavirus 2 epidemic. Arbidol was administered at a dose of 200 mg, three times a day, with a 10-d course to adults not receiving any other drugs. Due to the shortage of beds at the time, not every patient could be admitted immediately. We looked for the early stages of the sudden outbreak, places of limited medical resources, limited ward beds, and delayed admission; thus, some patients naturally fit into the control group who did not receive any antiviral drugs. Out of the 132 patients, 72 received arbidol treatment, and 60 did not. We compared the disease course of the two groups and explored the predictors of extended disease duration.
RESULTS
Seventy-two patients commenced arbidol, and 60 patients did not receive arbidol treatment. The disease duration in the former group was shorter (23.42 ± 6.92 vs 29.60 ± 6.49, P < 0.001). Multivariate regression analysis showed that the risk of a prolonged course of disease increased by 7.158 times in the non-arbidol treatment group. Ferritin > 483.0 ng/mL and lactate dehydrogenase (LDH) > 237.5 U/L were found to be independent risk factors for protracted cases, with the risk of an extended disease duration increasing to 2.852 times and 5.946 times, respectively.
CONCLUSION
The duration course of moderate and severe COVID-19 patients is reduced by 6.183 d when arbidol is administered. Ferritin > 486.5 ng/mL and LDH > 239.5 U/L are independent risk factors for delayed recovery from COVID-19. Early oral administration of arbidol 200 mg t.i.d. with a 10-d course of treatment may be an effective management strategy in COVID-19 patients, particularly those with increased serum ferritin or elevated LDH.
Keywords: COVID-19, SARS-CoV-2, Arbidol, Treatment, Antiviral
Core Tip: This study indicated that the duration course of moderate and severe coronavirus disease 2019 (COVID-19) patients is reduced by 6.183 d when arbidol is administered. Ferritin > 486.5 ng/mL and lactate dehydrogenase (LDH) > 239.5 U/L are independent risk factors for delayed recovery from COVID-19. Early oral administration of arbidol 200 mg t.i.d. with a 10-d course of treatment may be an effective management strategy in COVID-19 patients, particularly those with increased serum ferritin or elevated LDH.
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in December 2019 and rapidly spread worldwide. The evidence gathered from the collective battle to prevent and contain the coronavirus disease 2019 (COVID-19) pandemic for more than a year has shown that most COVID-19 infections clinically manifest with headache, loss of sense of smell, nasal congestion, and malaise. Hospitalization is generally not required unless there is fever or abnormal findings on clinical imaging[1,2]. Approximately 1/5 to 1/10 of patients with old age or an underlying condition will require inpatient admission[3,4]. The intensive care unit (ICU) admission rate is 5% to 32%, and the mortality rate is 26%. Independent risk factors for mortality include old age, male sex, chronic comorbidities, and obesity[3,5]. Currently, there is no effective treatment for COVID-19. The commonly used drugs and efficacy of antiviral therapy remain controversial[6]. Arbidol is a promising repurposed antiviral agent, with a unique mechanism of action targeting the S protein/ACE2 interaction and inhibiting membrane fusion of the viral envelope[7]. Arbidol can be easily administered orally at home with few noted side effects and costs only US $50 for 10 d of treatment. The agent is currently approved in Russia and China for the treatment and prophylaxis of influenza and is of increasing interest for treating COVID-19 based on in vitro data suggesting activity against SARS[8]. Although arbidol has been widely used in China to treat COVID-19 patients, clinical trials to date have not clearly substantiated this approach[9].
Therefore, this study collected data on the efficacy of arbidol treatment in 132 moderate and severe COVID-19 patients from Jinyintan Hospital and Huoshenshan Hospital in Wuhan, China. As the pandemic in China was brought effectively under control in April 2020 with a significant decline in new cases, a retrospective study was conducted from February to March 2020 on the treatment of COVID-19 using arbidol.
