Abstract
Objective:
Avoidant/restrictive food intake disorder (ARFID) occurs across the weight spectrum, however research addressing the coexistence of ARFID with overweight/obesity (OV/OB) is lacking. We aimed to establish co-occurrence of OV/OB and ARFID and to characterize divergent neurobiological features of ARFID by weight.
Method:
Youth with full/subthreshold ARFID (12 with healthy weight [HW], 11 with OV/OB) underwent fasting brain fMRI scan while viewing food/non-food images (M age = 16.92 years, 65% female, 87% white). We compared groups on BOLD response to high-calorie foods (HCF) (vs. objects) in food cue processing regions of interest. Following fMRI scanning, we evaluated subjective hunger pre- vs. post-meal. We used a mediation model to explore the association between BMI, brain activation and hunger.
Results:
Participants with ARFID and OV/OB demonstrated significant hyperactivation in response to HCF (vs. objects) in the orbitofrontal cortex (OFC) and anterior insula compared with HW subjects with ARFID. Mediation analysis yielded a significant indirect effect of group (HW vs. OV/OB) on hunger via OFC activation (effect=18.39, SE=11.27, 95% CI [−45.09, −3.00]), suggesting that OFC activation mediates differences in hunger between ARFID participants with HW and OV/OB.
Conclusions:
Compared to youth with ARFID and HW, those with OV/OB demonstrate hyperactivation of brain areas critical for reward value of food cues. Postprandial changes in subjective hunger depend on BMI and are mediated by OFC activation to food cues. Whether these neurobiological differences contribute to selective hyperphagia in ARFID presenting with OV/OB and represent potential treatment targets is an important area for future investigation.
Keywords: Avoidant/restrictive food intake disorder, ARFID, functional magnetic resonance imaging, fMRI, obesity, reward, orbitofrontal cortex
Introduction
Avoidant/restrictive food intake disorder (ARFID) is a lifespan diagnosis that was first introduced in the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (APA, 2013). Clinical observations that ARFID spans the weight spectrum and can continue into or develop in adulthood led to the reformulation of Feeding Disorder of Infancy and Early Childhood (DSM-IV) to ARFID (DSM-5) (APA, 1994). While the majority of studies published since the introduction of ARFID to the DSM-5 have focused on young children, there is increasing evidence that ARFID occurs in adolescents and young adults, consistent with the framing of ARFID as a lifespan diagnosis in DSM-5 (Bourne, Bryant-Waugh, Cook, & Mandy, 2020; Goldberg et al., 2020; Hay et al., 2017; Murray et al., 2020). Individuals with ARFID do not fear weight gain or have body image disturbance; however, they demonstrate and endorse avoidant and/or restrictive (A/R) eating that can typically be attributed to one, two, or three primary ARFID presentations. These distinct ARFID profiles include sensory sensitivity (avoidance of specific food items due to their texture, smell, or taste), restrictive eating resulting from lack of interest in eating or food, and/or food avoidance due to fear of aversive consequences such as choking, vomiting, or gastrointestinal discomfort (APA, 2013).
Consistent with the clinical observation that individuals with ARFID can present with more than one ARFID prototype (Bryant-Waugh et al., 2019; Thomas et al., 2020; Zickgraf, Lane-Loney, Essayli, & Ornstein, 2019), Thomas et al. (2017) have suggested a three dimensional neurobiological model of ARFID in which the three separate core profiles exist along a continuum of severity and are not mutually exclusive. This model emphasizes the fact that an individual diagnosed with ARFID may present with a component of inadequate caloric consumption together with poor variety of food intake (e.g., the same patient may demonstrate poor caloric intake due to concerns about aversive consequences of eating together with restricted range of foods due to the sensory characteristics of food). In all cases, in order to meet the DSM-5 criteria for ARFID diagnosis, the A/R eating behavior should result in marked psychosocial impairment and/or medical problems requiring professional, medical attention (e.g., nutritional deficiencies, faltering growth) (APA, 2013; Eddy et al., 2019).
From a weight status standpoint, ARFID represents a heterogenous eating disorder. While some individuals with ARFID restrict food volume and/or variety and are underweight, others restrict primarily food variety, generally relying on processed, energy-dense foods (e.g., ice cream, crackers, pasta) and either maintain their weight in the normal range or develop overweight or obesity (Harshman et al., 2019; Kurz, van Dyck, Dremmel, Munsch, & Hilbert, 2015; Thomas & Eddy, 2019). This observation in the ARFID population is consistent with the more general literature directly addressing the relationship between adiposity and avoidant eating behaivor (referred to as food selectivity / neophobia / fussiness and picky eating behavior in different studies) which does not suggest a consistent association between the two (Brown et al., 2018; Knaapila et al., 2015; Rahill, Kennedy, Walton, McNulty, & Kearney, 2019; Sarin et al., 2019). Importantly, restrictive eating behavior associated with low dietary quality is likely to be overlooked in youth with normal weight, overweight, or obesity thus increasing the risk for severe nutritional deficiencies and associated sequelae (Benezech, Hartmann, Morfin, Bertrand, & Domenech, 2020; Chandran, Anderson, Kennedy, Kohn, & Clarke, 2015; Sharp, Berry, Burrell, Scahill, & McElhanon, 2020). Within analogue samples of individuals with A/R eating, while the fear prototype preferentially results in avoidance from animal protein, the food selectivity profile impacts a variety of food groups, but particularly fruits and vegetables (Zickgraf & Ellis, 2018). Hence, in individuals with ARFID and excess weight, food selectivity may augment the risk for all-cause mortality, cancer, and cardiovascular disease – conditions that have been shown to have reduced risk with fruit and vegetable consumption (Aune et al., 2017; Zurbau et al., 2020).
The heterogeneity in body weight among individuals with A/R eating provides a unique opportunity to parse the divergent presentations of ARFID by body mass index (BMI) status. More specifically, studying neurobiological aspects of appetite regulation in youth with ARFID across the weight spectrum may provide insight into why some individuals with ARFID maintain healthy weight while others develop overweight or obesity.
Youth with ARFID and OV/OB represent an under-investigated, highly vulnerable population. Coexistence of extreme dietary avoidance together with excess adiposity predisposes these individuals to the complications associated with each condition. Childhood obesity is a major public health concern; currently, one-third of youth in the United States meet criteria for either overweight or obesity (Ogden, Carroll, Kit, & Flegal, 2014). Excess weight during childhood and adolescence has critical health implications which include a heightened risk for metabolic (e.g., type 2 diabetes, hyperlipidemia, nonalcoholic fatty liver disease, cardiovascular disease) (Kumar & Kelly, 2017) and psychological (e.g., depression, anxiety, low quality of life) comorbidities (Hrabosky & Thomas, 2008; Rankin et al., 2016).
Human functional magnetic resonance imaging (fMRI) studies provide evidence that youth and adults with OV/OB demonstrate hyperactivation of food motivation neurocircuitry (e.g., insula, orbitofrontal cortex [OFC], and nucleus accumbens) in response to visual images of palatable foods (Devoto et al., 2018). These findings support the reward surfeit theory of overeating, postulating that increased responsivity of reward brain regions could drive overconsumption of high-calorie foods in the absence of a homeostatic need for caloric intake (Devoto et al., 2018; Stice & Burger, 2019). To our knowledge, there are no published studies investigating neural responsivity to visual food images in individuals with ARFID and excess weight. Such investigations could improve our understanding of the underlying neurobiological mechanisms contributing to the highly restricted hyperphagia of palatable, energy-dense foods observed in individuals with ARFID and OV/OB and assist in identifying potential therapeutic targets.
Here, we aimed to characterize the neurobiological features of A/R eating behavior by BMI status. To establish the coexistence of A/R eating behavior with OV/OB, participants were administered validated structured interviews and self-report questionnaires assessing the severity of ARFID and evaluating food neophobia, a behavioral trait in which a person refrains from tasting and experiencing unfamiliar or novel foods (Pliner & Hobden, 1992). To investigate the main aim of our study, we assessed brain fMRI blood-oxygenation level-dependent (BOLD) activation in response to visual images of high-calorie foods in youth with ARFID. We hypothesized that, compared to HW individuals with full or subthreshold ARFID, individuals with full or subthreshold ARFID and OV/OB would demonstrate hyperactivation of food motivation brain regions (i.e., OFC, anterior insula, amygdala, and nucleus accumbens). Finally, in an exploratory analysis, we tested whether group differences (HW vs. OV/OB) in appetite status pre- vs. post-meal were related to hyperactivation in these same regions.
