Abstract
T-cell receptor (TCR) engagement results in the activation of Src family (Lck and Fyn) and ZAP-70 protein tyrosine kinases, leading to tyrosine phosphorylation of multiple cellular substrates including the complex adapter protein c-Cbl. Moreover, Cbl is tyrosine phosphorylated upon engagement of growth factor receptors, cytokine receptors, and immunoreceptors and functions as a negative regulator of tyrosine kinase signalling pathways. Cbl associates via its phosphotyrosine binding (PTB) domain to the ZAP-70 pY292 negative regulatory phosphotyrosine. We recently demonstrated that the oncogenic Cbl mutant, 70Z Cbl, requires its PTB domain to upregulate NFAT in unstimulated Jurkat T cells. Here, we demonstrate that kinase-dead but not wild-type forms of Fyn, Lck, and ZAP-70 block 70Z Cbl-mediated NFAT activation. Moreover, 70Z Cbl does not upregulate NFAT in the ZAP-70-deficient P116 Jurkat T-cell line. The requirement for Fyn, Lck, and ZAP-70 is not due to tyrosine phosphorylation of 70Z Cbl, as mutation of all tyrosines in, or deletion of, the C-terminal region of 70Z Cbl (amino acids 655 to 906) blocks 70Z Cbl tyrosine phosphorylation but enhances 70Z Cbl-mediated NFAT activation. Further, 70Z Cbl does not cooperate with ZAP-70 Y292F to upregulate NFAT, indicating that 70Z Cbl and ZAP-70 do not activate parallel signalling pathways. Finally, the upregulation of NFAT observed upon ZAP-70 overexpression is blocked by Cbl in a PTB domain-dependent manner. We conclude that oncogenic 70Z Cbl acts as a dominant negative to block the PTB domain-dependent negative regulatory role of endogenous Cbl on ZAP-70, leading to constitutive ZAP-70 signalling and activation of transcription factors.
Engagement of the T-cell receptor (TCR)–CD3 complex and either CD4 or CD8 coreceptor by foreign antigen or antibodies leads to the activation of Src family tyrosine kinases Lck and Fyn, which phosphorylate tandem tyrosine residues in the immunoreceptor tyrosine-based activation motifs (ITAMs) of the invariant CD3 and ζ subunits of the TCR complex (reviewed in reference 64). Tyrosine phosphorylation of the ITAMs leads to the recruitment of ZAP-70/Syk family tyrosine kinases through their tandem SH2 domains. Subsequent activation of the ZAP-70 tyrosine kinase is critically dependent on phosphorylation of tyrosine Y493 in the activation loop of the catalytic domain by Src family kinases Lck and/or Fyn. Activation of Src- and ZAP-70 family kinases following antigen receptor cross-linking leads to the phosphorylation of critical cellular substrates including Tec family tyrosine kinases, phospholipase Cγ, p95 Vav, and various adapter proteins such as linker for activation of T cells (LAT), SLP-76, and p85 phosphatidylinositol 3′-kinase (PI3K) (reviewed in references 45 and 64). ZAP-70/Syk family kinases play a crucial role in antigen receptor signal transduction in lymphocytes. For instance, mice that are genetically deficient in ZAP-70 or Syk show a developmental arrest in T or B lymphocytes, respectively (61), and cell lines that fail to express ZAP-70 and Syk are unable to mobilize Ca2+ in response to antigen receptor engagement (58, 68). In addition to their role in antigen receptor signal transduction pathways, ZAP-70/Syk family tyrosine kinases have also been implicated in Fc receptor (FcR) (9, 23, 47), DAP-12-coupled NK cell receptor (34), and integrin (54) signal transduction pathways. Understanding the regulation of ZAP-70/Syk family tyrosine kinases is therefore of considerable interest.
One of the most prominent substrates of TCR-activated protein tyrosine kinases (PTKs) is a 120-kDa phosphoprotein that has previously been identified as the c-Cbl proto-oncogene product (14). Moreover, Cbl is also prominently tyrosine phosphorylated following engagement of numerous growth factor receptors, cytokine receptors, and immunoreceptors as well as following integrin ligation (reviewed in reference 53). c-Cbl is a ubiquitously expressed 906-amino-acid (aa) complex adapter protein that lacks any obvious catalytic domain (3). It contains an N-terminal phosphotyrosine binding (PTB) domain (31, 32, 36), a Zn2+-coordinating C3HC4 ring finger motif (7), a proline-rich region that includes a Grb2 SH3 binding site (13), a C-terminal region that contains several tyrosines that, upon ligand-induced phosphorylation, serve as docking sites for the SH2 domains of Crk(L) and p85 PI3K adapter proteins (55, 63), and a putative leucine zipper at the extreme C terminus (3). Cbl belongs to a family of related molecules that also includes Cbl-b (21) and a third family member (42). Cbl homologs have also been identified in Drosophila melanogaster (D-Cbl) (19, 35) and Caenorhabditis elegans (Sli-1) (69). The N-terminal half of the protein, which includes the PTB domain and the ring finger motif, is highly conserved among all family members.
Cbl physically associates with members of different families of tyrosine kinases, including receptor tyrosine kinases, Src family tyrosine kinases, and ZAP-70/Syk family tyrosine kinases (reviewed in reference 53). Notably, recent evidence from in vitro experiments and coexpression studies in heterologous Cos-7 cells indicate that Cbl requires its PTB domain to associate with the ZAP-70 pY292 and Syk pY323 phosphotyrosine residues (12, 30–32). These tyrosines are located in the interdomain B region of ZAP-70/Syk, between the C-terminal SH2 domain and the catalytic domain. Indeed, the crystal structure of the Cbl PTB domain has revealed that the Cbl PTB domain consists of a four-helix bundle, a tandem EF-hand domain, and a divergent SH2 domain which functionally cooperate to bind to a ZAP-70 pY292 phosphopeptide (36). ZAP-70 Y292 and the corresponding tyrosine in Syk have previously been identified as negative regulatory tyrosines (22, 24, 71). Interestingly, the ZAP-70 Y292F mutation does not affect the interaction of ZAP-70 with phosphorylated ITAMs in the receptor, nor does it affect ZAP-70 tyrosine phosphorylation, ZAP-70 kinase activity, or the ability of ZAP-70 to reconstitute B-cell receptor-mediated Ca2+ mobilization in Syk-deficient DT40 cells (24, 71). In contrast, mutation of tyrosine Y492, a negative regulatory tyrosine that is located in the activation loop of the catalytic domain in ZAP-70, leads to an increase in ZAP-70 kinase activity (24, 67), indicating that negative regulation of ZAP-70 function by tyrosine Y292 and that by Y492 involve distinct mechanisms. It has been proposed that the ZAP-70 pY292 phosphotyrosine recruits a protein that negative regulates the signalling function of ZAP-70 (24, 71).
Several lines of evidence indicate that Cbl functions as an evolutionary conserved negative regulator of both receptor and nonreceptor PTK signalling pathways. First, genetic studies of C. elegans indicate that the G315E loss-of-function allele of Sli-1 rescues vulval development induced by a reduction-of-function allele of the Let23 epidermal growth factor receptor (EGFR) homolog (69). Second, in D. melanogaster, overexpression of D-Cbl under the control of the sevenless promoter in transgenic flies inhibits the sevenless PTK-induced development of the R7 photoreceptor neuron (35). Third, antisense-mediated repression of Cbl enhances EGFR-mediated activation of the Jak-Stat signalling pathway (62). Fourth, in the RBL 2H3 mast cell line, overexpression of mammalian Cbl inhibits FcɛRI-induced Syk tyrosine kinase activity and serotonin release (44). Finally, c-Cbl-deficient mice show hyperplastic changes in breast and lymphoid tissues, enhanced TCR-induced tyrosine phosphorylation of multiple cellular substrates including ZAP-70, SLP-76, and LAT, and enhanced positive selection of CD4+ transgenic thymocytes (38, 39).
Further support for negative regulation of receptor and nonreceptor PTKs by Cbl is derived from studies of transforming Cbl mutants. The 70Z Cbl oncoprotein, which was originally identified from the 70Z/3 pre-B-lymphoma cell line, contains an internal deletion of 17 aa that affects the N-terminal region of the C3HC4 ring finger motif (1). Stable overexpression of 70Z Cbl in fibroblasts leads to enhanced recruitment of critical signalling molecules to hyperphosphorylated platelet-derived growth factor receptor (PDGFR) and EGFR (5, 60). Moreover, transient overexpression of 70Z Cbl in Jurkat T cells leads to constitutive upregulation of NFAT and AP-1 transcriptional activity (29, 63), similar to what has been observed upon overexpression of ZAP-70 Y292F (24, 71). The molecular mechanism underlying the oncogenic activity of 70Z Cbl is not clear, but it has been attributed to its increased basal and activation-induced phosphotyrosine content and/or to a dominant negative mode of action (1, 5, 60, 63). We (63) and others (70) recently demonstrated that activation of NFAT and AP-1 by oncogenic 70Z Cbl in unstimulated Jurkat T cells requires its PTB domain but not its association with Crk(L) or p85 PI3K adapter proteins. These findings raised the possibility that ZAP-70 may be required for 70Z Cbl-mediated NFAT activation. Thus, we previously hypothesized that 70Z Cbl acts as a dominant negative by inhibiting the functional interaction between the PTB domain of endogenous Cbl and the ZAP-70 pY292 phosphotyrosine residue, thereby relieving ZAP-70 kinase from the negative regulatory role of endogenous Cbl (63). In this study, we tested this hypothesis and demonstrate that oncogenic 70Z Cbl functionally interacts with the ZAP-70 pY292 phosphotyrosine leading to enhanced ZAP-70 signalling and that negatively regulation of ZAP-70 function by wild-type (wt) Cbl is dependent on an intact Cbl PTB domain.
MATERIALS AND METHODS
Cell lines and antibodies.
