Abstract
Background:
Inflammation is associated with coronary artery disease (CAD) and myocardial infarction (MI). Patients with gout are at increased risk of MI, and colchicine is associated with a reduced risk of MI. The objective of this study was to determine whether colchicine prevents incident development of CAD in patients with gout.
Methods:
This retrospective study followed a cohort of male patients with gout without known CAD at the time of diagnosis of gout in the VA New York Harbor Healthcare System. The association between colchicine use and development of incident CAD, defined as evidence of ischemia or obstructive CAD on stress test or angiography, was determined using an inverse probability weighted (IPW) Cox proportional hazard model.
Results:
Among 178,877 patients, 1638 met criteria of gout, of whom 722 without known CAD at baseline (446 colchicine users and 276 nonusers) were followed for a median of 96 months (57 to 117). A trend toward association between use of colchicine and reduced incident CAD was observed but not statistically significant (IPW hazard ratio [HR], 0.49; 0.23–1.05). In patients without chronic kidney disease, use of colchicine was associated with a lower rate of incident CAD (interaction P = 0.005, IPW HR, 0.31; 0.14–0.70). Colchicine was also associated with a lower rate of the composite of incident CAD and MI (IPW HR, 0.37; 0.16–0.83).
Conclusions:
In male patients with gout and no known CAD, a trend of reduced incident CAD was observed with use of colchicine that was not statistically significant. Larger, prospective studies will be required to assess the primary prevention benefit of colchicine definitively.
RÉSUMÉ
Introduction:
L’inflammation est associée à la coronaropathie et à l’infarctus du myocarde (IM). Les patients atteints de la goutte sont exposés à un risque accru d’IM. Néanmoins, la colchicine est associée à la diminution de ce risque d’IM. L’objectif de la présente étude était de déterminer si la colchicine prévient l’incidence de la coronaropathie chez les patients atteints de goutte.
Méthodes:
Cette étude rétrospective portait sur le suivi d’une cohorte de patients de sexe masculin atteints de goutte, mais sans coronaropathie connue au moment du diagnostic de la goutte, du VA New York Harbor Healthcare System. L’association entre l’utilisation de la colchicine et l’incidence de la coronaropathie, définie par les signes d’ischémie ou de coronaropathie obstructive à l’épreuve d’effort ou à l’angiographie, était déterminée à l’aide du modèle à risques proportionnels de Cox pondéré par l’inverse de la probabilité (IPW, de l’anglais inverse probability weighted).
Résultats:
Parmi les 17S S77 patients, 163S patients répondaient aux critères de la goutte. Parmi ces derniers, 722 patients qui n’avaient pas de coronaropathie connue au début (446 utilisateurs de colchicine et 276 non-utilisateurs) ont eu un suivi médian de 96 mois (de 57 à 117). On observait une tendance à l’association entre l’utilisation de la colchicine et la réduction de l’incidence de coronaropathie, mais cette tendance n’était pas significative sur le plan statistique (rapport de risque [RR] IPW, 0,49; 0,23–1,05). Chez les patients non atteints de maladie rénale chronique, l’utilisation de la colchicine était associée à un taux plus faible de l’incidence de coronaropathie (interaction p = 0,005, RR IPW, 0,31; 0,14–0,70). La colchicine était également associée à un taux plus faible du critère composite de l’incidence de coronaropathie et d’IM (RR IPW, 0,37; 0,16–0,S3).
Conclusions:
Chez les patients de sexe masculin atteints de goutte et n’ayant pas de coronaropathie connue, on observait une tendance à la réduction de l’incidence de coronaropathie lors de l’utilisation de la colchicine, mais cette réduction n’était pas significative sur le plan statistique. De plus vastes études prospectives qui porteront sur l’évaluation définitive des avantages de la colchicine en prévention primaire seront nécessaires.
