Abstract
Background:
The present study investigated the effects of the dual orexin receptor antagonist (DORA) almorexant, a sleep-modulating drug, on the sleep-disrupting effects of methamphetamine in adult rhesus monkeys.
Methods:
Monkeys were fitted with primate collars to which actigraphy monitors were attached. To determine the effects of methamphetamine on daytime activity and sleep-like parameters, monkeys were given acute injections of vehicle or methamphetamine (0.03, 0.1 or 0.3 mg/kg, i.m.) in the morning (9:00h) (n=4 males). We then determined the ability of almorexant to alter the daytime and/or sleep-like effects of the largest (effective) dose of methamphetamine. Vehicle or almorexant (1, 3 or 10 mg/kg, i.m.) were administered in the evening (16:30h, 1.5h before “lights off”) following morning (9:00h) administration of methamphetamine (0.3 mg/kg, i.m.), or as a pretreatment (8:30h) before methamphetamine injections (9:00h) (n=4 males). The ability of almorexant (10 mg/kg) to improve sleep-like behaviors also was assessed in a group of monkeys quantitatively identified with short-duration sleep (n=2 males, 2 females).
Results:
Morning methamphetamine administration dose-dependently impaired sleep in rhesus monkeys (0.3 mg/kg significantly increased sleep latency and decreased sleep efficiency). Administration of almorexant, both as a pretreatment or as an evening treatment, improved methamphetamine-induced sleep impairment in a dose dependent manner. Morning pretreatment with almorexant also blocked the daytime stimulant effects of methamphetamine. Evening, but not morning, treatment with almorexant in a group of monkeys with baseline short-duration sleep improved sleep measures.
Conclusions:
Our findings indicate that orexin receptor systems are involved in methamphetamine-induced hyperarousal and sleep disruption.
Keywords: actigraphy, DORA, methamphetamine, orexin, rhesus monkeys, sleep
Introduction
Stimulants are important therapeutic tools in the management of psychiatric disorders, such as hypersomnia, narcolepsy and attention deficit/hyperactivity disorders (ADHD) (Arnsten, 2006; Arnulf et al., 2019; Kornum et al., 2017). However, they also induce several unwanted side effects, from sleep-wake disturbances to addiction and dependence (Greenhill, 2006; Ogeil and Phillips, 2015).
Data from the National Surveys on Drug Use and Health (2015–2016) show that approximately 6.6% of U.S. adults in the past year (annual average) used prescription stimulants (Compton et al., 2019). Stimulant drugs are frequently prescribed in the context of sleep-wake disorders (Arnulf et al., 2019; Kornum et al., 2017). In addition, individuals using psychostimulant substances for wakefulness-promoting purposes show a progressively growing nocturnal pattern of drug use, particularly for shift workers or individuals doing extended shifts, as in the medical field (Deng et al., 2018; Fallah et al., 2018). Over the past decades, it has become relatively common to skip one night’s sleep because of job or academic demands (National Sleep Foundation, 2019), and psychostimulants are used frequently as alertness-enhancing drugs when irregular work/rest patterns cause excessive sleepiness (Deng et al., 2018; Fallah et al., 2018). In addition, among young adults, late-night parties are often associated with the recreational use of stimulant drugs, which continues to expand markedly in this population (Schepis et al., 2020). Yet, the mechanisms underlying the relationship between stimulant use and sleep impairment remain poorly understood.
Several neurotransmitter systems are involved in the regulation of sleep-wake cycles and could be mediating the effects of stimulant drugs on sleep. One in particular is positioned at the interface of sleep and reward: the orexin/hypocretin system (Tsujino and Sakurai, 2009). Orexin neuropeptides play important roles in multiple physiological functions, particularly in sleep-wake regulation (Peyron and Kilduff, 2017) and in reward processes (Aston-Jones et al., 2010; Baimel et al., 2015). Moreover, of the two orexin receptor subtypes identified, orexin-1 (OX1) receptors appear to be involved in the abuse-related effects of stimulants (Brodnik et al., 2020; Foltin and Evans, 2018), whereas orexin-2 (OX2) receptors have been associated with the sleep-promoting properties of orexin antagonists (Jacobson et al., 2017). However, although OX1 receptor antagonists do not exert robust sleep-inducing effects (Morairty et al., 2012), non-selective orexin antagonists, referred to as “dual orexin receptor antagonists” (DORAs) have shown characteristics of an ideal sleep aid in primates (Winrow et al., 2011), and also are more effective in inducing sleep in rodents than selective OX2 receptor antagonists alone (Morairty et al., 2012).
Together, these findings suggest the important possibility that orexin receptor systems could be involved in sleep disruption induced by the administration of stimulant drugs. We have previously demonstrated that methamphetamine self-administration impairs actigraphy-based nighttime sleep in rhesus monkeys even when self-administration sessions were conducted in the morning (Berro et al., 2017a,b), an effect that also has been recently reported in long-term recreational methamphetamine users (Hermann et al., 2017). However, the extent to which experimenter-administered methamphetamine induces sleep impairment in naïve rhesus monkeys remained unknown. Therefore, the aim of the present study was to investigate the effects of acute non-contingent administration of methamphetamine on sleep-like parameters in adult naïve rhesus monkeys, and the effects of the DORA almorexant on the daytime stimulant and sleep-disrupting effects of methamphetamine. Almorexant was administered as a pretreatment before morning methamphetamine injections, or as an evening treatment following morning methamphetamine injections. Sleep-like parameters and daytime activity were measured using actigraphy. The effects of almorexant on sleep in the absence of methamphetamine were also investigated using a novel approach in which the ability of almorexant to alter sleep-like behaviors was assessed in a group of monkeys quantitatively identified with short-duration sleep (increased sleep latency, decreased sleep efficiency compared to normal sleepers).
