Abstract
Background:
Endocrine disrupting chemical (EDC) exposure is ubiquitous. EDC exposure during critical windows of development may interfere with the body’s endocrine system, affecting growth. Previous human studies have examined one EDC at a time in relation to infant growth. By studying mixtures, the human experience can be better approximated.
Aims:
We investigated the association of prenatal exposure to persistent EDCs (per- and polyfluoroalkyl substances (PFAS), polychlorinated biphenyls (PCBs), and organochlorine pesticides (OCPs)) as mixtures with postnatal body size among female offspring.
Subjects:
We used a sub-sample of the Avon Longitudinal Study of Parents and Children (N=425), based in the United Kingdom.
Study design:
We quantified 52 EDCs in maternal serum collected during pregnancy. We used Bayesian kernel machine regression with a random intercept to examine the association of prenatal concentrations of EDC mixtures with longitudinal postnatal body size measures for each EDC class separately (PFAS, PCBs, and OCPs) and for all three classes combined.
Outcome measures:
Weight and height measures at 0, 2, 9, and 19 months were obtained by health professionals as part of routine child health surveillance.
Results:
The mixture representing all three classes combined (31 chemicals) (n=301) was inversely associated with postnatal body size. Holding all EDCs in the 31-chemical mixture at the 75th percentile compared to the 50th percentile was associated with 0.15 lower weight-for-age z-score (95% credible interval: −0.26, −0.03). Weak inverse associations were also seen for height-for-age and body mass index-for-age scores.
Conclusions:
These results suggest that prenatal exposure to mixtures of persistent EDCs may affect postnatal body size.
Keywords: ALSPAC, per- and polyfluoroalkyl substance, polychlorinated biphenyl, organochlorine pesticide, early childhood growth, postnatal body size
Introduction
Endocrine disrupting chemicals (EDCs) may interfere with the body’s endocrine system, potentially producing adverse developmental, reproductive, neurological, and immune effects (1). Environmentally persistent EDCs are normally highly resistant to degradation, tend to bioaccumulate in animals and humans, and have been used throughout the 20th and 21st centuries for a variety of purposes (2–4). Exposure to several persistent EDCs have declined in the general population following several countries banning or severely restricting the production, handling, and disposal of numerous polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs), as well as certain per- and polyfluoroalkyl substances (PFAS). Still, almost all humans have detectable serum concentrations of some of these persistent chemicals (5, 6). Moreover, many persistent EDCs can cross the placental barrier, enabling potential fetal exposure, and the amount of EDCs found in cord serum may be substantial in relation to a developing fetus’s size (7–10).
While birth weight is a well-studied outcome in relation to prenatal exposure to EDCs (select references: (11–17)), growth in the years following birth has been studied less, though the influence of prenatal exposure to EDCs on growth may persist after birth. The first two years of life are a particularly important period of change as the greatest variations in rates of weight gain are usually seen during this time when infants show accelerated or diminished growth to compensate for intrauterine restraint or enhancement of fetal growth (18).
Postnatal growth has been examined in a few previous studies regarding prenatal EDC exposure. No studies have found an association of PCBs with growth measures in the first few years following birth (19–22). All studies to examine OCPs found associations of dichlorodiphenyltrichloroethane, dichlorodiphenyldichloroethylene, or hexachlorobenzene with early growth: four studies found a positive association (19–21, 23) and one small study found an inverse association (22). Studies of PFAS and growth through age 3 have been especially mixed, with some studies finding children with higher prenatal PFAS exposure to be heavier in the years after birth (24), while other studies found lower body mass index (BMI) (25, 26) or height (27) postnatally or no association (22, 28).
Because only a few studies have focused on growth after birth, and the available studies have used a variety of outcome measures (e.g., height, weight, BMI z-score, rapid growth), it is difficult to see patterns emerging across chemical classes. Previous studies have only examined one EDC at a time in relation to growth, and it is thought that this could have led to inconsistent conclusions on their effects on health. Because humans are exposed to many chemicals, as opposed to any one chemical in isolation, examining combined exposures or “mixtures” of chemicals allows for a better approximation of the human experience (29). Typically, a mixture is defined as a combination of three or more independent chemicals or chemical groups (30).
Our aim was to investigate the association of maternal gestational concentrations of persistent EDCs (PFAS, PCBs, and OCPs) and postnatal body size (weight-, height-, and BMI-for-age scores) at 0, 2, 9, and 19 months using a mixtures approach.
Methods
Study population
The Avon Longitudinal Study of Parents and Children (ALSPAC) is an ongoing prospective birth cohort of 14,541 pregnancies. ALSPAC enrolled pregnant women with an expected delivery date between 1 April 1991 and 31 December 1992 from three health districts in the former county of Avon in the United Kingdom (UK). Information was collected on parents and children through clinic visits, interviews, and mailed questionnaires. Details on ALSPAC recruitment and study methods have been described elsewhere (31, 32). The present analysis uses exposure data from a nested case–control study that was established within the ALSPAC cohort to explore associations of prenatal maternal concentrations of various suspected EDCs and age at menarche among the daughters. Details of the nested case–control study (N=448) are described elsewhere (33). Where possible, the nested case-control study data were reweighted in analyses to represent the full cohort.
The ALSPAC website contains details of all available data through a fully searchable data dictionary and variable search tool (http://www.bris.ac.uk/alspac/researchers/our-data/). We obtained ethical approval for the study from the ALSPAC Ethics and Law Committee, the Local Research Ethics Committees, and the Centers for Disease Control and Prevention (CDC) Institutional Review Board. Mothers provided written informed consent for participation in the study. Consent for biological samples has been collected in accordance with the Human Tissue Act (2004). Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time.
Exposure assessment
Fasting blood samples were collected from mothers during pregnancy at enrollment in 1991–1992 at a median of 15 (interquartile range (IQR): 10–28) weeks gestation. Samples were processed and frozen for later analysis. Maternal serum samples were held in storage facilities at the University of Bristol until they were transferred under controlled conditions and analyzed at the National Center for Environmental Health of the CDC (Atlanta, GA) in 2009–2010. Laboratory analyses included low- and high-concentration pooled quality control materials, standards, reagent blanks, and study samples. Concentrations below the limit of detection (LOD) were imputed by dividing the LOD by the square root of 2 prior to statistical analysis. The persistent EDCs detected in greater than 75% of mothers were included in the main analyses.
Per- and polyfluoroalkyl substances
Eight PFAS were quantified (Table S1) in serum via on-line solid-phase extraction coupled to isotope dilution high-performance liquid chromatography-tandem mass spectrometry (34). LODs are presented in Table S1. Coefficients of variation (CVs) were largely below 10%.
Organochlorine pesticides and polychlorinated biphenyls
We quantified nine OCPs and 35 PCBs (Table S1) in serum using gas chromatography isotope dilution high resolution mass spectrometry (35). PCB congeners 138 and 158 could not be separated and were quantified as a summed concentration hereafter referred to as PCB138. Similarly, PCB congeners 196 and 203 could not be separated and were quantified as a summed concentration hereafter referred to as PCB196. LODs for PCBs and OCPs are dependent on the size of the sample available, thus an individual LOD was reported for each individual result rather than an overall LOD. CVs were generally below 10%. PCB and OCP concentrations were adjusted for lipids.
