Abstract
Latent fingerprints (LFPs) are one of the most important forms of evidence in crime scenes due to the uniqueness and permanence of the friction ridges in fingerprints. Therefore, an efficient method to detect LFPs is crucial in forensic science. However, there remain several challenges with traditional detection strategies including low sensitivity, low contrast, high background, and complicated processing steps. In order to overcome these drawbacks, we present an approach for developing latent fingerprints using stabilized CsPbBr3 perovskite nanocrystals (NCs) as solid-state nanopowders. We demonstrate the superior optical stability of CsPbBr3 NCs with respect to absorption, photoluminescence (PL), and fluorescence lifetime. We then used these highly stable, fluorescent CsPbBr3 NCs as a powder dusting material to develop LFPs on diverse surfaces. The stable optical properties and hydrophobic surface of the CsPbBr3 NC nanopowder permitted high resolution images from which unique features of friction ridge arrangements with first, second, and third-level LFP details can be obtained within minutes.
Keywords: Lead bromide nanocrystals, solid-state nanopowder, forensic science, latent fingerprint, Fluorescence
Graphical Abstract

Bright and stable fluorescent cesium lead bromide perovskite nanocrystals are synthesized via the hot injection method. The stable fluorescence and hydrophobic surface of nanopowders obtained by purification of the perovskite nanocrystals result in high sensitivity selectivity, and contrast imaging of developed latent fingerprints.
1. Introduction
Lead halide perovskites nanocrystals (NCs) with a crystal structure of APbX3 (where A+ = methylammonium (MA+), cesium (Cs+), or foramidinium (FA+); X− = Cl−, Br−, or I−) represent an emerging class of materials with excellent photophysical and optoelectronic properties [1–6]. Indeed, perovskite NCs are characterized with tunable exciton binding energy, high absorption coefficient, narrow photoluminescent (PL) emission wavelength, and nearly unitary PL quantum yield (~100%). Such superior optoelectronic properties make them appropriate as light emitters for applications in light-emitting diodes (LEDs), lasing, and display technologies [7–12].
Tuning the size and halide composition in lead halide perovskite NCs provides an effective means of changing the optoelectronic properties of these materials [13,14]. One of the most striking features of perovskite NCs is the tunability of the bandgap, which can be readily controlled through post-synthetic approaches such as a halide ion exchange [1,15]. The halide ion of the perovskite NCs can also be exchanged (from Cl to I) in solution or solid-state phases. The bandgap of the perovskite NCs can thus be tuned from 1.6—3.2 eV as a function of halide composition (the corresponding emission wavelength ranges from 400—750 nm across the entire visible range) [6,16,17]. This facile bandgap tuning of perovskite NCs is particularly advantageous for controlling the emission wavelength and emissive color of these fluorescent nanomaterials. The surface of lead halide perovskite NCs needs to be passivated by surface-capping molecules, which impact the luminescent properties [18–21]. The dynamic binding nature of the native passivating ligands including oleate (X-type) and oleylamine (L-type) under equilibrium conditions does not guarantee long-term stability. However, the development of zwitterion ligand stabilization and utilization of N-bromosuccinimide as an additional bromide source improves long-term colloidal stability of perovskite NCs [4,22,23]. Additionally, the PL properties of NCs are severely degraded through purification with harsh polar solvents such as ethanol or acetone. To retain the overall integrity and preserve the PL properties of NCs throughout the purification process, less polar solvents such as methyl or ethyl acetates are employed as antisolvents [24–27].
Unique features of friction ridge skin emerge during embryo development and are retained until death [28]. Fingerprints result from contact of friction ridge skin with a surface. The impression of the friction ridge skin with distinct features is termed a latent fingerprint (LFP) [29,30]. LFPs on a surface are composed of a variety components including intrinsic (amino acids, proteins, lipids, and metabolites) and extrinsic (food, cosmetics, drugs, and explosive contaminants) constituents, in addition to distinct features of individuals [28,31,32]. Therefore, the amount of information contained within LFPs makes them a crucial piece of evidence in forensic science. Since LFPs are not readily visible to the naked eye at crime scenes, a large number of detection strategies have been developed such as powder dusting, chemical fuming, metal deposition, and fluorescence staining [33–37]. Amidst these LFP detection techniques, powder (metallic, magnetic, or luminescent) dusting is one of the most common and effective methods due to its simplicity and effectiveness on a variety of surfaces [28,33]. Several different types of organic and inorganic powders have been used for LFP detection. Despite the ease of use and effectiveness of powders for LFP detection, several challenges remain. Paramount among these are increasing the contrast and selectivity in addition decreasing background interference [38–40]. Fluorescent powder detection approaches afford higher contrast and improved sensitivity compared to non-fluorescent detection methods. Nevertheless, heterogeneous particle sizes and poor adhesion properties limit the LFP detection selectivity and sensitivity of powders. Furthermore, complicated patterns, diverse colors and intrinsic fluorescence of substrates contribute to high background interference [33,38–41]. In order to overcome these challenges, nanomaterials have been developed for LFP detection due to their exceptional physical and chemical properties including large surface area, small size, and high quantum yield [42,43]. In particular, fluorescent nanomaterials such as quantum dots, fluorescent silica nanoparticles, fluorescent nanodiamonds, and up-conversion nanoparticles have received attention due to their high sensitivity, contrast, and selectivity arising from their optical and surface properties [39–41,44,45]. Recently, Li et al. reported LFP detection using perovskite NCs with tunable color achieved via anion-exchange. The fluorescence intensity of the perovskite NCs showed a dependence on histidine concentration, which was exploited to obtain additional information from the developed LFPs due to the relation between metabolites, including histidine, and general human traits including gender, age, and health status [46]. Furthermore, by integrating perovskite NC LFP detection, cloud computing, and an AI deep neural network, the identity of the fingerprint could be rapidly ascertained [47]. Despite the exciting potential of perovskite NC-based LFP detection, further work is required to understand and optimize the optical properties and surface stabilization of perovskite NCs for use as powder dusting material for LFP detection.
Here, we demonstrate a strategy for LFP detection affording high-resolution images using bright and stable CsPbBr3 perovskite nanopowder. CsPbBr3 NCs dispersed in hexanes were converted to a solid-state nanopowder by treatment with antisolvent to eliminate the large excess of unbound surface-capping ligands oleic acid and oleyamine. Optical properties including absorption, emission, and fluorescent lifetime of CsPbBr3 NCs were evaluated after each purification step to ensure that they were unchanged. The resulting purified, optically stable, fluorescent CsPbBr3 NCs were applied to develop LFPs. Due to the intrinsic hydrophobic surface, uniform size, and high PL efficiency of the CsPbBr3 NCs, LFPs developed using the CsPbBr3 nanopowder provide high resolution images revealing unique features of friction ridge arrangements on various surfaces including porous and nonporous substrates. In addition, LFPs developed with CsPbBr3 NCs retained distinct features over time, and similarly, high resolution images of aged LFPs could be obtained with CsPbBr3 NCs.
