Abstract
Purpose:
Fatigue is a component of frailty and may undermine functional well-being and independent living. The prevalence of fatigue and its impact on functional limitations among older adults with cancer remains understudied.
Methods:
Using participants enrolled in the Cancer and Aging Resilience Evaluation (CARE), a prospective registry of patients (≥60y) with cancer, who underwent a geriatric assessment (GA) at the first visit with oncology, we examined the presence of fatigue based on self-report of moderate to severe fatigue on PROMIS global health 10-item instrument at the time of GA. We examined the association of fatigue with impairments in Instrumental Activities of Daily Living (IADL) and Activities of Daily Living (ADL) adjusting for age, sex, race/ethnicity, education, cancer type and stage, pain, comorbidities, and time from cancer.
Results:
We included 374 older adults with cancer with a median age of 70y; 56% were male and 23% black. Diagnoses included colorectal (33%) and pancreatic cancers (25%), with most patients with advanced stage disease (71% stage III/IV). Overall, 210 (58%) patients reported significant fatigue. Patients reporting significant fatigue had an increased odds of IADL (adjusted Odds Ratio, aOR 1.9; 95% CI 1.1–3.2) or ADL impairment (aOR 3.6; 95% CI 1.4–9.3), as compared to those without, after adjusting for aforementioned confounders.
Conclusions:
Over half of older adults with cancer reported moderate to severe fatigue that was independently associated with functional status limitations. Further understanding of the multifaceted aspects of fatigue and development of interventions combating fatigue in this population is urgently needed.
Keywords: fatigue, functional status, geriatric oncology, aging, cancer, geriatric assessment
Introduction
Older adults represent the vast majority of newly diagnosed cancer as well as cancer deaths in the US [1]. In addition, the fastest growing cancer population is among adults aged 85 years and older [2]. Older adults comprise a heterogeneous population with respect to health status as well as social support that is not routinely captured in traditional oncologic evaluations [3 4]. Older adults vary in their preferences regarding oncologic treatment plans. Although prolonging life is important to older patients with cancer, 44% value other outcomes as more important than survival [5]. Over half of older adults with cancer would rather live a shorter life than lose their ability to take care of themselves and nearly three quarters with serious illness would not choose a life-prolonging therapy if it resulted in severe functional impairment [5 6].
Cancer-related fatigue is commonly defined as a subjective sense of tiredness or exhaustion related to cancer or its treatment that is disproportionate to recent activity [7]. Fatigue is common and often described as the most distressing symptom associated with cancer and cancer treatments, which can persist for years after treatment [8 9]. Fatigue is one of the five components of the frailty phenotype originally proposed by Fried et al [10 11]. Moreover, fatigue is associated with many adverse outcomes including functional limitations, disability, and mortality in the general older adult population and has been proposed as an early indicator of aging [12]. However, the underlying physiologic mechanisms of cancer-related fatigue and how this is similar or different from age-related fatigue has not yet been elucidated. As such, no mechanistically driven fatigue interventions exist [13]. Furthermore, the burden of fatigue and its impact on functional well-being among the growing number of older adults with cancer remains poorly understood.
In this study, we aimed to 1) describe the burden of fatigue among older adults with cancer, 2) evaluate its association with other geriatric assessment (GA) identified impairments, frailty, health-related quality of life (HRQoL), and self-reported healthcare utilization, and 3) examine its relationship with functional status impairments.
Methods
Study Population
This is a cross-sectional analysis of participants enrolled in a prospective study of older adults with cancer at the University of Alabama at Birmingham (Cancer & Ageing Resilience Evaluation, UAB CARE Registry). The development and integration of CARE registry into routine clinical practice at UAB is described elsewhere [14]. Adults 60 years and older completed a patient-reported GA assessment at the time of initial consultation with a medical oncologist. For the purpose of this analysis, we included patients enrolled in the CARE registry between September 2017 and January 2020 with a GI malignancy who underwent GA prior to any planned systemic therapy. The institutional review board at UAB approved this study and all participants provided written consent prior to participation (IRB-300000092).
Geriatric Assessment
The CARE GA tool represents a modified, patient-reported version of the Cancer and Aging Research Group (CARG) GA tool originally pioneered by Dr. Hurria.[15] The CARE GA tool includes a systematic assessment of falls, functional status, nutrition, social support, psychological health, patient-reported cognitive complaints, social activities, patient-reported eastern cooperative oncology group (ECOG) performance status, polypharmacy and comorbid conditions (using the Older Americans Resources and Services [OARS] comorbidity tool and dichotomized as <3 or ≥3) [3 14 16]. Previously published domain-specific cut-offs were utilized [3 14 17]. The individual domains along with the cut-offs are detailed in the Supplement. We used 44 items included in the GA to develop a frailty index based on the principles of deficit accumulation; standard threshold scoring was applied (frail >0.35) [18–20]. Frailty indices using this method have been associated with increased mortality, increased severe chemotherapy toxicities, and reduced HRQoL [19–21].
