Abstract
Background
Anaemia is a prevalent health problem worldwide. Some types are preventable or controllable with iron supplementation (pills or drops), fortification (sprinkles or powders containing iron added to food) or improvements to dietary diversity and quality (e.g. education or counselling).
Objectives
To summarise the evidence from systematic reviews regarding the benefits or harms of nutrition‐specific interventions for preventing and controlling anaemia in anaemic or non‐anaemic, apparently healthy populations throughout the life cycle.
Methods
In August 2020, we searched MEDLINE, Embase and 10 other databases for systematic reviews of randomised controlled trials (RCTs) in anaemic or non‐anaemic, apparently healthy populations. We followed Cochrane methodology, extracting GRADE ratings where provided. The primary outcomes were haemoglobin (Hb) concentration, anaemia, and iron deficiency anaemia (IDA); secondary outcomes were iron deficiency (ID), severe anaemia and adverse effects (e.g. diarrhoea, vomiting).
Main results
We included 75 systematic reviews, 33 of which provided GRADE assessments; these varied between high and very low.
Infants (6 to 23 months; 13 reviews)
Iron supplementation increased Hb levels and reduced the risk of anaemia and IDA in two reviews. Iron fortification of milk or cereals, multiple‐micronutrient powder (MMNP), home fortification of complementary foods, and supplementary feeding increased Hb levels and reduced the risk of anaemia in six reviews. In one review, lipid‐based nutrient supplementation (LNS) reduced the risk of anaemia. In another, caterpillar cereal increased Hb levels and reduced IDA prevalence. Food‐based strategies (red meat and fortified cow's milk, beef) showed no evidence of a difference (1 review).
Preschool and school‐aged children (2 to 10 years; 8 reviews)
Daily or intermittent iron supplementation increased Hb levels and reduced the risk of anaemia and ID in two reviews. One review found no evidence of difference in Hb levels, but an increased risk of anaemia and ID for the intermittent regime. All suggested that zinc plus iron supplementation versus zinc alone, multiple‐micronutrient (MMN)‐fortified beverage versus control, and point‐of‐use fortification of food with iron‐containing micronutrient powder (MNP) versus placebo or no intervention may increase Hb levels and reduce the risk of anaemia and ID. Fortified dairy products and cereal food showed no evidence of a difference on the incidence of anaemia (1 review).
Adolescent children (11 to 18 years; 4 reviews)
Compared with no supplementation or placebo, five types of iron supplementation may increase Hb levels and reduce the risk of anaemia (3 reviews). One review on prevention found no evidence of a difference in anaemia incidence on iron supplementation with or without folic acid, but Hb levels increased. Another suggested that nutritional supplementation and counselling reduced IDA. One review comparing MMN fortification with no fortification observed no evidence of a difference in Hb levels.
Non‐pregnant women of reproductive age (19 to 49 years; 5 reviews)
Two reviews suggested that iron therapy (oral, intravenous (IV), intramuscular (IM)) increased Hb levels; one showed that iron folic acid supplementation reduced anaemia incidence; and another that daily iron supplementation with or without folic acid or vitamin C increased Hb levels and reduced the risk of anaemia and ID. No review reported interventions related to fortification or dietary diversity and quality.
Pregnant women of reproductive age (15 to 49 years; 23 reviews)
One review apiece suggested that: daily iron supplementation with or without folic acid increased Hb levels in the third trimester or at delivery and in the postpartum period, and reduced the risk of anaemia, IDA and ID in the third trimester or at delivery; intermittent iron supplementation had no effect on Hb levels and IDA, but increased the risk of anaemia at or near term and ID, and reduced the risk of side effects; vitamin A supplementation alone versus placebo, no intervention or other micronutrient might increase maternal Hb levels and reduce the risk of maternal anaemia; MMN with iron and folic acid versus placebo reduced the risk of anaemia; supplementation with oral bovine lactoferrin versus oral ferrous iron preparations increased Hb levels and reduced gastrointestinal side effects; MNP for point‐of‐use fortification of food versus iron and folic acid supplementation might decrease Hb levels at 32 weeks' gestation and increase the risk of anaemia; and LNS versus iron or folic acid and MMN increased the risk of anaemia.
Mixed population (all ages; 22 reviews)
Iron supplementation versus placebo or control increased Hb levels in healthy children, adults, and elderly people (4 reviews). Hb levels appeared to increase and risk of anaemia and ID decrease in two reviews investigating MMN fortification versus placebo or no treatment, iron fortified flour versus control, double fortified salt versus iodine only fortified salt, and rice fortification with iron alone or in combination with other micronutrients versus unfortified rice or no intervention. Each review suggested that fortified versus non‐fortified condiments or noodles, fortified (sodium iron ethylenediaminetetraacetate; NaFeEDTA) versus non‐fortified soy sauce, and double‐fortified salt versus control salt may increase Hb concentration and reduce the risk of anaemia. One review indicated that Hb levels increased for children who were anaemic or had IDA and received iron supplementation, and decreased for those who received dietary interventions. Another assessed the effects of foods prepared in iron pots, and found higher Hb levels in children with low‐risk malaria status in two trials, but no difference when comparing food prepared in non‐cast iron pots in a high‐risk malaria endemicity mixed population.
There was no evidence of a difference for adverse effects. Anaemia and malaria prevalence were rarely reported. No review focused on women aged 50 to 65 years plus or men (19 to 65 years plus).
Authors' conclusions
Compared to no treatment, daily iron supplementation may increase Hb levels and reduce the risk of anaemia and IDA in infants, preschool and school‐aged children and pregnant and non‐pregnant women. Iron fortification of foods in infants and use of iron pots with children may have prophylactic benefits for malaria endemicity low‐risk populations. In any age group, only a limited number of reviews assessed interventions to improve dietary diversity and quality. Future trials should assess the effects of these types of interventions, and consider the requirements of different populations.
Plain language summary
Interventions throughout life for the prevention or treatment of anaemia
What is the issue?
Anaemia (low iron levels in the blood) is a health problem worldwide, caused by nutritional (e.g. nutrient deficiencies) or non‐nutritional (e.g. diseases or genetic disorders) factors. Its health consequences include fatigue, loss of productivity and adverse pregnancy and child outcomes.
Why is this important?
Iron deficiency (ID) is a common cause of nutritional anaemia, resulting from a lack of iron in the diet or reduced absorption of iron in the body (e.g. components in coffee, tea or cocoa inhibit iron absorption, while beverages and foods high in vitamin C, such as fruits and vegetables, enhance iron absorption). Some types of anaemia are preventable or controllable with iron supplementation (via capsules or drops), fortification (food enriched with sprinkles or powders containing iron) or improvements to diet diversity and quality (e.g. education or counselling).
What evidence did we find?
Infants (6 to 23 months)
Two reviews suggested that iron supplementation increased haemoglobin (Hb) levels, and reduced the risk of anaemia and iron deficiency anaemia (IDA) compared with placebo, no intervention or other interventions. Six reviews suggested that iron fortification of milk or cereals, multiple‐micronutrient powder (MMNP), home fortification of complementary foods and supplementary feeding increased Hb levels and reduced the risk of anaemia. In one review apiece, lipid‐based nutrient supplementation (LNS) reduced the risk of anaemia, while caterpillar cereal increased Hb levels and reduced IDA prevalence.
Preschool and school‐aged children (2 to 10 years)
Two reviews suggested that daily or intermittent (e.g. 1 to 3 times per week) iron supplementation increased Hb levels and reduced the risk of anaemia and ID. For daily versus intermittent iron supplementation, one review found no difference in Hb levels, but an increased risk of anaemia and ID for the intermittent regime. One review apiece found higher Hb levels and reduced risk of anaemia and ID for zinc plus iron supplementation versus zinc alone, multiple‐micronutrient (MMN)‐fortified beverages, and point‐of‐use fortification of food with iron‐containing micronutrient powder (MNP).
Adolescent children (11 to 18 years)
Three reviews for prevention or treatment suggested that intermittent iron supplementation alone or in combination with other micronutrients, iron supplementation with or without folic acid supplementation, or other micronutrient supplementation increased Hb levels and reduced the risk of anaemia. One review suggested that nutritional supplementation and counselling reduced IDA. In one review for prevention, iron supplementation with or without folic acid appeared to increase Hb levels but have no effect on the incidence of anaemia.
Non‐pregnant women of reproductive age (19 to 49 years)
Two reviews suggested that iron therapy (oral, intravenous, intramuscular) increased Hb levels. One review found that intravenous iron increased Hb levels compared with oral iron, and another that daily iron supplementation with or without folic acid or vitamin C increased Hb levels and reduced the risk of anaemia and ID.
Pregnant women of reproductive age (15 to 49 years)
In one review, daily iron supplementation with or without folic acid increased Hb levels in the third trimester or at delivery, and in the postpartum period, and reduced the risk of anaemia, IDA and ID in the third trimester or at delivery. Six reviews suggested that intravenous iron versus oral iron or intramuscular iron increased Hb levels. In one review, vitamin A supplementation alone versus placebo, no intervention or other micronutrient increased Hb levels and reduced the risk of anaemia for the mother. One review found that supplementation with oral bovine lactoferrin versus oral ferrous iron preparations increased Hb levels and reduced gastrointestinal side effects. In one review, compared to iron or folic acid and MMNs, LNS increased the risk of anaemia.
Mixed population (all ages)
Iron supplementation versus placebo or control increased Hb levels in healthy children, adults, and elderly people in four reviews. In two reviews, MMN fortification versus placebo or no treatment increased Hb levels in children, as did iron supplementation, but Hb levels decreased for those receiving dietary interventions. Intravenous iron resulted in higher Hb levels than oral iron in one review. In another, vitamin B12 or folic acid supplementation did not increase Hb levels. Each review suggested that iron fortification of food, iron‐fortified soy sauce, double‐fortified salt with iron and iodine, and fortified condiments or noodles increased Hb levels and reduced the risk of anaemia. In one review, foods prepared in iron pots showed the potential to increase Hb levels in children.
No review focused on older adult women (50 to 65 years plus) or men (19 to 65 years plus), and anaemia and malaria prevalence were rarely reported.
What does this mean?
Compared to no treatment, daily iron supplementation may increase Hb levels and reduce the risk of anaemia and IDA in infants, preschool and school‐aged children and pregnant and non‐pregnant women. Iron fortification of foods in infants and use of iron pots with children may have benefits for low‐risk populations. Many trials reported the effects of supplementations, but very few reviews focused on fortification or improving diet diversity and quality. Future trials should focus on different types of interventions to increase food variety and dietary quality.
Background
Description of the condition
Anaemia is defined as a decreased level of red blood cells, abnormal red blood cell morphology, or an inadequate amount of haemoglobin in red blood cells which, consequently, leads to an insufficient supply of oxygen in the body. It results from decreased red blood cell production (erythropoiesis), increased destruction, blood loss, or a combination of these factors. The underlying cause of anaemia (e.g. nutritional deficiencies, diseases, or genetic disorders) is frequently used to classify anaemia into nutritional and non‐nutritional anaemia (WHO 2017). Causes and treatment of non‐nutritional anaemia have been discussed in other Cochrane Reviews (Fortin 2018; Gordon 2021; Siegfried 2012). One of the most common causes of anaemia is iron deficiency (ID), which is estimated to account for approximately 50% of all anaemia cases (Stevens 2013; Stoltzfus 2004). However, more recent estimates suggest that anaemia due to ID accounts for less than 50%, depending on the country‐specific context (Petry 2016a). Anaemia of chronic disease, another common type of anaemia, is multifactorial and its diagnosis generally requires the presence of chronic inflammation (i.e. infection, autoimmune disease, kidney disease, or cancer) (Weiss 2005). Numerous other nutritional and non‐nutritional factors, in combination or isolation, have been associated with anaemia, such as vitamin deficiencies (including folate, vitamin B12, and vitamin A), inflammation, infectious diseases (i.e. malaria; soil‐transmitted helminthiasis, especially hookworm infection; HIV; cancer; and tuberculosis), as well as genetic or acquired impairment of haemoglobin synthesis, and production and survival of red blood cells (Camaschella 2015; Lopez 2016). In the state of infection or inflammation, iron absorption is decreased as an innate immune response to restrict iron availability for pathogens (Hurrell 2012). Anaemia may also be the result of physiological or pathophysiological acute or chronic blood losses. In menstruating women and adolescent girls, periods are the most common cause of iron deficiency anaemia (IDA), which, in some cases, may be excessive (i.e. menorrhagia, metrorrhagia) (WHO/CDC 2008). In men and post‐menopausal women, bleeding in the gastrointestinal tract may be a common cause of anaemia (Lopez 2016). The health consequences of anaemia include fatigue during the early stages of the disease, coupled with a negative effect on productivity due to weakness, loss of energy, and dizziness; such loss of productivity also has an important impact on social and economic development (Bager 2014; Horton 2003). In addition, anaemia is associated with adverse pregnancy and child outcomes (GBDPC 2016). Maternal anaemia may lead to greater blood loss during delivery, increased risk of postpartum haemorrhage, and maternal mortality (Brabin 2001a). Anaemic mothers are at greater risk of delivering preterm babies and of having a low‐birthweight infant (Allen 2000; Haider 2013). Anaemia also negatively impacts the cognitive and motor development of children, and severe anaemia increases the risk of child mortality (Brabin 2001b).
Anaemia is a significant public health problem, with prevalence highest in South Asia and Central and West Africa (Stevens 2013). Estimates from the World Health Organization (WHO) indicate that 800 million children and women were anaemic in 2011 (Stevens 2013; WHO 2015). Although anaemia can occur throughout the life cycle, young children and pregnant women are the most vulnerable, with estimated prevalences of 43% and 38%, respectively (WHO 2015). Anaemia prevalence decreased globally from 33% to 29% in non‐pregnant women, from 43% to 38% in pregnant women, and from 47% to 43% in children between 1995 and 2011 (Stevens 2013). Other studies reported that between 1993 and 2013, the global prevalence of anaemia improved by only 0.2% to 0.3% points (Kassebaum 2014; Mason 2013). This slow progress, coupled with the overall burden of anaemia, has lead to anaemia's inclusion in the global nutrition targets to improve maternal, infant, and child nutrition agreed by the World Health Assembly in 2012 (WHO 2014a); the second of the six global goals aims for a 50% reduction of anaemia in women of reproductive age by 2015 (WHO 2014b). In addition, anaemia is indirectly included in the Sustainable Development Goals (SDGs); according to the second goal on ending hunger, target 2.2 aims to end all forms of malnutrition by 2030, by addressing, in particular, the nutritional needs of children under five years of age, adolescent girls, pregnant and lactating women, and older people (UN 2015).
Blood haemoglobin (Hb) concentration is most commonly used as an indicator of anaemia, since it is relatively easy and inexpensive to measure. Whilst it alone cannot determine the underlying cause of anaemia, in combination with other measurements, Hb concentration can provide important information about the severity of ID (WHO/CDC 2007). Blood Hb concentration levels currently used by the WHO to define anaemia are: less than 110 g/L for children under five years of age and pregnant women; less than 115 g/L for children aged 5 to 11 years; 120 g/L for children aged 12 to 14 years and non‐pregnant women; and less than 130 g/L for men (WHO 2011). In this overview of reviews, we use the anaemia cut‐offs defined by the WHO to summarise the benefits or harms of nutrition‐specific interventions for preventing and controlling anaemia throughout the life cycle.
Description of the interventions
The reasons for anaemia development are diverse, but poor nutrition is one of its main causes (WHO 2017). ID is a common nutritional deficiency worldwide and jointly responsible for the high and persistent prevalence of anaemia. However, various other micronutrients may be lacking in inadequate and imbalanced diets and contribute to micronutrient deficiencies and the emergence of anaemia (WHO 2015; WHO 2017). Micronutrient deficiencies, alone or in combination, manifest when requirements cannot be satisfied through adequate provision, intake, or absorption of nutrients. To counter nutritional anaemia, several different approaches for dietary improvement have been implemented at the population level, or more directly at vulnerable groups, such as infants, young children, and pregnant women. First, nutrition‐specific interventions that address the immediate determinants of anaemia (e.g. poor diet); and second, nutrition‐sensitive interventions that address the underlying causes of anaemia (e.g. diseases or infections) (Ruel 2013). This overview of reviews focused on the former, and included food‐based strategies to control micronutrient malnutrition and increase the intake of micronutrients through supplementation, food fortification, and enhancement of food diversity and quality (WHO/FAO 2006; Zimmermann 2007). Nutrition‐sensitive interventions (i.e. addressing food insecurity, providing access to adequate health services, ensuring a safe and hygienic environment) were outside the scope of this review. Due to the multifactorial causation of anaemia, ideally a multisectoral approach, such as the Strengthening Partnerships, Results, and Innovations in Nutrition Globally project (SPRING) supported by USAID would be necessary to address anaemia in all its forms (SPRING/USAID 2017).
Supplements are taken orally and are intended to supplement the diet with varies micronutrients, alone or in combination, at higher doses, to immediately improve nutritional deficiencies and anaemia (Stoltzfus 1998). Fortification refers to the addition of nutrients to food (e.g. in the form of powders) and beverages, and is another practical way to improve the diet of target populations (WHO/FAO 2006). These interventions show less immediate impact but are more sustainable and cost‐effective over the long term (Baltussen 2004; WHO/FAO 2006). Iron supplementation and fortification have been intensively used and various studies have shown a positive effect on iron status (Man 2021). However, adverse affects such as an increased risk of illness (e.g. diarrhoea or inflammation in the gastrointestinal tract), decreased growth or influence on children's development have been reported (Lönnerdal 2017; Paganini 2017).
Cultural norms influence the client’s perspective on their food choices and eating patterns. Nutrition education, counselling, and promotion aim to increase the intake of foods that are naturally high in certain micronutrients with high bioavailability (i.e. the degree to which the micronutrient is absorbed from the diet and available for the body's functions), and have a high content of factors to improve absorption coupled with a low content of inhibiting factors for micronutrient absorption (WHO 2017). Increasing food diversity is the most desirable and long‐lasting intervention, but efforts to improve dietary quality and to encourage behaviour change may take a long time (WHO/FAO 2006). While improving dietary diversity and quality are important interventions across the life course, cost and availability of animal products, fruits and vegetables are often the limiting factors for theses interventions. Supplementation or fortification may be the intervention of choice if more immediate impact on iron status or anaemia is required.
This overview of reviews focuses on the prevention and control of anaemia at all stages of the life cycle, and includes the nutrition‐specific interventions listed below.
-
Supplementation
Daily or intermittent oral iron, vitamins, or any other mineral (especially vitamin B12, folate, vitamin A, or provitamin A, but also vitamin C, vitamin E, zinc) supplementation alone or in combination
-
Fortification
Fortification of foods with vitamins and minerals (e.g. iron, folate, vitamin B12, zinc, vitamin A) alone or in combination
Use of multiple‐micronutrient powders (MMNPs; e.g. sprinkles or point of use fortification/home fortification added to prepared foods at the time of consumption)
Provision of supplementary foods containing macronutrients (e.g. protein supplementation) alone or in combination with micronutrients (e.g. lipid‐based nutrient supplementation (LNS))
Provision of fortified complementary foods
Provisions of fortified staple foods or beverages (i.e. water) with micronutrients
Provision of micronutrient, biofortified foods with increased contents of micronutrients (e.g. iron, zinc, vitamin A)
-
Improving dietary diversity and quality
Increasing food variety through nutrition education and provision of foods rich in minerals and vitamins such as fruits, vegetables, and iron‐rich foods (i.e. red meat, proteins)
Nutrition education and use of iron‐pot cooking and fish‐shaped iron ingots
General nutrition education and counselling (e.g. increasing the intake of micronutrient absorption factors and decreasing inhibitors of micronutrient absorption)
For the purpose of this overview review, we focused on apparently healthy populations with or without anaemia and excluded populations with acute or chronic infections (e.g. malaria, helminth infection, cancer, tuberculosis), inflammation or inherited anaemia (i.e. sickle cell anaemia, thalassaemia).
How the intervention might work
Supplementation
Supplements in the form of capsules or drops are provided to target populations (WHO/FAO 2006). In this way, micronutrients can be given in the desired quantity and in combination with high bioavailability.
Iron supplementation is used widely, either to prevent ID and anaemia in populations at risk (e.g. pregnant women and young children), or to improve the haemoglobin status of people with existing anaemia. Four different iron preparations are used frequently for oral supplementation: ferrous sulphate, ferric sulphate, ferrous gluconate, and ferrous fumarate. The efficiency of iron supplementation greatly depends on the prevalence of ID and anaemia in the area, and interventions have been implemented in both low‐ and middle‐income countries. Populations at high risk of anaemia may especially benefit from iron supplementation; for example, supplementation during pregnancy can reduce the risk of maternal anaemia and ID; however, benefits for infants, such as a reduced risk of being born premature or with a low birthweight, are less clear (Peña‐Rosas 2015a). Oral iron therapy is often limited due to low adherence and the development of side effects, such as nausea and epigastric pain (Beutler 2003). Alternatively, other iron supplementation regimes, such as lower dosage or intermittent supplementation, can be used to reduce the occurrence of side effects (Cavalli‐Sforza 2005). In areas with an anaemia prevalence of 40% or higher, the WHO recommends the following doses of elemental iron given daily, for three consecutive months in a year, to prevent ID and anaemia: 10 mg to 12.5 mg for infants and young children aged 6 months to 23 months; 30 mg for preschool‐aged children (24 to 59 months); and 30 mg to 60 mg for school‐aged children (60 months and older), menstruating adult women and adolescent girls (WHO 2016a; WHO 2016b). In settings with a lower anaemia prevalence, the WHO recommends an intermittent regime (one supplement of elemental iron per week for three consecutive months, followed by three months without supplementation, and then three months with supplementation) at the following doses: 25 mg for preschool‐aged children (24 to 59 months), 45 mg for school‐aged children (60 months and older), and 60 mg for menstruating women and adolescent girls (WHO 2017). For pregnant women in areas with a lower anaemia prevalence (less than 20%), the recommended elemental iron supplementation is 120 mg (with folic acid) once a week throughout pregnancy to prevent the development of anaemia (WHO 2017). A comprehensive systematic review showed that there was no evidence of a difference in the prevalence of anaemia for women receiving intermittent oral iron supplementation during pregnancy compared with daily supplementation; additionally, intermittent supplementation was associated with fewer side effects (Peña‐Rosas 2015a).
In addition to iron, various other micronutrients are important for proper function of hematopoesis, and deficiencies may contribute to the development of anaemia. Primarily, folic acid, vitamin A, and vitamin B12 supplements, given alone or in combination with iron supplementation, are used to prevent and control for nutritional deficiencies in conjunction with anaemia. Folic acid plays a central role in erythropoiesis, and pregnant women especially are at high risk of folic acid deficiency (Fishman 2000). The WHO recommends daily folic acid supplementation of 400 μg with 30 mg to 60 mg elemental iron, or 2800 μg folic acid with 120 mg iron once a week for menstruating women as well as pregnant women to prevent maternal anaemia, puerperal sepsis, low birthweight, and preterm birth (WHO 2016c). Vitamin A acts on several stages of iron metabolism; it increases iron uptake, iron mobilisation, and erythropoiesis (Fishman 2000). Supplementation during pregnancy is associated with reduced maternal anaemia for women living in areas with a vitamin A deficiency (McCauley 2015). Likewise, vitamin B12 plays a crucial role in erythropoiesis, and severe vitamin B12 deficiency can lead to the development of megaloblastic anaemia (Fishman 2000). Vitamin B12 is only produced by microorganisms, thus putting vegetarians, vegans, and populations in settings with low intake of animal products at increased risk of vitamin B12 deficiency. There is no consistent recommendation for the daily dosage of vitamin B12 supplementation, but commonly 2.4 μg/day is recommended for an adult; a pregnant women should add 0.2 μg/day of vitamin B12 to the estimated daily requirement (De Benoist 2008; Van Sande 2013). Other vitamins and minerals (e.g. vitamin C, vitamin E, zinc, or copper) are also required for normal enzyme and hematopoietic function, and deficiencies in isolation or combination with other vitamins and minerals may contribute to the development of nutritional anaemia (Fishman 2000). Micronutrients interact in the body to maintain normal physiological functions and poor diets frequently lack several micronutrients at the same time, suggesting that micronutrient deficiencies often occur together. A cost‐effective way of delivering micronutrients, especially for pregnant women, is through multiple‐micronutrient (MMN) supplementation. The international MMN preparation, UNIMMAP, is used frequently and contains one recommended daily allowance (RDA) of 15 vitamins and minerals (vitamin A, vitamin B1, vitamin B2, niacin, vitamin B6, vitamin B12, folic acid, vitamin C, vitamin D, vitamin E, copper, selenium, and iodine with 30 mg of iron and 15 mg of zinc) (UNICEF 1999). While MMN supplementation during pregnancy has been shown to improve birth outcomes, such as low birthweight and small‐for‐gestational weight, there is no clear evidence for a risk reduction of anaemia (Da Silva Lopes 2017; Haider 2017).
Fortification
Fortification enriches food with nutrients in order to improve the nutritional status of populations at risk of micronutrient deficiencies (WHO/FAO 2006). Mass fortification approaches can reach a large proportion of the population by adding micronutrients, such as iron, folic acid, vitamin B12, or vitamin A, to commonly consumed foods (e.g. cereals, salt, milk) (WHO 2017). In contrast, targeted fortification aims to improve the diet of a particular subpopulation that is unable to consume high quantities of staple foods (i.e. infants and young children), or who have higher nutritional requirements (i.e. pregnant women, infants, children, elderly), or both (WHO 2017). Targeted fortification can include the fortification of complementary foods (primarily with iron, zinc, and calcium) for infants during the transition from exclusive breastfeeding to solid foods (PAHO 2003). Nutrients can be added to food prior to consumption, in the form of MNPs or sprinkles (point‐of‐use fortification), or consumed in the form of lipid‐based supplements which contain micronutrients, energy, protein, and essential fatty acids (WHO 2017). Instead of adding nutrients directly to foods, biofortification (through breeding techniques and genetic modifications) has been used to increase the nutrient content (i.e. iron, zinc, provitamin A, amino acids, or protein) of crops (e.g. cereals, legumes, tubers) during plant growth (WHO/FAO 2006). Iron fortification can include the addition of iron as salt or chelates, or the addition of iron‐rich components, such as meat, to food products (Prentice 2017). Iron fortification produces some technical difficulties as the addition of the most bioavailable forms is more expensive, causes unwanted flavour and colour changes, and may react with other food components (Hurrell 2002). Hence, less reactive and less expensive iron forms are chosen for fortification, but these forms are also less bioavailable (Hurrell 2002; Zimmermann 2007). Iron doses used for fortification are lower compared with supplementation and, accordingly, body iron levels increase much slower; however, fortification may be overall the safer intervention (Prentice 2017). Most commonly, wheat and maize flour, infant formula, and cereals are fortified with iron (WHO 2016d; WHO/FAO 2006). Other micronutrients, such as folic acid or B vitamins, are also commonly added to wheat flour. Vitamin A has been successfully added to milk or sugar to prevent vitamin A deficiency (Dary 2002; Hombali 2019).
Improving dietary diversity and quality
Dietary diversity refers to the intake of different food or food groups over a defined period of time, and is an essential component of diet quality (FAO 2011; Ruel 2003). Diet quality itself is defined as nutrient adequacy in terms of adequate intake of macro‐ and micronutrients and diet variety at the household or individual level (FAO 2011). Insufficient dietary intake or poor bioavailability, especially of iron, vitamin A, vitamin B12, and folate, are the major causes of nutritional anaemia (WHO 2017). Nutrition education and counselling (e.g. meal preparation, increased intake of micronutrient absorption factors and decreased intake of inhibitors), combined with the provision of foods rich in minerals and vitamins such as fruits, vegetables and iron‐rich foods (i.e. red meat, proteins), aim to stimulate behaviour change and to improve dietary diversity and quality (Allen 2008). These food‐based approaches are potentially simple and sustainable methods for preventing and treating not only IDA but also micronutrient malnutrition, though implementation may be challenging due to limited availability, access, and safety of food (WHO 2017). When educating people in different areas of the world, health educators need to understand micronutrient nutrition and also regional and local variations in the diet, different cultural practices, different methods of food processing and meal preparation, and economic constraints (Sharifirad 2011; WHO 2017). Furthermore, these interventions need to take into account the special requirements of subpopulations and vulnerable groups (i.e. young children, pregnant women, elderly).
Bioavailability of iron refers to total iron in plant or animal food that is available to the body after digestion and absorption and depends on the form of iron present in theses foods (Zhang 2021). Heme iron is found only in animal‐based products and has a high bioavailability of approximately 25% to 30%, while the bioavailability of non‐heme iron in plant and animal products ranges from 1% to 10% (Beck 2014; Heath 2002). Examples of iron‐rich foods include foods of animal (meat and organs, such as liver from cattle, fish, fowl, etc.) and non‐animal (spinach, legumes, and green leafy vegetables) origin. The availability of dietary iron can be influenced by various dietary factors, and it is important to promote the consumption of foods that enhance the absorption and utilisation of iron and reduce the intake of inhibitors. Ascorbic acid (vitamin C) enhances iron absorption through its iron reducing and chelating effects (Teucher 2004). On the other hand, food components such as phytate (e.g. in cereals) and calcium can inhibit iron absorption (Lynch 2000). Tea and coffee, but also red wine and cocoa contain a high amount of polyphenol which have inhibitory effects on iron absorption in the gastrointestinal tract (Milman 2020). Consumption of black tea during or after a meal reduced iron absorption by 79% to 84% and consumption of coffee by 39% (Hurrell 1999; Morck 1983). Additionally, milk proteins, egg proteins, and albumin negatively influence iron absorption (Hurrell 2010). Therefore, iron in food or supplements is best absorbed in combination with foods or beverages naturally containing vitamin C and by avoiding iron inhibitors (Teucher 2004).
Providing nutrition knowledge is a key step in behaviour change to establish adequate nutrition and iron intake. Previous studies showed that nutrition education programmes can improve a study population's knowledge, attitude, and eating behaviour, as well as haemoglobin levels (Alaofè 2009; Kapur 2003; Nandi 2016; Sharifirad 2011; Yusoff 2012). In some regions, fish‐shaped iron ingots named "Happy Fish" or "Lucky Iron Fish" are commonly accepted for continuous use in soup or boiling drinking water (Adish 1999; Armstrong 2017). Cooking with iron ingots has been shown to release sufficient iron to provide 40% to 75% of the daily iron requirement for women of reproductive age. The duration of boiling iron fish coupled with the water's acidity increases iron release; any toxicity with daily use has not been reported (Armstrong 2017; Charles 2011). However, some studies reported the production of reactive oxygen species with iron‐containing cookware or the risk of iron overload (Alves 2019). Another concern has been the low acceptability of iron pots due to rusting and pot weight which could limit the potential of the intervention (Prinsen 2002).
The dietary requirements of vitamin A (retinol) and pro‐vitamin A (carotenoids) can be attained by consumption of dark green leafy vegetables, orange/yellow fruits and vegetables, as well as animal products such as meat, liver, margarine, fish and fish oils, and dairy products. The fat‐soluble vitamin needs to be consumed with lipids to improve its absorption and it is recommended that cooking time is reduced to preserve the activity of pro‐vitamin A (WHO 2017).
Meat, fish, poultry, and dairy products are the best sources of vitamin B12. Folate‐rich foods include dark green leafy vegetables, fruits and fruit juices, dairy products, beans, nuts, and grains.
Why it is important to do this overview
Anaemia is a major public health problem worldwide. Anaemia prevalence fluctuates according to various factors, including age, living area, sex, and socioeconomic status. Through interventions, improvement in iron status and anaemia have been made, but the progress is slow and countries are not on track to meet the nutrition target for anaemia (Global Nutrition Report 2020). Some types of anaemia are preventable and controllable with effective interventions. However, a limited number of studies have looked at the variety of nutrition‐specific interventions for controlling anaemia and ID throughout the life cycle. Overview reviews can provide summaries of research relevant to a decision. Thus, we summarised different nutrition‐specific interventions at any stage of life in this Cochrane overview Review. It is important to assess the current evidence base to help clarify the best nutrition‐specific intervention for preventing anaemia in order to reduce the socioeconomic burden of the condition.
Objectives
To summarise the evidence from systematic reviews regarding the benefits or harms of nutrition‐specific interventions for preventing and controlling anaemia in anaemic or non‐anaemic, apparently healthy populations throughout the life cycle.
Methods
Criteria for considering reviews for inclusion
We considered all published systematic reviews of randomised controlled trials (RCTs) of nutrition interventions for preventing and controlling anaemia.
We included both Cochrane Reviews and non‐Cochrane Reviews provided they had used a systematic approach, only included RCTs, and assessed the methodological quality of the included trials. We considered systematic reviews with and without meta‐analyses but excluded meta‐analyses without systematic reviews. If the systematic reviews included RCTs and non‐RCTs, we included the systematic reviews which presented results from RCTs separately. We listed eligible systematic reviews in preparation (e.g. published protocols and titles) in Appendix 1 to be included in future updates of this overview of reviews.
Types of participants
Anaemic or non‐anaemic, apparently healthy populations (see directly below)
Infants (aged 6 months to 23 months)
Preschool and school‐aged children (aged 2 years to 10 years)
Adolescent children (aged 11 to 18 years)
-
Adult women
Non‐pregnant women of reproductive age (aged 19 to 49 years)
Pregnant women of reproductive age (aged 15 to 49 years)
Older adult women (aged 50 to 65 years and above)
Adult men (aged 19 to 65 years and above)
Mixed population (all ages)
We assigned age groups according to the time when the interventions commenced. Where age groups overlapped but data were presented separately, we extracted data according to age groups and calculated the mean age of the participants. If this was not the case, we used the mean age of the participants to assign the review into one of the prespecified groups in such a way that most participants (e.g. 60%) fell within this group. If this was not possible or none of the age groups dominated, we assigned this review to “mixed population”.
We excluded infants younger than six months of age, since exclusive breastfeeding is recommended from birth until aged six months. In addition, we excluded populations at risk of anaemia due to acute or chronic infections (e.g. malaria, helminth infection, cancer, tuberculosis, HIV infection), acquired bone marrow disorders, inflammation or inherited anaemia (i.e. blood disorders such as sickle cell anaemia or thalassaemia), and reviews with studies conducted in populations comprising only individuals with undernutrition (i.e. wasting, stunting, underweight).
Types of interventions
We considered all types of nutrition‐specific interventions (i.e. interventions that address the immediate determinants of nutrition) to prevent or correct anaemia, including the following.
-
Supplementation
Daily or intermittent oral iron, vitamins, or any other mineral (especially vitamin B12, folate, vitamin A, or provitamin A, but also vitamin C, vitamin E, zinc) supplementation alone or in combination
-
Fortification
Fortification of foods with vitamins and minerals (e.g. iron, folate, vitamin B12, zinc, vitamin A) alone or in combination
Use of multiple‐micronutrient powders (MMNPs; e.g. sprinkles or point‐of‐use fortification/home fortification added to prepared foods at the time of consumption)
Provision of supplementary foods containing macronutrients (e.g. protein supplementation) alone or in combination with micronutrients (e.g. lipid‐based nutrient supplementation (LNS))
Provision of fortified complementary foods
Provision of fortified staple foods or beverages (i.e. water) with micronutrients
Provision of micronutrient, biofortified foods with increased contents of micronutrients (e.g. iron, zinc, vitamin A, protein)
-
Improving dietary diversity and quality
Increasing food variety through nutrition education and provision of foods rich in minerals and vitamins, such as fruits, vegetables, and iron‐rich foods (i.e. red meat, proteins)
Nutrition education and use of iron‐pot cooking and fish‐shaped iron ingots
General nutrition education and counselling (e.g. increasing the intake of micronutrient absorption factors and decreasing inhibitors of micronutrient absorption)
Nutrition‐sensitive interventions, i.e. interventions that address the underlying determinants of nutrition, such as food insecurity, inadequate healthcare services or an unsafe and unhygienic environment, were outside the scope of this review.
Types of comparisons
We considered all types of comparisons, such as comparison of the intervention with placebo, another intervention (e.g. other minerals and vitamins), co‐intervention (provided the co‐intervention is the same in both the intervention and control groups), or no intervention (to correct anaemia levels directly or indirectly) or a control group defined by trial authors.
Types of outcomes
We excluded reviews that did not report relevant outcomes from this overview, as preventing and controlling anaemia was a key focus for this overview.
Primary outcomes
Haemoglobin concentration (Hb; in g/L)
Anaemia (defined per the WHO Hb cut‐off for age group (WHO 2011), and adjusted by altitude, smoking)
Iron deficiency anaemia (IDA; defined by the presence of anaemia plus ID, and diagnosed with an indicator of iron status selected by trial authors)
Secondary outcomes
Iron deficiency (ID; defined by trial authors and measured using indicators of iron status, such as ferritin or transferrin)
Severe anaemia (defined per the WHO Hb cut‐off for age group (WHO 2011))
Adverse effect (any adverse effects, including side effects, all gastrointestinal symptoms, diarrhoea, vomiting, constipation, as defined by trial authors)
Search methods for identification of reviews
We first searched the following sources in July 2018. We ran top‐up searches of each source in August 2020 and September 2020 apart from DARE and POPLINE, which have both ceased publication.
Cochrane Database of Systematic Reviews (CDSR; 2020 Issue 8) part of the Cochrane Library (searched 25 August 2020)
MEDLINE via Ovid (1946 to August 2020 week 2)
MEDLINE In‐Progress and other Non‐Indexed Citations via Ovid (searched 25 August 2020)
MEDLINE Epub Ahead of Print via Ovid (current issue; searched 25 August 2020)
Embase via Ovid (1974 to 25 August 2020)
CINAHL via EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; (1937 to 25 August 2020))
Database of Abstract of Reviews of Effects (DARE; 2015, Issue 4. Final Issue) part of the Cochrane Library (searched 24 July 2018)
Campbell Collaboration Online Library (www.campbellcollaboration.org/better-evidence.html; searched 15 September 2020)
3ie International Initiative for Impact Evaluation (www.3ieimpact.org; searched 15 September 2020)
Epistemonikos (www.epistemonikos.org; searched 25 August 2020)
POPLINE (www.popline.org; searched 24 July 2018)
PROSPERO (www.crd.york.ac.uk/prospero; searched 25 August 2020)
The search strategies are listed in Appendix 2.
We searched for other relevant reviews in the reference lists of the included reviews, as well as the references of relevant narrative reviews and guidelines. We did not apply any restrictions on language or publication date.
Data collection and analysis
The methods we used for data collection and analysis, as described in successive sections, are based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
In successive sections, we report only the methods that we used in this review. Please see our protocol, Da Silva Lopes 2018, and Table 1 for additionally planned but unused methods.
1. Unused methods.
| Section in the protocol (Da Silva Lopes 2018) | Planned method in the protocol (Da Silva Lopes 2018) | Reason for non‐use |
| Data extraction and management | Where any information from the reviews is unclear or missing, we will access the published papers of the individual trials. If we cannot obtain the details from the published papers, we will request the information from the individual review authors or authors of the original papers. | We planned to request the information from the individual review authors or authors of the original papers if we could not obtain the details from the published papers, but this was not necessary. |
Selection of reviews
In order to identify all relevant published systematic reviews of RCTs assessing the effects of nutrition‐specific interventions to prevent and control anaemia throughout the life cycle, six review authors (KL, YT, NY, MS, OR, and WM) independently screened titles and abstracts, and assessed the full texts of all identified systematic reviews for eligibility. We assessed the reviews' objectives and methods, including outcomes and participants, for relevance and included only those reviews that met the criteria listed above (under Criteria for considering reviews for inclusion).
Where systematic reviews were similar in relation to research question, participants and interventions, we chose the most comprehensive review, provided there was an overlap between the underlying studies included in the individual reviews.
We resolved any disagreements through discussion until we reached a consensus, or, if necessary, we consulted another review author (EO).
The selection process is reported in the PRISMA flow diagram (Moher 2009).
Data extraction and management
We generated a data extraction form and pilot tested it. After verification, five review authors (KL, YT, NY, MS, and OR) independently extracted data from the included reviews on the following.
Characteristics of included systematic reviews: date of search; numbers of participants and included trials; review objective(s); type of participants; setting (countries, anaemia and malaria prevalence); interventions; comparisons; relevant outcomes with definition and information for any adjustments; GRADE assessment of relevant outcomes
Risk of bias assessment in included systematic reviews: method used; domains assessed; judgements
Characteristics of interventions: population (mean age, anaemia status/prevalence, known micronutrient deficiencies); prevention or treatment; dosage or form of application (including compound, formulation); frequency; start and duration of intervention; adherence to intervention
Results of included reviews: comparison; outcome; numbers of trials and participants; results (from meta‐analysis or narrative description)
We presented the review details and results in tables according to age group and type of intervention. Another review author (EO) verified the extracted data. We resolved any discrepancies through discussion until we reached a consensus, or, if necessary, by consulting another review author (EO).
Where any information from the reviews was unclear or missing, we accessed the published papers of the individual trials.
Assessment of methodological quality of included reviews
We assessed both the quality of evidence in the included reviews (by assessing the risk of bias of the included trials in each review and the GRADE certainty ratings of the evidence, if provided), and the methodological quality of the systematic reviews (using AMSTAR: A MeaSurement Tool to Assess systematic Reviews; Shea 2007a; Shea 2007b; Shea 2009).
Quality of the evidence in included reviews
Five review authors (KL, YT, NY, MS, and OR) independently assessed the quality of the evidence in the included reviews. We summarised the methods used to assess random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential sources of bias. If provided in the included systematic reviews, we also extracted GRADE ratings for our primary and secondary outcomes, to assess the certainty of the evidence.
Quality of included reviews
Five review authors (KL, YT, NY, MS, and OR) independently assessed the methodological quality of the included reviews using AMSTAR (Shea 2007a; Shea 2007b; Shea 2009). AMSTAR assesses the degree to which review methods avoided bias by evaluating the methods against 11 distinct criteria (shown below).
Was an a priori design provided?
Was there duplicate study selection and data extraction?
Was a comprehensive literature search performed?
Was the status of publication (i.e. grey literature) used as an inclusion criterion?
Was a list of studies (included and excluded) provided?
Were the characteristics of the included studies provided?
Was the scientific quality of the included studies assessed and documented?
Was the scientific quality of the included studies used appropriately in formulating conclusions?
Were the methods used to combine the findings of studies appropriate?
Was the likelihood of publication bias assessed?
Was the conflict of interest stated?
Each item on AMSTAR is rated as yes (clearly done), no (clearly not done), cannot answer, or not applicable (Shea 2007a; Shea 2007b; Shea 2009). We used this assessment to interpret the results of the reviews. We resolved any discrepancies through discussion until we reached a consensus, or, if necessary, by consulting another review author (EO).
Data synthesis
We provided a narrative summary of the data from the individual reviews for our primary and secondary outcomes and presented these summaries using tables. We presented data according to age group. Within each age category, we summarised the results from the different systematic reviews according to the types of interventions (supplementation, fortification, improving dietary diversity and quality). We described the results separately for interventions versus placebo or no intervention, and interventions versus another intervention. We investigated heterogeneity in relation to setting and population characteristics (e.g. prevalence of anaemia, malaria or other infectious diseases, baseline anaemia status, micronutrient deficiencies), features of the intervention (e.g. type, compound, dose, frequency, duration), and comparator (e.g. placebo, co‐interventions, other interventions, no intervention). If the authors of the individual reviews had not adjusted the data on anaemia for altitude and smoking, we presented the data in the form provided and noted this in the 'Results' and 'Discussion' sections of the review.
Results
Description of included reviews
For this overview of reviews, we searched for Cochrane and non‐Cochrane systematic reviews of RCTs of nutrition‐specific interventions to control or prevent anaemia at any stage of life. In total, we identified 9588 records from database searching. After removal of 2719 duplicates, we screened 6869 titles and abstracts. We excluded 6607 records at this stage and screened 262 full texts against our inclusion and exclusion criteria (see Criteria for considering reviews for inclusion). We excluded 107 clearly irrelevant records plus an additional 62 records that we decided did not meet the inclusion criteria following closer inspection; we summarise these 62 studies, with reasons for their exclusion, in Table 2. Eighteen ongoing reviews have been listed in Appendix 1. Finally, we included 75 systematic reviews in the review. The selection process is shown in Figure 1.
2. Excluded reviews: reasons for exclusion.
| Review | Title | Reason for exclusion |
| Agha 2014 | Interventions to reduce and prevent obesity in pre‐conceptual and pregnant women: a systematic review and meta‐analysis | No relevant outcomes for anaemia |
| Allen 2009 | Provision of multiple rather than two or fewer micronutrients more effectively improves growth and other outcomes in micronutrient‐deficient children and adults | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Ashman 2017 | The effectiveness of nutrition interventions for pregnant indigenous women: a systematic review | No relevant outcomes for anaemia |
| Athe 2014 | Impact of iron‐fortified foods on Hb concentration in children (< 10 years): a systematic review and meta‐analysis of randomized controlled trials | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Athe 2020 | Meta‐analysis approach on iron fortification and its effect on pregnancy and its outcome through randomized, controlled trials | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Bairwa 2017 | Directly observed iron supplementation for control of iron deficiency anemia | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Best 2011 | Can multi‐micronutrient food fortification improve the micronutrient status, growth, health, and cognition of schoolchildren? A systematic review | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Brown 2009 | Preventive zinc supplementation among infants, preschoolers, and older prepubertal children | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Butler 2006 | Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials | Systematic review included participants with megaloblastic anaemia, which was outside the scope of this overview of reviews |
| Cancelo‐Hidalgo 2013 | Tolerability of different oral iron supplements: a systematic review | Included different trial designs and results have not been presented separately |
| Câmara 2011 | The use of games in health education in order to prevent iron deficiency anemia in infancy: the role of the nurse ‐ literature systematic review | No relevant outcomes for anaemia |
| Chang 2018 | Safety and tolerability of prescription omega‐3 fatty acids: a systematic review and meta‐analysis of randomized controlled trials | Systematic review included participants with dyslipidaemia, which was outside the scope of this overview of reviews |
| Da Cunha 2019 | Effect of vitamin A supplementation on iron status in humans: a systematic review and meta‐analysis | Included different trial designs and results have not been presented separately |
| Das 2013b | Micronutrient fortification of food and its impact on woman and child health: a systematic review | Included different trial designs and results have not been presented separately |
| De Barros 2016 | Adherence to and acceptability of home fortification with vitamins and minerals in children aged 6 to 23 months: a systematic review | No relevant outcomes for anaemia |
| Dror 2012 | Interventions with vitamins B6, B12 and C in pregnancy | No relevant outcomes for anaemia |
| Eaton 2019 | Effectiveness of provision of animal‐source foods for supporting optimal growth and development in children 6 to 59 months of age | No relevant outcomes for anaemia |
| Falkingham 2010 | The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta‐analysis | No relevant outcomes for anaemia |
| Fishman 2000 | The role of vitamins in the prevention and control anaemia | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Gavaravarapu 2017 | Role of education and communication interventions in promoting micronutrient status in India – what research in the last two decades informs | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Ghanchi 2019 | Guts, germs, and iron: a systematic review on iron supplementation, iron fortification, and diarrhea in children aged 4‐59 months | No relevant outcomes for anaemia |
| Gera 2007b | Effect of iron supplementation on physical performance in children and adolescents: systematic review of randomized controlled trials | No relevant outcomes for anaemia |
| Girard 2012a | Nutrition education and counselling provided during pregnancy: effects on maternal, neonatal and child health outcomes | Included different trial designs and results have not been presented separately |
| Girard 2012b | The effects of household food production strategies on the health and nutrition outcomes of women and young children: a systematic review | Included different trial designs and results have not been presented separately |
| Guo 2015 | Daily iron supplementation on cognitive performance in primary‐school‐aged children with and without anemia: a meta‐analysis | No relevant outcomes for anaemia |
| Gurusamy 2014 | Iron therapy in anaemic adults without chronic kidney disease | Systematic review included non‐healthy participants, which was outside the scope of this overview of reviews |
| Haider 2018 | The effect of vegetarian diets on iron status in adults: a systematic review and meta‐analysis | Included different trial designs and results have not been presented separately |
| Kong 2016 | Limitations of studies on school‐based nutrition education interventions for obesity in China: a systematic review and meta‐analysis | Included different trial designs and results have not been presented separately |
| Iannotti 2006 | Iron supplementation in early childhood: health benefits and risks | Systematic review included high‐risk populations (e.g. participants with HIV, tuberculosis), which was outside the scope of this overview of reviews |
| Iglesias 2019 | Prevalence of anemia in children from Latin America and the Caribbean and effectiveness of nutritional interventions: systematic review and meta‐analysis | Included different trial designs and results have not been presented separately |
| Iqbal 2019 | Maternal and neonatal outcomes related to iron supplementation or iron status: a summary of meta‐analyses | Overview of systematic reviews |
| Jackson 2016 | Is higher consumption of animal flesh foods associated with better iron status among adults in developed countries? A systematic review | Included different trial designs and results have not been presented separately |
| Lewkowitz 2019 | Intravenous compared with oral iron for the treatment of iron‐deficiency anemia in pregnancy: a systematic review and meta‐analysis | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Lohner 2012 | Effect of folate supplementation on folate status and health outcomes in infants, children and adolescents: a systematic review | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Martínez 2020 | Efficacy and tolerability of oral iron protein succinylate: a systematic review of three decades of research | Included different trial designs and results have not been presented separately |
| Mayo‐Wilson 2014b | Preventive zinc supplementation for children, and the effect of additional iron: a systematic review and meta‐analysis | Systematic review published as a Cochrane Review, Mayo‐Wilson 2014a, which has been included in this overview of reviews |
| McDonagh 2015a | Routine iron supplementation and screening for iron deficiency anemia in children ages 6 to 24 months: a systematic review to update the US Preventive Services Task Force Recommendation | Included different trial designs and results have not been presented separately |
| McDonagh 2015b | Routine iron supplementation and screening for iron deficiency anemia in pregnant women: a systematic review to update the US Preventive Services Task Force Recommendation | Included different trial designs and results have not been presented separately |
| McDonagh 2015c | Screening and routine supplementation for iron deficiency anemia: a systematic review | Included different trial designs and results have not been presented separately |
| Michelazzo 2013 | The influence of vitamin A supplementation on iron status | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Middleton 2013 | Nutrition interventions and programs for reducing mortality and morbidity in pregnant and lactating women and women of reproductive age: a systematic review | Included different trial designs and results have not been presented separately |
| Middleton 2018 | Omega‐3 fatty acid addition during pregnancy | No relevant outcomes for anaemia |
| Miles 2019 | Intravenous iron therapy for non‐anaemic, iron‐deficient adults | Systematic review included high‐risk populations (not‐healthy participants), which was outside the scope of this overview of reviews |
| Milne 2009 | Protein and energy supplementation in elderly people at risk from malnutrition | No relevant outcomes for anaemia |
| Mirmiran 2012 | Iron, iodine and vitamin a in the middle East; a systematic review of deficiency and food fortification | Included different trial designs and results have not been presented separately |
| Oddo 2019 | Potential interventions targeting adolescent nutrition in Indonesia: a literature review | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Oh 2020 | Vitamin and mineral supplementation during pregnancy on maternal, birth, child health and development outcomes in low‐ and middle‐income countries: a systematic review and meta‐analysis | Included different trial designs and results have not been presented separately |
| Oliveira 2016 | Vitamin A supplementation for postpartum women | No relevant outcomes for anaemia |
| Osungbade 2012 | Preventive treatments of iron deficiency anaemia in pregnancy: a review of their effectiveness and implications for health system strengthening | Included different trial designs and results have not been presented separately |
| Pachón 2015 | Evidence of the effectiveness of flour fortification programs on iron status and anemia: a systematic review | Included different trial designs and results have not been presented separately |
| Pasricha 2009 | Risks of routine iron and folic acid supplementation for young children | No relevant outcomes for anaemia |
| Pasricha 2014 | Iron supplementation benefits physical performance in women of reproductive age: a systematic review and meta‐analysis | No relevant outcomes for anaemia |
| Sachdev 2005 | Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials | No relevant outcomes for anaemia |
| Sguassero 2012 | Community‐based supplementary feeding for promoting the growth of children under five years of age in low and middle income countries | No relevant outcomes for anaemia |
| Shao 2019 | The efficacy of ferumoxytol for iron deficiency anemia: a meta‐analysis of randomized controlled trials | Systematic review included high‐risk populations (cancer patients), which was outside the scope of this overview of reviews |
| Smith 2017 | Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta‐analysis of individual patient data from 17 randomised trials in low‐income and middle‐income countries | No relevant outcomes for anaemia |
| Sun 2018 | Effect of dietary intervention treatment on children with iron deficiency anemia in China: a meta‐analysis | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Szajewska 2010 | Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: a systematic review of randomized controlled trials | No relevant outcomes for anaemia |
| Tam 2020 | Micronutrient supplementation and fortification interventions on health and development outcomes among children under‐five in low‐ and middle‐income countries: a systematic review and meta‐analysis | Methodological quality of trials was not assessed, making it difficult to interpret the results |
| Vonderheid 2019 | A systematic review and meta‐analysis on the effects of probiotic species on iron absorption and iron status | Included different trial designs and results have not been presented separately |
| Xu 2019 | Supplementing fortified soybean powder reduced anemia in infants and young children aged 6‐24months | Included different trial designs and results have not been presented separately |
| Yadav 2020 | Comparison of different doses of daily iron supplementation for anemia prophylaxis in pregnancy: a systematic review | Methodological quality of trials was not assessed, making it difficult to interpret the results |
1.
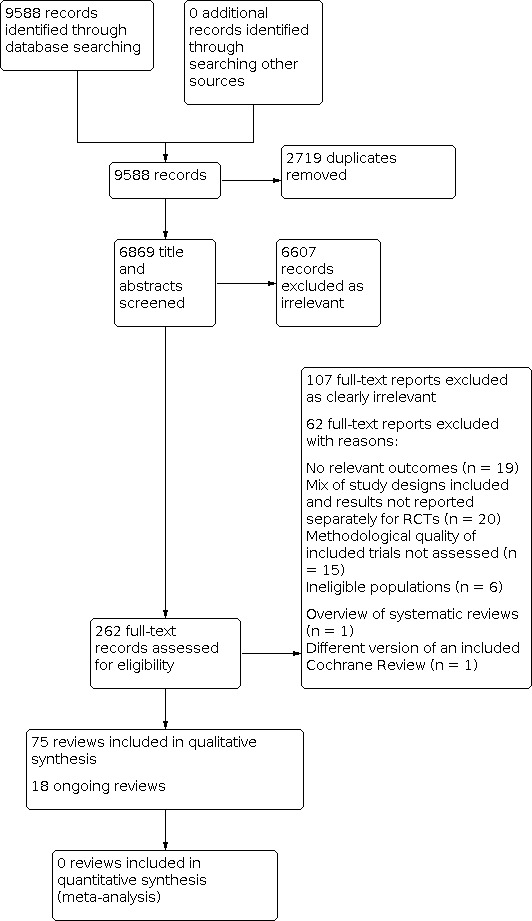
Study flow diagram.
Objectives and scope of included reviews
We summarised the key characteristics of the included reviews in Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8. Reviews aimed to assess nutrition‐specific interventions to prevent or control anaemia at different stages of life. All reviews included one or more of our primary outcomes: haemoglobin (Hb) concentration, anaemia and iron deficiency anaemia (IDA), and our secondary outcomes: iron deficiency (ID), severe anaemia and adverse effects. Although two reviews met our inclusion criteria, they did not contribute any data (Abe 2016; De‐Regil 2015).
3. Characteristics of included systematic reviews: infants (aged 6 to 23 months).
| Review | Date of search | Number of included trials (number of participants included) | Review question/objective | Trial designs included | Participants | Setting, anaemia and malaria prevalence | Intervention and comparison | Relevant outcomes (definition used in the review, adjusted for smoking and altitude) |
GRADE assessment of relevant outcomes Method used to assess risk of bias and summary |
| Supplementation | |||||||||
|
Abdullah 2013 Efficacy of oral iron therapy in improving the developmental outcome of pre‐school children with non‐anaemic iron deficiency: a systematic review |
January 2011 | 2 trials (249 children) | To evaluate the efficacy of oral Fe therapy in children of pre‐school age (1–5 years) with NAID (normal Hb, low Fe status) in improving developmental outcomes and to evaluate the efficacy of oral Fe therapy in terms of haematological outcomes and incidence of side‐effects of Fe therapy in children of pre‐school age with NAID | RCTs Quasi‐RCTs |
The participants were Fe deficient (serum ferritin < 12 μg/L) but non‐anaemic (Hb > 110 g/L) children who were otherwise healthy and aged 1–5 years | Turkey, Indonesia Anaemia and malaria prevalence: not reported |
Intervention: oral Fe therapy (≥ 2 mg elemental Fe/kg body weight per day administered for ≥ 3 months) with or without other interventions aimed at improving Fe level (such as dietary counselling, vitamin C, folic acid) Comparison: placebo or no treatment |
End‐of‐trial levels of Hb (g/L) Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. Both trials were assessed at moderate risk of bias |
|
Das 2019a Preventive lipid‐based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes |
October 2018 | 17 trials (23,200 children) | To assess the effects and safety of preventive LNS given with complementary foods on health, nutrition and developmental outcomes of non‐hospitalised infants and children 6 to 23 months of age, and whether or not they are more effective than other foods, including FBF or MNP | RCTs Quasi‐RCTs |
Non‐hospitalised infants and young children aged 6 to 23 months of age in stable (i.e. not in any emergency‐affected country or emergency settings according to WHO definition) | Ghana (2 trials), Malawi (4 trials), Democratic Republic of Congo, Bangladesh (3 trials), Burkina Faso, Chad, Haiti, Peru, Kenya, Guatemala, and the south‐western part of Intibucá, Honduras, bordering El Salvador Anaemia and malaria prevalence: not reported |
Intervention: LNS with complementary food at point‐of‐use Comparison: no intervention, placebo, or compared with other foods/supplements or nutrition intervention |
Anaemia (as defined by trialists)
Any adverse effects, including allergic reactions, as diagnosed by clinical assessment (atopic dermatitis, urticaria, oedema (oral), ophthalmic pruritus, allergic rhinitis, asthma, anaphylaxis) Adjustments: not reported |
GRADE: LNS plus complementary feeding compared with no intervention: anaemia = low, adverse effects = moderate; LNS plus complementary feeding compared with MNP: anaemia = low Cochrane RoB 1 tool. Overall, most trials were at low risk of bias for random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias. Most trials were assessed at high risk of bias for blinding of participants and personnel due to the nature of the intervention. |
|
Dekker 2010 Zinc supplementation in children is not associated with decreases in hemoglobin concentrations |
May 2009 | 21 trials (3869 children) | To evaluate the effect of zinc supplementation on haemoglobin concentrations among apparently healthy children | RCTs | Apparently healthy children from birth‐15 years (mean age at baseline = 32 months, the majority of trials commenced between 6‐23 months) |
Latin America (8 trials), Africa (3 trials), Asia (10 trials) Anaemiac and malaria prevalence: 3 trials were conducted among anaemic children and 3 among children with malaria |
Intervention: zinc supplementation Comparison: placebo |
Hb (g/L) Adjustments: not reported |
GRADE: not assessed Jadad level‐of‐evidence score for RCTs. 11 trials with high Jadad scores |
|
Pasricha 2013 Effect of daily iron supplementation on health in children aged 4‐23 months: a systematic review and meta‐analysis of randomised controlled trials |
February 2013 | 33 trials (42,015 children) | To review the evidence for benefit and safety of daily iron supplementation in children aged 4–23 months | RCTs Cluster‐RCTs Quasi‐RCTs |
Healthy children aged 4–23 months | Benin, Chile, Costa Rica, France, Ghana, Guatemala, India (2 trials), Indonesia (5 trials), Kenya, Nepal (2 trials), Pakistan, Sweden and Honduras, Tanzania (2 trials), Thailand, Togo, Turkey (4 trials), UK, USA (6 trials), Vietnam (2 trials) Anaemia and malaria prevalence: some trials conducted in malaria‐endemic areas |
Intervention: daily oral iron supplementation (alone or with co‐intervention) Comparison: control or co‐intervention alone |
Hb (g/L) Anaemia (defined by trial investigators) IDA (defined by trial investigators) ID Adverse effect (any side effects, vomiting, diarrhoea, constipation) Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. Many trials did not adequately report methodology for randomisation and concealment of allocation, and only 9 trials were considered at low risk of bias |
|
Petry 2016b The effect of low dose iron and zinc intake on child micronutrient status and development during the first 1000 days of life: a systematic review and meta‐analysis |
October 2015 | 90 trials | To evaluate the potential of interventions delivering daily doses of iron and zinc in concentrations up to approximately the RNI in diets with low bioavailability during the first 1000 days of life on child micronutrient status and health | RCTs Quasi‐RCTs (and quasi experimental, but rarely included) Cluster‐RCTs |
Pregnant women or lactating women, children aged 6–23 months ; 74 trials included children, 17 pregnant women, 1 lactating women |
Not described | Intervention: iron or zinc
supplementation, fortification or biofortification Comparison in fortification trials: unfortified foods or regular diets, same micronutrient but without iron or zinc Comparison in supplementation trials: no supplements, placebo, a lower concentration of iron or zinc, same micronutrients without iron or zinc |
Hb (g/dL) Anaemia (%; defined as Hb < 110 g/L) IDA (%; defined as Hb < 105 g/L or < 110 g/L and serum ferritin < 10 μg/L or < 12 μg/L) ID (%; defined as serum ferritin < 10 μg/L or < 12 μg/L) Diarrhoea Adjustments: not reported Data only available for children and iron interventions |
GRADE: Hb = moderate, anaemia = low, IDA = high, ID = high, diarrhoea = not assessed Assessment based on random sequence generation, adequacy of blinding of trial participants and personnel and completeness of outcomes assessment. Only GRADE results are presented |
|
Pratt 2015 A review of the strategies used to reduce the prevalence of iron deficiency and iron deficiency anaemia in infants aged 6‐36 months |
October 2014 | 8 trials (8109 children) | To compare the effectiveness of several strategies used to reduce the prevalence of ID and IDA in infants aged 6–36 months | RCTs
Cluster‐RCTs Randomised effectiveness trial |
6 and 36 months of age, either healthy or diagnosed with ID or IDA | Mexico (3 trials), Cambodia (1 trial), The Kyrygyz Republic (1 trial), Brazil (1 trial), USA (1 trial), New Zealand (1 trial) Anaemia and malaria prevalence: not reported |
Interventions: any strategy or method used to reduce the prevalence of ID and IDA Comparison: control or other current regimens to increase Hb status and reduce the prevalence of ID and IDA |
Hb (g/L) Anaemia (as defined by trialists) ID (as defined by trialists, based on biomarker of iron status, e.g. ferritin < 12 lg/L for preschool children) | GRADE: not assessed Modified Critical Appraisal Skills Programme (CASP) tool. In all trials, participants were randomised to treatments and were blinded |
| Fortification | |||||||||
|
Dewey 2009 Systematic review and meta‐analysis of home fortification of complementary foods |
November 2007 | 16 trials (6113 children) | To evaluate the efficacy and effectiveness of home fortification of complementary foods | RCTs Cluster‐RCTs (2 non‐randomised trials) |
Infant and young children (anaemic at baseline in treatment trials and non‐anaemic in prevention trials) |
Ghana (5 trials), China (2 trials), India (1 trial), Mongolia (1 trial), South Africa (1 trial), Bangladesh (1 trial), Pakistan (1 trial), Canada (1 trial), Cambodia (1 trial), Malawi (1 trial), Haiti (1 trial) Malaria prevalence: several trials conducted in populations with high rates of malaria |
Intervention: home fortification of complementary foods with MNPs (sprinkles), crushable tablets and lipid‐based or soy‐based products Comparison: non‐intervention group or placebo or fortified wheat soy blend without sprinkles or iron drops or complementary foods alone or sprinkles iron only |
Hb (g/L) Anaemia (Hb < 100 g/L) ID (ferritin < 12 μg/L) Diarrhoea Adjustments: not reported |
GRADE: not assessed Tool used to assess risk of bias was not described. 7 trials were rated at low risk of bias, 5 at very low risk of bias and 1 at high risk of bias. 1 non‐randomised trial was rated at low risk of confounding and 1 of moderate risk of confounding. |
|
Eichler 2012 Effects of micronutrient fortified milk and cereal food for infants and children: a systematic review |
February 2011 | 18 trials (5468 infants and children) | To specifically assess the impact of micronutrient fortified milk and cereal food on the health of infants and children compared to non‐fortified food in RCTs | RCTs Cluster‐RCTs |
Infants and children from 6 months to 5 years of age. Mean age of participants ranged from 6 to 23 months at inclusion |
Asia (2 trials), Africa (5 trials), South and Middle America (5 trials), Europa (6 trials) Anaemia and malaria prevalence: not reported |
Intervnetion: micronutrient‐fortified milk or cereal food Comparison: non‐fortified food; additional, other nutritional approaches, if such approaches were applied in the intervention and control group |
Hb (g/dL) Anaemia Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. Only 2 trials were rated at low risk of bias for random sequence generation and allocation concealment. Blinding was rated at low risk in 13 trials |
|
Matsuyama 2017 Effect of fortified milk on growth and nutritional status in young children: a systematic review and meta‐analysis |
June 2014 | 15 trials | To investigate the effect of fortified milk products compared with control milk in young children's growth and nutritional status outcome, such as body size and composition, and/or biochemical markers | RCTs Cluster‐RCTs |
Children (mean age at baseline = 6 to 22.4 months, 1 trial = 29 to 31 months at baseline) | Low‐income to high‐income economies (India, Indonesia, Mexico, Vietnam, Malysia, Thailand, Netherlands, Poland, Portugal, UK, Sweden, New Zealand) Anaemia and malaria prevalence: not reported |
Intervention: fortified milk Comparison: cow's milk or non‐ or low‐fortified milk |
Hb (g/L) Anaemia (Hb < 110 g/L) Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. About two‐thirds of the trials were adequate for random sequence generation, allocation concealment and blinding. |
|
Salam 2013 Effectiveness of micronutrient powders (MNP) in women and children |
November 2012 | 17 trials | To estimate the effect of MNPs on the health outcomes of women and children | RCTs Cluster‐RCTs |
Children aged 6 months to 11 years (most trials 6 months to 6 years, 2 trials up to 11 years) *50% of the trials were conducted in children aged 6 to 23 months, 75% of the trials were conducted children aged 6 months to 6 years |
Low‐income countries Anaemia and malaria prevalence: not reported |
Intervention: MNP Comparison: no intervention or control |
Hb (g/L) Anaemia IDA Diarrhoea Adjustments: not reported |
GRADE: Hb = moderate, anaemia = moderate, IDA = moderate, diarrhoea = moderate, recurrent diarrhoea = moderate Each trial was assessed and graded according to the CHERG adaptation of the GRADE technique |
|
Suchdev 2020 Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age |
July 2019 | 29 trials (33,147 participants) | To assess the effects and safety of home (point‐of‐use) fortification of foods with multiple MNPs) on nutrition, health, and developmental outcomes in children under two years of age | RCTs Cluster‐RCTs Quasi‐RCTs |
Infants and young children aged 6 to 23 months at the start of the intervention (apparently healthy children from the general population, although some may be at risk of having highly prevalent diseases such as malaria, diarrhoea or even undernutrition) | Low‐income countries in Asia, Africa and the Caribbean where anaemia is a public health problem (that is, > 40% of the population are affected) Malaria prevalence: 27 trials conducted in malaria‐endemic areas |
Intervention: MNPs including at least the 3 micronutrients iron, zinc and vitamin A (given to whole families, added to the family meal) Comparison: no intervention, placebo or usual supplementation: 1) home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo; 2) versus iron‐only supplement; 3) versus iron and folic acid supplements; 4) versus the same multiple micronutrients as in supplements |
Hb (g/L) Anaemia (defined as Hb values lower than 110 g/L) ID (defined by trialists) Diarrhoea Side effects (such as staining of teeth, vomiting, stool discolouration, constipation, coughing) Adjustments: not reported |
GRADE: MNP versus placebo or no intervention: Hb = low, anaemia = moderate, ID = high; MNP versus iron supplements intervention: Hb = very low, anaemia = low Cochrane RoB 1 tool. Overall, random sequence generation was adequate in 22 trials and allocation concealment in 21 trials. 10 trials were at high risk of bias for blinding of participants and personnel and 9 for blinding of outcome assessment. |
| Improving dietary diversity and quality | |||||||||
|
Kristjansson 2015 Food supplementation for improving the physical and psychosocial health of socio‐economically disadvantaged children aged three months to five years |
January 2014 | 32 trials (21 RCTs and 11 CBAs) | To assess the effectiveness of supplementary feeding interventions, alone or with co‐intervention, for improving the physical and psychosocial health of disadvantaged children aged 3 months to 5 years | RCTs Cluster RCTs CBAs (data extracted for RCTs only) |
Children aged 3 months to 5 years; > 60% of children were under 2 years | 29 trials were from low‐ and middle‐income countries; 3 were from high‐income countries Anaemia and malaria prevalence: not reported |
Intervention: supplementary feeding (provision of energy and macronutrients) with or without added micronutrients Comparison: non‐feeding control |
Hb Anaemia (CBAs only) Adjustments: not reported |
GRADE: not assessed for relevant outcomes Cochrane RoB 1 tool. The quality of RCTs was moderate. Attrition bias was problematic, ranging from 1% to 78%. Many trials did not mention blinding. |
|
Shapiro 2019 A systematic review investigating the relation between animal‐source food consumption and stunting in children aged 6‐60 months in low and middle‐income countries |
June 2017 | 21 studies (7 RCTs, 7 cross‐sectional studies, 7 longitudinal cohort studies) | To examine the relation between ASF consumption and stunting in children aged 6–60 months in low‐ and middle‐income countries. To examine the relation between ASF consumption and other indicators of growth and development (length/height, weight, head circumference, and anaemia) |
RCTs Cross‐sectional studies Longitudinal cohort studies (data extracted for RCTs only) |
Children aged 6 to 60 months (RCTs: 6 to 9 months) in low‐ and middle‐income countries | Low‐income countries: 10 trials Low‐middle income countries: 7 trials Upper‐middle income countries: 3 trials Multi‐site trial conducted in 4 countries (1 low and 3 middle‐income countries): 1 trial (malaria treatment reported in one trial, anaemia prevalence not reported) |
Intervention: consumption of ASFs Comparison: comparator or control group (e.g. non‐ASF, such as a PSF, or no intervention) |
Hb Anaemia adjustments: not reported |
GRADE: not assessed Quality assessment tools from the NHLBI: NHLBI Quality Assessment of Controlled Intervention Studies, NHLBI Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies. Three of the 7 RCTs were rated as good, 2 were rated as fair, and 2 were rated as poor. |
ASF: animal‐source foods; CBA: controlled before‐after trials; CASP: Critical Appraisal Skills Programme; CHERG: Child Health Epidemiology Reference Group; FBF: fortified blended foods; Fe: chemical symbol for iron; Hb: haemoglobin; ID: iron deficiency; IDA: iron deficiency anaemia; LNS: lipid‐based nutrient supplements; MNP: micronutrient powder; NAID: non‐anaemic iron deficiency; NHLBI: National Heart, Lung, and Blood Institute; PSF: plant‐source foods; RCTs: randomised controlled trials; RNI: recommended nutrient intake; WHO: World Health Organization.
