Abstract
Neuroinflammation represents a central feature in the development of Alzheimer’s disease (AD). The resident innate immune cells of the brain are the principal players in neuroinflammation, and their activation leads to a defensive response aimed at promoting β-amyloid (Aβ) clearance. However, it is now widely accepted that the peripheral immune system—by virtue of a dysfunctional blood–brain barrier (BBB)—is involved in the pathogenesis and progression of AD; microglial and astrocytic activation leads to the release of chemokines able to recruit peripheral immune cells into the central nervous system (CNS); at the same time, cytokines released by peripheral cells are able to cross the BBB and act upon glial cells, modifying their phenotype. To successfully fight this neurodegenerative disorder, accurate and sensitive biomarkers are required to be used for implementing an early diagnosis, monitoring the disease progression and treatment effectiveness. Interestingly, as a result of the bidirectional communication between the brain and the periphery, the blood compartment ends up reflecting several pathological changes occurring in the AD brain and can represent an accessible source for such biomarkers. In this review, we provide an overview on some of the most promising peripheral biomarkers of neuroinflammation, discussing their pathogenic role in AD.
Keywords: Alzheimer’s disease, neuroinflammation, peripheral markers, monocytes, cytokines, chemokines, TSPO, delirium
1. Alzheimer’s Disease and the Quest for Biomarkers
Alzheimer’s disease (AD), widely recognized as the most common cause of dementia, is an age-related neurodegenerative disorder characterized by progressive cognitive deterioration, affecting both memory and other aspects of cognitive functioning. Age being the main risk factor for the disease, the marked increase in life expectancy is likely to determine a dramatic rise in the number of AD patients over the years, with substantial consequences on the public health worldwide [1]. The overwhelming impact that the increasing incidence of AD will have on global health can be mainly attributed to the inadequate and lacking understanding of its pathogenesis; the sporadic form of AD is influenced by various genetic and environmental factors and possesses a multifactorial etiology [2]. Despite being well-established that tau neurofibrillary tangles (NFT) and β-amyloid (Aβ) plaques are the hallmarks defining the neuropathology of this disease, it is not yet completely clear how much they contribute to neuronal degeneration spreading or if they may be just byproducts of ongoing damage [3]; this has been fully recognized and has led to the idea that other pathological processes can significantly contribute to AD pathogenesis as well [4]. Neuroinflammation, neuronal and synaptic degeneration, cerebral homeostasis disruption, metabolic disorders, vascular dysfunction and oxidative stress are just a few examples [5,6,7,8,9,10,11]. Furthermore, in spite of all the advances in modern medicine, a definitive diagnosis of AD is still based on the post-mortem detection of characteristic pathologic brain lesions—Aβ plaques and neurofibrillary tangles—similar to those described by Alzheimer himself in 1907 [12,13]. Additionally, the routine medical practice—based mostly on clinical and neuropsychological assessments in vivo—makes it possible to diagnose merely probable or possible AD [14]. Attempts have been made in the last few years to get to an in vivo neuropathological diagnosis of the disease, independent from the clinical symptoms [15]; however, the latest recommendation is to opt of a comprehensive approach, encompassing both the clinical assessment and in vivo neuropathological evaluation [16]. The goal is to diagnose AD as early as possible, in the prodromal phase (Mild Cognitive Impairment), when patients harbor pre-symptomatic pathological changes but maintain good brain functionality, in order to make use of treatments able to counteract or delay the appearance of symptoms [17]. As a matter of fact, therapies currently administered to patients with more advanced diseases are just symptomatic and, thus, unable to block the pathophysiological events leading to full-blown AD. Differently, the so-called “disease-modifying therapies” able to act on the cause and evolution of the disease by interfering with its pathogenesis are more effective when the brain damage is still restrained [18]. Under these circumstances, it is indisputable that, to successfully fight this neurodegenerative disorder, we require accurate and sensitive diagnostic and prognostic tools to be used for implementing an early diagnosis, the monitoring of disease progression and assessing the treatment effectiveness.
1.1. The Urgency for Diagnostic and Prognostic Biomarkers in Alzheimer’s Disease
Universally, the term biomarker refers to whatever feature that can be objectively measured and that is able to define the status of a biological entity at a given time [19]. The ideal biomarker for Alzheimer’s disease should closely reflect the succession of pathological events and prove potentially useful for a wide variety of applications. First and foremost, biomarkers are expected to be helpful for the early and differential diagnosis of AD. They should be able to reveal or suggest the existence of the fundamental alterations of the AD brain even during the preclinical asymptomatic phases of the disease; a better understanding of the biomarker signature preceding the clinical manifestation would enable the differentiation between the individuals at risk for AD and elderly people experiencing the normal aging process. At the same time, biomarkers should be able to correlate with the disease intensification—with the purpose of discerning between the different stages of AD pathology and predicting the conversion and progression to further cognitive decline—and to discriminate AD from other dementias. Furthermore, biomarkers should enable a more effective drug development in AD. They could have a role in the identification and screening of new therapeutic targets or potential therapeutic agents and in the monitoring of treatment efficacy and dosage, as well in clinical trials’ subject selections [20]. For the purpose of this review, the interest is focused on biological fluids-based biomarkers.
1.2. Blood-Based Biomarkers: Interplay between the Brain and the Periphery
Given its intimate contact with the central nervous system (CNS), the cerebrospinal fluid (CSF) represents a reasonable source for AD biomarkers. As a matter of fact, the CSF reflects the status of the CNS, and, therefore, the pathological changes in the brain—determining alterations in the levels of various substances—are able to affect its composition accordingly [21]. Both research and clinical practices have largely benefitted from biomarker discovery studies in the CSF over the years; on the one hand, those studies exceptionally contributed to the understanding of the natural history of AD [22]; on the other hand, the measurement of AD specific biomarkers (Aβ, Tau and pTau) in the CSF has been included in the criteria for the in vivo diagnosis of this disease [23]. However, the high invasiveness of CSF collection by lumbar puncture—together with the risks and costs associated with the procedure—has extensively limited its application in the standard clinical routine, given the reluctance of patients to undergo invasive and expensive testing repeatedly.
The limitations of CSF have prompted a switch of attention towards more accessible biological sources for biomarker research. The current focus is primarily on blood, whose collection is a routine procedure undeniably less invasive, inexpensive, replicable at regular intervals and easy to implement in large populations compared to lumbar puncture. As a consequence, blood-based biomarkers appear to be easily implementable in clinical practice to monitor the disease progression or treatment efficacy over time [24]. The rationale behind blood being a suitable source of biomarkers for this neurodegenerative disease lays in the evidence that the functionally impaired blood–brain barrier (BBB) of AD patients elicits an increased leakage of molecules from the CSF; those molecules end up reflecting the pathological changes occurring in the AD brain in the periphery, assuming the role of CNS informative blood biomarkers [25].
However, the analysis of blood biomarkers comes with an array of detection challenges, imputable to the nature of the sample and to the complexity of AD pathology, and it could often be difficult to correlate the changes in the CNS with those observed in the blood. First of all, the biomarkers’ concentrations are likely to be considerably low compared to the CSF, as they undergo substantial dilutions when entering into the bloodstream; in addition, given the pathological heterogeneity of AD, the BBB integrity can be affected differently, with consequences on the degree of crossing analytes. Moreover, brain proteins crossing the BBB may experience proteolysis in peripheral fluids. Secondly, it should be taken into account that AD can be accompanied by a systemic inflammatory response able to influence the blood levels of proteins indicative of the disease process; as a consequence, the fraction biomarkers attributable to the brain may be concealed by the one produced in the periphery. Finally, the blood owes a complex matrix—characterized by an abundant pool of antibodies and other molecules—that might interfere with the measurements of the biomarkers of interest, attenuating the sensitivity and specificity of the detection assays [24,26,27].
2. Neuroinflammation in the Pathogenesis of Alzheimer’s Disease
The term neuroinflammation refers to the broad range of inflammatory responses that originate in the CNS secondary to insults, injury or disease. Neuroinflammation has been described in AD since the first characterization of the disorder [12] and is now well-recognized as a central feature in its development, contributing to the pathogenesis just as much as the pathological hallmarks [4]. The pathological accumulation of Aβ and NFT in the brain is considered the principal trigger for neuroinflammatory responses in AD, inducing the activation of resident glial cells [28]. In any case, it should be noted that neuroinflammation priming is not a process specific for AD, since other protein aggregates (α-synuclein, TDP-43, etc.; collectively known as DAMP (danger-associated molecular patterns) and recognized by Toll-like receptors) are able to start such a response.
2.1. Microglia
Microglia are the resident innate immune cells of the CNS. They are ubiquitously distributed in the brain and act as the first line of defense, playing a fundamental role in its surveillance [29]. In AD, the microglia are able to identify and bind Aβ—through a range of different receptors—and become activated [30]. When activated, microglia go through morphological changes, localize in the vicinity of senile plaques and start releasing inflammatory mediators [5]. It should be noted that microglial activation is a complex process, producing multiple phenotypes according to the evolution of the inflammatory response, likely to have either beneficial or detrimental roles and effects. The classical (M1) form of microglial activation is characterized by the elevated production of proinflammatory cytokines—including tumor necrosis factor-alpha (TNF-α); interleukins IL-1β, IL-6, IL-12 and IL-18; interferon gamma (IFN-γ); chemokines like the monocyte chemotactic protein 1 (MCP-1) and neurotoxic agents—and is accompanied by impaired phagocytic capacity. On the other hand, the alternative (M2) activation phenotype is characterized by the release of several anti-inflammatory cytokines—interleukins IL-4, IL-10, IL-13 and transforming growth factor-beta (TGF-β)—tissue repair promotion and enhanced phagocytic ability [31,32]. Throughout AD progression, microglia switch from the M2 to M1 activation phenotype. The immediate activation of microglia in AD leads to a defensive response (acute inflammatory response) aimed at promoting Aβ clearance, thereby reducing its accumulation [33]. However, the long-standing exposure to Aβ—due to its increased production and accumulation over time—and inflammatory mediators (a result of the proinflammatory environment) induce a functional impairment in the microglia, whose phagocytic capacity becomes largely insufficient [34]. The failure of microglial phagocytosis increases the amyloid burden in the brain and facilitates direct Aβ-induced neurotoxicity [35]. During its turn, neuronal damage contributes to the release of further proinflammatory cytokines by activated microglia. This long-standing and self-perpetuating (chronic) neuroinflammatory response is often detrimental, resulting in neurodegeneration and brain function impairment [36].