2. Experimental section
2.1. Materials and Methods
All chemicals were used as received without further purification. Cesium carbonate (Cs2CO3, 99.9%, Catalog #: 202126), oleic acid (OA, 90%, Catalog #: 364525), oleylamine (OLAm, technical grade, 70%, Catalog #: O7805), 1-octadecence (1-ODE, 90%, Catalog #: O806), lead bromide (PbBr2, 98%, Catalog #: 211141), and Ethylacetate (99.5 %, Catalog #: 676810) were purchased from Sigma-Aldrich (St. Louis, US). The commercial green fluorescent powder (2 oz, Catalog #: SILL703) used to compare the LFP detection efficiency was purchased from ADORAMA (New York, US).
2.2. Synthesis of CsPbBr3 NCs.
In a typical reaction, cesium oleate (Cs-OA) was initially prepared by following a previously reported method [6]. Briefly, 0.37 g of Cs2CO3, 3.4 mL of OA, and 3.6 mL of 1-ODE were loaded in a 50 mL three-neck round bottom flask. The mixture was degassed at 100 °C under vacuum, and heated to 150 °C under argon flow until all the solid was fully dissolved. In parallel, 0.83 g of PbBr2, 6 mL OA, 6 mL of OLAm, and 6 mL of 1-ODE were loaded into a 50 mL three-neck round bottom and degassed under vacuum for 1 hour at 100 °C. After the complete dissolution of PbBr2 forming lead oleate (Pb-OA), the temperature was further increased to 170 °C, followed by swift injection of 4 mL of Cs-OA (already-prepared) to Pb-OA precursor solutions under nitrogen flow. The reaction mixture was maintained for 10 s and then immediately quenched by placing the flask into an ice bath.
For purification of nanocrystals, 5 mL of ODE was added and the reaction product was centrifuged at 7000 rpm for 10 minutes to collect precipitate. The supernatant was discarded, and the pale greenish pellet was kept and washed with 2 mL of hexanes and 2 mL of ethyl acetate (defined as 1 cycle of purification, denoted as 1X). This solution was again spun down at 7000 rpms for 10 minutes and the precipitate was resuspended in hexanes (1X). For additional purification, the reprecipitation of nanocrystals with ethyl acetate was repeated up to three times (3X).
2.3. Collection and Development of LFPs using CsPbBr3 NCs.
Three donors provided sebaceous fingerprints by the following procedure. Donors gently rubbed their fingertips over their forehead and nose region and then pressed their finger onto the surfaces of different substrates including aluminum foil, ceramic, cover glass, paper, plastic petri-dish, and wood with minimal pressure. To develop the LFPs, CsPbBr3 perovskite nanopowder was carefully introduced onto the substrates. Excess nanopowder was dusted off and the nanopowder labeled LFPs on the substrate were blown with compressed air for 30 s to remove nonspecific or weakly bound nanopowder. This process improved the clarity of the fluorescence images of developed LFP. Developed LFPs using nanopowder were imaged in a UVP BioSpectrum Imaging System (UVP, Upland, US) equipped with a BioLite MultiSpectral Light Source to obtain fluorescence images. Fluorescence images were processed in Fiji [48].
2.4. Characterization.
Powder X-ray diffraction (XRD) measurements were performed using a Bruker D8-Focus (Bruker, Billerica, US). Bragg-Brentano X-ray Powder Diffractometer with a Cu Kα radiation source (λ = 1.5418 Å) in the range of 10—40°. High-resolution transmission electron microscopy (TEM) images were obtained using a FEI Tecnai G2 F20 ST instrument (FEI company, Hillsboro, US) at accelerating voltage 200 kV. Steady-state absorption spectra were measured using a Hitachi U-4100 UV–Vis-NIR spectrophotometer (Hitachi, Tokyo, Japan). Photoluminescent emission spectra were obtained using a Horiba PTI Quanta-Master series spectrofluorometer (Horiba, Kyoto, Japan) with a Xenon arc lamp as the light source and a photomultiplier tube (PMT) as the detector. Time-resolved photoluminescence emission decay was collected using a Horiba Jobin Yvon (Horiba, Kyoto, Japan) equipped with a time correlated single photon counting (TCSPC) system. Samples were subjected to 370 nm excitation using nanoLED IBH laser. The air velocity of the compressed air duster was obtained with an ANEMOMASTER (KANOMAX, Andover, US) anemometer. A Nikon D80 camera (Nikon, Tokyo, Japan) with an AF-Micro Nikkor 105 mm 1:2.8 lens was used to obtain bright-field optical images of developed LFPs. Fluorescence images of developed LFPs were recorded with a UVP BioSpectrum Imaging System with an SYBR Green (515–570 nm) emission filter under illumination with a BioLite MultiSpectral Light Source (excitation filter 455 nm, Olympus camera C-4040Z, Olympus Korea Co., Ltd., Seoul, South Korea).
3. Results and discussion
3.1. Characterization of CsPbBr3 NCs.
Colloidal CsPbBr3 perovskite nanocrystals were prepared through hot colloidal synthesis at 170 °C and characterized using transmission electron microscopy (TEM), powder X-ray diffraction (XRD) and photoluminescence spectroscopy (PL) to determine the size, crystal structure, and optical properties of the prepared nanocrystals.
A representative transmission electron microscopy (TEM) image of CsPbBr3 perovskite NCs prepared through the hot injection method is shown in Fig. 1A. The CsPbBr3 NCs are monodisperse with a cuboidal shape (Fig. 1A). The inset of Fig. 1A is a size histogram of the perovskite NCs demonstrating a narrow size distribution of 10.6 ± 0.4 nm. Fig. S1 in Supporting Information shows additional high-resolution TEM images of the CsPbBr3 NCs with distinctive lattice fringes. The high resolution TEM images further reveal the d-spacing value of 0.41 nm between (110) crystallographic planes, attesting to the crystalline nature of the all-inorganic perovskite NCs, in line with previously reported results [16,49].
Fig. 1.

(A) TEM image, (B) distorted orthorhombic crystal structure, and (C) absorption (red) and emission (green) spectra of the synthesized CsPbBr3 nanocrystals (NCs). Figure 1A inset, histogram of 50 CsPbBr3 nanocrystal sizes obtained from TEM images.
Fig. 1B shows the crystal structures of CsPbBr3 NCs. Colloidal CsPbBr3 NCs generally have a distorted orthorhombic crystal structure, whereas bulk CsPbBr3 adopts different polymorphic crystal phases as a function of temperature. The cubic phase is thermodynamically preferred at high temperature (> 400K) whereas the orthorhombic phase is more favorable at ambient temperature (~ 300K) [50,51]. For NCs, the addition of surface-capping ligands oleic acid and oleyamine give rise to dimensional confinement, further inducing lattice distortion of the crystal structure to form the orthorhombic phase [52,53]. Indeed, powder X-ray diffraction patterns of the CsPbBr3 NCs indicate an orthorhombic phase (Fig. S1, Supporting Information, PDF# 01-072-7929). Sharp diffraction reflection peaks were observed at 2 theta values of 15, 22, and 30 degrees, corresponding to (110), (200), and (220) crystallographic planes, respectively.
