Abstract
Background
While many studies have described the impact of prenatal opioid exposure on development, possible mechanisms for how opioids exert developmental impairments remain elusive. Emerging evidence indicates disruptions in the maternal gut microbiome can alter offspring development; however, no studies to date have examined the impact of maternal opioid treatment on maternal-offspring microbiome dysbiosis.
Methods
A mouse model of prenatal methadone exposure (PME) was employed to assess the impact of maternal opioid treatment on the microbiome of methadone-treated dams (MD) and their offspring. Fecal samples were collected from dams (n=8 per treatment), one male and one female offspring per dam (n=8 offspring per sex per treatment) for 16s rRNA sequencing.
Results
Methadone treatment significantly increased the microbial diversity and led to an expansion in family level bacterial abundance. Correlational analysis revealed significant positive associations between dam and offspring measures of diversity indicating methadone-induced shifts in the microbial communities are shared between dam and offspring. Sixteen features in dams and 10 features in offspring were significantly differentially abundant between treatment groups with many features corresponding to the Lachnospiraceae NK4A136 genus. Of the six features identified as differentially abundant in both MD and PME offspring, all were assigned to the Lachnospiraceae NK4A136 group, and the abundances demonstrated strong positive correlations between dam and offspring.
Conclusions
These preliminary findings indicate that maternal opioid treatment during pregnancy alters the composition of the maternal microbiome, and this opioid-induced shift is similarly observed in offspring which could contribute to the impaired developmental phenotypes previously described.
Keywords: Prenatal, Microbiome, Development, Methadone, Pregnancy, Opioid
1. Introduction
As the opioid crisis continues to flourish, the population of children born exposed to opioids during pregnancy has steadily risen. From 2010 to 2017, maternal opioid use disorder at delivery increased 131%, and neonatal opioid withdrawal syndrome increased by 82% in the U.S. (Hirai et al., 2021). Opioid exposure during gestation has diverse and enduring consequences on development including impairments in attention, cognition, and motor skills (Monnelly et al., 2019; Yeoh et al., 2019). It remains unclear how opioids impact fetal and childhood development.
We developed a mouse model of prenatal methadone exposure (PME; (Grecco et al., 2021)) which exemplifies the growing proportion of prenatal opioid-exposure cases resulting from treatment of opioid use disorder in reproductive-age women (Tolia et al., 2015). Although pregnancy and maternal care were unaffected by methadone treatment to dams, PME produced impairments in offspring physical, behavioral, and neurological development (Grecco et al., 2021).
There are numerous mechanisms by which opioid exposure may induce these maladaptive phenotypes in offspring. One possibility is through an opioid-induced disruption in the maternal-offspring gut microbiome. The bacterial composition of the microbiome shapes development and an array of biological processes (Smith and Wissel, 2019). Disruptions in the maternal gut microbiome alter neuronal development, metabolic phenotypes, and the immune response of offspring likely through the production of gut microbiota–derived metabolites such as short-chain fatty acids or other neuromodulators (Kimura et al., 2020; Lee et al., 2021; Nyangahu et al., 2018; Vuong et al., 2020). As opioids induce gut microbiota dysbiosis (Gicquelais et al., 2020; Wang et al., 2018), maternal opioid use may disrupt the maternal microbiota during pregnancy which impairs the development of opioid-exposed offspring through the direct transfer of gut dysbiosis or indirectly via the effects of gut microbiota–derived metabolites on fetal development. Here, we assess the gut microbiome of methadone-treated dams (MD) and PME male and female offspring to identify shifts in microbial communities that may contribute to the impairments in offspring development we previously characterized.
2. Materials and Methods
2.1. Animals
Experimental protocols followed NIH guidelines and were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. A full description of the PME model can be found in (Grecco et al., 2021). Female C57BL/6J mice received either saline or an increasing oxycodone dose ramp to model recreational opioid dependence. Oxycodone-dependent mice then transitioned to methadone (10 mg/kg s.c. b.i.d.) while saline-treated animals continued receiving saline. These treatments continued throughout mating, pregnancy, and weaning. This dose of methadone produces plasma levels within the therapeutic range and dependency in both dams and offspring (Grecco et al., 2021).