4. Characteristics of included systematic reviews: preschool and school‐aged children (aged 2 to 10 years).
| Review | Date of search | Number of included trials (number of participants included) | Review question/objective | Trial designs included | Participants | Setting, anaemia and malaria prevalence | Intervention and comparison | Relevant outcomes (definition used in the review, adjusted for smoking and altitude) |
GRADE assessment of relevant outcomes Method used to assess risk of bias and summary |
| Supplementation | |||||||||
|
Low 2013 Effects of daily iron supplementation in primary‐school‐aged children: systematic review and meta‐analysis of randomized controlled trials |
July 2013 | 32 trials (7089 children) | To review of the effects of daily iron supplementation, a commonly used strategy to combat anaemia | RCTs Cluster‐RCTs |
Primary school–aged children (5–12 years) | Low or middle‐income countries, except for 1 trial Malaria: 9 trials conducted in endemic areas Anaemia prevalence: not reported |
Intervention: daily iron supplementation Comparison: placebo or control |
Hb (g/L) Anaemia (Hb < 120 g/L or as defined by trial authors) IDA ID Adverse effects (gastrointestinal upset, constipation, vomiting) Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. Only 4 trials were considered at low overall risk of bias. Many trials did not report the randomisation method (20 trials), allocation concealment (25 trials) or blinding (18 trials). |
|
De‐Regil 2011 Intermittent iron supplementation for improving nutrition and development in children under 12 years of age |
May 2011 | 33 trials (13,114 children) | To assess the effects of intermittent iron supplementation, alone or in combination with other vitamins and minerals, on nutritional and developmental outcomes in children from birth to 12 years of age compared with a placebo, no intervention or daily supplementation | RCTs Cluster‐RCTs Quasi‐RCTs |
Children under 12 years of age | Low and middle‐income countries in Asia, Africa and Latin America Malaria: 6 trials conducted in endemic areas, most trials did not report on malaria Anaemia prevalence: not reported |
Intervention: intermittent supplementation with iron alone or with other nutrients Comparison: placebo or no intervention or daily supplementation |
Hb (g/L) Anaemia (Hb below a cut‐off defined by trialists, taking into account the age and altitude) IDA (defined by the presence of anaemia plus ID, diagnosed with an indicator of iron status selected by trialists) ID (as measured by trialists by using indicators of iron status, such as ferritin or transferrin) Diarrhoea Any other adverse side effects (as measured by trialists, such as stained teeth, headache, stomach ache, discomfort, constipation) Adjustments: not reported |
GRADE: intermittent iron supplementation versus placebo or no intervention: Hb = low, anaemia = moderate, IDA = no data, ID = very low; intermittent iron supplementation versus daily iron supplementation: Hb = low, anaemia = low, IDA = no data, ID = very low, other outcomes = not assessed Cochrane RoB 1 tool. Many trials were rated at unclear risk of bias for, random sequence generation, allocation concealment and attrition rates. In half of the trials, blinding of participants and personnel was rated at high risk of bias. Overall, less than one‐third of the trials were rated at low risk of bias. |
|
Mayo‐Wilson 2014a Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age |
January 2013 | 80 trials (205,401 participants) | To assess the effects of zinc supplementation for preventing mortality and morbidity, and for promoting growth, in children aged 6 months to 12 years of age | RCTs Cluster‐RCTs Cross‐over RCTs |
Children aged 6 months to 12 years of age (mean age = 28 months) | 73 trials (91%) were conducted in
low‐ or middle‐income countries: Asia (37 trials), Latin America and the Caribbean (26 trials) and sub‐Saharan Africa (10 trials); 7 conducted in North America or Europe Anaemia and malaria prevalence: measured in some trials, but not specified |
Intervention: zinc supplementation Comparison: placebo, no intervention |
Blood Hb concentration Prevalence of anaemia Prevalence of ID Side effects (participants with ≥ 1 side effect, vomiting episodes, participants with ≥ 1 vomiting episode) Adjustments: not reported |
GRADE: side effect participants with ≥ 1 vomiting episode = high, other outcomes = not assessed Cochrane RoB 1 tool. One‐third of the trials were at low risk of bias for random sequence generation and allocation concealment. The remaining trials were at unclear risk of bias. 80% of the trials were at low risk of bias for blinding. Selective reporting was unclear in 50% of the trials and high risk in 40%. |
|
Thompson 2013 Effects of daily iron supplementation in 2‐ to 5‐year‐old children: systematic review and meta‐analysis |
April 2012 | 15 trials | To summarise the evidence for effects of daily iron supplementation administered to children 2 to 5 years of age | RCTs Cluster‐RCTs |
Children from 2 to 5 years of age | Mainly low‐middle‐income countries Anaemia and malaria prevalence: 4 trials in malaria endemic areas |
Intervention: oral iron supplement Comparison: placebo or other supplementations |
Hb (g/L) Anemia (defined by authors) Adjustments: not reported |
GRADE: Hb = high, anaemia = very low Cochrane RoB 1 tool. 13 trials at unclear risk of bias for random sequence generation and allocation concealment. Blinding was adequate in 11 trials. |
| Fortification | |||||||||
|
Aaron 2015 Multiple‐micronutrient fortified non‐dairy beverage interventions reduce the risk of anemia and iron deficiency in school‐aged children in low‐middle income countries: a systematic review and meta‐analysis |
February 2015 | 10 trials (4645 participants) | To evaluate the nutritional impacts of MMN‐fortified beverages in the context of low‐middle‐income countries | RCTs | Apparently healthy (school‐aged) children and women of reproductive age | School setting in low‐middle‐income countries: Bangladesh, Botswana, India, Nigeria, the Philippines, South Africa, and Tanzania Anaemia and malaria prevalence: not reported |
Intervention: non‐dairy MMN‐fortified beverages Comparison: iso‐caloric non‐fortified, non‐intervention controls, MMN‐fortified non‐caloric beverage or unfortified non‐caloric control |
Hb (g/L) Anaemia (Hb < 110 to 120 g/L) ID (ferritin < 27 to 45 pmol/L) IDA (Hb < 110 to 120 g/L and ferritin < 27 to 45 pmol/L) Adjustments: not reported |
GRADE: Hb = moderate, anaemia = moderate, ID = moderate, IDA = low Risk of bias tool not stated, but trial bias was assessed by publication bias, randomisation methods, type of blinding (single or double), the percentage of loss to follow‐up (low versus high) and subgroup analyses. Methodological quality was high in 2 trials, moderate in 7 and low in 1 |
|
Das 2013a Systematic review of zinc fortification trials |
October 2012 | 11 trials (771 women and children) | To assess impact of food fortification with zinc on the health and nutrition of women and children | RCTs Quasi‐RCTs |
Woman and children (newborn, infants and school‐aged children) Hb data only available for school‐aged children |
4 trials were conducted in low‐income countries, while the rest were from high‐income countries. Anaemia and malaria prevalence: not reported |
Intervention: fortified food with zinc as the only micronutrient Comparison: no intervention group, with a regular diet or unfortified foods |
Serum haemoglobin Adjustments: not reported |
GRADE = not assessed Risk of bias tool not stated, but risk of bias was assessed for the following domains: sequence allocation, allocation concealment, blinding, incomplete outcome data addressed, selective reporting. In 2 trials, risk of bias was low; most trials were at unclear or high risk of bias |
|
De‐Regil 2017 Point‐of‐use fortification of foods with micronutrient powders containing iron in children of preschool and school age |
December 2016 and April 2017 | 13 trials (5810 participants) | To assess the effects of point‐of‐use fortification of foods with iron‐containing MNP alone, or in combination with other vitamins and minerals on nutrition, health and development among children at preschool (24 to 59 months) and school (5 to 12 years) age | RCTs Quasi‐RCTs Cluster‐RCTs |
Children aged 24 months (2 years) to 59 months (< 5 years of age) and 5 to 12 years of age at the time of receiving the intervention with MNP | Low‐ and middle‐income populations, with the authors of 7 trials reporting that participants were of low socioeconomic status 3 trials conducted in malaria‐endemic areas Anaemia prevalence: range = 7.3% to 92% among the 9 trials reporting these data that did not exclude participants with anaemia |
Intervention: provision of MNP for point‐of‐use fortification given at any dose, frequency and duration Comparison: no intervention, placebo or usual supplementation |
Hb (g/L) Anaemia (defined as Hb < 110 g/L for children aged 24 to 59 months and < 115 g/L for children aged 5 to 11.9 years, adjusted by altitude where appropriate) IDA (defined by the presence of anaemia plus ID, diagnosed with an indicator of iron status as selected by trialists) ID (defined by using ferritin concentrations < 15 μg/L) Adverse effects (any, as defined by trialists) Diarrhoea (3 liquid stools or more per day) Adjustments: not reported |
GRADE: Hb = low, anaemia = moderate, ID = moderate, adverse effects = moderate, diarrhoea = low Cochrane RoB 1 tool. 9 of the 13 trials were considered at low risk of bias. In most trials, the main limitation was the lack of blinding at all levels. |
|
Eichler 2019 Health effects of micronutrient fortified dairy products and cereal food for children and adolescents: A systematic review |
January 2018 | 24 studies (9367 children and adolescents) | To assess the impact of MN fortified dairy products and cereal food on the health of children and adolescents (aged 5 to 15 years) compared with non‐fortified food | RCTs Cluster‐RCTs |
Children (aged 5 to 12 years) and adolescents (aged 12 to 15 years) of both sexes and from all risk groups. Studies with mixed population groups were included only if the majority of participants were within the age range of 5 to 15 years | Low‐ and middle‐income countries Anaemia and malaria prevalence: not reported |
Intervention: centrally‐processed fortified dairy products and fortified cereals, using any fortification strategy Comparison: non‐fortified food |
Hb (g/dL; conversion to g/L with factor 10) Anaemia rate (< 11.5 g/dL (5 to 11 years of age)); < 12.0 g/dL (12 to 15 years of age)) IDA ID (ferritin level) Adverse events Adjustments: not reported |
GRADE: Hb = very low, anaemia = very low, IDA = very low, ID = very low, adverse events = low Cochrane RoB 1 tool for RCTs. Only 4 of 24 studies were judged as having a low risk of bias in at least 5 of 6 assessed domains. |
Fe: ferrum (iron); Hb: haemoglobin; ID: iron deficiency; IDA: iron deficiency anaemia; MMN: multiple micronutrient; MN: micronutrient; MNP: micronutrient powders; RCT: randomised controlled trial; WHO: World Health Organization.
5. Characteristics of included systematic reviews: adolescent children (aged 11 to 18 years).
| Review | Date of search | Number of included trials (number of participants included) | Review question/objective | Trial designs included | Participants | Setting, anaemia and malaria prevalence | Intervention and comparison | Relevant outcomes (definition used in the review, adjusted for smoking and altitude) |
GRADE assessment of relevant outcomes Method used to assess risk of bias and summary |
| Supplementation | |||||||||
|
Fernández‐Gaxiola 2019 Intermittent iron supplementation for reducing anaemia and its associated impairments in adolescent and adult menstruating women |
February 2018 | 25 trials (10,996 women) | To assess the effects of intermittent oral iron supplementation, alone or in combination with other nutrients, on anaemia and its associated impairments in menstruating women, compared with no intervention, a placebo or daily supplementation | RCTs Quasi‐RCTs Cluster‐RCTs |
Menstruating women, that is women beyond menarche and prior to menopause who are not pregnant or lactating or have any condition that impedes the presence of menstrual periods, regardless of their baseline iron status/anaemia status, ethnicity, country of residence or level of endurance. (> 60% of the included trials included women under 18 years) |
LMIC (15 trials) Europe (1 trial) Latin America (4 trials) Africa (5 trials) Asia (15 trials) Malaria: 5 trials conducted in endemic areas Anaemia prevalence: 1 trial prevalent, but percentage not reported |
Intervention: intermittent dosage of iron alone or with other vitamins and minerals Comparison: placebo or no intervention or the same supplements provided on a daily basis |
Hb (g/L) Anaemia (Hb concentration below a cut‐off defined by trialists, adjusted by altitude and smoking as appropriate) IDA (defined by the presence of anaemia plus ID diagnosed with an indicator of iron status selected by trialists) ID (defined by trialists using indicators of iron status such as ferritin or transferrin) Any adverse side effects (e.g. nausea, vomiting, constipation, gastrointestinal discomfort, as defined by the trialists) Adjustments: not reported |
GRADE: intermittent iron supplementation versus no supplementation or placebo: Hb = moderate, anaemia = low IDA = low, ID = low, any adverse side effects = moderate; intermittent iron supplementation versus daily iron: Hb = low, anaemia = moderate, IDA = no trials, ID = very low, any adverse side effects = low Cochrane RoB 1 tool Overall, most trials (23/25) were considered to be at high risk of bias due to lack of description of methods used for randomisation and allocation concealment and lack of blinding |
|
Neuberger 2016 Oral iron supplements for children in malaria‐endemic areas |
MEDLINE (August 2015); Other databases (February 2015) | 35 trials (31,955 children) | To evaluate the effects and safety of iron supplementation, with or without folic acid, in children living in areas with hyperendemic or holoendemic malaria transmission | RCTs Cluster‐RCTs |
Children (less than 18 years of age), with or without anaemia, and with or without malaria or parasitaemia | Areas that are malaria‐endemic, and where children may benefit from iron treatment; the baseline rate of malaria parasitaemia
(reported in 11 of 19 trials) ranged from 0% to 70% of children (mean 45%) Anaemia prevalence: not reported |
Intervention: iron supplementation, with or without folic acid or antimalarial treatment Comparison: placebo or no treatment or antimalarial treatment (only when the intervention is iron plus antimalarial) |
Hb (g/dL) Anaemia as defined in trial Adjustments: not reported | GRADE: not assessed The quality of studies was assessed by the Cochrane RoB 1 tool; subgroup analyses performed. Publication bias assessed by funnel plots. Around 57% of studies were at low risk of bias related to allocation concealment, 74.5% of studies to random sequence generation, and 77.1% to blinding. |
|
Salam 2016 Interventions to improve adolescent nutrition: a systematic review and meta‐analysis |
December 2014 | 31 trials (MN supplementation) 10 trials (nutrition in pregnant adolescents) |
To ascertain the effectiveness of interventions to promote nutrition among adolescents comprising of MN supplementation, nutrition interventions for pregnant adolescents, and interventions to prevent obesity | RCTs Quasi‐RCTs CBA trials |
Adolescent population (11 to 19 years old)
Low‐income, pregnant adolescents (13 to 20 years old) (only data for adolescent population was extracted) |
MN supplementation: 23/31 trials conducted in LMICs
Nutrition in pregnancy: prenatal clinics in urban areas in Chile, Ecuador, USA, Canada Anaemia and malaria prevalence: not reported |
Interventions: interventions to promote nutrition among adolescent (e.g. micronutrient supplementation, nutrition in pregnancy) Comparison: control (not specified) |
Hb (g/L) Anaemia (as defined by trial authors) IDA Adjustments: not reported |
GRADE: MN supplementation: anaemia = moderate; nutrition in pregnancy: anaemia = low, other outcomes = not assessed Cochrane RoB 1 tool, but only GRADE is reported |
|
Salam 2020 Effects of preventive nutrition interventions among adolescents on health and nutritional status in low‐ and middle‐income countries |
February 2019 | 10 studies (10,802 adolescents) | To assess the impact of preventive nutrition interventions on health and nutritional status of adolescents aged 10 to 19 years in LMICs | RCTs Cluster‐RCTs Quasi‐RCTs CBA ITS |
Adolescents aged 10 to 19 years | School setting in LMICs: China, India, Sri Lanka, Bangladesh, Indonesia Anaemia and malaria prevalence: not reported |
Intervention: MN supplementation/fortification Comparison: placebo/no supplementation/no fortification |
Anaemia; adjustment: not reported | GRADE: anaemia = low Study quality assessed by Cochrane RoB 1 tool for RCTs and EPOC risk of bias tool for non‐randomised studies. The included studies were judged to be at unclear risk of bias due to insufficient information regarding sequence generation, allocation concealment, and selective reporting. |
CBA: controlled before‐and‐after trials; EPOC: Effective Practice and Organisation of Care; Hb: haemoglobin; ID: iron deficiency; IDA: iron deficiency anaemia; ITS: interrupted time series; LMICs: low‐ and middle‐income countries; MN: micronutrient; RCTs: randomised controlled trials.
6. Characteristics of included systematic reviews: non‐pregnant women of reproductive age (aged 19 to 49 years).
| Review | Date of search | Number of included trials (number of participants included) | Review question/objective | Trial designs included | Participants | Setting, anaemia and malaria prevalence | Intervention and comparison | Relevant outcomes (definition used in the review, adjusted for smoking and altitude) |
GRADE assessment of relevant outcomes Method used to assess risk of bias and summary |
| Supplementation | |||||||||
|
Abe 2016 Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby |
September 2015 | 2 trials (52 women) | To evaluate the effects of MMN supplementation in breastfeeding mothers on maternal and infant outcomes | RCTs | Non‐pregnant mothers who exclusively fed breast milk or practiced mixed feeding (breast milk and formula). HIV‐positive women were excluded | Brazil, USA Anaemia and malaria prevalence: not reported |
Intervention: MMN supplements of 3 or more micronutrients Comparison: placebo, no supplementation or supplementation with 2 or fewer micronutrients, irrespective of dosage of micronutrient |
Anaemia (maternal haemoglobin level < 12 g/dL or maternal serum ferritin < 15 μg/L) Adverse effects Adjustments: not reported |
GRADE: intended but not assessed due to lack of outcomes Cochrane RoB 1 tool. Most domains were rated as unclear overall due to lack of information in both trial reports |
|
Houston 2018 Efficacy of iron supplementation on fatigue and physical capacity in non‐anaemic iron‐deficient adults: a systematic review of randomised controlled trials |
October 2016 | 18 trials (from 20 reports) (1170 participants) | To identify the effects of iron therapy on fatigue and physical capacity in IDNA adults | RCTs | Adults (≥ 18 years) who were iron deficient but non‐anaemic; 15 trials included only women, with > 60% within this age group |
North America (8 trials), Europe (7 trials), Australia (2 trials), Asia (1 trial) Anaemia and malaria prevalence: not reported |
Intervention: oral, IM or IV iron supplementation Comparison: placebo or active therapy |
Hb (g/L) Anaemia (Hb < 130 g/L for males, < 120 g/L for females) Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. Overall, 1 trial was rated at low risk of bias while the remaining trials were rated at unclear risk of bias. 13 trials were adequate for performance bias and 10 for detection bias. Randomisation was adequate in 6 trials and allocation concealment in 5 trials. |
|
Lassi 2020 Effects of preconception care and periconception interventions on maternal nutritional status and birth outcomes in low‐ and middle‐income countries: a systematic review |
May 2019 | 45 trials (10 trials for iron supplementation, including 8955 participants; data and information only extracted for those trials) | To synthesise the current evidence on the effectiveness of preconception care interventions relating to the delayed age at first pregnancy; optimising inter‐pregnancy intervals | RCTs Quasi‐experimental Natural experiment |
Women of reproductive age | Low‐ and middle‐ income countries: Bangladesh (2 trials), India (2 trials), Indonesia (3 trials), Nepal (1 trials), Mali (1 trials), Tanzania (1 trials) Anaemia and malaria prevalence: not reported |
Intervention: periconceptional iron folic acid supplementation Comparison: placebo |
Anaemia Adjustments: not reported |
GRADE: anaemia ‐ RCTs = very low, anaemia ‐ weekly supplementation = very low, anaemia ‐ daily supplementation = very low Risk of bias assessment comprised of Cochrane RoB 1 and EPOC criteria. Eight trials were assessed unclear or low risk of bias in selection bias and attrition bias. Two trials were assessed as high risk of bias in selection bias or attrition bias each. |
|
Low 2016 Daily iron supplementation for improving anaemia, iron status and health in menstruating women |
November 2015 | 67 trials (8506 women) | To establish the evidence for effects of daily supplementation with iron on anaemia and iron status, as well as on physical, psychological and neurocognitive health, in menstruating women | RCTs Cluster‐RCTs Quasi‐RCTs |
Menstruating women, that is, women beyond menarche and prior to menopause who were not pregnant or lactating or had any condition that impeded the presence of menstrual periods | Trials were conducted in numerous countries of differing cultural and economic backgrounds Malaria: 1 trial conducted in endemic area Anaemia prevalence: not reported |
Intervention: daily oral iron supplementation with or without a co‐intervention (folic acid or vitamin C) Comparison: no supplemental iron |
Hb (g/L) Anaemia (Hb concentrations below a cut‐off defined by trial authors) IDA (defined by the presence of anaemia plus iron deficiency, diagnosed with an indicator of iron status selected by trialists) Iron deficiency (as measured by trial authors using indicators of iron status such as ferritin or transferrin) Any adverse side effects (as measured by trial authors such as abdominal pain, vomiting, nausea, heartburn, diarrhoea, constipation) Adjustments: not reported |
GRADE: Hb = high, anaemia = moderate, IDA = not assessed, ID = moderate, any adverse side effect = low Cochrane RoB 1 tool. Overall, trial methods were not well described. 14 trials used adequate methods for random sequence generation and 15 for allocation concealment. Blinding of participants was not attempted in 8 trials (unlikely to impact on laboratory outcomes). Overall, only 10 trials were assessed as being at low overall risk of bias. |
|
Sultan 2019 Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta‐analysis |
November 2017 | 15 studies (2182 participants) | To compare oral versus IV iron therapy to treat postpartum anaemia | RCTs | Women with a postdelivery Hb level of < 12 g/dL | India (4 trials), Greece (2 trials), USA (2 trials), Norway, Romania, Egypt, UK, Spain, Denmark, Pakistan Anaemia and malaria prevalence: not reported |
Intervention: IV iron Comparison: oral iron |
Hb (g/dL) Treatment‐related side effects Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. Eight trials were low risk for random sequence generation and 7 unclear. Allocation concealment was only adequate in 6 trials. Performance bias was high risk in 14 trials and unclear in 1. Detection bias was high risk in 6 trials, low risk in 7 and unclear in 1 trial. |
EPOC: Effective Practice and Organisation of Care; Hb: haemoglobin; IDA: iron deficiency anaemia; IDNA: iron‐deficient non‐anaemic; IM: intramuscular; IV: intravenous; MMN: multiple micronutrient; RCTs: randomised controlled trials.
7. Characteristics of included systematic reviews: pregnant women (aged 15 to 49 years).
| Review | Date of search | Number of included trials (number of participants included) | Review question/objective | Trial designs included | Participants | Setting, anaemia and malaria prevalence | Intervention and comparison | Relevant outcomes (definition used in the review, adjusted for smoking and altitude) |
GRADE assessment of relevant outcomes Method used to assess risk of bias and summary |
| Supplementation | |||||||||
|
Abu Hashim 2017 Lactoferrin or ferrous salts for iron deficiency anemia in pregnancy: a meta‐analysis of randomized trials |
February 2017 | 4 trials (600 women) | To evaluate the efficacy of daily oral bovine lactoferrin versus daily oral ferrous iron preparations for treatment of IDA during pregnancy | RCTs | Pregnant women with IDA (Hb < 11 g/dL) | Italy (3 trials), Egypt (1 trial) Anaemia and malaria prevalence: not reported |
Intervention: oral bovine lactoferrin Comparison: oral ferrous iron preparations |
Change in Hb level (g/dL) after at least 4 weeks of treatment
Rates of gastrointestinal side effects during the treatment period (epigastric discomfort, nausea, vomiting, diarrhoea, constipation, abdominal colicky pain and dark stools) Adjustments: not reported |
GRADE: Hb levels = low, gastrointestinal side effects = moderate Cochrane RoB 1 tool. Risk of bias was mostly unclear for the important domains of random sequence generation, allocation concealment and blinding. Other domains were at low risk of bias |
|
Bhutta 2012 Is it time to replace iron folate supplements in pregnancy with multiple micronutrients? |
September 2011 | 7 trials (18,595 pregnant women) | To compare the effects of MMN supplements during pregnancy versus iron folate for the prevention of maternal anaemia | RCTs Cluster‐RCTs |
Healthy pregnant women | All trials were from low or middle‐income settings Anaemia and malaria prevalence: not reported |
Intervention: MMN supplementation Comparison: iron folate supplementation |
Maternal Hb Maternal anaemia in the third trimester Adjustments: not reported |
GRADE: Hb = low, anaemia = moderate Cochrane RoB 1 tool. 6 trials assessed at low risk of bias and 1 trial assessed at high risk of bias |
|
Buppasiri 2015 Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes |
September 2014 | 25 trials, of which, 23 contributed data (18,578 pregnant women) | To determine the effect of calcium supplementation on maternal, fetal and neonatal outcomes (other than for preventing or treating hypertension), including the occurrence of side effects | RCTs | Pregnant women who received any calcium supplementation compared with placebo or no treatment | Argentina, Australia, Columbia, Ecuador, Egypt, Gambia, Guatemala, Hong Kong, India, Iran, Mexico, South Africa, USA and Vietnam Anaemia and malaria prevalence: not reported |
Intervention: calcium supplementation during pregnancy Comparison: placebo or no treatment |
Maternal anaemia (as defined by the trial authors) Adjustments: not reported |
GRADE: not assessed for relevant outcomes Cochrane RoB 1 tool. Most of the trials (17 out of 25) were rated at low risk of bias for both sequence generation and allocation concealment |
|
Daru 2016 Systematic review of randomized trials of the effect of iron supplementation on iron stores and oxygen carrying capacity in pregnancy |
January 2015 | 23 trials (3525 women) | To assess the effect on serum ferritin (iron stores) and Hb (oxygen‐carrying capacity) following iron supplementation in pregnant women with anaemia and NAID | RCTs | Pregnant women at any gestation with NAID or IDA, or both | Not reported Anaemia and malaria prevalence: not reported |
Intervention: iron supplementation (oral, including fortified water, intravenous or intramuscular) Comparison: placebo, or oral or intramuscular iron preparations |
Hb Adjustments: not reported |
GRADE: not assessed Jadad method used to assess trial quality (a score of > 3 equated to good quality). 12 trials were of high quality and 11 of low quality |
|
Das 2018 Lipid‐based nutrient supplements for maternal, birth, and infant developmental outcomes |
May 2018 | 3 RCTs and 1 cluster‐RCT (8018 pregnant women) | To assess the effects of LNS for maternal, birth and infant outcomes in pregnant women | RCTs Cluster‐RCTs |
Women with singleton pregnancy of any age and parity | Stable community settings: Ghana, Malawi, Burkina Faso, Bangladesh Anaemia and malaria prevalence: not reported | Intervention: LNS Comparison: no intervention, placebo, IFA, MMNs or nutritional counselling |
Hb Anaemia (Hb less than 110 g/L) Adverse effects Adjustments: not reported |
GRADE: anaemia = moderate Cochrane RoB 1 tool |
|
De‐Regil 2015 Effects and safety of periconceptional oral folate supplementation for preventing birth defects |
August 2015 | 5 trials (7391 women) | To examine whether periconceptional folate supplementation reduces the risk of neural tube and other congenital anomalies (including cleft palate) without causing adverse outcomes in mothers or babies | RCTs | Pregnant women ≤ 12 weeks' gestation at the time of intervention | Settings: 4 trials from 9 high‐income countries, 1 trial from 1 lower‐ middle‐income country Anaemia and malaria prevalence: not reported |
Intervention: periconceptional folate or folic acid supplementation alone or in combination with other vitamins or minerals Comparison: no treatment or placebo or other micronutrients without folate |
Maternal anaemia at or near term (Hb < 110 g/L at 34 weeks' gestation or more) Adjustments: not reported |
GRADE: not assessed for relevant outcomes Cochrane RoB 1 tool. Trials were rated at unclear or low risk of bias for allocation concealment and blinding |
|
Govindappagari 2019 Treatment of iron deficiency anemia in pregnancy with intravenous versus oral iron: systematic review and meta‐analysis |
October 2017 | 11 trials (1190 participants) | To study benefits of IV iron over oral iron for treatment of anaemia in pregnancy | RCTs | Pregnant women with IDA | India (7 trials), France (1 trial), Turkey (1 trial), Egypt (1 trial), multi‐country (1 trial) Anaemia and malaria prevalence: not reported |
Intervention: IV iron Comparison: oral iron |
Hb in response to treatment Hb after 4 weeks of treatment Adverse effects Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. The domains random sequence generation, incomplete outcome data, selective reporting and other bias were adequate in all trials. All trials were high risk of bias for performance and detection bias due to lack of blinding. Allocation concealment was unclear in most trials. |
|
Haider 2011 Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes |
December 2009 | 14 trials (17 reports) | To evaluate the evidence of the impact of MMN supplements during pregnancy, in comparison with standard iron‐folate supplements, on specific maternal and pregnancy outcomes of relevance to the Lives Saved Tool | RCTs Cluster‐RCTs |
Pregnant women (any gestation) | All trials were from low‐income or middle‐income settings Anaemia and malaria prevalence: not reported |
Intervention: MMN supplementation (at least 5 MMNs, including the UNIMMAP formulation or those with comparable composition) Comparison: maternal iron‐folate supplementation |
Maternal anaemia in the third trimester Adjustments: not reported |
GRADE: anaemia = high Cochrane RoB 1 tool. Overall, 9 trials were of high quality and 5 moderate. Some of the trials had limitations based on trial design and execution such as large losses to follow‐up, insufficient power to detect differences in small‐for‐gestational age and mortality, and missing compliance data. |
|
Haider 2013 Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta‐analysis |
May 2012 | 48 trials (17,793 women) 44 cohort trials (1,851,682 women) |
To summarise evidence on the associations of maternal anaemia and prenatal iron use with maternal haematological and adverse pregnancy outcomes; and to evaluate potential exposure‐response relations of dose of iron, duration of use, and Hb concentration in prenatal period with pregnancy outcomes | RCTs Cluster‐RCTs Cohort trials Data only extracted for RCTs |
Pregnant women | 27 trials conducted in high‐income countries (4861 women), 21 in low‐ or middle‐income countries (12,932 women) Malaria endemicity: endemic in 7 trials, non‐endemic in 29 trials Baseline anaemia: anaemic in 7 trials, non‐anaemic in 26 trials |
Intervention: daily oral iron or iron and folic acid (both supplementation and fortification) Comparison: placebo, no iron, or no iron and folic acid |
Mean Hb concentration (g/L) Anaemia (Hb < 110 g/L) IDA (Hb < 110 g/L and serum ferritin < 12 µg/L) ID (defined as serum ferritin < 12 μg/L) in the second or third trimester or at delivery and in the postpartum period Adjustments: not reported |
GRADE: not assessed Risk of bias tool not stated, but trial quality was assessed using the following domains: randomisation technique, concealment of allocation, blinding, and loss to follow‐up. 18 trials deemed to be of high quality (adequate for randomisation and allocation concealment plus either blinding or loss to follow‐up under 20%) |
|
Imdad 2012 Routine iron/folate supplementation during pregnancy: effect on maternal anaemia and birth outcomes |
June 2011 | 30 trials | To assess the impact of routine iron supplementation on maternal anaemia and perinatal outcomes | RCTs Cluster‐RCTs Quasi‐RCTs |
Pregnant women | High‐income and low‐income countries Anaemia and malaria prevalence: not reported |
Intervention: prenatal iron or iron plus folic acid Comparison: placebo or no intervention |
Maternal anaemia at term (Hb < 110 g/L) Severe anaemia (at term or any time during second or third trimester) Maternal IDA (Hb < 110 g/L) Adjustments: not reported |
GRADE: anaemia at term = moderate Assessed trial limitations using GRADE. Limitations for trials reporting anaemia at term: trials with unclear or inadequate sequence generation and high loss to follow‐up |
|
Keats 2019 Multiple‐micronutrient supplementation for women during pregnancy |
February 2018 | 21 trials (142,496 women) | To evaluate the benefits of oral MMN supplementation during pregnancy on maternal, fetal and infant health outcomes | RCTs Cluster‐RCTs |
Pregnant women at any length of gestation at the time of enrolment in the trial | All trials, except 1, were from low‐ and middle‐income settings Malaria: 5 trials reported malaria prophylaxis Anaemia prevalence: not reported |
Intervention and comparison:
|
Maternal anaemia (third trimester (Hb < 110 g/L) Side‐effects of MMN supplements (no data) Adjustments: not reported |
GRADE: not assessed for relevant outcomes Cochrane RoB 1 tool. The risk of bias was generally low with at least 50% of the judgements at low risk of bias for 2 domains (allocation concealment and incomplete outcome data) and at least 75% of the judgements at low risk of bias for the remaining 5 domains. |
|
Lassi 2013 Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes |
December 2012 | 31 trials (17,771 women) | To assess the effectiveness of oral folic acid supplementation alone or with other micronutrients versus no folic acid (placebo or same micronutrients but no folic acid) during pregnancy on haematological and biochemical parameters during pregnancy and on pregnancy outcomes | RCTs Cluster‐RCTs Quasi‐RCTs |
Pregnant women of any age and parity | The majority of trials were conducted in Europe, Africa and Asia. 1 trial was conducted in South America, 1 in Australia and 1 in New Zealand Anaemia and malaria prevalence: not reported |
Interventions:
Comparison: placebo or same micronutrients but no folic acid |
Mean pre‐delivery Hb Pre‐delivery anaemia (< 10 g/dL, Hb or haematocrit below 30%) Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. All included trials were conducted over 30 to 45 years ago. The reviewers found poor subjective and objective compliance with random allocation, adequate concealment and blinding. |
|
McCauley 2015 Vitamin A supplementation during pregnancy for maternal and newborn outcomes |
March 2015 | 19 trials (over 310,000 women) | To review the effects of supplementation of vitamin A, or one of its derivatives, during pregnancy, alone or in combination with other vitamins and micronutrients, on maternal and newborn clinical outcomes | RCTs Cluster‐RCTs Quasi‐RCTs |
Pregnant women | Africa (7 trials), Indonesia (6 trials), Bangladesh (2 trials), Nepal (1 trial), China (1 trial), India (1 trial) Anaemia and malaria prevalence: malaria prevalence reported in 3 trials |
Intervention: vitamin A (or one of its derivatives) supplementation, alone or in combination with other supplements Comparison: placebo or no treatment |
Maternal anaemia (Hb < 11.0 g/dL) Neonatal anaemia (as defined by investigator) Adjustments: not reported |
GRADE: maternal anaemia = moderate Cochrane RoB 1 tool. One‐third of the trials were at low risk of bias for random sequence generation and half of the trials were adequate for allocation concealment. The risk of performance bias was low in 80% of the trials, but only 25% of the trials for detection bias. Selective reporting bias was unclear in 18 of the 19 trials. |
|
Peña‐Rosas 2015b Daily oral iron supplementation during pregnancy |
February 2015 | 61 trials (44 with 43,274 women contributing data) | To assess the effects of daily oral iron supplements for pregnant women, either alone or in conjunction with folic acid, or with other vitamins and minerals as a public health intervention in antenatal care | RCTs Cluster‐RCTs Quasi‐RCTs |
Pregnant women of any gestational age and parity | Europe (24 trials), Americas (11 trials), Africa (4 trials), Iran (4 trials), Hong Kong (1 trial), China (4 trials), Australia (3 trials), Asia (8 trials) Anaemia and malaria prevalence: 23 trials conducted in malaria‐risk areas, anaemia prevalence unclear |
Intervention: any supplements containing iron Comparison: same supplements without iron or no treatment or placebo (no iron or placebo) |
Maternal Hb concentration at or near term (in g/L, at 34 weeks’ gestation or more) Maternal Hb concentration within 6 weeks postpartum (in g/L) Maternal anaemia at term (Hb < 110 g/L at 37 weeks’ gestation or more) Maternal anaemia at or near term (Hb < 110 g/L at 34 weeks’ gestation or more) Moderate anaemia at postpartum (Hb between 80 and 109 g/L) Maternal IDA at term (as defined by trialists at 37 weeks’ gestation or more) Maternal IDA at or near term (Hb < 110 g/L and at least 1 additional laboratory indicator at 34 weeks’ gestation or more) Maternal ID at term (as defined by trialists, based on any indicator of iron status at 37 weeks’ gestation or more) Maternal ID at or near term (as defined by trialists, based on any indicator of iron status at 34 weeks’s gestation or more) Severe anaemia at any time during second or third trimesters (Hb < 70 g/L) Maternal severe anaemia at or near term (Hb < 70 g/ L at 34 weeks’ gestation or more) Severe anaemia postpartum (Hb < 80 g/L) Side effects (any reported throughout intervention period) Diarrhoea (as defined by trialists) Constipation (as defined by trialists) Vomiting (as defined by trialists) Adjustments: not reported |
GRADE: any supplements containing iron versus same supplements without iron or no treatment or placebo (no iron or placebo): maternal anaemia at term = low, maternal ID at term = low, maternal severe anaemia at any time during second and third trimester = very low, side effects = very low; any supplements containing iron and folic acid versus same supplements without iron nor folic acid (no iron nor folic acid or placebo): maternal anaemia at term = moderate, maternal ID at term = low, maternal severe anaemia at any time during second and third trimester = very low, side effects = moderate, other outcomes = not assessed Cochrane RoB 1 tool. Only 25 trials were at low risk of bias for random sequence generation and 22 for allocation concealment. Blinding was adequate in about half of the trials. Almost all trials were of unclear risk of bias for selective reporting |
|
Peña‐Rosas 2015a Intermittent oral iron supplementation during pregnancy |
July 2015 | 21 trials (5490 women) | To assess the benefits and harms of intermittent supplementation with iron alone or in combination with folic acid or other vitamins and minerals to pregnant women on neonatal and pregnancy outcomes | RCTs Quasi‐RCTs Cluster‐RCTs |
Pregnant women | Argentina (1 trial), Bangladesh (1 trial), China (1 trial), Guatemala (2 trials), India (4 trials), Indonesia (2 trials), Iran (5 trials), Malawi (1 trial), Malaysia (1 trial), Mexico (3 trials), Pakistan (2 trials), South Korea (1 trial), Sri Lanka (1 trial),Thailand (1 trial), and Vietnam (1 trial) Malaria prevalence: all countries with some malaria risk Anaemia prevalence: not reported |
Intervention: oral supplements of iron, or iron + folic acid, or iron + vitamins and minerals, given as a public health strategy on an intermittent basis Comparison: placebo or no supplementation, or the same supplements provided daily |
Maternal Hb concentration at or near term (g/L, at 34 weeks’ gestation or more) Maternal anaemia at term (Hb < 110 g/L at 37 weeks' gestation or more) Maternal anaemia at or near term (Hb < 110 g/L at 34 weeks’ gestation or more) Moderate anaemia at any time during second and third trimester (Hb between 70 and 99 g/L) Maternal IDA at term (Hb < 110 g/L and at least 1 additional laboratory indicator at 37 weeks’ gestation or more) Maternal IDA at term or near term (Hb < 110 g/L and at least 1 additional laboratory indicator at 34 weeks’ gestation or more) Maternal ID at or near term (as defined by trialists, based on any indicator of iron status at 34 weeks’ gestation or more) Severe anaemia at any time during second or third trimesters (Hb < 70 g/L) Severe anaemia at or near term (Hb < 70 g/L at 34 weeks’ gestation or more) Severe anaemia at term (Hb < 70 g/L at 37 weeks’ gestation or more) Severe anaemia at postpartum (Hb < 80 g/L) Side effects (any reported throughout the intervention period) Diarrhoea (as defined by trialists) Constipation (as defined by trialists) Vomiting (as defined by trialists) Adjustments: some trials adjusted for altitude | GRADE: any intermittent iron regimen (with or without other vitamins and minerals) versus daily regimen (with same vitamins and minerals): maternal anaemia at term = very low, maternal severe anaemia at any time during second and third trimester = very low, maternal IDA at term = very low, side effects (any reported throughout the intervention period) = very low, other outcomes = not assessed Cochrane RoB 1 tool. About half of the trials were at low risk of bias for random sequence generation, but 5 trials were at high risk of bias. Allocation concealment was adequate in only 3 trials and at high risk in 9 trials. All trials, except 1, were at high risk of performance bias. Contrary, all trials, except 1, were at low risk of detection bias. |
|
Qassim 2018 Safety and efficacy of intravenous iron polymaltose, iron sucrose and ferric carboxymaltose in pregnancy: a systematic review |
June 2016 | 47 studies (2635 in IS group, 276 in FCM group, 164 in IPM group) | To evaluate the efficacy and safety of IV IPM, IS and FCM in the management of antenatal IDA | RCTs Quasi‐RCTs Observational studies |
Pregnant women with IDA | Any countries: India, USA, Pakistan and several countries Anaemia and malaria prevalence: not reported |
Intervention: IV IPM, IS and FCM Comparison: any other comparator |
Hb (g/L); adjustment: not reported | GRADE: not assessed Study quality assessed by Cochrane RoB 1 tool for RCT studies and RoBANS for non‐randomised studies. One‐quarter of the included trials had high risk of bias in at least one domain, whereas 12 of 26 observational studies had high risk of bias in at least one domain. |
|
Qassim 2019 Intravenous or oral iron for treating iron deficiency anaemia during pregnancy: systematic review and meta‐analysis |
January 2019 | 15 studies (1938 participants) | To compare the effects on perinatal maternal and neonatal outcomes of intravenous and oral iron therapy as first‐line treatment of IDA in pregnant women | RCTs | Pregnant women who initially had low haemoglobin levels (< 110 g/L) or were at high risk of developing IDA | Any country: Singapore, France, Turkey, Australia, India, Thailand and several countries Anaemia and malaria prevalence: not reported |
Intervention: IV iron therapy Comparison: oral iron |
Hb (g/L); adjustment: not reported | GRADE: Hb = low Cochrane RoB 1 tool used to assess risk of bias. All studies were at high risk of bias due to lack of blinding participants or study personnel. |
|
Radhika 2019 Parenteral versus oral iron for treatment of iron deficiency anaemia during pregnancy and post‐partum: a systematic review |
August 2011 to March 2018 | 18 studies (1633 antenatal women) and 8 studies (713 post‐partum women) | To confirm the safety and efficacy of intravenous iron sucrose compared to oral iron for treatment of IDA in antenatal and post‐partum women | RCTs | Antenatal and post‐partum women diagnosed with IDA | Developing countries Anaemia and malaria prevalence: not reported |
Intervention: parenteral iron (IV IS) Comparison: oral iron |
Hb (g/L); adjustment: not reported | GRADE: Hb = high in pregnant women, moderate in post‐partum women The quality of studies was assessed by Cochrane RoB 1 tool; subgroups and sensitivity analyses performed. Publication bias assessed by testing the intercept of Egger regression. All studies had loss to follow‐up and dropout rates less than 20%. |
|
Reveiz 2011 Treatments for iron‐deficiency anaemia in pregnancy |
June 2011 | 23 trials (3198 women) | To assess the effects of different treatments for anaemia in pregnancy attributed to ID (defined as haemoglobin < 11 g/dL or other equivalent parameters) on maternal and neonatal morbidity and mortality | RCTs | Pregnant women with a diagnosis of anaemia (Hb levels < 11 g/dL, or other tests for anaemia as described by trialists) attributed to ID | Not reported Anaemia and malaria prevalence: not reported |
Interventions:
Comparison: placebo or no intervention or other type of intervention |
Hb Anaemia Side effects Nausea and vomiting Constipation Diarrhoea Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. Half of the trials were at low risk of bias for random sequence generation and about half of the trials were at unclear risk of bias. Allocation concealment was rated at low risk of bias in about one‐third of the trials and two‐thirds were unclear. Blinding was rated at high risk of bias in more than half of the trials and only one‐third were deemed to be at low risk for performance and detection bias |
|
Rumbold 2015 Vitamin C supplementation in pregnancy |
31 March 2015 | 29 trials (24,300 women) | To identify all published and unpublished randomised and quasi‐randomised controlled trials investigating vitamin C supplementation in pregnancy and to investigate the benefits and hazards of vitamin C supplementation in pregnancy | RCTs Quasi‐RCTs |
All pregnant women | High‐income countries: Australia, Canada, the Netherlands, UK and USA Low‐ and middle‐income countries: Brazil, India, Iran, Latvia, Mexico, Peru, South Africa, Turkey, Uganda, Vietnam, Venezuela Anaemia and malaria prevalence: not reported |
Intervention: vitamin C supplementation, alone or in combination with other separate supplements Comparison: placebo, no placebo or other supplements |
Maternal Hb (no data reported for this outcome) Maternal anaemia (no data reported for this outcome) Side effects Adjustments: not reported |
GRADE: not assessed for relevant outcomes Cochrane RoB 1 tool. About two‐thirds of trials were at low risk of bias for random sequence generation, allocation concealment and blinding of participants and personnel. |
|
Shi 2015 Intravenous iron sucrose versus oral iron in the treatment of pregnancy with iron deficiency anaemia: a systematic review |
January 2014 | 6 trials (576 women) | To assess the efficacy and safety of IV IS in pregnancy with IDA | RCTs | Pregnant women diagnosed with IDA | India (4 trials), not reported (2 trials) Anaemia and malaria prevalence: not reported |
Intervention: IV IS Comparison: oral iron supplement |
Mean Hb concentration (g/dL) Adverse events Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. 5 trials were deemed adequate for random sequence generation and 3 for allocation concealment. All trials were rated at high risk of performance bias and 1 trial was rated at high risk of detection bias. The remaining trials were rated at unclear risk of bias |
|
Thorne‐Lyman 2012 Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta‐analysis |
November 2010 and June 2011 | 17 trials | To consolidate knowledge about the effects of supplementation on multiple outcomes related to maternal, perinatal and infant health to inform policy in low‐income countries and to identify research priorities | RCTs Cluster‐RCTs |
Pregnant women (anaemic or non‐anaemic women, 3 trials included HIV‐positive women) | Low‐middle income countries: India, South Africa, Ghana, Indonesia, Tanzania, Malawi, China, Nepal, and Bangladesh Anaemia and malaria prevalence: 3 trials did malaria prophylaxis |
Intervention: supplementation with vitamin A or carotenoids (or both) Comparison: placebo and multivitamins alone |
Mean Hb Maternal anaemia (< 11 g/dL) Severe maternal anaemia (< 8.0 or 8.5 g/dL) Adjustments: not reported |
GRADE: Hb = moderate, maternal anaemia = high, severe maternal anaemia = low Trial bias was assessed by a slightly modified version of the CHERG’s GRADE tool. Methodological quality was deemed high in 10 trials, moderate in three trials and low in four trials |
| Fortification | |||||||||
|
Suchdev 2015 Multiple micronutrient powders for home (point‐of‐use) fortification of foods in pregnant women |
January 2015 | 2 trials (1172 pregnant women) | To assess the effects of prenatal home (point‐of‐use) fortification of foods with MNP on maternal and newborn health | Cluster‐RCTs | Pregnant women of any gestational age and parity | Rural setting in Bangladesh and Mexico Anaemia and malaria prevalence: malaria not endemic |
Intervention: MNP for point‐of‐use fortification of semi‐solid foods containing at least 3 micronutrients, with 1 of them being iron, provided to women during pregnancy Comparison: no intervention or placebo, iron and folic acid supplements, iron‐only supplements, folic acid‐only supplements or same MMNs in supplements |
Hb concentration (g/L) at 32 weeks’ gestation Any anaemia at 32 weeks’ gestation Maternal Hb (g/L) at term or near term Maternal anaemia at term or near term (Hb < 110 g/L at 34 weeks’ gestation or more) Adjustments: not reported |
GRADE: maternal anaemia at term or near term = very low, other relevant outcomes = not assessed Cochrane RoB 1. Both trials were at unclear of bias for random sequence generation. Allocation concealment was low risk of bias. The risk of performance and detection bias was high in both trials. |
CHERG: Child Health Epidemiology Reference Group; FCM: ferric carboxymaltose; Hb: haemoglobin; HIV: Human Immunodeficiency Virus; ID: iron deficiency; IDA: iron deficiency anaemia; IFA: iron folic acid; IPM: iron polymaltose; IS: iron sucrose; IV: intravenous; LNS: lipid‐based nutrient supplements; MMNs: multiple micronutrients; MNP: micronutrient powder; NAID: non‐anaemic iron deficiency; RCTs: randomised controlled trials; RoBANS: Risk of Bias Assessment tool for Non‐randomized Studies; UNIMMAP: United Nations International Multiple Micronutrient Preparation.
8. Characteristics of included systematic reviews: mixed populations.
| Review | Date of search | Number of included trials (number of participants included) | Review question/objective | Trial designs included | Participants | Setting, anaemia and malaria prevalence | Intervention and comparison | Relevant outcomes (definition used in the review, adjusted for smoking and altitude) |
GRADE assessment of relevant outcomes Method used to assess risk of bias and summary |
| Supplementation | |||||||||
|
Arabi 2020 The effect of vitamin D supplementation on hemoglobin concentration: a systematic review and meta‐analysis |
January 2019 | 14 trials (10 to 276 participants) | To investigate the effect of vitamin D supplements on haemoglobin concentration in participants aged 17.5 to 68 years old | RCTs | Aged 17.5 to 68 years old (including RCTs with healthy adults, anaemic patients, chronic kidney disease patients, heart failure patients, hypertensive patients, critically ill patients and athletes) | USA (5 trials), Germany (3 trials), Poland (2 trials), Spain, India, Norway, Columbia Anaemia and malaria prevalence: not reported |
Intervention: oral vitamin D supplements; such as cholecalciferol, ergocalciferol and calcitriol Comparison: placebo, Fortified dry milk with 15 mg iron, ampoules normal saline + ampoules iron |
Haemoglobin levels, iron markers (levels of ferritin, serum iron, and transferrin saturation) | GRADE: not assessed Cochrane RoB 1 tool used. Low risk of bias in 9 trials, moderate in 2 trials |
|
Basutkar 2019 Vitamin D supplementation in patients with iron deficiency anaemia: a systematic review and a meta‐analysis |
January 2018 | 4 trials (429 participants) | To estimate the efficacy of vitamin D supplementation on patients with IDA | RCTs | Patients with IDA (20 to 45 years) | Norway, USA, India (2 trials), community centres and outpatient settings (3 trials); (patients with IDA) | Intervention: vitamin D supplementation Comparison: placebo |
Change in serum ferritin and haemoglobin levels | GRADE: haemoglobin = high, serum ferritin = high Cochrane RoB 1 tool |
|
Casgrain 2012 Effect of iron intake on iron status: a systematic review and meta‐analysis of randomized controlled trials |
February 2012 | 41 trials | To assess the effects of baseline iron status, sex, menopausal status, duration of intervention, iron form, and daily dose on the change in iron status in response to iron supplementation | RCTs | Healthy adult population (mean age ≥ 18 years, with any baseline iron status; excluding highly trained athletes, regular blood donors, and individuals receiving erythropoietin, with chronic disease, or with GI infections) | Switzerland, Kuwait, USA (15 trials), Spain, Mexico (2 trials), UK (5 trials), Thailand (2 trials), Brazil, Sri Lanka, Sweden (2 trials), Philippines (2 trials), Finland, New Zealand, Australia, China, South Africa, Vietnam (2 trials), Japan Anaemia and malaria prevalence: not reported |
Intervention: iron supplement, fortified food, or rich natural dietary sources Comparison: placebo or no dietary intervention |
Hb (g/dL) Adjustments: not reported |
GRADE: not assessed Scale designed by the EURRECA Network of Excellence (on the basis of Cochrane methods). Most trials were rated at high risk of bias (24 trials) and the remainder at unclear risk of bias because of insufficient information for an accurate assessment (17 trials). |
|
Gera 2007a Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials |
February or April 2003 | 55 trials (some trials contributed to > 1 analytic component) | To evaluate the effect of iron supplementation on Hb in children through a systematic review of trials | RCTs Cluster‐RCTs |
Children (aged from birth to 19 years, 31 trials in infants and preschool children under 6 years, 25 trials among older children) None of the age groups dominated (i.e. > 60%) |
Asia (22 trials), Africa (11 trials), Europe (10 trials), South America (8 trials), North America (4 trials) Malaria: 11 analytic components in malaria‐endemic areas versus 80 not Anaemia prevalence: not reported |
Intervention: iron supplementation through the oral or the parenteral route or as formula, milk, or cereals fortified with iron Comparison: placebo (trials in which other micronutrients and drugs were simultaneously administered were included if the only difference between the experimental and control groups was iron supplementation) |
Hb (g/dL) Adjustments: not reported |
GRADE: not assessed Methodological quality assessment (A, B, C or D) using the following domains: randomisations, allocation concealment, follow‐up and blinding. Allocation concealment was adequate in 12 analytic components versus 73 others; attrition was < 10% in 57 versus > 10% in 34; and 23 were double‐blinded versus 25 others |
|
Gera 2009 Effect of combining multiple micronutrients with iron supplementation on Hb response in children: systematic review of randomized controlled trials |
January or February 2006 | 30 trials (50 analytic components as some trials contributed to > 1 comparison) | To study the effect of combining multiple (2 or more) micronutrients with Fe supplementation on Hb response, when compared with placebo and with Fe supplementation, in children | RCTs | Children (aged from birth to 18 years) None of the age groups dominated (i.e. > 60%) |
Majority conducted in developing countries, Africa (14 trials), Asia (11 trials), South America (4 trials), North America (1 trial) Malaria: 11 analytic components in malaria‐endemic areas versus 22 not Anaemia prevalence: not reported |
Intervention: iron supplementation in combination with 2 or more other micronutrients Comparison: placebo (trials in which other drugs were also simultaneously administered were included if the only difference between the trial and the control groups was supplementation with 2 or more micronutrients, for the second objective, and Fe plus 2 or more micronutrients for the first objective) |
Hb (g/dL) Adjustments: not reported |
GRADE: not assessed Methodological quality assessment (A, B, C or D) using the following domains: randomisations, allocation concealment, follow‐up and blinding. Allocation concealment was adequate in 12 analytic components versus 21 others; attrition was < 10% in 18 versus > 10% in 14; and 23 were double blinded versus 10 others |
|
Silva Neto 2019 Effects of iron supplementation versus dietary iron on the nutritional iron status: systematic review with meta‐analysis of randomized controlled trials |
Not reported | 12 trials (6 trials: 730 children, 5 trials: 164 adolescents or adults, 1 trial: 85 pregnant women) | To compare the effects of a dietary intervention versus iron supplementation on Hb and other serum biochemical parameters related to the iron nutritional status of humans, in a standard treatment period (defined as 12 weeks or more follow‐up) | RCTs | Any (no restrictions based on the participants’ sex, age or race) | Low and high‐income countries: USA (3 trials), Vietnam (2 trials), Australia (1 trials), Chile (1 trial), India (1 trial), Kenya (1 trial), Mexico (1 trial), New Zealand (1 trial), Sweden (1 trial) Anaemia and malaria prevalence: not reported |
Intervention: fortified foods or dietary plan; dietary intervention (i.e. an intervention that provided a fortified food product or a dietary plan offering at least half of the daily recommended dietary allowances) Comparison: iron supplementation |
Hb (g/L) Final prevalence of anaemia Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. 7 of the included trials were assessed as being at high risk of bias and only 5 at low risk of bias |
|
Smelt 2018 The effect of vitamin B12 and folic acid supplementation on routine haematological parameters in older people: an individual participant data meta‐analysis |
April 2016 | 7 trials (from 32 reports) (1306 participants) | To evaluate the effect of vitamin B12 and folic acid supplementation on haematological parameters in the elderly | RCTs | Community‐dwelling elderly (aged 60 + years); specific populations (e.g. trials in patients with diabetes or with renal failure) were excluded | England (2 trials), Switzerland (1 trial), Denmark (1 trial), the Netherlands (1 trial), Australia (1 trial), Greece (1 trial) Anaemia and malaria prevalence: not reported |
Intervention: vitamin B12 or folic acid (all dosages and all forms of administration) Comparison: placebo |
Hb (g/dL) Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. All trials were rated at low risk of bias for random sequence generation, allocation concealment and blinding of participants and personnel. |
|
Tay 2015 Systematic review and meta‐analysis: what is the evidence for oral iron supplementation in treating anaemia in elderly people? |
January 2014 | 3 trials (440 participants) | To determine if oral iron therapy is effective in elderly people with IDA | RCTs | Anaemic elderly people aged 65 years and over | Anaemia and malaria prevalence: participants were elderly patients with anaemia | Intervention: oral iron Comparison: no oral supplementation or placebo |
Hb (g/L) Adverse effects Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. Overall, risk of bias was rated as high in 1 trial, moderate in 1 trial and low in 1 trial. |
|
Tolkien 2015 Ferrous sulfate supplementation causes significant gastrointestinal side‐effects in adults: a systematic review and meta‐analysis |
March 2014 | 43 trials (6831 participants) | To quantify the odds of GI side effects in adults related to current gold standard oral iron therapy, namely ferrous sulphate | RCTs Cross‐over RCTs |
Adults (including pregnant women in 7 trials) | No setting reported Anaemia and malaria prevalence: not reported, but 1 trial included anaemic participants |
Intervention: supplementation with ferrous sulphate Comparison: placebo or IV iron |
Hb GI side effects Individual side effects Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. Random sequence generation was rated at low risk of bias in 25 trials and high risk of bias in 2. Allocation concealment was rated at low risk of bias in 19 trials and high risk of bias in 2 trials. Blinding was rated at low risk of bias in 17 trials and high risk of bias in 24. |
| Fortification | |||||||||
|
Das 2019b Food fortification with multiple micronutrients: impact on health outcomes in general population |
August 2018 | 43 studies (48 reports) (19,585 participants, including 17,878 children) | To assess the impact of food fortification with MMNs on health outcomes in the general population, including men, women and children | RCTs Cluster‐RCTs Quasi‐randomised trials CBA studies ITS studies |
Men, women, and children. Specific populations such as older people, pregnant women, women of reproductive age, and children at school through institutions were included. Critically‐ill people, anaemic people or people diagnosed with any specific diseases were excluded. | Healthy people in high‐income and low‐ and middle‐income countries Anaemia and malaria prevalence: not reported |
Intervention: MMN fortification (3 or more micronutrients) by any food vehicle Comparison: a single micronutrient or no fortification |
Serum Hb level (g/dL) Anaemia (Hb < 11 g/dL) IDA (Hb < 11 g/dL with serum ferritin < 15 μg/L Potential adverse outcomes Adjustments: not reported |
GRADE: Hb = low, anaemia = low, IDA = low, ID = low Cochrane RoB 1 tool for RCTs 5 trials: overall low risk of bias, 34 trials: overall high risk of bias. Cochrane EPOC for non‐RCTs and CBA studies, 4 trials: high risk |
|
Field 2020 Wheat flour fortification with iron for reducing anaemia and improving iron status in populations |
September 2019 | 9 trials (3166 participants) | To determine the benefits and harms of wheat flour fortification with iron alone or with other vitamins and minerals on anaemia, iron status and health‐related outcomes in populations over two years of age | RCTs Cluster‐RCTs Quasi‐RCTs |
General population from any country aged two years and above | India (2 trials), Brazil, Kuwait, Phillipines, Pakistan, Sri Lanka, Bangladesh, South Africa Anaemia prevalence: not reported Malaria prevalence: 2 trials reported to be conducted in non‐malaria‐endemic areas; the remaining studies did not report on malaria |
Intervention and comparison:
|
Hb concentration (g/L) Anaemia (defined as haemoglobin below WHO cut‐off for age and adjusted for altitude as appropriate) ID (as defined by trialists, based on a biomarker of iron status) Adverse side effects For children aged 2 to 11 years old: Diarrhoea (3 liquid stools in per day) Respiratory infections (as measured by trialists) |
GRADE: wheat flour fortified with iron alone versus unfortified wheat flour: Hb = very low, anaemia = low, ID = moderate; wheat flour fortified with iron in combination with other micronutrients versus unfortified wheat flour: Hb = low, anaemia = low, ID = moderate; wheat flour fortified with iron in combination with other micronutrients versus fortified wheat flour with same micronutrients (but not iron): Hb = low, anaemia = very low, ID = very low Cochrane RoB 1 tool for RCTs. Most of the included trials were assessed as low or unclear risk of bias for key elements of selection, performance or reporting bias. |
|
Finkelstein 2019 Iron biofortification interventions to improve iron status and functional outcomes |
August 2018 | 3 trials (633 adolescents and adults) | To inform public health programmes and to incorporate biofortification as a strategy to target iron deficiency in at‐risk populations | RCTs | General population (including pregnant or lactating women) | Phillipines, India, Rwanda Anaemia and malaria prevalence: not reported |
Intervention: providing iron‐biofortified staple crops that were not genetically modified Comparison: conventional crops |
Anaemia (Hb < 120 g/L) ID (serum ferritin < 15.0 μg/L) |
GRADE: not assessed Cochrane RoB 1 tool for RCTs. Most of the included trials were assessed as low or unclear risk of bias for key elements of selection, performance or reporting bias. |
|
Garcia‐Casal 2018 Fortification of maize flour with iron for controlling anaemia and iron deficiency in populations |
December 2017 and January 2018 | 5 trials (2610 participants in RCTs, 849 in uncontrolled before‐after trials) | To assess the effects of iron fortification of maize flour, corn meal and fortified maize flour products on anaemia and iron status in the general population | RCTs Cluster‐RCTs Uncontrolled before‐after trials (data only extracted for RCTs) |
General population older than 2 years of age (including pregnant women), from any country | Kenya, Mexico (2 trials), Brazil, Zambia Anaemia prevalence: < 20% in 3 trials, > 40% in 1 trial and not reported in 1 trial Malaria prevalence: 2 trials in malaria settings, 3 trials did not report on malaria endemicity |
Intervention and comparison:
|
Hb (g/L) Anaemia (defined as Hb below WHO cut‐off, adjusted for altitude and smoking, as appropriate) IDA (as defined by trialists) ID (as defined by trialists, based on a biomarker of iron status) Any adverse effects (including constipation, nausea, vomiting, heartburn or diarrhoea, as measured by trialists) Adjustments: adjusted for altitude as appropriate |
GRADE: provision of maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products: Hb = very low, anaemia = very low, ID = very low Cochrane EPOC risk of bias tool. Overall risk of bias was low in 1 trial, and high in the remaining trials due to high risk or unclear risk of bias for random sequence generation, allocation concealment and blinding. |
|
Gera 2012 Effect of iron‐fortified foods on hematologic and biological outcomes: systematic review of randomized controlled trials |
March 2012 | 60 trials (11,750 iron‐fortified participants and 9077 control participants) (85 analytic components as some trials contributed to > 1 comparison) |
The objectives were to evaluate: i) the effect of iron fortification on Hb and serum ferritin and the prevalence of ID and anaemia; ii) the possible predictors of a positive Hb response; iii) the effect of iron fortification on zinc and iron status; and iv) the effect of iron‐fortified foods on mental and motor development, anthropometric measures, and infections. | RCTs Quasi‐RCTs Cluster‐RCTs |
Apparently healthy (non‐diseased) individuals, families, or communities irrespective of age and sex considerations | Most of the trials were from low‐ and middle‐income countries: Asia (41 trials), Africa (13 trials), South America (14 trials), Europe (9 trials), Australia (1 trial), North America (7 trials) Malaria: 6 analytic components in malaria‐endemic areas versus 71 not Anaemia prevalence: not reported |
Intervention: additional dietary iron through the route of food fortification or bifortification Comparison: similar food without iron fortification |
Hb (g/dL) Anemia (%, as defined in individual trials, accounted for age and sex differences for defining Hb cut‐off concentrations) ID (%, as defined in individual trials) Adverse effects (any, as defined in individual trials) Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. Allocation concealment was adequate in 25 analytic components versus 52 others; sequence generation was adequate in 14 versus 63 others; blinding was adequate in 52 versus 25 others; selective outcomes was judged yes in 62 versus 15 others |
|
Hess 2016 Micronutrient fortified condiments and noodles to reduce anemia in children and adults—a literature review and meta‐analysis |
December 2013 | 14 trials (8674 participants) | To investigate existing evidence on the impact of micronutrient‐fortified condiments and noodles on haemoglobin, anaemia, and functional outcomes in children and adults (aged 5 to 50 years) | RCTs Cluster‐RCTs |
Children and adults from 5 to 50 years | 11 trials in Asia: India (4 trials), Vietnam (3 trials), China (2 trials), Cambodia (1 trial), Thailand (1 trial) 3 trials in Africa (Morocco, Ghana, South Africa) Malaria prevalence: not reported Anaemia prevalence: median of 46% at baseline |
Intervention: micronutrient‐fortified condiments or noodles product. Fortified condiments included: condiments, salt, seasonings, soy sauce, fish sauce, bouillon, sprinkles and powder. Micronutrients for fortification included: iron, vitamins, zinc, iodine, folate, calcium, phosphorus, magnesium and selenium. Comparison: non‐fortified condiments and noodles |
Hb (g/dL) Anaemia rate (between 11 g/dL and 13 g/dL, depending on age) Adjustments: not reported |
GRADE: not assessed Risk of bias assessment conducted according to 'CRD’s Guidance for Undertaking Reviews in Health Care': adequate sequence generation, allocation concealment and blinding; incomplete outcome data addressed; no selective reporting. The risk of bias for the investigated outcomes of “haemoglobin change” and “anaemia rates” was unclear in most of the trials. |
|
Huo 2015 Effect of NaFeEDTA‐fortified soy sauce on anemia prevalence in China: a systematic review and meta‐analysis of randomized controlled trials |
June 2014 | 16 trials (16,819 participants) | To assess the effect of NaFeEDTA‐fortified soy sauce on anaemia prevalence in the Chinese population | RCTs Cluster‐RCTs |
Any Chinese population in which anaemia is a public health problem | Chinese population: 5 trials in poor rural villages, 8 trials in schools, and 3 trials in hospitals Anaemia: all trials included anaemia‐risk populations Malaria prevalence: not reported |
Intervention: NaFeEDTA‐fortified soy sauce Comparison: non‐fortified soy sauce groups |
Anemia rate and Hb concentrations (according to WHO‐defined cut‐off values for different populations) Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. Random sequence generation was adequate in 10 trials and allocation concealment in 13 trials. Performance bias was low in 12 trials, and detection and reporting bias were low in all trials. |
|
Peña‐Rosas 2019 Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition |
December 2018 | 17 studies (10,483 participants) | To determine the benefits and harms of rice fortification with vitamins and minerals (iron, vitamin A, zinc or folic acid) on micronutrient status and health‐related outcomes in the general population | RCTs Cluster‐RCTs Quasi‐RCTs Non‐randomised controlled trials, observational studies (cohort studies, CBA studies, ITS studies) |
General population older than two years of age (including pregnant women) | Any countries; anaemia prevalence: 5% to 62% in children, 21% in women, and 34% in teenagers; malaria prevalence: not reported but one study conducted in a malaria‐endemic area | Intervention: rice fortified with at least one micronutrient or a combination of several micronutrients (iron, folic acid, zinc, vitamin A or other vitamins and minerals) Comparison: unfortified rice or no intervention |
Hb (g/L), anaemia (WHO cut‐off or as defined by authors), ID, diarrhoea, and any adverse effects; adjustment: yes | GRADE: Hb = low, anaemia = low, ID = low, diarrhoea = very low, any adverse effects (hookworm infection risk) = low The EPOC risk of bias tool used for risk of bias assessment. Funnel plots used to assess the reporting bias. All CBA studies had a high risk or unclear risk of bias in most domains. |
|
Ramírez‐Luzuriaga 2018 Impact of double‐fortified salt with iron and iodine on hemoglobin, anemia, and iron deficiency anemia: a systematic review and meta‐analysis |
Not reported | 14 trials (45,995 participants) | To assess the impact of DFS on biomarkers of iron status, and the risk of anaemia and IDA | RCTs Quasi‐RCTs Cluster‐RCTs |
Any participants | Low‐ and middle‐income countries: India (10 trials), Morocco (2 trials), Côte D’Ivoire (1 trial), Ghana (1 trial) Anaemia and malaria prevalence: not reported |
Intervention: DFS Comparison: control salt (iodised salt) |
Hb (g/L) Anaemia IDA Adjustments: not reported |
GRADE: not assessed EPHPP quality assessment tool; 3 trials were rated at overall strong quality (strong for selection bias, trial design, confounders, blinding, data collection methods, withdrawals and dropouts, intervention integrity and robustness of the analysis), 3 moderate and 8 weak |
|
Sadighi 2019 Systematic review and meta‐analysis of the effect of iron‐fortified flour on iron status of populations worldwide |
February/ March 2019 | 101 studies (52 controlled trials, 49 before‐after design) | To assess the effectiveness of iron‐fortified flour on iron status | Controlled trials and before–after studies (data only extracted for RCTs) |
Males or females of all ages | Cameroon, Chile, China, Costa Rica, Côte d’Ivoire, Denmark, India, Iran, Jordan, Kazakhstan, Kenya, Kuwait, Mongolia, Morocco, Norway, South Africa, Sri Lanka, Tajikistan, Thailand, UK, USA, Uzbekistan, Venezuela, Vietnam, and Zambia | Intervention: dietary fortification of flour (e.g. wheat, maize, or rice), either in a raw form or in a cooking process, with iron or with iron and other micronutrients Comparison: any |
Hb level (g/L) Anaemia IDA ID Adjustments: no reported |
GRADE: not assessed Cochrane EPOC statement. Of the 52 trials included in the meta‐analysis, 37 were of overall low risk of bias and the remaining 15 of high risk. |
|
Tablante 2019 Fortification of wheat and maize flour with folic acid for population health outcomes |
June 2018 | 5 trials (1182 participants) (3 non‐RCTs, 2 ITS) | To evaluate the health benefits and safety of folic acid fortification of wheat and maize flour (i.e. alone or in combination with other micronutrients) on folate status and health outcomes in the overall population, compared to wheat or maize flour without folic acid (or no intervention). | RCTs Non‐RCTs ITS |
Participants from the general population, who were two years of age and older (including pregnant and lactating women), and from any country | General population; Anaemia and malaria prevalence: not reported |
Intervention: wheat flour or wheat flour products fortified with folic acid (plus other vitamins and minerals) Comparison: unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals) |
Hb level (g/L) Anaemia |
GRADE: Hb = low, anaemia = low Cochrane RoB 1 tool |
|
Yadav 2019 Meta‐analysis of efficacy of iron and iodine fortified salt in improving iron nutrition status |
August 2016 | 10 studies (3219 participants) | To study the efficacy of DFS as compared to IS in improving iron nutrition status | RCTs | Participants of all age and gender | India (5 trials), Marocco (2 trials), Ghana (2 trials), Cote d’Ivoire (anaemia and malaria prevalence: not reported) | Intervention: DFS (iron and iodine) Comparison: IS |
Hb Prevalence of anaemia Prevalence of IDA Prevalence of ID Adjustments: not reported |
GRADE: not assessed Cochrane RoB 1 tool. Allocation concealment was adequate in 5 trials, blinding of participants and study personnel in 8 trials, and 7 trials addressed incomplete outcome data. Random sequence generation was mentioned in only 1 trial. Details about blinding of outcome assessment were not provided in any of the trials. |
| Improving dietary diversity and quality | |||||||||
|
Geerligs 2003 Food prepared in iron cooking pots as an intervention for reducing iron deficiency anaemia in developing countries: a systematic review |
May 2002 | 3 trials (784 participants) | To complete a systematic review of the effect of preparing food cooked in iron pots on haemoglobin concentrations and to assess compliance with pot use | RCTs | People in low‐income countries (minimum age was set at 4 months) | Low‐income countries: Ethiopia, Brazil, Malawi Malaria: 1 trial conducted in malaria‐endemic area Anaemia prevalence: not reported |
Intervention: food prepared in cast iron pots Comparison: food prepared in non‐cast iron pots |
Change in Hb concentration Adjustments: not reported |
GRADE: not assessed Delphi list used as a means for quality assessment. Random sequence generation was adequate in all trials, allocation concealment only described in 1 trial. 2 trials reported blinding of assessors, but none of the trials blinded care provider or participants. |
CBA: Controlled before‐after; CRD: Centre for Reviews and Dissemination; DFS: double‐fortified salt; EPOC: Effective Practice and Organisation of Care; EPHPP: Effective Public Health Practice Project; EURRECA: EURopean micronutrient RECommendations Aligned; Fe: ferrum (iron); GI: gastrointestinal; Hb: haemoglobin; ID: iron deficiency; IDA: iron deficiency anaemia; IDNA: iron‐deficient non‐anaemic; IS: iodine fortified salt; ITS: interrupted time series; IV: intravenous; MMN: multiple micronutrients; NaFeEDTA: sodium iron ethylenediaminetetraacetate; RCTs: randomised controlled trials; WHO: World Health Organization.
Among the 75 included systematic reviews:
54 reviews reported Hb concentration;
45 reviews reported anaemia;
18 reviews reported IDA;
4 reviews reported severe anaemia;
23 reviews reported ID; and
23 reviews reported adverse effects.