MATERIALS AND METHODS
Case definition
Diagnostic criteria: Reverse transcriptase-polymerase chain reaction (RT-PCR) was used for testing SARS-CoV-2 positivity. Clinical classifications were as follows: Mild clinical forms had no evidence of pneumonia on computed tomography (CT) imaging; moderate clinical forms, including fever, respiratory symptoms, and pneumonia lesions, could be found on radiology imaging. The inclusion criteria for severe adult patients were: (1) Shortness of breath and respiration rate ≥ 30 times/m; (2) oxygen saturation (SPO2) ≤ 93% at rest; and (3) arterial blood oxygen partial pressure (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa), and pulmonary imaging showed marked lesion progression > 50% within 24-48 h. Critically ill patients must meet one of the following conditions: (1) Onset of respiratory failure and requiring mechanical ventilation; (2) shock symptoms; and (3) multiple organ failure requiring ICU monitoring and management.
Participants
Patients from Jinyintan Hospital and Huoshenshan Hospital, two infectious disease hospitals in Wuhan, China, were recruited for the study. Overall, 132 patients above 18 years old who met the diagnostic criteria for moderate to severe presentation of COVID-19 and the arbidol treatment program were included[9]. Mild clinical forms and critically ill patients were excluded.
Data collection
The study included demographics, chest CT, blood test results, time to commence arbidol treatment during the disease, and duration of the disease. All patients received treatment in isolation wards. Vital signs, oxygen saturation, biochemistry, full blood count, C-reactive protein (CRP), procalcitonin (PCT), erythrocyte sedimentation rate (ESR), ferritin, serum amyloid A, and other blood tests and chest imaging were closely monitored. The course of the disease was defined as the number of days since the onset of illness as per the patient’s chief complaint plus the number of days for admission.
RT-PCR assay
RT-PCR was used for virus detection from nasopharyngeal swabs, sputum or other lower respiratory tract secretions, blood, and feces.
Ethics statement
The institutional ethics board of Jinyintan Hospital approved this study. Due to the nature of retrospective chart review, the need for informed consent from individual patients was waived (No. KY-2020-71.01).
Treatment plan
The treatment plan was determined by the doctor on duty, and the directions for arbidol use were 200 mg for adults, three times a day, with a 10-d course of treatment and not receiving any other antiviral drugs. We compared the disease course of the two groups and explored the predictors of long disease duration.
Discharge criteria
Patients who met the following conditions were discharged: (1) Body temperature returned to normal for more than 3 d; (2) significant reduction of respiratory symptoms; and (3) pulmonary imaging showed significant improvement in acute exudative lesions and two consecutive sputum and NP swabs and other respiratory specimens tested negative for nucleic acid (with an interval of 24 h+).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics (version 23.0) to compare the baseline data of the two groups and the disease course. Various factors of long and short disease duration were compared. An independent sample t test was used to compare the normally distributed continuous variables (mean ± SD), and continuous variables not fully conforming to a normal distribution (median, IQR) were compared using the Mann-Whitney U test. Categorical variables are expressed as numbers (%) and were compared by χ² test or Fisher’s exact test.
The receiver operating characteristic (ROC) curve was used to calculate the cutoff points of various chance factors in long disease duration cases. We used single and multifactor analyses to study the independent risk factors that affect the course and duration of disease. A two-tailed α of less than 0.05 was considered statistically significant.
RESULTS
Effects of using arbidol
To analyze the effects of arbidol, we formed two study groups: An intervention group (72 patients who used arbidol) and a control group (60 patients who did not receive any antiviral drugs). The mean duration of the disease course was 25.73 ± 7.45 d. The patients in the arbidol group had a shorter course of disease than the patients in the non-antiviral treatment group (23.42 ± 6.92 vs 29.60 ± 6.49, P < 0.001). It took less time for two nucleic acid testing results to turn negative (21.61 ± 6.95 vs 27.59 ± 6.39, P < 0.001) and for chest CT improvement in the intervention group (18.33 ± 5.94 vs 24.28 ± 5.35, P < 0.001) (Table 1).
Table 1.
Comparison of recovery time of various indicators between arbidol therapy group and non–antiviral treatment control group
|
|
Arbidol treatment (n = 72)
|
None-antiviral treatment (n = 60)
|
P
value
|
Difference (d)
|
| Disease duration (d)1 | 23.42 ± 6.92 | 29.60 ± 6.49 | < 0.001 | 6.183 |
| Days before 2 NAT negative results1 | 21.61 ± 6.95 | 27.59 ± 6.39 | < 0.001 | 5.983 |
| Days before significantly improved chest CT outcome1 | 18.33 ± 5.94 | 24.28 ± 5.35 | < 0.001 | 5.949 |
t test. NAT: Nucleic acid testing; CT: Computed tomography.