Methods
Participants
Twenty-three individuals with full or subthreshold ARFID, ages 11–23 years (12 individuals with HW and 11 with OV/OB matched for age and sex), were recruited as part of an ongoing multidisciplinary study of the neurobiology of ARFID (National Institute of Mental Health R01MH108595). Participants were recruited primarily from the Greater Boston area from both treatment settings (e.g., eating disorder clinics, student health centers, adolescent medicine programs) and non-treatment settings (e.g., social media, clinical research websites, flyers, word of mouth). A minority of the participants was recruited from other states and overall the vast majority of the sample was urban/suburban.
HW was defined as BMI between the 10–85th percentiles for age and sex for subjects younger than 20 years according to the Centers for Disease Control growth charts (Kuczmarski et al., 2002); and as BMI between 18.5–25.0 kg/m2 for subjects older than 20. Subjects with OV/OB had BMI percentiles greater than 85th for age and sex (participants younger than 20) or BMI greater than 25.0 kg/m2 (participants older than 20). Additionally, we calculated percent median BMI (%mBMI) (BMI [kg/m2]*100/median BMI for age and gender) across ages. Subjects were eligible to participate in the study if they met criteria for ARFID as determined by the Eating Disorder Assessment for DSM-5 (EDA-5) (Sysko et al., 2015); or if they endorsed significant A/R eating behavior on the Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children - Present and Lifetime Version 2013 Working Draft; KSADS-PL (Kaufman et al., 1997). Exclusion criteria included an active eating disorder other than ARFID (determined by EDA-5) or clinically significant non-A/R disordered eating as evidenced by an Eating Disorder Examination-Questionnaire global score ≥4 (Fairburn & Beglin, 1994). Additional exclusion criteria included active suicidal ideation, substance or alcohol use disorders, history of psychosis (both assessed by KSADS-PL), and intellectual disability (IQ < 70) by clinical history. Pregnancy, breastfeeding, use of hormones within eight weeks of the baseline visit, contraindications to MRI, gastrointestinal surgery which could affect appetite regulation, and hematocrit < 30% (potentially preventing blood acquisition for laboratory testing) were additional exclusion criteria.
Procedure
The study was approved by the Partners HealthCare Institutional Review Board. All participants 18 years or older and guardians of younger participants provided their written informed consent prior to participation. Participants younger than 18 years provided assent. One person who attended a screening visit did not consent and elected to not participate in the study. All subjects who provided their consent or assent to participate in the study completed the main visit. Study participants received between $100–250 payment for participation (the variation resulting from the number of study components they chose to complete). Participants were admitted to the Massachusetts General Hospital for a screening visit. At screening, a nurse practitioner obtained a detailed medical history, physical exam, and anthropometric measurements. Additionally, subjects were given the KSADS-PL, EDA-5, and EDE-Q. Eligible participants then returned within a mean of 56.3 days for the main study visit which included neuroimaging following an overnight fast, appetite evaluation pre- and post-meal which was consumed following fMRI scanning, and A/R eating behavior evaluation. At both the screening and main visits, we triply obtained anthropometric measures using a stadiometer and calibrated scale, with the subjects wearing socks, gowns, and MRI-safe pants for these measurements.
Evaluation of A/R eating behavior
The PARDI is a semi-structured interview developed to diagnose pica, ARFID, and rumination disorder in children and adults and it has been shown to have adequate reliability and validity in preliminary studies (Bryant-Waugh et al., 2019). In addition to providing an overall severity score on a scale from 0–6, it evaluates the presence and severity of the three DSM-5 ARFID profiles (i.e., sensory sensitivity, lack of interest in eating or food, and fear of aversive consequences). Scores on the three ARFID profiles also range from 0–6, with higher scores indicating greater severity. The PARDI diagnostic algorithm differentiates between full ARFID and subthreshold ARFID. In order to meet criteria for full ARFID, participants had to fulfill one or more of the four components of criterion A (i.e., weight loss, nutritional deficiency, dependence on oral nutrition supplements, marked interference with psychosocial functioning), and also fulfill criteria B (the eating disturbance is not explained by lack of available food or a cultural practice), C (which rules out anorexia nervosa, bulimia nervosa, and significant shape/weight concerns), and D (the eating disturbance cannot be fully explained by another medical condition or psychiatric disorder). Participants were diagnosed as having subthreshold ARFID if they did not reach sufficient severity in any of the four components of criterion A but demonstrated clinically significant food restriction by volume and/or variety and met criteria B, C, and D. Doctoral-level psychologists and bachelors-level research assistants performed and reviewed the PARDI interview. Each assessor received intensive training prior to conducting interviews and interpreting PARDI results. Raters discussed the interpretation and scoring of each PARDI interview. Percent agreement based on second independent ratings of eight randomly selected PARDIs from the current sample was 87.5%.
We measured food neophobia severity using the Food Neophobia Scale (FNS), a widely used method that has been shown to provide reliable and valid results (Pliner & Hobden, 1992; Ritchey, Frank, Hursti, & Tuorila, 2003). The FNS is a self-report questionnaire comprising 10 statements concerning willingness to try novel foods on a 7-point Likert scale. The final score is a continuous variable ranging from 10 to 70, with a higher score representing greater reluctance to taste novel or unfamiliar foods. In the validation study of the FNS (Pliner & Hobden, 1992), the mean score for healthy individuals was 34.51 with a standard deviation (SD) of 11.86, thus a score above 58.23 (> 2 SD above the mean) would be considered high.
Functional MRI paradigm
At the main visit, we conducted MRI scanning pre-meal after an overnight fast. Participants completed a well-established fMRI food motivation paradigm (Holsen et al., 2012), programmed through Presentation software (Neurobehavioral Systems, Albany, CA, USA). During scanning, participants viewed 100 high-calorie food stimuli (e.g., doughnuts, pizza), 100 low-calorie food stimuli (e.g., vegetables, grilled fish), 100 non-food-related objects, and 100 fixation stimuli (blurred images) in a block design, with each stimulus presented for 3 seconds. Subjects were instructed to press a button when pictures changed to ensure attention. The task included a total of five 4-minute runs with five images in each block and 16 blocks in each run.
MRI acquisition parameters
MRI data were acquired using a Siemens 3T Trio scanner (Siemens, Erlangen, Germany) at the Athinoula A. Martinos Center for Biomedical Imaging. Head movements were restricted with foam cushions. Whole-brain functional imaging was performed using a gradient-echo EPI pulse sequence (33 contiguous oblique-axial slices, 4-mm thick, TR/TE = 2000/30 ms, flip angle = 90°, FOV = 200 × 200 mm, 120 total images per run). A sagittal 3D SPGR (T1-weighted) sequence was also acquired (TR/TE=2350/3.39 ms, flip angle=7°, FOV=256 × 256 mm, effective slice thickness=1.33 mm with 128 slices).
fMRI data analysis
We analyzed fMRI data using Statistical Parametric Mapping software (SPM12; Wellcome Trust Centre for Neuroimaging). Volumes were realigned and unwarped with phase correction provided from the fieldmap, normalized to the Montreal Neurological Institute MNI152 brain template, re-sampled to 3 mm isotropic, and smoothed with a 6 mm Gaussian kernel. Outliers in global mean image time series (threshold: 3.5 SD) and movement (threshold: 0.8 mm, scan-to-scan movement) were detected using ART (http://www.nitrc.org/projects/artifact_detect/) and entered as nuisance regressors in the single-subject level GLM. For the block design, each stimulus type was modeled using a boxcar function convolved with a canonical hemodynamic response function. Contrasts of interest (high-calorie food > non-food objects) from the first-level analysis were tested using linear contrasts and SPM t-maps, then submitted to second-level random effects group analysis. The contrast of high-calorie food > non-food objects was chosen based on hypotheses related to fMRI heightened responses to high-calorie foods among participants with ARFID and OV/OB. Effects of interest at the group level were examined using a two-sample t-test (participants with OV/OB > HW). Multiple comparisons for a priori region of interest (ROI) analyses using small volume correction were controlled using a combination of cluster extent (k≥20 in each ROI) and voxel-level threshold of p<0.05, FWE-corrected. ROIs were selected a priori and defined anatomically using the AAL3 atlas (Rolls, Huang, Lin, Feng, & Joliot, 2020) and included the nucleus accumbens, OFC, amygdala, and anterior insula. Average parameter estimates within each region of interest (ROI) for each participant were extracted from clusters within each ROI meeting statistical thresholds using the Region of Interest Extraction Toolbox (REX) and exported to SPSS (v19, Chicago, IL) for graphical depiction. In addition to a priori ROI analyses, we examined group differences (participants with OV/OB > HW) at the whole brain level, with a cluster extent of k>50 and cluster-level threshold of p<0.05 FWE-corrected.
