Abstract
Social hierarchies are ubiquitous features of virtually all animal groups. The varying social ranks of members within these groups have profound effects on both physical and emotional health, with lower‐ranked individuals typically being the most adversely affected by their respective ranks. Thus, reliable measures of social dominance in preclinical rodent models are necessary to better understand the effects of an individual's social rank on other behaviors and physiological processes. In this review, we outline the primary methodologies used to assess social dominance in various rodent species: those that are based on analyses of agonistic behaviors, and those that are based on resource competition. In synthesizing this review, we conclude that assays based on resource competition may be better suited to characterize social dominance in a wider variety of rodent species and strains, and in both males and females. Lastly, albeit expectedly, we demonstrate that similarly to many other areas of preclinical research, studies incorporating female subjects are lacking in comparison to those using males. These findings emphasize the need for an increased number of studies assessing social dominance in females to form a more comprehensive understanding of this behavioral phenomenon.
Keywords: agonistic behavior, hierarchy, resource competition, social rank
This review describes and discusses approaches to assess social ranks in rodent species.
1. INTRODUCTION
The investigation of social hierarchies dates back to Thorlief Schjelderup‐Ebbe's work in the 1920s characterizing social hierarchies in populations of chickens. Throughout this work, Schjelderup‐Ebbe observed a strict, linear relationship of inter‐group aggression, suggesting that each bird maintained a different social rank as a function of their rank within this aggressive order (the finding responsible for the colloquialism “pecking order” when referring to any social hierarchy). 1 It has since been well demonstrated that these hierarchies are an integral part of virtually all group‐living animal species, and that a subject's rank within a given hierarchy may be characterized by quantifying their access to various resources and/or exhibition of aggressive behaviors, with higher‐ranking, dominant animals acquiring larger proportions of said resources 2 , 3 and/or exhibiting higher levels of aggression toward their lower‐ranking counterparts 4 and even colony intruders. 5
Conceptually, the analyses of hierarchical relationships between rodents can be divided into two main categories: (1) Analysis of agonistic interactions and (2) analysis of differences in access to various types of resources, including territory, mates, standard chow, palatable food, and/or water. Agonistic behaviors can be directly measured in rodents, and highly aggressive rodents do have a tendency for increased access to resources. 5 , 6 , 7 , 8 However, the unequal distribution of resources in humans is not exclusively regulated by aggression of higher‐ranking individuals. 9 , 10 Similarly, increased access to resources in rodents does not always necessitate overt aggression on the part of the high‐ranking subject, suggesting that the assessment of aggression alone is not sufficient to identify a rodent's social rank. It should be noted, though, that the measures obtained during the assessment of differences in access to resources are often indirect. For example, higher scent marking or increased ultrasonic vocalizations in dominant animals (see Sections 4 and 8i) could reflect increased access to territory and mates, but these measures are not necessarily directly proportional to such access. In addition, the behaviors assessed in these resource competition tasks could reflect a state of the animal, rather than a trait: for example, competition for food may not exclusively reflect social rank, as performance in this assay can also be affected by an individual's sensitivity to food deprivation and/or their innate motivation to consume a given resource.
In the sections below, we describe the most frequently used tests of social hierarchy and dominance in rodents, as well as the relationships between them in various rodent species/strains and across both sexes (for a summary of this information, refer to Figure 1). Following the description of these assays, we synthesize this information to provide strategies for future studies and highlight opportunities to develop an improved understanding of social rank among both male and female rodents.
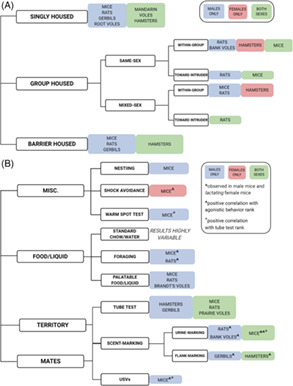
FIGURE 1.
Summary of species and sexes used in each measure of social dominance. (A) Agonistic behavior. (B) Resource competition
2. AGONISTIC BEHAVIORS
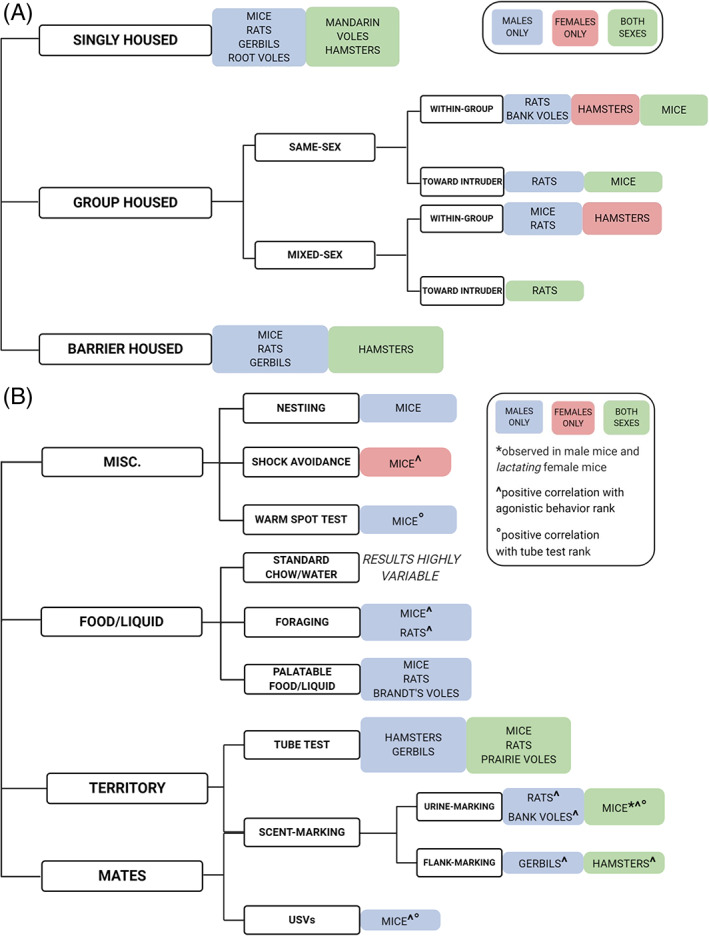
One of the most commonly used methods to assess social rank among rodents is the analysis of agonistic behaviors (for schematic of common agonistic behaviors, see Figure 2(A)). Agonistic encounters consist of both offensive and defensive behaviors exchanged between two animals. 11 , 12 Offensive behaviors typically include lateral attacks, chasing, biting and barbering, whereas defensive behaviors typically include flight, freezing, and exhibition of submissive (lying on back) or defensive (upright with paws raised) postures. 12 , 13 , 14 , 15 , 16 Social rank is often determined following the observation of these behaviors during social interactions. However, experimenters have also used weight loss, the extent of barbering, and the number, severity, and location of scars/wounds following social interactions as indirect measures of dominance. Specifically, an animal is considered subordinate if it exhibits greater weight loss and/or has a higher number of severe wounds, while an animal is considered dominant if it experiences less weight loss and has a lower number of less severe wounds. Additionally, in rats, the wounds of subordinates are often located primarily on the tail, back, and flank, in comparison to dominants, for which wounds are primarily located on the head and snout. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 For barbering, also termed fur trimming, whisker trimming, the Dalila effect, or overgrooming, a mouse is considered dominant if all fur and whiskers remain intact, and subordinate if fur loss and/or whisker shortening/loss are observed. 35 , 36 , 37 , 38 , 39 , 40 Notably, barbering is one of the only agonistic behaviors readily observed in female C57BL/6J mice. 35 , 36 , 38 , 41 On the other hand, barbering has also been shown to be unrelated to social dominance, rather, attributed to factors ranging from an unenriched environment to a manifestation of an obsessive–compulsive‐like disorder or stereotypic behavior. 42 , 43 In fact, barbering is one agonistic behavior that is not thought to occur in a naturalistic setting, but is limited to laboratory mice. 43 , 44
FIGURE 2.
Schematics of experimental setup for agonistic behavioral analyses. (A) Common examples of agonistic behaviors exhibited by dominant and subordinate subjects. Often conducted in a standard homecage or neutral arena. (B) Example of visible burrow system (VBS), a group‐housed, mixed‐sex design that promotes strict, despotic hierarchies among male subjects. In addition to the agonistic behaviors outlined in part (A), dominant and subordinate subjects in the VBS also spend differing amounts of time on the surface/in the tunnel system
To assess social dominance relations between singly housed animals, a pair of subjects, typically same‐sex, is placed in a neutral cage or arena to allow for social interaction, during which agonistic behaviors are scored. This design is especially useful for more aggressive species or strains, in that the length of agonistic interactions can be controlled by the experimenter. This model has been used to characterize social rank in male mice, 45 , 46 male gerbils, 47 male and female rats, 13 , 14 , 48 , 49 male and female mandarin voles, 50 male root voles, 51 and male and female hamsters. 52 , 53 , 54 , 55 , 56 , 57 , 58 These studies have revealed that dominant‐subordinate relationships are readily formed for all male mice, rats, hamsters, gerbils, and mandarin voles under these conditions, and that these relationships are typically stable over time. In contrast, female rats are rarely reported to form these relationships, due to less frequent and severe agonistic interactions. 13 , 14 On the other hand, female hamsters and mandarin voles are naturally more aggressive, which results in the observation of strict dominant‐subordinate agonistic relationships during same‐sex interactions. 50 , 54 , 55 It must be noted that while these studies identify potential dominant‐subordinate relationships between pairs of subjects, it cannot be conclusively stated that these relationships are reflective of the social hierarchies that emerge in socially‐housed animals.
Thus, agonistic behaviors have also been analyzed in rodents that are housed in the same cage, but separated by barriers or partitions to prevent constant, direct physical contact. When these partitions are removed by the experimenter, subjects are able to interact directly, allowing for the observation of agonistic behaviors. 32 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 This assay has been used to identify dominance hierarchies in male Long Evans rats, 32 , 66 male BALB/cJ mice, 59 male NMRI mice, 63 male CD1 mice, 64 male large vesper mice (Calomys callosus), 65 male and female hamsters, 60 , 61 , 62 , 68 and male gerbils. 67 As in single‐housed designs, these experiments are particularly useful when investigating social rank in highly aggressive species and strains, that if housed in standard group‐housing conditions, would present the risk of severe injury or death of subordinate subjects.