2.2. Sample Collection and DNA Extraction
Fresh fecal samples were collected on the day of weaning from methadone-treated or saline-treated dams (MD & SD) and one male and one female PME or prenatal saline exposed (PSE) offspring per dam. Total genomic DNA was extracted using the Qiagen QIAamp PowerFecal DNA Kit (Cat. No. 51804) from each individual sample (no sample pooling was performed). Extracted DNA samples were stored at −80°C until amplification.
2.3. 16S rRNA Amplification, Sequencing, and Preprocessing
DNA samples were assessed using a TapeStation (Agilent Technologies) and quantified with Qubit (ThermoFisher Scientific). 16S sequencing libraries were prepared using the QIAseq 16S/ITS Screen Panel kit (Qiagen) following the manufacturer’s instructions. All nine variable regions of the bacterial 16S gene and the fungal ITS gene were targeted. The kit uses an ultraclean production Master Mix and PCR Water to prevent reagent contamination. Each indexed library was quantified and its quality evaluated by TapeStation. Final libraries were sequenced on an Illumina MiSeq with 2 X 300bp paired-end reads.
Sequencing reads generated were checked using FastQC (Babraham Bioinformatics) for data quality. As QIAseq 16S/ITS Screen Panel interrogates all variable regions of 16S, the FASTQ files derived from each sample were first demultiplexed into separate FASTQ files that contain reads from each targeted 16S region amplicon (V1V2, V2V3, V3V4, V4V5, V5V7, V7V9). Microbial compositions were investigated on each amplified 16S region for all samples using DADA2 version 0.16 (Callahan et al., 2016). Pseudo-pooling was used for sample inference and an amplicon sequence variant (ASV) table was generated with the DADA2 function "makeSequenceTable". Silva reference database v132 was used for taxonomy assignment. The Smart Control included in the QIAseq 16S/ITS kit was used as a negative control for microbial composition.
The ASV files with abundance values from the V3V4 region were used for analyses due to the reported greater phylogenetic resolution (Xu et al., 2017). The remaining analyses were completed using MicrobiomeAnalyst (Dhariwal et al., 2017). The preprocessed data are found in Supplementary File 1. Features were filtered and normalized using the default settings in MicrobiomeAnalyst.
2.4. Community Profiling Analysis and Statistics
The alpha diversity was calculated at the feature level using Chao1 index and Observed metric and compared using two-way ANOVA with treatment (methadone/saline) and age (dam/offspring) as factors (GraphPad Prism 8). A principal coordinate analysis was employed at the feature level via the Jensen-Shannon Divergence distance method to compare beta diversity. Abundance profiling was completed at the family taxonomy level (although genus level comparisons are also provided). As not all microbial families were identified in both dam and offspring samples, two-sided Student’s t-tests were used to assess abundance differences. The level of significance was set at p<0.05 for all community profiling analyses. As initial analyses of offspring revealed no sex effects or interactions with sex for community profiling, subsequent analyses were collapsed on sex.
2.5. Hierarchical Clustering and Comparative Analyses
Hierarchical clustering by treatment and heatmap visualization were completed at the feature level using the Pearson Correlation coefficient and Ward clustering algorithm followed by differential abundance analysis using DEseq2 (log2 fold change (FC) of MD vs SD and PME vs PSE). A linear discriminant analysis (LDA) effect size (LEfSe) was further completed in offspring for biomarker discovery (Log LDA cutoff ≥ ±4.0). Only features that fell below the strict FDR<0.05 were considered significant for feature level comparisons.
2.6. Maternal-Offspring Correlations
To identify potential relationships between dam and offspring microbiota, Pearson’s r correlation coefficients (p<0.05) were calculated for alpha diversity metrics, family level abundances, and abundances of ASVs.