Assessment of subjective appetite
Following fMRI scanning and 10 minutes prior to receiving a meal, appetite was assessed while fasting on the dimensions Hunger, Fullness, and Desire to Eat a Favorite Food on visual analog scales (VAS), a widely utilized measure for assessing subjective appetite. Participants responded on an electronic scale of 0 to 100, with 0 corresponding to a response of “not at all” and 100 referring to “extremely strong”. Participants then received an approximately 400kcal meal standardized for macronutrient content (~20% protein, ~20% fat, ~60% carbohydrate), composed under the supervision of a registered dietician. We asked the participants to eat the entire meal over 15 minutes and to complete the VAS assessment following meal completion.
Statistical analysis
We conducted analyses in STATA/IC V15.1 (College Station, TX). All descriptive statistics are reported as mean±SEM and percentages unless otherwise noted. We compared clinical characteristics and scores on psychological evaluation using the Student t-test or Wilcoxon Rank Sum test depending on data distribution (parametric and non-parametric, respectively). We used Chi-squared or Fisher’s exact tests to compare categorical demographic variables. Given the sequential nature of our study design (fMRI scanning preceding appetite evaluation), we conducted a mediation model using 5000 bootstrapped analyses (Hayes, 2013) (PROCESS macro, v.3.4.1 in SPSS Statistics 26( to measure the indirect effect of brain activation (intermediate variable) on change in hunger pre-vs. post-meal (outcome) in the two groups (with BMI as the predictor variable) (Cole & Maxwell, 2003; De Los Reyes, 2017; Maxwell & Cole, 2007). In this mediation analysis we specifically examined the brain activation in ROIs found to be significant in the fMRI analyses.
Results
Participant characteristics
Table 1 summarizes participant characteristics and the evaluation of A/R eating behavior. Participants with HW did not differ significantly in age, sex, race, or ethnicity from those with OV/OB. By design, participants with ARFID and HW had significantly lower BMI, BMI Z score, and % median BMI compared with those with OV/OB. There were no statistically significant differences in the severity of ARFID symptoms, scores on the three ARFID profiles, or DSM-5 ARFID criteria A1-A3; however, individuals with OV/OB were more likely (compared with HW participants) to meet diagnostic criteria by psychological impairment (criterion A4). Average food neophobia scores did not differ significantly between groups and were more than 2 SD above the mean for healthy individuals (Pliner & Hobden, 1992), thus supporting clinically meaningful food neophobia in both HW participants and those with OV/OB. Table 2 summarizes the current psychiatric diagnoses from the KSADS-PL interview (Kaufman et al., 1997) as well as any medications participants were taking that could potentially affect appetite and weight (Gurbuz et al., 2016; Saunders, Igel, Shukla, & Aronne, 2016).
Table 1.
Demographic characteristics, clinical features and eating behavior of participants with full or subthreshold avoidant/restrictive food intake disorder with vs. without overweight/obesity.
| Healthy weight (n=12) | Overweight/obesity (n=11) | p | |
|---|---|---|---|
|
| |||
| Age, years | 16.06±0.94 | 17.86±1.4 | 0.29a |
| Female | 7 (58%) | 8 (73%) | 0.67b |
| Race | |||
| White | 11 (92%) | 9 (82%) | 0.48c |
| Black/African American | 0 | 1 (9%) | |
| Asian | 0 | 0 | |
| American Indian/Alaska Native | 0 | 0 | |
| Native Hawaiian/other Pacific | 0 | 0 | |
| Islander | |||
| More than one race | 1 (8.3%) | 1 (9%) | |
| Ethnicity | |||
| Hispanic | 1 (8%) | 1 (9%) | 0.94c |
| Non-Hispanic | 11 (92%) | 10 (90%) | |
| Anthropometric measurements | |||
| BMI, kg/m2 | 19.1±0.63 | 30.96±1.19 | <0.0001a |
| BMI z/score | −0.47±0.14 | 1.8±0.12 | <0.0001d |
| % median BMI | 94.47±1.8 | 152.51±6.84 | <0.0001d |
| Evaluation of A/R eating | |||
| PARDI severity | 1.92±0.2 | 2.48±0.22 | 0.08a |
| PARDI sensory sensitivity | 1.4±0.3 | 1.65±0.31 | 0.56a |
| PARDI fear of aversive consequences | 0.16±0.14 | 0.27±0.2 | 0.55d |
| PARDI lack of interest | 1.42±0.49 | 1.71±0.35 | 0.20d |
| eDSM-5 criteria met for ARFID (A1-A4) based on PARDI | |||
| A1 | 2 (17%) | 0 | 0.16c |
| A2 | 3 (25%) | 3 (27%) | 0.90c |
| A3 | 6 (50%) | 2 (18%) | 0.11c |
| A4 | 5 (42%) | 9 (81%) | 0.05c |
| Met criteria for full ARFID | 8 (67%) | 9 (82%) | |
| Food Neophobia Scale | 59.91±2.69 | 61.9±1.9f | 0.71d |
Abbreviations: PARDI; Pica, ARFID, and Rumination Disorder Interview
Calculated with Student t-test
Calculated with Fisher’s exact test
Calculated with Chi-squared test
Calculated with Wilcoxon rank-sum (Mann-Whitney) test
Criteria A1-A4 have 0 for no and 1 for yes if the participant met criteria in that way.
A1 - Low weight (BMI < 10th percentile), significant weight loss, and/or failure to grow.
A2 - Nutritional deficiency (diagnosed by healthcare professional via blood tests).
A3 - Dependence on nutritional supplements (i.e., prescribed vitamins or high-energy drinks).
A4 - Psychosocial impairment (one or more PARDI impairment items ≥ 4).
One participant with obesity did not complete the FNS
Table 2.
KSADS-PL current diagnoses, psychiatric pharmacotherapy and other medications potentially affecting weight of participants with full or subthreshold avoidant/restrictive food intake disorder with vs. without overweight/obesity
| Healthy weight (n=12) | Overweight/obesity (n=11) | |
|---|---|---|
|
| ||
| Psychiatric comorbidities (current) | ||
| Major depressive disorder | 0 | 2 (18%) |
| Generalized anxiety disorder | 3 (25%) | 6 (54%) |
| Panic disorder | 1 (8.3%) | 5 (45%) |
| Social anxiety disorder | 1 (8.3%) | 1 (9%) |
| Autism spectrum disorder | 1 (8.3%) | 1 (9%) |
| Obsessive compulsive disorder | 1 (8.3%) | 0 |
| ADHD | 4 (33%) | 1 (9%) |
| More than one psychiatric diagnosis (not including ARFID) | 2 (17%) | 4 (36%) |
| Psychiatric pharmacotherapy | ||
| Antidepressantsa | 5 (42%) | 5 (45%) |
| Antipsychoticsb | 2 (17%) | 0 |
| Anxiolyticsc | 1 (8.3%) | 0 |
| Stimulantsd | 4 (33%) | 0 |
| More than one psychiatric medication | 5 (42%) | 1 (9%) |
| Other medications | ||
| Metformine | 1 (8.3%) | 0 |
| Cyproheptadinef | 1 (8.3%) | 0 |
Abbreviations: KSADS-PL, Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children - Present and Lifetime Version.
Antidepressants included sertraline, fluoxetine, citalopram, bupropion and trazadone.
Antipsychotics included risperidone and olanzapine and were specifically prescribed for the treatment of ARFID, to facilitate eating and alleviate the cognitive symptoms of ARFID (Brewerton & D’Agostino, 2017).
Anxiolytics included clonazepam.
Stimulants included methylphenidate and dextroamphetamine/amphetamine.
Metformin was prescribed to address weight gain caused by antipsychotic treatment.
Cyproheptadine prescribed to increase appetite (Harrison et al., 2019).
