Abstract
Introduction.
The optimal management for immune related adverse events (irAEs) in patients who do not respond or become intolerant to steroids is unclear. Guidelines suggest additional immunosuppressants based on case reports and expert opinion.
Methods.
We examined patients with lung cancers at MSK treated with immune checkpoint blockade (ICB) from 2011-2020. Pharmacy records were queried to identify patients who received systemic steroids as well as an additional immunosuppressant (eg TNFα inhibitor, mycophenolate mofetil). Patient records were manually reviewed to examine baseline characteristics, management, and outcomes.
Results.
Among 2,750 patients with lung cancers treated with ICB, 51 (2%) received both steroids and an additional immunosuppressant for a severe irAE (TNFα inhibitor (73%), mycophenolate mofetil (20%)). The most common events were colitis (53%), pneumonitis (20%), hepatitis (12%), and neuromuscular (10%). At 90 days after start of an additional immunosuppressant, 57% were improved from their irAE, 18% were unchanged, and 25% were deceased. Improvement was more common in hepatitis (5/6) and colitis (18/27) but less common in neuromuscular (1/5) and pneumonitis (3/10). Of patients who died, 8/13 were attributable directly to the irAE and 4/13 were related to toxicity from immunosuppression (3, infection-related deaths; 1, drug-induced liver injury leading to acute liver failure).
Conclusions.
Steroid-refractory/resistant immune related adverse events are rare. While existing treatments help patients with hepatitis and colitis, many patients with other irAEs remain refractory and/or experience toxicities from immunosuppression. A more precise understanding of the pathophysiology of specific irAEs is needed to guide biologically-informed treatments for severe irAEs.
Keywords: Immune related adverse events, immune checkpoint blockade, non-small cell lung cancer, small cell lung cancer
Introduction
Immune checkpoint blockade therapy (ICB) restores and promotes antitumor immunity leading to years of benefit in a growing number of patients. In many, the homeostatic role of PD-1 and CTLA-41 pathways in preventing autoimmunity is thrown off balance, leading to immune related adverse events (irAEs). Most (non-endocrine) irAEs improve with prompt recognition, halting ICB, and steroid treatment.
Severe irAEs are rare but can be persistent/life-threatening. The management of patients with a severe irAE necessitating steroids and an additional immunosuppressant is uncertain. Consensus guidelines2–4 recommend the addition of a second immunosuppressant based on autoimmune disease management and published case reports.
To address this issue, we examined the treatments and outcomes of patients with lung cancers who experienced a steroid-refractory/resistant irAE at a single institution in detail.
Materials and methods
Patients
This retrospective study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSK). Our study population included all patients with lung cancer who started ICB between June 1, 2011 and March 1, 2019 at our institution who experienced a steroid refractory/resistant irAE prior to March 1, 2020 and followed through November 29, 2020. To identify these patients, pharmacy records were queried for those who received steroids and an additional immunosuppressant (n=229) (Supplementary Table S1). Cases were manually reviewed. We excluded dermatologic irAEs (n=8), which have been previously reported5. Best overall response (BOR) was per investigator assessment. irAE grade was determined using Common Terminology Criteria for Adverse Events (CTCAE) v5.0.
Outcomes
The primary objective of this study was to determine the use and benefit of additional immunosuppressants. We defined steroid-refractory as the addition of an immunosuppressant due to initial non-response and steroid-resistant as the addition of an immunosuppressant after initial response and inability to taper off systemic steroids. Patient outcomes were summarized at 90 days after the start of the additional immunosuppressant. See the Supplementary Methods for additional details.
Statistical analysis
Descriptive statistics was used to summarize the irAE course. Overall survival was measured from date of the steroid-refractory/resistant irAE. Linear least squares fit was used to describe the relationship between body mass index (BMI) and duration of systemic steroids. All 95% confidence interval estimates reflect an alpha level of 0.025 in each tail.
Results
Characteristics of steroid-refractory/resistant irAEs
A total of 2,750 patients with lung cancer received ICB between June 1, 2011 and March 1, 2019. Of this group, 51 (2%) had a non-dermatologic irAE that required an additional non-steroidal immunosuppressant (median follow up time from ICB start 21 months, interquartile range [IQR] 9.5-35.5) (Table 1). Most patients (n=32, 63%) received PD-(L)1 blockade without CTLA-4 blockade.
