Abstract
Background:
Obesity and cigarette smoking are two leading preventable causes of death. Previous research suggests that comorbid smoking and obesity likely share neurobehavioral underpinnings; however, the influence of body mass index (BMI) on resting-state functional connectivity (rsFC) in smokers remains unknown. In this study, we explore how BMI affects rsFC and associations between rsFC and smoking-related behavior.
Methods:
Treatment-seeking cigarette smokers (N=87; 54% men) completed a BOLD resting-state fMRI scan session. We grouped smokers into BMI groups (N=23 with obesity, N=33 with overweight, N=31 lean) and used independent components analysis (ICA) to identify the resting state networks commonly associated with cigarette smoking: salience network (SN), right and left executive control networks (ECN) and default mode network (DMN). Average rsFC values were extracted (p<0.001, k=100) to determine group differences in rsFC and relationship to self-reported smoking and dependence.
Results:
Analyses revealed a significant relationship between BMI and connectivity in the SN and a significant quadratic effect of BMI on DMN connectivity. Heavier smoking was related to greater rsFC in the SN among lean and obese groups but reduced rsFC in the overweight group.
Conclusions:
Findings build on research suggesting an influence of BMI on the neurobiology of smokers. In particular, dysfunction of SN-DMN-ECN circuitry in smokers with overweight may lead to a failure to modulate attention and behavior and subsequent difficulty quitting smoking. Future research is needed to elucidate the mechanism underlying the interaction of BMI and smoking and its impact on treatment.
Keywords: cigarette smoking, obesity, overweight, resting-state functional connectivity
1. INTRODUCTION
Cigarette smoking and obesity are the two leading causes of premature morbidity and mortality worldwide (Goodchild et al., 2018; Janssen et al., 2020; Seidell & Halberstadt, 2015). Individuals who smoke cigarettes and have obesity incur four times the risk of all-cause mortality than lean individuals who have never smoked (Freedman et al., 2006). For example, in the United States alone, between 30% – 40% of people with obesity smoke cigarettes (Hales et al., 2018) compared to less than 15% of the general population (Creamer, 2019). Obese smokers also have a higher daily smoking rate and smoke for a greater number of years (Dare et al., 2015). Furthermore, smokers with overweight and obesity comprise 70% of treatment-seeking smokers, and when they achieve cessation, they tend to gain the most weight after quitting and find the weight gain more intolerable (Bush et al., 2008; LaRowe et al., 2009; A. Levine et al., 2007; M. D. Levine et al., 2013). As a result, smokers with overweight and obesity are more likely to relapse than lean smokers (Audrain-McGovern & Benowitz, 2011; Borrelli et al., 1999). Interventions targeting smoking and overweight independently have modest success at best (Aubin et al., 2012; Spring et al., 2009), which may be because behavior change interventions ignore potential neurobiological mechanisms shared by smoking and excess food intake (Aubin et al., 2012; Bush et al., 2008; LaRowe et al., 2009; A. Levine et al., 2007; M. D. Levine et al., 2013; Spring et al., 2009). Upon quitting, smokers lose a primary reinforcer (Caggiula et al., 2009; Chaudhri et al., 2006; Perkins et al., 2006), and highly-palatable food increases in motivational salience (Audrain-McGovern et al., 2004; Berridge, 2009; Robinson & Berridge, 2008), leading to overeating and post-cessation weight gain (Perkins, 1992, 1993; Perkins et al., 1990). Conversely, caloric restriction is associated with increased smoking (Cheskin et al., 2005; Raffoul et al., 2018; Strauss & Mir, 2001), elevated craving, and brain response to cigarette cues (Jenks & Higgs, 2011). Identifying the influence of overweight and obesity on neural activity in smokers could help inform neurobiologically-informed interventions and improve treatment outcomes for this at-risk population.
Resting-state functional connectivity (rsFC) describes the associations between distinct brain regions based on the intrinsic fluctuation in ongoing activity in the brain at rest (Biswal et al., 1995; Fox et al., 2005; Zou, Wu, Stein, Zang, & Yang, 2009). The executive control network (ECN), default mode network (DMN), and salience network (SN), are three resting-state networks linked to aberrant reward-related behaviors (for reviews, see Fedota & Stein, 2015; Sutherland et al., 2012; and Donofry et al., 2019). The ECN, comprising regions of the dorsolateral prefrontal cortex and the lateral posterior parietal cortex, mediates cognitive control functions such as inhibitory control, planning, and decision making (Laird et al., 2011a). The DMN consists of the posterior cingulate cortex, prefrontal cortex, angular gyri and parahippocampus and is involved in internally-focused (rather than task-focused) processes and mental imagery (Laird et al., 2011a). Functionally connected regions of the salience network (SN) include the anterior ventral insula and anterior cingulate cortex and have been shown to guide neurocircuit activation (particularly that of the ECN and DMN (Fedota & Stein, 2015; Sutherland et al., 2012)) to modulate behavior (Peters et al., 2016). Neurobiological research has revealed that chronic cigarette smoking is associated with alterations in functional connectivity between brain regions (Claus & Weywadt, 2020; Janes et al., 2012; Vergara et al., 2017). In smokers, SN connectivity is positively correlated with response to smoking cues (Janes et al., 2015) and may potentially reflect a stable trait of elevated cue reactivity. Connectivity within the SN and broader brain networks is associated with the severity of nicotine dependence (Claus et al., 2013) and craving (Lerman et al., 2014; Moran-Santa Maria et al., 2015; Sutherland et al., 2012). Further, resting-state FC has been shown to predict treatment outcomes in smokers (Wilcox et al., 2017). Thus, identifying factors, such as body mass index, that influence brain network function in smokers may point to brain-based treatment targets to improve smoking cessation.
Research on rsFC in obesity in nonsmokers has focused on neurocircuitry related to reward and cognitive control, yet findings are inconsistent, possibly due to methodological and sampling differences. Few studies have identified differences in rsFC within the ECN, but connectivity among frontal regions positively correlates with BMI in healthy adults (Filbey & Yezhuvath, 2017). Within the SN, research suggests that rsFC strength increases as BMI increases (Figley et al., 2016; García-García et al., 2013; Hogenkamp et al., 2016; Kullmann et al., 2013). Within the DMN, higher weight is associated with reduced rsFC (Doucet et al., 2018). This pattern of rsFC dysfunction in the ECN, SN, and DMN may manifest in a disrupted ability to integrate information from both external and internal sources, resulting in dysfunction in inhibition and metacognition. Obesity in smokers may result in a unique pattern of rsFC compared to those at lower BMIs, but there is no research on the effect of elevated BMI on rsFC in this population.
In a prior study, we found that BMI was negatively associated with activation of the right dorsolateral prefrontal cortex (dlPFC) during smoking cue exposure, with significantly reduced response in cigarette smokers with overweight and obesity compared to lean. In addition, greater commission errors on an inhibitory task (i.e., Go/NoGo) were correlated with reduced neural response to smoking cues in the right dlPFC amongst smokers with obesity but not those who were overweight or lean. Together, these findings provide preliminary evidence that obesity in treatment-seeking smokers is related to neurobiological alterations in inhibitory control over cue-potentiated behaviors. The current exploratory study used resting-state BOLD imaging data to extend these findings. Specifically, we examined the effects of overweight and obesity on resting-state intra-network functional connectivity in cigarette smokers and examined whether rsFC was associated with self-reported smoking behavior and cigarette dependence. Research suggests that BMI is associated with increased SN and decreased DMN rsFC in nonsmokers (Figley et al., 2016; García-García et al., 2013; Hogenkamp et al., 2016; Kullmann et al., 2013), and elevations in SN rsFC in response to smoking cues may be related to greater dependence (Claus et al., 2013). Thus, we hypothesize greater SN and reduced DMN rsFC in smokers at higher BMIs. However, given the paucity of literature on the neural function of those comorbid for obesity and smoking, these analyses are primarily exploratory.