Jurkat E6.1, P116 (68), and Jurkat-TAg (57) cell lines were maintained in RPMI medium supplemented with 10% fetal bovine serum (FBS) at a cell density of 0.1 × 106 to 1 × 106 cells/ml. HuTK− and CV-1 cells were maintained in Dulbecco modified Eagle medium (DMEM)–10% FBS. The antibodies used were monoclonal antibody (MAb) 4G10 (antiphosphotyrosine; Upstate Biotechnology), c15 (anti-Cbl; Santa Cruz), 12CA5 (anti-hemagglutinin epitope [HA]; Boehringer Mannheim) and 9E10 (anti-Myc ascites) and rabbit antiserum raised against the linker region of ZAP-70 (67).
cDNA constructs.
pSX SRα, pSX HA Cbl, and pSX HA 70Z (13) as well as the G306E and Y700F/Y731F/Y774F (here referred to as Y3F) mutant derivatives of pSX HA Cbl and pSX HA 70Z (63) were previously described. Y674F/Y700F/Y731F/Y735F/Y774F (here referred to as Y5F) mutant derivatives of pSX HA Cbl and pSX HA 70Z were made by replacing the BglII-NotI fragment of pSX HA Cbl or pSX HA 70Z with that from pAlter Max HA Cbl Y5F constructs (gifts from A. Tsygankov) (15). Y869/871F mutant derivatives were made by using a Quick Change site-directed mutagenesis kit (Stratagene) and oligonucleotides 5′-GAGTCAGGGGTTCTCCTTCCAGGACATCC-3′ (sense) and 5′-GGATGTCCTGGAAGGAGAACCCCTGACTC-3′ (antisense) for Y869/871F, followed by sequencing and subcloning of the 3′ SacII-KpnI fragment of the mutagenized pSX HA Cbl constructs into pSX HA Cbl and pSX HA 70Z. Y674F/Y700F/Y731F/Y735F/Y774F/Y869F/Y871F (here referred to as Y7F) mutant derivatives of pSX HA Cbl and pSX HA 70Z were made by replacing the 3′ SacII-KpnI fragment of pSX HA Cbl Y5F or pSX HA 70Z Y5F with that from the mutagenized pSX HA Cbl Y869F/Y871F construct. pSC65, pSC65 HA Cbl, and pSC65 HA 70Z (43) Y3F mutant derivatives of pSC 65 HA Cbl and pSC65 HA 70Z were described previously (63). The Y5F and Y7F mutant derivatives of pSC65 HA Cbl and pSC65 HA 70Z were made by replacing the 3′ BglII-KpnI fragment of pSC65 HA Cbl or 70Z with that from the respective pSX HA Cbl mutant derivatives. pSX HA Cbl 1-655 and pSC65 HA Cbl 1-655 were previously described (43). pSX HA 70Z 1-655 and pSC65 HA 70Z 1-655 were made by replacing the 3′ BglII-KpnI fragment of pSX HA 70Z or pSC65 HA 70Z with that from pSX HA Cbl 1-655. pSC65 HA 70Z G306E was made by replacing the PshAI-BglII fragment from pSC65 HA Cbl with that from pSX HA 70Z G306E (63).
pXS mFyn Myc and pXS mFyn Myc K295R (kinase dead) were described previously (16). pSX mLck Myc K273E (kinase dead) was made by subcloning the XbaI-EcoRI fragment of pGEM4Z mLck Myc K273E (generous gift from J. Ashwell) (10) into the XbaI-EcoRI sites of pSX SRα. pSX mLck Myc was made by replacing the SacII-EcoRI fragment of pSX mLck (67) with that from pGEM4Z mLck Myc K273E. pSX ZAP-70 Myc and its Y292F, K369R (kinase dead), Y492F, and Y493F mutant derivatives were described previously (67).
Expression of recombinant vaccinia virus.
Recombinant vaccinia virus was made by standard procedures. Briefly, near-confluent CV-1 cells were infected in 25-cm2 flasks for 2 h with wild-type WR′ strain TK+ vaccinia virus at a multiplicity of infection of 0.25 and transfected overnight with 20 μg of the appropriate constructs, using Lipofectin in Optimem medium (GibcoBRL) followed by an additional 24 h of culture in DMEM–10% FBS. Infected and transfected cells were harvested by centrifugation and lysed by repeated cycles of freeze-thawing and sonication. Blue recombinant TK− plaques were purified by three rounds of plaque purification on confluent HuTK− cells in 1% low-melting-point agarose–1× basal medium Eagle (GibcoBRL)–5% FBS and three rounds of amplification in DMEM–10% FBS in the continuous presence of bromodeoxyuridine (25 μg/ml; Sigma). Crude viral stocks were titered on HuTK− cells and used to infect Jurkat T cells at a multiplicity of infection of 5. After 15 h, infected Jurkat T cells were harvested. Cell viability was routinely determined by trypan blue exclusion and always exceeded 95%.
OKT3 stimulation, immunoprecipitation, SDS-PAGE, and immunoblotting.
Jurkat T cells were washed once in ice-cold RPMI medium without FBS and resuspended at 108 cells/ml. Generally, 1 × 107 to 2 × 107 cells were preincubated at 37°C for 5 min, before cross-linking of CD3 by addition of OKT3 ascites fluid (1:100). Cells were incubated at 37°C for the indicated time periods and solubilized for 30 min on ice in lysis buffer containing 150 mM NaCl, 25 mM Tris (pH 7.5), 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 10 μg each of aprotonin and leupeptin per ml, and 1% Triton X-100 (TX-100). Lysates were clarified by centrifugation and immunoprecipitated for 2 to 3 h at 4°C with appropriate antibodies preabsorbed to protein A. Immunoprecipitates were washed twice in ice-cold lysis buffer and once in ice-cold phosphate-buffered saline before boiling for 5 min in Laemmli sample buffer. Immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, transferred to nitrocellulose membranes (Schleicher & Schuell), and immunoblotted according to standard procedures. Goat anti-mouse- or anti-rabbit-horseradish peroxidase conjugates (Amersham Life Sciences) were used as secondary reagents, and immunoblots were developed by using enhanced chemiluminescence (Amersham Life Sciences) and exposed to X-ray films (Kodak).
Transient transfection and NFAT reporter gene assays.
Secreted alkaline phosphatase (SEAP) reporter gene constructs composed of multimers of the NFAT DNA binding site were kindly provided by G. Crabtree (57). The pNFAT luc reporter construct was obtained from David McKean (Mayo Clinic, Rochester, Minn.). In general, 107 Jurkat-TAg cells were transfected with 5 μg of reporter construct and 10 μg of test construct by electroporation in a Bio-Rad gene pulser (310 V, 200 Ω, 960 μF). Transfected cells were cultured in bulk for 24 h and left unstimulated or stimulated in duplicate with immobilized OKT3 (1 μl of ascites fluid/well) or phorbol myristate acetate (PMA; 10 ng/ml; Sigma) plus ionomycin (1 μg/ml; Calbiochem) in 1 ml of phenol red-deficient RPMI medium–10% FBS at a density of 3 × 106 transfected cells/ml. After stimulation for 15 h, cell cultures were incubated for 1 h at 65°C to inactivate endogenous phosphatases, and supernatants were assayed in duplicate at 37°C for SEAP activity, using p-nitrophenolphosphate (Sigma) at 1.8 mg/ml in diethanolamine bicarbonate (pH 10.0) as a substrate. Absorbance at 405 nm was determined in an MR 5000 microtiterplate reader (Dynatech) usually between 6 and 12 h of incubation. Luciferase assays were performed as instructed by the manufacturer (Promega) in a Microlumat LB96P luminometer (EG&G Berthold). Presented data are representative of at least three independent experiments.
RESULTS
Src family tyrosine kinase-activated ZAP-70 is required for NFAT activation by 70Z Cbl.
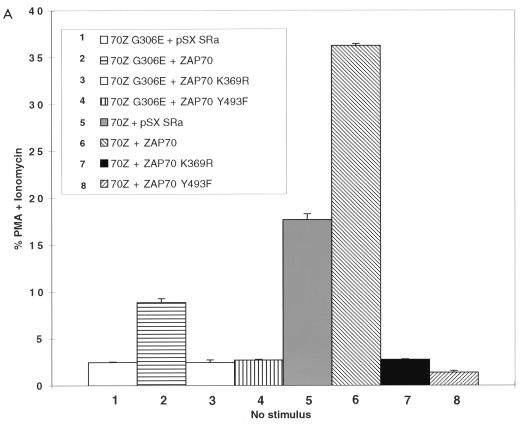
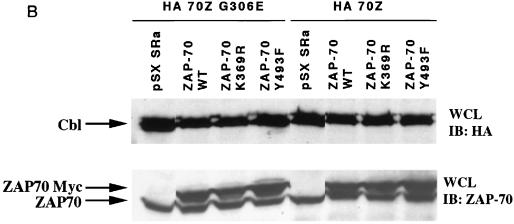
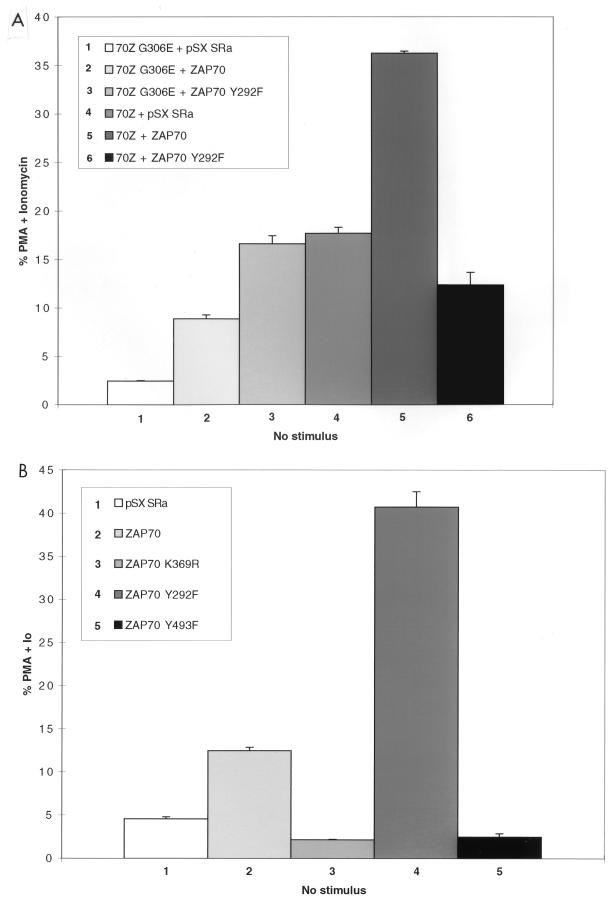
To determine whether 70Z Cbl-mediated NFAT activation was dependent on the ZAP-70 kinase, we decided to perform coexpression studies in Jurkat-TAg cells, which stably express the simian virus 40 (SV40) large T antigen (TAg) (57). Previously published reports have demonstrated that overexpression of dominant negative ZAP-70 constructs can block TCR-mediated NFAT activation in this system (40, 48). Thus, we cotransfected Jurkat-TAg cells with either 70Z Cbl or its inactive G306E mutant derivative together with empty vector, wt ZAP-70 or kinase-dead ZAP-70 (ZAP-70 K369R). Consistent with recent findings (63, 70), overexpression of 70Z Cbl but not its G306E mutant derivative led to constitutive upregulation of NFAT in unstimulated Jurkat T cells (Fig. 1A; compare columns 1 and 5). We therefore used the 70Z G306E mutant construct as a negative control in subsequent experiments. Coexpression of wt ZAP-70 but not kinase-dead ZAP-70 with the inactive 70Z Cbl G306E mutant led to a two- to threefold increase over basal NFAT activation in Jurkat T cells (Fig. 1A, columns 2 and 3). In separate experiments, overexpression of ZAP-70 alone induced a similar two- to threefold increase in basal NFAT activation (see Fig. 4B). Moreover, kinase dead ZAP-70 but not wt ZAP-70 inhibited OKT3-induced NFAT activity (data not shown), confirming that the kinase dead ZAP-70 was acting as a dominant negative (40, 48). Importantly, whereas coexpression of wt ZAP-70 enhanced 70Z Cbl-mediated NFAT activation, kinase-dead ZAP-70 completely blocked NFAT activation by 70Z Cbl (Fig. 1A, columns 6 and 7). Immunoblotting whole cell lysates with anti-ZAP-70 and anti-HA antibodies revealed similar expression levels of transfected proteins (Fig. 1B).