Pre-meal (fasting) fMRI activation in response to high-calorie food images
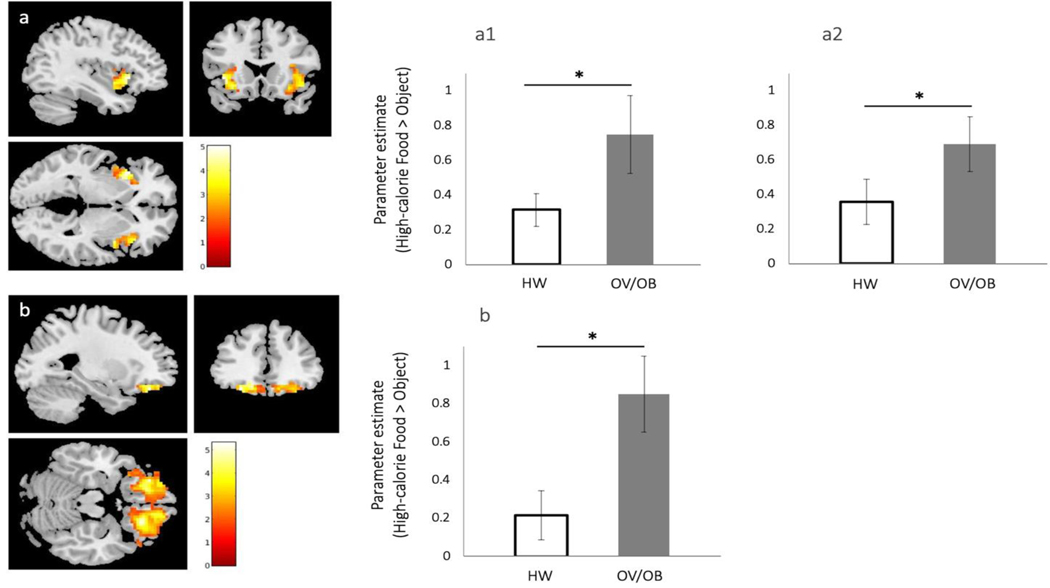
Pre-meal fMRI BOLD activation in response to contrasts of high-calorie foods vs. non-food objects was compared between groups in pre-defined ROIs. Compared with participants with ARFID and HW, subjects with ARFID and OV/OB demonstrated significant fasting BOLD hyperactivation in a large bilateral cluster in the OFC (maximum voxel located in the left OFC) and bilateral anterior insula (Table 3; figure 1a-b). Additionally, hyperactivation was found in the right amygdala at a trend level (Table 3). There were no significant differences in BOLD activation in the nucleus accumbens. Whole brain exploratory analysis contrasting images of high-calorie food vs. non-food objects confirmed greater activation in participants with OV/OB compared with HW subjects in the OFC and anterior insula, and revealed additional regions including the striate area/Brodmann area 17 and anterior cingulate cortex (Table 3).
Table 3.
Group differences (overweight/obesity > healthy weight) in BOLD response to high-calorie foods > non-food objects in a priori ROIs and at whole-brain cluster level.
| a priori ROIs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ROI | Hemisphere | K(E) | x | y | z1 | t | Uncorrected p-value2 | Peak-level PFWE-corr3 | Cohen’s d’ |
|
| |||||||||
| OFC | L | 632 | −27 | 38 | −19 | 5.33 | <0.001 | 0.019 | 1.14 |
| aINS | L | 232 | −39 | 20 | −1 | 5.04 | <0.001 | 0.015 | 0.69 |
| R | 243 | 42 | 8 | −7 | 4.58 | <0.001 | 0.036 | 0.78 | |
| Amygdala | R | 30 | 24 | 2 | −16 | 3.40 | 0.001 | 0.092 | 0.09 |
|
| |||||||||
| Whole-brain cluster
level | |||||||||
| ROI | Hemisphere | K(E) | x | y | z1 | t | Uncorrected p-value4 | Peak-level PFWE-corr5 | Cohen’s d’ |
|
| |||||||||
| ACC | L | 73 | −12 | 29 | 17 | 5.17 | 0.001 | 0.009 | 1.47 |
| R | 67 | 15 | 29 | 5 | 6.64 | 0.001 | 0.013 | 1.69 | |
|
| |||||||||
| Striate area/ BA 17 | L R |
144 131 |
−6 12 |
−97 −94 |
−10 −4 |
6.09 6.05 |
<0.001 <0.001 |
<0.001
<0.001 |
0.02 0.07 |
|
| |||||||||
| aINS | R | 107 | 39 | −1 | 5 | 5.51 | <0.001 | 0.001 | 1.11 |
|
| |||||||||
| OFC | L | 175 | −21 | 32 | −13 | 5.42 | <0.001 | <0.001 | 1.27 |
| R | 59 | 18 | 32 | −13 | 5.50 | 0.001 | 0.023 | 1.50 | |
Coordinates are presented in MNI space
Voxel-wise t-test significance level p<0.05 uncorrected for multiple comparisons within a hypothesized ROI; ROIs listed represent regions of significantly activated clusters within the a priori hypothesized ROI
FWE rate (family-wise error rate) used for SVC (small volume correction): Peak-level significance level (FWE-corrected within the search volume of interest); p values for ROIs reaching p(FWE-corrected)<0.05 are bolded
Voxel-wise t-test significance level p<0.05 uncorrected
FWE rate (family-wise error rate) used for cluster-level significance level; p values for ROIs reaching p(FWE-corrected)<0.05 are bolded
ACC: anterior cingulate cortex; BA: Brodmann area; aINS: anterior insula; OFC: orbitofrontal cortex
Figure 1. BOLD response to high-calorie vs. non-food objects in participants with ARFID and overweight/obesity compared with ARFID and healthy weight.
For each ROI, figures show SPM maps of BOLD activation at pFWE<0.05 in response to high-calorie food images vs. non-food objects. The right panel depicts bar graphs with error bars (indicating SEM) for the mean parameter estimates extracted from the ROI cluster for each group (participants with ARFID and overweight/obesity, OV/OB; and participants with ARFID and healthy weight, HW). For p-values and AAL3 coordinates see Table 4. (a) Anterior insula, aINS (a1, right aINS; a2, left aINS); (b) left OFC (cluster spans both hemispheres with maximum voxel corresponding to the left hemisphere).
Since psychiatric pharmacotherapy can potentially affect appetite and fMRI responses to visual food images (Mathews et al., 2012; Saunders et al., 2016), we visually inspected the within-group distribution of parameter estimates extracted from clusters identified in the between-group comparison of fMRI data. Specifically, within each group (participants with HW and those with OV/OB), we plotted individual subject parameter estimates for participants taking at least one psychiatric or weight affecting medication (i.e., metformin or cyproheptadine) vs. those who did not receive any medications. This examination revealed significant overlap between participants taking and those not taking medications, suggesting absence of potential bias related to pharmacotherapy in between-group differences in BOLD response to high-calorie foods (vs. objects).
Exploratory analysis of the relationship between BMI status, pre-meal BOLD response to food cues, and change in subjective appetite pre- vs. post-meal
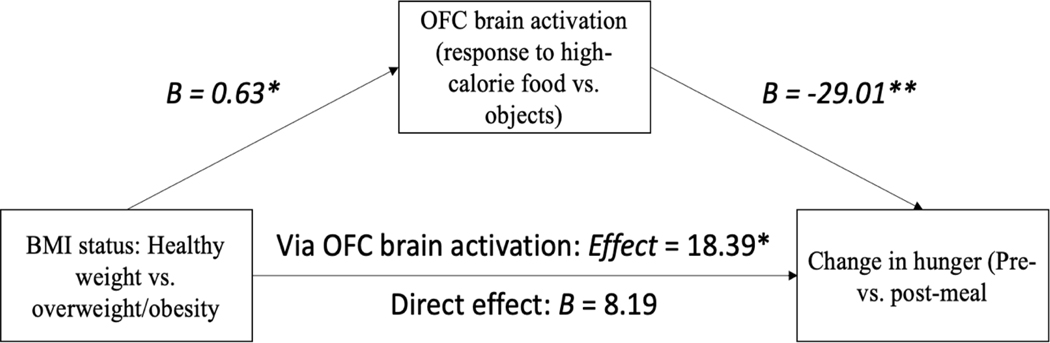
Since both subjective hunger and level of fMRI activation to high-calorie foods have been found to predict caloric consumption at a subsequent buffet (Mehta et al., 2012; Stice & Burger, 2019), we explored the association between pre-meal BOLD response to high-calorie foods and changes in subjective appetite scores pre- vs. post-meal as measured by the VAS. BOLD response in the OFC was selected for this analysis based on its function in reward processing, particularly with respect to valuation of primary rewards such as external food cues (Fuhrer, Zysset, & Stumvoll, 2008; Siep et al., 2009). Of note, there were no significant differences between groups (HW and OV/OB) in caloric consumption at the meal served following fMRI scanning. First, we examined linear relationships between changes in appetite pre- vs. post meal (as measured on the VAS) and pre-meal OFC activation in response to high-calorie foods vs. non-food objects, with BMI category (HW or OV/OB) as the predicting variable. Linear regression analysis established that BMI category, pre-meal BOLD response in the OFC, and the interaction between these two variables explained 39% of the variation in change in hunger pre- vs. post meal (R2=0.389, adjusted R2 = 0.293, p=0.02), indicating that the change in hunger following the meal was predicted by pre-meal OFC BOLD response and dependent on BMI status (interaction term p=0.045).