Materials and Methods
Subjects
Five adult male and 2 adult female rhesus monkeys (Macaca mulatta) aged 9–25 and weighing 8–16 kg served as subjects for the studies. All monkeys were experimentally naïve prior to the beginning of the studies. Monkeys were housed individually, but had visual, auditory and olfactory contact with other monkeys throughout the study, as well as access to chew toys and a mirror in their cage. Monkeys were maintained on a 12h light/12h dark cycle (lights on at 6h), at a temperature of 21±2°C, with water available ad libitum and monkey diet available once/day, supplemented by fresh fruit and forage (seeds and dry fruit), and were weighed monthly during physical examinations. Monkeys were fitted with collars (Primate Products) prior to the initiation of the studies. All of the procedures and animal maintenance were in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011), with review and approval via the Institutional Animal Care and Use Committees of the University of Mississippi Medical Center.
Drugs
Methamphetamine (±) HCl was supplied by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC, USA). Methamphetamine was dissolved in 0.9% sterile physiological saline. Almorexant (MedKoo, NC, USA) was dissolved in 25% (2-Hydroxypropyl)-β-cyclodextrin (Sigma, MO, USA) w/v in saline. All drugs were administered intramuscularly (i.m.) at a volume of 0.1 ml/kg. Doses are based on the salt forms of the drugs.
Actigraphy-based daytime activity and sleep parameters
Actiwatch sensors (Mini Mitter, Bend, OR, USA) were used to assess daytime and nighttime activity, as previously described (Andersen et al., 2010, 2013). Briefly, the Actiwatch device consisted of an omni-directional sensor that is sensitive to motion (recorded as activity counts). The monitors were programmed to record the intensity, amount, and duration of movement over the preceding 60 s (i.e. epoch length=60 s). Monkeys had been adapted to wearing the activity monitors attached to primate collars (Primate Products), and collars were placed on monkeys while under ketamine (10 mg/kg) during physical examinations. Daytime activity data generated activity counts/hour. Nighttime activity data generated the following sleep-like behavior parameters: sleep efficiency (i.e., the percentage of the 12h dark phase – 6pm to 6am – spent sleeping) and sleep latency (i.e., the time between “lights off” at 18h and the first 5min with no detected movement). All parameters were calculated using the Actiware Sleep 3.4 software program (Mini-Mitter, Bend, OR, USA). Sleep measurements were automatically calculated from the underlying activity counts using a temporal smoothing algorithm on the basis that sleeping or wakefulness are continuous behaviors. The Actiware analysis software automatically inferred sleep-like parameters from activity counts during each 60-s epoch by comparing the sum of the activity counts in that epoch and neighboring epochs to a predefined criterion.
Study design
Before the beginning of drug treatments, Actiwatches were attached to the monkeys’ collars and baseline sleep-like behavior and daytime activity were measured for 1 week. Actigraphy recording continued for the duration of the treatments. Four male monkeys completed Experiments 1 through 3, and 4 different monkeys (2 females and 2 males) completed Experiment 4 (see Table 1 for Individual subject data). During a first set of treatments, each monkey was administered an acute intramuscular injection of saline (vehicle) or methamphetamine (0.03 – 0.3 mg/kg) at 9:00h (Experiment 1). Doses were chosen based on previous studies investigating the pharmacokinetic profile of methamphetamine in rhesus monkeys (Banks et al., 2016). During a second set of treatments, each monkey was administered an acute morning (9:00h) injection of methamphetamine (0.3 mg/kg, dose determined to have significant sleep-disrupting effects during the first treatments). Vehicle or almorexant (1, 3 or 10 mg/kg, i.m.) were then administered as a pretreatment (8:30h) before morning (9:00h) administration of methamphetamine (Experiment 2), or in the evening (16:30h, 1.5h before “lights off”) following morning (9:00h) methamphetamine injections (Experiment 3).
Table 1.
Individual-subject baseline sleep-like parameters
| Subject | Age | Symbol on Graphs | Sleep Latency (min) | Sleep Efficiency (%) |
|---|---|---|---|---|
| Experiments 1–3, Figures 1–3 | ||||
| 271–2002 (M) | 19 | Circle | 1.2 (0 – 9) | 94.5 (92.4 – 96.2) |
| 205–2002 (M) | 19 | Triangle | 18.1 (8 – 21) | 85.7 (80.1 – 89.2) |
| 385–2001 (M) | 20 | Square | 10.9 (8 – 20) | 86.6 (83.5 – 89.3) |
| 248–2005 (M) | 16 | Rhombus | 73.3 (71 – 170) | 68.5 (62.9 – 72) |
| Mean ± SEM= | 25.9 ± 16.2 | 83.8 ± 5.5 | ||
| Experiment 4, Figure 4 | ||||
| 248–2005 (M) | 16 | Rhombus | 83.8 (33 – 324) | 63.1 (59.8 – 73.3) |
| 98–003 (M) | 25 | Hexagon | 35.2 (13 – 45) | 56.9 (54 – 62.2) |
| BG-93 (F) | 10 | Inverted triangle | 75.2 (18 – 164) | 53.1 (49.3 – 56.5) |
| BH-91 (F) | 9 | Crossed square | 72.2 (12 – 166) | 64.4 (56 – 68.3) |
| Mean ± SEM= | 66.6 ± 10.7 | 59.4 ± 2.7 | ||
M = male rhesus monkey; F = female rhesus monkey. Individual data are expressed as mean (range) for a 7-day period of baseline sleep recording. Grouped data are expressed as mean ± SEM.
In order to determine the sleep-promoting effects of morning vs evening almorexant in the absence of methamphetamine (Experiment 4), vehicle or almorexant (10 mg/kg, i.m.) were administered at 8:30h and at 16:30h following morning (9:00h) administration of vehicle (saline) in a group of monkeys in which sleep latency was longer and sleep efficiency values were less than typical values from our colony, respectively (see Table 1 and Figure 5).
A 2-week baseline period was given between each Experiment. During each set of treatments, at least 2 days were given between each treatment. For Experiment 1, each treatment (vehicle or one of the 3 doses of methamphetamine) was determined twice and averaged together for analysis. For all other Experiments, each data-point was singly determined for all monkeys. All monkeys were housed in the same colony room. All treatments within an Experiment were carried out contemporaneously and baseline sleep data were collected during the same week for all monkeys. During the treatment phase, the order of drug treatments, including vehicle treatments, and doses was randomized and counterbalanced across monkeys, minimizing any potential influences of one monkey’s sleep alterations over others.
