Abstract
We provide a primer to assist in the difficult transition of Helicobacter pylori therapy guidelines to those that adhere to the principles of antimicrobial stewardship. This transition will entail abandonment of many of the principles that heretofore formed the basis of treatment guidelines and recommendations. The goals of antimicrobial stewardship include optimization of the use of antibiotics while reducing antimicrobial resistance. The critical outcome measure is absolute cure rate which largely restricts comparative trials to those which reliably produce high cure rates (e.g., ~95%). Therapies that fail to achieve at least a 90% cure rate should be abandoned as unacceptable. Because only optimized therapies should be prescribed, guidance on the principles and practices of optimization will we required. Therapies that contain antibiotics which do not contribute to outcome should be eliminated. Surveillance, one of the fundamental elements of antimicrobial stewardship, must be done to provide ongoing assurance that the recommended therapies remain effective. It is yet not widely recognized when utilizing otherwise highly successful therapies, the routine test of cure data is an indirect, surrogate method for susceptibility testing. To systematically guide therapy, test of cure data should be collected, shared and integrated into local antimicrobial stewardship programs to provide guidance regarding best practices to both prescribers and public health individuals. Treatment recommendations should be compatible with those of the American Society of Infectious Disease white paper on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens which include criteria for ethical active-controlled superiority studies of antibacterial agents.
Keywords: Helicobacter pylori, antimicrobial stewardship, guidance, therapy, susceptibility testing, test of cure
Introduction
Any attempt to modernize the guidelines regarding management of Helicobacter pylori infections and bring them into line with guidelines for treatment other infectious diseases must adhere to the principles of antimicrobial stewardship 1 and take into consideration guidelines promulgated by the Infectious Disease community such as the American Society of Infectious Diseases 2. The objectives of antimicrobial stewardship program are to optimize the use of antibiotics, to improve patient outcomes, to reduce antimicrobial resistance, to prolong the lifespan of existing antibiotics, to reduce health-care-associated infection, and to save health-care costs 3
In the past, the development of H. pylori therapy was based largely on trial and error. The primary approach to development of management guidelines for H. pylori therapy has been based on comparisons of differences between therapies (i.e., a “better than” approach) will little regard for the absolute cure rates obtained 4. In contrast, the principles of antimicrobial stewardship judge therapies primarily on their ability to reliably achieve prespecified cure rates. Antimicrobial stewardship is often a new concept to gastroenterologists who are most familiar with treatment of diseases where curative therapies are not available, treatment response is moderate, and placebo responses to therapy are common. In contrast, H. pylori infections can be cured and there is no placebo response which obviates the need for a placebo control group.
Fundamentally, antimicrobial stewardship can be considered as a coherent set of actions which: 1) promote using antibiotics responsibly, 2) are designed to improve and measure appropriate use of antimicrobial agents and promote selection of optimal drug regimes, and 3) promote using antimicrobials in ways that ensure sustainable access to effective therapy for all that need them 2, 4. Here, we attempt of provide a primer to assist in what will likely prove to be a difficult transition to guidelines based on the principles of antimicrobial stewardship.
A. Principle 1. Promotion of use of antimicrobial responsibility.
Use of antibiotics responsibly requires that antibiotics be used in accordance with the general principles of both antimicrobial therapy (Table 1) 5, 6 and antimicrobial stewardship 1, 7. Optimal drug regimens should be used. Optimization encompasses all relevant parameters of therapy, including the choice and details of administration of antibiotics (i.e., drugs, doses, dosing, formulations, route of administration, formulation, administration in relation to meals, duration of therapy, etc.). Only antimicrobials to which the infection is susceptible should be used. Exceptions to this statement include drugs for which resistance can be overcome such as by the concomitant use of clavulanic acid to inhibit B-lactamase. H. pylori infections are unusual in that it has been possible to overcome metronidazole resistance the details of which will be discussed below.
Table 1.
Principles of antimicrobial therapy for H. pylori infection
| General principles of antimicrobial therapy * | |
| Obtain an accurate diagnosis | |
| Understand the difference between empiric and definitive therapy | |
| Understand drug characteristics that are peculiar to antimicrobial agents (such as pharmacodynamics and efficacy at the site of infection) | |
| Account for host characteristics that influence antimicrobial activity | |
| Recognize the adverse effects of antimicrobial agents on the host | |
| Clinical definitions of outcome ** | |
| Terms | Definition |
| Optimized (optimal) therapy | Reliably achieves ≥95% cure rates in adherent patients with susceptible infection. |
| Conditionally acceptable therapy | Reliably achieves 90% to 94% cure rates in adherent patients with susceptible infection. |
| Optimal duration of therapy | Days of therapy required to reliably achieve an optimal result |
| Doses and frequency of drug administration | Those that will reliably achieve an optimal result |
| Recommendation for use of empiric therapy ** | |
| Empiric therapy should be restricted to therapies locally proven to be highly effective. | |
| Treatment effectiveness should always be confirmed by a test of cure. | |
| Test of cure data and any other direct and indirect antimicrobial susceptibility data should be collected and shared with other clinicians and integrated into local and the regional antimicrobial stewardship programs. | |
| Only treatment outcomes should form the basis of the recommendations regarding which therapies to use and which to avoid locally. | |
B. Principle 2. Improve and measure appropriate use of antimicrobial agents by promoting selection of the optimal drug regimen.
The definition of an optimized therapy is one that reliably achieves the highest cure rate possible, preferably 100%. Factors such as tolerability, cost, convenience, etc. become important considerations when choosing among different optimized therapies. Cure of H. pylori infections has often proved difficult and, operationally, an optimized therapy may be defined as one that will reliable achieve cure rates ≥95% (Table 1) 4. Unacceptable therapies are those that fail to reliably achieve cure rates of at least 90%.
Issues to consider when considering optimal cure rates for a specific geographic region or population.
It is generally easy to determine that an infectious disease, such as pneumonia or meningitis, has been cured. In contrast, H. pylori infections are generally silent and recrudescence may occur during the year following an apparently successful therapy. The accuracy of determining cure is dependent on the type, number, accuracy and timing of the tests used. For practical purposes, determination of cure must also be assessed within a reasonable time after the end of therapy. Based on the results of the original treatment trials submitted to the US Food and Drug Administration (FDA), 4 weeks or more after the end of therapy was chosen as the standard. For practical purposes, usually only one noninvasive test, either the urea breath test (UBT) or the stool antigen test, is utilized with the knowledge that neither is 100% sensitive nor specific. For example, in countries where atrophic gastritis is common, the proportion of false positive UBT tests will increase, especially if citric acid is not used. Inadvertent or unrecognized use of antibiotics or other drugs (e.g., proton pump inhibitors or PPIs) may also cause false negative tests. The fact that false positive and false negative tests occur (i.e., the ability to identify when 95% cure rates have been obtained) mean that it may be useful to consider adjusting the operational optimal cure rate, such as using ≥93% rather than ≥95%. We have chosen to use the ≥95% definition and to define cure rates of 90% to 94% as conditionally acceptable and cure rates below 90% as being unacceptable Table 1 4.