Study characteristics
The included reviews were conducted between 2003 (Geerligs 2003) and 2020 (Arabi 2020; Field 2020; Lassi 2020; Salam 2020; Suchdev 2020). The number of trials in the included reviews ranged from two (Abdullah 2013; Abe 2016; Suchdev 2015) to 90 (Petry 2016b). Of the 55 reviews, 54 included randomised controlled trials (RCTs) and Suchdev 2015 included only cluster‐RCTs. Thirty‐two included RCTs and cluster‐RCTs, 18 reviews quasi‐RCTs, and two reviews included cross‐over RCTs (Mayo‐Wilson 2014a; Tolkien 2015). Das 2019a included RCTs and quasi‐RCTs. Five reviews (Das 2019b; Field 2020; Peña‐Rosas 2019; Sadighi 2019; Tablante 2019) included randomised and non‐randomised trials, including observational studies (see Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8).
Populations
The number of participants in the included reviews ranged from 52 in Abe 2016 to over 310,000 in McCauley 2015.
Infants (aged 6 to 23 months)
Thirteen reviews included infants aged 6 to 23 months at the start of the interventions (Abdullah 2013; Das 2019a; Dekker 2010; Dewey 2009; Eichler 2012; Kristjansson 2015; Matsuyama 2017; Pasricha 2013; Petry 2016b; Pratt 2015; Salam 2013; Shapiro 2019; Suchdev 2020). Abdullah 2013, De‐Regil 2017 and Petry 2016b included apparently healthy infants. Dekker 2010, Dewey 2009, Eichler 2012, Kristjansson 2015, Matsuyama 2017 and Pasricha 2013 assessed mixed populations of anaemic and non‐anaemic infants with or without known micronutrient deficiencies. Das 2019a included non‐hospitalised infants and young children aged 6 months to 23 months. In Pratt 2015 and Salam 2013, infant's anaemia status and known micronutrient deficiencies at baseline were not reported.
Preschool and school‐aged children (aged 2 to 10 years)
Eight reviews included children aged 2 to 10 years of age at the start of the interventions (Aaron 2015; Das 2013a; De‐Regil 2011; De‐Regil 2017; Eichler 2019; Low 2013; Mayo‐Wilson 2014a; Thompson 2013). Das 2013a, De‐Regil 2011, De‐Regil 2017, Low 2013, Mayo‐Wilson 2014a and Thompson 2013 included a mixed population of anaemic and non‐anaemic children with or without known micronutrient deficiencies. In Aaron 2015, children's anaemia status and known micronutrient deficiencies at baseline were not reported.
Adolescent children (aged 11 to 18 years)
Four reviews included adolescent children aged 11 to 18 years (Fernández‐Gaxiola 2019; Neuberger 2016; Salam 2016; Salam 2020). Fernández‐Gaxiola 2019 included anaemic and non‐anaemic menstruating women and some included trials reported on ID at baseline. Neuberger 2016 included a mixed population of anaemic and non‐anaemic children. In Salam 2016, two trials included anaemic girls while the remaining 29 trials did not report the children's anaemia status and known micronutrient deficiencies at baseline. Salam 2020 did not describe the children's anaemia status.
Non‐pregnant women of reproductive age (aged 19 to 49 years)
Five reviews included non‐pregnant women between 19 and 49 years of age (Abe 2016; Houston 2018; Lassi 2020; Low 2016; Sultan 2019). In Abe 2016, the population consisted of non‐pregnant mothers of unknown anaemia or micronutrient deficiency status at baseline who either exclusively breastfed or practised mixed feeding. Houston 2018 included iron‐deficient but non‐anaemic adult women. Lassi 2020 included women of reproductive age with unknown anaemia or micronutrient deficiency status at baseline. Low 2016 included a mixed population of anaemic and non‐anaemic and iron‐deficient menstruating women between 13 and 45 years, but only three of the 67 trials included adolescent girls. Sultan 2019 targeted women with a postdelivery and Hb level less than 12g/dL.
Pregnant women of reproductive age (aged 15 to 49 years)
Twenty‐three reviews included pregnant women (Abu Hashim 2017; Bhutta 2012; Buppasiri 2015; Daru 2016; Das 2018; De‐Regil 2015; Govindappagari 2019; Haider 2011; Haider 2013; Imdad 2012; Keats 2019; Lassi 2013; McCauley 2015; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Qassim 2018; Qassim 2019; Radhika 2019; Reveiz 2011; Rumbold 2015; Shi 2015; Suchdev 2015; Thorne‐Lyman 2012). The majority of reviews included pregnant women of any gestational age; only two reviews did not report the gestational age of women (Imdad 2012; Shi 2015). Ten reviews included a mixed population of anaemic and non‐anaemic pregnant women (Abu Hashim 2017; Daru 2016; Das 2018; Keats 2019; McCauley 2015; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Reveiz 2011; Shi 2015; Thorne‐Lyman 2012). Four reviews included a small number of women with known ID (Daru 2016; Haider 2013; Keats 2019; Reveiz 2011). Some women with vitamin A deficiency were included in two reviews (Keats 2019; McCauley 2015) and vitamin C deficiency in one review (Rumbold 2015). Anaemic status at baseline was not reported in 10 reviews (Bhutta 2012; Buppasiri 2015; Das 2018; De‐Regil 2015; Haider 2011; Haider 2013; Imdad 2012; Lassi 2013; Rumbold 2015; Suchdev 2015), and known micronutrient deficiencies were not reported in 17 reviews (Abu Hashim 2017; Bhutta 2012; Buppasiri 2015; Das 2018; De‐Regil 2015; Govindappagari 2019; Haider 2011; Haider 2013; Lassi 2013; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Qassim 2018; Qassim 2019; Radhika 2019; Shi 2015; Suchdev 2015; Thorne‐Lyman 2012).
Older adult women (aged 50 to 65 years and above)
None of the included reviews included older adult women only.
Adult men (aged 19 to 65 years and above)
None of the included reviews included adult men only.
Mixed population (all ages)
Twenty‐two reviews included a mixed population where it was not possible to include the reviews in any of the specific age groups above (Arabi 2020; Basutkar 2019; Casgrain 2012; Das 2019b; Field 2020; Finkelstein 2019; Garcia‐Casal 2018; Geerligs 2003; Gera 2007a; Gera 2009; Gera 2012; Hess 2016; Huo 2015; Peña‐Rosas 2019; Ramírez‐Luzuriaga 2018; Sadighi 2019; Silva Neto 2019; Smelt 2018; Tablante 2019; Tay 2015; Tolkien 2015; Yadav 2019). Field 2020 included a general population aged two years and above. Gera 2007a and Gera 2009 included children of any age between birth and 19 years. Garcia‐Casal 2018, Silva Neto 2019 and Hess 2016 included children and adults. Casgrain 2012 and Tolkien 2015 included adults, while Smelt 2018 and Tay 2015 focused on older adults. Basutkar 2019 included patients with IDA aged 20 to 45 years. The remaining 11 reviews included any population (Arabi 2020; Das 2019b; Finkelstein 2019; Geerligs 2003; Gera 2012; Huo 2015; Peña‐Rosas 2019; Ramírez‐Luzuriaga 2018; Sadighi 2019; Tablante 2019; Yadav 2019). All reviews reported to have included a mixed population of anaemic and non‐anaemic participants, except Ramírez‐Luzuriaga 2018 where anaemia status at baseline was not reported. ID at baseline was described in seven reviews (Basutkar 2019; Casgrain 2012; Geerligs 2003; Gera 2007a; Gera 2012; Huo 2015; Silva Neto 2019). Some participants were vitamin B12 deficient and folate deficient in Smelt 2018. In Gera 2009, known micronutrient deficiency was reported, but not further described. In Garcia‐Casal 2018, all included trials were conducted in areas with high prevalence of micronutrient deficiencies, especially iron. The remaining four reviews did not report on known micronutrient deficiencies (Hess 2016; Ramírez‐Luzuriaga 2018; Tay 2015; Tolkien 2015).
Setting
Infants (aged 6 to 23 months)
Of the 13 included reviews for this age group, seven included trials conducted in low‐ and middle‐income countries (Abdullah 2013; Suchdev 2020; Das 2019a; Dekker 2010; Kristjansson 2015; Salam 2013; Shapiro 2019) and five in low‐, middle‐, and high‐income countries (Dewey 2009; Eichler 2012; Matsuyama 2017; Pasricha 2013; Pratt 2015); one review did not report the setting of the included trials (Petry 2016b). In Suchdev 2020, Dekker 2010, Dewey 2009 and Pasricha 2013, the included trials were conducted in anaemia and malaria prevalent settings. Anaemia and malaria prevalence was not described in nine reviews (Abdullah 2013; Das 2019a; Eichler 2012; Kristjansson 2015; Matsuyama 2017; Petry 2016b; Pratt 2015; Salam 2013; Shapiro 2019).
Preschool and school‐aged children (aged 2 to 10 years)
Four reviews included trials that were conducted in low‐ and middle‐income countries (Aaron 2015; De‐Regil 2011; De‐Regil 2017; Thompson 2013) and four included trials from low‐, middle‐, and high‐income countries (Das 2013a; Eichler 2019; Low 2013; Mayo‐Wilson 2014a). De‐Regil 2011, Low 2013 and Thompson 2013 included trials that were conducted in malaria‐endemic areas, but did not report on anaemia prevalence. De‐Regil 2017 and Mayo‐Wilson 2014a included trials conducted in anaemia and malaria prevalence settings, while Aaron 2015, Das 2013a and Eichler 2019 did not report on anaemia and malaria prevalence in the included trials.
Adolescent children (aged 11 to 18 years)
Fernández‐Gaxiola 2019 and Salam 2016 included trials conducted in low‐, middle‐, and high‐income countries. Neuberger 2016 and Salam 2020 included trials conducted in low‐ and middle‐income countries. In Fernández‐Gaxiola 2019, five of the 25 included trials were conducted in malaria‐endemic areas and one RCT reported anaemia prevalence. Neuberger 2016 included a mixed adolescents, with or without anaemia, and with or without malaria or parasitaemia. Salam 2016 and Salam 2020 did not report anaemia and malaria prevalence in the included trials.
Non‐pregnant women of reproductive age (aged 19 to 49 years)
Four reviews included trials conducted in low‐, middle, and high‐income countries, and none of the reviews reported anaemia and malaria prevalence in the included trials (Abe 2016; Houston 2018; Low 2016; Sultan 2019). Lassi 2020 included trials conducted in low‐ and middle‐income countries, and none described anaemia and malaria prevalence.
Pregnant women of reproductive age (aged 15 to 49 years)
Nine reviews included trials conducted in low‐ and middle‐income countries (Abu Hashim 2017; Bhutta 2012; Das 2018; Haider 2011; McCauley 2015; Radhika 2019; Shi 2015; Suchdev 2015; Thorne‐Lyman 2012), and 12 in low‐, middle‐, and high‐income countries (Buppasiri 2015; De‐Regil 2015; Govindappagari 2019; Haider 2013; Imdad 2012; Keats 2019; Lassi 2013; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Qassim 2018; Qassim 2019; Rumbold 2015); two reviews did not report the setting of the included trials (Daru 2016; Reveiz 2011). Trials included in Haider 2013 were conducted in anaemia and malaria prevalent settings. McCauley 2015, Peña‐Rosas 2015a, Peña‐Rosas 2015b, and Thorne‐Lyman 2012 included trials conducted in malaria‐endemic areas, but did not report on anaemia prevalence. Suchdev 2015 reported that malaria was not endemic in the areas where the trials were conducted. Seventeen reviews did not report on anaemia and malaria prevalence in the included trials (Abu Hashim 2017; Bhutta 2012; Buppasiri 2015; Das 2018; Daru 2016; De‐Regil 2015; Govindappagari 2019; Haider 2011; Imdad 2012; Keats 2019; Lassi 2013; Qassim 2018; Qassim 2019; Radhika 2019; Reveiz 2011; Rumbold 2015; Shi 2015).
Mixed population (all ages)
Six reviews included trials conducted in low‐ and middle‐income countries (Garcia‐Casal 2018; Geerligs 2003; Hess 2016; Huo 2015; Ramírez‐Luzuriaga 2018; Yadav 2019), 11 in low‐, middle‐, and high‐income countries (Arabi 2020; Basutkar 2019; Casgrain 2012; Das 2019b; Field 2020; Finkelstein 2019; Gera 2007a; Gera 2009; Gera 2012; Sadighi 2019; Silva Neto 2019), and one review, Smelt 2018, included trials conducted in high‐income countries. Four reviews did not report the setting of the included trials (Peña‐Rosas 2019; Tablante 2019; Tay 2015; Tolkien 2015). Geerligs 2003 included one trial conducted in a malaria‐endemic area, but did not report on anaemia prevalence in the included trials. In Garcia‐Casal 2018, two trials reported 40% or higher prevalence on anaemia and three less than 20%, and two trials were conducted in malaria‐endemic areas. Trials included in five reviews were conducted in settings where anaemia is prevalent, but the reviews did not report on malaria prevalence (Gera 2007a; Gera 2009; Gera 2012; Hess 2016; Huo 2015). In Field 2020, two trials conducted in non‐malaria‐endemic areas, while other trials did not report on malaria. In Peña‐Rosas 2019, one trial conducted in malaria‐endemic areas but malaria prevalence was not reported. Nine reviews did not report on anaemia and malaria prevalence in the included trials (Arabi 2020; Casgrain 2012; Das 2019b; Finkelstein 2019; Ramírez‐Luzuriaga 2018; Silva Neto 2019; Smelt 2018; Tablante 2019; Yadav 2019). Tay 2015 and Tolkien 2015 did not report on anaemia or malaria prevalence, but the trials included anaemic participants.
Interventions
We summarise the key characteristics of the interventions included in the reviews in Table 9; Table 10; Table 11; Table 12; Table 13 and Table 14.
9. Characteristics of interventions: infants (aged 6 to 23 months).
| Review | Intervention | Prevention or treatment | Population (mean age, baseline anaemia status/prevalence, known micronutrient deficiencies) | Dose (mean range) or composition or form of application (including compound, formulation) | Frequency | Start of intervention or duration (or both) | Adherence to intervention |
| Supplementation | |||||||
|
Abdullah 2013 Efficacy of oral iron therapy in improving the developmental outcome of pre‐school children with non‐anaemic iron deficiency: a systematic review |
Oral iron | Treatment | Non‐anaemic iron‐deficient children aged 1 to 5 years (mean age = 6 months to 30 months) | Oral ferrous sulphate (3 mg/kg per day) | Once or twice daily | Duration: 3 to 4 months | Reported in 1 trial |
|
Das 2019a Preventive lipid‐based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes |
LNS with complementary food at point‐of‐use | Prevention | 12 trials: children aged 6 months to 18 months (mean age: 5.9 months to 9.9 months); 4 trials: children aged 6 months to 24 months (mean age: not specified); 1 trial: children aged 6 months to 36 months (mean age: 24 months) Baseline anaemia status: not reported Known MN deficiencies: not reported |
10 trials: SQ LNS, 110 to 120 kcal/day (20 g dose); 4 trials: MQ LNS, 250 to 500 kcal/day (45 g to 90 g dose); 2 trials, SQ and MQ LNS; 1 trial: SQ for children aged 6 to 12 months and MQ for children aged 12 to 18 months | Daily | Start of intervention: 5.5 months to 6 months. However most studies were not described. Duration: 7 months to 18 months | Not reported |
|
Dekker 2010 Zinc supplementation in children is not associated with decreases in hemoglobin concentrations |
Zinc | Treatment | Children 0 to 15 years (mean age at baseline = 32 months, the majority of the trials commenced between 6 and 23 months; 3 trials conducted among anaemic children, 3 trials among children with malaria, 1 among HIV‐1–infected children, 1 among children suffering from diarrhoea, 1 among children with protein‐energy malnutrition, and 1 trial among children who were zinc deficient) | Typically 10 mg or 20 mg zinc per day | Daily | Duration: 4 months to 15 months | Not reported |
|
Pasricha 2013 Effect of daily iron supplementation on health in children aged 4‐23 months: a systematic review and meta‐analysis of randomised controlled trials |
Oral iron | Prevention | Children aged 4 to 23 months (8 trials included children with anaemia, ID or IDA, most trials unknown) | Typically provided as ferrous salts (ferrous sulphate in 22 trials) Dose: 12.5 mg or less, 12.6 mg to 30 mg, 31 mg to 59 mg, > 60 mg per day |
Daily | Duration: 1 week to 14 months (majority between 3 and 6 months) | Adherence not reported in 11 trials. In another 11 trials, there was no difference in adherence between iron and control group. |
|
Petry 2016b The effect of low dose iron and zinc intake on child micronutrient status and development during the first 1000 days of life: a systematic review and meta‐analysis |
Iron supplementation, fortification or biofortification | Prevention | Children were 6– to 23 months old (74 trials, apparently healthy) Baseline anaemia status/prevalence: apparently health, but may suffer from anaemia Known MN deficiencies: apparently healthy, but may suffer from ID or zinc deficiency |
Iron dose: ≤ 15 mg/day Supplements defined as compounds, which are routinely consumed separately from a normal meal, including tablets, pills, drops, capsules, syrups, drinks, biscuits, and LNS |
Daily or 3 or more times per week | Not reported | Not reported |
|
Pratt 2015 A review of the strategies used to reduce the prevalence of iron deficiency and iron deficiency anaemia in infants aged 6‐36 months |
MN sprinkles, iron‐fortified milk, iron supplementation and food‐based strategies | Prevention | Infants aged 3 to 36 months Baseline anaemia status/prevalence: not reported Known MN deficiencies: not reported |
MN sprinkles: iron dose of 12.5 mg (2 trials) Iron‐fortified milk: ferrous gluconate 5.28 mg to 5.8 mg (2 trials) Iron supplementation: 10 mg to 12.5mg (2 trials) | Fortification: daily Supplementation: daily or weekly |
Duration: average 6 months | Not reported |
| Fortification | |||||||
|
Dewey 2009 Systematic review and meta‐analysis of home fortification of complementary foods |
Home fortification of complementary foods with MNP (sprinkles), crushable tablets and lipid‐based or soy‐based products | Prevention and treatment | Infant and young children aged 4 to 36 months (anaemic at baseline in 5 treatment trials and non‐anaemic in 11 prevention trials) Baseline anaemia status/prevalence: reported, Known MN deficiencies: not reported |
Sprinkles: 12.5 mg/day to 80 mg/day; Iron drops: 12.5 mg/day to 40 mg/day Sprinkles + folic acids + MMN + fortified supplement: 12.5 mg/day to 30 mg/day |
Daily or several times per week | Duration: 6 weeks to 20 months | Only few trials reported. Adherence was high when reported |
|
Eichler 2012 Effects of micronutrient fortified milk and cereal food for infants and children: a systematic review |
MN fortified milk or cereal food | Prevention | Infants and children from 6 months to 5 years of age (mean age ranged from 6 months to 23 months at inclusion, upper age limit was 3 years in 1 trial, median of anaemia rates at baseline was 36% from 9 trials) | MN food with 1.8 mg/day to 27.5 mg/day iron | Daily | Mean follow‐up period: 8.25 months (2.2 to 12 months) | Not reported |
|
Matsuyama 2017 Effect of fortified milk on growth and nutritional status in young children: a systematic review and meta‐analysis |
Fortified milk (most common with iron, vitamin C, zinc, fatty acids, vitamin D, probiotics or synbiotics) | Prevention | Children (mean age at baseline = 6 months to 22.4 months, 1 trial = 29 months to 31 months at baseline, some trials included anaemic children at baseline) | Dose: not reported Type of iron used: ferrous sulphate (3 trials), ferrous gluconate (2), ferrous lactate (1), unclear in remaining trials |
Not specified | Duration: 4 to 12 months | Adherance checked in most included trials |
|
Salam 2013 Effectiveness of micronutrient powders (MNP) in women and children |
MNP | Prevention | Children aged 6 months to 11 years (most trials children aged 6 months to 6 years, 2 trials up to 11 years) Baseline anaemia status/prevalence: not reported Known MN deficiencies: not reported |
MNP: vitamins and minerals, most trials used 12.5 mg iron (range = 2.5 mg to 30 mg) as ferrous fumarate | Daily | Duration: 2 to 24 months | Not reported |
|
Suchdev 2020 Point‐of‐use fortification of foods with micronutrient powders containing iron in children of preschool and school age |
MNPs, including at least iron, zinc and vitamin A | Prevention | Apparently healthy infants and young children aged 6 months to 23 months Baseline anaemia status/prevalence: mixed status Known MN deficiencies: not reported |
MNP with 12.5 mg elemental iron in 14 trials, 10 mg in 8 trials, 6 mg in 1 trial, 30 mg in 1 trial. MNP with 9 mg of ferrous orthophosphate, 6 mg of ferrous lactate and 2.5 mg of NaFeEDTA in 1 trial each | Daily | Duration: 2 months to 44 months | No studies reported data on outcome adherence |
| Improving dietary diversity and quality | |||||||
|
Kristjansson 2015 Food supplementation for improving the physical and psychosocial health of socio‐economically disadvantaged children aged three months to five years |
Supplementary feeding (provision of energy and macronutrients) with or without added micronutrients | Prevention | Children aged 3 months to 5 years (> 60% of children were under 2 years, high proportion of children had low weight‐for‐age z‐scores or height‐for‐age z‐scores) | Variet of food was provided (milk, bread, fruit, vegetables, rice and lentils, or provided a fortified cookie, etc.) 11 trials: ready‐to‐use therapeutic feeding acid with or without other foods 1 trial: bread with milk 4 trials: cereal, flours or vegetable mixture, usually with milk 7 trials: locally available foods such as fruit, vegetables, rice and lentils, or provided a fortified cookie 2 trials: iron‐fortified cereal 1 trial: meat 1 trial: hot lunches, nutritious snacks, and vitamin supplementation 16 trials: fortified foods |
Daily | Duration: 3 months to 32 months | Method of adherence reported in most trials |
|
Shapiro 2019 A systematic review investigating the relation between animal‐source food consumption and stunting in children aged 6‐60 months in low and middle‐income countries |
Animal‐source food consumption versus control (e.g. non‐ASF or no intervention) | Prevention | Children aged 6‐9 months Baseline anaemia status: not reported Know micronutrient deficiencies: not reported |
Animal‐source food (beef, fish, fish powder, pork, egg, caterpillar cereal) | Not specified | 5 months to 12 months | Not reported |
ID: iron deficiency; IDA: iron deficiency anaemia; LNS: lipid‐based nutrient supplements; MN: micronutrient; MMN: multiple micronutrient; MNP: micronutrient powders; MQ: medium quantity; NaFeEDTA: sodium iron ethylenediaminetetraacetic acid; SQ: small quantity.
10. Characteristics of interventions: preschool and school‐aged children (aged 2 to 10 years).
| Review | Intervention | Prevention or treatment | Population (mean age, baseline anaemia status/prevalence, known micronutrient deficiencies) | Dose (mean range) or composition or form of application (including compound, formulation) | Frequency | Start of intervention or duration (or both) | Adherence to intervention |
| Supplementation | |||||||
|
Low 2013 Effects of daily iron supplementation in primary‐school‐aged children: systematic review and meta‐analysis of randomized controlled trials |
Oral iron | Prevention or treatment | Children 5 years to 12 years Baseline anaemia status/prevalence: some trials included anaemic children Known MN deficiencies: some trials included ID or IDA children |
Oral iron supplementation: 5 mg/day to 400 mg/day or 3 mg/kg/day to 10 mg/kg/day (most trials ferrous sulfate, but also iron citrate, ferrous fumarate, iron polymaltose, ferrous gluconate, ferrous dextran) | Daily | Duration: 1 month to 12 months | Reported in 21 trials, adherence ranged from 80% to 90% in most trials |
|
De‐Regil 2011 Intermittent iron supplementation for improving nutrition and development in children under 12 years of age |
Intermittent supplementation with iron alone or with other nutrients | Prevention or treatment | Children under 12 years of age Baseline anaemia status/prevalence: 7 trials included only anaemic children, 3 trials only non‐anaemic children, in the remaining trials, the baseline prevalence of anaemia ranged between 15% and 90% Known MN deficiencies: some trials included children with ID |
Total elemental iron per week: 7.5 mg to 200 mg (ferrous sulphate in almost all trials, most trials supplemented only with iron, 1 trial in combination with 30 mg vitamin C, 5 trials in combination with folic acid, 4 trials with MMNs) | Twice/week (9 trials), 3 times/week (2 trials), once/week (remaining trials) | Duration: 6 weeks to 3 months (15 trials), > 3 months to 1 year (18 trials) | Adherence tends to be higher in children receiving intermittent iron supplementation compared with those receiving daily iron supplements (not statistically significant) |
|
Mayo‐Wilson 2014a Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age |
Orally administered zinc given as a supplement | Prevention | Children of 6 months to 12 years of age (mean age = 28 months) Baseline anaemia status/prevalence: 1 trial included anaemic children Known MN deficiencies: median of mean baseline zinc concentration was 72.5 μg/dL |
Zinc supplements provided zinc as a solution or syrup (46 trials), pill or tablet (17 trials), capsule (6 trials), or powder (2 trials) Zinc dose administered daily dose equivalents of < 5 mg (5 trials), 5 mg to < 10 mg (19 trials), 10 mg to < 15 mg (30 trials), 15 mg to < 20 mg (8 trials), and 20 mg or more (12 trials) | Ranging from daily to weekly | Trials provided zinc for < 2 months (8 trials), 2 months to < 6 months (22 trials), 6 months to < 12 months (33 trials), and 11 months or more (16 trials) | Poor or non‐compliance for some participants reported in 4 trials |
|
Thompson 2013 Effects of daily iron supplementation in 2‐ to 5‐year‐old children: systematic review and meta‐analysis |
Oral iron | Prevention or treatment | Children 2 years to 5 years Baseline anaemia status/prevalence: 6 trials included anaemic children, 7 trials mixed or unknown Known MN deficiencies: children with ID in 4 trials |
Iron: 5 mg/day to 50 mg/day (most trials used ferrous sulphate, some used sodium iron edetate, ferric ammonium citrate, ferrous gluconate) | At least 5 days per week | Duration: 28 days to 15 months | Adherence reported in 2 trials: consumption ranged from 80% to 97% |
| Fortification | |||||||
|
Aaron 2015 Multiple‐micronutrient fortified non‐dairy beverage interventions reduce the risk of anemia and iron deficiency in school‐aged children in low‐middle income countries: a systematic review and meta‐analysis |
Non‐dairy MMN‐fortified beverages | Prevention | 9 trials: school‐aged children (mean age = 5 years to 18 years); 1 trial: pregnant women (mean age = 25 years) Baseline anaemia status/prevalence: not reported, most studies excluded children with low Hb level (60 g/L to 80 g/L) Known MN deficiencies: not reported |
MMN contained vitamin A, vitamin C, iron and zinc in all trials and also vitamin B12, vitamin E, folate, niacin, vitamin B6 and B2 and iodine in most trials (incomplete information on quantities and chemical forms of micronutrients) | Daily | Duration: 8 weeks to 8.5 months Start of intervention for pregnant women: 12 weeks to 34 weeks' gestation and duration 8 weeks |
Reported in 4 trials, but not further specified |
|
Das 2013a Systematic review of zinc fortification studies |
Food fortification (bread, porridge, cereal) with zinc | Prevention | 3 trials: school children (2 years to 11 years) Baseline anaemia status/prevalence: healthy or unknown Known MN deficiencies: healthy or with asymptomatic zinc deficiency |
Zinc oxide in cereals (3.75 mg/oz) and zinc acetate in bread (400 mg/loaf) or porridge (5 mg/100 g) | Not reported | Duration: 3 months to 9 months | Not reported |
|
De‐Regil 2017 Point‐of‐use fortification of foods with micronutrient powders containing iron in children of preschool and school age |
MNP for point‐of‐use fortification | Prevention | Preschool and school‐aged children (6 trials: only children up to 59 months of age, 4 trials: age 5 years and older, 3 trials: both younger and older than 59 months of age) Baseline anaemia status/prevalence: 7 trials excluded children with low Hb values Known MN deficiencies: not reported |
3 trials: formulation of 14 vitamins and minerals 2 trials: formulation with 6 vitamins and minerals Remaining trials: different formulations (range = 2 to 18 vitamin and minerals) |
Daily | Duration: 8 weeks to 12 weeks | Reported in 1 trial (no difference between intervention and control group) |
|
Eichler 2019 Health effects of micronutrient fortified dairy products and cereal food for children and adolescents: a systematic review |
Centrally‐processed fortified dairy products and fortified cereals, using any fortification strategy | Prevention | 24 trials: children (aged 5 years to 12 years) and adolescents (aged 12 to 15 years) (mean age: 6 years to 13.3 years, more than 60% fell within the age group of 2 years to 10 years) Baseline anaemia status: not reported Known MN deficiencies: not reported |
Centrally‐processed fortified dairy products and fortified cereals, using any fortification strategy. Dairy products included fortified fresh milk; centrally processed milk or other dairy products (such as yoghourt, milk powder and cheese). Cereals included, for example, fortified wheat flour or maize (corn) | Not reported | Not reported | Not reported |
Hb: haemoglobin; ID: iron deficiency; IDA: iron deficiency anaemia; MN: micronutrient; MMNs: multiple micronutrients, MNP: micronutrient powder.
11. Characteristics of interventions: adolescent children (aged 11 to 18 years).
| Review | Intervention | Prevention or treatment | Population (mean age, baseline anaemia status/prevalence, known micronutrient deficiencies) | Dose (mean range) or composition or form of application (including compound, formulation) | Frequency | Start of intervention or duration (or both) | Adherence to intervention |
| Supplementation | |||||||
|
Fernández‐Gaxiola 2019 Intermittent iron supplementation for reducing anaemia and its associated impairments in adolescent and adult menstruating women |
Oral iron alone or with other vitamins and minerals | Prevention or treatment | Menstruating women (range 6 years to 49 years, but > 60% of the trials included women under 18 years, most trials involved a mix of anaemic and non‐anaemic women) | 60 mg iron (10 mg to maximum 120 mg), ferrous sulphate (in most trials) | Once a week (5 trials twice a week) | Duration: 3 months or less (13 trials), 3.5 months (3 trials), 4 months (6 trials), 5 months (1 trial), 6 months (1 trial), 12 months (1 trial) | Reported in 6 trials, no evidence of a difference between groups |
|
Neuberger 2016 Oral iron supplements for children in malaria‐endemic areas |
Oral iron Oral iron with or without folic acid Oral iron with antimalarial prophylaxis | Treatment or prevention | Children less than 18 years of age Baseline anaemia status: not reported. Known MN deficiencies: not reported |
Mean iron supplementation dose: 2 mg/kg/day | Daily | Mean duration of treatment: 4.5 months (1 to 12 months) | Adherence reported in 20 trials and the average overall adherence to all trial drugs was good (89%) |
|
Salam 2016 Interventions to improve adolescent nutrition: a systematic review and meta‐analysis |
MN supplementation and nutrition in pregnancy | Prevention or treatment | Adolecent population (11 years to 19 years old, most trials included girls, 9 included boys and girls) Baseline anaemia status/prevalence: 2 trials included anaemic girls, 29 not reported Known MN deficiencies: unknown |
MN supplementation: 13 trials = iron/iron folic acid supplementation alone 9 trials = iron/iron folic acid in combination with other MNs 2 trials = multiple MNs alone 2 trials = zinc supplementation 5 trials: supplemented with calcium and vitamin D Nutrition in pregnancy: mainly provision of MN supplementation such as calcium and zinc, in addition to the routine iron folic acid supplementation or nutritional education sessions |
MN supplementation: daily or weekly Nutrition in pregnancy: not reported |
MN supplementation duration: not reported Nutrition in pregnancy start: between 20 weeks to 27 weeks' gestation Duration: until delivery |
Not reported |
|
Salam 2020 Effects of preventive nutrition interventions among adolescents on health and nutritional status in low‐ and middle‐income countries |
MN supplementation/fortification (any MN alone or in combination) | Prevention | 10 studies: 10,802 adolescents aged 10 years to 19 years Baseline anaemia status; not reported This review excluded hospitalised adolescents and adolescents with any pre‐existing health condition |
Supplementaion of iron, calcium, folic acid, zinc, vitamin A, vitamin D | Daily Weekly |
10 weeks to 2 years | Not reported |
MN: micronutrient
12. Characteristics of interventions: non‐pregnant women of reproductive age (aged 19 to 49 years).
| Review | Intervention | Prevention or treatment | Population (mean age, baseline anaemia status/prevalence, known micronutrient deficiencies) | Dose (mean range) or composition or form of application (including compound, formulation) | Frequency | Start of intervention or duration (or both) | Adherence to intervention |
| Supplementation | |||||||
|
Abe 2016 Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby |
MMNs | Prevention | Non‐pregnant mothers who exclusively fed breast milk or practiced mixed feeding Baseline anaemia status/prevalence: not reported Known MN deficiencies: not reported |
Trial 1: 18 mg of iron (ferrous fumarate), 15 mg of zinc (zinc oxide), 2 mg of copper (cupric oxide), 162 mg of calcium (calcium phosphate dibasic), and other minerals and vitamins Trial 2: vitamin A 8000 IU, vitamin D 400 IU, vitamin E 30 IU, vitamin C 90 mg, folic acid 0.8 mg, thiamin 1.7 mg, riboflavin 2 mg, niacin 20 mg, vitamin B6 4 mg, vitamin B12 8 μg, calcium 200 mg, iodine 150 μg, iron 45 mg, and magnesium 100 mg |
Daily | Duration: 6 weeks to 15 weeks postpartum | Not reported |
|
Houston 2018 Efficacy of iron supplementation on fatigue and physical capacity in non‐anaemic iron‐deficient adults: a systematic review of randomised controlled trials |
Oral, IM or IV iron supplementation | Prevention/treatment | Adults (≥ 18 years) (age range = 17 years to 55 years); 15 trials included only women with > 60% within this age group Baseline anaemia status/prevalence: non‐anaemic Known MN deficiencies: iron deficient |
Oral iron: 16 mg/day to 200 mg/day
IV iron: 200 mg/day to 1000 mg/day IM: 100 mg per day |
Daily | Duration: iron therapy was 46 days (mean = ± 30 days; range = 1 day to 112 days), follow‐up 57 days (mean = ± 24 days; range = 28 days to 112 days) | Adherence with the trial intervention was reported in 13 trials (RR 1.0, 95% CI 0.99 to 1.01; 13 trials, 958 participants) |
|
Lassi 2020 Effects of preconception care and periconception interventions on maternal nutritional status and birth outcomes in low‐ and middle‐income countries: a systematic review |
Iron folic acid supplementation versus placebo | Prevention | Preconceptional women of reproductive age in low‐ and middle‐ income countries (mean age not reported) Baseline anaemia status: not reported Known MN deficiencies: not reported |
Different dosages of iron and folic acid: 60 mg elemental iron and 0.25 mg folic weekly (3 trials), 120 mg iron and 3.5 mg folic acid (1 trial), 100 mg iron and folate 500 μg daily or weekly (1 trial), daily 60 mg elemental iron and 0.5 mg folic acid (1 trial), weekly 65 mg of elemental iron with 0.25 mg folic acid (1 trial), once daily or weekly 350 mg iron and 1.5 mg folic acid (1 trail), daily or twice weekly 60 mg iron and 0.5 mg folic acid (1 trial), weekly 200 mg ferrous fumarate and 200 mg folic acid (1 trial) | Daily (1 trial), weekly (6 trials), daily and weekly (3 trials) | Duration: 8 weeks to more than 12 weeks (1 trial 8 weeks, 2 trials 10 weeks, 7 trials more than 12 weeks) | Adherence was an outcome in some trials, but not results have been reported. |
|
Low 2016 Daily iron supplementation for improving anaemia, iron status and health in menstruating women |
Oral iron alone or with folic acid or vitamin C | Prevention | Menstruating women (13 years to 45 years, 3 trials included adolescent girls only) Baseline anaemia status/prevalence: anaemic and non‐anaemic participants Known MN deficiencies: iron deficiency reported in some trials |
Elemental iron dose: 1 mg/day to 300 mg/day 33 trials = ferrous sulphate, 1 trial =: ferrous sulphate and carbonyl iron, 2 trials = carbonyl iron, 5 trials = ferrous fumarate, 1 trial = ferric pyrophosphate and ferrous fumarate together, remaining trials = variety of different iron formulations |
Daily (at least 5 days/week) | Duration: 1 week to 24 weeks | Adherence was not reported in any form in 34 of the trials. Participants randomised to iron did not appear to have poorer adherence compared with those randomised to placebo. |
|
Sultan 2019 Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta‐analysis |
IV iron versus oral iron | Treatment | Postpartum women (age not reported); Hb upper cut‐off values for inclusion: 8 g/dL (4 studies), 8.5 g/dL (1 study), 9 g/dL (3 studies), 10 g/dL (5 studies), 10.5 g/dL (1 study), postpartum haemorrhage (1 study) Known MN deficiencies: not reported |
IV formulation: 300 mg to 600 mg ferric sucrose (9 trials), 1000 mg to 3000 mg ferric carboxy maltose (5 trials), 1000 mg iron dextran (1 trial), 1200 mg iron maltoside (1 trial); oral iron: 200 mg/day to 975 mg/day ferrous sulphate (9 trials), ferrous ascorbate (2 trials), iron protein succinylate (2 trials), ferrous fumarate (1 trial), not described (1 trial) | IV: alternate days (5 trials), consecutive days (4 trials), weekly (3 trials), single dose (2 trials), not stated (1 trial); oral iron: daily | Start: within 48 h after delivery (11 trials), up to 10 days (2 trials), not described (2 trials); duration: 14 days to 12 weeks (6‐week trial period in 6 trials) | Adherence assessed in 10 trials, data reported in 3. Two trials reported higher adherence in the IV group (98% versus 84% and 100% versus 84%) and 1 trial equal adherence in intervention and control group (100%) |
CI: confidence interval; Hb: haemoglobin; IM: intramuscular; IV: intravenous; MN: micronutrient; MMNs: multiple micronutrients; RR: risk ratio.
13. Characteristics of interventions: pregnant women (aged 15 to 49 years).
| Review | Intervention | Prevention or treatment | Population (mean age, baseline anaemia status/prevalence, known micronutrient deficiencies) | Dose (mean range) or composition or form of application (including compound, formulation) | Frequency | Start of intervention or duration (or both) | Adherence to intervention |
| Supplementation | |||||||
|
Abu Hashim 2017 Lactoferrin or ferrous salts for iron deficiency anemia in pregnancy: a meta‐analysis of randomized trials |
Bovine lactoferrin | Treatment | Pregnant women between 12 and 36 weeks' gestation Baseline anaemia status/prevalence: mild IDA (diagnosed in the second or third trimester according to the WHO (Hb < 11 g/dL)) Known MN deficiencies: not reported |
3 trials: bovine lactoferrin oral dose of 1 capsule of 100 mg, twice a day before meals, for 4 weeks 1 trial: bovine lactoferrin oral dose of 1 capsule of 250 mg, daily, for 8 consecutive weeks |
Once (1 trial) or twice (3 trials) a day | Start: second or third trimester Duration: 4 weeks (3 trials) or 8 consecutive weeks (1 trial) |
Not reported |
|
Bhutta 2012 Is it time to replace iron folate supplements in pregnancy with multiple micronutrients? |
MMN | Prevention | Healthy pregnant women at any gestation Baseline anaemia status/prevalence: not reported Known MN deficiencies: not reported |
MMN supplement formula UNIMMAP (30 mg iron, 400 μg folic acid, 15 mg zinc, 2 mg copper, 65 μg selenium, 800 μg RE vitamin A, 1.4 mg vitamin B1, 1.4 mg vitamin B2, 18 mg niacin, 1.9 mg vitamin B6, 2.6 μg vitamin B12, 70 mg vitamin C, 5 μg vitamin D, 10 mg vitamin E and 150 μg iodine) or similar to UNIMMAP (small variations in IFA) | At least 6 days/week | Start: 28 weeks' gestation at the latest Duration: until delivery |
Not reported |
|
Buppasiri 2015 Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes |
Calcium supplementation | Prevention | Pregnant women (any trimester, most trials in second trimester; 3 trials only adolescent pregnant women with mean age of 17.0 years) Baseline anaemia status/prevalence: not reported Known MN deficiencies: not reported |
Various types of calcium: calcium carbonate, calcium gluconate, calcium lactate and combined (range = 1000 to 2000 mg/day; 3 trials < 1000 mg/day (range = 300 mg to 600 mg); 1 trial 600 mg at 22 to 32 weeks’ gestational age and then 1200 mg from 32 weeks until delivery | Daily | Start and duration: 11 trials at 20 weeks’ gestational age (or after) until delivery; 5 trials < 20 weeks' gestational age until delivery; remaining trials were unclear | No data available for compliance |
|
Daru 2016 Systematic review of randomized trials of the effect of iron supplementation on iron stores and oxygen carrying capacity in pregnancy |
Iron (oral, including fortified water, IV or IM) | Prevention and/or treatment | Pregnant women at any gestation Baseline anaemia status/prevalence: IDA in 18 trials Known MN deficiencies: NAID in 5 trials |
Pregnant women with IDA: 200 mg IV iron (up to 400 mg) or 120 mg oral iron Pregnant women with NAID: 30 mg to 80 mg oral iron |
Pregnant women with IDA: weekly Pregnant women with NAID: weekly or daily |
Start: not specified Duration: until 28 weeks' gestation |
Not reported |
|
Das 2018 Lipid‐based nutrient supplements for maternal, birth, and infant developmental outcomes |
LNS | Prevention | Pregnant women at 20 weeks' gestation or less (mean age: 21.9 to 26.6 years) Baseline anaemia status: not reported Known MN deficiencies: not reported |
The energy content of LNS was 118 kcal/day, LNS with 372 kcal/day | Daily | The interventions began during pregnancy and lasted up to six months postpartum | Not reported |
|
De‐Regil 2015 Effects and safety of periconceptional oral folate supplementation for preventing birth defects |
Periconceptional folate or folic acid supplementation alone or in combination with other vitamins or minerals | Prevention | All women who became pregnant or were 12 or less week's pregnant at the time of the intervention Baseline anaemia status/prevalence: not reported Known MN deficiencies: not reported |
Folic acid supplementation doses ranged from 0.4 mg/day to 4.0 mg/day | Daily (2 to 3 equal doses) | Start and duration: periconceptional period (supplementation started before pregnancy and discontinued after 12 weeks of pregnancy) | Adherence reported, but not further specified |
|
Govindappagari 2019 Treatment of iron deficiency anemia in pregnancy with intravenous versus oral iron: systematic review and meta‐analysis |
IV iron versus oral iron | Treatment | 11 trials: pregnant women with IDA (mean age: not reported); mean baseline haemoglobin before treatment was < 8.0 g/dL in 5 studies and > 8.0 g/dL in 6 studies Known MN: not reported |
IV iron: iron sucrose (8 trials), FCM, LMW iron dextran Oral iron: 100 mg to 200 mg elemental iron as ferrous sulphate (6 trials), ferrous fumerate (3 trials), ferrous ascorbate (1 trial), iron polymaltose complex (1 trial) |
IV iron: infused in split doses every other day (maximum daily dose of 200 mg, IV iron FCM at a dose of 1000 mg once/week, LMW iron dextran as a one‐time, total dose infusion) Oral iron: daily |
Start: first trimester Duration: at least 4 weeks |
Not reported |
|
Haider 2011 Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes |
MMN (at least 5 MNs, including the UNIMMAP formulation or those with comparable composition) | Prevention | Pregnant women (any gestation) Baseline anaemia status/prevalence: not reported Known MN deficiencies: not reported |
UNIMMAP (30 mg iron, 400 µg folic acid, 15 mg zinc, 2 mg copper, 65 µg selenium, 800 µg RE vitamin A, 1.4 mg vitamin B1, 1.4 mg vitamin B2, 18 mg niacin, 1.9 mg vitamin B6, 2.6 µg vitamin B12, 70 mg vitamin C, 5 µg vitamin D, 10 mg vitamin E and 150 µg iodine) used in 12 trials, remaining trials were comparable to UNIMMAP except for a small variation in dose of iron and folic acid used | Daily | Start: any gestation Duration: any |
Missing compliance data in included trials |
|
Haider 2013 Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta‐analysis |
Oral iron supplementation (with or without folic acid or other MNs), iron fortification (2 trials) | Prevention | Pregnant women (any gestation) Baseline anaemia status/prevalence: not reported Known MN deficiencies: ID reported in 1 trial |
Oral iron or iron and folic acid, 10 mg to 240 mg daily (1 trial used a daily dose of 900 mg) | Daily | Start: early (< 21 weeks' gestation) in the majority of trials, late (from 22 weeks' gestation) Duration: 7 to 8 weeks (up to 30 weeks during pregnancy) |
Not reported |
|
Imdad 2012 Routine iron/folate supplementation during pregnancy: effect on maternal anaemia and birth outcomes |
Oral iron or iron with folic acid | Prevention | Pregnant women (gestational age not reported) Baseline anaemia status/prevalence: not reported Known MN deficiencies: not reported |
Iron: 20 mg/day to 300 mg/day | Daily | Start: no later than 28 weeks' gestation Duration: not specified |
Not reported |
|
Keats 2019 Multiple‐micronutrient supplementation for women during pregnancy |
MMN with iron and folic acid | Prevention | Pregnant women (ranging from early pregnancy to 36 weeks' gestation) Baseline anaemia status/prevalence: anaemia at baseline reported in 2 trials Known MN deficiencies: ID reported in 1 trial and vitamin A deficiency reported in 2 trials |
Oral supplementation, composition of the MMN supplement was different in all included trials (18 included iron and folic acid in the MMN supplement) | Daily (1 trial 6 days/week, 1 trial twice/week) | Start: from enrolment (first, second, or third trimester) Duration: until delivery (11 trials) or 4 (1 trial), 6 (1 trial), 12 (5 trials), or 24 (2 trials) weeks after delivery |
Not reported |
|
Lassi 2013 Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes |
Folic acid with or without iron or other vitamins and minerals | Prevention | Pregnant women (any age and parity) Baseline anaemia status/prevalence: not reported Known MN deficiencies: not reported |
Most trials supplemented women with folic acid in combination with iron Folic acid: 10 µg to 400 µg, iron: 60 mg to 5 g |
Daily | Start: from 8 weeks' gestation (most trials from at least 20 weeks' gestation) Duration: during pregnancy |
Not reported |
|
McCauley 2015 Vitamin A supplementation during pregnancy for maternal and newborn outcomes |
Vitamin A alone or in combination with other supplements | Prevention | Pregnant women (any gestational age) Baseline anaemia status/prevalence: 2 trials included anaemic women Known MN deficiencies: all trials conducted in populations considered to be moderately deficient in vitamin A, 1 trial in women with severe vitamin A deficiency, 2 trials (UK and USA) considered not deficient in vitamin A |
1 trial: IM 600,000 IU vitamin A palmitate in oil at parturition 18 trials: 5750 IU to 444,000 IU vitamin A capsules |
Daily or weekly | Start: from enrolment Duration: 8‐12 weeks (up to 6 weeks postpartum) |
Complicane assessed in only 1 trial |
|
Peña‐Rosas 2015b Daily oral iron supplementation during pregnancy |
Daily iron (any supplements containing iron) | Prevention | Pregnant women (any gestational age) Baseline anaemia status/prevalence: 24 trials had non‐anaemic women; the remaining trials were unclear but may have included women with mild or moderate anaemia Known MN deficiencies: not reported |
Dose range: 9 mg to 900 mg of elemental iron (18 trials 60 mg) trials that provided daily dose of folic acid: 0.01 mg to 5 mg 13 trials: ferrous sulphate; 6 trials: ferrous fumarate; 1 trial: ferrous iron; 6 trials: ferrous gluconate; the remaining trials: ferrous betainate hydrochloride, heme iron from porcine blood, ferritin in a micro granulated gastric resistant capsule, chelated iron aminoates, iron EDTA |
Daily | Start: 12 weeks gestation (before 20 weeks in most trials, 13 trials at or after 20 weeks' gestation) Duration: until delivery (or postpartum) |
One third of the trials reported compliance. Compliance in the iron and control groups seemed to be similar. |
|
Peña‐Rosas 2015a Intermittent oral iron supplementation during pregnancy |
Intermittent iron (with or without other vitamins and minerals) | Prevention | Pregnant women (any gestational age) Baseline anaemia status/prevalence: 9 trials had non‐anaemic women; 1 trial had women who were anaemic at baseline; the remaining trials may have included some women with moderate or mild anaemia at baseline Known MN deficiencies: not reported |
Dose range: 80 mg to 300 mg elemental iron per week (dose in the daily supplementation comparison group ranged from 40 mg to 120 mg elemental iron daily) In trials with folic acid: 0.4 mg/week to 3.5 mg/week |
Weekly (on 1 day each week) | Start: before 20 weeks' gestation (9 trials); in the remaining trials, gestational age at the start of supplementation was mixed or unclear Duration: at least 10 weeks (some trials until delivery) |
Reported in some trials, but not further described |
|
Qassim 2018 Safety and efficacy of intravenous iron polymaltose, iron sucrose and ferric carboxymaltose in pregnancy: a systematic review |
IV IPM, IS and FCM | Treatment | IS (2635 pregnant women; 41 studies), FCM (276 pregnant women; 4 studies) and IPM (164 pregnant women; 3 studies) Known MN deficiencies: not reported |
IV IPM, IS, or FCM | Daily | Start of intervention: any gestational weeks | Not reported |
|
Qassim 2019 Intravenous or oral iron for treating iron deficiency anaemia during pregnancy: systematic review and meta‐analysis |
IV iron therapy | Treatment | Pregnant women who initially had low haemoglobin levels (< 110 g/L) or were at high risk of developing IDA Baseline mean haemoglobin levels (range, 60 g/L to 109 g/L) and mean gestation at enrolment (range, 22 to 33.3 weeks); Known MN deficiencies: not reported |
IV IPM, IS, or FCM | Daily | Start of intervention: 22 to 33.3 gestational weeks | Not reported |
|
Radhika 2019 Parenteral versus oral iron for treatment of iron deficiency anaemia during pregnancy and post‐partum: a systematic review |
Parenteral iron (IV IS) | Treatment | 18 studies (1633 antenatal women) and 8 studies (713 post‐partum women) Known MN deficiencies: not reported |
IV IS versus oral iron (ferrous sulphate, ferrous ascorbate or fumarate) | Daily | Antenatal and post‐partum period | Not reported |
|
Reveiz 2011 Treatments for iron‐deficiency anaemia in pregnancy |
Any iron intervention (oral, oral iron plus adjuncts, IM, IV, blood transfusion, recombinant erythropoietin) | Treatment | Pregnant women (any gestational age) Baseline anaemia status/prevalence and known micronutrient deficiencies: pregnant women with a diagnosis of anaemia attributed to ID |
Oral iron: 20 mg to 300 mg IM iron: total dose of iron (mg) = weight (kg) x Hb deficit (g/dL) x 4.4 + 500, 2 injections of 250 mg elemental iron IV iron: weight in kg x (target Hb ‐ actual Hb) x 0.25 + 500 (maximum total dose 200 mg to 300 mg or 500 mg elemental iron) |
Oral iron: daily or weekly IM iron: alternate days IV iron: every other day, twice weekly |
Start: between 16 to 20, 26 and 34 weeks' gestation Duration: 4 to 16 weeks (oral iron up to delivery) |
Compliance reported in 1 trial comparing oral iron polymaltose complex versus ferrous sulphate; found no evidence of a difference |
|
Rumbold 2015 Vitamin C supplementation in pregnancy |
Vitamin C supplementation | Prevention | All pregnant women of any gestation (9 trials recruited women who were at “high” or “increased” risk of pre‐eclampsia, 2 trials included women with established pre‐eclampsia) Baseline anaemia status/prevalence: not reported Known MN deficiencies: 1 trial had women at high risk of ID, and 1 trial had 11 participants with vitamin C deficiency |
12 trials: vitamin C alone 15 trials: vitamin C in addition to vitamin E, or vitamin C and vitamin E in addition to allopurinol, or aspirin and fish oil 6 trials: additional supplements containing iron, folic acid, vitamin B and/or calcium or a “standard prenatal vitamin” were given to all women (i.e. in the vitamin C group and the control group) The most common daily dosage of vitamin C was 1000 mg/day (15 trials), 500 mg/day (6 trials), 100 mg/day (4 trials), 2000 mg/day (2 trials), 400 mg (1 trial), and 250 mg/day for 6 days and thereafter 250 mg/week (vaginally, 1 trial) | Daily | Start: in the second trimester Duration: not reported in many trials |
Not reported |
|
Shi 2015 Intravenous iron sucrose versus oral iron in the treatment of pregnancy with iron deficiency anaemia: a systematic review |
IV iron sucrose | Treatment | Pregnant women (gestational age = not reported) Baseline anaemia status/prevalence: diagnosed IDA Known MN deficiencies: not reported |
IV iron sucrose total dose from formula: weight × (11 or 12 g/dL – actual Hb) × 0.24 + 500 mg Oral elemental iron: 100 mg to 300 mg daily (as ferrous sulphate, iron polymaltose complex or ferrous fumerate) |
IV: every other day Oral iron: daily |
Start: not specified Duration: 4 to 6 weeks |
Not reported |
|
Thorne‐Lyman 2012 Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta‐analysis |
Supplementation with vitamin A or carotenoids, or both | Prevention and treatment | Pregnant women (any gestational age) Baseline anaemia status/prevalence: 3 trials included anaemic women, 3 trials included anaemic and non‐anaemic Known MN deficiencies: not reported |
Dose: vitamin A 3333 IU to 10 000 IU per day (2 trials with HIV‐positive women: vitamin A 5000 IU per day and 30 mg beta‐carotene as well as a 200,000 IU dose of vitamin A at delivery) | 11 trials: daily; 4 trials: weekly; 2 trials: unclear | Duration: no description Start: between 12 to 39 weeks' gestation |
Not reported |
| Fortification | |||||||
|
Suchdev 2015 Multiple micronutrient powders for home (point‐of‐use) fortification of foods in pregnant women |
MNP for point‐of‐use fortification of semi‐solid foods containing at least 3 MMNs, with 1 of them being iron | Prevention | Pregnant women (any gestation and parity) Baseline anaemia status/prevalence: not reported, but women with severe anaemia were excluded Known MN deficiencies: not reported |
Trial 1: MNP (60 mg of elemental iron as ferrous fumarate, 400 μg of folic acid, 30 mg of vitamin C, and 5 mg of zinc) versus 60 mg elemental iron and 400 μg folic acid Trial 2: MNP (15 mg elemental iron, 400 μg folic acid and 5 additional micronutrients (zinc, iodine, vitamin E, vitamin C, vitamin B12) versus 1 tablet (15 mg elemental iron, 400 μg folic acid and the same 5 additional MNs) |
Trial 1: daily Trial 2: daily for 6 months then weekly |
Start: 14 to 22 weeks' gestation (trial 1), before 24 weeks' gestation (trial 2) Duration: until 32 weeks' gestation (trial 1), until 3 months postpartum (trial 2) |
Maternal adherence reported in 1 trial (MNP versus iron or folic acid supplement): RR 0.76, 95% CI 0.66 to 0.87 |
CI: confidence interval; EDTA: ethylenediaminetetraacetate; FCM: ferric carboxy maltose; Hb: haemoglobin; ID: iron deficiency; IDA: iron deficiency anaemia; IFA: iron or folic acid; IM: intramuscular; IPM: iron polymaltose; IV: intravenous; IS: iron sucrose; LMW: low molecular weight; LNS: lipid‐based nutrient supplementation; MN: micronutrient; MNP: micronutrient powder; NAID: non‐anaemic iron deficiency; RR: risk ratio; UNIMMAP: United Nations International Multiple Micronutrient Preparation; WHO: World Health Organization.
14. Characteristics of interventions: mixed populations.
| Review | Intervention | Prevention or treatment | Population (mean age, baseline anaemia status/prevalence, known micronutrient deficiencies) | Dose (mean range) or composition or form of application (including compound, formulation) | Frequency | Start of intervention or duration, or both | Adherence to intervention |
| Supplementation | |||||||
|
Arabi 2020 The effect of vitamin D supplementation on hemoglobin concentration: a systematic review and meta‐analysis |
Oral vitamin D supplements | Prevention and treatment | 14 trials: participants aged 17.5 to 68 years old (including RCTs with healthy adults, anaemic patients, chronic kidney disease patients, heart failure patients, hypertensive patients, critically ill patients and athletes) Baseline anaemia status: not reported Known MN deficiencies: not reported |
Vitamin D fortified food with cholecalciferol (4 trials), oral vitamin D (cholecalciferol) supplements (8 trials), supplemented with ergocalciferol (1 trial), with calcitriol (1 trial). The minimum vitamin D dosage was 20 IU and maximum was 500,000 IU. | Daily | Duration: 3 hours to 36 months | Not reported |
|
Basutkar 2019 Vitamin D supplementation in patients with iron deficiency anaemia: A systematic review and a meta‐analysis |
Vitamin D supplementation | Treatment | Patients with iron deficiency anemia (20 to 45 years) Known MN deficiencies: not reported | Vitamin D and calcium containing snack bar, 10 mcg and 25 mcg of Vitamin D, iron plus vitamin D supplementation | Daily | Duration: 12 (3 months) to 16 weeks | Not reported |
|
Casgrain 2012 Effect of iron intake on iron status: a systematic review and meta‐analysis of randomized controlled trials |
Oral iron, fortified food, or rich natural dietary sources | Prevention | Healthy adults (≥ 18 years) Baseline anaemia status/prevalence: anaemic and non‐anaemic Know micronutrient deficiencies: any baseline iron status (iron deficient in many trials) |
Iron supplementation: 5 mg to 240 mg as iron fumarate, ferrous sulphate (mainly), ferric polymaltose Fortification with iron: 1.42 mg to 27.9 mg (fortified wheat‐based snacks, rice, food bar, fish sauce) |
Daily or weekly | Duration: 3 to 24 weeks | Not reported |
|
Gera 2007a Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials |
Oral iron, parenteral route or as formula, milk, or cereals fortified with iron | Prevention | Children (0 to 19 years, no age group dominated, i.e. > 60%) Baseline anaemia status/prevalence: anaemic and non‐anaemic (mean baseline Hb < 11 g/dL in 37 analytic components, Hb ≥ 11 in 54 analytic components) Know MN deficiencies: iron deficiency |
Iron: 5 mg to 120 mg/day or 1 mg/kg/day to 4 mg/kg/day (compound not reported) | Daily 2 trials: weekly |
Duration: 1 week to 12 months | Most of the included trials do not provide relevant compliance data. |
|
Gera 2009 Effect of combining multiple micronutrients with iron supplementation on Hb response in children: systematic review of randomized controlled trials |
Oral iron in combination with 2 or more MNs | Prevention | Children (0 to 18 years, no age group dominated, i.e. > 60%) Baseline anaemia status/prevalence: anaemic and non‐anaemic (mean baseline Hb < 11 g/dL in 15 analytic components, Hb ≥ 11 in 18 analytic components) Know MN deficiencies: yes, but not specified |
Iron: 5 mg to 60 mg per day (compound not reported) | Daily to once a week | Duration: 3 weeks to 12 months | Not reported |
|
Silva Neto 2019 Effects of iron supplementation versus dietary iron on the nutritional iron status: Systematic review with meta‐analysis of randomized controlled trials |
Iron supplementation versus dietary intervention (fortification or dietary plan) | 3 trials: prevention, 6 trials: treatment, 3 trials: N/A | 6 trials infants and children (age = 0.25 years to 7.3 years, males and females) 5 trials: adults (mean age = 18.5 to 29 years, females) 1 trial: pregnant women (mean age = 25 years) Baseline anaemia status/prevalence: iron deficiency anaemia (6 trials = yes, 3 trials = no, 3 trials = no information) Known MN deficiencies: iron deficiency |
Dietary plan (4 trials) or fortified food (8 trials): iron dose = 7 mg to 35.4 mg Iron supplementation: 2.5 mg to 105 mg |
Daily (at least 5 times per week) | Not reported | Not reported |
|
Smelt 2018 The effect of vitamin B12 and folic acid supplementation on routine haematological parameters in older people: an individual participant data meta‐analysis |
Vitamin B12 or folic acid supplementation | Prevention | Older people (60.3 to 80 years) Baseline anaemia status/prevalence: number of individuals with anaemia was small Know MN deficiencies: vitamin B12 deficiency and folate deficiency in some trials |
Vitamin B12 (0.01 mg to 1 mg) or folic acid (0.8 mg to 5 mg) supplementation, including tablet, capsule and intramuscular | 6 trials: daily 1 trial: weekly |
Duration: 4 weeks to 3 years | Not reported |
|
Tay 2015 Systematic review and meta‐analysis: what is the evidence for oral iron supplementation in treating anaemia in elderly people? |
Oral iron | Prevention | Elderly people after hip or knee arthroplasty (mean age range = 70 to 83 years, men and women) Baseline anaemia status/prevalence: participants were anaemic after surgery, but none of the participants were anaemic on admission Known MN deficiencies: not reported |
Ferrous sulphate 200 mg | 2 trials: 3 times daily 1 trial: twice daily |
Duration: 4 weeks to 6 weeks Start of intervention for elderly people: after hip or knee arthroplasty |
1 trial reported poor compliance. |
|
Tolkien 2015 Ferrous sulfate supplementation causes significant gastrointestinal side‐effects in adults: a systematic review and meta‐analysis |
Oral ferrous sulphate | Prevention and treatment | Oral iron versus placebo (20 trials, 3168 participants): adults, including pregnant women (18 to 58.6 years), baseline Hb status in 12 trials = 10.4 g/dL to 15.25 g/dL (not reported for the remaining trials), 19 trials in healthy non‐anaemic individuals, 1 trial in anaemic participants Oral iron versus IV iron (23 trials, 3663 participants): adults, including pregnant women (15 to 66 years), baseline Hb status = 7.6 g/dL to 12.4 g/dL Known MN deficiencies: not reported |
Oral iron versus placebo: oral dose 20 mg/Fe/day to 222 mg/Fe/day Oral iron versus IV iron: oral dose 100 mg/Fe/day to 400 mg/Fe/day (ferrous sulphate) |
Daily | Duration oral iron versus placebo: 1 to 26 weeks Duration oral iron versus IV iron: 4 to 26 weeks |
Not reported |
| Fortification | |||||||
|
Das 2019b Food fortification with multiple micronutrients: impact on health outcomes in general population |
Multiple micronutrient (MMN) fortification (3 or more MNs) by any food vehicle | Prevention | 36 trials: children (29 trials: preschool and school‐aged children, 4 trials: infants, 3 trials: children aged 1 to 3 years) 3 trials: pregnant women 3 trials: adults 1 trial: elderly population over 70 years old mean age: not reported Baseline anaemia status: not reported Known micronutrient deficiencies: not reported |
MMN fortification (3 or more MNs) by any food vehicle: rice and flour (12 trials), dairy products (9 trials), non‐dairy beverages (13 trials), biscuits (6 trials), salt (2 trials) | Daily or weekly | Duration: 8 weeks to 1 year; 29 trials: less than 6 months, 14 trials: between 6 months and 1 year | Not reported |
|
Field 2020 Wheat flour fortification with iron for reducing anaemia and improving iron status in populations |
Fortification of wheat flour with iron alone or in combination with other micronutrients | Prevention | 9 trials: 6 trials included children aged 6 to 11 years, 1 trial included children aged 6 to 12 years, 1 trial included children aged 6 to 13 years, and 2 trials included children aged 6 to 15 years old. Another trial included children aged 9 months to 11 years, primary school children aged 6 to 11 years, and non‐pregnant women. 2 trials included adult women. One trial targeted adolescent girls aged 15.2 ± 2.4 years Baseline anaemia status: varied; low (< 20%) in 2 trials, moderate in 4 trials, high in 2 trials, 1 trial did not specify prevalence Known MN deficiencies: not reported |
Any form of wheat flour iron fortification independent of length of intervention, extraction rate of wheat flour, iron compounds used, preparation of the iron‐flour premix, and fortification levels achieved in the wheat flour or derivative foods Iron compounds: NaFeEDTA ferrous sulphate, elemental iron, ferrous fumarate Amount of elemental iron added to flour: 41 mg iron/kg to 60 mg iron/kg flour (3 trials), < 40 mg iron/kg flour (2 trials), > 60 mg iron/kg flour (2 trials), 80 mg/kg for electrolytic iron and reduced iron and 40 mg/kg for ferrous fumarate (1 trial), unknown (1 trial) |
Daily | 3 to 8 months (8 trials) 24 months (1 trial) |
Adherence was measured in some studies through 24‐hour recalls and in some cases weighing of food remains in the meals. |
|
Finkelstein 2019 Iron biofortification interventions to improve iron status and functional outcomes |
Iron‐biofortified staple crops | Prevention | 1 trial: male and female adolescents aged 12 to 16 years old 2 trials: adults females (18 to 45 years) Baseline anaemia status: 28% to 37% anaemic at baseline Known MN deficiencies: 34% to 86% to iron deficient at baseline |
Crop: rice, pearl millet, beans Iron content: 10 mg/kg to 86 mg/kg dry per crop, iron intake from staple 1.8 mg/d to 17.6 mg/d Percentage of total dietary iron: 18% to 90% |
Daily | Duration: 4 to 9 months | Not reported |
|
Garcia‐Casal 2018 Fortification of maize flour with iron for controlling anaemia and iron deficiency in populations |
Maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products | Prevention | General population older than 2 years of age without critical illness or severe comorbidities (children = 6 months to 14 years, adolescents = 10 to 19 years, women = 20 to 49 years) Baseline anaemia status/prevalence: < 20% in 3 trials, > 40% in 1 trial and not reported in 1 trial Know MN deficiencies: all trials conducted in settings with a high prevalence of MN deficiencies, especially iron |
3 trials: 2.8 mg to 5.6 mg elemental iron per 100 g maize flour 1 trial: 9.8 mg reduced iron per 100 g flour 1 trial: 42.4 mg ferrous fumarate per 100 g maize flour |
Not specified | Duration: 6 to 10 months | Not reported |
|
Gera 2012 Effect of iron‐fortified foods on hematologic and biological outcomes: systematic review of randomized controlled trials |
Iron food fortification or biofortification | Prevention | Apparently healthy (non‐diseased) individuals, families, or communities Baseline anaemic status/prevalence: Hb concentration ≤ 120 g/L in 49 of 80 (57%) analytic components Known MN deficiencies: iron deficiency (serum ferritin was ≤ 20 μg/L in 22 of 47 (47%)) |
Computed additional iron intake: ≤ 10 mg in 49 trials (63%) and > 10 mg in 29 trials (37%) Cereal‐based fortification (36 trials; 42%): salt (12 trials; 14%), sauces (fish and soy; 9 trials; 11%), and milk (9 trials; 11%) Ferrous sulphate (24 trials; 28%), NaFeEDTA (17 trials; 20%), electrolytic iron (11 trials; 13%), ferric pyrophosphate (7 trials; 8%), hydrogen‐reduced iron (3 trials; 3%), and heme (3 trials; 3%) or ferric orthophosphate (3 trials; 3%), ferrous fumarate (6 trials; 7%), amino acid chelates (2 trials; 2%), iron gluconate (1 trial; 1%), or ammonium citrate (1 trial; 1%) |
Daily in 50 analytic components, intermittent in 35 | Duration: up to 7 months in 44 trials (51%), 7 to 12 months in 30 trials (35%), and 12 months in 11 (13%) trials | Compliance directly observed: 21 analytic components versus 56 others |
|
Hess 2016 Micronutrient fortified condiments and noodles to reduce anemia in children and adults—a literature review and meta‐analysis |
MN (iron, vitamins, zinc, iodine, folate, calcium, phosphorus, magnesium, selenium) fortified condiments or noodles product | Prevention | Children and adults from 5 to 50 years Baseline anaemia status/prevalence: anaemia rate at baseline 46% Known MN deficiencies: not reported |
Salt: 1 mg to 2 mg iron/g salt; masala powder: 25 μg NaFeEDTA/g masala; soy sauce: 0.3 mg to 4 mg NaFeEDTA/mL soy sauce; noodles: 20.6 mg NaFeEDTA/100 g noodles; fish sauce: 1 mg Fe/mL fish sauce | Not specified | Duration: follow‐up mostly under 1 year (range = 2.4 months to 2 years) | Adherence to intervention was not reported in included trials |
|
Huo 2015 Effect of NaFeEDTA‐fortified soy sauce on anemia prevalence in China: a systematic review and meta‐analysis of randomized controlled trials |
NaFeEDTA‐fortified soy sauce (prevention) | Prevention | Any population in which anaemia is a public health problem (Chinese, aged 3 to 55 years, 3‐ to 6‐year‐old children, 9 trials focusing on teenagers, 3 trials on pregnant women, and 1 trial covered all groups of children > 3 years) Baseline anaemia status/prevalence: anaemic and non‐anaemic Known MN deficiencies: iron deficiency |
Iron in NaFeEDTA ranged from 2.3 to 20 mg/day/person, iron dosages were < 4 mg/day in 8 trials and ≥ 4 mg/day in 7 trials | Daily | Duration: 3 to 18 months | Not reported |
|
Peña‐Rosas 2019 Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition |
Rice fortified with iron alone or in combination with other MNs Rice fortified with vitamin A alone or in combination with other MNs | Treatment or prevention | Population older than 2 years of age, including pregnant women Baseline anaemia status: 5% to 62% in children, 21% in women, and 34% in teenagers Known MN deficiencies: not reported |
Rice fortified with elemental iron, vitamin A, zinc, folic acid, thiamin, riboflavin, niacin, pyridoxine, cobalamin. The amount of elemental iron per 100 g of rice ranged from 0.2 mg to 112.8 mg; vitamin A: 0.15 mg to 2.1 mg; zinc: 2 mg to 18 mg; ferrous sulphate: 18 mg/g | Daily | Duration: 2 weeks to 4 years | Not reported |
|
Ramírez‐Luzuriaga 2018 Impact of double‐fortified salt with iron and iodine on hemoglobin, anemia, and iron deficiency anemia: a systematic review and meta‐analysis |
DFS | Prevention | Any participants (subgroup analysis for children aged < 5 years, school‐aged children, non‐pregnant, non‐lactating women of childbearing age, men, pregnant women) Baseline anaemia status/prevalence: not reported Known MN deficiencies: not reported |
Most trials used concentrations of 1 mg to 3 mg elemental Fe/g salt The 3 main iron sources used for salt fortification were ferrous sulphate, ferrous fumarate and ferric pyrophosphate | Not specified | Duration: mostly > 6 months | Not reported |
|
Sadighi 2019 Systematic review and meta‐analysis of the effect of iron‐fortified flour on iron status of populations worldwide |
Fortification of flour (e.g. wheat,maize, or rice), either in a raw form or in a cooking process, with iron or with iron and other MNs | Prevention | 19 trials of infants/toddlers (4 to 36 months), 42 of children (3 to 19 years), 31 of women (15 to 49 years), and 2 of people of all ages Baseline anaemia status/prevalence: not reported Known MN deficiencies: not reported |
Fortification vehicles: 61 trials wheat flour, maize flour in 7 trials, wheat and maize flours in 7 trials, rice flour in 4 trials, wheat and corn flours in 4 trials, maize and soy flours in 2 trials, corn flour in 1 trial, maize, beans, bambara nuts, and groundnuts flours in 1 trial, rice and soybeans flours in 1 trial, rye flour in 1 trial, wheat and soybean flours in 1 trial, and unknown flour in 4 trials; iron alone added to flour: 31 trials, iron with other micronutrients: 63 trials | Not specified | Mean duration: 20.6 months (SD: 25.5, range: 2 to 144) | Not reported |
|
Tablante 2019 Fortification of wheat and maize flour with folic acid for population health outcomes |
Wheat flour fortified with folic acid plus other vitamins and minerals | Prevention | Male and female children (5 to 12 years) (cluster‐RCT reported relevant outcomes: Bangladesh has had more than a 75% decrease in the incidence of malaria cases between 2000 and 2014) | Wheat flour chapattis were fortified with 0.15 mg of folic acid per 100 g of flour (1.5 ppm), along with other MNs (cluster‐RCT reported relevant outcomes) | Daily | Duration: over a six‐month period | Not reported |
|
Yadav 2019 Meta‐analysis of efficacy of iron and iodine fortified salt in improving iron nutrition status |
DFS (iron and iodine) versus iodine only IS | Prevention | Children 1 to 5 years (1 study), school children 5 to 18 years (6 studies), non‐pregnant and non‐lactating females 15 to 45 years (1 study), healthy and nonpregnant females 18 to 55 years (1 study), male and female participants 10 to 65 years (1 study) Baseline anaemia status/prevalence: not reported Known MN deficiencies: not reported |
1 mg/g salt ferrous sulfate (3 trials), 2 mg/g to 3 mg/g salt ferric pyrophosphate (3 trials), 1 mg/g to 2 mg/g salt ferrous fumarate (4 trials) | Not specified | Duration: 6 to 18 months | Not reported |
| Improving dietary diversity and quality | |||||||
|
Geerligs 2003 Food prepared in iron cooking pots as an intervention for reducing iron deficiency anaemia in developing countries: a systematic review |
Consumption or use of food prepared in iron or aluminium pot | Prevention | People in developing countries (minimum age was set at 4 months) Baseline anaemia status/prevalence: high prevalence of anaemia Known MN deficiencies: high prevalence of iron deficiency |
Use of iron or aluminium pots | Daily | Duration: 5 to 12 months | Daily compliance reported in 3 trials Trial 1: initial 10 weeks of iron pot use 80% to 85%, subsequent 10 weeks 68% to 70% Trial 2: 2 of 22 people stopped using iron pots after 4 to 5 months Trial 3: iron pot use 34.7% after 3 weeks, and 31.1% after 20 weeks |
DFS: double‑fortified salt; IDA: iron deficiency anaemia; IS: iodine‐fortified salt; MN: micronutrient; MMNs: multiple micronutrients; NaFeEDTA: sodium iron ethylenediaminetetraacetate; SD: standard deviation.