Data analysis
Baseline data were combined across a 7-day period for a period immediately preceding each Experiment (1–4). The same monkeys were the subjects for Experiments 1–3, and no significant changes in baseline sleep parameters were observed over the course of the experiments for these monkeys. Sleep and daytime activity data were analyzed using one-way repeated-measures (RM) analysis of variance (ANOVA) with treatment as the factor. Multiple comparisons were conducted using Bonferroni t-test. Sleep-like measures and daytime activity data are presented as normalized data (percentage of baseline). In order to confirm that monkeys used in Experiment 4 (almorexant alone tests), referred to as a “short-duration sleep” cohort, differed in their sleep parameters compared with the larger colony, actigraphy data first were analyzed for monkeys from the housing rooms from which the short-duration sleep monkeys were chosen (N=26). These data were analyzed for skewness/kurtosis (i.e., confirming to a normal distribution) by computing d’Agostino and Pearson tests of normality (K2). Because both sleep latency and sleep efficiency data sets passed normality tests (see Results), the mean values for the short-duration sleep cohort during baseline assessments were compared with the colony statistics using a one-sample t-test. This comparison was also conducted with the cohort of monkeys from the first three experiments. All graphical data presentations were created and all statistical tests were performed using Prism 8 (GraphPad Software, vers. 8.4.3). Significance was accepted at an alpha of 0.05.
Results
Experiment 1. Effects of acute morning methamphetamine administration on actigraphy-based sleep parameters
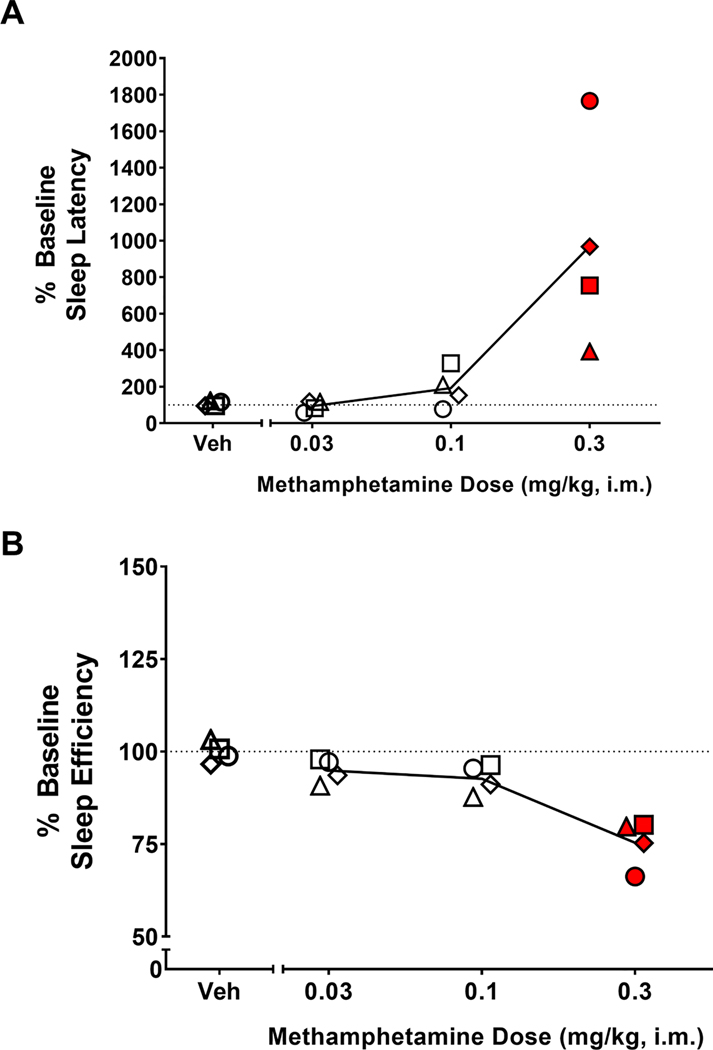
Table 1 shows individual-subject baseline sleep-like parameters. The dose-dependent effects of methamphetamine on sleep-like parameters are illustrated in Figure 1. The ANOVA showed a significant treatment effect for both sleep latency [F(4,12)=11.36, p<0.001] (Figure 1A) and sleep efficiency [F(4,12)=14.74, p<0.001] (Figure 1B). For both measures, the Bonferroni tests showed that the 0.3 mg/kg dose of methamphetamine differed significantly from vehicle treatment (p<0.01 and p<0.001, respectively), with average sleep latencies increased and average sleep efficiency values correspondingly decreased relative to averages observed after vehicle administration. The vehicle averages were not significantly different from average baseline values for any measure.
Figure 1.
Effects of acute morning methamphetamine administration on actigraphy-based sleep parameters. Dose-response curve of the effects of methamphetamine on sleep parameters. Sleep latency (A) and sleep efficiency (B) in the nights after morning (9:00h) administration of vehicle (Veh) or methamphetamine (0.03, 0.1 or 0.3 mg/kg, i.m.) in male rhesus monkeys (N=4). Actigraphy-based sleep-like measures are presented as normalized data (percentage of baseline). Data are normalized sleep parameters for individual monkeys (symbols) and the mean for each sleep parameter (lines). Dotted lines represent baseline sleep parameters (100%). Red-filled symbols represent doses of methamphetamine for which the mean sleep parameters were significantly different from vehicle (Bonferroni t-tests, p<0.05).
