Abstract
Background:
Repetitive Transcranial Magnetic Stimulation (rTMS) has shown initial promise in combating age-related cognitive decline and dementia. The nature and severity of cognitive aging, however, varies markedly between individuals.
Objective/Hypothesis:
We hypothesized that the distinct constellation of brain changes responsible for individual differences in cognitive aging might influence the response to rTMS.
Methods:
Cognitive effects of rTMS were evaluated using a rat model of cognitive aging in which aged rats are classified as Aged-Impaired (AI) or -Unimpaired (AU) relative to young (Y) according to their performance in the Morris water maze. Several weeks later, following presentation of a sample odor in an olfactory recognition task, rats received either sham (Y, n = 9; AU, n = 8; AI, n = 9) or intermittent Theta Burst Stimulation (Y, n = 8; AU, n = 8; AI, n = 9). Memory was tested 24 hours later.
Results:
Recognition memory in the sham and stimulated conditions depended on pre-treatment cognitive status in the aged rats. Y and AU sham rats displayed robust odor recognition, whereas sham-treated AI rats exhibited no retention. In contrast, rTMS treated AI rats showed robust retention, comparable in magnitude to Y, whereas the AU stimulated scored at chance.
Conclusion:
Our results are consistent with a perspective that the unique neurobiology associated with variability in cognitive aging modulates the response to rTMS. Protocols with documented efficacy in young adults may have unexpected outcomes in aging or neurodegenerative conditions, requiring individualized approaches.
Keywords: intermittent theta-burst stimulation, iTBS, memory, cognitive aging, state dependency
INTRODUCTION
The world’s population is aging; by 2050, the number of individuals over 60 and 80 is expected to double and triple, respectively [1, 2]. Even in the absence of neurodegenerative disease, advanced age is often accompanied by cognitive deficits that significantly compromise independence and the quality of life. Memory decline can be an early feature of Alzheimer’s disease and is one of the most commonly reported concerns among older adults [3]. However, despite intense research effort, progress on safe and effective strategies for promoting successful cognitive outcomes in aging has been limited. In the search for treatments that maintain cognitive function and slow age-related impairment caused by neurological diseases, novel approaches such as repetitive Transcranial Magnetic Stimulation (rTMS) have generated substantial interest.
The effects of rTMS on neuronal activity can persist beyond the period of direct stimulation, thought to be mediated by long-term potentiation (LTP) and depression (LTD)-like effects [4] implicated in learning and memory. Evidence that rTMS can enhance cognitive performance in young animals and humans [5–7] has generated excitement about potential applications in cognitive aging. However, while some reports have found beneficial rTMS effects on cognition in both aged animals [8–10] and older adults [11–13], others demonstrated either no benefit [14, 15] or detrimental effects [16] following treatment. Discrepant results have also been reported in patients with mild cognitive impairment and Alzheimer’s disease [17–21].
Aging is accompanied by a wide variety of neurobiological alterations that might influence the effects of rTMS, including baseline alterations in Ca2+ homeostasis and neuronal excitability [22–24], changes in neuronal morphology [25], brain atrophy [26, 27], and network-level disruptions [28, 29]. The nature and magnitude of these alterations can also vary tremendously among older individuals. Heterogeneity in cognitive status in aging has been related to disruptions in hippocampal excitability [30, 31] and decreased inhibitory interneuron protein expression [32], potentially contributing to the different results obtained across studies of rTMS.
Taken together, this background suggests that individual differences in neurocognitive aging might potentially influence the cognitive response to non-invasive brain stimulation. Here, we directly tested that hypothesis taking advantage of a rat model that features the robust interindividual differences described in cognitive aging.
MATERIALS AND METHODS
Animals
Young (n=17; 6–7 months of age) and aged (n=34; 24–25 months of age) male Long–Evans rats (Charles River Laboratories) were individually housed and maintained in a climate-controlled vivarium on a 12-hour light/dark cycle at the National Institute on Aging (NIA, Baltimore, MD). Standard rat chow and water were available ad libitum throughout the experiments. All procedures were approved by the Animal Care and Use Committee of the Intramural Research Program of the NIA.
Background behavioral characterization
The overall experimental design is schematically illustrated in Figure 1A. To establish the baseline cognitive status of the animals, rats were initially tested in a ‘place’ version of the Morris water maze task as previously described [33]. Training continued over 8 consecutive days, three training trials per day. Every other day, the third trial was a probe in which the platform was inaccessible for 30 seconds, interspersed with four-probe trials (one on the last trial of every other day). A learning index (LI) score was calculated for each animal from their average proximity (in cm) to the hidden escape location across the last three probe trials. Lower LI scores indicate better task performance. The LI measure has provided a reliable metric for classifying aged rats as either AU or AI, with a cut off based on the normative distribution of scores for many hundreds of young rats in previous research [33–36]. Aged rats that performed on par with young animals were denoted Aged Unimpaired (AU), while rats that scored greater than the young were classified as Aged Impaired (AI)[32]. To control for non-mnemonic deficits, rats were tested in a single session of a hippocampus-independent cued water maze protocol the following day. No animals that performed outside the normal range on this version of the task were included in the present experiments.
FIGURE.