Approaches to improve cure rates of H. pylori therapies
Initial attempts to cure H. pylori infections discovered that although the infection could be suppressed, it was difficult or impossible to cure with a single antibiotic 8. Cure of H. pylori infections is impeded by many, often physical, factors which impair antimicrobial action and increase the likelihood of at least a small population of resistant organisms being present 9. For example, the organisms reside within the highly acid stomach which is physically outside of the body and thus poorly accessible to blood borne antibiotics and the immune system. H. pylori are also typically present in vast numbers, resulting in an inoculum effect 9. It is thought that the inoculum effect is largely responsible for the failure of dual therapy with PPI clarithromycin, metronidazole, or levofloxacin as their effectiveness is undermined by emergence of resistance during therapy 10. It was subsequently discovered that the addition of PPI to raise the pH and reduce washout, and amoxicillin to prevent emergence of resistant during therapy would allow the development of triple therapies consisting of a PPI amoxicillin and clarithromycin, metronidazole, or levofloxacin. (see below).
A proportion of H. pylori attach to the surface of gastric cells leading to a biofilm phenomenon and some are present intracellular by requiring the use of antibiotics capable of penetrating into cells 9. Finally, H. pylori can become dormant in part because they can replicate only when the pH is approximately 6. Such a high pH is infrequently present in the stomach and is difficult to obtain with standard PPIs. Overall, this results in a persister effect 11, 12 which is often managed by increasing the duration of therapy. The optimal duration of therapy remains unclear and is complicated by the fact that full antisecretory activity of PPIs requires 3 to 4 days. This makes the actual duration of effective therapy shorter than the days it is administered. Overcoming these problems is largely responsible for the almost universal recommendation for standardization of the duration of therapy at 14 days. However, pharmaceutical companies often have chosen to shorten the recommended duration of therapy to obtain a marketing advantage at the expense of reduced effectiveness. Initially, this was possible because FDA approval was based on the healing of ulcers rather than on the cure rates achieved by the therapy 4, 10.
H. pylori gastritis is an infectious disease and cure of an infectious disease is predicated on the choice of effective antibiotics. The difficulty in obtaining gastric samples for culture and acceptance of “ownership” of H. pylori therapy by Gastroenterology rather than by Infectious Diseases eventually resulted in a divergence of methods to judge the effectiveness of therapy. Traditionally, for Infectious Diseases, cure rate is the primary determinate of effectiveness. Instead, Gastroenterology focused on the results of comparisons of what were relatively ineffective, empirically administered therapies. For example, sequential therapy was judged as superior to clarithromycin triple therapy based on trials in which the cure rates with clarithromycin-containing triple therapy were low. They were accepted at face value, despite the knowledge that with clarithromycin susceptible infections, 14-day triple therapy would reliably achieve 95% cure rates. Meta-analyses were conducted and the study- or population-specific results were accepted, and generalized (i.e., sequential therapy was superior to triple therapy) 13 without the caveat that the superiority over triple therapy was obtained in populations in which the prevalence of clarithromycin resistance was increased and that combined clarithromycin and metronidazole resistance was absent. Subsequent attempts to use sequential therapy in other populations failed and it was eventually considered obsolete 14. This is an example, of how the Gastroenterology approach (comparisons of relative effectiveness) failed when compared to the Infectious Disease approach (ability to reliably achieve high cure rates).
To reduce further emergence, selection, and spread of antimicrobial resistance to prolong the lifespan of existing antibiotics
The potential of emergence or selection of antimicrobial resistance differs according to the classes of antibiotics. The world health organization (WHO) has developed the Access, Watch, and Reserve (AWaRe) classification to the recommend on the use of antibiotics according to their potential of emergence of antimicrobial resistance (Table 2) 3, 15.
Table 2.
WHO AWaRe Committee on Selection and Use of Essential Medicines15
| Group | Characteristics of antibiotics or antibiotic classes | Antibiotics used for H. pylori eradication |
|---|---|---|
| Access | 1. Activity against a wide range of commonly encountered susceptible pathogens 2. Showing lower resistance potential 3. Should be widely available and affordable 4. Essential first-choice or second-choice empirical treatment options |
amoxicillin, tetracycline, metronidazole, doxycycline, |
| Watch | 1. Higher risk of selection of bacterial resistance 2. The highest priority agents among the critically important antimicrobials for human medicine 3. Should be prioritized as key targets of stewardship programs and monitoring |
clarithromycin, ciprofloxacin, levofloxacin, rifabutin |
| Reserve | 1. Should be reserved for treatment of confirmed or suspected infections due to multi drug-resistant organisms 2. Treated as “last-resort” options 3. Should be tailored to highly specific patients and settings to preserve their effectiveness |
minocycline |
The 2019 WHO AWaRe classification of antibiotics for evaluation and monitoring of use. Geneva: World Health Organization; 2019. (WHO/EMP/IAU/2019.11). License: CCBY-NC-SA3.0IGO 15
Antibiotics in the “Access group” have lower resistance potential than those in the other two groups. They should be widely available and affordable. Amoxicillin, tetracycline, and metronidazole are classified as the “Access group”. In contrast, clarithromycin and levofloxacin have higher resistance potential and are classified as the “Watch” group. They should be prioritized as key targets of stewardship program and monitoring. These recommendations should be taken into account in clinical treatment guidelines for H. pylori infection. For example, bismuth quadruple therapy containing tetracycline and metronidazole should be considered as first-line therapy in mass eradication programs for gastric cancer prevention.
Empiric therapy for H. pylori infections.