Several studies have also assessed agonistic behaviors and social rank in pair‐ or group‐housed rodents allowing for continuous, direct social interaction. These designs allow for agonistic interactions to be analyzed under two potential conditions: (1) Among the group and colony members only, or (2) following the introduction of an intruder rodent. In the former example, animals exhibiting the highest number of offensive behaviors toward their own cagemates are considered dominant, 69 whereas in the resident‐intruder model, animals exhibiting the highest number of offensive behaviors toward the intruder are considered dominant. 21 , 70
Analysis of spontaneous agonistic interactions and evaluation of wounds or barbering among cagemates has been conducted in group‐housed male mice, 16 , 27 , 28 , 30 , 33 , 38 , 39 , 46 , 69 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 female mice, 16 , 38 , 39 , 86 , 91 , 92 male rats, 4 , 14 , 22 , 23 , 24 , 31 , 32 , 34 , 93 , 94 , 95 , 96 , 97 , 98 female rats, 14 male bank voles, 99 and female hamsters. 100 , 101 The majority of these studies observed strict linear dominance hierarchies in male mice, male rats, male voles, and female hamsters; whereas markedly less strict hierarchies—or no hierarchies at all—were observed among female mice and rats. In this context, linear refers to the structure in which there is an alpha (dominant over all cagemates), beta (dominant over all cagemates except alpha), and so forth, within the hierarchy. 86 In contrast, studies using the resident‐intruder model have demonstrated that despotic, or exclusive, dominance hierarchies are formed in male rats and male and female mice, 21 , 91 , 102 , 103 , 104 where despotic refers to a single animal maintaining dominance over all other cagemates, with no differences in rank between these subordinates. 86
Additional studies of social rank have been conducted using a mixed‐sex design. These models are typically employed to potentiate agonistic interactions among male subjects and/or to provide more naturalistic housing conditions (refer to Figure 2(B) for a schematic of the visual burrow system, or VBS, a system often used in these types of experiments). As in same‐sex studies, social rank can be determined by analyzing the spontaneous agonistic behaviors among the colony members 6 , 29 , 105 , 106 , 107 , 108 or the agonistic behaviors of colony members toward a stranger, “intruder” rodent in a resident‐intruder test. 109 , 110 The vast majority of mixed‐sex colony studies analyzing spontaneous agonistic encounters within a colony have revealed that strict, despotic social hierarchies are readily established among male rats and mice, while no hierarchies are observed among female rats and mice. 5 , 6 , 7 , 8 , 15 , 17 , 25 , 26 , 86 , 87 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 In contrast, female hamsters housed in mixed‐sex pairs establish strong dominant‐subordinate relationships, with females typically maintaining dominance over the male. 130 However, a resident‐intruder study using mixed‐sex colonies of Long Evans rats has demonstrated that one male and one female in each colony exhibit social dominance evidenced by increased aggression toward an intruder rat introduced to the colony. 109 Importantly, though, a female was only identifiable as dominant when all male colony members were removed from the cage during the resident‐intruder test. 109 This finding demonstrates that the lack of observed social ranks among females in previous VBS studies is likely attributable to the testing conditions used: It seems that for this assay, all males must be removed from the environment during testing for a dominant female to emerge. These data suggest that social hierarchies can, in fact, form among female rats, but that the tests traditionally used to assess dominance in males may prove insufficient in detecting these dynamics in females.
Overall, these studies demonstrate that agonistic behaviors serve as a useful measure of social dominance in males of many rodent species, as well as in female hamsters and mandarin voles, and even female rats under certain testing conditions. As such, this method has proven generally less reliable in female mice, rats (when tested in the presence of males), and gerbils, suggesting that different measures should be utilized when assessing social rank in these populations. Lastly, it must be noted that severe injury or death of subordinate subjects is a considerable risk in studies using more aggressive strains and species. Therefore, in these subjects, single‐housing, or modified group‐housing settings where subjects are separated by partitions need to be used, in that they allow for constant supervision of agonistic encounters.
3. TUBE TEST
The tube test was first developed by Lindzey and colleagues in 1961 to characterize social dominance in male and female mice, 131 and has since been employed to assess social rank in different mouse strains and other rodent species (see Figure 3(A) for tube test schematic).
FIGURE 3.
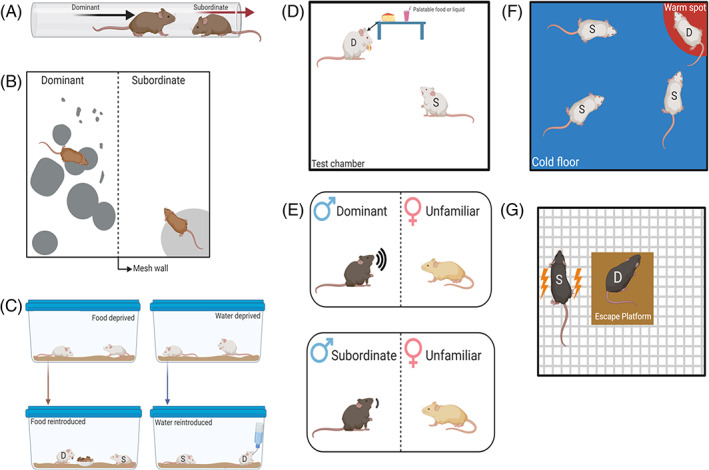
Schematics of experiment setup for resource competition assays. (A) Tube test. (B) Scent‐marking. (C) Standard chow/water competition. (D) Palatable food competition. (E) 70‐mHz ultrasonic vocalizations. (F) Warm spot test. (G) Shock avoidance
For this assay, experimenters use a clear, plastic tube, typically 30 cm in length for smaller rodents. The appropriate diameter is determined so that there is only room for one subject to pass through the tube at a given time. 131 , 132 Before testing, rodents are first habituated to the apparatus and trained to cross through the tube individually. Subjects are often presented with a food reward during this training process to promote crossing through the full length of the tube. 131 , 133 , 134 , 135 Animals are then paired to undergo testing, during which each subject is placed on opposite ends of the tube and allowed to approach its conspecific toward the center of the tube. The subject that subsequently forces its competitor out of the tube is considered the winner (dominant), and the subject forced out of the tube the loser (subordinate). If examining social rank among a group of 3 or more subjects, multiple tube tests are conducted using a round‐robin design to ensure that tests take place between all members of said group. 132
The tube test has been used to evaluate social rank in male and female mice, 36 , 37 , 39 , 40 , 41 , 45 , 70 , 131 , 132 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 male and female prairie voles, 150 male and female rats, 133 , 134 , 151 , 152 male hamsters, 135 and male gerbils. 135 In these models, a point system is often used to express the social rank of a subject relative to other group members. For example, the winner of each test can be assigned 1 point, and the loser 0 points. Following the completion of all tests between group members, each subjects' points are added together to determine its social rank within the group. Therefore, in a group of four subjects, the most dominant animal would obtain 3 total points, and the most subordinate 0 points following tube tests with all other group members. 37 , 131 , 132 Alternatively, David's Score (DS) can be used to calculate social rank following tube tests. DS is a slightly more complex measure that calculates dominance score based on a subject's proportion of wins to losses following repeated social interactions (for detailed information on DS calculation, refer to Reference 153).
Importantly, social ranks determined using this measure have been shown to positively correlate with ranks determined from other dominance measures, such as the warm spot test, urine‐marking, and ultrasonic vocalization measures. 37 , 132 , 138 , 142 , 143 The relationship between tube test ranks and food/water competition ranks remains less clear, however, and tends to vary between species and the type of reward used. For example, a negative correlation between tube test and food competition ranks has been reported in male DBA and albino mice when the food reward is standard rodent chow. 45 In contrast, in studies using male Lister rats 151 or male ICR mice, 145 ranks obtained following palatable food/liquid competition were positively correlated with tube test ranks. 145 , 151 These inconsistencies are also observed in water competition tasks, in which no relationship between water competition rank and tube test rank is seen in male gerbils, though this relationship is positively correlated in male hamsters. 135
Contradictory results have also been obtained for the relationship between tube test rank and ranks determined from displays of agonistic behaviors, with certain studies revealing a positive correlation, 37 , 40 , 142 and others finding a negative correlation or no relationship between these measures. 41 , 45 Of note, the studies that observed positive correlations between agonistic behavior rank and tube test rank were conducted using male C57BL/6 mice. In contrast, the studies that observed a negative correlation 45 or no relationship between these measures 41 were conducted using male DBA and albino mice 45 or male and female C57BL/6 mice, 41 respectively. Taken together, these findings suggest a potential role of sex and/or strain in the correlation between social ranks determined from the tube test and those determined from agonistic behavior measures.
Overall, the tube test serves as a useful model for assessing social dominance in males and females and across various rodent species, proving especially useful if the subject population is less prone to agonistic behaviors. Even among rodents that readily exhibit aggression, the tube test presents certain advantages, in that animals are not susceptible to injury as they would be if using assays such as agonistic behavioral assessment. It must be considered, though, that species, strain, and sex can determine the generalizability of tube test results to other dominance measures.
4. SCENT‐MARKING
Patterns in scent‐marking, or urine‐ and flank‐marking, in rodents have also been used as measures of social dominance (for schematic of scent‐marking assay, refer to Figure 3(B)). Urine‐marking is most often used to characterize the social rank of male mice, 28 , 37 , 84 , 85 , 138 , 142 , 154 , 155 , 156 but it has also been used to assess dominance in in female mice, 157 male bank voles, 99 , 158 , 159 and male root voles. 51
In this assay, two subjects are placed in a neutral, clean cage separated into two compartments. The rodents remain in their respective compartments for a test period allowing for urine collection, ranging from 2 to 22 h. 28 , 37 , 84 , 85 , 142 , 158 However, urine‐marking can also be assessed in a single subject, placed in a test cage alone for 3 min to 2 h. 51 , 154 , 155 , 156 , 157 This design also allows for the assessment of counter‐marking, or scent‐marking over an experimenter‐presented urine sample from a conspecific, which can denote social rank as well. 155 , 157
Regardless of the setup used for testing, cages are lined with filter paper so that urine markings can be subsequently analyzed by visualization with UV light. The number of urine marks reflects social rank, with a higher number of marks indicating dominance, and a lower number of marks indicating subordinance. 28 , 85 , 99 , 156 Counter‐marking studies have also revealed that subjects identified as dominant based on agonistic behaviors will urine‐mark over virtually any urine sample presented, regardless of the rank of the animal from which the sample was collected, while subordinate subjects will not. 155 , 157 Additional studies have shown that dominants, again the rank of which is determined based on displays of agonistic behavior, typically scent‐mark throughout the entirety of the test arena, with concentrations close to the partition, while the urine marks of subordinates are often confined to the corners of the arena. 28 , 158 , 159 Interestingly, in the one study using females, urine‐marking patterns of dominants were similar to those observed in dominant males, in that female dominants, identified based on agonistic behavior analysis, exhibited a higher number of urine‐marks than subordinates, while also exhibiting counter‐marking behaviors. 155 , 157 Each of the studies mentioned above has demonstrated that social rank determined by urine‐marking positively correlates with that determined by agonistic behavior analysis or the tube test, with the exception of the study using male root voles. In this experiment, no relationship was observed between social rank determined by agonistic behavior analysis and social rank determined by urine‐marking, 51 a finding suggesting that urine‐marking assays may not be a reliable measure of social dominance in all species. Notably, of the one study conducted using female mice, urine‐marking was compared between breeding and nonbreeding females, 157 demonstrating that additional urine‐marking studies in females of the same breeding status should be conducted to further investigate the effect of social rank on this behavior in females.