To evaluate the optical properties of the NCs, the absorption and photoluminescent (PL) emission spectra of the as-synthesized CsPbBr3 NCs dispersed in hexanes were recorded (Fig. 1C). Band-edge absorption was observed at 500 nm, whereas the emission maximum peak appeared at 510 nm. The sharp excitonic absorption feature along with PL emission spectra with full-width at half maximum of 31.4 nm indicated that the colloidal CsPbBr3 NCs had a narrow size distribution, as observed in the TEM images (Fig. 1A and S1, Supporting Information).
3.2. Assessment of Optical Properties of Purified CsPbBr3 NCs.
Solid-state powder samples are typically used for fingerprint detection owing to better dispersion of fluorescent materials over the substrate. In order to utilize the CsPbBr3 NCs as fluorescent probes for LFPs detection, we needed a dried solid-state nanopowder. The colloidal CsPbBr3 NCs prepared through the hot injection method have a high density of surface-passivating ligands oleic acid and oleyamine, which make them challenging to use to develop LFPs. Native NCs with hydrophobic surface ligands tend to agglomerate and form large particulates instead of being homogeneously dispersed across the fingerprints. Thus, the CsPbBr3 NCs must be purified and passivated by treating with the mild antisolvent ethylacetate. Treating with ethylacetate eliminates excess surface-passivating ligands on the surface of NCs while maintaining the optical properties of CsPbBr3 NCs [54].
In order to evaluate the effect of purification on the optical properties of NCs, the absorption and emission spectra were monitored as a function of the number of purification cycles (denoted as 1—3X). As-obtained NCs dispersed in hexanes were defined as 1X since the NCs were collected through a reprecipitation and purification processes. To understand the solid state optical properties of NCs, NC films were prepared by spin-coating purified NCs (3X) on a fluorine-doped tin oxide substrate. Fig. 2A depicts the absorption spectra of the NCs through successive rounds of purification (1—3X) and the NC film (3X). The band-edge absorption features of CsPbBr3 NCs either dispersed in hexanes or in NC film remain at the same absorption wavelength around 500 nm regardless of the purification cycle and or condition of the sample. This indicates that the anitsolvent treatment has a minimal impact on the surface and corresponding optical properties of NCs. Digital photographs taken of CsPbBr3 NCs (samples for 1—3X and film) under ambient light conditions further confirm the colloidal stability of the NCs over successive purification cycles (Fig. 2A Inset).
Fig. 2.
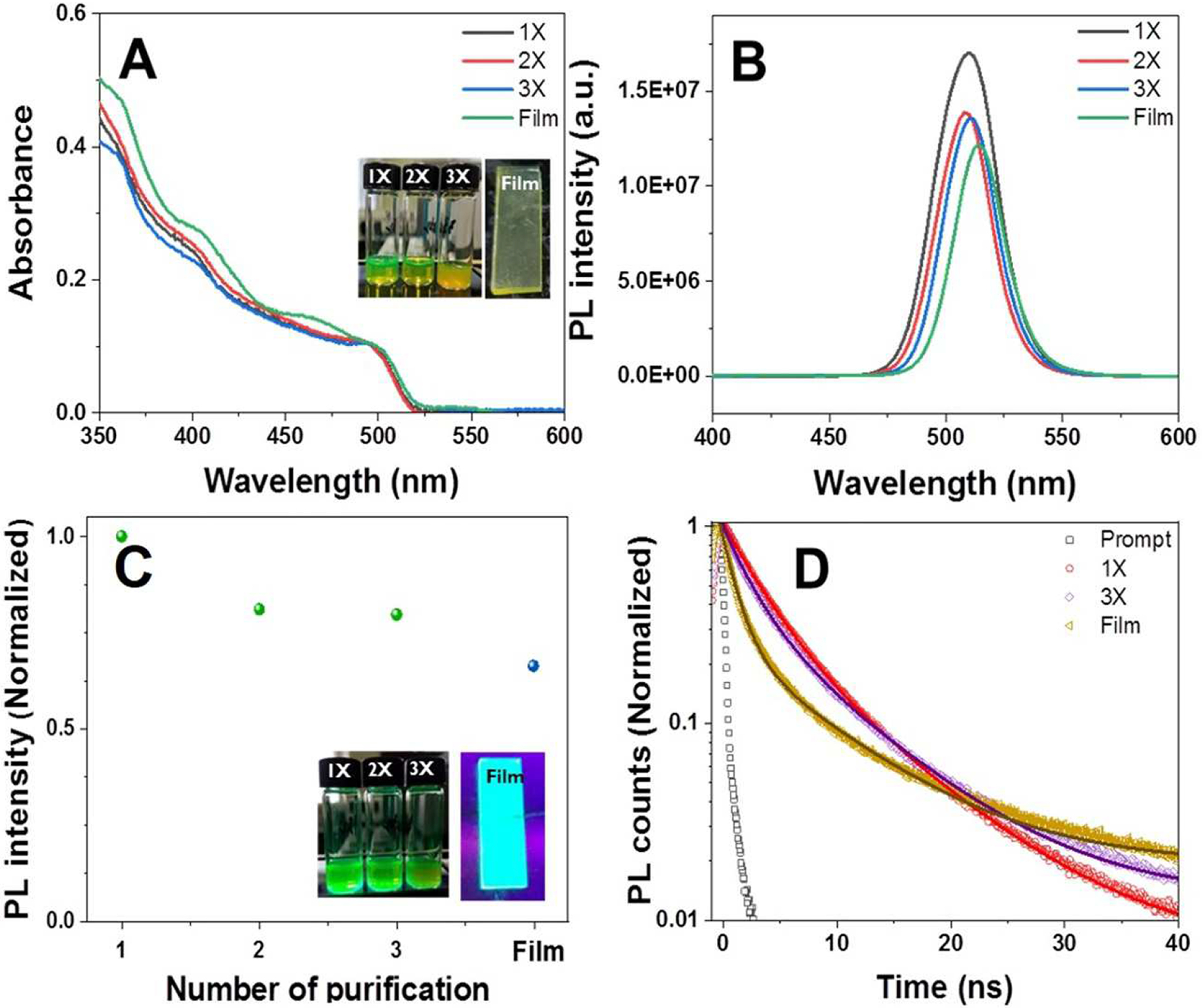
(A) Absorption spectra, (B) PL emission spectra, (C) normalized PL emission intensity (normalized to PL emission intensity of 1X), and (D) PL decay dynamics and biexponential kinetic fitting of CsPbBr3 NCs monitored at 510 nm after 370 nm pulsed laser excitation of each successive purified sample (samples 1–3X) and deposited NC film. Insets of 2A and 2C show digital photographs of 1–3X samples as well as NC film taken under ambient light (2A) and UV excitation (2C, 365 nm).
