Abstract
This research assessed the molecular mechanism of procyanidins (PCs) against neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and its metabolite 1-methyl-4-phenylpyridinium (MPP+) induced Parkinson’s disease (PD) models. In vitro, PC12 cells were incubated with PCs or deprenyl for 24 h, and then exposed to 1.5 mM MPP+ for 24 h. In vivo, zebrafish larvae (AB strain) 3 days post-fertilization (dpf) were incubated with deprenyl or PCs in 400 μM MPTP for 4 days. Compared with MPP+/MPTP alone, PCs significantly improved antioxidant activities (e.g., glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalase (CAT)), and decreased levels of reactive oxygen species (ROS) and malondialdehyde (MDA). Furthermore, PCs significantly increased nuclear Nrf2 accumulation in PC12 cells and raised the expression of NQO1, HO-1, GCLM, and GCLC in both PC12 cells and zebrafish compared to MPP+/MPTP alone. The current study shows that PCs have neuroprotective effects, activate the nuclear factor-erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway and alleviate oxidative damage in MPP+/MPTP-induced PD models.
Keywords: procyanidins, Parkinson’s disease, PC12 cells, zebrafish, Nrf2/ARE pathway
1. Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative syndrome caused by the absence of dopaminergic neurons in the substantia nigra. Its clinical manifestations include motor retardation, tremor, stiffness and postural instability [1,2,3,4,5,6]. Effective methods to prevent or reverse neuronal degeneration in PD patients are still unclear. Hence, the discovery and development of novel antiparkinsonian drugs remains a top priority in the search for PD treatments.
Although the pathogenesis of PD remains unclear, previous studies have demonstrated that oxidative stress plays a key role in the loss of dopaminergic neurons [7,8]. Dopamine can undergo auto- or enzyme-catalyzed oxidation to lead the production of reactive oxygen species (ROS) and electrophilic quinone molecules [9], both processes that may underlie the vulnerability of dopaminergic neurons to oxidative and electrophilic stress [10,11,12]. Hence, a possible method for the prevention or treatment of PD may be to supplement treatment with antioxidants to eliminate excessive ROS. Indeed, natural bioactive compounds are advantageous in that they are often safe for consumers and have very few (if any) adverse reactions [13]. Therefore, antioxidant compounds from natural resources may aid in the treatment of PD.
Procyanidins (PCs) are natural phenolic compound of flavonoids, including oligomer of monomer catechin and epicatechins [14]. PCs are natural nutrients and antioxidants with antioxidant effects stronger than vitamin C and E [15]. The Nrf2/ARE pathway is a significant antioxidant pathway. Nrf2 is released from Keap1 by ROS or through an electrophilic reaction. Thereafter, the compound then binds to the antioxidant response element (ARE) in the nucleus and mediates expression of peroxiredoxin and phase II detoxification enzymes (HO-1, GCLC, GCLM and NQO-1), which act as scavengers of ROS and protect cells from oxidative damage [16].
The embryonic zebrafish brain contains several clusters of dopaminergic, noradrenergic and adrenergic neurons in locations similar to those found in the adult brain [17,18,19]. By 3 days post-fertilization (dpf), dopaminergic neurons are observed in the ventral diencephalon, the pretectum, the locus coeruleus, the olfactory bulb and the retina [20]. Compared to experimental mice models, zebrafish models are associated with shorter experimental periods, lower cost and reduced complexity in gene manipulation [21]. Rat pheochromocytoma cells (PC12 cells) are very similar to neurons in morphology, structure and function [22]. They are highly consistent with primary cultured nerve cells, and have the advantages of easy and stable culture [23]. PC12 cells have been widely used as models for studying nerve cells [24,25,26].
The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) has been shown to cause a syndrome in humans that resembles Parkinson’s disease. MPTP causes selective degeneration of dopaminergic neurons in the substantia nigra, and patients exhibit symptoms including tremors, immobility and a shuffling gait [27]. MPTP is metabolized to 1-methyl-4-phenylpyridinium (MPP+) in glial cells in the brain. After release from the glia, MPP+ is transported into dopaminergic neurons via the dopamine transporter (DAT) [28] and accumulates in the mitochondria, where it is believed to cause cell death by disrupting respiratory enzymes and causing oxidative damage [29,30]. Previous studies have found that PCs prevent some aging processes of female rats [31] and have certain protective effects on nerves [32,33]. However, whether PCs can prevent or reverse neuronal degeneration in PD by regulating the Nrf2/ARE pathway remains unclear. This study employed MPP+-treated PC12 cells [34,35] and MPTP-treated zebrafish [36] to assess the protective effects and potential mechanism of PCs on a model of PD.
2. Results
2.1. Effects of PCs on MPP+-Induced Cytotoxicity of PC12 Cells
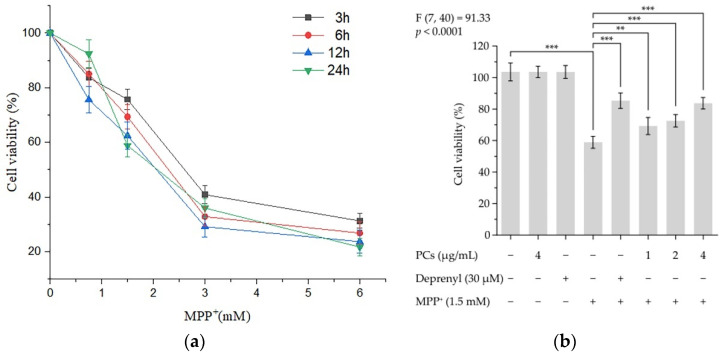
Incubation of PC12 cells to 1.5 mM MPP+ for 24 h resulted in cell viability of 58.88% (Figure 1a). Pretreatment of cells with PCs protected PC12 cells from MPP+-induced damage by increasing the cell viability (Figure 1b). There was no marked difference in cell survival rate between the 4 μg/mL PCs group and 30 μM deprenyl group (positive control group) (Figure 1b).
Figure 1.
PCs on MPP+-induced cytotoxicity of PC12 cells. Cell viability was detected by CCK-8 assays. (a) Cytotoxic effects of MPP+ at different concentrations in PC12 cells; (b) PC-attenuated MPP+ induced decreases in cell viability. F and p values of the one-way analysis of variance are presented above the chart. The results of Tukey’s post hoc test are presented for selected comparisons: **, p < 0.01; ***, p < 0.001; the error bars are standard deviation (SD).