Atherosclerosis is an inflammatory disorder with immune cell infiltrates in fatty streaks, atheroma formation, and acute plaque rupture.1–4 Inflammation-driven upregulation of adhesion molecules on endothelial cells and activation of neutrophils also play important roles in the development of coronary artery disease (CAD) and myocardial infarction (MI).5–7 Low-grade systemic inflammation is associated with an up to 4-fold increase in the risk of future cardiovascular events,8 and the inflammatory pathway to cardiovascular events includes the cytokines interleukin (IL)-1β and IL-6.9,10
Colchicine has been used for millennia to prevent and treat acute gout flares and has anti-inflammatory actions on the same myeloid and endothelial cell types that are implicated in the development of atherogenesis. Colchicine inhibits leukocyte migration, division, phagocytosis, and vesicle transport; reduces neutrophil deformability and tyrosine kinase signalling; and suppresses leukocyte vascular adhesion via altered selectin expression on both endothelial cells and leukocytes.5,11,12 Colchicine also inhibits caspase-1 activation, suppressing inflammasome-mediated IL-1β activation and subsequent generation of IL-6.13,14
Colchicine use may reduce the risk of MI in gout and in patients without gout.15–17 Low-dose colchicine after MI reduces the rate of future major adverse cardiovascular events, driven partly by urgent coronary revascularization.18 Whether cardioprotective effects of colchicine derive from a decrease in incident development of CAD or from plaque stabilization or suppression of inflammation-associated thrombosis that occurs in MI, remains uncertain and has important implications for care. For example, if prolonged use of colchicine has the ability to prevent the development of CAD, its good safety profile and relatively inexpensive cost would make it a good candidate for long-term use in patients with defined risk factors who have yet to develop CAD. On the other hand, if colchicine does not prevent CAD but can help mitigate the impact of acute events, it would be better suited either for daily prophylactic use in patients with established CAD at high risk for events or for acute administration in the setting of acute vascular injury. The aim of this study was to assess the rate of incident CAD between colchicine users and nonusers in a well-defined cohort of patients with gout who did not have clinical manifestations of CAD at baseline.
Methods
All patients with an ICD-9 code for gout and/or hyperuricemia (274.xx or 790.6) treated between January 1, 2000, and December 31, 2009, at the New York Harbor Health Care System of the US Department of Veterans Affairs (VA) were identified by electronic search. To ensure that all enrolled patients had gout, the 7819 charts identified were manually reviewed, and only patients with at least 1 of the following were considered for ongoing inclusion: presence of microscopically identified monosodium urate crystals; ≥ 6 of 12 1977 American Rheumatology Association (ARA) gout classification criteria,19 or 4 to 5 of 12 criteria (allopurinol permitted as a hyperuricemia surrogate) plus a diagnosis of gout by a primary care physician; or a recorded diagnosis of gout by a rheumatologist. If all of the information required to make the diagnosis of gout as described here was not present in the electronic medical record (EMR), the patient was excluded. Patients were excluded if they were < 45 years of age (to eliminate persons unlikely to develop incident CAD) or > 85 years of age (to reduce risk of dropout owing to death from other causes). Also excluded were subjects of female sex (0.8%), given the almost exclusively male population of VA patients with gout and differing clinical presentations of gout between women and men, and subjects with a late index date that would preclude adequate study follow-up (< 3 months before end of study period). Finally, patients with any pre-existing diagnosis of cardiovascular disease (by ICD-9 code or chart review)—including CAD or angina (including positive stress test or evidence of ≥ 50% stenosis on coronary angiography); MI; previous coronary revascularization with coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI); peripheral vascular disease; congestive heart failure; cerebrovascular accident or transient ischemic attack, at or before the index date—were excluded.
Colchicine users were identified using local VA pharmacy database records and defined as patients who had filled colchicine prescriptions for at least 1 period of > 30 consecutive days. Patients who had filled colchicine prescriptions only for ≤ 30 consecutive days, whether on 1 or more occasions, were excluded from the analysis as acute-only users. Because the VA is an integrated system, virtually all patients receive and fill their prescriptions within the searchable record. Nonusers were defined as patients with gout with no colchicine prescriptions ever within the study period. The study index date was defined as the date of diagnosis of gout.
The EMR of each patient meeting study criteria was further reviewed to identify demographics, presence of cardiovascular risk factors and comorbidities, medication use, anthropometric data, and laboratory data closest to the time of study enrollment. Direct chart review was also conducted to assess for the primary outcome and development of non-MI incident CAD, defined as any of the following incident events: diagnostic evidence of ischemia on cardiac stress test or evidence of ≥ 50% diameter stenosis in a coronary artery ≥ 2 mm in calibre by visual assessment on coronary angiography or coronary revascularization. Because some patients may present with MI as the initial evidence of previously occult CAD, we also assessed subjects for new-onset CAD as any of the aforementioned outcomes plus first-ever MI.20 The records of patients who were lost to follow-up in the internal hospital VA EMR were also assessed using the VA national EMR data access system.