Table 1:
Baseline patient characteristics and treatment details
| Characteristic | Patients (n=51) |
|---|---|
| Median age [IQR] - yr | 64 [59-71] |
| Sex - no. (%) | |
| Female | 29 (57%) |
| Male | 22 (43%) |
| Cigarette smoking status - no. (%) | |
| Former or current | 43 (84%) |
| Never | 8 (16%) |
| Histology - no. (%) | |
| Adenocarcinoma | 36 (71%) |
| Non-adenocarcinoma | 8 (16%) |
| Small cell lung cancer | 7 (14%)* |
| PD-L1 expression by TPS - no./total no. (%) | |
| 0% | 11/33 (33%) |
| 1-49% | 7/33 (21%) |
| ≥ 50% | 15/33 (45%)* |
| Unknown or not applicable | 18 |
| Line of therapy - no. (%) | |
| 1 or early stage | 28 (55%) |
| 2 | 13 (25%) |
| 3 or higher | 10 (20%) |
| Past medical history - no. (%) | |
| Autoimmune condition^ | 7 (14%) |
| Prior thoracic radiation therapy | 11 (22%) |
| Treatment - no. (%) | |
| Anti-PD-(L)1 monotherapy | 25 (49%) |
| Anti-PD-(L)1+CTLA-4 combination | 19 (37%) |
| Anti-PD-(L)1+chemotherapy | 5 (10%) |
| Anti-PD-(L)1+other (non-chemotherapy) | 2 (4%) |
Percentages may not add up to 100 due to rounding
Autoimmune condition including thyroiditis (n=3), inflammatory bowel disease (n=2), psoriasis (n=2), reactive airways disease (n=1), type 2 diabetes (n=1). IQR = interquartile range, PD-(L)1 = programmed cell death protein (ligand) 1, TPS = tumor proportion score, CTLA-4 = cytotoxic T-lymphocyte-associated protein 4
The most common steroid-refractory/resistant irAEs were diarrhea/colitis (n=27, 53%), pneumonitis (n=10, 20%), and hepatitis (n=6, 12%) (Figure 1A). Prior thoracic radiation therapy was enriched in patients with pneumonitis compared to the remaining patients (50% [5/10] vs 15% [6/41], Fisher’s p=0.03).
Figure 1. Steroid refractory/resistant irAEs.

A. The duration of the steroid refractory/ resistant irAE, systemic steroids, and additional immunosuppressant in 51 patients with lung cancer grouped by type of irAE ordered by duration of irAE. Boxes to the left show (1) baseline autoimmune condition (Bl Ai), (2) prior thoracic radiation therapy (TRT), (3) best overall response (BOR), and (4) treatment received (PD-1 blockade monotherapy [IOx1], combined checkpoint blockade [IOx2], or chemo-immunotherapy [c+IO]). ITP: immune thrombocytopenic purpura; PR: partial response; SD: stable disease; PD: progressive disease, MMF: mycophenolate mofetil, IVIG: intravenous immunoglobulin, ICB: immune checkpoint blockade. B. The proportion of patients who improved from their steroid refractory/ resistant irAE at 90 days after the start of the second immunosuppressant grouped by type of irAE and treatment. The size of the circle represents the number of patients. The size of the arc within each circle represents the % improved.
The time from ICB start to the start of irAE differed. It was most variable for pneumonitis (median 9 months or 12 doses, range 0.6-20 months or 1-38 doses) and colitis (median 3 months or 5 doses, range 0.2-20 months or 1-35 doses) and sooner for hepatitis (median 1 month or 1 dose, range 0.3-4 months or 1-8 doses).
Of living patients, 53% (19/36) had irAE-related symptoms that lasted >6 months. Five of 18 (28%) patients with colitis, 2/4 patients with pneumonitis and 3/3 patients with neuromuscular irAEs had intermittent/persistent symptoms for >1 year.
Characteristics of irAE treatment
The second immunosuppressant was given for grade 3+ irAE in 88% (45/51) of cases. In 67% (34/51), the immunosuppressant was added due to primary non-response, in 15 patients (29%) it was given due to dependence on steroids and in 2 patients (4%) the drug was given upfront. The median time from steroid start to starting a second immunosuppressant in steroid-refractory and steroid-resistant cases was 15 days (IQR 4-31) and 150 days (IQR 103-277), respectively. Of note, 7/8 patients with colitis had symptom resolution with 1-3 doses of infliximab.