2. METHODS
2.1. Participants
Participants were recruited from the local community for smoking cessation studies (Franklin et al., 2009, 2011) via advertisements, online listservs, and word of mouth. All eligible and interested participants provided informed consent before participation. Telephone screens and an in-person screening visit included medical and psychiatric evaluations to assess eligibility. Exclusion criteria included severe Axis I DSM-IV psychiatric diagnosis requiring hospitalization, substance use disorder other than nicotine use disorder (NUD), pregnancy, history of head trauma or injury causing loss of consciousness greater than three minutes or associated with skull fracture or inter-cranial bleeding, or irremovable ferromagnetic objects on or within their body.
A total of 216 individuals were screened as part of the two treatment studies (Franklin et al., 2009, 2011), of which 87 (39 male, 40 female) met criteria for participation. The two studies, investigating baclofen and varenicline as smoking cessation aids and examining their effect on brain function, had identical criteria for participation and baseline scanning visit procedures. The studies were conducted concurrently. Demographics and variables of interest did not differ between studies. Data for the current study were acquired prior to medication assignment. Given that these data were derived from smoking cessation studies, non-smoking participants were excluded, and information regarding weight history and eating behavior was not assessed. Participants were separated into three groups depending on their body mass index (BMI). Lean participants (n=31) were defined as those with a BMI less than 25.0, overweight participants (OW; n=33) had a BMI of 25.01 – 29.99, and participants with BMIs of 30 or greater were classified as obese (OB; n=23). All procedures were conducted at the University of Pennsylvania Perelman School of Medicine, Center for the Studies of Addiction and approved by the University’s Institutional Review Board and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to participation.
2.2. Procedures
Before the scanning session, participants completed self-report measures and a computerized neurobehavioral battery. Then, approximately 25 minutes before the scan session, participants smoked one of their own cigarettes to maintain individual and characteristic pharmacological, physiological, and psychological states and standardize time since they last smoked. Participants were also offered a light snack (i.e., granola bar) before scanning.
2.3. Measures
2.3.1. Demographics.
Demographic characteristics were obtained using a comprehensive background questionnaire.
2.3.2. Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al. 1998).
The MINI is a structured diagnostic measure administered to determine a diagnosis of substance use disorders or severe psychiatric symptoms (e.g., psychosis, dementia, acute suicidal or homicidal ideation, mania or depression). The MINI was administered by a doctoral- or masters-level clinician. Current diagnoses of severe psychiatric illness were exclusionary.
2.3.3. Smoking history questionnaire (SHQ).
A laboratory-developed smoking history questionnaire assessed smoking history, which included the Fagerström Test for Cigarette Dependence (FTCD; (Fagerström, 2012) to measure severity and duration of nicotine dependence.
2.3.4. Craving withdrawal questionnaire (CWQ).
The CWQ is a nine-item measure that measures cigarette craving and subjective withdrawal symptoms (Franklin et al., 2007, 2018; Ketcherside et al., 2020). Ratings are acquired while participants are in the scanner, both immediately before and following the smoking and nonsmoking stimulus presentations.
2.4. Image Acquisition
Magnetic resonance imaging (MRI) scanning was conducted on a Siemens 3.0 Tesla Trio whole-body scanner (Siemens AG, Erlangen, Germany) using an 8 channel (N = 68) and 32 channel (N = 19) head coil. We examined whether data acquisition differences affected findings by comparing variances between coil types using a homogeneity of variance test and found that the variances were not significantly different. Data were acquired using the following parameters: To co-register functional data, a T1-weighted three-dimensional high-resolution magnetization-prepared rapid acquisition with gradient echo (MPRAGE) scan was acquired (repetition time (TR)/ echo time (TE)/ inversion time (TI) = 1620/3.09/950 ms, flip angle = 15°, bandwidth = 150 HZ/Px, voxel size = 0.977 × 0.977 × 1.0 mm, matrix = 192 × 256, slices= 160, slice thickness = 1.0 mm). BOLD resting-state fMRI images were acquired using T2* weighted gradient-echo planar imaging (EPI) (TE = 30 ms, TR = 2080 ms, 64 × 64 matrix with 35 slices, slice thickness = 3.3 mm, flip angle = 90°, voxel size = 3.4 × 3.4 × 3.3 mm and 22 × 22 cm2 FOV).
2.5. Preprocessing
Preprocessing for the resting-state data was carried out using Data Processing Assistant for Resting-State fMRI (DPARSF) and Data Processing and Analysis of Brain Imaging (REST) toolbox, based on SPM12 (http://www.fil.ion.ucl.ac.uk/spm) under the Matlab R2020 environment. Images were slice-time corrected, realigned (each of the six affine motion parameters was less than 2mm), coregistered and segmented with each participant’s anatomical image, band-pass filtered (0.01–0.08 Hz), smoothed at FWHM 6mm kernel and normalized to the standard MNI template. In addition, each subject’s data were examined for mean Frame Displacement (Power et al., 2012) and did not exceed more than 0.5mm. Head micro-motion (≤ 0.2 mm) was addressed by calculating the sum of the absolute values of the differentiated head motion estimates (by backwards differences) at each time point. White matter, cerebrospinal fluid and global mean signals were regressed out. Frame Displacement did not differ between groups, and thus, it was not included as a covariate in analyses.
Following preprocessing, data were analyzed with the Group ICA for fMRI Toolbox (GIFT v4.0b, icatb.sourceforge.net) using the Infomax algorithm (Bell & Sejnowski, 1995). Participants’ data were reduced by Principle Component Analysis (PCA), concatenated into a group data set followed by further PCA reduction, and decomposed into a group of independent components across all subjects using the Infomax algorithm. Component data were then back reconstructed into subject-level time course and spatial maps. The number of independent components was estimated as 20 using the default parameter setting. To derive reliable components, ICA was iteratively applied 50 times using the Icasso toolbox (Himberg & Hyvarinen, 2003). Finally, the components’ spatial maps were converted into z-score activation maps, thresholded at p <0.001 at FWE, k =100. Voxel-wise z-scores were calculated by dividing the estimated spatial maps by the noise standard deviation. These z-scores were extracted for the statistical analysis described below.
2.6. Component Identification
A systemic procedure was used to inspect and select the resting-state networks (RSNs) from the estimated 20 components. The RSNs were identified by visual inspection and spatial template matching technique with the standard templates (Laird et al., 2011b). Using this technique, one or more components were identified by comparing them with the spatial templates. We first excluded the components representing visual, auditory, and sensory-motor artifacts according to spatial topology. Secondly, non-artifactual components that showed a high correlation coefficient with the reference components described in Laird (2011) were chosen for further analysis. Of these, two showed high correlations with executive control network (ECN) components (Right, ICN 8, r = 0.58 and Left, ICN 11, r = 0.55), one salience network (SN, ICN 19) component (r = 0.43) and a component representing the default mode network (DMN, ICN 17, r = 0.50). The spatial maps of the networks are shown in Figure 1.