Traditionally, most infectious disease therapy is given empirically and is highly successful. Most H. pylori therapy is also given empirically but typically is not highly successful 16, 17. The difference is that with traditional infectious diseases, only proven locally effective, optimized susceptibility-based therapies are used empirically. This is based on the general rule, when the local antimicrobial susceptibility pattern is stable, effective, optimized susceptibility-based therapies can be used empirically until resistance arises. Continuing use of the therapy is predicated on the monitoring of local and regional antimicrobial susceptibility and treatment outcomes. Any changes that are observed are then communicated to clinicians along with recommendations regarding modification of therapy. Surveillance is one of the fundamental elements of antimicrobial stewardship that also provides ongoing assurance that the recommended empiric therapies remain effective. For simple infectious diseases cultures may not be obtained unless there is poor response to therapy cultures. In contrast, with serious acute infections, or chronic infections with the potential for bad outcomes (e.g. tuberculosis), immediate treatment may be indicated. However, cultures are also obtained so that the therapy can be rapidly revised, if needed. H. pylori gastritis is a chronic infection, typically acquired in childhood, that has been present for years (often decades) before it is diagnosed. As such, there are few, if any indications requiring emergency initiation of treatment; therapy can be safely delayed until the best treatment has been identified.
There are many H. pylori therapies that will reliably achieve high cure rates with susceptible infections 18, 19. However, currently most H. pylori infections receive empiric therapy and the clinician does not know, or even have access to treatment guidance based on local or regional antimicrobial susceptibility patterns.
Susceptibility testing
To date, H. pylori has remained excluded from local and regional antimicrobial surveillance programs and few hospitals or clinics offer susceptibility testing. Although the paucity of susceptibility testing and data has been recognized as a serious national problem and has prompted calls for action, nothing has been done 6. Although the Centers for Medicare and Medicaid Services (CMS) finalized a new regulation requiring all hospitals participating in its programs to establish antimicrobial stewardship programs by March 30, 2020 which included a requirement for susceptibility testing, there has been no action, suggesting that H. pylori infections have been exempted 20, 21. The continued use of this approach has been justified by the fact that susceptibility testing for H. pylori is largely unavailable. Notably, none of the Gastroenterology, Infectious Disease, or Helicobacter pylori study groups has stepped up and attempted to solve the problem. Currently, a few major reference laboratories have begun to offer H. pylori culture and susceptibility testing, plus molecular-based H. pylori testing is now commercially available for commonly used antibiotics using fresh or formalin fixed gastric biopsies or stools 22, 23.
In retrospect, and as discussed in detail below, clinicians have long had the data needed to provide guidance regarding which therapies to use and which to avoid. These data are available as the test of cure data obtained to confirm H. pylori eradication which provide an indirect assessment of local antibiotic resistance, as defined by the World Health Organization as an antimicrobial resistance surveillance program 4.
Test of cure as an indirect currently available method of susceptibility testing.
Traditionally, treatment of an H. pylori infection consists of diagnosis and treatment, followed a 4 or more weeks later by a test of evaluate whether the infection was cured (the test of cure). This has become increasingly important as cure rates have fallen. The importance of these data has been greatly underestimated and there are many examples of test of cure data routinely collected and even linked to individual treatments without understanding how it could best be utilized 17, 24. Fundamentally, if the regimen is one that reliably provides high cure rates with susceptible infections, the test of cure data provides an indirect assessment of susceptibility/resistance and can be used systematically to guide therapy 17, 24. It is important to collect and share these data with other physicians but especially with those with charged with collecting and disseminating local resistance/susceptibility data for infectious diseases. The data, along with monitoring of prescription practices, should be integrated into local antimicrobial stewardship programs designed to provide guidance regarding best practices to both prescribers and public health individuals. One can imagine that, if the European Registry on Helicobacter pylori management had designed their program to collect and disseminate their test of cure results to the participants and the local public health individuals, the overall outcome in terms of the proportion of H. pylori infectious cured would likely have markedly improved 16, 17. Table 1 4 provides recommendations on how to use test of cure data to promote rational empiric therapy of H. pylori infections.
Comparison of therapies in the era of antimicrobial stewardship.
The recent era that employed trial and error as the main method for discovery of H. pylori therapies relied primarily on comparison of therapies, especially by meta-analyses, to judge outcome and provide treatment guidance. Assessing therapies by attempting to determine which was superior independent of actual cure rates has been superseded by employing the principles of antimicrobial stewardship which judge therapies in terms of outcome or cure with the goal of 100%. Because antimicrobial stewardship requires that clinicians prescribe only those therapies that reliably achieve high cure rates, differences between regimens or factors such as duration that fail to achieve high cure rates are no longer of interest 4. Only therapies that reliably achieve high cure rates (e.g., 94% vs. 96%) will be compared and most often the comparison will use a non-inferiority design 25. Because the differences in cure rate of acceptable therapies are small, future comparative studies will likely focus primarily on factors such as tolerability, cost, convenience, duration, etc.
Effect of the introduction of the antimicrobial stewardship on currently popular therapies.
H. pylori therapies can be divided into those that 1) are susceptibility based, 2) have been optimized for an intended population, 3) local susceptibility patterns are considered, 4) therapies that contain antibiotics that play no role in outcome, and 5) therapies they contain an antimicrobial for which resistance might be overcome (Table 3) 26. Using antimicrobial stewardship should simplify these grouping to no more than three categories (Table 3).
Table 3.
Classification of H. pylori therapies.
| Currently used classifications* | |
|---|---|
| Type | Currently used H. pylori therapies |
| A1 | Susceptibility-based therapy: regimen optimized for the population treated. |
| A2 | Indirect susceptibility-based therapy: based on results of test-of-cure in the population and using a regimen optimized for the population treated. |
| B1 | Susceptibility-based therapy: regimen not yet optimized for the population treated. |
| B2 | Indirect susceptibility-based therapy: based on results of test-of-cure in the population treated that use regimens not optimized for the population treated. |
| C1 | Regimens optimized for another population and prescribed without reference to local susceptibility patterns. |
| C2 | Unoptimized regimens given without reference to local susceptibility patterns. |
| D | Therapies include antimicrobial(s) that do not contribute to cure of the infection. |
| X | Special regimens where an antibiotic is prescribed despite resistance based on evidence that the antimicrobial resistance may be overcome. |
| Classification using antimicrobial stewardship | |
| A | Directly or indirectly susceptibility-based therapy optimized for the population treated. |
| D | Therapies which include antimicrobial(s) that do not contribute to cure of the infection. |
| X | Special regimens where an antibiotic is prescribe despite resistance based on evidence that the antimicrobial resistance may be overcome. |
Adapted from reference 26, with permission
Antimicrobial stewardship is predicated on the use of only optimized, directly or indirectly susceptibility-guided therapy. New guidelines must focus on the need to optimize current therapies to reliably achieve high cure rates. This will involve guidance regarding all the specific details of therapy, including the drugs, doses, formulations, frequency of administration, administration in relation to meals, duration, adjuvants, etc.