FIG. 1.
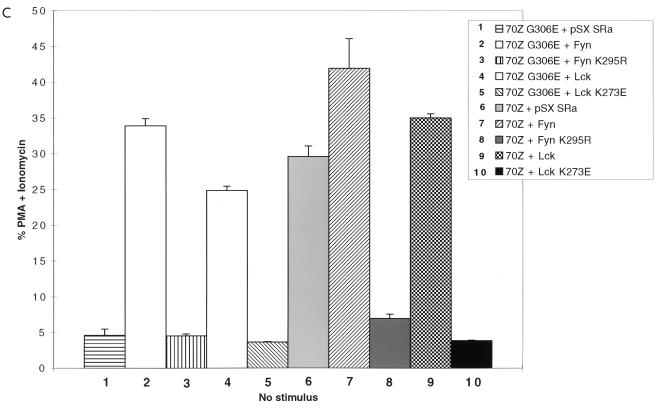
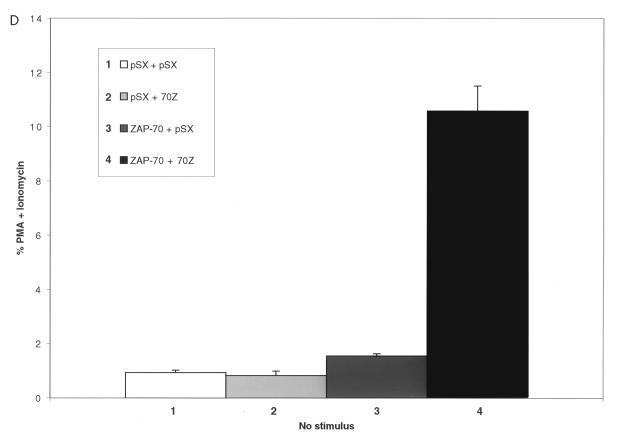
Src family-activated ZAP-70 is required for NFAT activation by 70Z Cbl. (A) Jurkat-TAg cells were transiently cotransfected with HA-70Z G306E or HA-70Z and either Myc epitope-tagged wt, K369R (kinase dead), or Y493F ZAP-70 constructs. After 24 h, cell were left unstimulated or stimulated with PMA plus ionomycin as described in Materials and Methods. Following 15 h of stimulation, SEAP reporter gene activity was assayed and expressed relative to the response induced by PMA plus ionomycin in each group of transfected cells. (B) Whole-cell lysates (WCL) from the transient transfections described in panel A were lysed in 1% TX-100 lysis buffer at the end of the 15-h stimulation period, and expression of ZAP-70 and Cbl was analyzed by standard immunoblotting (IB) techniques. (C) Jurkat-TAg cells were transiently cotransfected with HA-70Z G306E or HA-70Z and either pSX SRa (vector), wt Fyn Myc, Fyn Myc K295R (kinase dead), wt Lck Myc, or Lck Myc K273E (kinase dead) and further treated as for panel A. (D) P116 Jurkat T cells were transiently cotransfected with vector (pSX), HA-70Z, and/or ZAP-70 as indicated together with an NFAT luciferase reporter construct and an SV40 TAg expression plasmid. Twenty-four hours after transfection, cells were left unstimulated or stimulated with PMA plus ionomycin for 15 h, and luciferase activity determined as described in Materials and Methods.
FIG. 4.
70Z Cbl does not synergize with the ZAP-70 Y292F mutant to upregulate NFAT. Jurkat-TAg cells were transiently cotransfected with the indicated expression constructs and further treated as for Fig. 1A. Immunoblotting of whole-cell lysates confirmed similar expression levels of transfected proteins among different groups (data not shown). Io, ionomycin.
Following TCR engagement, activation of the ZAP-70 kinase is absolutely dependent on recruitment of ZAP-70 to tyrosine phosphorylated ITAM docking sites and subsequent phosphorylation of ZAP-70 Tyr493 in the activation loop of the catalytic domain by Src family kinases Lck and/or Fyn (8, 20, 67). To determine whether activation of ZAP-70 by Src family PTKs was required for 70Z Cbl-mediated NFAT activation, the effect of the ZAP-70 Y493F mutation on 70Z Cbl-mediated NFAT activation was also evaluated. The ZAP-70 Y493F mutation blocked 70Z Cbl-mediated NFAT activation as efficiently as kinase-dead ZAP-70 (Fig. 1A, columns 7 and 8; Fig. 1B), indicating an absolute requirement for Src family tyrosine kinase-mediated activation of ZAP-70 during the induction of NFAT by oncogenic 70Z Cbl proteins. The specificity of the Y493F-mediated blockade is indicated by the fact that the ZAP-70 Y492F mutation, which enhances NFAT activation in unstimulated Jurkat T cells (reference 71 and Fig. 4B), does not block 70Z Cbl-induced NFAT activation (data not shown). To further corroborate these results, we directly tested the requirement of Src family PTK activity for 70Z Cbl-mediated NFAT activation by coexpression of 70Z Cbl with wt or kinase-dead forms of Fyn and Lck. Coexpression of wt but not kinase-dead forms of Fyn and Lck with 70Z G306E constitutively upregulated NFAT activity in unstimulated Jurkat T cells (Fig. 1C, columns 1 to 5). More importantly, 70Z Cbl-mediated NFAT activation was blocked by overexpression of kinase-dead but not wt forms of Fyn and Lck (Fig. 1C, columns 6 to 10). These findings are consistent with a role for Fyn and Lck in the tyrosine phosphorylation of ITAMs, the recruitment of ZAP-70 to the phosphorylated receptor, and the activation of ZAP-70 catalytic activity through phosphorylation of Tyr493.
Finally, to further support the requirement of the ZAP-70 kinase for 70Z Cbl-mediated NFAT activation, we transiently transfected 70Z Cbl, either in the absence or in the presence of cotransfected ZAP-70, into the ZAP-70-deficient P116 Jurkat T-cell line (68). We used an NFAT luciferase reporter construct for these studies, as initial results demonstrated that the sensitivity of the SEAP reporter assay was insufficient for this purpose. Indeed, even in the presence of cotransfected SV40 TAg, PMA-plus-ionomycin-induced NFAT luciferase activity was >100-fold lower in P116 cells compared to Jurkat-TAg cells (data not shown). Our results indicated that 70Z Cbl-mediated NFAT activation was observed only when P116 cells were cotransfected with ZAP-70 (Fig. 1D), providing genetic evidence that ZAP-70 is required for 70Z Cbl-mediated NFAT activation. Similar studies using Lck-deficient JCAM1.6 Jurkat T cells were not possible, most likely due to insufficient expression levels of transfected proteins because of a further 10-fold decrease in the transient transfection efficiency of JCAM1.6 relative to P116 Jurkat T cells (data not shown). Nonetheless, our findings provide supporting genetic evidence for the previous conclusion that functional ZAP-70 is required for 70Z Cbl-mediated NFAT activation.
Several models may explain the requirement of activated ZAP-70 for 70Z Cbl-mediated NFAT activation. First, Src family and ZAP-70 tyrosine kinases may act upstream of and phosphorylate 70Z Cbl which, in turn, may be sufficient to upregulate NFAT. Indeed, Andoniou et al. have suggested that the transforming ability of 70Z Cbl may be due to its increased phosphotyrosine content (1). Consistent with this hypothesis, disruption of the 70Z Cbl PTB domain blocks both increased tyrosine phosphorylation of 70Z Cbl as well as 70Z Cbl-mediated upregulation of NFAT (63). Using site-directed mutagenesis of Tyr700, Tyr731, and Tyr774, we previously ruled out the possibility that 70Z Cbl activates NFAT through increased association with the SH2 domain containing Crk(L) and p85 PI3K adapter proteins (63). However, we did not rule out the possibility that 70Z Cbl action is mediated through increased phosphorylation at other tyrosine phosphorylation sites. Second, as wt but not oncogenic 70Z Cbl has been shown to act as a negative regulator of the ZAP-70-related Syk tyrosine kinase (44), 70Z Cbl may regulate ZAP-70 signalling by blocking the negative regulatory role of endogenous Cbl on ZAP-70. Indeed, the data presented in Fig. 1 indicate that 70Z Cbl is unable to negatively regulate ZAP-70 (see also Fig. 5). Third, it is possible that 70Z Cbl and ZAP-70 generate parallel signals that synergistically activate NFAT. To distinguish between these models, we tested whether 70Z Cbl-mediated NFAT activation depends on (i) tyrosine phosphorylation of 70Z Cbl and (ii) synergism with the ZAP-70 Y292F mutation.