Figure 1:
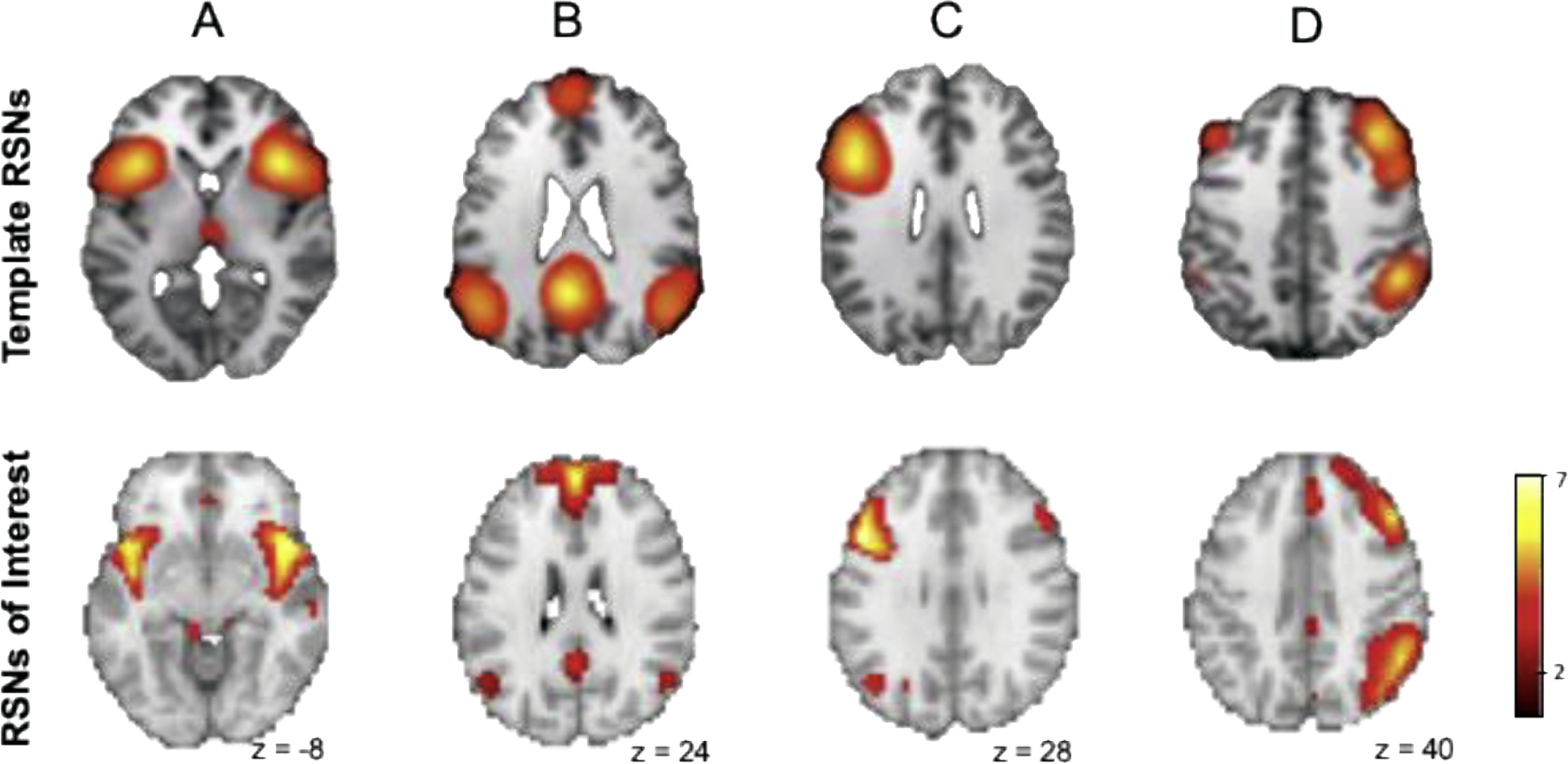
Spatial maps of the resting state networks (RSNs) of interest in comparison to template RSNs: (A) salience network, (B) default mode network, (C) left executive control network, (D) right executive control network. Maps are visualized using a threshold of p <0.001 family wise error-corrected, k = 100. Template RSNs are shown from the most representative slice per Laird et al, 2011.
2.7. Statistical Analysis
SPSS version 25 software was used to examine relationships between rsFC and smoking variables. First, means and standard deviations were calculated. Scores for variables > 3 SD from the mean were excluded. None of our participants met criteria for exclusion on any measure. Next, partial Pearson correlations were used to determine relationships between BMI and descriptive and behavioral variables. Relationships between the four component z-scores and BMI were examined using linear regressions. 3-group (lean, overweight and obesity) MANOVAs were conducted to compare group differences in rsFC for all components; Bonferroni correction was used to account for multiple comparisons in post hoc analyses. Given apparent nonlinear relationships between connectivity and BMI group based on visual inspection, post hoc quadratic regression analyses were conducted. To determine if the relationship between rsFC and smoking behavior differed depending on BMI status, three MANCOVAs were conducted with CPD, Pack Years, and FTCD as covariates of interest.
3. RESULTS
3.1. Demographic and Descriptive Data
Participant characteristics are reported in Table 1. BMI was not significantly correlated with age, education, Pack Years, number of cigarettes per day (CPD), craving scores, or cigarette dependence (FTCD). BMI was significantly different across groups (F(2, 84)=147.18, p<0.001). The sample identified as 32.2% White, 54% Black, 5.7% Asian, and 8% Multiracial. 6.9% of participants identified as of Hispanic ethnicity and 93.1% as Non-Hispanic. Weight groups did not differ in racial or ethnic background, nor the balance of male and female participants.
Table 1:
Comparisons of descriptive data across groups.
| Lean | Overweight | Obese | F | Sig. | ||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |||
| BMI | 21.9 | 2.2 | 27.6 | 1.2 | 33.9 | 4.0 | 147.2 | > 0.001 |
| Education | 13.6 | 2.0 | 13.8 | 2.1 | 14.5 | 2.8 | 1.1 | 0.35 |
| Age | 36.9 | 13.0 | 40.9 | 11.0 | 38.6 | 11.6 | 0.9 | 0.41 |
| CPD | 14.5 | 5.9 | 14.4 | 6.9 | 12.5 | 4.6 | 0.9 | 0.40 |
| PackYears | 13.0 | 9.7 | 14.1 | 9.4 | 11.8 | 9.9 | 0.4 | 0.68 |
| FTCD | 4.9 | 1.6 | 4.7 | 1.6 | 4.5 | 1.6 | 0.5 | 0.63 |
| % Female | 50.0% | 33.3% | 60.1% | 0.11 | ||||
| % White | 28% | 33% | 35% | |||||
| % Black | 52% | 58% | 52% | 0.89 | ||||
| % Asian | 7% | 6% | 4% | |||||
| % Multiracial | 13% | 3% | 9% | |||||
BMI = body mass index, CPD = cigarettes per day, FTCD = Fagerström Test for Cigarette Dependence
3.2. rsFC and BMI
Regression analyses revealed a significant negative association between BMI and connectivity z-scores in the SN (β= −0.21, t(84) = −2.01, p<0.05). There were no significant relationships between BMI and rsFC in other components. A MANOVA showed a significant difference between groups in connectivity over all components (Roy’s Largest Root=0.13, p=0.04, η2=0.12). The main effect of BMI group revealed trend-level differences between groups in the left ECN (p=0.08) and SN (p=0.05).