Outcome assessment
Birth weight (g) was abstracted from infant medical records. Trained ALSPAC staff measured crown to heel length (cm) using a Harpenden neonatometer (Holtain Ltd., Crymych, UK) and head circumference (cm) using a lasso tape measure within 24 hours of birth. Weight and height at roughly 2, 9, and 19 months were obtained by health professionals as part of the routine child health surveillance program in the UK. These weight and height measurements were extracted from health visitor records, which form part of standard child care in the UK. We calculated standard deviation (SD) scores (z-scores) of weight, height, and BMI on the basis of the British growth reference centiles for females from 1990 (36) with Excel macros provided on the internet (www.healthforallchildren.co.uk). The final clinical estimate of the expected date of delivery was abstracted from the obstetric records and used to calculate gestational age at birth.
Covariates
Covariate information was collected by clinical staff or through self-report in questionnaires completed by the mother during or immediately after pregnancy. Covariates under examination included gestational age at biological sample collection, age at measurement, maternal age at delivery, maternal pre-pregnancy BMI, maternal education, parity, and smoking during pregnancy.
Statistical analyses
The analytic dataset included daughters who had EDC measurements at three or four time points (N=425; 23 excluded). Descriptive analyses were conducted to report mother-daughter dyad characteristics and body size measures across time points. The persistent EDCs under study were modeled as natural log-transformed continuous variables. We ran single-chemical linear regression models to examine independent associations between each chemical and weight-for-age z-score at 19 months. We also ran multi-chemical linear regression models to examine associations between each chemical in a class (e.g., PFAS or PCBs) and weight-for-age score at 19 months, independent of other chemicals in the class (e.g., adjusting for other chemicals in the class). To account for the study design, we reweighted the sample to represent the full cohort of girls. All models adjust for parity (categorized as nulliparous/multiparous), pre-pregnancy BMI (kg/m2, linear), maternal age at delivery (years, linear), maternal education (categorized as <O-level [ordinary level: required, completed at age 16], O-level, or > O-level), prenatal smoking (categorized as any/none), and gestational age at sample collection (categorized as ≤20 weeks or >20 weeks).
We also analyzed the data using the multiple measurement time points and accounting for clustering within child. Before utilizing a mixtures approach, we used single-chemical linear mixed models to examine independent associations between each chemical and weight-for-age z-scores while accounting for the longitudinal nature of the data. We also used multi-chemical linear mixed models to examine associations between each chemical in a class and weight-for-age scores, independent of other chemicals in the class. The full linear mixed models included the same covariates as the models for the outcomes at 19 months plus a time variable representing the age (in months, initially included as linear and quadratic terms) when weight measurements were taken (roughly 0, 2, 9 or 19 months). The linear mixed-effects models also included an intercept, a subject-specific random intercept and a random slope for age at the measurement (as a linear term) (Supplemental Methods, Models 1 and 2). As before, we reweighted the sample to represent the full cohort. This full model was used to test for the necessity of including the quadratic term for age at measurement and the random-effects (random intercept and random slope for age at measurement in months) and to assess various random-effects covariance structures for the R matrix (autoregressive(1), compound symmetric, Toeplitz, Toeplitz with two bands, unstructured, and variance components). We also considered models that included an interaction between age at measurement and EDC concentrations (see Supplemental Methods for details on model selection). Our final model included a subject-specific random intercept with a Toeplitz with two bands covariance structure for the R matrix (Supplemental Methods, Model 2).
To estimate how prenatal mixtures are associated with repeated measures of postnatal size-for-age scores, we used Bayesian kernel machine regression (BKMR). BKMR is a flexible approach for estimating the joint health effects of exposure to multiple concurrent risk factors (37). BKMR allowed us to evaluate the association of the EDC mixture with postnatal body size measures (collected at 0, 2, 9, and 19 months), accounting for the correlated nature of the data through use of a random intercept (specified using the id argument within the main argument kmbayes of the R package bkmr) (38). Within BKMR, we used hierarchical variable selection when examining multiple classes of chemicals (i.e., PFAS, PCBs, and OCPs) in the same mixture, which provided group importance scores (posterior inclusion probabilities (PIPs)) for pre-defined mutually exclusive groups of variables. Further, we estimated the importance of a chemical given that the group containing the chemical was important (conditional PIPs) (37–39). Currently, the bkmr package does not allow for weighting, so we were unable to reweight our nested case-control data back to the full cohort, which limits generalizability. We ran the Markov Chain Monte Carlo sampler for 10,000 iterations. Natural log-transformed EDC concentrations were centered and scaled by the standard deviation. Mixtures under study included each chemical class separately (PFAS, PCBs, and OCPs) and all three chemicals classes combined. These models included the same covariates used in the linear mixed effects models but continuous covariates were centered and scaled.
In sensitivity analyses, we considered BKMR models that excluded the observation at birth, and used weight-for-age scores that were adjusted for gestational age (see Supplemental Methods). We also did sensitivity analyses using weighted quantile sum (WQS) regression to construct a weighted index estimating the mixture effect associated with all predictor variables on weight-for-age at each measurement time point in separate models (40, 41). WQS regression is a complementary method to BKMR and can also estimate the overall effect of a mixture, while identifying the most important chemicals within the mixture.
SAS software 9.4 (Cary, NC) was used for descriptive analyses and single- and multi-chemical models. R software 3.5.0 (Vienna, Austria) was used for WQS regression and BKMR analyses.
Results
Descriptive statistics
There were 425 ALSPAC mother-daughter dyads with 3 or 4 postnatal body size measures (Figure S1), of these, 354 had weight measurements at 19 months. From the 425 ALSPAC mother-daughter dyads with ≥3 measures, 347 had complete covariate data. An additional 46 mother-daughter dyads were missing information on lipids, PCBs, or OCPs, bringing the analytic dataset for mixture analyses of all three chemical classes to 301 dyads.
ALSPAC mothers were predominantly higher-educated, non-smokers who were 25 years or older at the time of delivery (Table 1). On average, daughters were 3.39 kg (SD: 0.50 kg) at birth and 11.54 kg (SD: 1.41 kg) at 19 months of age (Table 2). To examine characteristics of mother-daughter dyads, we stratified the sample by weight-for-age scores at 19 months (n=354) (Table 3). Daughters born to mothers with under-/normal weight pre-pregnancy BMI were more likely to have z-scores <0 at 19 months (81.3%) compared to z-scores >1 (71.3%). A lower birth weight (<2800 g) was more frequently observed among daughters with an z-score <0 at 19 months (22.0%), compared to those with an z-score >1 (percent suppressed due to small cell size).
Table 1.
Characteristics of the Avon Longitudinal Study of Parents and Children (ALSPAC) sub-study population by weight-for-age z-score at 19 months.