Experiment 2. Effects of pretreatment with almorexant on methamphetamine-induced sleep impairment and increased daytime activity
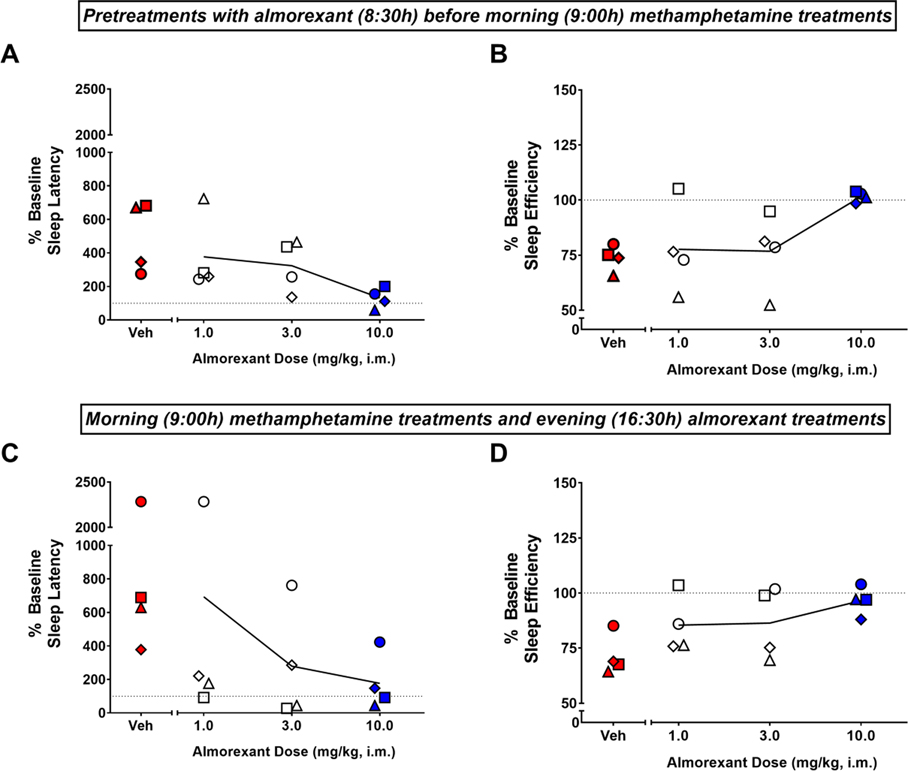
The effects of pretreatment (8:30h) with almorexant on sleep impairment induced by morning (9:00h) methamphetamine administration are shown in Figures 2A and 2B. The ANOVA showed a significant treatment effect for both sleep latency [F(4,12)=7.507, p<0.01] (Figure 2A) and sleep efficiency [F(4,12)=5.817, p<0.01] (Figure 2B). The Bonferroni tests showed that morning methamphetamine administration (0.3 mg/kg) preceded by vehicle treatment significantly increased average sleep latencies and decreased average sleep efficiency values compared to the corresponding averages under baseline conditions (p<0.05). For both sleep measures, the Bonferroni tests also showed that when given as a pretreatment before morning methamphetamine administration, the 10 mg/kg dose of almorexant differed significantly from vehicle treatment (p<0.05), with almorexant decreasing average sleep latencies and increasing average sleep efficiency values relative to the averages observed with vehicle.
Figure 2.
Effects of morning pretreatment (top panel) or evening treatment (bottom panel) with almorexant on methamphetamine-induced sleep impairment. Sleep latency (A, C) and sleep efficiency (B, D) obtained on the nights after morning (9:00h) administration of methamphetamine (0.3 mg/kg, i.m.) and morning administration (8:30h, top panel) or evening administration (16:30h, bottom panel) of vehicle (Veh) or almorexant (1, 3 or 10 mg/kg, i.m.) in male rhesus monkeys (N=4). Actigraphy-based sleep-like measures are presented as normalized data (percentage of baseline). Data are normalized sleep parameters for individual monkeys (symbols) and the mean for each sleep parameter (lines). Dotted lines represent baseline sleep parameters (100%). Red-filled symbols represent mean sleep parameters significantly different from baseline (Bonferroni t-tests, p<0.05). Blue-filled symbols represent mean sleep parameters significantly different from vehicle (Bonferroni t-tests, p<0.05).
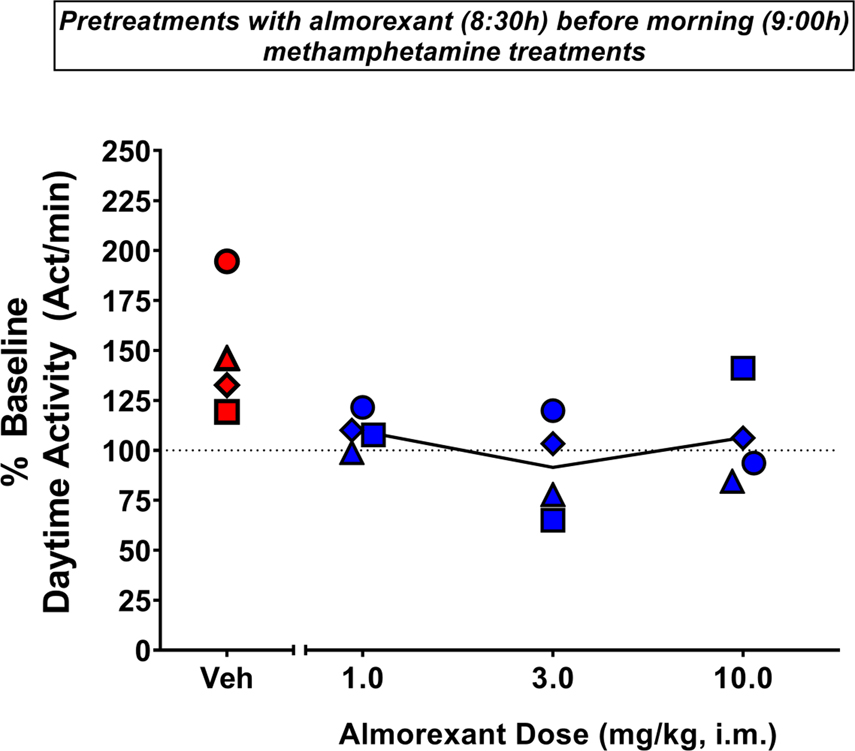
The effects of pretreatment with almorexant on increased daytime activity induced by morning methamphetamine administration are shown in Figure 3. The ANOVA revealed that a significant treatment effect [F(4,12)=4.140, p<0.05]. The Bonferroni tests showed that morning methamphetamine administration (0.3 mg/kg) preceded by vehicle treatment significantly increased daytime activity compared to baseline (p<0.05). The Bonferroni tests also showed that all doses of almorexant differed significantly from vehicle treatment (p<0.05), with almorexant decreasing average daytime activity values relative to vehicle averages when given as a pretreatment before morning methamphetamine administration.
Figure 3.