Given possible nonlinear relationships between BMI and rsFC, post hoc quadratic regressions were also conducted. There was a significant quadratic effect of BMI on connectivity z-scores in the DMN (β = −0.23, t(84) = −2.02, p=0.047), with smokers in the overweight range showing the greatest rsFC (Figure 2). There were no quadratic effects of BMI on rsFC in any other components.
Figure 2:
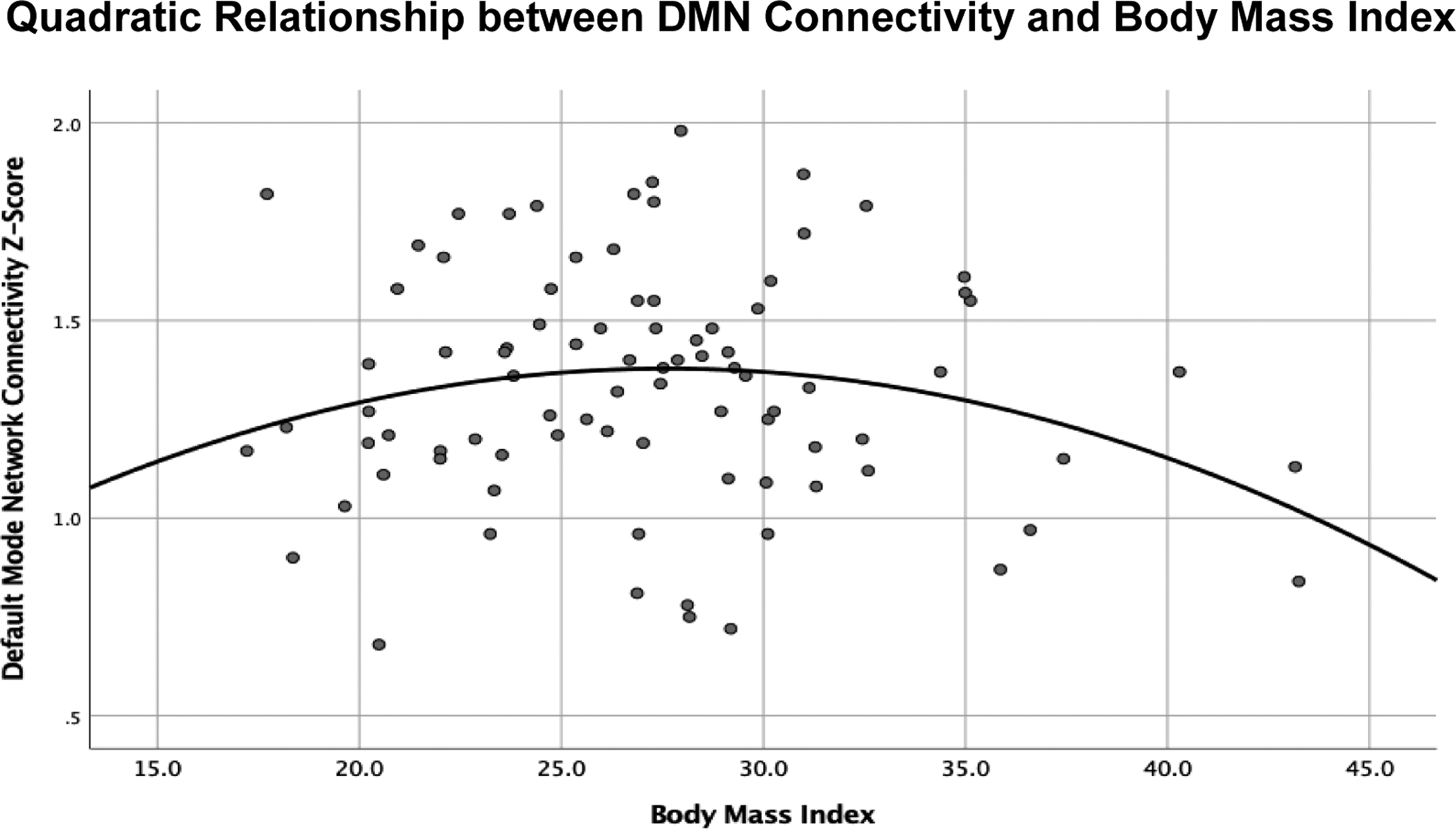
Quadratic regression determining nonlinear relationships between resting state functional connectivity and body mass index (BMI), showed a significant quadratic effect of BMI on default mode network (DMN) connectivity, with highest intranetwork connectivity in smokers in the overweight range.
3.3. rsFC and Self-Reported Smoking Behavior
Correlations run between z-scores for components of interest and CPD, FTCD, and Pack Years revealed a significant negative correlation between Pack Years and both right (r = −0.39, p<0.001) and left ECN (r = −0.35, p=0.002). No relationships were found between rsFC and CPD or FTCD.
3.4. Differences in BMI groups as a Function of Self-Reported Smoking Behavior
Three multivariate ANOVAs were conducted to determine differences in rsFC across all components between BMI groups as a function of CPD, Pack Years, and FTCD. Analyses of the Group × CPD model revealed a main effect of group (Roy’s Largest Root=0.20, p=0.006, η2=0.17) and an interaction effect of Group × CPD in rsFC over all components (Roy’s Largest Root=0.25, p=0.001, η2=0.20). The omnibus model was significantly related to connectivity z-values in the SN (p=0.001), but not to the right or left ECN or to DMN rsFC. There was a significant interaction effect of Group × CPD only in the SN (Figure 3; p<0.001) such that higher CPD was related to greater connectivity in the SN among lean smokers and those with obesity, while those with overweight showed reduced connectivity with heavier daily smoking. A significant main effect of BMI group revealed differences between groups in the SN (p=0.001); there was no main effect of CPD.