2.2. Astrocytes
Astrocytes are the most abundant glial cells within the brain, part of the resident innate immune system of the CNS and active players in the neuroinflammatory response [37]. Astrocytes constitute a structural part of the BBB, with their processes make extensive contact with—and providing an almost complete coverage of—brain microvessels [38]. In AD, astrocytes are intimately associated with Aβ deposits, with their processes surrounding and even penetrating into the plaques to isolate the neighboring healthy tissue. Astrocytes accumulating around senile plaques are markedly hypertrophic and reactive, showing a striking upregulation of the glial fibrillary acidic protein (GFAP) [39,40]. Reactive astrocytes are involved in Aβ clearance across the BBB through Aquaporin 4 (AQP4) [41] and degradation [42]. Upon exposure to Aβ, reactive astrocytes release proinflammatory cytokines (mainly IFN-γ, IL-1β, IL6, TNFα and TGFβ) and upregulate the production of reactive oxygen species (ROS) and nitric oxide (NO) [43]; those mediators act either in an autocrine and a paracrine way, modulating the microglial activation [44] and self-perpetuating reactive gliosis. The persistent (chronic) activation of astrocytes results in atrophy and degeneration, decreased end feet coverage of cerebral microvessels and altered AQP4 perivascular localization [45]; as a consequence, Aβ clearance through the BBB is compromised, with a subsequent increase of the Aβ burden in the brain [46].
2.3. BBB Integrity and Permeability Are Compromised in AD Pathology
Proinflammatory mediators released by chronically activated glial cells in the AD brain could compromise the integrity and permeability of the BBB [47]. The BBB comprises various elements, collectively known as the neurovascular unit (NVU), including—besides endothelial cells—pericytes, astrocytes and oligodendrocytes [48]. The neuroinflammation and infiltration of toxic aggregates lead to the dysfunction of these components, resulting in a homeostatic imbalance conceivably playing a role in the progression of AD [49]. The reduction of pericytes has also already been demonstrated in AD [50,51], together with the CSF increase of the soluble marker of pericytes damage, PDGFR-β [52]. Oligodendrocytes are vulnerable to neuroinflammation and Aβ oligomers, and the resulting BBB dysfunction and myelin breakdown further amplifies the AD-related damage [53]. Furthermore, besides their role in maintaining the integrity of the NVU/BBB, oligodendrocyte progenitor cells may be a source of further Aβ secretion [54]. Under physiological conditions, the BBB completely separates the CNS from the peripheral immune system [55]; however, proinflammatory cytokines, chemokines, NO, ROS and metalloproteinases (MMPs) are able to increase the BBB permeability, ultimately leading to the withdrawal of the immune privilege of the brain. In particular, proinflammatory cytokines and oxidative stress modulate the BBB permeability by acting on the tight junctions in the cerebrovascular endothelial cells [56,57], while MMPs digest the endothelial basal lamina necessary for BBB integrity [58]. A major contributor to this process is also cerebral amyloid angiopathy (CAA), a condition characterized by the accumulation of Aβ in the cerebrovasculature; amyloid-laden vessels—often associated with degenerated smooth muscle cells, pericytes and endothelial cells—show reduced integrity, with implications for BBB permeability [59].
3. Involvement of Peripheral Immune System in the Development of Alzheimer’s Disease
Neuroinflammation was originally defined as a CNS-limited process; however, evidence increasingly shows that the peripheral immune system is involved in the pathogenesis and progression of AD. This may be counterintuitive at first, since AD is not a “classic” inflammatory disorder. Nevertheless, the existence of an immune–brain axis (see below, Section 3.2.1), the mechanism of inflammaging (Section 3.2.2) and the two-way relationship with delirium (Section 3.2.3) are just some of the evidence that changed our perception about a potential “peripheral” contribution to a central process. As aforementioned, microglial and astrocytic activation leads to the release of soluble inflammatory mediators that, despite being released locally, are able to cross the BBB, diffuse into the bloodstream and migrate to the periphery, thereby recruiting peripheral immune cells [60]. At the same time, the CNS is particularly responsive to peripheral immune system activation; proinflammatory cytokines—released by peripheral cells in response to pathogens and injury—are transported by the blood flow towards the BBB; here, they cross into the brain parenchyma and act upon glial cells, increasing their susceptibility to disease [61].
3.1. Peripheral Immune Cells Come to the Rescue of Functionally Impaired Glial Cells
3.1.1. Monocytes/Macrophages
Circulating monocytes are predominantly recruited in the AD brain in a CCR2/CCL2-dependent manner. CCR2 is a monocytic surface receptor for CCL2 (also known as MCP-1), one of the most effective chemotactic factors for monocytes, upregulated near Aβ deposits as a result of the release from plaque-associated microglia [62]. Noteworthy, the process can be potentiated by the concurrent intervention of soluble Aβ, proven to be a powerful chemotactic stimulus both in vitro blood–brain barrier models and in AD transgenic mice [60,63]. In addition, the translocator protein 18 kDa (TSPO) may give an additional contribution; indeed, previous ex vivo human studies have demonstrated both the presence of functional TSPO receptors on monocyte surfaces [64] and the ability of various TSPO ligands to influence their chemotaxis [65].
The contribution of circulating monocytes to AD pathogenesis is still controversial. In vivo studies using mouse models of AD showed that the recruited monocytes are able to cross the BBB in areas of greater permeability, reach the perivascular space and, from there, infiltrate the brain parenchyma, associating with the regions of increased Aβ deposition [66]. Upon recognition of the Aβ peptides, infiltrating monocytes appear to differentiate into macrophages and to contribute to the clearance of Aβ from both the brain vasculature and parenchyma, thereby restricting Aβ plaques and reducing the degree of CAA [63,67]. On the other hand, monocytes isolated from AD patients exhibit reduced differentiation into macrophages—together with decreased phagocytosis and the lysosomal degradation of Aβ—compared to monocytes from age-matched healthy controls [68].
3.1.2. Lymphocytes
The role of the adaptive immune system in AD is far from completely elucidated; however, the ablation of T- and B-lymphocytes results in a significant acceleration of amyloid pathogenesis and worsening of neuroinflammation [69]. A number of reports have confirmed an increase of CD4+ and CD8+ T cells in the brains, blood and CSF of AD patients [70,71]. In addition, the number of B cells was found significantly increased in the blood of AD patients compared to healthy controls (given their role in antibody secretion, direct tissue infiltration is not necessarily required) [72]. T cells can interact with microglia and modulate their phagocytic and secretory phenotypes, either by infiltrating the brain through the damaged BBB or exerting their function from the periphery (via cytokine secretion) [73]. On the other hand, B cells can be induced by Th2 modulation to produce immunoglobulins—and, among them, anti-Aβ antibodies. Anti-Aβ antibodies have proven able to promote Aβ clearance, thereby reducing its deposition in plaques via different mechanisms; they can bind Aβ in the brain and favor its degradation by microglial cells [74] or facilitate its efflux through the BBB [75]; alternatively, they can sequester Aβ in the peripheral blood, lowering its free level, and induce its release from the brain (peripheral sink effect) [76]. Furthermore, these naturally occurring anti-Aβ auto-antibodies (NAb) may have a role in preventing Aβ toxicity and aggregation [77,78].
3.2. Systemic Inflammation Enhances CNS Susceptibility to Disease
3.2.1. Immune–Brain Axis
Circulating proinflammatory cytokines, released by peripheral immune cells in response to pathogens and injury, are able to communicate with the CNS by crossing into the brain parenchyma—either passively, through leaky areas of the barrier, or by active transport systems (receptors on epithelial cells) [79]; alternatively, they can stimulate the afferent vagus nerve to induce signaling within the brain [80]. This increased inflammatory signaling within the brain is able to act upon the resident immune cells, promoting their activation and shift towards a proinflammatory phenotype. A single challenge induces only a transient activation, with the glial cells returning to their normal resting state shortly thereafter [81].
Interestingly, an increasing amount of data indicate that the gut microbiota may play a major role in priming “neuro”-inflammation and oxidative stress [82]. Furthermore, psychoactive substance release and decreased levels of neurotrophic metabolites have also been related to altered microbiota compositions and may participate in AD pathogenesis as well, besides potentially inducing direct cognitive and behavioral effects [83]. In particular, immune cells are deeply involved in regulating the gut microbiota homeostasis, and significant changes in the host mononucleate phenotype result from the exposure to the vast array of soluble mediators released in the gut (microbiota-related TLR ligands and cytokines, mainly) [84]. These changes participate in AD pathogenesis, promoting an inflammatory “inclination” that is presumably part of the process known as “inflammaging” (see below) [85]. Thus, the possibility of targeting the gut microbiota is nowadays considered as promising and has led to the development of nutritional interventions, besides microbiota transfer, which has also been shown in experimental animal models to reduce AD-related pathology [86].
3.2.2. Inflammaging and Glial Priming
Throughout the aging process, the longstanding exposure to internal and external damaging agents promotes a chronic low-grade activation in the immune system, with a consequent increase in its proinflammatory status (inflammaging) [87]. Likewise, the resident immune cells of the brain undergo a progressive shift toward a proinflammatory activated state with aging; the persistent stimulation over time makes them hypervigilant, enhancing their sensitivity and response to inflammatory stimuli (priming) [88]. As a consequence, a certain degree of neuroinflammation would already be detectable in the prodromal phase of AD, even before Aβ and NFT deposition [89].
3.2.3. Sickness Behavior and Delirium
The existence of an intercommunication between the brain and the periphery is clearly supported by the occurrence of behavioral symptoms—a syndrome known as sickness behavior—in response to proinflammatory cytokines released in the framework of systemic inflammation [90]. It is now clear that the same systemic inflammatory signals can have severe deleterious effects on brain functions when occurring in old age or in the presence of a preexisting neuronal vulnerability. Delirium represents an extreme form of sickness behavior characterized by disturbances in multiple aspects of cognitive functioning ascribable to neuronal and synaptic dysfunctions [91]. These symptoms allegedly reflect an exaggerated response of the CNS to systemic inflammatory stimuli due to the microglial priming [92] archetypal of advanced age and dementia, indeed recognized as the most common risk factors for delirium [93,94]. At the same time, delirium can affect the course of dementia by exacerbating cognitive impairment [95]. It is now recognized that delirium and dementia share overlapping clinical features and common pathogenic mechanisms, to the point that it is possible to speculate that both disorders represent different stages of a common process [96].