In contrast to urine‐marking, flank‐marking has been used to characterize social rank in male hamsters, 53 , 60 , 62 , 160 , 161 , 162 female hamsters, 52 , 61 and male gerbils. 121 Flank‐marking involves the rubbing of an animal's dorsolateral flank glands on objects and/or areas within their environment. 161 As such, this behavior is typically scored as the total number of flank‐marks exhibited by each subject during social interaction, 61 , 62 when placed in an open field, 121 or when placed in the empty, dirty homecage of a conspecific. 52 , 161 It has been consistently demonstrated that male and female hamsters and male gerbils that exhibit the most flank‐marks are also dominant in agonistic encounters. Notably, while female gerbils also possess flank glands, they appear to use scent‐marking behavior not to communicate dominance, but rather as a means to defend the nest during gestation and lactation, 163 a finding suggesting that while flank‐marking is a useful measure for social dominance in male and female hamsters and male gerbils, this assay would not be useful in assessing dominance for female gerbils.
Collectively, these data suggest that while scent‐marking assays can prove useful in characterizing social rank, their validity is dependent upon the sex and species—and potentially breeder status—of subjects used.
5. STANDARD FOOD AND WATER ACCESS PRIORITY/COMPETITION
Resource competition assays, or food and water access priority/competition, are additional means of characterizing social dominance in rodents. These tasks almost always involve food or water deprivation, most often for 22–23 h periods, 135 , 164 , 165 , 166 , 167 , 168 or food restriction, during which subjects are maintained at approximately 80%–85% free‐feeding body weight. 91 , 169 , 170 Animals are then given access to standard rodent chow or water for a short period of time, typically 5 min, 45 , 164 , 165 , 166 , 171 , 172 to induce resource competition in a water‐ or food‐deprived state. In fact, only one study of food/water competition has been conducted in non‐deprived conditions. 100 Overall, these assays have been conducted in the homecage of group‐housed subjects, 96 , 135 , 173 , 174 or in a neutral test cage, allowing for testing between novel conspecifics 45 , 165 , 166 , 167 , 168 , 169 , 171 , 172 , 175 , 176 , 177 or cagemates 169 , 176 (see Figure 3(C) for schematic of standard chow/water competition assay).
The way in which food or water is presented in most tests allows for only one subject to access the resource at a given time, and the dominant can be identified as the subject that maintains control of the resource. While one of the first publications on food competition described using subjects' changes in body weight after testing as an indirect measure of amount of food consumed, and thus social dominance, 178 no subsequent studies have relied on this measure. Specifically, social dominance has since been determined by measuring the total amount of food or water consumed or taken, 100 the number of instances in which a subject successfully gains access to the resource, 109 , 171 , 174 , 177 the number of offensive (dominant) or defensive (subordinate) behaviors exhibited during competition, 179 the time spent consuming or maintaining control of the resource, 45 , 96 , 109 , 164 , 165 , 166 , 168 , 172 , 173 , 175 or the order of access, with dominants accessing food/water first. 109 , 135
An additional study has employed an operant self‐administration model, in which rats were trained to lever press for the delivery of a food pellet. For testing, pairs were then placed in the self‐administration chamber and allowed to compete for the delivery of food pellets, and the subject consuming the greatest number of reinforcers was considered dominant. 170 Lastly, modified tube test procedures have been used to assess food competition for standard pellets of chow. 91 However, in this assay, dominance was determined based on the number of aggressive behaviors exhibited while competing for the food pellets. 91
Food and water access priority tasks have been used to assess social dominance in male rats, 96 , 109 , 164 , 165 , 166 , 167 , 170 , 172 , 173 , 175 , 176 , 179 female rats, 109 , 166 , 167 male mice, 45 , 174 female mice, 91 male hamsters, 135 , 169 female hamsters, 100 and male gerbils. 135 The results of this assay tend to vary across studies, however, suggesting that factors such as species, strain, housing conditions, experimental setup, and cohort variability may affect the outcome of resource competition. For example, one study using singly‐housed Wistar rats documented the emergence of stable dominant‐subordinate relationships, 166 while another using singly‐housed albino rats did not. 167 Additionally, in a study by Candland and Bloomquist, no stable hierarchies were observed among large groups of rats or hamsters (n = 10), 169 suggesting that group size may also impact the results of food competition tasks. Further, a subsequent study demonstrated that resource competition tasks can serve as effective measures of social rank in larger group sizes, and that larger groups merely require a greater number of tests to reach stability. 172
Similarly, the generalizability of these assays' results to other measures of social dominance varies across species. Specifically, social ranks obtained from food/water access priority have been shown to positively correlate with social ranks based on agonistic interactions in male mice, 45 but not in male rats 96 , 109 or female rats. 109 Additionally, positive correlations between tube test social ranks and water competition social ranks have been observed in hamsters, but not gerbils. 135 Perhaps these differing outcomes also reflect potential effects of sex, species, and/or strain on an animal's sensitivity to food/water deprivation or food restriction. For example, these manipulations have been demonstrated to serve as stressors, 180 , 181 , 182 , 183 inducing anxiety‐like behavior 184 and increases in plasma corticosterone levels, 185 which in turn could differentially affect animals' motivation to compete for food and water. Overall, these food/water competition tasks seem to vary substantially between species, strain, and overall experimental design, suggesting that they may not serve as the most reliable measure of social dominance in rodents.
6. FORAGING
Patterns in food/water consumption under ad libitum conditions in group‐housed animals may serve as a more reliable measure of social dominance. For example, it has been repeatedly shown that dominant male rats (based on exhibition of agonistic behaviors) consume significantly more food/water and have more frequent bouts of consumption than their subordinate cagemates. 5 , 6 , 7 , 8 Based on these previous findings, a study by Lee and colleagues 90 analyzed patterns of food and water intake in male mice of differing social ranks. They found that mice considered dominant based on exhibition of agonistic behaviors had a significantly higher number of eating and drinking bouts than subordinates. 90 Additionally, periods of quiescence, or inactivity, were significantly shorter in dominant mice compared to subordinates. 90 Overall, these data suggest that additional studies testing food and water intake under ad libitum conditions should be performed in females, as well as other rodent species and strains, to determine whether these measures are indicative of social rank in additional subject populations.
7. PALATABLE FOOD AND LIQUID COMPETITION
Palatable food and liquid competition assays have also been used to assess social dominance in male mice, 186 , 187 , 188 rats, 109 , 151 , 173 , 179 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 and Brandt's voles, 204 with fairly consistent results across strains and species, in that dominant‐subordinate relationships are consistently observed following testing (schematic of palatable food/liquid competition illustrated in Figure 3(D)). Only one study has been conducted using females, in which female Long Evans rats were co‐housed with male Long Evans rats for the entirety of the study, except during palatable food competition. 109 This assay has yet to be conducted in singly‐housed or same‐sex group‐housed females.
Palatable food competition can be conducted within the homecage, and assess dominance relationships between cagemates, 109 , 173 , 190 , 193 , 194 , 195 , 196 , 197 , 198 , 199 as well as in neutral environments to assess dominance among novel conspecifics 179 , 191 , 200 , 201 , 202 or cagemates. 151 , 186 , 187 , 189 , 192 , 203 , 204 Generally, foods and liquids with high levels of sucrose, such as chocolate, sweetened condensed milk, or graham crackers, are used as the object of competition, as rodents have an innately high preference for sucrose. 205 , 206 However, palatable foods with lower sucrose content, such as cheese, have also been used successfully in these assays. 187 Regardless of the tastant presented, all competition assays require an initial habituation period in which subjects are exposed to the palatable food or liquid prior to competition tasks to avoid neophobia during testing and to ensure that all animals exhibit a similar preference for the food under noncompetitive conditions. As a caveat, it should be noted that motivation to consume a given reinforcer (e.g., through the use of a progressive ratio schedule of operant reinforcement) has not been examined, and thus should be considered as a potential factor contributing to each subject's performance.
For testing under non‐deprived conditions, subjects are either transferred to a neutral test cage, or the standard chow and water bottles of the homecage are removed, so that only the palatable food/liquid is available for consumption. During these food competition tests, the amount of food presented, how it is presented, and how social dominance is scored, vary across studies. For example, one set of studies involve the presentation of one sucrose pellet every 2 min for a 1 h test session, resulting in the delivery of 30 total pellets per session to each group. 195 , 196 , 197 , 198 , 199 Thus, the rat consuming the highest number of sucrose pellets during a 1 h session is considered to be the dominant subject. 195 , 196 , 197 , 198 , 199 A pair of rodents can also be tested under modified tube test conditions, with each subject approaching a 0.4 g piece of cheese placed in the center of a plastic tube, and the mouse consuming the cheese being identified as dominant. 187 Lastly, food competition tasks can involve the presentation of a predetermined amount of food (for example, 3 g graham cracker crumbs 201 or eight pieces of chocolate cereal 179 ) in a central location within a neutral cage or the subjects' homecage. Importantly, the food is presented so that only a single subject can access or consume the reward at a given time. The food is typically made available for 10–15 min, 179 , 201 , 204 or until all the food has been consumed. With this design, the dominant can be identified using several measures: time spent eating food, 201 time spent maintaining possession of food, 109 , 186 , 188 amount of food consumed, 179 and order of access, with dominants accessing food first. 109 , 190 , 204
Similarly, palatable liquid competition tests also vary in regard to the amount of solution presented, how it is presented, and how dominance is scored. For example, in a study by Askew and colleagues, pairs of rats were trained to compete for sweetened condensed milk solution in an operant self‐administration model, such that the animal with the highest number of active lever presses, reinforcers earned, and solution consumed was identified as dominant. 191 Additionally, a pair of rodents can be tested under modified tube test conditions, with each subject approaching a feeder containing sweetened milk in the center of a plastic tube. 192 , 200 , 202 As with palatable food competition tests, the sippers are designed so that only a single subject can access and consume the reward at a given time. Thus, the animal that spends the higher amount of time at the feeder during the test period is considered dominant. 192 , 200 , 202 Lastly, a standard bottle/sipper containing palatable liquid can be presented in the homecage or a neutral test cage, with social rank being determined based on the volume of solution consumed or the amount of time spent consuming solution 151 , 189 , 193 , 194 .
It must be noted, though, that several studies incorporate food or water deprivation prior to competition tasks, with periods of deprivation ranging from 8 to 23 h, 109 , 173 , 186 , 187 , 192 , 203 , 204 while an additional study chose to employ food restriction, maintaining subjects at 95% body weight throughout the entire experiment. 191 Both food/water deprivation and food restriction strategies are thought to increase the motivation to consume the food/liquid presented during testing, though many palatable food/liquid competition studies do not incorporate food or water deprivation given rodents' innate, high motivation to consume most substances used in these tasks.