Figure 3:
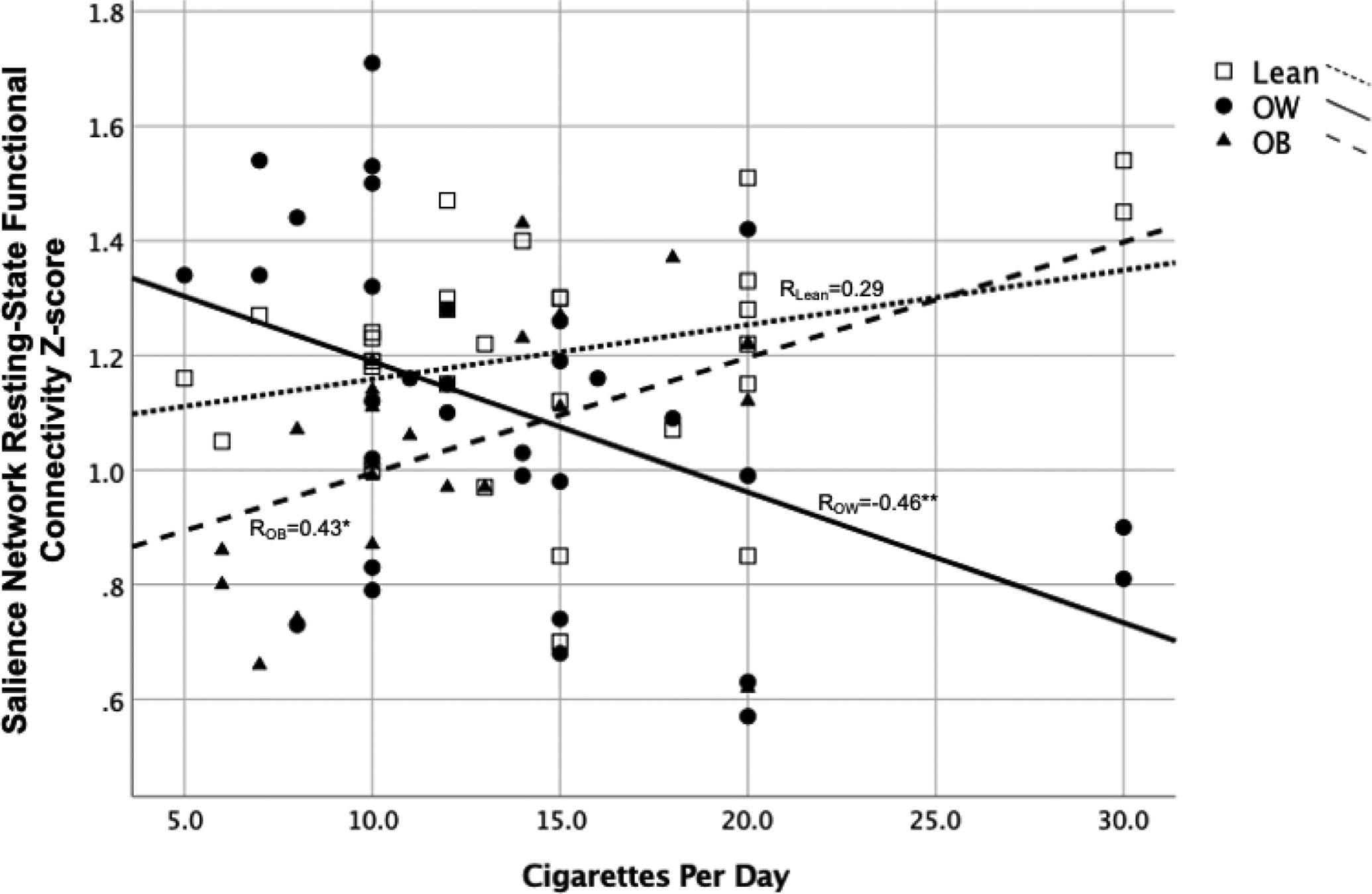
Significant interaction effect of Group × Cigarettes per Day (CPD) in the salience network (SN) (p<0.001) such that higher CPD was related to greater resting state functional connectivity (rsFC) in the SN among lean smokers and those with obesity, while those with overweight showed reduced rsFC with heavier daily smoking. OB = obesity, OW = overweight, * = p<0.05, ** = p<0.01
Analyses of the Group × Pack Years model revealed a main effect of group (Roy’s Largest Root=0.29, p=0.029, η2=0.13) and a Group × Pack Years interaction on rsFC over all components (Roy’s Largest Root=0.14, p=0.04, η2=0.12). The omnibus model was significantly related to connectivity z-values in the right (p=0.001) and left ECN (p=0.005). However, there was no significant interaction effect of Group × Pack Years in any component, nor main effects of BMI group or Pack Years.
Multivariate analysis of the Group × FTCD model revealed no significant interaction effect of BMI group × FTCD nor main effect of group in rsFC over all components. The omnibus model was significantly related to rsFC z-values in the SN (p=0.027), but not to the right or left ECN or to DMN connectivity. There was a significant interaction effect of Group × FTCD only in the SN (p=0.037) such that higher dependence was related to greater rsFC in the SN among lean smokers and those with obesity, while those with overweight showed reduced rsFC with greater FTCD. There was no main effect of BMI group or FTCD.
4. DISCUSSION
Comorbid chronic cigarette smoking and obesity is increasingly common globally (Janssen et al., 2020) and has significant adverse health effects. Despite decades of neuroimaging research on chronic cigarette smoking and obesity independently, the neurobiology underlying these co-occurring conditions remains understudied. Here, we show that BMI influences resting-state functional connectivity in chronic cigarette smokers, particularly in the salience and default mode networks. Further, differences in the relationship between connectivity and self-reported smoking behavior were shown among groups, with higher rates of daily smoking and greater dependence significantly associated with greater rsFC in the SN among lean smokers and those with obesity. In contrast, among smokers with overweight, higher CPD and dependence were linked to lower rsFC in the SN. Together, these findings show that lean smokers and smokers with obesity may have elevated connectivity in the SN, while those with overweight have a unique relationship between SN connectivity and smoking behavior. Results suggest that alterations in rsFC with BMI may be linked to differences in severity of nicotine dependence, which could translate to more difficulty quitting (Kozlowski et al., 1993).
There are conflicting findings in the extant literature as to whether SN or DMN rsFC is reduced (Li et al., 2017; Weiland et al., 2015; Wetherill et al., 2019) or elevated in smokers as compared to nonsmokers (Claus & Weywadt, 2020; Wang et al., 2020). Given research suggesting elevation of DMN and SN connectivity may occur with increasing BMI (Donofry et al., n.d.; Figley et al., 2016; Hogenkamp et al., 2016; Kullmann et al., 2013), we propose that these differences in results in smokers may partially be driven by the effects of BMI on this population. While we could not directly compare to nonsmokers, BMI may differentially impact rsFC in smokers. Broadly, the DMN is implicated in internally-focused self-referential processes, and DMN rsFC is also linked to improved performance on measures of executive function and memory (Foret et al., 2020; O’Shea et al., 2018). Notably, greater DMN connectivity is also associated with poorer cognitive function among those with metabolic syndrome (Foret et al., 2020). The SN is responsible for adjusting attention and motivation based on subjectively relevant stimuli, whether external or interoceptive (Seeley et al., 2007), by “toggling” activity between the ECN and DMN (Peters et al., 2016). It is theorized that dysregulated rsFC between these networks may underlie the maintenance of nicotine addiction through hypersensitivity to drug cues, somatic symptoms of withdrawal, and the cognitive impairments associated with acute abstinence (Sutherland et al., 2012). Sutherland and colleagues hypothesize that acute nicotine abstinence may enhance connectivity in the DMN. Given that participants smoked just before the scanning session, the elevated connectivity in the DMN seen in smokers with overweight may mean that they experience somatic withdrawal sooner than lean smokers or those with obesity. Although Sutherland and colleagues do not explicitly implicate hypoconnectivity within the SN as potentially influential in the cycle of drug use and withdrawal, our finding of a correlation between reduced rsFC in the SN and heavier daily smoking in smokers with overweight may reflect further dysfunction of the SN-DMN-ECN circuitry, leading to a failure to modulate attention and behavior appropriately. If overweight dampens the effect of smoking on DMN connectivity, this would further contribute to SN-DMN-ECN circuit dysfunction that may maintain smoking behavior. Consequently, smokers with overweight BMIs may be more likely to have difficulty maintaining abstinence.