3.2.4. The Cholinergic Anti-Inflammatory Pathway
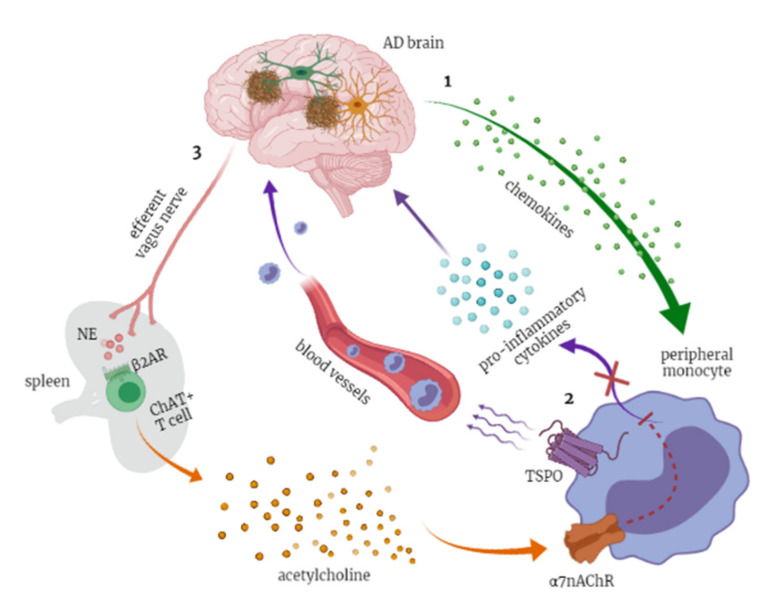
Persistent inflammatory stimuli could result in chronic inflammation, a common risk factor for AD, if immune homeostasis is not restored. In this regard, it has been demonstrated that the CNS is able to monitor and regulate inflammatory responses in real time by means of the cholinergic anti-inflammatory pathway (CAIP) [97]. The so-called inflammatory reflex is triggered when peripheral inflammatory stimuli signal the nucleus tractus solitarius (NTS) by interacting with their receptors on the vagus nerve afferent fibers; once processed in the brain, the outbound signals are transmitted to the spleen via the efferent vagal nerve and splenic nerve. Upon activation, the splenic nerve releases norepinephrine (NE), which interacts with β2-adrenergic receptors expressed on choline acetyltransferase (ChAT)-positive T cells in the spleen, increasing acetylcholine (ACh) synthesis and release. Finally, the binding of ACh to α7 nicotinic acetylcholine receptors (α7nAChRs) expressed on macrophages inhibits proinflammatory cytokine synthesis and release, resulting in alleviated systemic inflammation [98] (Figure 1). Pharmacological intervention on the cholinergic anti-inflammatory pathway is a real possibility; for one, the acetylcholinesterase inhibitor Donepezil is able to act on α7nAChR expressed by peripheral blood mononuclear cells (PBMC) and mediate an immunomodulatory response [78].
Figure 1.
(1) Beta-amyloid oligomers and their subsequent deposition in plaque fuel neuroinflammation, leading microglia to produce chemokines that lure monocytes into the brain. The chemotaxis process is regulated, among other players, by the TSPO, expressed by monocytes and well-known markers of activated microglia. (2) Besides entering the blood–brain barrier, peripheral monocytes produce proinflammatory cytokines that represent the afferent arm of the “inflammatory reflex”. (3) The efferent arm of this reflex is known as the “cholinergic anti-inflammatory pathway” (CAIP) and is represented by a vagal efflux indirectly stimulating the activation of the α7-nicotinic cholinergic receptor (α 7nAChR) expressed by peripheral monocytes and shutting off inflammasome and cytokine production. Created with BioRender.com.
4. Blood-Based Biomarkers of Neuroinflammation
The evidence outlined above of an involvement of the peripheral immune system in AD pathogenesis inevitably offers the possibility of a theoretical approach to the blood compartment as a source of potential biomarkers. A selection of such targets is provided in this section, although, at the moment, none of them has a tangible clinical use. This may be mainly due to the fact that these markers of immune system perturbation may indeed change in the blood following inflammatory events other than AD. Nevertheless, these markers represent very interesting opportunities for a better understanding of AD pathogenesis, and in the future, they may form part of the panels for precision medicine, identifying selected patients for targeting drugs.
4.1. Cytokines as Biomarkers of Immune System Activation
Cytokines released by immunocompetent cells—whether they are peripheral immune cells or resident glial cells—promote the bidirectional crosstalk between the AD brain and the periphery. Despite that the actual source of circulating cytokines has yet to be completely elucidated, assessing their peripheral profile could help shed light on the evolution of the inflammatory response throughout AD progression. To date, the results on circulating cytokines in AD patients are controversial—probably on account of the different methodological approaches used for their peripheral detection.
4.1.1. Pro-Inflammatory Cytokines
IL-6, IL-1β and TNF-α can be considered among the principal mediators of the proinflammatory response in AD. In the CNS, these inflammatory mediators are released as a result of glial exposure to Aβ [43,99], while, in the periphery, they are commonly produced by monocytes/macrophages during the acute phase of the immune response [100]. Both increased [101,102,103,104] and unchanged [105,106,107] peripheral levels of those proinflammatory cytokines have been reported in AD patients compared to healthy controls. On the other hand, the concentrations of IL-6 and IL-1β were found significantly lower in MCI patients compared to AD ones [102,108], while the TNF-α concentration appeared to be disease stage-dependent, with decreased levels in patients with mild-to-moderate AD compared to those with severe AD [109]. Finally, a significant correlation was observed in AD patients between the IL-6 levels in matched CSF and blood samples [110].
4.1.2. Anti-Inflammatory Cytokines
IL-10, IL-4 and TGF-β may be regarded as the anti-inflammatory cytokines putatively able to limit proinflammatory activation in AD. Their main activities concern the suppression of macrophage activation and the reduction of proinflammatory cytokine synthesis [111,112,113,114]; moreover, while IL-10 and IL-4 inhibit Th1 lymphocytes while fostering a Th2 response—with a subsequent induction of IgG secretion by B cells [115,116], TGF-β acts as a potent suppressor of both Th1 and Th2 cells [117]. In the CNS, IL-10 acts as a modulator of glial activation; it mediates the crosstalk between the microglia and astrocytes and inhibits the excessive production of proinflammatory mediators, thereby exerting neuroprotective effects [118]. IL-4 exerts a neuroprotective effect by eliciting neuroprotective phenotypes in astrocytes and the microglia, with critical effects on higher brain functions, such as learning and memory [119,120,121]. Finally, TGF-β promotes neuroprotection by means of microglia-mediated Aβ degradation [122]. Studies evaluating the levels of IL-10 and IL-4 in the periphery of patients with AD are not consistent; however, unaltered levels—compared to healthy controls—have been reported in most studies [123,124,125]. On the other hand, AD patients showed significantly elevated TGF-β peripheral levels [126,127], with higher concentrations in patients with mild-to-moderate AD and lower concentrations in patients with severe AD [128].
4.2. Chemokines as Biomarkers for Peripheral Immune Cells Recruitment
Chemokines are chemotactic cytokines essential for the activation and migration of specific subsets of leukocytes in order to elicit targeted and specialized immune responses. In AD, the development of neuroinflammation is accompanied by a generalized upregulation of plaque-associated chemokines and chemokine receptors, leading to peripheral monocyte recruitment and glial cell activation [62,129].
4.2.1. Monocyte Chemotactic Protein 1 (MCP-1)
Monocyte chemotactic protein 1 (MCP-1)—also known as CC chemokine ligand 2 (CCL2)—was the first chemokine associated with AD and plays a pivotal role in the recruitment and accumulation of immune cells at the level of senile plaques. The inflammatory responses mediated by MCP-1 are linked to Aβ pathology, as the Aβ-induced upregulation of MCP-1 has been demonstrated in the microglia, astrocytes and human monocytes [130,131]. It is plausible that plaque-associated MCP1 production is related to an initial attempt of glial cells to eliminate Aβ deposits by means of resident and peripheral phagocyte recruitment [132,133]; however, its overexpression in the late stages of AD may induce inflammatory states with detrimental effects on the brain [134]. The clinical data of AD patients have shown an increase of CCL2 both in the CSF and the periphery compared to the healthy controls [135,136] and a strong positive correlation between the matched samples [110]. In particular, in line with the hypothesized role in the early stage of the disease, higher MCP-1 concentrations were found, especially in MCI patients [137].
Despite most chemokines contributing to AD by recruiting peripheral immune cells and promoting glial activation, emerging data hypothesize for some of them an additional anti-inflammatory and neuroprotective role.
4.2.2. CXCL8/IL-8
Interleukin 8 (IL-8)—also known as CXCL8—is a chemokine produced in response to Aβ and proinflammatory stimuli, both in the CNS and the periphery [138,139], and has been found to be increased in the blood and CSF of AD patients [140,141]. Together with its principal function of neutrophils and T-lymphocyte recruitment [142,143], CXCL8 also shows a neuroprotective effect by inhibiting Aβ-induced apoptosis and upregulating the brain-derived neurotrophic factor (BDNF) [144].
4.2.3. CX3CL1 (Fractalkine)
Soluble Fractalkine is a chemoattractant for NK cells predominantly expressed in the CNS by neurons and glial cells [145]. It appears that this chemokine is also able to exert an anti-inflammatory function by mediating the neuronal regulation of microglial activation and overproduction of inflammatory mediators, thereby preventing neurotoxicity [146]. The central and peripheral levels of Fractalkine have been reported to be significantly elevated in patients with MCI compared to patients with severe AD [147,148], supporting the idea that, during AD pathogenesis, Fractalkine may lose control over microglial activation, paving the way to neurodegeneration.
4.3. YKL-40 as Biomarker for Astrocytes (and Macrophages) Activation
YKL-40, also known as chitinase 3-like protein-1 (CHI3L1), is a secreted glycoprotein belonging to the human chitinase family, named after its three N-terminal amino acids—tyrosine (Y), lysine (K) and leucine (L)—and its molecular weight of 40 kDa [149]. YKL-40 is expressed in a wide variety of tissues, including the CNS, and is upregulated in a number of inflammatory conditions in response to the proinflammatory cytokines TNF-α and IL-1β [150,151]. In the periphery, YKL-40 is expressed principally by activated macrophages and exerts a significant impact on their alternative (M2) differentiation [152,153]. The pattern of YKL-40 expression in the CNS has long been a subject of debate; despite being initially associated with the macrophages/microglia lineage, evidence suggests that, during neuroinflammatory processes, its synthesis is enhanced predominantly in reactive astrocytes and only occasionally in the microglia [154].
In the AD brain, YKL-40 is expressed by reactive astrocytes around amyloid plaques and blood vessels with CAA [155,156]. It has been suggested that YKL-40 transcription is induced in astrocytes by proinflammatory cytokines released from macrophages, as a result of the inflammatory processes taking place in the CNS or in the periphery [151]. Despite that the precise function of YKL-40 in AD remains to be elucidated, it seems to be involved in Aβ phagocytosis and amyloid plaque formation, with a still undetermined beneficial or detrimental role [157,158].
Since the very first analysis carried out by Craig-Schapiro, several studies have consistently demonstrated increased CSF levels of YKL-40 in patients with AD compared with healthy controls [155,159]; in particular, the CSF YKL-40 level is already increased during the preclinical and MCI phases of AD [160]—a hint that immune system activation occurs early in the disease—and increases longitudinally over time along the AD continuum [161]. Furthermore, higher baseline levels of CSF YKL-40 in preclinical and MCI patients are associated with an increased risk of progression to AD [161]. Similarly, the plasma levels of YKL-40 are elevated in AD patients compared to the MCI and controls; however, mild AD patients show significantly higher levels compared to moderate/severe AD [162]. In addition, plasma YKL-40 does not seem to demonstrate a prognostic utility for cognitive decline [155].