We then conducted a mediation analysis testing whether pre-meal BOLD response in the OFC (intermediate variable) mediated the relationship between BMI status (predictor variable) and changes in subjective hunger pre- vs. post-meal (outcome variable) through different levels of fMRI activation. We computed indirect effects of each of 5,000 bootstrapped samples using the PROCESS macro (Hayes, 2013). We found that the two groups (participants with ARFID and HW and those with OV/OB) differed significantly in pre-meal OFC BOLD response (B=0.63, p=0.01), which in turn, was associated with change in hunger; such that greater pre-meal OFC BOLD response was associated with a smaller decrease in hunger pre- vs. post-meal (B= −29.01, p=0.009). The analysis also yielded a significant indirect effect of group on change in hunger via pre-meal OFC BOLD response (effect=18.39, SE=11.27, 95% CI [−45.09, −3.00]), suggesting that pre-meal OFC BOLD response to high-calorie foods mediated the difference in hunger status pre- vs. post-meal between participants with HW and OV/OB (Figure 2). There was no direct effect of BMI status on change in hunger.
Figure 2. Exploratory mediation analysis of the association between BMI status, pre-meal OFC activation in response to high-calorie foods and changes in subjective appetite scores pre- vs. post-meal.
Note: * p < .05, ** p < .01
Discussion
In this study we have shown that, among individuals with full or subthreshold ARFID, compared to HW individuals, those with OV/OB demonstrate significant fasting fMRI hyperactivation in brain regions associated with food cue processing and reward in response to visual cues of palatable foods (i.e., OFC and anterior insula), thus supporting divergent neurobiological underpinnings of A/R eating behavior by weight status. We also found that fasting OFC BOLD response to high-calorie foods vs. objects mediated differences in hunger state pre- vs. post-meal between the two groups, suggesting that differential brain activation in HW vs. OV/OB triggered by visual food stimuli impacts subjective appetite. Future studies are needed to examine the relation between activation of the OFC and food intake in HW compared to OV/OB individuals with ARFID.
Youth who demonstrate pervasive A/R eating and attain excess weight consume a strikingly limited variety of processed, palatable, energy-dense foods. The main aim of our study was to characterize the food motivation circuitry in these individuals to better understand the neurobiological mechanisms underlying the exaggerated, but selective, consumption of palatable foods. Consistent with our hypothesis, we found that when viewing high-calorie foods vs. objects after an overnight fast, participants with ARFID and OV/OB (compared with HW participants) demonstrated significantly greater activation in hypothesized regions (i.e., OFC, anterior insula) and in regions associated with attentional control and emotion regulation (anterior cingulate cortex) and visual processing (striate area/Broadman area 17). The OFC plays a critical role in neural processing of external sensory stimuli and determining their reward value (Kringelbach & Rolls, 2004). It is closely interconnected with the insular cortex, a gustatory processing center also involved in integration of external food cues with internal signals of energy bioavailability (e.g., peripheral glucose levels) and visceral signals such as gastric distention (Frank, Kullmann, & Veit, 2013). The anterior insula also receives input from the amygdala, anterior cingulate cortex, and striatum thus playing a pivotal role in forming conscious perception and subjective feeling of the integrated interoceptive and external sensory signals (Nieuwenhuys, 2012). We also detected trend-level differences in fMRI activation in the amygdala, a brain region critically involved in saliency and stimulus-reward learning (Janak & Tye, 2015).
There is ample evidence showing that visual food stimuli, compared with non-food objects, elicit an fMRI BOLD response in specific brain regions associated with reward value (OFC), gustatory processing (insula), saliency and stimulus-reward learning (amygdala) (van der Laan, de Ridder, Viergever, & Smeets, 2011). Since these responses are more pronounced in states of hunger, we specifically selected a study design assessing visual food processing following an overnight fast (Siep et al., 2009). Numerous fMRI studies have shown that adults and children with obesity demonstrate differential hyperactivation of food-motivation and reward brain areas in response to palatable food images, compared with healthy-weight individuals (Bruce et al., 2010; Devoto et al., 2018). These perturbations in neural processing of food stimuli are thought to represent reward dysfunction contributing to overeating behavior beyond homeostatic needs (Stice & Burger, 2019), and fMRI hyperactivation in response to appetitive visual cues in adolescents is predictive of future weight gain (Yokum, Gearhardt, Harris, Brownell, & Stice, 2014). Taken together, these studies support a unique neurobiological signature related to processing of visual food cues in youth with OV/OB, and provide a potential neural mechanism predisposing them to overeating, developing and maintaining obesity.
Our study did not include a group of participants with OV/OB and without ARFID. Therefore, we are unable to determine if the pattern of brain activation we observed is unique to individuals with OV/OB who endorse A/R eating or alternatively, it is related solely to having excess weight. Nonetheless, the participants in this study endorsed A/R eating, were diagnosed as having full/subthreshold ARFID by PARDI, were found to have high scores on the food neophobia scale and demonstrated psychological impairment directly related to their eating behavior. We demonstrate here that OV/OB can occur in the setting of ARFID, an entity commonly perceived as associated with underweight. We speculate that within ARFID, a distinctive pattern of neurocircuitry activation in response to energy-dense, palatable visual food images could lead to the attainment and maintenance of OV/OB despite high levels of A/R eating. Future studies comparing fMRI brain activation in youth with obesity with and without ARFID would be important to test whether the former phenotype is characterized by unique fMRI responses to food cues that could explain excessive weight gain in the setting of A/R eating behavior. Our study design, which included a consecutive evaluation of fMRI scanning followed by appetite evaluation pre- vs. post meal, allowed us to explore the relationship between BMI status, pre-meal OFC activation, and change in hunger pre- vs. post-meal using a mediation model (Cole & Maxwell, 2003; De Los Reyes, 2017). We found that OFC activation in response to high-calorie foods vs. objects mediated differences in hunger state pre- vs. post-meal between the two groups (HW and OV/OB). This finding suggests that differential fasting brain activation triggered by visual food stimuli affects subjective appetite in response to food intake in youth with ARFID and HW vs. OV/OB. Subjective hunger, as measured by the visual analogue scale, has been previously shown to correlate with consumption of kilocalories at a meal buffet (Mehta et al., 2012). Moreover, increased fMRI responsivity of food motivation brain areas to palatable visual food stimuli predicted subsequent greater ad lib consumption of high-calorie foods (Stice & Burger, 2019). Our findings suggest that OFC brain activation in response to visual food stimuli could indirectly modulate prandial effects on subjective hunger. Future studies of youth with ARFID and OV/OB should include an evaluation of food consumption to better characterize the association between A/R eating behavior, subjective appetite, and brain activation.
To our knowledge, our study is the first to present the results of the diagnostic PARDI evaluation in a modest size group of youth with OV/OB. Youth with ARFID who carry excess weight represent an under-investigated and vulnerable population. Healthcare providers may be less likely to screen for avoidant or restrictive eating in children or adolescents who have obesity, and therefore may not recognize the potential malnutrition and marked psychosocial impairment that accompany ARFID symptoms (Bourne et al., 2020). The paucity of research describing youth with ARFID and OV/OB further contributes to the lack of awareness among primary care providers encountering these individuals. To date, only two studies assessed the prevalence of OV/OB in a cohort of individuals with ARFID; one study reported that 27% of pediatric outpatients with selective/neophobic presentation of ARFID had overweight or obesity (Zickgraf, Murray, Kratz, & Franklin, 2019) and another study reported 4% prevalence of overweight and 1.7% of obesity in children with ARFID (Kurz et al., 2015). With the exception of these two studies, and reports of ARFID with OV/OB in the setting of autism spectrum disease (Sharp et al., 2020), there is no literature specifically addressing the co-occurrence of ARFID and obesity. There is, however, evidence that highly selective eating and food neophobia occur in the setting of body weight within the normal range and in the presence of OV/OB (Brown et al., 2018; Sarin et al., 2019; Zickgraf, Murray, et al., 2019). While operationalization of diagnostic criteria for ARFID, selective eating and food neophobia is currently ongoing (Eddy et al., 2019), it is clear that all of these eating behaviors have a component of A/R eating and all can involve an increased risk for psychological impairment and poor dietary quality intake (Lobos & Januszewicz, 2019; Zucker et al., 2015).
