Introduction
Ustekinumab has been shown to be effective for the treatment of ulcerative colitis (UC), however >40% of patients have suboptimal clinical response after induction and maintenance dosing every 8 weeks (q8w).1, 2 The best management approach for these patients is unclear. Many undergo empiric dose intensification to q4w or q6w, a non-standardized decision due to limited data supporting therapeutic drug monitoring of ustekinumab.3 In Crohn’s disease (CD), approximately 50% of patients undergo ustekinumab dose intensification, which appears to be effective based on prior work from our group and others.4−8 However, similar data in UC is lacking. In this real-world multicenter cohort study, we sought to identify predictors and outcomes of ustekinumab dose intensification in UC.
Methods
Study design
This retrospective cohort study included adults with UC (ICD-10-CM 51x) initiating ustekinumab at Brigham and Women’s Hospital, Massachusetts General Hospital, or the University of North Carolina, Chapel Hill between 1/1/2016–11/1/2020. Patients with prior colectomy or those treated primarily for non-UC indications were excluded. Electronic health records were reviewed for clinical data. Disease activity was documented using either the simple clinical colitis activity index (SCCAI) or partial Mayo score. (Supplementary Methods).
Independent variables
Independent variables included demographics, UC duration, extraintestinal manifestations, substance use, endoscopic extent/severity, prior/current UC medications, primary documented justification for intensification, intravenous (IV) reinduction, dose interval, and the most recent body mass index, albumin, C-reactive protein, and bowel frequency recorded within 12 weeks prior to intensification (Supplementary Methods).
Outcomes
The primary outcome was corticosteroid-free clinical remission (i.e. “remission,” SCCAI/Mayo < 3 points and no oral/IV corticosteroid use for ≥4 weeks) at evaluation 12–16 weeks after intensification. Secondary outcomes were clinical response (i.e. “response,” reduction in SCCAI/Mayo by ≥3 points from baseline) at 12–16 weeks and time-to-intensification. See Supplementary Methods for additional endpoints.
Statistical analysis
Logistic and Cox Proportional Hazards regression were used to identify variables associated with remission and time-to-intensification, respectively. Variables with p<0.10 on univariable analysis were included in multivariable analyses. Covariates with p<0.05 on multivariable analysis were considered significant (Supplementary Methods).
Results
A total of 108 patients with UC initiated ustekinumab: 56.5% were female, 91.7% had prior anti-TNF exposure, 39.8% had >2 prior biologic exposures, and 57.4% were taking oral corticosteroids (Supplementary Table 1). Among these, 39.6% (40/101 with SCCAI/Mayo data) achieved remission 12–16 weeks after induction.
42.6% (46/108) required intensification to q4w (n=33) or q6w (n=13) after a median of 95 days (IQR 65–208 days) primarily for no/minimal response to induction (22/46) or loss of response (LOR; 20/46) (Figure 1A). IV reinduction doses were administered to 4/46 preceding intensification. At 12–16 weeks after intensification, 55.0% (22/40 with SCCAI/Mayo data) achieved remission and 67.5% (27/40) achieved response. 30.0% (12/40) had drug discontinuation or colectomy within 16 weeks after intensification (Figure 1B). Among these, 10/12 had no/minimal response to induction and 2/12 had LOR. Over a median follow-up of 230 days (IQR 137–623 days) after intensification, 56.3% (9/16 with pre/post-intensification data) had improvement in endoscopic inflammation and fecal calprotectin (Supplementary Table 2A), 10.0% (4/40) had IBD-related hospitalization, and 5.0% (2/40) had adverse events (urinary tract infection and C. difficile infection).
Figure 1. Reasons for dose-intensification (A) and outcomes after dose-intensification (B).