The effect of drug administration in relation to meals has largely been ignored, although it may be important. For example, in Shanghai, several different bismuth-containing 4 drug regimens have been shown to be reliably highly successful despite a very high prevalence of resistance to commonly prescribed antibiotics 27, 28. In those studies, the PPI and bismuth were given 30 minutes before meals and the antibiotics 30 minutes after meals. It is unclear that this timing actually was critical in achieving the reported success, but it emphasized that the parameter still needs to be evaluated. Logically, administration of the PPI 30 minutes before meals makes sense, although the effect is likely only important when PPIs are first begun 29.
There was considerable interest in drugs, doses and duration of therapy in the early days of therapy when the primary goal was to find a regimen that was reliably effective (reviewed in 8, 30–35), There was special interest in bismuth triple and quadruple therapy (reviewed in 36–38). Duration of therapy was the subject of a 2013 Cochrane review 39. The approach was to use meta-analysis. Treatments were therefore those that were done. The review was not designed to examine optimized therapies and the presence of resistance was not taken into effect resulting in the majority of the cure rates being below 90% with the majority between 70% and 80% 39. This tendency continues even today. An excellent example is fluoroquinolone triple therapy for which most studies have been either 7 or 10 days. For example a 2006 meta-analysis comparing 7- and 10- day levofloxacin triple therapy showed 10 days was superior but neither achieve a cure rate of 90% or greater 40. In 2011, Miehlke et al. compared 7 and 14 day moxifloxacin triple therapy and showed that with susceptible infections it was possible to achieve high cure rates (e.g., 95% vs. 78.9% 14-day vs. 7-day) with fluoroquinolone triple therapy 41. This observation has been confirmed showing that with susceptible infections 14-day fluoroquinolone triple therapy can reliable achieve high cure rates 27, 42. With antimicrobial stewardship the regimen would be further optimized and therapies of less than 14 days abandoned. Instead, comparative studies have continued to be done and recently analyzed in a new meta-analysis based on comparing which of two unacceptable regimens was superior 43.
Duration of therapy is an especially important when attempting to overcome the persister effect 11. The effects of duration of therapy are especially evident with amoxicillin-containing regimens 4. That the duration of two therapies was for the same number of days may not always mean they were equivalent. For example, PPIs require at least 3 days to achieve full effect, so that a comparison of 7-day triple therapy with a PPI vs. vonoprazan, which becomes effective on day, one would clearly be biased toward vonoprazan.
Factorial design in drug development and interpretation of treatment trials.
Treatment failures occurring with therapies that contain 2 or more antibiotics are difficult to interpret. With current triple therapies and bismuth quadruple therapy, resistance to amoxicillin or tetracycline is still very rare and allows one to deal with a two-component model. Resistance to clarithromycin and levofloxacin act as if that resistance transforms clarithromycin or levofloxacin triple therapies into a PPI-amoxicillin dual therapy 44. Metronidazole resistance also markedly decreases the cure rate in triple therapy, but the outcome is less predictable as it is both dose and duration dependent 45. Because the outcome with PPI-amoxicillin dual therapy is affected by both the antisecretory potency of PPIs, and the duration of therapy, an increase in either, or both, will produce an improved outcome. For example, increasing clarithromycin resistance in triple therapy resulted in a decline in cure rates, this effect was blunted by use of new generation PPIs which have relative higher antisecretory potency, resulting in an increase in cure rates in the patients with clarithromycin resistance 46, 47. Whether there was also an improvement in cure rates among those in the clarithromycin susceptible population is unlikely, as relative potency has been shown to have at best a minor effect when clarithromycin resistance is low 48. The more complex the therapy the greater the need for a factorial design. This is especially important when amoxicillin is included, for example, in Asia high dose amoxicillin plus a PPI can produce very high cure rates 49, 50. An example is a series of studies in China that used 3 grams of amoxicillin, metronidazole, a potent PPI, and bismuth and proved highly effective 27, 51–53. However, effectiveness was undiminished as the dosage of metronidazole was reduced and then eliminated. Use of a factorial design in complicated therapies is often the key to understanding how the therapy actually works. 51, 52, 54.
Bismuth quadruple therapy and metronidazole resistance
Metronidazole-containing bismuth quadruple therapy is unique in that it appears that metronidazole resistance can be partially or completely overcome by increasing the dose and duration of therapy (Class X therapy, Table 3) 26, 36, 55. The mechanism remains unknown as do the parameters critical to optimize bismuth, tetracycline, metronidazole, PPI quadruple therapy. The problem is that metronidazole resistance as assessed in vitro does not correlate well with its effectiveness in vivo, especially when used as a component of a triple or quadruple therapy. The ability to separate metronidazole susceptible strains from resistant strains is also difficult, as the commonly use Etest tends to disagree with the results of agar dilution in up to one-fourth of cases 56. The original triple therapy combination of bismuth, metronidazole and tetracycline produced high cure rates. Omeprazole was added as it improved efficacy in the presence of metronidazole resistance 57. Increasing the duration of therapy to 14 days and the dose of metronidazole to 1.6 to 2 grams of metronidazole was thought to provide an optimal regimen 36, 55. An issue of the optimal duration of therapy arose when the commercial 3-in-1 formulation (Pylera®) was introduced. Pylera® was investigated to the FDA as a 10-day regimen to gain a marketing advantage over the already approved 14-day regimens. No comparative studies were done.
In a population with a low prevalence of metronidazole resistance, the use of bismuth quadruple therapy with duration ranging from 4 to 14-days have been shown to be effective 58, 59. However, bismuth quadruple therapy is one of the less tolerable of the H. pylori therapies 55 and, for populations where metronidazole resistance is low, a metronidazole-containing triple therapy would likely be preferred as it is effective, has fewer side effects and is cheaper. One can reasonably conclude that for patients with metronidazole susceptible infections both 10 and 14-day durations are too long. The optimal doses and durations of bismuth quadruple therapy in the presence of metronidazole resistance are unknown, but the bulk of current evidence is consistent with 14-day therapy 27, 36. Earlier studies that explored different doses and dosing intervals clearly appear to show that the dose and frequency of administration of tetracycline and bismuth have not been optimized (i.e., twice daily dosing and lower doses of tetracycline and bismuth appear equally effective) 38, 60. In a small study the cure rates with 14-day therapy with PPI-tetracycline was 26%, increasing to 48% with the addition of bismuth, confirming the requirement for metronidazole 61.