The nutritional deficiencies potentially evolving as a result of pervasive A/R eating behavior can occur in the absence of underweight and specifically in the setting of excess weight. Case reports of scurvy, spinal cord injury and blindness due to selective eating have been reported in patients with an average or overweight BMI (Chandran et al., 2015; Chiarello, Marini, Ballerini, & Ricca, 2018; Harrison, Warburton, Lux, & Atan, 2020; Sharp et al., 2020; Zaenglein, Martin, Carlson, & Williams, 2020). More commonly reported is the poor dietary quality associated with A/R eating. Youth with ARFID across the weight spectrum are characterized by lower consumption of vitamin B12, vitamin K, vegetables and protein and increased intake of processed foods (Harshman et al., 2019). Consumption of ultra-processed food has been shown to increase the risk for obesity (Mendonca et al., 2016) and it is associated with body fat mass during childhood and adolescence (Costa, Del-Ponte, Assuncao, & Santos, 2018). It is also known that the presence of adiposity increases the risk for metabolic comorbidities in children and adults (Kumar & Kelly, 2017; Richard, White, Elks, & Stephens, 2000). Importantly, food selectivity (as measured by the FNS), was recently found to be associated with reduced dietary quality and adverse alteration in health-related biomarkers independent of adiposity measures (Sarin et al., 2019) and other large-scale studies have shown a direct association with lower dietary quality and increased adiposity (Knaapila et al., 2015) . Overall it can be concluded that selective eating is associated with greater risk of unwanted metabolic consequences independent of BMI status. In youth with ARFID and obesity, A/R eating behavior can potentially augment the deleterious metabolic consequences engendered by adiposity, thus warranting future research of this population.
ARFID and obesity are also both associated separately with increased risk for psychiatric comorbidities. In a recent study examining the prevalence of psychiatric diagnoses in youth with ARFID across the BMI spectrum, approximately half the sample met criteria for concurrent and lifetime psychiatric disorder (e.g., anxiety, depression and neurodevelopmental, disruptive and conduct behavior) and 9% of participants demonstrated current suicidality (Kambanis et al., 2020). The association between obesity and psychiatric comorbidities is well established (Hrabosky & Thomas, 2008). Childhood obesity is tightly linked with depression which can be aggravated by stigma, teasing, and bullying that youth with excess weight commonly experience (Rankin et al., 2016). Anxiety and low self-esteem are also frequently reported in these individuals (Sagar & Gupta, 2018). In our study, we have shown that compared with HW individuals, participants with OV/OB were more likely to meet diagnostic criteria for ARFID by psychological impairment. Additionally, more than half of the participants with OV/OB met criteria for concurrent anxiety disorder and nearly 20% met criteria for depression. Nearly half were treated with antidepressants. Our findings and the literature discussed above suggest that both ARFID and obesity independently heighten the risk for psychiatric sequela, thus necessitating additional research to better understand the interplay between the two in youth with ARFID and obesity.
The categorization of ARFID patients based on their weight status could have important therapeutical implications. Currently, sparse evidence exists to guide the behavioral/psychological therapy for ARFID in general and let alone in the case of ARFID associated with OV/OB (Magel, Hewitt, Dimitropoulos, von Ranson, & McMorris, 2020; Thomas, Wons, & Eddy, 2018). More specifically, to the best of our knowledge there are no studies addressing behavioral therapeutic strategies for individuals with ARFID and OV/OB, with the exception of a handful of case reports presenting the results of cognitive behavioral therapy (CBT) in youth with ARFID and OV/OB (Aloi, Sinopoli, & Segura-Garcia, 2018; Thomas & Eddy, 2019). Current CBT for ARFID is individually tailored based on the core symptoms on presentation and growing evidence supports its efficacy in children and adolescents (Thomas et al., 2020). However, individuals with ARFID and OV/OB may need modified CBT to prevent further weight gain in addition to alleviating the psychological impairment and reducing the risk for nutritional deficiencies. From a pharmacological therapeutic standpoint, individuals with ARFID and obesity may also require customized approach that would take their weight status into consideration. While antipsychotics have been used in the treatment of ARFID to alleviate the psychological burden associated with the disorder (Brewerton & D’Agostino, 2017), they could be deleterious to patients with obesity and promote additional weight gain and metabolic disorders (Saunders et al., 2016). Similarly, when antidepressants are needed as part of the pharmacological treatment, familiarity with the effects of the different classes would be critical to avoid weight gain (Gill et al., 2020). In conclusion, awareness and further research addressing the co-existence of ARFID and OV/OB is critically needed to develop therapeutical strategies dedicated for individuals with ARFID and excess weight.
The limitations of this study include the relatively small sample size. Importantly, since the study did not include a control group of individuals with OV/OB and without ARFID, we cannot exclude the possibility that the neural response observed in our participants reflected mechanisms linked to excess weight. Future studies including a control group of youth with OV/OB who do not endorse avoidant or restrictive eating will be important to characterize the neurobiological responses that are unique to individuals with ARFID and obesity. Almost all of the participants in this study were white. The current available literature provides very little information about the epidemiology of ARFID and its prevalence in different ethnicities (Bryant-Waugh, 2019). Future studies targeted to assess the neurobiology of ARFID in more diverse populations will be important to conduct. The main strength of our study is that it is the first to present an fMRI investigation of neural processing of visual food images in individuals with ARFID. Although this study included a relatively small sample size, we were able to detect a significant mediation of the effect of high-calorie food images on hunger state pre- vs. post meal by pre-meal OFC brain activation, a finding that could have implications concerning caloric consumption and should be further investigated. Given the significantly increased risk for psychological and metabolic comorbidities directly associated with OV/OB and with A/R eating separately, it will be important to conduct additional studies to better understand the consequences of having both conditions.
In conclusion, ARFID and OV/OB, both associated with metabolic and psychological adverse sequela, coexist in youth, warranting future investigation of this understudied, vulnerable population. Youth with ARFID demonstrate divergent fMRI activation in response to visual food stimuli of high-calorie content depending on their weight status, suggesting a potential neurobiological mechanism underlying exaggerated overconsumption of processed, energy-dense food by youth with ARFID and OV/OB. Better understanding of food-motivation neural pathways in these individuals could aid in developing tailored treatment programs, including targeted cognitive behavioral therapy and pharmacotherapy affecting central appetite.