Infants (aged 6 to 23 months)
Five reviews assessed supplementation interventions (Abdullah 2013; Das 2019a; Dekker 2010; Pasricha 2013; Petry 2016b). In Abdullah 2013, the intervention included iron supplementation with 3 mg/kg/day ferrous sulphate for three to four months. Das 2019a assessed the effects of daily small quantity lipid‐based nutrient supplementation (LNS) (110 to 120 kcal/day; 20 g dose) and medium quantity LNS (250 to 500 kcal/day; 45 g to 90 g dose) for 7 to 18 months. Pasricha 2013 also investigated the effects of supplementation with daily iron, with iron doses ranging from 12.5 mg to > 60 mg (ferrous sulphate in most trials), for 1 week to 14 months. Petry 2016b assessed daily iron supplements of not more than 15 mg iron/day. One review assessed daily zinc supplementation (typically 10 mg to 20 mg of zinc), with the duration of the intervention ranging from 4 to 15 months (Pasricha 2013).
Five reviews assessed fortification interventions (Dewey 2009; Eichler 2012; Matsuyama 2017; Salam 2013; Suchdev 2020). Suchdev 2020 included trials assessing the effect of daily micronutrient powders (MNPs) with 12.5 mg elemental iron for 2 to 12 months. Salam 2013 also investigated daily MNP interventions with 12.5 mg iron as ferrous fumarate (range 2.5 mg to 30 mg) for 2 to 24 months. In Dewey 2009, trials included daily home fortification of complementary foods with at least 12.5 mg iron in MNP (sprinkles), crushable tablets, and lipid‐based or soy‐based products for 6 weeks up to 20 months. Matsuyama 2017 included trials assessing the effect of fortified milk (most common with iron, vitamin C, zinc, fatty acids, vitamin D, probiotics or synbiotics) given for 4 to 12 months without specifying the iron dose and frequency of the interventions. Another review examined daily micronutrient‐fortified milk or cereal food with 1.8 mg/day to 27.5 mg/day iron, with a mean follow‐up period of 8.25 months (Eichler 2012).
One review included supplementation and fortification interventions and included daily micronutrient sprinkles with 12.5 mg iron, iron‐fortified milk with 5.28 mg to 5.8 mg ferrous gluconate, daily or weekly iron supplementation with 10 mg to 12.5 mg iron and food‐based strategies for an average duration of six months (Pratt 2015).
Two reviews focused on improving dietary diversity and quality (Kristjansson 2015; Shapiro 2019). Kristjansson 2015 assessed them through daily supplementary feeding (provision of energy and macronutrients), with or without added micronutrient, for 3 to 32 months. Shapiro 2019 evaluated the effects of consumption of animal‐source foods compared with no animal‐source foods, such as a plant‐source foods or no intervention.
Preschool and school‐aged children (aged 2 to 10 years)
Four reviews assessed supplementation interventions (De‐Regil 2011; Low 2013; Mayo‐Wilson 2014a; Thompson 2013). Three reviews assessed oral iron supplementation (De‐Regil 2011; Low 2013; Thompson 2013). De‐Regil 2011 included trials that used 7.5 mg to 200 mg elemental iron (ferrous sulphate in most trials) weekly, or two to three times per week, for a period of 6 weeks to 12 months. Low 2013 investigated the effects of daily 5 mg to 400 mg of elemental iron (ferrous sulphate in most trials) for one to 12 months. Thompson 2013 also assessed 5 mg to 50 mg of daily ferrous sulphate (in most trials) given at least five times per week for a period of 28 days to 15 months.
Four reviews assessed fortification interventions (Aaron 2015; Das 2013a; De‐Regil 2017; Eichler 2019). In Aaron 2015, trials investigated the effects of daily, non‐dairy, multiple‐micronutrient (MMN)‐fortified beverages for a duration of 8 weeks to 8.5 months. Das 2013a assessed food fortification with zinc, including 3.75 mg/oz of zinc oxide in cereals, 400 mg/loaf of zinc acetate in bread and 5 mg zinc in 100 mg porridge, for a duration of three to nine months. De‐Regil 2017 included trials assessing daily MNP for point‐of‐use fortification for 8 to 12 weeks. Eichler 2019 evaluated the effects of the centrally‐processed fortified dairy products and fortified cereals, using any fortification strategy.
Adolescent children (aged 11 to 18 years)
Four reviews examined supplementation interventions (Fernández‐Gaxiola 2019; Neuberger 2016; Salam 2016; Salam 2020). Fernández‐Gaxiola 2019 included trials assessing the effects of oral iron alone or with other vitamins and minerals, using mostly 10 mg to 120 mg of ferrous sulphate; the intervention period ranged between 3 months or less and up to 12 months. Neuberger 2016 evaluated the effects of oral iron supplementation with or without folic acid and oral iron supplementation with antimalarial prophylaxis. Salam 2016 investigated daily or weekly micronutrient supplementation, where most trials used iron or iron and folic acid alone or in combination with other micronutrients. That review also assessed the effect of micronutrient supplementation for adolescent pregnant women commencing between 20 and 27 weeks' gestation until delivery. Salam 2020 also investigated daily or weekly micronutrient supplementation alone, such as iron, calcium, folic acid, zinc, vitamin A, and vitamin D or in combination with other micronutrients.
Non‐pregnant women of reproductive age (aged 19 to 49 years)
Five reviews assessed supplementation interventions (Abe 2016; Houston 2018; Lassi 2020; Low 2016; Sultan 2019). In Abe 2016, included trials assessed the effect of daily, MMN supplementation with 18 mg to 45 mg iron for 6 to 15 weeks postpartum. Houston 2018 assessed daily iron therapy for 46 days, including oral supplementation with 16 mg/day to 200 mg/day iron, intravenous (IV) with 200 mg/day to 1000 mg/day iron, and intramuscular (IM) with 100 mg/day iron. Lassi 2020 evaluated the effects of daily and weekly iron folic acid supplementation. This review included a variety of studies which used different dosages of iron and folic acid supplementation. Low 2016 investigated daily oral iron supplementation alone or with folic acid or vitamin C, with 1 mg to 300 mg of elemental iron, where half of the trials used ferrous sulphate and the remaining trials a variety of different iron formulations. The interventions lasted between 1 and 24 weeks. Sultan 2019 investigated the effects of the iron IV formulation compared with oral iron supplementation.
Pregnant women of reproductive age (aged 15 to 49 years)
Twenty‐two reviews assessed supplementation interventions in pregnant women (Abu Hashim 2017; Bhutta 2012; Buppasiri 2015; Daru 2016; Das 2018; De‐Regil 2015; Govindappagari 2019; Haider 2011; Haider 2013; Imdad 2012; Keats 2019; Lassi 2013; McCauley 2015; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Qassim 2018; Qassim 2019; Radhika 2019; Reveiz 2011; Rumbold 2015; Shi 2015; Thorne‐Lyman 2012). Any form of iron supplementation or treatment was assessed in 11 reviews (Daru 2016; Govindappagari 2019; Haider 2013; Imdad 2012; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Qassim 2018; Qassim 2019; Radhika 2019; Reveiz 2011; Shi 2015). Daru 2016 studied iron treatment (oral, including fortified water, IV or IM) for pregnant women with ID anaemia using weekly 200 mg to 400 mg IV iron or 120 mg oral iron and 30 mg to 80 mg of oral iron for women with non‐anaemic ID on a weekly or daily basis until 28 weeks' gestation. Govindappagari 2019 included trials using IV iron (iron sucrose, ferric carboxymaltose, low molecular weight iron dextran) infused in split doses every other day, with a maximum daily dose of 200 mg. In Haider 2013, included trials assessed the effect of daily iron supplementation (10 mg to 240 mg) with or without folic acid, starting before 21 weeks' gestation up to 30 weeks' gestation. Imdad 2012 also looked at trials assessing iron supplementation with or without folic acid using 20 mg/day to 300 mg/day from no later than 28 weeks' gestation; however, the length of the interventions was not reported. Peña‐Rosas 2015b included trials using daily iron supplements, with doses ranging from 9 mg to 900 mg, and that commenced before 20 weeks' gestation until delivery or postpartum. Peña‐Rosas 2015a assessed intermittent iron supplementation, and iron doses in the included trials ranged from 80 to 300 mg elemental iron given once a week, starting before 20 weeks' gestation, and lasting for at least 10 weeks or until delivery. Qassim 2018 and Qassim 2019 included trials involving administration of daily IV iron (ferric carboxymaltose, iron polymaltose or iron sucrose). Radhika 2019 also focused on daily IV iron sucrose. Reveiz 2011 included trials assessing any iron intervention (oral, oral iron plus adjuncts, IM, IV, blood transfusion, recombinant erythropoietin) using different iron doses: daily or weekly oral iron 20 mg to 300 mg; IM iron dose depending on body weight and Hb deficit and injected on alternating day; IV maximum total dose of iron ranged from 200 mg to 500 mg injected every other day or twice weekly. The interventions started between 16 and 34 weeks' gestation and lasted for 4 to 16 weeks or up to delivery. Shi 2015 looked at trials comparing IV iron sucrose treatment every other day with 100 mg to 300 mg daily elemental iron as ferrous sulphate, iron polymaltose complex or ferrous fumarate for a duration of four to six weeks without specifying the starting time.
MMN supplementation was assessed in three reviews (Bhutta 2012; Haider 2011; Keats 2019). Trials included in Bhutta 2012 assessed the effect of MMN supplementation using the United Nations International Multiple Micronutrient Antenatal Preparation (UNIMMAP) formulation at least six days a week, starting at 28 weeks' gestation at the latest, and lasting until delivery. Das 2018 included trials assessed daily LNS with 118 kcal/day or 372 kcal/day. Haider 2011 investigated daily MMN supplementation interventions using UNIMMAP or comparable formulations, starting at any gestational week and for any length. Keats 2019 included trials on daily supplementation of MMN with iron and folic acid, starting from enrolment (any trimester) and lasting until delivery and up to 24 weeks after delivery. McCauley 2015 and Thorne‐Lyman 2012 investigated vitamin A supplementation during pregnancy. In McCauley 2015, trials assessed daily or weekly vitamin A, alone or in combination with other supplements, mainly as capsules of 5750 IU to 444,000 IU of vitamin A, starting from trial enrolment for a duration of 8 to 12 weeks up to 6 weeks postpartum. Thorne‐Lyman 2012 investigated daily or weekly supplementation with vitamin A (3333 IU/day to 10,000 IU/day) or carotenoids (or both) starting between 12 to 39 weeks' gestation. In De‐Regil 2015, the included trials assessed daily periconceptional folate or folic acid supplementation alone or in combination with other vitamins or minerals, using 0.4 mg to 4.0 mg folic acid, starting at preconception until 12 weeks' gestation. Lassi 2013 also focused on daily folic acid supplementation with 10 μg to 400 μg folic acid, with or without iron or other vitamins and minerals, starting from eight weeks' gestation or from at least 20 weeks' gestation and continuing throughout pregnancy. One review focused on calcium supplementation, with daily doses ranging from 300 mg to 2000 mg, using calcium carbonate, calcium gluconate or calcium lactate; the interventions started at around 20 weeks' gestation and lasted until delivery (Buppasiri 2015). Rumbold 2015 investigated daily vitamin C supplementation alone or in combination with other vitamins and minerals; the included trials commonly used 1000 mg/day (250 mg to 2000 mg) starting in the second trimester. Another review assessed the effect of bovine lactoferrin, 100 mg twice a day or 250 mg once a day, starting in the second or third trimester with intervention lengths of four to eight weeks (Abu Hashim 2017). Only one review focused on fortification and assessed daily MNP for point‐of‐use fortification of semi‐solid foods starting at 14 to 24 weeks' gestation until 32 weeks' gestation or three months postpartum (Suchdev 2015).
Mixed population (all ages)
Nine reviews assessed supplementation interventions (Arabi 2020; Basutkar 2019; Casgrain 2012; Gera 2007a; Gera 2009; Silva Neto 2019; Smelt 2018; Tay 2015; Tolkien 2015). Casgrain 2012 included trials assessing 3‐week to 24‐week long iron interventions in healthy adults, including daily or weekly iron supplementation using 5 mg to 240 mg iron, mainly as ferrous sulphate, iron fortification of rice, wheat‐based snacks or fish sauce with 1.42 mg to 27.9 mg iron, or rich natural dietary sources such as meat. Gera 2007a investigated iron interventions for children from birth to 19 years old, including oral iron supplementation, parenteral route or as formula, milk, or cereals fortified with 5 mg/day to 120 mg/day iron for a period of 1 week to 12 months. Gera 2009 focused on iron supplementation for children from birth to 18 years old, with 5 mg/day to 60 mg/day iron in combination with two or more MNs, for a duration of 3 weeks to 12 months. Another review assessed daily iron supplementation compared with dietary intervention (fortification or dietary plan) for infants, children, adults and pregnant women (Silva Neto 2019). In Tay 2015, the included trials assessed the effect of two to three times daily iron supplementation of 200 mg ferrous sulphate for elderly people after hip or knee arthroplasty for a duration of four to six weeks. Tolkien 2015 also investigated the effects of daily iron supplementation (20 mg/day to 222 mg/day ferrous sulphate) versus placebo or daily iron supplementation (100 mg/day to 400 mg/day ferrous sulphate) versus IV iron for adults; the interventions lasted for 1 week up to 26 weeks.
Twelve reviews assessed fortification interventions (Das 2019b; Field 2020; Finkelstein 2019; Garcia‐Casal 2018; Gera 2012; Hess 2016; Huo 2015; Peña‐Rosas 2019; Ramírez‐Luzuriaga 2018; Sadighi 2019; Tablante 2019; Yadav 2019). Garcia‐Casal 2018 assessed the effects of iron fortification of maize flour on anaemia and iron status in the general population using 2.8 mg to 5.6 mg elemental iron per 100 g flour, 9.8 mg reduced iron per 100 g flour, or 42.4 mg ferrous fumerate per 100 g flour for 6 to 10 months. Gera 2012 included trials looking at the effect of daily or intermittent iron fortification in individuals, families or communities using mainly less than 10 mg of ferrous sulphate for up to 12 months. Hess 2016 assessed micronutrient‐fortified condiments or noodle products using various iron doses in children and adults with a follow‐up period of 2.4 months to 2 years. In Huo 2015, the included trials assessed the effect of sodium iron ethylenediaminetetraacetate (NaFeEDTA)‐fortified soy sauce in any population in which anaemia was a public health problem, with iron doses in NaFeEDTA ranging from 2.3 mg/day/person to 20 mg/day/person for an intervention period of 3 to 18 months. Another review assessed double‐fortified salt with elemental iron doses of 1 mg to 3 mg using mostly ferrous sulphate, ferrous fumarate and ferric pyrophosphate, for up to six months in any participant (Ramírez‐Luzuriaga 2018). One review focused on improving dietary diversity and quality through increasing the daily consumption or use of food prepared in iron or aluminium pots with the intervention lasting 5 to 12 months (Geerligs 2003).
Methodological quality of included reviews
Quality of the evidence in included reviews
Below, we summarise the risk of bias assessment of the included trials and the GRADE assessment of the certainty of the evidence in the included reviews. Further details can be found in Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8.
Infants (aged 6 to 23 months)
Seven reviews used the Cochrane RoB 1 tool (Higgins 2011) to assess the methodological quality of the included trials (Abdullah 2013; Das 2019a; Eichler 2012; Kristjansson 2015; Matsuyama 2017; Pasricha 2013; Suchdev 2020). Dekker 2010 used the Jadad level‐of‐evidence score for RCTs (Jadad 1996), whereas Pratt 2015 used a Modified Critical Appraisal Skills Programme (CASP) tool (CASP 2013). In Petry 2016b, the methodological quality of the trials was based on the assessment of random sequence generation, adequacy of blinding of trial participants and personnel and completeness of outcomes assessment. In Salam 2013, each trial was assessed and graded according to the Child Health Epidemiology Reference Group's (CHERG) adaptation of the GRADE technique (Walker 2010). Dewey 2009 did not describe the tool used for quality assessment. Shapiro 2019 used the National Heart, Lung and Blood Institute (NHLBI) Quality Assessment of Controlled Intervention Studies for RCTs, and the NHLBI Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies (NHLBI). Four reviews performed GRADE assessment for one or more of our primary and secondary outcomes (Das 2019a; Petry 2016b; Salam 2013; Suchdev 2020). Nine reviews did not perform GRADE assessment (Abdullah 2013; Dekker 2010; Dewey 2009; Eichler 2012; Kristjansson 2015; Matsuyama 2017; Pasricha 2013; Pratt 2015; Shapiro 2019).
Preschool and school‐aged children (aged 2 to 10 years)
Six reviews used the Cochrane RoB 1 tool (Higgins 2011) to assess the methodological quality of the included trials (De‐Regil 2011; De‐Regil 2017; Eichler 2019; Low 2013; Mayo‐Wilson 2014a; Thompson 2013). Aaron 2015 assessed trial quality by publication bias, method of randomisation, type of blinding, the percentage of loss to follow‐up and subgroup analyses. Das 2013a assessed bias using the following domains: sequence allocation, allocation concealment, blinding, incomplete outcome data addressed and selective reporting. Six reviews performed GRADE assessment for one or more of our primary and secondary outcomes (Aaron 2015; De‐Regil 2011; De‐Regil 2017; Eichler 2019; Mayo‐Wilson 2014a; Thompson 2013). Two reviews did not perform GRADE assessment (Das 2013a; Low 2013).
Adolescent children (aged 11 to 18 years)
All four reviews used the Cochrane RoB 1 tool (Higgins 2011) to assess the methodological quality of the included trials (Fernández‐Gaxiola 2019; Neuberger 2016; Salam 2016; Salam 2020). Three reviews performed GRADE assessment for one or more of our primary and secondary outcomes (Fernández‐Gaxiola 2019; Salam 2016; Salam 2020). Neuberger 2016 did not perform GRADE assessment.
Non‐pregnant women of reproductive age (aged 19 to 49 years)
Four reviews used the Cochrane RoB 1 tool (Higgins 2011) to assess the methodological quality of the included trials (Abe 2016; Houston 2018; Low 2016; Sultan 2019). Lassi 2020 used the Cochrane RoB 1 tool (Higgins 2011) and EPOC criteria (EPOC 2019). Low 2016 and Lassi 2020 performed GRADE assessment for our primary and secondary outcomes. Abe 2016 intended to do GRADE assessment but was not able to due to the lack of outcomes. Houston 2018 and Sultan 2019 did not perform GRADE assessment.
Pregnant women of reproductive age (aged 15 to 49 years)
Nineteen reviews used the Cochrane RoB 1 tool (Higgins 2011) to assess the methodological quality of the included trials (Abu Hashim 2017; Bhutta 2012; Buppasiri 2015; Das 2018; De‐Regil 2015; Govindappagari 2019; Haider 2011; Keats 2019; Lassi 2013; McCauley 2015; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Qassim 2018; Qassim 2019; Radhika 2019; Reveiz 2011; Rumbold 2015; Shi 2015; Suchdev 2015). Daru 2016 used the Jadad method for quality assessment (Jadad 1996). Haider 2013 assessed trial quality using the following domains: randomisation technique, concealment of allocation, blinding and loss to follow‐up. Imdad 2012 only used the GRADE tool to assess trial limitations. Thorne‐Lyman 2012 assessed trial bias using a modified version of the CHERG's GRADE tool (Walker 2010). Twelve reviews performed GRADE assessment for one or more of our primary and secondary outcomes (Abu Hashim 2017; Bhutta 2012; Das 2018; Haider 2011; Imdad 2012; McCauley 2015; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Qassim 2019; Radhika 2019; Suchdev 2015; Thorne‐Lyman 2012). Eleven reviews did not perform GRADE assessment (Buppasiri 2015; Daru 2016; De‐Regil 2015; Govindappagari 2019; Haider 2013; Keats 2019; Lassi 2013; Qassim 2018; Reveiz 2011; Rumbold 2015; Shi 2015).
Mixed population (all ages)
Twelve reviews used the Cochrane RoB 1 tool (Higgins 2011) to assess the methodological quality of the included trials (Arabi 2020; Basutkar 2019; Field 2020; Finkelstein 2019; Gera 2012; Huo 2015; Silva Neto 2019; Smelt 2018; Tablante 2019; Tay 2015; Tolkien 2015; Yadav 2019), and three reviews (Garcia‐Casal 2018; Peña‐Rosas 2019; Sadighi 2019) used Cochrane EPOC's risk of bias tool (EPOC 2019). Das 2019b applied the Cochrane RoB 1 tool for RCTs (Higgins 2011) and Cochrane EPOC's risk of bias tool for non‐RCTs and CBA studies (EPOC 2019). Casgrain 2012 used a scale designed by the EURopean micronutrient RECommendations Aligned (EURRECA) Network of Excellence (on the basis of Cochrane methods) to assess trial quality (Higgins 2011). Gera 2007a and Gera 2009 assessed the methodological quality (A, B, C or D) using the following domains: randomisations, allocation concealment, follow‐up and blinding. In Geerligs 2003, the Delphi list was used as a mean for quality assessment (Verhagen 1998). Hess 2016 used the CRD Guidance for Undertaking Reviews in Health Care (CDR 2009) and assessed risk of bias using the following domains: adequate sequence generation, allocation concealment, blinding, incomplete outcome data addressed, and no selective reporting. Ramírez‐Luzuriaga 2018 used the Effective Public Health Practice Project (EPHPP) quality assessment tool (Jackson 2005). In this category, only six reviews performed GRADE assessment (Basutkar 2019; Das 2019b; Field 2020; Garcia‐Casal 2018; Peña‐Rosas 2019; Tablante 2019).
Quality of included reviews
We assessed the methodological quality of the reviews using AMSTAR (Shea 2007a; Shea 2007b; Shea 2009). Some reviews had specific methodological limitations that could create bias: conflict of interest disclosure not stated (56 reviews), publication bias not assessed (38 reviews), included and excluded trials not listed (35 reviews), and review protocol not provided (21 reviews).
We summarise the assessment of the methodological quality of the included reviews in Table 15; Table 16; Table 17; Table 18; Table 19 and Table 20.
15. AMSTAR ratings for each systematic review: infants (aged 6 to 23 months).
| Study and review title | 1.* | 2.* | 3.* | 4.* | 5.* | 6.* | 7.* | 8.* | 9.* | 10.* | 11.* | Total score (out of a maximum of 11) |
| Supplementation | ||||||||||||
|
Abdullah 2013 Efficacy of oral iron therapy in improving the developmental outcome of pre‐school children with non‐anaemic iron deficiency: a systematic review |
No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | 7 |
|
Das 2019a Preventive lipid‐based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
|
Dekker 2010 Zinc supplementation in children is not associated with decreases in hemoglobin concentrations |
No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | No | 6 |
|
Pasricha 2013 Effect of daily iron supplementation on health in children aged 4‐23 months: a systematic review and meta‐analysis of randomised controlled trials |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 |
|
Petry 2016b The effect of low dose iron and zinc intake on child micronutrient status and development during the first 1000 days of life: a systematic review and meta‐analysis |
No | Cannot answer | Yes | No | No | No | Yes | Yes | Yes | Yes | No | 5 |
|
Pratt 2015 A review of the strategies used to reduce the prevalence of iron deficiency and iron deficiency anaemia in infants aged 6‐36 months |
No | No | Yes | No | No | Yes | Yes | No | Yes | No | No | 4 |
| Fortification | ||||||||||||
|
Dewey 2009 Systematic review and meta‐analysis of home fortification of complementary foods |
No | Yes | No | No | No | Yes | Yes | No | Yes | Yes | No | 5 |
|
Eichler 2012 Effects of micronutrient fortified milk and cereal food for infants and children: a systematic review |
Yes | No | Yes | No | No | Yes | Yes | No | Yes | No | No | 5 |
|
Matsuyama 2017 Effect of fortified milk on growth and nutritional status in young children: a systematic review and meta‐analysis |
No | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | No | 6 |
|
Salam 2013 Effectiveness of micronutrient powders (MNP) in women and children |
No | Yes | Yes | Yes | No | Yes | Yes | No | No | No | No | 5 |
|
Suchdev 2020 Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 |
| Improving dietary diversity and quality | ||||||||||||
|
Kristjansson 2015 Food supplementation for improving the physical and psychosocial health of socio‐economically disadvantaged children aged three months to five years |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
|
Shapiro 2019 A systematic review investigating the relation between animal‐source food consumption and stunting in children aged 6‐60 months in low and middle‐income countries |
Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | No | 7 |
| AMSTAR: A Measurement Tool to Assess Reviews | ||||||||||||
*Criteria for AMSTAR:
1. A priori design provided 2. Duplicate study selection and data extraction 3. Comprehensive literature search performed 4. Status of publication used as an inclusion criterion 5. List of studies (included and excluded) provided 6. Characteristics of included studies provided 7. Quality of included studies assessed and documented 8. Quality of included studies used appropriately in formulating conclusions 9. Appropriate methods used to combine the findings of the studies 10. Likelihood of publication bias assessed 11. Conflict of interest stated
16. AMSTAR ratings for each systematic review: preschool and school‐aged children (aged 2 to 10 years).
| Study and review title | 1.* | 2.* | 3.* | 4.* | 5.* | 6.* | 7.* | 8.* | 9.* | 10.* | 11.* | Total score (out of a maximum of 11) |
| Supplementation | ||||||||||||
|
Low 2013 Effects of daily iron supplementation in primary‐school‐aged children: systematic review and meta‐analysis of randomized controlled trials |
Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | 8 |
|
De‐Regil 2011 Intermittent iron supplementation for improving nutrition and development in children under 12 years of age |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
|
Mayo‐Wilson 2014a Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 |
|
Thompson 2013 Effects of daily iron supplementation in 2‐ to 5‐year‐old children: systematic review and meta‐analysis |
Yes | Cannot answer | Yes | Cannot answer | No | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Fortification | ||||||||||||
|
Aaron 2015 Multiple‐micronutrient fortified non‐dairy beverage interventions reduce the risk of anemia and iron deficiency in school‐aged children in low‐middle income countries: a systematic review and meta‐analysis |
No | Yes | No | No | No | Yes | Yes | No | Yes | Yes | No | 5 |
|
Das 2013a Systematic review of zinc fortification trials |
No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | No | 6 |
|
De‐Regil 2017 Point‐of‐use fortification of foods with micronutrient powders containing iron in children of preschool and school age |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
|
Eichler 2019 Health effects of micronutrient fortified dairy products and cereal food for children and adolescents: a systematic review |
Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 8 |
| AMSTAR: A Measurement Tool to Assess Reviews | ||||||||||||
*Criteria for AMSTAR:
1. A priori design provided 2. Duplicate study selection and data extraction 3. Comprehensive literature search performed 4. Status of publication used as an inclusion criterion 5. List of studies (included and excluded) provided 6. Characteristics of included studies provided 7. Quality of included studies assessed and documented 8. Quality of included studies used appropriately in formulating conclusions 9. Appropriate methods used to combine the findings of the studies 10. Likelihood of publication bias assessed 11. Conflict of interest stated
17. AMSTAR ratings for each systematic review: adolescent children (aged 11 to 18 years).
| Study and review title | 1.* | 2.* | 3.* | 4.* | 5.* | 6.* | 7.* | 8.* | 9.* | 10.* | 11.* | Total score (out of a maximum of 11) |
| Supplementation | ||||||||||||
|
Fernández‐Gaxiola 2019 Intermittent iron supplementation for reducing anaemia and its associated impairments in adolescent and adult menstruating women |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
|
Neuberger 2016 Oral iron supplements for children in malaria‐endemic areas |
Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | 9 |
|
Salam 2016 Interventions to improve adolescent nutrition: a systematic review and meta‐analysis |
No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | No | 6 |
|
Salam 2020 Effects of preventive nutrition interventions among adolescents on health and nutritional status in low‐ and middle‐income countries |
Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | 8 |
| AMSTAR: A Measurement Tool to Assess Reviews | ||||||||||||
*Criteria for AMSTAR:
1. A priori design provided 2. Duplicate study selection and data extraction 3. Comprehensive literature search performed 4. Status of publication used as an inclusion criterion 5. List of studies (included and excluded) provided 6. Characteristics of included studies provided 7. Quality of included studies assessed and documented 8. Quality of included studies used appropriately in formulating conclusions 9. Appropriate methods used to combine the findings of the studies 10. Likelihood of publication bias assessed 11. Conflict of interest stated
18. AMSTAR ratings for each systematic review: non‐pregnant women of reproductive age (aged 19 to 49 years).
| Study and review title | 1.* | 2.* | 3.* | 4.* | 5.* | 6.* | 7.* | 8.* | 9.* | 10.* | 11.* | Total score (out of a maximum of 11) |
| Supplementation | ||||||||||||
|
Abe 2016 Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Not applicable | Yes | Yes | 9 |
|
Houston 2018 Efficacy of iron supplementation on fatigue and physical capacity in non‐anaemic iron‐deficient adults: a systematic review of randomised controlled trials |
Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | 8 |
|
Lassi 2020 Effects of preconception care and periconception interventions on maternal nutritional status and birth outcomes in low‐ and middle‐income countries: a systematic review |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
|
Low 2016 Daily iron supplementation for improving anaemia, iron status and health in menstruating women |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 |
|
Sultan 2019 Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta‐analysis |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| AMSTAR: A Measurement Tool to Assess Reviews | ||||||||||||
*Criteria for AMSTAR:
1. A priori design provided 2. Duplicate study selection and data extraction 3. Comprehensive literature search performed 4. Status of publication used as an inclusion criterion 5. List of studies (included and excluded) provided 6. Characteristics of included studies provided 7. Quality of included studies assessed and documented 8. Quality of included studies used appropriately in formulating conclusions 9. Appropriate methods used to combine the findings of the studies 10. Likelihood of publication bias assessed 11. Conflict of interest stated
19. AMSTAR ratings for each systematic review: pregnant women (aged 15 to 49 years).
| Study and review title | 1.* | 2.* | 3.* | 4.* | 5.* | 6.* | 7.* | 8.* | 9.* | 10.* | 11.* | Total score (out of a maximum of 11) |
| Supplementation | ||||||||||||
|
Abu Hashim 2017 Lactoferrin or ferrous salts for iron deficiency anemia in pregnancy: a meta‐analysis of randomized trials |
No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 8 |
|
Bhutta 2012 Is it time to replace iron folate supplements in pregnancy with multiple micronutrients? |
No | Yes | Yes | Yes | No | No | Yes | No | Yes | No | No | 5 |
|
Buppasiri 2015 Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
|
Daru 2016 Systematic review of randomized trials of the effect of iron supplementation on iron stores and oxygen carrying capacity in pregnancy |
Yes | Yes | Yes | Yes | No | Yes | Yes | No | Not applicable | No | No | 6 |
|
Das 2018 Lipid‐based nutrient supplements for maternal, birth, and infant developmental outcomes |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
|
De‐Regil 2015 Effects and safety of periconceptional oral folate supplementation for preventing birth defects |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | 9 |
|
Govindappagari 2019 Treatment of iron deficiency anemia in pregnancy with intravenous versus oral iron: systematic review and meta‐analysis |
Yes | Yes | Yes | No | No | Yes | Yes | Cannot answer | Yes | No | No | 6 |
|
Haider 2013 Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta‐analysis |
Yes | Yes | No | Cannot answer | Yes | Yes | Yes | No | Yes | Yes | No | 7 |
|
Haider 2011 Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes |
Yes | Cannot answer | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 8 |
|
Imdad 2012 Routine iron/folate supplementation during pregnancy: effect on maternal anaemia and birth outcomes |
No | Yes | Yes | No | Yes | Yes | Yes | Not applicable | Yes | No | No | 6 |
|
Keats 2019 Multiple‐micronutrient supplementation for women during pregnancy |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 |
|
Lassi 2013 Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
|
McCauley 2015 Vitamin A supplementation during pregnancy for maternal and newborn outcomes |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 |
|
Peña‐Rosas 2015b Daily oral iron supplementation during pregnancy |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 |
|
Peña‐Rosas 2015a Intermittent oral iron supplementation during pregnancy |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 |
|
Qassim 2018 Safety and efficacy of intravenous iron polymaltose, iron sucrose and ferric carboxymaltose in pregnancy: a systematic review |
No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | 6 |
|
Qassim 2019 Intravenous or oral iron for treating iron deficiency anaemia during pregnancy: systematic review and meta‐analysis |
Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | 8 |
|
Radhika 2019 Parenteral versus oral iron for treatment of iIron deficiency anaemia during pregnancy and post‐partum: a systematic review |
No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 8 |
|
Reveiz 2011 Treatments for iron‐deficiency anaemia in pregnancy |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not applicable | Yes | 10 |
|
Rumbold 2015 Vitamin C supplementation in pregnancy |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 |
|
Shi 2015 Intravenous iron sucrose versus oral iron in the treatment of pregnancy with iron deficiency anaemia: a systematic review |
No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 8 |
|
Thorne‐Lyman 2012 Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta‐analysis |
No | No | No | No | No | Yes | Yes | No | Yes | No | Yes | 4 |
| Fortification | ||||||||||||
|
Suchdev 2015 Multiple micronutrient powders for home (point‐of‐use) fortification of foods in pregnant women |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not applicable | No | 9 |
| AMSTAR: A Measurement Tool to Assess Reviews | ||||||||||||
*Criteria for AMSTAR:
1. A priori design provided 2. Duplicate study selection and data extraction 3. Comprehensive literature search performed 4. Status of publication used as an inclusion criterion 5. List of studies (included and excluded) provided 6. Characteristics of included studies provided 7. Quality of included studies assessed and documented 8. Quality of included studies used appropriately in formulating conclusions 9. Appropriate methods used to combine the findings of the studies 10. Likelihood of publication bias assessed 11. Conflict of interest stated
20. AMSTAR ratings for each systematic review: mixed populations.
| Study and review title | 1.* | 2.* | 3.* | 4.* | 5.* | 6.* | 7.* | 8.* | 9.* | 10.* | 11.* | Total score (out of a maximum of 11) |
| Supplementation | ||||||||||||
|
Arabi 2020 The effect of vitamin D supplementation on hemoglobin concentration: a systematic review and meta‐analysis |
No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | 8 |
|
Basutkar 2019 Vitamin D supplementation in patients with iron deficiency anaemia: a systematic review and a meta‐analysis |
Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
|
Casgrain 2012 Effect of iron intake on iron status: a systematic review and meta‐analysis of randomized controlled trials |
No | Yes | Yes | No | No | Yes | Yes | No | Yes | No | Yes | 6 |
|
Gera 2009 Effect of combining multiple micronutrients with iron supplementation on Hb response in children: systematic review of randomized controlled trials |
Yes | Cannot answer | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | 7 |
|
Gera 2007a Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials |
Yes | No | Cannot answer | Yes | No | Yes | Yes | No | Yes | Yes | No | 6 |
|
Silva Neto 2019 Effects of iron supplementation versus dietary iron on the nutritional iron status: systematic review with meta‐analysis of randomized controlled trials |
Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | No | 7 |
|
Smelt 2018 The effect of vitamin B12 and folic acid supplementation on routine haematological parameters in older people: an individual participant data meta‐analysis |
No | Cannot answer | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | 6 |
|
Tay 2015 Systematic review and meta‐analysis: what is the evidence for oral iron supplementation in treating anaemia in elderly people? |
No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 9 |
|
Tolkien 2015 Ferrous sulfate supplementation causes significant gastrointestinal side‐effects in adults: a systematic review and meta‐analysis |
No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 8 |
| Fortification | ||||||||||||
|
Das 2019b Food fortification with multiple micronutrients: impact on health outcomes in general population |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
|
Field 2020 Wheat flour fortification with iron for reducing anaemia and improving iron status in populations |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
|
Finkelstein 2019 Iron biofortification interventions to improve iron status and functional outcomes |
Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | No | 7 |
|
Garcia‐Casal 2018 Fortification of maize flour with iron for controlling anaemia and iron deficiency in populations |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
|
Gera 2012 Effect of iron‐fortified foods on hematologic and biological outcomes: systematic review of randomized controlled trials |
Yes | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | No | 7 |
|
Hess 2016 Micronutrient fortified condiments and noodles to reduce anemia in children and adults — a Literature review and meta‐analysis |
Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | 8 |
|
Huo 2015 Effect of NaFeEDTA‐fortified soy sauce on anemia prevalence in China: a systematic review and meta‐analysis of randomized controlled trials |
No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Cannot answer | No | 6 |
|
Peña‐Rosas 2019 Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 |
|
Ramírez‐Luzuriaga 2018 Impact of double‐fortified salt with iron and iodine on hemoglobin, anemia, and iron deficiency anemia: a systematic review and meta‐analysis |
No | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | No | 6 |
|
Sadighi 2019 Systematic review and meta‐analysis of the effect of iron‐fortified flour on iron status of populations worldwide |
No | No | Yes | No | No | Yes | Yes | No | Yes | Yes | No | 5 |
|
Tablante 2019 Fortification of wheat and maize flour with folic acid for population health outcomes |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
|
Yadav 2019 Meta‐analysis of efficacy of iron and iodine fortified salt in improving iron nutrition status |
No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | 7 |
| Improving dietary diversity and quality | ||||||||||||
|
Geerligs 2003 Food prepared in iron cooking pots as an intervention for reducing iron deficiency anaemia in developing countries: a systematic review |
No | Cannot answer | Yes | Yes | No | Yes | Yes | Yes | Not applicable | Not applicable | No | 5 |
| AMSTAR: A Measurement Tool to Assess Reviews | ||||||||||||
*Criteria for AMSTAR:
1. A priori design provided 2. Duplicate study selection and data extraction 3. Comprehensive literature search performed 4. Status of publication used as an inclusion criterion 5. List of studies (included and excluded) provided 6. Characteristics of included studies provided 7. Quality of included studies assessed and documented 8. Quality of included studies used appropriately in formulating conclusions 9. Appropriate methods used to combine the findings of the studies 10. Likelihood of publication bias assessed 11. Conflict of interest stated
Effect of interventions
Infants (aged 6 to 23 months) (13 reviews)
Thirteen systematic reviews assessed interventions to prevent or control anaemia in infants aged 6 months to 23 months (Abdullah 2013; Das 2019a; Dekker 2010; Dewey 2009; Eichler 2012; Kristjansson 2015; Matsuyama 2017; Pasricha 2013; Petry 2016b; Pratt 2015; Salam 2013; Shapiro 2019; Suchdev 2020). GRADE assessments were provided in four out of 13 reviews. The effects of the interventions are summarised in Table 21.
21. Results of included systematic reviews: infants (aged 6 to 23 months).
| Review | Comparison | Outcome | Number of studies; number of participants | Results | GRADE assessment |
| Supplementation | |||||
|
Abdullah 2013 Efficacy of oral iron therapy in improving the developmental outcome of pre‐school children with non‐anaemic iron deficiency: a systematic review |
Iron supplementation versus no treatment or placebo | Post‐treatment Hb level (g/L) | 2 trials; 68 children | Trial results not combined: MD 11.5, 95% CI 5.1 to 17.9 (P < 0.01); 1 trial, 28 children MD 2.7, 95% CI −1.7 to 7.1; 1 trial, 40 children |
Not assessed |
|
Das 2019a Preventive lipid‐based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes |
LNS plus complementary feeding compared with no intervention | Anaemia (Hb < 10 g/dL) | 5 trials: 2332 children | RR 0.79, 95% CI 0.69 to 0.90; significant reduction in anaemia for children receiving LNS plus complementary feeding compared with no intervention | Low |
| Adverse effects Defined as deaths, hospitalisations, congenital abnormalities and life‐threatening conditions requiring an immediate hospital visit | 3 trials: 3382 children | RR 0.86, 95% CI 0.74 to 1.01; no evidence of a difference | Moderate | ||
| LNS plus complementary feeding compared with MNP | Anaemia (Hb < 10 g/dL) | 2 trials: 557 children | RR 0.38, 95% CI 0.21 to 0.68; significant reduction in anaemia for children receiving LNS plus complementary feeding | Low | |
|
Dekker 2010 Zinc supplementation in children is not associated with decreases in hemoglobin concentrations |
Zinc supplementation versus placebo or control | Hb (g/L) | 21 trials; 3869 children | WMD 0.79, 95% CI −0.62 to 2.21; no evidence of a difference | Not assessed |
|
Pasricha 2013 Effect of daily iron supplementation on health in children aged 4‐23 months: a systematic review and meta‐analysis of randomised controlled trials |
Daily oral iron supplements versus control | Hb (g/L) | 26 trials; 5479 children | MD 7.22, 95% CI 4.87 to 9.57 (P < 0.001); significant increase in Hb concentration for children receiving daily iron | Not assessed |
| Anaemia | 17 trials; 4825 children | RR 0.61, 95% CI 0.50 to 0.74 (P < 0.001); significant reduction in anaemia for children receiving daily iron | Not assessed | ||
| IDA | 6 trials; 2145 children | RR 0.14, 95% CI 0.10 to 0.22 (P < 0.001); significant reduction in IDA for children receiving daily iron | Not assessed | ||
| ID | 9 trials; 2464 children | RR 0.30, 95% CI 0.15 to 0.60 (P = 0.001); significant reduction in ID for children receiving daily iron | Not assessed | ||
| Adverse effect: 'any side effect' | 3 trials; 912 children | RR 1.10, 95% CI 0.98 to 1.25; no evidence of a difference | Not assessed | ||
| Adverse effect: 'vomiting' | 3 trials; 1020 children | RR 1.38, 95% CI 1.10 to 1.73 (P = 0.006); significant increase in vomiting for children receiving daily iron | Not assessed | ||
| Adverse effect: 'diarrhoea (prevalence)' | 6 trials; 1697 children | RR 1.03, 95% CI 0.86 to 1.23; no evidence of a difference | Not assessed | ||
| Adverse effect: 'diarrhoea (incidence)' | 5 trials; number of participants: not reported | RR 0.98, 95% CI 0.88 to 1.09; no evidence of a difference | Not assessed | ||
| Adverse effect: 'constipation' | 2 trials; 570 children | RR 0.54, 95% CI 0.05 to 5.83; no evidence of a difference | Not assessed | ||
|
Petry 2016b The effect of low dose iron and zinc intake on child micronutrient status and development during the first 1000 days of life: a systematic review and meta‐analysis |
Children 6 months to 23 months: daily iron administration (≤ 15 mg/day) versus control | Hb (g/L) | 30 trials; 6569 children | MD 4.10,95% CI 2.80 to 5.30 (P < 0.001); significant increase in Hb concentration for children receiving daily iron intervention | Moderate |
| Anaemia | 22 trials; 5647 children | RR 0.59, 95% CI 0.49 to 0.70 (P < 0.001); significant decrease in anaemia for children receiving daily iron intervention | Low | ||
| IDA | 8 trials; 3464 children | RR 0.20, 95% CI 0.11 to 0.37 (P < 0.001); significant decrease in IDA for children receiving daily iron intervention | High | ||
| ID | 13 trials; 3698 children | RR 0.22, 95% CI 0.14 to 0.35 (P < 0.001); significant decrease in ID for children receiving daily iron intervention | High | ||
| Diarrhoea | 8 trials; number of participants: not reported | No beneficial effect of iron on diarrhoea | Not assessed | ||
|
Pratt 2015 A review of the strategies used to reduce the prevalence of iron deficiency and iron deficiency anaemia in infants aged 6‐36 months |
Iron supplementation versus control | Hb (g/L) | 1 trial; 391 children | A statistically significant difference in mean Hb levels for children receiving daily 12.5 mg iron (P = 0.046), but not for the group receiving weekly supplements | Not assessed |
| Anaemia prevalence | 2 trials; 675 children | Trial 1: at 9 months, 21% of infants were anaemic, but no differences between groups for occurrence of anaemia Trial 2: dose–response effect in the group given daily, but not weekly supplements |
Not assessed | ||
| ID | 1 trial; 284 children | At 9 months, 81% of infants had ID, but no differences between groups for occurrence of ID | Not assessed | ||
| Iron‐fortified milk versus control | Hb (g/L) | 1 trial; 115 children | Hb was positively associated with treatment (P < 0.001) | Not assessed | |
| Anaemia prevalence | 2 trials; 910 children | Trial 1: decline from 41.4% to 12.1% in intervention group and no decline in control group Trial 2: decline in intervention group from 44.5% to 12.7% to 4.0%, and in control group from 42.6% to 19.7% to 9.4%, from baseline, to 6 and to 12 months |
Not assessed | ||
| Micronutrient sprinkles versus control | Hb (g/L) | 2 trials; 3633 children | Trial 1: + 6.1 g/L in intervention group compared with + 2.2 g/L in control group, from baseline to 12 and to 18 months, P < 0.001 Trial 2: + 7 g/L in intervention group compared with + 2 g/L in control group, from baseline to 2 months, P < 0.001 |
Not assessed | |
| Anaemia prevalence | 2 trials; 3633 children | Trial 1: reduction of 20.6% in the intervention group (reduction of moderate anaemia by 27.1%), from baseline to 6 months, P < 0.001 Trial 2: reduction from 72% to 52% in the intervention group, increase from 72% to 75% in the control group, from baseline to 2 months, P < 0.001 |
Not assessed | ||
| Food‐based strategies: red meat, fortified cow's milk versus control |
Hb (g/L) | 1 trial; 225 children | No evidence of intervention effects on haemoglobin | Not assessed | |
| Efficacy of different strategies: iron supplement, iron and folic acid supplement, multiple micronutrient supplements, fortified complementary food or fortified water |
Hb (g/L) | 1 trial; 2666 children | All treatments: significant increase in Hb | Not assessed | |
| Anaemia prevalence | 1 trial; 2666 children | Anaemia prevalence significantly more reduced in multiple micronutrient supplement (72%) and iron and folic acid supplementation (69%) groups than fortified complementary food (45%) group | Not assessed | ||
| Fortification | |||||
|
Dewey 2009 Systematic review and meta‐analysis of home fortification of complementary foods |
Home fortification treatment versus iron drops (treatment) | Hb (g/L) | 3 trials; 1263 children | MD −0.91, 95% CI −11.96 to 10.14; no evidence of a difference | Not assessed |
| Anaemia | 3 trials; 1263 children | RR 1.04, 95% CI 0.76 to 1.41; no evidence of a difference | Not assessed | ||
| Diarrhoea | 2 trials; 808 children | SMD −0.34, 95% CI −0.71 to 0.03; no evidence of a difference | Not assessed | ||
| Home fortification versus no intervention or placebo (prevention) | Hb (g/L) | 8 trials; 2649 children | MD 5.06, 95% CI 2.29 to 7.83; significant increase in Hb concentration for children receiving home fortification | Not assessed | |
| Anaemia | 8 trials; 4331 children | RR 0.54, 95% CI 0.46 to 0.64; significant reduction in anaemia for children receiving home fortification | Not assessed | ||
| ID | 3 trials; 1210 children | RR 0.44, 95% CI 0.22 to 0.86; significant reduction in ID for children receiving home fortification | Not assessed | ||
| Diarrhoea | 5 trials; 1195 children | RR 1.07, 95% CI 0.78 to 1.47; no evidence of a difference | Not assessed | ||
|
Eichler 2012 Effects of micronutrient fortified milk and cereal food for infants and children: a systematic review |
Iron fortification of milk and cereals versus non‐fortified food | Hb (g/L) | 13 trials; 2274 children | MD 6.20, 95% CI 3.40 to 8.90; significant increase in Hb concentration for children receiving iron‐fortified milk and cereals | Not assessed |
| Anaemia | 11 trials; 3100 children | RR 0.50, 95% CI 0.33 to 0.75; significant reduction in anaemia for children receiving iron‐fortified milk and cereals | Not assessed | ||
|
Matsuyama 2017 Effect of fortified milk on growth and nutritional status in young children: a systematic review and meta‐analysis |
Fortified milk versus control milk | Hb (g/L) | 9 trials; number of participants: not reported | MD 5.89, 95% CI −0.24 to 12.02; no evidence of a difference | Not assessed |
| Anaemia | 9 trials; number of participants: not reported | OR 0.32, 95% CI 0.15 to 0.66 (P = 0.000); significant reduction in anaemia for children receiving fortified milk | Not assessed | ||
|
Salam 2013 Effectiveness of micronutrient powders (MNP) in women and children |
MNP versus control or no intervention | Hb (g/L) | 14 trials; 9132 children | SMD 0.98, 95% 0.55 to 1.40 (P < 0.001); significant improvement in Hb for children receiving MNP | Moderate |
| Anaemia | 11 trials; 2524 children | RR 0.66, 95% CI 0.57 to 0.77 (P < 0.001); significant reduction in anaemia for children receiving MNP | Moderate | ||
| IDA | 7 trials; 1390 children | RR 0.43, 95% CI 0.35 to 0.52, significant reduction in IDA for children receiving MNP | Moderate | ||
| Diarrhoea | 4 trials; 3371 children | RR 1.04, 95% CI 1.01 to 1.06 (P = 0.002); significant increase in diarrhoea for children receiving MNP | Moderate | ||
| Recurrent diarrhoea | 1 trial; number of participants: not reported | RR 2.86, 95% Cl 0.12 to 69.0; no evidence of a difference | Moderate | ||
|
Suchdev 2020 Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age |
Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo | Hb (g/L) | 20 trials; 1,050,947 children | MD 2.74, 95% CI 1.95 to 3.53 (P < 0.001); significant increase in Hb concentration for children receiving MNP | Low |
| Anaemia | 16 trials; 9927 children | RR 0.82, 95% CI 0.76 to 0.90 (P < 0.001); significant reduction in anaemia for children receiving MNP | Moderate | ||
| ID | 7 trials; 1634 children | RR 0.47, 95% CI 0.39 to 0.567 (P < 0.001); significant reduction in ID for children receiving MNP | High | ||
| Diarrhoea | 5 trial; 5579 children | OR 1.05, 95% CI 0.82 to 1.35; no evidence of a difference | Not assessed | ||
| Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement | Hb (g/L) | 2 trials; 278 children | MD −2.81, 95% CI −10.84 to 5.22; no evidence of a difference | Very low | |
| Anaemia | 1 trial; 145 children | RR 0.89, 95% CI 0.58 to 1.39; no evidence of a difference | Low | ||
| Diarrhoea | 1 trial; 262 children | RR 0.52, 95% CI 0.38 to 0.72 (P < 0.001); significant reduction in diarrhoea for children receiving MNP | Not assessed | ||
| Vomiting | 1 trial; 262 children | RR 0.58, 95% CI 0.35 to 0.95 (P = 0.029); significant reduction in vomiting for children receiving MNP | Not assessed | ||
| Staining of teeth | 2 trials; 395 children | RR 0.37, 95% CI 0.16 to 0.82 (P = 0.02); significant reduction in teeth staining for children receiving MNP | Not assessed | ||
| Stool discolouration | 2 trials; 395 children | RR 0.80, 95% CI 0.66 to 0.98 (P = 0.04); significant reduction in stool discolouration for children receiving MNP | Not assessed | ||
| Improving dietary diversity and quality | |||||
|
Kristjansson 2015 Food supplementation for improving the physical and psychosocial health of socio‐economically disadvantaged children aged three months to five years |
Supplementary feeding versus control | Change in Hb (g/L) | 5 trials; 300 children | SMD 0.49, 95% CI 0.07 to 0.91 (P = 0.002); significant increase in Hb concentration for children receiving supplementary feeding | Not assessed |
|
Shapiro 2019 A systematic review investigating the relation between animal‐source food consumption and stunting in children aged 6‐60 months in low and middle‐income countries |
Caterpillar cereal versus usual diet | Hb (g/dL) | 1 trial; 175 children | Mean (SD) caterpillar cereal: 10.7 (1.6), usual diet: 10.1 (1.8) (P < 0.05) | Not assessed |
| IDA prevalence | 1 trial; 175 children | Caterpillar cereal: 26%, usual diet control: 50% (P < 0.01) | Not assessed | ||
| Beef versus fortified rice‐soy cereal | Hb (g/dL) | 1 trial; 1602 children | No significant difference in Hb levels between intervention and control group | Not assessed | |
| Food fortified with fish powder versus food with or without vitamins and minerals | Hb (g/dL) | 1 trial; 190 children | No significant difference in Hb levels between intervention and control group | Not assessed | |
CI: confidence interval; Hb: haemoglobin; ID: iron deficiency; IDA: iron deficiency anaemia; LNS: lipid‐based nutrient supplements; MD: mean difference; MNP: micronutrient powders; OR: odds ratio; RR: risk ratio; SMD: standard mean difference; WMD: weighted mean difference.
Supplementation (6 reviews)
Six systematic reviews assessed supplementation interventions for infants aged 6 months to 23 months (Abdullah 2013; Das 2019a; Dekker 2010; Pasricha 2013; Petry 2016b; Pratt 2015). Four reviews addressed prevention (Das 2019a; Pasricha 2013; Petry 2016b; Pratt 2015), and two treatment (Abdullah 2013; Dekker 2010). See Table 21.
Iron supplementation
Four reviews assessed iron supplementation in infants aged 6 months to 23 months (Abdullah 2013; Pasricha 2013; Petry 2016b; Pratt 2015).
Prevention
Oral iron supplementation alone or with co‐intervention versus control or co‐intervention
One systematic review included 33 RCTs randomising 42,015 healthy infants aged 4 months to 23 months (Pasricha 2013). This review assessed the effect and safety of oral iron supplementation alone or in combination with co‐interventions versus control or co‐intervention alone (dose: 12.5 mg or less, 12.6 mg to 30 mg, 31 mg to 59 mg, > 60 mg; frequency: per day). The outcomes related to anaemia informed guidelines for anaemia control: Hb (g/L), anaemia, IDA, and safety. IDA was defined by trial investigators and ID diagnosed with an adverse effect (any side effects, vomiting, diarrhoea, constipation). Twenty‐six RCTs assessed the effects of this intervention on Hb level and reported iron supplementation increased Hb level (mean difference (MD) 7.22 g/L, 95% confidence interval (CI) 4.87 to 9.57; 5479 infants; GRADE: not assessed). Seventeen RCTs assessed the effects of iron supplementation on anaemia and reported that this intervention decreased the incidence of anaemia (risk ratio (RR) 0.61, 95% CI 0.50 to 0.74; 4825 infants; GRADE: not assessed). The intervention reduced the incidence of IDA (RR 0.14, 95% CI 0.10 to 0.22; 6 RCTs, 2145 infants; GRADE: not assessed) and ID (RR 0.30, 95% CI 0.15 to 0.60; 9 RCTs, 2464 infants; GRADE: not assessed). Regarding adverse effects, daily oral iron supplementation increased vomiting (RR 1.38, 95% CI 1.10 to 1.73; 3 RCTs, 1020 infants), but there was no clear evidence of an effect on other adverse effects: "any side‐effects" (RR 1.10, 95% CI 0.98 to 1.25; 3 RCTs, 912 infants), diarrhoea (prevalence) (RR 1.03, 95% CI 0.86 to 1.23; 6 RCTs, 1697 infants), diarrhoea (incidence) (RR 0.98, 95% CI 0.88 to 1.09; 5 trials), and constipation (RR 0.54, 95% CI 0.05 to 5.83; 2 trials, 570 infants).
Iron supplementation versus control or other intervention to increase Hb status and reduce the prevalence of ID and IDA
Eight RCTs and cluster‐RCTs comprising 8109 children aged 6 months to 36 months, more than 60% of whom were under the age of two years, were included in this review (Pratt 2015). This review assessed the effects on several strategies or methods used to reduce the prevalence of ID and IDA. ID was defined by trialists, based on biomarker of iron status, for example, ferritin < 12 μg/L for preschool‐aged children. The outcomes Hb, anaemia and ID were reported. Only one RCT reported that daily 12.5 mg iron supplementation resulted in higher Hb levels compared with control (P = 0.046, 391 infants). However, there was no clear evidence of difference for the group receiving iron supplements weekly. Two RCTs (675 children) reported on anaemia and in one RCT, 21% of infants were anaemic at nine months; however, there was no clear evidence of a difference in anaemia prevalence between the groups for the occurrence of anaemia. The second RCT reported a dose‐response effect in the group receiving daily supplements, but not in the group receiving weekly iron. At nine months, although 81% of infants had ID, there was no clear evidence of a difference between groups for the occurrence of ID (1 RCT, 284 infants). Comparing the efficiency of different strategies, Hb levels increased in all treatments, and anaemia prevalence reduced more in the MMN supplement group (72%) and iron and folic acid supplementation group (69%) than complementary fortified food (45%) (1 RCT, 2666 children).
Daily iron administration versus control
In total, 90 trials were included in one systematic review assessing the effect of iron and zinc supplementation (Petry 2016b). Thirty‐three RCTs, cluster‐RCTs, and quasi‐RCTs comprising 7772 infants assessed the effects of iron supplementation. Additionally, 47 RCTs, cluster‐RCTs, and quasi‐RCTs assessed the effects of zinc supplementation on children aged 6 months to 23 months. This review assessed the effects of iron supplementation on Hb, anaemia, IDA, ID, and safety (dose: ≤ 15 mg/day). IDA was defined as Hb < 105 g/L or < 110 g/L and serum ferritin < 10 μg/L or < 12 μg/L. ID was defined as serum ferritin < 10 μg/L or < 12 μg/L. Daily iron supplementation increased Hb concentrations for infants compared with control (MD 4.10 g/L, 95% CI 2.80 to 5.30; 30 trials, 6569 infants; moderate‐certainty evidence). This intervention decreased anaemia (RR 0.59, 95% CI 0.49 to 0.70; 22 trials, 5647 infants; low‐certainty evidence), IDA (RR 0.20, 95% CI 0.11 to 0.37; 8 RCTs, 3464 infants; high‐certainty evidence), and ID (RR 0.22, 95% CI 0.14 to 0.35; 13 trials, 3698 infants; high‐certainty evidence). There was no clear evidence of a difference in the incidence of diarrhoea between the intervention and control group (8 trials).
Treatment
Oral iron therapy versus placebo or no treatment
Two RCTs comprising 249 non‐anaemic, iron‐deficient infants and children aged 6 months to 30 months (mean age 17 months) were included in this systematic review (Abdullah 2013). This review assessed the effects of iron supplementation (dose: ≥ 2 mg elemental Fe/kg body weight; frequency: per day administered for ≥ 3 months) compared with placebo or no treatment for children's outcomes: mental and psychomotor development. Due to high heterogeneity between the two trials, post‐treatment results were reported separately. In one trial, iron supplementation increased Hb level (MD 11.50 g/L, 95% CI 5.10 to 17.90; 28 children; GRADE: not assessed). The second trial reported no clear evidence between intervention and control group (MD 2.70 g/L, 95% CI −1.70 to 7.10; 40 children; GRADE: not assessed).
Prevention
Seventeen trials comprising 23,200 children aged 6 months to 23 months were included in this review (Das 2019a). This review assessed the effects of LNS plus complementary feeding compared with no intervention, and LNS plus complementary feeding compared with micronutrient powders (MNPs).
LNS plus complementary feeding versus no intervention
LNS plus complementary feeding reduced the risk of anaemia by 21% (RR 0.79, 95% CI 0.69 to 0.90; 5 trials, 2332 children; low‐certainty evidence). There was no clear evidence of a difference in the adverse effects between the groups (RR 0.86, 95% CI 0.74 to 1.01; 3 trials, 3382 children; low‐certainty evidence).
LNS plus complementary feeding versus MNP
There was a reduction in the risk of anaemia for children receiving LNS plus complementary feeding compared with MNP (RR 0.38, 95% CI 0.21 to 0.68; 2 trials, 557 children; low‐certainty evidence).
Treatment
Zinc supplementation versus placebo
Twenty‐one RCTs randomising 3869 children aged 0 to 15 years were included in this review (Dekker 2010). Although the mean age at baseline was 32 months, the majority of the trials commenced between 6 months to 23 months. This review assessed the effect of zinc supplementation (dose: 10 mg or 20 mg zinc; frequency: per day) compared with no treatment or placebo. The intervention showed no clear evidence of a difference in Hb levels between intervention and control groups (weighted mean difference (WMD) 0.79 g/L, 95% CI −0.62 to 2.21; 21 trials, 3869 children; GRADE not assessed).
Fortification (6 reviews)
Six systematic reviews assessed fortification interventions for infants 6 months to 23 months (Dewey 2009; Eichler 2012; Matsuyama 2017; Pratt 2015; Salam 2013; Suchdev 2020).
Five reviews focused on prevention (Eichler 2012; Matsuyama 2017; Pratt 2015; Salam 2013; Suchdev 2020), and one on prevention and treatment (Dewey 2009). See Table 21.
Prevention
Two reviews assessed iron fortification in infants aged 6 months to 23 months (Eichler 2012; Pratt 2015).
Iron‐fortified milk versus control
Eight RCTs and cluster‐RCTs comprised 8109 children aged 6 months to 36 months and under two years, and of whom, over 60% were included in this review (Pratt 2015). This review assessed the effects of several strategies or methods used to reduce the prevalence of ID and IDA and the outcomes of Hb and anaemia prevalence were reported. ID was defined by trialists, based on biomarker of iron status, for example, ferritin < 12 μg/L for preschool‐aged children. Hb levels in infants receiving iron‐fortified milk versus control were positively associated with the treatment (P < 0.001, 1 trial, 115 children; GRADE: not assessed). Two RCTs reported on anaemia prevalence (2 trials, 910 children; GRADE: not assessed). One RCT reported a decline from 41.4% to 12.1% in the intervention group and no decline in the control group, and one RCT reported a decline in anaemia prevalence from baseline to 6 months and 12 months (intervention group: 44.5% to 12.7% to 4.0% and control group: 42.6% to 19.7% to 9.4%).
Iron‐fortified milk and cereals versus non‐fortified food
Eighteen RCTs and cluster‐RCTs comprising 5468 children aged six months to five years (mean age = 6 months to 23 months at inclusion) were included in this review (Eichler 2012). This review assessed the effects on micronutrient‐fortified milk or cereals on Hb and anaemia. Iron‐fortified milk or cereals increased Hb levels (MD 6.20 g/L, 95% CI 3.40 to 8.90; 13 trials, 2274 children; GRADE: not assessed) and reduced anaemia (RR 0.50, 95% CI 0.33 to 0.75; 11 trials, 3100 children; GRADE: not assessed) compared with placebo or no intervention.
Prevention
Three reviews assessed MNP interventions in infants 6 months to 23 months (Suchdev 2020; Pratt 2015; Salam 2013).
MNP, including at least iron, zinc and vitamin A versus placebo or no intervention, or iron supplementation
Eight RCTs comprising 3748 children aged 6 months to 23 months were included in this review (Suchdev 2020). This review assessed the effects and the safety of MNP on Hb, anaemia prevalence, and ID (ID was defined by trialists) and compared the intervention with placebo or no intervention, or iron supplementation. MNP, including at least iron, zinc and vitamin A increased Hb concentration (MD 5.87, 95% CI 3.25 to 8.49; 6 RCTs, 1447 children; moderate‐certainty evidence), reduced anaemia (RR 0.69, 95% CI 0.60 to 0.78; 6 trials, 1447 children; moderate‐certainty evidence), reduced ID (RR 0.49, 95% CI 0.35 to 0.67; 4 trials 586 children; high‐certainty evidence) compared with placebo or no intervention, but no clear evidence of a difference was seen in diarrhoea between groups (RR 1.33, 95% CI 1.00 to 1.78; 206 children).
There was no difference between the MNP intervention group and the iron supplementation group on Hb levels (MD −2.36 g/L, 95% CI −10.30 to 5.58; 2 trials, 278 children; low‐certainty evidence) and anaemia (RR 0.89, 95% CI 0.58 to 1.39; 1 trial, 145 children; low‐certainty evidence). This intervention reduced the incidence of diarrhoea (RR 0.52, 95% CI 0.38 to 0.72; 1 trial, 262 children), the incidence of vomiting (RR 0.58, 95% CI 0.35 to 0.95; 1 trial, 262 children), the incidence of staining of teeth (RR 0.37, 95% CI 0.16 to 0.82; 2 trials, 395 children), and the incidence of stool discolouration (RR 0.80, 95% CI 0.66 to 0.98; 2 trials, 395 children) compared with iron supplementation.
MNP versus no intervention or control
Seventeen RCTs comprising children aged 6 months to 11 years (majority conducted in infants 6 to 23 months) were included in this review (Salam 2013). This review assessed the effects and safety of MNP on Hb, anaemia prevalence, ID, and the incidence of diarrhoea. MNP versus control or no intervention increased Hb concentration (standardised mean difference (SMD) 0.98, 95% CI 0.55 to 1.40; 14 trials, 9132 children; moderate‐certainty evidence), reduced anaemia (RR 0.66, 95% CI 0.57 to 0.77; 11 trials, 2524 children; moderate‐certainty evidence), reduced IDA (RR 0.43, 95% CI 0.35 to 0.52; 7 trials, 1390 children; moderate‐certainty evidence), but increased the incidence of diarrhoea (RR 1.04, 95% CI 1.01 to 1.06; 4 trials, 3371 children; moderate‐certainty evidence). There was no clear evidence of a difference between groups for the outcome recurrent diarrhoea (RR 2.86, 95% CI 0.12 to 69.0, 1 trial; moderate‐certainty evidence).
Mironutrient sprinkles versus control
Two RCTs (3633 children) assessed the effects of micronutrient sprinkles versus control and reported results on Hb levels separately (Pratt 2015). One trial reported that Hb levels increased in the intervention group compared with the control group from baseline to 12 to 18 months (6.10 g/L intervention group versus 2.20 g/L control group, P < 0.001; 2 trials, 3633 children; GRADE: not assessed). The second trial reported that Hb levels increased in the intervention group compared with the control group from baseline to two months (7.00 g/L intervention group versus 2.00 g/L control group).
Prevention
Fortified milk versus control milk (cow's milk or non‐ or low‐fortified milk)
Fifteen RCTs including individual RCTs and cluster‐RCTs comprising children (mean age at baseline was 6 months to 22.4 months) were included in this review (Matsuyama 2017). This review assessed the effects of fortified milk (dose: not reported; frequency: not reported) on Hb and anaemia compared with cow's milk or non‐ or low‐fortified milk. There was no clear evidence of a difference between fortified milk and control milk on Hb level (MD 5.89, 95% CI −0.24 to 12.02; 9 trials, number of participants: not reported; GRADE assessment: not assessed). While fortified milk reduced anaemia (odds ratio (OR) 0.32, 95% CI 0.15 to 0.66; 9 trials, number of participants: not reported; GRADE assessment: not assessed).
Prevention and treatment
Home fortification of complementary foods versus iron drops or placebo or no intervention
Fourteen RCTs, cluster‐RCTs and two non‐RCTs comprising 6113 infants and children aged four months to 36 months were included in this review (Dewey 2009). This review assessed the effects and safety of home fortification on Hb, anaemia, ID, and diarrhoea. ID was defined as ferritin < 12 μg/L. The intervention was compared with iron drops or placebo or no intervention. There was no clear evidence of a difference between home fortification of complementary foods and iron drops on Hb levels (MD ‐0.91, 95% CI ‐11.96 to 10.14; 3 trials, 1263 children), anaemia (RR 1.04, 95% CI 0.76 to 1.41; 3 trials, 1263 children) and diarrhoea (SMD ‐0.34, 95% CI ‐0.71 to 0.03; 2 trials, 808 children).
Home fortification of complementary foods compared with placebo or no intervention increased Hb levels (MD 5.06 g/L, 95% CI 2.29 to 7.83; 8 RCTs, 2649 children), reduced anaemia (RR 0.54, 95% CI 0.46 to 0.64; 8 trials, 4331 children), reduced ID (RR 0.44, 95% CI 0.22 to 0.86; 3 trials, 1210 children), but resulted in no difference in the incidence of diarrhoea between intervention and control group (RR 1.07, 95% CI 0.78 to 1.47; 5 trials, 1195 children).
Improving dietary diversity and quality (3 reviews)
Three systematic reviews assessed preventive interventions to improve dietary diversity and quality for infants 6 months to 23 months (Kristjansson 2015; Pratt 2015; Shapiro 2019). See Table 21.
Prevention
Food‐based strategies: red meat, fortified cow's milk versus control
One RCT (225 children) assessed the effects of food‐based strategies: red meat and fortified cow's milk versus control and found no evidence of a difference in Hb levels between intervention and control group (Pratt 2015).
Caterpillar cereal versus usual diet
One RCT (175 children) assessed the effects of caterpillar cereal compared with usual diet. This review showed that caterpillar cereal increased Hb levels (mean (SD) caterpillar cereal: 10.70 g/dL (1.6), usual diet: 10.10 g/dL (1.8) (P < 0.05); GRADE: not assessed) and reduced IDA prevalence (caterpillar cereal: 26%, usual diet: 50% (P < 0.01); GRADE: not assessed) (Shapiro 2019).
Beef versus fortified rice‐soy cereal
One RCT (1602 children) evaluated the effects of beef compared with fortified rice‐soy cereal. This review showed no evidence of a difference in Hb levels (Shapiro 2019).
Food fortified with fish powder versus food with or without vitamins and minerals
One RCT (190 children) assessed the effects of food fortified with fish powder compared with food with or without vitamins and minerals. They found no evidence of a difference in HB levels (Shapiro 2019).
Prevention
Twenty‐one RCTs and 11 controlled before‐after (CBA) trials compromising children aged three months to five years (60% of the trials included children under 2 years of age) were included (Kristjansson 2015). This review assessed the effects of supplementary feeding alone or in combination with added micronutrient on Hb concentration and anaemia. Supplementary feeding was associated with an increase in Hb levels (change in Hb levels: SMD 0.49, 95% CI 0.07 to 0.91; 5 trials, 300 children; GRADE: not assessed). The review included no results for anaemia.
Preschool and school‐aged children (aged 2 to 10 years) (8 reviews)
Eight systematic reviews assessed interventions to prevent or control anaemia in preschool and school‐aged children aged 2 to 10 years (Aaron 2015; Das 2013a; De‐Regil 2011; De‐Regil 2017; Eichler 2019; Low 2013; Mayo‐Wilson 2014a; Thompson 2013). GRADE assessments were provided in six out of eight reviews. The effects of interventions are summarised in Table 22.