Of 51 patients, 17 (33%) started the second immunosuppressant in the hospital, and 2/6 patients with pneumonitis and 3/5 with colitis improved within a week (all received infliximab) (Supplementary Figure 1, Supplementary Table 2).
At 90 days after the start of the second immunosuppressant, 5/6 (83%) patients with hepatitis improved, and these patients received mycophenolate mofetil for a median of 3 months (range 2-5) (Figure 1B).
In patients with colitis/diarrhea, 100% received infliximab – 67% (18/27) improved and the majority (10/18, 55%) needed 1 dose. The remaining 8 patients needed 0.5-2.7 months of treatment (median 1.4, up to 3 doses).
ICB re-challenge
Most patients (n=47, 92%) discontinued ICB because of irAEs. The remaining 4 patients were re-challenged (n=1 hepatitis, n=3 colitis). One patient experienced recurrence of their steroid-refractory colitis while the others continued to benefit from ICB for 20-39 months.
Of all patients, two had pre-existing inflammatory bowel disease, both not on active treatment. Both developed steroid-refractory/resistant colitis – while 1 patient died (cause unknown), the other recovered with 1 dose of infliximab and was re-challenged with ICB for 20 months, with ongoing benefit at database lock.
Challenges of steroid-refractory/resistant irAE treatment
While 57% (29/51) of patients improved from their irAE at 90 days after starting the additional immunosuppressant, 18% (9/51) were unchanged/worsened and 25% (13/51) died (Figure 2A). Of the patients who died, 4/13 were related to immunosuppression toxicity. Three died from infection. In particular, one patient died from fungemia likely secondary to high dose corticosteroids and had no evidence of disease at the time of autopsy. One patient who received infliximab for colitis died from biopsy-diagnosed infliximab-related hepatotoxicity. Those who died from immunosuppressive therapy received more systemic steroids than those who did not (max median 525 vs 132 mg/day prednisone equivalent, Mann Whitney U p=0.004, total median 5.9k vs 2.3k mg prednisone equivalent, p=0.004).
Figure 2. Side effects of systemic steroids.
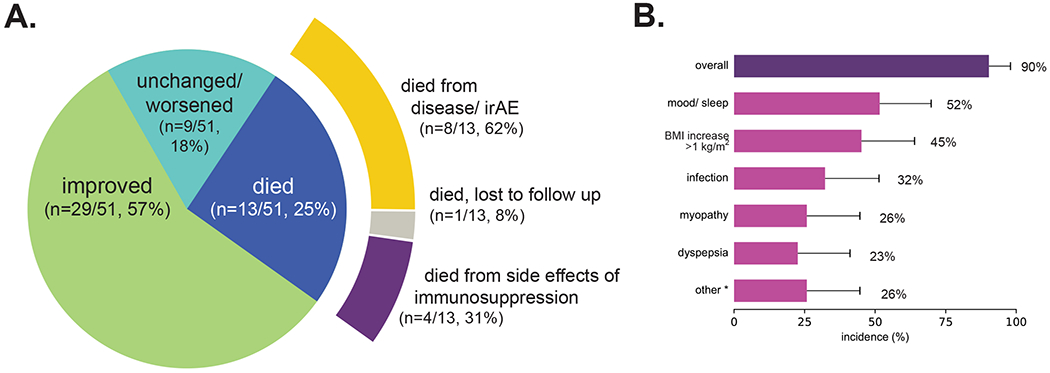
A. The proportion of patients who experienced each outcome and the most likely cause of death 90 days after the start of the second immunosuppressant. B. Side effects of systemic steroids reported by patients who received treatment for at least 30 days (n=31). Overall 90% experienced a side effect. The most common side effects were altered mood/ sleep (52%), increase in body mass index (BMI) of greater than 1 kg/m2 (45%), infection (32%), myopathy (26%), and dyspepsia (23%). * Other side effects included hyperglycemia without diabetes (n=3), hypertension (n=2), bruising (n=2), symptomatic compression fracture (n=1), and acne (n=1). Error bars reflect 95% CI estimates of the population proportion.