2.2. PCs Reduced MPP+-Induced Oxidative Stress and Increased Antioxidant Enzyme Activity
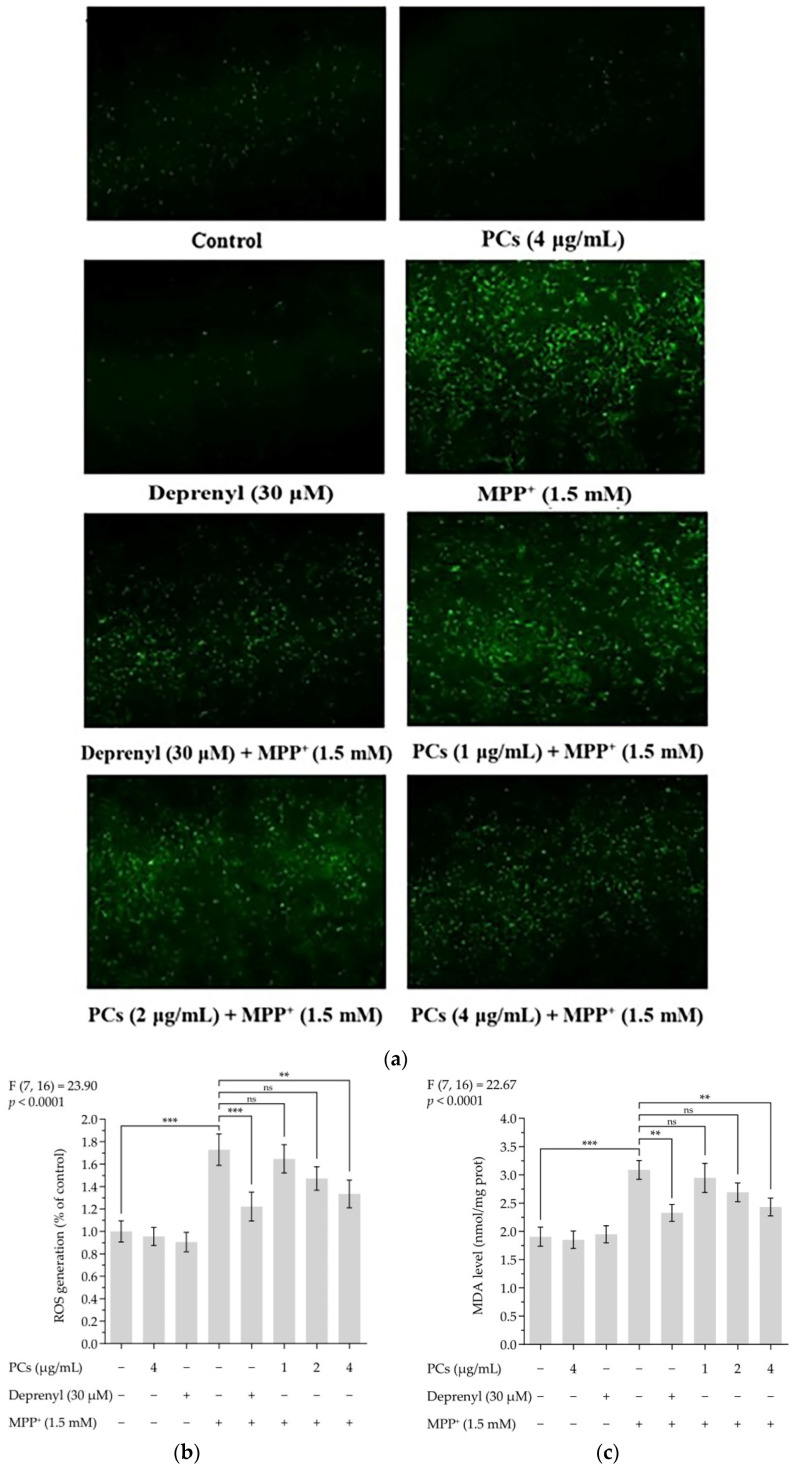
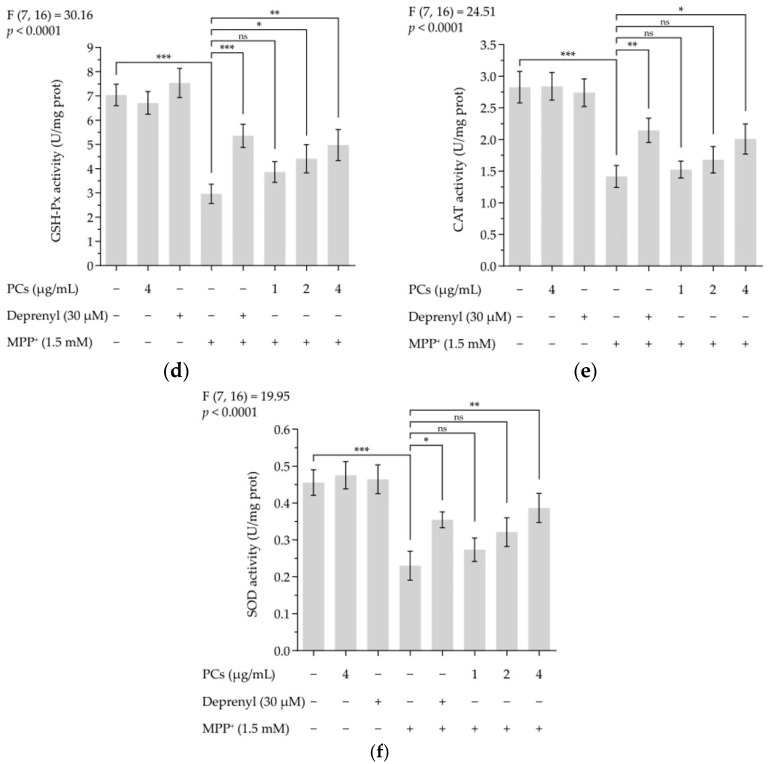
ROS and malondialdehyde (MDA) levels indicate the severity of oxidative damage. Exposing PC12 cells to 1.5 mM MMP+ for 24 h increased ROS and MDA levels, which were inhibited by PCs at 4 μg/mL (Figure 2a–c). To further evaluate the effect of PCs on antioxidant capabilities of MPP+-treated PC12 cells, we measured glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD) activity and observed that MPP+ treatment suppressed GSH-Px, CAT, and SOD activity. However, these MPP+ effects were rescued by PCs exposure at 4 μg/mL (Figure 2d–f).
Figure 2.
PCs reduced MPP+-induced oxidative stress and increased antioxidant enzyme activity. (a) ROS levels were measured by fluorescent microscopy and imaging analysis; (b) ROS levels were measured by image J software; (c) MDA levels; (d) GSH-Px activity; (e) CAT activity; (f) SOD activity. F and p values of the one-way analysis of variance are presented above each chart. The results of Tukey’s post hoc test are presented for selected comparisons: ns, p > 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001; the error bars are SD.
2.3. Effects of PCs on Nrf2/ARE Pathway in MPP+-Induced PC12 Cells
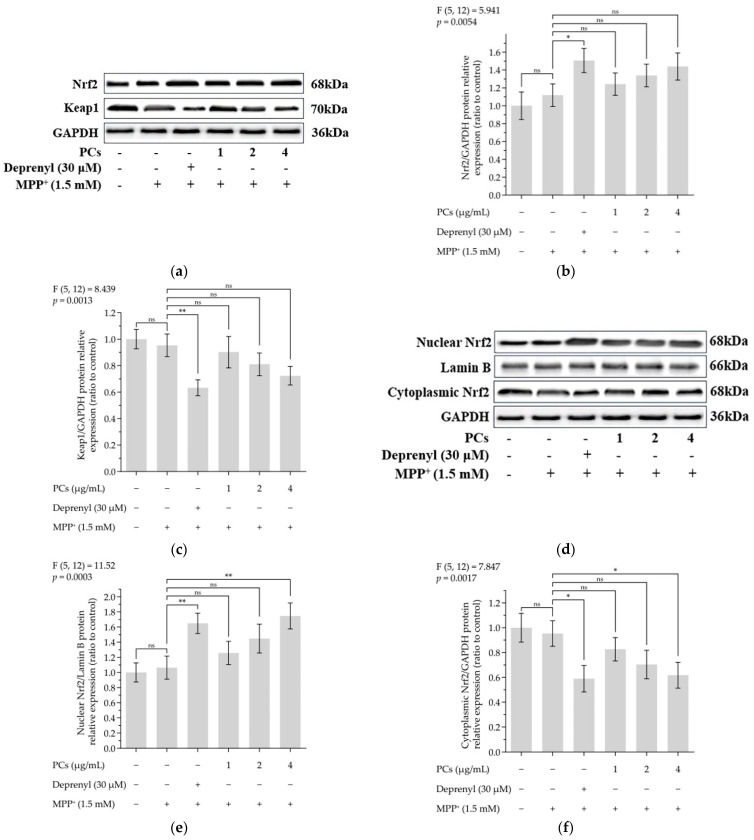
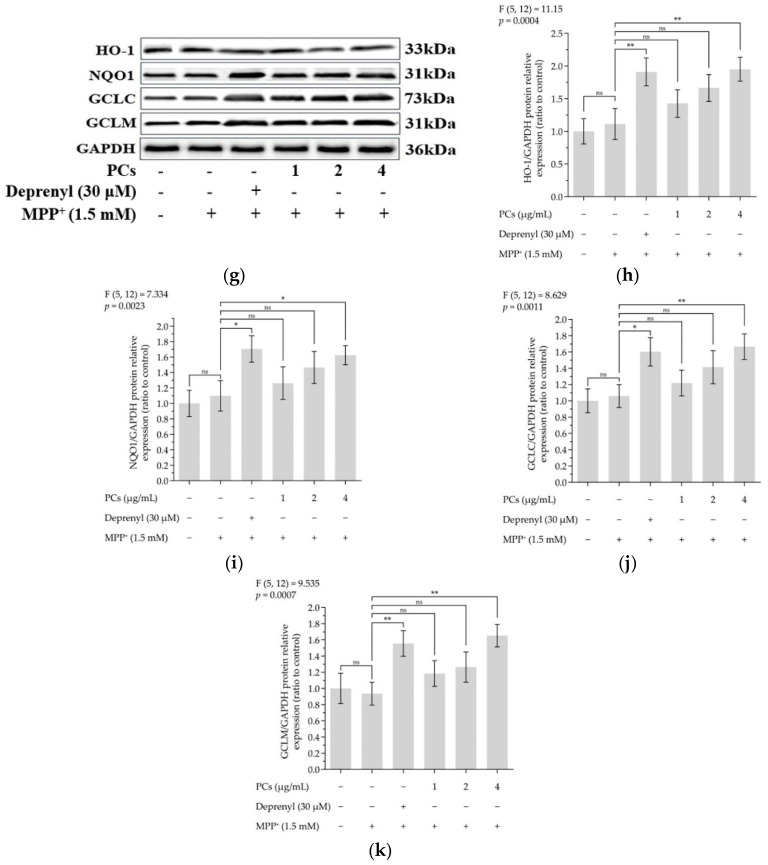
The expression of nuclear factor-erythroid 2-related factor 2 (Nrf2) was upregulated, and the level of Keap1 was downregulated following PC treatment (Figure 3a–c). PCs (4 μg/mL) facilitated nuclear localization of Nrf2 under MPP+ lesion conditions (Figure 3d–f). Additionally, Western blot data showed a marked upregulation in the expression of heme oxygenase 1 (HO-1), quinone oxidoreductase 1 (NQO1), glutamate-cysteine ligase catalytic subunit (GCLC) and glutamate-cysteine ligase modifier subunit (GCLM) expression after PC (4 μg/mL) treatment, compared with MPP+ exposure alone (Figure 3g–k).