Overall, palatable food and liquid competition tasks have proven reliable in assessing social dominance in various rodent species, with either dominant‐subordinate relationships or linear hierarchies being observed in nearly all groups examined for each study. Nevertheless, in one study that compared social rank based on agonistic behaviors to social rank based on palatable food competition, results were not consistent. 109 Notably, this study tested mixed‐sex groups of male and female rats that were only housed with same‐sex animals during the palatable food competition test, 109 suggesting that factors such as housing condition and breeding/maternal status, may influence an individual's performance in this assay. Lastly, as with many other assays outlined in this review, more studies that incorporate female subjects are warranted to determine whether this assay is a suitable measure of dominance for this sex.
8. ADDITIONAL MEASURES
8.1. Ultrasonic vocalizations
Two studies have measured 70 kHz ultrasonic vocalizations (USVs), often termed “courtship songs,” of male mice to determine social dominance, as this USV frequency is used by male mice during mating behaviors. 207 , 208 More specifically, when presented with a female, dominant male mice (rank determined based on display of agonistic behaviors) emit significantly more and longer‐lasting 70 kHz USVs, and have shorter latencies to emit the first USV when compared to subordinate males, 37 , 208 demonstrating the potential of 70 kHz USVs to serve as a measure of social dominance in male mice (refer to Figure 3(E) for schematic of this USV assay).
While rats are also known to emit USVs of various frequencies, the purpose of these USVs is completely different from that in mice. 207 For example, subordinate rats have been shown to exhibit 22 kHz frequency USVs during agonistic interactions in which they are being defeated by a dominant, as well as in response to other aversive stimuli. 207 Additionally, while these 22 kHz frequency USVs may serve as a potential measure of social rank, it can be challenging to identify the precise location of a given USV's source, 209 suggesting that USVs may not be the most reliable measure of social rank in rats since such data are required to be collected while subjects are in close proximity and undergoing agonistic interactions.
8.2. Warm spot test
A more recent study by Zhou and colleagues has developed an additional method of assessing social dominance: the warm spot test. 143 This assay involves two test cages, the floors of which are cooled to 0°C. A group of four male mice is placed in one of the cooled cages for 30 min before being transferred to an additional cooled test cage. In this additional cage, a nestlet 5 cm in diameter is placed in a corner that has been heated to 34°C. Mice remain in this test cage for 20 min while their behavior is recorded. As animals have an innate drive to stay warm, 210 , 211 this assay can be considered a type of resource competition. Thus, dominance is scored based on the amount of time each animal spends in the warm spot of the cage, such that the most dominant animal spends the most time in this area, while the most subordinate animal spends the least amount of time in this area 143 (see Figure 3(F) for schematic of warm spot test). This method warrants future studies examining its reliability in females as well as in other rodent species and strains.
8.3. Shock avoidance
Social rank has also been examined in singly‐housed male C57BL/6 mice using a shock avoidance model. 212 Briefly, a pair of subjects is placed in a neutral apparatus equipped with a grid floor that administers a footshock and an escape platform on which an animal can jump to avoid shock. Because the platform is designed to accommodate only one mouse, a subject is considered to be dominant if it maintains control of the escape platform throughout the test session 212 (see Figure 3(G) for schematic of shock avoidance assay). To our knowledge, this assay has only been used as a measure of social dominance once, with male C57BL/6 mice, so the generalizability of this assay to females as well as to other species and strains of rodents remains unknown.
8.4. Nesting
It has also been demonstrated that dominant female mice (bred on a mixed 129 x Black Swiss background) express different nesting behaviors than their subordinate counterparts. Specifically, subjects identified as dominant based on frequency of offensive agonistic behaviors consistently constructed all nests while also “corralling” subordinate cagemates to these nest areas. 91 This finding suggests that nesting behaviors may serve as a potential measure for social dominance, at least in certain strains of female mice. Though as with the shock avoidance assay outlined above, the applicability of this assay for males and different rodent species or strains remains unknown. Additionally, it must be noted that nesting behavior has been used extensively in other areas of research to assess general well‐being, 213 and even in models of autism spectrum and obsessive–compulsive disorders. 214 These data suggest that much more work is needed to determine whether this behavior is a manifestation of an animal's social rank, an animal model of stereotypies associated with certain human neurological disorders, or a different phenomenon altogether.
9. CONCLUSIONS
The assays outlined above can be thought of to belong to two broad categories used in measuring social dominance: (1) those based on agonistic interactions, or “aggressive” dominance and (2) those based on resource competition, or “competitive” dominance. 215 While it may seem initially surprising that the ranks obtained from these two types of measures do not always positively correlate, the authors refer to an observation made by J. P. Scott in his 1966 review on agonistic behavior in rodents: The agonistic behavior measure is assessing animal‐directed behavior, 216 and can be interpreted as the subject's attempt to decrease subsequent competition for resources. 217 In contrast, the resource competition measure is assessing object‐directed behavior, in that in that a conspecific represents a direct obstacle to a particular resource of interest. 216 Therefore, it can be argued that resource competition assays do not disregard agonistic behavior, rather, that they incorporate the assessment of additional behaviors involved in the drive to obtain resources. 216 Additionally, the propensity to display overt agonistic behaviors varies as a product of sex, species, and strain, suggesting that resource competition assays may serve as a more widely applicable measure of social dominance due to their capacity to be employed in a variety of subject populations.
One of the primary findings of this review was that while a vast amount of the literature has been conducted in the area of social dominance in rodents, the use of female subjects—with the exception of hamsters—remains grossly underrepresented. While this deficit is in part attributable to the less aggressive nature of most female rodents in comparison to males, 218 it is also likely due to the historical disregard of sex as a biological variable in virtually all fields of research. 219 , 220 Therefore, increased studies using resource competition assays, which do not require the display of aggressive behavior, are greatly needed to better characterize to social dominance in females.
We also feel it is important to interpret the findings from these experiments within the larger context of the naturalistic ecology of the rodent species being studied. To quote a review on ecological validity regarding social interaction tests in rodents, “even though not all experiments have to be ecologically valid, ecological perspective is crucial for the design and interpretation of research programs.” 221 In designing and conducting this type of experiment, researchers must appreciate the role of each rodent species' ecology in their social behaviors, as well as how these species' behaviors are potentially altered by years of inbreeding, the laboratory setting, and different housing conditions. 221 , 222
Lastly, the authors would like to emphasize the importance of conservative interpretation of data obtained from these assays, particularly if attempting to make translational applications. We suggest that the measures outlined here are most useful when applied in reference to the specific rodent species used for a given study, rather than as a model for a human condition. Assessments of social dominance best serve to inform researchers about that specific species' behavior and how an individual's social rank may affect other variables with high translational value (i.e., stress responsivity or depressive‐like behavior). Considering all of these factors in both experimental design and data interpretation will contribute greatly to progress in understanding social hierarchies among various rodent species.
CONFLICT OF INTEREST
Authors declare no conflict of interests.
ACKNOWLEDGMENTS
While writing this review, authors were supported by NIH Grants RO1 AA019793, RO1 AA025024 and T32 AA007468. All figures were created using the BioRender software.
Fulenwider HD, Caruso MA, Ryabinin AE. Manifestations of domination: Assessments of social dominance in rodents. Genes, Brain and Behavior. 2022;21:e12731. 10.1111/gbb.12731
Funding information National Institute on Alcohol Abuse and Alcoholism, Grant/Award Numbers: RO1 AA019793, RO1 AA025024, T32 AA007468
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Utevsky AV, Platt ML. Status and the brain. PLoS Biol. 2014;12(9):e1001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chase ID, Seitz K. Self‐structuring properties of dominance hierarchies a new perspective. Adv Genet. 2011;75:51‐81. [DOI] [PubMed] [Google Scholar]
- 3. Drews C. The concept and definition of dominance in animal behaviour. Behaviour. 1993;125(3–4):228‐313. [Google Scholar]
- 4. Baenninger LP. The reliability of dominance orders in rats. Anim Behav. 1966;14(2):367‐371. [DOI] [PubMed] [Google Scholar]
- 5. Blanchard DC, Blanchard RJ. Behavioral correlates of chronic dominance‐subordination relationships of male rats in a seminatural situation. Neurosci Biobehav Rev. 1990;14(4):455‐462. [DOI] [PubMed] [Google Scholar]
- 6. Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103(1):70‐82. [DOI] [PubMed] [Google Scholar]