FIG. 5.
Cbl negatively regulates ZAP-70 function in a PTB domain-dependent manner. Jurkat-TAg cells were transiently cotransfected with the indicated expression constructs and further treated as for Fig. 1A. Immunoblotting of whole-cell lysates confirmed similar expression levels of transfected proteins among different groups (data not shown).
Tyrosine phosphorylation of 70Z Cbl in the C-terminal region is not required for NFAT activation.
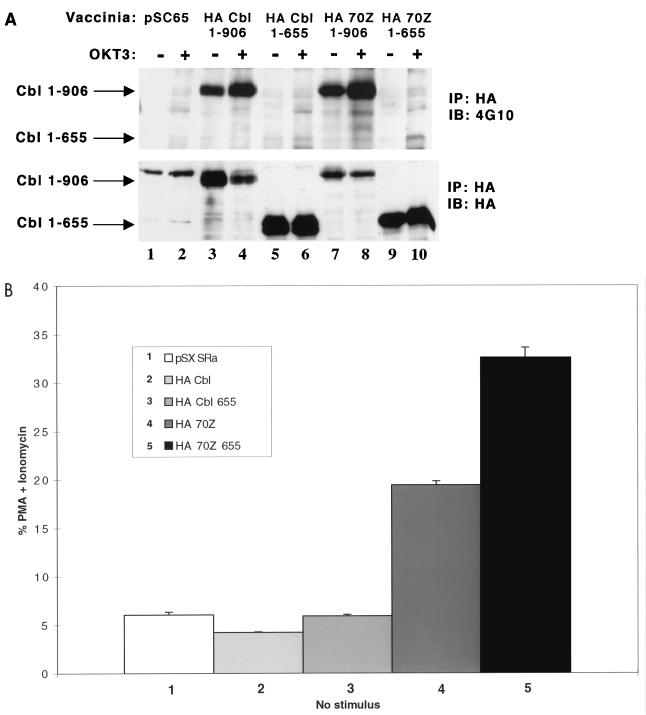
We previously reported that truncated wt Cbl 1-655 proteins (proteins consisting of aa 1 to 655) do not show detectable tyrosine phosphorylation (43, 63). Cbl contains seven tyrosine residues in the C-terminal region (aa 655 to 906). Tyr700, Tyr731, and Tyr774 undergo phosphorylation in vivo as they bind to the SH2 domains of Crk(L) and p85 PI3K adapter proteins following receptor activation (63). Whether the other tyrosines (Tyr674, Tyr735, Tyr869, and Tyr871) undergo phosphorylation is not known, but we previously reported residual tyrosine phosphorylation of wt and 70Z Cbl Y3F triple mutants under both basal and OKT3-stimulated conditions (63). To further assess the role of 70Z Cbl tyrosine phosphorylation in the regulation of NFAT, we generated wt and 70Z Cbl 1-655 truncation constructs and analyzed the tyrosine phosphorylation and NFAT-inducing activities of wt and 70Z Cbl 1-655 constructs. As shown in Fig. 2A, antiphosphotyrosine blotting revealed that basal and OKT3-induced tyrosine phosphorylation of wt Cbl 1-655 truncation mutants could not be reliably detected (compare lanes 5 and 6 to lanes 1 to 4). Similarly, basal and activation-induced tyrosine phosphorylation of the 70Z Cbl 1-655 truncation was virtually eliminated compared to the full-length 70Z Cbl protein (compare lanes 9 and 10 to lanes 7 and 8). Scanning densitometry revealed that the level of tyrosine phosphorylation of the wt and 70Z 1-655 truncation mutants was less than 1% of that observed for full-length wt and 70Z Cbl (data not shown). As illustrated in Fig. 2B, even though tyrosine phosphorylation of 70Z Cbl was virtually eliminated by deletion of the C-terminal region, the 70Z Cbl 1-655 truncation mutant did not block and even enhanced NFAT activation compared to full-length 70Z Cbl. These findings indicate that the C-terminal region (aa 655 to 906) of 70Z Cbl is not absolutely required for and negatively regulates 70Z Cbl-mediated NFAT activation.
FIG. 2.
Removal of the 70Z Cbl C-terminal region (aa 655 to 906) blocks 70Z Cbl tyrosine phosphorylation but enhances 70Z Cbl-mediated NFAT activation. (A) Jurkat E6.1 T cells were infected with the indicated recombinant vaccinia virus constructs and stimulated in the presence or absence of OKT3. Cells were lysed in 1% TX-100 lysis buffer, and postnuclear lysates were immunoprecipitated (IP) with anti-HA antibody followed by SDS-PAGE and immunoblotting (IB) with antiphosphotyrosine MAb 4G10 (B) Jurkat-TAg cells were transiently transfected with the indicated expression constructs and further treated as for Fig. 1A. Immunoblotting of whole-cell lysates confirmed similar expression levels of transfected proteins among different groups (data not shown).
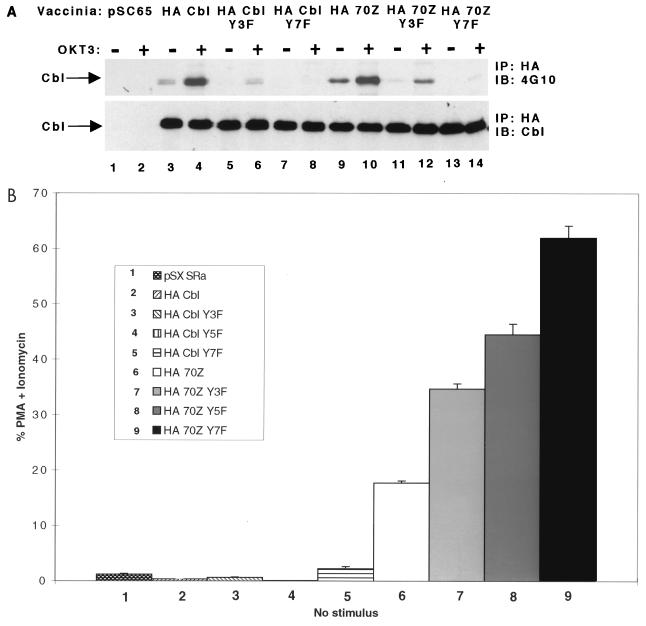
To further assess the effect of tyrosine phosphorylation on 70Z Cbl-mediated NFAT activation, we mutated all seven tyrosines that are present in the C-terminal region (aa 655 to 906) and evaluated tyrosine phosphorylation and NFAT activation. We previously reported that Tyr700, Tyr731, and Tyr774 represent the major phosphorylation sites in c-Cbl but that wt and 70Z Cbl Y3F triple tyrosine mutants still display residual basal and activation-induced tyrosine phosphorylation (63). We therefore tested whether mutation of two additional tyrosines (Y674F and Y735F) in the Y5F constructs or mutation of all seven tyrosines (including Y869F and Y871F) in the Y7F constructs eliminated Cbl tyrosine phosphorylation. As illustrated in Fig. 3A, and in agreement with previous findings (15, 63), mutation of the Crk(L) and p85 PI3K binding sites in wt and 70Z Cbl greatly reduced basal and OKT3-induced tyrosine phosphorylation relative to their unmutated counterparts (Fig. 3A; compare lanes 5 and 6 and lanes 11 and 12 with lanes 3 and 4 and lanes 9 and 10, respectively). Importantly, the residual basal and activation-induced tyrosine phosphorylation observed in the wt and 70Z Cbl Y3F mutants was virtually eliminated in the wt and 70Z Cbl Y7F mutants (Fig. 3A, lanes 7, 8, 13, and 14). Scanning densitometry revealed that the level of tyrosine phosphorylation in the wt and 70Z Cbl Y7F mutants was less than 2% of that observed in the unmutated constructs (data not shown). Compared to the Y3F and Y7F constructs, the wt and 70Z Cbl Y5F constructs displayed intermediate levels of tyrosine phosphorylation (data not shown). These results demonstrate that tyrosine phosphorylation of wt and 70Z Cbl is essentially eliminated by simultaneous mutation of all seven tyrosines in the C-terminal region (aa 655 to 906).
FIG. 3.
Tyrosine phosphorylation of 70Z Cbl in the C-terminal region (aa 655 to 906) negatively regulates 70Z Cbl-mediated NFAT activation. (A) Jurkat E6.1 T cells were infected with the indicated recombinant vaccinia constructs and further treated as for Fig. 2A. (B) Jurkat-TAg cells were transiently cotransfected with the indicated expression constructs and further treated as for Fig. 1A. Immunoblotting (IB) of whole-cell lysates confirmed similar expression levels of transfected proteins among different groups (data not shown). IP, immunoprecipitation.
Importantly, and in agreement with the data obtained with the 1-655 truncation constructs, the Y7F and, to a lesser extent, Y5F and Y3F mutations not only blocked but even enhanced NFAT activation by 70Z Cbl (Fig. 3B). Although we initially reported that the 70Z Cbl Y3F mutation showed a relatively small but not consistently observed increase in basal NFAT activation (63), additional experiments revealed that the 70Z Y3F mutant showed an approximately 50% increase in NFAT activation relative to 70Z Cbl in four of six experiments. Taken together, these findings demonstrate that simultaneous mutation of all seven tyrosines in the C-terminal region of 70Z Cbl, which blocks tyrosine phosphorylation of 70Z Cbl, does not inhibit but actually enhances 70Z Cbl-mediated NFAT activation. We conclude that the requirement of Fyn, Lck, and ZAP-70 kinases for 70Z Cbl-mediated NFAT activation is not due to tyrosine phosphorylation of 70Z Cbl.
70Z Cbl functionally interacts with the ZAP-70 pY292 phosphotyrosine residue.