Fatigue
As part of the GA, patients were asked to complete the National Institutes of Health’s 10-item Patient-Reported Outcomes Measurement Information System (PROMIS®) global health scale (PROMIS Global 10) short-form to assess HRQoL.[22] PROMIS Global 10 incorporates a question on fatigue: “In the past 7 days, how would you rate your fatigue on average?” Response range from none, mild, moderate, severe, to very severe. Consistent with prior reports [23 24], we classified presence of significant fatigue as self-report of at least moderate or higher degree of fatigue whereas a response of none or mild fatigue was categorized as no significant fatigue.
Functional Status
We measured functional status using the Older Americans Resources and Services (OARS) assessment [25]. Specifically, we used a 6-item measure of instrumental activities of daily living (IADL) that included an assessment of driving/traveling, shopping, preparing meals, house-work, taking medicines, and handling money, and a 3-item measure of activities of daily living (ADL) that included getting in/out of bed, ability to dress/undress, and to take a bath or shower. The response to each item included the ability to perform the task without help, with some help, or unable to perform the independently. We defined any limitation (either some help or completely unable) within any of the IADL and ADL measures as being an impairment within that domain.
Other covariates
We used PROMIS® global-10 to measure HRQoL, which includes separate scoring for physical and mental health subscales and has been tested previously in large samples of adults in the US [22 26]. The item responses were converted to T-scores with a standardized mean score of 50 and a standard deviation (SD) of 10. The minimal clinically relevant difference for PROMIS ranges from 2 to 6 points, and a score of 40 or less (1 standard deviation) is considered impaired for the physical and mental subscales [27]. We measured healthcare utilization by asking participants whether they had been seen in the emergency room (ER) or hospitalized in the past year. Race, ethnicity, education level, employment, and marital status self-reported. We abstracted cancer stage, cancer type, date of diagnosis, and treatment status from the electronic medical record.
Statistical Analyses
We used descriptive statistics to summarize the demographic and clinical characteristics of the study participants. We measured the proportion of patients with significant fatigue and computed the 95% confidence interval (95%CI) using exact binomial (Clopper-Pearson) method [28]. We examined group differences in demographic, clinical, geriatric assessment domain impairments, frailty, HRQoL, and healthcare utilization using Analysis of Variance/Wilcoxon Rank Sum test for continuous variables and Chi-squared test/Fisher’s exact test for categorical variables. We used logistic regression models to examine the association between fatigue and limitations in IADL and ADL in separate models adjusting a priori for potential confounders within the literature including age, sex, race/ethnicity, education, cancer type and stage, pain, multi-morbidity, and time from cancer diagnosis. All statistical tests were two-sided and the level of significance was set at 0.05. All analyses used SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics
Overall, 650 adults were diagnosed with a GI malignancy at age ≥60y were seen at the UAB GI Oncology clinic for initial consultation during the study period; of these 556 (85%) were enrolled in the CARE registry. Of these, 364 participants underwent GA evaluation prior to starting anti-cancer therapy and were included in the current study (Figure 1). Mean age at study enrollment was 70.1y (SD, 7.26y); 56% of the cohort was male and 23% were Black (Table 1). The most common tumor types included colorectal (22.1%) and pancreatic (17.8%) and majority of participants had stage III/IV disease (70%).
Figure 1:
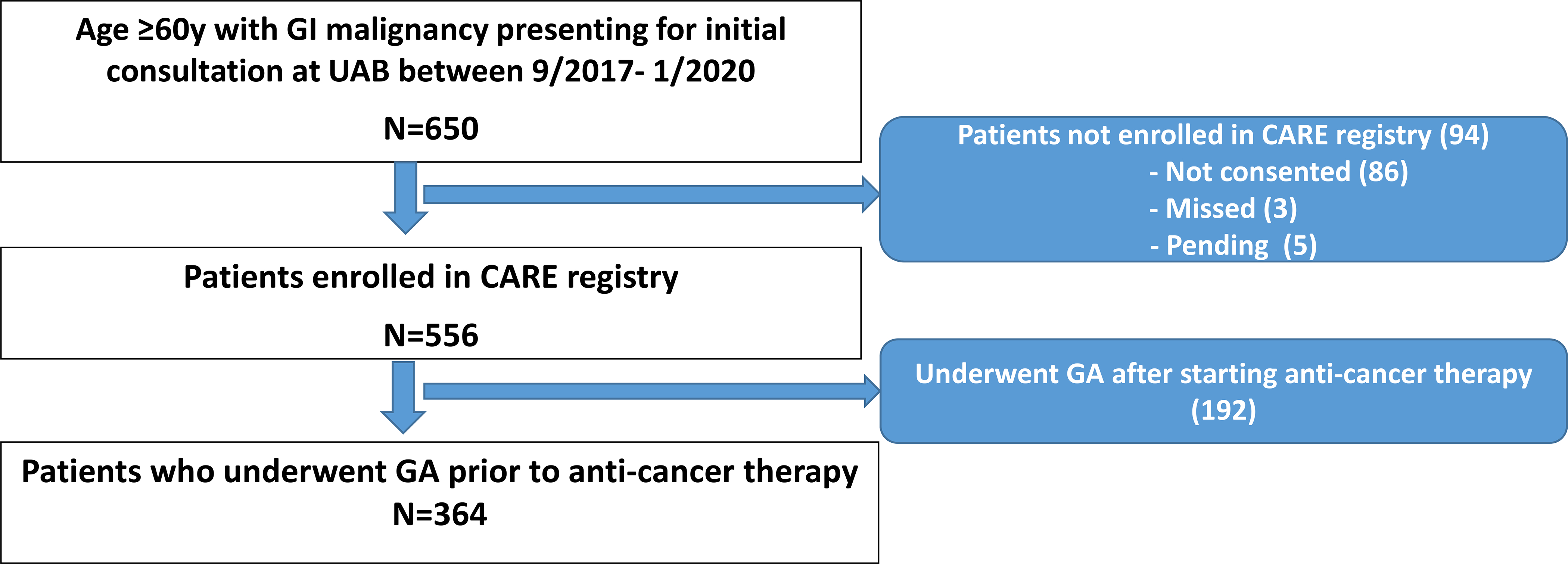
Flow Chart showing the process of study cohort selection. Overall, 650 patients were seen for initial consultation at the UAB GI Oncology clinic between 9/2017 and 1/2020, of which 364 were enrolled in CARE registry and underwent geriatric assessment prior to starting systemic anti-cancer therapy.
Table 1.
Patient Demographics and Characteristics
| All patients (n= 364) | None/mild fatigue (n=154) | Moderate-very Severe fatigue (n=210) | p * | |
|---|---|---|---|---|
| Age, mean (SD) | 70.1 (7.3) | 69.7 (7.1) | 70.5 (7.3) | 0.34 |
|
Age, n (%) 60–64 65–69 70–74 75–79 80+ |
97 (26.6) 84 (23.1) 83 (22.8) 55 (15.1) 45 (12.4) |
43 (27.9) 36 (23.4) 36 (23.4) 23 (14.9) 16 (10.4) |
54 (25.7) 48 (22.9) 47 (22.4) 32 (15.2) 29 (13.8) |
0.90 |
|
Sex, n (%) Male |
205 (56.3) | 82 (53.2) | 123 (58.6) | 0.31 |
|
Race, n (%) White Black Other |
274 (75.3) 85 (23.4) 5 (1.4) |
119 (77.3) 32 (20.8) 3 (1.9) |
155 (73.8) 53 (25.2) 2 (1.0) |
0.46 |
|
Ethnicity, n (%) Hispanic |
8 (2.2) | 3 (1.9) | 5 (2.4) | 0.78 |
|
Educational level, n (%) Less than high school High school graduate Some college Associate/bachelors Advanced degree |
65 (17.9) 96 (26.4) 67 (18.4) 101 (27.7) 35 (9.6) |
21 (13.6) 43 (27.9) 24 (15.6) 46 (29.9) 20 (13.0) |
44 (21.0) 53 (25.2) 43 (20.5) 55 (26.2) 15 (7.1) |
0.11 |
|
Marital status, n (%) Single Widowed/divorced Married |
26 (7.1) 104 (28.6) 234 (64.3) |
10 (6.5) 37 (24.0) 107 (69.5) |
16 (7.6) 67 (31.9) 127 (60.5) |
0.20 |
|
Employment, n (%) Retired Disabled Part-time (<32hr/wk) Full-time (>32hr/wk) Other |
222 (61.0) 50 (13.7) 10 (2.7) 38 (10.4) 44 (12.1) |
99 (64.3) 8 (5.2) 5 (3.2) 25 (16.2) 17 (11.0) |
123 (58.6) 42 (20.0) 5 (2.4) 13 (6.2) 27 (12.9) |
<.001 |
|
Cancer type, n (%) Colorectal Pancreatic Hepatobiliary Gastroesophageal Other |
121 (33.2) 90 (24.7) 61 (16.8) 39 (10.7) 53 (14.6) |
56 (36.4) 36 (23.4) 25 (16.2) 14 (9.1) 23 (14.9) |
65 (31.0) 54 (25.7) 36 (17.1) 25 (11.9) 30 (14.3) |
0.79 |
|
Cancer stage, n (%) I/II III IV |
107 (29.4) 93 (25.5) 164 (45.1) |
44 (28.6) 52 (33.8) 58 (37.7) |
63 (30.0) 41 (19.5) 106 (50.5) |
.006 |
p-value from the bivariate comparison of those with and without fatigue
Abbreviations: SD, Standard deviation
Association of fatigue with demographic and clinical characteristics
Overall, 12.1% of participants reported no fatigue, 30.2% reported mild, 35.7% moderate, 16.8% severe, and 5.2% very severe fatigue (Figure 2). Based on this, 57.8% (95%CI 52.4–62.8%) of the patients reported moderate to severe fatigue. We found no associations between moderate to severe fatigue and sex, race/ethnicity, marital status, or cancer type. However, patients with moderate to severe fatigue were more likely to be diagnosed with advanced stage cancer (stage IV 51% vs. 39% p=.005) and to be on disability (20% vs. 5%, p <.001) (Table 1).