- 7. Tamashiro KL, Hegeman MA, Nguyen MM, et al. Dynamic body weight and body composition changes in response to subordination stress. Physiol Behav. 2007;91(4):440‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58(1–2):113‐121. [DOI] [PubMed] [Google Scholar]
- 9. Cheng JT. Dominance, prestige, and the role of leveling in human social hierarchy and equality. Curr Opin Psychol. 2020;33:238‐244. [DOI] [PubMed] [Google Scholar]
- 10. Qu C, Ligneul R, Van der Henst JB, Dreher JC. An integrative interdisciplinary perspective on social dominance hierarchies. Trends Cogn Sci. 2017;21(11):893‐908. [DOI] [PubMed] [Google Scholar]
- 11. Kudryavtseva NN. Agonistic behavior: a model, experimental studies, and perspectives. Neurosci Behav Physiol. 2000;30(3):293‐305. [DOI] [PubMed] [Google Scholar]
- 12. Bell R, Hobson H. 5‐HT1A receptor influences on rodent social and agonistic behavior: a review and empirical study. Neurosci Biobehav Rev. 1994;18(3):325‐338. [DOI] [PubMed] [Google Scholar]
- 13. Seward JP. Aggressive behavior in the rat. I. General characteristics; age and sex differences. J Comp Psychol. 1945;38(4):175‐197. [Google Scholar]
- 14. Grant EC, Chance MRA. Rank order in caged rats. Anim Behav. 1958;1(3–4):183‐194. [Google Scholar]
- 15. Brown RZ. Social behavior, reproduction, and population changes in the house mouse (Mus musculus L.). Ecol Monogr. 1953;23(3):217‐240. [Google Scholar]
- 16. Bartolomucci A, Chirieleison A, Gioiosa L, Ceresini G, Parmigiani S, Palanza P. Age at group formation alters behavior and physiology in male but not female CD‐1 mice. Physiol Behav. 2004;82(2–3):425‐434. [DOI] [PubMed] [Google Scholar]
- 17. Blanchard DC, Cholvanich P, Blanchard RJ, et al. Serotonin, but not dopamine, metabolites are increased in selected brain regions of subordinate male rats in a colony environment. Brain Res. 1991;568(1–2):61‐66. [DOI] [PubMed] [Google Scholar]
- 18. Herman JP, Tamashiro KL. The visible burrow system: a view from across the hall. Physiol Behav. 2017;178:103‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hilakivi‐Clarke L, Lister RG. Social status and voluntary alcohol consumption in mice: interaction with stress. Psychopharmacology. 1992;108(3):276‐282. [DOI] [PubMed] [Google Scholar]
- 20. Pellis SM, Pellis VC. Role reversal changes during the ontogeny of play fighting in male rats: attack vs defense. Aggress Behav. 1991;17:179‐189. [Google Scholar]
- 21. Kaliste‐Korhonen E, Eskola S. Fighting in NIH/S male mice: consequences for behaviour in resident‐intruder tests and physiological parameters. Lab Anim. 2000;34(2):189‐198. [DOI] [PubMed] [Google Scholar]
- 22. Pohorecky LA, Skiandos A, Zhang X, Rice KC, Benjamin D. Effect of chronic social stress on delta‐opioid receptor function in the rat. J Pharmacol Exp Ther. 1999;290(1):196‐206. [PubMed] [Google Scholar]
- 23. Pohorecky LA. Acute novel stressors modify ethanol intake of psychosocially stressed rats. Pharmacol Biochem Behav. 2010;95(4):390‐400. [DOI] [PubMed] [Google Scholar]
- 24. Pohorecky LA, Blakley GG, Ma EW, et al. Social housing influences the composition of volatile compounds in the preputial glands of male rats. Horm Behav. 2008;53(4):536‐545. [DOI] [PubMed] [Google Scholar]
- 25. Scott KA, de Kloet AD, Smeltzer MD, et al. Susceptibility or resilience? Prenatal stress predisposes male rats to social subordination, but facilitates adaptation to subordinate status. Physiol Behav. 2017;178:117‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watanabe Y, McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Effects of chronic social stress on tyrosine hydroxylase mRNA and protein levels. Brain Res Mol Brain Res. 1995;32(1):176‐180. [DOI] [PubMed] [Google Scholar]
- 27. McKinney TD, Desjardins C. Intermale stimuli and testicular function in adult and immature house mice. Biol Reprod. 1973;9(4):370‐378. [DOI] [PubMed] [Google Scholar]
- 28. Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182(4115):939‐941. [DOI] [PubMed] [Google Scholar]
- 29. Selmanoff MK, Goldman BD, Ginsburg BE. Serum testosterone, agonistic behavior, and dominance in inbred strains of mice. Horm Behav. 1977;8(1):107‐119. [DOI] [PubMed] [Google Scholar]
- 30. Benton D, Goldsmith JF, Gamal‐el‐Din L, Brain PF, Hucklebridge FH. Adrenal activity in isolated mice and mice of different social status. Physiol Behav. 1978;20(4):459‐464. [DOI] [PubMed] [Google Scholar]
- 31. Raab A, Dantzer R, Michaud B, et al. Behavioural, physiological and immunological consequences of social status and aggression in chronically coexisting resident‐intruder dyads of male rats. Physiol Behav. 1986;36(2):223‐228. [DOI] [PubMed] [Google Scholar]
- 32. Pohorecky LA, Blakley GG, Kubovcakova L, Krizanova O, Patterson‐Buckendahl P, Kvetnansky R. Social hierarchy affects gene expression for catecholamine biosynthetic enzymes in rat adrenal glands. Neuroendocrinology. 2004;80(1):42‐51. [DOI] [PubMed] [Google Scholar]
- 33. DeFries JC, McClearn GE. Social dominance and Darwinian fitness in the laboratory mouse. Am Nat. 1970;104(938):408‐411. [Google Scholar]
- 34. Blakley G, Pohorecky LA. Psychosocial stress alters ethanol's effect on open field behaviors. Pharmacol Biochem Behav. 2006;84(1):51‐61. [DOI] [PubMed] [Google Scholar]
- 35. Kalueff AV, Minasyan A, Keisala T, Shah ZH, Tuohimaa P. Hair barbering in mice: implications for neurobehavioural research. Behav Processes. 2006;71(1):8‐15. [DOI] [PubMed] [Google Scholar]
- 36. Garfield AS, Cowley M, Smith FM, et al. Distinct physiological and behavioural functions for parental alleles of imprinted Grb10. Nature. 2011;469(7331):534‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334(6056):693‐697. [DOI] [PubMed] [Google Scholar]
- 38. Long SY. Hair‐nibbling and whisker‐trimming as indicators of social hierarchy in mice. Anim Behav. 1972;20(1):10‐12. [DOI] [PubMed] [Google Scholar]
- 39. Strozik E, Festing MF. Whisker trimming in mice. Lab Anim. 1981;15(4):309‐312. [DOI] [PubMed] [Google Scholar]
- 40. Sugimoto H, Ikeda K, Kawakami K. Atp1a3‐deficient heterozygous mice show lower rank in the hierarchy and altered social behavior. Genes Brain Behav. 2018;17(5):e12435. [DOI] [PubMed] [Google Scholar]
- 41. Garner JP, Dufour B, Gregg LE, Weisker SM, Mench JA. Social and husbandry factors affecting the prevalence and severity of barbering (‘whisker trimming’) by laboratory mice. Appl Anim Behav Sci. 2004;89:263‐282. [Google Scholar]
- 42. Garner JP, Weisker SM, Dufour B, Mench JA. Barbering (fur and whisker trimming) by laboratory mice as a model of human trichotillomania and obsessive‐compulsive spectrum disorders. Comp Med. 2004;54(2):216‐224. [PubMed] [Google Scholar]
- 43. Bechard A, Meagher R, Mason G. Environmental enrichment reduces the likelihood of alopecia in adult C57BL/6J mice. J Am Assoc Lab Anim Sci. 2011;50(2):171‐174. [PMC free article] [PubMed] [Google Scholar]
- 44. Sarna JR, Dyck RH, Whishaw IQ. The Dalila effect: C57BL6 mice barber whiskers by plucking. Behav Brain Res. 2000;108(1):39‐45. [DOI] [PubMed] [Google Scholar]
- 45. Lindzey G, Manosevitz M, Winston H. Social dominance in the mouse. Psychon Sci. 1966;5(11):451‐452. [Google Scholar]
- 46. Fitchett AE, Barnard CJ, Cassaday HJ. There's no place like home: cage odours and place preference in subordinate CD‐1 male mice. Physiol Behav. 2006;87(5):955‐962. [DOI] [PubMed] [Google Scholar]
- 47. Shimozuru M, Kikusui T, Takeuchi Y, Mori Y. Social‐defeat stress suppresses scent‐marking and social‐approach behaviors in male Mongolian gerbils (Meriones unguiculatus). Physiol Behav. 2006;88(4–5):620‐627. [DOI] [PubMed] [Google Scholar]
- 48. van der Kooij MA, Hollis F, Lozano L, et al. Diazepam actions in the VTA enhance social dominance and mitochondrial function in the nucleus accumbens by activation of dopamine D1 receptors. Mol Psychiatry. 2018;23(3):569‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hollis F, van der Kooij MA, Zanoletti O, Lozano L, Canto C, Sandi C. Mitochondrial function in the brain links anxiety with social subordination. Proc Natl Acad Sci U S A. 2015;112(50):15486‐15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qiao X, Yan Y, Wu R, et al. Sociality and oxytocin and vasopressin in the brain of male and female dominant and subordinate mandarin voles. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2014;200(2):149‐159. [DOI] [PubMed] [Google Scholar]
- 51. Borowski Z, Malinowska A, Ksiazek A. Relationships between dominance, testosterone level and scent marking of males in a free‐living root vole (Microtus oeconomus) population. Physiol Behav. 2014;128:26‐31. [DOI] [PubMed] [Google Scholar]
- 52. Johnston RE. The causation of two scent‐marking behaviour patterns in female hamsters (Mesocricetus auratus). Anim Behav. 1977;25(2):317‐327. [DOI] [PubMed] [Google Scholar]
- 53. Ferris CF, Axelson JF, Shinto LH, Albers HE. Scent marking and the maintenance of dominant/subordinate status in male golden hamsters. Physiol Behav. 1987;40(5):661‐664. [DOI] [PubMed] [Google Scholar]
- 54. Payne AP, Swanson HH. Agonistic behaviour between pairs of hamsters of the same and opposite sex in a neutral observation area. Behaviour. 1970;36(4):260‐269. [PubMed] [Google Scholar]
- 55. Bath KG, Johnston RE. Dominant‐subordinate relationships in hamsters: sex differences in reactions to familiar opponents. Horm Behav. 2007;51(2):258‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morrison KE, Swallows CL, Cooper MA. Effects of dominance status on conditioned defeat and expression of 5‐HT1A and 5‐HT2A receptors. Physiol Behav. 2011;104(2):283‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Morrison KE, Bader LR, Clinard CT, Gerhard DM, Gross SE, Cooper MA. Maintenance of dominance status is necessary for resistance to social defeat stress in Syrian hamsters. Behav Brain Res. 2014;270:277‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muller L, Weinert D. Individual recognition of social rank and social memory performance depends on a functional circadian system. Behav Processes. 2016;132:85‐93. [DOI] [PubMed] [Google Scholar]
- 59. Lee CT, Naranjo JN. The effects of castration and androgen on the social dominance of BALB/cJ male mice. Physiol Psychol. 1974;2(1):93‐98. [Google Scholar]
- 60. Drickamer LC, Vandenbergh JG, Colby DR. Predictors of dominance in the male golden hamster (Mesocricetus auratus). Anim Behav. 1973;21(3):557‐563. [DOI] [PubMed] [Google Scholar]
- 61. Drickamer LC, Vandenbergh JG. Predictors of social dominance in the adult female golden hamster (Mesocricetus auratus). Anim Behav. 1973;21:564‐570. [DOI] [PubMed] [Google Scholar]
