Abstract
The clinical utility of the chemotherapeutic drug cisplatin is significantly limited by its nephrotoxicity, which is characterized by electrolytic disorders, glomerular filtration rate decline, and azotemia. These alterations are consequences of a primary tubulopathy causing injury to proximal and distal epithelial cells, and thus tubular dysfunction. Oxidative stress plays a role in cisplatin nephrotoxicity and cytotoxicity, but its relative contribution to overall toxicity remains unknown. We studied the relation between the degree of oxidative reduction (provided by antioxidant treatment) and the extent of nephrotoxicity amelioration (i.e., nephroprotection) by means of a regression analysis of studies in animal models. Our results indicate that a linear relation exists between these two parameters, and that this relation very nearly crosses the value of maximal nephroprotection at maximal antioxidant effect, suggesting that oxidative stress seems to be a pivotal and mandatory mechanism of cisplatin nephrotoxicity, and, hence, an interesting, rationale-based target for clinical use. Our model also serves to identify antioxidants with enhanced effectiveness by comparing their actual nephroprotective power with that predicted by their antioxidant effect. Among those, this study identified nanoceria, erythropoietin, and maltol as highly effective candidates affording more nephroprotection than expected from their antioxidant effect for prospective clinical development.
Keywords: cisplatin, nephrotoxicity, prevention, antioxidants, preclinical, linear fit
1. Introduction
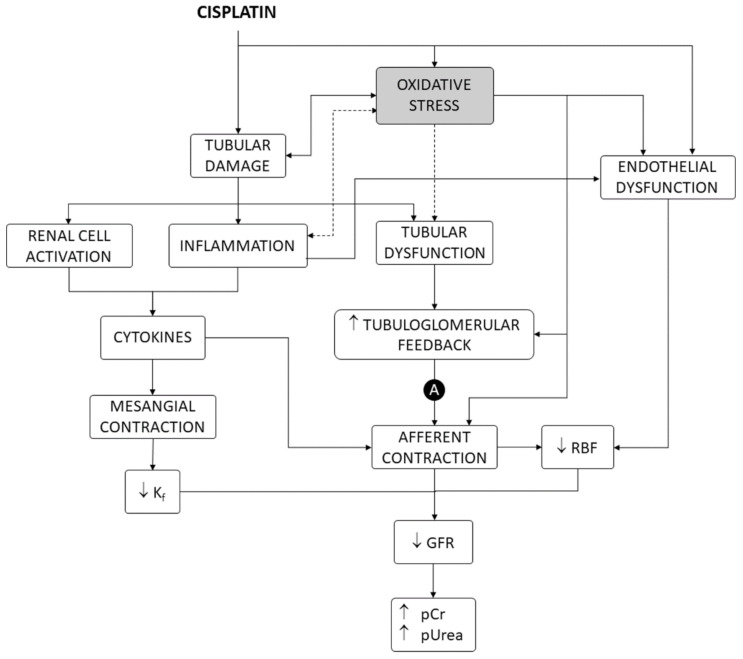
Cisplatin is one of the most potent and widely used chemotherapeutic drugs for the treatment of a variety of solid cancers [1], but its dosage and clinical utility are limited by nephrotoxicity [2]. Nephrotoxicity occurs in 25–35% of adult [3] and 70% of pediatric [4] therapeutic courses. Direct effects on the renal vasculature are involved [2], but cisplatin nephrotoxicity mostly shows a tubular damage pattern of dysfunction and derangement, producing electrolytic disturbances (i.e., most typically hypomagnesemia and hypokalemia), acute tubular injury (ATI), and acute kidney injury (AKI), with elevated plasma creatinine (pCr) and urea (pUrea) levels [2,5,6,7], which may occasionally progress to chronic fibrotic nephropathy [8,9]. As shown in Figure 1, tubular damage causes a reduction in glomerular filtration rate (GFR) by a number of mechanisms, including activation of the tubuloglomerular feedback (TGF) mechanism and renal vasoconstriction induced by inflammation and factors released by activated renal cells [2,10].
Figure 1.
Mechanisms of cisplatin nephrotoxicity, including oxidative stress as a contributing factor. A, autoregulation of renal blood flow and intraglomerular blood pressure. GFR, glomerular filtration rate. Kf, ultrafiltration coefficient. pCr, plasma creatinine concentration. pUrea, plasma urea concentration. RBF, renal blood flow.
This pathophysiological pattern results from cisplatin accumulation in proximal (mainly the S3 segment) [11,12] and distal tubule cells [2,13], which causes diverse cellular alterations, chiefly including inhibition of membrane transporters [2,14], interference with metabolic pathways [15], and cell death [16,17]. Tubular cell death shows apoptotic and nonapoptotic phenotypes, depending on the level of exposure to cisplatin [16]. While lower concentrations induce apoptosis, higher concentrations cause a necrotic-like phenotype [18,19]. Inside the cells, cisplatin becomes aquated and turns into a potent nucleophilic that binds to numerous targets, most prominently nucleic acids and many proteins [18,20]. Cisplatin cytotoxicity has been traditionally explained by formation of inter- and intra-strand adducts with nuclear DNA, which activates DNA repair mechanisms that, when overwhelmed, in turn, activate apoptosis. This cytotoxic mechanism is very effective in rapidly dividing cells, because nonrepaired DNA activates the p53–p21 cyclin-dependent kinase 2 (cdk2) pathway to make death/life decisions at cell division checkpoints [21].