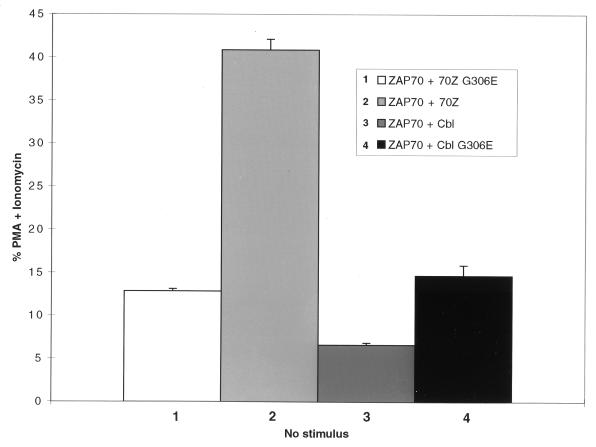
Lupher and colleagues have previously demonstrated that the Cbl PTB domain binds the ZAP-70 pY292 phosphotyrosine residue in vitro and upon coexpression in heterologous Cos cells in vivo (31, 32). As previously indicated, mutation of the ZAP-70 Tyr292 residue generates a mutant form of the ZAP-70 that constitutively upregulates NFAT in unstimulated Jurkat T cells (71). We previously hypothesized that 70Z Cbl acts as a dominant negative by disrupting the functional interaction of endogenous Cbl with ZAP-70 (63). This model predicts that overexpression of activated ZAP-70 Y292F does not cooperate with 70Z Cbl overexpression to upregulate NFAT compared to the overexpression of either construct alone. However, it is also possible that ZAP-70 and 70Z Cbl activate parallel pathways that synergistically activate NFAT. To distinguish between these two possibilities, coexpression studies were performed with 70Z Cbl or the inactivated 70Z Cbl G306E mutant, as a control, together with various ZAP-70 constructs. Coexpression of the 70Z G306E construct with ZAP-70 Y292F resulted in constitutive NFAT activation that was increased relative to coexpression of 70Z Cbl G306E with wt ZAP-70 or the vector control (Fig. 4A; compare columns 1, 2, and 3). This is consistent with previously published data demonstrating that overexpression of ZAP-70 Y292F alone upregulates NFAT activity (reference 71 and Fig. 4B). Importantly, coexpression of ZAP-70 Y292F with 70Z Cbl did not lead to enhanced NFAT activation relative to the expression of either construct alone (Fig. 4A; compare columns 3, 4, and 6). In contrast, coexpression of wt ZAP-70 enhanced NFAT activation by 70Z Cbl (Fig. 4A; compare columns 4 and 5) even though NFAT activation induced by wt ZAP-70 (in the presence of 70Z G306E) was less than that induced by ZAP-70 Y292F (Fig. 4A; compare columns 2 and 3). It should be noted that the slightly decreased NFAT activation observed upon coexpression of 70Z Cbl with ZAP-70 Y292F (Fig. 4A, column 6) relative to the expression of 70Z G306E with ZAP-70 Y292F (Fig. 4A, column 3) was not consistently observed. We suggest the following interpretation for our findings. Upon overexpression of ZAP-70, a fraction of the TCR-associated ZAP-70 pool escapes negative regulation because of now limiting amounts of endogenous Cbl (Fig. 5), leading to two- to threefold upregulation of NFAT activity in unstimulated Jurkat T cells. Cooverexpression of 70Z Cbl further enhances NFAT activation by blocking the functional interaction of those ZAP-70 molecules that do interact with endogenous Cbl. In contrast to cells overexpressing wt ZAP-70, the great majority of TCR-associated ZAP-70 proteins in cells overexpressing the ZAP-70 Y292F mutant will carry the Y292F mutation. This will essentially block the effect of 70Z Cbl, leading to lack of cooperativity between 70Z Cbl and ZAP-70 Y292F in the induction of NFAT. It should be noted that the upregulation of NFAT by overexpression of ZAP-70 Y292F depends on intact SH2 and kinase domains (71), indicating that the ZAP-70 Y292F protein must be recruited to phosphorylated ITAMs and activated by Src family kinases to upregulate NFAT activity. The data presented in Fig. 4 provide evidence that 70Z Cbl functionally interacts with the ZAP-70 pY292 phosphotyrosine residue in vivo and demonstrate that the 70Z Cbl oncoprotein does not synergize with the ZAP-70 Y292F mutant to upregulate NFAT. Taken together with the results presented in Fig. 1 to 3, these findings indicate that the requirement of ZAP-70 for 70Z Cbl-mediated NFAT activation is due to the fact that 70Z Cbl enhances ZAP-70 signalling via a functional interaction of the 70Z Cbl PTB domain with the ZAP-70 pY292 phosphotyrosine residue. Importantly, these findings are consistent with the model that overexpression of 70Z Cbl enhances ZAP-70 function by relieving ZAP-70 from the negative regulatory role of endogenous c-Cbl, which depends on the association of the c-Cbl PTB domain with the ZAP-70 pY292 phosphotyrosine residue.
Cbl negatively regulates NFAT activation induced by overexpression of ZAP-70.
Finally, we evaluated whether Cbl can negatively regulate ZAP-70 signalling in Jurkat T cells. We have previously reported that overexpression of Cbl does not reproducibly and significantly inhibit NFAT or AP-1 activation following optimal stimulation with immobilized anti-CD3 MAbs (63). We reasoned that in Jurkat T cells, the amount of Cbl may be sufficient to effectively suppress signalling by endogenous ZAP-70 (see also above). Therefore, we hypothesized that the limited NFAT activation observed under conditions of ZAP-70 overexpression (Fig. 1 and 4) might result from a limiting amount of c-Cbl protein. Under these conditions, simultaneous overexpression of Cbl might have an effect on ZAP-70-induced NFAT activation. Indeed, the negative regulation of Syk tyrosine kinase by Cbl in RBL 2H3 mast cells that was previously reported by our laboratory was also observed under conditions of Syk overexpression (44). To test this idea, Jurkat-TAg cells were cotransfected with ZAP-70 and either the inactivated 70Z G306E (as a negative control), 70Z, wt Cbl, or the Cbl G306E mutant. As already shown in Fig. 1 and 4, overexpression of ZAP-70 in the presence of 70Z G306E resulted in a two- to threefold enhancement of NFAT activation relative to expression of the inactive 70Z G306E alone. Most importantly, under conditions where coexpression of ZAP-70 with 70Z Cbl resulted in enhanced NFAT activation relative to the negative control (Fig. 5; compare columns 1 and 2), coexpression of ZAP-70 with wt Cbl resulted in an approximately twofold inhibition of NFAT activation (Fig. 5; compare columns 1 and 3). Moreover, Cbl-mediated inhibition of ZAP-70-induced NFAT activation was blocked by the Cbl G306E mutation (Fig. 5; compare columns 1, 3, and 4). These findings directly demonstrate that Cbl negatively regulates the signalling function of ZAP-70 in Jurkat T cells in a PTB domain-dependent manner.
DISCUSSION
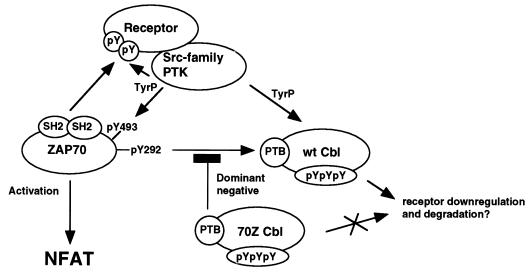
The c-Cbl proto-oncogene product is a ubiquitously expressed complex adapter protein that functions as an evolutionarily conserved negative regulator of receptor and nonreceptor PTKs (53). One of its transforming mutants, 70Z Cbl, contains an internal deletion of 17 aa, which affects the N-terminal part of the ring finger motif (1). The molecular mechanism underlying its oncogenic activity is not clear, but it has been attributed to its increased phosphotyrosine content and/or to a dominant negative mode of action (1, 5, 60, 63). We previously reported that upregulation of NFAT and AP-1 activity by overexpression of oncogenic 70Z Cbl in unstimulated Jurkat T cells depends on an intact PTB domain but not on its association with the SH2 domain-containing Crk(L) and p85 PI3K adapter proteins (63). As the PTB domain of Cbl is known to associate with the ZAP-70 pY292 phosphotyrosine residue in vitro and upon coexpression in heterologous Cos cells in vivo (31, 32), we hypothesized that ZAP-70 is required for 70Z Cbl-mediated NFAT activation. Here, we demonstrate that (i) Src family kinase activated ZAP-70 is required for 70Z Cbl-mediated NFAT activation, (ii) 70Z Cbl fails to activate NFAT in ZAP-70-deficient P116 Jurkat T cells, (iii) tyrosine phosphorylation of 70Z Cbl is not required for and negatively regulates 70Z Cbl-mediated NFAT activation, (iv) 70Z Cbl-mediated NFAT activation is mediated through a functional interaction of the 70Z Cbl PTB domain with the ZAP-70 pY292 phosphotyrosine leading to enhanced ZAP-70 signalling, (v) NFAT activation induced by overexpression of ZAP-70 is inhibited by wt Cbl, and (vi) negative regulation of ZAP-70 by Cbl requires an intact PTB domain. We conclude that oncogenic 70Z Cbl acts as a dominant negative to block the functional interaction between the ZAP-70 pY292 phosphotyrosine and the PTB domain of endogenous Cbl proteins. Overexpression of 70Z Cbl thereby relieves ZAP-70 from a negative regulatory mechanism and results in constitutive but Src family tyrosine kinase dependent ZAP-70 signalling (Fig. 6).
FIG. 6.
Model for the regulation of ZAP-70 signalling by wt and 70Z Cbl. Src family kinases Fyn and/or Lck phosphorylate tandem tyrosines in the ITAMs of the receptor. ZAP-70 is recruited to the phosphorylated receptor through its tandem SH2 domains. Lck (or Fyn) then phosphorylates ZAP-70 on Tyr493 in the activation loop of the catalytic domain. This leads to ZAP-70 kinase activation and auto- or transphosphorylation on multiple tyrosines, including Tyr292. Phosphorylation of ZAP-70 Tyr292 recruits Cbl to the activated receptor complex through its PTB domain. This interaction negatively regulates ZAP-70 function, perhaps by regulating ubiquitination, downregulation, and/or lysosomal degradation of the activated receptor complex. Overexpression of the oncogenic 70Z Cbl mutant blocks the functional interaction of ZAP-70 pY292 with endogenous Cbl in a PTB domain-dependent manner, thereby relieving ZAP-70 from a negative regulatory mechanism, resulting in enhanced ZAP-70 signalling.