4.4. TREM2 as Biomarker of Phagocytic Activity
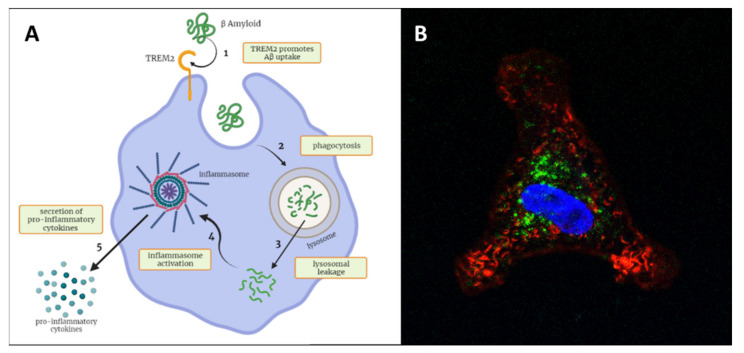
The Triggering receptor expressed on myeloid cells 2 (TREM2) is a transmembrane receptor expressed on the cell surface of myeloid cells (i.e., monocytes and macrophages)—including microglial cells in the CNS [163,164]—and involved in the regulation of phagocytosis [165]. The increased expression of TREM2 has been confirmed in peripheral monocytes of AD patients compared to controls [166] and of MCI patients progressing to AD (converter) compared to MCI nonconverters and to AD patients [167]. In the AD brain, TREM2 is expressed by amyloid-associated microglia and peripherally recruited monocytes/macrophages [168,169,170]; upon binding oligomeric Aβ [171], it mediates the myeloid cell recruitment and accumulation around plaques [169,172], as well as amyloid uptake and degradation [173,174] (Figure 2). In light of these premises, it is possible to speculate a protective role of TREM2 in the early stages of AD.
Figure 2.
(A) Mononucleate cells phagocytize beta-amyloid by TREM2 involvement (1). Due to the beta sheet-rich structure, the autophagy–lysosomal system (2) eventually results in incompetence for a full degradation and (3) leakage of the toxic peptide activates the inflammasome (4) with the secretion of proinflammatory cytokines (5), fueling downstream neuroinflammation. (B) In vitro phagocytosis assay; fluorescence micrograph showing beta-amyloid (in green) phagocytized by a THP-1 cell (acute monocytic leukemia line) (courtesy of Virginia Rodriguez-Menendez, UNIMIB, Italy). Created with BioRender.com.
Moreover, TREM2 undergoes proteolytic cleavage and is released as a soluble form (sTREM2) [175]. The sTREM2 CSF levels are significantly elevated in both MCI and AD patients compared to healthy controls [176,177] and change in a disease stage–dependent manner, peaking at the early symptomatic phase of the disease and decreasing during AD progression [178,179]. The existing studies have generally reported no significant difference in the plasma levels of sTREM2 between AD patients and healthy controls [179,180]; however, a positive correlation was observed between the CSF and plasma sTREM2 levels [177]. Accumulating evidence suggests that sTREM2 is able to exert a neuroprotective effect [181] and possesses many functional aspects resembling those of the full-length membrane-bound protein, including the specific interaction with oligomeric Aβ and subsequent reduction of amyloid deposition by means of microglial recruitment and Aβ uptake and degradation [182].
4.5. TSPO as Classic Biomarker of Neuroinflammation with a Putative Role in Peripheral Monocytes Recruitment
The translocator protein 18 kDa (TSPO) is a transmembrane protein widely expressed throughout the body originally discovered as a peripheral binding site for benzodiazepines [183]. Although TSPO is primarily located on the outer mitochondrial membrane, it has also been identified on the plasma membrane of various cell types, including glia and monocytes/macrophages [64,184,185]. In the CNS under physiological conditions, the TSPO levels are very low; however, they experience a dramatic increase in response to neuroinflammation as a result of glial activation and peripheral monocyte recruitment [186]. Its marked upregulation in a broad spectrum of neuropathological conditions has made TSPO a leading biomarker of neuroinflammation. To this end, the TSPO levels can be assessed using in vitro and in vivo imaging techniques with high selective tracers [187].
AD and MCI patients have been found to show significantly increased TSPO expression compared with age-matched healthy controls [188]. In particular, the TSPO levels seem to increase in glial cells before the appearance of AD-associated brain lesions and, better yet, predict the subsequent neurodegeneration in the same areas [189]. Furthermore, longitudinal studies suggest that TSPO levels increase over time throughout AD progression from MCI to full-blown dementia [190] and that astrocytes upregulate TSPO earlier in AD pathology compared to the microglia [191].
Despite its wide employment in imaging studies, the precise contribution of TSPO to the neuroinflammation in AD remains unclear. Of note, TSPO ligands have been shown to modulate chemotaxis in peripheral monocytes [65], and diazepam-binding inhibitor (DBI)—the endogenous ligand for TSPO—has been found upregulated in the CSF and serum of AD patients [192,193]. Intriguingly, serum DBI displayed a further increase in patients with delirium [193]. In light of the aforementioned common pathogenic mechanism (neuroinflammation) between delirium and AD, it is possible to speculate on a possible involvement of the DBI/TSPO system in peripheral monocyte recruitment in the CNS (Figure 1).
5. Conclusions
Neuroinflammation is a central feature in the development of AD—contributing to its pathogenesis just as much as Aβ plaques and NFT—and this may be especially relevant when thinking of the partial failure of anti-amyloid approaches. Apart from merely mirroring the pathogenic changes happening into the AD CNS, the peripheral immune system contributes to neuroinflammation by means of phagocytic cells and inflammatory mediators able to access the brain parenchyma through a functionally impaired BBB. According to this vision, peripheral biomarkers of neuroinflammation should be considered as true core markers of AD, accessible for implementing the diagnosis and the clinical follow-up of patients. Further studies with novel sensitive techniques (e.g., Simoa) comparing the central and peripheral compartments are therefore advisable, analogously to what has been recently made, for example, for phosphorylated tau [194].
Finally, effective disease-modifying therapies for AD are still a substantially unmet need, and focusing on the dysregulated peripheral inflammatory responses represents an interesting line of research. As a matter of fact, modulating cytokine/chemokine production, monocyte chemotaxis and neuro-invasion and Aβ phagocytosis could eventually slow down AD-related neurodegeneration. Theoretically, we may even think to target neuroinflammation right from the periphery by means of drugs that do not cross the BBB (e.g., Etanercept [195]). Last, but not least, in light of the clinical and pathogenic liaisons with the neurobehavioral syndrome of delirium, targeting peripheral inflammatory processes could also be interesting for specifically treating the behavioral symptoms of AD [196]. At the moment, clinical studies are required to clarify if drugs able to modulate these processes may eventually succeed in mitigating the clinical phenotype and delaying the conversion in prodromal AD patients.
Author Contributions
Conceptualization, F.A., E.C., C.F. and L.T.; writing—original draft preparation, F.A., E.C. and L.T. and writing—review and editing, F.A., E.C., L.T., C.P.Z., F.D.R., I.A. and C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Education, University and Research (MIUR), grant: PRIN 2017PFYK27 (AGAINST-AD) (L.T.). The APC was funded by the University of Milano-Bicocca, grant: 2020-ATE-0018 (L.T.).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hebert L.E., Bienias J.L., Aggarwal N.T., Wilson R.S., Bennett D.A., Shah R.C., Evans D.A. Change in risk of Alzheimer disease over time. Neurology. 2010;75:786–791. doi: 10.1212/WNL.0b013e3181f0754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong R.A. What causes alzheimer’s disease? Folia Neuropathol. 2013;51:169–188. doi: 10.5114/fn.2013.37702. [DOI] [PubMed] [Google Scholar]
- 3.Carter J., Lippa C.F. Beta-amyloid, neuronal death and Alzheimer’s disease. Curr. Mol. Med. 2001;1:733–737. doi: 10.2174/1566524013363177. [DOI] [PubMed] [Google Scholar]
- 4.Zhang B., Gaiteri C., Bodea L.G., Wang Z., McElwee J., Podtelezhnikov A.A., Zhang C., Xie T., Tran L., Dobrin R., et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153:707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilimann I., Grothe M., Heinsen H., Alho E.J., Grinberg L., Amaro E., Dos Santos G.A., da Silva R.E., Mitchell A.J., Frisoni G.B., et al. Subregional basal forebrain atrophy in Alzheimer’s disease: A multicenter study. J. Alzheimers Dis. 2014;40:687–700. doi: 10.3233/JAD-132345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selkoe D.J. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 8.Arranz A.M., De Strooper B. The role of astroglia in Alzheimer’s disease: Pathophysiology and clinical implications. Lancet Neurol. 2019;18:406–414. doi: 10.1016/S1474-4422(18)30490-3. [DOI] [PubMed] [Google Scholar]
- 9.Klupp E., Grimmer T., Tahmasian M., Sorg C., Yakushev I., Yousefi B.H., Drzezga A., Förster S. Prefrontal hypometabolism in Alzheimer disease is related to longitudinal amyloid accumulation in remote brain regions. J. Nucl. Med. 2015;56:399–404. doi: 10.2967/jnumed.114.149302. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki Y., Kanekiyo T. Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2017;18:1965. doi: 10.3390/ijms18091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tönnies E., Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017;57:1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alzheimer A., Stelzmann R.A., Schnitzlein H.N., Murtagh F.R. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin. Anat. 1995;8:429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 13.Hyman B.T., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Carrillo M.C., Dickson D.W., Duyckaerts C., Frosch M.P., Masliah E., et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubois B., Villain N., Frisoni G.B., Rabinovici G.D., Sabbagh M., Cappa S., Bejanin A., Bombois S., Epelbaum S., Teichmann M., et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021;20:484–496. doi: 10.1016/S1474-4422(21)00066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris J.C., Blennow K., Froelich L., Nordberg A., Soininen H., Waldemar G., Wahlund L.O., Dubois B. Harmonized diagnostic criteria for Alzheimer’s disease: Recommendations. J. Intern. Med. 2014;275:204–213. doi: 10.1111/joim.12199. [DOI] [PubMed] [Google Scholar]
- 18.Galimberti D., Scarpini E. Treatment of Alzheimer’s disease: Symptomatic and disease-modifying approaches. Curr. Aging Sci. 2010;3:46–56. doi: 10.2174/1874609811003010046. [DOI] [PubMed] [Google Scholar]
- 19.FDA-NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet] Food and Drug Administration; Silve Spring, MD, USA: 2016. [PubMed] [Google Scholar]