Baseline variables were compared between the colchicine user and nonuser groups using the χ2 test and 2-sample Student’s t-test for categorical and continuous variables, respectively. Incidence rates in each group were defined as rate of incident CAD per patient-years of observation. Because of baseline differences in several key baseline characteristics, inverse probability weighting was used for the analyses. Standardized mean difference was calculated between groups to verify the balance of covariates. Standardized differences of less than 10% for a given covariate indicate balance. To measure the association between colchicine use and development of incident CAD, we performed a weighted Cox proportional hazard model with exposure of colchicine use as a time-varying covariate and set the start of follow-up time for both groups as the date of diagnosis of gout. Subgroup analyses were also performed using a weighted Cox proportional hazard model in the setting of each of the CAD risk factors separately (diabetes mellitus, hypertension, hyperlipidemia, chronic kidney disease (CKD), and tobacco use) and with the addition of the risk factor as an interaction term. Significance level was set at a 2-sided α level of 0.05. These analyses were performed by R version 3.5.1.
This study was approved by the Institutional Review Boards of the VA New York Harbor Health Care System and New York University School of Medicine.
Results
Subject identification
Among 178,877 patients with active medical records within the defined study period, 7819 patients (4.4%) with possible diagnoses of gout (ICD-9 code for gout and/or hyperuricemia) were identified (Fig. 1). Manual chart review of the 7819 patients confirmed a final study cohort of 722 subjects (9.2%) who met all inclusion and exclusion criteria (446 colchicine users and 276 nonusers) and were followed for a median of 96 months (interquartile range [IQR]: 57–117). The median duration of colchicine use was 23 months (IQR: 9–52).
Figure 1.
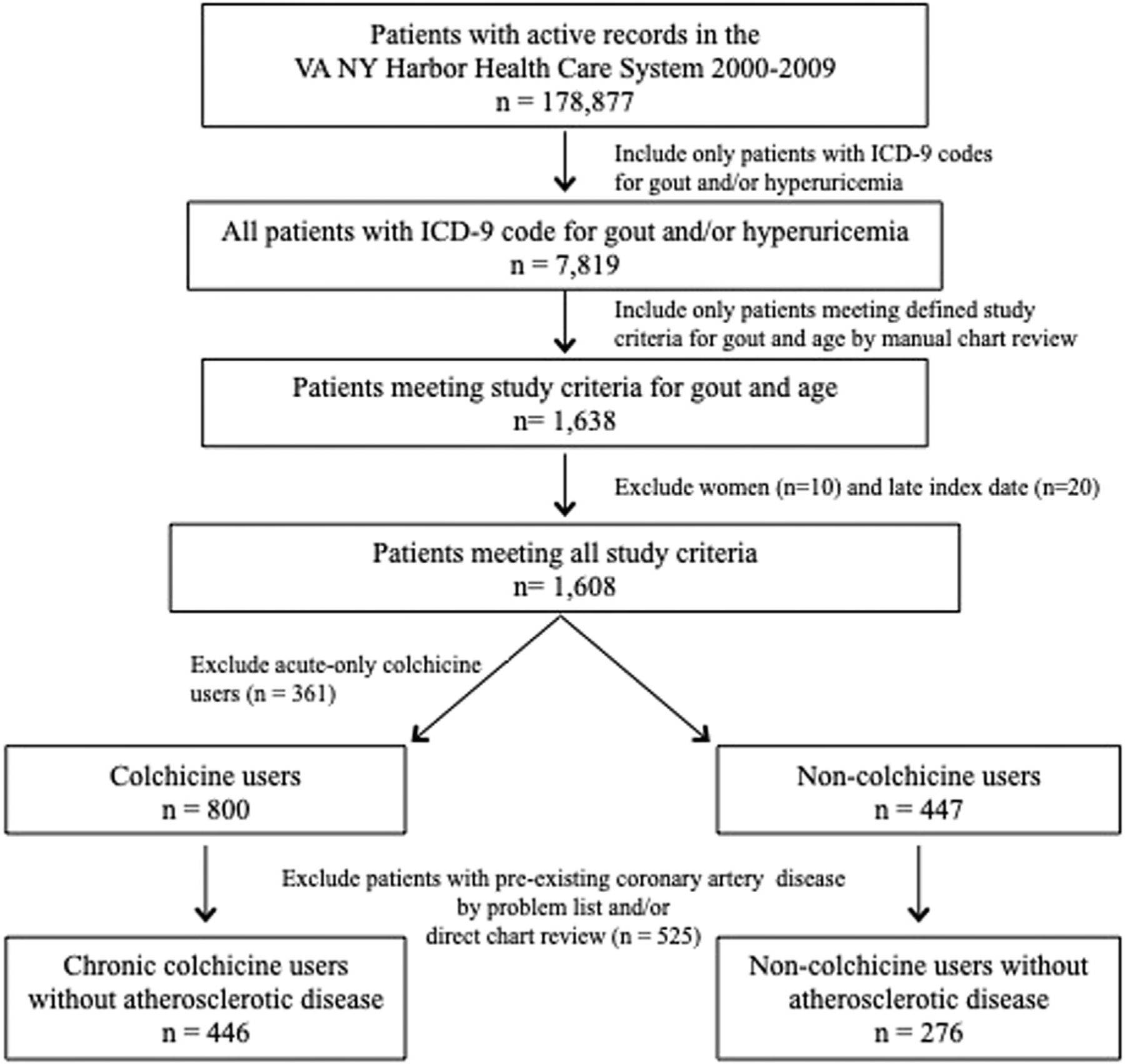
Study population.
Baseline characteristics