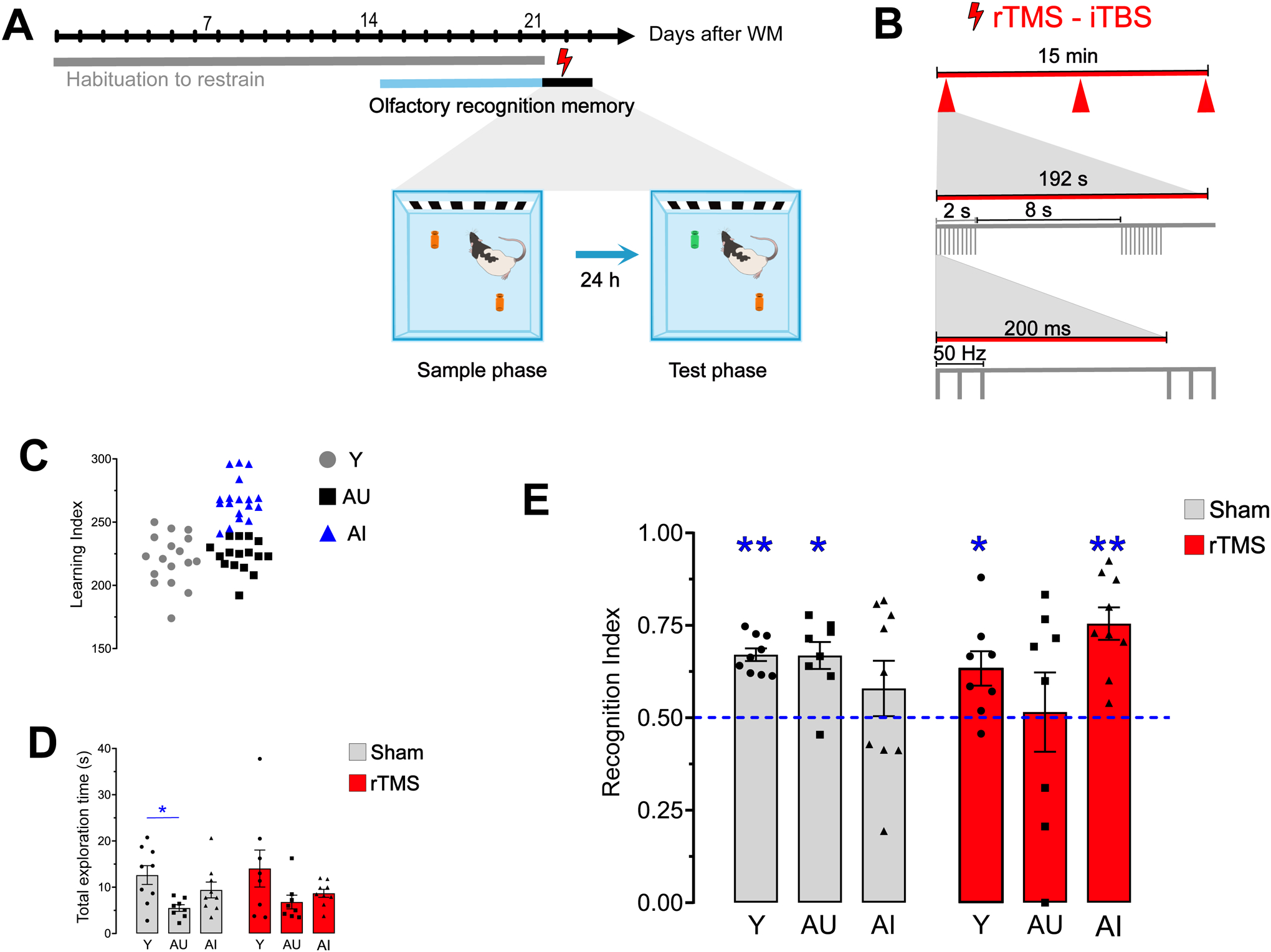
A) Experimental design. The effect of repetitive Transcranial Magnetic Stimulation – intermittent Theta Burst Stimulation (rTMS-iTBS) on long-term memory was assessed using the Novel Odor Recognition memory task in awake rats after a 3-week habituation period (represented by the blue bar). B) iTBS protocol. C) Distribution of Learning Index scores for individual young, Aged-Unimpaired (AU), and Aged-Impaired (AI) rats, derived from performance on the Morris water maze. D) Graphs showing no statistically significant differences in total exploration time between sham and stimulated animals. E) Graphs of Recognition Index scores in sham and stimulated animals: young (Y) and AU sham-treated groups displayed above chance memory for the sample odorant, whereas AI did not. The Y and AI groups that received post-sample rTMS demonstrated robust 24-hour memory, whereas AU rats exhibited no recognition. One-sample t-test compared to chance, 0.5 (i.e., no retention), *p < 0.05, **p < 0.001. Error bars = standard error.
Restraint protocol for delivering Transcranial Magnetic Stimulation in awake rats
Approximately one week after water maze testing, rats underwent a 3-week, graded habituation to the physical restraint used during subsequent rTMS administration. To reduce stress and facilitate consistent positioning of the TMS coil, snuggle sacks were custom-made from disposable medical arm sleeves and self-adhering elastic wrap. Animals were initially accommodated to walking through the sleeve, where over subsequent days they were held in the narrow end using an elastic wrap, adjusted for a snug fit. Animals were gradually habituated in this manner across sessions until they consistently accepted 15 minutes of restraint. Earlier sessions were terminated when animals displayed significant struggling. The dorsal surface of the rat’s head was left exposed, facilitating appropriate positioning of the TMS coil. Beginning the second week of habituation, the TMS unit (described below) was turned on during restraint in order to acclimate animals to the noise produced by the stimulator. All procedures were performed by the same experimenter in a quiet room, under red-light illumination, to minimize stress.