- 20.The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group Consensus report of the Working Group on: “Molecular and Biochemical Markers of Alzheimer’s Disease”. Neurobiol. Aging. 1998;19:109–116. [PubMed] [Google Scholar]
- 21.Brinker T., Stopa E., Morrison J., Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10. doi: 10.1186/2045-8118-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkins J.M., Trushina E. Application of Metabolomics in Alzheimer’s Disease. Front. Neurol. 2017;8:719. doi: 10.3389/fneur.2017.00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira D., Perestelo-Pérez L., Westman E., Wahlund L.O., Sarría A., Serrano-Aguilar P. Meta-Review of CSF Core Biomarkers in Alzheimer’s Disease: The State-of-the-Art after the New Revised Diagnostic Criteria. Front. Aging Neurosci. 2014;6:47. doi: 10.3389/fnagi.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampel H., O’Bryant S.E., Molinuevo J.L., Zetterberg H., Masters C.L., Lista S., Kiddle S.J., Batrla R., Blennow K. Blood-based biomarkers for Alzheimer disease: Mapping the road to the clinic. Nat. Rev. Neurol. 2018;14:639–652. doi: 10.1038/s41582-018-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zipser B.D., Johanson C.E., Gonzalez L., Berzin T.M., Tavares R., Hulette C.M., Vitek M.P., Hovanesian V., Stopa E.G. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Thambisetty M., Lovestone S. Blood-based biomarkers of Alzheimer’s disease: Challenging but feasible. Biomark. Med. 2010;4:65–79. doi: 10.2217/bmm.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galasko D., Golde T.E. Biomarkers for Alzheimer’s disease in plasma, serum and blood—Conceptual and practical problems. Alzheimers Res. Ther. 2013;5:10. doi: 10.1186/alzrt164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serrano-Pozo A., Mielke M.L., Gómez-Isla T., Betensky R.A., Growdon J.H., Frosch M.P., Hyman B.T. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am. J. Pathol. 2011;179:1373–1384. doi: 10.1016/j.ajpath.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 30.Bamberger M.E., Harris M.E., McDonald D.R., Husemann J., Landreth G.E. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J. Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michelucci A., Heurtaux T., Grandbarbe L., Morga E., Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J. Neuroimmunol. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Czeh M., Gressens P., Kaindl A.M. The yin and yang of microglia. Dev. Neurosci. 2011;33:199–209. doi: 10.1159/000328989. [DOI] [PubMed] [Google Scholar]
- 33.Bolmont T., Haiss F., Eicke D., Radde R., Mathis C.A., Klunk W.E., Kohsaka S., Jucker M., Calhoun M.E. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J. Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hickman S.E., Allison E.K., El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng J.G., Zhou X.Q., Mrak R.E., Griffin W.S. Progressive neuronal injury associated with amyloid plaque formation in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1998;57:714–717. doi: 10.1097/00005072-199807000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank-Cannon T.C., Alto L.T., McAlpine F.E., Tansey M.G. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol. Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sofroniew M.V., Vinters H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 39.Wisniewski H.M., Wegiel J. Spatial relationships between astrocytes and classical plaque components. Neurobiol. Aging. 1991;12:593–600. doi: 10.1016/0197-4580(91)90091-W. [DOI] [PubMed] [Google Scholar]
- 40.Orre M., Kamphuis W., Osborn L.M., Jansen A.H.P., Kooijman L., Bossers K., Hol E.M. Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiol. Aging. 2014;35:2746–2760. doi: 10.1016/j.neurobiolaging.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Rolyan H., Feike A.C., Upadhaya A.R., Waha A., Van Dooren T., Haass C., Birkenmeier G., Pietrzik C.U., Van Leuven F., Thal D.R. Amyloid-β protein modulates the perivascular clearance of neuronal apolipoprotein E in mouse models of Alzheimer’s disease. J. Neural. Transm. 2011;118:699–712. doi: 10.1007/s00702-010-0572-7. [DOI] [PubMed] [Google Scholar]
- 42.Wyss-Coray T., Loike J.D., Brionne T.C., Lu E., Anankov R., Yan F., Silverstein S.C., Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat. Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 43.Hu J., Akama K.T., Krafft G.A., Chromy B.A., Van Eldik L.J. Amyloid-beta peptide activates cultured astrocytes: Morphological alterations, cytokine induction and nitric oxide release. Brain Res. 1998;785:195–206. doi: 10.1016/S0006-8993(97)01318-8. [DOI] [PubMed] [Google Scholar]
- 44.Von Bernhardi R., Eugenín J. Microglial reactivity to beta-amyloid is modulated by astrocytes and proinflammatory factors. Brain Res. 2004;1025:186–193. doi: 10.1016/j.brainres.2004.07.084. [DOI] [PubMed] [Google Scholar]
- 45.Yang J., Lunde L.K., Nuntagij P., Oguchi T., Camassa L.M., Nilsson L.N., Lannfelt L., Xu Y., Amiry-Moghaddam M., Ottersen O.P., et al. Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2011;27:711–722. doi: 10.3233/JAD-2011-110725. [DOI] [PubMed] [Google Scholar]
- 46.Iram T., Trudler D., Kain D., Kanner S., Galron R., Vassar R., Barzilai A., Blinder P., Fishelson Z., Frenkel D. Astrocytes from old Alzheimer’s disease mice are impaired in Aβ uptake and in neuroprotection. Neurobiol. Dis. 2016;96:84–94. doi: 10.1016/j.nbd.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Zenaro E., Piacentino G., Constantin G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2017;107:41–56. doi: 10.1016/j.nbd.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Procter T.V., Williams A., Montagne A. Interplay between brain pericytes and endothelial cells in dementia. Am. J. Pathol. 2021 doi: 10.1016/j.ajpath.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Anwar M.M., Özkan E., Gürsoy-Özdemir Y. The role of extracellular matrix alterations in mediating astrocyte damage and pericyte dysfunction in Alzheimer’s disease: A comprehensive review. Eur. J. Neurosci. 2021 doi: 10.1111/ejn.15372. [DOI] [PubMed] [Google Scholar]
- 50.Sengillo J.D., Winkler E.A., Walker C.T., Sullivan J.S., Johnson M., Zlokovic B.V. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z., Toga A.W., Jacobs R.E., Liu C.Y., Amezcua L., et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montagne A., Nation D.A., Sagare A.P., Barisano G., Sweeney M.D., Chakhoyan A., Pachicano M., Joe E., Nelson A.R., D’Orazio L.M., et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71–76. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai Z., Xiao M. Oligodendrocytes and Alzheimer’s disease. Int. J. Neurosci. 2016;126:97–104. doi: 10.3109/00207454.2015.1025778. [DOI] [PubMed] [Google Scholar]
- 54.Nihonmatsu-Kikuchi N., Yu X.J., Matsuda Y., Ozawa N., Ito T., Satou K., Kaname T., Iwasaki Y., Akagi A., Yoshida M., et al. Essential roles of plexin-B3. Commun. Biol. 2021;4:870. doi: 10.1038/s42003-021-02404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daneman R., Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong D., Dorovini-Zis K., Vincent S.R. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp. Neurol. 2004;190:446–455. doi: 10.1016/j.expneurol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Van Wetering S., van Buul J.D., Quik S., Mul F.P., Anthony E.C., ten Klooster J.P., Collard J.G., Hordijk P.L. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J. Cell Sci. 2002;115:1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 58.Gurney K.J., Estrada E.Y., Rosenberg G.A. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol. Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Vinters H.V. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.STR.18.2.311. [DOI] [PubMed] [Google Scholar]
- 60.Fiala M., Zhang L., Gan X., Sherry B., Taub D., Graves M.C., Hama S., Way D., Weinand M., Witte M., et al. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood–brain barrier model. Mol. Med. 1998;4:480–489. doi: 10.1007/BF03401753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krstic D., Madhusudan A., Doehner J., Vogel P., Notter T., Imhof C., Manalastas A., Hilfiker M., Pfister S., Schwerdel C., et al. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J. Neuroinflamm. 2012;9:151. doi: 10.1186/1742-2094-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishizuka K., Kimura T., Igata-yi R., Katsuragi S., Takamatsu J., Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer’s disease. Psychiatry Clin. Neurosci. 1997;51:135–138. doi: 10.1111/j.1440-1819.1997.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 63.Simard A.R., Soulet D., Gowing G., Julien J.P., Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 64.Ferrarese C., Appollonio I., Frigo M., Perego M., Pierpaoli C., Trabucchi M., Frattola L. Characterization of peripheral benzodiazepine receptors in human blood mononuclear cells. Neuropharmacology. 1990;29:375–378. doi: 10.1016/0028-3908(90)90097-B. [DOI] [PubMed] [Google Scholar]
- 65.Sacerdote P., Locatelli L.D., Panerai A.E. Benzodiazepine induced chemotaxis of human monocytes: A tool for the study of benzodiazepine receptors. Life Sci. 1993;53:653–658. doi: 10.1016/0024-3205(93)90275-8. [DOI] [PubMed] [Google Scholar]
- 66.Stalder A.K., Ermini F., Bondolfi L., Krenger W., Burbach G.J., Deller T., Coomaraswamy J., Staufenbiel M., Landmann R., Jucker M. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J. Neurosci. 2005;25:11125–11132. doi: 10.1523/JNEUROSCI.2545-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michaud J.P., Bellavance M.A., Préfontaine P., Rivest S. Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep. 2013;5:646–653. doi: 10.1016/j.celrep.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 68.Fiala M., Lin J., Ringman J., Kermani-Arab V., Tsao G., Patel A., Lossinsky A.S., Graves M.C., Gustavson A., Sayre J., et al. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2005;7:221–232. doi: 10.3233/JAD-2005-7304. discussion 255–262. [DOI] [PubMed] [Google Scholar]