Colchicine users and nonusers were not different in age, race, ethnicity, and body mass index (Table 1). Users and nonusers also did not differ in rates of diabetes mellitus, hypertension, CKD, and tobacco use or use of allopurinol, nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, statins, and β-blockers. Compared with nonusers, colchicine users had higher rates of hyperlipidemia and use of angiotensin converting-enzyme inhibitors (ACEis). Serum urate concentration was only available at baseline in 414 patients (62.8% of colchicine users and 48.6% of colchicine nonusers). In patients with available data, there was a slight but statistically significantly higher serum urate concentration among colchicine users vs nonusers (8.25 ± 1.95 vs 7.64 ± 2.42, P = 0.006). After weighting, the standardized mean differences were less than 10%, indicating that the treatment arms were balanced (Table 1).
Table 1.
Baseline characteristics of gout patients using vs not using colchicine before and after inverse probability weighting
| Variable | Colchicine (n = 446) | No colchicine (n = 276) | P value | After IPW | P value | Standardized mean difference | |
|---|---|---|---|---|---|---|---|
| Colchicine | No colchicine | ||||||
| Age, years | 64 ± 9 | 64 ± 10 | 0.92 | 64.5 ± 9.3 | 64.8 ± 9.2 | 0.75 | 0.03 |
| Race | 0.68 | 0.99 | 0.003 | ||||
| White | 234 (52.6%) | 141 (51.3%) | 280.2 (53.2) | 282.9 (53.3) | |||
| Black | 205 (46.1%) | 128 (46.5%) | 239.2 (45.4) | 239.7 (45.2) | |||
| Asian | 6 (1.3%) | 6 (2.2%) | 7.6 (1.4) | 7.6 (1.4) | |||
| Ethnicity | 0.72 | 0.95 | 0.006 | ||||
| Hispanic | 48 (10.8%) | 27 (9.9%) | 52.0 (9.8) | 52.0 (9.8) | |||
| Non-Hispanic | 397 (89.2%) | 245 (90.1%) | 478.3 (90.2) | 478.3 (90.2) | |||
| Body mass index, kg/m2 | 30.2 ± 5.8 | 30.2 ± 6.5 | 0.91 | 30.2 ± 6.4 | 30.2 ± 6.4 | 0.97 | 0.003 |
| Diabetes mellitus | 106 (23.8%) | 67 (24.3%) | 0.88 | 130.2 (24.6) | 130.2 (24.6) | 0.82 | 0.021 |
| Hypertension | 363 (81.6%) | 222 (80.4%) | 0.70 | 448.9 (84.7) | 448.9 (84.7) | 0.83 | 0.02 |
| Systolic blood pressure, mm Hg | 137 ± 20 | 140 ± 20 | 0.07 | 137.8 ± 20.6 | 137.8 ± 20.6 | 0.92 | 0.01 |
| Diastolic blood pressure, mm Hg | 80 ± 11 | 81 ± 12 | 0.23 | 79.82 ± 12.8 | 79.82 ± 12.8 | 0.95 | 0.006 |
| Hyperlipidemia | 224 (50.9%) | 118 (42.9%) | 0.04 | 273.2 (51.5) | 273.2 (51.5) | 0.93 | 0.008 |
| Chronic kidney disease | 116 (26.2%) | 72 (26.6%) | 0.92 | 151.4 (28.6) | 151.4 (28.6) | 0.92 | 0.01 |
| Serum creatinine, mg/dL | 1.26 ± 0.50 | 1.27 ± 0.60 | 0.73 | 1.26 ± 0.54 | 1.26 ± 0.54 | 0.96 | 0.004 |
| Glomerular filtration rate, mL/min | 71 ± 23 | 72 ± 24 | 0.74 | 71.4 ± 22.1 | 71.4 ± 22.1 | 0.99 | 0.001 |
| History of tobacco use | 330 (74.3%) | 197 (73.2%) | 0.75 | 400.4 (75.5) | 400.4 (75.5) | 0.98 | 0.003 |