22. Results of included systematic reviews: preschool and school‐aged children (aged 2 to 10 years).
| Review | Comparison | Outcome | Number of studies; number of participants | Results | GRADE assessment |
| Supplementation | |||||
|
Low 2013 Effects of daily iron supplementation in primary‐school‐aged children: systematic review and meta‐analysis of randomized controlled trials |
Iron supplementation versus placebo or control | Hb (g/L) | 28 trials; 6545 children | MD 8.38, 95% CI 6.21 to 10.56 (P < 0.001), significant increase in Hb concentration for children receiving iron supplementation | Not assessed |
| Anaemia | 7 trials; 1763 children | RR 0.50, 95% CI 0.39 to 0.64 (P < 0.001), significant reduction in anaemia for children receiving iron supplementation | Not assessed | ||
| IDA | 2 trials; 334 children | RR 0.12, 95% CI 0.02 to 0.66 (P = 0.01), significant reduction in IDA for children receiving iron supplementation | Not assessed | ||
| ID | 4 trials; 1020 children | RR 0.21, 95% CI 0.07 to 0.63 (P = 0.006), significant reduction in IDA for children receiving iron supplementation | Not assessed | ||
| Adverse event: 'gastrointestinal upset' | 4 trials; 576 children | RR 1.30, 95% CI 0.89 to 1.91, no evidence of a difference | Not assessed | ||
| Adverse event: 'constipation' | 2 trials; 202 children | RR 3.44, 95% CI 0.66 to 19.68, no evidence of a difference | Not assessed | ||
| Adverse event: 'vomiting' | 2 trials; 202 children | RR 0.86, 95% CI 0.13 to 5.67, no evidence of a difference | Not assessed | ||
|
De‐Regil 2011 Intermittent iron supplementation for improving nutrition and development in children under 12 years of age |
Intermittent supplementation with iron alone or with other nutrients versus placebo or no intervention | Hb (g/L) | 19 trials; 3032 children | MD 5.20, 95 % CI 2.51 to 7.88 (P < 0.001), significant increase in Hb concentration for children receiving intermittent iron supplementation versus placebo or no intervention | Low |
| Anaemia | 10 trials; 1824 children | RR 0.51, 95 % CI 0.37 to 0.72 (P < 0.001), significant reduction in anaemia for children receiving intermittent iron supplementation versus placebo or no intervention | Moderate | ||
| ID | 3 trials; 431 children | RR 0.24, 95 % CI 0.06 to 0.91 (P = 0.036), significant reduction in ID for children receiving intermittent iron supplementation versus placebo or no intervention | Very low | ||
| Any side effects | 1 trial; 53 children | RR 3.87, 95% CI 0.19 to 76.92, no evidence of a difference | Not assessed | ||
| Intermittent iron supplementation versus daily iron supplementation | Hb (g/L) | 19 trials; 2851 children | MD −0.60, 95% CI −1.54 to 0.35, no evidence of a difference | Low | |
| Anaemia | 6 trials; 980 children | RR 1.23, 95% CI 1.04 to 1.47 (P = 0.017), significant reduction in anaemia for children receiving intermittent iron supplementation versus daily iron | Low | ||
| ID | 1 trial; 76 children | RR 4.00, 95% CI 1.23 to 13.05, (P = 0.022), significant increase in ID for children receiving intermittent iron supplementation versus daily iron | Very low | ||
| Diarrhoea | 2 trials; 122 children | RR 1.17, 95% CI 0.60 to 2.28, no evidence of a difference | Not assessed | ||
| Any side effects | 4 trials; 895 children | RR 0.60, 95% CI 0.19 to 1.87, no evidence of a difference | Not assessed | ||
|
Mayo‐Wilson 2014a Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age |
Zinc versus no zinc | Blood Hb concentration | 26 trials; 6024 children | SMD 0.05, 95% CI ‐0.00 to 0.10, no evidence of a difference | Not assessed |
| Prevalence of anaemia | 13 trials; 4287 children | RR 1.00, 95% CI 0.95 to 1.06, no evidence of a difference | Not assessed | ||
| Prevalence of ID | 10 trials; 3149 children | RR 0.99, 95% CI 0.89 to 1.10, no evidence of a difference | Not assessed | ||
| Side effect: 'participants with ≥ 1 side effect' | 3 trials; 850 children | RR 1.13, 95% CI 1.00 to 1.27, no evidence of a difference | Not assessed | ||
| Side effect: 'vomiting episodes' | 5 trials; 4095 children | RR 1.68, 95% CI 1.61 to 1.75 (P < 0.001), significant increase in vomiting episodes for children receiving zinc supplementation | Not assessed | ||
| Side effect: 'participants with ≥ 1 vomiting episode' | 5 trials; 35192 children | RR 1.29, 95% CI 1.14 to 1.46 (P < 0.001), significant increase in ≥ 1 vomiting episode for children receiving zinc supplementation | High | ||
| Zinc versus zinc plus iron | Blood Hb concentration | 8 trials; 1341 children | SMD −0.23, 95% CI −0.34 to −0.12 (P < 0.001), difference favouring zinc plus iron | Not assessed | |
| Prevalence of anaemia | 3 trials; 482 children | RR 0.78, 95% CI 0.67 to 0.92 (P = 0.003), significant reduction anaemia prevalence for children receiving zinc plus iron | Not assessed | ||
| Prevalence of ID | 2 trials; 248 children | RR 0.12, 95% CI 0.04 to 0.32 (P < 0.001), reduction in ID prevalence for children receiving zinc plus iron | Not assessed | ||
|
Thompson 2013 Effects of daily iron supplementation in 2‐ to 5‐year‐old children: systematic review and meta‐analysis |
Iron supplementation versus control | Hb (g/L) | 9 trials; 1690 children | MD 6.97, 95% CI 4.21 to 9.72, significant increase in Hb concentration for children receiving iron supplementation | High |
| Anaemia | 1 trial; 359 children | 144/183 (79%) anaemic in iron group, 142/176 (81%) anaemic in control group; no evidence of a difference | Very low | ||
| Fortification | |||||
|
Aaron 2015 Multiple‐micronutrient fortified non‐dairy beverage interventions reduce the risk of anemia and iron deficiency in school‐aged children in low‐middle income countries: a systematic review and meta‐analysis |
Non‐dairy MMN‐fortified beverages versus control | Hb (g/L) | 8 trials; 3835 children | MD 2.76, 95% CI 1.19 to 4.33 (P = 0.004), significant increase in Hb concentration for children receiving MMN‐fortified beverages | Moderate |
| Anaemia | 6 trials; 2828 children | RR 0.63, 95% CI 0.54 to 0.73 (P < 0.001), significant reduction in anaemia for children receiving MMN‐fortified beverages | Moderate | ||
| ID | 7 trials; 2523 children | RR 0.32, 95% CI 0.23 to 0.45 (P < 0.001), significant reduction in ID for children receiving MMN‐fortified beverages | Moderate | ||
| IDA | 3 trials; 1649 children | RR 0.13, 95% CI 0.07 to 0.25 (P < 0.001), significant reduction in IDA for children receiving MMN‐fortified beverages | Low | ||
|
Das 2013a Systematic review of zinc fortification trials |
Zinc fortification versus control (with a regular diet or unfortified foods) | Serum Hb | 1 trial; 19 children | SMD 0.28, 95% CI −0.62 to 1.19, no evidence of a difference | Not assessed |
|
De‐Regil 2017 Point‐of‐use fortification of foods with micronutrient powders containing iron in children of preschool and school age |
Point‐of‐use fortification of foods with MNP versus no intervention or placebo | Hb (g/L) | 11 trials; 2746 children | MD 3.37, 95% CI 0.94 to 5.80 (P = 0.007), significant decrease in Hb concentration for children receiving MNP | Low |
| Anaemia | 10 trials; 2448 children | RR 0.66, 95% CI 0.49 to 0.88 (P = 0.005), significant decrease in anaemia for children receiving MNP | Moderate | ||
| IDA | 3 trials; 918 children | RR 0.28, 95% CI 0.07 to 1.10, no evidence of a difference | Not assessed | ||
| ID | 5 trials; 1364 children | RR 0.35, 95% CI 0.27 to 0.47, significant decrease in iron deficiency for children receiving MNP | Moderate | ||
| Adverse effects | 1 trial; 90 children | RR 1.09, 95% CI 0.16 to 7.42. no evidence of a difference | Moderate | ||
| Diarrhoea | 2 trials; 366 children | RR 0.97, 95% CI 0.53 to 1.78, no evidence of a difference | Low | ||
|
Eichler 2019 Health effects of micronutrient fortified dairy products and cereal food for children and adolescents: a systematic review |
Fortified dairy products and cereal food versus no fortification with MN | Hb g/L | 14 trials; 4855 children and adolescents | MD 0.90, 95% CI −0.10 to 1.80, no evidence of a difference | Very low |
| Anaemia | 12 trials; 1149 children | RR 0.87, 95% CI 0.76 to 1.01, no evidence of a difference | Very low | ||
| IDA | 5 trials; 148 children | RR 0.38, 95% CI 0.18 to 0.81, significant reduction in IDA for children receiving fortification | Very low | ||
| ID | 8 trials; 519 children | RR 0.62, 95% CI 0.40 to 0.97, significant reduction in ID for children receiving fortification | Very low | ||
| Adverse events | 3 trials | Three studies reported that no significant adverse events were related to the study food or to the fortification | Low | ||
CI: confidence interval; Hb: haemoglobin; ID: iron deficiency; IDA: iron deficiency anaemia; MD: mean difference; MMN: multiple‐micronutrient; MN: micronutrient; MNP: micronutrient powders; OR: odds ratio; RR: risk ratio; SMD: standardised mean difference.
Supplementation (4 reviews)
Four systematic reviews assessed supplementation interventions for preschool and school‐aged children aged 2 to 10 years (De‐Regil 2011; Low 2013; Mayo‐Wilson 2014a; Thompson 2013). See Table 22. One was a prevention (Mayo‐Wilson 2014a), and three were prevention or treatment reviews (De‐Regil 2011; Low 2013; Thompson 2013).
Prevention
Zinc versus no zinc or zinc plus iron
Eighty RCTs, cluster‐RCTs and cross‐over RCTs randomising over 205,401 children aged 6 months to 12 years of age were included in the Mayo‐Wilson 2014a systematic review, which assessed the effects of zinc supplementation (dose: < 5 mg to 20 mg daily; frequency: from daily to weekly) versus no zinc or zinc plus iron for preventing mortality and morbidity, and for promoting growth. They did not report the definition of ID. An increase in vomiting episodes (RR 1.68, 95% CI 1.61 to 1.75; 5 trials, 4095 children) and in ≥ 1 vomiting episode (RR 1.29, 95% CI 1.14 to 1.46; 5 trials, 35,192 children; high‐certainty evidence) was found for children receiving zinc compared to those not receiving zinc. There was no evidence of a difference in blood Hb concentration (SMD 0.05, 95% CI ‐0.00 to 0.10; 26 trials, 6024 children; GRADE: not assessed), prevalence of anaemia (RR 1.00, 95% CI 0.95 to 1.06; 13 trials, 4287 children; GRADE: not assessed), prevalence of ID (RR 0.99, 95% CI 0.89 to 1.10; 10 trials, 3149 children), and ≥ 1 side effect (RR 1.13, 95% CI 1.00 to 1.27; 3 trials, 850 children) between the intervention and control groups. There was an increase in blood Hb concentration (SMD −0.23, 95% CI −0.34 to −0.12; 8 trials, 1341 children; GRADE: not assessed) and a reduction in the prevalence of anaemia (RR 0.78, 95% CI 0.67 to 0.92; 3 trials, 482 children; GRADE: not assessed), and the prevalence of ID (RR 0.12, 95% CI 0.04 to 0.32; 2 trials, 248 children) for children receiving zinc plus iron compared to those receiving zinc alone.
Prevention or treatment
Three reviews assessed iron supplementation in preschool and school‐aged children aged 2 to 10 years (De‐Regil 2011; Low 2013; Thompson 2013).
Daily iron supplementation versus placebo or control
Thirty‐two RCTs and cluster‐RCTs randomising over 7089 primary school children aged 5 to 12 years were included in the Low 2013 systematic review. This review evaluated the effects of daily iron supplementation (dose: 5 mg/day to 400 mg/day or 3 mg/kg/day to 10 mg/kg/day; frequency: daily), a commonly used strategy to combat anaemia in primary school‐aged children. They did not report the definition of IDA and ID. Iron supplementation compared to placebo or control resulted in an increase in Hb concentration (MD 8.38 g/L, 95% CI 6.21 to 10.56; 28 trials, 6545 children; GRADE: not assessed) and a reduction in anaemia (RR 0.50, 95% CI 0.39 to 0.64; 7 trials, 1763 children; GRADE: not assessed), reduction in IDA (RR 0.12, 95% CI 0.02 to 0.66; 2 trials, 334 children; GRADE: not assessed), and reduction in ID (RR 0.21, 95% CI 0.07 to 0.63; 4 trials, 1020 children). There was no evidence of a difference in adverse events of gastrointestinal upset (RR 1.30, 95% CI 0.89 to 1.91; 4 trials, 576 children), constipation (RR 3.44, 95% CI 0.66 to 19.68; 2 trials, 202 children) and vomiting (RR 0.86, 95% CI 0.13 to 5.67; 2 trials, 202 children) for children receiving iron supplementation compared with those receiving placebo or control.
Intermittent iron supplementation
Thirty‐three RCTs, cluster‐RCTs and quasi‐RCTs comprising 13,114 children under 12 years of age were included in the systematic review by De‐Regil 2011. The review assessed the effects of intermittent iron supplementation (dose: 7.5 mg/week to 200 mg/week; frequency: twice, 3 times or once a week), alone or in combination with other vitamins and minerals, on nutritional and developmental outcomes in children from birth to 12 years of age compared with placebo, no intervention or daily supplementation. IDA was defined by the presence of anaemia and ID, diagnosed with an indicator of iron stats selected by trialists. Trialists measured ID by using indicators of iron status, such as ferritin or transferrin. An increase in Hb concentration was seen for children receiving intermittent supplementation with iron alone or with other nutrients compared to placebo or no intervention (MD 5.20 g/L, 95 % CI 2.51 to 7.88; 19 trials, 3032 children; low‐certainty evidence). There was a reduction in anaemia (RR 0.51, 95 % CI 0.37 to 0.72; 10 trials, 1824 children; moderate‐certainty evidence) and ID (RR 0.24, 95 % CI 0.06 to 0.91; 3 trials, 431 children; very low‐certainty evidence) for children receiving intermittent iron supplementation compared with those receiving placebo or no intervention. In one trial, there was no evidence of a difference in any side effects between the intervention and control groups (RR 3.87, 95% CI 0.19 to 76.92; 53 children). For children receiving intermittent iron supplementation compared with children receiving daily iron supplementation, no difference was seen in Hb concentration (MD −0.60 g/L, 95% CI −1.54 to 0.35; 19 trials, 2851 children; low‐certainty evidence), diarrhoea (RR 1.17, 95% CI 0.60 to 2.28; 2 trials, 122 children), and any side effects (RR 0.60, 95% CI 0.19 to 1.87; 4 trials, 895 children). However, there was an increase in anaemia (RR 1.23, 95% CI 1.04 to 1.47; 6 trials, 980 children; low‐certainty evidence) and ID (RR 4.00, 95% CI 1.23 to 13.05; 1 trial, 76 children; very low‐certainty evidence) for children receiving intermittent iron supplementation compared to those receiving daily iron supplementation.
Oral iron supplementation
Fifteen RCTs and cluster‐RCTs were included in the Thompson 2013 review, which summarised the evidence for effects of daily iron supplementation (dose: 5 mg/day to 50 mg/day; frequency: at least 5 days per week) administered to children aged two to five years. In this systematic review, nine trials reported Hb concentration and found an increase in Hb concentration for children receiving iron supplementation compared to the control group (MD 6.97 g/L, 95% CI 4.21 to 9.72; 9 trials, 1690 children; high‐certainty evidence). In one trial, the intervention showed no clear evidence of a difference in reducing anaemia compared to the control group (79% anaemic in the iron group versus 81% anaemic in the control group; very low‐certainty evidence).
Fortification (4 reviews)
Four systematic reviews assessed fortification interventions for preschool and school‐aged children aged 2 to 10 years (Aaron 2015; Das 2013a; De‐Regil 2017; Eichler 2019; see Table 22). All systematic reviews focused on prevention.
Prevention
MMN‐fortified beverages versus control
Ten RCTs randomising over 4645 apparently healthy school‐aged children and women of reproductive age were included in the review by Aaron 2015, which evaluated the nutritional impacts of MMN‐fortified beverages (dose: not reported; frequency: daily) in the context of low‐middle‐income countries. They defined ID as ferritin levels less than 27 pmol/L to 45 pmol/L and IDA as Hb less than 110 g/L to 120 g/L and ferritin levels less than 27 pmol/L to 45 pmol/L. They found an increase in Hb concentration (MD 2.76 g/L, 95% CI 1.19 to 4.33; 8 trials, 3835 children; moderate‐certainty evidence) and a reduction in anaemia (RR 0.63, 95% CI 0.54 to 0.73; 6 trials, 2828 children; moderate‐certainty evidence), ID (RR 0.32, 95% CI 0.23 to 0.45; 7 trials, 2523 children; moderate‐certainty evidence), and IDA (RR 0.13, 95% CI 0.07 to 0.25; 3 trials, 1649 children; low‐certainty evidence) when non‐dairy MMN‐fortified beverages were compared with control.
Prevention
Zinc‐fortified food versus control (regular diet or unfortified food)
Eleven RCTs and quasi‐RCTs comprising 771 women and children (newborn, infants and school‐aged children) were included in the review by Das 2013a. This review investigated the impact of food fortification with zinc (dose: 3.75 mg to 400 mg; frequency: not reported) on the health and nutrition of women and children. Only one trial reported serum Hb for school‐aged children and found no difference between the intervention and control groups with regular diet or unfortified foods (SMD 0.28, 95% CI −0.62 to 1.19; 19 children; GRADE: not assessed).
Prevention
Centrally‐processed fortified dairy products and fortified cereals versus non‐fortified food
Eichler 2019 included 24 studies comprising 9367 children and adolescents in this review and found no evidence of a difference between the centrally‐processed fortified dairy products and fortified cereals (dose: not reported; frequency: not reported) and control groups in Hb levels (MD 0.90 g/L, 95% CI ‐0.10 to 1.80; 14 trials, 4855 children; very low‐certainty evidence), incidence of anaemia (RR 0.87, 95% CI 0.76 to 1.01; 12 trials, 1149 children; very low‐certainty evidence), and adverse events (3 trials). While centrally‐processed fortified dairy products reduced the incidence of IDA (RR 0.38, 95% CI 0.18 to 0.81; 5 trials, 148 children; very low‐certainty evidence), and incidence of ID (RR 0.62, 95%CI 0.40 to 0.97; 8 trials, 519 children; very low‐certainty evidence).
Prevention
Point‐of‐use fortification of foods with MNP versus no intervention or placebo
Thirteen RCTs, quasi‐RCTs, and cluster‐RCTs randomising 5810 children aged 24 to 59 months and children aged 5 to 12 years receiving point‐of‐use fortification of foods with MNP were included in this review (De‐Regil 2017). The review assessed the effects of point‐of‐use fortification of foods with iron‐containing MNP alone, or in combination with other vitamins and minerals (dose: not reported; frequency: daily), on nutrition, health and development among children of preschool (24 to 59 months) and school age (5 to 12 years). IDA was defined by the presence of anaemia and ID, diagnosed with an indicator of iron status as selected by trialists. ID was defined as ferritin concentrations less than 15 μg/L. Point‐of‐use fortification of foods with MNP increased Hb concentration (MD 3.37 g/L, 95% CI 0.94 to 5.80; 11 trials, 2746 children; low‐certainty evidence) and reduced the prevalence of anaemia (RR 0.66, 95% CI 0.49 to 0.88; 10 trials, 2448 children; moderate‐certainty evidence), and ID (RR 0.35, 95% CI 0.27 to 0.47; 5 trials, 1364 children; moderate‐certainty evidence) compared with no intervention or placebo. No evidence of a difference was seen between the groups for IDA (RR 0.28, 95% CI 0.07 to 1.10; 3 trials, 918 children; GRADE: not assessed), adverse events (RR 1.09, 95% CI 0.16 to 7.42; 1 trial, 90 children; moderate‐certainty evidence) and diarrhoea (RR 0.97, 95% CI 0.53 to 1.78; 2 trials, 366 children; low‐certainty evidence).
Improving dietary diversity and quality
None of the included reviews assessed interventions to improve dietary diversity and quality for preschool and school children.
Adolescent children (aged 11 to 18 years) (4 reviews)
Four systematic reviews assessed interventions to prevent or control anaemia in adolescent children aged 11 to 18 years (Fernández‐Gaxiola 2019; Neuberger 2016; Salam 2016; Salam 2020). GRADE assessments were provided in three out of four reviews. The effects of the interventions are summarised in Table 23.
23. Results of included systematic reviews: adolescent children (aged 11 to 18 years).
| Review | Comparison | Outcome | Number of studies; number of participants | Results | GRADE assessment |
| Supplementation | |||||
|
Fernández‐Gaxiola 2019 Intermittent iron supplementation for reducing anaemia and its associated impairments in adolescent and adult menstruating women |
Intermittent iron supplementation (alone or with any other micronutrients) versus no supplementation or placebo | Hb (g/L) | 15 trials; 2886 women | MD 5.19, 95% CI 3.07 to 7.32 (P < 0.001), significant increase in Hb levels for women receiving intermittent iron supplementation | Moderate |
| Anaemia | 11 trials; 3135 women | RR 0.65, 95% CI 0.49 to 0.87 (P = 0.0038), significant reduction in anaemia for women receiving intermittent iron supplementation | Low | ||
| IDA | 1 trial; 97 women | RR 0.07, 95% CI 0.00 to 1.16, no evidence of a difference | Low | ||
| ID | 3 trials; 624 women | RR 0.50, 95% CI 0.24 to 1.04, no evidence of a difference | Low | ||
| Any adverse side effect | 3 trials; 630 women | RR 1.98, 95% CI 0.31 to 12.72, no evidence of a difference | Moderate | ||
| Intermittent iron supplementation versus daily iron supplementation | Hb (g/L) | 10 trials; 2127 women | MD 0.43, 95% CI −1.44 to 2.31, no evidence of a difference | Low | |
| Anaemia | 8 trials; 1749 women | RR 1.09, 95% CI 0.93 to 1.29, no evidence of a difference | Moderate | ||
| ID | 1 trial; 198 women | RR 4.30, 95% CI 0.56 to 33.20, no evidence of a difference | Very low | ||
| Any adverse side effect | 6 trials; 1166 women | RR 0.41, 95% CI 0.21 to 0.82 (P = 0.011), significant reduction in any adverse side effects for women receiving intermittent iron supplementation versus iron supplementation | Low | ||
|
Neuberger 2016 Oral iron supplements for children in malaria‐endemic areas |
Iron versus placebo/no treatment | Hb (g/L) | 16 trials, 5261 children 7 trials, 2481 anaemic children at baseline 9 trials, 2780 non anaemic children at baseline 12 trials, 2462 children |
MD 7.50, 95% CI 4.80 to 10.10, significant increase in Hb concentration at the end of treatment for children receiving iron supplementation MD 9.50, 95% CI 3.80 to 15.10, significant increase in Hb concentration at the end of treatment for anaemic children receiving iron supplementation MD 6.10, 95% CI 3.80 to 8.50, significant increase in Hb concentration at the end of treatment for non anaemic children receiving iron supplementation MD 6.70, 95% CI 4.20 to 9.20 (P<0.001), significant improvement in haemoglobin from the baseline at end of treatment |
Not assessed |
| Anaemia (as defined in the trial) | 15 trials, 3784 children | RR 0.63, 95% CI 0.49 to 0.82, significant reduction in anaemia for children receiving iron supplementation | Not assessed | ||
| Iron plus folic acid versus placebo | Hb (g/L) | 1 trial, 124 children | MD 9.00, 95% CI 5.10 to 12.90, significant increase in Hb concentration at the end of treatment for children receiving iron plus folic acid supplementation | Not assessed | |
| Anaemia (as defined in the trial) | 3 trials, 633 children | RR 0.49, 95% CI 0.25 to 0.99, significant reduction in anaemia for children receiving iron plus folic acid supplementation | Not assessed | ||
| Iron plus antimalarial versus placebo | Hb (g/L) | 1 trial, 151 children | MD 9.10, 95% CI 4.70 to 13.50, significant increase in Hb concentration at the end of treatment for children receiving iron plus antimalarial supplementation | Not assessed | |
| Anaemia (as defined in the trial) | 2 trials, 295 children 1 trial, 420 children |
RR 0.44, 95% CI 0.28 to 0.70, significant reduction in anaemia at the end of treatment for children receiving iron plus antimalarial supplementation RR 0.37, 95% CI 0.26 to 0.54, significant reduction in anaemia at the end of follow‐up for children receiving iron plus antimalarial supplementation |
Not assessed | ||
|
Salam 2016 Interventions to improve adolescent nutrition: a systematic review and meta‐analysis |
Iron or iron folic acid supplementation alone or in combination with other micronutrient supplementation versus control | Hb (g/L) | Not reported | MD 1.94, 95% CI 1.48 to 2.41, significant increase in Hb concentration for adolescents receiving supplementation | Not assessed |
| Anaemia | 11 trials; 11,861 adolescents | RR 0.69, 95% CI 0.62 to 0.76, significant reduction in anaemia for adolescents receiving supplementation | Moderate | ||
| Nutritional supplementation and counselling versus control | IDA | 1 trial; 14 pregnant adolescents | RR 0.34, 95% CI 0.13 to 0.89, significant reduction in IDA for pregnant adolescents receiving nutritional supplementation and counselling | Low | |
|
Salam 2020 Effects of preventive nutrition interventions among adolescents on health and nutritional status in low‐ and middle‐income countries |
Daily iron supplementation with or without folic acid versus placebo/no supplementation/no fortification | Anaemia | 1 trial, 1160 participants | RR 1.04, 95% CI 0.88 to 1.24, no significant reduction in anaemia for participants receiving daily iron supplementation with or without folic acid versus placebo/no supplementation/no fortification | Low |
| Weekly iron supplementation with or without folic acid versus placebo/no supplementation/no fortification | Anaemia | 1 trial, 1274 participants | RR 1.07, 95% CI 0.91 to 1.26, no significant reduction in anaemia for participants receiving weekly iron supplementation with or without folic acid versus placebo/no supplementation/no fortification | Low | |
| Iron supplementation with or without folic acid versus no supplementation | Hb (g/L) | 4 trials, 1020 participants | MD 0.42 g/L, 95% CI 0.13 to 0.71, significant increase in Hb concentration for participants receiving iron supplementation with or without folic acid compared to no supplementation | Low | |
| Fortification | |||||
|
Salam 2020 Effects of preventive nutrition interventions among adolescents on health and nutritional status in low‐ and middle‐income countries |
MMN fortification versus no fortification | Hb (g/L) | 2 trials, 1102 participants | MD −0.10 g/L, 95% CI ‐0.88 to 0.68, no significant increase in Hb concentration for participants receiving MMN fortification compared to no fortification | Low |
CI: confidence interval; Hb: haemoglobin; ID: iron deficiency; IDA: iron deficiency anaemia; MD: mean difference; MMN: multiple micronutrient; RR: risk ratio.
Supplementation (4 reviews)
Four systematic reviews assessed iron supplementation for adolescent children aged 11 to 18 years (Fernández‐Gaxiola 2019; Neuberger 2016; Salam 2016; Salam 2020). One review focused on prevention and three on prevention or treatment (Table 23).
Prevention
Iron supplementation with or without folic acid versus placebo/no supplementation/no fortification
Salam 2020 assessed the effects of iron supplementation with or without folic acid supplementation (dose: not reported; frequency: daily and weekly) on the health and nutritional status of adolescents aged 10 to 19 years.
Assessing the effects of daily iron supplementation with or without folic acid for girls aged 10 to 17 years old, found no evidence of a difference in the incidence of anaemia (RR 1.04, 95% CI 0.88 to 1.24; 1 trial, 1160 children; low‐certainty evidence). Also, comparing weekly iron supplementation with or without folic acid for girls aged 10 to 17 years old, showed no evidence of a difference in the incidence of anaemia (RR 1.07, 95% CI 0.91 to 1.26; 1 trial, 1274 children; low‐certainty evidence). Four trials showed that iron supplementation with or without folic acid improved Hb concentrations among adolescents compared with no supplementation (MD 0.42 g/L, 95% CI 0.13 to 0.71; 4 trials, 1020 children; low‐certainty evidence).
Prevention or treatment
Intermittent iron supplementation (alone or with any other micronutrients) versus no supplementation, placebo, or daily iron
Two reviews assessed iron supplementation (Fernández‐Gaxiola 2019; Neuberger 2016). Twenty‐five RCTs, quasi‐RCTs, and cluster‐RCTs comprising 10,996 menstruating women (range = 6 years to 49 years, but more than 60% of the trials included women under 18 years) were included in the Fernández‐Gaxiola 2019 systematic review. Most trials involved a mix of anaemic and non‐anaemic women. This review assessed the effects and safety of oral iron alone or with other vitamins and minerals (dose: 60 mg to 120 mg; frequency: once or twice a week) on Hb levels, anaemia, IDA, ID and safety. IDA was defined by the presence of anaemia and ID diagnosed with an indicator of iron status selected by trialists. ID was defined by trialists using indicators of iron status such as ferritin or transferrin. Fifteen trials assessed the effects of intermittent iron supplementation alone or with any other micronutrient on Hb level and found an increase in Hb levels (MD 5.19 g/L, 95% CI 3.07 to 7.32; 15 trials, 2886 women; moderate‐certainty evidence) and decreased the incidence of anaemia (RR 0.65, 95% CI 0.49 to 0.87; 11 trials, 3135 women; low‐certainty evidence) compared with no supplementation or placebo. There was no evidence of a difference in the incidence of IDA (RR 0.07, 95% CI 0.00 to 1.16; 1 trial, 97 women; low‐certainty evidence), ID (RR 0.50, 95% CI 0.24 to 1.04; 3 trials, 624 women; low‐certainty evidence), or any adverse side effect (RR 1.98, 95% CI 0.31 to 12.72; 3 trials, 630 women; moderate‐certainty evidence) between the intermittent iron supplementation and control groups.
Comparing intermittent iron supplementation (alone or with any other micronutrient) versus daily iron supplementation, intermittent iron supplementation reduced any adverse side effects (RR 0.41, 95% CI 0.21 to 0.82; 6 trials, 1166 women; low‐certainty evidence), but there was no difference in Hb levels (MD 0.43 g/L, 95% CI −1.44 to 2.31; 10 trials, 2127 women; low‐certainty evidence), the incidence of anaemia (RR 1.09, 95% CI 0.93 to 1.29; 8 trials, 1749 women; moderate‐certainty evidence), or ID (RR 4.30, 95% CI 0.56 to 33.20; 1 trial, 198 women; very low‐certainty evidence).
Iron supplementation versus placebo/no treatment
Neuberger 2016 included 31 trials comprising 12,963 children living in areas with hyperendemic or holoendemic malaria transmission, and assessed the effects of iron supplementation compared with placebo or no treatment (mean dose: 2 mg/kg/day; frequency: daily).
16 trials for iron supplementation showed an increase in Hb concentration at the end of treatment for children (MD 7.50 g/L, 95% CI 4.80 to 10.10; 16 trials, 5261 children; GRADE: not assessed), for anaemic children (MD 0.95 g/dL, 95% CI 0.38 to 1.51; 7 trials, 2481 anaemic children; GRADE: not assessed), and for non‐anaemic children (MD 6.10 g/L, 95% CI 3.80 to 8.50; 9 trials, 2780 non‐anaemic children; GRADE not assessed). 12 trials showed that iron supplementation improved Hb levels at the end of treatment from baseline compared with no supplementation (MD 6.70 g/L, 95% CI 4.20 to 9.20 (P < 0.0001); 12 trials, 2462 children; GRADE: not assessed).
15 trials showed a reduction in the incidence of anaemia for children (RR 0.63, 95% CI 0.49 to 0.82; 15 trials, 3784 children; GRADE: not assessed).
Prevention or treatment
Iron plus folic acid supplementation versus placebo
Neuberger 2016 included six trials comprising 19,456 children living in areas with hyperendemic or holoendemic malaria transmission and assessed the effects of iron plus folic acid supplementation compared with placebo or no treatment (mean dose: 2 mg/kg/day; frequency: daily).
One trial showed an increase in Hb concentration (MD 0.90, 95% CI 0.51 to 1.29; 1 trial, 124 children; GRADE: not assessed) and three trials showed a reduction in the incidence of anaemia (RR 0.49, 95% CI 0.25 to 0.99; 3 trial, 633 children; GRADE: not assessed).
Prevention or treatment
Iron supplementation plus antimalarial supplementation versus placebo
Neuberger 2016 included four trials comprising 1915 children living in areas with hyperendemic or holoendemic malaria transmission, and assessed the effects of iron plus folic acid supplementation compared with placebo or no treatment (mean dose: 2 mg/kg/day; frequency: daily).
One trial showed that the iron plus antimalarial supplementation increased in Hb concentration compared with placebo (MD 9.10 g/L, 95% CI 4.70 to 13.50; 1 trial, 151 children; GRADE: not assessed). Two trials showed a reduction in the incidence of anaemia at the end of treatment (RR 0.44, 95% CI 0.28 to 0.70; 2 trials, 295 children; GRADE: not assessed), and one trial showed a reduction in the incidence of anaemia at the end of follow‐up (RR 0.37, 95% CI 0.26 to 0.54; 1 trial, 420 children; not assessed).
Iron or iron folic acid supplementation alone or in combination with other micronutrient supplementation
One review assessed iron or iron folic acid supplementation alone or in combination with other micronutrient supplementation for adolescents (Salam 2016).
Thirty‐one trials for micronutrient supplementation in an adolescent population (11 to 19 years old) and 10 trials for nutrition in pregnant adolescents (13 to 20 years old) were included in this systematic review by Salam 2016. For the adolescent population, most trials included girls, and nine trials included boys and girls. This review assessed the effects of interventions (dose: not reported; frequency: daily or weekly) on Hb, anaemia, and IDA. They did not report the definition of IDA.
There was an increase in Hb concentration (MD 1.94 g/L, 95% CI 1.48 to 2.41, number of participants: not reported; GRADE: not assessed) and reduction in anaemia (RR 0.69, 95% CI 0.62 to 0.76; 11 trials, 11,861 adolescents; moderate‐certainty evidence) for adolescents receiving micronutrient supplementation compared with control.
Nutritional supplementation
One review assessed nutritional supplementation for adolescents (Salam 2016).
Prevention or treatment
Nutritional supplementation and counselling versus control
One trial reported a reduction in IDA for pregnant adolescents receiving nutritional supplementation and counselling compared with control (RR 0.34, 95% CI 0.13 to 0.89; 1 trial, 14 pregnant adolescents; low‐certainty evidence).
Fortification (1 review)
One review assessed MMN fortification (dose: not reported; frequency: daily or weekly) for adolescents (Salam 2020).
Prevention
MMN fortification versus no fortification
Salam 2020 evaluated the effects of MMN for adolescents aged 10 to 19 years old. There is no evidence of a difference in Hb concentration compared with no fortification (MD ‐0.10 g/L, 95% CI ‐0.88 to 0.68; 2 trials, 1102 participants; low‐certainty evidence).
Improving dietary diversity and quality
None of the included reviews assessed interventions aimed at improving dietary diversity and quality for adolescent children aged 11 to 18 years.
Non‐pregnant women of reproductive age (aged 19 to 49 years) (5 reviews)
Five systematic reviews assessed interventions to prevent or control anaemia in non‐pregnant women of reproductive age (aged 19 to 49 years) (Abe 2016; Houston 2018; Lassi 2020; Low 2016; Sultan 2019). GRADE assessments were provided in two out of five reviews. The effects of the interventions are summarised in Table 24.
24. Results of included systematic reviews: non‐pregnant women of reproductive age (aged 19 to 49 years).
| Review | Comparison | Outcome | Number of studies; number of participants | Results | GRADE assessment |
| Supplementation | |||||
|
Abe 2016 Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby |
No data | No data | No data | No data | No data |
|
Houston 2018 Efficacy of iron supplementation on fatigue and physical capacity in non‐anaemic iron‐deficient adults: a systematic review of randomised controlled trials |
Iron therapy (oral, IV, IM) versus control | Hb (g/L) | 12 trials; 298 participants | MD 4.01, 95% CI 1.22 to 6.81 (P = 0.005) | Not assessed |
| Anaemia | 2 trials; 327 participants | Anaemia was less common in patients randomised to receive iron supplementation | Not assessed | ||
| Gastrointestinal intolerance | 3 trials; 262 participants | Significantly increased in 1 trial using IM iron administration but not in the 2 trials that used oral administration | Not assessed | ||
| Nausea | 4 trials; 540 participants | 2 trials using IV administration of iron reported significantly increased nausea, whereas nausea was not increased in patients who received iron by oral administration | Not assessed | ||
| Constipation | 1 trial; 24 participants | 1 participant in the intervention group suffered by constipation compared with 0 in the control group | Not assessed | ||
| Diarrhoea | 2 trials; 114 participants | 2 participants in the intervention group suffered from diarrhoea compared with 3 in the control group | Not assessed | ||
|
Lassi 2020 Effects of preconception care and periconception interventions on maternal nutritional status and birth outcomes in low‐ and middle‐income countries: a systematic review |
Iron folic acid supplementation versus placebo | Anaemia | 6 trials; 3430 women | RR 0.66, 95% CI 0.53 to 0.81, significant reduction in anaemia for women receiving iron folic acid supplementation | Very low |
| Anaemia ‐ weekly supplementation | 6 trials; 2661 women | RR 0.70, 95% CI 0.55 to 0.88, significant reduction in anaemia for women receiving weekly iron folic acid supplementation | Very low | ||
| Anaemia ‐ daily supplementation | 2 trials; 1532 women | RR 0.49, 95% CI 0.21 to 1.12, significant reduction in anaemia for women receiving daily iron folic acid supplementation | Very low | ||
|
Low 2016 Daily iron supplementation for improving anaemia, iron status and health in menstruating women |
Daily oral iron supplementation versus no daily oral iron supplementation | Hb at the end of therapy (g/L) | 51 trials; 6861 women | MD 5.30, 95% CI 4.14 to 6.45 (P < 0.001), significant increase in Hb concentration for women receiving daily oral iron | High |
| Anaemia at the end of therapy | 10 trials; 3273 women | RR 0.39, 95% CI 0.25 to 0.60 (P = 0.000017), significant reduction in anaemia for women receiving daily oral iron | Moderate | ||
| IDA at the end of therapy | 1 trial; 55 women | Not estimated | Not assessed | ||
| ID at the end of therapy | 7 trials; 1088 women | RR 0.62, 95% CI 0.50 to 0.76 (P < 0.001), significant reduction in ID for women receiving daily oral iron | Moderate | ||
| Any adverse side effect (total) | 7 trials; 901 women | RR 2.14, 95% CI 0.94 to 4.86, no evidence of a difference | Low | ||
|
Sultan 2019 Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta‐analysis |
IV iron versus oral iron | Hb (g/L) 1 week postpartum | 11 trials (1236 women) | MD 10.00, 95% CI 5.00 to 15.00 (P < 0.0001), significant increase in Hb levels for women receiving IV iron | Not assessed |
| Hb (g/L) 2 weeks postpartum | 7 trials (980 women) | MD 12.00, 95% CI 5.00 to 19.00 (P = 0.0007), significant increase in Hb levels for women receiving IV iron | Not assessed | ||
| Hb (g/L) 3 weeks postpartum | 2 trials (346 women) | MD 13.00, 95% CI 0.60 to 26.00 (P = 0.04), significant increase in Hb levels for women receiving IV iron | Not assessed | ||
| Hb (g/L) 4 weeks postpartum | 4 trials (583 women) | MD 7.00, 95% CI −3.00 to 16.00 (P = 0.18), no evidence of a difference | Not assessed | ||
| Hb (g/L) 6 weeks postpartum | 4 trials (385 women) | MD 9.00,95% CI 4.00 to 13.00 (P = 0.0003), significant increase in Hb levels for women receiving IV iron | Not assessed | ||
| Treatment‐related side effects: skin flushing | 4 trials (281 women) | OR 6.95, 95% CI 1.56 to 31.03 (P = 0.01), women receiving IV iron had increased skin flushing | Not assessed | ||
| Treatment‐related side effects: constipation | 8 trials (1535 women) | OR 0.08, 95% CI 0.03 to 0.21 (P < 0.00001), women receiving IV iron had decreased constipation | Not assessed | ||
| Treatment‐related side effects: dyspepsia | 3 trials (304 women) | OR 0.07, 95% CI 0.01 to 0.42 (P = 0.004), women receiving IV iron had decreased dyspepsia | Not assessed | ||
| Other treatment‐related side effects | 1 to 6 trials | No difference between IV iron and oral iron for nausea, muscle cramps, alanine transferase rise, aspartate transaminase rise, headache, anaphylaxis, urticaria, rash, infection | Not assessed | ||
CI: confidence interval; Hb: haemoglobin; ID: iron deficiency; IDA: iron deficiency anaemia; IM: intramuscular; IV: intravenous; MD: mean difference; RCTs: randomised controlled trials; RR: risk ratio.
Supplementation (5 reviews)
Five systematic reviews assessed the effects of supplementation interventions in non‐pregnant women of reproductive age (Abe 2016; Houston 2018; Lassi 2020; Low 2016; Sultan 2019). See Table 24.
Iron supplementation
Three reviews assessed iron supplementation for non‐pregnant women of reproductive age (Houston 2018; Low 2016; Sultan 2019). See Table 24.
Prevention
Daily oral iron supplementation with or without co‐intervention (folic acid or vitamin C) versus no supplemental iron
Sixty‐seven trials comprising 8506 menstruating women (13 to 45 years, 3 trials included adolescent girls only) were included in the systematic review by Low 2016. This review assessed the effects of daily supplementation with iron (dose: 1 mg to 300 mg of elemental iron; frequency: per day) on anaemia and iron status (Hb, anaemia, IDA, ID) as well as on physical, psychological and neurocognitive health. IDA was defined by the presence of anaemia plus iron deficiency, diagnosed with an indicator of iron status selected by trialists, ID was measured by trial authors using indicators of iron status such as ferritin or transferrin. Trials were conducted in numerous countries of differing cultural and economic backgrounds. Hb concentration increased for women receiving daily oral iron compared to those not receiving daily oral iron supplementation at the end of therapy (MD 5.30 g/L, 95% CI 4.14 to 6.45; 51 trials, 6861 women; high‐certainty evidence). At the end of therapy, there was a reduction in anaemia (RR 0.39, 95% CI 0.25 to 0.60; 10 trials, 3273 women; moderate‐certainty evidence), ID (RR 0.62, 95% CI 0.50 to 0.76; 7 trials, 1088 women; moderate‐certainty evidence), and IDA (not estimated; GRADE: not assessed), in women receiving daily oral iron compared with those not receiving daily oral iron supplementation. There was no evidence of a difference in any adverse side effect (RR 2.14, 95% CI 0.94 to 4.86; 7 trials, 901 women; low‐certainty evidence).
Treatment
Intravenous iron versus oral iron
Sultan 2019 included 15 trials comprising 2182 women with a postdelivery Hb level of < 12 g/dL and assessed IV iron (dose/frequency: different dosages) compared with oral iron.
There was an increase in Hb at 1 week postpartum (MD 10.00 g/L, 95% CI 5.00 to 15.00 (P < 0.0001); 11 trials, 1236 women; GRADE: not assessed), at 2 weeks postpartum (MD 12.00 g/L, 95% CI 5.00 to 19.00 (P = 0.0007); 7 trials, 980 women; GRADE: not assessed), at 3 weeks postpartum (MD 13.00 g/L, 95% CI 0.60 to 26.00 (P = 0.04); 2 trials, 346 women; GRADE: not assessed), at 6 weeks postpartum (MD 9.00 g/L, 95% CI 4.00 to 13.00 (P = 0.0003); 4 trials, 385 women; GRADE: not assessed), but there was no evidence of a difference at 4 weeks postpartum (MD 7.00 g/L, 95% CI ‐3.00 to 16.00 (P = 0.18), 4 trials, 583 women; GRADE: not assessed).
Four trials showed that IV iron increased skin flushing compared with oral iron (OR 6.95, 95% CI 1.56 to 31.03 (P = 0.01); 4 trials, 281 women; GRADE: not assessed). IV iron decreased constipation (OR 0.08, 95% CI 0.03 to 0.21 (P < 0.00001); 8 trials, 1535 women; GRADE: not assessed), and dyspepsia (OR 0.07, 95% CI 0.01 to 0.42 (P = 0.004); 3 trials, 304 women; GRADE: not assessed). However, there was no evidence of a difference in other treatment‐related side effects, including nausea, muscle cramps, alanine transferase rise, aspartate transaminase rise, headache, anaphylaxis, urticaria, rash, and infection between IV iron and oral iron.
Prevention and/or treatment
Iron therapy (oral, IV, IM) versus control
Eighteen RCTs comprising 1170 participants (age range = 17 to 55 years) who were iron deficient but non‐anaemic were included in the Houston 2018 systematic review. This review assessed the effects of iron therapy (oral (dose: 16 mg to 200 mg; frequency: daily), IV (dose: 200 mg to 1000 mg; frequency: daily), IM (dose: 100 mg; frequency: daily)) on fatigue and physical capacity in iron‐deficient non‐anaemic (IDNA) adults (15 trials included women only with more than 60% within this age group). Iron therapy increased Hb levels compared with control (MD 4.01 g/L, 95% CI 1.22 to 6.81; 12 trials, 298 women; GRADE: not assessed). Anaemia was less common in patients randomised to receive iron supplementation (2 trials, 327 women). There was no clear difference between the intervention group and the control group for the other outcomes: gastrointestinal intolerance (3 trials, 262 women), nausea (4 trials, 540 women), constipation (1 trial, 24 women), and diarrhoea (2 trials, 114 women). However, this systematic review reported increases in gastrointestinal intolerance and nausea in trials using IM or IV iron administration, but not in trials using oral administration.
Prevention
Iron folic acid supplementation versus placebo
Lassi 2020 included 10 trials comprising 8955 periconceptional women, and assessed the effects of iron folic acid supplementation (dose: different dosages; frequency: daily/weekly).
Comparing iron folic acid supplementation with placebo showed a reduction in the incidence of anaemia (RR 0.66, 95% CI 0.53 to 0.81; 6 trials, 3430 women; very low‐certainty evidence). Also, there was a reduction in the incidence of anaemia with weekly supplementation (RR 0.70, 95% CI 0.55 to 0.88; 6 trials, 2661 women; very low‐certainty evidence) and daily supplementation (RR 0.49, 95% CI 0.21 to 1.12; 2 trials, 1532 women; very low‐certainty evidence).
Prevention
Two RCTs comprising 52 breastfeeding women were included in the systematic review by Abe 2016. This review assessed the effects and safety of MMN supplementation (dose: 18 mg to 45 mg iron; frequency: daily) in breastfeeding mothers on maternal and infant outcomes, but no data were available for outcomes related to anaemia.
Fortification
None of the included reviews assessed fortification interventions for non‐pregnant women of reproductive age.
Improving dietary diversity and quality
None of the included reviews assessed interventions aimed at improving dietary diversity and quality for non‐pregnant women of reproductive age.
Pregnant women of reproductive age (aged 15 to 49 years) (23 reviews)
Twenty‐three systematic reviews assessed interventions to prevent or control anaemia in pregnant women of reproductive age (Abu Hashim 2017; Bhutta 2012; Buppasiri 2015; Daru 2016; Das 2018; De‐Regil 2015; Govindappagari 2019; Haider 2011; Haider 2013; Imdad 2012; Keats 2019; Lassi 2013; McCauley 2015; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Qassim 2018; Qassim 2019; Radhika 2019; Reveiz 2011; Rumbold 2015; Shi 2015; Suchdev 2015; Thorne‐Lyman 2012). One additional review, focusing on school‐aged children, also presented results for pregnant women, and is included here as well (Aaron 2015). GRADE assessments were provided in 12 out of 23 reviews. The results are summarised in Table 25.
25. Results of included systematic reviews: pregnant women (aged 15 to 49 years).
| Review | Comparison | Outcome | Number of studies; number of participants | Results | GRADE assessment |
| Supplementation | |||||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Abu‐Hashim‐2017 Lactoferrin or ferrous salts for iron deficiency anemia in pregnancy: a meta‐analysis of randomized trials |
Oral bovine lactoferrin versus oral ferrous iron preparations | Hb levels at 4 weeks (g/L) | 4 trials; 600 women | MD 7.70, 95% CI 0.40 to 15.50 (P = 0.04), significant increase in Hb level for women receiving oral bovine lactoferrin | Low |
| Gastrointestinal side effects: 'epigastric discomfort' | 2 trials; 328 women | OR 0.11, 95% CI 0.05 to 0.22 (P < 0.001), significant reduction in epigastric discomfort for women receiving oral bovine lactoferrin | Moderate | ||
| Gastrointestinal side effects: 'vomiting' | 2 trials; 328 women | OR 0.32, 95% CI 0.15 to 0.67 (P = 0.002), significant reduction in vomiting for women receiving oral bovine lactoferrin | Moderate | ||
| Gastrointestinal side effects: 'constipation' | 2 trials; 328 women | OR 0.22, 95% CI 0.12 to 0.40 (P < 0.001), significant reduction in constipation for women receiving oral bovine lactoferrin | Moderate | ||
| Gastrointestinal side effects: 'abdominal colicky pain' | 1 trial; 228 women | OR 0.21, 95% CI 0.12 to 0.39 (P < 0.001), significant reduction in abdominal colicky pain for women receiving oral bovine lactoferrin | Moderate | ||
| Gastrointestinal side effects: 'dark stool' | 1 trial; 228 women | OR 0.01, 95% CI 0.00 to 0.22 (P = 0.002), significant reduction in dark stool for women receiving oral bovine lactoferrin | Moderate | ||
| Gastrointestinal side effects: 'diarrhoea' | 1 trial; 228 women | OR 0.00, 95% CI 0.00 to 0.00, no evidence of a difference | Not assessed | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Bhutta‐2012 Is it time to replace iron folate supplements in pregnancy with multiple micronutrients? |
MMN versus iron folate | Maternal Hb (g/L) | 4 trials; number of participants: not reported | SMD −0.01, 95% CI −0.08 to 0.06, no evidence of a difference | Low |
| Maternal anaemia in the third trimester | 7 trials; not reported | RR 1.03, 95% CI 0.94 to 1.12, no evidence of a difference | Moderate | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Buppasiri‐2015 Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes |
Calcium supplementation versus placebo or no treatment | Maternal anaemia | 1 trial; 1098 women | RR 1.04, 95% CI 0.90 to 1.22, no evidence of a difference | Not assessed |
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Daru‐2016 Systematic review of randomized trials of the effect of iron supplementation on iron stores and oxygen carrying capacity in pregnancy |
IV iron versus oral iron supplementation in pregnant women with IDA | Hb | 6 trials; number of participants: not reported | Significant increase in Hb in the IV group compared with the oral group in 2 trials | Not assessed |
| IM versus oral iron supplementation in pregnant women with IDA | Hb | 1 trial; number of participants: not reported | No evidence of a difference | Not assessed | |
| IV versus IM iron supplementation in pregnant women with IDA | Hb | 1 trial; number of participants: not reported | Significant change in Hb in the IV group compared with the IM group | Not assessed | |
| Weekly oral iron supplementation versus daily in pregnant women with IDA | Hb | 3 trials; number of participants: not reported | Significant increase in Hb in daily arm in 1 trial | Not assessed | |
| Different doses of oral iron supplementation in pregnant women with IDA | Hb | 2 trials; number of participants: not reported | Significant change in Hb in the higher‐dose arm in 1 trial | Not assessed | |
| Alternative oral preparations versus a commonly used preparation in pregnant women with IDA | Hb | 3 trials; number of participants: not reported | No evidence of a difference | Not assessed | |
| Oral iron supplementation versus placebo in pregnant women with IDA or NAID | Hb | 6 trials; number of participants: not reported | Significant increase in Hb in the supplemented group compared with the placebo group in 2 trials | Not assessed | |
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Das‐2018 Lipid‐based nutrient supplements for maternal, birth, and infant developmental outcomes |
Lipid‐based nutrient supplements (LNS) versus iron folic acid (IFA) | Anaemia | 1 trial, 536 participants | RR 2.35, 95% CI 1.67 to 3.30, showing a two‐fold increase in the prevalence of anaemia in the LNS group compared to the IFA group | Moderate |
| Adverse effects | 1 trial, 881 participants | RR 1.34, 95% CI 0.93 to 1.94 (P = 0.11), did not find any significant difference in hospitalisation episodes between the LNS and IFA groups | Not assessed | ||
| Lipid‐based nutrient supplements (LNS) versus multiple micronutrients (MMN) | Anaemia | 1 trial, 557 participants | RR 1.40, 95% CI 1.07 to 1.82, showing increased anaemia in the LNS group when compared to the MMN group | Moderate | |
| Adverse effects | 1 trial, 879 participants | RR 1.18, 95% CI 0.83 to 1.68 (P = 0.36), did not find any significant difference in hospitalisation episodes between the LNS and MMN groups | Not assessed | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐De_x002d_Regil‐2015 Effects and safety of periconceptional oral folate supplementation for preventing birth defects |
Supplementation with any folate versus no intervention, placebo or other micronutrients without folate | Maternal anaemia at or near term (Hb <110 g/L at 34 weeks' gestation or more) | No trials reported on this outcome | No data | No data |
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Govindappagari‐2019 Treatment of iron deficiency anemia in pregnancy with intravenous versus oral iron: systematic review and meta‐analysis |
IV iron versus oral iron | Percentage of participants achieving desired Hb target after 4 Weeks | 7 trials, participants: not reported | OR 2.66, 95% CI 1.71 to 4.15 (P < 0.001), pregnant women receiving IV iron were more likely to reach their Hb target compared with those receiving oral iron | Not assessed |
| Increase in Hb after 4 weeks of treatment | 9 trials, participants: not reported | WMD 0.84, 95% CI 0.59 to 1.09 (P < 0.001), Hb increase was greater in subjects receiving IV compared with oral iron | Not assessed | ||
| Adverse effects in response to treatment | 11 trials, participants: not reported | OR 0.35, 95% CI 0.18 to 0.67 (P < 0.001), women receiving IV iron experienced significantly fewer adverse events compared with those receiving oral iron | Not assessed | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Haider‐2011 Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes |
MMN supplementation versus iron or folate supplementation | Maternal anaemia in third trimester | 4 trials; number of participants: not reported | RR 1.03, 95% CI 0.87 to 1.22, no evidence of a difference | High |
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Haider‐2013 Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta‐analysis |
Iron, with or without folic acid | Hb (g/L) in second trimester | 8 trials; number of participants: not reported | WMD −0.23, 95% CI −12.18 to 11.72, no evidence of a difference | Not assessed |
| Hb (g/L) in third trimester or at delivery | 36 trials; number of participants: not reported | WMD 4.59, 95% CI 3.72 to 5.46 (P <0.001), significantly higher mean Hb concentration for women receiving iron, with or without folic acid | Not assessed | ||
| Hb (g/L) in postpartum period | 12 trials; number of participants: not reported | WMD 6.79, 95% CI 0.22 to 13.36, significantly higher mean Hb concentration for women receiving iron, with or without folic acid | Not assessed | ||
| Anaemia in third trimester or at delivery | 20 trials; number of participants: not reported | RR 0.50, 95% CI 0.42 to 0.59 (P < 0.001), significant reduction in anaemia for women receiving iron, with or without folic acid | Not assessed | ||
| IDA in third trimester or at delivery | 6 trials; number of participants: not reported | RR 0.40, 95% CI 0.26 to 0.60 (P < 0.001), significant reduction in IDA for women receiving iron, with or without folic acid | Not assessed | ||
| ID in third trimester or at delivery | 8 trials; number of participants: not reported | RR 0.59, 95% CI 0.44 to 0.79 (P < 0.001), significant reduction in ID for women receiving iron, with or without folic acid | Not assessed | ||
| Iron only versus no iron or placebo | Hb (g/L) in third trimester or at delivery | 31 trials; number of participants: not reported | WMD 4.50, 95% CI 3.62 to 5.39, significantly higher mean Hb concentration for women receiving iron only | Not assessed | |
| Hb (g/L) in postpartum period | 8 trials; number of participants: not reported | WMD 7.01, 95% CI 0.36 to 13.66, significantly higher mean Hb concentration for women receiving iron only | Not assessed | ||
| Anaemia in third trimester or at delivery | 17 trials; number of participants: not reported | RR 0.56, 95% CI 0.48 to 0.65, significant reduction in anaemia for women receiving iron only | Not assessed | ||
| IDA in third trimester or at delivery | 5 trials; number of participants: not reported | RR 0.37, 95% CI 0.23 to 0.60, significant reduction in IDA for women receiving iron only | Not assessed | ||
| ID in third trimester or at delivery | 8 trials; number of participants: not reported | RR 0.59, 95% CI 0.44 to 0.79, significant reduction in ID for women receiving iron only | Not assessed | ||
| Iron with folic acid versus no iron and folic acid or placebo | Hb (g/L) in third trimester or at delivery | 9 trials; number of participants: not reported | WMD 10.41, 95% CI 5.36 to 15.46, significantly higher mean Hb concentration for women receiving iron with folic acid | Not assessed | |
| Anaemia in third trimester or at delivery | 5 trials; number of participants: not reported | RR 0.44, 95% CI 0.37 to 0.53, significant reduction in anaemia for women receiving iron with folic acid | Not assessed | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Imdad‐2012 Routine iron/folate supplementation during pregnancy: effect on maternal anaemia and birth outcomes |
Iron versus no iron | Anaemia at term | 18 trials/quasi‐trials; 8665 women | RR 0.31, 95% CI 0.22 to 0.44 (P <0.00001), significant reduction in anaemia for women receiving iron | Moderate |
| Severe anaemia at term | 11 trials; number of participants: not reported (only 1 trial contributed data, zero events in the rest of the trials) | RR 4.83, 95% CI 0.23 to 99.88, no evidence of a difference | Not assessed | ||
| Severe anaemia at any time during second or third trimester | 13 trials; number of participants: not reported | RR 0.25, 95% CI 0.03 to 2.48, no evidence of a difference | Not assessed | ||
| IDA at term | 7 trials; number of participants: not reported | RR 0.44, 95% CI 0.28 to 0.68, significant reduction in IDA for women receiving iron | Not assessed | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Keats‐2019 Multiple‐micronutrient supplementation for women during pregnancy |
MMN with iron and folic acid versus iron with or without folic acid | Maternal anaemia (third trimester Hb < 110 g/L) | 9 trials; 5912 participants | RR 1.04, 95% CI 0.94 to 1.15, no evidence of a difference | Not assessed |
| MMN with iron and folic acid versus placebo | Maternal anaemia (third trimester Hb < 110 g/L) | 1 trial; number of participants: not reported | RR 0.66, 95% CI 0.51 to 0.85 (P = 0.001), significant reduction in maternal anaemia for women receiving MMN with iron and folic acid versus placebo | Not assessed | |
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Lassi‐2013 Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes |
Folic acid versus no folic acid | Mean pre‐delivery Hb (g/L) | 12 trials; 1806 participants | MD −0.03, 95% CI −0.25 to 0.19, no evidence of a difference | Not assessed |
| Pre‐delivery anaemia | 8 trials; 4149 participants | RR 0.62, 95% CI 0.35 to 1.10, no evidence of a difference | Not assessed | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐McCauley‐2015 Vitamin A supplementation during pregnancy for maternal and newborn outcomes |
Vitamin A alone versus placebo or no treatment | Maternal anaemia | 3 trials; 3818 participants | RR 0.64, 95% CI 0.43 to 0.94 (P = 0.025), significant reduction in maternal anaemia for women receiving vitamin A alone | Moderate |
| Neonatal anaemia | 1 trial; 406 neonates | RR 0.99, 95% CI 0.92 to 1.08, no evidence of a difference | Not assessed | ||
| Vitamin A with other micronutrients versus micronutrient supplements without vitamin A | Maternal anaemia | 3 trials; 706 women | RR 0.86, 95% CI 0.68 to 1.09, no evidence of a difference | Low | |
| Neonatal anaemia | 2 trials; 1052 neonates | RR 0.75, 95% CI 0.38 to 1.51, no evidence of a difference | Not assessed | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Pe_x00f1_a_x002d_Rosas‐2015a Daily oral iron supplementation during pregnancy |
Any supplements containing iron versus same supplements without iron or no treatment or placebo (no iron or placebo) | Maternal Hb concentration at or near term (g/L) | 19 trials; 3704 women | MD 8.88, 95% CI 6.96 to 10.80 (P < 0.001), significant increase in Hb concentration for women receiving daily iron | Not assessed |
| Maternal Hb concentration within 6 weeks postpartum (g/L) | 7 trials; 956 women | MD 7.61, 95% CI 5.50 to 9.72 (P < 0.001), significant increase in Hb concentration for women receiving daily iron | Not assessed | ||
| Maternal anaemia at term (or near term) | 14 trials; 2199 women | RR 0.30, 95% CI 0.19 to 0.46 (P < 0.001), significant reduction in maternal anaemia for women receiving daily iron | Low | ||
| Moderate anaemia at postpartum | 3 trials; 766 women | RR 0.55, 95% CI 0.12 to 2.51, no evidence of a difference | Not assessed | ||
| Maternal IDA at term (or near term) | 6 trials; 1088 women | RR 0.33, 95% CI 0.16 to 0.69 (P = 0.003), significant reduction in maternal IDA for women receiving daily iron | Not assessed | ||
| Maternal ID at term (or near term) | 7 trials; 1256 women | RR 0.43, 95% CI 0.27 to 0.66 (P < 0.001), significant reduction in maternal ID for women receiving daily iron | Low | ||
| Maternal severe anaemia at any time during second and third trimester | 9 trials; 2125 women | RR 0.22, 95% CI 0.01 to 3.20, no evidence of a difference | Very low | ||
| Maternal severe anaemia at or near term | 8 trials; 1819 women | RR 0.47, 95% CI 0.01 to 44.11, no evidence of a difference | Not assessed | ||
| Severe anaemia at postpartum | 8 trials; 1339 women | RR 0.04, 95% CI 0.01 to 0.28 (P < 0.001), significant reduction of severe anaemia postpartum for women receiving daily iron | Not assessed | ||
| Side effects | 11 trials; 2423 women | RR 1.29, 95% CI 0.83 to 2.02, no evidence of a difference | Very low | ||
| Diarrhoea | 3 trials; 1088 women | RR 0.55, 95% CI 0.32 to 0.93 (P = 0.025), significant reduction in diarrhoea for women receiving daily iron | Not assessed | ||
| Constipation | 4 trials; 1495 women | RR 0.95, 95% CI 0.62 to 1.43, no evidence of a difference | Not assessed | ||
| Vomiting | 4 trials; 1392 women | RR 0.88, 95% CI 0.59 to 1.30, no evidence of a difference | Not assessed | ||
| Any supplements containing iron and folic acid versus same supplements without iron nor folic acid (no iron nor folic acid or placebo) | Maternal Hb concentration at or near term (g/L) | 3 trials; 140 women | MD 16.13, 95% CI 12.74 to 19.52 (P < 0.001), significant increase in Hb concentration for women receiving daily iron and folic acid | Not assessed | |
| Maternal Hb concentration within 6 weeks postpartum (g/L) | 2 trials; 459 women | MD 10.07, 95% CI 7.33 to 12.81 (P < 0.001), significant increase in Hb concentration for women receiving daily iron and folic acid | Not assessed | ||
| Maternal anaemia at term (or near term) | 3 trials; 346 women | RR 0.34, 95% CI 0.21 to 0.54 (P < 0.001), significant reduction in maternal anaemia for women receiving daily iron and folic acid | Moderate | ||
| Moderate anaemia at postpartum | 3 trials; 491 women | RR 0.33, 95% CI 0.17 to 0.65 (P < 0.001), significant reduction in maternal anaemia for women receiving daily iron and folic acid | Not assessed | ||
| Maternal IDA at term (or near term) | 1 trial; 131 women | RR 0.43, 95% CI 0.17 to 1.09, no evidence of a difference | Not assessed | ||
| Maternal ID at term (or near term) | 1 trial; 131 women | RR 0.24, 95% CI 0.06 to 0.99 (P = 0.049), significant reduction in ID for women receiving daily iron and folic acid | Low | ||
| Maternal severe anaemia at any time during second and third trimester | 4 trials; 506 women | RR 0.12, 95% CI 0.02 to 0.63 (P = 0.012), significant reduction in maternal severe anaemia for women receiving daily iron and folic acid | Very low | ||
| Maternal severe anaemia at or near term | 3 trials; 191 women | RR 0.14, 95% CI 0.01 to 2.63, no evidence of a difference | Not assessed | ||
| Severe anaemia at postpartum | 3 trials; 491 women | RR 0.05, 95% CI 0.00 to 0.76 (P = 0.031), significant reduction in severe anaemia at postpartum for women receiving daily iron and folic acid | Not assessed | ||
| Side effects | 1 trial; 456 women | RR 44.32, 95% CI 2.77 to 709.09 (P = 0.007), significant increase in side effects for women receiving daily iron and folic acid | Moderate | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Pe_x00f1_a_x002d_Rosas‐2015b Intermittent oral iron supplementation during pregnancy |
Any intermittent iron regimen (with or without other vitamins and minerals) versus daily regimen (with same vitamins and minerals) | Maternal Hb (g/L) at or near term | 8 trials; 1306 women | MD −2.57, 95% CI −5.18 to 0.04, no evidence of a difference | Not assessed |
| Maternal anaemia at term | 4 trials; 676 women | RR 1.22, 95% CI 0.84 to 1.80, no evidence of a difference | Very low | ||
| Maternal anaemia at or near term | 8 trials; 1385 women | RR 1.66, 95% CI 1.09 to 2.53 (P = 0.017), increase in anaemia at or near term for women receiving intermittent iron | Not assessed | ||
| Moderate anaemia at any time during second and third trimester | 9 trials; 1291 women | RR 2.50, 95% CI 0.84 to 7.48, no evidence of a difference | Not assessed | ||
| Maternal IDA at term | 1 trial; 156 women | RR 0.71, 95% CI 0.08 to 6.63, no evidence of a difference | Very low | ||
| Maternal IDA at term or near term | 2 trials; 278 women | RR 2.06, 95% CI 0.65 to 6.61, no evidence of a difference | Not assessed | ||
| Maternal ID at or near term | 3 trials; 587 women | RR 2.38, 95% CI 1.30 to 4.36 (P = 0.005), increase in ID at or near term for women receiving intermittent iron | Not assessed | ||
| Severe anaemia at any time during second and third trimester | 6 trials; 1240 women | RR 0.00, 95% CI 0.0 to 0.0, no events | Very low | ||
| Severe anaemia at or near term | 6 trials; 1050 women | RR 0.00, 95% CI 0.0 to 0.0, no events | Not assessed | ||
| Severe anaemia at term | 3 trials; 475 women | RR 0.00, 95% CI 0.0 to 0.0, no events | Not assessed | ||
| Severe anaemia at postpartum | 1 trial; 169 women | RR 0.43, 95% CI 0.04 to 4.64, no evidence of a difference | Not assessed | ||
| Side effects | 11 trials; 1777 women | RR 0.56, 95% CI 0.37 to 0.84 (P 0.006), significant reduction in side effects for women receiving intermittent iron | Very low | ||
| Diarrhoea | 5 trials; 613 women | RR 0.80, 95% CI 0.32 to 2.00, no evidence of a difference | Not assessed | ||
| Constipation | 6 trials; 733 women | RR 0.85, 95% CI 0.45 to 1.59, no evidence of a difference | Not assessed | ||
| Vomiting | 6 trials; 954 women | RR 1.30, 95% CI 0.79 to 2.15, no evidence of a difference | Not assessed | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Qassim‐2018 Safety and efficacy of intravenous iron polymaltose, iron sucrose and ferric carboxymaltose in pregnancy: a systematic review |
IV IPM, IS and FCM versus any other comparator | Hb (g/L) | IS (2635 pregnant women; 41 studies), FCM (276 pregnant women; 4 studies) and IPM (164 pregnant women; 3 studies) | All IV preparations resulted in significant improvements in haematological parameters, with a median increase of 21.80 g/L at 3 to 4 weeks and 30.10 g/L by delivery | Not assessed |
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Qassim‐2019 Intravenous or oral iron for treating iron deficiency anaemia during pregnancy: systematic review and meta‐analysis |
IV iron therapy versus oral iron | Hb (g/L) | 9 trials; 1009 women | MD 7.40 g/L, 95% CI 3.90 to 10.90, significant increase in Hb concentration at delivery for pregnant women receiving IV iron therapy compared to oral iron supplementation | Low |
| 6 trials; 849 neonates | MD −1.00 g/L, 95% CI −4.70 to 2.80, no significant increase in Hb concentration of neonates at delivery for pregnant women receiving IV iron therapy compared to oral iron supplementation | Low | |||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Radhika‐2019 Parenteral versus oral iron for treatment of iron deficiency anaemia during pregnancy and post‐partum: a systematic review |
IV iron sucrose versus oral iron | Antenatal haemoglobin (g/L) | 11 trials, 3460 pregnant women | MD 7.80 lower, 95% CI 10.00 lower to 5.70 lower, significant increase in Hb concentration for pregnant women receiving IV iron sucrose compared to oral iron supplementation | High |
| Antenatal haemoglobin level at 6 weeks (g/L) | 9 trials, 1147 pregnant women | MD 6.60 lower, 95% CI 10.40 lower to 2.90 lower, significant increase in Hb concentration at 6 weeks for pregnant women receiving IV IS compared to oral iron supplementation | High | ||
| Post‐partum haemoglobin (g/L) | 8 trials, 1370 post‐partum women | MD 8.30 lower, 95% CI 12.50 lower to 4.20 lower, significant increase in Hb concentration for pregnant women receiving IV IS compared to oral iron supplementation | Moderate | ||
| Post‐partum haemoglobin level at 6 weeks (g/L) | 3 trials, 234 post‐partum women | MD 7.10 lower, 95% CI 26.10 lower to 12.002 higher, no significant increase in Hb concentration at 6 weeks for pregnant women receiving IV IS compared to oral iron supplementation | Moderate | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Reveiz‐2011 Treatments for iron‐deficiency anaemia in pregnancy |
Oral iron versus placebo | Hb levels (g/L) | 2 trials; 215 women | MD 13.4, 95% CI 2.7 to 24.2 (P = 0.014), significant increase in Hb concentration for women receiving oral iron | Not assessed |
| Anaemia during 2nd trimester | 1 trial; 125 women | RR 0.38, 95% CI 0.26 to 0.55 (P < 0.001), significant reduction in anaemia during 2nd trimester for women receiving oral iron | Not assessed | ||
| Side effects | 1 trial; 51 women | RR 1.97, 95% CI 0.66 to 5.91, no evidence of a difference | Not assessed | ||
| Nausea and vomiting | 1 trial; 51 women | RR 4.5, 95% CI 0.54 to 37.54, no evidence of a difference | Not assessed | ||
| Constipation | 1 trial; 51 women | RR 1.13, 95% CI 0.32 to 4.01, no evidence of a difference | Not assessed | ||
| Oral iron plus vitamin A versus placebo | Hb levels (g/L) | 1 trial; 125 women | MD 13.0, 95% CI 11.1 to 14.9 (P < 0.001), significant increase in Hb concentration for women receiving oral iron plus vitamin A | Not assessed | |
| Anaemia during 2nd trimester | 1 trial; 125 women | RR 0.04, 95% CI 0.01 to 0.15 (P < 0.001), significant reduction in anaemia during 2nd trimester for women receiving oral iron plus vitamin A | Not assessed | ||
| Controlled‐release oral iron versus regular oral iron | Side effects | 1 trial; 49 women | RR 0.96, 95% CI 0.40 to 2.33, no evidence of a difference | Not assessed | |
| Nausea and vomiting | 1 trial; 49 women | RR 0.96, 95% CI 0.27 to 3.41, no evidence of a difference | Not assessed | ||
| Constipation | 1 trial; 49 women | RR 0.24, 95% CI 0.03 to 2.00, no evidence of a difference | Not assessed | ||
| IM iron sorbito‐citric acid versus IM dextran | Nausea or vomiting | 1 trial; 48 women | RR 0.92, 95% CI 0.06 to 13.87, no evidence of a difference | Not assessed | |
| IM iron dextran versus IV iron dextran | Nausea or vomiting | 1 trial; 49 women | RR 0.57, 95% CI 0.05 to 5.83, no evidence of a difference | Not assessed | |
| IM iron sorbitol citric acid versus IV iron dextran | Nausea or vomiting | 1 trial; 51 women | RR 0.52, 95% CI 0.05 to 5.38, no evidence of a difference | Not assessed | |
| IV iron versus placebo | Side effects | 1 trial; 54 women | RR 0.75, 95% CI 0.19 to 3.04, no evidence of a difference | Not assessed | |
| Nausea or vomiting | 1 trial; 54 women | RR 0.33, 95% CI 0.01 to 7.84, no evidence of a difference | Not assessed | ||
| Constipation | 1 trial; 54 women | RR 0.25, 95% CI 0.03 to 2.09, no evidence of a difference | Not assessed | ||
| IV iron versus regular oral iron | Maternal Hb at birth (g/L) | 1 trial; 90 women | MD 7.50, 95% CI 3.40 to 11.60 (P < 0.001), significant increase in Hb concentration at birth for women receiving IV iron | Not assessed | |
| Mean maternal Hb at 4 weeks (g/L) | 3 trials; 167 women | MD 4.40, 95% CI 0.50 to 8.20 (P = 0.027), significant increase in Hb concentration at 4 weeks for women receiving IV iron | Not assessed | ||
| Side effects | 1 trial; 51 women | RR 0.38, 95% CI 0.11 to 1.31, no evidence of a difference | Not assessed | ||
| Nausea or vomiting or epigastric discomfort | 3 trials; 244 women | RR 0.33, 95% CI 0.15 to 0.74 (P = 0.007), significant reduction in nausea or vomiting or epigastric discomfort for women receiving IV iron | Not assessed | ||
| Constipation | 2 trials; 151 women | RR 0.11, 95% CI 0.02 to 0.71 (P = 0.020), significant reduction in constipation for women receiving IV iron | Not assessed | ||
| Diarrhoea | 3 trials; 237 women | RR 0.16, 95% CI 0.03 to 0.86 (P = 0.033), significant reduction in diarrhoea for women receiving IV iron | Not assessed | ||
| IV iron versus controlled‐release oral iron | Side effects | 1 trial; 52 women | RR 0.40, 95% CI 0.12 to 1.37, no evidence of a difference | Not assessed | |
| Nausea or vomiting | 1 trial; 52 women | RR 0.10, 95% CI 0.01 to 1.82, no evidence of a difference | Not assessed | ||
| Constipation | 1 trial; 52 women | RR 0.93, 95% CI 0.06 to 14.03, no evidence of a difference | Not assessed | ||
| IM iron sorbitol citric acid versus oral iron | Mean maternal Hb at birth (g/L) | 1 trial; 200 women | MD 5.4, 95% CI 3.0 to 7.8 (P < 0.001), significant increase in Hb concentration at birth for women receiving IM iron | Not assessed | |
| Not anaemic at term | 1 trial; 200 women | RR 1.23, 95% CI 1.01 to 1.48 (P = 0.035), significant increase in not anaemic at term for women receiving IM iron | Not assessed | ||
| IM iron dextran versus oral iron plus vitamin C plus folic acid | Not anaemic at 6 weeks (packed cell volume > 33%) | 1 trial; 60 women | RR 11.0, 95% CI 1.51 to 79.96 (P = 0.018), significant increase in not anaemic at 6 weeks for women receiving IM iron | Not assessed | |
| IM iron sorbitol citric acid versus oral iron plus folic acid | Mean Hb at 36 weeks (g/L) | 1 trial; 150 women | MD −2.60, 95% CI −4.80 to −0.40 (P = 0.023), significant reduction in mean Hb at 36 weeks for women receiving IM iron | Not assessed | |
| Diarrhoea | 1 trial; 150 women | RR 0.09, 95% CI 0.01 to 1.62, no evidence of a difference | Not assessed | ||
| Constipation | 1 trial; 150 women | RR 0.06, 95% CI 0.00 to 1.00, no evidence of a difference | Not assessed | ||
| Oral iron daily versus oral iron twice weekly | Hb level at 4 weeks (g/L) | 1 trial; 160 women | MD 5.40, 95% CI 1.40 to 9.40 (P = 0.008), significant increase in Hb concentration at 4 weeks for women receiving oral iron daily | Not assessed | |
| Hb level at 8 weeks (g/L) | 1 trial; 129 women | MD 11.7, 95% CI 6.7 to 16.7 (P < 0.001), significant increase in Hb concentration at 8 weeks for women receiving oral iron daily | Not assessed | ||
| Hb level at 12 weeks (g/L) | 1 trial; 105 women | MD 12.7, 95% CI 6.8 to 18.6 (P < 0.001), significant increase in Hb concentration at 12 weeks for women receiving oral iron daily | Not assessed | ||
| Hb level at 16 weeks (g/L) | 1 trial; 102 women | MD 3.00, 95% CI −0.10 to 6.10, no evidence of a difference | Not assessed | ||
| Oral iron daily versus oral iron once week | Hb level at 16 weeks (g/L) | 1 trial; 97 women | MD 7.00, 95% CI 3.60 to 10.40 (P < 0.001), significant increase in Hb concentration at 16 weeks for women receiving oral iron daily | Not assessed | |
| Oral iron twice a week versus oral iron once a week | Hb level at 16 weeks (g/L) | 1 trial; 101 women | MD 4.00, 95% CI 0.30 to 7.70 (P = 0.035), significant increase in Hb concentration at 16 weeks for women receiving oral iron twice a week | Not assessed | |
| IV iron sucrose 500 mg versus IV iron sucrose 200 mg | Hb level at delivery (g/L) | 1 trial; 35 women | MD 5.00, 95% CI −1.80 to 11.80, no evidence of a difference | Not assessed | |
| IV iron sucrose 500 mg versus IM iron sorbitol | Maternal haemoglobin level at birth (g/L) | 1 trial; 40 women | MD 16.0, 95% CI 8.7 to 23.3 (P < 0.001), significant increase in Hb concentration at birth for women receiving IV iron sucrose 500 mg | Not assessed | |
| IV iron sucrose 200 mg versus IM iron sorbitol | Hb level at delivery (g/L) | 1 trial; 45 women | MD 11.00, 95% CI 4.90 to 17.10 (P < 0.001), significant increase in Hb concentration at birth for women receiving IV iron sucrose 200 mg | Not assessed | |
| Oral iron polymaltose complex (100 mg) versus ferrous sulphate (120 mg) | Hb level at 8 weeks (g/L) | 1 trial; 100 women | MD −0.50, 95% CI −3.00 to 2.00, no evidence of a difference | Not assessed | |
| Constipation at 8 weeks | 1 trial; 100 women | RR 0.30, 95% CI 0.14 to 0.64 (P = 0.002), significant reduction in constipation at 8 weeks for women receiving oral iron polymaltose complex | Not assessed | ||
| Diarrhoea | 1 trial; 100 women | RR 0.22, 95% CI 0.01 to 4.39, no evidence of a difference | Not assessed | ||
| Oral bovine lactoferrin versus ferrous sulphate | Mean Hb levels at 1 month (g/L) | 1 trial; 97 women | MD −3.00, 95% CI −5.20 to −0.80 (P = 0.008), significant decrease in Hb concentration at birth for women receiving oral bovine lactoferrin | Not assessed | |
| Ferrous sulphate (elementary iron) 20 mg versus 40 mg | Hb level at 8 weeks (or until birth) (g/L) | 1 trial; 114 women | MD −3.00, 95% CI −7.40 to 1.40, no evidence of a difference | Not assessed | |
| Rate of anaemia at 8 weeks | 1 trial; 114 women | RR 1.45, 95% CI 0.84 to 2.52, no evidence of a difference | Not assessed | ||
| Rate of moderate anaemia at 8 weeks | 1 trial; 114 women | RR 1.66, 95% CI 0.58 to 4.76, no evidence of a difference | Not assessed | ||
| Vomiting at 8 weeks | 1 trial; 120 women | RR 0.71, 95% CI 0.39 to 1.30, no evidence of a difference | Not assessed | ||
| Ferrous sulphate (elementary iron) 20 mg versus 80 mg | Hb level at 8 weeks (or until birth) (g/L) | 1 trial; 110 women | MD −8.00, 95% CI −12.70 to −3.30 (P < 0.001), significant reduction in Hb level at 8 weeks for women receiving 20 mg ferrous sulphate | Not assessed | |
| Rate of patients with anaemia at 8 weeks | 1 trial; 110 women | RR 1.56, 95% CI 0.87 to 2.79, no evidence of a difference | Not assessed | ||
| Rate of moderate anaemia at 8 weeks | 1 trial; 111 women | RR 1.76, 95% CI 0.44 to 7.01, no evidence of a difference | Not assessed | ||
| Vomiting at 8 weeks | 1 trial; 119 women | RR 0.53, 95% CI 0.30 to 0.93 (P = 0.027), significant reduction in vomiting at 8 weeks for women receiving 20 mg ferrous sulphate | Not assessed | ||
| Ferrous sulphate (elementary iron) 40 mg versus 80 mg | Hb level at 8 weeks (or until birth) (g/L) | 1 trial; 112 women | MD −5.00, 95% CI −9.30 to −0.70 (P = 0.022), significant reduction in Hb level at 8 weeks for women receiving 40 mg ferrous sulphate | Not assessed | |
| Rate of patients with anaemia at 8 weeks | 1 trial; 110 women | RR 1.56, 95% CI 0.87 to 2.79, no evidence of a difference | Not assessed | ||
| Rate of moderate anaemia at 8 weeks | 1 trial; 107 women | RR 1.64, 95% CI 0.41 to 6.5, no evidence of a difference | Not assessed | ||
| Vomiting at 8 weeks | 1 trial; 121 women | RR 0.75, 95% CI 0.46 to 1.21, no evidence of a difference | Not assessed | ||
| IV iron plus oral iron versus oral iron | Mean pre‐delivery maternal Hb (g/L) | 1 trial; 183 women | MD 4.80, 95% CI 2.10 to 7.50 (P < 0.001), significant increase in Hb level at 8 weeks for women receiving IV iron | Not assessed | |
| Mean maternal Hb after delivery (g/L) | 1 trial; 112 women | MD 3.90, 95% CI 0.20 to 7.60 (P = 0.037), significant reduction in Hb level at 8 weeks for women receiving IV iron | Not assessed | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Rumbold‐2015 Vitamin C supplementation in pregnancy |
Vitamin C supplementation alone or in combination with other supplements | Maternal Hb | No trials reported on the outcome | No data | Not assessed |
| Maternal anaemia | No trials reported on the outcome | No data | Not assessed | ||
| Side effects of supplementation: 'any side effect' | 1 trial; 707 women | RR 1.16, 95% CI 0.39 to 3.41, no evidence of a difference | Not assessed | ||
| Side effects of supplementation: 'abdominal pain' | 1 trial; 1877 women | RR 1.66, 95% CI 1.16 to 2.37 (P = 0.006), significant increase in abdominal pain for women receiving vitamin C supplementation | Not assessed | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Shi‐2015 Intravenous iron sucrose versus oral iron in the treatment of pregnancy with iron deficiency anaemia: a systematic review |
IV iron versus oral iron | Maternal haemoglobin (g/L) level at the end of treatment | 6 trials; 576 women | MD 8.50, 95% CI 3.10 to 13.90 (P = 0.002), significant increase in Hb concentration for women receiving IV iron | Not assessed |
| Adverse events at the end of treatment | 4 trials; 439 women | RR 0.50, 95% CI 0.34 to 0.73 (P < 0.001), significant reduction in adverse events for women receiving IV iron | Not assessed | ||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Thorne_x002d_Lyman‐2012 Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta‐analysis |
Vitamin A versus placebo or multivitamins | Hb (g/L) | 6 trials; 1034 pregnant women | MD 3.50, 95% CI 2.40 to 4.50 (P < 0.001), significant increase in Hb concentration for pregnant women receiving vitamin A supplementation | Moderate |
| Anaemia | 6 trials; 1587 pregnant women | RR 0.81, 95% CI 0.69 to 0.94 (P = 0.007), significant reduction in anaemia for pregnant women receiving vitamin A supplementation | High | ||
| Severe anaemia | 2 trials; 961 pregnant women | RR 0.93, 95% CI 0.59 to 1.45, no evidence of a difference | Low | ||
| Fortification | |||||
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Aaron‐2015 Multiple‐micronutrient fortified non‐dairy beverage interventions reduce the risk of anemia and iron deficiency in school‐aged children in low‐middle income countries: a systematic review and meta‐analysis |
Non‐dairy MMN‐fortified beverages versus iso‐caloric non‐fortified beverage | Hb | 1 trial; 439 pregnant women | Significant improvement in Hb (P = 0.015) in the intervention group compared with the control group | Not assessed |
| https://revman.cochrane.org/#/498917022710493686/dashboard/htmlView/2.175.57?revertEnabled=false#REF‐Suchdev‐2015 Multiple micronutrient powders for home (point‐of‐use) fortification of foods in pregnant women |
MNP for point‐of‐use fortification of foods versus iron and folic acid supplements | Hb concentration (g/L) at 32 weeks’ gestation | 1 trial; 405 women | MD −2.50, 95% CI −4.85 to −0.15 (P = 0.037), significant reduction in Hb concentration for women receiving MNP‐fortified food | Not assessed |
| Any anaemia at 32 weeks’ gestation | 1 trial; 405 women | RR 1.25, 95% CI 1.00 to 1.57 (P = 0.047), significant increase in anaemia for women receiving MNP fortified food | Not assessed | ||
| MNP for point‐of‐use fortification of foods versus same multiple micronutrients in supplements | Maternal Hb (g/L) at term or near term | 1 trial; 470 women | MD 1.00, 95% CI −1.77 to 3.77, no evidence of a difference | Not assessed | |
| Maternal anaemia at term or near term | 1 trial; 470 women | RR 0.92, 95% CI 0.53 to 1.59, no evidence of a difference | Very low | ||
CI: confidence interval; FCM: ferric carboxymaltose; Hb: haemoglobin; ID: iron deficiency; IDA: iron deficiency anaemia; IM: intramuscular; IPM: iron polymaltose; IS: iron sucrose; IV: intravenous; MD: mean difference; MMN: multiple micronutrient; MNP: micronutrient powders; OR: odds ratio; RR: risk ratio; SMD: standardised mean difference.