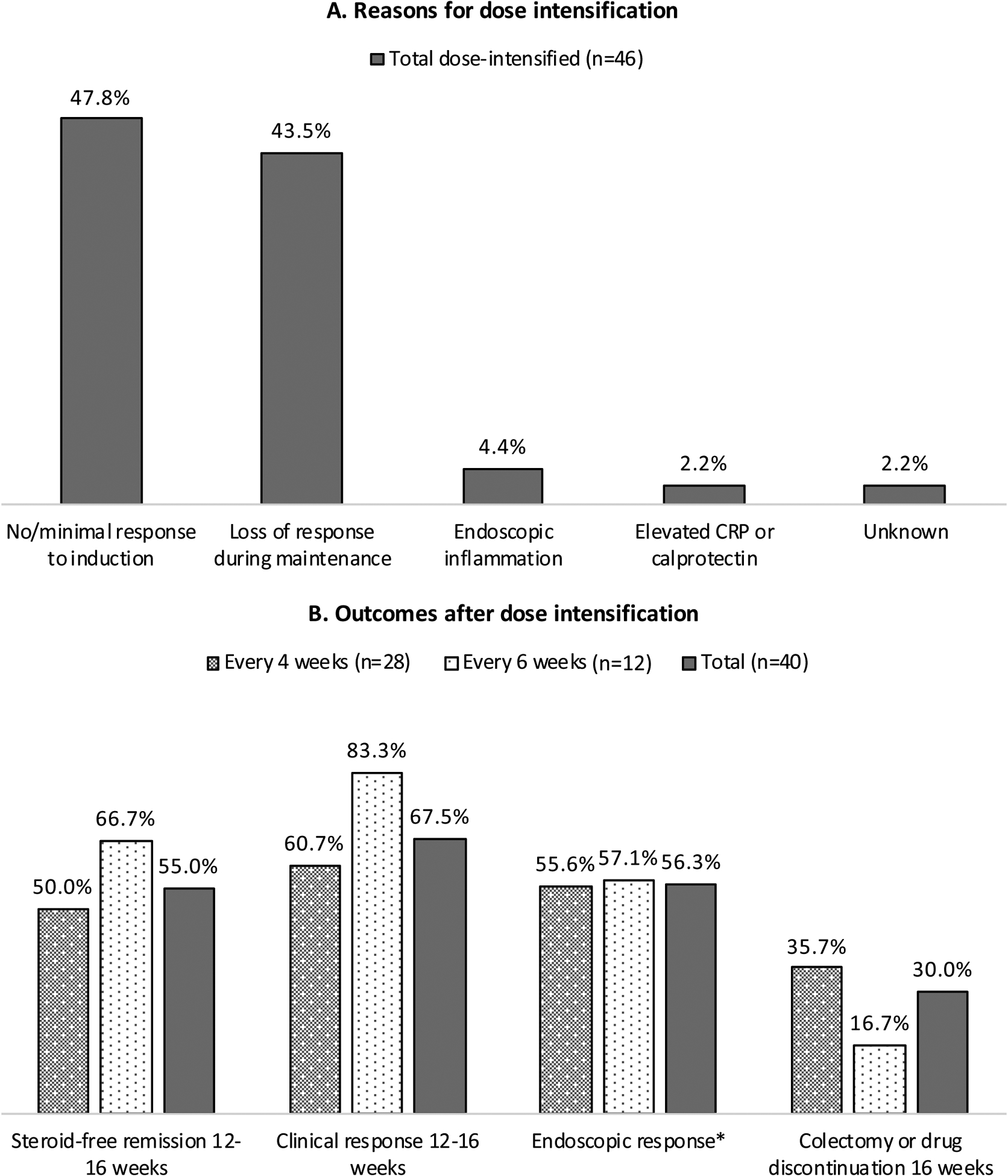
Clinical follow-up data after dose-intensification was available for 40/46 patients.
There were no significant differences between q4w and q6w outcomes using p<0.05 by Fisher’s exact test
*Post-intensification endoscopic data was available for n=9 for q4w, n=7 for q6w.
After multivariable analysis, no/minimal response to induction (OR 0.2, 95% CI 0.04–0.7) was inversely associated with remission after intensification. Bowel frequency (HR 1.1, 95% 1.02–1.2) and >2 prior biologic exposures (HR 2.5, 95% 1.1–5.8) were associated with time-to-intensification (Supplementary Table 2B).