The PL emission spectra of the NCs exhibits a small red-shift of 2 nm, from 510 nm to 512 nm, over successive purification cycles (1—3X) (Fig. 2B) owing to partial removal of surface-capping ligands (resulting in a change in the dielectric environment of the NCs). The PL emission intensity of the NCs gradually decreased with increasing number of rounds of purification (up to 3X) or deposition in a film on a substrate. For the sake of comparison, the PL intensity of the samples corresponding to 1—3X and film are normalized to the PL intensity of the 1X sample (Fig. 2C). The purified NCs (2—3X) retained around 80% of their original PL emission intensity indicating that mild antisolvent treatment with ethyl acetates can remove unreacted precursors and extra ligands without causing particulate agglomeration [25]. This mild purification process results in the formation of a stable nanopowder that retains the optical properties of the CsPbBr3 NCs and that can be utilized for the application of latent fingerprint detection as demonstrated below (Fig. 2C). The PL emission of the NC film decreased by ~ 66% as compared to the initial PL emission intensity (1X). This reduction of PL emission intensity in the NC film likely represents self-reabsorption and -emission processes among NCs since they are self-assembled in a dense film [55]. Inset of Fig. 2C confirms the visual color change of the NCs under UV excitation (365 nm) over the course of purification. Despite the 20% reduction in PL intensity following purification as a result of removal of surface-capping ligands from the NCs, the PL emission colors of the samples under UV excitation (365 nm) were maintained. The optical properties of CsPbBr3 NCs over the purification steps and within the NC film are summarized in Table S1 in Supporting Information.
The PL emission decay was analyzed by monitoring the intensity of the PL maximum wavelength at 510 nm following a pulse of 370 nm laser excitation (Fig. 2D), providing further insights into the surface effects on the charge carrier recombination dynamics. The PL decay was fitted using biexponential functions and the fitting parameters are summarized in Table S2 in Supporting Information. The time-resolved PL emission decay was collected for the NCs dispersions in hexanes after 1X or 3X purification rounds, and for the NC film. Two different decay time components were obtained from the fits; a fast time component (2—3 ns) and a slow time component (7—8 ns). The average lifetime (τave) was calculated from the kinetic fitting analysis (equation 1).
| (1) |
The average lifetime slightly increased with successive rounds of purification from 6.58±0.13 ns (for 1X) to 6.67±0.11 ns (for 3X). After three rounds of purification (3X), the slow component (A2) became the more dominant decay pathway (41%) as compared to that of the 1X sample (37%), due to the detachment of surface-passivating ligands (Table S2, Supporting Information). However, the average lifetimes of the 1X and 3X samples were similar, which indicates the perovskite NCs are stable and there is no significant surface modification of the nanocrystals through successive rounds of purification. It is worth noting that the NC film exhibited faster PL decay with an average lifetime of 3.4 ns due to self-assembly of NCs within the film, consistent with the decreased PL emission intensity (Fig. 2C). The relative stability of the PL emission and lifetime indicate that purification with ethyl acetate has a minimal impact on the optical properties of the NCs.
3.3. Development of Latent Fingerprint Using CsPbBr3 Perovskite NCs.
We next tested the CsPbBr3 NC nanopowder as a dusting material to develop LFPs. The fingerprint residue contains a mixture of different amino acids, salts, proteins, lipids, and metabolites [28,31]. Although it has commonly been thought that a large portion of the residue is water, it can be changed by contamination via touching other surfaces. In particular, fingers are commonly coated with a large amount of lipids due to unconscious touching of the oil rich regions of the body including the face, nose, and forehead. We thus obtained sebaceous fingerprints from 3 donors deposited on transparent plastic surfaces to evaluate the detection capability and reproducibility of the CsPbBr3 perovskite nanopowder. In order to generate oil rich fingerprints by contact on a transparent plastic surface, each donor washed his/her hands with soap and water to rinse away all other residues then rubbed their fingers over oily parts of their face such as forehead and nose. The oil coated fingertips were then gently touched to a petri dish. After treating the fingerprint with nanopowder, the excess powder was readily removed using an air duster (air velocity ~6.5 m/s) for 30 s. The specimens were subsequently exposed to a 455 nm light source and images were obtained with a camera. Well-defined ridges and furrows of fingerprints were clearly confirmed in all donor’s fingerprints labeled using the nanopowder due to the strong chemical interaction between the nanopowder and fingerprint residue (Fig. 3A–C). The intrinsic surface properties of CsPbBr3 NCs are primarily determined by the surface-passivating ligands oleic acid and oleyamine. Both of these ligand molecules are highly hydrophobic due to long alkyl chains in their molecular structures. Consequently, the hydrophobic interaction between surface ligands of the NCs and a large number of hydrophobic components associated with both sebaceous excretion and continuous inadvertent touching of oily parts of the body is considered as the main chemical interaction [56–58]. In a systematic study of the interactions between fingerprint residues and fluorescent poly(p-phenylenevinylene) (PPV) nanoparticles, Chen et al. confirmed the dominant role of hydrophobic interactions in selective and specific LFP development [58]. Thus, selectivity depends on the hydrophobic interactions between fingerprint residues and NC nanopowder. Owing to the high selectivity and sensitivity of the nanopowder, the high resolution fluorescence images clearly demonstrate different core shapes within each donor’s fingerprint (right side of Fig. 3C). Semi-quantitative analysis of the adhesion efficiency of nanopowders on fingerprints can be performed by plotting the profile of the resulting fluorescence image. Based on this analysis, the nanopowder primarily interacted with ridges rather than furrows in the fingerprint (Fig. 3 D‒F). To investigate the efficiency and practicality of CsPbBr3 nanopowder for LFP detection, LFPs were developed using either commercial green fluorescent powder or CsPbBr3 nanopowder. Due to the high nonspecific adhesion to the surface, images obtained with the commercial powder developed LFPs were of low quality and difficult to quantify. On the contrary, images obtained with the nanopowder developed fingerprints revealed clear ridges and furrows with unique features (Fig. S2 A and B, Supporting Information). The differences in fluorescence images of LPF developed with conventional powder or CsPbBr3 nanopowder were quantified by comparing the total fluorescence obtained from each image, in addition to the variation in intensity between the peaks and valleys that make up the latent fingerprint image. The overall fluorescence intensity of the fluorescence image of LFPs developed with the commercial powder is higher than those developed with the CsPbBr3 NC powder due to nonspecific adhesion to the substrate. However, since the CsPbBr3 perovskite nanopowder specifically interacted with ridges in the LFP via hydrophobic interactions, the fluorescence intensity ratio between ridges and valleys in the images was 4-fold higher for CsPbBr3 nanopowders than commercial powders (Fig. S2 C, D, and Table S3, Supporting Information). Due to this improved specificity and improved signal to background, the fluorescent images of CsPbBr3 nanopowder labeled LFP permits the identification of finer structures than images of commercial powder labeled LFP. Together, these results demonstrate the important role of the hydrophobic surface-passivating ligands and highly luminescent and stable optical properties of CsPbBr3 perovskite NC powders for efficient LFP development and imaging.
Fig. 3.

(A–C) High resolution fluorescence images of CsPbBr3 NCs labeled LFPs from three donors on transparent plastic. The fluorescence images were obtained with 455 nm excitation, a 15 s exposure, and an SYBR green (515–570 nm) emission filter. Different core shapes are identified on the right-side. (E, F) Normalized PL intensity along two orthogonal lines in (D): (E) vertical axis from top to bottom and (F) horizontal axis from left to right.