It has previously been suggested that v-Cbl, which contains an intact PTB domain, competitively inhibits c-Cbl binding to activated tyrosine kinases, thereby leading to enhanced tyrosine kinase signalling (59). We believe that 70Z Cbl, rather than acting merely as a competitive inhibitor, acts as a genuine dominant negative. Indeed, our recent studies demonstrate that 70Z Cbl can oligomerize with wt c-Cbl and that the Cbl oligomerization domain is absent from v-Cbl. Moreover, disruption of 70Z Cbl oligomerization blocks constitutive NFAT activation induced by the 70Z Cbl oncoprotein (64a). We therefore propose that the weakly oncogenic v-Cbl acts as a competitive inhibitor, inhibiting binding of the c-Cbl PTB domain to activated tyrosine kinases. In contrast, we propose that the 70Z Cbl oncoprotein acts as a genuine dominant negative in 70Z/wt Cbl hetero-oligomers and blocks the critical negative regulatory function of c-Cbl that is associated with the ring finger domain. The dominant negative activity of 70Z Cbl does not, however, affect a second negative regulatory function that is associated with Cbl tyrosine phosphorylation (see below). Thus, inactivation of this second negative regulatory function in the 70Z Y7F or 70Z 655 truncation mutants leads to enhanced NFAT activation relative to the 70Z Cbl oncoprotein.
Our finding that 70Z Cbl acts as a genuine dominant negative to block a critical negative regulatory function of c-Cbl on ZAP-70 signalling predicts that loss of endogenous c-Cbl function leads to enhanced ZAP-70 signalling. Indeed, it was recently reported that anti-CD3-stimulated thymocytes from c-Cbl-deficient mice show enhanced TCR signalling, as evidenced by increased tyrosine phosphorylation of ZAP-70, increased ZAP-70 kinase activity, and hyperphosphorylation of ZAP-70 substrates such as LAT and SLP-76 (38, 39). Moreover, Naramura et al. reported enhanced positive selection of CD4+ major histocompatibility complex class II-specific but not CD8+ major histocompatibility complex class I-specific transgenic thymocytes in c-Cbl-deficient mice (39). Thus, these findings are consistent with the possibility that c-Cbl negatively regulates signalling by ZAP-70 family tyrosine kinases. Our present findings also correlate well with previous studies from our lab demonstrating that wt but not 70Z Cbl is able to negatively regulate the ZAP-70-related Syk tyrosine kinase in RBL 2H3 mast cells (44). In that study, Cbl-mediated negative regulation of Syk correlated with Cbl-Syk complex formation which was dependent on the Cbl proline-rich domain. It was not investigated, however, whether Cbl-Syk complex formation and Cbl-mediated negative regulation of Syk in RBL 2H3 mast cells was also dependent on the Cbl PTB domain. Further studies are needed to determine whether differences exist in the regulation of ZAP-70 and Syk tyrosine kinases by c-Cbl. Inhibition of endogenous c-Cbl function by overexpression of oncogenic 70Z Cbl also provides an explanation for the observed hyperphosphorylation of the EGFR and PDGFR, and the increased recruitment of signalling molecules to these receptors that has been found in stable fibroblast cell lines overexpressing the 70Z Cbl oncoprotein (5, 60). Our finding that 70Z Cbl acts as a dominant negative also implies that the transforming activity of 70Z Cbl (1, 41) is not due to a gain-of-function mutation of the 70Z Cbl mutant but is due to loss of endogenous c-Cbl function in fibroblasts. A role for c-Cbl in suppressing tumorigenesis is further suggested by the finding that c-Cbl-deficient mice show signs of hyperplastic changes in breast and hematopoietic tissues, although it should be noted that c-Cbl-deficient mice did not show overt tumors (38). Based on our findings, we conclude that overexpression of 70Z Cbl provides a convenient tool to block and study the function of endogenous c-Cbl in a variety of cells.
Our findings indicate that overexpression of 70Z Cbl results in low-level activation of signal transduction pathways that are fully activated by optimal engagement of the TCR-CD3 complex. This finding explains our previous observation that overexpression of 70Z Cbl does not enhance NFAT activation induced by optimal stimulation of the TCR-CD3 complex (63). In the same study, we also could not detect a significant and reproducible effect of c-Cbl overexpression on anti-CD3-induced NFAT or AP-1 activation (63). A possible explanation for this finding is that endogenous Cbl protein levels in our Jurkat-TAg cells are already in excess of the amount needed to downregulate TCR-induced ZAP-70 activation. One prediction of this model is that upon overexpression of ZAP-70, Cbl might become limiting, leading to enhanced ZAP-70 signalling. Indeed, in Jurkat T cells (this study) and RBL 2H3 mast cells (44), the inhibitory function of Cbl is revealed under conditions of ZAP-70 and Syk overexpression, respectively. It should be noted, however, that the effect of Cbl on anti-CD3-induced AP-1 activation is controversial, as Rellahan et al. (50) reported that Cbl overexpression inhibited anti-CD3-induced AP-1 activity. Whether these differences are due to different levels of Cbl expression, the use of different expression vectors, or other differences remains to be determined.
The finding that overexpression of 70Z Cbl in unstimulated Jurkat T cells leads to constitutive activation of transcription factors (29, 63) suggests an important role for c-Cbl proteins in suppressing signalling in the apparent absence of TCR engagement. The activation state of tyrosine kinase signalling pathways depends on an intricate balance between PTKs and phosphatases. Activation may result not only from induction of PTK activity but also from the inhibition of tyrosine phosphatases. The latter is most clearly illustrated by the fact that treatment of Jurkat T cells with pervanadate, an inhibitor of tyrosine phosphatases, potently activates TCR signal transduction pathways (51). Thus, in unstimulated Jurkat T cells, Src family PTKs might be activated to a limited extent due to a low level intrinsic kinase activity. Random clustering events that involve the receptor and Src family PTKs may lead to low-level ITAM tyrosine phosphorylation and the recruitment and activation of a small fraction of ZAP-70. Indeed, based on the observation that overexpression of Syk tyrosine kinase results in NFAT activation in ZAP-70/Syk-deficient P116 but not TCRαβ-deficient J.RT3 Jurkat T-cell lines, it has previously been hypothesized that Jurkat T cells contain a low level of tyrosine-phosphorylated ITAMs (68). Our findings suggest that one important role for c-Cbl in unstimulated T cells might be to prevent inappropriate activation of signalling pathways downstream of ZAP-70 by negatively regulating ZAP-70 function.
What is the molecular mechanism underlying the negative regulation of ZAP-70 by c-Cbl? The signalling function of ZAP-70 likely depends on multiple factors including its specific enzymatic activity, the expression levels of the activated enzyme, and the duration of ZAP-70 activation. We have been unable to detect any significant differences in the level or kinetics of tyrosine phosphorylation of cellular substrates (except for Cbl itself), ZAP-70 and phospholipase Cγ1 tyrosine phosphorylation, ZAP-70 kinase activity, or TCRζ-associated ZAP-70 kinase activity between vector-, wt Cbl-, or 70Z Cbl-expressing Jurkat T cells in either transient transfection or vaccinia virus overexpression systems (data not shown). Similarly, overexpression of ZAP-70 Y292F, which disrupts the interaction of ZAP-70 with the Cbl PTB domain in vitro and in vivo (31, 32), does not detectably affect ZAP-70 tyrosine phosphorylation, ZAP-70 kinase activity, recruitment of ZAP-70 to phosphorylated ITAMs, or the ability of ZAP-70 to reconstitute Ca2+ mobilization in Syk-deficient DT40 cells compared to wt ZAP-70 (24, 71). In striking contrast, the Y492F mutation enhances the specific enzymatic activity of ZAP-70 (references 24 and 67 and data not shown). Although we cannot exclude the possibility that the effect of ZAP-70 Y292F or 70Z Cbl overexpression on the specific enzymatic activity of ZAP-70 is too subtle to be detected by biochemical methods, these observations suggest that the effect of ZAP-70 Y292F and 70Z Cbl overexpression on NFAT activation results primarily from an effect on the expression levels of the activated ZAP-70 enzyme and/or the duration of ZAP-70 activation. This effect is likely to be subtle but may accumulate over time. In this context, it is important to note that activation of NFAT requires prolonged signalling and sustained capacitative Ca2+ entry (49). The increase in ZAP-70 kinase activity observed during the first few minutes of TCR engagement may therefore be only a small fraction of the total ZAP-70 kinase activity that is necessary for NFAT activation.
Growth factor receptors, cytokine receptors, and immunoreceptors undergo rapid ligand-induced receptor internalization through clathrin-coated pits followed by either receptor recycling to the cell surface or lysosomal targeting and degradation. Many of these receptors have also been shown to undergo ubiquitination, and several model systems in yeast and mammalian species suggest a causal relation between ligand-induced receptor ubiquitination and downregulation (reviewed in references 4 and 18). Several recent observations suggest a role for c-Cbl in ligand-induced downregulation, ubiquitination, and/or lysosomal degradation of receptor tyrosine kinases. First, Wang and colleagues originally suggested a role for Cbl in the ubiquitination pathway by showing the ubiquitination of colony-stimulating factor 1 receptor and Cbl in response to colony-stimulating factor 1 stimulation of macrophages (65, 66). Moreover, it was demonstrated that overexpression of Cbl enhances the ubiquitination and degradation of activated PDGFR and EGFR tyrosine kinases (28, 37). Second, Cbl is efficiently tyrosine phosphorylated in response to ErbB1 (EGFR) but not ErbB2-4 engagement (17, 27), which correlates with the ability of ErbB1 but not ErbB2-4 receptors to undergo rapid ligand-induced downregulation (2, 26). Moreover, mutational analysis of the EGFR has suggested a correlation between EGFR-Cbl complex formation and receptor downregulation (28). In the latter study, it was proposed that Cbl promotes the endocytic sorting of internalized EGFRs to the lysosomal pathway and that inhibition of Cbl function, by overexpression of v-Cbl, enhances recycling of internalized receptors back to the cell surface.