3. Results
3.1. Microbial Community Diversity
Alpha diversity was evaluated using the Chao1 index (microbial evenness) and Observed metric (microbial richness). Analyses revealed opioid treatment and age effects on the Chao1 (ANOVA: Treatment, F(1,44)=5.97, p=0.019; Age, F(1,44)=82.7, p<0.0001; Interaction, F(1,44)=0.0727, p=0.79; Figure 1A, top) and Observed metric (ANOVA: Treatment, F(1,44)=7.202, p=0.010; Age, F(1,44)=87.77, p<0.0001; Interaction, F(1,44)=0.0426, p=0.84; Figure 1A, bottom) indicating methadone treatment increased both the overall microbiome evenness and richness in MD and PME offspring. Chao1 (r=0.5841, p=0.0004; Figure 1B, top) and Observed (r=0.6015, p=0.0003; Figure 1B, bottom) metrics for dams and their offspring were positively correlated suggesting maternal opioid treatment produces shared alterations in microbial diversity of dams and offspring. Beta diversity (microbial similarity/dissimilarity within groups) assessment revealed distinct clustering by treatment for dams (PERMANOVA: F=2.42, R2=0.15, p=0.026; Figure 1C top) but this clustering was lost in offspring (PERMANOVA: F=1.76, R2=0.055, p=0.11; Figure 1C bottom). Similar to the alpha diversity, taxonomical analysis at the family level revealed an overall increase in the abundance of nearly all bacterial families identified in MD and PME offspring relative to controls. In dams, methadone increased Erysipelotrichaceae, Peptostreptococcaceae, Akkermansiaceae, Lactobacillaceae, Sutterellaceae, Eubacterium Coprostanoligenes Group, Anaerovoracaceae, Monoglobaceae, and Eggerthellaceae (Figure 1D, top; Supplementary File 2 contains full test statistics). In offspring, methadone increased Rikenellaceae, Peptococcaceae, and Saccharimonadaceae (Figure 1D, bottom; Supplementary File 2 contains full test statistics). Genus level comparisons also revealed several differences in genera of MD and PME offspring (provided in Supplementary File 2). Of the 21 bacterial families identified in both dams and offspring, seven families including Ruminococcaceae, Rikenellaceae, Bacteroidaceae, Erysipelotrichaceae, Acholeplasmataceae, Peptococcaceae, and the unassigned category were positively correlated (Figure 1E contains full statistics) further supporting common opioid-induced gut dysbiosis in dams and their offspring.
Figure 1. Microbial Community Profiling.
A Methadone treatment increased the alpha diversity as measured by the Chao1 (ANOVA: Treatment, p=0.019; Age, p<0.0001; top) and Observed metrics (ANOVA: Treatment, p=0.010; Age, p<0.0001; bottom). B Both the Chao1 (Pearson’s r=0.5841, p=0.0004; top) and the Observed (Pearson’s r=0.6015, p=0.0003; bottom) alpha diversity metrics showed strong correlation between dams and their offspring indicating the level of microbial diversity in dams is related to microbial diversity in offspring. C Principal coordinate analysis for beta diversity of samples demonstrated significant clustering by treatment group in dams (PERMANOVA: F=2.42, R2=0.15, p=0.026; top) but not in offspring (PERMANOVA: F=1.76, R2=0.055, p=0.11; bottom). D Family level taxonomical analysis revealed an expansion of bacerial abundance across nearly all families present in samples of MD (top) and PME offspring (bottom) compared to saline controls (Families in red text reached the level of significance). E Correlational analysis revealed seven families that were significantly positively correlated between dams and offspring further underscoring the possibility that microbial communtities between dam and offspring are related. n=8 dams per treatment. n=8 offspring per sex per exposure group. *p<0.05. Box plots indicate 25th to 75th percentiles with whisker characterizing the minimum and maximum value. Stacked columns for each of the group show the mean of abundance of a given family of bacteria from seqeuncing data for each treatment group.