3.4. Analysis of Unique Details of Latent Fingerprint Using CsPbBr3 Perovskite NCs.
Since the fingerprint structure has distinct features, which is not only different from person to person, but also between twins, it is used as direct biometric information for individual credentials, personal identification, and civil security [30,40,59,60]. The unique features of fingerprints are distinguished by three levels of detail at different scales, namely level 1, level 2, and level 3, to discriminate individual identity [39,61,62]. Level 1 delineates global level features such as overall appearance including core shape (loop, whorl, and arch) and ridge flow of fingerprints. Although level 1 can be used for classification and indexing for fingerprints, it is insufficient for precise matching of personal identification. The minute details of the fingerprint such as ridge endings, bifurcation, islands, short ridges, lakes, and crossovers are defined as level 2 at the local level. These minute details permit individual identification due to the number of ridge details (150 different local ridge characteristics) as well as high stability and integrity depending on impression conditions. Level 3 is defined as attributes of intra-ridge details, including shape, edge contours, width, pores, curvature, and other permanent minutiae such as incipient ridges and dots. With this background in mind, we analyzed the fluorescence images of developed LFPs using perovskite CsPbBr3 NC powders. High-resolution fluorescence images of nanopowder-treated LFPs exhibit different core shapes and ridge flows (Fig. 4A and S3, Supporting Information). In addition, magnified fluorescence images of developed LFPs reveal level 1, 2, and 3 details of friction ridges including core shape, independent ridges, bifurcations, ending ridges, crossovers, bridges, scars, and pores (right side of Fig. 4A and S3, Supporting Information). The fluorescence images of nanopowder-labeled LFPs from donor 3 also show different ridge-line flow, core shape, local distribution, and a number of additional features. Since level 2 and 3 details correspond to specific ridge flows, including the starting position, endpoint, length, and width of ridges, these features in the fingerprint enable individual identification.
Fig. 4.

(A–H) High resolution and magnified fluorescence images of developed LFPs using CsPbBr3 NCs. An 455 nm excitation, a 15 s exposure, and an SYBR green (515–570 nm) emission filter were used to obtain these fluorescence images. (A) High resolution image shows overall ridge pattern in the fingerprint (level 1). (B–H) Magnified fluorescence images describe level 2 and level 3 features such as bifurcation, independent ridge, ending ridge, crossover, bridge (level 2), scar, and pores (level 3).
3.5. Practical Application of CsPbBr3 Perovskite Nanopowders for Latent Fingerprint Detection.
We further investigated the practical application of CsPbBr3 perovskite nanopowder to develop LFPs since diverse conditions such as deposition surface and age of fingerprints can significantly influence to the subsequent effectiveness of LFP detection [28,63,64]. In order to evaluate the applicability of the nanopowder for practical LFP detection, a series of porous (e.g., paper and wood) and nonporous (aluminum foil, ceramic tile, glass, and plastic) substrates were used. Bright-field optical images of developed LFPs taken with a digital camera under room light were captured on glass, transparent plastic, and wood surfaces. However, nanopowder-treated LFPs on other substrates were not readily observed in bright-field optical images (Fig. 5). Upon excitation with 455 nm light, the developed LFPs clearly reveal unique details of fingerprints due to the high sensitivity and selectivity regardless of the surface type.
Fig. 5.

Bright-field and fluorescence images of CsPbBr3 nanopowder labeled LFPs on various surfaces. (A) Aluminum foil, (B) ceramic, (C) glass, (D) colored paper (E) transparent plastic, (F) wood. A Nikon D80 camera with an AF-Micro Nikkor 105 mm 1:2.8 lens was used to obtain the bright-field optical images of CsPbBr3 NCs treated LFPs. The high resolution fluorescence images of CsPbBr3 nanopowder developed LFPs were recorded with a SYBR Green (515–570 nm) emission filter, under illumination of 455 nm excitation wavelength for 15 s exposure in a UVP BioSpectrum Imaging System.
Significant changes in the composition of fingerprint residues can occur over time by oxidation and decomposition mechanisms. These effects can influence the development of LFPs as a function of age [31,32]. To assess the effect of aging on the development of LFPs, we prepared three LFPs on glass coverslips, which were kept in an environmentally controlled chamber for 3 and 14 days. High resolution fluorescent images of aged LFPs were obtained with 455 nm excitation (Fig. 6A–C). Even though the fluorescence intensity of nanopowder-labeled LFPs decreased slightly over time, ridge details sufficient for individual identification could still be obtained from the fluorescence images. We further explored the long-term stability of nanopowder developed fingerprints, the photostability of the nanopowder, and the interaction between the fingerprint and the nanopowder. Nanopowder developed LFPs on cover glass were stored under ambient conditions for 14 days. Fluorescence images of the developed LFPs were acquired after 3 and 14 days. The fluorescence intensity of images diminished compared with the freshly developed fingerprint, but the fingerprint ridge patterns and unique features remained visible (Fig. 6D–E).
Fig. 6.

High resolution fluorescence images of developed LFPs on glass. Measurement of aging effect for LFPs: (A) As prepared, (B) after 3 days, and (C) after 14 days aged LFPs. Stability assessment of CsPbBr3 nanopowder developed LFPs as function of time: (D) developed on day 1, (E) developed after 3 days, and (F) developed after 14 days. The 455 nm excitation, a 15 s exposure, and an SYBR green (515–570 nm) emission filter were used to record the fluorescence images.
4. Conclusion
Bright and stable fluorescent CsPbBr3 perovskite NCs were synthesized via the hot injection method. The resulting NCs have an uniform size and shape as well as narrow PL emission line-width. The NCs retained their PL emission intensity during purification, demonstrating the optical stability of the NCs. The stable fluorescence, and hydrophobic surface of nanopowders obtained by purification of the NCs, result in high sensitivity, selectivity, and contrast in developed LFPs. High-resolution fluorescence images of LFPs reveal unique features of fingerprint at levels 1, 2, and 3, which are sufficient to unambiguously identify individuals. Therefore CsPbBr3 perovskite nanopowder is an effective fluorescent probe material for powder dusting method in LFPs detection on various substrates. Although the application of CsPbBr3 perovskite nanopowder for LFP detection shows promising potential as a powder dusting material, further work will be required to improve aspects of this approach. The toxicity associated with the CsPbBr3 perovskites could be reduced by switching to lead free perovskites or by encapsulating the nanocrystals with a silica shell [65–68]. Further work involving encapsulation or alternative surface stabilization approaches will be required to improve the PL emission intensity over time or in real crime scenes [69,70].
Supplementary Material
Highlights.
Lead halide perovskite nanocrystals were synthesized via hot injection
Perovskite nanocrystals remain highly optically stable after purification
Perovskite nanocrystals afford sensitive and specific latent fingerprint detection
ACKNOWLEDGMENT
The authors acknowledge to the fingerprint donors. This research was supported by the NHLBI and NCI DIR programs of the National Institutes of Health.
Funding Sources
The NHLBI intramural programs of the National Institutes of Health. Grants ZIAHL006087-09 to KCN.