Figure 1 illustrates how the prevalence of metronidazole resistance affects the outcome of bismuth quadruple therapy. These data also show that studies done populations with low resistance to metronidazole will always achieve high cure rates, which provides no guidance regarding the issue of possible differences in outcome between 10 and 14-day therapy for patients with metronidazole resistant infections. It is also important to note that none of the components have been formally optimized and the presence of a “big pocket” pharmaceutical company tends to reduce objectivity. It is reasonable to conclude that in the absence of metronidazole resistance, one should use metronidazole triple therapy 27. If metronidazole resistance is high or unknown, we would recommend 14-day therapy 27, 36. Innumerable studies of 10 day Pylera® done in low or unknown metronidazole prevalence populations or meta-analyses of such populations do not directly address, and cannot settle, the question about what is the optimal duration of therapy in the presence (or absence of metronidazole resistance). The only way to settle the optimal duration of bismuth quadruple therapies is via head to head comparisons (e.g., 10 vs. 14-day therapy) in populations with a prevalence of metronidazole resistance. This has yet to be done. As noted above, there are considerable data that the doses and possibly dosing intervals of bismuth and tetracycline can be reduced which would likely make the therapy both more tolerable and less expensive. Optimization for both susceptible and particularly metronidazole resistant infections should be a priority.
figure 1.
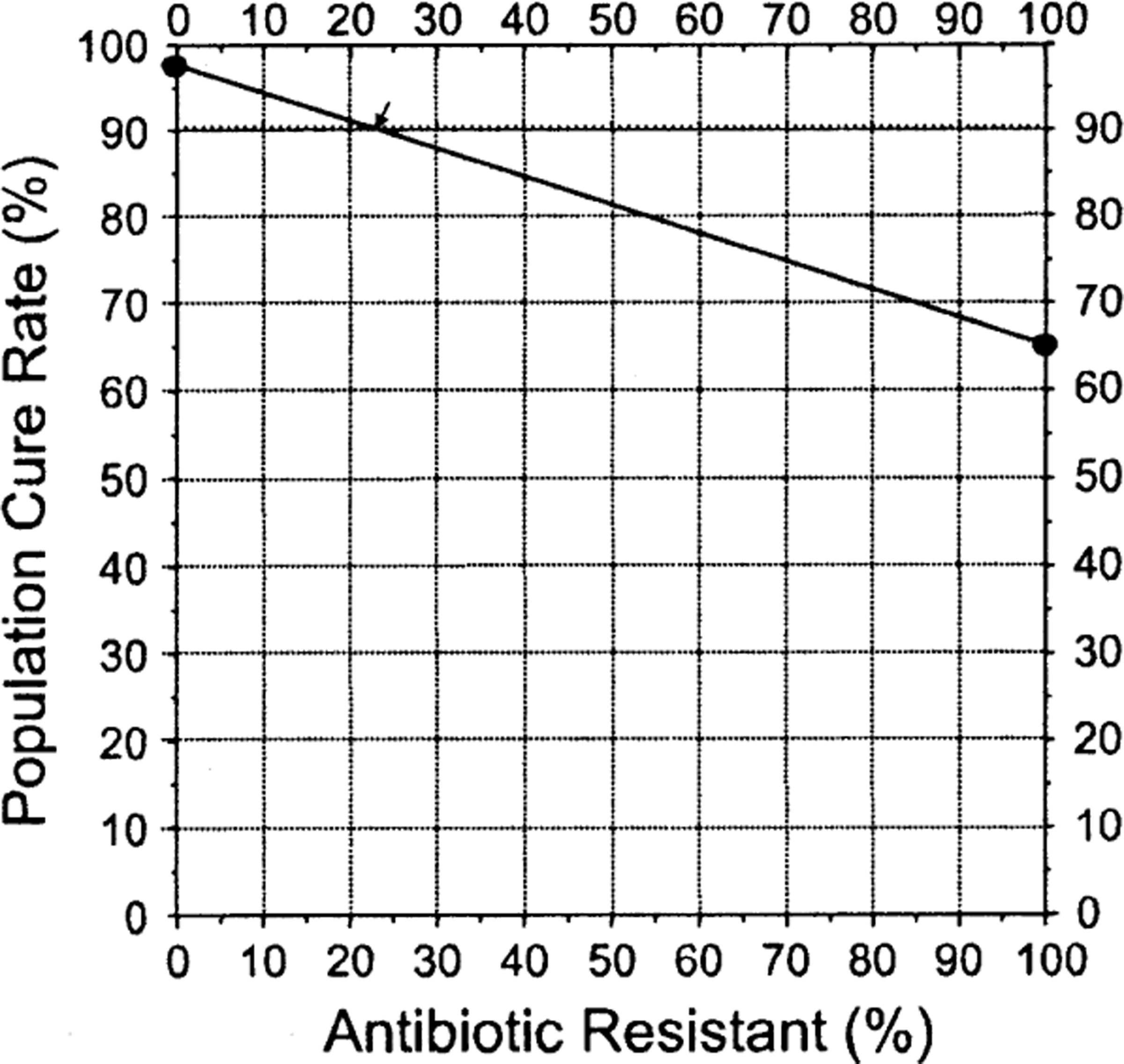
H. pylori nomogram showing the effects of increasing metronidazole resistance with 14-day high dose bismuth quadruple therapy. The proportion with resistance is directly related to the cure rate with susceptible and with resistant infections and the proportion with resistance. With a highly effective therapy the cure rate likely would remain above 90 until the proportion with resistance exceeded 20%.
C. Principle 3. Promotion of the use of antimicrobials in ways that ensure sustainable access to effective therapy for all that need them
Fundamentally, this principle is about reducing or preventing the misuse of antibiotics and includes Principle 2 or the need to optimize therapies. One of the most common ways physicians misuse antibiotics is to prescribe antibiotics for conditions in which they cannot produce a benefit (e.g., antibiotics for the common cold). In relation to therapy for H. pylori infections, the most common reason that poor results are achieved is because of either an incorrect or unoptimized regimen (i.e., to not adhere to the principles of antimicrobial stewardship. For example, administration of clarithromycin triple therapy for 7 days in a susceptible population in the US achieves a lower cure rate than when administered for 14 days (e.g., 86% rather than 95%) 62. Another example would be to administer 14-day triple therapy while ignoring the increase in clarithromycin, metronidazole, and levofloxacin resistance, resulting in a cure rate of 74%. Unnecessary treatment failures not only misuse antibiotics, but are also expensive in terms of resources expended to repeat diagnostic studies, to administer and pay for new therapy, and to provide appropriate follow-up. All patients who fail treatment are also eligible for retreatment, which would double the total number receiving antibiotics unnecessarily.