Several immunoreceptors, including the TCR and FcγR, are also known to be ubiquitinated (reviewed in reference 4). In addition, it was recently reported that Cbl regulates the degradation of Syk in heterologous Cos-7 cells (30). A possible role for Cbl in targeting activated ZAP-70 molecules to lysosomes could provide an explanation for our inability to detect the steady-state association between ZAP-70 and wt or 70Z Cbl by direct immunoblotting in unstimulated or TCR-stimulated Jurkat T cells. Analogous to the proposed role of v-Cbl in promoting recycling of internalized EGFRs back to the cell surface (28), it is possible that overexpression of 70Z Cbl in Jurkat T cells leads to decreased lysosomal targeting and enhanced recycling of activated TCR complexes. The increased 70Z Cbl-mediated NFAT activation observed upon elimination of 70Z Cbl tyrosine phosphorylation and disruption of 70Z Cbl complex formation with Crk(L) and p85 PI3K adapter proteins (this study and reference 70) may further decrease lysosomal targeting and enhance TCR recycling. Such as model would be consistent with the requirement for tyrosine kinases in receptor downregulation and lysosomal targeting (6, 11, 25, 33, 56) and the role of PI3K in the regulation of postendocytic vesicle trafficking events (52). Whether tyrosine phosphorylation of c-Cbl regulates activation of the small GTPase Rap1 through its association with Crk(L)-C3G complexes has yet to be determined. However, it is interesting that Rap1 has been reported to colocalize with lysosome-associated membrane proteins to late endosomes/lysosomes in macrophage and fibroblast cell lines by confocal microscopy (46). A role for c-Cbl in TCR downregulation and lysosomal targeting might also explain the increased TCR expression levels on Cbl-deficient thymocytes (38, 39). It seems possible that regulation of Cbl function in vivo sets the threshold for T-cell activation and determines the responsiveness of T cells toward antigens.
In summary, we have demonstrated that c-Cbl negatively regulates ZAP-70 signalling in a PTB domain-dependent manner and that the oncogenic 70Z Cbl blocks the negative regulation of ZAP-70 by c-Cbl via a functional interaction with the ZAP-70 pY292 phosphotyrosine, leading to enhanced ZAP-70 signalling. We conclude that 70Z Cbl acts as a dominant negative to inhibit the negative regulation of ZAP-70 by endogenous c-Cbl. Our findings indicate that c-Cbl plays a crucial role in preventing inappropriate activation of transcription factors in T cells.
ACKNOWLEDGMENTS
We thank G. R. Crabtree, A. Tsygankov, and J. Ashwell for providing constructs used in this study, R. T. Abraham and R. L. Wange for helpful discussions, and W. Zhang, A. M. Weissman, and R. L. Wange for critical reading of the manuscript.
J.E.M.L. is supported by a postdoctoral fellowship award from the Cancer Research Institute.
REFERENCES
- 1.Andoniou C E, Thien C B, Langdon W Y. Tumour induction by activated abl involves tyrosine phosphorylation of the product of the cbl oncogene. EMBO J. 1994;13:4515–4523. doi: 10.1002/j.1460-2075.1994.tb06773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baulida J, Kraus M H, Alimandi M, Di Fiore P P, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- 3.Blake T J, Shapiro M, Morse H C, Langdon W Y. The sequences of the human and mouse c-cbl proto-oncogenes show v-cbl was generated by a large truncation encompassing a proline-rich domain and a leucine zipper-like motif. Oncogene. 1991;6:653–657. [PubMed] [Google Scholar]
- 4.Bonifacino J S, Weissman A M. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonita D P, Miyake S, Lupher M L, Jr, Langdon W Y, Band H. Phosphotyrosine binding domain-dependent upregulation of the platelet-derived growth factor receptor alpha signaling cascade by transforming mutants of Cbl: implications for Cbl’s function and oncogenicity. Mol Cell Biol. 1997;17:4597–4610. doi: 10.1128/mcb.17.8.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnerot C, Briken V, Brachet V, Lankar D, Cassard S, Jabri B, Amigorena S. syk protein tyrosine kinase regulates Fc receptor gamma-chain-mediated transport to lysosomes. EMBO J. 1998;17:4606–4616. doi: 10.1093/emboj/17.16.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borden K L, Freemont P S. The RING finger domain: a recent example of a sequence-structure family. Curr Opin Struct Biol. 1996;6:395–401. doi: 10.1016/s0959-440x(96)80060-1. [DOI] [PubMed] [Google Scholar]
- 8.Chan A C, Dalton M, Johnson R, Kong G H, Wang T, Thoma R, Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley M T, Costello P S, Fitzer-Attas C J, Turner M, Meng F, Lowell C, Tybulewicz V L, DeFranco A L. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Oro U, Sakaguchi K, Appella E, Ashwell J D. Mutational analysis of Lck in CD45-negative T cells: dominant role of tyrosine 394 phosphorylation in kinase activity. Mol Cell Biol. 1996;16:4996–5003. doi: 10.1128/mcb.16.9.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Oro U, Vacchio M S, Weissman A M, Ashwell J D. Activation of the Lck tyrosine kinase targets cell surface T cell antigen receptors for lysosomal degradation. Immunity. 1997;7:619–628. doi: 10.1016/s1074-7613(00)80383-0. [DOI] [PubMed] [Google Scholar]
- 12.Deckert M, Elly C, Altman A, Liu Y C. Coordinated regulation of the tyrosine phosphorylation of Cbl by Fyn and Syk tyrosine kinases. J Biol Chem. 1998;273:8867–8874. doi: 10.1074/jbc.273.15.8867. [DOI] [PubMed] [Google Scholar]
- 13.Donovan J A, Ota Y, Langdon W Y, Samelson L E. Regulation of the association of p120cbl with Grb2 in Jurkat T cells. J Biol Chem. 1996;271:26369–26374. doi: 10.1074/jbc.271.42.26369. [DOI] [PubMed] [Google Scholar]
- 14.Donovan J A, Wange R L, Langdon W Y, Samelson L E. The protein product of the c-cbl protooncogene is the 120-kDa tyrosine-phosphorylated protein in Jurkat cells activated via the T cell antigen receptor. J Biol Chem. 1994;269:22921–22924. [PubMed] [Google Scholar]
- 15.Feshchenko E A, Langdon W Y, Tsygankov A Y. Fyn, Yes, and Syk phosphorylation sites in c-Cbl map to the same tyrosine residues that become phosphorylated in activated T cells. J Biol Chem. 1998;273:8323–8331. doi: 10.1074/jbc.273.14.8323. [DOI] [PubMed] [Google Scholar]
- 16.Gauen L K, Zhu Y, Letourneur F, Hu Q, Bolen J B, Matis L A, Klausner R D, Shaw A S. Interactions of p59fyn and ZAP-70 with T-cell receptor activation motifs: defining the nature of a signalling motif. Mol Cell Biol. 1994;14:3729–3741. doi: 10.1128/mcb.14.6.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graus-Porta D, Beerli R R, Daly J M, Hynes N E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hicke L. Ubiquitin-dependent internalization and down-regulation of plasma membrane proteins. FASEB J. 1999;11:1215–1226. doi: 10.1096/fasebj.11.14.9409540. [DOI] [PubMed] [Google Scholar]
- 19.Hime G R, Dhungat M P, Ng A, Bowtell D D. D-Cbl, the Drosophila homologue of the c-Cbl proto-oncogene, interacts with the Drosophila EGF receptor in vivo, despite lacking C-terminal adaptor binding sites. Oncogene. 1997;14:2709–2719. doi: 10.1038/sj.onc.1201223. [DOI] [PubMed] [Google Scholar]
- 20.Iwashima M, Irving B A, van Oers N S, Chan A C, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 21.Keane M M, Rivero-Lezcano O M, Mitchell J A, Robbins K C, Lipkowitz S. Cloning and characterization of cbl-b: a SH3 binding protein with homology to the c-cbl proto-oncogene. Oncogene. 1995;10:2367–2377. [PubMed] [Google Scholar]
- 22.Keshvara L M, Isaacson C, Yankee T M, Sarac R, Harrison M L, Geahlen R L. Syk- and Lyn-dependent phosphorylation of Syk on multiple tyrosines following B cell activation includes a site that negatively regulates signaling. J Immunol. 1998;161:5276–5283. [PubMed] [Google Scholar]
- 23.Kiefer F, Brumell J, Al-Alawi N, Latour S, Cheng A, Veillette A, Grinstein S, Pawson T. The Syk protein tyrosine kinase is essential for Fcγ receptor signalling in macrophages and neutrophils. Mol Cell Biol. 1998;18:4209–4220. doi: 10.1128/mcb.18.7.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong G, Dalton M, Wardenburg J B, Straus D, Kurosaki T, Chan A C. Distinct tyrosine phosphorylation sites in ZAP-70 mediate activation and negative regulation of antigen receptor function. Mol Cell Biol. 1996;16:5026–5035. doi: 10.1128/mcb.16.9.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauritsen J P, Christensen M D, Dietrich J, Kastrup J, Odum N, Geisler C. Two distinct pathways exist for down-regulation of the TCR. J Immunol. 1998;161:260–267. [PubMed] [Google Scholar]
- 26.Lenferink A E, Pinkas-Kramarski R, van de Poll M L, van Vugt M J, Klapper L N, Tzahar E, Waterman H, Sela M, van Zoelen E J, Yarden Y. Differential endocytic routing of homo- and hetero-dimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J. 1998;17:3385–3397. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levkowitz G, Klapper L N, Tzahar E, Freywald A, Sela M, Yarden Y. Coupling of the c-Cbl protooncogene product to ErbB-1/EGF-receptor but not to other ErbB proteins. Oncogene. 1996;12:1117–1125. [PubMed] [Google Scholar]
- 28.Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon W Y, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y C, Elly C, Langdon W Y, Altman A. Ras-dependent, Ca2+-stimulated activation of nuclear factor of activated T cells by a constitutively active Cbl mutant in T cells. J Biol Chem. 1997;272:168–173. doi: 10.1074/jbc.272.1.168. [DOI] [PubMed] [Google Scholar]