- 69.Marsh S.E., Abud E.M., Lakatos A., Karimzadeh A., Yeung S.T., Davtyan H., Fote G.M., Lau L., Weinger J.G., Lane T.E., et al. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc. Natl. Acad. Sci. USA. 2016;113:E1316–E1325. doi: 10.1073/pnas.1525466113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Togo T., Akiyama H., Iseki E., Kondo H., Ikeda K., Kato M., Oda T., Tsuchiya K., Kosaka K. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 2002;124:83–92. doi: 10.1016/S0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 71.Lueg G., Gross C.C., Lohmann H., Johnen A., Kemmling A., Deppe M., Groger J., Minnerup J., Wiendl H., Meuth S.G., et al. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer’s disease. Neurobiol. Aging. 2015;36:81–89. doi: 10.1016/j.neurobiolaging.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 72.Söllvander S., Ekholm-Pettersson F., Brundin R.M., Westman G., Kilander L., Paulie S., Lannfelt L., Sehlin D. Increased Number of Plasma B Cells Producing Autoantibodies Against Aβ42 Protofibrils in Alzheimer’s Disease. J. Alzheimers Dis. 2015;48:63–72. doi: 10.3233/JAD-150236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Town T., Tan J., Flavell R.A., Mullan M. T-cells in Alzheimer’s disease. Neuromol. Med. 2005;7:255–264. doi: 10.1385/NMM:7:3:255. [DOI] [PubMed] [Google Scholar]
- 74.Lue L.F., Walker D.G. Modeling Alzheimer’s disease immune therapy mechanisms: Interactions of human postmortem microglia with antibody-opsonized amyloid beta peptide. J. Neurosci. Res. 2002;70:599–610. doi: 10.1002/jnr.10422. [DOI] [PubMed] [Google Scholar]
- 75.Deane R., Sagare A., Hamm K., Parisi M., LaRue B., Guo H., Wu Z., Holtzman D.M., Zlokovic B.V. IgG-assisted age-dependent clearance of Alzheimer’s amyloid beta peptide by the blood-brain barrier neonatal Fc receptor. J. Neurosci. 2005;25:11495–11503. doi: 10.1523/JNEUROSCI.3697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeMattos R.B., Bales K.R., Cummins D.J., Dodart J.C., Paul S.M., Holtzman D.M. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lafaye P., Achour I., England P., Duyckaerts C., Rougeon F. Single-domain antibodies recognize selectively small oligomeric forms of amyloid beta, prevent Abeta-induced neurotoxicity and inhibit fibril formation. Mol. Immunol. 2009;46:695–704. doi: 10.1016/j.molimm.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Conti E., Tremolizzo L., Santarone M.E., Tironi M., Radice I., Zoia C.P., Aliprandi A., Salmaggi A., Dominici R., Casati M., et al. Donepezil modulates the endogenous immune response: Implications for Alzheimer’s disease. Hum. Psychopharmacol. 2016;31:296–303. doi: 10.1002/hup.2538. [DOI] [PubMed] [Google Scholar]
- 79.Banks W.A., Kastin A.J., Broadwell R.D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 80.Watkins L.R., Goehler L.E., Relton J.K., Tartaglia N., Silbert L., Martin D., Maier S.F. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: Evidence for vagal mediation of immune-brain communication. Neurosci. Lett. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-R. [DOI] [PubMed] [Google Scholar]
- 81.Hoogland I.C., Houbolt C., van Westerloo D.J., van Gool W.A., van de Beek D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scassellati C., Galoforo A.C., Esposito C., Ciani M., Ricevuti G., Bonvicini C. Promising Intervention Approaches to Potentially Resolve Neuroinflammation And Steroid Hormones Alterations in Alzheimer’s Disease and Its Neuropsychiatric Symptoms. Aging Dis. 2021;12:1337–1357. doi: 10.14336/AD.2021.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma V.K., Singh T.G., Garg N., Dhiman S., Gupta S., Rahman M.H., Najda A., Walasek-Janusz M., Kamel M., Albadrani G.M., et al. Dysbiosis and Alzheimer’s Disease: A Role for Chronic Stress? Biomolecules. 2021;11:678. doi: 10.3390/biom11050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Olst L., Roks S.J.M., Kamermans A., Verhaar B.J.H., van der Geest A.M., Muller M., van der Flier W.M., de Vries H.E. Contribution of Gut Microbiota to Immunological Changes in Alzheimer’s Disease. Front. Immunol. 2021;12:683068. doi: 10.3389/fimmu.2021.683068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim M.S., Kim Y., Choi H., Kim W., Park S., Lee D., Kim D.K., Kim H.J., Hyun D.W., Lee J.Y., et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut. 2020;69:283–294. doi: 10.1136/gutjnl-2018-317431. [DOI] [PubMed] [Google Scholar]
- 87.Franceschi C., Capri M., Monti D., Giunta S., Olivieri F., Sevini F., Panourgia M.P., Invidia L., Celani L., Scurti M., et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 88.Norden D.M., Godbout J.P. Review: Microglia of the aged brain: Primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nordengen K., Kirsebom B.E., Henjum K., Selnes P., Gísladóttir B., Wettergreen M., Torsetnes S.B., Grøntvedt G.R., Waterloo K.K., Aarsland D., et al. Glial activation and inflammation along the Alzheimer’s disease continuum. J. Neuroinflamm. 2019;16:46. doi: 10.1186/s12974-019-1399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Konsman J.P., Parnet P., Dantzer R. Cytokine-induced sickness behaviour: Mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/S0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 91.Maldonado J.R. Acute Brain Failure: Pathophysiology, Diagnosis, Management, and Sequelae of Delirium. Crit. Care Clin. 2017;33:461–519. doi: 10.1016/j.ccc.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 92.Murray C., Sanderson D.J., Barkus C., Deacon R.M., Rawlins J.N., Bannerman D.M., Cunningham C. Systemic inflammation induces acute working memory deficits in the primed brain: Relevance for delirium. Neurobiol. Aging. 2012;33:603.e3–616.e3. doi: 10.1016/j.neurobiolaging.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elie M., Cole M.G., Primeau F.J., Bellavance F. Delirium risk factors in elderly hospitalized patients. J. Gen. Intern. Med. 1998;13:204–212. doi: 10.1046/j.1525-1497.1998.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bugiani O. Why is delirium more frequent in the elderly? Neurol. Sci. 2021;42:3491–3503. doi: 10.1007/s10072-021-05339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fong T.G., Jones R.N., Shi P., Marcantonio E.R., Yap L., Rudolph J.L., Yang F.M., Kiely D.K., Inouye S.K. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eikelenboom P., Hoogendijk W.J. Do delirium and Alzheimer’s dementia share specific pathogenetic mechanisms? Dement. Geriatr. Cogn. Disord. 1999;10:319–324. doi: 10.1159/000017162. [DOI] [PubMed] [Google Scholar]
- 97.Tracey K.J. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 98.Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Yang H., Ulloa L., Al-Abed Y., et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 99.Patel N.S., Paris D., Mathura V., Quadros A.N., Crawford F.C., Mullan M.J. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer’s disease. J. Neuroinflamm. 2005;2:9. doi: 10.1186/1742-2094-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arango Duque G., Descoteaux A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu Y.Y., Hsu J.L., Wang H.C., Wu S.J., Hong C.J., Cheng I.H. Alterations of the Neuroinflammatory Markers IL-6 and TRAIL in Alzheimer’s Disease. Dement. Geriatr. Cogn. Dis. Extra. 2015;5:424–434. doi: 10.1159/000439214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Forlenza O.V., Diniz B.S., Talib L.L., Mendonça V.A., Ojopi E.B., Gattaz W.F., Teixeira A.L. Increased serum IL-1beta level in Alzheimer’s disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2009;28:507–512. doi: 10.1159/000255051. [DOI] [PubMed] [Google Scholar]
- 103.Fillit H., Ding W.H., Buee L., Kalman J., Altstiel L., Lawlor B., Wolf-Klein G. Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci. Lett. 1991;129:318–320. doi: 10.1016/0304-3940(91)90490-K. [DOI] [PubMed] [Google Scholar]
- 104.Alvarez A., Cacabelos R., Sanpedro C., García-Fantini M., Aleixandre M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol. Aging. 2007;28:533–536. doi: 10.1016/j.neurobiolaging.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 105.Van Duijn C.M., Hofman A., Nagelkerken L. Serum levels of interleukin-6 are not elevated in patients with Alzheimer’s disease. Neurosci. Lett. 1990;108:350–354. doi: 10.1016/0304-3940(90)90666-W. [DOI] [PubMed] [Google Scholar]
- 106.Pirttila T., Mehta P.D., Frey H., Wisniewski H.M. Alpha 1-antichymotrypsin and IL-1 beta are not increased in CSF or serum in Alzheimer’s disease. Neurobiol. Aging. 1994;15:313–317. doi: 10.1016/0197-4580(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 107.Yasutake C., Kuroda K., Yanagawa T., Okamura T., Yoneda H. Serum BDNF, TNF-alpha and IL-1beta levels in dementia patients: Comparison between Alzheimer’s disease and vascular dementia. Eur. Arch. Psychiatry Clin. Neurosci. 2006;256:402–406. doi: 10.1007/s00406-006-0652-8. [DOI] [PubMed] [Google Scholar]
- 108.Bermejo P., Martín-Aragón S., Benedí J., Susín C., Felici E., Gil P., Ribera J.M., Villar A.M. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer’s disease. Immunol. Lett. 2008;117:198–202. doi: 10.1016/j.imlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 109.Paganelli R., Di Iorio A., Patricelli L., Ripani F., Sparvieri E., Faricelli R., Iarlori C., Porreca E., Di Gioacchino M., Abate G. Proinflammatory cytokines in sera of elderly patients with dementia: Levels in vascular injury are higher than those of mild-moderate Alzheimer’s disease patients. Exp. Gerontol. 2002;37:257–263. doi: 10.1016/S0531-5565(01)00191-7. [DOI] [PubMed] [Google Scholar]
- 110.Sun Y.X., Minthon L., Wallmark A., Warkentin S., Blennow K., Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2003;16:136–144. doi: 10.1159/000071001. [DOI] [PubMed] [Google Scholar]
- 111.Fiorentino D.F., Zlotnik A., Mosmann T.R., Howard M., O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 112.Stein M., Keshav S., Harris N., Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Te Velde A.A., Huijbens R.J., Heije K., de Vries J.E., Figdor C.G. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990;76:1392–1397. doi: 10.1182/blood.V76.7.1392.1392. [DOI] [PubMed] [Google Scholar]
- 114.Gong D., Shi W., Yi S.J., Chen H., Groffen J., Heisterkamp N. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13:31. doi: 10.1186/1471-2172-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D.H., Kastelein R., Moore K.W., Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hsieh C.S., Heimberger A.B., Gold J.S., O’Garra A., Murphy K.M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc. Natl. Acad. Sci. USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Defrance T., Vanbervliet B., Brière F., Durand I., Rousset F., Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J. Exp. Med. 1992;175:671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ledeboer A., Brevé J.J., Poole S., Tilders F.J., Van Dam A.M. Interleukin-10, interleukin-4, and transforming growth factor-beta differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia. 2000;30:134–142. doi: 10.1002/(SICI)1098-1136(200004)30:2<134::AID-GLIA3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 119.Garg S.K., Kipnis J., Banerjee R. IFN-gamma and IL-4 differentially shape metabolic responses and neuroprotective phenotype of astrocytes. J. Neurochem. 2009;108:1155–1166. doi: 10.1111/j.1471-4159.2009.05872.x. [DOI] [PubMed] [Google Scholar]