A second method of misuse is to prescribe antibiotics which provide no benefit toward curing the infection. One impetus for doing this is that addition of an extra antibiotic may result in higher cure rates (as seen with concomitant therapy) 63. Antibiotic misuse occurs when two or more antibiotics are prescribed when one would suffice. The intentional use of an extra antibiotic is especially common in the four-drug therapies that contain both clarithromycin and metronidazole (e.g., concomitant, sequential therapy, reverse concomitant therapy). Functionally, concomitant therapy is the simultaneous use of both metronidazole and clarithromycin triple therapies. The therapy will be effective if the infection is susceptible to one or both antibiotics and fail only when dual resistance is present. However, in every case, at least one antibiotic has no effect on outcome 4. Using this approach, successful therapy with 14-day concomitant therapy containing 1 gm of metronidazole and clarithromycin would produce 14,000 kg of unneeded antibiotic per 1 million successful treatments and 28,000 kg per 1 million treatment failures 4. WHO defines the defined daily dose as the assumed average maintenance dose per day for a drug used for its main indication in adults. One defined daily dose is 0.5 g for clarithromycin and 1.5 g for metronidazole which would result in 10 to 28 million excess defined daily doses of either clarithromycin or metronidazole 64. Another example is vonoprazan, clarithromycin, amoxicillin triple therapy when 80% are cured by the vonoprazan-amoxicillin dual therapy and 88% of those receiving clarithromycin receive it unnecessarily (Supplemental Figure 1) 50.
What is the basis for the recommendation to stop empiric use of an antibiotic exceeds 15%?
Maastricht IV suggested that when resistance to an antibiotic exceeded 15%, that antibiotic should no longer be used empirically 65. The number, 15%, was an arbitrary choice and not evidence-based. Based on the principles of antimicrobial stewardship, the choice would more likely be 0% or 2%. We suggest a more practical rule which would base the recommendation on cure rates such as “when the cure rate falls to 90% or below). For many infections, one can estimate that point if one has estimates of the cure rate with both susceptible and resistant infections with the formula [(proportion with susceptible infections) X (cure rate with susceptible infections) + (proportion with resistant infections) X (cure rate with resistant infections)]. An alternate approach is to use the H. pylori nomogram which allows on to rapidly visualize the proportion of the population with resistance at which the cure rate falls below 90% 44 (Figures 1 and supplemental figures 1 and 2). The exercise also shows the fallacy of attempting to try to use a one size fits all answer as the results are dependent on the details of the therapy such as PPI dose which is shown in Supplemental Figure 3. The exercise also illustrates the importance of knowing the cure rate with susceptible and with resistant infections, as well as the relative effects of different degrees of acid suppression for the local population, as this markedly affects the effectiveness of amoxicillin-antisecretory dual therapy arm of the regimen (Supplemental Figures 1 and 2).
Conclusion:
New guidelines for treatment of H. pylori infections should aim at developing therapies that deal with the problems unique to H. pylori using the principles of antimicrobial stewardship. Recommendations should also be compatible with those of the American Society of Infectious Disease white paper on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens (Table 4) 2, 4 which includes criteria for ethical active-controlled superiority studies of antibacterial agents (Table 5) 2, 4. Most prior H. pylori treatment trials fail to meet these guidelines.
Table 4.
Guidelines to implement antimicrobial stewardship for treatment of H. pylori infections
| Therapies must be optimized to reliably achieve high cure rates. |
| Optimization should include information regarding the effects of resistance to the different components and should be confirmed when used in a different region. |
| Surveillance of treatment success should be instituted. This should include tests of cure and, preferably, with susceptibility testing available for treatment failures. |
| Treatment of H. pylori should be integrated with ongoing or planned prescription and treatment monitoring programs utilized for other bacterial infections. |
| Data from sites where culture and susceptibility testing and/or molecular testing are done locally should be published and kept up to date. |
| Susceptibility testing should be reimbursed as for other bacterial pathogens and the results data should be submitted to local and central repositories responsible for monitoring resistance among bacterial pathogens. |
| To avoid unethical studies, studies should adhere to the guidelines of the Infectious Diseases Society of America regarding conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. |
From reference 4 with permission.
Table 5.
American Society of Infectious Diseases criteria for ethical active-controlled superiority studies of antibacterial agents2.
| 1 | The control (i.e., the comparator drug) is active against most, or all, of the bacterial strains likely to be encountered in the study. |
| 2 | All available drugs that could be used as comparators for the study are inadequately active against the strains likely to be encountered, such that there is no alternative effective therapy possible; or |
| 3 | The infection under study is almost universally non-fatal, such that rescue therapy can be instituted rapidly enough to preclude serious sequelae upon recognition that the strain causing the infection is resistant to the comparator drug (e.g., uncomplicated urinary tract infection). The susceptibility of etiologic bacteria is almost never known at the time an infected patient is enrolled in a clinical trial that evaluates initial antimicrobial treatment. Therefore, the comparator drugs chosen for study in antibacterial clinical trials are selected because they are anticipated to be effective against all, or almost all, strains likely to be encountered during conduct of the study. |
From reference 4, with permission.
Supplementary Material
What You Need to Know.
Background:
Until recently H. pylori therapy has been developed based on trial and error with a focus on comparative analyses of clinical trials with the better therapy recommended irrespective of the actual cure rates. Recognition that H. pylori gastritis was an infectious disease and that it was not exempt from the traditions and practices used for other antimicrobial therapies resulted in the ongoing transition to utilize the principles of antimicrobial stewardship.
Findings:
The goals of antimicrobial stewardship include optimization of the use of antibiotics while reducing antimicrobial resistance. The critical outcome measure is absolute cure rate which largely restricts choices to those that reliable produce high cure rates (e.g., ~95%). Assessment of antimicrobial susceptibility is also available as the results of testing for cure. We provide a primer to assist in what will likely prove to be a difficult transition to guidelines based on the principles of antimicrobial stewardship
Implications for patient care:
Currently, overall H pylori cure rates are unsatisfactorily low compared to other infectious diseases. Development and implementation of guidelines based on the general principles of antimicrobial therapy, the principles of antimicrobial stewardship, and the recommendations of the American Society of Infectious Disease should improve treatment outcome of H. pylori infections and reduce unintentional misuse of antibiotics thus reducing the imprint of H pylori therapy on global antimicrobial resistance.