| Distribution by weight-for-age z-score | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | <0 n=134 |
0–1 n=134 |
>1 n=86 |
|||
|
| ||||||
| n | % | n | % | n | % | |
| Maternal educationa | ||||||
| < O-level | 23 | 18.4 | 26 | 21.0 | 14 | 17.5 |
| O-level | 39 | 31.2 | 48 | 38.7 | 24 | 30.0 |
| >O-level | 63 | 50.4 | 50 | 40.3 | 42 | 52.5 |
| Maternal pre-pregnancy BMI, kg/m2 | ||||||
| <25 (under/normal weight) | 100 | 81.3 | 91 | 76.5 | 57 | 71.3 |
| ≥25 (overweight/obese) | 23 | 18.7 | 28 | 23.5 | 23 | 28.8 |
| Prenatal smoking | ||||||
| Any | 22 | 17.2 | 31 | 24.0 | 18 | 21.4 |
| None | 106 | 82.8 | 98 | 76.0 | 66 | 78.6 |
| Physical activity | ||||||
| Any | 76 | 65.5 | 70 | 60.3 | 57 | 73.1 |
| None | 40 | 34.5 | 46 | 39.7 | 21 | 26.9 |
| Maternal age at delivery, years | ||||||
| <25 | 26 | 19.4 | 32 | 23.9 | 17 | 19.8 |
| 25–29 | 44 | 32.8 | 61 | 45.5 | 31 | 36.0 |
| ≥30 | 64 | 47.8 | 41 | 30.6 | 38 | 44.2 |
| Child birth order | ||||||
| First born | 62 | 48.1 | 73 | 57.9 | 35 | 42.7 |
| Second born or later | 67 | 51.9 | 53 | 42.1 | 47 | 57.3 |
| Birth weight, g | ||||||
| <2800 | 29 | 22.0 | 9 | 6.8 | --b | --b |
| ≥2800 | 103 | 78.0 | 124 | 93.2 | 81 | --b |
Abbreviations: BMI, body mass index; kg/m2, kilograms per meter-squared; g, grams
<O-level=none, Certificate of Secondary Education, and vocational education, which are equivalent to no diploma or a GED in the United States. O-levels (ordinary levels) are required and completed at the age of 16. >O-level=A-levels (advanced levels) completed at 18, which are optional, but required to get into university; and a university degree.
Suppressed due to small cell sizes
Table 2.
Mean (standard deviation) weight, height, and body mass index (BMI) across four time points in the Avon Longitudinal Study of Parents and Children (ALSPAC) sub-study population (N=425 mother-daughter dyads).
| n | Age (months) Mean (SD) |
n | Weight (kg) Mean (SD) |
Weight z-score Mean (SD) |
n | Height (cm) Mean (SD) |
Height z-score Mean (SD) |
n | BMI (kg/m2) Mean (SD) |
BMI z-score Mean (SD) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth | 424 | 0 (0) | 417 | 3.39 (0.50) | −0.05 (1.09) | 363 | 50.34 (2.16) | 0.06 (1.15) | 356 | 13.35 (1.26) | 0.20 (1.05) |
| 2 months | 406 | 1.71 (0.30) | 402 | 4.82 (0.62) | −0.01 (1.04) | 361 | 56.95 (2.37) | 0.47 (1.15) | 357 | 14.88 (1.41) | −0.40 (1.16) |
| 9 months | 392 | 9.17 (0.94) | 383 | 8.89 (1.00) | 0.23 (1.04) | 332 | 71.61 (2.60) | 0.49 (1.10) | 323 | 17.47 (1.48) | −0.04 (1.11) |
| 19 months | 358 | 19.39 (3.14) | 354 | 11.54 (1.41) | 0.32 (1.01) | 287 | 83.19 (3.94) | 0.33 (1.06) | 283 | 16.82 (1.53) | 0.06 (1.12) |
Table 3.
Adjusteda singleb- and multic-chemical associations of maternal serum concentrations of persistent endocrine disrupting chemicals (EDCs) and weight-for-age z-score at 0, 2, 9 and 19 months in the Avon Longitudinal Study of Parents and Children (ALSPAC) sub-study (N=347 mother-daughter dyads) using a linear mixed model with a random intercept. Beta estimates represent the change in weight-for-age z-scores for 10% higher chemical concentrationsd.
| Single-chemical modelsbe | Multi-chemical modelsce | |||
|---|---|---|---|---|
|
|
||||
| β | 95% CI | β | 95% CI | |
|
Per- and polyfluoroalkyl substances (PFAS) (ng/mL) | ||||
| PFOA | −0.03 | −0.06, 0.00 | −0.03 | −0.07, 0.01 |
| PFOS | −0.02 | −0.05, 0.01 | −0.01 | −0.06, 0.04 |
| PFHxS | ||||
| PFNA | 0.01 | −0.03, 0.05 | ||
| MeFOSAA | ||||
| EtFOSAA | ||||
|
Polychlorinated biphenyls (PCBs) (ng/g lipid) | ||||
| PCB28 | ||||
| PCB74 | 0.07 | 0.01, 0.13 | ||
| PCB99 | −0.05 | −0.13, 0.03 | ||
| PCB105 | −0.01 | −0.04, 0.01 | 0.06 | −0.03, 0.14 |
| PCB118 | −0.09 | −0.19, 0.01 | ||
| PCB138f | −0.01 | −0.04, 0.01 | ||
| PCB146 | −0.01 | −0.04, 0.02 | ||
| PCB153 | −0.02 | −0.05, 0.01 | 0.03 | −0.21, 0.27 |
| PCB156 | −0.02 | −0.14, 0.09 | ||
| PCB170 | −0.02 | −0.06, 0.01 | 0.02 | −0.16, 0.20 |
| PCB172 | 0.01 | −0.02, 0.04 | 0.03 | −0.02, 0.08 |
| PCB177 | −0.02 | −0.07, 0.03 | ||
| PCB178 | 0.01 | −0.03, 0.05 | ||
| PCB180 | −0.03 | −0.06, 0.01 | −0.12 | −0.36, 0.12 |
| PCB183 | 0.05 | −0.02, 0.12 | ||
| PCB187 | −0.03 | −0.13, 0.06 | ||
| PCB194 | ||||
| PCB195 | ||||
| PCB196f | −0.01 | −0.05, 0.02 | 0.08 | −0.02, 0.18 |
| PCB199 | 0.02 | −0.02, 0.07 | ||
| PCB206 | −0.01 | −0.04, 0.01 | −0.04 | −0.09, 0.02 |
|
Organochlorine pesticides (OCPs) (ng/g lipid) | ||||
| HCB | −0.02 | −0.06, 0.01 | ||
| β-HCH | 0.01 | −0.01, 0.04 | ||
| p,p’-DDE | ||||
| p,p’-DDT | ||||
Abbreviations: CI, confidence interval; ng/mL, nanogram per milliliter; ng/g lipid, nanogram per gram lipid
Adjusted for parity, pre-pregnancy BMI, maternal age at delivery, education, prenatal smoking, age at measurement, and gestational age at sample collection.
Single-chemical linear regression models were run to examine associations between each chemical and weight-for-age z-scores. Betas represent a change of 10% higher chemical concentrations.
Multi-chemical linear regression models were run to examine associations between each chemical in a class (e.g., PFAS) and weight-for-age z-scores, independent of other chemicals in the class (i.e., adjusting for other chemicals in the class). Betas represent a change of 10% higher chemical concentrations.
10% change in chemical concentrations calculated as β*ln(1.1)
Only β values ≥|0.01| are displayed.
PCB congeners 138 and 158 could not be separated and were quantified as a summed concentration, referred to as PCB138. Similarly, PCB congeners 196 and 203 could not be separated and were quantified as a summed concentration, referred to as PCB196.
Median serum concentrations (and interquartile ranges) of PFAS, PCBs, and OCPs are presented in Table S2. Of the 52 chemicals measured, 31 were detected in more than 75% of mothers. Correlation was high among the 31 chemicals (Figure 1), with strong intra- and inter-class correlation among PCBs and OCPs.
Figure 1.