Effects of pretreatment with almorexant on methamphetamine-induced increased daytime activity. Daytime activity (9:00h-18:00h) after pretreatment (8:30h) with vehicle (Veh) or almorexant (1, 3 or 10 mg/kg, i.m.) before morning (9:00h) administration of methamphetamine (0.3 mg/kg, i.m.) in male rhesus monkeys (N=4). Actigraphy-based activity measures are presented as normalized data (percentage of baseline). Data are daytime activity for individual monkeys (symbols) and the mean for each sleep parameter (lines). Dotted lines represent baseline sleep parameters (100%). Red-filled symbols represent mean daytime activity data significantly different from baseline (Bonferroni t-tests, p<0.05). Blue-filled symbols represent mean daytime activity data significantly different from vehicle (Bonferroni t-tests, p<0.05).
Experiment 3. Effects of evening treatment with almorexant on methamphetamine-induced sleep impairment
The effects of evening (1.5h before “lights off”) treatment with almorexant on sleep impairment induced by morning methamphetamine administration are shown in Figures 2C and 2D. The ANOVA showed a significant treatment effect for both sleep latency [F(4,12)=4.849, p<0.05] (Figure 2C) and sleep efficiency [F(4,12)=6.567, p<0.01] (Figure 2D). The Bonferroni tests showed that morning methamphetamine administration (0.3 mg/kg) followed by vehicle treatment significantly increased average sleep latencies and decreased average sleep efficiency values compared to corresponding averages under baseline condittions (p<0.05 and p<0.01, respectively). For both sleep measures, the Bonferroni tests also showed that the 10 mg/kg dose of almorexant treatment resulted in significantly different values compared with those obtained after vehicle treatment (p<0.05), with almorexant decreasing average sleep latencies and increasing average sleep efficiency values relative to corresponding vehicle averages following morning methamphetamine administration.
Experiment 4. Effects of almorexant in monkeys with short-duration sleep
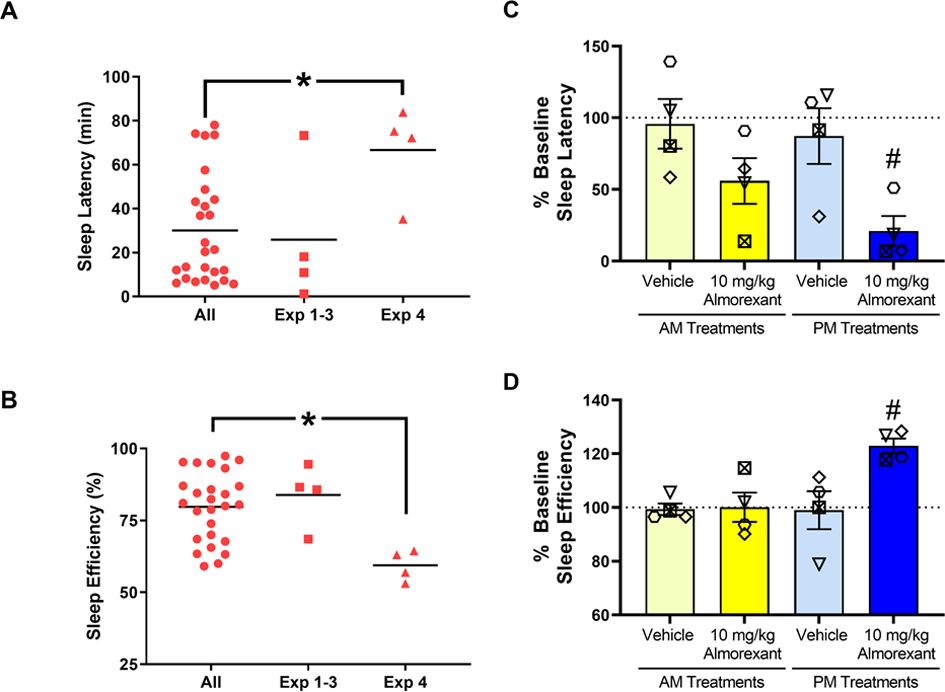
Individual monkey baseline sleep parameters are presented in Table 1. In order to establish whether morning/evening almorexant had sleep-improving effects in the absence of methamphetamine, we selected a group of monkeys with baseline sleep values indicating short-duration sleep compared with the larger pool of available monkeys in our colony (N=26). Analyses of baseline actigraphy data from the larger pool indicated that for both sleep latency values (mean= 30.1, SEM= 4.85) and sleep efficiency values (mean= 79.2, SEM= 2.36), the scores conformed to a normal distribution (K2 = 3.82 and 3.52, respectively; p’s > 0.10). The group of monkeys in Experiment 4 (2 males, 2 females) showed a significantly lower baseline sleep values compared to the overall pool of monkeys (Figures 4A and 4B, one-sample t-test, sleep latency: [t(3)=3.40, p<0.05]; sleep efficiency: [t(3)=7.65, p<0.05]). For the comparison of baseline sleep parameters from Experiments 1–3 with the overall pool of monkeys, no statistically significant differences were observed (Figures 4A and 4B, one-sample t-test, sleep latency: [t(3)=0.261, p>0.1]; sleep efficiency: [t(3)=0.753, p>0.1).
Figure 4.
Effects of almorexant on baseline sleep measures. (A) Sleep latency and (B) sleep efficiency for the group of monkeys with baseline sleep values indicating short-duration sleep (N=4, Exp. 4) compared with the larger pool of available monkeys in our colony (N=26, All) and with monkeys in Experiments 1–3 (N=4, Exp. 1–3). Sleep latency (C) and sleep efficiency (D) in the nights after morning (AM, 8:30h) or evening (PM, 16:30h) treatment with vehicle (Veh) or an effective dose of almorexant (10 mg/kg, i.m.) in male rhesus monkeys (N=4). Actigraphy-based sleep-like measures for (C) and (D) are presented as normalized data (percentage of baseline) for individual monkeys (symbols) and the mean for each sleep parameter (bars). Data in bar charts are expressed as mean ± SEM. Dotted lines represent baseline sleep parameters (100%). * p<0.05 compared to All; # p<0.05 compared to respective (AM/PM) Vehicle.