Acknowledgments
Support: Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center.
Footnotes
Both authors were involved with concept; acquisition of data; analysis and interpretation of data; drafting of the manuscripts; and critical revision of the manuscript for important intellectual content.
Writing Assistance: none
Potential conflicts: Dr. Graham is a consultant for RedHill Biopharma and Phathom Pharmaceuticals regarding novel H. pylori therapies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dyar OJ, Huttner B, Schouten J, et al. What is antimicrobial stewardship? Clin Microbiol. Infect 2017;23:793–798. [DOI] [PubMed] [Google Scholar]
- 2.America IDSo. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clinical Infectious Diseases 2012;55:1031–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health O. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries: a WHO practical toolkit. Geneva: World Health Organization, 2019. https://creativecommons.org/licenses/by-nc-sa/3.0/igo. Assessed 7/15/2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham DY. Transitioning of Helicobacter pylori therapy from trial and error to antimicrobial stewardship. Antibiotics (Basel) 2020;9. doi 10.3390/antibiotics9100671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc 2011;86:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Serag HB, Kao JY, Kanwal F, et al. Houston Consensus Conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol 2018;16:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charani E, Holmes A. Antibiotic Stewardship-Twenty Years in the Making. Antibiotics. (Basel) 2019;8. doi antibiotics8010007 [pii]; 10.3390/antibiotics8010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borsch GM, Graham DY. Helicobacter pylori. In: Collen MJ, Benjamin SB, eds. Pharmacology of Peptic Ulcer Disease, In: Handbook of Experimental Pharmacology Vol 99. Berlin: Springer-Verlag, 1991:107–148. [Google Scholar]
- 9.Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology 1998;115:1272–1277. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins RJ. Current FDA-approved treatments for Helicobacter pylori and the FDA approval process. Gastroenterology 1997;113:S126–S130. [DOI] [PubMed] [Google Scholar]
- 11.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat. Clin. Pract. Gastroenterol. Hepatol 2008;5:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michiels JE, Van den Bergh B, Verstraeten N, et al. Molecular mechanisms and clinical implications of bacterial persistence. Drug Resistance Updates 2016;29:76–89. [DOI] [PubMed] [Google Scholar]
- 13.Zullo A, De F V, Hassan C, et al. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut 2007;56:1353–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gisbert JP, Calvet X, O’connor A, et al. Sequential therapy for Helicobacter pylori eradication: A critical review. J. Clin. Gastroenterol 2010;44:313–325. [DOI] [PubMed] [Google Scholar]
- 15.The 2019 WHO AWaRe classification of antibiotics for evaluation and monitoring of use. Geneva: World Health Organization. Volume WHO/EMP/IAU/2019.11 Licence: CCBY-NC-SA3.0IGO Geneva: World Health Organization. [Google Scholar]
- 16.Graham DY, El-Serag HB. European Registry on Helicobacter pylori management shows that gastroenterology has largely failed in its efforts to guide practitioners. Gut 2021;70:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyssen OP, Bordin D, Tepes B, et al. European Registry on Helicobacter pylori management (Hp-EuReg): patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2021;70:40–54. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto H, Shiotani A, Graham DY. Current and Future Treatment of Helicobacter pylori Infections. Adv. Exp. Med Biol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liou J-M, Chen P-Y, Kuo Y-T, et al. Toward population specific and personalized treatment of Helicobacter pylori infection. J Biomed Sci 2018;25:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A Rule by the Centers for Medicare & Medicaid Services. Federal Register, 2019. https://federalregister.gov/d/2019-20736 <https://federalregister.gov/d/2019-20736. Accessed 7/15/2020
- 21.Graham DY, El-Serag HB. CMS’s new rule for antibiotic stewardship: The case of H. pylori. GI and Hepatology News 2020. http://federalregister.gov/d/2019-20736 <https://federalregister.gov/d/2019-20736. Accessed 7/15/2020. [Google Scholar]
- 22.Graham DY. Molecular-based Helicobacter pylori susceptibility testing is almost ready for prime time. Gastroenterology 2021;(in press). [DOI] [PubMed] [Google Scholar]
- 23.Argueta EA, Alsamman MA, Moss SF, Agata EMC. Impact of antimicrobial resistance rates on eradication of Helicobacter pylori in a United States population. Gastroenterology 2021;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ginnebaugh BD, Baker J, Watts L, et al. S1348 Triple Therapy for Primary Treatment of Helicobacter pylori: A 19-Year U.S. Single Center Experience. Am J. Gastroenterol 2020;115:S680–S681. [Google Scholar]
- 25.Riedner G, Rusizoka M, Todd J, et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N. Engl. J Med 2005;353:1236–1244. [DOI] [PubMed] [Google Scholar]
- 26.Graham DY, Megraud F. Classification system for Helicobacter pylori therapies: Compared and contrasted to traditional infectious disease therapy. Helicobacter 2021;26:e12773. [DOI] [PubMed] [Google Scholar]
- 27.Yu L, Luo L, Long X, et al. Susceptibility-guided therapy for Helicobacter pylori infection treatment failures. Therap.Adv.Gastroenterol 2019;12:1756284819874922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Q, Long X, Ji Y, et al. Randomised controlled trial: susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment Pharmacol Ther 2019;49:1385–1394. [DOI] [PubMed] [Google Scholar]
- 29.Graham DY. Optimal PPI Dosing for Improving GERD Symptoms: Is Timing Everything? Dig. Dis Sci 2019;64:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Axon AT. Campylobacter pylori--therapy review. Scand. J. Gastroenterol. Suppl 1989;160:35–38. [DOI] [PubMed] [Google Scholar]
- 31.Axon AH pylori and gastroduodenal disease. Practitioner 1991;235:733–736. [PubMed] [Google Scholar]
- 32.Axon AT. Helicobacter pylori infection. J. Antimicrob. Chemother 1993;32 Suppl A:61–68. [DOI] [PubMed] [Google Scholar]
- 33.Chiba N, Rao BV, Rademaker JW, et al. Meta-analysis of the efficacy of antibiotic therapy in eradicating Helicobacter pylori. Am. J. Gastroenterol 1992;87:1716–1727. [PubMed] [Google Scholar]