Olfactory recognition memory test
A recently adapted implicit odor recognition task was used to assess the effects of rTMS on long-term memory [37](Figure 1A). Square arenas (60 × 60 cm) with dark brown plexiglass walls and lined with corncob bedding were placed in the same dim-light illuminated room where animals were restrained for rTMS delivery. A video camera was mounted above the arenas, and activity during test sessions was digitized with Any-Maze Software (Version 5.3; Stoelting Co., Wood Dale, IL). Once a day for five consecutive days, animals were placed individually in the empty open-field arena for 3 minutes immediately after the restraint period. This phase of the protocol aimed to habituate animals to the overall testing environment and thereby increase the relative salience of the odor stimuli used to test memory. In subsequent sessions, odorants were presented in two identical amber glass vials (2.5 cm in diameter and 5 cm in height), fixed to the arena floor with Velcro (10 cm from the wall at the back corners). The position of the vials was constant, and they contained a small cotton plug infused with a suprathreshold concentration of either freshly diluted commercial orange or lime extract (McCormick, Hunt Valley, MD; 200 ul in 1ml deionized water). Odors were selected based on earlier standardization experiments in which rats explored odor pairs from a battery of perceptually distinct scents. Young and old rats spent comparable amounts of time exploring each of the two odorants chosen for the current study, confirming that, on average, animals fail to display prepotent preference or aversion for the individual test stimuli. Specifically, young (n=8) and aged (n=16) animals directed equivalent amounts of exploration to both orange and lime: young 7.19±4.62 s for orange and 6.86±3.16 s for lime; aged 5.51±2.81 s for orange and 5.02±2.26 s for lime (F (3,44) = 2.245, p = 0.305). The arena was cleaned with 70% ethanol after each animal. Different odor vials, thoroughly cleaned with ethanol, were used for each animal.
In the sample phase, both vials contained the same odorant (counter-balanced for lime and orange across animals within groups). Rats were placed in the arena facing the wall opposite the vials and allowed to freely explore for 10 minutes. They were removed immediately after and quickly restrained for rTMS or sham treatment (see below). Long-term memory was tested 24 h later. During the memory test, one vial contained the sample odorant, and the other held the novel odor (with the left/right position of the novel stimulus counter-balanced). Rats explored freely for 5 min during the memory test. Video recordings were scored manually by two independent raters blinded to baseline cognitive status and odor identities using BORIS software (Version 7.9.19, University of Torino, Italy; [38]).
Odor exploration was defined as a rat orienting its snout within 1 cm of one of either stimulus vial opening. Contacting other parts of the body or sitting on the vials was not considered odor-directed exploratory behavior. Rats with cumulative odor exploration totaling less than 2 s during the memory test, or that failed to explore both odorants during the sample phase, were excluded from the analysis. A Recognition Index (RI) was calculated, as previously described for object recognition [39], where RI = cumulative exploration of the novel odor/total odor exploration (sample plus novel) during the memory test. By this measure, 0.5 indicates no odor preference, reflecting a lack of memory for the sample stimulus.
repetitive Transcranial Magnetic Stimulation
Immediately after the sample presentation (i.e., overlapping the presumptive period of memory consolidation), animals were manually restrained as described earlier for either rTMS or sham treatment. rTMS was delivered using a Magstim Rapid2 stimulator with a 70-mm figure-eight coil (The Magstim Company, Whitland, Dyfed, UK). Restrained rats in the stimulated groups were manually positioned with the coil centered dorsomedially between the eyes and ears and the coil handle perpendicular to the rat’s body axis [40]. For the sham groups, the coil was placed adjacent and facing away from the animal’s head, ensuring that auditory conditions were similar, but no electromagnetic stimulation was delivered. Rats received 3, 192-second bouts of intermittent Theta Burst Stimulation over the course of a 15 minute session (iTBS, Fig. 1B). Each bout of iTBS consisted of 20 trains of 3, 50-Hz pulse bursts repeated at 5 Hz for 2 seconds, with a 10-second inter-train interval [41]. In total, stimulated rats received 600 pulses in each 192-second bout, for a combined total of 1800 pulses. Each iTBS block was 192 s (~3 min), 3 min apart from each other. Stimulation intensity was set to 15 % of maximum output [9]. The stimulation protocol was adapted from earlier work in rats [42–44], using an intensity of stimulation just below that expected to elicit an evoked motor response [45, 46] and adjusted to accommodate the longest interval rats consistently tolerated restraint without signs of distress during habituation. No evidence of overt seizures was noted in any animal in the present experiments.
Statistical analysis
All statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, II, USA, version 22). The Kolmogorov-Smirnov test was used to test for normal distribution of the data, and Levene’s test was used to test for equality of variances for all variables. Two-way analysis of variance (ANOVA) was performed with age, treatment, and interaction terms (age*treatment) as independent factors, investigating differences between sham vs. treated groups and whether the effects of stimulation on RI differed as a function of age. The same analysis was performed replacing the age variable with the cognitive status variable to investigate differences between sham vs. treated groups and whether the effects of stimulation on RI differed as a function of pre-treatment cognitive status. Memory retention in sham and stimulated rats were also investigated using one-sample t-tests, comparing RI scores to 0.5 (i.e., comparing investigation preference for the novel odor to chance) [47–49].