- 120.Shimizu E., Kawahara K., Kajizono M., Sawada M., Nakayama H. IL-4-induced selective clearance of oligomeric beta-amyloid peptide(1-42) by rat primary type 2 microglia. J. Immunol. 2008;181:6503–6513. doi: 10.4049/jimmunol.181.9.6503. [DOI] [PubMed] [Google Scholar]
- 121.Derecki N.C., Cardani A.N., Yang C.H., Quinnies K.M., Crihfield A., Lynch K.R., Kipnis J. Regulation of learning and memory by meningeal immunity: A key role for IL-4. J. Exp. Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wyss-Coray T., Lin C., Yan F., Yu G.Q., Rohde M., McConlogue L., Masliah E., Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat. Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 123.Bonotis K., Krikki E., Holeva V., Aggouridaki C., Costa V., Baloyannis S. Systemic immune aberrations in Alzheimer’s disease patients. J. Neuroimmunol. 2008;193:183–187. doi: 10.1016/j.jneuroim.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 124.Swardfager W., Lanctôt K., Rothenburg L., Wong A., Cappell J., Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 125.Choi C., Jeong J.H., Jang J.S., Choi K., Lee J., Kwon J., Choi K.G., Lee J.S., Kang S.W. Multiplex analysis of cytokines in the serum and cerebrospinal fluid of patients with Alzheimer’s disease by color-coded bead technology. J. Clin. Neurol. 2008;4:84–88. doi: 10.3988/jcn.2008.4.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Malaguarnera L., Motta M., Di Rosa M., Anzaldi M., Malaguarnera M. Interleukin-18 and transforming growth factor-beta 1 plasma levels in Alzheimer’s disease and vascular dementia. Neuropathology. 2006;26:307–312. doi: 10.1111/j.1440-1789.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 127.Anuradha U., Kumar A., Singh R.K. The clinical correlation of proinflammatory and anti-inflammatory biomarkers with Alzheimer disease: A meta-analysis. Neurol. Sci. 2021 doi: 10.1007/s10072-021-05343-7. in press. [DOI] [PubMed] [Google Scholar]
- 128.Motta M., Imbesi R., Di Rosa M., Stivala F., Malaguarnera L. Altered plasma cytokine levels in Alzheimer’s disease: Correlation with the disease progression. Immunol. Lett. 2007;114:46–51. doi: 10.1016/j.imlet.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 129.Xia M., Qin S., McNamara M., Mackay C., Hyman B.T. Interleukin-8 receptor B immunoreactivity in brain and neuritic plaques of Alzheimer’s disease. Am. J. Pathol. 1997;150:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- 130.Meda L., Bernasconi S., Bonaiuto C., Sozzani S., Zhou D., Otvos L., Mantovani A., Rossi F., Cassatella M.A. Beta-amyloid (25-35) peptide and IFN-gamma synergistically induce the production of the chemotactic cytokine MCP-1/JE in monocytes and microglial cells. J. Immunol. 1996;157:1213–1218. [PubMed] [Google Scholar]
- 131.Johnstone M., Gearing A.J., Miller K.M. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J. Neuroimmunol. 1999;93:182–193. doi: 10.1016/S0165-5728(98)00226-4. [DOI] [PubMed] [Google Scholar]
- 132.Peterson P.K., Hu S., Salak-Johnson J., Molitor T.W., Chao C.C. Differential production of and migratory response to beta chemokines by human microglia and astrocytes. J. Infect. Dis. 1997;175:478–481. doi: 10.1093/infdis/175.2.478. [DOI] [PubMed] [Google Scholar]
- 133.Kiyota T., Gendelman H.E., Weir R.A., Higgins E.E., Zhang G., Jain M. CCL2 affects β-amyloidosis and progressive neurocognitive dysfunction in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2013;34:1060–1068. doi: 10.1016/j.neurobiolaging.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sokolova A., Hill M.D., Rahimi F., Warden L.A., Halliday G.M., Shepherd C.E. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer’s disease. Brain Pathol. 2009;19:392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim S.M., Song J., Kim S., Han C., Park M.H., Koh Y., Jo S.A., Kim Y.Y. Identification of peripheral inflammatory markers between normal control and Alzheimer’s disease. BMC Neurol. 2011;11:51. doi: 10.1186/1471-2377-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Corrêa J.D., Starling D., Teixeira A.L., Caramelli P., Silva T.A. Chemokines in CSF of Alzheimer’s disease patients. Arq. Neuropsiquiatr. 2011;69:455–459. doi: 10.1590/S0004-282X2011000400009. [DOI] [PubMed] [Google Scholar]
- 137.Galimberti D., Fenoglio C., Lovati C., Venturelli E., Guidi I., Corrà B., Scalabrini D., Clerici F., Mariani C., Bresolin N., et al. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol. Aging. 2006;27:1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 138.Ehrlich L.C., Hu S., Sheng W.S., Sutton R.L., Rockswold G.L., Peterson P.K., Chao C.C. Cytokine regulation of human microglial cell IL-8 production. J. Immunol. 1998;160:1944–1948. [PubMed] [Google Scholar]
- 139.Meda L., Bonaiuto C., Szendrei G.I., Ceska M., Rossi F., Cassatella M.A. beta-Amyloid(25-35) induces the production of interleukin-8 from human monocytes. J. Neuroimmunol. 1995;59:29–33. doi: 10.1016/0165-5728(95)00021-S. [DOI] [PubMed] [Google Scholar]
- 140.Alsadany M.A., Shehata H.H., Mohamad M.I., Mahfouz R.G. Histone deacetylases enzyme, copper, and IL-8 levels in patients with Alzheimer’s disease. Am. J. Alzheimers Dis. Other Dement. 2013;28:54–61. doi: 10.1177/1533317512467680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Galimberti D., Schoonenboom N., Scheltens P., Fenoglio C., Bouwman F., Venturelli E., Guidi I., Blankenstein M.A., Bresolin N., Scarpini E. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch. Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- 142.Detmers P.A., Lo S.K., Olsen-Egbert E., Walz A., Baggiolini M., Cohn Z.A. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J. Exp. Med. 1990;171:1155–1162. doi: 10.1084/jem.171.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Y.J., Guo D.W., Tian L., Shang D.S., Zhao W.D., Li B., Fang W.G., Zhu L., Chen Y.H. Peripheral T cells derived from Alzheimer’s disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-alpha-dependent. Neurobiol. Aging. 2010;31:175–188. doi: 10.1016/j.neurobiolaging.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 144.Ashutosh, Kou W., Cotter R., Borgmann K., Wu L., Persidsky R., Sakhuja N., Ghorpade A. CXCL8 protects human neurons from amyloid-β-induced neurotoxicity: Relevance to Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2011;412:565–571. doi: 10.1016/j.bbrc.2011.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang D., Shi F.D., Jung S., Pien G.C., Wang J., Salazar-Mather T.P., He T.T., Weaver J.T., Ljunggren H.G., Biron C.A., et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- 146.Zujovic V., Benavides J., Vigé X., Carter C., Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. doi: 10.1002/(SICI)1098-1136(20000215)29:4<305::AID-GLIA2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 147.Perea J.R., Lleó A., Alcolea D., Fortea J., Ávila J., Bolós M. Decreased CX3CL1 Levels in the Cerebrospinal Fluid of Patients With Alzheimer’s Disease. Front. Neurosci. 2018;12:609. doi: 10.3389/fnins.2018.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim T.S., Lim H.K., Lee J.Y., Kim D.J., Park S., Lee C., Lee C.U. Changes in the levels of plasma soluble fractalkine in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 2008;436:196–200. doi: 10.1016/j.neulet.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 149.Rejman J.J., Hurley W.L. Isolation and characterization of a novel 39 kilodalton whey protein from bovine mammary secretions collected during the nonlactating period. Biochem. Biophys. Res. Commun. 1988;150:329–334. doi: 10.1016/0006-291X(88)90524-4. [DOI] [PubMed] [Google Scholar]
- 150.Yeo I.J., Lee C.K., Han S.B., Yun J., Hong J.T. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol. Ther. 2019;203:107394. doi: 10.1016/j.pharmthera.2019.107394. [DOI] [PubMed] [Google Scholar]
- 151.Bonneh-Barkay D., Bissel S.J., Kofler J., Starkey A., Wang G., Wiley C.A. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol. 2012;22:530–546. doi: 10.1111/j.1750-3639.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rehli M., Niller H.H., Ammon C., Langmann S., Schwarzfischer L., Andreesen R., Krause S.W. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J. Biol. Chem. 2003;278:44058–44067. doi: 10.1074/jbc.M306792200. [DOI] [PubMed] [Google Scholar]
- 153.Xu N., Bo Q., Shao R., Liang J., Zhai Y., Yang S., Wang F., Sun X. Chitinase-3-Like-1 Promotes M2 Macrophage Differentiation and Induces Choroidal Neovascularization in Neovascular Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci. 2019;60:4596–4605. doi: 10.1167/iovs.19-27493. [DOI] [PubMed] [Google Scholar]
- 154.Bonneh-Barkay D., Wang G., Starkey A., Hamilton R.L., Wiley C.A. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J. Neuroinflamm. 2010;7:34. doi: 10.1186/1742-2094-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Craig-Schapiro R., Perrin R.J., Roe C.M., Xiong C., Carter D., Cairns N.J., Mintun M.A., Peskind E.R., Li G., Galasko D.R., et al. YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol. Psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Llorens F., Thüne K., Tahir W., Kanata E., Diaz-Lucena D., Xanthopoulos K., Kovatsi E., Pleschka C., Garcia-Esparcia P., Schmitz M., et al. YKL-40 in the brain and cerebrospinal fluid of neurodegenerative dementias. Mol. Neurodegener. 2017;12:83. doi: 10.1186/s13024-017-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Choi J.Y., Yeo I.J., Kim K.C., Choi W.R., Jung J.K., Han S.B., Hong J.T. K284-6111 prevents the amyloid beta-induced neuroinflammation and impairment of recognition memory through inhibition of NF-κB-mediated CHI3L1 expression. J. Neuroinflamm. 2018;15:224. doi: 10.1186/s12974-018-1269-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 158.Lananna B.V., McKee C.A., King M.W., Del-Aguila J.L., Dimitry J.M., Farias F.H.G., Nadarajah C.J., Xiong D.D., Guo C., Cammack A.J., et al. Chi3l1 /YKL-40 is controlled by the astrocyte circadian clock and regulates neuroinflammation and Alzheimer’s disease pathogenesis. Sci. Transl. Med. 2020;12:eaax3519. doi: 10.1126/scitranslmed.aax3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shen X.N., Niu L.D., Wang Y.J., Cao X.P., Liu Q., Tan L., Zhang C., Yu J.T. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatry. 2019;90:590–598. doi: 10.1136/jnnp-2018-319148. [DOI] [PubMed] [Google Scholar]