Figure 3.
Effects of PCs on Nrf2/ARE pathway in MPP+-induced PC12 cells. (a) Protein levels of Nrf2 and Keap-1, as determined by Western blotting; (b) Nrf2/GAPDH protein relative expression (ratio to control); (c) Keap1/GAPDH protein relative expression (ratio to control); (d) protein expression levels of nuclear Nrf2 and cytoplasmic Nrf2, as determined by Western blotting; (e) nuclear Nrf2/LaminB protein relative expression (ratio to control); (f) cytoplasmic Nrf2/GAPDH protein relative expression (ratio to control); (g) protein levels of HO-1, NQO1, GCLC and GCLM, as determined by Western blotting; (h) HO-1/GAPDH protein relative expression (ratio to control); (i) NQO1/GAPDH protein relative expression (ratio to control); (j) GCLC/GAPDH protein relative expression (ratio to control); (k) GCLM/GAPDH protein relative expression (ratio to control). F and p values of the one-way analysis of variance are presented above each chart. The results of Tukey’s post hoc test are presented for selected comparisons: ns, p > 0.05; *, p < 0.05; **, p < 0.01; the error bars are SD.
2.4. Nrf2/ARE Signaling Is Related to the Neuroprotective and Antioxidant Effects Mediated by PCs
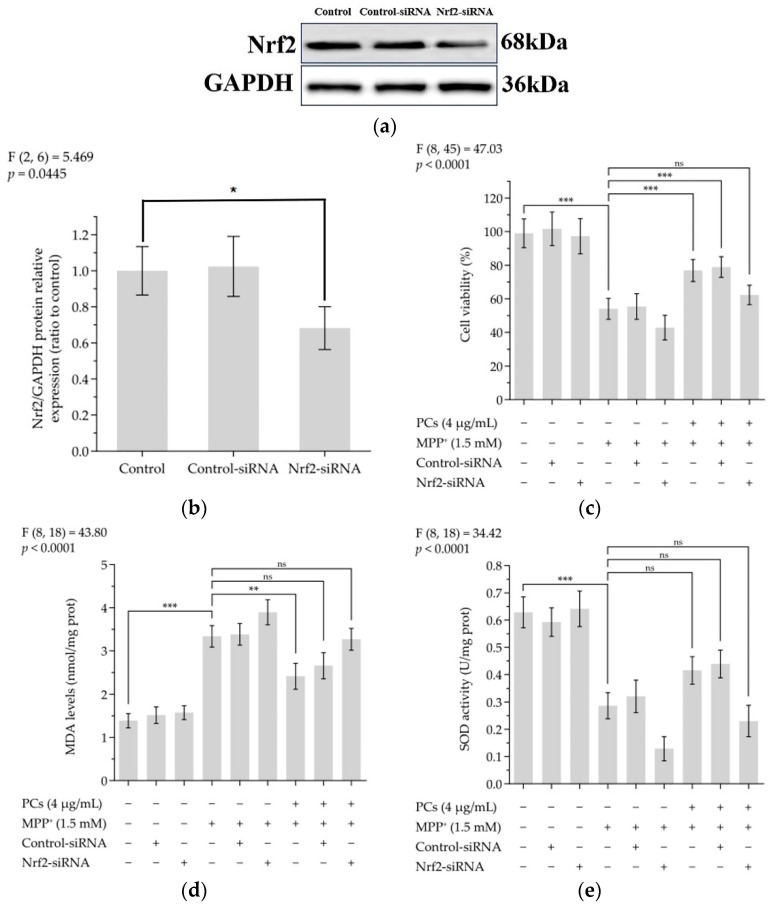
The knockdown of Nrf2 by siRNA effectively abolished the expression of Nrf2 (Figure 4a,b). Indeed, Nrf2 siRNA treatment abrogated the protective actions of PCs against MPP+-treated oxidative damage, as evidenced by the reduced cell viability in the Nrf2 siRNA-transfected group (Figure 4c). In addition, the effects of PCs on MDA and SOD were reversed by Nrf2 knockdown under MPP+ lesioning conditions (Figure 4d,e).
Figure 4.
Nrf2/ARE signaling is related to the neuroprotective and antioxidant effects mediated by PCs. (a) Knockout efficiency was detected by determination of Nrf2 protein expression using Western blotting; (b) Nrf2/GAPDH protein relative expression (ratio to control); (c) Cell viability; (d) MDA levels; (e) SOD activity. F and p values of the one-way analysis of variance are presented above each chart. The results of Tukey’s post hoc test are presented for selected comparisons: ns, p > 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001; the error bars are SD.
2.5. Effects of PCs on Zebrafish Larvae Motility upon MPTP Treatment
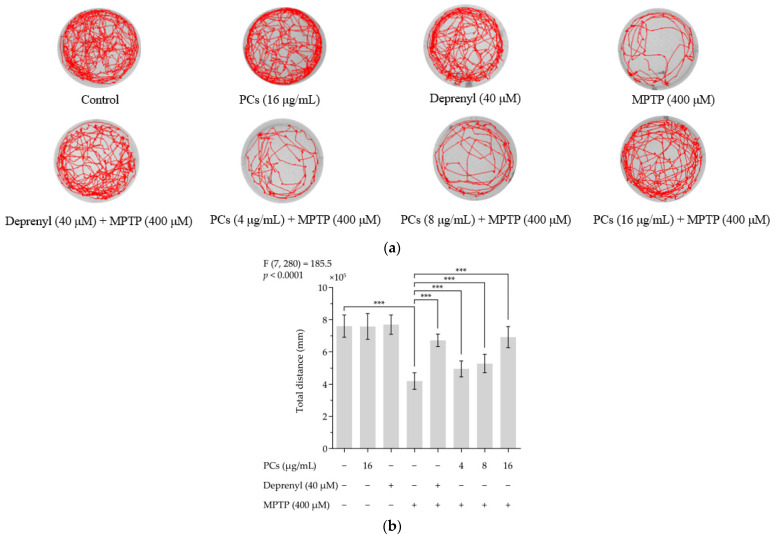
MPTP reduced the total distance zebrafish swam. PC (4, 8 and 16 μg/mL) treatment rescued MPTP-induced locomotive deficits and increased total distance traveled relative to the MPTP group (Figure 5a,b). Furthermore, deprenyl appeared to offer protection from MPTP-induced damage and was used as a positive control. Notably, PCs (16 μg/mL) and deprenyl (40 μM) alone did not affect locomotion behavior of normal zebrafish larvae.
Figure 5.
Effects of PCs on zebrafish larvae motility upon MPTP treatment. (a) Typical patterns of swimming traces of zebrafish larvae in each group; (b) Average total distance of zebrafish larvae in each group. F and p values of the one-way analysis of variance are presented above the chart. The results of Tukey’s post hoc test are presented for selected comparisons: ***, p < 0.001; the error bars are SD.