RESULTS
Spatial learning and memory capture divergent cognitive trajectories in aging
As in many previous reports [50], we found greater variability in spatial learning and memory with age. Sixteen aged animals had LI scores ≤ 240 and were classified as AU, and 18 were AI (LI scores > 240, Figure 1C).
rTMS did not affect total exploration time
We first tested the possibility that rTMS might affect exploratory behavior non-specifically, independent of the memory demands of the task. The overall pattern of results showed that total exploration time was lower in aged rats than young, as might be expected as a consequence of physical aging. However, total exploration time failed to differ across stimulation conditions or in relation to cognitive status determined in the water maze (Fig. 1D, see Table 1 for descriptive statistics). Specifically, sham-treated young rats explored more than the sham AU group (F (2, 23) = 4.55, p = 0.021; Tukey post hoc: young vs. AU: p = 0.015; young vs. AI: p = 0.349; and AU vs. AI: p = 0.240) in the test phase. In the rTMS condition, total exploration time was not statically different among the groups (F (2,22) = 2.32, p = 0.121. Tukey post hoc: young vs. AU: p = 0.119; young vs. AI: p = 0.275; and AU vs. AI: p = 0.847). Analysis of exploration time within age groups across the sham and rTMS conditions showed no statistical differences (young sham vs. young rTMS: t (15) = −0.327, p = 0.748, AU sham vs. AU rTMS: t (14) = −0.798, p = 0.438, and AI sham vs. AI rTMS: t (16) = 0.376, p = 0.712).
Table 1:
Descriptive statistics of the novel odor recognition task metrics.
| Young | Aged | AU | AI | ||
|---|---|---|---|---|---|
| Sham | N | 9 | 17 | 8 | 9 |
| Time novel (s) | 8.49 ± 1.44 | 4.47 ± 0.04 | 3.53 ± 0.35 | 5.3 ± 1.01 | |
| Time familiar (s) | 4.14 ± 0.66 | 3.09 ± 0.70 | 1.97 ± 0.44 | 4.01 ± 1.21 | |
| Total exploration time (s) | 12.63 ± 2.02 | 7.56 ± 1.05 | 5.5 ± 0.71 | 9.4 ± 1.71 | |
| Recognition Index | 0.67 ± 0.02 | 0.62 ± 0.04 | 0.67 ± 0.03 | 0.58 ± 0.07 | |
| rTMS | N | 8 | 17 | 8 | 9 |
| Time novel (s) | 8.28 ± 1.91 | 5.21 ± .079 | 3.62 ± 1.19 | 6.62 ± 0.85 | |
| Time familiar (s) | 5.76 ± 2.28 | 2.58 ± 0.43 | 3.18 ± 0.79 | 2.05 ± 0.37 | |
| Total exploration time (s) | 14.04 ± 3.99 | 7.8 ± 0.83 | 6.81 ± 1.48 | 8.68 ± 0.87 | |
| Recognition Index | 0.63 ± 0.04 | 0.64 ± 0.06 | 0.51 ± 0.10 | 0.75 ± 0.04 |
Mean ± std error. AU: aged-unimpaired rats based on water maze performance; AI: aged-impaired rats based on water maze performance; rTMS: repetitive Transcranial Magnetic Stimulation; Recognition Index (novel odor exploration/total exploration time).
rTMS effect on odor recognition memory in aged rats depends on pre-treatment cognitive status
RI scores were normally distributed across groups (Kolmogorov-Smirnov test). A two-way ANOVA examining age and treatment as between-subject factors failed to detect significant main effects. However, the interaction between treatment and group was significant, both when the young group was included in the analysis (F (2,45) = 3.796, p = 0.03), and when the AU and AI subgroups were considered alone (F (1,30) = 5.410, p = 0.027).
The observed interaction effect directly documents that the influence of rTMS on 24-hour recognition memory differed in the unimpaired and impaired aged subgroups, i.e., in aged rats distinguished by their pre-treatment cognitive status. The difference was evident in statistical comparisons of RI scores relative to chance (i.e., in one-sample t-tests). In the sham condition, both the Y and AU groups displayed significant retention, focusing their exploration on the novel odor at levels significantly above chance (one-sample t test compared with 0.5, young: t (8) = 9.88, p < 0.001, mean difference 0.17, 95 % confidence interval [CI, 0.13, 0.21]; AU: t (7) = 4.62, p = 0.002, mean difference 0.17, 95 % CI [0.08, 0.25]). AI rats, in contrast, failed to exhibit reliable memory for the sample odor (t (8) = 1.06, p = 0.32, mean difference 0.08, 95 % CI [−0.09, 0.25]). Correlations between performance measures on the olfactory memory test and LI scores were not statistically significant in the aged group. Nonetheless, the pattern of results for odor recognition memory in sham controls mirrored the variability in cognitive outcomes documented in the same animals in the water maze.
Findings from the aged rats that received rTMS during the immediate post sample interval, by comparison, were essentially the opposite seen in the sham condition. Notably, the AI-rTMS group displayed statistically robust retention (t (8) = 5.82, p < 0.001, mean difference 0.25, 95 % CI [0.15, 0.35]), comparable in magnitude to Y rats, whereas the aged group with intact spatial memory failed to exhibit reliable retention following rTMS (t (7) = 0.144, p = 0.89, mean difference 0.15, 95 % CI [−0.02, 0.26]; Fig. 1E). Although young rats that received rTMS also showed significant odor recognition, there was no hint of an improvement above Y-sham values, and in fact, stimulation appeared to increase inter-subject variability (Leven’s test, F = 4.58, p = 0.049). These results confirm that the effects of rTMS on memory were dependent on pre-treatment cognitive status in aged rats (Fig. 1E).