Supplementation (22 reviews)
Twenty‐two reviews assessed supplementation interventions in pregnant women (Abu Hashim 2017; Bhutta 2012; Buppasiri 2015; Daru 2016; Das 2018; De‐Regil 2015; Govindappagari 2019; Haider 2011; Haider 2013; Imdad 2012; Keats 2019; Lassi 2013; McCauley 2015; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Qassim 2018; Qassim 2019; Radhika 2019; Reveiz 2011; Rumbold 2015; Shi 2015; Thorne‐Lyman 2012). See Table 25.
Iron supplementation
The effect of iron supplementation during pregnancy was assessed in 11 reviews (Daru 2016; Govindappagari 2019; Haider 2013; Imdad 2012; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Qassim 2018; Qassim 2019; Radhika 2019; Reveiz 2011; Shi 2015). See Table 25.
Prevention
Daily iron supplementation
Fourty‐eight RCTs and cluster‐RCTs randomising 17,793 women were included in one review assessing prenatal iron use (dose: 10 mg to 240 mg (1 trial used a daily dose of 900 mg); frequency: daily) in pregnant women of any gestational age (Haider 2013). IDA was defined as Hb less than 110 g/L and serum ferritin less than 12 μg/L, and ID was defined as serum ferritin less than 12 μg/L. Iron interventions with or without folic acid increased Hb concentration in the third trimester or at delivery (WMD 4.59 g/L, 95% CI 3.72 to 5.46; 36 trials; GRADE: not assessed) and in the postpartum period (WMD 6.79 g/L, 95%CI 0.22 to 13.36; 12 trials; GRADE: not assessed), but not in the second trimester (WMD −0.23 g/L, 95% CI −12.18 to 11.72; 8 trials; GRADE: not assessed). The intervention also reduced anaemia in (RR 0.50, 95% CI 0.42 to 0.59; 20 trials; GRADE: not assessed), IDA (RR 0.40, 95% CI 0.26 to 0.60; 6 trials; GRADE: not assessed), and ID in the third trimester or at delivery (RR 0.59, 95% CI 0.44 to 0.79; 8 trials; GRADE: not assessed). Women receiving iron only compared with no iron or placebo showed increased Hb concentrations in the third trimester or at delivery (WMD 4.50 g/L, 95% CI 3.62 to 5.39; 31 trials; GRADE: not assessed) and in the postpartum period (WMD 7.01 g/L, 95% CI 0.36 to 13.66; 8 trials; GRADE: not assessed), as well as reduced anaemia (RR 0.56, 95% CI 0.48 to 0.65; 17 trials; GRADE: not assessed), IDA (RR 0.37, 95% CI 0.23 to 0.60; 5 trials; GRADE: not assessed) and ID in the third trimester or at delivery (RR 0.59, 95% CI 0.44 to 0.79; 8 trials; GRADE: not assessed). Iron with folic acid increased Hb levels (WMD 10.41 g/L, 95% CI 5.36 to 15.46; 9 trials; GRADE: not assessed) and reduced anaemia (RR 0.44, 95% CI 0.37 to 0.53; 5 trials; GRADE: not assessed) in the third trimester or at delivery compared with no iron and folic acid or placebo.
Another review, Imdad 2012, included 30 RCTs, cluster‐RCTs and quasi‐RCTs to assess the impact of routine iron supplementation (dose: 20 mg to 300 mg; frequency: daily) in pregnant women. Women receiving iron supplementation had a reduced risk of anaemia at term (RR 0.31, 95% CI 0.22 to 0.44; 18 trials, 8665 women; moderate‐certainty evidence) and IDA (defined as Hb less than 110 g/L) at term (RR 0.44, 95% CI 0.28 to 0.68; 7 trials; GRADE: not assessed) compared with women receiving no iron. There was no evidence of a difference for severe anaemia at any time during the second and third trimester (RR 0.25, 95% CI 0.03 to 2.48; 13 trials; GRADE: not assessed) or at term (RR 4.83, 95% CI 0.23 to 99.88; 11 trials; GRADE: not assessed).
Peña‐Rosas 2015b conducted a comprehensive review assessing the effects of daily oral iron supplementation (dose: 9 mg to 900 mg; frequency: daily) for pregnant women of any gestational age, which included 61 RCTs, cluster‐RCTs and quasi‐RCTs, randomising a total of 43,274 women. Maternal IDA at term was defined by trialists at 37 weeks’ gestation or more, and maternal ID at term was defined by trialists, based on any indicator of iron status at 37 weeks’ gestation or more. Any supplements containing iron compared with the same supplements without iron or no treatment or placebo increased maternal Hb concentration at or near term (MD 8.88 g/L, 95% CI 6.96 to 10.80; 19 trials, 3704 women; GRADE: not assessed) and within six weeks postpartum (MD 7.61 g/L, 95% CI 5.50 to 9.72; 7 trials, 956 women; GRADE: not assessed). The intervention reduced maternal anaemia (RR 0.30, 95% CI 0.19 to 0.46; 14 trials, 2199 women; low‐certainty evidence), IDA (RR 0.33, 95% CI 0.16 to 0.69; 6 trials, 1088 women; GRADE: not assessed), ID (RR 0.43, 95% CI 0.27 to 0.66; 7 trials, 1256 women; low‐certainty‐evidence) at term (or near term), and severe anaemia postpartum (RR 0.04, 95% CI 0.01 to 0.28; 8 trials, 1339 women; GRADE: not assessed). There was no evidence of a difference between the groups for the outcomes of moderate anaemia at postpartum (RR 0.55, 95% CI 0.12 to 2.51; 3 trials, 766 women; GRADE: not assessed), maternal severe anaemia at any time during the second and third trimester (RR 0.22, 95% CI 0.01 to 3.20; 9 trials, 2125 women; very low‐certainty evidence), maternal severe anaemia at or near term (RR 0.47, 95% CI 0.01 to 44.11; 8 trials, 1819 women; GRADE: not assessed). There was also no evidence of a difference between the iron supplementation and control groups for side effects (RR 1.29, 95% CI 0.83 to 2.02; 11 trials, 2423 women; very low‐certainty evidence). While diarrhoea was reduced for women receiving iron supplementation compared with the same supplements without iron or no treatment or placebo (RR 0.55, 95% CI 0.32 to 0.93; 3 trials, 1088 women; GRADE: not assessed), there were no differences between the groups for constipation (RR 0.95, 95% CI 0.62 to 1.43; 4 trials, 1495 women; GRADE: not assessed) and vomiting (RR 0.88, 95% CI 0.59 to 1.30; 4 trials, 1392 women; GRADE: not assessed). In a second comparison, any supplements containing iron and folic acid versus the same supplements without iron nor folic acid (no iron nor folic acid or placebo) showed increased maternal Hb levels at or near term (MD 16.13 g/L, 95% CI 12.74 to 19.52; 3 trials, 140 women; GRADE: not assessed) and within six weeks postpartum (MD 10.07 g/L, 95% CI 7.33 to 12.81; 2 trials, 459 women; GRADE: not assessed). Any supplements containing iron and folic acid also resulted in a reduction of maternal anaemia at term (or near term) (RR 0.34, 95% CI 0.21 to 0.54; 3 trials, 346 women; moderate‐certainty evidence), moderate anaemia at postpartum (RR 0.33, 95% CI 0.17 to 0.65; 3 trials, 491 women; GRADE: not assessed), maternal ID at term (or near term) (RR 0.24, 95% CI 0.06 to 0.99; 1 trial, 131 women; low‐certainty evidence), maternal severe anaemia at any time during second and third trimester (RR 0.12, 95% CI 0.02 to 0.63; 4 trials, 506 women; very low‐certainty evidence) and severe anaemia at postpartum (RR 0.05, 95% CI 0.00 to 0.76; 3 trials, 491 women; GRADE: not assessed). There were no differences between the intervention and control groups for maternal IDA at term (or near term) (RR 0.43, 95% CI 0.17 to 1.09; 1 trial, 131 women; GRADE: not assessed) and maternal severe anaemia at or near term (RR 0.14, 95% CI 0.01 to 2.63; 3 trials, 191 women; GRADE: not assessed). However, the intervention increased the risk of side effects (RR 44.32, 95% CI 2.77 to 709.09; 1 trial, 456 women; moderate‐certainty evidence).
Intermittent iron supplementation
Peña‐Rosas 2015a included 21 RCTs, cluster‐RCTs and quasi‐RCTs of 5490 pregnant women of any gestational age to examine intermittent iron supplementation with or without other vitamins and minerals (dose: 80 mg to 300 mg elemental iron; frequency: weekly). Maternal IDA at term was defined as Hb less than 110 g/L and at least one additional laboratory indicator at 37 weeks’ gestation or more, and maternal ID at term was defined by trialists, based on any indicator of iron status at 37 weeks’ gestation or more. Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with the same vitamins and minerals) increased maternal anaemia at or near term (RR 1.66, 95% CI 1.09 to 2.53; 8 trials, 1385 women; GRADE: not assessed) and increased maternal ID at or near term (RR 2.38, 95% CI 1.30 to 4.36; 3 trials, 587 women; GRADE: not assessed). There was no evidence of a difference between the groups for maternal Hb at or near term (MD −2.57, 95% CI −5.18 to 0.04; 8 trials, 1306 women; GRADE: not assessed), maternal anaemia at term (RR 1.22, 95% CI 0.84 to 1.80; 4 trials, 676 women; very low‐certainty evidence), moderate anaemia at any time during the second and third trimester (RR 2.50, 95% CI 0.84 to 7.48; 9 trials, 1291 women; GRADE: not assessed), maternal IDA at term (RR 0.71, 95% CI 0.08 to 6.63; 1 trial, 156 women; very low‐certainty evidence) or near term (RR 2.06, 95% CI 0.65 to 6.61; 2 trials, 278 women; GRADE: not assessed) and severe anaemia at postpartum (RR 0.43, 95% CI 0.04 to 4.64; 1 trial, 169 women; GRADE: not assessed). There were no events reported for severe anaemia at any time during second and third trimesters (6 trials, 1240 women), severe anaemia at or near term (6 trials, 1050 women) and severe anaemia at term (3 trials, 475 women). Women receiving intermittent iron supplementation experienced fewer side effects (RR 0.56, 95% CI 0.37 to 0.84; 11 trials, 1777 women; very low‐certainty evidence) and there were no differences between the groups for diarrhoea (RR 0.80, 95% CI 0.32 to 2.00; 5 trials, 613 women; GRADE: not assessed), constipation (RR 0.85, 95% CI 0.45 to 1.59; 6 trials, 733 women; GRADE: not assessed) or vomiting (RR 1.30, 95% CI 0.79 to 2.15; 6 trials, 954 women; GRADE: not assessed).
Treatment
Oral, IV or IM iron interventions
Govindappagari 2019 included 11 RCTs with 1190 pregnant women with IDA to assess the impact of IV iron (dose: a maximum daily dose of 200 mg; frequency: infused in split doses every other day). Women receiving IV iron were more likely to reach their Hb target (OR 2.66, 95% CI 1.71 to 4.15; 7 trials; GRADE: not assessed), showed increased Hb levels after 4 weeks of treatment (WMD 0.84, 95% CI 0.59 to 1.09; 9 trials; GRADE: not assessed), and experienced fewer adverse events compared with those receiving oral iron (OR 0.35, 95% CI 0.18 to 0.67; 11 trials; GRADE: not assessed).
Qassim 2018 included 21 RCTs involving administration of IV iron (ferric carboxymaltose, iron polymaltose or iron sucrose) to manage antenatal IDA (dose: different dosages; frequency: daily). All IV preparations showed improvements in haematological parameters, with an overall median increase of 21.8 g/L at 3 to 4 weeks and 30.1 g/L by delivery. Qassim 2019 also assessed the effects of IV and oral iron therapy for IDA in pregnant women with 15 trials (dose: different dosages; frequency: daily). Women receiving IV iron therapy showed increases in Hb concentration (MD 7.40, 95% CI 3.90 to 10.90; 9 trials, 1009 women; low‐certainty evidence), but no influence in Hb concentration of neonates at delivery (MD ‐1.00, 95% CI ‐4.70 to 2.80; 6 trials, 849 neonates; low‐certainty evidence) compared to oral iron supplementation.
Radhika 2019 included 18 RCTs with 1633 antenatal women with IDA to assess the effects of IV iron sucrose compared with oral iron therapy (dose: not reported; frequency: daily). Cumulative analysis showed increases in Hb concentration for women receiving IV iron sucrose compared to oral iron supplementation at all time points (MD 0.78 g/L 95% CI 0.57 to 1.00; 11 trials, 3460 women; high‐certainty evidence) and at the end of six weeks' evaluation (MD 0.66 g/L, 95% CI 0.29 to 1.04; 9 trials, 1147 women; high‐certainty evidence). For postpartum women, the cumulative analysis showed increases in Hb concentration for those receiving IV iron sucrose compared to oral iron supplementation from the first week to six weeks of observation (MD 0.83 g/L, 95% CI 0.42 to 1.25; 8 trials, 1370 women; moderate‐certainty evidence), but no statistical difference at six weeks.
In Shi 2015, six RCTs and a total of 576 pregnant women with diagnosed IDA were included to assess the efficacy and safety of IV iron sucrose (dose: weight × (11 g/dL or 12 g/dL – actual Hb) × 0.24 + 500 mg; frequency: every other day) compared with oral iron supplementation (dose: 100 mg to 300 mg elemental iron; frequency: daily). Women receiving IV iron showed increased Hb levels at the end of treatment (MD 8.50, 95% CI 3.10 to 13.90; 6 trials, 576 women; GRADE: not assessed) and fewer adverse events at the of treatment (RR 0.50, 95% CI 0.34 to 0.73; 4 trials, 439 women; GRADE: not assessed) compared with oral iron supplementation.
Another review included 23 RCTs, randomising 3198 pregnant women of any gestational age with diagnosed anaemia attributed to ID, to examine the effects of different treatments for anaemia during pregnancy (dose/frequency: daily or weekly oral iron 20 mg to 300 mg; IM iron dose depending on body weight and Hb deficit and injected on alternating day; IV maximum total dose of iron ranged from 200 mg to 500 mg injected every other day or twice weekly) (Reveiz 2011). Women receiving oral iron supplementation compared with placebo showed increased Hb levels (MD 13.40 g/L, 95% CI 2.70 to 24.20; 2 trials, 215 women; GRADE: not assessed) and reduced anaemia during the second trimester (RR 0.38, 95% CI 0.26 to 0.55; 1 trial, 125 women; GRADE: not assessed). Only one trial with 51 women assessed side effects, nausea and vomiting or constipation for this comparison, and found no differences between the groups. Oral iron plus vitamin A versus placebo also resulted in an increase in Hb levels (MD 13.00 g/L, 95% CI 11.10 to 14.90; 1 trial, 125 women; GRADE: not assessed) and a reduction in anaemia during the second trimester (RR 0.04, 95% CI 0.01 to 0.15; 1 trial, 125 women; GRADE: not assessed). There were no differences for side effects, nausea and vomiting or constipation for the comparisons controlled‐release oral iron versus regular oral iron (1 trial, 49 women), IV iron versus placebo (1 trial, 54 women) and IV iron versus controlled‐release oral iron (1 trial, 52 women). There were also no differences for nausea or vomiting for the comparisons IM iron sorbitol‐citric acid versus IM dextran (1 trial, 48 women), IM iron dextran versus IV iron dextran (1 trial, 49 women) and IM iron sorbitol citric acid versus IV iron dextran (1 trial, 51 women). IV iron versus regular oral iron increased maternal Hb levels at birth (MD 7.50 g/L, 95% CI 3.40 to 11.60; 1 trial, 90 women; GRADE: not assessed) and mean maternal Hb levels at four weeks (MD 4.40 g/L, 95% CI 0.50 to 8.20; 3 trials, 167 women; GRADE: not assessed), and reduced nausea or vomiting or epigastric discomfort (RR 0.33, 95% CI 0.15 to 0.74; 3 trials, 244 women; GRADE: not assessed), constipation (RR 0.11, 95% CI 0.02 to 0.71; 2 trials, 151 women; GRADE: not assessed) and diarrhoea (RR 0.16, 95% CI 0.03 to 0.86; 3 trials, 237 women; GRADE: not assessed), but not side effects (RR 0.38, 95% CI 0.11 to 1.31; 1 trial, 51 women; GRADE: not assessed). IM iron sorbitol‐citric acid versus oral iron increased mean maternal Hb concentration at birth (MD 5.40 g/L, 95% CI 3.00 to 7.80; 1 trial, 200 women; GRADE: not assessed) and the relative risk of being not anaemic at term (RR 1.23, 95% CI 1.01 to 1.48; 1 trial, 200 women; GRADE: not assessed). IM iron dextran led to an increase in not being anaemic at six weeks compared with oral iron with vitamin C and folic acid (RR 11.0, 95% CI 1.51 to 79.96; 1 trial, 60 women; GRADE: not assessed). IM iron sorbitol citric acid decreased mean Hb levels at 36 weeks' gestation (MD −2.60 g/L, 95% CI −4.80 to −0.40; 1 trial, 150 women; GRADE: not assessed), but had no effect on diarrhoea or constipation, compared with oral iron plus folic acid. One trial assessed the effect of daily compared to twice a week oral iron and found an increase in Hb levels at four weeks (MD 5.40 g/L, 95% CI 1.40 to 9.40; 1 trial, 160 women; GRADE: not assessed), eight weeks (MD 11.70 g/L, 95% CI 6.70 to 16.70; 1 trial, 129 women; GRADE: not assessed) and 12 weeks (MD 12.70 g/L, 95% CI 6.80 to 18.60; 1 trial, 105 women; GRADE: not assessed) but not at 16 weeks. Oral iron daily versus oral iron once a week increased Hb levels (MD 7.00 g/L, 95% CI 3.60 to 10.40; 1 trial, 97 women; GRADE: not assessed) as did oral iron twice a week versus oral iron once a week (MD 4.00 g/L, 95% CI 0.30 to 7.70; 1 trial, 101 women; GRADE: not assessed). There was no improvement in Hb levels at delivery for IV iron sucrose 500 mg versus IV iron sucrose 200 mg. IV iron sucrose 500 mg increased Hb levels at birth (MD 16.00 g/L, 95% CI 8.70 to 23.30; 1 trial, 40 women; GRADE: not assessed) compared with IM iron sorbitol, as did IV iron sucrose 200 mg (MD 11.00 g/L, 95% CI 4.90 to 17.10; 1 trial, 45 women; GRADE: not assessed) compared with IM iron sorbitol. Oral iron polymaltose complex (100 mg) compared with ferrous sulphate (120 mg) improved constipation at eight weeks (RR 0.30, 95% CI 0.14 to 0.64; 1 trial, 100 women; GRADE: not assessed), but not Hb levels at eight weeks or diarrhoea. Oral bovine lactoferrin compared with ferrous sulphate decreased mean Hb levels at one month (MD −3.00, 95% CI −5.20 to −0.80; 1 trial, 97 women; GRADE: not assessed). At eight weeks, there was no difference in Hb levels, the rate of anaemia, the rate of moderate anaemia or vomiting for women receiving ferrous sulphate (elementary iron) 20 mg compared with 40 mg of iron (1 trial). However, when compared to 80 mg of ferrous sulphate, the intervention of 20 mg ferrous sulphate resulted in decreased Hb levels at eight weeks (MD −8.00 g/L, 95% CI −12.70 to −3.30; 1 trial, 110 women; GRADE: not assessed) and reduced vomiting (RR 0.53, 95% CI 0.30 to 0.93; 1 trial, 119 women; GRADE: not assessed) but no difference in the rate of anaemia at eight weeks and the rate of moderate anaemia at eight weeks. The intervention of 40 mg ferrous sulphate resulted in a reduction of Hb levels at eight weeks (MD −5.00 g/L, 95% CI −9.30 to −0.70; 1 trial, 112 women; GRADE: not assessed) but no difference in the rate of anaemia at eight weeks and the rate of moderate anaemia at eight weeks and vomiting at eight weeks when compared with 80 mg ferrous sulphate. The combination of IV iron and oral iron increased the mean pre‐delivery maternal Hb levels (MD 4.80 g/L, 95% CI 2.10 to 7.50; 1 trial, 183 women; GRADE: not assessed) and mean maternal Hb levels after delivery (MD 3.90 g/L 95% CI 0.20 to 7.60; 1 trial, 112 women; GRADE: not assessed) compared with oral iron alone.
Prevention and/or treatment
Oral, IV or IM iron interventions
Daru 2016 included 23 RCTs and a total of 3525 pregnant women at any gestation with non‐anaemic ID (NAID) or IDA and assessed the effects of iron interventions (oral, including fortified water, IV or IM) on iron stores and oxygen carrying capacity in pregnancy (dose/frequency: for pregnant women with ID anaemia using weekly 200 mg to 400 mg IV iron or 120 mg oral iron and 30 mg to 80 mg of oral iron for women with NAID on a weekly or daily basis). In three out of six trials, higher Hb levels were observed in pregnant women with IDA receiving IV iron compared with oral iron supplementation. One trial compared IV versus IM iron supplementation in pregnant women with IDA and observed higher Hb levels in the IV group. Daily oral iron resulted in higher Hb levels compared with weekly oral iron supplementation in pregnant women with IDA in one out of three trials. Different doses of oral iron supplementation in pregnant women with IDA were compared in two trials and one trial found an increase in Hb concentration in the higher dose arm. IM versus oral iron supplementation (1 trial) and alternative oral preparations versus a commonly used preparation in pregnant women with IDA showed no evidence of a difference between the groups. Oral iron supplementation versus placebo in pregnant women with IDA or NAID was assessed in six trials and two trials found increased Hb levels in the supplementation group.
Vitamin A supplementation
Two systematic reviews assessed vitamin A supplementation during pregnancy (McCauley 2015; Thorne‐Lyman 2012).
Prevention
Vitamin A alone or with other micronutrients versus placebo or no treatment or micronutrient supplements without vitamin A
One review included 19 trials with over 310,000 pregnant women of any gestational age to assess the effects of vitamin A supplementation (dose: mainly as capsules of 5750 IU to 444,000 IU of vitamin A; frequency: daily or weekly) during pregnancy (McCauley 2015). Vitamin A supplementation alone reduced maternal anaemia (RR 0.64, 95% CI 0.43 to 0.94; 3 trials, 3818 women; moderate‐certainty evidence) compared with placebo or no treatment. However, vitamin A supplementation with other micronutrient versus micronutrient supplements without vitamin A showed no clear effect on maternal anaemia (RR 0.86, 95% CI 0.68 to 1.09; 3 trials, 706 women; low‐certainty evidence).
Prevention and/or treatment
Vitamin A versus placebo or other MNs
Thorne‐Lyman 2012 included 17 RCTs and cluster‐RCTs and investigated the effect of vitamin A supplementation during pregnancy (dose: 3333 IU to 10,000 IU; frequency: daily or weekly). Vitamin A supplementation during pregnancy resulted in an increase in maternal Hb concentration (MD 3.50 g/L, 95% CI 2.40 to 4.50; 6 trials, 1034 women; moderate‐certainty evidence) and a reduction in maternal anaemia (RR 0.81, 95% CI 0.69 to 0.94; 6 trials, 1587 women; high‐certainty evidence), but not severe anaemia (RR 0.93, 95% CI 0.59 to 1.45; 2 trials, 961 women; low‐certainty evidence) compared with placebo or multivitamins.
Prevention
One review included 29 RCTs and quasi‐RCTs with a total of 24,300 pregnant women of any gestational age to assess the effects of vitamin C supplementation during pregnancy (dose: commonly used 1000 mg/day (250 mg to 2000 mg); frequency: daily) (Rumbold 2015). None of the included trials reported on the outcomes of maternal Hb concentration and maternal anaemia. Vitamin C supplementation, alone or in combination with other supplements, did not increase the outcome of "any side effect" (RR 1.16, 95% CI 0.39 to 3.41; 1 trial 707 women; GRADE: not assessed), but did increase the outcome of "abdominal pain" (RR 1.66, 95% CI 1.16 to 2.37; 1 trial, 1877 women; GRADE: not assessed).
Folic acid supplementation
Two reviews evaluated the effect of folic acid supplementation during pregnancy (De‐Regil 2015; Lassi 2013).
Prevention
Folate supplementation versus no intervention, placebo, or other micronutrients without folate
De‐Regil 2015 included five RCTs with a total of 7391 pregnant women of less than 12 weeks' gestation at the time of the intervention. The review examined whether or not preconceptional folate supplementation (dose: 0.4 mg to 4.0 mg folic acid; frequency: daily) reduces the risk of adverse pregnancy outcomes. However, none of the included trials reported on maternal anaemia at or near term.
Thrity‐one RCTs, cluster‐RCTs and quasi‐RCTs randomising 17,771 pregnant women of any age and parity were included in the Lassi 2013 review, which assessed the effectiveness of oral folic acid supplementation (dose: 10 μg to 400 μg folic acid; frequency: daily) alone or with other micronutrients. There was no evidence of a difference between folic acid supplementation versus no folic acid supplementation during pregnancy for the outcomes of mean pre‐delivery Hb concentration (MD −0.03 g/L, 95% CI −0.25 to 0.19; 12 trials, 1806 women; GRADE: not assessed) and pre‐delivery anaemia (RR 0.62, 95% CI 0.35 to 1.10; 8 trials, 4149 women; GRADE: not assessed).
MMN supplementation
Three reviews assessed MMN supplementation with iron and folic acid during pregnancy (Bhutta 2012; Haider 2011; Keats 2019).
Prevention
MMN with iron and folic acid versus iron and folic acid alone
Seven RCTs and cluster‐RCTs of 18,595 healthy pregnant women of any gestation were included in the Bhutta 2012 review, which assessed MMN supplementation during pregnancy to prevent maternal anaemia (dose: United Nations International Multiple Micronutrient Antenatal Preparation (UNIMMAP) formulation; frequency: at least 6 days/week). MMN versus iron and folic acid supplementation showed no improvement in maternal Hb concentration (SMD −0.01 g/L, 95% CI −0.08 to 0.06; 4 trials; low‐certainty evidence) or reduced the risk of maternal anaemia in the third trimester (RR 1.03, 95% CI 0.94 to 1.12; 7 trials; moderate‐certainty evidence).
Haider 2011 included 14 RCTs and cluster‐RCTs and evaluated the impact of MMN supplements for pregnant women of any gestation (dose: UNIMMAP formulation; frequency: daily). MMN supplementation, including iron and folic acid versus iron or folate supplementation alone showed no effect on maternal anaemia in the third trimester (RR 1.03, 95% CI 0.87 to 1.22; 4 trials, high‐certainty evidence).
In Keats 2019, 21 RCTs and cluster‐RCTs randomising 142,496 pregnant women of any gestational age were included to assess the benefits of oral MMN supplementation (dose: different dosages; frequency: daily). Consistent with the two other reviews, no difference was observed for MMN with iron and folic acid compared with iron with or without folic acid supplementation on maternal anaemia (RR 1.04, 95% CI 0.94 to 1.15; 9 trials; GRADE: not assessed). However, MMN with iron and folic acid versus placebo showed a reduction of maternal anaemia (RR 0.66, 95% CI 0.51 to 0.85; 1 trial; GRADE: not assessed).
Prevention
Twenty‐five RCTs randomising 18,578 pregnant women were included in the Buppasiri 2015 systematic review. This review assessed the effect of calcium supplementation (dose: 300 mg to 2000 mg; frequency: daily) other than for preventing or treating hypertension and found no evidence of a difference in maternal anaemia for women receiving calcium supplementation during pregnancy compared with placebo or no treatment (RR 1.04, 95% CI 0.90 to 1.22; 1 trial, 1098 women; GRADE: not assessed).
Prevention
Das 2018 included 3 RCTs and 1 cluster‐RCT with 8018 pregnant women (dose: 118 kcal/day or 372 kcal/day; frequency: daily). One study showed an increase in the prevalence of maternal anaemia in the LNS group compared to iron and folic acid (RR 2.35, 95% CI 1.67 to 3.30; 1 trial, 536 women; moderate‐certainty evidence), but no difference in adverse effects (hospitalisation episode) between the groups (RR 1.34, 95% CI 0.93 to 1.94; 1 trial, 881 women; GRADE: not assessed). Compared to MMN, the same study showed an increase in the prevalence of maternal anaemia in the LNS group (RR 1.40, 95% CI 1.07 to 1.82; 1 trial, 557 women; moderate‐certainty evidence), but no difference in adverse effects (hospitalisation episode) between the groups (RR 1.18, 95% CI 0.83 to 1.68; 1 trial, 879 women; GRADE: not assessed).
Treatment
One review including four RCTs and 600 pregnant women with IDA assessed the efficacy of oral bovine lactoferrin (dose/frequency: 100 mg twice a day or 250 mg once a day) (Abu Hashim 2017). Oral bovine lactoferrin supplementation, compared with oral ferrous iron preparations, increased Hb levels at four weeks (MD 7.70 g/L, 95% CI 0.40 to 15.50; 4 trials, 600 women; low‐certainty evidence) and reduced the gastrointestinal side effects of 'epigastric discomfort' (OR 0.11, 95% CI 0.05 to 0.22; 2 trials, 328 women; moderate‐certainty evidence), 'vomiting' (OR 0.32, 95% CI 0.15 to 0.67; 2 trials, 328 women; moderate‐certainty evidence), 'constipation' (OR 0.22, 95% CI 0.12 to 0.40; 2 trials, 328 women; moderate‐certainty evidence), 'abdominal colicky pain' (OR 0.21, 95% CI 0.12 to 0.39; 1 trial, 228 women; moderate‐certainty evidence), 'dark stool' (OR 0.01, 95% CI 0.00 to 0.22; 1 trial, 228 women; moderate‐certainty evidence), but not 'diarrhoea' (OR 0.0, 95% CI 0.0 to 0.0; 1 trial, 228 women; GRADE: not assessed).
Fortification (2 reviews)
Two reviews assessed fortification interventions in pregnant women (Aaron 2015; Suchdev 2015).
Prevention
Non‐dairy MMN‐fortified beverages versus iso‐caloric non‐fortified beverage
Aaron 2015 included 10 trials with a total of 4645 participants and evaluated the impact of MMN‐fortified beverages on Hb levels in school‐aged children and women of reproductive age (dose: not reported; frequency: daily). There was a clear improvement in Hb levels for pregnant women receiving non‐dairy MMN‐fortified beverages compared with iso‐caloric non‐fortified beverages (1 trial, 439 women).
MNP for point‐of‐use fortification of foods versus iron and folic acid supplementation or same MMN as supplements
Suchdev 2015 included two cluster‐RCTs, randomising 1172 pregnant women of any age and gestation, and assessed the effects of prenatal home fortification of foods with MNP on maternal Hb concentration and anaemia (dose: different dosages; frequency: daily or weekly). Women receiving MNP for point‐of‐use fortification of foods showed reduced Hb levels at 32 weeks' gestation (MD −2.50 g/L, 95% CI −4.85 to −0.15; 1 trial, 405 women; GRADE: not assessed) and increased risk of any anaemia at 32 weeks' gestation (RR 1.25, 95% CI 1.00 to 1.57; 1 trial, 405 women; GRADE: not assessed) compared with women receiving iron and folic acid supplements. There were no clear differences in maternal Hb levels at term or near term (MD 1.00 g/L, 95% CI −1.77 to 3.77; 1 trial, 470 women; GRADE: not assessed) and maternal anaemia at term or near term (RR 0.92, 95% CI 0.53 to 1.59; 1 trial, 470 women; very low‐certainty evidence) for women receiving MNP for point‐of‐use fortification of foods compared with women receiving the same MMN in supplements.
Improving dietary diversity and quality
None of the included reviews assessed interventions to improve dietary diversity and quality during pregnancy.
Older adult women (aged 50 to 65 years and above)
None of the included reviews focused only on older adult women aged 50 to 65 years and above.
Adult men (aged 19 to 65 years and above)
None of the included reviews focused only on adult men aged 19 to 65 years and above.
Mixed population (all ages) (22 reviews)
Twenty‐two systematic reviews assessed interventions to prevent or control anaemia in mixed populations (Arabi 2020; Basutkar 2019; Casgrain 2012; Das 2019b; Field 2020; Finkelstein 2019; Garcia‐Casal 2018; Geerligs 2003; Gera 2007a; Gera 2009; Gera 2012; Hess 2016; Huo 2015; Peña‐Rosas 2019; Ramírez‐Luzuriaga 2018; Sadighi 2019; Silva Neto 2019; Smelt 2018;Tablante 2019; Tay 2015; Tolkien 2015; Yadav 2019). GRADE assessments were provided in 6 out of 22 reviews. The results are summarised in Table 26.
26. Results of included systematic reviews: mixed populations.
| Review | Comparison | Outcome | Number of studies; number of participants | Results | GRADE assessment |
| Supplementation | |||||
|
Arabi 2020 The effect of vitamin D supplementation on hemoglobin concentration: a systematic review and meta‐analysis |
Vitamin D supplements versus control | Hb (g/L) | 1 trial, 205 healthy adults; 2 trials, 466 anaemic patients |
SMD 0.13, 95% CI ‐0.16 to 0.42 (P = 0.38), vitamin D supplementation leads to a non‐significant reduction in haemoglobin levels; SMD 0.02, 95% CI −0.20 to 0.24 (P = 0.84), vitamin D supplementation leads to a non‐significant reduction in haemoglobin levels |
Not assessed |
| Ferritin | 3 trials, 303 healthy adults; 2 trials, 466 anaemic patients |
SMD −0.17, 95% CI −0.72 to 0.39 (P = 0.56) SMD −0.18, 95% CI −0.36 to 0.01 (P = 0.06) |
Not assessed | ||
|
Basutkar 2019 Vitamin D supplementation in patients with iron deficiency anaemia: A systematic review and a meta‐analysis |
Vitamin D supplementation versus control groups administered placebo | Hb (g/L) | 4 trials, 407 participants | MD −0.05, 95% CI −0.39 to 0.28 (P < 0.18), vitamin D supplementation had no statistically significant impact on the outcomes of haemoglobin | High |
| Ferritin | 3 trials, 396 participants | MD 1.70, 95% CI −9.12 to 12.53 (P < 0.21), Vitamin D supplementation had no statistically significant impact on the outcomes of serum ferritin | High | ||
| Casgrain 2012 Effect of iron intake on iron status: a systematic review and meta‐analysis of randomized controlled trials |
Iron supplementation versus placebo or control | Hb (g/L) | 37 trials, 49 arms; 3577 participants | MD 5.10, 95% CI 3.70 to 6.50 (P < 0.001), significant increase in Hb concentration for participants receiving iron supplementation | Not assessed |
| Gera 2007 Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials |
Iron supplementation versus placebo | Hb (g/L) | 55 trials; 12,198 participants | WMD 7.40, 95% CI 6.10 to 8.70 (P < 0.001), significant increase in Hb concentration for children receiving iron supplementation | Not assessed |
| Gera 2009 Effect of combining multiple micronutrients with iron supplementation on Hb response in children: systematic review of randomized controlled trials |
Iron and multiple micronutrient supplementation versus placebo or no treatment | Hb (g/L) | 23 trials; 4981 participants | WMD 6.50, 95% CI 5.00 to 8.00 (P < 0.001), significant increase in Hb concentration for children receiving iron and multiple micronutrients versus placebo or no treatment | Not assessed |
| Iron and multiple‐micronutrient supplementation versus iron supplementation | Hb (g/L) | 13 trials; 1483 participants | WMD 1.40, 95% CI 0.00 to 2.80 (P = 0.044), significant increase in Hb concentration for children receiving iron and multiple micronutrients versu2`s iron alone | Not assessed | |
| Silva 2018 Effects of iron supplementation versus dietary iron on the nutritional iron status: systematic review with meta‐analysis of randomized controlled trials |
Children: dietary intervention versus iron supplementation | Hb (g/L) | Iron status: anaemia or deficient = 3 trials; 425 children |
MD 3.19, 95% CI 1.31 to 5.07 (P < 0.001), significant increase in Hb concentration for children with anaemia or iron deficiency anaemia receiving supplementation | Not assessed |
| Iron status: non‐anaemia or sufficient = 3 trials; 305 children | MD −6.58, 95% CI −11.52 to −1.64 (P = 0.009), significant reduction in Hb concentration for non‐anaemic children receiving dietary intervention | Not assessed | |||
| Final prevalence of anaemia | 3 trials; number of participants: not reported | 3 trials reported final anaemia prevalence after supplementation versus fortification: 4.3% versus 9.7%, 42.5% versus 54.9%, and 6.6% versus 9.7% | Not assessed | ||
| Adolescents and adults: dietary plan versus iron supplement only | Hb (g/L) | 5 trials; 165 participants | MD 0.04, 95% CI −2.50 to 2.58, no evidence of a difference | Not assessed | |
| Pregnant women: dietary plan versus iron supplement only | Hb (g/L) | 1 trial; number of participants: not reported | 113.3 (± 8.8) for supplementation versus 112.1 (± 8.4) for fortified food, significant increase in Hb for pregnant women receiving supplementation | Not assessed | |
|
Smelt 2018 The effect of vitamin B12 and folic acid supplementation on routine haematological parameters in older people: an individual participant data meta‐analysis |
Vitamin B12 supplementation versus placebo | Hb (g/L) | 4 trials; 343 participants | MD 0.00, 95% CI −0.19 to 0.18, no evidence of a difference | Not assessed |
| Folic acid supplementation versus placebo | Hb (g/L) | 3 trials; 929 participants | MD −0.09, 95% CI −0.19 to 0.01, no evidence of a difference | Not assessed | |
|
Tay 2015 Systematic review and meta‐analysis: what is the evidence for oral iron supplementation in treating anaemia in elderly people? |
Oral iron supplementation versus no oral supplementation or placebo | Hb (g/L) | 3 trials; 438 elderly people | MD 3.50, 95 % CI 1.20 to 5.90 (P = 0.003), significant increase in Hb concentration for elderly people taking oral iron | Not assessed |
| Adverse effects | 3 trials; 440 participants | 36 participants with oral iron supplementation reported adverse events (constipation, diarrhoea, abdominal pain, indigestion, nausea and vomiting) | Not assessed | ||
|
Tolkien 2015 Ferrous sulfate supplementation causes significant gastrointestinal side‐effects in adults: a systematic review and meta‐analysis |
Adults, including pregnant women: oral iron supplementation versus placebo | Incidence of GI side effects | 20 trials; 3168 participants | OR 2.32, 95% CI 1.74 to 3.08 (P < 0.0001), significant increase in the incidence of GI side effects for participants receiving oral iron versus placebo | Not assessed |
| Adults, including pregnant women: oral iron supplementation versus IV iron | Hb | 20 trials; number of participants: not reported | Increase in Hb for oral iron supplementation was lower than for IV iron | Not assessed | |
| Incidence of GI side effects | 23 trials; number of participants: not reported | OR 3.05, 95% CI 2.07 to 4.48 (P < 0.0001), significant increase in the incidence of GI side effects for participants receiving oral iron versus IV iron | Not assessed | ||
| Adults, including pregnant women: oral iron supplementation versus placebo or IV iron | Constipation | 27 trials; number of participants: not reported | Incidence in the oral iron group was 12%, 95% CI 10% to 15% | Not assessed | |
| Nausea | 30 trials; number of participants: not reported | Incidence in the oral iron group was 11%, 95% CI 8% to 14% | Not assessed | ||
| Diarrhoea | 25 trials; number of participants: not reported | Incidence in the oral iron group was 8%, 95% CI 6% to 11% | Not assessed | ||
| Pregnant women only: oral iron supplementation versus placebo or IV iron | Incidence of GI side effects | 5 trials; 561 pregnant women | OR 9.44, 95% CI 2.23 to 39.93 (P = 0.002), increase in the incidence of GI side effects for participants receiving oral iron versus IV iron | Not assessed | |
| Fortification | |||||
|
Das 2019b Food fortification with multiple micronutrients: impact on health outcomes in general population |
MMN fortification versus placebo/no intervention | Serum Hb level (g/L) | 20 trials; 6985 participants | MD 3.01 g/L, 95% CI 2.14 to 3.87 (P < 0.001), significant increase in Hb concentration for participants receiving MMN fortification | Low |
| Anaemia (Hb < 11 g/dL) | 11 trials; 3746 participants | RR 0.68, 95% CI 0.56 to 0.84 (P < 0.001), significant decrease in anaemia for participants receiving MMN fortification | Low | ||
| IDA (Hb < 11 g/dL with serum ferritin < 15 μg/L) | 6 trials; 2189 participants | RR 0.28, 95% CI 0.19 to 0.39 (P < 0.001), significant decrease in IDA for participants receiving MMN fortification | Low | ||
| ID (serum ferritin < 5 μg/L) | 11 trials; 3289 participants | RR 0.44, 95% CI 0.32 to 0.60 (P < 0.001), significant decrease in ID for participants receiving MMN fortification | Low | ||
|
Field 2020 Wheat flour fortification with iron for reducing anaemia and improving iron status in populations |
Wheat flour fortified with iron alone versus unfortified wheat flour | Hb (g/L) | 7 trials; 2355 participants | MD 3.30 g/L, 95% CI 0.86 to 5.74 (P = 0.008), significant increase in Hb concentration for participants receiving fortified flour | Very low |
| Anaemia | 5 trials; 2200 participants | RR 0.81, 95% CI 0.61 to 1.07, no evidence of a difference | Low | ||
| ID | 3 trials; 633 participants | RR 0.43, 95% CI 0.17 to 1.07, no evidence of a difference | Moderate | ||
| Wheat flour fortified with iron in combination with other micronutrients versus unfortified wheat | Hb (g/L) | 3 trials; 384 participants | MD 3.29 g/L, 95% CI ‐0.78 to 7.36, no evidence of a difference | Low | |
| Anaemia | 2 trials; 322 participants | RR 0.95, 95% CI 0.69 to 1.31, no evidence of a difference | Low | ||
| ID | 3 trials; 387 participants | RR 0.74, 95% CI 0.54 to 1.00, no evidence of a difference | Moderate | ||
| Wheat flour fortified with iron in combination with other micronutrients versus fortified wheat flour with same micronutrients (but not iron) | Hb (g/L) | 2 trials; 488 participants | MD 0.81 g/L, 95% CI ‐1.28 to 2.89, no evidence of a difference | Low | |
| Anaemia | 1 trial; 127 participants | RR 0.24, 95% CI 0.08 to 0.71 (P = 0.009), significant decrease in anaemia for participants receiving fortified flour | Very low | ||
| ID | 1 trial; 127 participants | RR 0.42, 95% CI 0.18 to 0.97 (P = 0.04), significant decrease in ID for participants receiving fortified flour | Very low | ||
|
Finkelstein 2019 Iron biofortification interventions to improve iron status and functional outcomes |
Iron‐biofortified staple crops versus conventional crops | Anaemia (Hb < 120 g/L) | 3 trials; 597 participants | OR 0.83, 95% CI 0.58 to 1.19, no evidence of a difference | Not assessed |
| ID (serum ferrin < 15.0 μg/L) | 3 trials; 603 participants | OR 0.86, 95% CI 0.61 to 1.23, no evidence of a difference | Not assessed | ||
|
Garcia‐Casal 2018 Fortification of maize flour with iron for controlling anaemia and iron deficiency in populations |
Maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing iron nor any other vitamin and minerals) | Hb (g/L) | 3 trials; 1144 participants | MD 1.25, 95% CI −2.36 to 4.86, no evidence of a difference | Very low |
| Anaemia | 2 trials; 1027 participants | RR 0.90, 95% CI 0.58 to 1.40, no evidence of a difference | Very low | ||
| IDA | 1 trial; 515 participants | RR 1.04, 95% CI 0.58 to 1.88, no evidence of a difference | Not assessed | ||
| ID | 2 trials; 1102 participants | RR 0.75, 95% CI 0.49 to 1.15, no evidence of a difference | Very low | ||
| Gera 2012 Effect of iron‐fortified foods on hematologic and biological outcomes: systematic review of randomized controlled trials |
Fortification with iron versus control | Hb (g/L) | 54 trials (77 analytic components); 19,161 participants | WMD 4.20, 95% CI 2.80 to 5.60 (P < 0.001), significant increase in Hb concentration for participants receiving fortification | Not assessed |
| Anaemia at end of fortification | 33 trials; 13,331 participants | RR 0.59, 95% CI 0.48 to 0.71 (P < 0.001), significant reduction in anaemia for participants receiving fortification | Not assessed | ||
| ID | 21 trials; 5765 participants | RR 0.48, 95% CI 0.38 to 0.62 (P < 0.001), significant reduction in ID for participants receiving fortification | Not assessed | ||
| Hess 2016 Micronutrient fortified condiments and noodles to reduce anemia in children and adults—a literature review and meta‐analysis |
Fortified condiments and noodles versus non‐fortified condiments or noodles | Hb (g/L) | 13 trials (14 comparisons); 8845 participants | WMD 6.80, 95% CI 5.10 to 8.50, significant increase in Hb concentration for participants receiving fortified food | Not assessed |
| Anaemia prevalence | 10 trials (11 comparisons); 5498 participants | RR 0.59, 95% CI 0.44 to 0.80, significant reduction in anaemia for participants receiving fortified food | Not assessed | ||
| Huo 2015 Effect of NaFeEDTA‐fortified soy sauce on anemia prevalence in China: a systematic review and meta‐analysis of randomized controlled trials |
NaFeEDTA‐fortified soy sauce versus non‐fortified soy sauce | Hb (g/L) | 12 trials; 8071 participants | MD 8.81 g/L, 95% CI 5.96 to 11.67 (P < 0.001), significant increase in Hb concentration for participants receiving fortified soy sauce | Not assessed |
| Anaemia rates | 16 trials; 16,819 participants | OR 0.25, 95% CI 0.19 to 0.35 (P < 0.001), significant reduction in anaemia for participants receiving fortified soy sauce | Not assessed | ||
|
Peña‐Rosas 2019 Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition |
Rice fortified with iron alone or in combination with other micronutrients versus unfortified rice or no intervention | Hb (g/L) | 11 trials, 2163 participants | MD 1.83 g/L, 95% CI 0.66 to 3.00, significant increase in Hb concentration for participants consuming rice fortified with iron or in combination with other micronutrients | Low |
| Anaemia (WHO cut‐off) | 7 trials, 1634 children | RR 0.72, 95% CI 0.54 to 0.97, significant reduction in anaemia for children consuming rice fortified with iron or in combination with other micronutrients | Low | ||
| ID | 8 trials, 1733 participants | RR 0.66, 95% CI 0.51 to 0.84, significant reduction in ID for participants consuming rice fortified with iron or in combination with other micronutrients | Low | ||
| Diarrhoea | 1 trial, 258 children | RR 0.3.52, 95% CI 0.18 to 67.39, no significant reduction in diarrhoea for children consuming rice fortified with iron or in combination with other micronutrients | Very low | ||
| Adverse effect (hookworm infection risk) | 1 trial, 785 participants | RR 1.78, 95% CI 1.18 to 2.70, significant increase the hookworm infection risk for participants consuming rice fortified with iron or in combination with other micronutrients | Low | ||
| Adverse effect (abdominal pain more than three days) | 1 trial, 234 children | RR 0.77, 95% CI 0.42 to 1.42, no significant increase the risk of abdominal pain more than three days for children given rice fortified with iron or in combination with other micronutrients | Not reported | ||
| Rice fortified with vitamin A alone or in combination with other micronutrients versus unfortified rice or no intervention | Hb (g/L) | 1 trial, 74 participants | MD 10.00 g/L, 95% CI 8.79 to 11.21, significant increase in Hb concentration for participants receiving rice fortified with vitamin A alone or in combination with other micronutrients | Low | |
|
Ramírez‐Luzuriaga 2018 Impact of double‐fortified salt with iron and iodine on hemoglobin, anemia, and iron deficiency anemia: a systematic review and meta‐analysis |
DFS versus control salt | Hb (g/L) | 14 trials; 45,759 participants | MD 3.01, 95% CI 1.79 to 4.24 (P < 0.001), SMD 0.21, 95% CI 0.12 to 0.29 (P < 0.001), significant increase in Hb concentration for participants receiving DFS | Not assessed |
| Anaemia | 10 trials; 42,103 participants | RR 0.84, 95% CI 0.78 to 0.92 (P < 0.001), significant reduction in anaemia for participants receiving DFS | Not assessed | ||
| IDA | 4 trials; 831 participants | RR 0.37, 95% CI 0.25 to 0.54 (P < 0.001), significant reduction in IDA for participants receiving DFS | Not assessed | ||
|
Sadighi 2019 Systematic review and meta‐analysis of the effect of iron‐fortified flour on iron status of populations worldwide |
Iron‐fortified flour versus control | Hb (g/L) | 46 trials (10,353 infants/toddlers, children, women) | MD 2.63 g/L, 95% CI 1.31 to 3.95 (P < 0.001), significant increase in Hb concentration for participants receiving iron‐fortified flour | Not assessed |
| Prevalence of anaemia | 27 trials (6950 infants/toddlers, children, women) | MD −0.08 (−8.1 %), 95% CI −0.117 to −0.044 (P < 0.001), significant reduction in anaemia prevalence for participants receiving iron‐fortified flour | Not assessed | ||
| Prevalence of IDA | 15 trials (4260 infants/toddlers, children, women) | MD −0.209 (−20.9%), 95% CI −0.384 to −0.034 (P = 0.019), significant reduction in IDA prevalence for participants receiving iron‐fortified flour | Not assessed | ||
| Prevalence of ID | 23 trials (5371 infants/toddlers, children, women) | MD −0.120 (−12%), 95% CI −0.189 to −0.051 (P = 0.001), significant reduction in ID prevalence for participants receiving iron‐fortified flour | Not assessed | ||
|
Tablante 2019 Fortification of wheat and maize flour with folic acid for population health outcomes |
Wheat flour fortified with folic acid and other micronutrients versus unfortified wheat flour | Hb g/L | 1 trial, 334 children | MD 0.00 g/L (2.08 lower to 2.08 higher), there were no significant effects of fortified wheat flour flatbread, compared to unfortified wheat flour flatbread, on haemoglobin concentrations | Low |
| Anaemia | 1 trial, 334 children | RR 1.07, 95% CI 0.74 to 1.55 (P = 0.72), there were no significant effects of fortified wheat flour flatbread, compared to unfortified wheat flour flatbread, on anaemia | Low | ||
|
Yadav 2019 Meta‐analysis of efficacy of iron and iodine fortified salt in improving iron nutrition status |
Double‑fortified salt (iron and iodine) (DFS) versus iodine only fortified salt (IS) | Hb g/L | 10 trials | MD 4.40, 95% CI 1.60 to 7.10 (P < 0.01), significant increase in Hb levels for participants consuming DFS | Not assessed |
| Anaemia | 7 trials (1526 participants) | Risk difference (RD) −0.16, 95% CI −0.26 to −0.06 (P < 0.001), significant risk reduction in anaemia for participants consuming DFS | Not assessed | ||
| IDA | Not reported | RD −0.08, 95% CI −0.28 to 0.11, no evidence of a difference | Not assessed | ||
| ID | 5 trials (1306 participants) | RD −0.20, 95% CI: −0.32 to −0.08 (P < 0.001), significant risk reduction in iron deficiency for participants consuming DFS | Not assessed | ||
| Improving dietary diversity and quality | |||||
| Geerligs 2003 Food prepared in iron cooking pots as an intervention for reducing iron deficiency anaemia in developing countries: a systematic review |
Food prepared in cast iron pots versus food prepared in non‐cast iron pots | Change in Hb concentration | 3 trials (784 participants) | 2 of the 3 trials found a difference in haemoglobin at the end of the trial, with children eating food prepared in iron pots having a significantly higher haemoglobin. trial 1: Hb 13 g/L higher in iron pot group after 12 months (P < 0.001), Malaria endemicity very low trial 2: Hb 13 g/L higher in iron pot group after 8 months (P = 0.02), Malaria endemicity none trial 3: Hb 0.2 g/L higher in iron pot group after 5 months for those aged 1‐11 years (parasite rate 45.3%), Hb 3 g/L higher in iron pot group after 5 months for those > 12 years of age (parasite rate 17.5%), not significant |
Not assessed |
CI: confidence interval; DFS: double‐fortified salt; GI: gastrointestinal, Hb: haemoglobin; IS: iodine‐fortified salt; IV: intravenous; MD: mean difference; MMN: multiple micronutrient; OR: odds ratio; RR: risk ratio; SMD: standardised mean difference; WMD: weighted mean difference.
Supplementation (9 reviews)
Nine systematic reviews assessed supplementation interventions in mixed populations (Arabi 2020; Basutkar 2019; Casgrain 2012; Gera 2007a; Gera 2009; Silva Neto 2019; Smelt 2018; Tay 2015; Tolkien 2015). Five systematic reviews focused on prevention (Casgrain 2012; Gera 2007a; Gera 2009; Smelt 2018; Tay 2015), one on treatment (Basutkar 2019), and three reviews on prevention and/or treatment (Arabi 2020; Silva Neto 2019; Tolkien 2015). See Table 26.
Iron supplementation
Iron supplementation in mixed population was assessed in six reviews (Casgrain 2012; Gera 2007a; Gera 2009; Silva Neto 2019; Tay 2015; Tolkien 2015).
Prevention
Iron supplementation versus placebo or control
Forty‐one RCTs randomising 3577 healthy adults aged 18 years and over were included in the systematic review by Casgrain 2012. The review assessed the effects of iron supplementation versus placebo or control on Hb in healthy adults. There was an increase in Hb concentration for participants receiving iron supplementation compared with those receiving placebo or control (MD 5.10 g/L, 95% CI 3.70 to 6.50, 49 arms, 3577 participants, GRADE: not assessed).
Another review, Gera 2007a, included 55 RCTs and cluster‐RCTs randomising over 12,198 children from birth to 19 years of age and compared oral or parenteral iron supplementation (dose: 5 mg/day to 120 mg/day or 1 mg/kg/day to 4 mg/kg/day) with placebo. This review evaluated the effect of oral iron supplementation on Hb in children and found an increase in Hb concentration for the intervention group (WMD 7.40 g/L, 95% CI 6.10 to 8.70; 55 trials, 12,198 children; GRADE: not assessed).
In Tay 2015, three RCTs comprising 440 anaemic elderly people over 64 years of age were included in this systematic review. This review assessed the effectiveness of oral iron supplementation on Hb and adverse effects in elderly people with IDA. The review found higher levels of Hb (MD 3.50 g/L, 95 % CI 1.20 to 5.90; 3 trials, 438 elderly people; GRADE: not assessed) for elderly people taking oral iron supplementation but no differences were observed in adverse effects between the oral iron supplementation and no supplementation or placebo groups.
Iron with MMN versus placebo, no treatment, or iron supplementation
Thirty RCTs were included in the systematic review by Gera 2009, which examined the effects of multiple (2 or more) MNs with iron supplementation (dose: 5 mg to 60 mg; frequency: per day) versus placebo, no treatment or iron supplementation alone in children from birth to 18 years of age. Hb levels increased for children receiving iron with MMN compared with placebo or no treatment (WMD 6.50 g/L, 95% CI 5.00 to 8.00; 23 trials, 4981 participants; GRADE: not assessed). Hb slightly increased for children receiving iron with MMN compared with iron supplementation alone (WMD 1.40 g/L, 95% CI 0.00 to 2.80; 13 trials, 1483 participants; GRADE: not assessed).
Prevention and/or treatment
Dietary intervention and dietary plan or fortified foods versus iron supplementation
Twelve RCTS including 730 children, 164 adolescent or adults and 85 pregnant women, irrespective of age, sex or race, were included in the systematic review by Silva Neto 2019. This review compared the effects of a dietary intervention versus iron supplementation on Hb and other serum biochemical parameters related to iron nutritional status, in a standard treatment period (defined as 12 weeks or more follow‐up). Hb concentration increased for children with anaemia or IDA (MD 3.19 g/L, 95% CI 1.31 to 5.07; 425 children; GRADE: not assessed) who received supplementation but decreased for non‐anaemic children (MD −6.58 g/L; 95% CI −11.52 to −1.64; 3 trials, 305 children, GRADE: not assessed) who received dietary interventions. In three RCTs, the prevalence of anaemia was lower after iron supplementation compared with fortification (supplementation versus fortification: 4.3% versus 9.7%, 42.5% versus 54.9%, 6.6% versus 9.7%). Five RCTs evaluated Hb concentrations for adolescents and adults and found no difference between the dietary plan group and the iron supplementation group (MD 0.04 g/L, 95% CI −2.50 to 2.58; 165 participants; GRADE: not assessed). In one RCT, there was an increase in Hb levels for pregnant women receiving iron supplementation compared to those receiving dietary intervention (113.30 (± 8.80) g/L versus 112.40 (± 8.40) g/L; GRADE: not assessed).
Oral iron supplementation versus placebo or IV iron
Forty‐three RCTs and cross‐over RCTs randomising 6831 adults (including pregnant women) were included in the Tolkien 2015 review. This review evaluated the effect of ferrous sulphate supplementation on Hb, gastrointestinal side effects, and individual side effects in adults. Oral iron supplementation increased the risk of gastrointestinal side effects (OR 2.32, 95% CI 1.74 to 3.08; 20 trials, 3168 participants) compared with placebo. Adults (including pregnant women) receiving IV iron showed higher Hb levels compared with those receiving oral iron supplementation (20 trials). The risk of gastrointestinal side effects increased (OR 3.05, 95% CI 2.07 to 4.48; 23 trials) for adults receiving oral iron supplementation compared with those receiving IV iron. Adults (including pregnant women) receiving oral iron supplementation experienced higher incidences of constipation (12%, 95% CI 10% to 15%; 27 trials), nausea (11%, 95% CI 8% to 14%; 30 trials), and diarrhoea (8%, 95% CI 6% to 11%; 25 trials), compared to those receiving IV iron. An increase in the incidence of gastrointestinal side effects (OR 9.44, 95% CI 2.23 to 33.93; 5 trials, 561 pregnant women) was observed for pregnant women receiving oral iron compared with IV iron.
