Introduction
Patients with nonalcoholic fatty liver disease (NAFLD) are at an increased risk of cardiovascular disease. Hydoxy-3-Methyglutaryl-coenzyme reductase inhibitors, “statins”, reduce the risk of cardiovascular events.1 Studies have shown statins are safe among patients with liver disease, including those with compensated cirrhosis,2 and their use is associated with lower mortality, hepatic decompensation, and possibly hepatocellular carcinoma.3,4 Despite these data, statins are under prescribed among patients with liver disease due to concerns about hepatotoxicity.5 This study aimed to assess prevalence and patient factors associated with indicated statin use in patients with NAFLD in a real-world cohort.
Methods
Adults with NAFLD enrolled in TARGET-NASH, across 60 sites in the U.S., with an indication for statin therapy were included. Statin indication was based on the 2013 American College of Cardiology guidelines for primary and secondary prevention of cardiovascular disease: 1) atherosclerotic cardiovascular disease (ASCVD), 2) low density lipoprotein (LDL-C) ≥190 mg/dL, 3) history of diabetes and age 40–75 years, or 4) 10-year ASCVD risk score ≥7.5%.6
Medical records available within 3 years prior to enrollment were reviewed for co-morbidities and liver disease severity; statin use within six months of enrollment was determined (Supplemental materials). Patients were classified as having nonalcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), or compensated or decompensated NAFLD cirrhosis according to clinical assessments previously described.7 Unadjusted and adjusted logistic regression models were fit to assess the association between patient demographic and clinical characteristics with statin use.
Results
This analysis included 2,214 patients with at least one statin indication. Median age was 62 years, 80.2% were white, and 61.1% female. At enrollment, 26.2% had compensated and 20.1% had decompensated cirrhosis, 73.2% had hypertension, 83.2% type 2 diabetes, and 62.6% dyslipidemia. Diabetes plus age 40–75 years was the most common indication for statin use (81.4%) (Table S1).