Figure 2:
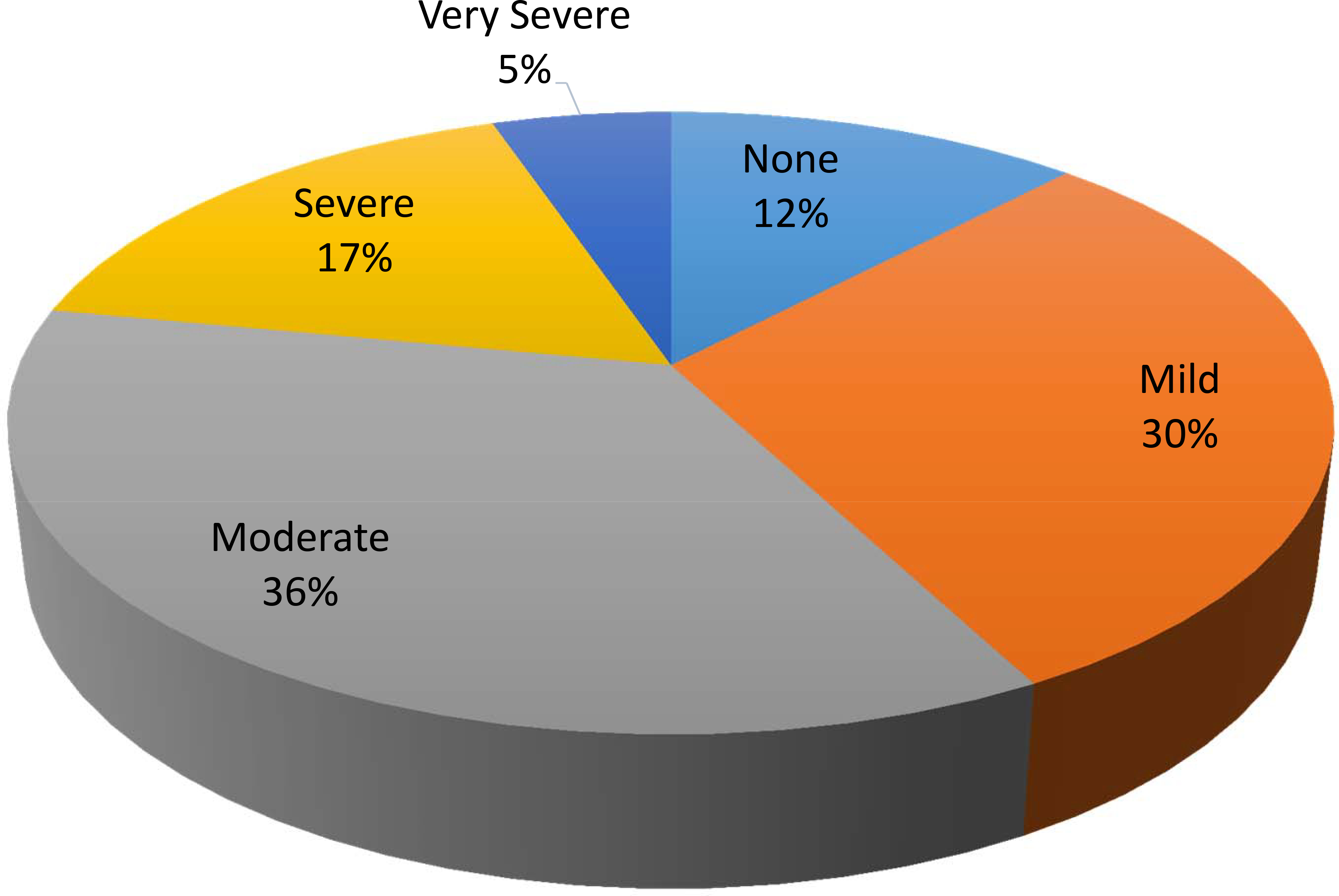
Original responses of study participants to the single item fatigue question, “In the past 7 days, how would you rate your fatigue on average?” Overall, 58% reported having at least moderate or higher-grade fatigue.