- 62. Johnston RE. Scent marking by male Golden hamsters (Mesocricetus auratus) III. Behavior in a seminatural environment. Z Tierpsychol. 1975;37(2):213‐221. [PubMed] [Google Scholar]
- 63. D'Amato FR, Rizzi R, Moles A. A model of social stress in dominant mice: effects on sociosexual behaviour. Physiol Behav. 2001;73(3):421‐426. [DOI] [PubMed] [Google Scholar]
- 64. Moles A, Cooper SJ. Opioid modulation of sucrose intake in CD‐1 mice: effects of gender and housing conditions. Physiol Behav. 1995;58(4):791‐796. [DOI] [PubMed] [Google Scholar]
- 65. Povoa CP, Brandeburgo MA. Study of the social hierarchy and territoriality of Calomys callosus Rengger, 1830 (Rodentia: Cricetidae). Braz J Biol. 2007;67(3):429‐432. [DOI] [PubMed] [Google Scholar]
- 66. Steensland P, Blakely G, Nyberg F, Fahlke C, Pohorecky LA. Anabolic androgenic steroid affects social aggression and fear‐related behaviors in male pair‐housed rats. Horm Behav. 2005;48(2):216‐224. [DOI] [PubMed] [Google Scholar]
- 67. Yamaguchi H, Kikusui T, Takeuchi Y, Yoshimura H, Mori Y. Social stress decreases marking behavior independently of testosterone in Mongolian gerbils. Horm Behav. 2005;47(5):549‐555. [DOI] [PubMed] [Google Scholar]
- 68. Huck UW, Lisk RD, McKay MV. Social dominance and reproductive success in pregnant and lactating golden hamsters (Mesocricetus auratus) under seminatural conditions. Physiol Behav. 1988;44(3):313‐319. [DOI] [PubMed] [Google Scholar]
- 69. Bartolomucci A, Palanza P, Gaspani L, et al. Social status in mice: behavioral, endocrine and immune changes are context dependent. Physiol Behav. 2001;73(3):401‐410. [DOI] [PubMed] [Google Scholar]
- 70. Lyon KA, Rood BD, Wu L, Senft RA, Goodrich LV, Dymecki SM. Sex‐specific role for dopamine receptor D2 in dorsal raphe serotonergic neuron modulation of defensive acoustic startle and dominance behavior. eNeuro. 2020;7(6):1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee W, Khan A, Curley JP. Major urinary protein levels are associated with social status and context in mouse social hierarchies. Proc Biol Sci. 2017;284(1863):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee W, Hiura LC, Yang E, Broekman KA, Ophir AG, Curley JP. Social status in mouse social hierarchies is associated with variation in oxytocin and vasopressin 1a receptor densities. Horm Behav. 2019;114:104551. [DOI] [PubMed] [Google Scholar]
- 73. Martinez‐Cue C, Rueda N, Garcia E, Davisson MT, Schmidt C, Florez J. Behavioral, cognitive and biochemical responses to different environmental conditions in male Ts65Dn mice, a model of down syndrome. Behav Brain Res. 2005;163(2):174‐185. [DOI] [PubMed] [Google Scholar]
- 74. Van Loo PLP, de Groot AC, Van Zutphen BFM, Baumans V. Do male mice prefer or avoid each Other's company? Influence of hierarchy, kinship, and familiarity. J Appl Anim Welf Sci. 2001;4(2):91‐103. [Google Scholar]
- 75. Williamson CM, Lee W, Curley JP. Temporal dynamics of social hierarchy formation and maintenance in male mice. Anim Behav. 2016;115:259‐272. [Google Scholar]
- 76. Williamson CM, Franks B, Curley JP. Mouse social network dynamics and community structure are associated with plasticity‐related brain gene expression. Front Behav Neurosci. 2016;10:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Williamson CM, Romeo RD, Curley JP. Dynamic changes in social dominance and mPOA GnRH expression in male mice following social opportunity. Horm Behav. 2017;87:80‐88. [DOI] [PubMed] [Google Scholar]
- 78. Williamson CM, Lee W, Romeo RD, Curley JP. Social context‐dependent relationships between mouse dominance rank and plasma hormone levels. Physiol Behav. 2017;171:110‐119. [DOI] [PubMed] [Google Scholar]
- 79. Williamson CM, Klein IS, Lee W, Curley JP. Immediate early gene activation throughout the brain is associated with dynamic changes in social context. Soc Neurosci. 2019;14(3):253‐265. [DOI] [PubMed] [Google Scholar]
- 80. Machida T, Yonezawa Y, Noumura T. Age‐associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Horm Behav. 1981;15(3):238‐245. [DOI] [PubMed] [Google Scholar]
- 81. Bronson FH. Establishment of social rank among grouped male mice: relative effects on circulating FSH, LH, and corticosterone. Physiol Behav. 1973;10(5):947‐951. [DOI] [PubMed] [Google Scholar]
- 82. Jones RB, Nowell NW. A comparison of the aversive and female attractant properties of urine from dominant and subordinate male mice. Anim Learn Behav. 1974;2(2):141‐144. [DOI] [PubMed] [Google Scholar]
- 83. Nevison CM, Armstrong S, Beynon RJ, Humphries RE, Hurst JL. The ownership signature in mouse scent marks is involatile. Proc Biol Sci. 2003;270(1527):1957‐1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nevison CM, Barnard CJ, Beynon RJ, Hurst JL. The consequences of inbreeding for recognizing competitors. Proc Biol Sci. 2000;267(1444):687‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Drickamer LC. Urine marking and social dominance in male house mice (Mus musculus domesticus). Behav Processes. 2001;53(1–2):113‐120. [DOI] [PubMed] [Google Scholar]
- 86. Uhrich J. The social hierarchy in albino mice. J Comp Psychol. 1938;25(2):373‐413. [Google Scholar]
- 87. Lockwood JA, Turney TH. Social dominance and stress‐induced hypertension: strain differences in inbred mice. Physiol Behav. 1981;26(3):547‐549. [DOI] [PubMed] [Google Scholar]
- 88. Cohn DW, Gabanyi I, Kinoshita D, de Sa‐Rocha LC. Lipopolysaccharide administration in the dominant mouse destabilizes social hierarchy. Behav Processes. 2012;91(1):54‐60. [DOI] [PubMed] [Google Scholar]
- 89. So N, Franks B, Lim S, Curley JP. A social network approach reveals associations between mouse social dominance and brain gene expression. PLoS One. 2015;10(7):e0134509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lee W, Yang E, Curley JP. Foraging dynamics are associated with social status and context in mouse social hierarchies. Peer J. 2018;6:e5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ragnauth AK, Devidze N, Moy V, et al. Female oxytocin gene‐knockout mice, in a semi‐natural environment, display exaggerated aggressive behavior. Genes Brain Behav. 2005;4(4):229‐239. [DOI] [PubMed] [Google Scholar]
- 92. Williamson CM, Lee W, DeCasien AR, Lanham A, Romeo RD, Curley JP. Social hierarchy position in female mice is associated with plasma corticosterone levels and hypothalamic gene expression. Sci Rep. 2019;9(1):7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pohorecky LA. Housing and rank status of male Long‐Evans rats modify ethanol's effect on open‐field behaviors. Psychopharmacology. 2006;185(3):289‐297. [DOI] [PubMed] [Google Scholar]
- 94. Takahashi LK, Lore RK. Intermale aggression of subordinate resident long‐Evans rats. Behav Processes. 1983;8(1):21‐32. [DOI] [PubMed] [Google Scholar]
- 95. Mitchell PJ, Redfern PH. Chronic treatment with clomipramine and mianserin increases the hierarchical position of subdominant rats housed in triads. Behav Pharmacol. 1992;3(3):239‐247. [PubMed] [Google Scholar]
- 96. Baenninger LP. Social Dominane orders in the rat: “Spontaneuous,” food, and water competition. J Comp Physiol Psychol. 1970;71(2):202‐209. [Google Scholar]
- 97. Ellison G, Levy A, Lorant N. Alcohol‐preferring rats in colonies show withdrawal, inactivity, and lowered dominance. Pharmacol Biochem Behav. 1983;18(Suppl 1):565‐570. [DOI] [PubMed] [Google Scholar]
- 98. Pohorecky LA, Baumann MH, Benjamin D. Effects of chronic social stress on neuroendocrine responsiveness to challenge with ethanol, dexamethasone and corticotropin‐releasing hormone. Neuroendocrinology. 2004;80(5):332‐342. [DOI] [PubMed] [Google Scholar]
- 99. Rozenfeld FM, Rasmont R. Odour cue recognition by dominant male bank voles, Clethrionomys glareolus . Anim Behav. 1991;41:839‐850. [Google Scholar]
- 100. Chelini MO, Palme R, Otta E. Social stress and reproductive success in the female Syrian hamster: endocrine and behavioral correlates. Physiol Behav. 2011;104(5):948‐954. [DOI] [PubMed] [Google Scholar]
- 101. Fritzsche P, Riek M, Gattermann R. Effects of social stress on behavior and corpus luteum in female golden hamsters (Mesocricetus auratus). Physiol Behav. 2000;68(5):625‐630. [DOI] [PubMed] [Google Scholar]
- 102. Haemisch A, Voss T, Gartner K. Effects of environmental enrichment on aggressive behavior, dominance hierarchies, and endocrine states in male DBA/2J mice. Physiol Behav. 1994;56(5):1041‐1048. [DOI] [PubMed] [Google Scholar]
- 103. Lore RK, Stipo‐Flaherty A. Postweaning social experience and adult aggression in rats. Physiol Behav. 1984;33(4):571‐574. [DOI] [PubMed] [Google Scholar]
- 104. Takahashi LK. Postweaning environmental and social factors influencing the onset and expression of agonistic behavior in Norway rats. Behav Processes. 1986;12(3):237‐260. [DOI] [PubMed] [Google Scholar]
- 105. Kruczek M, Styrna J. Semen quantity and quality correlate with bank vole males' social status. Behav Processes. 2009;82(3):279‐285. [DOI] [PubMed] [Google Scholar]
- 106. Kruczek M. Male rank and female choice in the bank vole Clethrionomys glareolus . Behav Processes. 1997;40(2):171‐176. [DOI] [PubMed] [Google Scholar]
- 107. Kruczek M, Zatorska M. Male rank affects reproductive success and offspring performance in bank voles. Physiol Behav. 2008;94(4):611‐615. [DOI] [PubMed] [Google Scholar]
- 108. Berdoy M, Smith P, Macdonald DW. Stability of social status in wild rats: age and the role of settled dominance. Behaviour. 1995;132(3/4):193‐212. [Google Scholar]
- 109. Blanchard DC, Fukunaga‐Stinson C, Takahashi LK, Flannelly KJ, Blanchard RJ. Dominance and aggression in social groups of male and female rats. Behav Processes. 1984;9(1):31‐48. [DOI] [PubMed] [Google Scholar]
- 110. Blanchard RJ, Takahashi LK, Blanchard DC. The development of intruder attack in colonies of laboratory rats. Anim Learn Behav. 1977;5(4):365‐369. [Google Scholar]
- 111. Blanchard RJ, Flannelly KJ, Blanchard DC. Life‐span studies of dominance and aggression in established colonies of laboratory rats. Physiol Behav. 1988;43(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 112. Stefanski V, Knopf G, Schulz S. Long‐term colony housing in Long Evans rats: immunological, hormonal, and behavioral consequences. J Neuroimmunol. 2001;114(1–2):122‐130. [DOI] [PubMed] [Google Scholar]
- 113. Stefanski V, Engler H. Social stress, dominance and blood cellular immunity. J Neuroimmunol. 1999;94(1–2):144‐152. [DOI] [PubMed] [Google Scholar]