- 34.van der Hulst RWM, Keller JJ, Rauws EA, et al. Treatment of Helicobacter pylori infection in humans: a review of the world literature. Helicobacter 1996;1:6–19. [DOI] [PubMed] [Google Scholar]
- 35.Wermeille J, Zelger G, Cunningham M. The eradication treatments of Helicobacter pylori. Pharm. World Sci 1998;20:1–17. [DOI] [PubMed] [Google Scholar]
- 36.Graham DY, Dore MP, Lu H. Understanding treatment guidelines with bismuth and non-bismuth quadruple Helicobacter pylori eradication therapies. Expert Rev. Anti Infect Ther 2018;16:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016;65:870–878. [DOI] [PubMed] [Google Scholar]
- 38.Fischbach LA, Goodman KJ, Feldman M, et al. Sources of variation of Helicobacter pylori treatment success in adults worldwide: a meta-analysis. Int. J. Epidemiol 2002;31:128–139. [DOI] [PubMed] [Google Scholar]
- 39.Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane. Database. Syst. Rev 2013;12:CD008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saad RJ, Schoenfeld P, Kim HM, et al. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. Am. J. Gastroenterol 2006;101:488–496. [DOI] [PubMed] [Google Scholar]
- 41.Miehlke S, Krasz S, Schneider-Brachert W, et al. Randomized trial on 14 versus 7 days of esomeprazole, moxifloxacin, and amoxicillin for second-line or rescue treatment of Helicobacter pylori Iinfection. Helicobacter 2011;16:420–426. [DOI] [PubMed] [Google Scholar]
- 42.Liao J, Zheng Q, Liang X, et al. Effect of fluoroquinolone resistance on 14-day levofloxacin triple and triple plus bismuth quadruple therapy. Helicobacter 2013;18:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gisbert JP. Optimization Strategies Aimed to Increase the Efficacy of Helicobacter pylori Eradication Therapies with Quinolones. Molecules 2020;25. doi 10.3390/molecules25215084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: Effect of resistance, duration, and CYP2C19 genotype. Helicobacter 2015;21:85–90. [DOI] [PubMed] [Google Scholar]
- 45.Bardhan K, Bayerdorffer E, Veldhuyzen van Zanten SJ, et al. The HOMER Study: the effect of increasing the dose of metronidazole when given with omeprazole and amoxicillin to cure Helicobacter pylori infection. Helicobacter 2000;5:196–201. [DOI] [PubMed] [Google Scholar]
- 46.McNicholl AG, Linares PM, Nyssen OP, et al. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment. Pharmacol. Ther 2012;36:414–425. [DOI] [PubMed] [Google Scholar]
- 47.Graham DY, Lu H, Dore MP. Relative potency of proton-pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter 2019;24:e12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gisbert JP, Pajares R, Pajares JM. Evolution of Helicobacter pylori therapy from a meta-analytical perspective. Helicobacter 2007;12 Suppl 2:50–58. [DOI] [PubMed] [Google Scholar]
- 49.Gao CP, Zhang D, Zhang T, et al. PPI-amoxicillin dual therapy for Helicobacter pylori infection: An update based on a systematic review and meta-analysis. Helicobacter 2020;25:e12692. [DOI] [PubMed] [Google Scholar]
- 50.Graham DY, Lu H, Shiotani A. Vonoprazan-containing Helicobacter pylori triple therapies contribution to global antimicrobial resistance. J Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 51.Luo L, Ji Y, Yu L, et al. 14-Day High-Dose Amoxicillin- and Metronidazole-Containing Triple Therapy With or Without Bismuth as First-Line Helicobacter pylori Treatment. Dig Dis Sci 2020;65:3639–3646. [DOI] [PubMed] [Google Scholar]
- 52.Yu L, Luo L, Long X, et al. High-dose PPI-amoxicillin dual therapy with or without bismuth for first-line Helicobacter pylori therapy: A randomized trial. Helicobacter 2019;24:e12596. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Chen Q, Liang X, et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut 2015;64:1715–1720. [DOI] [PubMed] [Google Scholar]
- 54.Chen Q, Zhang W, Fu Q, et al. Rescue therapy for Helicobacter pylori eradication: A randomized non-inferiority trial of amoxicillin or tetracycline in bismuth quadruple therapy. Am J Gastroenterol 2016;111:1736–1742. [DOI] [PubMed] [Google Scholar]
- 55.Graham DY, Lee SY. How to effectively use bismuth quadruple therapy: The good, the bad, and the ugly. Gastroenterol Clin North Am 2015;44:537–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osato MS, Reddy R, Reddy SG, et al. Comparison of the Etest and the NCCLS-approved agar dilution method to detect metronidazole and clarithromycin resistant Helicobacter pylori. Int. J. Antimicrob. Agents 2001;17:39–44. [DOI] [PubMed] [Google Scholar]
- 57.Borody TJ, Andrews P, Fracchia G, et al. Omeprazole enhances efficacy of triple therapy in eradicating Helicobacter pylori. Gut 1995;37:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Boer WA. How to achieve a near 100% cure rate for H. pylori infection in peptic ulcer patients. A personal viewpoint. J. Clin. Gastroenterol 1996;22:313–316. [DOI] [PubMed] [Google Scholar]
- 59.Lai JY, de Boer WA, Driessen WM, et al. Long-term follow-up after cure of Helicobacter pylori infection with 4 days of quadruple therapy. Aliment. Pharmacol. Ther 1996;10:645–650. [DOI] [PubMed] [Google Scholar]
- 60.Roghani HS, Massarrat S, Pahlewanzadeh MR, et al. Effect of two different doses of metronidazole and tetracycline in bismuth triple therapy on eradication of Helicobacter pylori and its resistant strains. Eur. J Gastroenterol Hepatol 1999;11:709–712. [DOI] [PubMed] [Google Scholar]
- 61.Al-Assi MT, Genta RM, Graham DY. Short report: omeprazole-tetracycline combinations are inadequate as therapy for Helicobacter pylori infection. Aliment. Pharmacol. Ther 1994;8:259–262. [DOI] [PubMed] [Google Scholar]
- 62.Laine L, Estrada R, Trujillo M, et al. Randomized comparison of differing periods of twice-a-day triple therapy for the eradication of Helicobacter pylori. Aliment. Pharmacol. Ther 1996;10:1029–1033. [DOI] [PubMed] [Google Scholar]
- 63.Dang BN, Graham DY. Helicobacter pylori infection and antibiotic resistance: a WHO high priority? Nat. Rev. Gastroenterol Hepatol 2017;7:383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.ATC-DDD Tool Kit. Geneva: World Health Organization. https://www.who.int/tools/atc-ddd-toolkit. Accessed 1/18/2021. [Google Scholar]
- 65.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646–664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