Prior studies have suggested that individuals with overweight are unique in their processing of salient stimuli (Davis et al., 2004; Verdejo-Román et al., 2017) and that relationships between BMI and insula function may be quadratic rather than linear (Dietrich et al., 2016). Horstmann and colleagues (2015) proposed that dopaminergic function may vary according to BMI, with overweight individuals demonstrating the lowest tonic dopamine (DA) levels compared to lean or those with obesity. While speculative, it is possible that the alterations of DA tone seen in overweight smokers may differentially predispose them to maintenance or even escalation of smoking or overeating and may explain the unique relationship between reduced rsFC in the SN and increased daily smoking and dependence. It has been suggested that DA responsivity to salient stimuli (like cigarettes or highly-palatable food) is regulated by tonic levels of DA, such that when tonic DA is low, the amplitude of phasic DA release is high (Floresco et al., 2003; Grace, 1991) thereby enhancing response. Reductions in DA are associated with less deactivation of the DMN during cognitive tasks (Nagano-Saito et al., 2008); likewise, increased DA signaling is associated with reduced connectivity within the DMN (Conio et al., 2020). Notably, nicotine has been shown to amplify phasic DA in this way via nicotinic acetylcholine receptor desensitization in the striatum, further enhancing the reinforcing efficacy of salient stimuli in general (Exley & Cragg, 2008). Smokers with overweight may demonstrate low tonic (Horstmann et al., 2015) and high phasic DA release, which may, in turn, be associated with functional connectivity disturbance and increased smoking. Overconsumption is likely driven by a dynamic neurobehavioral system that may change with increasing body weight, such that different types of dysfunction may drive smoking when an individual is overweight versus when they are lean or have obesity.
4.2. Limitations
This study, the first of its kind, examined the effects of overweight and obesity on resting state functional connectivity in smokers as it related to self-reported smoking behavior. This was a secondary analysis of data from two treatment studies and did not enroll nonsmokers. It will be important to determine if BMI influences connectivity differently in smokers versus nonsmokers in future research. Because this was not a prospective examination, we could not analyze additional variables of interest that might specifically be relevant to a population with obesity, such as hunger ratings, weight loss, dieting, weight stability/suppression, or dietary intake. Recent research suggests that nicotine, acting through nicotinic acetylcholine receptors (nAChRs), regulates food intake circuits in the hypothalamus (Calarco and Picciotto, 2020), and as such, future research should consider these variables and examine hypothalamic connectivity. Additionally, we lacked adequate measures to control for hormonal status among female participants. However, lack of control over hormonal variability would, if anything, increase error variance, theoretically reducing the ability to detect differences. Future studies of the impact of BMI would benefit from comparing rsFC in both abstinent and sated smoking states. It is possible that groups differed in the nicotine content of their preferred cigarette brand or the number of cigarettes smoked since awakening, which may influence nicotine levels at the time of scanning. However, in our paradigm, we attempted to minimize this by having individuals smoke to satiety prior to the scan, and consequently, this study cannot examine differences related to withdrawal. In addition, given this was a treatment-seeking sample, the results may not be generalizable to smokers as a whole; however, since the goal of the study is to identify treatment targets, treatment seekers may be the most representative group. Nonetheless, future research would ideally focus on comparing treatment-seeking smokers to those not actively interested in quitting. Finally, sex affects rsFC in smokers (Wetherill et al., 2014), which we could not examine with the current study’s limited sample size. An interaction between BMI status and sex is conceivable and should be considered a future area of study. Despite these limitations, our findings show that smokers demonstrated variable connectivity in the SN and DMN as BMI increases, potentially reflecting dysfunction in these circuits that may influence response to salient smoking cues. Further, lower rsFC in the SN in smokers with overweight may be linked to heavier smoking in contrast to other groups. Future research should test this by prospectively following smokers during quit attempts to determine if BMI differences confer greater difficulty with smoking cessation. Rigorous study of the influence of BMI on the neurobiology of smokers as compared to nonsmokers is also necessary to clearly determine the interaction between these two conditions. Doing so will allow more precise targeting of treatment and understanding of potential barriers to cessation.
4.3. Conclusions
In summary, cigarette smoking and obesity are the two leading causes of premature morbidity and mortality worldwide (Goodchild et al., 2018; Janssen et al., 2020; Seidell & Halberstadt, 2015) and have overlapping pathophysiology, yet no study to date had investigated the effect of obesity or increasing BMI on functional connectivity in smokers. The current study is the first to demonstrate that individuals with comorbid smoking and obesity show a different pattern of rsFC compared to their overweight counterparts, particularly in regions linked to executive control and motivational salience. Additional research is needed in order to tailor treatment to smokers who have obesity who are at greater risk of experiencing significant adverse health-related outcomes.
Highlights.
Cigarette smokers show reduced resting-state functional connectivity within the salience network as BMI increases.
Smokers show an inverted U-shaped relationship between BMI and default mode network rsFC.
Heavier smoking is related to greater connectivity in the SN among lean and obese groups.
Among the overweight group, heavier smoking is associated with reduced connectivity in the salience network.
Acknowledgements
The authors would like to thank Melanie Maron, M.S., Wetherill Lab Manager for supervision of staff and study procedures.
Funding
Funding for this study was provided by NIH NIDA grants, R01DA030394 and R01DA029845.
Dr. Ely is supported by NIDA grant 5T32DA028874-10. Dr. Rao is supported by the NIMH grant R01 MH107571 and NIA grant R21AG051981.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no financial interests or conflicts of interest.
REFERENCES
- Aubin H-J, Farley A, Lycett D, Lahmek P, & Aveyard P (2012). Weight gain in smokers after quitting cigarettes: Meta-analysis. BMJ, 345. 10.1136/bmj.e4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, & Benowitz NL (2011). Cigarette Smoking, Nicotine, and Body Weight. Clinical Pharmacology & Therapeutics, 90(1), 164–168. 10.1038/clpt.2011.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Tercyak KP, Epstein LH, Goldman P, & Wileyto EP (2004). Applying a behavioral economic framework to understanding adolescent smoking. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors, 18(1), 64–73. 10.1037/0893-164X.18.1.64 [DOI] [PubMed] [Google Scholar]
- Bell AJ, & Sejnowski TJ (1995). An Information-Maximization Approach to Blind Separation and Blind Deconvolution. Neural Computation, 7(6), 1129–1159. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2009). “Liking” and “wanting” food rewards: Brain substrates and roles in eating disorders. Physiology & Behavior, 97(5), 537–550. 10.1016/j.physbeh.2009.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli B, Spring B, Niaura R, Kristeller J, Ockene JK, & Keuthen NJ (1999). Weight suppression and weight rebound in ex-smokers treated with fluoxetine. Journal of Consulting and Clinical Psychology, 67(1), 124–131. 10.1037/0022-006X.67.1.124 [DOI] [PubMed] [Google Scholar]