Overall, 55.8% of patients with at least one indication used a statin, with the highest use among patients with clinical ASCVD (63.0%) (Figure 1a). Patients on an indicated statin were older (63 vs 61 years old; p<0.0001), with more cardiovascular comorbidities, and were less often female (58.9% vs 63.8%; p<0.019) (Table S1). The proportions of patients who received an indicated statin were lower in patients with more advanced liver disease: 60.8%, 61.6%, 55.1%, and 42.2%, respectively in patients with NAFL, NASH, compensated cirrhosis, and decompensated cirrhosis (Figure S1).
Figure 1a.
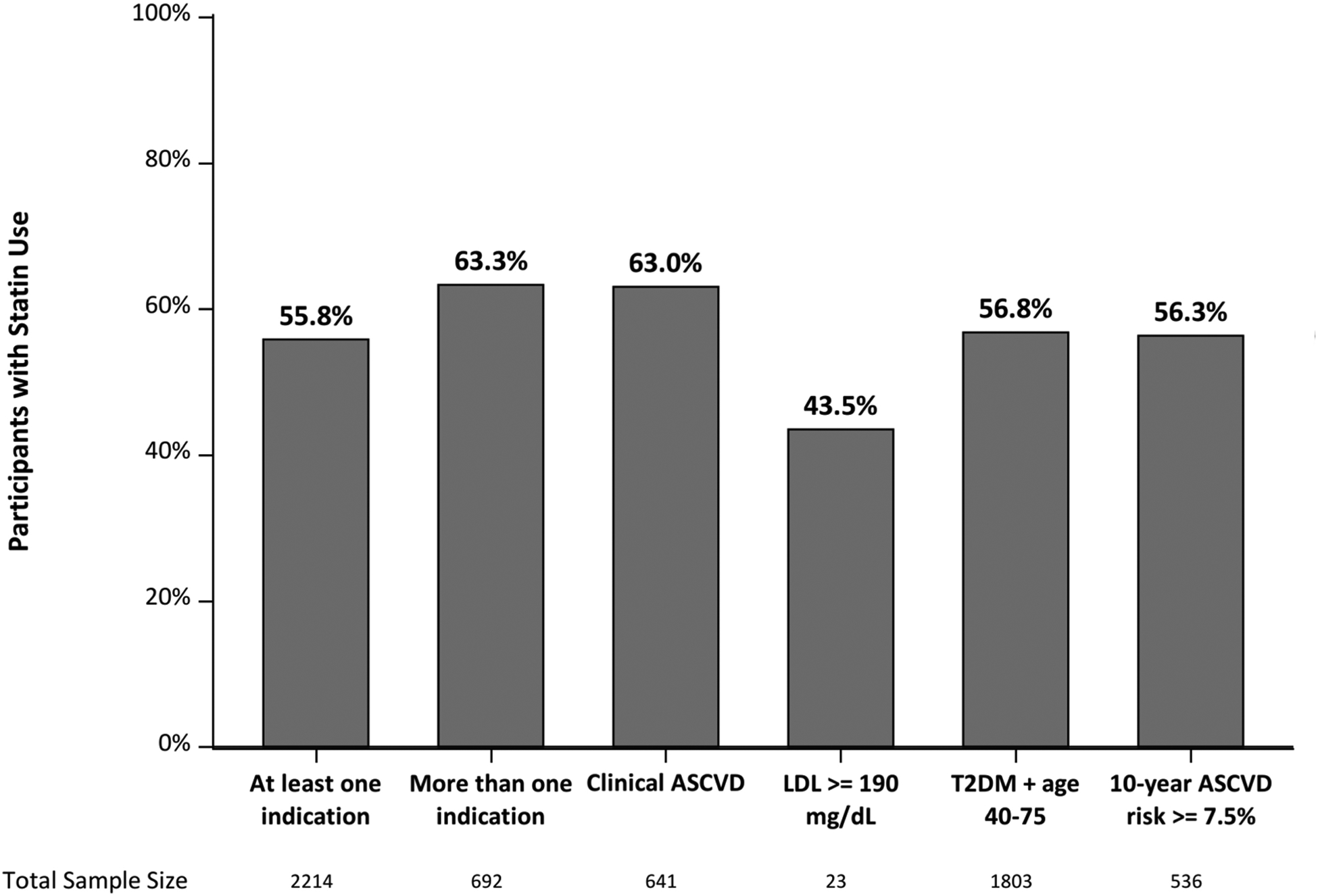
Percentage of patients in TARGET-NASH prescribed statin by statin indications.
Abbreviations: atherosclerotic cardiovascular disease (ASCVD), body mass index (BMI), history (Hx), lower and upper confidence limits (LCL and UCL), milligrams per deciliter (mg/dL), type 2 diabetes mellitus (T2DM)
In a multivariable analysis adjusting for demographics, liver disease severity, and other clinical characteristics, age ≥65 (OR 1.44, 95%CI 1.11–1.88), no cirrhosis versus decompensated cirrhosis (OR 1.88,95% CI 1.34–2.65), compensated versus decompensated cirrhosis (OR 1.44, 95%CI 1.04–1.99), history of hypertension (OR 1.32, 95%CI 1.03–1.69), type 2 diabetes (OR 1.96, 95%CI 1.44–2.67), dyslipidemia (OR 5.42, 95%CI 4.34–6.77), and clinical ASCVD (OR 1.49, 95%CI 1.16–1.92) were independently associated with higher odds of statin use (Figure 1b). There was a non-significant trend towards higher statin use in patients without cirrhosis compared to those with compensated cirrhosis (OR 1.31, 95%CI 1.00–1.72). Female sex (OR 0.70, 95%CI 0.56–0.88) and platelet count <100,000/μL (OR 0.7, 95%CI 0.50–0.97) were associated with lower odds of statin use.
Figure 1b.