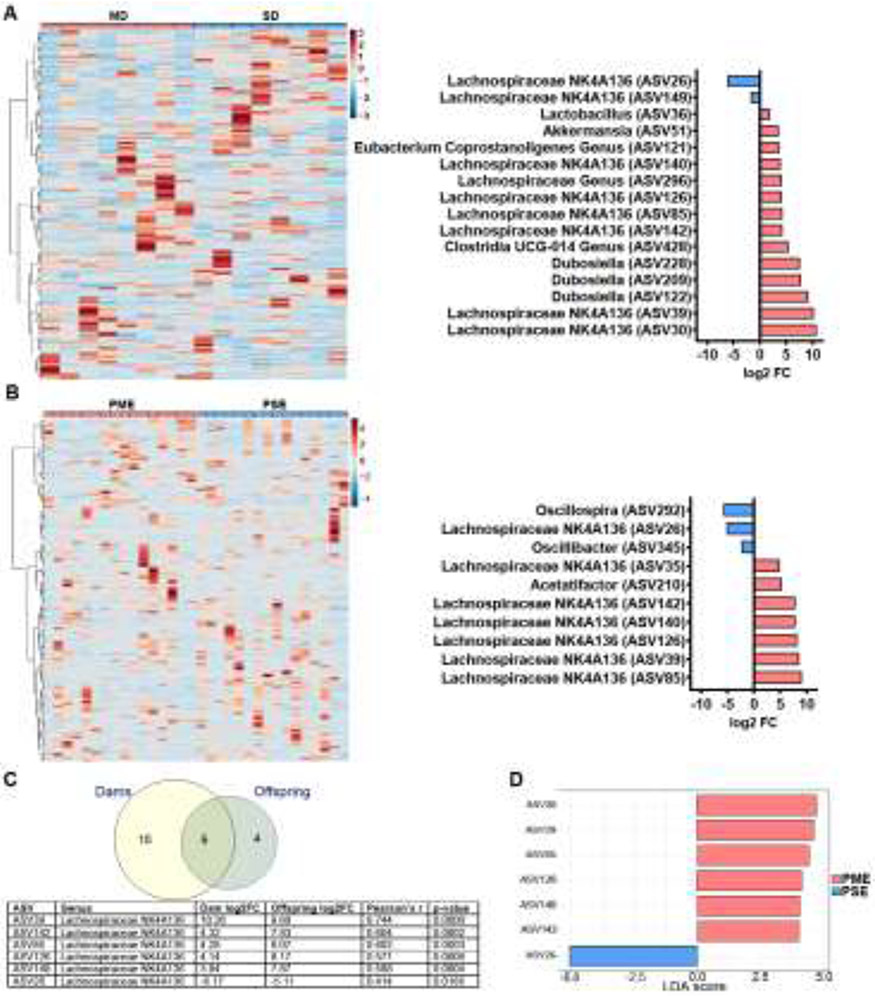
3.2. Feature Level Comparative Analysis
Clustered heatmaps demonstrate the variation in ASV abundance for MD and SD (Figure 2A, left) and PME and PSE offspring (Figure 2B, left). Of the 344 ASVs observed in dams, 16 were differentially abundant (FDR<0.05; Figure 2A, right) with 8/16 ASVs assigned to the Lachnospiraceae NK4A136 genus. Of 271 ASVs identified in offspring, 10 were differentially abundant (FDR<0.05; Figure 2B, right; Supplementary File 3 for full DEseq2 output) with 7/10 ASVs also assigned to Lachnospiraceae NK4A136. Six differentially abundant ASVs were discovered in both dams and offspring, and these six were all assigned to the Lachnospiraceae NK4A136 genus (Figure 2C). The abundance of these six overlapping ASVs in dams was positively correlated with the abundance in offspring (see Figure 2C for statistics). Given our extensive characterization of the phenotypic differences in PME offspring, we performed a biomarker analysis to determine the features that may be most relevant for differentiating exposure groups to facilitate the identification of bacterial groups that contribute to the aberrant development of PME offspring. The LEfSe analysis revealed seven features (LDA score ≥ ±4.0, FDR<0.05; Supplementary File 4 for full LEfSe output) that may serve as potentially relevant biomarkers to differentiate PME and PSE microbiome profiles (Figure 2D). All seven features were assigned to the Lachnospiraceae NK4A136 genus indicating these particular bacteria may represent a novel microbial feature associated with PME.
Figure 2. Comparative Analysis and Biomarker Identification.