Bivariate association of fatigue with ADL/IADL and additional GA impairments
The prevalence of ADL and IADL impairment in the overall cohort was 17.7% (95%CI 13.9–22.1%) and 50.7% (95%CI 45.4–56.1%), respectively. Patients reporting moderate to severe fatigue were more likely to report functional impairments in terms of both IADL (64.7% vs. 32.0%; p <.001) and ADL limitations (27.5% vs. 4.6%; p<.001). Patients with moderate to severe fatigue were also more likely to report multiple GA impairments. These impairments included ≥1 fall in the last 6 months (28.4% vs. 9.5%, p <.001), limitations in walking one block (71.2% vs. 29.8%; p <.001), ≥3 comorbid conditions (62.4% vs. 38.8%; p<.001), malnutrition (66.7% vs. 31.9%; p<.001), cognitive complaints (10.9% vs. 0.7%; p<.001), limitations in social activities (36.1% vs. 7.9%; p<.001), anxiety (26.7% vs. 7.4%; p<.001), depression (20.8% vs. 2.0%; p<.001), moderate/severe pain (63.3% vs. 20.4%; <.001), and frailty (53.8% vs. 9.7%; p<.001). Finally, patients reporting moderate to severe fatigue were more likely to report hospitalizations (72.2% vs. 54.3%, p<.001) and ER visits (65.0% vs. 46.4%, p<.001) in the 12 months prior to study participation (Table 2).
Table 2.
Geriatric Assessment, Health-Related Quality of Life, and Healthcare Utilization differences in those with and without moderate/severe fatigue.
| Overall (n= 364) | None / Mild fatigue (n=154) | Moderate / Severe fatigue (n=210) | p | |
|---|---|---|---|---|
| Geriatric Assessment Domain | ||||
| Any IADL dependence, n(%) | 178 (50.7) | 48 (32.0) | 130 (64.7) | <.001 |
| Any ADL dependence, n(%) | 63 (17.7) | 7 (4.6) | 56 (27.5) | <.001 |
| Falls (≥1), n(%) | 70 (20.3) | 14 (9.5) | 56 (28.4) | <.001 |
| Reported limitations in walking one block, n(%) | 191 (53.7) | 45 (29.8) | 146 (71.2) | <.001 |
| Impaired ecog ps (≥2), n(%) | 120 (33.7) | 13 (8.6) | 107 (52.2) | <.001 |
| Medications (≥9 daily), n(%) | 87 (24.6) | 23 (15.1) | 64 (31.7) | <.001 |
| Comorbidities (≥3), n(%) | 185 (52.3) | 59 (38.8) | 126 (62.4) | <.001 |
| Malnutrition, n(%) | 173 (52.0) | 45 (31.9) | 128 (66.7) | <.001 |
| Cognitive complaints (mod/sev.), n(%) | 23 (6.5) | 1 (0.7) | 22 (10.9) | <.001 |
| Limitations in social activities, n(%) | 85 (24.0) | 12 (7.9) | 73 (36.1) | <.001 |
| Anxiety (mod/sev.), n(%) | 65 (18.5) | 11 (7.4) | 54 (26.7) | <.001 |
| Depression (mod/sev.), n(%) | 45 (12.8) | 3 (2.0) | 42 (20.8) | <.001 |
| Pain (mod/sev), n(%) | 162 (45.1) | 31 (20.4) | 131 (63.3) | <.001 |
| Frailty Index (frail), n(%) | 128 (35.2) | 15 (9.7) | 113 (53.8) | <.001 |
| Health-Related Quality of Life | ||||
| Overall self-reported health (fair/poor), n (%) | 159 (44.0) | 25 (16.2) | 134 (64.7) | <.001 |
| Physical Health sub-score, mean (SD) | 42.9 (10.8) | 51.8 (7.7) | 36.2 (7.4) | <.001 |
| Mental Health sub-score, mean (SD) | 47.9 (9.6) | 54.0 (8.0) | 43.3 (8.0) | <.001 |
| Healthcare Utilization | ||||
| Hospitalized in the last year, n(%) | 230 (64.6) | 82 (54.3) | 148 (72.2) | <.001 |
| ER visit in the last year, n(%) | 200 (57.0) | 70 (46.4) | 130 (65.0) | <.001 |
p-value from the bivariate comparison of those with moderate/severe versus none/mild fatigue
Abbreviations: IADL, instrumental activities of daily living; ADL; activities of daily living; ecog ps, eastern cooperative oncology group performance status; mod/sev, moderate/severe; ER, emergency room.
Multivariable analyses adjusted for age, sex, education, cancer type and stage, pain, comorbid conditions, and time from cancer diagnosis, revealed higher odds of IADL and ADL impairment in those with moderate to severe fatigue (OR=1.9, 95%CI 1.1–3.2, p=0.03; OR=3.6, 95%CI 1.4–9.3, p=0.007, respectively), compared with those with no/mild fatigue (Table 3).
Table 3.