The effects of morning (8:30h) or evening (16:30h) administration of 10 mg/kg almorexant are shown in Figures 4C and 4D. The ANOVA showed a significant treatment effect for both sleep latency [F(4,12)=8.99, p<0.01] (Figure 4C) and sleep efficiency [F(4,12)=4.932, p<0.05] (Figure 4D). The Bonferroni tests showed that evening (PM), but not morning (AM), treatment with 10 mg/kg almorexant significantly increased average sleep latencies and decreased average sleep efficiency values compared to both baseline values (p<0.05) and values following evening (PM) vehicle administration (p<0.05). Importantly, morning (AM) almorexant administration did not significantly improve sleep measures in the absence of methamphetamine.
Discussion
The present study shows for the first time that acute morning administration of methamphetamine in naïve rhesus monkeys dose-dependently impaired sleep parameters in rhesus monkeys, with the dose of 0.3 mg/kg significantly increasing sleep latency and decreasing sleep efficiency. Administration of the DORA almorexant, both as a morning pretreatment or as an evening treatment, improved methamphetamine-induced sleep impairment in a dose-dependent manner. Morning pretreatment with almorexant also blocked the daytime stimulant effects of methamphetamine at all doses tested. Evening treatment with almorexant (10 mg/kg) in a group of monkeys with baseline short-duration sleep (i.e., in the absence of methamphetamine) “normalized” sleep measures, i.e., decreased sleep latency and improved sleep efficiency. However, morning administration of almorexant in this same group of monkeys did not have sleep-improving effects, contrary to that observed when animals were given methamphetamine following almorexant pretreatment.
Our findings showing that morning administration of methamphetamine induces sleep impairment in rhesus monkeys are in agreement with our previous studies. We have demonstrated previously that methamphetamine self-administration impairs actigraphy-based nighttime sleep in rhesus monkeys even when self-administration sessions were conducted in the morning (Berro et al., 2017a,b). The present study establishes that methamphetamine-induced disruption of sleep is not dependent on the drug being available in a self-administration context. Of note, the experimental design used in the present study has some unique advantages for investigating stimulant-induced sleep impairment. By administering methamphetamine response-independently, we were able to standardize the methamphetamine dosing, which is a challenge in self-administration studies, as the animals control drug intake with contingent drug delivery. Also, by giving methamphetamine as a single injection, we controlled for the potential effect that a pretreatment with almorexant might have had on methamphetamine intake, which would ultimately affect sleep outcomes due to decreasing methamphetamine exposure, as seen in previous studies from our group (Berro et al., 2017b). Finally, the present study also shows for the first time that we can replicate our previous findings showing that methamphetamine disrupts sleep, even in the absence of drug-associated cues, which we also have shown to contribute to methamphetamine-induced sleep impairment in the context of drug self-administration (Berro et al., 2016).
Accumulating evidence suggests that the orexin system is a potential therapeutic target in psychostimulant use disorders (James et al., 2017) and insomnia (Janto et al., 2018). Consistent with the role of orexin in regulating reward processes in general, selective OX1 receptor antagonists and DORAs, but not selective OX2 antagonists, have been shown to block the abuse-related effects of stimulants in rodents (Gentile et al., 2018; James et al., 2017; Steiner et al., 2013). While OX1 receptor antagonists do not exert robust sleep-inducing effects, selective OX2 antagonists promote sleep in rodents (Morairty et al., 2012). Although these initial findings suggest that the two subtypes represent distinct targets for stimulant abuse vs. sleep disorders, studies suggest that a synergistic effect may exist between antagonism of OX1 and OX2 receptors, particularly regarding their sleep-promoting effects. In that regard, DORAs are more effective in inducing sleep in rodents than selective OX2 receptor antagonists (Morairty et al., 2017). In addition, anatomical localization of orexin receptors in the rodent brain suggests that both receptors may be involved in the promotion of wakefulness (Lindemann et al., 2008; Marcus et al., 2001). Therefore, in the present study we investigated the effects of a DORA on sleep disruption induced by acute methamphetamine administration. While we were not able to directly address the contribution of OX1 vs OX2 receptors on stimulant-induced sleep impairment, we hoped to add to the literature indicating that DORAs are potential candidates for the treatment of insomnia and/or sleep disruption.
DORAs have shown characteristics of an ideal sleep aid in primates (Winrow et al., 2011), and seem to facilitate sleep by reducing wake drive without altering normal sleep architecture (Coleman et al., 2017; Morairty et al., 2012). On the other hand, conventional sleep aids (e.g., benzodiazepine-type drugs) induce sleep via generalized inhibition, markedly changing sleep architecture (suppressing slow wave sleep and rapid eye movement – REM – sleep; Arbon et al., 2015). Although the effects of DORAs on sleep quality and sleep architecture have been well established in several species (Gotter et al., 2013; Winrow et al., 2011), only recently have studies started to investigate the potential of DORAs for treatment of sleep disruption in the context of psychostimulant use and abuse. In the only preliminary study published on the topic to date, Suchting and colleagues (2020) investigated the effects of the DORA suvorexant on stress, sleep, cue reactivity, and inhibitory control as factors related to relapse in 20 non-treatment seeking individuals with cocaine use disorder. The study found improvements in all these domains with administration of suvorexant as a sleep aid before bedtime (22h), including improvements in actigraphy-based sleep, compared to placebo (Suchting et al., 2020). The authors emphasize that given the study design and sample size, they were unable to test causation (Suchting et al., 2020). Although drug accumulation may be expected given the chronic drug regimen and the 12-hour half-life of oral suvorexant in humans (Bennett et al., 2014), research suggesting that sleep impairment may be a risk factor for relapse to drug use (Brower and Perron, 2010) indicate that suvorexant may have exerted a beneficial effect on craving simply by improving sleep.