A, B Hierarchical clustered heatmaps at the ASV level demonstrating the variation in abundance by treatment group (left) and log2FC values for the ASV which demonstrated differential abundance analysis (represented as MD/PME vs SD/PSE; FDR<0.05; right) for dams (A) and offspring (B). For both dams and offspring, many of the differentially abundant ASVs were assigned to the Lachnospiraceae NK4A136 genus. C Of the 16 and 10 significant differentially abundant ASVs identified in dams and offspring, respectively, six of these ASVs were present in both the dams and the offspring. Additionally, all six ASVs were all assigned to the Lachnospiraceae NK4A136 genus and the abundances demonstrated moderately strong positive correlations between dam and offspring. D A LEfSe analysis further identified seven features (LDA score ≥ ± 4.0, FDR<0.05) all corresponding to the Lachnospiraceae NK4A136 genus suggesting this genus may serve as potentially relevant biomarker that plays the largest role in differentiating PME and PSE gut microbiome profiles. n=8 dams per treatment. n=8 offspring per sex per exposure group.
4. Discussion
This study illustrates that maternal opioid administration during the perinatal period produces microbial dysbiosis in dams with shared alterations observed in the microbiome of opioid-exposed offspring. We also identified the Lachnospiraceae NK4A136 group as notable genus that is differentially abundant in both dams and offspring and may represent a novel biomarker for further investigation.
Although the mechanism by which opioids disrupt the microbiota is unknown, activation of opioid receptors within the gastrointestinal system slows transit possibly producing gut dysbiosis (Akbarali and Dewey, 2019). This change in the bacterial community may promote proinflammatory changes or alter production of bacterial metabolites (Banerjee et al., 2016; Ren and Lotfipour, 2020; Wang and Roy, 2017). Although very little is known about the specific Lachnospiraceae NK4A136 genus we identified, aside from their relation to host metabolism, these anaerobic fermenters break down dietary carbohydrates in gut producing short-chain fatty acids (SCFAs; e.g. acetate, propionate, butyrate) (Wu et al., 2020). These colonic-derived SCFAs enter systemic circulation and are transported into the brain where they influence neurogenesis, microglia functioning, serotonin metabolism, and synaptic transmission which are hypothesized mechanisms by which gut dysbiosis impacts neurodevelopment and dysfunction (Silva et al., 2020).
Investigations of opioid effects on microbial diversity in the rodent microbiome are sparse. Our data contrast with two studies demonstrating no impact (Banerjee et al., 2016; Lee et al., 2018) and another reporting a reduction in alpha diversity (Wang et al., 2018) following morphine treatment. However, these studies administered morphine for 5-6 days, while we utilize a 60-day chronic methadone treatment. Interestingly, an increase in alpha diversity was described within a population of individuals with substance use disorder, the majority reported heroin use (Xu et al., 2017). A gradual increase in bacterial abundance was associated with longer substance use indicating the effect on the microbiome may be dependent on length of exposure. Our findings indicate that chronic opioid treatment leads to an expansion of the microbiota in both opioid-treated dams and their offspring. One must consider that the adverse or favorable effects of the microbiome on the host depend not only on the exact species present and their quantity, but also the physiology of the host and the host’s environment. (Smith and Wissel, 2019). While an increase in alpha diversity could be considered beneficial in some contexts, in a model of chronic opioid use and dependence, an increase in microbial diversity may be linked to pathology.