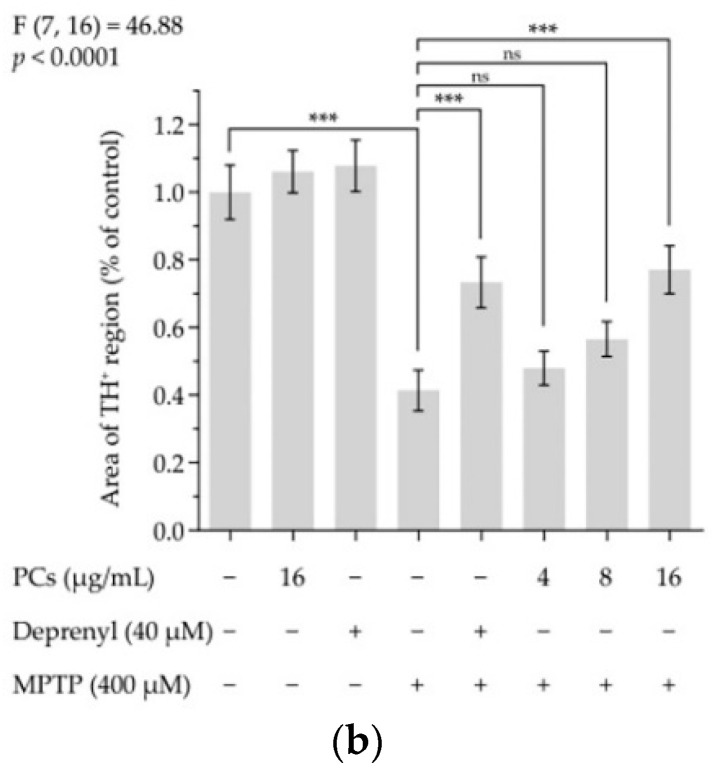
2.6. Effects of PCs on MPTP-Induced Dopaminergic Neuron Injury in Zebrafish
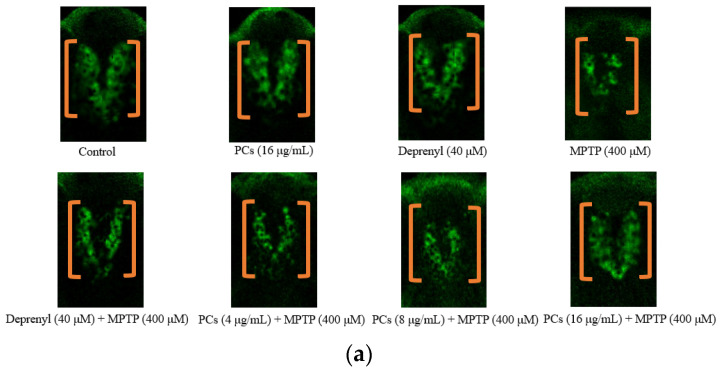
Zebrafish embryos incubated with MPTP demonstrated a statistically significant decrease in tyrosine hydroxylase (TH) density (Figure 6a,b). Nevertheless, treatment with 16 μg/mL of PCs significantly reversed the reduction in TH+ cell density (Figure 6a,b).
Figure 6.
Effects of PCs on MPTP-induced dopaminergic neuron injury in zebrafish. (a) Representative pictures of the dopaminergic neurons in the brains of zebrafish; (b) number of TH+ neurons was measured by ImageJ. F and p values of the one-way analysis of variance are presented above the chart. The results of Tukey’s post hoc test are presented for selected comparisons on the chart: ns, p > 0.05; ***, p < 0.001; the error bars are SD.
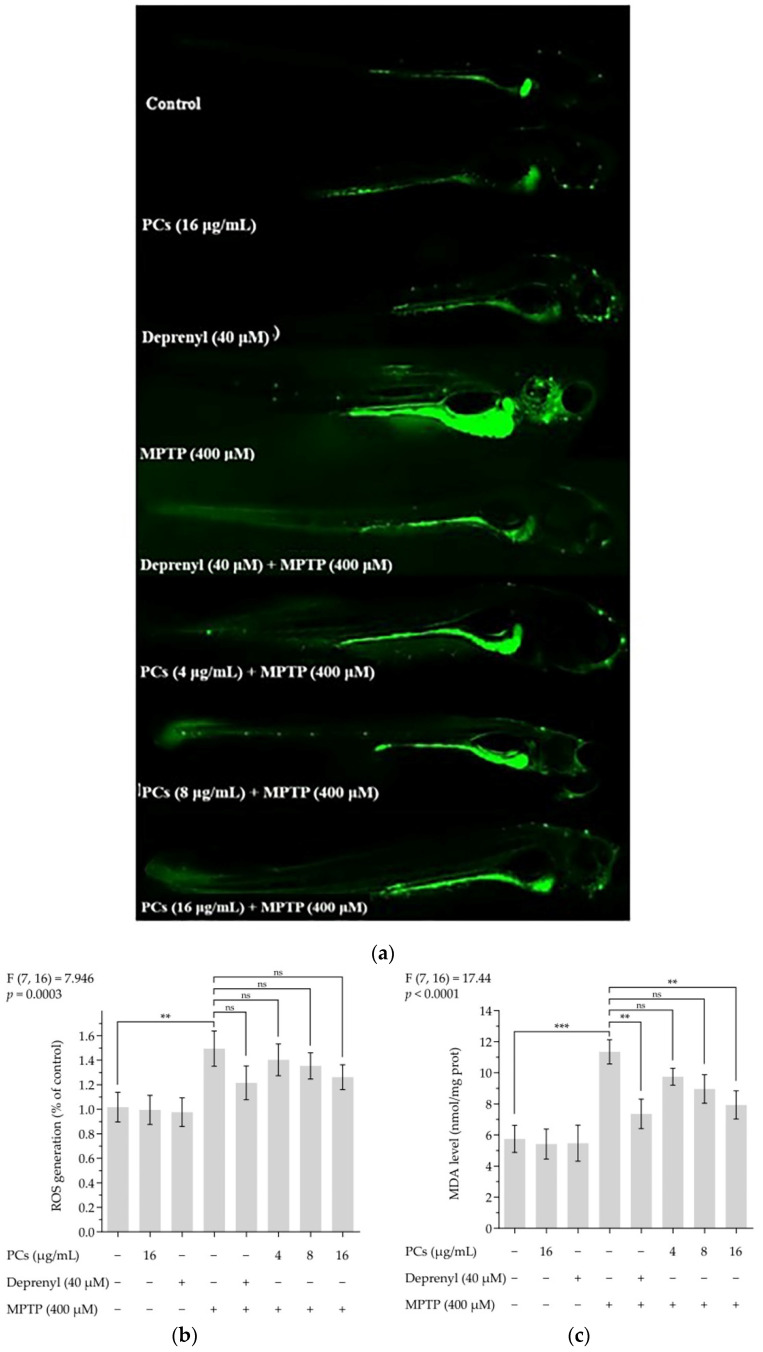
2.7. Effects of PCs on Oxidative Stress of Zebrafish Larvae Treated with MPTP
Treatment of zebrafish larvae with PCs decreased MPTP-induced increased intracellular ROS formation (Figure 7a,b). Furthermore, the lipid peroxidation assay results showed that PC exposure (16 μg/mL) blocked the MPTP-induced MDA levels in zebrafish larvae (Figure 7c). Additionally, the MPTP-induced reduction in GSH-Px activity was reversed by PC (16 μg/mL) treatment (Figure 7d), and PCs (4, 8, and 16 μg/mL) increased MPTP-induced decreases in CAT and SOD activity (Figure 7e,f).
Figure 7.
Effects of PCs on oxidative stress of zebrafish larvae treated with MPTP. (a) ROS levels were measured by fluorescent microscopy and imaging analysis; (b) ROS levels were measured via image J software; (c) MDA levels; (d) GSH-Px activity; (e) CAT activity; (f) SOD activity. F and p values of the one-way analysis of variance are presented above each chart. The results of Tukey’s post hoc test are presented for selected comparisons: ns, p > 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001; the error bars are SD.