Multivariable model of associations of moderate/severe fatigue with impairments in instrumental activities of daily living and activities of daily living
| Variable | Adjusted OR | 95 % CI of OR | p |
|---|---|---|---|
| Instrumental Activities of Daily Living Model | |||
| IADL impairment | 1.9 | 1.1–3.2 | 0.032 |
| Age group 60–64 65–69 70–74 75–79 80+ |
Ref 0.9 0.9 1.0 1.6 |
Ref 0.4–1.9 0.5–1.9 0.4–2.5 0.6–3.9 |
0.85 0.93 0.34 0.34 |
| Sex Female |
0.8 |
0.5–1.3 |
0.34 |
| Education Less than HS HS graduate Some college Associate/Bachelors Advanced Degree |
Ref 0.8 1.6 1.2 0.8 |
Ref 0.3–1.8 0.6–4.0 0.5–2.8 0.3–2.3 |
0.59 0.35 0.65 0.66 |
| Pain 0–3 >3 |
Ref 5.6 |
Ref 3.1–10.2 |
<0.001 |
| Comorbidity 0–2 ≥3 |
Ref 2.3 |
Ref 1.4–4.0 |
0.002 |
| Cancer type Colorectal Pancreatic Hepatobiliary Gastroesophageal Other |
Ref 0.8 1.0 2.2 0.8 |
Ref 0.4–1.6 0.4–2.1 0.9–5.5 0.4–1.8 |
0.52 0.92 0.10 0.59 |
| Cancer stage 0-II III IV |
Ref 0.5 1.3 |
Ref 1.1 2.4 |
0.9 0.46 |
| Days from diagnosis to GA | 1.0 | 1.0 | 0.35 |
| Activities of Daily Living Model | |||
| ADL impairment | 3.6 | 1.4–9.3 | 0.007 |
| Age group 60–64 65–69 70–74 75–79 80+ |
Ref 1.0 1.0 1.1 1.7 |
Ref 0.5–2.0 0.5–2.0 0.5–2.6 0.7–4.2 |
0.90 0.96 0.85 0.25 |
| Sex Female |
0.83 |
0.5–1.4 |
0.25 |
| Education Less than HS HS graduate Some college Associate/Bachelors Advanced Degree |
Ref 0.7 1.4 1.2 0.8 |
Ref 0.3–1.7 0.6–3.7 0.5–2.8 0.3–2.4 |
0.46 0.45 0.65 0.73 |
| Pain 0–3 >3 |
Ref 5.9 |
Ref 3.3–10.5 |
<0.001 |
| Comorbidity 0–2 ≥3 |
Ref 2.4 |
Ref 1.4–4.1 |
0.001 |
| Cancer type Colorectal Pancreatic Hepatobiliary Gastroesophageal Other |
Ref 0.8 0.9 2.0 0.8 |
Ref 0.4–1.6 0.4–2.0 0.8–5.2 0.3–1.8 |
0.54 0.82 0.15 0.55 |
| Cancer stage 0-II III IV |
Ref 0.5 1.4 |
Ref 0.3–1.1 0.7–2.6 |
0.09 0.32 |
| Days from diagnosis to GA | 1.0 | 1.0–1.0 | 0.49 |
Abbreviations: IADL, Instrumental Activities of Daily Living; ADL, Activities of Daily Living; OR, Odds Ratio
Discussion
In this study, we show that over half of the older adults with GI malignancies report moderate to severe fatigue. Further, fatigue is associated with multiple GA identified impairments including functional limitations, reduced physical and mental HRQoL, and higher rates of prior healthcare utilization.
The prevalence of fatigue in our study is consistent with previous literature on cancer-related fatigue. Prior estimates report 60–96% of patients with cancer undergoing treatment experience fatigue.[8 9] In the few other studies that have explored fatigue in the older adult cancer population, a similar prevalence was found [29 30]. In a study by Respini et al of 80 older adults undergoing chemotherapy, 72.7% reported fatigue; there was an association between fatigue and depressive symptoms. Interestingly, they found that female patients had higher fatigue levels than males in their population, whereas we found no sex differences in our population [29]. In a prior study of older adults with cancer by Luciani et al, fatigue was associated with ADL and IADL impairment; the authors suggested that fatigue was a mediator of functional dependence. However, they did not account for comorbid conditions, pain, or cancer types as our analyses included and they had a smaller sample population [30]. In addition, in a recent by Pisu et al of the most impactful factors of HRQoL in older adults with cancer, fatigue was the strongest predictor of both physical and mental HRQoL [31]. Similarly, results in the broader gerontology literature have shown similar association of fatigue with functional status limitations [32 33]. Moreover, prior studies have clearly shown that fatigue rarely occurs in isolation and often occurs as part of a symptom cluster that includes pain, emotional distress, and sleep disturbances [7]. Thus, our findings demonstrating uniform and strong associations with all GA impairments measured is not surprising, and should raise attention to the potential negative impact of cancer-related fatigue in the older adult population [7]. As functional independence is a priority for older adults and strongly associated with increased chemotherapy toxicities and reduced survival, further understanding the determinants of functional limitations and developing targeted interventions is critical [34].