Prevention
Vitamin B12 or folic acid supplementation versus placebo
Seven RCTs randomising 1306 participants over 60 years of age were included in the Smelt 2018 review. The review examined the effect of vitamin B12 and folic acid supplementation on haematological parameters in an elderly population. There was no difference in Hb concentration (MD 0.00 g/L, 95% CI −0.19 to 0.18; 4 trials, 343 participants) between the vitamin B12 supplementation group and placebo group. Similarly, there was no difference in Hb concentration (MD −0.09 g/L, 95% CI −0.19 to 0.01; 3 trials, 929 participants) between elderly participants allocated to receive folic acid supplementation or placebo.
Vitamin D supplementation
Vitamin D supplementation in mixed population was assessed in two reviews (Arabi 2020; Basutkar 2019).
Treatment
Another review, Basutkar 2019, included 4 RCTs randomising over 429 patients with IDA, evaluated the efficacy of vitamin D supplementation with placebo on Hb concentration and ferritin concentration. This review found no difference in Hb concentration (MD ‐0.05, 95% CI ‐0.39 to 0.28; 4 trials, 407 participants; high‐certainty evidence) and ferritin concentration (MD 1.70, 95% CI ‐9.12 to 12.53; 3 trials, 396 participants; high‐certainty evidence) between the vitamin D supplementation group and placebo group.
Prevention and/or treatment
Fourteen RCTs were included in the systematic review by Arabi 2020, which examined the effects of vitamin D supplementation on haemoglobin concentration in participants aged 17.5 to 68 years. There was no difference in Hb concentration (SMD 0.13, 95% CI ‐0.16 to 0.42; 1 RCT, 205 healthy adults; SMD 0.02, 95% CI ‐0.20 to 0.24; 2 RCTs, 466 anaemic patients) and ferritin concentration (SMD ‐0.17, 95% CI ‐0.72 to 0.39; 3 RCTs, 303 healthy adults; SMD ‐0.18, 95% CI ‐0.36 to 0.01; 2 RCTs, 466 anaemic patients) for participants receiving vitamin D supplementation compared with the control group.
Fortification (12 reviews)
Twelve systematic reviews assessed fortification interventions in mixed populations (Das 2019b; Field 2020; Finkelstein 2019; Garcia‐Casal 2018; Gera 2012; Hess 2016; Huo 2015; Peña‐Rosas 2019; Ramírez‐Luzuriaga 2018; Sadighi 2019; Tablante 2019; Yadav 2019). One review includes is prevention and treatment (Peña‐Rosas 2019), and the others are all prevention reviews (Das 2019b; Field 2020; Finkelstein 2019; Garcia‐Casal 2018; Gera 2012; Hess 2016; Huo 2015; Ramírez‐Luzuriaga 2018; Sadighi 2019; Tablante 2019; Yadav 2019). See Table 26.
Prevention
Ten reviews assessed iron‐fortified foods in mixed populations (Field 2020; Finkelstein 2019; Garcia‐Casal 2018; Gera 2012; Huo 2015; Peña‐Rosas 2019; Ramírez‐Luzuriaga 2018; Sadighi 2019; Tablante 2019; Yadav 2019).
Wheat flour fortified with iron alone or in combination with other micronutrients versus unfortified wheat flour or fortified with the same micronutrients but not iron
Nine RCTs randomising 3166 participants over two years of age were included in the systematic review by Field 2020, which examined the effect of wheat flour fortified with iron alone versus unfortified wheat flour, wheat flour fortified with iron in combination with other micronutrients versus unfortified wheat, wheat flour fortified with iron in combination with other micronutrients versus fortified with the same micronutrients but not iron, on Hb concentration, the risk of anaemia and ID among a general population over two years of age. ID was defined by trialists, based on a biomarker of iron status. Compared with unfortified wheat flour, the intervention with wheat flour fortified with iron alone increased Hb concentrations (MD 3.30 g/L, 95% CI 0.86 to 5.74; 7 trials, 2355 participants; very low‐certainty evidence) but found no difference in the reduction of anaemia (RR 0.81, 95% CI 0.61 to 1.07; 5 trials, 2200 participants; low‐certainty evidence) or ID (RR 0.43, 95% CI 0.17 to 1.07; 3 trials, 633 participants; moderate‐certainty evidence). There was no difference in Hb concentration (MD 3.29 g/L, 95% CI ‐0.78 to 7.36; 3 trials, 384 participants; low‐certainty evidence), the risk of anaemia (RR 0.95, 95% CI 0.69 to 1.31; 2 trials, 322 participants; low‐certainty evidence) and ID (RR 0.74, 95% CI 0.54 to 1.00; 3 trials, 378 participants; moderate‐certainty evidence), between wheat flour fortified with iron in combination with other micronutrients and unfortified wheat. Wheat flour fortification with iron in combination with other micronutrients, as compared to fortified with the same micronutrients but not iron, reduced the risk of anaemia (RR 0.24, 95% CI 0.08 to 0.71; 1 trial, 127 participants; very low‐certainty evidence) and ID (RR 0.42, 95% CI 0.18 to 0.97; 1 trial, 127 participants; very low‐certainty evidence), but no difference was found for Hb concentration (MD 0.81 g/L, 95% CI ‐1.28 to 2.89; 2 trials, 488 participants; low‐certainty evidence).
Iron‐biofortified staple crops versus conventional crops
Three RCTs comprising 633 adolescents and adults (including pregnant or lactating women) were included in the Finkelstein 2019 systematic review. This review assessed the effects of iron biofortified staple crops versus conventional crops on improving iron status and functional outcomes. ID was defined as serum ferritin < 15.0 μg/L. There was no difference in the risk of anaemia (RR 0.83, 95% CI 0.58 to 1.19; 3 trials, 597 participants) and ID (RR 0.86, 95% CI 0.61 to 1.23; 3 trials, 603 participants) for participants receiving iron‐biofortified staple crops compared with conventional crops.
Maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products
Three RCTs and cluster‐RCTs including 2610 participants and two uncontrolled before‐after trials involving 849 participants were included in the Garcia‐Casal 2018 review, which assessed the effects of iron fortification of maize flour, corn meal and fortified maize flour products for anaemia and iron status in the general population. Results from the RCTs showed no effect of maize flour or maize flour products fortified with iron plus other vitamins and minerals versus unfortified maize flours or maize flour products on Hb concentration (MD 1.25 g/L, 95% CI −2.36 to 4.86; 3 trials, 1144 participants; very low‐certainty evidence), anaemia (RR 0.90, 95% CI 0.58 to 1.40; 2 trials, 1027 participants; very low‐certainty evidence), IDA (RR 1.04, 95% CI 0.58, 1.88; 1 trial, 515 participants), or ID (RR 0.75, 95% CI 0.49 to 1.15; 2 trials, 1102 participants; very low‐certainty evidence). Adverse events were not reported in any of the included trials.
Fortification with iron versus control
Sixty RCTs, cluster‐RCTs, and quasi‐RCTs comprising 11,750 iron‐fortified participants and 9077 control participants, irrespective of age and sex, were included in the Gera 2012 review which assessed the effect of iron fortification on Hb and serum ferritin and the prevalence of ID and anaemia. ID was defined in individual trials. Participants receiving iron‐fortified food showed an increase in Hb concentration (WMD 4.20 g/L, 95% CI 2.80 to 5.60; 54 RCTs, 19,161 participants) compared with control. The interventions also reduced anaemia (RR 0.59, 95% CI 0.48 to 0.71; 33 trials, 13,331 participants; GRADE: not assessed) and ID (RR 0.48, 95% CI 0.38 to 0.62; 21 trials, 5765 participants, GRADE: not assessed) compared with control.
Sodium iron ethylenediaminetetraacetate (NaFeEDTA)‐fortified soy sauce versus non‐fortified soy sauce
Sixteen RCTs and cluster‐RCTS comprising 16,819 participants were included in the Huo 2015 systematic review, which examined the effect of NaFeEDTA‐fortified soy sauce on anaemia prevalence in the Chinese population. The intervention led to an increase in Hb concentration (MD 8.81 g/L, 95% CI 5.96 to 11.67; 12 trials, 8071 population, GRADE: not assessed) for participants receiving NaFeEDTA‐fortified soy sauce compared with those receiving non‐fortified soy sauce. The review also reported a reduction in anaemia rates for participants receiving fortified soy sauce (OR 0.25, 95% CI 0.19 to 0.35; 16 trials, 16,819 participants, GRADE: not assessed).
Double‐fortified salt with iron and iodine versus control salt or iodine only fortified salt
Fourteen RCTs, cluster‐RCTs, and quasi‐RCTs randomising over 45,995 participants of any age were included in the Ramírez‐Luzuriaga 2018 systematic review. This review assessed the impact of double‐fortified salt with iron and iodine on biomarkers of iron status, the risk of anaemia and IDA. There was an increase in Hb concentration for participants receiving double‐fortified salt (MD 3.01 g/L, 95% CI 1.79 to 4.24; 14 RCTs, 45,759 participants). Also, the intervention reduced the risk of anaemia (RR 0.84, 95% CI 0.78 to 0.92; 10 trials, 42,103 participants; GRADE: not assessed) and IDA (RR 0.37, 95% CI 0.25 to 0.54; 4 trials, 831 participants; GRADE: not assessed) for participants receiving double‐fortified salt compared with those receiving control salt.
Another review, Yadav 2019, included 10 RCTs randomising over 3219 participants of all age and gender, and assessed the efficacy of double‐fortified salt with iron and iodine as compared to iodine‐fortified salt in improving iron nutrition status. The intervention of double‐fortified salt increased Hb concentrations (MD 0.44 g/L, 95% CI 0.16 to 0.71; 10 trials, GRADE: not assessed), and reduced the risk of having anaemia (risk difference (RD) ‐0.16, 95% CI ‐0.26 to ‐0.06; 7 trials, 1526 participants, GRADE: not assessed) and ID (RD ‐0.20, 95% CI ‐0.32 to ‐0.08; 5 trials, 1306 participants; GRADE: not assessed). There was no difference in the reduction of IDA (RD ‐0.08, 95% CI ‐0.28 to 0.11; GRADE: not assessed) for participants consuming double‐fortified salt.
Iron‐fortified flour versus control
One hundred and one controlled trials and before‐after studies that aimed to assess the effectiveness of iron‐fortified flour on iron status of women, children, and infants/toddlers were included in the systematic review by Sadighi 2019. This review reported that iron‐fortified flour led to increases of Hb concentration (MD 2.63 g/L, 95% CI 1.31 to 3.95; 46 trials, 10,353 participants; GRADE: not assessed), and decreases in prevalence of anaemia (MD −8.1%, 95% CI −11.7% to −4.4%; 27 trials, 6950 participants; GRADE: not assessed), ID (MD −12.0%, 95% CI −18.9% to −5.1%; 23 trials, 5371 participants; GRADE: not assessed), and IDA (MD −20.9%, 95% CI −38.4% to −3.4%; 15 trials, 4260 participants, GRADE: not assessed).
Wheat flour fortified with folic acid and other micronutrients versus unfortified wheat flour
Five RCTs, non‐RCTs, and ITS studies including 1182 general population participants aged over two years (including pregnant and lactating women) were included in the Tablante 2019 systematic review. This review evaluated the health benefits and safety of folic acid fortification on wheat and maize flour (alone or in combination with other micronutrients), compared with wheat or maize flour without folic acid, on folate status and health outcomes in the overall population. There were no effects of fortified wheat flour flatbread, compared to unfortified wheat flour flatbread, on Hb concentrations (MD 0.00 g/L, 95% CI ‐2.08 to 2.08; 1 trial, 334 children; low‐certainty evidence), and anaemia (RR 1.07, 95% CI 0.74 to 1.55; 1 trial, 334 children; low‐certainty evidence).
Prevention or treatment
Seventeen RCTs, cluster‐RCTs, quasi‐RCTs, non‐RCTs, CBA studies, and ITS studies, including 10,483 general population participants older than two years of age (including pregnant women) were included in the Peña‐Rosas 2019 systematic review which assessed the benefits and harms of rice fortification with vitamins and minerals (iron, vitamin A, zinc or folic acid) on micronutrient status and health‐related outcomes in the general population. Compared with unfortified rice or no intervention, fortification of rice with iron (alone or in combination with other micronutrients) resulted in increased Hb concentrations (MD 1.83 g/L, 95% CI 0.66 to 3.00; 11 trials, 2163 participants; low‐certainty evidence) and reduced the risk of having anaemia (RR 0.72, 95% CI 0.54 to 0.97; 7 trials, 1634 children; low‐certainty evidence) and ID (RR 0.66, 95% CI 0.51 to 0.84; 8 trials, 1733 participants; low‐certainty evidence). The review also reported no different reduction in diarrhoea (RR 3.52, 95% CI 0.18 to 67.39; 1 trial, 258 children; very low‐certainty evidence) and abdominal pain more than three days (RR 0.77, 95% CI 0.42 to 1.42; 1 trial, 234 children) for children consuming rice fortified with iron or in combination with other micronutrients.
Fortification of rice with vitamin A (in combination with other micronutrients), compared with unfortified rice or no intervention, increased Hb concentration (MD 10.00 g/L, 95% CI 8.79 to 11.21; 1 trial, 74 participants; low‐certainty evidence).
Prevention
Two reviews assessed micronutrient‐fortified foods in mixed populations (Das 2019b; Hess 2016).
Micronutrient fortification versus placebo/no intervention
Forty‐three RCTs, cluster‐RCTs, quasi‐randomised trials, CBA studies, and ITS studies comprising 19,585 healthy men, women and children were included in the Das 2019b systematic review. This review evaluated the impact of micronutrient fortification versus placebo/no intervention on serum Hb level, the risk of anaemia, IDA and ID. IDA was defined as Hb < 11 g/dL with serum ferritin < 15 μg/L. There was an increase in serum Hb concentration (MD 3.01 g/L, 95% CI 2.14 to 3.87; 20 trials, 6985 participants, low‐certainty evidence) and a decrease in the risk of anaemia (RR 0.68, 95% CI 0.56 to 0.84; 11 trials, 3,746 participants; low‐certainty evidence), IDA (RR 0.28, 95% CI 0.19 to 0.39; 6 trials, 42,189 participants; low‐certainty evidence) and ID (RR 0.44, 95% CI 0.32 to 0.60; 11 trials, 3,289 participants; low‐certainty evidence) for participants receiving micronutrient fortification compared with placebo/no intervention.
Fortified condiments or noodles versus non‐fortified condiments or noodles
Fourteen RCTs and cluster‐RCTS randomising over 8674 children and adults aged 5 to 50 years were included in the systematic review by Hess 2016. This review investigated the impact of micronutrient‐fortified condiments and noodles on Hb, anaemia and functional outcomes. The prevalence of anaemia was reported as 46% at baseline in this review. There was a positive effect of the intervention on Hb concentration (WMD 6.80 g/L, 95% CI 5.10 to 8.50; 13 trials, 8845 participants; GRADE: not assessed). Participants receiving fortified foods compared with those receiving non‐fortified foods showed a reduction in the prevalence of anaemia (RR 0.59, 95% CI 0.44 to 0.80; 10 trials, 5498 participants, GRADE: not assessed).
Improving dietary diversity and quality (one review)
Food prepared in iron pots
Prevention
Three RCTs comprising 784 children aged at least four months were included in the Geerligs 2003 systematic review. The review assessed the effect of preparing food cooked in cast iron pots on Hb concentrations. In this review, the results of the three RCTs were reported separately due to high heterogeneity. Two RCTs found higher Hb levels in children eating food prepared in cast iron pots (MD 13.00 g/L, 2 RCTs) in non‐malaria‐endemic areas. Another trial reported no difference in Hb concentrations (MD 0.20 g/L) between children aged 1 to 11 years eating food prepared in cast iron pots compared with children eating food prepared in non‐cast iron pots, as well as for children older than 12 years (MD 3.00 g/L).
Discussion
This overview of reviews identified 75 systematic reviews assessing the effects of various interventions to prevent or control anaemia at different stages of life. Half of the included reviews were rated as being of high methodological quality and the remaining reviews as medium quality.
Summary of main results
Of the 75 included systematic reviews, 13 reviews included infants aged 6 months to 23 months at the start of the interventions, eight reviews included children 2 years to 10 years of age, four reviews included adolescents aged 11 to 18 years, five reviews included non‐pregnant women between 19 and 49 years of age, 23 reviews included pregnant women aged 15 to 49 years, and 22 reviews included mixed populations. We did not identify any reviews that included only older adult women aged 50 to 65 years or above or only adult men aged 19 to 65 years or above. Few reviews reported on anaemia and malaria prevalence. GRADE assessments were provided in only 33 out of 75 reviews. These varied between high and very low, meaning we are not certain about the effects of nutrition‐specific interventions for controlling anaemia and iron deficiency.
Infants (aged 6 to 23 months)
Supplementation
Of the six systematic reviews that assessed supplementation interventions for infants, four investigated the effect of iron supplementation. Oral iron (alone or in combination with co‐interventions) supplementation compared with placebo, no treatment or other interventions showed a positive effect on Hb levels (1 review, moderate‐certainty evidence). The intervention also reduced the risk of anaemia by 31% (low‐certainty evidence), IDA by 80% (high‐certainty evidence) and ID by 78% (high‐certainty evidence). Adverse effects were less frequently reported, but an increase in vomiting was reported in one review, while the other reviews did not see any differences in adverse effects between the intervention and control groups. One review investigated the effect of zinc supplementation and found no clear evidence of a difference in Hb levels between zinc supplementation and placebo. LNS plus complementary feeding resulted in a reduction of anaemia compared with no intervention or MNP (low‐certainty evidence), but there was no evidence of difference in adverse effects between the groups (moderate‐certainty evidence).
Fortification
Six systematic reviews assessed fortification intervention for infants. Iron‐fortified milk or cereals was investigated in two reviews and were found to increase Hb levels and reduce anaemia. MNP (3 reviews) compared with placebo or no intervention increased Hb levels (2 reviews, moderate‐certainty evidence; 1 review, GRADE not assessed) and reduced the risk of anaemia (2 reviews, moderate‐certainty evidence; 1 review, outcome not reported). This intervention also reduced the risk of IDA by 57% (moderate‐certainty evidence) and ID by 51% (high‐certainty evidence). MNP supplementation may result in a small increase in the incidence of diarrhoea (4%; moderate‐certainty evidence) but not recurrent diarrhoea. Comparing MNP supplementation with iron supplementation, no differences in Hb levels (low‐certainty evidence) and anaemia (low‐certainty evidence) were observed, but MNP supplementation reduced the incidence of adverse effects (incidence of diarrhoea, vomiting, staining of teeth and stool discolouration). One review investigating the effect of home fortification of complementary foods found no differences in Hb levels, anaemia and diarrhoea when compared with iron drops, but increases in Hb levels, and 46% reduction in anaemia and a 56% reduction in ID when compared with placebo or no intervention.
Improving dietary diversity and quality
Improving dietary diversity and quality in infants was assessed in three systematic reviews. Food‐based strategies (red meat, fortified cow's milk, beef, fortified food with fish powder) versus control showed no difference in Hb levels between the groups, while caterpillar cereal increased Hb levels and reduced IDA prevalence. The intervention 'supplementary feeding alone or in combination with added micronutrient' was associated with an increase in Hb levels in infants.
Preschool and school‐aged children (aged 2 to 10 years)
Supplementation
Four reviews assessed supplementation interventions for preschool and school‐aged children. Three of them investigated the effects of iron supplementation. Daily oral iron supplementation increased Hb levels and reduced the risk of anaemia by 50%, IDA by 88% and ID by 79% compared with placebo or control. There were no differences in adverse effects (gastrointestinal upset, constipation, and vomiting). Intermittent iron supplementation alone or with other nutrients increased Hb levels (low‐certainty evidence) and reduced the risk of anaemia by 49% (moderate‐certainty evidence) and ID by 76% (low‐certainty evidence) compared with placebo or no intervention. No differences in side effects were observed between the groups. Comparing intermittent iron supplementation with daily iron supplementation, no differences were observed between the groups for the outcomes of Hb levels (low‐certainty evidence), diarrhoea, and any side effects. However, children receiving intermittent iron showed a 23% increased risk of anaemia (low‐certainty evidence), 30% increased risk of ID (very low‐certainty evidence) compared with daily iron. Zinc supplementation for preschool and school‐aged children was investigated in one review. Zinc supplementation compared with no zinc supplementation showed no differences in Hb levels, prevalence of anaemia, prevalence of ID or ≥ 1 side effect. However, the intervention increased the risk of vomiting episodes by 68% and increased risk of ≥ 1 vomiting episode by 29% (high‐certainty evidence). Children receiving zinc plus iron showed higher Hb levels and a 22% reduction in the prevalence of anaemia and an 88% reduction in the prevalence of ID compared with zinc alone.
Fortification
Four reviews assessed fortification interventions. MMN‐fortified beverages for preschool and school‐aged children increased Hb levels (moderate‐certainty evidence) and reduced the risk of anaemia by 37% (moderate‐certainty evidence), the risk of ID by 68% (moderate‐certainty evidence), and the risk of IDA by 87% (low‐certainty evidence) compared with children in the control group. Comparing fortified dairy products and cereal food with no fortification showed no clear evidence of a difference in Hb levels, risk of anaemia, and adverse events, while fortified dairy products and cereal food reduced the risk of IDA by 62% (very low‐certainty evidence), and ID by 38% (very low‐certainty evidence). Children receiving zinc‐fortified food compared with children receiving a regular diet or unfortified food showed no differences in Hb levels. Point‐of‐use fortification of foods with iron‐containing MNP increased Hb levels (low‐certainty evidence) and reduced the prevalence of anaemia by 34% (moderate‐certainty evidence), and ID by 65% (moderate‐certainty evidence) compared with no intervention or placebo. No difference was observed for IDA and diarrhoea (low‐certainty evidence).
Improving dietary diversity and quality
None of the included reviews assessed interventions to improve dietary diversity and quality for preschool and school‐aged children.
Adolescent children (aged 11 to 18 years)
Supplementation
Four reviews assessed supplementation interventions for adolescent children. Intermittent iron supplementation alone or with other vitamins and minerals increased Hb levels (moderate‐certainty evidence) and reduced the risk of anaemia by 35% (low‐certainty evidence) compared with no supplementation or placebo. There were no differences between intervention and control groups for the outcomes of IDA (low‐certainty evidence), ID (low‐certainty evidence), or any adverse effects (moderate‐certainty evidence). Comparing this intervention to daily iron supplementation, there were no differences in Hb levels (low‐certainty evidence), incidence of anaemia (moderate‐certainty evidence), or ID (very low‐certainty evidence), but there was a 79% reduction in the risk of adverse effects (low‐certainty evidence). Iron supplementation, iron plus folic acid and iron plus antimalarial supplementation increased Hb levels for children with or without anaemia compared with placebo. These interventions reduced the incidence of anaemia; iron supplementation reduced by 37%, iron plus folic acid reduced by 51%, and iron plus antimalarial supplementation reduced by 56%. Iron supplementation with or without folic acid increased Hb levels (low‐certainty evidence), but there was no clear evidence of an effect in the incidence of anaemia (low‐certainty evidence). Also, comparing MMN to no fortification, there was no clear evidence of an effect in Hb levels (low‐certainty evidence). Another review compared micronutrient supplementation with control and found an increase in Hb levels and a 31% reduction in the risk of anaemia (moderate‐certainty evidence) in the intervention group.
Fortification
None of the included reviews assessed fortification interventions for adolescent children.
Improving dietary diversity and quality
None of the included reviews assessed interventions to improve dietary diversity and quality for adolescent children.
Non‐pregnant women of reproductive age (aged 19 to 49 years)
Supplementation
Five systematic reviews assessed supplementation interventions for non‐pregnant women. Iron therapy (oral, intravenous (IV), intramuscular (IM)) increased Hb levels compared with control but no differences were observed for the outcomes of anaemia, gastrointestinal intolerance, nausea, constipation or diarrhoea. Compared to placebo, iron folic acid supplementation reduced the incidence of anaemia by 34% (very low‐certainty evidence). Also, weekly use of this intervention reduced the incidence of anaemia by 30% (very low‐certainty evidence), and daily use reduced it by 51% (very low‐certainty evidence). In another review, daily iron supplementation with or without co‐intervention (folic acid or vitamin C) resulted in increased Hb levels (high‐certainty evidence), a 61% reduced risk of anaemia (moderate‐certainty evidence), a 35% reduced risk of IDA, and a 38% reduced risk of ID (moderate‐certainty evidence) compared with no iron supplementation. The intervention showed no differences between the groups for any adverse side effects. In the review that compared IV iron to oral iron, IV iron increased Hb levels. Another review assessed MMN supplementation, but reported no outcomes related to anaemia.
Fortification
None of the included reviews assessed fortification interventions for non‐pregnant women of reproductive age.
Improving dietary diversity and quality
None of the included reviews assessed interventions to improve dietary diversity and quality for non‐pregnant women of reproductive age.
Pregnant women of reproductive age (aged 15 to 49 years)
Supplementation
Twenty‐two reviews assessed supplementation interventions for pregnant women. Eleven reviews assessed iron supplementation. In one review, daily iron supplementation with or without folic acid increased Hb levels in the third trimester or at delivery and in the postpartum period. Also, the risk of anaemia was reduced by 50%, IDA by 60%, and ID in the third trimester or at delivery by 41%. The comparison, iron only versus no iron or placebo resulted in increased Hb levels in the third trimester or at delivery and in the postpartum period, and reduced the risk of anaemia by 44% and ID by 41%. Iron with folic acid versus no iron and folic acid supplements also increased Hb levels and reduced the risk of anaemia by 56% in the third trimester or at delivery. In another review, iron supplementation reduced the risk of anaemia at term by 69% (moderate‐certainty evidence) and IDA at term by 56%. Any supplement containing iron compared with the same supplement without iron or no treatment or placebo increased Hb levels at or near term and postpartum, and reduced the risk of anaemia by 70% (low‐certainty evidence), IDA by 67%, ID by 57% (low‐certainty evidence) at or near term, and severe anaemia postpartum by 96%. In the same review, iron and folic acid versus the same supplement without iron and folic acid showed an increase in Hb levels at or near term and postpartum, and a reduction in the risk of anaemia at or near term by 66% (moderate‐certainty evidence), ID at or near term by 76% (low‐certainty evidence), and severe anaemia at any time during second and third trimester by 82% (low‐certainty evidence) and postpartum by 95%, but an increase in the risk of side effects (moderate‐certainty evidence). In another review assessing intermittent iron supplementation, the intervention increased the risk of anaemia at or near term by 66% and ID by 138%, and reduced the risk of side effects by 44% (very low‐certainty evidence), but had no effect on Hb levels and IDA. Five reviews investigating different iron treatments for pregnant women with IDA, found that IV iron compared with oral iron or IM iron increased Hb levels, as did daily versus weekly oral iron and different oral iron doses, and oral iron versus placebo. Another review assessed various different treatments for IDA in pregnancy and found positive effects on Hb levels for oral iron versus placebo, oral iron plus vitamin A versus placebo, IV iron versus oral iron, IM iron versus oral iron (with or without folic acid), daily versus oral iron once or twice per week, oral iron twice per week versus once per week, IV iron sucrose 200 mg or 500 mg versus IM, and IV iron plus oral iron versus oral iron. The same review showed a reduction in the risk of anaemia during the second trimester of 62% for oral iron versus placebo and 96% for oral iron plus vitamin A versus placebo, and an improvement in the outcome 'not anaemic at term' for IM iron versus oral iron (with or without vitamin C and folic acid). IV versus oral iron for pregnant women with IDA was assessed in four reviews and was found to increase Hb levels at the end of treatment and reduce the risk of adverse events.
Two reviews assessed vitamin A supplementation. Vitamin A supplementation alone versus placebo or no intervention resulted in a 36% reduced risk of maternal anaemia (moderate‐certainty evidence). Vitamin A supplementation versus placebo or other micronutrient increased maternal Hb levels (moderate‐certainty evidence), and reduced the risk of maternal anaemia by 19%, but not severe anaemia (low‐certainty evidence). Two reviews assessed folic acid supplementation during pregnancy and found no difference in pre‐delivery Hb levels and anaemia compared with no folic acid supplementation. Three reviews assessed MMN supplementation with iron and folic during pregnancy and found no effects on maternal Hb concentration (low‐certainty evidence) and anaemia (moderate to high‐certainty evidence) in the third trimester, compared with iron and folic supplementation alone. However, MMN with iron and folic acid compared with placebo reduced the risk of anaemia by 34%. One review assessing the effect of calcium supplementation found no evidence of a difference in anaemia when compared with placebo or no treatment. The supplementation with oral bovine lactoferrin increased Hb levels (low‐certainty evidence) and reduced gastrointestinal side effects compared with oral ferrous iron preparations. One review assessing the effect of LNS found an increase in the prevalence of anaemia when compared with IFA or MMN treatment, with no difference in hospitalisation episodes.
Fortification
Two reviews assessed MMN fortification for pregnant women. Non‐dairy MMN‐fortified beverages improved Hb levels for pregnant women compared with iso‐caloric non‐fortified beverages. MNP for point‐of‐use fortification of food reduced Hb levels at 32 weeks' gestation and increased the risk of anaemia compared with iron and folic acid supplementation. No differences were observed in Hb levels at term or near term and anaemia (very low‐certainty evidence) for the fortification intervention compared with MMN supplementation.
Improving dietary diversity and quality
None of the included reviews assessed interventions to improve dietary diversity and quality for pregnant women of reproductive age.
Mixed population (all ages)
Supplementation
Of the nine reviews assessing supplementation interventions for mixed populations, six reviews investigated the effect of iron supplementation. Iron supplementation versus placebo or control, as investigated in three reviews, increased Hb levels in healthy adults, children and elderly people, but there was no difference between the groups for adverse events. Iron with MMN increased Hb levels in children compared with placebo or no treatment and slightly increased Hb levels compared with iron supplementation alone. Another review assessing an iron intervention found an increase in Hb levels for children with anaemia or IDA receiving iron supplementation and a decrease in Hb levels for those receiving dietary interventions. The same review showed lower prevalences of anaemia for participants receiving iron supplementation compared with fortification, and in adolescents and adults there was no difference in Hb levels between the dietary plan group and the iron supplementation group. One review found an increased risk (132%) of gastrointestinal side effects for participants receiving oral iron compared with placebo. The same review showed an increase in Hb levels for IV iron compared with oral iron supplementation and an increased risk of gastrointestinal side effects. Compared with placebo, vitamin B12 or folic acid supplementation in an elderly population showed no differences in Hb levels between the groups. There was no difference in Hb levels between the groups comparing vitamin D supplementation with placebo.
Fortification
Twelve reviews assessed fortification interventions for mixed populations. Iron fortification of food resulted in an increase in Hb levels and reduced the risk of anaemia by 41% and ID by 52% compared with control. MMN fortification compared with placebo or no intervention showed in an increase in Hb levels and reduced the risk of anaemia, IDA, and ID. However, wheat or maize flour fortified with iron plus other vitamins and minerals versus unfortified wheat or maize flours showed no effect on Hb levels, anaemia, IDA and ID. Rice fortification with iron alone or in combination with other micronutrients resulted in an increase in Hb levels and decreased the risk of anaemia and ID compared with unfortified rice or no intervention. Another review assessed NaFeEDTA‐fortified soy sauce in a Chinese population and found an increase in Hb levels and a 75% reduction of anaemia rates compared with non‐fortified soy sauce. Double‐fortified salt with iron and iodine increased Hb levels and also reduced the risk of anaemia by 16% and IDA by 63% compared with control salt. Fortified condiments or noodles compared with non‐fortified condiments or noodles increased Hb levels and reduced the risk of anaemia by 41%.
Improving dietary diversity and quality
One review assessed the effects of foods prepared in iron pots, and found higher Hb levels in children with low‐risk malaria status in two trials, but another trial found no difference when compared with consuming food prepared in non‐cast iron pots in a high‐risk malaria endemicity mixed population. Adverse events have not been reported in the review. Using iron pots may have prophylactic benefits for malaria endemicity low‐risk populations.
Overall completeness and applicability of evidence
The objective of this overview of reviews was to summarise the benefits or harms of nutrition interventions for preventing and controlling anaemia in anaemic or non‐anaemic, apparently healthy populations throughout the life cycle. Anaemia prevalence fluctuates according to various factors, including age, living area, sex and socioeconomic status. This overview of reviews summarised extensive evidence on the effects of three types of interventions: 1) supplementation for infants (6 reviews), preschool and school‐aged children (4 reviews), adolescent children (4 reviews), non‐pregnant women (5 reviews), pregnant women (22 reviews), and mixed populations (9 reviews); 2) fortification for infants (5 reviews), preschool and school‐aged children (4 reviews), pregnant women (1 review), and mixed populations (12 reviews); and 3) improving dietary diversity and quality for infants (2 reviews), preschool and school‐aged children (1 review), and mixed populations (1 review). In total, we included 75 systematic reviews, which included one or more of our primary outcomes (Hb concentration, anaemia or IDA) and secondary outcomes (ID, severe anaemia, or adverse effects). The number of trials included in these systematic reviews ranged from 2 to 90 trials, and the number of participants ranged from 52 to over 310,000. At least 67 of the included reviews were conducted in low‐ and middle‐income countries, and we synthesised the data from numerous countries of differing cultural and economic backgrounds.
Although we comprehensively summarised the data from various systematic reviews, there was a limited number of systematic reviews regarding some factors. First, none of the systematic reviews included adult men aged over 19 years and older adult women aged over 50 years. Thus, we could not conclude the benefits or harms of nutrition interventions for theses populations. However, 42% of children younger than five years of age, 30% of non‐pregnant women, and 40% of pregnant women aged 15 to 49 years were estimated as anaemic (WHO 2020a; WHO 2020b). This overview provided a comprehensive summary of the effects and harms of supplementation, fortification, and interventions to improve dietary diversity and quality for these high‐risk populations. Second, most systematic reviews focused on supplementation (50 reviews) and fortification (22 reviews) interventions; only three systematic reviews focused on interventions to improve dietary diversity and quality. Third, as of June 2021, reviews included in this overview were conducted between 2003 and 2019. Even though Cochrane recommends that Cochrane Reviews should be updated periodically depending on the continuing importance of the review question, sufficiency of new evidence or new methods, 12 of 20 included Cochrane Reviews conducted their searches before July 2015 (Buppasiri 2015; De‐Regil 2011; Kristjansson 2015; Lassi 2013; Mayo‐Wilson 2014a; McCauley 2015; Peña‐Rosas 2015b; Reveiz 2011; Rumbold 2015; Shi 2015; Suchdev 2015; Suchdev 2020); we found some new relevant studies that had been published since 2015. These reviews did not include any recent controlled trials assessing the effects of supplementation and fortification to prevent or correct anaemia. Additionally, only 37% of the included reviews actively reported adverse events. This may affect the completeness of the evidence, as conclusions can only be drawn based on the available data.
This overview of reviews summarised the demonstrated effects and the certainty of the evidence of nutrition‐specific interventions across reviews and participant groups, including infants, preschool or school‐aged children, adolescent children, non‐pregnant adult women aged between 19 and 49 years, pregnant women, and mixed populations. Where systematic reviews were similar in relation to research question, participants and interventions, we chose the most comprehensive review. The results of this summary can help policymakers and healthcare professionals to determine the implementation and evaluation of nutrition‐specific interventions. We also believe this overview of reviews highlights areas that need further research.
Aside from nutritional factors, other non‐nutritional factors, such as inflammation, infections or genetic disorders can cause iron deficiency or anaemia. Therefore, interventions need to address the various causes of the condition in an a multisectoral approach. The treatment or prevention of non‐nutritional anaemia has been discussed in related Cochrane Reviews which complement our overview review. Gordon 2021 compared different forms of iron administration (IV, oral) to treat IDA in people with inflammatory bowel disease. Another review investigated the effects of micronutrient supplementation in pregnant women with HIV infection, and in this context assessed maternal haematological parameters (Siegfried 2012). In an overview review of Cochrane Reviews, Fortin 2018 assessed red blood cell transfusion to treat or prevent complications in sickle cell disease, an inherited blood disorder which results in anaemia due to abnormal red blood cells.
Certainty of the evidence
We assessed the quality of the included reviews using the AMSTAR tool (Shea 2007a; Shea 2007b; Shea 2009), which is the recommended approach for systematic reviews. Of these, 26 Cochrane Reviews were of high quality, because they all followed a similar and prescribed process (Abe 2016; Buppasiri 2015; Das 2018; Das 2019a; Das 2019b; Suchdev 2020; De‐Regil 2011; De‐Regil 2015; De‐Regil 2017; Garcia‐Casal 2018; Fernández‐Gaxiola 2019; Keats 2019; Lassi 2013; Kristjansson 2015; Low 2016; Mayo‐Wilson 2014a; McCauley 2015; Neuberger 2016; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Peña‐Rosas 2019; Reveiz 2011; Rumbold 2015; Shi 2015; Suchdev 2015; Tablante 2019). Systematic reviews should be conducted using a rigorous method to minimise the potential for bias in the review process (Higgins 2021). However, some reviews had specific methodological limitations that could create bias: conflict of interest disclosure not stated (56 reviews), publication bias not assessed (38 reviews), included and excluded trials not listed (35 reviews), and review protocol not provided (21 reviews) (Table 15; Table 16; Table 17; Table 18; Table 19; Table 20). Although supplementation trials might be sponsored by commercial companies, 76% of included systematic reviews did not disclose their conflict of interest. In addition, the AMSTAR tool was developed to assess the methodological quality of systematic reviews using the reports of systematic reviews (Shea 2007a; Shea 2007b; Shea 2009), but not the actual undertaking or conduct of the review process (Faggion 2015; Wegewitz 2016). Therefore, the assessment of methodological quality highly depends on the accurate and thorough reporting in the systematic review. The results presented in this overview of reviews should be used with caution, considering the risks of these potential biases.
All included reviews assessed the risk of bias in the included trials using several assessment tools such as the Cochrane RoB 1 tool (Higgins 2011), the Jadad level‐of‐evidence score for RCTs (Jadad 1996), and the Modified Critical Appraisal Skills Programme (CASP) tool (CASP 2013). About 70% of the included reviews used the Cochrane RoB 1 tool (Higgins 2011). They included a variety of trials, varying between low and high risk of bias (Table 3; Table 4; Table 5; Table 6; Table 7; Table 8). These reviews included individual trials at high risk of bias due to no or insufficient description of methods for random sequence generation and allocation concealment, as well as lack of blinding.
Of the 75 included reviews, 33 assessed the certainty of the evidence for relevant outcomes using the GRADE approach (Aaron 2015; Abu Hashim 2017; Basutkar 2019; Bhutta 2012; Das 2018; Das 2019a; Das 2019b; De‐Regil 2011; De‐Regil 2017; Eichler 2019; Field 2020; Garcia‐Casal 2018; Fernández‐Gaxiola 2019; Haider 2011; Imdad 2012; Lassi 2020; Low 2016; Mayo‐Wilson 2014a; McCauley 2015; Petry 2016b; Peña‐Rosas 2015a; Peña‐Rosas 2015b; Peña‐Rosas 2019; Qassim 2019; Radhika 2019; Salam 2013; Salam 2016; Salam 2020; Suchdev 2015; Suchdev 2020; Tablante 2019; Thompson 2013; Thorne‐Lyman 2012). Although six reviews planned to assess the certainty of the evidence, they did not assess it for relevant outcomes (Abe 2016; Buppasiri 2015; De‐Regil 2015; Keats 2019; Kristjansson 2015; Rumbold 2015). GRADE is a rating of the certainty that the true effect lies on one side of a particular threshold or in a particular range. High‐certainty evidence indicates a low likelihood that the effect will be different enough from what the research found to affect a decision (Hultcrantz 2017). In this overview of reviews, the certainty of the evidence for the estimates of relevant outcomes ranged between very low (for example, the effects of MNP for the point‐of‐use fortification of semi‐solid foods for pregnant women; Suchdev 2015) and high (for example, for the effect of daily oral iron supplementation for non‐pregnant women to improve Hb; Low 2016). In general, the certainty of the evidence varied between low and moderate.
Potential biases in the overview process
We considered several potential biases at all stages of the overview process and made efforts to reduce them in different ways. For example, we included outcomes in the search strategy in order to limit the number of references to the relevant reviews for this broad research question. At least two review authors independently assessed the reviews for inclusion according to the eligibility criteria, extracted data and assessed the quality of the included reviews using AMSTAR (Shea 2007a; Shea 2007b; Shea 2009). We resolved disagreements through discussion with a third review author, where necessary. We included both Cochrane Reviews and non‐Cochrane Reviews that included individual RCTs, cluster‐RCTs, quasi‐RCTs, controlled before‐after trials or cross‐over trials, in order to limit the risk of bias that may be reported by observational data and narrative reviews. We considered systematic reviews that assessed the methodological quality of the included trials, both with and without meta‐analyses, but did not include meta‐analyses without systematic reviews. We listed 18 eligible ongoing systematic reviews in Appendix 1 to be included in future updates of this overview of reviews. Therefore, we would like to check all related findings of these ongoing reviews, as these may make some interesting updates to the results in further versions of this overview of reviews. Furthermore, we highly encourage international readers to provide feedback on the interpretation of the results, in order to improve future updates of this review.
Agreements and disagreements with other studies or reviews
We did not identify other overview reviews or network meta‐analyses assessing nutrition‐specific interventions for preventing and controlling anaemia at any stage of life. In this overview of reviews, one possible explanation for the agreements and disagreements with other reviews is the differences in trial settings, or methodological quality. For example, a pooled analysis of a Cochrane Review assessing the effectiveness of home fortification of foods with MNPs for health and nutrition in children under two years of age shows there may be an increase in Hb concentration and likely reduction in anaemia and ID (Suchdev 2020). The findings of that review agree with evidence from a systematic review and meta‐analysis in which a MNP intervention likely increased Hb levels (SMD 0.98, 95% CI 0.55 to 1.44; 9132 children), and reduced anaemia and ID by 34% and 57%, respectively (Salam 2013). However, the findings from both reviews on the adverse effect of the intervention on the incidence of diarrhoea, disagreed; Salam 2013 found that MNPs likely increase the incidence of diarrhoea by 4%, whereas Suchdev 2020 did not find any association with the adverse effect, diarrhoea. These discordant findings are due to differences in settings, where provision of the same intervention from one setting to another might influence the effectiveness of the intervention when conditions and socioeconomic status are culturally different. Though both reviews restricted the sites to low‐ and middle‐income countries, Suchdev 2020 included trials conducted in anaemia‐ and malaria‐prevalent settings, whereas this was not described in Salam 2013. Furthermore, Suchdev 2020 included individual RCTs, cluster‐RCTs and quasi‐RCTs in their review, whereas Salam 2013 included only RCTs and cluster‐RCTs.
There were also similar findings in other interventions for different types of population. For example, daily iron supplementation compared with placebo or control resulted in an increase in Hb concentration for infants aged 6 to 23 months (Petry 2016b), preschool and school children aged 2 to 10 years (Low 2013), non‐pregnant women aged 19 to 49 years (Low 2016), pregnant women aged 15 to 49 years (Haider 2013), and a mixed population of elderly people over 64 years old (Tay 2015). The intervention may decrease anaemia by 41% in infants (Petry 2016b), 50% in preschool and school‐aged children (Low 2013), 41% in non‐pregnant reproductive‐aged women (Low 2016), and 44% in the third trimester or at delivery (Haider 2013); reduced IDA by 80% in infants (Petry 2016b), 88% in preschool and school‐aged children (Low 2013), 35% in non‐pregnant reproductive‐aged women (Low 2016), and 63% in the third trimester or at delivery (Haider 2013); and reduced ID by 78% in infants (Petry 2016b), 79% in preschool and school‐aged children (Low 2013), 38% in non‐pregnant reproductive‐aged women (Low 2016), and 41% in the third trimester or at delivery (Haider 2013). Furthermore, there was no evidence of a difference in any adverse side effect (e.g. diarrhoea, constipation, or vomiting) of the intervention, regardless of the types of trial participants or their ages.
Authors' conclusions
Implications for practice.
We carefully summarised the effects of supplementation interventions, fortification interventions and interventions to improve dietary diversity and quality in various different populations in order to treat or prevent anaemia. In most cases, the population consisted of a mix of anaemic and non‐anaemic participants, and anaemia and malaria prevalence in the setting were rarely reported in included reviews. Supplementation interventions in particular have been intensively studied in infants, preschool and school‐aged children, pregnant women, and mixed populations. Daily iron supplementation (12.5 mg to 15 mg iron/day) versus no supplementation, no treatment, or placebo has the potential to increase Hb levels in infants aged 6 to 23 months by 4.1 g/L to 7.22 g/L, and reduce the risk of anaemia by 39% to 41% and IDA by 80% to 86%. In preschool and school‐aged children aged 2 to 10 years, daily (5 mg/day to 400 mg/day) or intermittent (7.5 mg/week to 200 mg/week) iron supplementation increased Hb levels by 5.2 g/L to 8.4 g/L, and reduced the risk of anaemia by 49% to 50% and IDA by 88%. In non‐pregnant women, daily oral iron (1 mg/day to 300 mg/day) versus no iron supplementation also increased Hb levels by 4.0 g/L to 5.3 g/L, and reduced the risk of anaemia by 61% and IDA by 38%. For pregnant women, this intervention (10 mg/day iron to 300 mg/day iron) increased Hb levels in the third trimester or at delivery by 4.5 g/L to 13.4 g/L, and reduced the risk of anaemia by 50% to 70% and IDA by 56% to 67%. Iron plus folic acid versus no iron and folic acid or placebo had a greater impact on the increase of Hb levels at or near term (10.41 g/L to 16.13 g/L). In addition, vitamin A supplementation (3333 IU/day to 444,000 IU/day) can improve Hb levels by 3.5 g/L and reduce the risk of anaemia by 19% to 36%. For mixed populations, oral iron (5 mg/day to 120 mg/day) versus placebo or control resulted in an increase in Hb levels (3.5 g/L to 7.4 g/L). Intermittent and daily iron supplementation showed no differences in Hb levels and risk of anaemia in preschool and school‐aged children and adolescent children, which provides the opportunity to use either regimen for these populations. However, in pregnant women, intermittent iron supplementation increased anaemia at or near term and ID, indicating that intermittent regimen may pose a risk to pregnant women. Food fortification increased Hb levels and reduced anaemia. MNP fortification led to increases in Hb levels and reductions in the risk of anaemia and IDA in infants, preschool and school‐aged children and pregnant women, compared with no intervention or placebo. No differences were found when comparing MNP with iron supplementation, indicating that MNP or iron supplementation can be used to improve Hb levels and anaemia. Interventions aimed to improve dietary diversity and quality were mixed and largely depended on the particular intervention and population. MNP or iron fortification of foods or beverages can have a positive impact on Hb levels and reduce the risk of anaemia in infants, preschool and school‐aged children, pregnant women, and mixed populations. Furthermore, there was no evidence of a difference in any adverse side effect (e.g. diarrhoea, constipation, or vomiting) of the intervention, regardless of the types of trial participants or their ages.
We highlighted nutrition‐specific interventions for apparently healthy populations in this overview review. However, nutritional anaemia accounts only for a portion of all anaemia cases and multiple factors ‐ nutritional and non‐nutritional ‐ can cause anaemia within one population. Furthermore, this review did not include populations at risk of anaemia due to acute or chronic infections, acquired bone marrow disorders, inflammation or inherited anaemia. However, treatment and prevention of infectious diseases (e.g. helminth infection or malaria) is important and should be addressed, in addition to the interventions described in this overview review. Programmes need to include nutrition‐specific and nutritional‐sensitive interventions and involve multiple sectors to tackle the condition. Furthermore, population characteristics (e.g. age, baseline anaemia status and micronutrient deficiencies) as well as local conditions (e.g. anaemia and malaria prevalence) need to be carefully assessed in order to choose the most suitable intervention.
Implications for research.
We identified several systematic reviews focusing on interventions for infants, preschool and school‐aged children, adolescent children, non‐pregnant and pregnant women of reproductive age, and mixed populations. Additionally, we identified only a small number of reviews assessing the effects of different interventions to prevent or treat anaemia in adolescent children, non‐pregnant women, women aged 50 to 65 years, and men aged 19 to 65 years. Future trials should focus on these specific populations in order to assess the effects of different interventions to treat or prevent anaemia. Furthermore, in any age group, only a limited number of reviews assessed interventions to improve dietary diversity and quality. These interventions should be prioritised to create a long‐lasting and sustainable impact on iron status and anaemia at any stage of life. Research efforts should be focused on different types of interventions to increase the variety of foods and dietary quality as well as take into account the special requirements of different populations. Most of the included reviews did not report adverse events which could have led to selection, publication, and reporting bias in this overview review. There is also a need for trials to assess adverse events and side effects as many trials did not evaluate the safety of the interventions. Future research should also focus on understanding how effects of interventions differ by the type of the intervention or characteristics of the population or setting. More than half of the included reviews did not perform any GRADE assessment. Therefore, future systematic reviews should be conducted well and assess the certainty in the evidence of relevant outcomes. In addition to further systematic reviews, non‐nutritional interventions and programmes including multiple interventions (e.g. nutrition‐specific and nutrition‐sensitive interventions implemented in parallel) should be considered and evaluated due to the multifactorial causation of anaemia.
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2022 | Amended | Removing additional spaces that had been introduced into the text of the review by a bug. |
History
Protocol first published: Issue 8, 2018 Review first published: Issue 9, 2021
| Date | Event | Description |
|---|---|---|
| 20 August 2018 | Amended | Correcting Maria García‐Casal's DOI. See Declarations of interest. |
Notes
Typo corrected in the abstract.
Acknowledgements
We thank Juan Pablo Peña‐Rosas and Luz Maria De‐Regil for their contribution to the design of this review.
In addition, we thank Cochrane Developmental, Psychosocial and Learning Problems for their support in the preparation of this overview of reviews.
We also thank Windy MV Wariki (WW) from the Faculty of Medicine, Sam Ratulangi University, Manado, Indonesia, for initial help in screening and data extraction.
The CRG Editorial Team are grateful to the following reviewers for their time and comments: Reina Engle‐Stone, Department of Nutrition, University of California, Davis; Denish Moorthy, Senior Technical Advisor, USAID Advancing Nutrition; and Emma Sydenham.
Appendices
Appendix 1. Protocols for future assessment and possible inclusion in this review
| Reference | Protocol title |
| An 2020 | Effects of probiotics/prebiotics/synbiotics supplementation on iron status and anemia |
| Andersen 2016 | Child iron supplementation or fortification for anemia, growth, infection, and developmental outcomes: a systematic review and meta‐analysis of randomized trials |
| Butwick 2017 | A systematic review of the efficacy of oral versus intravenous iron therapy for the treatment of postpartum anemia |
| Da Rocha 2017 | The effects of iron supplementation versus dietary iron on iron reserves in children: a systematic review and meta‐analysis |
| Dubey 2019 | A systematic review and meta‐analysis on the effect of food fortification with micronutrients (iron/folic acid/vitamin B12, or combination) on anemia among pregnant women |
| Gadhave 2020 | Effect of meal supplementation during the antenatal period on the improvement in maternal and birth weight |
| Hansen 2020 | Benefits and risks of daily oral iron supplementation in pregnancy in iron replete non‐anaemic women: a systematic review |
| Hare 2018 | Health outcomes of iron supplementation and/or food fortification in iron‐replete children aged 4‐24 months: a systematic review and meta‐analysis of randomised controlled trials |
| Jere 2020 | The effects of B vitamins and/or iron on haematological parameters and nutrient biomarkers of anaemia in children in low‐ and middle‐income countries: a systematic review and meta‐analysis of randomized controlled trials |
| Moore 2014 | A systematic review and meta‐analysis of the evidence for the regulation of dietary iron bioavailability by vitamin C and phytate, in the context of hepcidin, and subsequent effects on iron status |
| Nikooyeh 2018 | Nutritional impacts of home‐fortification strategies in children younger than 5 year‐old: a systematic review and meta‐analysis |
| Pasricha 2011 | Daily iron supplementation for improving anaemia and health in children |
| Rahman 2019 | Systematic review of randomised controlled trials for effectiveness of intervention for prevention of anaemia in pregnancy |
| Rogozinska 2018 | Iron treatments (Fe) in reproductive age women with Iron Deficient Anaemia (FRIDA): a systematic review with network meta‐analysis of randomised controlled trials |
| Rosli 2019 | Iron polymaltose complex for iron deficiency anaemia in children |
| Tsang 2015 | Folate supplementation in women of reproductive age |
| Zamalloa 2017 | The impact of counseling and education on incidence rates of anemia and iron deficiency in children under 3 years |
| Zandonadi 2017 | Food strategy for the prevention and treatment of iron deficiency anemia in childhood: a systematic review |
Appendix 2. Search strategies
Cochrane Database of Systematic Reviews (CDSR), in the Cochrane Library
#1 [mh anemia] #2 (anaem* or anem* or non‐anemic* or non‐anaemic*):ti,ab #3 (iron* near/3 (deficien* or status)):ti,ab #4 (haemoglobin or hemoglobin or Hb):ti,ab #5 (ferric or ferrous or ferritin* or Fe):ti,ab #6 {or #1‐#5} #7 [mh "Nutrition therapy"] #8 [mh "Dietary Supplements"] #9 [mh "Diet Therapy"] or [mh Diet] #10 [mh Micronutrients] #11 [mh "foods, specialized"] #12 [mh Biofortification] #13 [mh Iron] or [mh Zinc] or [mh "vitamin A"] #14 [mh "iron, dietary"] #15 [mh Lipids] #16 [mh "Iron Chelating Agents"] #17 [mh "ferric compounds"] or [mh "ferrous compounds"] #18 (*nutrient* or nutrition* or diet* or food* or feeding or supplement* or complementary or *fortif* or vitamin):ti,ab #19 ("point of use" or "ready to use" or RUSF* or RUTF* or FBF*):ti,ab #20 (iron* or zinc* or "vitamin A" or retinol*):ti,ab #21 (counsel* or education* or teach* or class*):ti #22 [mh "health education"] or [mh "health promotion"] #23 [mh Weaning] or wean*;ti,ab #24 {or #7‐#23} #25 #6 and #24, in Cochrane Reviews, Cochrane Protocols #26 #6 and #24 with Cochrane Library publication date Between Jul 2018 and Aug 2020, in Cochrane Reviews, Cochrane Protocols
MEDLINE Ovid
1 exp Anemia/ 2 (anaem$ or anem$ or non‐anemic$ or non‐anaemic$).tw,kf. 3 (iron$ adj3 (deficien$ or status)).tw,kf. 4 (haemoglobin or hemoglobin or Hb).tw,kf. 5 (ferric or ferrous or ferritin$ or Fe).tw,kf. 6 or/1‐5 7 exp NUTRITION THERAPY/ 8 Dietary Supplements/ 9 Micronutrients/ 10 exp foods, specialized/ 11 diet/ 12 food, fortified/ 13 Biofortification/ 14 iron/ or zinc/ or vitamin A/ 15 (nutrition$ or diet$).ti. 16 (micronutrient$ or micro‐nutrient$).tw,kf. 17 (multinutrient$ or multi‐nutrient$ or multi$ nutrient$).tw,kf. 18 (multimicro‐nutrient$ or multimicronutrient$).tw,kw. 19 (MNPs or MMPs or Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem).tw,kf. 20 (multivitamin$ or multi‐vitamin$).tw,kf. 21 (trace adj (element$ or mineral$ or nutrient$)).tw,kf. 22 iron, dietary/ 23 Iron Chelating Agents/ 24 ferric compounds/ or ferrous compounds/ 25 (iron$ or zinc$ or "vitamin A" or retinol$).tw,kf. 26 (folic$ or folate$ or folvite$ or folacin$ or pteroylglutamic$).tw,kf. 27 exp Lipids/ 28 (fatty acid$ or Docosahexaenoic acid$ or Eicosapentaenoic Acid$ or PUFA$ or lipid$ or omega 3$ or omega 6$).tw,kf. 29 (soy$ or wheat‐soy$ or corn‐soy$ or peanut or groundnut or whey or sesame or cashew or chickpea or oil$).tw,kf. 30 ((fortif$ or supplement$) adj3 (blend$ or diet$ or food$ or nutrition$)).tw,kf. 31 ((fortif$ or supplement$) adj3 (cereal$ or condiment$ or flour$ or legume$ or rice$ or salt$ or sauce$ or milk$ or formula)).tw,kf. 32 ("point of use" or point‐of‐use or "ready to use" or "ready ‐to‐ use" or RUSF$1 or RUTF$1) .tw,kf. 33 Weaning/ 34 (complementary adj3 (feed$ or food$ or nutrition$)).tw,kf. 35 weaning.tw,kf. 36 (biofortif$ or bio‐fortif$).tw,kf. 37 or/7‐36 38 6 and 37 39 Anemia, Iron‐Deficiency/dh, pc, th [Diet Therapy, Prevention & Control, Therapy] 40 38 or 39 41 Nutritional Sciences/ 42 diet/ 43 Diet Therapy/ 44 Nutrition Therapy/ 45 or/41‐44 46 Health Education/ 47 Health Knowledge, Attitudes, Practice/ 48 health promotion/ 49 Counseling/ 50 or/46‐49 51 45 and 50 52 ((nutrition$ or diet$ or food or feeding) adj3 (counsel$ or education$ or teach$ or class$)).tw,kf. 53 51 or 52 54 6 and 53 55 40 or 54 56 exp animals/ not humans/ 57 55 not 56 58 meta‐analysis.pt. 59 Meta‐Analysis as Topic/ 60 systematic$ review$.tw. 61 (metanalysis or metaanalysis or meta analysis).tw. 62 (metaregression or meta‐regression or meta regression).tw. 63 (metasynthesis or meta‐synthesis or meta synthesis).tw. 64 (realist review or realist synthesis or rapid review or pragmatic review or umbrella review).tw. 65 (Medline or Pubmed or Embase or Cinahl or Cochrane).ab. 66 ((literature or database$ or bibliographic) adj3 search$).ab. 67 ((inclusion or selection or predefined or predetermined) adj5 (studies or articles or reports)).ab. 68 or/58‐67 69 57 and 68 70 limit 57 to systematic reviews 71 69 or 70 72 (201807* or 201808* or 201809* or 201810* or 201811* or 201812* or 2019* or 2020*).dt,ez,da. 73 71 and 72
MEDLINE In‐Progress and other Non‐Indexed Citations Ovid
1 (anaem$ or anem$ or non‐anemic$ or non‐anaemic$).tw,kf. 2 (iron$ adj3 (deficien$ or status)).tw,kf. 3 (haemoglobin or hemoglobin or Hb).tw,kf. 4 (ferric or ferrous or ferritin$ or Fe).tw,kf. 5 or/1‐4 6 (nutrition$ or diet$).ti. 7 (micronutrient$ or micro‐nutrient$).tw,kf. 8 (multimicro‐nutrient$ or multimicronutrient$).tw,kf. 9 (MNPs or MMPs or Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem).tw,kf. 10 (multivitamin$ or multi‐vitamin$).tw,kf. 11 (trace adj (element$ or mineral$ or nutrient$)).tw,kf. 12 (iron$ or zinc$ or "vitamin A" or retinol$).tw,kf. 13 (folic$ or folate$ or folvite$ or folacin$ or pteroylglutamic$).tw,kf. (2861) 14 (fatty acid$ or Docosahexaenoic acid$ or Eicosapentaenoic Acid$ or PUFA$ or lipid$ or omega 3$ or omega 6$).tw,kf. 15 (soy$ or wheat‐soy$ or corn‐soy$ or peanut or groundnut or whey or sesame or cashew or chickpea or oil$).tw,kf. 16 ((fortif$ or supplement$) adj3 (blend$ or diet$ or food$ or nutrition$)).tw,kf. 17 ((fortif$ or supplement$) adj3 (cereal$ or condiment$ or flour$ or legume$ or rice$ or salt$ or sauce$ or milk$ or formula)).tw,kf.) 18 ("point of use" or point‐of‐use or "ready to use" or "ready ‐to‐ use" or RUSF$1 or RUTF$1 or FBF$1).tw,kf. 19 (complementary adj3 (feed$ or food$ or nutrition$)).tw,kf. 20 weaning.tw,kf. 21 (biofortif$ or bio‐fortif$).tw,kf. 22 ((nutrition$ or diet$ or food or feeding) adj3 (counsel$ or education$ or teach$ or class$)).tw,kf. 23 or/6‐22 24 5 and 23 25 systematic$ review$.tw. 26 (metanalysis or metaanalysis or meta analysis).tw. 27 (metaregression or meta‐regression or meta regression).tw. 28 (metasynthesis or meta‐synthesis or meta synthesis).tw. 29 (realist review or realist synthesis or rapid review or pragmatic review or umbrella review).tw. 30 (Medline or Pubmed or Embase or Cinahl or Cochrane).ab. 31 ((literature or database$ or bibliographic) adj3 search$).ab. 32 ((inclusion or selection or predefined or predetermined) adj5 (studies or articles or reports)).ab. 33 or/25‐32 34 24 and 33 35 (201807* or 201808* or 201809* or 201810* or 201811* or 201812* or 2019* or 2020*).dt,ez,da. 36 34 and 35
MEDLINE Epub Ahead of Print Ovid
1 (anaem$ or anem$ or non‐anemic$ or non‐anaemic$).tw,kf. 2 (iron$ adj3 (deficien$ or status)).tw,kf. 3 (haemoglobin or hemoglobin or Hb).tw,kf. 4 (ferric or ferrous or ferritin$ or Fe).tw,kf. 5 or/1‐4 6 (nutrition$ or diet$).ti. 7 (micronutrient$ or micro‐nutrient$).tw,kf. 8 (multimicro‐nutrient$ or multimicronutrient$).tw,kf. 9 (MNPs or MMPs or Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem).tw,kf. 10 (multivitamin$ or multi‐vitamin$).tw,kf. 11 (trace adj (element$ or mineral$ or nutrient$)).tw,kf. 12 (iron$ or zinc$ or "vitamin A" or retinol$).tw,kf. 13 (folic$ or folate$ or folvite$ or folacin$ or pteroylglutamic$).tw,kf. (2861) 14 (fatty acid$ or Docosahexaenoic acid$ or Eicosapentaenoic Acid$ or PUFA$ or lipid$ or omega 3$ or omega 6$).tw,kf. 15 (soy$ or wheat‐soy$ or corn‐soy$ or peanut or groundnut or whey or sesame or cashew or chickpea or oil$).tw,kf. 16 ((fortif$ or supplement$) adj3 (blend$ or diet$ or food$ or nutrition$)).tw,kf. 17 ((fortif$ or supplement$) adj3 (cereal$ or condiment$ or flour$ or legume$ or rice$ or salt$ or sauce$ or milk$ or formula)).tw,kf.) 18 ("point of use" or point‐of‐use or "ready to use" or "ready ‐to‐ use" or RUSF$1 or RUTF$1 or FBF$1).tw,kf. 19 (complementary adj3 (feed$ or food$ or nutrition$)).tw,kf. 20 weaning.tw,kf. 21 (biofortif$ or bio‐fortif$).tw,kf. 22 ((nutrition$ or diet$ or food or feeding) adj3 (counsel$ or education$ or teach$ or class$)).tw,kf. 23 or/6‐22 24 5 and 23 25 systematic$ review$.tw. 26 (metanalysis or metaanalysis or meta analysis).tw. 27 (metaregression or meta‐regression or meta regression).tw. 28 (metasynthesis or meta‐synthesis or meta synthesis).tw. 29 (realist review or realist synthesis or rapid review or pragmatic review or umbrella review).tw. 30 (Medline or Pubmed or Embase or Cinahl or Cochrane).ab. 31 ((literature or database$ or bibliographic) adj3 search$).ab. 32 ((inclusion or selection or predefined or predetermined) adj5 (studies or articles or reports)).ab. 33 or/25‐32 34 24 and 33
Embase Ovid
1 exp anemia/ 2 (anaem$ or anem$ or non‐anemic$ or non‐anaemic$).tw,kw. 3 (iron$ adj3 (deficien$ or status)).tw,kw. 4 (haemoglobin or hemoglobin or Hb).tw,kw. 5 (ferric or ferrous or ferritin$ or Fe).tw,kw. (138543) 6 or/1‐5 7 exp diet therapy/ 8 dietary supplement/ 9 diet/ 10 exp trace element/ 11 functional food/ 12 fortified food/ 13 biofortification/ 14 iron/ 15 zinc/ 16 retinol/ 17 (nutrition$ or diet$).ti. 18 (micronutrient$ or micro‐nutrient$).tw,kw. 19 (multinutrient$ or multi‐nutrient$ or multi$ nutrient$).tw,kw. 20 (multimicro‐nutrient$ or multimicronutrient$).tw,kw. 21 (MNPs or MMPs or Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem).tw,kw. 22 (multivitamin$ or multi‐vitamin$).tw,kw. 23 (trace adj (element$ or mineral$ or nutrient$)).tw,kw. 24 iron intake/ 25 iron chelating agent/ 26 (iron$ or zinc$ or "vitamin A" or retinol$).tw,kw. 27 (folic$ or folate$ or folvite$ or folacin$ or pteroylglutamic$).tw,kw. 28 exp lipid/ 29 (fatty acid$ or Docosahexaenoic acid$ or Eicosapentaenoic Acid$ or PUFA$ or lipid$ or omega 3$ or omega 6$).tw,kw. 30 (soy$ or wheat‐soy$ or corn‐soy$ or peanut or groundnut or whey or sesame or cashew or chickpea or oil$).tw,kw. 31 ((fortif$ or supplement$) adj3 (blend$ or diet$ or food$ or nutrition$)).tw,kw. 32 ((fortif$ or supplement$) adj3 (cereal$ or condiment$ or flour$ or legume$ or rice$ or salt$ or sauce$ or milk$ or formula)).tw,kw. 33 ("point of use" or point‐of‐use or "ready to use" or "ready ‐to‐ use" or RUSF$1 or RUTF$1 or FBF$1).tw,kw. 34 weaning/ 35 weaning.tw,kw. 36 (complementary adj3 (feed$ or food$ or nutrition$)).tw,kw. 37 (biofortif$ or bio‐fortif$).tw,kw. 38 or/7‐37 39 6 and 38 40 nutritional science/ 41 diet/ 42 exp diet therapy/ 43 nutrition/ 44 or/40‐43 45 health education/ 46 health promotion/ 47 counseling/ 48 or/45‐47 49 44 and 48 50 nutrition education/ 51 nutritional counseling/ (2180) 52 ((nutrition$ or diet$ or food or feeding) adj3 (counsel$ or education$ or teach$ or class$)).tw,kw. 53 or/49‐52 54 6 and 53 55 39 or 54 56 meta analysis/ 57 "systematic review"/ 58 systematic$ review$.tw. 59 (metanalysis or metaanalysis or meta analysis).tw. 60 (metaregression or meta‐regression or meta regression).tw. 61 (metasynthesis or meta‐synthesis or meta synthesis).tw. 62 (realist review or realist synthesis or rapid review or pragmatic review or umbrella review).tw. 63 (Medline or Pubmed or Embase or Cinahl or Cochrane).ab. 64 ((literature or database$ or bibliographic) adj3 search$).ab. 65 ((inclusion or selection or predefined or pre‐defined or predetermined or pre‐determined) adj5 (studies or articles or reports)).ab. 66 or/56‐65 67 55 and 66 68 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 69 human/ or normal human/ or human cell/ 70 68 and 69 71 68 not 70 72 67 not 71 73 limit 72 to dc=20180701‐20200824
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S1 (MH "Anemia+") S2 TI (anaem* or anem* or non‐anemic* or non‐anaemic*) OR AB (anaem* or anem* or non‐anemic* or non‐anaemic*) S3 TI (iron* N3 (deficien* or status)) OR AB (iron* N3 (deficien* or status)) S4 TI (haemoglobin or hemoglobin or Hb) OR AB (haemoglobin or hemoglobin or HB S5 TI (ferric or ferrous or ferritin* or Fe) OR AB (ferric or ferrous or ferritin* or Fe) S6 S1 OR S2 OR S3 OR S4 OR S5 S7 (MH "Nutrition+") S8 (MH "Dietary Supplements+") OR (MH "Food, Fortified") OR (MH "Food, Formulated") OR (MH "Food, Genetically Modified") S9 (MH "Food/TU") S10 (MH "Micronutrients") S11 (MH "Diet") S12 (MH "Biofortification") S13 (MH "Iron") OR (MH "Iron Compounds") S14 (MH "Zinc") S15 (MH "Vitamin A") S16 TI (nutrition* or diet*) S17 TI (micronutrient* or micro‐nutrient* OR micro nutrient*) OR AB (micronutrient* or micro‐nutrient* OR micro nutrient*) S18 TI(multinutrient* or multi‐nutrient* or multi nutrient*" OR AB(multinutrient* or multi‐nutrient* or multi nutrient*) S19 TI (multimicro‐nutrient* or multimicronutrient*) OR AB (multimicro‐nutrient* or multimicronutrient*) S20 TI (MNPs or MMPs or Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem) OR AB (MNPs or MMPs or Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem) S21 TI (multivitamin* or multi‐vitamin* or multi vitamin*) OR AB (multivitamin* or multi‐vitamin* or multi vitamin*) S22 TI (trace N1 (element* or mineral*or nutrient*) OR AB (trace N1 (element* or mineral*or nutrient*) S23 TI (iron* or zinc*or "vitamin A" or retinol*) OR AB (iron* or zinc*or "vitamin A" or retinol*) S24 TI (folic* or folate* or folvite* or folacin* or pteroylglutamic*) OR AB(folic* or folate* or folvite* or folacin* or pteroylglutamic*) S25 (MH "Lipids") S26 TI (fatty acid* or Docosahexaenoic acid* or Eicosapentaenoic Acid* or PUFA* or lipid* or omega 3* or omega 6* OR omega‐ 3* OR omega‐6*) OR AB (fatty acid* or Docosahexaenoic acid* or Eicosapentaenoic Acid* or PUFA* or lipid* or omega 3* or omega 6* OR omega‐ 3* OR omega‐6*) S27 TI (soy* or wheat‐soy* or corn‐soy* or peanut or groundnut or whey or sesame or cashew or chickpea or oil*) OR AB (soy* or wheat‐soy* or corn‐soy* or peanut or groundnut or whey or sesame or cashew or chickpea or oil*) S28 TI ((fortif* or supplement*) N3 (blend* or diet* or food* or nutrition*) OR AB ((fortif* or supplement*) N3 (blend* or diet* or food* or nutrition*) S29 TI ((fortif* or supplement*) N3 (cereal* or condiment* or flour* or legume* or rice* or salt* or sauce* or milk* or formula)) OR AB((fortif* or supplement*) N3 (cereal* or condiment* or flour* or legume* or rice* or salt* or sauce* or milk* or formula)) S30 TI ("point of use" or point‐of‐use or "ready to use" or "ready ‐to‐ use" or RUSF*1 or RUTF*1 or FBF*1) OR AB ("point of use" or point‐of‐use or "ready to use" or "ready ‐to‐ use" or RUSF*1 or RUTF*1 or FBF*1) S31 (MH "Weaning") S32 TI (complementary N3 (feed* or food* or nutrition*) OR AB (complementary N3 (feed* or food* or nutrition*) S33 TI(weaning) OR AB(weaning) S34 TI(biofortif* or bio‐fortif* OR AB (biofortif* or bio‐fortif*) S35 (MH "Diet Therapy") S36 (MH "Health Promotion") S37 (MH "Nutritional Counseling") S38 (MH "Nutrition Education") S39 TI ((nutrition* or diet* or food or feeding) N3 (counsel* or education* or teach* or class*)) OR AB ((nutrition* or diet* or food or feeding) N3 (counsel* or education* or teach* or class*)) S40 S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 S41 S6 AND S40 S42 (MH "Anemia, Iron Deficiency/DH/PC/TH") S43 S41 OR S42 S44 (MH "Systematic Review") S45 (MH "Meta Analysis") S46 (MH "Meta Synthesis") S47 TI (systematic* review*) OR AB (systematic* review*) S48 TI (metanalysis or metaanalysis or meta analysis OR meta‐ analysis) OR AB (metanalysis or metaanalysis or meta analysis OR meta‐ analysis) S49 TI (metaregression or meta‐regression or meta regression) OR AB (metaregression or meta‐regression or meta regression) S50 TI (metasynthesis or meta‐synthesis or meta synthesis) OR AB (metasynthesis or meta‐synthesis or meta synthesis) S51 (realist review or realist synthesis or rapid review or pragmatic review or umbrella review) S52 AB (Medline or Pubmed or Embase or Cinahl or Cochrane) S53 AB ((literature or database* or bibliographic) N3 search*) S54 AB ((inclusion or selection or predefined or predetermined) N5 (studies or articles or reports)) S55 S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 S56 S43 AND S55 S57 EM 20180701‐ S58 S56 AND S57
Database of Abstract of Reviews of Effects, in the Cochrane Library
#1 [mh anemia] #2 (anaem* or anem* or non‐anemic* or non‐anaemic*):ti,ab #3 (iron* near/3 (deficien* or status)):ti,ab #4 (haemoglobin or hemoglobin or Hb):ti,ab #5 (ferric or ferrous or ferritin* or Fe):ti,ab #6 {or #1‐#5} in Other Reviews
Epistemonikos (www.epistemonikos.org)
(title:((anem* OR anaem* OR non‐anaem* OR non‐anem* OR "iron defic*")) OR abstract:((anem* OR anaem* OR non‐anaem* OR non‐anem* OR "iron defic*"))) AND (title:((supplement* OR complement* OR food OR feeding OR fortif* OR nutrient* OR micronutrient* OR nutrition* OR MMN* OR RTUF OR RUSF OR iron* OR zinc OR "Vitamin A" OR educat* OR class* OR counsel*)) OR abstract:((supplement* OR complement* OR food OR feeding OR fortif* OR nutrient* OR micronutrient* OR nutrition* OR MMN* OR RTUF OR RUSF OR iron* OR zinc OR "Vitamin A" OR educat* OR class* OR counsel*)))
POPLINE (www.popline.org)
Taxonomy term IDs from the <em class="placeholder">Keyword</em> vocabulary:FOOD SUPPLEMENTATION OR Taxonomy term IDs from the <em class="placeholder">Keyword</em> vocabulary:CHILD NUTRITION OR Taxonomy term IDs from the <em class="placeholder">Keyword</em> vocabulary:INFANT NUTRITION OR Taxonomy term IDs from the <em class="placeholder">Keyword</em> vocabulary:MATERNAL NUTRITION OR Taxonomy term IDs from the <em class="placeholder">Keyword</em> vocabulary:IRON OR Taxonomy term IDs from the <em class="placeholder">Keyword</em> vocabulary:HEALTH EDUCATION OR Taxonomy term IDs from the <em class="placeholder">Keyword</em> vocabulary:EDUCATION OR Taxonomy term IDs from the <em class="placeholder">Keyword</em> vocabulary:VITAMIN A OR Taxonomy term IDs from the <em class="placeholder">Keyword</em> vocabulary:VITAMINS AND MINERALS OR Taxonomy term IDs from the <em class="placeholder">Keyword</em> vocabulary:SUPPLEMENTARY FEEDING OR Taxonomy term IDs from the <em class="placeholder">Keyword</em> vocabulary:WEANING))) AND ((("meta analysis" OR "meta\‐analysi"s OR "SYSTEMATIC REVIEW" OR metaregression OR "meta\‐regression" OR "meta regression" OR metasynthesis OR "meta\‐synthesis" OR "meta synthesis")))
PROSPERO (www.crd.york.ac.uk/prospero)
#1 (iron deficien* OR iron status):CS #2 (haemoglobin OR hemoglobin):CS #3 (ferric OR ferrous OR ferritin OR Fe):CS #4 (anaem* OR anem* OR non‐anaem* OR non‐anem*):CS #5 #4 OR #3 OR #2 OR #1 #6 (diet* OR nutrition OR food* OR feed* OR supplement* OR micro* OR multi* OR biofortif* OR educat* OR teach* OR class*):IV #7 #5 AND #6 #8 (diet* OR nutrition OR food* OR feed* OR supplement* OR micro* OR multi* OR biofortif* OR educat* OR teach* OR class*):IV WHERE CD FROM 25/07/2018 TO 25/08/2020 #9 #5 AND #8 CS = condition studied IV= intervention
Campbell Collaboration Online Library of Systematic Reviews (www.campbellcollaboration.org/better‐evidence.html)
We conducted the following individual searches:
Title: Anaemia, Anemia, Iron, Supplementation, Fortification
Keywords: Anaemia, Anemia, Iron, Supplementation, Fortification
3ie Database of Systematic Reviews (developmentevidence.3ieimpact.org/)
We limited the search to systematic reviews and individually searched for the following terms:
Anaemia, Anemia, Iron, Supplementation, Fortification
Differences between protocol and review
Three additional authors joined the review team: Noyuri Yamaji, Maiko Suto, and Md. Obaidur Rahman.