Acknowledgements
All persons who have made substantial contributions to the work reported in the manuscript (e.g., technical help, writing and editing assistance, general support), but who do not meet the criteria for authorship, are named in the Acknowledgements and have given us their written permission to be named. If we have not included an Acknowledgements, then that indicates that we have not received substantial contributions from non-authors.
This statement is signed by all the authors (a photocopy of this form may be used if there are more than 10 authors):
| Author’s name (typed) | Author’s signature | Date |
|---|---|---|
| Keir C. Neuman |  |
06. 17. 2021 |
| Haksung Jung |  |
06. 18. 2021 |
| Junsang Cho |  |
06. 16. 2021 |
ABBREVIATIONS
- LFPs
latent fingerprints
- NCs
nanocrystals
- LHP
Lead halide perovskites
- PL
photoluminescence
- TEM
transmission electron microscopy
- Cs2CO3
Cesium carbonate
- OA
oleic acid
- OLAm
oleylamine
- 1-ODE
1-octadecence
- PbBr2
lead bromide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information.
High resolution TEM images and powder XRD of CsPbBr3 perovskite NCs, fluorescence images of developed LFPs.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Akkerman QA, D’Innocenzo V, Accornero S, Scarpellini A, Petrozza A, Prato M, Manna L, Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc 137 (2015) 10276–10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Akkerman QA, Rainò G, Kovalenko MV, Manna L, Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater 17 (2018) 394–405. [DOI] [PubMed] [Google Scholar]
- [3].Shamsi J, Dang Z, Bianchini P, Canale C, Di Stasio F, Brescia R, Prato M, Manna L, Colloidal Synthesis of Quantum Confined Single Crystal CsPbBr3 Nanosheets with Lateral Size Control up to the Micrometer Range. J. Am. Chem. Soc 138 (2016) 7240–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].De Roo J, Ibáñez M, Geiregat P, Nedelcu G, Walravens W, Maes J, Martins JC, Van Driessche I, Kovalenko MV, Hens Z, Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 10 (2016) 2071–2081. [DOI] [PubMed] [Google Scholar]
- [5].Lignos I, Stavrakis S, Nedelcu G, Protesescu L, deMello AJ, Kovalenko MV, Synthesis of Cesium Lead Halide Perovskite Nanocrystals in a Droplet-Based Microfluidic Platform: Fast Parametric Space Mapping. Nano Lett. 16 (2016) 1869–1877. [DOI] [PubMed] [Google Scholar]
- [6].Protesescu L, Yakunin S, Bodnarchuk MI, Krieg F, Caputo R, Hendon CH, Yang RX, Walsh A, Kovalenko MV, Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 15 (2015) 3692–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin K, Xing J, Quan LN, de Arquer FPG, Gong X, Lu J, Xie L, Zhao W, Zhang D, Yan C, Li W, Liu X, Lu Y, Kirman J, Sargent EH, Xiong Q, Wei Z, Perovskite Light-Emitting Diodes with External Quantum Efficiency Exceeding 20 Per Cent. Nature 562 (2018) 245–248. [DOI] [PubMed] [Google Scholar]
- [8].Xiao Z, Kerner RA, Zhao L, Tran NL, Lee KM, Koh T-W, Scholes GD, Rand BP, Efficient Perovskite Light-Emitting Diodes Featuring Nanometre-Sized Crystallites. Nat. Photonics 11 (2017) 108–115. [Google Scholar]
- [9].Zhu H, Fu Y, Meng F, Wu X, Gong Z, Ding Q, Gustafsson MV, Trinh MT, Jin S, Zhu XY, Lead Halide Perovskite Nanowire Lasers with Low Lasing Thresholds and High Quality Factors. Nat. Mater 14 (2015) 636–642. [DOI] [PubMed] [Google Scholar]
- [10].Eaton SW, Lai M, Gibson NA, Wong AB, Dou L, Ma J, Wang L-W, Leone SR, Yang P, Lasing in Robust Cesium Lead Halide Perovskite Nanowires. Proc. Natl. Acad. Sci. U. S. A 113 (2016) 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang H-C, Lin S-Y, Tang A-C, Singh BP, Tong H-C, Chen C-Y, Lee Y-C, Tsai T-L, Liu R-S, Mesoporous Silica Particles Integrated with All-Inorganic CsPbBr3 Perovskite Quantum-Dot Nanocomposites (MP-PQDs) with High Stability and Wide Color Gamut Used for Backlight Display. Angew. Chem. Int. Ed 55 (2016) 7924–7929. [DOI] [PubMed] [Google Scholar]
- [12].Lu M, Zhang Y, Wang S, Guo J, Yu WW, Rogach AL, Metal Halide Perovskite Light-Emitting Devices: Promising Technology for Next-Generation Displays. Adv. Funct. Mater 29 (2019) 1902008. [Google Scholar]
- [13].Braham EJ, Cho J, Forlano KM, Watson DF, Arròyave R, Banerjee S, Machine Learning-Directed Navigation of Synthetic Design Space: A Statistical Learning Approach to Controlling the Synthesis of Perovskite Halide Nanoplatelets in the Quantum-Confined Regime. Chem. Mater 31 (2019) 3281–3292. [Google Scholar]
- [14].Cho J, DuBose JT, Kamat PV, Charge Carrier Recombination Dynamics of Two-Dimensional Lead Halide Perovskites. J. Phys. Chem. Lett 11 (2020) 2570–2576. [DOI] [PubMed] [Google Scholar]
- [15].Parobek D, Dong Y, Qiao T, Rossi D, Son DH, Photoinduced Anion Exchange in Cesium Lead Halide Perovskite Nanocrystals. J. Am. Chem. Soc 139 (2017) 4358–4361. [DOI] [PubMed] [Google Scholar]
- [16].Cho J, Banerjee S, Ligand-Directed Stabilization of Ternary Phases: Synthetic Control of Structural Dimensionality in Solution-Grown Cesium Lead Bromide Nanocrystals. Chem. Mater 30 (2018) 6144–6155. [Google Scholar]
- [17].Cho J, Choi Y-H, O’Loughlin TE, De Jesus L, Banerjee S, Ligand-Mediated Modulation of Layer Thicknesses of Perovskite Methylammonium Lead Bromide Nanoplatelets. Chem. Mater 28 (2016) 6909–6916. [Google Scholar]
- [18].ten Brinck S, Infante I, Surface Termination, Morphology, and Bright Photoluminescence of Cesium Lead Halide Perovskite Nanocrystals. ACS Energy Lett. 1 (2016) 1266–1272. [Google Scholar]