Citations
- Aloi M, Sinopoli F, & Segura-Garcia C. (2018). A case report of an adult male patient with Avoidant/Restrictive Food Intake Disorder treated with CBT. Psychiatr Danub, 30(3), 370–373. doi: 10.24869/psyd.2018.370 [DOI] [PubMed] [Google Scholar]
- APA. (1994). Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Association. [Google Scholar]
- APA. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, . . . Tonstad S. (2017). Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol, 46(3), 1029–1056. doi: 10.1093/ije/dyw319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezech S, Hartmann C, Morfin D, Bertrand Y, & Domenech C. (2020). Is it leukemia, doctor? No, it’s scurvy induced by an ARFID! Eur J Clin Nutr. doi: 10.1038/s41430-020-0640-5 [DOI] [PubMed] [Google Scholar]
- Bourne L, Bryant-Waugh R, Cook J, & Mandy W. (2020). Avoidant/restrictive food intake disorder: A systematic scoping review of the current literature. Psychiatry Res, 288, 112961. doi: 10.1016/j.psychres.2020.112961 [DOI] [PubMed] [Google Scholar]
- Brewerton TD, & D’Agostino M. (2017). Adjunctive Use of Olanzapine in the Treatment of Avoidant Restrictive Food Intake Disorder in Children and Adolescents in an Eating Disorders Program. J Child Adolesc Psychopharmacol, 27(10), 920–922. doi: 10.1089/cap.2017.0133 [DOI] [PubMed] [Google Scholar]
- Brown CL, Perrin EM, Peterson KE, Brophy Herb HE, Horodynski MA, Contreras D, . . . Lumeng JC (2018). Association of Picky Eating With Weight Status and Dietary Quality Among Low-Income Preschoolers. Acad Pediatr, 18(3), 334–341. doi: 10.1016/j.acap.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, . . . Savage CR (2010). Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes (Lond), 34(10), 1494–1500. doi: 10.1038/ijo.2010.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant-Waugh R. (2019). Avoidant/Restrictive Food Intake Disorder. Child Adolesc Psychiatr Clin N Am, 28(4), 557–565. doi: 10.1016/j.chc.2019.05.004 [DOI] [PubMed] [Google Scholar]
- Bryant-Waugh R, Micali N, Cooke L, Lawson EA, Eddy KT, & Thomas JJ (2019). Development of the Pica, ARFID, and Rumination Disorder Interview, a multi-informant, semi-structured interview of feeding disorders across the lifespan: A pilot study for ages 10–22. Int J Eat Disord, 52(4), 378–387. doi: 10.1002/eat.22958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran JJ, Anderson G, Kennedy A, Kohn M, & Clarke S. (2015). Subacute combined degeneration of the spinal cord in an adolescent male with avoidant/restrictive food intake disorder: A clinical case report. Int J Eat Disord, 48(8), 1176–1179. doi: 10.1002/eat.22450 [DOI] [PubMed] [Google Scholar]
- Chiarello F, Marini E, Ballerini A, & Ricca V. (2018). Optic neuropathy due to nutritional deficiency in a male adolescent with Avoidant/Restrictive Food Intake Disorder: a case report. Eat Weight Disord, 23(4), 533–535. doi: 10.1007/s40519-017-0409-6 [DOI] [PubMed] [Google Scholar]
- Cole DA, & Maxwell SE (2003). Testing mediational models with longitudinal data: questions and tips in the use of structural equation modeling. J Abnorm Psychol, 112(4), 558–577. doi: 10.1037/0021-843X.112.4.558 [DOI] [PubMed] [Google Scholar]
- Costa CS, Del-Ponte B, Assuncao MCF, & Santos IS (2018). Consumption of ultra-processed foods and body fat during childhood and adolescence: a systematic review. Public Health Nutr, 21(1), 148–159. doi: 10.1017/S1368980017001331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Reyes A. (2017). Inaugural Editorial: Making the Journal of Clinical Child & Adolescent Psychology Your “Home Journal”. J Clin Child Adolesc Psychol, 46(1), 1–10. doi: 10.1080/15374416.2016.1266649 [DOI] [PubMed] [Google Scholar]
- Devoto F, Zapparoli L, Bonandrini R, Berlingeri M, Ferrulli A, Luzi L, . . . Paulesu E. (2018). Hungry brains: A meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neurosci Biobehav Rev, 94, 271–285. doi: 10.1016/j.neubiorev.2018.07.017 [DOI] [PubMed] [Google Scholar]
- Eddy KT, Harshman SG, Becker KR, Bern E, Bryant-Waugh R, Hilbert A, . . . Thomas JJ (2019). Radcliffe ARFID Workgroup: Toward operationalization of research diagnostic criteria and directions for the field. Int J Eat Disord, 52(4), 361–366. doi: 10.1002/eat.23042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG, & Beglin SJ (1994). Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord, 16(4), 363–370. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7866415 [PubMed] [Google Scholar]
- Frank S, Kullmann S, & Veit R. (2013). Food related processes in the insular cortex. Front Hum Neurosci, 7, 499. doi: 10.3389/fnhum.2013.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer D, Zysset S, & Stumvoll M. (2008). Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring), 16(5), 945–950. doi: 10.1038/oby.2008.33 [DOI] [PubMed] [Google Scholar]
- Gill H, Gill B, El-Halabi S, Chen-Li D, Lipsitz O, Rosenblat JD, . . . McIntyre RS (2020). Antidepressant Medications and Weight Change: A Narrative Review. Obesity (Silver Spring). doi: 10.1002/oby.22969 [DOI] [PubMed] [Google Scholar]
- Goldberg HR, Katzman DK, Allen L, Martin S, Sheehan C, Kaiserman J, . . . Kives S. (2020). The Prevalence of Children and Adolescents at Risk for Avoidant Restrictive Food Intake Disorder in a Pediatric and Adolescent Gynecology Clinic. J Pediatr Adolesc Gynecol, 33(5), 466–469. doi: 10.1016/j.jpag.2020.06.004 [DOI] [PubMed] [Google Scholar]
- Gurbuz F, Gurbuz BB, Celik GG, Yildirim V, Ucakturk SA, Seydaoglu G, . . . Yuksel B. (2016). Effects of methylphenidate on appetite and growth in children diagnosed with attention deficit and hyperactivity disorder. J Pediatr Endocrinol Metab, 29(1), 85–92. doi: 10.1515/jpem-2015-0171 [DOI] [PubMed] [Google Scholar]
- Harrison ME, Norris ML, Robinson A, Spettigue W, Morrissey M, & Isserlin L. (2019). Use of cyproheptadine to stimulate appetite and body weight gain: A systematic review. Appetite, 137, 62–72. doi: 10.1016/j.appet.2019.02.012 [DOI] [PubMed] [Google Scholar]
- Harrison R, Warburton V, Lux A, & Atan D. (2020). Blindness Caused by a Junk Food Diet. Ann Intern Med, 172(8), 575–576. doi: 10.7326/L20-0016 [DOI] [PubMed] [Google Scholar]
- Harshman SG, Wons O, Rogers MS, Izquierdo AM, Holmes TM, Pulumo RL, . . . Thomas JJ (2019). A Diet High in Processed Foods, Total Carbohydrates and Added Sugars, and Low in Vegetables and Protein Is Characteristic of Youth with Avoidant/Restrictive Food Intake Disorder. Nutrients, 11(9). doi: 10.3390/nu11092013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay P, Mitchison D, Collado AEL, Gonzalez-Chica DA, Stocks N, & Touyz S. (2017). Burden and health-related quality of life of eating disorders, including Avoidant/Restrictive Food Intake Disorder (ARFID), in the Australian population. J Eat Disord, 5, 21. doi: 10.1186/s40337-017-0149-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis : a regression-based approach. New York: The Guilford Press. [Google Scholar]
- Holsen LM, Lawson EA, Blum J, Ko E, Makris N, Fazeli PK, . . . Goldstein JM (2012). Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight-restored women with anorexia nervosa. J Psychiatry Neurosci, 37(5), 322–332. doi: 10.1503/jpn.110156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabosky JI, & Thomas JJ (2008). Elucidating the relationship between obesity and depression: Recommendations for future research. [Google Scholar]
- Janak PH, & Tye KM (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284–292. doi: 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambanis PE, Kuhnle MC, Wons OB, Jo JH, Keshishian AC, Hauser K, . . . Thomas JJ (2020). Prevalence and correlates of psychiatric comorbidities in children and adolescents with full and subthreshold avoidant/restrictive food intake disorder. Int J Eat Disord, 53(2), 256–265. doi: 10.1002/eat.23191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, . . . Ryan N. (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry, 36(7), 980–988. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Knaapila AJ, Sandell MA, Vaarno J, Hoppu U, Puolimatka T, Kaljonen A, & Lagstrom H. (2015). Food neophobia associates with lower dietary quality and higher BMI in Finnish adults. Public Health Nutr, 18(12), 2161–2171. doi: 10.1017/S1368980014003024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, & Rolls ET (2004). The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol, 72(5), 341–372. doi: 10.1016/j.pneurobio.2004.03.006 [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, . . . Johnson CL (2002). 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11(246), 1–190. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12043359 [PubMed] [Google Scholar]