Of 31 patients who received at least 3 weeks of prednisone ≥20mg, most (90%, 28/31) had a side effect brought to clinical attention (Figure 2B). The most common were altered mood/sleep (52%, 16/31), an increase in body mass index (BMI) of greater than 1 kg/m2 (45%, 14/31; R2=0.2 with duration of use, Supplementary Figure 2), and infection (32%, 10/31). One patient developed compression fracture that necessitated hospitalization for pain crisis.
Discussion
We systematically examined patients who received ICB between 2011-2019 with almost two years of follow-up treated by multidisciplinary physicians, nurse practitioners, and nurses with expertise in irAE management. Overall 2% of patients required additional immunosuppressive therapies beyond steroids, termed steroid-refractory/resistant irAEs. This represents approximately 1/5 patients who have an irAE necessitating steroids.6 Most steroid-refractory/resistant irAEs lasted for months and were challenging to treat.
While patients with steroid-refractory hepatitis and colitis overall did well and responded to mycophenolate mofetil and infliximab respectively, the other irAEs were associated with symptoms lasting months and/or significant mortality. Greater than half of cases needed a prednisone equivalent of >20 mg for 3 weeks and had steroid-associated side effects.
Our findings of success of mycophenolate mofetil for immune-related hepatitis is similar to the literature7. While published case series mostly report positive outcomes from TNFα inhibition8, 9 for colitis, we found 67% of patients experienced benefit at 90 days. Almost a third of patients had intermittent/persistent diarrhea lasting for greater than a year. Almost all patients with colitis improved with one dose of TNFα inhibition. This supports early initiation of TNFα inhibition in lieu of continuing systemic steroids for months.
Our study adds granularity to the observation that high doses of steroids for irAEs can lead to adverse outcomes. Mirroring prior cases10, we identified 3 patients who died from infection likely due to steroids who otherwise benefitted from ICB (n=1 had no evidence of disease on autopsy, n=2 with radiological tumor shrinkage). One study11 found those with steroid-refractory irAEs treated with TNFα inhibition had worse survival, although the researchers did not have details to distinguish the impact of steroids versus TNFα inhibition and lacked data to control for survivor bias. Several series of immune related colitis7, 9, 12 reported earlier symptom resolution with TNFα inhibition and no significant impact on cancer outcomes. Additionally, in contrast to targeted immunosuppressives that uncouple the efficacy and toxicity of ICB13, prolonged/high-dose systemic steroids may offset ICB benefit14. These findings suggest further refinement to steroid dosing and duration.
While our study cohort is large, a limitation is it is single institution experience in treating these specific irAEs. Additionally, steroids side effects were identified in notes rather than by patient survey and likely an underestimation.
Treatment of steroid-refractory/resistant irAEs should be organ-specific, based on irAE pathophysiology. One framework may be to prospectively examine targeted immunosuppressants similar to dermatologic irAE management5. While reports of this approach to irAEs exist, mechanistic studies15 driving rational drug selection are needed.
In summary, our data indicate that steroid-refractory/resistant irAEs are a rare (but growing) challenge. While hepatitis and colitis may be straightforward to treat, management of most irAEs require further investigation. Personalized immunomodulatory treatments to address severe irAEs will be critical if we are to realize the true clinical benefit of immune checkpoint blockade in our patients.
Supplementary Material
Funding
Supported by Memorial Sloan Kettering Cancer Center Support Grant/Core [P30-CA008748]; the Druckenmiller Center for Lung Cancer Research at Memorial Sloan Kettering Cancer Center; the National Institutes of Health [T32-CA009207 to JL, K30-UL1TR00457 to JL, U01AR077511 to MEL, MDH, DYML, and JK]; the Conquer Cancer Foundation [Young Investigator Award to JL]; the Damon Runyon Cancer Research Foundation [CI-98-18 to MDH]. MDH is a member of the Parker Institute for Cancer Immunotherapy.
Conflict of Interest Statements
J.L. has received honoraria from Targeted Oncology and Physicians’ Education Resource.