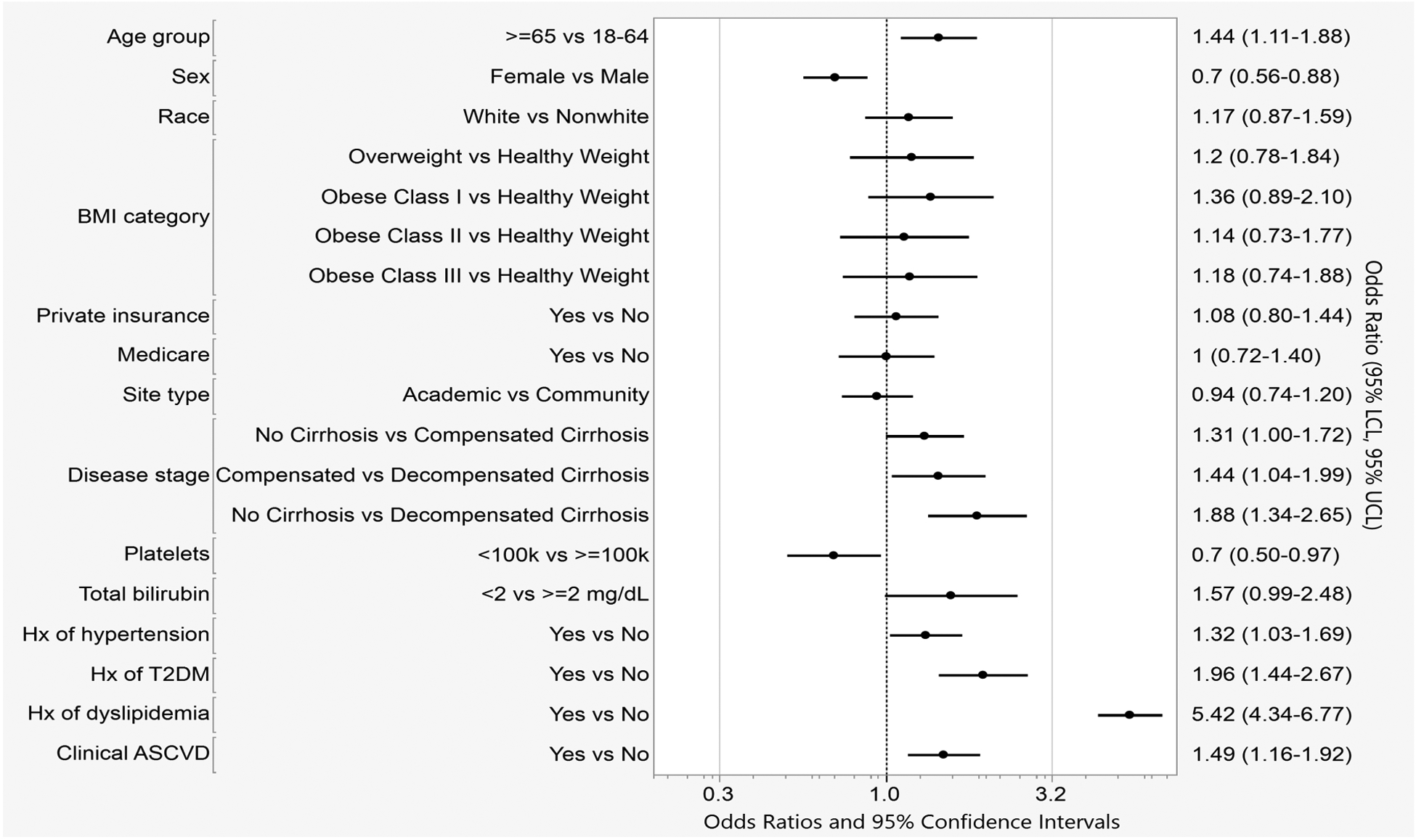
Multivariate model showing odds of statin use in TARGET-NASH patients with indications for statin therapy.
Effect estimates are adjusted for all other variables in the model.
Abbreviations: atherosclerotic cardiovascular disease (ASCVD), body mass index (BMI), history (Hx), lower and upper confidence limits (LCL and UCL), milligrams per deciliter (mg/dL), type 2 diabetes mellitus (T2DM)
Discussion
In this study, only 56% of patients with NAFLD were taking guideline-recommended statin therapy. Older patients as well as those with dyslipidemia or hypertension were more likely to be on a statin. Statin use decreased as NAFLD became more advanced, likely reflecting safety concerns in patients with decompensated cirrhosis.8 Women were less likely to be on an indicated statin compared to men, even after adjusting for cardiovascular risk factors and liver disease severity.