| Active tobacco use | 98 (22.0%) | 60 (22.1%) | 0.97 | 116.9 (22.0) | 116.9 (22.0) | 0.98 | 0.003 |
| Allopurinol use | 102 (22.9%) | 54 (19.6%) | 0.29 | 110.9 (20.9) | 110.9 (20.9) | 0.93 | 0.009 |
| NSAID use | 184 (41.3%) | 117 (42.4%) | 0.76 | 229.7 (43.3) | 229.7 (43.3) | 0.99 | < 0.001 |
| Aspirin use | 90 (20.2%) | 64 (23.2%) | 0.34 | 114.0 (21.5) | 114.0 (21.5) | 0.89 | 0.012 |
| Statin use | 122 (27.4%) | 80 (29.2%) | 0.59 | 164.4 (31.0) | 164.4 (31.0) | 0.92 | 0.009 |
| ACE inhibitor use | 174 (39.0%) | 80 (29.0%) | 0.006 | 209.0 (39.4) | 209.0 (39.4) | 0.85 | 0.018 |
| β-blocker use | 124 (27.8%) | 63 (22.8%) | 0.14 | 146.5 (27.6) | 146.5 (27.6) | 0.99 | < 0.001 |
| Any antihypertensive use | 317 (71.1%) | 175 (63.4%) | 0.03 | 387.1 (73.0) | 387.1 (73.0) | 0.86 | 0.017 |
Continuous data are shown as mean ± standard deviation and compared using the independent Student’s t-test. Categorical data are shown as frequency (proportion) and compared using the χ2 test.
ACE, angiotensin-converting enzyme; IPW, inverse probability weighting; NSAID, nonsteroidal anti-inflammatory drug.
Cardiac outcomes
There was no difference in the rate of the primary outcome of non-MI incident CAD between the colchicine user and nonuser groups. A trend toward an association between colchicine use and reduced incident CAD was observed after inverse probability weighting, but this was not statistically significant (hazard ratio [HR], 0.49; 0.23–1.05). Colchicine use, however, did have a statistically significantly lower risk of the composite outcome of incident CAD with inclusion of MI (HR, 0.37; 0.16–0.83) (Table 2).
Table 2.
Development of incident coronary artery disease in colchicine users vs nonusers
| Colchicine (n = 446) | No colchicine (n = 276) | ||||||
|---|---|---|---|---|---|---|---|
| Event | Event rate | Events/1000 patient-years | Inverse probability weighting HR (95% CI) | Event rate | Events/1000 patient-years | Inverse probability weighting HR (95% CI) | P value |
| Incident stable CAD | 40 (9.0%) | 12.3 (9.0–16.7) | 0.49 (0.23–1.05) | 18 (6.5%) | 11.6 (7.3–18.4) | Reference | 0.24 |
| Incident stable CAD + myocardial infarction | 40 (9.0%) | 12.3 (9.0–16.7) | 0.37 (0.16–0.83) | 19 (6.9%) | 12.3 (7.9–19.2) | Reference | 0.32 |
Data are shown as frequency (proportion) and compared using χ2 test.
CAD, coronary artery disease; CI, confidence interval; HR, hazard ratio.