Guidelines for management of cancer-related fatigue suggest early recognition and management [7]. Patients found to have fatigue should undergo a comprehensive history and evaluation with a focus on the treatable contributing factors, such as pain, anemia, emotional distress, malnutrition, medication side effect, and deconditioning. Many potential therapeutic treatments exist for cancer-related fatigue and clinical trials have explored a variety of interventions. Overall, physical activity and exercise-based interventions have shown the most promise both during and post cancer treatment [7 35]. However, the optimal type, intensity, and timing of exercise/physical activity interventions remains unknown [35]. Psychosocial interventions, such as cognitive behavioral therapy and mindfulness-based stress reduction, have also demonstrated improvements in fatigue [36]. In addition, pharmacologic approaches involving psychostimulants (such as methylphenidate) can be considered after ruling out other causes of fatigue and treating the above reversible causes [37]. However, the use of psychostimulants is potentially problematic in the older adult population, and if utilized, a management philosophy of “start low and go slow” is recommended to reduce the risk of unintended side effects. Few to no intervention studies have focused specifically on the older adult cancer population, and given older adults with cancer are potentially at higher risk of fatigue and related adverse events, such as functional decline, a more focused approach to interventions in this population is warranted.
Our study has limitations. The cross-sectional nature of our study limits any determination of causality or directionality of our findings. Our sample derives from a single site in the Southeastern US and consists of only GI malignancies, and our findings may not be representative of other populations. Although a high proportion of the eligible population enrolled in CARE (over 90%)[38], there remains some concern for potential selection bias. In addition, the fatigue reported in this older adult cancer population is both a combination of aging-related fatigue as well as cancer-related fatigue. Without a non-cancer control population and/or longitudinal analyses, differentiating these two important and distinct causes of fatigue is not possible. Lastly, the CARE survey is patient-reported GA and although a standard approach in geriatric oncology, the survey is not validated against objective testing.
In conclusion, fatigue is highly prevalent in older adults with GI malignancies and is associated with numerous adverse outcomes. Fatigue may undermine functional independence in older adults with cancer, which is a high priority outcome for this vulnerable population. Further exploration of fatigue as a potential mediator of functional decline in longitudinal assessments in patients undergoing chemotherapy is needed and development of interventions tailored to the older adult is necessary to mitigate and manage fatigue.
Supplementary Material
Funding:
Supported in part by the National Institutes of Health (K08CA234225 – GW and 1K02AG062498- TWB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement: The authors have no conflicts to disclose relevant to the content of this article.
Availability of data and material: All data is available per written individual request to University of Alabama at Birmingham (UAB).
Code availability: Any software programming code is available per written individual request to University of Alabama at Birmingham (UAB).
Ethics approval: Ethical considerations were presented to and approved by the UAB IRB per review (IRB-300000092). Study subjects were determined to be exposed to minimal risk.
Consent to participate: All enrolled participants have been consented by IRB-approved study personnel, according to UAB IRB rules and regulations.
Consent for publication: All authors reviewed and agreed to submission with an intent for publication.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: a cancer journal for clinicians 2020;70(1):7–30 doi: 10.3322/caac.21590[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Miller KD, Dale W, et al. Cancer statistics for adults aged 85 years and older, 2019. CA: a cancer journal for clinicians 2019;69(6):452–67 doi: 10.3322/caac.21577[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 3.Jolly TA, Deal AM, Nyrop KA, et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist 2015;20(4):379–85 doi: 10.1634/theoncologist.2014-0247[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams GR, Pisu M, Rocque GB, et al. Unmet social support needs among older adults with cancer. Cancer 2019;125(3):473–81 doi: 10.1002/cncr.31809[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 5.Soto-Perez-de-Celis E, Li D, Su CY, et al. Patient-defined goals and preferences among older adults with cancer starting chemotherapy J Clin Oncol 36, 2018 (suppl 15; abstr 10009) 2018 [Google Scholar]
- 6.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. The New England journal of medicine 2002;346(14):1061–6 doi: 10.1056/NEJMsa012528[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-Related Fatigue, Version 2.2015. Journal of the National Comprehensive Cancer Network : JNCCN 2015;13(8):1012–39 doi: 10.6004/jnccn.2015.0122[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014;11(10):597–609 doi: 10.1038/nrclinonc.2014.127[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist 2007;12Suppl 1:4–10 doi: 10.1634/theoncologist.12-S1-4[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 10.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology. Series A, Biological sciences and medical sciences 2001;56(3):M146–56 [DOI] [PubMed] [Google Scholar]