There were several limitations to this study. The majority of patients were seen in academic gastroenterology or hepatology practices, statin use may be lower in other settings. Clinical information was extracted from clinic notes, which depends on accurate and complete documentation. The pragmatic NAFLD diagnoses utilized may be inaccurate in classifying disease severity; however, this reflects real world practice where biopsies are uncommonly done. Finally, follow-up was not long enough to determine the effect on cardiovascular or hepatic outcomes.
Similar to other studies of statin use in the general population and in NAFLD patients, this analysis of a large national real-world sample showed that guideline-recommended statins continue to be underutilized and were not prescribed in 40% of NAFLD patients with clear indications. Ongoing studies on the potential benefit of statins in preventing cirrhosis complications may broaden statin indications beyond cardiovascular disease in the future. Further steps are needed to educate providers and patients on statin safety and its benefits in preventing cardiovascular disease in at-risk NAFLD patients.
Supplementary Material
Funding:
Marina Serper receives funding from the National Institutes of Health award (1K23DK1158907-03). Mary Thomson receives funding from the AASLD Advanced/Transplant Hepatology Award.
Financial Support Statement:
TARGET-NASH is a collaboration among academic and community investigators and the pharmaceutical industry. Target RWE is the sponsor of TARGET-NASH. We thank the study staff, nurses, health care providers, and participants at each study center for their contributions to this work.
Abbreviations:
- ASCVD
atherosclerotic cardiovascular disease
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- BMI
body mass index
- IQR
interquartile range
- LDL-C
low density lipoproteins
- HCC
hepatocellular carcinoma
- HDL-C
high density lipoproteins
- NAFLD
nonalcoholic fatty liver disease
- NAFL
nonalcoholic fatty liver
- NASH
nonalcoholic steatohepatitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
MT: No conflicts of interest to disclose.
MS: Consulting fees, Gilead, Inc.
VK: None.
LMW: None.
HT: Gilead: speaker, grant research, advisor, stock shareholder
Intercept: grant research
RFM: None.
MR is on the scientific advisory boards of Allergan, Astra-Zeneca, Bristol-Myers Squibb, Eli Lilly, Gilead Sciences, Inventiva, Intercept Pharma, Novartis, NovoNordisk, Servier Laboratories, Target Pharmasolutions, and Terra Firma and receives investigator-initiated support from Boehringer Ingelheim, Nutricia/Danone and Sanofi–Aventis.
DG, ARM: Employee of Target RWE.
MWF: Receives personal fees from Target RWE as an independent contractor consultant, serving in the role of Chief Medical Officer. He is a stockholder in Target RWE.
RR: Research grants (paid to the University of Pennsylvania) HCV-TARGET, TARGET-HCC, TARGET-NASH.
AL: receives research grants (to University of Michigan) from and serves as advisor to Target RWE, also serves on DSMB for Novo Nordisk.
ClinicalTrials.gov Identifier: NCT02815891
REFERENCES
- 1.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis JH, Mortensen ME, Zweig S, et al. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 2007;46:1453–63. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan DE, Serper MA, Mehta R, et al. Effects of Hypercholesterolemia and Statin Exposure on Survival in a Large National Cohort of Patients With Cirrhosis. Gastroenterology 2019;156:1693–1706 e12. [DOI] [PubMed] [Google Scholar]
- 4.Kim RG, Loomba R, Prokop LJ, et al. Statin use and risk of cirrhosis and related complications in patients with chronic liver diseases: a systematic review and meta-analysis. Clinical Gastroenterology and Hepatology 2017;15:1521–1530. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Ben M, Baratta F, Polimeni L, et al. Under-prescription of statins in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 2017;27:161–167. [DOI] [PubMed] [Google Scholar]
- 6.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934. [DOI] [PubMed] [Google Scholar]
- 7.Barritt ASt, Gitlin N, Klein S, et al. Design and rationale for a real-world observational cohort of patients with nonalcoholic fatty liver disease: The TARGET-NASH study. Contemp Clin Trials 2017;61:33–38. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan DE. The Use of Statins in Patients With Cirrhosis. Gastroenterol Hepatol (N Y) 2018;14:485–487. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