The present study demonstrates that the DORA almorexant blocked the sleep-disrupting effects induced by acute methamphetamine injections. Although a different protocol compared to the study by Suchting and colleagues (2020), in which volunteers were chronic cocaine users, our study further indicates the potential of DORAs for the treatment of stimulant-induced sleep impairment. Our findings also show that morning administration of almorexant blocks the sleep-disrupting effects of a subsequent methamphetamine injection. In order to rule out the possibility that pretreatment with almorexant blocked the nighttime effects of methamphetamine because of a long duration of action, we also investigated the effects of almorexant on sleep in the absence of methamphetamine in a group of monkeys quantitatively identified with short-duration sleep. Our results show that morning administration of almorexant in this group of monkeys was not effective in inducing significant sleep-promoting effects. While 2 monkeys showed improved sleep latency after morning almorexant administration, no significant differences were observed in the group analysis for both sleep latency and sleep efficiency. On the other hand, evening administration of almorexant significantly improved sleep in monkeys with short-duration sleep. Therefore, the effects of morning almorexant on methamphetamine-induced sleep impairment likely are not due to almorexant having significant exposure levels during the nighttime. Instead, antagonism of orexin receptors seems to block the downstream effects of methamphetamine that are responsible for its sleep-disrupting effects.
In accordance with studies showing altered dopaminergic neuroplasticity following sleep deprivation (Volkow et al., 2012); Wiers et al., 2016), the mesolimbic dopaminergic system has been proposed as the main pathway mediating stimulant-induced sleep impairment and hyperarousal. However, our previous findings suggest that alterations in mesolimbic dopaminergic neurotransmission are not sufficient to explain sleep disruption induced by methamphetamine (Berro et al., 2017b). In this regard, the GABAA receptor modulator temazepam did not improve sleep-like measures in rhesus monkeys self-administering methamphetamine, yet this drug attenuated methamphetamine-induced dopamine levels in the nucleus accumbens (Berro et al., 2017b). Considering that the half-life of methamphetamine in rhesus monkeys is relatively short (~3 hours; Banks et al., 2016), the fact that sleep disruption is observed more than 9 hours after methamphetamine administration suggests a downstream mechanism that might not be related directly to the drug-induced increases in mesolimbic dopamine levels. Our findings with morning almorexant administration suggest that orexin receptor blockade prevents the downstream effects of methamphetamine that are responsible for its sleep disrupting, or arousal-inducing, effects.
In fact, pretreatment with almorexant also blocked the methamphetamine-induced increase in daytime activity, even at doses that were not effective at improving sleep disruption induced by methamphetamine. Because the present study’s activity data were averaged across the 12h light cycle, it is possible that the effects of methamphetamine on general daytime activity were more prominent during the hours immediately following drug injection. Moreover, almorexant was administered shortly before methamphetamine in the daytime study, raising the possibility that lower doses of almorexant were effective at blocking methamphetamine-induced daytime activity due to pharmacokinetic factors. It is also possible that different mechanisms mediate the daytime stimulant vs sleep disrupting effects of methamphetamine, and that orexin systems mediate both effects. If this is the case, then our results suggest that the mechanisms involved in methamphetamine-induced hyperarousal are more sensitive to methamphetamine than those regulating sleep.
In summary, our findings indicate that orexin receptor systems are involved in methamphetamine-induced hyperarousal and sleep disruption, and that DORAs may block the sleep-disrupting effects of stimulant drugs. While limited by a small sample size, the effects observed in the present study are consistent across experimental protocols. Although further studies are needed to investigate whether DORAs would also block sleep impairment in the context of chronic methamphetamine use and abuse, the present findings provide important insights into clinical practice regarding patients on stimulant prescription. In addition to narcolepsy and hypersomnia (Arnulf et al., 2019; Kornum et al., 2017), stimulant medications are important tools in the management of ADHD, which is prevalent in up to 9% of children (Merikangas et al., 2011) and 8% of college students in the U.S. (Ascherman and Shaftel, 2017). Our findings indicate that DORAs may be useful in the management of insomnia induced by stimulant use and misuse, but further studies to investigate the effects of DORAs on chronic stimulant use are needed.
Highlights.
Morning methamphetamine administration impaired evening sleep in rhesus monkeys
Almorexant improved methamphetamine-induced sleep impairment
Almorexant was effective both as a pretreatment or as an evening treatment
Pretreatment with almorexant blocked methamphetamine’s daytime stimulant effects
Evening treatment with almorexant improved sleep in monkeys with short sleep
Acknowledgments
We thank Dr. Eric Vallender for assistance with statistical analyses and helpful discussion about these data. The authors also thank the Veterinary staff from the UMMC Center for Comparative Research for their exceptional care of our animals. This work was supported by the National Institutes of Health [DA011792, DA043204, DA046778 and DA049886].
Role of Funding Source
This work was supported by the National Institutes of Health [DA011792, DA043204, DA046778 and DA049886]. The funding source had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the article for publication.