We identified several reviews that included mixed populations (e.g. men and women), which we could not allocate to one of the prespecified age groups. Therefore, we created a new category, 'mixed populations', as a type of participants.
We included reviews with other trial designs if the results for RCTs were reported separately.
We excluded reviews where our primary or secondary outcomes were not included or prespecified. This became necessary because we already included a high number of systematic reviews. Including reviews without our primary or secondary outcomes would have increased the number of included reviews dramatically, making our overview review confusing, unclear and difficult to convey to the reader which interventions prevent or control anaemia.
We searched the final issue of DARE in 2018, after which no new content was added to this database. The POPLINE website was retired on 1 September 2019 so was no longer available for the top‐up search.
We assessed risk of bias using AMSTAR (Shea 2007a; Shea 2007b; Shea 2009), however, we did not show the overall rating scores.
Contributions of authors
Maria N García‐Casal (MNGC) contributed to the design of the review. Katharina da Silva Lopes (KL), Yo Takemoto (YT), and Erika Ota (EO) drafted the protocol. KL, Noyuri Yamaji (NY), Md Obaidur Rahman (OR), Maiko Suto (MS), and YT extracted review characteristics and data, and assessed the methodological quality of reviews independently. KL wrote the manuscript with the help of NY, OR, MS and EO. All authors provided critical comments and valuable suggestions.
The contact author, Erika Ota, is the guarantor for the review.
Sources of support
Internal sources
-
Department of Nutrition and Food Safety, World Health Organization (WHO), Switzerland
Dr Maria Nieves García‐Casal is a full‐time member of staff at the WHO.
External sources
-
Department of Nutrition and Food Safety, WHO, Switzerland
Provided financial support for the development of this overview of systematic reviews
-
Bill & Melinda Gates Foundation, USA
The WHO gratefully acknowledges the financial contribution of the Bill & Melinda Gates Foundation towards the development of overviews of systematic reviews of the evidence on the effects of nutrition interventions.
-
MHLW Healthy Next Generation Program Grant Number JPMH20DA0601, Japan
This work was supported by MHLW Program Grant.
Declarations of interest
Katharina da Silva Lopes: none known
Noyuri Yamaji: none known
Md Obaidur Rahman: none known
Maiko Suto: none known
Yo Takemoto: none known
Maria Nieves García‐Casal (MNGC) is a full‐time member of staff at the Department of Nutrition and Food Safety, World Health Organization (WHO). MNGC declares no other known conflicts of interest.
Erika Ota's institution received funding (2017/725990) for this work from the Evidence and Programme Guidance, Department of Nutrition for Health and Development, WHO, Switzerland.
EO, KL and MNGC are authors of included reviews (EO: Abe 2016, Rumbold 2015, Suchdev 2020; KL: Suchdev 2020; MNGC: Garcia‐Casal 2018, Peña‐Rosas 2015b, Peña‐Rosas 2019). EO and MNGC were not involved in review selection, data extraction and assessment of methodological quality using AMSTAR (Shea 2007a; Shea 2007b; Shea 2009). KL was involved in these tasks but not in relation to her own reviews; in those cases, these tasks were performed by two other independent review authors (OR, NY).
Disclaimer: The review authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the WHO.
Edited (no change to conclusions)
References
References to included reviews
Aaron 2015
- Aaron GJ, Dror DK, Yang Z. Multiple-micronutrient fortified non-dairy beverage interventions reduce the risk of anemia and iron deficiency in school-aged children in low-middle income countries: a systematic review and meta-analysis (i-iv). Nutrients 2015;7(5):3847-68. [DOI: 10.3390/nu7053847] [PMC4446783] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abdullah 2013
- Abdullah K, Kendzerska T, Shah P, Uleryk E, Parkin PC. Efficacy of oral iron therapy in improving the developmental outcome of pre-school children with non-anaemic iron deficiency: a systematic review. Public Health Nutrition 2013;16(8):1497-506. [DOI: 10.1017/S1368980012003709] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abe 2016
- Abe SK, Balogun OO, Ota E, Takahashi K, Mori R. Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD010647. [DOI: 10.1002/14651858.CD010647.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abu Hashim 2017
- Abu Hashim H, Foda O, Ghayaty E. Lactoferrin or ferrous salts for iron deficiency anemia in pregnancy: a meta-analysis of randomized trials. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2017;219:45-52. [DOI: 10.1016/j.ejogrb.2017.10.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Arabi 2020
- Arabi SM, Ranjbar G, Bahrami LS, Vafa M, Norouzy A. The effect of vitamin D supplementation on hemoglobin concentration: a systematic review and meta-analysis. Nutrition Journal 2020;19(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basutkar 2019
- Basutkar RS, Tsundue T, Siva H, Rose A, Sivasankaran P. Vitamin D supplementation in patients with iron deficiency anaemia: a systematic review and a meta-analysis. Systematic Reviews in Pharmacy 2019;10(1):1-10. [DOI: 10.5530/srp.2019.1.1] [www.sysrevpharm.org/fulltext/196-1568986036.pdf?1614009224] [DOI] [Google Scholar]
Bhutta 2012
- Bhutta ZA, Imdad A, Ramakrishnan U, Martorell R. Is it time to replace iron folate supplements in pregnancy with multiple micronutrients? Paediatric and Perinatal Epidemiology 2012;26 Suppl 1:27-35. [DOI: 10.1111/j.1365-3016.2012.01313.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Buppasiri 2015
- Buppasiri P, Lumbiganon P, Thinkhamrop J, Ngamjarus C, Laopaiboon M, Medley N. Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD007079. [DOI: 10.1002/14651858.CD007079.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Casgrain 2012
- Casgrain A, Collings R, Harvey LJ, Hooper L, Fairweather-Tait SJ. Effect of iron intake on iron status: a systematic review and meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition 2012;96(4):768-80. [DOI: 10.3945/ajcn.112.040626] [PMID: ] [DOI] [PubMed] [Google Scholar]
Daru 2016
- Daru J, Cooper NA, Khan KS. Systematic review of randomized trials of the effect of iron supplementation on iron stores and oxygen carrying capacity in pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2016;95(3):270-9. [DOI: 10.1111/aogs.12812] [PMID: ] [DOI] [PubMed] [Google Scholar]
Das 2013a
- Das JK, Kumar R, Salam RA, Bhutta ZA. Systematic review of zinc fortification trials. Annals of Nutrition & Metabolism 2013;62 Suppl 1:44-56. [DOI: 10.1159/000348262] [PMID: ] [DOI] [PubMed] [Google Scholar]
Das 2018
- Das JK, Hoodbhoy Z, Salam RA, Bhutta AZ, Valenzuela-Rubio NG, Weise Prinzo Z, et al. Lipid-based nutrient supplements for maternal, birth, and infant developmental outcomes. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD012610. [DOI: 10.1002/14651858.CD012610.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Das 2019a
- Das JK, Salam RA, Hadi YB, Sadiq Sheikh S, Bhutta AZ, Weise Prinzo Z, et al. Preventive lipid-based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD012611. [DOI: 10.1002/14651858.CD012611.pub3] [PMC6497129] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Das 2019b
- Das JK, Salam RA, Mahmood SB, Moin A, Kumar R, Mukhtar K, et al. Food fortification with multiple micronutrients: impact on health outcomes in general population. Cochrane Database of Systematic Reviews 2019, Issue 12. Art. No: CD011400. [DOI: 10.1002/14651858.CD011400.pub2] [PMC6917586] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dekker 2010
- Dekker LH, Villamor E. Zinc supplementation in children is not associated with decreases in hemoglobin concentrations. Journal of Nutrition 2010;140(5):1035-40. [DOI: 10.3945/jn.109.119305] [PMID: ] [DOI] [PubMed] [Google Scholar]
De‐Regil 2011
- De-Regil LM, Jefferds ME, Sylvetsky AC, Dowswell T. Intermittent iron supplementation for improving nutrition and development in children under 12 years of age. Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No: CD009085. [DOI: 10.1002/14651858.CD009085.pub2] [PMC4547491] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2015
- De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD007950. [DOI: 10.1002/14651858.CD007950.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2017
- De-Regil LM, Jefferds ME, Pena-Rosas JP. Point-of-use fortification of foods with micronutrient powders containing iron in children of preschool and school-age. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD009666. [DOI: 10.1002/14651858.CD009666.pub2] [PMC6486284] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dewey 2009
- Dewey KG, Yang Z, Boy E. Systematic review and meta-analysis of home fortification of complementary foods. Maternal & Child Nutrition 2009;5(4):283-321. [DOI: 10.1111/j.1740-8709.2009.00190.x] [DOI] [Google Scholar]
Eichler 2012
- Eichler K, Wieser S, Rüthemann I, Brügger U. Effects of micronutrient fortified milk and cereal food for infants and children: a systematic review. BMC Public Health 2012;12:506. [DOI: 10.1186/1471-2458-12-506] [PMC3444335] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eichler 2019
- Eichler K, Hess S, Twerenbold C, Sabatier M, Meier F, Wieser S. Health effects of micronutrient fortified dairy products and cereal food for children and adolescents: A systematic review. PloS One 2019;14(1):e0210899. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernández‐Gaxiola 2019
- Fernández-Gaxiola AC, De-Regil LM. Intermittent iron supplementation for reducing anaemia and its associated impairments in adolescent and adult menstruating women. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD009218. [DOI: 10.1002/14651858.CD009218.pub3] [PMC6360921] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Field 2020
- Field MS, Mithra P, Estevez D, Pena-Rosas JP. Wheat flour fortification with iron for reducing anaemia and improving iron status in populations. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD011302. [DOI: 10.1002/14651858.CD011302.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finkelstein 2019
- Finkelstein JL, Fothergill A, Hackl LS, Haas JD, Mehta S. Iron biofortification interventions to improve iron status and functional outcomes. Proceedings of the Nutrition Society 2019;78(2):197-207. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garcia‐Casal 2018
- Garcia-Casal MN, Peña‐Rosas JP, De‐Regil LM, Gwirtz JA, Pasricha SR. Fortification of maize flour with iron for controlling anaemia and iron deficiency in populations. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD010187. [DOI: 10.1002/14651858.CD010187.pub2] [PMC6517107] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geerligs 2003
- Geerligs PD, Brabin BJ, Omari AA. Food prepared in iron cooking pots as an intervention for reducing iron deficiency anaemia in developing countries: a systematic review. Journal of Human Nutrition and Dietetics 2003;16(4):275-81. [DOI: 10.1046/j.1365-277x.2003.00447.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gera 2007a
- Gera T, Sachdev HP, Nestel P, Sachdev SS. Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials. Journal of Pediatric Gastroenterology and Nutrition 2007;44(4):468-86. [DOI: 10.1097/01.mpg.0000243440.85452.38] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gera 2009
- Gera T, Sachdev HP, Nestel P. Effect of combining multiple micronutrients with iron supplementation on Hb response in children: systematic review of randomized controlled trials. Public Health Nutrition 2009;12(6):756-73. [DOI: 10.1017/S1368980008003145] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gera 2012
- Gera T, Sachdev HS, Boy E. Effect of iron-fortified foods on hematologic and biological outcomes: systematic review of randomized controlled trials. American Journal of Clinical Nutrition 2012;96(2):309-24. [DOI: 10.3945/ajcn.111.031500] [PMID: ] [DOI] [PubMed] [Google Scholar]
Govindappagari 2019
- Govindappagari S, Burwick RM. Treatment of iron deficiency anemia in pregnancy with intravenous versus oral iron: systematic review and meta-analysis. American Journal of Perinatology 2019;36(4):366-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Haider 2011
- Haider BA, Yakoob MY, Bhutta ZA. Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes. BMC Public Health 2011;11 Suppl 3:S19. [DOI: 10.1186/1471-2458-11-S3-S19] [PMC3231892] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haider 2013
- Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ 2013;346:f3443. [DOI: 10.1136/bmj.f3443] [PMC3689887] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hess 2016
- Hess S, Tecklenburg L, Eichler K. Micronutrient fortified condiments and noodles to reduce anemia in children and adults-a literature review and meta-analysis. Nutrients 2016;8(2):88. [DOI: 10.3390/nu8020088] [PMC4772051] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Houston 2018
- Houston BL, Hurrie D, Graham J, Perija B, Rimmer E, Rabbani R, et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open 2018;8(4):e019240. [DOI: 10.1136/bmjopen-2017-019240] [PMC5892776] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huo 2015
- Huo JS, Yin JY, Sun J, Huang J, Lu ZX, Regina MP, et al. Effect of NaFeEDTA-fortified soy sauce on anemia prevalence in China: a systematic review and meta-analysis of randomized controlled trials. Biomedical and Environmental Sciences 2015;28(11):788-98. [DOI: 10.3967/bes2015.110] [PMID: ] [DOI] [PubMed] [Google Scholar]
Imdad 2012
- Imdad A, Bhutta ZA. Routine iron/folate supplementation during pregnancy: effect on maternal anaemia and birth outcomes. Paediatric and Perinatal Epidemiology 2012;26 Suppl 1:168-77. [DOI: 10.1111/j.1365-3016.2012.01312.X] [PMID: ] [DOI] [PubMed] [Google Scholar]
Keats 2019
- Keats EC, Haider BA, Tam E, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD004905. [DOI: 10.1002/14651858.CD004905.pub6] [PMC6418471] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kristjansson 2015
- Kristjansson E, Francis DK, Liberato S, Benkhalti Jandu M, Welch V, Batal M, et al. Food supplementation for improving the physical and psychosocial health of socio-economically disadvantaged children aged three months to five years. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD009924. [DOI: 10.1002/14651858.CD009924.pub2] [PMC6885042] [PMID: 25739460] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lassi 2013
- Lassi ZS, Salam RA, Haider BA, Bhutta ZA. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD006896. [DOI: 10.1002/14651858.CD006896.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lassi 2020
- Lassi ZS, Kedzior SG, Tariq W, Jadoon Y, Das JK, Bhutta ZA. Effects of preconception care and periconception interventions on maternal nutritional status and birth outcomes in low- and middle-income countries: a systematic review. Nutrients 2020;12(3):606. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Low 2013
- Low M, Farrell A, Biggs BA, Pasricha SR. Effects of daily iron supplementation in primary-school-aged children: systematic review and meta-analysis of randomized controlled trials. CMAJ: Canadian Medical Association Journal [Journal De L'Association Medicale Canadienne] 2013;185(17):E791-802. [DOI: 10.1503/cmaj.130628] [PMC3832580] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Low 2016
- Low MS, Speedy J, Styles CE, De-Regil LM, Pasricha SR. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD009747. [DOI: 10.1002/14651858.CD009747.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuyama 2017
- Matsuyama M, Harb T, David M, Davies PS, Hill RJ. Effect of fortified milk on growth and nutritional status in young children: a systematic review and meta-analysis. Public Health Nutrition 2017;20(7):1214-25. [DOI: 10.1017/S1368980016003189] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayo‐Wilson 2014a
- Mayo-Wilson E, Junior JA, Imdad A, Dean S, Chan XH, Chan ES, et al. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD009384. [DOI: 10.1002/14651858.CD009384.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
McCauley 2015
- McCauley ME, Van den Broek N, Dou L, Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD008666. [DOI: 10.1002/14651858.CD008666.pub3] [PMC7173731] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neuberger 2016
- Neuberger A, Okebe J, Yahav D, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD006589. [DOI: 10.1002/14651858.CD006589] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasricha 2013
- Pasricha SR, Hayes E, Kalumba K, Biggs BA. Effect of daily iron supplementation on health in children aged 4-23 months: a systematic review and meta-analysis of randomised controlled trials. Lancet. Global Health 2013;1(2):e77-86. [DOI: 10.1016/S2214-109X(13)70046-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2015a
- Peña-Rosas JP, De-Regil LM, Gomez Malave H, Flores-Urrutia MC, Dowswell T. Intermittent oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD009997. [DOI: 10.1002/14651858.CD009997.pub2] [PMC7092533] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peña‐Rosas 2015b
- Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD004736. [DOI: 10.1002/14651858.CD004736.pub5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peña‐Rosas 2019
- Peña-Rosas JP, Mithra P, Unnikrishnan B, Kumar N, De-Regil LM, Nair NS, et al. Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD009902. [DOI: 10.1002/14651858.CD009902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petry 2016b
- Petry N, Olofin I, Boy E, Donahue Angel M, Rohner F. The effect of low dose iron and zinc intake on child micronutrient status and development during the first 1000 days of life: a systematic review and meta-analysis. Nutrients 2016;8(12):773. [DOI: 10.3390/nu8120773] [PMC5188428] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pratt 2015
- Pratt O. A review of the strategies used to reduce the prevalence of iron deficiency and iron deficiency anaemia in infants aged 6-36 months. Nutrition Bulletin 2015;40(4):257-67. [Google Scholar]
Qassim 2018
- Qassim A, Mol BW, Grivell RM, Grzeskowiak LE. Safety and efficacy of intravenous iron polymaltose, iron sucrose and ferric carboxymaltose in pregnancy: A systematic review. Australian & New Zealand Journal of Obstetrics & Gynaecology 2018;58:22-39. [DOI] [PubMed] [Google Scholar]
Qassim 2019
- Qassim A, Grivell RM, Henry A, Kidson‐Gerber G, Shand A, Grzeskowiak LE. Intravenous or oral iron for treating iron deficiency anaemia during pregnancy: systematic review and meta‐analysis. Medical Journal of Australia 2019;211(8):367-73. [DOI] [PubMed] [Google Scholar]
Radhika 2019
- Radhika AG, Sharma AK, Perumal V, Sinha A, Sriganesh V, Kulshreshtha V, et al. Parenteral versus oral iron for treatment of iron deficiency anaemia during pregnancy and post-partum: a systematic review. Journal of Obstetrics and Gynecology of India 2019;69(1):13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramírez‐Luzuriaga 2018
- Ramírez-Luzuriaga MJ, Larson LM, Mannar V, Martorell R. Impact of double-fortified salt with iron and iodine on hemoglobin, anemia, and iron deficiency anemia: a systematic review and meta-analysis. Advances in Nutrition 2018;9(3):207-18. [DOI: 10.1093/advances/nmy008] [PMC5952925] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reveiz 2011
- Reveiz L, Gyte GM, Cuervo LG, Casasbuenas A. Treatments for iron-deficiency anaemia in pregnancy. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No: CD003094. [DOI: 10.1002/14651858.CD003094.pub3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rumbold 2015
- Rumbold A, Ota E, Nagata C, Shahrook S, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD004072. [DOI: 10.1002/14651858.CD004072.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadighi 2019
- Sadighi J, Nedjat S, Rostami R. Systematic review and meta-analysis of the effect of iron-fortified flour on iron status of populations worldwide. Public Health Nutrition 2019;22(18):3465-84. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salam 2013
- Salam RA, MacPhail C, Das JK, Bhutta ZA. Effectiveness of micronutrient powders (MNP) in women and children. BMC Public Health 2013;13(Suppl 3):S22. [10.1186/1471-2458-13-S3-S22] [24564207] [PMC3847468] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salam 2016
- Salam RA, Hooda M, Das JK, Arshad A, Lassi ZS, Middleton P, et al. Interventions to improve adolescent nutrition: a systematic review and meta-analysis. Journal of Adolescent Health 2016;59(4S):S29-39. [DOI: 10.1016/j.jadohealth.2016.06.022] [PMC5026685] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salam 2020
- Salam RA, Das JK, Irfan O, Ahmed W, Sheikh SS, Bhutta ZA. Effects of preventive nutrition interventions among adolescents on health and nutritional status in low‐and middle‐income countries: A systematic review. Campbell Systematic Reviews 2020;16(2):e1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shapiro 2019
- Shapiro MJ, Downs SM, Swartz HJ, Parker M, Quelhas D, Kreis K, et al. A systematic review investigating the relation between animal-source food consumption and stunting in children aged 6-60 months in low and middle-income countries. Advances in Nutrition (Bethesda, Md.) 2019;10(5):827-47. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2015
- Shi Q, Leng W, Wazir R, Li J, Yao Q, Mi C, et al. Intravenous iron sucrose versus oral iron in the treatment of pregnancy with iron deficiency anaemia: a systematic review. Gynecologic and Obstetric Investigation 2015;80(3):170-8. [DOI: 10.1159/000376577] [PMID: ] [DOI] [PubMed] [Google Scholar]
Silva Neto 2019
- Silva Neto LG, Santos Neto JE, Bueno NB, De Oliveira SL, Ataide TD. Effects of iron supplementation versus dietary iron on the nutritional iron status: Systematic review with meta-analysis of randomized controlled trials. Critical Reviews in Food Science and Nutrition 2019;59(16):2553-61. [DOI: 10.1080/10408398.2018.1459469] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smelt 2018
- Smelt AF, Gussekloo J, Bermingham LW, Allen E, Dangour AD, Eussen SJ, et al. The effect of vitamin B12 and folic acid supplementation on routine haematological parameters in older people: an individual participant data meta-analysis. European Journal of Clinical Nutrition 2018;72(6):785-95. [DOI: 10.1038/s41430-018-0118-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Suchdev 2015
- Suchdev PS, Peña-Rosas JP, De-Regil LM. Multiple micronutrient powders for home (point-of-use) fortification of foods in pregnant women. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD011158. [DOI: 10.1002/14651858.CD011158.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suchdev 2020
- Suchdev PS, Jeerds ME, Ota E, da Silva Lopes K, De-Regil LM. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database of Systematic Reviews 2020, Issue 2. Art. No: CD008959. [DOI: 10.1002/14651858.CD008959.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sultan 2019
- Sultan P, Bampoe S, Shah R, Guo N, Estes J, Stave C, et al. Oral vs intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. American Journal of Obstetrics and Gynecology 2019;221(1):19-29.e3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tablante 2019
- Tablante EC, Pachón H, Guetterman HM, Finkelstein JL. Fortification of wheat and maize flour with folic acid for population health outcomes. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD012150. [DOI: 10.1002/14651858.CD012150.pub2] [PMC6599881] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tay 2015
- Tay HS, Soiza RL. Systematic review and meta-analysis: what is the evidence for oral iron supplementation in treating anaemia in elderly people? Drugs & Aging 2015;32(2):149-58. [DOI: 10.1007/s40266-015-0241-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thompson 2013
- Thompson J, Biggs BA, Pasricha SR. Effects of daily iron supplementation in 2- to 5-year-old children: systematic review and meta-analysis. Pediatrics 2013;131(4):739-53. [DOI: 10.1542/peds.2012-2256] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thorne‐Lyman 2012
- Thorne-Lyman AL, Fawzi WW. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatric and Perinatal Epidemiology 2012;26(Suppl 1):36-54. [DOI: 10.1111/j.1365-3016.2012.01284.x] [PMC3843354] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tolkien 2015
- Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PloS One 2015;10(2):e0117383. [DOI: 10.1371/journal.pone.0117383] [PMC4336293] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yadav 2019
- Yadav K, Goel AD, Yadav V, Upadhyay RP, Palepu S, Pandav CS. Meta-analysis of efficacy of iron and iodine fortified salt in improving iron nutrition status. Indian Journal of Public Health 2019;63(1):58-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to excluded reviews
Agha 2014
- Agha M, Agha RA, Sandall J. Interventions to reduce and prevent obesity in pre-conceptual and pregnant women: a systematic review and meta-analysis. PloS one 2014;9(5):e95132. [DOI: 10.1371/journal.pone.0095132] [PMC4020754] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2009
- Allen LH, Peerson JM, Olney DK. Provision of multiple rather than two or fewer micronutrients more effectively improves growth and other outcomes in micronutrient-deficient children and adults. Journal of Nutrition 2009;139(5):1022-30. [DOI: 10.3945/jn.107.086199] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ashman 2017
- Ashman AM, Brown LJ, Collins CE, Rollo ME, Rae KM. Factors associated with effective nutrition interventions for pregnant indigenous women: a systematic review. Journal of the Academy of Nutrition and Dietetics 2017;117(8):1222-53.e2. [DOI: 10.1016/j.jand.2017.03.012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Athe 2014
- Athe R, Rao MVV, Nair KM. Impact of iron-fortified foods on Hb concentration in children (< 10 years): a systematic review and meta-analysis of randomized controlled trials. Public Health Nutrition 2014;17(3):579-86. [DOI: 10.1017/S1368980013000062] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Athe 2020
- Athe R, Dwivedi R, Pati S, Mazumder A, Banset U. Meta-analysis approach on iron fortification and its effect on pregnancy and its outcome through randomized, controlled trials. Journal of Family Medicine and Primary Care 2020;9(2):513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bairwa 2017
- Bairwa M, Ahamed F, Sinha S, Yadav K, Kant S, Pandav CS. Directly observed iron supplementation for control of iron deficiency anemia. Indian Journal of Public Health 2017;61(1):37. [DOI] [PubMed] [Google Scholar]
Best 2011
- Best C, Neufingerl N, Del Rosso JM, Transler C, Van den Briel T, Osendarp S. Can multi-micronutrient food fortification improve the micronutrient status, growth, health, and cognition of schoolchildren? A systematic review. Nutrition Reviews 2011;69(4):186-204. [DOI: 10.1111/j.1753-4887.2011.00378.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brown 2009
- Brown KH, Peerson JM, Baker SK, Hess SY. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food and Nutrition Bulletin 2009;30(1 Suppl):S12-40. [DOI: 10.1177/15648265090301S103] [PMID: ] [DOI] [PubMed] [Google Scholar]
Butler 2006
- Butler CC, Vidal-Alaball J, Cannings-John R, McCaddon A, Hood K, Papaioannou A, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials. Family Practice 2006;23(3):279-85. [DOI: 10.1093/fampra/cml008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Câmara 2011
- Câmara MC, Barreto AM, Medeiros AP, Lucena IF, Moura JV, Oliveira TF. The use of games in health education in order to prevent iron deficiency anemia in infancy: the role of the nurse - literature systematic review [A utilização da brincadeira na educação em saúde como prevenção da anemia ferropriva na infância: atuação do enfermeiro – estudo de revisão integrativa]. Nursing Journal [Revista de Enfermagem] 2011;5(5):1280-5. [DOI: 10.5205/reuol.1302-9310-2-LE.0505201127] [DOI] [Google Scholar]
Cancelo‐Hidalgo 2013
- Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, Haya-Palazuelos J, Ciria-Recasens M, Manasanch J, et al. Tolerability of different oral iron supplements: a systematic review. Current Medical Research and Opinion 2013;29(4):291-303. [DOI: 10.1185/03007995.2012.761599] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chang 2018
- Chang CH, Tseng PT, Chen NY, Lin PC, Lin PY, Chang JP, et al. Safety and tolerability of prescription omega-3 fatty acids: a systematic review and meta-analysis of randomized controlled trials. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2018;129:1-12. [DOI: 10.1016/j.plefa.2018.01.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Da Cunha 2019
- Da Cunha MS, Campos Hankins NA, Arruda SF. Effect of vitamin A supplementation on iron status in humans: a systematic review and meta-analysis. Critical Reviews in Food Science and Nutrition 2019;59(11):1767-81. [DOI: 10.1080/10408398.2018.1427552] [PMID: ] [DOI] [PubMed] [Google Scholar]
Das 2013b
- Das JK, Salam RA, Kumar R, Bhutta ZA. Micronutrient fortification of food and its impact on woman and child health: a systematic review. Systematic Reviews 2013;2:67. [DOI: 10.1186/2046-4053-2-67] [PMC3765883] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Barros 2016
- De Barros SF, Cardoso MA. Adherence to and acceptability of home fortification with vitamins and minerals in children aged 6 to 23 months: a systematic review. BMC Public Health 2016;16:299. [DOI: 10.1186/s12889-016-2978-0] [PMC4823916] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dror 2012
- Dror DK, Allen LH. Interventions with vitamins B6, B12 and C in pregnancy. Paediatric and Perinatal Epidemiology 2012;26 Suppl 1:55-74. [DOI: 10.1111/j.1365-3016.2012.01277.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Eaton 2019
- Eaton JC, Rothpletz‐Puglia P, Dreker MR, Iannotti L, Lutter C, Kaganda J, et al. Effectiveness of provision of animal-source foods for supporting optimal growth and development in children 6 to 59 months of age. Cochrane Database of Systematic Reviews 2019, Issue 2. Art. No: CD012818. [DOI: 10.1002/14651858.CD012818.pub2] [PMC6380771] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falkingham 2010
- Falkingham M, Abdelhamid A, Curtis P, Fairweather-Tait S, Dye L, Hooper L. The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta-analysis. Nutrition Journal 2010;9:4. [DOI: 10.1186/1475-2891-9-4] [PMC2831810] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fishman 2000
- Fishman SM, Christian P, West KP. The role of vitamins in the prevention and control of anaemia. Public Health Nutrition 2000;3(2):125-50. [DOI: 10.1017/s1368980000000173] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gavaravarapu 2017
- Gavaravarapu SM, Archana Konapur A, Saha S. Role of education and communication interventions in promoting micronutrient status in India – what research in the last two decades informs. Journal of Communication in Healthcare 2017;10(4):238-49. [DOI: 10.1080/17538068.2017.1338407] [DOI] [Google Scholar]
Gera 2007b
- Gera T, Sachdev HP, Nestel P. Effect of iron supplementation on physical performance in children and adolescents: systematic review of randomized controlled trials. Indian Pediatrics 2007;44(1):15-24. [PMID: ] [PubMed] [Google Scholar]
Ghanchi 2019
- Ghanchi A, James PT, Cerami C. Guts, germs, and iron: a systematic review on iron supplementation, iron fortification, and diarrhea in children aged 4-59 months. Current Developments in Nutrition 2019;3(3):nzz005. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Girard 2012a
- Girard AW, Olude O. Nutrition education and counselling provided during pregnancy: effects on maternal, neonatal and child health outcomes. Paediatric and Perinatal Epidemiology 2012;26 Suppl 1:191-204. [DOI: 10.1111/j.1365-3016.2012.01278.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Girard 2012b
- Girard AW, Self JL, McAuliffe C, Olude O. The effects of household food production strategies on the health and nutrition outcomes of women and young children: a systematic review. Paediatric and Perinatal Epidemiology 2012;26 Suppl 1:205-22. [DOI: 10.1111/j.1365-3016.2012.01282.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guo 2015
- Guo XM, Liu H, Qian J. Daily iron supplementation on cognitive performance in primary-school-aged children with and without anemia: a meta-analysis. International Journal of Clinical and Experimental Medicine 2015;8(9):16107-11. [PMC4659009] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2014
- Gurusamy KS, Nagendran M, Broadhurst JF, Anker SD, Richards T. Iron therapy in anaemic adults without chronic kidney disease. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD010640. [DOI: 10.1002/14651858.CD010640.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haider 2018
- Haider LM, Schwingshackl L, Hoffmann G, Ekmekcioglu C. The effect of vegetarian diets on iron status in adults: a systematic review and meta-analysis. Critical Reviews in Food Science and Nutrition 2018;58(8):1359-74. [DOI: 10.1080/10408398.2016.1259210] [PMID: ] [DOI] [PubMed] [Google Scholar]
Iannotti 2006
- Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. American Journal of Clinical Nutrition 2006;84(6):1261-76. [DOI: 10.1093/ajcn/84.6.1261] [PMC3311916] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iglesias 2019
- Iglesias Vãzquez L, Valera E, Villalobos M, Tous M, Arija V. Prevalence of anemia in children from Latin America and the Caribbean and effectiveness of nutritional interventions: systematic review and meta-analysis. Nutrients 2019;11(1):183. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iqbal 2019
- Iqbal S, Ekmekcioglu C. Maternal and neonatal outcomes related to iron supplementation or iron status: a summary of meta-analyses. Journal of Maternal-Fetal & Neonatal Medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians 2019;32(9):1528-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jackson 2016
- Jackson J, Williams R, McEvoy M, MacDonald-Wicks L, Patterson A. Is higher consumption of animal flesh foods associated with better iron status among adults in developed countries? A systematic review. Nutrients 2016;8(2):89. [DOI: 10.3390/nu8020089] [PMC4772052] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kong 2016
- Kong K, Liu J, Tao Y. Limitations of studies on school-based nutrition education interventions for obesity in China: a systematic review and meta-analysis. Asia Pacific Journal of Clinical Nutrition 2016;25(3):589-601. [DOI: 10.6133/apjcn.092015.19] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lewkowitz 2019
- Lewkowitz AK, Gupta A, Simon L, Sabol BA, Stoll C, Cooke E, et al. Intravenous compared with oral iron for the treatment of iron-deficiency anemia in pregnancy: a systematic review and meta-analysis. Journal of Perinatology 2019;39(4):519-32. [DOI: 10.1038/s41372-019-0320-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lohner 2012
- Lohner S, Fekete K, Berti C, Hermoso M, Cetin I, Koletzko B, et al. Effect of folate supplementation on folate status and health outcomes in infants, children and adolescents: a systematic review. International Journal of Food Sciences and Nutrition 2012;63(8):1014-20. [DOI: 10.3109/09637486.2012.683779] [PMID: ] [DOI] [PubMed] [Google Scholar]
Martínez 2020
- Martínez Francés A, Leal Martínez-Bujanda J. Efficacy and tolerability of oral iron protein succinylate: a systematic review of three decades of research. Current Medical Research and Opinion 2020;36(4):613-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mayo‐Wilson 2014b
- Mayo-Wilson E, Imdad A, Junior J, Dean S, Bhutta ZA. Preventive zinc supplementation for children, and the effect of additional iron: a systematic review and meta-analysis. BMJ Open 2014;4(6):e004647. [DOI: 10.1136/bmjopen-2013-004647] [PMC4067863] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonagh 2015a
- McDonagh M, Blazina I, Dana T, Cantor A, Bougatsos C. Routine iron supplementation and screening for iron deficiency anemia in children ages 6 to 24 months: a systematic review to update the US Preventive Services Task Force Recommendation; March 2015. Available at www.ncbi.nlm.nih.gov/books/NBK285660. [NBK285660] [PMID: ] [PubMed]
McDonagh 2015b
- McDonagh M, Cantor A, Bougatsos C, Dana T, Blazina I. Routine iron supplementation and screening for iron deficiency anemia in pregnant women: a systematic review to update the US Preventive Services Task Force Recommendation [Internet]; March 2015. Available at www.ncbi.nlm.nih.gov/books/NBK285986. [NBK285986] [PMID: ] [PubMed]
McDonagh 2015c
- McDonagh MS, Blazina I, Dana T, Cantor A, Bougatsos C. Screening and routine supplementation for iron deficiency anemia: a systematic review. Pediatrics 2015;135(4):723-33. [DOI: 10.1542/peds.2014-3979] [PMID: ] [DOI] [PubMed] [Google Scholar]
Michelazzo 2013
- Michelazzo FB, Oliveira JM, Stefanello J, Luzia LA, Rondó PHC. The influence of vitamin A supplementation on iron status. Nutrients 2013;5(11):4399-413. [DOI: 10.3390/nu5114399] [MC3847738] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Middleton 2013
- Middleton P, Lassi Z, Tran TS, Bhutta ZA, Bubner T, Flenady V, et al. Nutrition interventions and programs for reducing mortality and morbidity in pregnant and lactating women and women of reproductive age: a systematic review. Journal of Paediatrics and Child Health 2013;49:71. [DOI: 10.1111/jpc.12131] [DOI] [Google Scholar]
Middleton 2018
- Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD003402. [DOI: 10.1002/14651858.CD003402.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miles 2019
- Miles LF, Litton E, Imberger G, Story D. Intravenous iron therapy for non-anaemic, iron-deficient adults. Cochrane Database of Systematic Reviews 2019, Issue 12. Art. No: CD013084. [DOI: 10.1002/14651858.CD013084.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milne 2009
- Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD003288. [DOI: 10.1002/14651858.CD003288.pub3] [PMC7144819] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirmiran 2012
- Mirmiran P, Golzarand M, Serra-Majem L, Azizi F. Iron, iodine and vitamin a in the middle East; a systematic review of deficiency and food fortification. Iranian Journal of Public Health 2012;41(8):8-19. [PMC3469033] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Oddo 2019
- Oddo VM, Roshita A, Rah JH. Potential interventions targeting adolescent nutrition in Indonesia: a literature review. Public Health Nutrition 2019;22(1):15-27. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oh 2020
- Oh C, Keats EC, Bhutta ZA. Vitamin and mineral supplementation during pregnancy on maternal, birth, child health and development outcomes in low- and middle-income countries: a systematic review and meta-analysis. Nutrients 2020;12(2):491. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliveira 2016
- Oliveira JM, Allert R, East CE. Vitamin A supplementation for postpartum women. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No: CD005944. [DOI: 10.1002/14651858.CD005944.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osungbade 2012
- Osungbade KO, Oladunjoye AO. Preventive treatments of iron deficiency anaemia in pregnancy: a review of their effectiveness and implications for health system strengthening. Journal of Pregnancy 2012;2012(454601):1-7. [DOI: 10.1155/2012/454601] [PMC3400371] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pachón 2015
- Pachón H, Spohrer R, Mei Z, Serdula MK. Evidence of the effectiveness of flour fortification programs on iron status and anemia: a systematic review. Nutrition Reviews 2015;73(11):780-95. [DOI: 10.1093/nutrit/nuv037] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasricha 2009
- Pasricha S, Shet A, Sachdev HP, Shet AS. Risks of routine iron and folic acid supplementation for young children. Indian Pediatrics 2009;46(10):857-66. [PMID: ] [PubMed] [Google Scholar]
Pasricha 2014
- Pasricha SR, Low M, Thompson J, Farrell A, De-Regil LM. Iron supplementation benefits physical performance in women of reproductive age: a systematic review and meta-analysis. Journal of Nutrition 2014;144(6):906-14. [DOI: 10.3945/jn.113.189589] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sachdev 2005
- Sachdev HP, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutrition 2005;8(2):117-32. [DOI: 10.1079/phn2004677] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sguassero 2012
- Sguassero Y, De Onis M, Bonotti AM, Carroli G. Community-based supplementary feeding for promoting the growth of children under five years of age in low and middle income countries. Cochrane Database of Systematic Reviews 2012, Issue 6. Art. No: CD005039. [DOI: 10.1002/14651858.CD005039.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shao 2019
- Shao Y, Luo W, Xu H, Zhang L, Guo Q. The efficacy of ferumoxytol for iron deficiency anemia: a meta-analysis of randomized controlled trials. Acta Haematologica 2019;142(3):125-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 2017
- Smith ER, Shankar AH, Wu LS, Aboud S, Adu-Afarwuah S, Ali H, et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet. Global health 2017;5(11):e1090-100. [DOI: 10.1016/S2214-109X(17)30371-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sun 2018
- Sun J, Zhang L, Cui J, Li S, Lu H, Zhang Y, et al. Effect of dietary intervention treatment on children with iron deficiency anemia in China: a meta-analysis. Lipids in Health and Disease 2018;17(1):108. [DOI: 10.1186/s12944-018-0749-x] [PMC5944174] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Szajewska 2010
- Szajewska H, Ruszczynski M, Chmielewska A. Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: a systematic review of randomized controlled trials. American Journal of Clinical Nutrition 2010;91(6):1684-90. [DOI: 10.3945/ajcn.2010.29191] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tam 2020
- Tam E, Keats EC, Rind F, Das JK, Bhutta ZA. Micronutrient Supplementation and Fortification Interventions on Health and Development Outcomes among Children Under-Five in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020;12(2):289. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vonderheid 2019
- Vonderheid SC, Tussing-Humphreys L, Park C, Pauls H, OjiNjideka Hemphill N, LaBomascus B, et al. A systematic review and meta-analysis on the effects of probiotic species on iron absorption and iron status. Nutrients 2019;11(12):2938. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2019
- Xu J, Li Y, Huo J, Sun J, Huang J. Supplementing fortified soybean powder reduced anemia in infants and young children aged 6-24 months. Nutrition Research 2019;63:21-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yadav 2020
- Yadav K, Arjun MC, Jacob OM, Kant S, Ahamed F, Ramaswamy G. Comparison of different doses of daily iron supplementation for anemia prophylaxis in pregnancy: A systematic review. Journal of Family Medicine and Primary Care 2020;9(3):1308-16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adish 1999
- Adish AA, Esrey SA, Gyorkos TW, Jean-Baptiste J, Rojhani A. Effect of consumption of food cooked in iron pots on iron status and growth of young children: a randomised trial. Lancet 1999;353(9154):712-6. [DOI: 10.1016/S0140-6736(98)04450-X] [PMID: ] [DOI] [PubMed] [Google Scholar]
Alaofè 2009
- Alaofè H, Zee J, Dossa R, O'Brien HT. Education and improved iron intakes for treatment of mild iron-deficiency anemia in adolescent girls in southern Benin. Food and Nutrition Bulletin 2009;30(1):24-36. [DOI: 10.1177/156482650903000103] [PMID: ] [DOI] [PubMed] [Google Scholar]
Allen 2000
- Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. American Journal of Clinical Nutrition 2000;71(Suppl 5):1280S-4S. [DOI: 10.1093/ajcn/71.5.1280s] [PMID: ] [DOI] [PubMed] [Google Scholar]
Allen 2008
- Allen LH. To what extent can food-based approaches improve micronutrient status? Asia Pacific Journal of Clinical Nutrition 2008;17 Suppl 1:103-5. [PMID: ] [PubMed] [Google Scholar]
Alves 2019
- Alves C, Saleh A, Alaofè H. Iron-containing cookware for the reduction of iron deficiency anemia among children and females of reproductive age in low- and middle-income countries: a systematic review. PloS one 2019;14(9):e0221094. [DOI: 10.1371/journal.pone.0221094] [PMC6719866] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
An 2020
- An P, Liu Y, Xu T, Zhang X, Min J, Wang F. Effects of probiotics/prebiotics/synbiotics supplementation on iron status and anemia. PROSPERO 2020. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020182785] [CRD42020182785]
Andersen 2016
- Andersen C, Noor R, Fawzi W, Mozaffarian D. Child iron supplementation or fortification for anemia, growth, infection, and developmental outcomes: a systematic review and meta-analysis of randomized trials. PROSPERO 2016. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42016039948] [CRD42016039948]
Armstrong 2017
- Armstrong GR, Dewey CE, Summerlee AJ. Iron release from the Lucky Iron Fish®: safety considerations. Asia Pacific Journal of Clinical Nutrition 2017;26(1):148-55. [DOI: 10.6133/apjcn.102015.14] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bager 2014
- Bager P. Fatigue and acute/chronic anaemia. Danish Medical Journal 2014;61(4):B4824. [PMID: ] [PubMed] [Google Scholar]
Baltussen 2004
- Baltussen R, Knai C, Sharan M. Iron fortification and iron supplementation are cost-effective interventions to reduce iron deficiency in four subregions of the world. Journal of Nutrition 2004;134(10):2678-84. [DOI: 10.1093/jn/134.10.2678] [PMID: ] [DOI] [PubMed] [Google Scholar]
Beck 2014
- Beck KL, Conlon CA, Kruger R, Coad J. Dietary determinants of and possible solutions to iron deficiency for young women living in industrialized countries: a review. Nutrients 2014;6(9):3747-76. [DOI: 10.3390/nu6093747] [PMC4179187] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beutler 2003
- Beutler E, Hoffbrand AV, Cook JD. Iron deficiency and overload. Hematology 2003;2003:40-61. [DOI: 10.1182/asheducation-2003.1.40] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brabin 2001a
- Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. Journal of Nutrition 2001;131(2S-2):604S-14S; discussion 614S-5S. [DOI: 10.1093/jn/131.2.604S] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brabin 2001b
- Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. Journal of Nutrition 2001;131(2S-2):636S-45S; discussion 646S-8S. [DOI: 10.1093/jn/131.2.636S] [PMID: ] [DOI] [PubMed] [Google Scholar]
Butwick 2017
- Butwick A, Bampoe S, Sultan P. A systematic review of the efficacy of oral vs intravenous iron therapy for the treatment of postpartum anemia. PROSPERO 2017. [Available from: www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42017080234] [CRD42017080234]
Camaschella 2015
- Camaschella C. Iron-deficiency anemia. New England Journal of Medicine 2015;372(19):1832-43. [DOI: 10.1056/NEJMra1401038] [PMID: ] [DOI] [PubMed] [Google Scholar]
CASP 2013
- Critical Appraisal Skills Programme (CASP). CASP Checklists. casp-uk.net/casp-tools-checklists (accessed 31 July 2015).
Cavalli‐Sforza 2005
- Cavalli-Sforza T, Berger J, Smitasiri S, Viteri F. Weekly iron-folic acid supplementation of women of reproductive age: impact overview, lessons learned, expansion plans, and contributions toward achievement of the millennium development goals. Nutrition Reviews 2005;63(12 Pt 2):S152-8. [PMID: ] [PubMed] [Google Scholar]
CDR 2009
- Centre for Reviews and Dissemination (CDR). Systematic reviews. CRD’s guidance for undertaking reviews in health care; 2009. Available at www.york.ac.uk/media/crd/Systematic_Reviews.pdf.
Charles 2011
- Charles CV, Summerlee AJ, Dewey CE. Iron content of Cambodian foods when prepared in cooking pots containing an iron ingot. Tropical Medicine & International Health 2011;16(12):1518-24. [DOI: 10.1111/j.1365-3156.2011.02878.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Da Rocha 2017
- Da Rocha TA, Silva Neto LG, Bezerra Bueno N, Dos Santos Neto JE, De Oliveira SL. The effects of iron supplementation versus dietary iron on iron reserves in children: a systematic review and meta-analysis. PROSPERO 2017. [Available from: www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42017075118] [CRD42017075118]
Dary 2002
- Dary O, Mora JO, International Vitamin A Consultative Group. Food fortification to reduce vitamin A deficiency: International Vitamin A Consultative Group recommendations. Journal of Nutrition 2002;132(Suppl 9):2927S-33S. [DOI: 10.1093/jn/132.9.2927S] [PMID: ] [DOI] [PubMed] [Google Scholar]
Da Silva Lopes 2017
- Da Silva Lopes K, Ota E, Shakya P, Dagvadorj A, Balogun OO, Peña-Rosas JP, et al. Effects of nutrition interventions during pregnancy on low birth weight: an overview of systematic reviews. BMJ Global Health 2017;2(3):e000389. [DOI: 10.1136/bmjgh-2017-000389] [PMC5623264] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Benoist 2008
- De Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food and Nutrition Bulletin 2008;29(Suppl 2):S238-44. [DOI: 10.1177/15648265080292S129] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dubey 2019
- Dubey S, Sahay MR, Verma P, Pati S, Singh Kshatri J, Kanungo S. A systematic review and meta-analysis on the effect of food fortification with micronutrients (iron/folic acid/vitamin B12, or combination) on anemia among pregnant women. PROSPERO 2019. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019146388] [CRD42019146388]
EPOC 2019
- Cochrane Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/suggested_risk_of_bias_criteria_for_epoc_reviews.pdf (accessed April 2019).
Faggion 2015
- Faggion CM Jr. Critical appraisal of AMSTAR: challenges, limitations, and potential solutions from the perspective of an assessor. BMC Medical Research Methodology 2015;15:63. [DOI: 10.1186/s12874-015-0062-6] [PMC4535290] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
FAO 2011
- Food and Agriculture Organization of the United Nations. Guidelines for measuring household and individual dietary diversity; 2011. Available at www.fao.org/publications/card/en/c/5aacbe39-068f-513b-b17d-1d92959654ea. [ISBN 9789251067499]
Fortin 2018
- Fortin PM, Hopewell S, Estcourt LJ. Red blood cell transfusion to treat or prevent complications in sickle cell disease: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD012082. [DOI: 10.1002/14651858.CD012082.pub2] [PMC6513377] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gadhave 2020
- Gadhave S, Babu GR, Deepa R, Lobo E, Saldanha ND. Effect of meal supplementation during the antenatal period on the improvement in maternal and birth weight. PROSPERO 2020. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020175138] [CRD42020175138]
GBDPC 2016
- Global Burden of Disease Pediatrics Collaboration, Kyu HH, Pinho C, Wagner JA, Brown JC, Bertozzi-Villa A, Charlson FJ, et al. Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the Global Burden of Disease 2013 Study. JAMA Pediatrics 2016;170(3):267-87. [DOI: 10.1001/jamapediatrics.2015.4276] [PMC5076765] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Global Nutrition Report 2020
- Global Nutrition Report Team. 2020 Global Nutrition Report: Action on Equity to End Malnutrition. Bristol (UK): Development Initiatives, 2020. [Available at globalnutritionreport.org/reports/2020-global-nutrition-report/] [Google Scholar]
Gordon 2021
- Gordon M, Sinopoulou V, Iheozor-Ejiofor Z, Iqbal T, Allen P, Hoque S, et al. Interventions for treating iron deficiency anaemia in inflammatory bowel disease. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013529. [DOI: 10.1002/14651858.CD013529.pub2] [PMC8092475 (available on 2022-01-20)] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haider 2017
- Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD004905. [DOI: 10.1002/14651858.CD004905.pub5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2020
- Hansen R, Schroll JB, Sejer EP, Holm C. Benefits and risks of daily oral iron supplementation in pregnancy in iron replete non-anaemic women: a systematic review. PROSPERO 2020. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020186210] [CRD42020186210]
Hare 2018
- Hare D, Braat S, Cardoso B, Morgan C, Szymlek-Gay E, Biggs BA. Health outcomes of iron supplementation and/or food fortification in iron-replete children aged 4-24 months: a systematic review and meta-analysis of randomised controlled trials. PROSPERO 2018. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018093744] [CRD42018093744] [DOI] [PMC free article] [PubMed]
Heath 2002
- Heath AL, Fairweather-Tait SJ. Clinical implications of changes in the modern diet: iron intake, absorption and status. Best Practice & Research. Clinical Haematology 2002;15(2):225-41. [PMID: ] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hombali 2019
- Hombali AS, Solon JA, Venkatesh BT, Nair NS, Peña-Rosas JP. Fortification of staple foods with vitamin A for vitamin A deficiency. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD010068. [DOI: 10.1002/14651858.CD010068.pub2] [PMC6509778] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horton 2003
- Horton S, Ross J. The economics of iron deficiency. Food Policy 2003;28(1):51-75. [DOI: 10.1016/S0306-9192(02)00070-2] [DOI] [Google Scholar]
Hultcrantz 2017
- Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. Journal of Clinical Epidemiology 2017;87:4-13. [DOI: 10.1016/j.jclinepi.2017.05.006] [PMC6542664] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurrell 1999
- Hurrell RF, Reddy M, Cook JD. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. British Journal of Nutrition 1999;81(4):289-95. [PMID: ] [PubMed] [Google Scholar]
Hurrell 2002
- Hurrell RF. Fortification: overcoming technical and practical barriers. Journal of Nutrition 2002;132(4 Suppl):806S-12S. [DOI: 10.1093/jn/132.4.806S] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hurrell 2010
- Hurrell R, Egli I. Iron bioavailability and dietary reference values. American Journal of Clinical Nutrition 2010;91(5):1461S-7S. [DOI: 10.3945/ajcn.2010.28674F] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hurrell 2012
- Hurrell RF. Influence of inflammatory disorders and infection on iron absorption and efficacy of iron-fortified foods. Nestle Nutrition Institute Workshop Series 2012;70:107-16. [DOI: 10.1159/000337673] [PMC4353607] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2005
- Jackson N, Waters E, Guidelines for Systematic Reviews in Health Promotion and Public Health Taskforce. Criteria for the systematic review of health promotion and public health interventions. Health Promotion International 2005;20(4):367-74. [DOI: 10.1093/heapro/dai022] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17(1):1-12. [DOI: 10.1016/0197-2456(95)00134-4] [8721797] [DOI] [PubMed] [Google Scholar]
Jere 2020
- Jere M, Amenyah S, McNulty H, Hoey L, Price R, Gairhre S. The effects of B vitamins and/or iron on haematological parameters and nutrient biomarkers of anaemia in children in low- and middle-income countries: a systematic review and meta-analysis of randomized controlled trials. PROPERO 2020. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020166563] [CRD42020166563]
Kapur 2003
- Kapur D, Sharma S, Agarwal KN. Effectiveness of nutrition education, iron supplementation or both on iron status in children. Indian pediatrics 2003;40(12):1131-44. [PMID: ] [PubMed] [Google Scholar]
Kassebaum 2014
- Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123(5):615-24. [DOI: 10.1182/blood-2013-06-508325] [PMC3907750] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lönnerdal 2017
- Lönnerdal B. Excess iron intake as a factor in growth, infections, and development of infants and young children. American Journal of Clinical Nutrition 2017;106(Suppl 6):1681S-7S. [DOI: 10.3945/ajcn.117.156042] [PMC5701711] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2016
- Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet 2016;387(10021):907-16. [DOI: 10.1016/S0140-6736(15)60865-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lynch 2000
- Lynch SR. The effect of calcium on iron absorption. Nutrition Research Reviews 2000;13(2):141-58. [DOI: 10.1079/095442200108729043] [PMID: ] [DOI] [PubMed] [Google Scholar]
Man 2021
Mason 2013
- Mason J, Martorell R, Saldanha L, Shrimpton R. Reduction of anaemia. Lancet Global Health 2013;1(1):e4-6. [DOI: 10.1016/S2214-109X(13)70009-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Milman 2020
- Milman NT. A review of nutrients and compounds, which promote or inhibit intestinal iron absorption: making a platform for dietary measures that can reduce iron uptake in patients with genetic haemochromatosis. Journal of Nutrition and Metabolism 2020;2020:7373498. [DOI: 10.1155/2020/7373498] [PMC7509542] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [PMC2707599] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2014
- Moore JB, Heffernan A, Allcock D, Holmes M, Evans C. A systematic review and meta-analysis of the evidence for the regulation of dietary iron bioavailability by vitamin C and phytate, in the context of hepcidin, and subsequent effects on iron status. PROSPERO 2014. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42014010453] [CRD42014010453]
Morck 1983
- Morck TA, Lynch SR, Cook JD. Inhibition of food iron absorption by coffee. American Journal of Clinical Nutrition 1983;37(3):416-20. [DOI: 10.1093/ajcn/37.3.416] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nandi 2016
- Nandi A, Ashok A, Kinra S, Behrman JR, Laxminarayan R. Early childhood nutrition is positively associated with adolescent educational outcomes: evidence from the Andhra Pradesh Child and Parents Study (APCAPS). Journal of Nutrition 2016;146(4):806-13. [DOI: 10.3945/jn.115.223198] [PMC4807645] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NHLBI
- National Heart, Lung and Blood Institute (NHLBI). Study Quality Assessment Tools. Available at www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
Nikooyeh 2018
- Nikooyeh B, Neyestani TR. Nutritional impacts of home-fortification strategies in children younger than 5 year-old: a systematic review and meta-analysis. PROSPERO 2018. [Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018109279] [CRD42018109279]
Paganini 2017
- Paganini D, Zimmermann MB. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: a review. American Journal of Clinical Nutrition 2017;106(Suppl 6):1688S-93S. [DOI: 10.3945/ajcn.117.156067] [PMC5701709] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PAHO 2003
- Pan American Health Organization (PAHO). Guiding Principles for Complementary Feeding of the Breastfed Child. Washington (DC): PAHO, 2003. [www.who.int/nutrition/publications/guiding_principles_compfeeding_breastfed.pdf] [Google Scholar]
Pasricha 2011
- Pasricha SR. Daily iron supplementation for improving anaemia and health in children. PROSPERO 2011. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42011001208] [CRD42011001208]
Petry 2016a
- Petry N, Olofin I, Hurrell RF, Boy E, Wirth JP, Moursi M, et al. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients 2016;8(11):E693. [DOI: 10.3390/nu8110693] [PMC5133080] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prentice 2017
- Prentice AM, Mendoza YA, Pereira D, Cerami C, Wegmuller R, Constable A, et al. Dietary strategies for improving iron status: balancing safety and efficacy. Nutrition Reviews 2017;75(1):49-60. [DOI: 10.1093/nutrit/nuw055] [PMC5155616] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prinsen 2002
- Prinsen Geerligs P, Brabin B, Mkumbwa A, Broadhead R, Cuevas LE. Acceptability of the use of iron cooking pots to reduce anaemia in developing countries. Public Health Nutrition 2002;5(5):619-24. [DOI: 10.1079/PHN2002341] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rahman 2019
- Rahman RA, Idris IB. Systematic review of randomised controlled trials for effectiveness of interventions for prevention of anaemia in pregnancy. PROSPERO 2019. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020184283] [CRD42020184283]
Rogozinska 2018
- Rogozinska E, Daru J, Nicolaides M, Robinson S, Saborido CM, Zamora J, et al. Iron treatments (Fe) in reproductive age women with Iron Deficient Anaemia (FRIDA): a systematic review with network meta-analysis of randomised controlled trials. PROSPERO 2018. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018100822] [CRD42018100822]
Rosli 2019
- Rosli RR, Noor NM. Iron polymaltose complex for iron deficiency anaemia in children. PROSPERO 2019. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019145020] [CRD42019145020]
Ruel 2003
- Ruel MT. Operationalizing dietary diversity: a review of measurement issues and research priorities. Journal of Nutrition 2003;133(11 Suppl 2):3911S-26S. [DOI: 10.1093/jn/133.11.3911S] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ruel 2013
- Ruel MT, Alderman H, Maternal and Child Nutrition Study Group. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet 2013;382(9891):536-51. [DOI: 10.1016/S0140-6736(13)60843-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharifirad 2011
- Sharifirad G, Golshiri P, Shahnazi H, Shakouri S, Hassanzadeh A. PRECEDE educational model for controlling iron-deficiency anaemia in Talesh, Iran. Journal of the Pakistan Medical Association 2011;61(9):862-5. [PMID: ] [PubMed] [Google Scholar]
Shea 2007a
- Shea BJ, Bouter LM, Peterson J, Boers M, Andersson N, Ortiz Z, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PLoS One 2007;2(12):e1350. [DOI: 10.1371/journal.pone.0001350] [PMC2131785] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shea 2007b
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007;7:10. [DOI: 10.1186/1471-2288-7-10] [PMC1810543] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shea 2009
- Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. Journal of Clinical Epidemiology 2009;62(10):1013-20. [DOI: 10.1016/j.jclinepi.2008.10.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Siegfried 2012
- Siegfried N, Irlam JH, Visser ME, Rollins NN. Micronutrient supplementation in pregnant women with HIV infection. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No: CD009755. [DOI: 10.1002/14651858.CD009755] [PMID: ] [DOI] [PubMed] [Google Scholar]
SPRING/USAID 2017
- Strengthening Partnerships, Results, and Innovations in Nutrition Globally (SPRING), USAID. Six key actions to reduce anaemia from learning to practice; September 2017. www.spring-nutrition.org/publications/briefs/six-key-actions-reduce-anemia.
Stevens 2013
- Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Global Health 2013;1(1):e16-25. [DOI: 10.1016/S2214-109X(13)70001-9] [PMC4547326] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoltzfus 1998
- Stoltzfus RJ, Dreyfuss ML, editor(s), International Nutritional Anemia Consultative Group (INACG), World Health Organization (WHO), United Nations Childrens Fund (UNICEF). Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anemia. Washington (DC): ILSI Press, 1998. [ISBN 1-57881-020-5] [motherchildnutrition.org/nutrition-protection-promotion/pdf/mcn-guidelines-for-iron-supplementation.pdf] [Google Scholar]
Stoltzfus 2004
- Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anaemia. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors(s). Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Vol. 1. Geneva (CH): World Health Organization, 2004:163–210. [ISBN 92 4 158031 3] [apps.who.int/iris/bitstream/handle/10665/42792/9241580348_eng_Volume1.pdf?sequence=1] [Google Scholar]
Teucher 2004
- Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. International Journal for Vitamin and Nutrition Research 2004;74(6):403-19. [DOI: 10.1024/0300-9831.74.6.403] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tsang 2015
- Tsang B, Sandalinas F, De-Regil LM. Folate supplementation in women of reproductive age. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD011766. [DOI: 10.1002/14651858.CD011766] [DOI] [Google Scholar]
UN 2015
- United Nations. Transforming our world: the 2030 Agenda for Sustainable Development. sustainabledevelopment.un.org/content/documents/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf (accessed 25 June 2018).
UNICEF 1999
- United Nations Children's Fund (UNICEF), World Health Organization (WHO), United Nations University (UNU). Composition of a multi-micronutrient supplement to be used in pilot programmes among pregnant women in developing countries: report of a United Nations Children's Fund (UNICEF), World Health Organization (WHO) and United Nations University workshop. apps.who.int/iris/bitstream/handle/10665/75358/UNICEF-WHO-multi-micronutrients.pdf?sequence=1&isAllowed=y (accessed 25 June 2018).
Van Sande 2013
- Van Sande H, Jacquemyn Y, Karepouan N, Ajaji M. Vitamin B12 in pregnancy: maternal and fetal/neonatal effects—a review. Open Journal of Obstetrics and Gynecology 2013;3(7):599-602. [DOI: 10.4236/ojog.2013.37107] [DOI] [Google Scholar]
Verhagen 1998
- Verhagen AP, De Vet HC, De Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. Journal of clinical epidemiology 1998;51(12):1235-41. [DOI: 10.1016/s0895-4356(98)00131-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walker 2010
- Walker N, Fischer-Walker C, Bryce J, Bahl R, Cousens S, CHERG Review Groups on Intervention Effects. Standards for CHERG reviews of intervention effects on child survival. International Journal of Epidemiology 2010;39(Suppl 1):i21-31. [DOI: 10.1093/ije/dyq036 ] [PMCID: PMC2845875] [PMID: 20348122 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wegewitz 2016
- Wegewitz U, Weikert B, Fishta A, Jacobs A, Pieper D. Resuming the discussion of AMSTAR: what can (should) be made better? BMC Medical Research Methodology 2016;16(1):111. [DOI: 10.1186/s12874-016-0183-6] [PMC5002206] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2005
- Weiss G, Goodnough LT. Anemia of chronic disease. New England Journal of Medicine 2005;352(10):1011-23. [DOI: 10.1056/NEJMra041809] [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO/CDC 2007
- World Health Organization, Centers for Disease Control and Prevention. Assessing the Iron Status of Populations. 2nd edition. Geneva: World Health Organization, 2007. [ISBN 978 92 4 159610 7] [www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596107.pdf] [Google Scholar]
WHO/CDC 2008
- World Health Organization, Centers for Disease Control and Prevention. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. Geneva: World Health Organization, 2008. [ISBN 978 92 4 159665 7] [apps.who.int/iris/bitstream/handle/10665/43894/9789241596657_eng.pdf?sequence=1] [Google Scholar]
WHO/FAO 2006
- World Health Organization, Food and Agriculture Organization of the United Nations. Guidelines on Food Fortification with Micronutrients. Geneva: World Health Organization, 2006. [ISBN 92 4 159401 2] [apps.who.int/iris/bitstream/handle/10665/43412/9241594012_eng.pdf?sequence=1] [Google Scholar]
WHO 2011
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. www.who.int/vmnis/indicators/haemoglobin/en/ (accessed 15 September 2020).
WHO 2014a
- World Health Organization. Global nutrition targets 2025: to improve maternal, infant and young child nutrition. www.who.int/nutrition/global-target-2025/en/ (accessed 25 June 2018).
WHO 2014b
- World Health Organization. Global nutrition targets 2025: anaemia policy brief. apps.who.int/iris/bitstream/handle/10665/148556/WHO_NMH_NHD_14.4_eng.pdf?sequence=1 (accessed 25 June 2018).
WHO 2015
- World Health Organization. The Global Prevalence of Anaemia in 2011. Geneva: World Health Organization, 2015. [ISBN 978 92 4 156496 0 ] [apps.who.int/iris/bitstream/handle/10665/177094/9789241564960_eng.pdf?sequence=1] [Google Scholar]
WHO 2016a
- World Health Organization. Guideline: Daily Iron Supplementation in Infants and Children. Geneva: World Health Organization, 2016. [ISBN 978 92 4 154952 3] [apps.who.int/iris/bitstream/handle/10665/204712/9789241549523_eng.pdf?sequence=1] [Google Scholar]
WHO 2016b
- World Health Organization. Guideline: Daily Iron Supplementation in Adult Women and Adolescent Girls. Geneva: World Health Organization, 2016. [ISBN 978 92 4 151019 6] [apps.who.int/iris/bitstream/handle/10665/204761/9789241510196_eng.pdf?sequence=1] [PubMed] [Google Scholar]
WHO 2016c
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: World Health Organization, 2016. [ISBN 978 92 4 154991 2] [apps.who.int/iris/bitstream/handle/10665/250796/9789241549912-eng.pdf?sequence=1] [Google Scholar]
WHO 2016d
- World Health Organization. WHO Guideline: Fortification of Maize Flour and Corn Meal with Vitamins and Minerals. Geneva: World Health Organization, 2016. [ISBN-13: 978-92-4-154993-6] [www.ncbi.nlm.nih.gov/books/NBK402401/] [PMID: ] [PubMed] [Google Scholar]
WHO 2017
- World Health Organization. Nutritional Anaemias: Tools for Effective Prevention and Control. Geneva: World Health Organization, 2017. [ISBN 978-92-4-151306-7] [http://apps.who.int/iris/bitstream/handle/10665/259425/9789241513067-eng.pdf?sequence=1] [Google Scholar]
WHO 2020a
- World Health Organization. Anaemia in women of reproductive age. www.who.int/data/gho/data/themes/topics/indicator-groups/indicator-group-details/GHO/prevalence-of-anaemia-in-women-of-reproductive-age (accessed 10 June 2021).
WHO 2020b
- World Health Organization. Prevalence of anaemia in children aged 6–59 months. www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-anaemia-in-children-under-5-years-(-) (accessed 10 June 2021).
Yusoff 2012
- Yusoff H, Daud WN, Ahmad Z. Nutrition education and knowledge, attitude and hemoglobin status of Malaysian adolescents. Southeast Asian Journal of Tropical Medicine and Public Health 2012;43(1):192-200. [PMID: ] [PubMed] [Google Scholar]
Zamalloa 2017
- Zamalloa CO, Fuentes MS, Valladares DM. The impact of counseling and education on incidence rates of anemia and iron deficiency in children under 3 years. PROSPERO 2017. [Available from www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42017064706] [CRD42017064706]
Zandonadi 2017
- Zandonadi R, Lima V, Pineli L, Chiarello M. Food strategy for the prevention and treatment of iron deficiency anemia in childhood: a systematic review. PROSPERO 2017. [Available from: www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42017075195] [CRD42017075195]
Zhang 2021
- Zhang YY, Stockmann R, Ng K, Ajlouni S. Opportunities for plant-derived enhancers for iron, zinc, and calcium bioavailability: a review. Comprehensive Reviews in Food Science and Food Safety 2021;20(1):652-85. [DOI: 10.1111/1541-4337.12669] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zimmermann 2007
- Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet 2007;370(9586):511-20. [DOI: 10.1016/S0140-6736(07)61235-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Da Silva Lopes 2018
- Da Silva Lopes K, Takemoto Y, Garcia-Casal MN, Ota E. Nutrition-specific interventions for preventing and controlling anaemia throughout the life cycle: an overview of systematic reviews. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD013092. [DOI: 10.1002/14651858.CD013092] [DOI] [PMC free article] [PubMed] [Google Scholar]