- 114. Turney TH, Hunt EF, Money VM. Systolic blood pressure during the formation of a social dominance hierarchy in C57BL/6j mice. Physiol Behav. 1983;31(3):299‐301. [DOI] [PubMed] [Google Scholar]
- 115. De Goeij DC, Dijkstra H, Tilders FJ. Chronic psychosocial stress enhances vasopressin, but not corticotropin‐releasing factor, in the external zone of the median eminence of male rats: relationship to subordinate status. Endocrinology. 1992;131(2):847‐853. [DOI] [PubMed] [Google Scholar]
- 116. Albeck DS, McKittrick CR, Blanchard DC, et al. Chronic social stress alters levels of corticotropin‐releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci. 1997;17(12):4895‐4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Boersma GJ, Smeltzer MD, Scott KA, Scheurink AJ, Tamashiro KL, Sakai RR. Stress coping style does not determine social status, but influences the consequences of social subordination stress. Physiol Behav. 2017;178:126‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Buwalda B, Koolhaas JM, de Boer SF. Trait aggressiveness does not predict social dominance of rats in the visible burrow system. Physiol Behav. 2017;178:134‐143. [DOI] [PubMed] [Google Scholar]
- 119. Choi DC, Nguyen MM, Tamashiro KL, Ma LY, Sakai RR, Herman JP. Chronic social stress in the visible burrow system modulates stress‐related gene expression in the bed nucleus of the stria terminalis. Physiol Behav. 2006;89(3):301‐310. [DOI] [PubMed] [Google Scholar]
- 120. Blanchard RJ, Blanchard DC, Flannelly KJ. Social stress, mortality and aggression in colonies and burrowing habitats. Behav Processes. 1985;11(2):209‐213. [DOI] [PubMed] [Google Scholar]
- 121. Thiessen DD, Owen K, Lindzey G. Mechanisms of territorial marking in the male and female Mongolian gerbil (Meriones unguiculatus). J Comp Physiol Psychol. 1971;77(1):38‐47. [DOI] [PubMed] [Google Scholar]
- 122. Lucas LR, Celen Z, Tamashiro KL, et al. Repeated exposure to social stress has long‐term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124(2):449‐457. [DOI] [PubMed] [Google Scholar]
- 123. Makinson R, Lundgren KH, Seroogy KB, Herman JP. Chronic social subordination stress modulates glutamic acid decarboxylase (GAD) 67 mRNA expression in central stress circuits. Physiol Behav. 2015;146:7‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36(2):85‐94. [DOI] [PubMed] [Google Scholar]
- 125. Melhorn SJ, Elfers CT, Scott KA, Sakai RR. A closer look at the subordinate population within the visible burrow system. Physiol Behav. 2017;178:110‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nguyen MM, Tamashiro KL, Melhorn SJ, Ma LY, Gardner SR, Sakai RR. Androgenic influences on behavior, body weight, and body composition in a model of chronic social stress. Endocrinology. 2007;148(12):6145‐6156. [DOI] [PubMed] [Google Scholar]
- 127. Tamashiro KL, Nguyen MM, Fujikawa T, et al. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav. 2004;80(5):683‐693. [DOI] [PubMed] [Google Scholar]
- 128. Weissbrod A, Shapiro A, Vasserman G, et al. Automated long‐term tracking and social behavioural phenotyping of animal colonies within a semi‐natural environment. Nat Commun. 2013;4:2018. [DOI] [PubMed] [Google Scholar]
- 129. Kozorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J Neurosci. 2004;24(30):6755‐6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Payne AP, Swanson HH. Hormonal control of aggressive dominance in the female hamster. Physiol Behav. 1971;6(4):355‐357. [DOI] [PubMed] [Google Scholar]
- 131. Lindzey G, Winston H, Manosevitz M. Social dominance in inbred mouse strains. Nature. 1961;191:474‐476. [DOI] [PubMed] [Google Scholar]
- 132. Fan Z, Zhu H, Zhou T, Wang S, Wu Y, Hu H. Using the tube test to measure social hierarchy in mice. Nat Protoc. 2019;14(3):819‐831. [DOI] [PubMed] [Google Scholar]
- 133. Wilson JW. Adaptation to the dominance tube. Psychonom Sci. 1968;10(3):119‐120. [Google Scholar]
- 134. Grossman SP. Aggression, avoidance, and reaction to novel environments in female rats with ventromedial hypothalamic lesions. J Comp Physiol Psychol. 1972;78(2):274‐283. [DOI] [PubMed] [Google Scholar]
- 135. Boice R. Social dominance in gerbils and hamsters. Psychonom Sci. 1969;16(3):127‐128. [Google Scholar]
- 136. Messeri P, Eleftheriou BE, Oliverio A. Dominance behavior: a phylogenetic analysis in the mouse. Physiol Behav. 1975;14(1):53‐58. [DOI] [PubMed] [Google Scholar]
- 137. Howerton CL, Garner JP, Mench JA. Effects of a running wheel‐igloo enrichment on aggression, hierarchy linearity, and stereotypy in group‐housed male CD‐1 (ICR) mice. Appl Anim Behav Sci. 2008;115:90‐103. [Google Scholar]
- 138. Kim B, Colon E, Chawla S, Vandenberg LN, Suvorov A. Endocrine disruptors alter social behaviors and indirectly influence social hierarchies via changes in body weight. Environ Health. 2015;14:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. van den Berg WE, Lamballais S, Kushner SA. Sex‐specific mechanism of social hierarchy in mice. Neuropsychopharmacology. 2015;40(6):1364‐1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yamaguchi Y, Lee YA, Kato A, Jas E, Goto Y. The roles of dopamine D2 receptor in the social hierarchy of rodents and primates. Sci Rep. 2017;7:43348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yamaguchi Y, Lee YA, Kato A, Goto Y. The roles of dopamine D1 receptor on the social hierarchy of rodents and nonhuman primates. Int J Neuropsychopharmacol. 2017;20(4):324‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Larrieu T, Cherix A, Duque A, et al. Hierarchical status predicts behavioral vulnerability and nucleus accumbens metabolic profile following chronic social defeat stress. Curr Biol. 2017;27(14):2202‐2210. [DOI] [PubMed] [Google Scholar]
- 143. Zhou T, Zhu H, Fan Z, et al. History of winning remodels thalamo‐PFC circuit to reinforce social dominance. Science. 2017;357(6347):162‐168. [DOI] [PubMed] [Google Scholar]
- 144. Kunkel T, Wang H. Socially dominant mice in C57BL6 background show increased social motivation. Behav Brain Res. 2018;336:173‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lee YA, Goto Y. The roles of serotonin in decision‐making under social group conditions. Sci Rep. 2018;8(1):10704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Stagkourakis S, Spigolon G, Williams P, Protzmann J, Fisone G, Broberger C. A neural network for intermale aggression to establish social hierarchy. Nat Neurosci. 2018;21(6):834‐842. [DOI] [PubMed] [Google Scholar]
- 147. Varholick JA, Pontiggia A, Murphy E, et al. Social dominance hierarchy type and rank contribute to phenotypic variation within cages of laboratory mice. Sci Rep. 2019;9(1):13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Varholick JA, Bailoo JD, Palme R, Wurbel H. Phenotypic variability between social dominance ranks in laboratory mice. Sci Rep. 2018;8(1):6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kingsbury L, Huang S, Wang J, et al. Correlated neural activity and encoding of behavior across brains of socially interacting animals. Cell. 2019;178(2):429‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Anacker AM, Smith ML, Ryabinin AE. Establishment of stable dominance interactions in prairie vole peers: relationships with alcohol drinking and activation of the paraventricular nucleus of the hypothalamus. Soc Neurosci. 2014;9(5):484‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Jupp B, Murray JE, Jordan ER, et al. Social dominance in rats: effects on cocaine self‐administration, novelty reactivity and dopamine receptor binding and content in the striatum. Psychopharmacology. 2016;233(4):579‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ji MJ, Zhang XY, Chen Z, Wang JJ, Zhu JN. Orexin prevents depressive‐like behavior by promoting stress resilience. Mol Psychiatry. 2019;24(2):282‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Gammell M, de Vries H, Jennings DJ, Carlin CM, Hayden TJ. David's score: a more appropriate dominance ranking method than Clutton‐Brock et al.’s index. Anim Behav. 2003;66:601‐605. [Google Scholar]
- 154. Fuxjager MJ, Knaebe B, Marler CA. A single testosterone pulse rapidly reduces urinary marking behaviour in subordinate, but not dominant, white‐footed mice. Anim Behav. 2015;100:8‐14. [Google Scholar]
- 155. Hurst JL. Urine marking in populations of wild house mice Mus domesticus Rutty. I. Communication between males. Anim Behav. 1990;40:209‐222. [Google Scholar]
- 156. Jemiolo B, Xie TM, Novotny M. Urine marking in male mice: responses to natural and synthetic chemosignals. Physiol Behav. 1992;52(3):521‐526. [DOI] [PubMed] [Google Scholar]
- 157. Hurst JL. Urine marking in populations of wild house mice Mus domesticus Rutty. II. Communication between females. Anim Behav. 1990;40:223‐232. [Google Scholar]
- 158. Horne TJ, Ylonen H. Heritabilities of dominance‐related traits in male Bank voles (Clethrionomys Glareolus). Evolution. 1998;52(3):894‐899. [DOI] [PubMed] [Google Scholar]
- 159. Horne TJ, Ylonen H. Female bank voles (Clethrionomys glareolus) preer dominant males; but what if there is no choice? Behav Ecol Sociobiol. 1996;38:401‐405. [Google Scholar]
- 160. Huck UW, Lisk RD, Gore AC. Scent marking and mate choice in the golden hamster. Physiol Behav. 1985;35(3):389‐393. [DOI] [PubMed] [Google Scholar]
- 161. Hyer MM, Rycek LM, Floody OR. Effects of apomorphine on mating behavior, flank marking and aggression in male hamsters. Pharmacol Biochem Behav. 2012;101(4):520‐527. [DOI] [PubMed] [Google Scholar]
- 162. Pan Y, Xu L, Young KA, Wang Z, Zhang Z. Agonistic encounters and brain activation in dominant and subordinate male greater long‐tailed hamsters. Horm Behav. 2010;58(3):478‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Wallace P, Owen K, Thiessen DD. The control and function of maternal scent marking the Mongolian gerbil. Physiol Behav. 1973;10(3):463‐466. [DOI] [PubMed] [Google Scholar]
- 164. Rosen J. Dominance behaviour as a function of post‐weaning gentling in the albino rat. Can J Psychol. 1958;12(4):229‐234. [DOI] [PubMed] [Google Scholar]
- 165. Kostowski W, Plewako M, Bidzinski A. Brain serotonergic neurons: their role in a form of dominance‐subordination behavior in rats. Physiol Behav. 1984;33(3):365‐371. [DOI] [PubMed] [Google Scholar]
- 166. Uyeno ET. Hereditary and environmental aspects of dominant behavior in the albino rat. J Comp Physiol Psychol. 1960;53(2):138‐141. [Google Scholar]
- 167. Seward JP. Aggressive behavior in the rat. III. The role of frustration. J Comp Psychol. 1945;38(4):225‐238. [Google Scholar]