Together, the findings encourage the perspective that rTMS has promise as a potentially effective tool against memory impairment in aging. However, outcomes can vary dramatically across individuals, and our results underscore the need for increased attention to the factors that dictate the response to treatment.
DISCUSSION
Brain stimulation techniques such as rTMS have attracted attention as safe, non-invasive interventions for enhancing memory and slowing age-related cognitive decline. Encouraging preliminary evidence also suggests that rTMS can improve function in mild cognitive impairment [51–53] and Alzheimer’s disease [21, 54–57]. However, firmly establishing clinical efficacy has been challenging due to a variety of design and participant sampling issues (e.g., for a review, see [20]). Studies in animal models are illuminating in this regard since they circumvent many life exposure differences and other influences that complicate interpretation in human data. Here, using a well-established rat model of cognitive aging, we report that the effects of rTMS on olfactory recognition are heterogeneous and depend on the pre-treatment status of memory. Specifically, 24-hour olfactory recognition profited from rTMS in aged rats with documented deficits in spatial memory. In contrast, the same stimulation protocol markedly impaired recognition memory in aged animals that scored as well as young adults in the water maze.
Increased interindividual variability is among the most reliable features of cognitive aging; many individuals show substantial impairment, whereas cognitive function is relatively preserved in others throughout life [58]. Age-related cognitive decline results at least in part from molecular changes that drive dysfunctional synaptic connectivity and disrupt neural network dynamics in vulnerable circuitry critical for normal function [59]. Although successful cognitive aging can reflect simply the absence of brain aging or ‘brain maintenance,’ it is increasingly evident that preserved function in late-life can also be supported by various compensatory or adaptive neural mechanisms, distinct from the substrates engaged in the young brain [60]. Based on this variability, it seems reasonable to suspect that the effects of brain stimulation might also vary across the neurocognitive aging spectrum. Our findings in rats are consistent with that prediction and provide a potential account of recent related observations in healthy older adults [61]. In that study, participants that were stratified as cognitively impaired or normal based on pre-treatment cognitive assessment showed drastically different LTP-like responses to the same iTBS protocol. Thus, the effects of rTMS appear conditioned on the state of neural substrates underlying plastic events in the brain, which are substantially variable even among healthy older adults.
Studies in animal models of accelerated aging and dementia have shown that rTMS can improve performance on hippocampus-dependent memory tasks [62–64]. The findings are congruent with those presented here, demonstrating a robust benefit of immediate post-sample rTMS on 24-hour recognition memory. Although the mechanisms-of-action of rTMS remain poorly understood, human neuroimaging of pre- and post-rTMS resting-state functional connectivity provide evidence of rapid network reorganization that extends substantially beyond the stimulated network [65]. At the cellular level, rTMS-induced network reorganization likely involves modulation of synaptic plasticity in local and distantly connected brain regions [66, 67]. Noteworthy in this context, results from in vitro and in vivo experimental models demonstrate that rTMS can increase LTP and rescue LTP deficits in animal models of dementia [64, 68–70]. Based on this background, it is tempting to speculate that the benefits of post-training rTMS in the present experiment might reflect a distributed neural network effect, mediated by compensating for or reversing the dysfunctional synaptic plasticity [71, 72] and age-related disruptions in hippocampal excitability [30, 31] reported in aged rats with spatial memory impairment.
Notably, under identical experimental conditions, rTMS markedly impaired 24-hour odor recognition in aged animals with intact pre-treatment spatial memory. Preserved cognitive function in late life is often assumed to result from simply limited brain aging, or ‘brain maintenance’ [73, 74], and by this view, one might expect that the effects of rTMS would be similar in Y and AU rats. However, successful aging is also accompanied by a variety of compensatory or adaptive processes, yielding plasticity, circuit dynamics, and cognitive strategies distinct from young. Since aging leads to reduced resilience to insults [75], rTMS may have worked as a perturbation that could not be overcome in cognitively intact animals that received treatment, disrupting the existing functional neural system. Our findings suggest that the constellation of neural adaptations that support positive cognitive outcomes also render the aged brain vulnerable to disruption after stimulation, under conditions that have little effect in younger individuals. Together the results underscore the importance of an individualized approach, recognizing that differences in background neurobiological context across the age and cognitive status spectrum can powerfully modulate the cognitive response to brain stimulation.
The limitations of research on non-invasive brain stimulation in experimental animal models are significant. Localizing the effective zone of stimulation is a major challenge, particularly using equipment designed for human clinical application, as is the case in most studies [76–78]. Still, it is noteworthy that recent modeling efforts, including direct cross-species comparisons, suggest that the effective field size of stimulation from standard clinical coils is relatively small [79]. There is also a need for increased attention to the time course and duration of rTMS benefits and to understanding the cell and circuit basis of stimulation effects. Nonetheless, the implications of preclinical animal work for future therapeutic applications should not be overlooked. Identifying the appropriate target population for treatment is likely to be critical, as protocols with documented safety in healthy young adults may have unexpected, adverse effects in brains compromised by aging or neurodegenerative disease. Future work aimed at identifying risk biomarkers for negative trajectories of cognitive aging will benefit the development and tracking of individualized interventions using non-invasive brain stimulation.