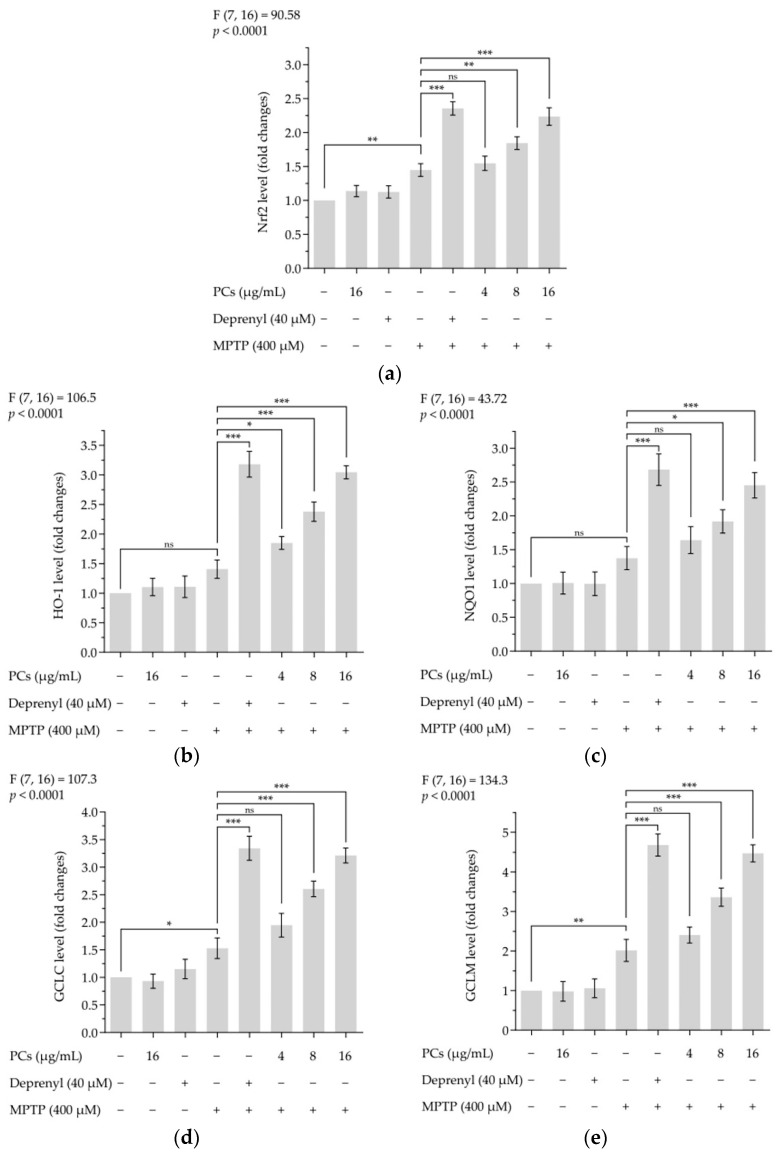
2.8. Effects of PCs on Nrf2/ARE Pathway in MPTP-Induced Zebrafish Larvae
Treatment of zebrafish larvae with PCs underscored the premise that activation of Nrf2/ARE pathways was involved in PC-mediated protective properties. The expression of Nrf2, HO-1, NQO1, GCLC and GCLM markers was upregulated by PCs treatment compared with MPTP exposure alone (Figure 8a–e).
Figure 8.
Effects of PCs on Nrf2/ARE pathway in MPTP-induced zebrafish larvae. (a) Nrf2 levels; (b) HO-1 levels; (c) NQO1 levels; (d) GCLC levels; (e) GCLM levels. F and p values of the one-way analysis of variance are presented above each chart. The results of Tukey’s post hoc test are presented for selected comparisons: ns, p > 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001; the error bars are SD.
3. Discussion
PCs exhibit strong radical scavenging abilities and antioxidant activity, and oxidative stress, caused by the excessive generation of ROS or/and the impaired antioxidant defense system, plays a critical role in PD [37,38,39]. However, whether PCs can play a neuroprotective role through antioxidant effects remains unclear. This research aimed to investigate the molecular mechanism of PCs against MPP+/MPTP-induced PD models.
In this study, compared with the MPP+/MPTP-alone group, PCs markedly raised the activity level of antioxidant enzymes (including GSH-Px, CAT and SOD) and decreased levels of ROS and MDA. The current findings are consistent with a previous study that suggests that a low PC supplement via diet improves the activities of GSH-Px, CAT and SOD in weaned piglets [40]. Another study also suggests that PCs significantly increase CAT and SOD activities and decrease MDA content, thus improving the quality of goat sperm [41]. These findings suggest that PCs have a protective effect on oxidative damage of nerve cells. Additionally, PCs improved cell viability compared with the MPP+-alone group in MPP+-induced PC12 cells, markedly increased total distance moved and decreased TH+ cell density relative to the MPTP group in MPTP-induced zebrafish larvae. These data indicate that PCs have a protective effect on nerve cells.
We further observed that PCs significantly increased nuclear Nrf2 accumulation compared with that of MPP+ alone in PC12 cells. Indeed, PCs markedly upregulated the expression levels of NQO1, HO-1, GCLM and GCLC [42]. Furthermore, Nfr2 gene silencing via Nrf2 siRNA was used to investigate the role of Nrf2/ARE activation in PCs-mediated neuroprotection against MPP+-induced oxidative damage: Nrf2-siRNA-transfected cells indicated a marked decrease in Nrf2 expression. This current study also found that Nrf2 knockout abolished both PCs-mediated protection against MPP+-treated impairments in cell viability and the antioxidant effects of PCs. These results are consistent with a previous study suggesting that improving activation of the Nrf2/ARE pathway contributes to neuroprotection [43].
The Nrf2/ARE pathway is a significant antioxidant pathway [44,45,46]. Normally, Nrf2 resides in the cytoplasm, where it is bound to the inhibitory protein, Keap1 [47,48]. When cells undergo oxidative stress, Nrf2 dissociates from Keap1, initiates the endogenous antioxidant defense system and subsequently translocates into the nucleus [46,49,50]. It then interacts with ARE to activate a series of cell-protective and antioxidant genes, including GCLC, GCLM, NQO1 and HO-1 [51,52,53,54]. In response to oxidative stress, Nrf2 dissociates from Keap1 in the cytosol and is then translocated into the nucleus, binding to the ARE sequence to activate transcription of cryoprotective genes [53,54,55,56,57,58]. The current results indicate that PCs can activate the Nrf2/ARE pathway, transfer Nrf2 from the cytoplasm to the nucleus, accumulate in the nucleus, upregulate the expression of GCLC, GCLM, NQO1 and HO-1 and improve the ability of cells to resist oxidative stress. Indeed, the Nrf2/ARE pathway may represent a pharmacological target of PCs for the prevention of PD.
4. Materials and Methods
4.1. Chemical Compounds and Reagents
MPP+ and MPTP agents were purchased from Sigma (St. Louis, MO, USA), and the Cell Counting Kit-8 was obtained from Beyotime (Shanghai, China). The PCs, 2′,7′-Dichlorofluorescin diacetate, MDA, GSH-Px, SOD and CAT diagnostic kits were all obtained from Solarbio (Beijing, China). The RNAiso Plus, PrimeScript™RT Reagent Kit with gDNA Eraser, and SYBR®Premix Ex Taq™ II were purchased from Takara (Shiga, Japan); the Nrf2-siRNA, control-siRNA, and Lipofectamine 2000 were obtained from GenePharma (Shanghai, China). Finally, the primary antibodies, Nrf2, HO-1, GCLC, GCLM, NAD(P)H: NQO1, Lamin B, GAPDH, Antityrosine Hydroxylase (TH) and corresponding secondary antibodies were obtained from Proteintech (Wuhan, China).
4.2. Cell Culture
PC12 cells were obtained from the National Collection of Authenticated Cell Cultures. The cells were maintained in DMEM supplemented with 10% fetal bovine serum and penicillin-streptomycin (100 U/mL; 100 μg/mL) in a humidified atmosphere incubator at 37 °C with 5% CO2 [59].
4.3. Cell Viability Assay
PC12 cells (1 × 104 cells/well) were incubated with 1, 2 or 4 μg/mL of PCs or deprenyl (30 μM) for 24 h, and then incubated with 1.5 mM MPP+ for 24 h. Next, 10 μL of Cell Counting Kit-8 solution was added into each well and incubated for 1 h. The absorbance was measured at 450 nm [60].
4.4. Fish Maintenance
Ethical approval for animal use was granted by the animal conservation and use committee of the experimental animal center, Zhejiang University (ZJU20200125). Adult zebrafish (AB strain) were obtained from the laboratory animal center of Zhejiang University (Hang Zhou, China) and maintained at 28 ± 1 °C under 14 h light/10 h dark cycles. The fish were fed Artemia nauplii twice daily [61]. To produce embryos, adult zebrafish were placed in breeding tanks overnight at a 1:1 male:female ratio. Spawning was triggered after the lights were turned on the next morning and completed in 2 h. Embryos were raised in embryo water (13.7 mM NaCl, 540 μM KCl, 25 μM Na2HPO4, 44 μM KH2PO4, 300 μM CaCl2, 100 μM MgSO4, 420 μM NaHCO3, pH 7.4) at 28 °C [62,63].