Discussion
Nearly 40% of UC patients in our multicenter study achieved remission after ustekinumab induction. However >40% required intensification, which was associated with higher daily bowel frequency and >2 prior biologic exposures. Similar to our findings in CD6, >50% of dose-intensified patients achieved corticosteroid-free remission, however patients with minimal/no response to induction had lower odds of remission after intensification. We observed no significant differences between q4w and q6w dosing, however larger studies are needed for this comparison.
The strengths of the study include utilization of an entire multicenter cohort of ustekinumab users to assess both predictors and comprehensive outcomes of dose intensification. Limitations include a small sample of dose-intensified patients precluding subgroup analyses. The variability in timing of colonoscopies also limits conclusions regarding endoscopic response. Long-term outcomes are lacking, which is largely due to the recent FDA approval of ustekinumab for UC in October 2019.
In summary, ustekinumab dose intensification appears to be safe and effective for patients with UC. This strategy may be more effective among patients with LOR to q8w dosing rather than those with no response after induction. Prospective studies may identify specific subpopulations that would benefit from different optimization strategies of ustekinumab in UC.
Supplementary Material
Grant Support:
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (5T32DK007533-35 to R.S.D.), Frederick Makrauer IBD Fund (to R.S.D.), and the Crohn’s & Colitis Foundation (567497 to E.L.B.)
Disclosures:
JRA serves as a consultant for Takeda, Janssen, Pfizer, Pandion, Servatus, Finch Therapeutics, Iterative Scopes and Artugen and has grant support from Merck. ELB has served as a consultant for AbbVie, Gilead, Pfizer, Takeda, and Target RWE. RSD, SS, JCP, and JM have no financial or personal conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Writing assistance: none
References
- 1.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. September 2019;381(13):1201–1214. doi: 10.1056/NEJMoa1900750 [DOI] [PubMed] [Google Scholar]
- 2.Amiot A, Filippi J, Abitbol V, et al. Effectiveness and safety of ustekinumab induction therapy for 103 patients with ulcerative colitis: a GETAID multicentre real-world cohort study. Aliment Pharmacol Ther. June 2020;51(11):1039–1046. doi: 10.1111/apt.15717 [DOI] [PubMed] [Google Scholar]
- 3.Battat R, Kopylov U, Bessissow T, et al. Association Between Ustekinumab Trough Concentrations and Clinical, Biomarker, and Endoscopic Outcomes in Patients With Crohn’s Disease. Clin Gastroenterol Hepatol. Sep 2017;15(9):1427–1434.e2. doi: 10.1016/j.cgh.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 4.Ollech JE, Normatov I, Peleg N, et al. Effectiveness of Ustekinumab Dose Escalation in Patients With Crohn’s Disease. Clin Gastroenterol Hepatol. Feb 2020;doi: 10.1016/j.cgh.2020.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopylov U, Hanzel J, Liefferinckx C, et al. Effectiveness of ustekinumab dose escalation in Crohn’s disease patients with insufficient response to standard-dose subcutaneous maintenance therapy. Aliment Pharmacol Ther. May 2020;doi: 10.1111/apt.15784 [DOI] [PubMed] [Google Scholar]
- 6.Dalal RS, Njie C, Marcus J, Gupta S, Allegretti JR. Predictors of Ustekinumab Failure in Crohn’s Disease After Dose-intensification. Inflammatory Bowel Diseases. 2020;doi: 10.1093/ibd/izaa282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fumery M, Peyrin-Biroulet L, Nancey S, et al. Effectiveness And Safety Of Ustekinumab Intensification At 90 Mg Every Four Weeks In Crohn’s Disease: A Multicenter Study. J Crohns Colitis. Sep 2020;doi: 10.1093/ecco-jcc/jjaa177 [DOI] [PubMed] [Google Scholar]
- 8.Haider SA, Yadav A, Perry C, et al. Ustekinumab dose escalation improves clinical responses in refractory Crohn’s disease. Therap Adv Gastroenterol. 2020;13:1756284820959245. doi: 10.1177/1756284820959245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