There are several limitations to these preliminary findings which require further investigation. The mechanisms by which opioids induce this shared disruption in the microbiome of offspring and dams are currently unclear but could result from direct opioid transfer through the breastmilk, direct transfer of microbial dysbiosis from the dam (e.g. vertical transmission via placental transfer, vaginal delivery, and/or breastfeeding), or other possible environmental variables of which we are currently unaware. Follow up studies including cross-fostering offspring following birth will likely lend insights into a mechanism. Further work will also be required to discern if these shifts in the microbiome of offspring persist beyond weaning and how this dysbiosis may contribute to developmental differences previously reported (Grecco et al., 2021)
5. Conclusion
The microbiome is responsive to numerous drugs of abuse and plays an important role in addiction-related pathology (Kiraly et al., 2016; Lee et al., 2018; Ridge et al., 2019; Simpson et al., 2020). As the maternal microbiome is known to modulate offspring development (Jasarevic and Bale, 2019), it is reasonable to assume that drug-exposed offspring will also be negatively impacted by drug-induced perturbations in the maternal microbiome. Our study using a translational model of PME supports this possibility. Further characterization of the prenatal opioid treatment-induced effects on the maternal-offspring microbiome interaction will be critical in understanding the developmental consequences of opioid exposure and for discovering novel treatment strategies for prenatal opioid exposure in the future.
Supplementary Material
Supplementary File 1_V3-V4 Full ASV Read Counts
Supplementary File 2_t Test Statistic for Bacterial Abundance
Supplementary File 3_Differential ASV Abundance DEseq
Supplementary File 4_LEfSe Output
Highlights.
Maternal opioid use during pregnancy is a growing concern.
Maternal methadone treatment disrupts the microbial diversity in dams and offspring.
16 features in dams and 10 features in offspring were differentially abundant.
Microbiome changes in dams and offspring demonstrate significant positive correlations.
The Lachnospiraceae NK4A136 group may represent a bacterial biomarker of interest associated with opioid exposure.
Acknowledgements
We thank the National Institute on Drug Abuse Drug Supply Program for generously providing the methadone and oxycodone utilized in the experiments of this manuscript. This work was supported by grants awarded by the NIH R01AA027214 (BKA), F30AA028687 (GGG), Indiana University (BKA), Indiana University Health (BKA), and the Stark Neurosciences Research Institute (BKA, GGG). We thank Drs. Jeffrey Alberts, Ardythe Morrow, Gregory Demas, and Jessica Cusick for their valuable feedback on the manuscript.
Funding Sources
This work was supported by grants awarded by the NIH R01AA027214 (BKA), F30AA028687 (GGG), Indiana University (BKA), Indiana University Health (BKA), and the Stark Neurosciences Research Institute (BKA, GGG).
Footnotes
Conflicts of Interest
The authors have no conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, Zhang L, Dauer P, Chen C, Dalluge J, Johnson T, Roy S, 2016. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol 9(6), 1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP, 2016. DADA2: High-resolution sample inference from Illumina amplicon data. Nature Methods 13(7), 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhariwal A, Chong J, Habib S, King IL, Agellon LB, Xia J, 2017. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res 45(W1), W180–W188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gicquelais RE, Bohnert ASB, Thomas L, Foxman B, 2020. Opioid agonist and antagonist use and the gut microbiota: associations among people in addiction treatment. Scientific Reports 10(1), 19471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecco GG, Mork BE, Huang J-Y, Metzger CE, Haggerty DL, Reeves KC, Gao Y, Hoffman H, Katner SN, Masters AR, Morris CW, Newell EA, Engleman EA, Baucum AJ, Kim J, Yamamoto BK, Allen MR, Wu Y-C, Lu H-C, Sheets PL, Atwood BK, 2021. Prenatal methadone exposure disrupts behavioral development and alters motor neuron intrinsic properties and local circuitry. eLife 10, e66230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai AH, Ko JY, Owens PL, Stocks C, Patrick SW, 2021. Neonatal Abstinence Syndrome and Maternal Opioid-Related Diagnoses in the US, 2010-2017. JAMA 325(2), 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Bale TL, 2019. Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Frontiers in neuroendocrinology 55, 100797. [DOI] [PubMed] [Google Scholar]