4.5. ROS Measurement
To measure ROS production, PC12 cells (2 × 104 cells/well) were exposed to 1, 2 or 4 μg/mL of PCs or deprenyl (30 μM) for 24 h, and then incubated with 1.5 mM MPP† for 24 h [64]. The cells were exposed to 10 μM 2’,7’-Dichlorofluorescin diacetate solution in dark conditions for 30 min; the dye solution was then removed, and the cells were washed with phosphate-buffered saline (PBS) 3 times. The images of the cells were observed using an Olympus laser scanning confocal microscope (Olympus, Tokyo capital, Japan). The fluorescence intensity of the cells was quantified using Image J software v1.8.0 (Olympus, Tokyo capital, Japan). The results are expressed as a percentage of area of the ROS regions in the control group.
To measure ROS production, the zebrafish larvae at 3 days post fertilization (dpf) were exposed to deprenyl (40 μM) or 4, 8 or 16 μg/mL of PCs with or without 400 μM MPTP for 4 days. Zebrafish larvae at 7 dpf were transferred to a 24-well plate (10 larvae per group), treated with 20 μM 2’,7’-Dichlorofluorescin diacetate solution and incubated for 60 min in the dark at 28.5 °C [65]. The larvae were then washed three times with embryo medium to remove excess 2’,7’-Dichlorofluorescin diacetate. The images of the stained larvae were observed using an Olympus laser scanning confocal microscope. The fluorescence intensity of the individual larva was quantified using Image J software. The results are expressed as a percentage of area of the ROS regions in the control group.
4.6. Assessment of MDA, GSH-Px, SOD, and CAT
PC12 cells (2 × 104 cells/well) were exposed to 1, 2 or 4 μg/mL of PCs or deprenyl (30 μM) for 24 h, and then incubated with 1.5 mM MPP+ for 24 h. Then, 1 mL of extract was added to 4 × 106 cells and cells were broken by ultrasonic centrifuging at 8000 rpm at 4 °C for 10 min. The supernatant was then put on ice for testing, and reagents were added for the determination of MDA, GSH-Px, SOD and CAT, respectively. Each index was repeated in triplicate.
The zebrafish larvae at 3 dpf were incubated with deprenyl (40 μM) or 4, 8 or 16 μg/mL of PCs with or without 400 μM MPTP for 4 days. Then, 0.05 g of zebrafish larvae tissue and 0.5 mL of extract were homogenized in an ice bath and then centrifuged at 4 °C for 10 min. The supernatant was put on ice for testing. Reagents were subsequently added for the determination of MDA, GSH-Px, SOD and CAT, respectively. Each index was repeated in triplicate.
4.7. Preparation of Whole Cell, Cytoplasmic, and Nuclear Protein
For whole-cell protein extraction, cells were collected and incubated with RIPA lysis buffer containing 1% PMSF and 1% protease inhibitor cocktail for 30 min on ice. Cell lysates were centrifuged, and the supernatant was collected and stored. For subcellular fractionation preparation, cell samples were processed using the nuclear and cytoplasmic protein extraction kit. The protein content was assayed using the BCA (Beyotime, Shanghai, China) assay.
4.8. Nrf2 siRNA Transfection
Nrf2-siRNA was used to knock down Nrf2. PC12 cells were transfected with Nrf2-siRNA (80 nM) or control-siRNA using Lipofectamine 2000, according to the manual.
4.9. Western Blotting
Protein samples were resolved by SDS-PAGE and transferred to polyvinylidene difuoride (PVDF) membranes. The blots were exposed to appropriate primary antibodies: Nrf2, Keap1, HO-1, NQO1, GCLC, GCLM, Lamin B, GAPDH and peroxidase-conjugated secondary antibodies. Protein bands were visualized using ECL (Beyotime, Shanghai, China) plus Western blotting detection reagents [36,43].
4.10. Locomotor Behavioral Test
The zebrafish larvae at 3 dpf were incubated with deprenyl (40 μM) or 4, 8 or 16 μg/mL of PCs with or without 400 μM MPTP for 4 days. The 7 dpf zebrafish were then placed into 24-well plates (1 fish per well and 12 larvae per group), and the total distance each fish swam over 10 min was recorded. Zebrafish behavior was monitored and analyzed using an automated video tracking system (Any-maze 4.73, Stoelting, Wood Dale, IL, USA) [66].
4.11. Total RNA Extraction, Reverse Transcription, and Quantitative Real-Time Polymerase Chain Reaction
RNA was extracted using RNAiso Plus following the manufacturer’s instructions. RNA was reverse transcribed using a PrimeScript™RT Reagent Kit with gDNA Eraser, following the manufacturer’s instructions. Quantitative real-time PCR analysis was performed on an Applied Biosystems ViiA™ 7 Real-Time PCR system using SYBR®Premix Ex Taq™ II (Takara, Shiga, Japan). β-actin was used as a reference gene and relative gene expression was calculated using the 2–ΔΔCt method. The primer sequences utilized in the research are listed in Table 1.
Table 1.
Sequences of primers for quantitative real-time PCR.
| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| β-Actin | CACTGAGGCTCCCCTGAATC | GGGTCACACCATCACCAGAG |
| Nrf2 | CTGCTGTCACTCCCAGAGTT | GCCGTAGTTTTGGGTTGGTG |
| HO-1 | AAGAGCTGGACAGAAACGCA | AGAAGTGCTCCAAGTCCTGC |
| GCLC | CTCCTCACAGTCACGGCATT | TGAATGGAGACGGGGTGTTG |
| GCLM | AAGCCAGACACTGACACACC | ATCTGGAGGCATCACACAGC |
| NQO1 | AAGCCTCTGTCCTTTGCTCC | TGCTGTGGTAATGCCGTAGG |
Nrf2, nuclear factor-erythroid 2-related factor 2; HO-1, heme oxygenase 1; GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modifier subunit; NQO1, quinone oxidoreductase 1.
4.12. Zebrafish Antityrosine Hydroxylase (TH) Whole-Mount Immunostaining
Zebrafish larvae at 1 dpf were incubated with deprenyl (40 μM) or 4, 8 or 16 μg/mL of PCs with or without 400 μM MPTP for 2 days (10 fish/group batches). Larvae were fixed with 4% paraformaldehyde in PBS for 30 min. After fixation, they were treated for whole-mount immunostaining of TH [67]. At room temperature, 2% (v/v) lamb serum and 0.1% (w/v) bovine serum albumin (BSA) were blocked in phosphate-buffered saline Tween-20 (PBST) for 1 h. They were then exposed in the blocking buffer to antityrosine hydroxylase antibody (1:200 diluted, Proteintech) for 2 h, and then rinsed 6 times with PBST. The final whole-mount immunostaining step was performed in staining buffer with 488 goat antirabbit (1:500) for 1 h and washed again with PBST. After sufficient color development, the zebrafish were flat mounted with 3.5% methylcellulose and imaged using an Olympus laser scanning confocal microscope.
4.13. Statistical Analysis
One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test were used to compare the means of different groups. Graphpad Prism v6.01 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis and plotting the graphs.
5. Conclusions
In conclusion, our findings indicate that PCs exert neuroprotective effects via activation of the Nrf2/ARE pathway and its downstream detoxification and antioxidant enzymes. Taken together, these insights suggest that PCs may be useful for treating PD.
Abbreviations
PCs: procyanidins; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MPP+: 1-methyl-4-phenylpyridinium; PD: Parkinson’s disease; dpf: days post-fertilization; GSH-Px: glutathione peroxidase; SOD: superoxide dismutase; CAT: catalase; ROS: reactive oxygen species; MDA: malondialdehyde; Nrf2: nuclear factor-erythroid 2-related factor 2; ARE: antioxidant response element; HO-1: heme oxygenase 1; NQO1: quinone oxidoreductase 1; GCLC: glutamate-cysteine ligase catalytic subunit; GCLM: glutamate-cysteine ligase modifier subunit; TH: tyrosine hydroxylase; SD: standard deviation; ANOVA: one-way analysis of variance.