CONCLUSION
Overall, our results show that rTMS treatment differently affects memory retention in aged rats based on pre-treatment cognitive status defined by spatial learning capacity. They support the view that rTMS may be a useful therapeutic in treating cognitive disorders of aging. However, individual differences in neurocognitive status can critically dictate the response to rTMS, highlighting the importance of individualized intervention strategies. Preclinical studies, especially research assessing longitudinal effects of rTMS, can significantly inform this area, moving us closer to the safe and effective therapeutic application of rTMS and related modalities.
The nature and severity of cognitive aging varies markedly between individuals
The effects of rTMS in aged rats depended on pre-treatment cognitive status
The neurobiology of cognitive aging modulates the response to rTMS
Our study highlights the importance of individualized intervention strategies
ACKNOWLEDGMENTS
This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging (NIH/NIA/IRP). We would like to thank Dr. Marta Woloszynowska-Fraser and Lynde Wangler for providing language help and proofreading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATIONS OF INTEREST: none
REFERENCES
- 1.Ortman JM, Velkoff VA, and Hogan H, An Aging Nation: The Older Population in the United States. Population Estimates and Projections. United States Census Bureau, 2014. [Google Scholar]
- 2.He W, Goodkind D, and Kowal P, An Aging World: 2015 International Population Reports. United States Census Bureau, 2016: p. P95/16–1. [Google Scholar]
- 3.National Association of Area Agencies on Aging, National Council on Aging and United Healthcare, in The 2015 United States of Aging Survey. 2015.
- 4.Huang YZ, et al. , The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin Neurophysiol, 2011. 122(5): p. 1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed Z and Wieraszko A, Modulation of learning and hippocampal, neuronal plasticity by repetitive transcranial magnetic stimulation (rTMS). Bioelectromagnetics, 2006. 27(4): p. 288–94. [DOI] [PubMed] [Google Scholar]
- 6.Shang Y, et al. , Repetitive transcranial magnetic stimulation effectively facilitates spatial cognition and synaptic plasticity associated with increasing the levels of BDNF and synaptic proteins in Wistar rats. Neurobiol Learn Mem, 2016. 134 Pt B: p. 369–78. [DOI] [PubMed] [Google Scholar]
- 7.Hoy KE, et al. , Enhancement of Working Memory and Task-Related Oscillatory Activity Following Intermittent Theta Burst Stimulation in Healthy Controls. Cereb Cortex, 2016. 26(12): p. 4563–4573. [DOI] [PubMed] [Google Scholar]
- 8.Wang HL, et al. , Chronic high-frequency repetitive transcranial magnetic stimulation improves age-related cognitive impairment in parallel with alterations in neuronal excitability and the voltage-dependent Ca2+ current in female mice. Neurobiol Learn Mem, 2015. 118: p. 1–7. [DOI] [PubMed] [Google Scholar]
- 9.Zhu H, et al. , The effects of repetitive transcranial magnetic stimulation on the cognition and neuronal excitability of mice. Electromagn Biol Med, 2019: p. 1–11. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, et al. , Repetitive transcranial magnetic stimulation applications normalized prefrontal dysfunctions and cognitive-related metabolic profiling in aged mice. PLoS One, 2013. 8(11): p. e81482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotelli M, et al. , Action and Object Naming in Physiological Aging: An rTMS Study. Front Aging Neurosci, 2010. 2: p. 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilakantan AS, et al. , Network-targeted stimulation engages neurobehavioral hallmarks of age-related memory decline. Neurology, 2019. 92(20): p. e2349–e2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SH, et al. , Effects of five daily high-frequency rTMS on Stroop task performance in aging individuals. Neurosci Res, 2012. 74(3–4): p. 256–60. [DOI] [PubMed] [Google Scholar]
- 14.Levkovitz Y and Segal M, Aging affects transcranial magnetic modulation of hippocampal evoked potentials. Neurobiol Aging, 2001. 22(2): p. 255–63. [DOI] [PubMed] [Google Scholar]
- 15.Vidal-Pineiro D, et al. , Task-dependent activity and connectivity predict episodic memory network-based responses to brain stimulation in healthy aging. Brain Stimul, 2014. 7(2): p. 287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beynel L, et al. , Effects of online repetitive transcranial magnetic stimulation (rTMS) on cognitive processing: A meta-analysis and recommendations for future studies. Neurosci Biobehav Rev, 2019. 107: p. 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iimori T, et al. , Effectiveness of the prefrontal repetitive transcranial magnetic stimulation on cognitive profiles in depression, schizophrenia, and Alzheimer’s disease: A systematic review. Prog Neuropsychopharmacol Biol Psychiatry, 2019. 88: p. 31–40. [DOI] [PubMed] [Google Scholar]
- 18.Anderkova L and Rektorova I, Cognitive effects of repetitive transcranial magnetic stimulation in patients with neurodegenerative diseases - clinician’s perspective. J Neurol Sci, 2014. 339(1–2): p. 15–25. [DOI] [PubMed] [Google Scholar]
- 19.Cheng CPW, et al. , Effects of repetitive transcranial magnetic stimulation on improvement of cognition in elderly patients with cognitive impairment: a systematic review and meta-analysis. Int J Geriatr Psychiatry, 2018. 33(1): p. e1–e13. [DOI] [PubMed] [Google Scholar]