The rates of the individual components of the primary outcome and MI were also not different between the 2 groups (Table 3). There remained no difference in the rate of development of incident CAD between colchicine users and nonusers by the presence of CAD risk factors, with the exception of CKD. In patients without CKD, colchicine was associated with a lower rate of incident CAD (Table 4).
Table 3.
Individual events comprising incident coronary artery disease and myocardial infarction
| Event | Colchicine (n = 446) | No colchicine (n = 276) | P value |
|---|---|---|---|
| Individual events of incident CAD | |||
| Positive ischemic stress test | 25 (5.6%) | 11 (4.0%) | 0.33 |
| Evidence of CAD on invasive angiography | 28 (6.3%) | 11 (4.0%) | 0.19 |
| Percutaneous coronary intervention (stent or angioplasty) | 11 (2.5%) | 6 (2.2%) | 0.80 |
| Coronary artery bypass graft surgery | 5 (1.1%) | 2 (0.7%) | 0.71 |
| Myocardial infarction | 8 (1.8%) | 3 (1.1%) | 0.55 |
Categorical data are shown as frequency (proportion) and compared using the χ2 test or Fisher’s exact test if the cell number is less than 5. CAD, coronary artery disease.
Table 4.
Development of incident CAD in colchicine users vs nonusers (reference) by the absence and presence of CAD risk factors
| CAD risk factor | Inverse probability weighting hazard ratio (95% CI) | Interaction P value |
|---|---|---|
| Diabetes mellitus | 0.23 | |
| No | 0.39 (0.17–0.91) | |
| Yes | 0.71 (0.27, 1.89) | |
| Hypertension | 0.88 | |
| No | 0.59 (0.10, 3.64) | |
| Yes | 0.51 (0.23, 1.1) | |
| Hyperlipidemia | 0.59 | |
| No | 0.44 (0.18, 1.04) | |
| Yes | 0.57 (0.23, 1.44) | |
| Chronic kidney disease | 0.005 | |
| No | 0.31 (0.14, 0.70) | |
| Yes | 2.09 (0.54, 7.99) | |
| Tobacco use | 0.38 | |
| No | 0.70 (0.24, 2.09) | |
| Yes | 0.43 (0.19, 0.97) |
CAD, coronary artery disease; CI, confidence interval.
Discussion
This is the first study to evaluate whether or not the potential underlying cardiovascular benefit of colchicine may be caused by a reduction in the development of incident CAD. The findings of this study suggest that colchicine use may not be associated with a reduction in the incidence of non-MI CAD in an all-comer gout population. However, the trend observed in favour of colchicine, together with the observation that use of colchicine was statistically significantly associated with a lower rate of incident CAD in patients without CKD, suggests that a larger study, or a longer follow-up period, may be needed further to establish or refute the primary prevention hypothesis. Inclusion of first MI events as a surrogate for previously unrecognized CAD revealed a lower risk in colchicine users compared with nonusers.
Anti-inflammatory therapy may play a therapeutic role in cardiovascular disease. Although low-dose methotrexate did not lower the rate of cardiovascular events when compared with placebo in patients with stable atherosclerosis, it also did not lower the concentrations of IL-1β, IL-6, or high-sensitivity C-reactive protein (hsCRP).21 In contrast however, the anti-IL-1β biologic agent canakinumab did reduce cardiovascular events in patients with previous MIs when compared with placebo, and this benefit was observed in the patients who achieved on-treatment lowering of hsCRP.9,10 However, canakinumab is expensive, carries increased risk of potentially severe infection, and is not approved for the management of cardiovascular disease.
Increasing data suggest a cardiovascular benefit of colchicine in patients at high risk of acute cardiovascular events. Our group conducted a cross-sectional study of 1288 patients with gout and demonstrated that patients who used colchicine experienced a statistically significant 64% reduction of MI, as well as lower mortality rates and CRP levels, compared with non-users.15 Solomon et al. reported a retrospective cohort study of 1002 patients with gout, using linked EMR and Medicare claims databases, in which use of colchicine was associated with a 49% lower risk for the combined outcome of MI, stroke, or transient ischemic attack, driven by statistically significant reductions in MI and stroke as well as a 73% reduction in all-cause mortality.16 In a prospective, randomized, observer-blinded endpoint study of patients with stable CAD already on antiplatelet agents and statin therapy, Nidorf et al. reported that the addition of low-dose daily colchicine resulted in a markedly reduced composite rate of acute coronary syndrome, out-of-hospital cardiac arrest, or noncardioembolic ischemic stroke on a median follow-up of 3 years (HR, 0.33; 95% confidence interval [CI], 0.18–0.59; P < 0.001).17 The reduction in this composite endpoint was largely driven by reductions in the rate of acute coronary syndrome. More recently, in a randomized, double-blind, placebo-controlled trial of patients with recent MI largely treated with PCI, the addition of low-dose daily colchicine to optimal standard medical therapy lowered the primary composite efficacy endpoint of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina leading to coronary revascularization on a median follow-up of 2 years (HR, 0.77; 95% CI, 0.61–0.96; P = 0.02).18 This primary endpoint was driven by reductions in stroke and urgent coronary revascularization.