Demographic characteristics
The baseline characteristics of patients in the intervention group and the non-antiviral treatment group were similar. No significant differences were found in the moderate/severe ratio, age, sex, underlying disease, comorbidities (abnormal heart and liver function, abnormal creatinine), LDH, white blood cell count, lymphocyte count, CRP, ESR, ferritin, IL-6, PCT, or serum amyloid A (P > 0.05) (Table 2).
Table 2.
Baseline data of patients, n (%)
|
|
Arbidol treatment (n = 72)
|
No antiviral treatment (n = 60)
|
P
value
|
| Age (yr) | 58.5 (45-65.8) | 58.5 (52-66) | 0.459 |
| Male gender2 | 46 (63.9) | 35 (58.3) | 0.514 |
| Underling illnesses2 | 23 (31.9) | 17 (28.3) | 0.653 |
| Abnormal liver function2 | 29 (40.3) | 22 (33.7) | 0.671 |
| Abnormal heart function3 | 3 (4.2) | 3 (5) | 0.819 |
| Abnormal creatinine3 | 1 (1.4) | 1 (1.7) | 0.896 |
| Lactate dehydrogenase1 | 202.5 (159-263) | 255 (191-265) | 0.054 |
| Leukocyte count1 | 4.90 (4.36-6.13) | 5.16 (4.10-6.88) | 0.654 |
| Lymphocyte count1 | 1.24 (1.14-1.33) | 1.135 (0.86-1.55) | 0.076 |
| C-reactive protein1 | 8.65 (3.175-.63.3) | 14.4 (3-67.7) | 0.805 |
| Erythrocyte sedimentation rate1 | 35 (8-57) | 32 (6-58) | 0.076 |
| Ferritin1 | 508 (320-672) | 605.5 (380-935.5) | 0.092 |
| Interleukin-61 | 8 (5.71-12) | 7.18 (6-8.62) | 0.337 |
| Procalcitonin (abnormal/normal)3 | 1 (2.7) | 0 (0) | > 0.999 |
| Serum amyloid A1 | 51.81 (18.33-218) | 65 (7.75-169.13) | 0.443 |
| Moderate cases2 | 51 (70.8) | 42 (70.0) | 0.917 |
| Severe cases2 | 21 (29.2) | 18 (30.0) | 0.917 |
Mann–Whitney U test.
Chi-square test.
Fisher’s exact test. Normal range of indicators: Lactate dehydrogenase 120-250 U/L, leukocyte count 3.5-9.5 × 109/L, lymphocyte count 1.1-3.2 × 109/L, procalcitonin < 0.5 ng/mL, serum amyloid A 0-10 mg/L, erythrocyte sedimentation rate 0-20 mm/h, C-reactive protein 0-5 mg/L, ferritin 4.63-204 ng/mL, and interleukin-6 0-7 pg/mL. Abnormal liver function: Alanine amiotransferase or aspartate aminotransferase is higher than the normal range. Abnormal cardiac function: Creatine kinase-MB, troponin I, or brain natriuretic peptide is above the normal range or combined with shortness of breath, oliguria, and edema. Abnormal renal function: Creatinine is higher than normal.
Independent risk factors for long course of disease
The mean duration of the disease course was 25.73 ± 7.45 d, and using this value as the cutoff, the groups were further divided into a short disease duration group [74 (56.1%) patients with disease duration < 25.7 d] and a long disease duration group [58 (43.9%) patients with disease duration > 25.7 d].