Author Contributions
Conceptualization, J.C. and J.Z. (Jiajin Zhu); methodology, J.C.; software, Y.C.; validation, Y.Z., J.Z. (Jiawen Zhao) and H.Y.; formal analysis, J.C.; investigation, Y.C.; resources, J.Z. (Jiajin Zhu); data curation, J.C.; writing—original draft preparation, J.C.; writing—review and editing, J.Z. (Jiajin Zhu); visualization, J.C.; supervision, D.L.; project administration, J.Z. (Jiajin Zhu); funding acquisition, J.Z. (Jiajin Zhu). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Animal Conservation and Use Committee of the Experimental Animal Center at Zhejiang University (protocol code ZJU20200125, date of approval: 2020-11-01).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Connolly B.S., Lang A.E. Pharmacological treatment of Parkinson disease: A review. JAMA. 2014;311:1670–1683. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 2.Fearnley J.M., Lees A.J. Aging and Parkinsons disease: Substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 3.de Lau L.M.L., Giesbergen P.C.L.M., de Rijk M.C., Hofman A., Koudstaal P.J., Breteler M.M.B. Incidence of parkinsonism and Parkinson disease in a general population: The Rotterdam Study. Neurology. 2004;63:1240–1244. doi: 10.1212/01.WNL.0000140706.52798.BE. [DOI] [PubMed] [Google Scholar]
- 4.Dorsey E.R., Constantinescu R., Thompson J.P., Biglan K.M., Holloway R.G., Kieburtz K., Marshall F.J., Ravina B.M., Schifitto G., Siderowf A., et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 5.Wang J., Xu H., Jiang H., Du X., Sun P., Xie J. Neurorescue effect of rosmarinic acid on 6-hydroxydopamine-lesioned nigral dopamine neurons in rat model of Parkinson’s disease. J. Mol. Neurosci. 2012;47:113–119. doi: 10.1007/s12031-011-9693-1. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong M.J., Okun M.S. Diagnosis and treatment of Parkinson disease: A review. JAMA. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 7.Solayman M., Islam M.A., Alam F., Khalil M.I., Kamal M.A., Gan S.H. Natural products combating neurodegeneration: Parkinson’s disease. Curr. Drug Metab. 2017;18:50–61. doi: 10.2174/1389200217666160709204826. [DOI] [PubMed] [Google Scholar]
- 8.Cenini G., Lloret A., Cascella R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid. Med. Cell. Longev. 2019;2019:2105607. doi: 10.1155/2019/2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spina M.B., Cohen G. Dopamine turnover and glutathione oxidation: Implications for Parkinson disease. Proc. Natl. Acad. Sci. USA. 1989;86:1398–1400. doi: 10.1073/pnas.86.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi H.J., Kim S.W., Lee S.Y., Hwang O. Dopamine-dependent cytotoxicity of tetrahydrobiopterin: A possible mechanism for selective neurodegeneration in Parkinson’s disease. J. Neurochem. 2003;86:143–152. doi: 10.1046/j.1471-4159.2003.01808.x. [DOI] [PubMed] [Google Scholar]
- 11.Imaizumi Y., Okada Y., Akamatsu W., Koike M., Kuzumaki N., Hayakawa H., Nihira T., Kobayashi T., Ohyama M., Sato S., et al. Mitochondrial dysfunction associated with increased oxidative stress and alpha-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol. Brain. 2012;5:1–13. doi: 10.1186/1756-6606-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruk J., Aboul-Enein H.Y., Kladna A., Bowser J.E. Oxidative stress in biological systems and its relation with pathophysiological functions: The effect of physical activity on cellular redox homeostasis. Free Radic. Res. 2019;53:497–521. doi: 10.1080/10715762.2019.1612059. [DOI] [PubMed] [Google Scholar]
- 13.Maleki S.J., Crespo J.F., Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124. doi: 10.1016/j.foodchem.2019.125124. [DOI] [PubMed] [Google Scholar]
- 14.Terra X., Fernández-Larrea J., Pujadas G., Ardèvol A., Bladé C., Salvadó J., Arola L., Blay M. Inhibitory effects of grape seed procyanidins on foam cell formation in vitro. J. Agric. Food Chem. 2009;57:2588–2594. doi: 10.1021/jf803450a. [DOI] [PubMed] [Google Scholar]
- 15.Bagchi D., Garg A., Krohn R.L., Bagchi M., Tran M.X., Stohs S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997;95:179–189. [PubMed] [Google Scholar]
- 16.Jiang Y.R., Mao S.Q., Huang W.S., Lu B.Y., Cai Z.X., Zhou F., Li M.Q., Lou T.T., Zhao Y.J. Phenylethanoid glycoside profiles and antioxidant activities of osmanthus fragrans lour. flowers by UPLC/PDA/MS and simulated digestion model. J Agr Food Chem. 2016;64:2459–2466. doi: 10.1021/acs.jafc.5b03474. [DOI] [PubMed] [Google Scholar]
- 17.Guo S., Wilson S.W., Cooke S., Chitnis A.B., Driever W., Rosenthal A. Mutations in the zebrafish unmask shared regulatory pathways controlling the development of catecholaminergic neurons. Dev. Biol. 1999;208:473–487. doi: 10.1006/dbio.1999.9204. [DOI] [PubMed] [Google Scholar]
- 18.Holzschuh J., Ryu S., Aberger F., Driever W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech. Develop. 2001;101:237–243. doi: 10.1016/S0925-4773(01)00287-8. [DOI] [PubMed] [Google Scholar]
- 19.McLean D.L., Fetcho J.R. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J. Comp. Neurol. 2004;480:38–56. doi: 10.1002/cne.20280. [DOI] [PubMed] [Google Scholar]
- 20.Rink E., Wullimann M.F. Development of the catecholaminergic system in the early zebrafish brain: An immunohistochemical study. Dev. Brain Res. 2002;137:89–100. doi: 10.1016/S0165-3806(02)00354-1. [DOI] [PubMed] [Google Scholar]
- 21.Kalueff A.V., Stewart A.M., Gerlai R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014;35:63–75. doi: 10.1016/j.tips.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos S.D., Verveer P.J., Bastiaens P.I. Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat. Cell Biol. 2007;9:324–330. doi: 10.1038/ncb1543. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg D.A., Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 24.Shui G., Bao Y.M., Bo J., An L.J. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur. J. Pharmacol. 2006;538:73–79. doi: 10.1016/j.ejphar.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 25.Cheng X.R., Zhang L., Hu J.J., Sun L., Du G.H. Neuroprotective effects of tetramethylpyrazine on hydrogen peroxide-induced apoptosis in PC12 cells. Cell Biol. Int. 2007;31:438–443. doi: 10.1016/j.cellbi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Jiang B., Liu J.H., Bao Y.M., An L.J. Hydrogen peroxide-induced apoptosis in pc12 cells and the protective effect of puerarin. Cell Biol. Int. 2003;27:1025–1031. doi: 10.1016/j.cellbi.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Langston J.W., Ballard P., Tetrud J.W., Irwin I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 28.Miller G.W., Gainetdinov R.R., Levey A.I., Caron M.G. Dopamine transporters and neuronal injury. Trends Pharmacol. Sci. 1999;20:424–429. doi: 10.1016/S0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- 29.Blum D., Torch S., Lambeng N., Nissou M.F., Benabid A.L., Sadoul R., Verna J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001;65:135–172. doi: 10.1016/S0301-0082(01)00003-X. [DOI] [PubMed] [Google Scholar]
- 30.Nicotra A., Parvez S.H. Cell death induced by MPTP, a substrate for monoamine oxidase B. Toxicology. 2000;153:157–166. doi: 10.1016/S0300-483X(00)00311-5. [DOI] [PubMed] [Google Scholar]
- 31.Grau-Bove C., Sierra-Cruz M., Miguens-Gomez A., Rodriguez-Gallego E., Beltran-Debon R., Blay M., Terra X., Pinent M., Ardevol A. A ten-day grape seed procyanidin treatment prevents certain ageing processes in female rats over the long term. Nutrients. 2020;12:3467. doi: 10.3390/nu12123647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Q., Chen Z.Y., Zhu B.R., Wang G.R., Jia Q., Li Y.M., Wu X.J. A-type cinnamon procyanidin oligomers protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice through inhibiting the p38 mitogen-activated protein kinase/P53/BCL-2 associated X protein signaling pathway. J. Nutr. 2020;150:1731–1737. doi: 10.1093/jn/nxaa128. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Huang N.Q., Chen M.J., Jin H., Nie J., Shi J.S., Ji F. Procyanidin protects against 6-hydroxydopamine-induced dopaminergic neuron damage via the regulation of the PI3K/Akt signalling pathway. Biomed. Pharmacother. 2019;114:108789. doi: 10.1016/j.biopha.2019.108789. [DOI] [PubMed] [Google Scholar]