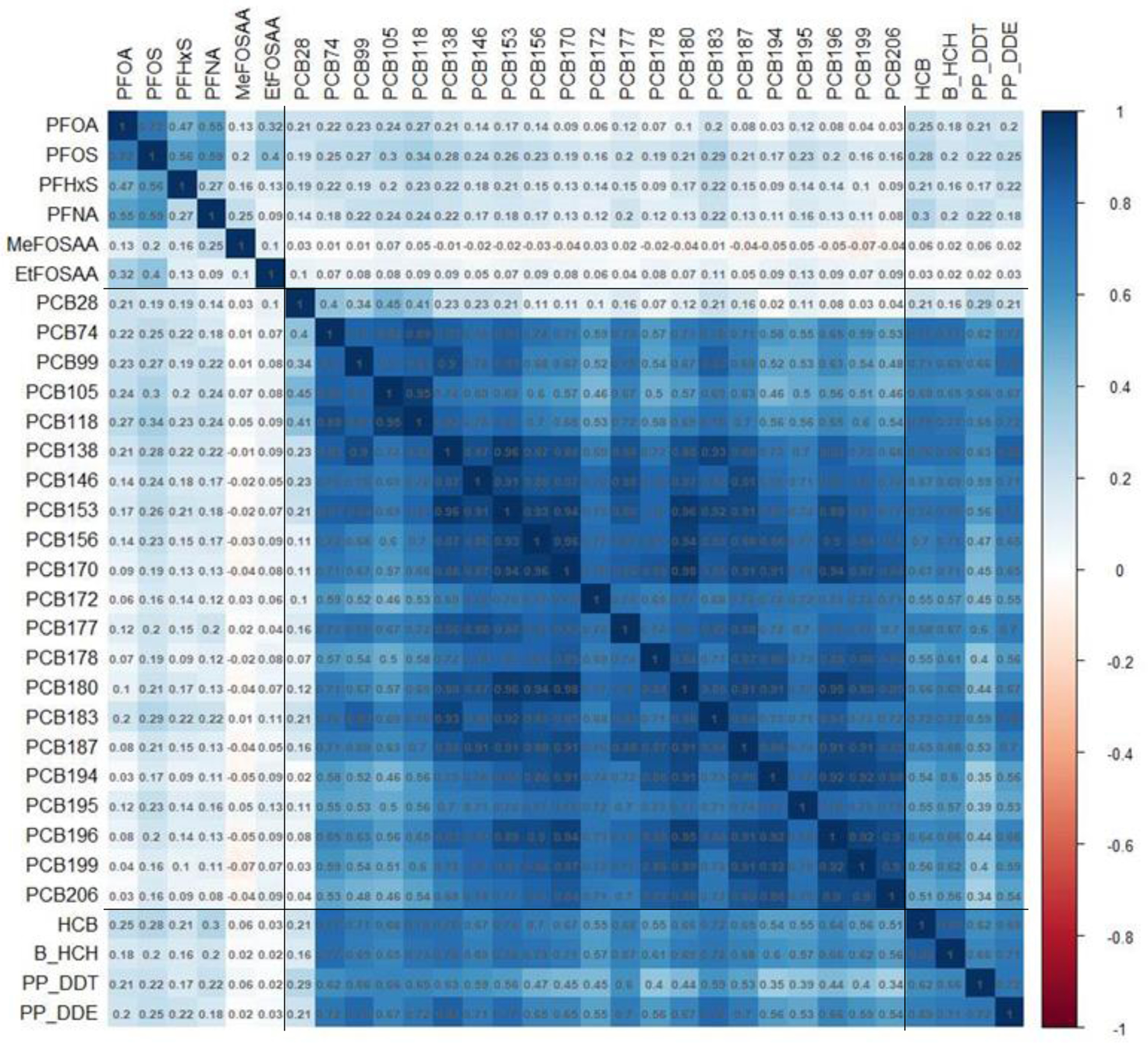
Correlation heatmap of serum concentrations of persistent endocrine disrupting chemicals across women during pregnancy in the Avon Longitudinal Study of Parents and Children (N=425). Spearman correlation coefficients presented for untransformed distributions, sectioned according to per- and polyfluoroalkyl substances (PFAS), polychlorinated biphenyl (PCB), and organochlorine pesticide (OCP) group membership. PCB and OCP concentrations were lipid-adjusted.
Single- and multi-chemical models
Weight-for-age scores at 19 months
Table S3 shows the results of single- and multi-chemical models for weight-for-age scores at roughly 19 months of age. While single-chemical associations were generally weak, they were consistent in direction: all chemicals were positively associated with weight-for-age scores in single-chemical models. For example, a 10% higher PCB199 concentration was associated with 0.03 higher (95% confidence interval [CI]: 0.00, 0.05) z-score for weight-for-age.
Weight-for-age scores at 0, 2, 9, and 19 months
Table 3 shows results from single- and multi-chemical mixed models for weight-for-age scores at 0, 2, 9, and 19 months old with a random intercept. While associations were weak in single-chemical mixed models, they were consistent in direction: almost all chemicals were inversely associated with weight-for-age scores (with the exception of PCB172). In the multi-chemical PFAS mixed model, 10% higher PFOA was inversely associated with weight-for-age scores (β: −0.03, 95% CI: −0.07, 0.01). In the multi-chemical PCB mixed model, 10% higher PCB74 was positively associated with weight-for-age scores (β: 0.07, 95% CI: 0.01, 0.13), while 10% higher PCB118 was inversely associated with weight-for-age scores (β: −0.09, 95% CI: −0.19, 0.01).
Bayesian Kernel Machine Regression
All three classes combined and weight-for-age scores
In the BKMR model with random intercept for the mixture of all three classes combined and weight-for-age scores at 0, 2, 9, and 19 months, OCPs and PCBs had the highest posterior inclusion probabilities (PIPs) of 0.61 and 0.60, respectively, making them the most important groups in the mixture (PIPPFAS: 0.54). Within the OCP group, β-HCH and p,p’-DDT had the highest PIPs (conditional PIP: 0.32 and 0.30, respectively). In the PCB group, PCB105 and PCB118 contributed the most (conditional PIP: 0.11 and 0.09, respectively). Within the PFAS class, PFOS and PFOA contributed the most to the model (conditional PIPs: 0.27 and 0.24, respectively).
Within the BKMR model, the independent natural log-transformed chemical associations all appeared relatively linear with the exception of β-HCH (Figure 2). Some chemicals had slightly positive associations (PFNA, PCB74, PCB105, PCB183, PCB194, HCB), some appeared to have negative associations (PFOA, PCB118, PCB153, p,p’-DDT), and the remainder showed no association with weight-for-age z-scores. We found an overall mixture effect, with higher exposure to the mixture associated with lower weight-for-age z-scores (Figure 3A). Holding all persistent EDCs at the 75th percentile compared to the median was associated with 0.15 lower weight-for-age z-score (estimate: −0.15, 95% credible interval: −0.26, −0.03). Comparing all persistent EDCs at the 75th percentile to the 25th percentile was associated with 0.27 lower weight-for-age z-score (estimate: −0.27, 95% credible interval: −0.42, −0.11).
Figure 2.