- Kimura I, Miyamoto J, Ohue-Kitano R, Watanabe K, Yamada T, Onuki M, Aoki R, Isobe Y, Kashihara D, Inoue D, Inaba A, Takamura Y, Taira S, Kumaki S, Watanabe M, Ito M, Nakagawa F, Irie J, Kakuta H, Shinohara M, Iwatsuki K, Tsujimoto G, Ohno H, Arita M, Itoh H, Hase K, 2020. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science (New York, N.Y.) 367(6481). [DOI] [PubMed] [Google Scholar]
- Kiraly DD, Walker DM, Calipari ES, Labonte B, Issler O, Pena CJ, Ribeiro EA, Russo SJ, Nestler EJ, 2016. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Scientific Reports 6(1), 35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Vuong HE, Nusbaum DJ, Hsiao EY, Evans CJ, Taylor AMW, 2018. The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 43(13), 2606–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Mu A, Wallace M, Gengatharan JM, Furst AJ, Bode L, Metallo CM, Ayres JS, 2021. Microbiota control of maternal behavior regulates early postnatal growth of offspring. Science Advances 7(5), eabe6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnelly VJ, Hamilton R, Chappell FM, Mactier H, Boardman JP, 2019. Childhood neurodevelopment after prescription of maintenance methadone for opioid dependency in pregnancy: a systematic review and meta-analysis. Developmental medicine and child neurology 61(7), 750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyangahu DD, Lennard KS, Brown BP, Darby MG, Wendoh JM, Havyarimana E, Smith P, Butcher J, Stintzi A, Mulder N, Horsnell W, Jaspan HB, 2018. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 6(1), 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Lotfipour S, 2020. The role of the gut microbiome in opioid use. Behavioural pharmacology 31(2&3), 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge EA, Pachhain S, Choudhury SR, Bodnar SR, Larsen RA, Phuntumart V, Sprague JE, 2019. The influence of the host microbiome on 3,4-methylenedioxymethamphetamine (MDMA)-induced hyperthermia and vice versa. Scientific Reports 9(1), 4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva YP, Bernardi A, Frozza RL, 2020. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Frontiers in Endocrinology 11(25). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S, Kimbrough A, Boomhower B, McLellan R, Hughes M, Shankar K, de Guglielmo G, George O, 2020. Depletion of the Microbiome Alters the Recruitment of Neuronal Ensembles of Oxycodone Intoxication and Withdrawal. eNeuro 7(3), ENEURO.0312–0319.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LK, Wissel EF, 2019. Microbes and the Mind: How Bacteria Shape Affect, Neurological Processes, Cognition, Social Relationships, Development, and Pathology. Perspectives on psychological science : a journal of the Association for Psychological Science 14(3), 397–418. [DOI] [PubMed] [Google Scholar]
- Tolia VN, Patrick SW, Bennett MM, Murthy K, Sousa J, Smith PB, Clark RH, Spitzer AR, 2015. Increasing Incidence of the Neonatal Abstinence Syndrome in U.S. Neonatal ICUs. New England Journal of Medicine 372(22), 2118–2126. [DOI] [PubMed] [Google Scholar]
- Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, Kazantsev M, Wilson CJ, Rendon T, Hsiao EY, 2020. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 586(7828), 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Meng J, Zhang L, Johnson T, Chen C, Roy S, 2018. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Scientific Reports 8(1), 3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Roy S, 2017. Gut Homeostasis, Microbial Dysbiosis, and Opioids. Toxicologic pathology 45(1), 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M-R, Chou T-S, Huang C-Y, Hsiao J-K, 2020. A potential probiotic-Lachnospiraceae NK4A136 group: Evidence from the restoration of the dietary pattern from a high-fat diet. Research Square. [Google Scholar]
- Xu Y, Xie Z, Wang H, Shen Z, Guo Y, Gao Y, Chen X, Wu Q, Li X, Wang K, 2017. Bacterial Diversity of Intestinal Microbiota in Patients with Substance Use Disorders Revealed by 16S rRNA Gene Deep Sequencing. Scientific reports 7(1), 3628–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh SL, Eastwood J, Wright IM, Morton R, Melhuish E, Ward M, Oei JL, 2019. Cognitive and Motor Outcomes of Children With Prenatal Opioid Exposure: A Systematic Review and Meta-analysis. JAMA Network Open 2(7), e197025–e197025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1_V3-V4 Full ASV Read Counts
Supplementary File 2_t Test Statistic for Bacterial Abundance
Supplementary File 3_Differential ASV Abundance DEseq
Supplementary File 4_LEfSe Output