- Bush T, Levine MD, Deprey M, Cerutti B, Zbikowski SM, McAfee T, Mahoney L, & Beebe L (2008). Prevalence of Weight Concerns and Obesity Among Smokers Calling a Quitline. Journal of Smoking Cessation, 4(5), 74–78. 10.1375/jsc.4.2.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, & Sved AF (2009). The role of nicotine in smoking: A dual-reinforcement model. Nebraska Symposium on Motivation. Nebraska Symposium on Motivation, 55, 91–109. 10.1007/978-0-387-78748-0_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, & Sved AF (2006). Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology, 184(3–4), 353–366. 10.1007/s00213-005-0178-1 [DOI] [PubMed] [Google Scholar]
- Cheskin LJ, Hess JM, Henningfield J, & Gorelick DA (2005). Calorie restriction increases cigarette use in adult smokers. Psychopharmacology, 179(2), 430–436. 10.1007/s00213-004-2037-x [DOI] [PubMed] [Google Scholar]
- Claus ED, Blaine SK, Filbey FM, Mayer AR, & Hutchison KE (2013). Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 38(12), 2363–2372. 10.1038/npp.2013.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, & Weywadt CR (2020). Resting-State Connectivity in Former, Current, and Never Smokers. Nicotine & Tobacco Research, 22(2), 180–187. 10.1093/ntr/nty266 [DOI] [PubMed] [Google Scholar]
- Conio B, Martino M, Magioncalda P, Escelsior A, Inglese M, Amore M, & Northoff G (2020). Opposite effects of dopamine and serotonin on resting-state networks: Review and implications for psychiatric disorders. Molecular Psychiatry, 25(1), 82–93. 10.1038/s41380-019-0406-4 [DOI] [PubMed] [Google Scholar]
- Creamer MR (2019). Tobacco Product Use and Cessation Indicators Among Adults—United States, 2018. MMWR. Morbidity and Mortality Weekly Report, 68. 10.15585/mmwr.mm6845a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dare S, Mackay DF, & Pell JP (2015). Relationship between Smoking and Obesity: A Cross-Sectional Study of 499,504 Middle-Aged Adults in the UK General Population. PLoS ONE, 10(4). 10.1371/journal.pone.0123579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Strachan S, & Berkson M (2004). Sensitivity to reward: Implications for overeating and overweight. Appetite, 42(2), 131–138. 10.1016/j.appet.2003.07.004 [DOI] [PubMed] [Google Scholar]
- Dietrich A, Hollmann M, Mathar D, Villringer A, & Horstmann A (2016). Brain regulation of food craving: Relationships with weight status and eating behavior. International Journal of Obesity, 40(6), 982–989. 10.1038/ijo.2016.28 [DOI] [PubMed] [Google Scholar]
- Donofry SD, Stillman CM, & Erickson KI (n.d.). A review of the relationship between eating behavior, obesity and functional brain network organization. Social Cognitive and Affective Neuroscience. 10.1093/scan/nsz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet GE, Rasgon N, McEwen BS, Micali N, & Frangou S (2018). Elevated Body Mass Index is Associated with Increased Integration and Reduced Cohesion of Sensory-Driven and Internally Guided Resting-State Functional Brain Networks. Cerebral Cortex, 28(3), 988–997. 10.1093/cercor/bhx008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, & Cragg SJ (2008). Presynaptic nicotinic receptors: A dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. British Journal of Pharmacology, 153(S1), S283–S297. 10.1038/sj.bjp.0707510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström K (2012). Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 14(1), 75–78. 10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- Fedota JR, & Stein EA (2015). Resting-state functional connectivity and nicotine addiction: Prospects for biomarker development. Annals of the New York Academy of Sciences, 1349(1), 64–82. 10.1111/nyas.12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figley CR, Asem JSA, Levenbaum EL, & Courtney SM (2016). Effects of Body Mass Index and Body Fat Percent on Default Mode, Executive Control, and Salience Network Structure and Function. Frontiers in Neuroscience, 10. 10.3389/fnins.2016.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, & Yezhuvath US (2017). A multimodal study of impulsivity and body weight: Integrating behavioral, cognitive, and neuroimaging approaches. Obesity (Silver Spring, Md.), 25(1), 147–154. 10.1002/oby.21713 [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, & Grace AA (2003). Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nature Neuroscience, 6(9), 968–973. 10.1038/nn1103 [DOI] [PubMed] [Google Scholar]
- Foret JT, Dekhtyar M, Birdsill AC, Tanaka H, & Haley AP (2020). Metabolic syndrome components moderate the association between executive function and functional connectivity in the default mode network. Brain Imaging and Behavior. 10.1007/s11682-020-00409-0 [DOI] [PubMed] [Google Scholar]
- Franklin TR, Harper D, Kampman K, Kildea-McCrea S, Jens W, Lynch KG, O’Brien CP, & Childress AR (2009). The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug and Alcohol Dependence, 103(1), 30–36. 10.1016/j.drugalcdep.2009.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Jagannathan K, Hager N, Fang Z, Xu S, Wong J, Childress AR, Detre JA, Rao H, & Wetherill R (2018). Brain substrates of early (4 h) cigarette abstinence: Identification of treatment targets. Drug and Alcohol Dependence, 182, 78–85. 10.1016/j.drugalcdep.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, & Childress AR (2011). Effects of Varenicline on Smoking Cue–Triggered Neural and Craving Responses. Archives of General Psychiatry, 68(5), 516–526. 10.1001/archgenpsychiatry.2010.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, & Childress AR (2007). Limbic Activation to Cigarette Smoking Cues Independent of Nicotine Withdrawal: A Perfusion fMRI Study. Neuropsychopharmacology, 32(11), 2301–2309. 10.1038/sj.npp.1301371 [DOI] [PubMed] [Google Scholar]
- Freedman DM, Sigurdson AJ, Rajaraman P, Doody MM, Linet MS, & Ron E (2006). The Mortality Risk of Smoking and Obesity Combined. American Journal of Preventive Medicine, 31(5), 355–362. 10.1016/j.amepre.2006.07.022 [DOI] [PubMed] [Google Scholar]
- García-García I, Jurado MA, Garolera M, Segura B, Marqués-Iturria I, Pueyo R, Vernet-Vernet M, Sender-Palacios MJ, Sala-Llonch R, Ariza M, Narberhaus A, & Junqué C (2013). Functional connectivity in obesity during reward processing. NeuroImage, 66, 232–239. 10.1016/j.neuroimage.2012.10.035 [DOI] [PubMed] [Google Scholar]
- Goodchild M, Nargis N, & d’Espaingnet ET (2018). Global economic cost of smoking-attributable diseases. Tobacco Control, 27, 58–64. 10.1136/tobaccocontrol-2016-053305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA (1991). Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience, 41(1), 1–24. 10.1016/0306-4522(91)90196-U [DOI] [PubMed] [Google Scholar]
- Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, & Ogden CL (2018). Differences in Obesity Prevalence by Demographic Characteristics and Urbanization Level Among Adults in the United States, 2013–2016. JAMA, 319(23), 2419–2429. 10.1001/jama.2018.7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg J, & Hyvarinen A (2003). Icasso: Software for investigating the reliability of ICA estimates by clustering and visualization. 2003 IEEE XIII Workshop on Neural Networks for Signal Processing (IEEE Cat. No.03TH8718), 259–268. 10.1109/NNSP.2003.1318025 [DOI] [Google Scholar]
- Hogenkamp PS, Zhou W, Dahlberg LS, Stark J, Larsen AL, Olivo G, Wiemerslage L, Larsson E-M, Sundbom M, Benedict C, & Schiöth HB (2016). Higher resting-state activity in reward-related brain circuits in obese versus normal-weight females independent of food intake. International Journal of Obesity, 40(11), 1687–1692. 10.1038/ijo.2016.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann A, Fenske WK, & Hankir MK (2015). Argument for a non-linear relationship between severity of human obesity and dopaminergic tone. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity, 16(10), 821–830. 10.1111/obr.12303 [DOI] [PubMed] [Google Scholar]