- 34.Kook Y.H., Ka M., Um M. Neuroprotective cytokines repress PUMA induction in the 1-methyl-4-phenylpyridinium (MPP+) model of Parkinson’s disease. Biochem. Bioph. Res. Commun. 2011;411:370–374. doi: 10.1016/j.bbrc.2011.06.151. [DOI] [PubMed] [Google Scholar]
- 35.Tang X.Q., Fang H.R., Li Y.J., Zhou C.F., Ren Y.K., Chen R.Q., Wang C.-Y., Hu B. Endogenous hydrogen sulfide is involved in asymmetric dimethylarginine-induced protection against neurotoxicity of 1-methyl-4-phenyl-pyridinium ion. Neurochem. Res. 2011;36:2176–2185. doi: 10.1007/s11064-011-0542-y. [DOI] [PubMed] [Google Scholar]
- 36.Chong C.M., Ma D., Zhao C., Franklin R.J.M., Zhou Z.Y., Ai N., Li C., Yu H., Hou T., Sa F., et al. Discovery of a novel neuroprotectant, BHDPC, that protects against MPP+/MPTP-induced neuronal death in multiple experimental models. Free Radical. Biol. Med. 2015;89:1057–1066. doi: 10.1016/j.freeradbiomed.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Kondo K., Klco J., Nakamura E., Lechpammer M., Kaelin W.G., Jr. Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/S1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 38.Razavi S.M., Gholamin S., Eskandari A., Mohsenian N., Ghorbanihaghjo A., Delazar A., Rashtchizadeh N., Keshtkar-Jahromi M., Argani H. Red grape seed extract improves lipid profiles and decreases oxidized low-density lipoprotein in patients with mild hyperlipidemia. J. Med. Food. 2013;16:255–258. doi: 10.1089/jmf.2012.2408. [DOI] [PubMed] [Google Scholar]
- 39.Kong X., Guan J., Gong S., Wang R. Neuroprotective effects of grape seed procyanidin extract on Ischemia-reperfusion brain injury. Chin. Med. Sci. J. 2017;32:92–99. doi: 10.24920/J1001-9294.2017.020. [DOI] [PubMed] [Google Scholar]
- 40.Fang L., Li M., Zhao L., Han S., Li Y., Xiong B., Jiang L. Dietary grape seed procyanidins suppressed weaning stress by improving antioxidant enzyme activity and mRNA expression in weanling piglets. J. Anim. Physiol. Anim. Nutr. 2020;104:1178–1185. doi: 10.1111/jpn.13335. [DOI] [PubMed] [Google Scholar]
- 41.Wen F., Li Y., Feng T., Du Y., Ren F., Zhang L., Han N., Ma S., Li F., Wang P., et al. Grape seed procyanidin extract (GSPE) improves goat sperm quality when preserved at 4 °C. Animals (Basel) 2019;9:810. doi: 10.3390/ani9100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang S., Tang Q., Jin J., Zheng G., Xu J., Huang W., Li X., Shang P., Liu H. Polydatin inhibits the IL-1β-induced inflammatory response in human osteoarthritic chondrocytes by activating the Nrf2 signaling pathway and ameliorates murine osteoarthritis. Food Funct. 2018;9:1701–1712. doi: 10.1039/C7FO01555K. [DOI] [PubMed] [Google Scholar]
- 43.Li C., Tang B., Feng Y., Tang F., Pui-Man Hoi M., Su Z., Ming-Yuen Lee S. Pinostrobin exerts neuroprotective actions in neurotoxin-induced Parkinson’s disease models through Nrf2 induction. J. Agric. Food Chem. 2018;66:8307–8318. doi: 10.1021/acs.jafc.8b02607. [DOI] [PubMed] [Google Scholar]
- 44.McMahon M., Thomas N., Itoh K., Yamamoto M., Hayes J.D. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J. Biol. Chem. 2004;279:31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- 45.Yu M., Li H., Liu Q., Liu F., Tang L., Li C., Yuan Y., Zhan Y., Xu W., Li W., et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell. Signal. 2011;23:883–892. doi: 10.1016/j.cellsig.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Itoh K., Igarashi K., Hayashi N., Nishizawa M., Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol. Cell. Biol. 1995;15:4184–4193. doi: 10.1128/MCB.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hybertson B.M., Gao B., Bose S.K., McCord J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi A., Kang M.-I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu M.-C., Ji J.-A., Jiang Z.-Y., You Q.-D. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: An update. Med. Res. Rev. 2016;36:924–963. doi: 10.1002/med.21396. [DOI] [PubMed] [Google Scholar]
- 51.Ungvari Z., Bagi Z., Feher A., Recchia F.A., Sonntag W.E., Pearson K., de Cabo R., Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidlin C.J., Dodson M.B., Madhavan L., Zhang D.D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019;134:702–707. doi: 10.1016/j.freeradbiomed.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slocum S.L., Kensler T.W. Nrf2: Control of sensitivity to carcinogens. Arch. Toxicol. 2011;85:273–284. doi: 10.1007/s00204-011-0675-4. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki T., Motohashi H., Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Peng S., Hou Y., Yao J., Fang J. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017;8:997–1007. doi: 10.1039/C7FO00054E. [DOI] [PubMed] [Google Scholar]
- 56.Mi Y., Zhang W., Tian H., Li R., Huang S., Li X., Qi G., Liu X. EGCG evokes Nrf2 nuclear translocation and dampens PTP1B expression to ameliorate metabolic misalignment under insulin resistance condition. Food Funct. 2018;9:1510–1523. doi: 10.1039/C7FO01554B. [DOI] [PubMed] [Google Scholar]
- 57.Nagle A.A., Reddy S.A., Bertrand H., Tajima H., Dang T.M., Wong S.C., Hayes J.D., Wells G., Chew E.H. 3-(2-oxoethylidene)indolin-2-one derivatives activate Nrf2 and inhibit NF-κB: Potential candidates for chemoprevention. ChemMedChem. 2014;9:1763–1774. doi: 10.1002/cmdc.201490031. [DOI] [PubMed] [Google Scholar]
- 58.Cullinan S.B., Gordan J.D., Jin J., Harper J.W., Diehl J.A. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: Oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng B.H., Guo Y.L., Li C.G., Ji B.Y., Pan Y.Y., Chen J., Bai B. Edaravone protected PC12 cells against MPP(+)-cytoxicity via inhibiting oxidative stress and up-regulating heme oxygenase-1 expression. J Neurol Sci. 2014;343:115–119. doi: 10.1016/j.jns.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 60.Gu J., Wang H.Y., Zhou L.J., Fan D.L., Shi L.L., Ji G.X., Gu A. Oxidative stress in bisphenol AF-induced cardiotoxicity in zebra fish and the protective role of N-acetyl N-cysteine. Sci. Total. Environ. 2020;731:139190. doi: 10.1016/j.scitotenv.2020.139190. [DOI] [PubMed] [Google Scholar]
- 61.Li M.Q., Zhou F., Xu T., Song H.X., Lu B.Y. Acteoside protects against 6-OHDA-induced dopaminergic neuron damage via Nrf2-ARE signaling pathway. Food and Chem. Toxicol. 2018;119:6–13. doi: 10.1016/j.fct.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y., Li G., Law H.C.H., Chen H., Lee S.M. Determination of oxyphylla a enantiomers in the fruits of alpinia oxyphylla by a chiral high-performance liquid chromatography-multiple reaction monitoring-mass spectrometry method and comparison of their in vivo biological activities. J. Agric. Food Chem. 2020;68:11170–11181. doi: 10.1021/acs.jafc.0c04031. [DOI] [PubMed] [Google Scholar]
- 63.Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F. Stages of embryonic-development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 64.Chen J., Chen Y.X., Zheng Y.F., Zhao J.W., Yu H.L., Zhu J.J., Li D. Neuroprotective effects and mechanisms of procyanidins in vitro and in vivo. Molecules. 2021;26:2963. doi: 10.3390/molecules26102963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin M., He Q., Zhang S., Cui Y., Han L., Liu K. Gastrodin suppresses pentylenetetrazole-induced seizures progression by modulating oxidative stress in zebrafish. Neurochem. Res. 2018;43:904–917. doi: 10.1007/s11064-018-2496-9. [DOI] [PubMed] [Google Scholar]
- 66.Zhao B., Ren B., Guo R., Zhang W., Ma S., Yao Y., Yuan T., Liu Z., Liu X. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem. Toxicol. 2017;109:505–516. doi: 10.1016/j.fct.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Z.J., Cheang L.C.V., Wang M.W., Li G.H., Chu I.K., Lin Z.X., Lee S.M.Y. Ethanolic extract of fructus Alpinia oxyphylla protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Cell. Mol. Neurobiol. 2012;32:27–40. doi: 10.1007/s10571-011-9731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.