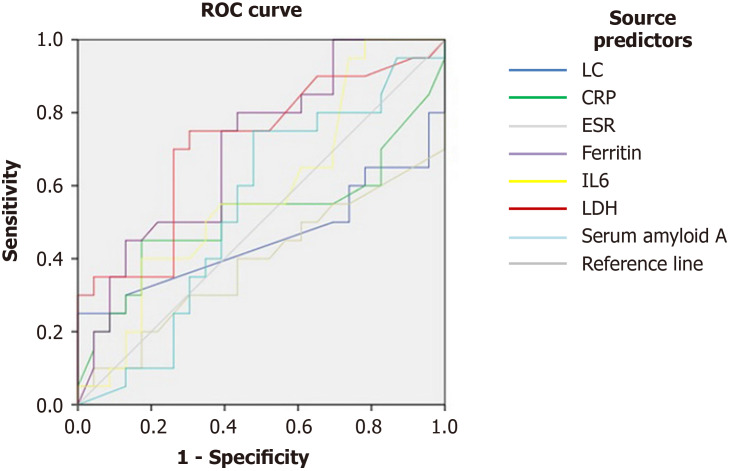
Taking into consideration the characteristics of various factors shown in the long disease duration ROC curve, we found that the LDH cutoff value of the long duration group was 239.5 U/L, the area under the ROC curve was 0.707, (0.595-0.820, P = 0.001), the sensitivity was 75%, and the specificity was 67.4%; the corresponding values of ferritin were 486.5 ng/mL, 0.708 (0.599-0.816, P = 0.001), 80%, and 56.5%. P-values of several other factors were greater than 0.05, offering little value in ascertaining the duration of the disease (Figure 1, Table 3).
Figure 1.
Receiver operating characteristic curves for predictors of long disease duration. ROC: Receiver operating characteristic; CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate; LDH: Lactate dehydrogenase.
Table 3.
Indicators in receiver operating characteristic curve
|
Test variable
|
AUC
|
95%CI
|
Cut-off
|
P
value
|
Sensitivity
|
Specificity
|
Youden's index
|
|
|
Lower range
|
Upper range
|
|||||||
| LC | 0.424 | 0.294 | 0.555 | 1.735 | 0.227 | |||
| CRP | 0.517 | 0.384 | 0.649 | 66.95 | 0.792 | |||
| ESR | 0.416 | 0.292 | 0.540 | 77.65 | 0.180 | |||
| Ferritin | 0.708 | 0.599 | 0.816 | 486.5 | 0.001 | 80% | 56.5% | 0.365 |
| IL6 | 0.597 | 0.476 | 0.718 | 5.935 | 0.123 | |||
| LDH | 0.707 | 0.595 | 0.820 | 239.5 | 0.001 | 75% | 67.4% | 0.424 |
| Serum amyloid A | 0.533 | 0.409 | 0.658 | 45.10 | 0.594 | |||
AUC: Area under curve; CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate; IL-6: Interleukin-6; LDH: Lactate dehydrogenase.
After evaluating variables by single-factor analysis of variance, we found that high LDH, high ferritin, and no arbidol treatment were risk factors for a long course of disease. Further multifactor analysis of variance confirmed that intervention, ferritin > 486.5 ng/mL, and LDH > 239.5 U/L were independent risk factors for a long course of disease (Table 4).
Table 4.
Single and multifactor analyses of variance identify high lactate dehydrogenase, ferritin, and no arbidol treatment as independent risk factors for long disease duration
|
|
Single factor
|
Multi factor
|
||
|
OR (95%CI)
|
P
value
|
OR (95%CI)
|
P
value
|
|
| Nontreatment | 8.167 (3.731-17.874) | < 0.001 | 7.158 (2.608-19.648) | < 0.001 |
| Ferritin (> 486.5 ng/mL) | 4.368 (1.895-10.066) | 0.001 | 2.852 (1.004-8.099) | 0.049 |
| LDH (> 239.5 U/L) | 8.167 (3.731,17.874) | < 0.001 | 5.946 (2.171-16.285) | 0.001 |
| Age (> 60 yr) | 0.699 (0.334-1.338) | 0.255 | ||
| Male gender | 0.628 (0.310-1.273) | 0.197 | ||
| Underlying disease (yes vs none) | 1.421 (0.674-2.996) | 0.356 | ||
| Abnormal liver function1 (yes vs none) | 1.229 (0.607-2.487) | 0.567 | ||
| Abnormal heart function2 (yes vs none) | 0.242 (0.027-2.132) | 0.201 | ||
| Abnormal creatinine2 (yes vs none) | 1.281 (0.078-20.922) | 0.862 | ||
Chi-square test.