- 20.Weiler M, et al. , Transcranial Magnetic Stimulation in Alzheimer’s Disease: Are We Ready? eNeuro, 2020. 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabbagh M, et al. , Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer’s disease. Alzheimers Dement, 2020. 16(4): p. 641–650. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Bodhinathan K, and Foster TC, Susceptibility to Calcium Dysregulation during Brain Aging. Front Aging Neurosci, 2009. 1: p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randall AD, Booth C, and Brown JT, Age-related changes to Na+ channel gating contribute to modified intrinsic neuronal excitability. Neurobiol Aging, 2012. 33(11): p. 2715–20. [DOI] [PubMed] [Google Scholar]
- 24.Disterhoft JF and Oh MM, Alterations in intrinsic neuronal excitability during normal aging. Aging Cell, 2007. 6(3): p. 327–36. [DOI] [PubMed] [Google Scholar]
- 25.Dickstein DL, et al. , Changes in the structural complexity of the aged brain. Aging Cell, 2007. 6(3): p. 275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terry RD and Katzman R, Life span and synapses: will there be a primary senile dementia? Neurobiol Aging, 2001. 22(3): p. 347–8; discussion 353–4. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, et al. , Aging of cerebral white matter. Ageing Res Rev, 2017. 34: p. 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews-Hanna JR, et al. , Disruption of large-scale brain systems in advanced aging, in Neuron. 2007: United States. p. 924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eyler LT, et al. , A review of functional brain imaging correlates of successful cognitive aging. Biol Psychiatry, 2011. 70(2): p. 115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes CA, Rao G, and McNaughton BL, Increased electrotonic coupling in aged rat hippocampus: a possible mechanism for cellular excitability changes. J Comp Neurol, 1987. 259(4): p. 549–58. [DOI] [PubMed] [Google Scholar]
- 31.Dickerson BC, et al. , Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol, 2004. 56(1): p. 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiegel AM, et al. , Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J Comp Neurol, 2013. 521(15): p. 3508–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallagher M, Burwell R, and Burchinal M, Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci, 1993. 107(4): p. 618–26. [DOI] [PubMed] [Google Scholar]
- 34.Rapp PR and Gallagher M, Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A, 1996. 93(18): p. 9926–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haberman R, Quigley C, and Gallagher M, Characterization of CpG island DNA methylation of impairment-related genes in a rat model of cognitive aging. Epigenetics, 2012. 7(9): p. 1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomás Pereira I and Burwell RD, Using the spatial learning index to evaluate performance on the water maze. Behav Neurosci, 2015. 129(4): p. 533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno-Castilla P, et al. , Hippocampal activity induced by increasing memory demand in an olfactory recognition task is altered in aged rats. Poster Sessions Wednesday/Thursday. Journal of Neurochemistry, 2019. 150(S1): p. 162–251. [Google Scholar]
- 38.Friard O and Gamba M, BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution, 2016. 7(11): p. 1325–1330. [Google Scholar]
- 39.Ennaceur A and Delacour J, A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res, 1988. 31(1): p. 47–59. [DOI] [PubMed] [Google Scholar]
- 40.Trippe J, et al. , theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp Brain Res, 2009. 199(3–4): p. 411–21. [DOI] [PubMed] [Google Scholar]
- 41.Huang YZ, et al. , Theta burst stimulation of the human motor cortex. Neuron, 2005. 45(2): p. 201–6. [DOI] [PubMed] [Google Scholar]
- 42.Benali A, et al. , Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci, 2011. 31(4): p. 1193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoppenrath K and Funke K, Time-course of changes in neuronal activity markers following iTBS-TMS of the rat neocortex. Neurosci Lett, 2013. 536: p. 19–23. [DOI] [PubMed] [Google Scholar]
- 44.Jazmati D, Neubacher U, and Funke K, Neuropeptide Y as a possible homeostatic element for changes in cortical excitability induced by repetitive transcranial magnetic stimulation. Brain Stimul, 2018. 11(4): p. 797–805. [DOI] [PubMed] [Google Scholar]
- 45.Mix A, et al. , Continuous and intermittent transcranial magnetic theta burst stimulation modify tactile learning performance and cortical protein expression in the rat differently. Eur J Neurosci, 2010. 32(9): p. 1575–86. [DOI] [PubMed] [Google Scholar]
- 46.Mix A, Hoppenrath K, and Funke K, Reduction in cortical parvalbumin expression due to intermittent theta-burst stimulation correlates with maturation of the perineuronal nets in young rats. Dev Neurobiol, 2015. 75(1): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 47.Scott GA, Mtetwa M, and Lehmann H, Novel odour recognition memory is independent of the hippocampus in rats. Exp Brain Res, 2013. 224(2): p. 199–209. [DOI] [PubMed] [Google Scholar]
- 48.Gaskin S, Tremblay A, and Mumby DG, Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus, 2003. 13(8): p. 962–9. [DOI] [PubMed] [Google Scholar]
- 49.Mumby DG, et al. , Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus, 2005. 15(8): p. 1050–6. [DOI] [PubMed] [Google Scholar]
- 50.Gallagher M, Burwell R, and Burchinal M, Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav Neurosci, 2015. 129(4): p. 540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drumond Marra HL, et al. , Transcranial Magnetic Stimulation to Address Mild Cognitive Impairment in the Elderly: A Randomized Controlled Study. Behav Neurol, 2015. 2015: p. 287843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padala PR, et al. , Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: A double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res, 2018. 261: p. 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turriziani P, et al. , Enhancing memory performance with rTMS in healthy subjects and individuals with Mild Cognitive Impairment: the role of the right dorsolateral prefrontal cortex. Front Hum Neurosci, 2012. 6: p. 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmed MA, et al. , Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer’s dementia. J Neurol, 2012. 259(1): p. 83–92. [DOI] [PubMed] [Google Scholar]