Footnotes
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Andersen ML, Diaz MP, Murnane KS, Howell LL, 2013. Effects of methamphetamine self-administration on actigraphy-based sleep parameters in rhesus monkeys. Psychopharmacology (Berl) 227:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL, 2010. Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology (Berl) 210:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbon EL, Knurowska M, Dijk DJ, 2015. Randomised clinical trial of the effects of prolonged-release melatonin, temazepam and zolpidem on slow-wave activity during sleep in healthy people. J Psychopharmacol 29(7):764–76. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, 2006. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology 31:2376–2383. [DOI] [PubMed] [Google Scholar]
- Arnulf I, Leu-Semenescu S, Dodet P, 2019. Precision Medicine for Idiopathic Hypersomnia. Sleep Med Clin 14:333–350. [DOI] [PubMed] [Google Scholar]
- Ascherman LI, Shaftel J, 2017. Facilitating transition from high school and special education to adult life: focus on youth with learning disorders, attention-deficit/hyperactivity disorder, and speech/language impairments. Child Adolesc Psychiatr Clin N Am 26(2):311–27. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA, 2010. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res 1314:74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, Tung LW, Borgland SL, 2015. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol 172(2):334–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Smith DA, Kisor DF, Poklis JL, 2016. Relationship between discriminative stimulus effects and plasma methamphetamine and amphetamine levels of intramuscular methamphetamine in male rhesus monkeys. Pharmacol Biochem Behav 141:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Bray D, Neville MW, 2014. Suvorexant, a dual orexin receptor antagonist for the management of insomnia. Pharm Therpeutics 39:264–266. [PMC free article] [PubMed] [Google Scholar]
- Berro LF, Andersen ML, Howell LL, 2017a. Assessment of tolerance to the effects of methamphetamine on daytime and nighttime activity evaluated with actigraphy in rhesus monkeys. Psychopharmacology (Berl) 234:2277–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berro LF, Andersen ML, Tufik S, Howell LL, 2016. Actigraphy-based sleep parameters during the reinstatement of methamphetamine self-administration in rhesus monkeys. Exp Clin Psychopharmacol 24:142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berro LF, Andersen ML, Tufik S, Howell LL, 2017b. GABAA receptor positive allosteric modulators modify the abuse-related behavioral and neurochemical effects of methamphetamine in rhesus monkeys. Neuropharmacology 123:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik ZD, Alonso IP, Xu W, Zhang Y, Kortagere S, España RA, 2020. Hypocretin receptor 1 involvement in cocaine-associated behavior: Therapeutic potential and novel mechanistic insights. Brain Res 1731:145894. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Perron BE, 2010. Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Med Hypotheses 74(5):928–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PJ, Gotter AL, Herring WJ, Winrow CJ, Renger JJ, 2017. The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu Rev Pharmacol Toxicol 57:509–533. [DOI] [PubMed] [Google Scholar]
- Compton WM, Han B, Blanco C, Johnson K, Jones CM, 2019. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the U.S. Am J Psychiatry 175(8): 741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng N, Kohn TP, Lipshultz LI, Pastuszak AW, 2018. The Relationship Between Shift Work and Men’s Health. Sex Med Rev 6(3):446–456. [DOI] [PubMed] [Google Scholar]
- Fallah G, Moudi S, Hamidia A, Bijani A, 2018. Stimulant use in medical students and residents requires more careful attention. Caspian J Intern Med; 9(1):87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Evans SM, 2018. Hypocretin/orexin antagonists decrease cocaine self-administration by female rhesus monkeys. Drug Alcohol Depend 188:318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile TA, Simmons SJ, Barker DJ, Shaw JK, España RA, Muschamp JW, 2018. Suvorexant, an orexin/hypocretin receptor antagonist, attenuates motivational and hedonic properties of cocaine. Addict Biol 23:247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter AL, Winrow CJ, Brunner J, Garson SL, Fox SV, Binns J, Harrell CM, Cui D, Yee KL, Stiteler M, Stevens J, Savitz A, Tannenbaum PL, Tye SJ, McDonald T, Yao L, Kuduk SD, Uslaner J, Coleman PJ, Renger JJ, 2013. The duration of sleep promoting efficacy by dual orexin receptor antagonists is dependent upon receptor occupancy threshold. BMC Neurosci 14:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill LL, 2006. The science of stimulant abuse. Pediatric Annals 35:552–556. [DOI] [PubMed] [Google Scholar]
- Herrmann ES, Johnson PS, Bruner NR, Vandrey R, Johnson MW, 2017. Morning administration of oral methamphetamine dose-dependently disrupts nighttime sleep in recreational stimulant users. Drug Alcohol Depend 178:291–295. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Chen S, Mir S, Hoyer D, 2017. Orexin OX2 Receptor Antagonists as Sleep Aids. Curr Top Behav Neurosci 33:105–136. [DOI] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G, 2017. A Decade of Orexin/Hypocretin and Addiction: Where Are We Now? Curr Top Behav Neurosci 33:247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janto K, Prichard JR, Pusalavidyasagar S, 2018. An Update on Dual Orexin Receptor Antagonists and Their Potential Role in Insomnia Therapeutics. J Clin Sleep Med 14(8):1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornum BR, Knudsen S, Ollila HM, Pizza F, Jennum PJ, Dauvilliers Y, Overeem S, 2017. Narcolepsy. Nat Rev Dis Primers 3:16100. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein JG, Borroni E, Moreau JL, Hoener MC, 2008. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther 324:948–956. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK, 2001. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435:6–25. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swendsen J, Avenevoli S, Case B, Georgiades K, Heaton L, Swanson S, Olfson M, 2011. Service utilization for lifetime mental disorders in U.S. adolescents: results of the National Comorbidity Survey Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 50(1):32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morairty SR, Revel FG, Malherbe P, Moreau JL, Valladao D, Wettstein JG, Kilduff TS, Borroni E, 2012. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS One 7:e39131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation. The 2019 sleep in America poll; 2019. [Google Scholar]
- Ogeil RP, Phillips JG, 2015. Commonly used stimulants: Sleep problems, dependence and psychological distress. Drug Alcohol Depend 153:145–51. [DOI] [PubMed] [Google Scholar]
- Peyron C, Kilduff TS, 2017. Mapping the Hypocretin/Orexin Neuronal System: An Unexpectedly Productive Journey. J Neurosci 37(9):2268–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Klare DL, Ford JA, McCabe SE, 2020. Prescription Drug Misuse: Taking a Lifespan Perspective. Subst Abuse 14:1178221820909352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MA, Lecourt H, Jenck F, 2013. The dual orexin receptor antagonist almorexant, alone and in combination with morphine, cocaine and amphetamine, on conditioned place preference and locomotor sensitization in the rat. Int J Neuropsychopharmacol 16:417–32. [DOI] [PubMed] [Google Scholar]
- Suchting R, Yoon JH, Miguel GGS, Green CE, Weaver MF, Vincent JN, Fries GR, Schmitz JM, Lane SD, 2020. Preliminary examination of the orexin system on relapse-related factors in cocaine use disorder. Brain Res 1731:146359. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T, 2009. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev 61(2):162–76. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Logan J, Benveniste H, Kim R, Thanos PK, Ferré S, 2012. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci 32(19):6711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers CE, Shumay E, Cabrera E, Shokri-Kojori E, Gladwin TE, Skarda E, Cunningham SI, Kim SW, Wong TC, Tomasi D, Wang GJ, Volkow ND, 2016. Reduced sleep duration mediates decreases in striatal D2/D3 receptor availability in cocaine abusers. Transl Psychiatry 6:e752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, Garson SL, Fox SV, Harrell CM, Stevens J, Reiss DR, Cui D, Coleman PJ, Renger JJ, 2011. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet 25:52–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.