M.G.K. receives personal fees from AstraZeneca, Pfizer, Regeneron, Novartis, Sanofi, and Daiichi-Sankyo; received honoraria for participation in educational programs from WebMD, OncLive, Physicians’ Education Resource, Prime Oncology, Intellisphere, Creative Educational Concepts, Peerview, i3 Health, Paradigm Medical Communications, AXIS, Carvive Systems, AstraZeneca, and Research to Practice; received travel support from AstraZeneca, Pfizer, Regeneron, and Genentech. Dr. Kris is an employee of Memorial Sloan Kettering. Memorial Sloan Kettering has received research funding from The National Cancer Institute (USA), The Lung Cancer Research Foundation, Genentech Roche, and PUMA Biotechnology for research conducted by Dr. Kris.
J.A.K. has received honoraria from Biodesix. He has units in Cireca.
M.E.L. receives institutional research funding from Lutris, Paxman, Novocure Inc, US Biotest, and Veloce; has been a compensated consultant for ADC Therapeutics America, Inc, Apricity Health, LLC, Azitra, Inc, Deciphera, Johnson and Johnson, NCODA, Novocure Inc, Kyowa Kirin, Inc, Janssen Research and Development LLC, Menlo Therapeutics, Novartis Pharmaceuticals Corp, QED Therapeutics, F. Hoffmann-La Roche AG, Amgen Inc, AstraZeneca Pharmaceuticals LP, Genentech Inc, Seattle Genetics, Lutris, Paxman Coolers, Teva Mexico, Parexel, OnQuality Pharmaceuticals Ltd, Oncodermatology, and Takeda Millenium.
B.D.S. receives institutional research funding from ADC therapeutics; has been a compensated consultant for Kite Gilead, Juno/Celgene, Insysus, Novartis, and Janssen.
M.D.H. receives institutional research funding from Bristol-Myers Squibb; has been a compensated consultant for Merck, Bristol-Myers Squibb, AstraZeneca, Genentech/Roche, Nektar, Syndax, Mirati, Shattuck Labs, Immunai, Blueprint Medicines, Achilles, Arcus and Natera; received travel support/honoraria from AstraZeneca, Eli Lilly, and Bristol-Myers Squibb; has options from Shattuck Labs, Immunai, and Arcus; has a patent filed by his institution related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208), which has received licensing fees from PGDx.
The remaining authors have no conflicts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eagar TN, Karandikar NJ, Bluestone JA, et al. The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance. European Journal of Immunology 2002;32:972–981. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology 2017;28:iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 4.Network NCC. Management of immunotherapy- related toxicities (Version 1.2020). Available at https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf.2020.
- 5.Phillips GS, Wu J, Hellmann MD, et al. Treatment Outcomes of Immune-Related Cutaneous Adverse Events. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019;37:2746–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen DH, Wei L, Bertino EM, et al. Incidence, Risk Factors, and Effect on Survival of Immune-related Adverse Events in Patients With Non–Small-cell Lung Cancer. Clinical Lung Cancer 2018;19:e893–e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horvat TZ, Adel NG, Dang T-O, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. Journal of Clinical Oncology 2015;33:3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in Patients With Cancer After Antibody Blockade of Cytotoxic T-Lymphocyte–Associated Antigen 4. Journal of Clinical Oncology 2006;24:2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arriola E, Wheater M, Karydis I, et al. Infliximab for IPILIMUMAB-Related Colitis—Letter. Clinical Cancer Research 2015;21:5642. [DOI] [PubMed] [Google Scholar]
- 10.Kyi C, Hellmann MD, Wolchok JD, et al. Opportunistic infections in patients treated with immunotherapy for cancer. Journal for ImmunoTherapy of Cancer 2014;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verheijden RJ, May AM, Blank CU, et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1–Treated Patients in the Dutch Melanoma Treatment Registry. Clinical Cancer Research 2020;26:2268. [DOI] [PubMed] [Google Scholar]
- 12.Johnson DH, Zobniw CM, Trinh VA, et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. Journal for immunotherapy of cancer 2018;6:103–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Ruiz E, Minute L, Otano I, et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 2019;569:428–432. [DOI] [PubMed] [Google Scholar]
- 14.Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706–3714. [DOI] [PubMed] [Google Scholar]
- 15.Dougan M, Luoma AM, Dougan SK, et al. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell 2021;184:1575–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