- [19].Smock SR, Williams TJ, Brutchey RL, Quantifying the Thermodynamics of Ligand Binding to CsPbBr3 Quantum Dots. Angew. Chem. Int. Ed 57 (2018) 11711–11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ripka EG, Deschene CR, Franck JM, Bae I-T, Maye MM, Understanding the Surface Properties of Halide Exchanged Cesium Lead Halide Nanoparticles. Langmuir 34 (2018) 11139–11146. [DOI] [PubMed] [Google Scholar]
- [21].Grisorio R, Conelli D, Giannelli R, Fanizza E, Striccoli M, Altamura D, Giannini C, Allegretta I, Terzano R, Suranna GP, A new route for the shape differentiation of cesium lead bromide perovskite nanocrystals with near-unity photoluminescence quantum yield. Nanoscale 12 (2020) 17053–17063. [DOI] [PubMed] [Google Scholar]
- [22].Paul S, Samanta A, N-Bromosuccinimide as Bromide Precursor for Direct Synthesis of Stable and Highly Luminescent Green-Emitting Perovskite Nanocrystals. ACS Energy Lett. 5 (2020) 64–69. [Google Scholar]
- [23].Krieg F, Ochsenbein ST, Yakunin S, ten Brinck S, Aellen P, Süess A, Clerc B, Guggisberg D, Nazarenko O, Shynkarenko Y, Kumar S, Shih C-J, Infante I, Kovalenko MV, Colloidal CsPbX3 (X = Cl, Br, I) Nanocrystals 2.0: Zwitterionic Capping Ligands for Improved Durability and Stability. ACS Energy Lett. 3 (2018) 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang Y, Siegler TD, Thomas CJ, Abney MK, Shah T, De Gorostiza A, Greene RM, Korgel BA, A “Tips and Tricks” Practical Guide to the Synthesis of Metal Halide Perovskite Nanocrystals. Chem. Mater 32 (2020) 5410–5423. [Google Scholar]
- [25].Swarnkar A, Marshall AR, Sanehira EM, Chernomordik BD, Moore DT, Christians JA, Chakrabarti T, Luther JM, Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 354 (2016) 92–95. [DOI] [PubMed] [Google Scholar]
- [26].Dahl JC, Wang X, Huang X, Chan EM, Alivisatos AP, Elucidating the Weakly Reversible Cs–Pb–Br Perovskite Nanocrystal Reaction Network with High-Throughput Maps and Transformations. J. Am. Chem. Soc 142 (2020) 11915–11926. [DOI] [PubMed] [Google Scholar]
- [27].Zhang D, Yu Y, Bekenstein Y, Wong AB, Alivisatos AP, Yang P, Ultrathin Colloidal Cesium Lead Halide Perovskite Nanowires. J. Am. Chem. Soc 138 (2016) 13155–13158. [DOI] [PubMed] [Google Scholar]
- [28].Holder EH, Robinson LO, Laub JH. The Fingerprint Sourcebook. Washington, DC: U.S. Dept. of Justice, Office of Justice Programs, National Institute of Justice; 2011. [Google Scholar]
- [29].Hazarika P, Jickells SM, Wolff K, Russell DA, Imaging of Latent Fingerprints through the Detection of Drugs and Metabolites. Angew. Chem. Int. Ed 47 (2008) 10167–10170. [DOI] [PubMed] [Google Scholar]
- [30].Hazarika P, Russell DA, Advances in Fingerprint Analysis. Angew. Chem. Int. Ed 51 (2012) 3524–3531. [DOI] [PubMed] [Google Scholar]
- [31].Girod A, Ramotowski R, Weyermann C, Composition of Fingermark Residue: A Qualitative and Quantitative Review. Forensic Sci. Int 223 (2012) 10–24. [DOI] [PubMed] [Google Scholar]
- [32].Cadd S, Islam M, Manson P, Bleay S, Fingerprint Composition and Aging: A Literature Review. Sci. Justice 55 (2015) 219–238. [DOI] [PubMed] [Google Scholar]
- [33].Sodhi GS, Kaur J, Powder Method for Detecting Latent Fingerprints: A Review. Forensic Sci. Int 120 (2001) 172–176. [DOI] [PubMed] [Google Scholar]
- [34].Jasuja OP, Singh G, Development of Latent Fingermarks on Thermal Paper: Preliminary Investigation into Use of Iodine Fuming. Forensic Sci. Int 192 (2009) e11–e16. [DOI] [PubMed] [Google Scholar]
- [35].Davis LWL, Kelly PF, King RSP, Bleay SM, Visualisation of Latent Fingermarks on Polymer Banknotes Using Copper Vacuum Metal Deposition: A Preliminary Study. Forensic Sci. Int 266 (2016) e86–e92. [DOI] [PubMed] [Google Scholar]
- [36].Frick AA, Busetti F, Cross A, Lewis SW, Aqueous Nile Blue: a Simple, Versatile and Safe Reagent for the Detection of Latent Fingermarks. Chem. Commun 50 (2014) 3341–3343. [DOI] [PubMed] [Google Scholar]
- [37].Lee HC, Gaensslen RE, Ramotowski RS, Berry J, Stoney DA, Olsen S, Robert D., Almog J, Menzel ER, Cantu A, Johnson JL, Jain A, Pankanti S, Hazen RJ, Phillips CE, Advances in Fingerprint Technology. 2nd ed.Florida: CRC Press; 2001. [Google Scholar]
- [38].Wang M, Li M, Yu A, Wu J, Mao C, Rare Earth Fluorescent Nanomaterials for Enhanced Development of Latent Fingerprints. ACS Appl. Mater. Interfaces 7 (2015) 28110–28115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim Y-J, Jung H-S, Lim J, Ryu S-J, Lee J-K, Rapid Imaging of Latent Fingerprints Using Biocompatible Fluorescent Silica Nanoparticles. Langmuir 32 (2016) 8077–8083. [DOI] [PubMed] [Google Scholar]
- [40].Wang M, Li M, Yu A, Zhu Y, Yang M, Mao C, Fluorescent Nanomaterials for the Development of Latent Fingerprints in Forensic Sciences. Adv. Funct. Mater 27 (2017) 1606243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jung H-S, Cho K-J, Ryu S-J, Takagi Y, Roche PA, Neuman KC, Biocompatible Fluorescent Nanodiamonds as Multifunctional Optical Probes for Latent Fingerprint Detection. ACS Appl. Mater. Interfaces 12 (2020) 6641–6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jessirie D, Hilton JK, Amanda VE, Nanotechnology as a New Tool for Fingermark Detection: A Review. Curr. Nanosci 7 (2011) 153–159. [Google Scholar]
- [43].Kumar P, Singh S, Gupta BK, Future Prospects of Luminescent Nanomaterial Based Security Inks: From Synthesis to Anti-counterfeiting Applications. Nanoscale 8 (2016) 14297–14340. [DOI] [PubMed] [Google Scholar]
- [44].Ryu S-J, Jung H-S, Lee J-K, Latent Fingerprint Detection Using Semiconductor Quantum Dots as a Fluorescent Inorganic Nanomaterial for Forensic Application. Bull. Korean Chem. Soc 36 (2015) 2561–2564. [Google Scholar]
- [45].Wang J, Wei T, Li X, Zhang B, Wang J, Huang C, Yuan Q, Near-Infrared-Light-Mediated Imaging of Latent Fingerprints Based on Molecular Recognition. Angew. Chem. Int. Ed 53 (2014) 1616–1620. [DOI] [PubMed] [Google Scholar]