- Janes AC, Farmer S, Peechatka AL, de Frederick BB, & Lukas SE (2015). Insula–Dorsal Anterior Cingulate Cortex Coupling is Associated with Enhanced Brain Reactivity to Smoking Cues. Neuropsychopharmacology, 40(7), 1561–1568. 10.1038/npp.2015.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, de Frederick BB, & Kaufman MJ (2012). Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug and Alcohol Dependence, 125(3), 252–259. 10.1016/j.drugalcdep.2012.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen F, Bardoutsos A, & Vidra N (2020). Obesity Prevalence in the Long-Term Future in 18 European Countries and in the USA. Obesity Facts, 13(5), 514–527. 10.1159/000511023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks RA, & Higgs S (2011). Reactivity to smoking- and food-related cues in currently dieting and non-dieting young women smokers. Journal of Psychopharmacology, 25(4), 520–529. 10.1177/0269881109359093 [DOI] [PubMed] [Google Scholar]
- Ketcherside A, Jagannathan K, Dolui S, Hager N, Spilka N, Nutor C, Rao H, Franklin T, & Wetherill R (2020). Baclofen-induced Changes in the Resting Brain Modulate Smoking Cue Reactivity: A Double-blind Placebo-controlled Functional Magnetic Resonance Imaging Study in Cigarette Smokers. Clinical Psychopharmacology and Neuroscience, 18(2), 289–302. 10.9758/cpn.2020.18.2.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, Porterc CQ, Orleansd CT, Popeb MA, & Heathertone T (1993). Predicting smoking cessation with self-reported measures of nicotine. [DOI] [PubMed]
- Kullmann S, Pape A-A, Heni M, Ketterer C, Schick F, Häring H-U, Fritsche A, Preissl H, & Veit R (2013). Functional Network Connectivity Underlying Food Processing: Disturbed Salience and Visual Processing in Overweight and Obese Adults. Cerebral Cortex, 23(5), 1247–1256. 10.1093/cercor/bhs124 [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, & Fox PT (2011a). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23(12), 4022–4037. 10.1162/jocn_a_00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, & Fox PT (2011b). Behavioral Interpretations of Intrinsic Connectivity Networks. Journal of Cognitive Neuroscience, 23(12), 4022–4037. 10.1162/jocn_a_00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe TL, Piper ME, Schlam TR, Fiore MC, & Baker TB (2009). Obesity and smoking: Comparing cessation treatment seekers with the general smoking population. Obesity (Silver Spring, Md.), 17(6), 1301–1305. 10.1038/oby.2009.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, & Stein EA (2014). Large-Scale Brain Network Coupling Predicts Acute Nicotine Abstinence Effects on Craving and Cognitive Function. JAMA Psychiatry, 71(5), 523–530. 10.1001/jamapsychiatry.2013.4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, & Weller A (2007). Measuring cortisol in human psychobiological studies. Physiology & Behavior, 90(1), 43–53. 10.1016/j.physbeh.2006.08.025 [DOI] [PubMed] [Google Scholar]
- Levine MD, Bush T, Magnusson B, Cheng Y, & Chen X (2013). Smoking-Related Weight Concerns and Obesity: Differences Among Normal Weight, Overweight, and Obese Smokers Using a Telephone Tobacco Quitline. Nicotine & Tobacco Research, 15(6), 1136–1140. 10.1093/ntr/nts226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yuan K, Guan Y, Cheng J, Bi Y, Shi S, Xue T, Lu X, Qin W, Yu D, & Tian J (2017). The implication of salience network abnormalities in young male adult smokers. Brain Imaging and Behavior, 11(4), 943–953. 10.1007/s11682-016-9568-8 [DOI] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, Hartwell KJ, Hanlon CA, Canterberry M, Lematty T, Owens M, Brady KT, & George MS (2015). Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers. Addiction Biology, 20(2), 407–414. 10.1111/adb.12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, & Dagher A (2008). Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(14), 3697–3706. 10.1523/JNEUROSCI.3921-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea DM, Langer K, Woods AJ, Porges EC, Williamson JB, O’Shea A, & Cohen RA (2018). Educational Attainment Moderates the Association Between Hippocampal Volumes and Memory Performances in Healthy Older Adults. Frontiers in Aging Neuroscience, 10. 10.3389/fnagi.2018.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA (1992). Effects of tobacco smoking on caloric intake. British Journal of Addiction, 87(2), 193–205. 10.1111/j.1360-0443.1992.tb02693.x [DOI] [PubMed] [Google Scholar]
- Perkins KA (1993). Weight gain following smoking cessation. Journal of Consulting and Clinical Psychology, 61(5), 768–777. 10.1037/0022-006X.61.5.768 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, & Caggiula A (2006). Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology, 184(3), 600–607. 10.1007/s00213-005-0103-7 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Sexton JE, & Pastor S (1990). Effects of smoking cessation on consumption of alcohol and sweet, high-fat foods. Journal of Substance Abuse, 2(3), 287–297. 10.1016/S0899-3289(10)80002-1 [DOI] [PubMed] [Google Scholar]
- Peters SK, Dunlop K, & Downar J (2016). Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Frontiers in Systems Neuroscience, 10, 104. 10.3389/fnsys.2016.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffoul A, Leatherdale ST, & Kirkpatrick SI (2018). Dieting predicts engagement in multiple risky behaviours among adolescent Canadian girls: A longitudinal analysis. Canadian Journal of Public Health, 109(1), 61–69. 10.17269/s41997-018-0025-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2008). The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1507), 3137–3146. 10.1098/rstb.2008.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, & Greicius MD (2007). Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. Journal of Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidell JC, & Halberstadt J (2015). The Global Burden of Obesity and the Challenges of Prevention. Annals of Nutrition and Metabolism, 66(Suppl. 2), 7–12. 10.1159/000375143 [DOI] [PubMed] [Google Scholar]
- Spring B, Howe D, Berendsen M, McFadden HG, Hitchcock K, Rademaker AW, & Hitsman B (2009). Behavioral intervention to promote smoking cessation and prevent weight gain: A systematic review and meta-analysis. Addiction (Abingdon, England), 104(9), 1472–1486. 10.1111/j.1360-0443.2009.02610.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss RS, & Mir HM (2001). Smoking and weight loss attempts in overweight and normal-weight adolescents. International Journal of Obesity, 25(9), 1381–1385. 10.1038/sj.ijo.0801683 [DOI] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, & Stein EA (2012). Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage, 62(4), 2281–2295. 10.1016/j.neuroimage.2012.01.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Román J, Vilar-López R, Navas JF, Soriano-Mas C, & Verdejo-García A (2017). Brain reward system’s alterations in response to food and monetary stimuli in overweight and obese individuals. Human Brain Mapping, 38(2), 666–677. 10.1002/hbm.23407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara VM, Liu J, Claus ED, Hutchison K, & Calhoun V (2017). Alterations of resting state functional network connectivity in the brain of nicotine and alcohol users. NeuroImage, 151, 45–54. 10.1016/j.neuroimage.2016.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xue T, Dong F, Li Y, Xie D, Liu C, Zhang M, Bi Y, Yuan K, & Yu D (2020). The changes of brain functional networks in young adult smokers based on independent component analysis. Brain Imaging and Behavior. 10.1007/s11682-020-00289-4 [DOI] [PubMed] [Google Scholar]
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, & Hutchison KE (2015). Reduced executive and default network functional connectivity in cigarette smokers. Human Brain Mapping, 36(3), 872–882. 10.1002/hbm.22672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Rao H, Hager N, Wang J, Franklin TR, & Fan Y (2019). Classifying and characterizing nicotine use disorder with high accuracy using machine learning and resting-state fMRI. Addiction Biology, 24(4), 811–821. 10.1111/adb.12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Calhoun VD, Rachakonda S, Claus ED, Littlewood RA, Mickey J, Arenella PB, & Hutchison KE (2017). Functional network connectivity predicts treatment outcome during treatment of nicotine use disorder. Psychiatry Research: Neuroimaging, 265, 45–53. 10.1016/j.pscychresns.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]