Limited data elucidate the potential mechanism of benefit on acute cardiovascular events with colchicine. This is the first study to evaluate the association between colchicine and incident CAD (ie, stable CAD). Although colchicine did not definitively reduce incident CAD in the entire study cohort, there was an observed benefit of colchicine in the three-fourths of patients without CKD on the development of incident CAD. The abnormal calcium-phosphorus metabolism and uremia in patients with CKD may independently lead to inflammation and oxidative stress, additive to the adverse effects derived from the presence of other traditional cardiovascular risk factors. Colchicine may not be potent enough to suppress inflammation in the setting of CKD or may not reduce noninflammatory metabolic risk factors associated with kidney disease. However, it is also possible that the benefit of colchicine may occur exclusively around the potential acute cardiovascular event, such as the prevention or stabilization of plaque rupture or prevention of secondary MI once plaque rupture has occurred. Indeed, our observation that colchicine reduced incident CAD when first MI was included in the incident CAD definition suggests a possible benefit of colchicine on MI per se rather than incident CAD itself. The reported ability of colchicine to inhibit both macrophage ingress and cytokine-mediated metalloproteinase release may be particularly relevant to the regulation of plaque rupture.22 Studies increasingly suggest an observed inflammatory component during MI, including an admixture of leukocytes and platelets rather than platelets alone (inflammothrombosis).23 As therapeutic doses of colchicine can reduce the formation of leukocyte-platelet, but not platelet-platelet aggregates,24 it is possible that colchicine exerts a mechanism of action during MI by inhibiting the interface between inflammation and thrombosis. On the other hand, the effects of colchicine on incident CAD and acute MI need not be mutually exclusive.
Limitations
First, our use of a male population with gout allowed us to study a cohort in which colchicine use was common, but it may limit generalizability in patients without gout and female patients with gout. We used strict criteria to capture a cohort of patients who unequivocally had gout and in whom the colchicine users and nonusers had similar demographics and apparent levels of risk. However, patients with gout whose records lacked sufficient data to meet these stringent criteria were excluded, potentially skewing the data. Second, it is possible that patients whose physicians prescribed colchicine might have had more severe gout and, hence, potentially greater cardiovascular risk25,26 than the control patients in whom colchicine use was deferred. Colchicine users had slightly but statistically significantly higher mean serum urate levels vs nonusers, of potential importance, as elevated serum urate levels—even in the absence of gout—may reprogram monocyte/macrophages to more active inflammatory profiles through metabolic and epigenetic effects.27 However, allopurinol prescription, another potential measure of gout severity, did not differ between the groups. If the colchicineuse group was at higher risk for incident CAD, a benefit of colchicine could have been obscured. Third, under current American College of Rheumatology guidelines, long-term use of colchicine for gout is recommended only during the initial period of urate lowering.28 However, we observed that many patients received long-term colchicine, and often independently of allopurinol, consistent with American College of Physicians recommendations to treat gout “to avoid symptoms” rather than to expressly lower serum urate.29 Although compliance with colchicine use could not be formally assessed, a sensitivity analysis looking at varying durations of colchicine use was performed and did not indicate any differences. Finally, despite the use of the VA EMR, which captures data from all VA centres, the number of outcome events was relatively small. However, the study was powered according to MI reductions seen in previous studies (49% to 67%), and the aim of this study was to understand whether the underlying mechanism of benefit observed in those previous studies relate to primary prevention of incident CAD.16–18 Nonetheless, the study may have lacked the power to observe a true but smaller-than-anticipated colchicine benefit despite the long follow-up. This is particularly important in the setting of the statistically significant observed association between use of colchicine and lower rate of incidence CAD in patients without CKD, when CKD was present in more than a quarter of the study cohort. In addition, owing to the rigourous definition of incident stable CAD that was used, and the retrospective study design, the outcome of interest may have been underidentified: for example, with the exclusion of subjects with only a clinical diagnosis of angina.