Fisher’s exact test. LDH: Lactate dehydrogenase; OR: Odds ratio.
DISCUSSION
All positive-strand RNA viruses of eukaryotes recombine the inner cell membrane to produce specific viral replication organelles. The broad-spectrum antiviral activity of arbidol indicates that it weakens the viral replication binding protein in the inner cell membrane[10]. In addition, a study indicated that SARS-CoV-2 executes the fusion of ACE2 on the surface of the cell membrane through the envelope spike protein. Molecular dynamics and structural studies have confirmed that the spike glycoprotein of SARS-CoV-2 is the drug target of arbidol. This shows that arbidol prevents the trimerization of the spike protein and inhibits host cell adhesion, making arbidol a potential repurposed drug[11].
Therefore, arbidol was widely used as an antiviral agent in the treatment of COVID-19 patients during the early epidemic in China[12]. However, there is no evidence that arbidol treatment is associated with improved outcomes, but some studies suggest that it tends to shorten the duration of positive RNA tests and increase the negative conversion rate[13]. Therefore, the role of arbidol in the treatment of COVID-19 is controversial.
This was a retrospective study of clinical data gathered from 132 patients diagnosed with COVID-19, including moderate to severe cases. The research data were collected by doctors on aid missions to Hubei province during the Wuhan lockdown. This data analysis represents the first batch of COVID-19 patients amidst the later global outbreak, as doctors from all over the country were called upon to leave Wuhan for their own provinces in order to achieve effective control; thus, only 2 to 3 mo of data were collected. Although the sample size of this study was relatively small, it contains real data on the actual effect of the use of arbidol against COVID-19 worldwide.
Due to the shortage of beds at the time, not every patient could be admitted immediately. Of the 132 patients, 72 received arbidol treatment, and 60 did not. The results of this study suggest that the duration course of COVID-19 was reduced by 6.183 days when arbidol was administered. This indicates the vital importance of the timing of administration for antiviral therapy. Since China exerted strict control over the pandemic, it was impossible to artificially set a control group for COVID-19 patients who opted not to have any antiviral treatment after they were admitted to the hospital. Therefore, we looked for the early stages of the outbreak of the epidemic, places of limited medical resources, limited ward beds, and delayed admission. As such, we were able to identify some patients who naturally fit into the control group because they did not receive any antiviral drugs. The results of the study show that not using arbidol could increase the risk of prolonging the course of COVID-19 to 7.158 times. This suggests that patients may benefit from treatment with arbidol. None of the 72 patients in this study developed any side effects following arbidol use, suggesting that high drug safety may make arbidol a suitable drug for at home use by patients.
In our study, the mean disease duration of COVID-19 was 25.73 ± 7.45 d. According to the characteristics of long disease duration in the ROC curve, ferritin > 486.5 ng/mL and LDH > 239.5 U/L were independent risk factors for a long course of disease. The risks for long duration increased to 2.582 times and 5.946 times, respectively.
In addition, a randomized controlled study of 100 COVID-19 patients included 50 patients in the hydroxychloroquine followed by KALETRA (lopinavir/ritonavir) group and 50 patients in the hydroxychloroquine followed by arbidol group. The length of hospital stay in the arbidol group was significantly less than that in the KALETRA group (7.2 d vs 9.6 d, P = 0.02). The results of the relevant parts of this report are quite similar[12].
CONCLUSION
This study indicates that the duration course of COVID-19 patients with moderate and severe cases is reduced by 6.183 d when arbidol is administered. Ferritin > 486.5 ng/mL and LDH > 239.5 U/L are independent risk factors for a long course of disease. Oral administration of arbidol 200 mg t.i.d. with a 10-d course of treatment is recommended for COVID-19 patients, particularly those with elevated ferritin and LDH. Nevertheless, future randomized controlled trials are desperately needed to confirm these findings and further study the mid- and long-term outcomes after discharge.
ARTICLE HIGHLIGHTS
Research background
To date, no treatment has proven to be effective for coronavirus disease 2019 (COVID-19) patients, and further research is necessary. Although arbidol has been widely used in China to treat COVID-19 patients, clinical trials to date have not clearly substantiated this approach.