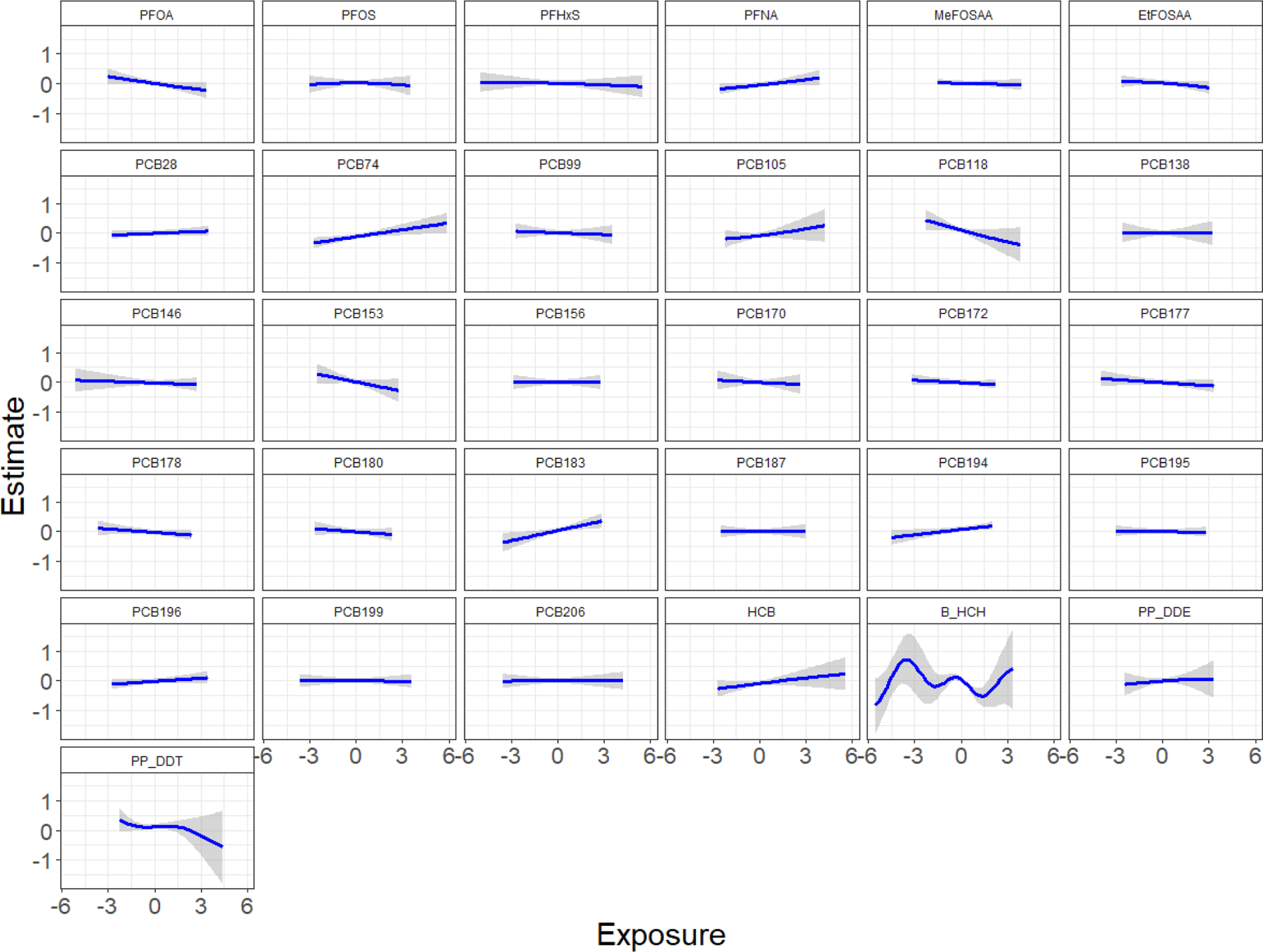
Chemical-specific effect estimates of mixture members on weight-for-age z-scores in ALSPAC mother-daughter dyads estimated by BKMR (n=301). Single chemical associations and 95% credible bands are presented with other chemicals fixed at their median. The model adjusted for maternal education, parity, pre-pregnancy body mass index, maternal age at delivery, prenatal smoking, age at measurement, and gestational week at sample collection. All chemical concentrations were natural log-transformed and standardized; PCB and OCP concentrations were lipid-adjusted.
Figure 3.
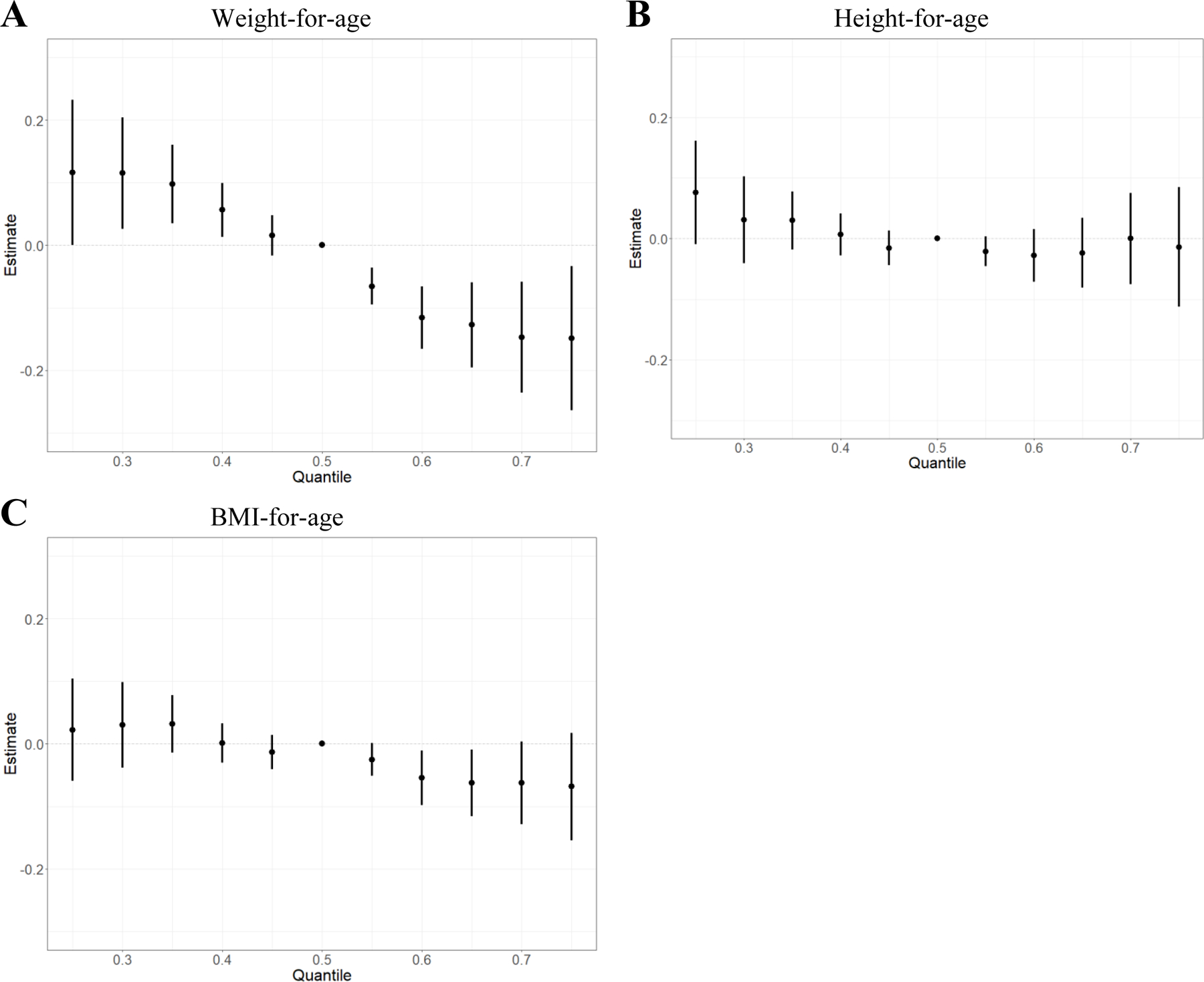
A Overall effect of the mixture on weight-for-age z-scores (estimates and 95% credible intervals), comparing the outcome when all exposures are at a particular quantile to the median (n=301). B Overall effect of the mixture on height-for-age z-scores (estimates and 95% credible intervals), comparing the outcome when all exposures are at a particular quantile to the median (n=300). C Overall effect of the mixture on body mass index (BMI)-for-age z-scores (estimates and 95% credible intervals), comparing the outcome when all concentrations are at a particular quantile to the median (n=300). Bayesian kernel machine regression models adjusted for maternal education, parity, pre-pregnancy body mass index, maternal age at delivery, prenatal smoking, age at measurement, and gestational age at sample collection, and used a random intercept to account for repeated measures at 0, 2, 9, and 19 months. All chemical concentrations were natural log-transformed and standardized; PCB and OCP concentrations were lipid-adjusted.