- 55.Cotelli M, et al. , Effect of transcranial magnetic stimulation on action naming in patients with Alzheimer disease. Arch Neurol, 2006. 63(11): p. 1602–4. [DOI] [PubMed] [Google Scholar]
- 56.Rutherford G, Lithgow B, and Moussavi Z, Short and Long-term Effects of rTMS Treatment on Alzheimer’s Disease at Different Stages: A Pilot Study. J Exp Neurosci, 2015. 9: p. 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Y, et al. , Adjunctive treatment with high frequency repetitive transcranial magnetic stimulation for the behavioral and psychological symptoms of patients with Alzheimer’s disease: a randomized, double-blind, sham-controlled study. Shanghai Arch Psychiatry, 2015. 27(5): p. 280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindenberger U, Human cognitive aging: corriger la fortune? Science, 2014. 346(6209): p. 572–8. [DOI] [PubMed] [Google Scholar]
- 59.Morrison JH and Baxter MG, The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci, 2012. 13(4): p. 240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rapp PR, Bañuelos C, and Myrum C, Neuroadaptive Trajectories of Healthy Mindspan: From Genes to Neural Networks, in The Cambridge Handbook of Cognitive Aging: A Life Course Perspective, Gutchess A and Thomas AK, Editors. 2020, Cambridge University Press: Cambridge. p. 62–81. [Google Scholar]
- 61.Sundman MH, et al. , Transcranial magnetic stimulation reveals diminished homoeostatic metaplasticity in cognitively impaired adults. Brain Commun, 2020. 2(2): p. fcaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma J, et al. , The Role of Hippocampal Structural Synaptic Plasticity in Repetitive Transcranial Magnetic Stimulation to Improve Cognitive Function in Male SAMP8 Mice. Cell Physiol Biochem, 2017. 41(1): p. 137–144. [DOI] [PubMed] [Google Scholar]
- 63.Wang F, et al. , The restoration after repetitive transcranial magnetic stimulation treatment on cognitive ability of vascular dementia rats and its impacts on synaptic plasticity in hippocampal CA1 area. J Mol Neurosci, 2010. 41(1): p. 145–55. [DOI] [PubMed] [Google Scholar]
- 64.Zhang N, et al. , Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience, 2015. 311: p. 284–91. [DOI] [PubMed] [Google Scholar]
- 65.Beynel L, Powers JP, and Appelbaum LG, Effects of repetitive transcranial magnetic stimulation on resting-state connectivity: A systematic review. Neuroimage, 2020. 211: p. 116596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ziemann U, TMS induced plasticity in human cortex. Rev Neurosci, 2004. 15(4): p. 253–66. [DOI] [PubMed] [Google Scholar]
- 67.Ziemann U, et al. , Consensus: Motor cortex plasticity protocols. Brain Stimul, 2008. 1(3): p. 164–82. [DOI] [PubMed] [Google Scholar]
- 68.Thickbroom GW, Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res, 2007. 180(4): p. 583–93. [DOI] [PubMed] [Google Scholar]
- 69.Wang F, et al. , Improvement of spatial learning by facilitating large-conductance calcium-activated potassium channel with transcranial magnetic stimulation in Alzheimer’s disease model mice. Neuropharmacology, 2015. 97: p. 210–9. [DOI] [PubMed] [Google Scholar]
- 70.Zhen J, et al. , Gamma rhythm low field magnetic stimulation alleviates neuropathologic changes and rescues memory and cognitive impairments in a mouse model of Alzheimer’s disease. Alzheimers Dement (N Y), 2017. 3(4): p. 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burke SN and Barnes CA, Neural plasticity in the ageing brain. Nat Rev Neurosci, 2006. 7(1): p. 30–40. [DOI] [PubMed] [Google Scholar]
- 72.Gray DT and Barnes CA, Distinguishing adaptive plasticity from vulnerability in the aging hippocampus. Neuroscience, 2015. 309: p. 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Düzel E, et al. , Functional phenotyping of successful aging in long-term memory: Preserved performance in the absence of neural compensation. Hippocampus, 2011. 21(8): p. 803–14. [DOI] [PubMed] [Google Scholar]
- 74.Nyberg L, et al. , Memory aging and brain maintenance. Trends Cogn Sci, 2012. 16(5): p. 292–305. [DOI] [PubMed] [Google Scholar]
- 75.Bickford PC, Flowers A, and Grimmig B, Aging leads to altered microglial function that reduces brain resiliency increasing vulnerability to neurodegenerative diseases. Exp Gerontol, 2017. 94: p. 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lippmann B, Barmashenko G, and Funke K, Effects of repetitive transcranial magnetic and deep brain stimulation on long-range synchrony of oscillatory activity in a rat model of developmental schizophrenia. European Journal of Neuroscience. n/a(n/a). [DOI] [PubMed] [Google Scholar]
- 77.Kloosterboer E and Funke K, Repetitive transcranial magnetic stimulation recovers cortical map plasticity induced by sensory deprivation due to deafferentiation. The Journal of Physiology, 2019. 597(15): p. 4025–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Charles James J and Funke K, Repetitive transcranial magnetic stimulation reverses reduced excitability of rat visual cortex induced by dark rearing during early critical period. Developmental Neurobiology, 2020. 80(11–12): p. 399–410. [DOI] [PubMed] [Google Scholar]
- 79.Alekseichuk I, et al. , Comparative modeling of transcranial magnetic and electric stimulation in mouse, monkey, and human. Neuroimage, 2019. 194: p. 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]