- 11.Tralongo P, Respini D, Ferrau F. Fatigue and aging. Critical reviews in oncology/hematology 2003;48(Suppl):S57–64 doi: 10.1016/j.critrevonc.2003.07.003[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 12.Avlund K Fatigue in older adults: an early indicator of the aging process? Aging clinical and experimental research 2010;22(2):100–15 doi: 10.1007/bf03324782[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 13.Berger AM, Mitchell SA, Jacobsen PB, Pirl WF. Screening, evaluation, and management of cancer-related fatigue: Ready for implementation to practice? CA: a cancer journal for clinicians 2015;65(3):190–211 doi: 10.3322/caac.21268[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 14.Williams GR, Kenzik KM, Parman M, et al. Integrating geriatric assessment into routine gastrointestinal (GI) consultation: The Cancer and Aging Resilience Evaluation (CARE). J Geriatr Oncol 2020;11(2):270–73 doi: 10.1016/j.jgo.2019.04.008[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer 2005;104(9):1998–2005 doi: 10.1002/cncr.21422[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 16.Williams GR, Deal AM, Lund JL, et al. Patient-Reported Comorbidity and Survival in Older Adults with Cancer. Oncologist 2018;23(4):433–39 doi: 10.1634/theoncologist.2017-0404[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams GR, Deal AM, Jolly TA, et al. Feasibility of geriatric assessment in community oncology clinics. Journal of geriatric oncology 2014;5(3):245–51 doi: 10.1016/j.jgo.2014.03.001[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 18.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. The journals of gerontology. Series A, Biological sciences and medical sciences 2007;62(7):722–7 [DOI] [PubMed] [Google Scholar]
- 19.Guerard EJ, Deal AM, Chang Y, et al. Frailty Index Developed From a Cancer-Specific Geriatric Assessment and the Association With Mortality Among Older Adults With Cancer. Journal of the National Comprehensive Cancer Network : JNCCN 2017;15(7):894–902 doi: 10.6004/jnccn.2017.0122[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 20.Williams GR, Deal AM, Sanoff HK, et al. Frailty and health-related quality of life in older women with breast cancer. Support Care Cancer 2019;27(7):2693–98 doi: 10.1007/s00520-018-4558-6[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer 2016;122(24):3865–72 doi: 10.1002/cncr.30269[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res 2009;18(7):873–80 doi: 10.1007/s11136-009-9496-9[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer 2014;120(3):425–32 doi: 10.1002/cncr.28434[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw 2015;13(8):1012–39 doi: 10.6004/jnccn.2015.0122[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fillenbaum GG, Smyer MA. The Development, Validity, and Reliability of the Oars Multidimensional Functional Assessment Questionnaire1. J Gerontol 1981;36(4):428–34 doi: 10.1093/geronj/36.4.428[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 26.Pergolotti M, Deal AM, Williams GR, et al. Activities, function, and health-related quality of life (HRQOL) of older adults with cancer. J Geriatr Oncol 2017. doi: 10.1016/j.jgo.2017.02.009[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol 2011;64(5):507–16 doi: 10.1016/j.jclinepi.2010.11.018[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26(4):404–13 doi: 10.1093/biomet/26.4.404[published Online First: Epub Date]|. [DOI] [Google Scholar]
- 29.Respini D, Jacobsen PB, Thors C, Tralongo P, Balducci L. The prevalence and correlates of fatigue in older cancer patients. Critical reviews in oncology/hematology 2003;47(3):273–9 doi: 10.1016/s1040-8428(02)00176-2[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 30.Luciani A, Jacobsen PB, Extermann M, et al. Fatigue and functional dependence in older cancer patients. Am J Clin Oncol 2008;31(5):424–30 doi: 10.1097/COC.0b013e31816d915f[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 31.Pisu M, Azuero A, Halilova KI, et al. Most impactful factors on the health-related quality of life of a geriatric population with cancer. Cancer 2018;124(3):596–605 doi: 10.1002/cncr.31048[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 32.Mueller-Schotte S, Bleijenberg N, van der Schouw YT, Schuurmans MJ. Fatigue as a long-term risk factor for limitations in instrumental activities of daily living and/or mobility performance in older adults after 10 years. Clin Interv Aging 2016;11:1579–87 doi: 10.2147/CIA.S116741[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardy SE, Studenski SA. Fatigue and function over 3 years among older adults. The journals of gerontology. Series A, Biological sciences and medical sciences 2008;63(12):1389–92 doi: 10.1093/gerona/63.12.1389[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nightingale G, Battisti NML, Loh KP, et al. Perspectives on functional status in older adults with cancer: An interprofessional report from the International Society of Geriatric Oncology (SIOG) nursing and allied health interest group and young SIOG. Journal of geriatric oncology 2020. doi: 10.1016/j.jgo.2020.10.018[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. The Cochrane database of systematic reviews 2008(2):CD006145 doi: 10.1002/14651858.CD006145.pub2[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 36.Daniels J, Kissane DW. Psychosocial interventions for cancer patients. Current opinion in oncology 2008;20(4):367–71 doi: 10.1097/CCO.0b013e3283021658[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 37.Minton O, Richardson A, Sharpe M, Hotopf M, Stone PC. Psychostimulants for the management of cancer-related fatigue: a systematic review and meta-analysis. Journal of pain and symptom management 2011;41(4):761–7 doi: 10.1016/j.jpainsymman.2010.06.020[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 38.Williams GR, Kenzik KM, Parman M, et al. Integrating geriatric assessment into routine gastrointestinal (GI) consultation: The Cancer and Aging Resilience Evaluation (CARE). J Geriatr Oncol 2019. doi: 10.1016/j.jgo.2019.04.008[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