Conclusions
The results of this study suggest that colchicine may not be associated with reduction in incident CAD among all-comer patients with gout without known previous cardiovascular disease, but may have a benefit on incident CAD in patients with gout without CKD. A larger study or a longer follow-up period may be needed to further establish or refute the primary-prevention hypothesis.
Acknowledgements
The authors thank Dr David Goldfarb and Dr Jeffrey Greenberg for helpful discussion. We dedicate this manuscript to the memory of Steven Sedlis, MD, Professor of Medicine, consummate mentor, and dedicated physician, who devoted his career to improving patient outcomes, educating the next generation of physicians, and advancing science. Dr Sedlis played a pivotal role with his contributions to background of this study.
Funding Sources
This manuscript was supported in part by an investigator-initiated grant from URL Pharma and Takeda, Inc (to M.H.P. and D.B.C.), a New York State Empire Clinical Research Investigator Program Award (to B.S., S.K., and M.H.P.), the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (iK2CX001074 to B.S.), and an American College of Rheumatology Student Preceptorship Award (to S.J.). Neither URL Pharma nor Takeda had any input into the design of the study or the content or the manuscript.
Disclosures
Dr Shah serves on the medical education advisory committee for Philips Volcano and Radux Device, and as a consultant for Terumo Medical. Dr Pillinger serves as a consultant for Horizon, SOBI, and Ampel Biosciences, and receives investigator-initiated grant support from Horizon and Hikma for studies unrelated to the current report. The other authors have no conflicts of interest to disclose.
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–25. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 2011;12:204–12. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res 2002;91: 281–91. [DOI] [PubMed] [Google Scholar]
- 5.Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest 1995;96: 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soehnlein O Multiple roles for neutrophils in atherosclerosis. Circ Res 2012;110:875–88. [DOI] [PubMed] [Google Scholar]
- 7.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 2010;122:1837–45. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003;107:363–9. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ; CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 2018;391:319–38. [DOI] [PubMed] [Google Scholar]
- 11.Taylor EW. The mechanism of colchicine inhibition of mitosis: I. kinetics of inhibition and the binding of H3-colchicine. J Cell Biol 1965;25(suppl):145–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terkeltaub RA. Colchicine update: 2008. Semin Arthritis Rheum 2009;38:411–9. [DOI] [PubMed] [Google Scholar]
- 13.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440: 237–41. [DOI] [PubMed] [Google Scholar]
- 14.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010;464:1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crittenden DB, Lehmann RA, Schneck L, et al. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol 2012;39:1458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon DH, Liu CC, Kuo IH, Zak A, Kim SC. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: a cohort study using electronic medical records linked with Medicare claims. Ann Rheum Dis 2016;75:1674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61:404–10. [DOI] [PubMed] [Google Scholar]
- 18.Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381: 2497–505. [DOI] [PubMed] [Google Scholar]
- 19.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977;20:895–900. [DOI] [PubMed] [Google Scholar]
- 20.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020–35. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Everett BM, Pradhan A, et al. CIRT Investigators. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slobodnick A, Shah B, Pillinger MH, Krasnokutsky S. Colchicine: old and new. Am J Med 2015;128:461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furman MI, Barnard MR, Krueger LA, et al. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol 2001;38:1002–6. [DOI] [PubMed] [Google Scholar]
- 24.Shah B, Allen N, Harchandani B, et al. Effect of colchicine on platelet-platelet and platelet-leukocyte interactions: a pilot study in healthy subjects. Inflammation 2016;39:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007;116:894–900. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum 2006;54:2688–96. [DOI] [PubMed] [Google Scholar]
- 27.Joosten LAB, Crisan TO, Bjornstad P, Johnson RG. Asymptomatic hyperuricemia: a silent activator of the innate immune system. Nat Rev Rheumatol 2020;16:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanna D, Khanna PP, Fitzgerald JD, et al. ; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res 2012;64:1447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qaseem A, Harris RP, Forciea MA. Clinical guidelines committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2017;166:58–68. [DOI] [PubMed] [Google Scholar]