All three classes combined and height- and BMI-for-age scores
In both the height- and BMI-for-age models, PCBs had the highest posterior inclusion probability (PIP) (PIPheight: 0.54 and PIPBMI: 0.45). In the height-for-age model, PCB153 contributed the most (conditional PIP: 0.17) to the mixture of all three classes combined, while PCB178 contributed the most (conditional PIP: 0.14) to the mixture in the BMI-for-age model. While associations were null, the overall mixture effect for all three classes combined and height-for-age (Figure 3B) and BMI-for-age (Figure 3C) z-scores showed slight inverse associations.
Class-specific mixtures and weight-for-age
BKMR models were run for each chemical class individually (i.e., separate models for PFAS, PCBs, and OCPs). In the PFAS model, PFOA and PFOS had the highest PIPs (PIPs: 0.57 and 0.54, respectively). We found an overall mixture effect for PFAS, with higher exposure to the mixture associated with lower weight-for-age z-scores (Figure S2A). Similarly, in the PCB model, we found an inverse association of exposure to the mixture with weight-for-age z-scores (Figure S2B), although it was less precisely estimated. In the OCP model, there was no overall mixture effect; median exposure to the OCP mixture was associated with the lowest weight-for-age z-scores (Figure S2C).
Sensitivity analyses
We conducted sensitivity analyses to examine the association between the overall mixture of chemicals and weight-for-age scores at various postnatal time points (separate time points, as opposed to all time points together in a mixed model). Using both WQS regression and BKMR, we found an inverse association of the overall mixture with birth weight (WQS: Table S4; BKMR: Figure S3A), which has previously been reported in ALSPAC (42), as well as weight-for-age scores at 2 months (WQS: Table S4; BKMR: Figure S3B). We found no association between the mixture of 31 chemicals and weight-for-age scores at 9 or 19 months (WQS: Table S4; BKMR: Figure S3C&D). See Supplemental Methods for further sensitivity analysis results.
Discussion
In this study of prenatal exposure to mixtures of persistent EDCs and postnatal body size at four time points through 19 months among British girls, we found an inverse association between a 31-chemical mixture and weight-for-age z-scores. This association seems to be driven by early postnatal weight-for-age. Further, weakly inverse associations were seen for height-for-age and BMI-for-age z-scores. These results suggest that prenatal exposure to mixtures of persistent EDCs may affect postnatal body size. We found that holding all 31 EDCs in the mixture at the 75th percentile compared to the 50th percentile was associated with 0.15 lower weight-for-age z-score (estimate: −0.15, 95% credible interval: −0.26, −0.03). At mean values for 19 months of age, a 0.15 lowering of the weight-for-age z-score corresponds to 0.18 kg lower weight (estimate: −0.18 kg, 95% credible interval: −0.31, −0.03 kg), which is a 1.6% decrease (estimate: −1.6%, 95% credible interval: −2.7, −0.3%). Although these associations with postnatal body size may be modest at the individual or clinical level, it is worth considering implications at the population level. A relatively small, subclinical effect size may be associated with considerable population burden if the exposure is common, like EDCs.
Previous studies under the single-chemical paradigm have identified no association between prenatal PCB exposure and postnatal growth (change in weight-for-age Z-score between birth and 2 years (19), rapid growth in the first 6 months of life (20), and elevated BMI at 14 months (20)). We observed an imprecise but inverse association of a mixture of 21 PCB congeners with weight-for-age scores through 19 months. An important difference between previous studies and this study is the use of mixture methods: it is possible that previous studies of PCBs and postnatal growth and body size have underestimated the risks of exposure to PCBs by examining only one congener at a time.
We observed no association between a four-chemical mixture of OCPs and weight-for-age scores. Previous studies of OCPs and postnatal growth measures under the single-chemical paradigm have noted positive associations between prenatal exposure to DDE (19–21), DDT (23), and HCB (21) and a variety of infant growth measures (change in weight-for-age z-score (19), BMI/overweight status (20, 21), BMI-for-age, weight-for-height, and weight-for-age (23)) within the first one to two years of life. We saw no association of the four-chemical OCP mixture with weight-for-age scores through 19 months using BKMR: low (e.g., 25th percentile) or high (e.g., 75th percentile) concentrations of the mixture were associated with higher weight-for-age scores than median concentrations. In our single-chemical models at 19 months, there were weakly positive associations for all OCPs and weight-for-age scores at 19 months, with β-HCH having the strongest association of the four.
A number of studies from Denmark (n=1,010), Ohio (n=334), and Taiwan (n=223) have found inverse associations (25–27) of prenatal PFAS exposure (mainly PFOA and PFOS) and postnatal growth measures (weight (25), height (27), BMI (25, 26)) at various time points in the first two years of life. Other studies from the Netherlands (n=148) and New York (n=1,954) have found no association (22, 28). We found an inverse association between the six-chemical PFAS mixture and weight-for-age scores through 19 months, and PFOA and PFOS were the most important contributors.
A previous study in the ALSPAC population examined prenatal concentrations of three PFAS (PFOA, PFOS, and PFHxS) and postnatal body size (24). The study found inverse associations of prenatal concentrations of PFOA, PFOS, and PFHxS with birth weight, while those in the highest tertile of prenatal PFOS concentrations were 364 g heavier at 20 months than those in the lowest tertile. In addition to PFOA, PFOS, and PFHxS, the present study examined PCBs, OCPs, and additional PFAS (PFNA, MeFOSAA, and EtFOSAA) using a mixtures approach. We saw results similar to Maisonet et al. (2012) in the present study: there were strong inverse associations with birth weight, and most chemicals were weakly associated with higher weight-for-age scores at 19 months in single- and multi-chemical models. While we observed an overall inverse association between prenatal exposure to mixtures of persistent EDCs and weight-for-age scores at four time points through 19 months, especially for PFAS mixtures, the association shrinks following birth and is null by 19 months. Essentially, we see a stronger effect of prenatal exposure to persistent EDCs on body size at time points closer to birth. This finding fits with previous ALSPAC studies that have examined prenatal PFAS and PCB exposure with body fatness at age 9 and have largely found no association (43, 44).
This is the first study to examine prenatal exposure to EDCs and postnatal body size using a mixtures approach. A few studies have examined prenatal exposure to mixtures of EDCs and birth size (42, 45, 46), though the influence of prenatal exposure to EDCs on weight may persist after birth (19–26), and the first two years of life are a particularly important period of change (18). While this was not a focus of the current study, there is mounting evidence that both prenatal and infant growth are associated with future obesity risk; rapid infant growth and obesity in infancy are associated with a higher risk of obesity later in life (47, 48).