- [46].Huynh C, Brunelle E, Halámková L, Agudelo J, Halámek J, Forensic Identification of Gender from Fingerprints. Anal. Chem 87 (2015) 11531–11536. [DOI] [PubMed] [Google Scholar]
- [47].Li M, Tian T, Zeng Y, Zhu S, Lu J, Yang J, Li C, Yin Y, Li G, Individual Cloud-Based Fingerprint Operation Platform for Latent Fingerprint Identification Using Perovskite Nanocrystals as Eikonogen. ACS Appl. Mater. Interfaces 12 (2020) 13494–13502. [DOI] [PubMed] [Google Scholar]
- [48].Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, Fiji: an open-source platform for biological-image analysis. Nat. Methods 9 (2012) 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cho J, Jin H, Sellers DG, Watson DF, Son DH, Banerjee S, Influence of Ligand Shell Ordering on Dimensional Confinement of Cesium Lead Bromide (CsPbBr3) Perovskite Nanoplatelets. J. Mater. Chem. C 5 (2017) 8810–8818. [Google Scholar]
- [50].Stoumpos CC, Malliakas CD, Peters JA, Liu Z, Sebastian M, Im J, Chasapis TC, Wibowo AC, Chung DY, Freeman AJ, Wessels BW, Kanatzidis MG, Crystal Growth of the Perovskite Semiconductor CsPbBr3: A New Material for High-Energy Radiation Detection. Cryst. Growth Des 13 (2013) 2722–2727. [Google Scholar]
- [51].Zhang M, Zheng Z, Fu Q, Chen Z, He J, Zhang S, Yan L, Hu Y, Luo W, Growth and Characterization of All-Inorganic Lead Halide Perovskite Semiconductor CsPbBr3 Single Crystals. CrystEngComm 19 (2017) 6797–6803. [Google Scholar]
- [52].Cottingham P, Brutchey RL, Compositionally Dependent Phase Identity of Colloidal CsPbBr3–xIx Quantum Dots . Chem. Mater 28 (2016) 7574–7577. [Google Scholar]
- [53].Cottingham P, Brutchey RL, On the Crystal Structure of Colloidally Prepared CsPbBr3 Quantum Dots. Chem. Commun 52 (2016) 5246–5249. [DOI] [PubMed] [Google Scholar]
- [54].Imran M, Ijaz P, Baranov D, Goldoni L, Petralanda U, Akkerman Q, Abdelhady AL, Prato M, Bianchini P, Infante I, Manna L, Shape-Pure, Nearly Monodispersed CsPbBr3 Nanocubes Prepared Using Secondary Aliphatic Amines. Nano Lett. 18 (2018) 7822–7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Park DH, Han JS, Kim W, Jang HS, Facile Synthesis of Thermally Stable CsPbBr3 Perovskite Quantum Dot-inorganic SiO2 Composites and Their Application to White Light-Emitting Diodes with Wide Color Gamut. Dyes Pigment. 149 (2018) 246–252. [Google Scholar]
- [56].Theaker BJ, Hudson KE, Rowell FJ, Doped hydrophobic silica nano- and micro-particles as novel agents for developing latent fingerprints. Forensic Sci. Int 174 (2008) 26–34. [DOI] [PubMed] [Google Scholar]
- [57].Shin-Il Kim B, Jin Y-J, Uddin MA, Sakaguchi T, Woo HY, Kwak G, Surfactant Chemistry for Fluorescence Imaging of Latent Fingerprints Using Conjugated Polyelectrolyte Nanoparticles. Chem. Commun 51 (2015) 13634–13637. [DOI] [PubMed] [Google Scholar]
- [58].Chen H, Ma R-l, Chen Y, Fan L-J, Fluorescence Development of Latent Fingerprint with Conjugated Polymer Nanoparticles in Aqueous Colloidal Solution. ACS Appl. Mater. Interfaces 9 (2017) 4908–4915. [DOI] [PubMed] [Google Scholar]
- [59].Xu L, Li Y, Wu S, Liu X, Su B, Imaging Latent Fingerprints by Electrochemiluminescence. Angew. Chem. Int. Ed 51 (2012) 8068–8072. [DOI] [PubMed] [Google Scholar]
- [60].Wang Y, Wang J, Ma Q, Li Z, Yuan Q, Recent Progress in Background-free Latent Fingerprint Imaging. Nano Res. 11 (2018) 5499–5518. [Google Scholar]
- [61].Maltoni D, Maio D, Jain AK, Prabhakar S, Handbook of Fingerprint Recognition. New York: Springer-Verlag; 2009. [Google Scholar]
- [62].Wang Y-L, Li C, Qu H-Q, Fan C, Zhao P-J, Tian R, Zhu M-Q, Real-Time Fluorescence In Situ Visualization of Latent Fingerprints Exceeding Level 3 Details Based on Aggregation-Induced Emission. J. Am. Chem. Soc 142 (2020) 7497–7505. [DOI] [PubMed] [Google Scholar]
- [63].Sears VG, Bleay SM, Bandey HL, Bowman VJ, A Methodology for Finger Mark Research. Sci. Justice 52 (2012) 145–160. [DOI] [PubMed] [Google Scholar]
- [64].Wang J, Ma Q, Liu H, Wang Y, Shen H, Hu X, Ma C, Yuan Q, Tan W, Time-Gated Imaging of Latent Fingerprints and Specific Visualization of Protein Secretions via Molecular Recognition. Anal. Chem 89 (2017) 12764–12770. [DOI] [PubMed] [Google Scholar]
- [65].Li J, Cao H-L, Jiao W-B, Wang Q, Wei M, Cantone I, Lü J, Abate A, Biological impact of lead from halide perovskites reveals the risk of introducing a safe threshold. Nat. Commun 11 (2020) 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kwak JI, Kim L, An Y-J, Sublethal toxicity of PbI2 in perovskite solar cells to fish embryos (Danio rerio and Oryzias latipes): Deformity and growth inhibition. Sci. Total Environ 771 (2021) 145388. [DOI] [PubMed] [Google Scholar]
- [67].Zheng W, Sun R, Liu Y, Wang X, Liu N, Ji Y, Wang L, Liu H, Zhang Y, Excitation Management of Lead-Free Perovskite Nanocrystals Through Doping. ACS Appl. Mater. Interfaces 13 (2021) 6404–6410. [DOI] [PubMed] [Google Scholar]
- [68].Zhong Q, Cao M, Hu H, Yang D, Chen M, Li P, Wu L, Zhang Q, One-Pot Synthesis of Highly Stable CsPbBr3@SiO2 Core–Shell Nanoparticles. ACS Nano 12 (2018) 8579–8587. [DOI] [PubMed] [Google Scholar]
- [69].Yang Z, Xu J, Zong S, Xu S, Zhu D, Zhang Y, Chen C, Wang C, Wang Z, Cui Y, Lead Halide Perovskite Nanocrystals–Phospholipid Micelles and Their Biological Applications: Multiplex Cellular Imaging and in Vitro Tumor Targeting. ACS Appl. Mater. Interfaces 11 (2019) 47671–47679. [DOI] [PubMed] [Google Scholar]
- [70].Wang Y, Varadi L, Trinchi A, Shen J, Zhu Y, Wei G, Li C, Spray-Assisted Coil–Globule Transition for Scalable Preparation of Water-Resistant CsPbBr3@PMMA Perovskite Nanospheres with Application in Live Cell Imaging. Small 14 (2018) 1803156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