- Kumar S, & Kelly AS (2017). Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin Proc, 92(2), 251–265. doi: 10.1016/j.mayocp.2016.09.017 [DOI] [PubMed] [Google Scholar]
- Kurz S, van Dyck Z, Dremmel D, Munsch S, & Hilbert A. (2015). Early-onset restrictive eating disturbances in primary school boys and girls. Eur Child Adolesc Psychiatry, 24(7), 779–785. doi: 10.1007/s00787-014-0622-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobos P, & Januszewicz A. (2019). Food neophobia in children. Pediatr Endocrinol Diabetes Metab, 25(3), 150–154. doi: 10.5114/pedm.2019.87711 [DOI] [PubMed] [Google Scholar]
- Magel CA, Hewitt K, Dimitropoulos G, von Ranson KM, & McMorris CA (2020). Who is treating ARFID, and how? The need for training for community clinicians. Eat Weight Disord. doi: 10.1007/s40519-020-01007-1 [DOI] [PubMed] [Google Scholar]
- Mathews J, Newcomer JW, Mathews JR, Fales CL, Pierce KJ, Akers BK, . . . Barch DM (2012). Neural correlates of weight gain with olanzapine. Arch Gen Psychiatry, 69(12), 1226–1237. doi: 10.1001/archgenpsychiatry.2012.934 [DOI] [PubMed] [Google Scholar]
- Maxwell SE, & Cole DA (2007). Bias in cross-sectional analyses of longitudinal mediation. Psychol Methods, 12(1), 23–44. doi: 10.1037/1082-989X.12.1.23 [DOI] [PubMed] [Google Scholar]
- Mehta S, Melhorn SJ, Smeraglio A, Tyagi V, Grabowski T, Schwartz MW, & Schur EA (2012). Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr, 96(5), 989–999. doi: 10.3945/ajcn.112.042341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca RD, Pimenta AM, Gea A, de la Fuente-Arrillaga C, Martinez-Gonzalez MA, Lopes AC, & Bes-Rastrollo M. (2016). Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr, 104(5), 1433–1440. doi: 10.3945/ajcn.116.135004 [DOI] [PubMed] [Google Scholar]
- Murray HB, Bailey AP, Keshishian AC, Silvernale CJ, Staller K, Eddy KT, . . . Kuo B. (2020). Prevalence and Characteristics of Avoidant/Restrictive Food Intake Disorder in Adult Neurogastroenterology Patients. Clin Gastroenterol Hepatol, 18(9), 1995–2002 e1991. doi: 10.1016/j.cgh.2019.10.030 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. (2012). The insular cortex: a review. Prog Brain Res, 195, 123–163. doi: 10.1016/B978-0-444-53860-4.00007-6 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, & Flegal KM. (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA, 311(8), 806–814. doi: 10.1001/jama.2014.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliner P, & Hobden K. (1992). Development of a scale to measure the trait of food neophobia in humans. Appetite, 19(2), 105–120. doi: 10.1016/0195-6663(92)90014-w [DOI] [PubMed] [Google Scholar]
- Rahill S, Kennedy A, Walton J, McNulty BA, & Kearney J. (2019). The factors associated with food fussiness in Irish school-aged children. Public Health Nutr, 22(1), 164–174. doi: 10.1017/S1368980018002835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin J, Matthews L, Cobley S, Han A, Sanders R, Wiltshire HD, & Baker JS (2016). Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc Health Med Ther, 7, 125–146. doi: 10.2147/AHMT.S101631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard AJ, White U, Elks CM, & Stephens JM (2000). Adipose Tissue: Physiology to Metabolic Dysfunction. In Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, & Wilson DP (Eds.), Endotext. South Dartmouth (MA). [PubMed] [Google Scholar]
- Ritchey PN, Frank RA, Hursti UK, & Tuorila H. (2003). Validation and cross-national comparison of the food neophobia scale (FNS) using confirmatory factor analysis. Appetite, 40(2), 163–173. doi: 10.1016/s0195-6663(02)00134-4 [DOI] [PubMed] [Google Scholar]
- Rolls ET, Huang CC, Lin CP, Feng J, & Joliot M. (2020). Automated anatomical labelling atlas 3. Neuroimage, 206, 116189. doi: 10.1016/j.neuroimage.2019.116189 [DOI] [PubMed] [Google Scholar]
- Sagar R, & Gupta T. (2018). Psychological Aspects of Obesity in Children and Adolescents. Indian J Pediatr, 85(7), 554–559. doi: 10.1007/s12098-017-2539-2 [DOI] [PubMed] [Google Scholar]
- Sarin HV, Taba N, Fischer K, Esko T, Kanerva N, Moilanen L, . . . Perola M. (2019). Food neophobia associates with poorer dietary quality, metabolic risk factors, and increased disease outcome risk in population-based cohorts in a metabolomics study. Am J Clin Nutr, 110(1), 233–245. doi: 10.1093/ajcn/nqz100 [DOI] [PubMed] [Google Scholar]
- Saunders KH, Igel LI, Shukla AP, & Aronne LJ (2016). Drug-induced weight gain: Rethinking our choices. J Fam Pract, 65(11), 780–788. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28087864 [PubMed] [Google Scholar]
- Sharp WG, Berry RC, Burrell L, Scahill L, & McElhanon BO (2020). Scurvy as a Sequela of Avoidant-Restrictive Food Intake Disorder in Autism: A Systematic Review. J Dev Behav Pediatr. doi: 10.1097/DBP.0000000000000782 [DOI] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, & Jansen A. (2009). Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res, 198(1), 149–158. doi: 10.1016/j.bbr.2008.10.035 [DOI] [PubMed] [Google Scholar]
- Stice E, & Burger K. (2019). Neural vulnerability factors for obesity. Clin Psychol Rev, 68, 38–53. doi: 10.1016/j.cpr.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sysko R, Glasofer DR, Hildebrandt T, Klimek P, Mitchell JE, Berg KC, . . . Walsh BT (2015). The eating disorder assessment for DSM-5 (EDA-5): Development and validation of a structured interview for feeding and eating disorders. Int J Eat Disord, 48(5), 452–463. doi: 10.1002/eat.22388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JJ, Becker KR, Kuhnle MC, Jo JH, Harshman SG, Wons OB, . . . Eddy KT (2020). Cognitive-behavioral therapy for avoidant/restrictive food intake disorder: Feasibility, acceptability, and proof-of-concept for children and adolescents. Int J Eat Disord. doi: 10.1002/eat.23355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JJ, & Eddy K. (2019). Cognitive-behavioral therapy for avoidant/restrictive food intake disorder : children, adolescents, and adults. Cambridge, United Kingdom ; New York, NY: Cambridge University Press. [Google Scholar]
- Thomas JJ, Lawson EA, Micali N, Misra M, Deckersbach T, & Eddy KT (2017). Avoidant/Restrictive Food Intake Disorder: a Three-Dimensional Model of Neurobiology with Implications for Etiology and Treatment. Curr Psychiatry Rep, 19(8), 54. doi: 10.1007/s11920-017-0795-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JJ, Wons OB, & Eddy KT (2018). Cognitive-behavioral treatment of avoidant/restrictive food intake disorder. Curr Opin Psychiatry, 31(6), 425–430. doi: 10.1097/YCO.0000000000000454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan LN, de Ridder DT, Viergever MA, & Smeets PA (2011). The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage, 55(1), 296–303. doi: 10.1016/j.neuroimage.2010.11.055 [DOI] [PubMed] [Google Scholar]
- Yokum S, Gearhardt AN, Harris JL, Brownell KD, & Stice E. (2014). Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity (Silver Spring), 22(12), 2544–2551. doi: 10.1002/oby.20882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaenglein A, Martin A, Carlson L, & Williams KE (2020). Pellagra secondary to selective eating in a child with autism. Pediatr Dermatol, 37(4), 698–700. doi: 10.1111/pde.14176 [DOI] [PubMed] [Google Scholar]
- Zickgraf HF, & Ellis JM (2018). Initial validation of the Nine Item Avoidant/Restrictive Food Intake disorder screen (NIAS): A measure of three restrictive eating patterns. Appetite, 123, 32–42. doi: 10.1016/j.appet.2017.11.111 [DOI] [PubMed] [Google Scholar]
- Zickgraf HF, Lane-Loney S, Essayli JH, & Ornstein RM (2019). Further support for diagnostically meaningful ARFID symptom presentations in an adolescent medicine partial hospitalization program. Int J Eat Disord, 52(4), 402–409. doi: 10.1002/eat.23016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickgraf HF, Murray HB, Kratz HE, & Franklin ME (2019). Characteristics of outpatients diagnosed with the selective/neophobic presentation of avoidant/restrictive food intake disorder. Int J Eat Disord, 52(4), 367–377. doi: 10.1002/eat.23013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker N, Copeland W, Franz L, Carpenter K, Keeling L, Angold A, & Egger H. (2015). Psychological and Psychosocial Impairment in Preschoolers With Selective Eating. Pediatrics, 136(3), e582–590. doi: 10.1542/peds.2014-2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbau A, Au-Yeung F, Blanco Mejia S, Khan TA, Vuksan V, Jovanovski E, . . . Sievenpiper JL (2020). Relation of Different Fruit and Vegetable Sources With Incident Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J Am Heart Assoc, 9(19), e017728. doi: 10.1161/JAHA.120.017728 [DOI] [PMC free article] [PubMed] [Google Scholar]