We only examined mother-daughter dyads in this sub-study, which was originally intended to investigate early menarche. While postnatal growth does not appear to differ by infant sex in the first six months of life (49), there is some evidence to suggest that EDCs may have differential effects on development by infant sex (50). For this reason, restricting to daughters is a prudent choice, though results cannot be generalized to male infants. Some previous studies that examined prenatal exposure to persistent EDCs with postnatal growth observed differences by infant sex (21–23, 25, 27). In many of these studies, male infants tended towards lower weight and BMI while female infants tended towards higher weight and BMI with higher prenatal exposure to persistent EDCs (DDE (22), DDT (23), PFOA and PFOS (25)). In other studies, higher DDE exposure was more strongly associated with rapid growth among male infants than female infants (21) and higher PFOA exposure was associated with lower weight and height among males than females at age 2 (27). Other studies report no difference by sex in the association of prenatal exposure to persistent EDCs with postnatal growth through three years of age (19, 20, 26, 28). Interestingly, differences by sex are common in studies of prenatal exposure to persistent organic pollutants and BMI during later childhood (5–12 years old), though the direction is not consistent (51–54).
This study has several strengths, including its prospective study design within a population-based birth cohort with frequent and thorough longitudinal data collection. Additionally, we have reliable biomonitoring measurements of more than 50 persistent EDCs collected at median 15 weeks gestation, several outcomes measured by health professionals at birth and during infancy, and extensive covariate data available for mothers and daughters.
This study also has limitations. Because we centered and scaled exposure variables, what appears to be the most important in this population might not be the same as what would appear to be the most important in a different population with a different distribution of exposures. Further, while we have detailed covariate data, there is always the possibility that we were not able to completely control for confounding by certain sensitive or self-reported variables, such as smoking and socioeconomic status, or possible other unmeasured covariates. Approaches to mixture analyses that involve regressing the outcome on several correlated exposures simultaneously can in some cases amplify rather than reduce confounding bias (“coexposure amplification bias”), particularly in cases of residual confounding (55). Our results could be biased if mother-daughter dyads with missing data were different from mother-daughter dyads included in the analyses. Mean values of body size outcomes and maternal characteristics for girls included in BKMR analyses were similar to the girls enrolled in the overall cohort, suggesting that selection bias is an unlikely explanation for our results. Additionally, we were unable to examine postnatal exposure to EDCs and its impact on infant growth, but this is an important question for future research.
Another limitation is that we were unable to weight BKMR analyses of this nested case-control study back to the full cohort, which limits generalizability. Nevertheless, results of the unweighted BKMR analyses were similar to weighted single- and multi-chemical mixed model results in terms of direction of effect. In the multi-chemical mixed model for PFAS, PFOA was most strongly inversely associated with weight-for-age scores; in the BKMR model for weight-for-age, PFOA was identified as one of the most important PFAS and was inversely associated with weight-for-age scores. Similarly, in the multi-chemical mixed model for PCBs, PCB118 was strongly inversely associated with weight-for-age scores, while PCB74 and PCB105 were positively associated with weight-for-age scores. Results of the BKMR model showed this as well: PCB118 was one of the most important PCBs in the mixture and had an inverse association with weight-for-age, while PCB74 and PCB105 showed positive associations. We were able to weight the WQS models in our sensitivity analyses examining each measurement time point individually (the R package, gWQS, does not yet allow for the analysis of correlated data). We found similar results to the equivalent BKMR models: the mixture of 31 chemicals is inversely associated with weight-for-age at birth and 2 months, but the associations substantially weaken at 9 and 19 months.
In conclusion, we found an inverse association between prenatal exposure to a mixture of persistent EDCs (PFAS, PCBs, OCPs) and longitudinal postnatal body size through 19 months as measured through weight-for-age z-scores. The observed association appears to be driven by early postnatal body size. The association of the 31-chemical EDC mixture with height-for-age and BMI-for-age scores was also inverse, but weaker than what was observed for weight-for age scores.
Supplementary Material
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Funding
The UK Medical Research Council and Wellcome (Grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and they will serve as guarantors for the contents of this paper. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf).
This work was specifically funded by the Centers for Disease Control and Prevention (AY5350).
This research was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC.
Abbreviations:
- EDCs
endocrine disrupting chemicals
- PFAS
per- and polyfluoroalkyl substances
- PCBs
polychlorinated biphenyls
- OCPs
organochlorine pesticides
- ALSPAC
Avon Longitudinal Study of Parents and Children
- BKMR
Bayesian kernel machine regression
- WQS regression
weighted quantile sum regression
- PIPs
posterior inclusion probabilities
Footnotes
Declarations of interest:
None
Disclosures
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Conflict of Interest Statement
None declared
References
- 1.National Institute of Environmental Health Sciences. Endocrine Disruptors Research Triangle Park, North Carolina2019 [updated May 10, 2019. Available from: https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm.
- 2.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Polychlorinated Biphenyls (PCBs) Atlanta, GA: 2000. [Available from: https://www.atsdr.cdc.gov/toxprofiles/tp17.pdf. [PubMed] [Google Scholar]
- 3.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for DDT, DDE, and DDD. In: Department of Health and Human Services PHS, editor. Atlanta, GA: 2002. [PubMed] [Google Scholar]
- 4.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for Perfluoroalkyls. (Draft for Public Comment). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2009. [Google Scholar]
- 5.Schoeters G, Govarts E, Bruckers L, Den Hond E, Nelen V, De Henauw S, et al. Three cycles of human biomonitoring in Flanders - Time trends observed in the Flemish Environment and Health Study. Int J Hyg Environ Health. 2017;220(2 Pt A):36–45. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention, National Center for Health Statistics (CDC). National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Department of Health and Human Services; 2017. [Google Scholar]
- 7.Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, et al. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspect 2004;112(11):1204–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kezios KL, Liu X, Cirillio PM, Kalantzi OI, Wang Y, Petreas MX, et al. Prenatal polychlorinated biphenyl exposure is associated with decreased gestational length but not birth weight: archived samples from the Child Health and Development Studies pregnancy cohort. Environmental health : a global access science source. 2012;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, Dowler JK. The transfer of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. Am J Public Health. 1984;74(4):378–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sala M, Ribas-Fito N, Cardo E, de Muga ME, Marco E, Mazon C, et al. Levels of hexachlorobenzene and other organochlorine compounds in cord blood: exposure across placenta. Chemosphere 2001;43(4–7):895–901. [DOI] [PubMed] [Google Scholar]
- 11.Sagiv SK, Rifas-Shiman SL, Fleisch AF, Webster TF, Calafat AM, Ye X, et al. Early-Pregnancy Plasma Concentrations of Perfluoroalkyl Substances and Birth Outcomes in Project Viva: Confounded by Pregnancy Hemodynamics? Am J Epidemiol 2018;187(4):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longnecker MP, Klebanoff MA, Brock JW, Guo X. Maternal levels of polychlorinated biphenyls in relation to preterm and small-for-gestational-age birth. Epidemiology. 2005;16(5):641–7. [DOI] [PubMed] [Google Scholar]