- 160.Antonell A., Mansilla A., Rami L., Lladó A., Iranzo A., Olives J., Balasa M., Sánchez-Valle R., Molinuevo J.L. Cerebrospinal fluid level of YKL-40 protein in preclinical and prodromal Alzheimer’s disease. J. Alzheimers Dis. 2014;42:901–908. doi: 10.3233/JAD-140624. [DOI] [PubMed] [Google Scholar]
- 161.Kester M.I., Teunissen C.E., Sutphen C., Herries E.M., Ladenson J.H., Xiong C., Scheltens P., van der Flier W.M., Morris J.C., Holtzman D.M., et al. Cerebrospinal fluid VILIP-1 and YKL-40, candidate biomarkers to diagnose, predict and monitor Alzheimer’s disease in a memory clinic cohort. Alzheimers Res. Ther. 2015;7:59. doi: 10.1186/s13195-015-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Choi J., Lee H.W., Suk K. Plasma level of chitinase 3-like 1 protein increases in patients with early Alzheimer’s disease. J. Neurol. 2011;258:2181–2185. doi: 10.1007/s00415-011-6087-9. [DOI] [PubMed] [Google Scholar]
- 163.Daws M.R., Lanier L.L., Seaman W.E., Ryan J.C. Cloning and characterization of a novel mouse myeloid DAP12-associated receptor family. Eur. J. Immunol. 2001;31:783–791. doi: 10.1002/1521-4141(200103)31:3<783::AID-IMMU783>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 164.Schmid C.D., Sautkulis L.N., Danielson P.E., Cooper J., Hasel K.W., Hilbush B.S., Sutcliffe J.G., Carson M.J. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J. Neurochem. 2002;83:1309–1320. doi: 10.1046/j.1471-4159.2002.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Takahashi K., Rochford C.D., Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J. Exp. Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hu N., Tan M.S., Yu J.T., Sun L., Tan L., Wang Y.L., Jiang T. Increased expression of TREM2 in peripheral blood of Alzheimer’s disease patients. J. Alzheimers Dis. 2014;38:497–501. doi: 10.3233/JAD-130854. [DOI] [PubMed] [Google Scholar]
- 167.Casati M., Ferri E., Gussago C., Mazzola P., Abbate C., Bellelli G., Mari D., Cesari M., Arosio B. Increased expression of TREM2 in peripheral cells from mild cognitive impairment patients who progress into Alzheimer’s disease. Eur. J. Neurol. 2018;25:805–810. doi: 10.1111/ene.13583. [DOI] [PubMed] [Google Scholar]
- 168.Fahrenhold M., Rakic S., Classey J., Brayne C., Ince P.G., Nicoll J.A.R., Boche D., MRC-CFAS TREM2 expression in the human brain: A marker of monocyte recruitment? Brain Pathol. 2018;28:595–602. doi: 10.1111/bpa.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jay T.R., Miller C.M., Cheng P.J., Graham L.C., Bemiller S., Broihier M.L., Xu G., Margevicius D., Karlo J.C., Sousa G.L., et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 2015;212:287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Frank S., Burbach G.J., Bonin M., Walter M., Streit W., Bechmann I., Deller T. TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia. 2008;56:1438–1447. doi: 10.1002/glia.20710. [DOI] [PubMed] [Google Scholar]
- 171.Lessard C.B., Malnik S.L., Zhou Y., Ladd T.B., Cruz P.E., Ran Y., Mahan T.E., Chakrabaty P., Holtzman D.M., Ulrich J.D., et al. High-affinity interactions and signal transduction between Aβ oligomers and TREM2. EMBO Mol. Med. 2018;10 doi: 10.15252/emmm.201809027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ulrich J.D., Finn M.B., Wang Y., Shen A., Mahan T.E., Jiang H., Stewart F.R., Piccio L., Colonna M., Holtzman D.M. Altered microglial response to Aβ plaques in APPPS1-21 mice heterozygous for TREM2. Mol. Neurodegener. 2014;9:20. doi: 10.1186/1750-1326-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhao Y., Wu X., Li X., Jiang L.L., Gui X., Liu Y., Sun Y., Zhu B., Piña-Crespo J.C., Zhang M., et al. TREM2 Is a Receptor for β-Amyloid that Mediates Microglial Function. Neuron. 2018;97:1023.e7–1031.e7. doi: 10.1016/j.neuron.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Claes C., Van Den Daele J., Boon R., Schouteden S., Colombo A., Monasor L.S., Fiers M., Ordovás L., Nami F., Bohrmann B., et al. Human stem cell-derived monocytes and microglia-like cells reveal impaired amyloid plaque clearance upon heterozygous or homozygous loss of TREM2. Alzheimers Dement. 2019;15:453–464. doi: 10.1016/j.jalz.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 175.Wunderlich P., Glebov K., Kemmerling N., Tien N.T., Neumann H., Walter J. Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and γ-secretase-dependent intramembranous cleavage. J. Biol. Chem. 2013;288:33027–33036. doi: 10.1074/jbc.M113.517540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Heslegrave A., Heywood W., Paterson R., Magdalinou N., Svensson J., Johansson P., Öhrfelt A., Blennow K., Hardy J., Schott J., et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol. Neurodegener. 2016;11:3. doi: 10.1186/s13024-016-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bekris L.M., Khrestian M., Dyne E., Shao Y., Pillai J.A., Rao S.M., Bemiller S.M., Lamb B., Fernandez H.H., Leverenz J.B. Soluble TREM2 and biomarkers of central and peripheral inflammation in neurodegenerative disease. J. Neuroimmunol. 2018;319:19–27. doi: 10.1016/j.jneuroim.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Suárez-Calvet M., Kleinberger G., Araque Caballero M., Brendel M., Rominger A., Alcolea D., Fortea J., Lleó A., Blesa R., Gispert J.D., et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol. Med. 2016;8:466–476. doi: 10.15252/emmm.201506123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu D., Cao B., Zhao Y., Huang H., McIntyre R.S., Rosenblat J.D., Zhou H. Soluble TREM2 changes during the clinical course of Alzheimer’s disease: A meta-analysis. Neurosci. Lett. 2018;686:10–16. doi: 10.1016/j.neulet.2018.08.038. [DOI] [PubMed] [Google Scholar]
- 180.Piccio L., Deming Y., Del-Águila J.L., Ghezzi L., Holtzman D.M., Fagan A.M., Fenoglio C., Galimberti D., Borroni B., Cruchaga C. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 2016;131:925–933. doi: 10.1007/s00401-016-1533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gispert J.D., Suárez-Calvet M., Monté G.C., Tucholka A., Falcon C., Rojas S., Rami L., Sánchez-Valle R., Lladó A., Kleinberger G., et al. Cerebrospinal fluid sTREM2 levels are associated with gray matter volume increases and reduced diffusivity in early Alzheimer’s disease. Alzheimers Dement. 2016;12:1259–1272. doi: 10.1016/j.jalz.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 182.Zhong L., Xu Y., Zhuo R., Wang T., Wang K., Huang R., Wang D., Gao Y., Zhu Y., Sheng X., et al. Soluble TREM2 ameliorates pathological phenotypes by modulating microglial functions in an Alzheimer’s disease model. Nat. Commun. 2019;10:1365. doi: 10.1038/s41467-019-09118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Braestrup C., Albrechtsen R., Squires R.F. High densities of benzodiazepine receptors in human cortical areas. Nature. 1977;269:702–704. doi: 10.1038/269702a0. [DOI] [PubMed] [Google Scholar]
- 184.Park C.H., Carboni E., Wood P.L., Gee K.W. Characterization of peripheral benzodiazepine type sites in a cultured murine BV-2 microglial cell line. Glia. 1996;16:65–70. doi: 10.1002/(SICI)1098-1136(199601)16:1<65::AID-GLIA7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 185.Kuhlmann A.C., Guilarte T.R. Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J. Neurochem. 2000;74:1694–1704. doi: 10.1046/j.1471-4159.2000.0741694.x. [DOI] [PubMed] [Google Scholar]
- 186.Cosenza-Nashat M., Zhao M.L., Suh H.S., Morgan J., Natividad R., Morgello S., Lee S.C. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol. Appl. Neurobiol. 2009;35:306–328. doi: 10.1111/j.1365-2990.2008.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen M.K., Guilarte T.R. Translocator protein 18 kDa (TSPO): Molecular sensor of brain injury and repair. Pharmacol. Ther. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bradburn S., Murgatroyd C., Ray N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res. Rev. 2019;50:1–8. doi: 10.1016/j.arr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 189.Cagnin A., Brooks D.J., Kennedy A.M., Gunn R.N., Myers R., Turkheimer F.E., Jones T., Banati R.B. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- 190.Kreisl W.C., Lyoo C.H., McGwier M., Snow J., Jenko K.J., Kimura N., Corona W., Morse C.L., Zoghbi S.S., Pike V.W., et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain. 2013;136:2228–2238. doi: 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tournier B.B., Tsartsalis S., Ceyzériat K., Fraser B.H., Grégoire M.C., Kövari E., Millet P. Astrocytic TSPO Upregulation Appears Before Microglial TSPO in Alzheimer’s Disease. J. Alzheimers Dis. 2020;77:1043–1056. doi: 10.3233/JAD-200136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ferrarese C., Appollonio I., Frigo M., Meregalli S., Piolti R., Tamma F., Frattola L. Cerebrospinal fluid levels of diazepam-binding inhibitor in neurodegenerative disorders with dementia. Neurology. 1990;40:632–635. doi: 10.1212/WNL.40.4.632. [DOI] [PubMed] [Google Scholar]
- 193.Conti E., Andreoni S., Tomaselli D., Storti B., Brovelli F., Acampora R., Da Re F., Appollonio I., Ferrarese C., Tremolizzo L. Serum DBI and biomarkers of neuroinflammation in Alzheimer’s disease and delirium. Neurol. Sci. 2021;42:1003–1007. doi: 10.1007/s10072-020-04608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Clark C., Lewczuk P., Kornhuber J., Richiardi J., Maréchal B., Karikari T.K., Blennow K., Zetterberg H., Popp J. Plasma neurofilament light and phosphorylated tau 181 as biomarkers of Alzheimer’s disease pathology and clinical disease progression. Alzheimers Res. Ther. 2021;13:65. doi: 10.1186/s13195-021-00805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Torres-Acosta N., O’Keefe J.H., O’Keefe E.L., Isaacson R., Small G. Therapeutic Potential of TNF-α Inhibition for Alzheimer’s Disease Prevention. J. Alzheimers Dis. 2020;78:619–626. doi: 10.3233/JAD-200711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lunardelli M.L., Crupi R., Siracusa R., Cocuzza G., Cordaro M., Martini E., Impellizzeri D., Di Paola R., Cuzzocrea S. Co-ultraPEALut: Role in Preclinical and Clinical Delirium Manifestations. CNS Neurol. Disord. Drug Targets. 2019;18:530–554. doi: 10.2174/1871527318666190617162041. [DOI] [PubMed] [Google Scholar]