Research motivation
This study mainly evaluated the efficacy of arbidol in patients with COVID-19 in the early stage of the severe acute respiratory syndrome coronavirus 2 epidemic.
Research objectives
This study aimed to evaluate the efficacy of arbidol in COVID-19 patients.
Research methods
Out of the 132 patients, 72 received arbidol treatment, and 60 did not. The disease course of the two groups was compared, and the predictors of extended disease duration were identified.
Research results
The disease duration in the arbidol treatment group was shorter. The risk of a prolonged course of disease increased by 7.158 times in the non-arbidol treatment group. Ferritin > 483.0 ng/mL and lactate dehydrogenase (LDH) > 237.5 U/L were found to be independent risk factors for protracted cases, with the risk of an extended disease duration increasing to 2.852 times and 5.946 times, respectively.
Research conclusions
Abidol can shorten the course of COVID-19 in moderate and severe patients. Ferritin > 486.5 ng/mL and LDH > 239.5 U/L are independent risk factors for delayed recovery from COVID-19.
Research perspectives
Early administration of arbidol may be an effective management strategy in some COVID-19 patients, particularly those with increased serum ferritin or elevated LDH.
Footnotes
Institutional review board statement: The study was reviewed and approved by the institutional ethics board of Jinyintan Hospital (No. KY-2020-71.01).
Informed consent statement: The ethics committee waived the requirement for written informed patient consent, because this was a retrospective study based on the assessment of medical records.
Conflict-of-interest statement: The authors declare no conflicts of interest for this manuscript.
Manuscript source: Unsolicited manuscript
Peer-review started: March 18, 2021
First decision: April 23, 2021
Article in press: July 14, 2021
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Delgado-Gallegos JL, Justo Arevalo S S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Xing YX
Contributor Information
Shuo Wei, Department of Infectious Diseases, Fujian Provincial Hospital, Fujian Medical University, Fuzhou 350000, Fujian Province, China.
Sha Xu, Department of Infectious Diseases, Jinyintan Hospital, Wuhan 430000, Hubei Province, China.
Yun-Hu Pan, Department of Respiratory Medicine, 907 Hospital of the Joint Logistics Team, Nanping 353000, Fujian Province, China. 18750975908@163.com.
Data sharing statement
No additional data are available.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A COVID-19 Lombardy ICU Network. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q, Huet K, Plzak J, Horoi M, Hans S, Rosaria Barillari M, Cammaroto G, Fakhry N, Martiny D, Ayad T, Jouffe L, Hopkins C, Saussez S COVID-19 Task Force of YO-IFOS. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institutes of Health. COVID-19 Management Guidelines. Available from: https://www.covid19treatmentguidelines.nih.gov/introduction/
- 7.Kadam RU, Wilson IA. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc Natl Acad Sci U S A. 2017;114:206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khamitov RA, Loginova SIa, Shchukina VN, Borisevich SV, Maksimov VA, Shuster AM. [Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures] Vopr Virusol. 2008;53:9–13. [PubMed] [Google Scholar]
- 9.National Health Commission of China. Guideline of management of COVID-19 (version 8). Available from: http://www.nhc.gov.cn/yzygj/s7653p/202008/0a7bdf12bd4b46e5bd28ca7f9a7f5e5a.shtml .
- 10.Blaising J, Polyak SJ, Pécheur EI. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vankadari N. Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int J Antimicrob Agents. 2020;56:105998. doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nojomi M, Yassin Z, Keyvani H, Makiani MJ, Roham M, Laali A, Dehghan N, Navaei M, Ranjbar M. Effect of Arbidol (Umifenovir) on COVID-19: a randomized controlled trial. BMC Infect Dis. 2020;20:954. doi: 10.1186/s12879-020-05698-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M, Wu T, Zuo Z, You Y, Yang X, Pan L, Hu Y, Luo X, Jiang L, Xia Z, Deng M. Evaluation of current medical approaches for COVID-19: a systematic review and meta-analysis. BMJ Support Palliat Care. 2021;11:45–52. doi: 10.1136/bmjspcare-2020-002554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.