- 13.Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, et al. The Navigation Guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect 2014;122(10):1028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govarts E, Nieuwenhuijsen M, Schoeters G, Ballester F, Bloemen K, de Boer M, et al. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European Birth Cohorts. Environ Health Perspect 2012;120(2):162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect 2007;115(11):1677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanck HM, Marcus M, Rubin C, Tolbert PE, Hertzberg VS, Henderson AK, et al. Growth in girls exposed in utero and postnatally to polybrominated biphenyls and polychlorinated biphenyls. Epidemiology. 2002;13(2):205–10. [DOI] [PubMed] [Google Scholar]
- 17.Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, et al. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect 2007;115(11):1670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. Bmj 2000;320(7240):967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iszatt N, Stigum H, Verner MA, White RA, Govarts E, Murinova LP, et al. Prenatal and Postnatal Exposure to Persistent Organic Pollutants and Infant Growth: A Pooled Analysis of Seven European Birth Cohorts. Environ Health Perspect 2015;123(7):730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendez MA, Garcia-Esteban R, Guxens M, Vrijheid M, Kogevinas M, Goni F, et al. Prenatal organochlorine compound exposure, rapid weight gain, and overweight in infancy. Environ Health Perspect 2011;119(2):272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valvi D, Mendez MA, Garcia-Esteban R, Ballester F, Ibarluzea J, Goni F, et al. Prenatal exposure to persistent organic pollutants and rapid weight gain and overweight in infancy. Obesity (Silver Spring). 2014;22(2):488–96. [DOI] [PubMed] [Google Scholar]
- 22.de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. First year growth in relation to prenatal exposure to endocrine disruptors - a Dutch prospective cohort study. International journal of environmental research and public health. 2014;11(7):7001–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coker E, Chevrier J, Rauch S, Bradman A, Obida M, Crause M, et al. Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort. Environ Int 2018;113:122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM, et al. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environmental health perspectives. 2012;120(10):1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen CS, Fei C, Gamborg M, Nohr EA, Sorensen TI, Olsen J. Prenatal exposures to perfluorinated chemicals and anthropometric measures in infancy. Am J Epidemiol 2010;172(11):1230–7. [DOI] [PubMed] [Google Scholar]
- 26.Shoaff J, Papandonatos GD, Calafat AM, Chen A, Lanphear BP, Ehrlich S, et al. Prenatal Exposure to Perfluoroalkyl Substances: Infant Birth Weight and Early Life Growth. Environ Epidemiol 2018;2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Adgent M, Su PH, Chen HY, Chen PC, Hsiung CA, et al. Prenatal Exposure to Perfluorocarboxylic Acids (PFCAs) and Fetal and Postnatal Growth in the Taiwan Maternal and Infant Cohort Study. Environ Health Perspect 2016;124(11):1794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeung EH, Bell EM, Sundaram R, Ghassabian A, Ma W, Kannan K, et al. Examining Endocrine Disruptors Measured in Newborn Dried Blood Spots and Early Childhood Growth in a Prospective Cohort. Obesity (Silver Spring). 2019;27(1):145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Institute of Environmental Health Sciences. 2018–2023 Strategic Plan: Advancing Environmental Health Science, Improving Health 2.0. In: Department of Health and Human Services, editor. Durham, NC: 2018. [Google Scholar]
- 30.National Institute of Environmental Health Sciences. Powering Research through Innovative Methods for mixtures in Epidemiology (PRIME) (R01) RFA-ES-17–001 2017. [Available from: https://grants.nih.gov/grants/guide/rfa-files/RFA-ES-17-001.html.
- 31.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort Profile: the ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013;42(1):111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, et al. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 2013;42(1):97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen KY, Maisonet M, Rubin C, Holmes A, Calafat AM, Kato K, et al. Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environment international. 2011;37(1):129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuklenyik Z, Needham LL, Calafat AM. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal Chem 2005;77(18):6085–91. [DOI] [PubMed] [Google Scholar]
- 35.Sjödin A, Jones RS, Lapeza CR, Focant J-F, McGahee EE, Patterson DG. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Analytical chemistry. 2004;76(7):1921–7. [DOI] [PubMed] [Google Scholar]
- 36.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Statistics in medicine. 1998;17(4):407–29. [PubMed] [Google Scholar]
- 37.Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bobb JF, Claus Henn B, Valeri L, Coull BA. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health. 2018;17(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coull B, Bobb J, Wellenius G, Kioumourtzoglou M, Mittleman M, Koutrakis P, et al. Part 1. Statistical Learning Methods for the Effects of Multiple Air Pollution Constituents. Research report (Health Effects Institute). 2015(183 Pt 1–2):5–50. [PubMed] [Google Scholar]
- 40.Czarnota J, Gennings C, Colt JS, De Roos AJ, Cerhan JR, Severson RK, et al. Analysis of Environmental Chemical Mixtures and Non-Hodgkin Lymphoma Risk in the NCI-SEER NHL Study. Environ Health Perspect 2015;123(10):965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat 2015;20(1):100–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marks KJ, Howards PP, Smarr MM, Flanders WD, Northstone K, Daniel JH, et al. Prenatal exposure to mixtures of persistent endocrine disrupting chemicals and birth size in a population-based cohort of British girls. Epidemiology. 2021;32(4):573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartman TJ, Calafat AM, Holmes AK, Marcus M, Northstone K, Flanders WD, et al. Prenatal Exposure to Perfluoroalkyl Substances and Body Fatness in Girls. Child Obes 2017;13(3):222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang A, Jeddy Z, Sjodin A, Taylor EV, Marks KJ, Hartman TJ. Prenatal exposure to Polychlorinated Biphenyls and body fatness in girls. Chemosphere. 2019;236:124315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods MM, Lanphear BP, Braun JM, McCandless LC. Gestational exposure to endocrine disrupting chemicals in relation to infant birth weight: a Bayesian analysis of the HOME Study. Environmental Health. 2017;16(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenters V, Portengen L, Rignell-Hydbom A, Jonsson BA, Lindh CH, Piersma AH, et al. Prenatal Phthalate, Perfluoroalkyl Acid, and Organochlorine Exposures and Term Birth Weight in Three Birth Cohorts: Multi-Pollutant Models Based on Elastic Net Regression. Environ Health Perspect 2016;124(3):365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monasta L, Batty GD, Cattaneo A, Lutje V, Ronfani L, Van Lenthe FJ, et al. Early-life determinants of overweight and obesity: a review of systematic reviews. Obes Rev 2010;11(10):695–708. [DOI] [PubMed] [Google Scholar]
- 48.Brisbois TD, Farmer AP, McCargar LJ. Early markers of adult obesity: a review. Obes Rev 2012;13(4):347–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hindmarsh PC, Geary MP, Rodeck CH, Kingdom JC, Cole TJ. Factors predicting ante- and postnatal growth. Pediatr Res 2008;63(1):99–102. [DOI] [PubMed] [Google Scholar]
- 50.Kjeldsen LS, Bonefeld-Jorgensen EC. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int 2013;20(11):8031–44. [DOI] [PubMed] [Google Scholar]
- 51.Tang-Peronard JL, Heitmann BL, Andersen HR, Steuerwald U, Grandjean P, Weihe P, et al. Association between prenatal polychlorinated biphenyl exposure and obesity development at ages 5 and 7 y: a prospective cohort study of 656 children from the Faroe Islands. Am J Clin Nutr 2014;99(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valvi D, Mendez MA, Martinez D, Grimalt JO, Torrent M, Sunyer J, et al. Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study. Environ Health Perspect 2012;120(3):451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warner M, Ye M, Harley K, Kogut K, Bradman A, Eskenazi B. Prenatal DDT exposure and child adiposity at age 12: The CHAMACOS study. Environ Res 2017;159:606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warner M, Wesselink A, Harley KG, Bradman A, Kogut K, Eskenazi B. Prenatal exposure to dichlorodiphenyltrichloroethane and obesity at 9 years of age in the CHAMACOS study cohort. Am J Epidemiol 2014;179(11):1312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weisskopf MG, Seals RM, Webster TF. Bias Amplification in Epidemiologic Analysis of Exposure to Mixtures. Environ Health Perspect 2018;126(4):047003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