- 30.Lupher M L, Jr, Rao N, Lill N L, Andoniou C E, Miyake S, Clark E A, Druker B, Band H. Cbl-mediated negative regulation of the Syk tyrosine kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J Biol Chem. 1998;273:35273–35281. doi: 10.1074/jbc.273.52.35273. [DOI] [PubMed] [Google Scholar]
- 31.Lupher M L, Jr, Reedquist K A, Miyake S, Langdon W Y, Band H. A novel phosphotyrosine-binding domain in the N-terminal transforming region of Cbl interacts directly and selectively with ZAP-70 in T cells. J Biol Chem. 1996;271:24063–24068. doi: 10.1074/jbc.271.39.24063. [DOI] [PubMed] [Google Scholar]
- 32.Lupher M L, Jr, Songyang Z, Shoelson S E, Cantley L C, Band H. The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the Tyr292 negative regulatory phosphorylation site of ZAP-70. J Biol Chem. 1997;272:33140–33144. doi: 10.1074/jbc.272.52.33140. [DOI] [PubMed] [Google Scholar]
- 33.Luton F, Legendre V, Gorvel J P, Schmitt-Verhulst A M, Boyer C. Tyrosine and serine protein kinase activities associated with ligand-induced internalized TCR/CD3 complexes. J Immunol. 1997;158:3140–3147. [PubMed] [Google Scholar]
- 34.McVicar D W, Taylor L S, Gosselin P, Willette-Brown J, Mikhael A I, Geahlen R L, Nakamura M C, Linnemeyer P, Seaman W E, Anderson S K, Ortaldo J R, Mason L H. DAP12-mediated signal transduction in natural killer cells. A dominant role for the syk protein-tyrosine kinase. J Biol Chem. 1998;273:32934–32942. doi: 10.1074/jbc.273.49.32934. [DOI] [PubMed] [Google Scholar]
- 35.Meisner H, Daga A, Buxton J, Fernandez B, Chawla A, Banerjee U, Czech M P. Interactions of Drosophila Cbl with epidermal growth factor receptors and role of Cbl in R7 photoreceptor cell development. Mol Cell Biol. 1997;17:2217–2225. doi: 10.1128/mcb.17.4.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng W, Sawasdikosol S, Burakoff S J, Eck M J. Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature. 1999;398:84–90. doi: 10.1038/18050. [DOI] [PubMed] [Google Scholar]
- 37.Miyake S, Lupher M L, Jr, Druker B, Band H. The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor alpha. Proc Natl Acad Sci USA. 1998;95:7927–7932. doi: 10.1073/pnas.95.14.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy M A, Schnall R G, Venter D J, Barnett L, Bertoncello I, Thien C B, Langdon W Y, Bowtell D D. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol Cell Biol. 1998;18:4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naramura M, Kole H K, Hu R-J, Gu H. Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc Natl Acad Sci USA. 1998;95:15547–15552. doi: 10.1073/pnas.95.26.15547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Northrop J P, Pustelnik M J, Lu A T, Grove J R. Characterization of the roles of SH2 domain-containing proteins in T-lymphocyte activation by using dominant negative SH2 domains. Mol Cell Biol. 1996;16:2255–2263. doi: 10.1128/mcb.16.5.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ojaniemi M, Langdon W Y, Vuori K. Oncogenic forms of Cbl abrogate the anchorage requirement but not the growth factor requirement for proliferation. Oncogene. 1998;16:3159–3167. doi: 10.1038/sj.onc.1201859. [DOI] [PubMed] [Google Scholar]
- 42.Ollendorff V, Mattei M, Fournier E, Adelaide J, Lopez M, Rosnet O, Birnbaum D. A third human CBL gene is on chromosome 19. Int J Oncol. 1998;13:1159–1161. doi: 10.3892/ijo.13.6.1159. [DOI] [PubMed] [Google Scholar]
- 43.Ota Y, Beitz L O, Scharenberg A M, Donovan J A, Kinet J P, Samelson L E. Characterization of Cbl tyrosine phosphorylation and a Cbl-Syk complex in RBL-2H3 cells. J Exp Med. 1996;184:1713–1723. doi: 10.1084/jem.184.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ota Y, Samelson L E. The product of the proto-oncogene c-Cbl: a negative regulator of the Syk tyrosine kinase. Science. 1997;276:418–420. doi: 10.1126/science.276.5311.418. [DOI] [PubMed] [Google Scholar]
- 45.Peterson E J, Clements J L, Fang N, Koretzky G A. Adaptor proteins in lymphocyte antigen-receptor signaling. Curr Opin Immunol. 1998;10:337–344. doi: 10.1016/s0952-7915(98)80173-8. [DOI] [PubMed] [Google Scholar]
- 46.Pizon V, Desjardins M, Bucci C, Parton R G, Zerial M. Association of Rap1a and Rap1b proteins with late endocytic/phagocytic compartments and Rap2a with the Golgi complex. J Cell Sci. 1994;107:1661–1670. doi: 10.1242/jcs.107.6.1661. [DOI] [PubMed] [Google Scholar]
- 47.Poole A, Gibbins J M, Turner M, van Vugt M J, van de Winkel J G J, Saito T, Tybulewicz V L, Watson S P. The Fc-receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian D, Mollenauer M N, Weiss A. Dominant-negative zeta-associated protein 70 inhibits T cell antigen receptor signaling. J Exp Med. 1996;183:611–620. doi: 10.1084/jem.183.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 50.Rellahan B L, Graham L J, Stoica B, DeBell K E, Bonvini E. Cbl-mediated regulation of T cell receptor-induced AP1 activation. Implications for activation via the Ras signaling pathway. J Biol Chem. 1997;272:30806–30811. doi: 10.1074/jbc.272.49.30806. [DOI] [PubMed] [Google Scholar]
- 51.Secrist J P, Burns L A, Karnitz L, Koretzky G A, Abraham R T. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J Biol Chem. 1993;268:5886–5893. [PubMed] [Google Scholar]
- 52.Shpetner H, Joly M, Hartley D, Corvera S. Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J Cell Biol. 1996;132:595–605. doi: 10.1083/jcb.132.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smit L, Borst J. The Cbl family of signal transduction molecules. Crit Rev Oncogenesis. 1998;8:359–379. doi: 10.1615/critrevoncog.v8.i4.50. [DOI] [PubMed] [Google Scholar]
- 54.Soede R D M, Wijnands Y M, Van Kouteren-Cobzaru I, Roos E. ZAP-70 tyrosine kinase is required for LFA-1-dependent T cell migration. J Cell Biol. 1998;142:1371–1379. doi: 10.1083/jcb.142.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 56.Sorkin A. Endocytosis and intracellular sorting of receptor tyrosine kinases. Front Biosci. 1998;3:729–738. doi: 10.2741/A316. [DOI] [PubMed] [Google Scholar]
- 57.Spencer D M, Wandless T J, Schreiber S L, Crabtree G R. Controlling signal transduction with synthetic ligands. Science. 1993;262:1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- 58.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thien C B, Langdon W Y. EGF receptor binding and transformation by v-Cbl is ablated by the introduction of a loss-of-function mutation from the Caenorhabditis elegans Sli-1 gene. Oncogene. 1997;14:2239–2249. doi: 10.1038/sj.onc.1201193. [DOI] [PubMed] [Google Scholar]
- 60.Thien C B, Langdon W Y. Tyrosine kinase activity of the EGF receptor is enhanced by the expression of oncogenic 70Z-Cbl. Oncogene. 1997;15:2909–2919. doi: 10.1038/sj.onc.1201468. [DOI] [PubMed] [Google Scholar]
- 61.Tybulewicz V L. Analysis of antigen receptor signalling using mouse gene targeting. Curr Opin Cell Biol. 1998;10:195–204. doi: 10.1016/s0955-0674(98)80142-7. [DOI] [PubMed] [Google Scholar]
- 62.Ueno H, Sasaki K, Miyagawa K, Honda H, Mitani K, Yazaki Y, Hirai H. Antisense repression of proto-oncogene c-Cbl enhances activation of the JAK-STAT pathway but not the ras pathway in epidermal growth factor receptor signaling. J Biol Chem. 1997;272:8739–8743. doi: 10.1074/jbc.272.13.8739. [DOI] [PubMed] [Google Scholar]
- 63.van Leeuwen J E M, Paik P K, Samelson L E. Activation of NFAT and AP1 by oncogenic 70Z Cbl requires an intact PTB domain but not Crk(L) or p85 PI3K association. J Biol Chem. 1999;274:5153–5162. doi: 10.1074/jbc.274.8.5153. [DOI] [PubMed] [Google Scholar]
- 64.van Leeuwen J E M, Samelson L E. T cell antigen-receptor signal transduction. Curr Opin Immunol. 1999;11:242–248. doi: 10.1016/s0952-7915(99)80040-5. [DOI] [PubMed] [Google Scholar]
- 64a.van Leeuwen, J. E. M., and L. E. Samelson. Unpublished data.
- 65.Wang Y, Yeung Y G, Langdon W Y, Stanley R J. c-Cbl is transiently tyrosine-phosphorylated, ubiquitinated, and membrane-targeted following CSF-1 stimulation of macrophages. J Biol Chem. 1996;271:17–20. doi: 10.1074/jbc.271.1.17. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Yeung Y G, Stanley R J. CSF-1 stimulated multiubiquitination of the CSF-1 receptor and of Cbl follows their tyrosine phosphorylation and association with other signaling proteins. J Cell Biochem. 1999;72:119–134. [PubMed] [Google Scholar]
- 67.Wange R L, Guitian R, Isakov N, Watts J D, Aebersold R, Samelson L E. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J Biol Chem. 1995;270:18730–18733. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- 68.Williams B L, Schreiber K L, Zhang W, Wange R L, Samelson L E, Leibson P J, Abraham R T. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol Cell Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoon C H, Lee J, Jongeward G D, Sternberg P W. Similarity of sli-1, a regulator of vulval development in C. elegans, to the mammalian proto-oncogene c-cbl. Science. 1995;269:1102–1105. doi: 10.1126/science.7652556. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Z, Elly C, Altman A, Liu Y C. Dual regulation of T cell receptor-mediated signaling by oncogenic Cbl mutant 70Z. J Biol Chem. 1999;274:4883–4889. doi: 10.1074/jbc.274.8.4883. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Q, Weiss A. Enhancement of lymphocyte responsiveness by a gain-of-function mutation of ZAP-70. Mol Cell Biol. 1996;16:6765–6774. doi: 10.1128/mcb.16.12.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]