- 168. Plewako M, Kostowski W. The effects of lesions of the locus coeruleus and treatment with drugs affecting brain noradrenergic neurotransmission on dominant‐subordinate behavior in rats competing for water. Pol J Pharmacol Pharm. 1984;36(5):555‐560. [PubMed] [Google Scholar]
- 169. Candland DK, Bloomquist DW. Interspecies comparisons of the reliability of dominance orders. J Comp Physiol Psychol. 1965;59:135‐137. [DOI] [PubMed] [Google Scholar]
- 170. Mitchell JA, Lewis RM, Wilson MC. The effects of d‐amphetamine on food competition in male rats. Pharmacol Biochem Behav. 1987;27(4):707‐714. [DOI] [PubMed] [Google Scholar]
- 171. Bruce RH. An experimental analysis of social factors affecting the performance of white rats. III. Dominance and cooperation motivated by water and food deprivation. J Comp Psychol. 1941;31(2):395‐412. [Google Scholar]
- 172. Becker G, Flaherty TB. Group size as a determinant of dominance‐hierarchy stability in the rat. J Comp Physiol Psychol. 1968;66(2):473‐476. [DOI] [PubMed] [Google Scholar]
- 173. Saxton KB, John‐Henderson N, Reid MW, Francis DD. The social environment and IL‐6 in rats and humans. Brain Behav Immun. 2011;25(8):1617‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Endo T, Kakeyama M, Uemura Y, et al. Executive function deficits and social‐behavioral abnormality in mice exposed to a low dose of dioxin in utero and via lactation. PLoS One. 2012;7(12):e50741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Becker G. Social dominance and subordination in the rat as a function of postweaning electrical stimulation. J Genet Psychol. 1965;107:349‐369. [DOI] [PubMed] [Google Scholar]
- 176. Becker GaE G. Early‐handling and social‐rearing effects on dominance‐subordination behavior in the adult rat. Psychonom Sci. 1969;15(1):27‐28. [Google Scholar]
- 177. Schumsky DaJ PD. Reliable paired comparison dominane orders in rats. Psychol Rec. 1966;16:473‐478. [Google Scholar]
- 178. Mowrer OH. Animal studies in the genesis of personality. Trans N Y Acad Sci. 1940;3:8‐11. [Google Scholar]
- 179. Timmer M, Cordero MI, Sevelinges Y, Sandi C. Evidence for a role of oxytocin receptors in the long‐term establishment of dominance hierarchies. Neuropsychopharmacology. 2011;36(11):2349‐2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Edalat P, Kavianpour M, Zarrabian S, Haghparast A. Role of orexin‐1 and orexin‐2 receptors in the CA1 region of hippocampus in the forced swim stress‐and food deprivation‐induced reinstatement of morphine seeking behaviors in rats. Brain Res Bull. 2018;142:25‐32. [DOI] [PubMed] [Google Scholar]
- 181. Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res. 1999;830(1):56‐71. [DOI] [PubMed] [Google Scholar]
- 182. Soblosky JS, Thurmond JB. Biochemical and behavioral correlates of chronic stress: effects of tricyclic antidepressants. Pharmacol Biochem Behav. 1986;24(5):1361‐1368. [DOI] [PubMed] [Google Scholar]
- 183. Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5(2):247‐251. [DOI] [PubMed] [Google Scholar]
- 184. Dietze S, Lees KR, Fink H, Brosda J, Voigt JP. Food deprivation, body weight loss and anxiety‐related behavior in rats. Animals. 2016;6(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Yadawa AK, Chaturvedi CM. Expression of stress hormones AVP and CRH in the hypothalamus of Mus musculus following water and food deprivation. Gen Comp Endocrinol. 2016;239:13‐20. [DOI] [PubMed] [Google Scholar]
- 186. Merlot E, Moze E, Bartolomucci A, Dantzer R, Neveu PJ. The rank assessed in a food competition test influences subsequent reactivity to immune and social challenges in mice. Brain Behav Immun. 2004;18(5):468‐475. [DOI] [PubMed] [Google Scholar]
- 187. Watanabe S. The dominant/subordinate relationship between mice modifies the approach behavior toward a cage mate experiencing pain. Behav Processes. 2014;103:1‐4. [DOI] [PubMed] [Google Scholar]
- 188. de Jong TR, Korosi A, Harris BN, Perea‐Rodriguez JP, Saltzman W. Individual variation in paternal responses of virgin male California mice (Peromyscus californicus): behavioral and physiological correlates. Physiol Biochem Zool. 2012;85(6):740‐751. [DOI] [PubMed] [Google Scholar]
- 189. Woodall KL, Domeney AM, Kelly ME. Selective effects of 8‐OH‐DPAT on social competition in the rat. Pharmacol Biochem Behav. 1996;54(1):169‐173. [DOI] [PubMed] [Google Scholar]
- 190. File SE. Effects of chlordiazepoxide on competition for a preferred food in the rat. Behav Brain Res. 1986;21(3):195‐202. [DOI] [PubMed] [Google Scholar]
- 191. Askew A, Gonzalez FA, Stahl JM, Karom MC. Food competition and social experience effects on V1a receptor binding in the forebrain of male Long‐Evans hooded rats. Horm Behav. 2006;49(3):328‐336. [DOI] [PubMed] [Google Scholar]
- 192. Cumming MJ, Thompson MA, McCormick CM. Adolescent social instability stress increases aggression in a food competition task in adult male long‐Evans rats. Dev Psychobiol. 2014;56(7):1575‐1588. [DOI] [PubMed] [Google Scholar]
- 193. Joly D, Sanger DJ. Social competition in rats: a test sensitive to acutely administered anxiolytics. Behav Pharmacol. 1991;2(3):205‐213. [PubMed] [Google Scholar]
- 194. Joly D, Sanger DJ. Social competition in dominant rats can be attenuated by anxiogenic drugs. Behav Pharmacol. 1992;3(1):83‐88. [DOI] [PubMed] [Google Scholar]
- 195. Millard A, Gentsch C. Competition for sucrose pellets in tetrads of male Wistar, Fischer or Sprague‐Dawley rats: is intra‐group ranking reflected in the level of anxiety? Behav Brain Res. 2006;168(2):243‐254. [DOI] [PubMed] [Google Scholar]
- 196. Gentsch C, Lichtsteiner M, Feer H. Competition for sucrose‐pellets in triads of male Wistar rats: the individuals' performances are differing but stable. Behav Brain Res. 1988;27(1):37‐44. [DOI] [PubMed] [Google Scholar]
- 197. Gentsch C, Lichtsteiner M, Feer H. Competition for sucrose‐pellets in triads of male Wistar rats: effects of three serotonergic drugs. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(5):639‐651. [DOI] [PubMed] [Google Scholar]
- 198. Gentsch C, Lichtsteiner M, Feer H. Competition for sucrose‐pellets in triads of male Wistar rats: effects of acute and subchronic chlordiazepoxide. Psychopharmacology. 1990;100(4):530‐534. [DOI] [PubMed] [Google Scholar]
- 199. Gentsch C, Lichtsteiner M, Feer H. Competition for sucrose‐pellets in triads of male Wistar rats: disinhibitory effect of individual housing in poor‐performing rats. Behav Brain Res. 1990;38(1):19‐24. [DOI] [PubMed] [Google Scholar]
- 200. Malatynska E, Pinhasov A, Crooke JJ, Smith‐Swintosky VL, Brenneman DE. Reduction of dominant or submissive behaviors as models for antimanic or antidepressant drug testing: technical considerations. J Neurosci Methods. 2007;165(2):175‐182. [DOI] [PubMed] [Google Scholar]
- 201. Hoshaw BA, Evans JC, Mueller B, Valentino RJ, Lucki I. Social competition in rats: cell proliferation and behavior. Behav Brain Res. 2006;175(2):343‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Leo GC, Caldwell GW, Crooke J, et al. The application of nuclear magnetic resonance‐based metabonomics to the dominant‐submissive rat behavioral model. Anal Biochem. 2005;339(1):174‐178. [DOI] [PubMed] [Google Scholar]
- 203. Malatynska E, Kostowski W. The effect of antidepressant drugs on dominance behavior in rats competing for food. Pol J Pharmacol Pharm. 1984;36(5):531‐540. [PubMed] [Google Scholar]
- 204. Li FH, Zhong WQ, Wang Z, Wang DH. Rank in a food competition test and humoral immune functions in male Brandt's voles (Lasiopodomys brandtii). Physiol Behav. 2007;90(2–3):490‐495. [DOI] [PubMed] [Google Scholar]
- 205. Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2(8):e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Sclafani A. Carbohydrate taste, appetite, and obesity: an overview. Neurosci Biobehav Rev. 1987;11(2):131‐153. [PubMed] [Google Scholar]
- 207. Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46(1):28‐34. [PubMed] [Google Scholar]
- 208. Nyby J, Dizinno GA, Whitney G. Social status and ultrasonic vocalizations of male mice. Behav Biol. 1976;18(2):285‐289. [DOI] [PubMed] [Google Scholar]
- 209. Brudzynski SM. Communication of adult rats by ultrasonic vocalization: biological, sociobiological, and neuroscience approaches. ILAR J. 2009;50(1):43‐50. [DOI] [PubMed] [Google Scholar]
- 210. Ganeshan K, Chawla A. Warming the mouse to model human diseases. Nat Rev Endocrinol. 2017;13(8):458‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kumar D, Mallick HN, Kumar VM. Ambient temperature that induces maximum sleep in rats. Physiol Behav. 2009;98(1–2):186‐191. [DOI] [PubMed] [Google Scholar]
- 212. Bevan W, Daves WF, Levy GW. The relation of castration, androgen therapy and pre‐test fighting experience to competitive aggression in male C57 BL/10 mice. Anim Behav. 1960;8(1–2):6‐12. [Google Scholar]
- 213. Jirkof P. Burrowing and nest building behavior as indicators of well‐being in mice. J Neurosci Methods. 2014;234:139‐146. [DOI] [PubMed] [Google Scholar]
- 214. Angoa‐Perez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM. Marble burying and nestlet shredding as tests of repetitive, compulsive‐like behaviors in mice. J Vis Exp. 2013;82:50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Syme GJ. Competitive orders as measures of social dominance. Anim Behav. 1974;22:931‐940. [Google Scholar]
- 216. Scott JP. Agonistic behavior of mice and rats: a review. Am Zool. 1966;6(4):683‐701. [DOI] [PubMed] [Google Scholar]
- 217. Blanchard DC, Blanchard RJ. What can animal aggression research tell us about human aggression? Horm Behav. 2003;44(3):171‐177. [DOI] [PubMed] [Google Scholar]
- 218. Hashikawa K, Hashikawa Y, Lischinsky J, Lin D. The neural mechanisms of sexually dimorphic aggressive behaviors. Trends Genet. 2018;34(10):755‐776. [DOI] [PubMed] [Google Scholar]
- 219. Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35(3):565‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Zucker I, Beery AK. Males still dominate animal studies. Nature. 2010;465(7299):690. [DOI] [PubMed] [Google Scholar]
- 221. Kondrakiewicz K, Kostecki M, Szadzinska W, Knapska E. Ecological validity of social interaction tests in rats and mice. Genes Brain Behav. 2019;18(1):e12525. [DOI] [PubMed] [Google Scholar]
- 222. Berdoy MaD LC. Comparative social organization and life history of Rattus and Mus . In: Wolff JaS PW, ed. Rodent Societies: an Ecological and Evolutionary Perspective. Chicago, IL: University of Chicago Press; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