Despite bearing a high and ready division capacity (for regeneration purposes), the proliferation rate of tubular epithelial cells is, however, very low under normal conditions [22]. Cell-cycle-independent mechanisms have been described, which might explain cisplatin cytotoxicity in target, nonproliferating epithelial cells, in which the drug accumulates [2,13]. Apoptotic and necrotic signaling is induced from damaged structures and organelles, such as mitochondria, endoplasmic reticulum, lysosomes, and others [16,21]. Cisplatin also induces oxidative stress in tubule epithelial cells in culture and in animal models [23,24,25] by accumulating in mitochondria and interfering with mitochondrial homeostasis and respiration [16,23]. Oxidative stress causes, or contributes to causing cell death, in general [26,27], and specifically after exposure to cisplatin [28,29,30]. In addition, oxidative stress also participates in other mechanisms of nephrotoxicity, such as renal vascular [31,32,33,34] and mesangial [35] contraction, endothelial dysfunction [36,37], inflammation [38,39,40], and TGF enhancement [32,33], leading to renal blood flow and GFR reduction and damage amplification [2,21] (Figure 1).
Oxidative stress has been proposed as a prominent event and mediator of cisplatin cytotoxicity and nephrotoxicity [2,13,21], but its relative weight among other pathophysiological mechanisms, and its hierarchical and causality relation with them, are mostly unknown. In this article, we studied and modeled the relation between the degree of reduction in oxidative stress and the degree of protection of cisplatin nephrotoxicity bestowed by exogenous antioxidants in a number of studies with animal models. A key role of oxidative stress in cisplatin nephrotoxicity was inferred from the linear relation between the antioxidant and nephroprotective effects, with almost complete prevention of nephrotoxicity at maximal antioxidant effect.
2. Materials and Methods
2.1. Data Mining
The data used for this study were obtained from the literature search carried out in our previous meta-analysis [41], in which preclinical studies reporting molecules or products preventing cisplatin nephrotoxicity were identified. Among them, only those articles meeting the following criteria were used: (1) evaluating antioxidant nephroprotectants, (2) conducted on experimental animals, (3) providing number of individuals per experimental group, (4) using cisplatin as the nephrotoxic agent, (5) written in English, (6) fully accessible for authors (through journal subscriptions, request to authors, or open access), (7) using pUrea or blood urea nitrogen (BUN) level as the parameter to estimate nephroprotection, and (8) using malonyldialdehyde (MDA) to evaluate oxidative stress, as previously described [42]. The subsequent mathematical analysis was performed only with those studies reporting statistically significant nephroprotective and antioxidant effects (with respect to the group that received cisplatin and no nephroprotectant). Publication bias was evaluated with the asymmetry tests of Begg and Mazumdar [43], and Egger et al. [44].
2.2. Mathematical Modeling
With the objective of evaluating a potential relation between the antioxidant and the nephroprotective activity of the nephroprotectants included in the study, the following parameters were defined:
Nephroprotection index (Enep):
where MaxNP is the value of the nephrotoxicity biomarker (i.e., pUrea or pBUN) at the maximum toxicity time in the nephroprotectant+cisplatin group; BasNP is the value of the nephrotoxicity biomarker at basal time point in the nephroprotectant+cisplatin group; MaxNA is the value of the nephrotoxicity biomarker at the maximum toxicity time point in the cisplatin group; and BasNA is the value of the nephrotoxicity biomarker at the basal time point in the cisplatin group. Thereof, MaxNP-BasNP corresponds to the increment in the level of the nephrotoxicity biomarker in the nephroprotectant+cisplatin group; and MaxNA-BasNA corresponds to the increment in the level of the same nephrotoxicity biomarker in the aminoglycoside group. Enep > 0 denotes nephroprotection (i.e., reduced cisplatin nephrotoxicity due to the action of the nephroprotectant), with the higher the value of Enep, the higher the nephroprotective effect. Enep = 1 represents total nephroprotection. Enep = 0 means there is no effect exerted by the nephroprotectant.
Antioxidant index (Eoxi):
where MaxOP is the value of the oxidative stress biomarker (i.e., MDA) at the maximum toxicity time point in the nephroprotectant+cisplatin group; BasOP is the value of the oxidative stress biomarker at the basal time point in the nephroprotectant+cisplatin group; MaxOA is the value of the oxidative stress biomarker at the maximum toxicity time point in the cisplatin group; and BasOA is the value of the oxidative stress biomarker at the basal time point in the cisplatin group. Thereof, MaxOP-BasOP corresponds to the increment in the level of the oxidative stress biomarker in the nephroprotectant+cisplatin-treated group; and MaxOA-BasOA corresponds to the increment in the level of the same oxidative stress biomarker in the cisplatin-treated group. Eoxi > 0 denotes antioxidant activity due to the protector, with the higher the value of Eoxi, the higher the antioxidant effect. Eoxi = 1 represents a complete antioxidant effect. Eoxi = 0 means no antioxidant effect is exerted by the nephroprotectant.
The Eoxi versus Enep relation was represented. We used an ordinary least squares (OLS) approach for building a linear regression model between Eoxi as an independent variable, and Enep as a dependent variable. Nonlinear models were also taken into account, but they did not improve the performance achieved by their linear counterparts. As on the basal state of Eoxi = 0, we should expect no nephroprotection effect (Enep = 0); we used this fact in the model assessment and supposed a zero-centered model that was tested with a proper model assessment using the Akaike information criterion (AIC). In particular, the final linear regression model was given by a weighted linear combination:
The model was assessed by measuring the statistical significance of the coefficients; the variability of the relationship between the predictors and the target value was determined by the corresponding coefficient [45].
As the presence of outliers was considerably high, two additional robust techniques were considered, namely the Huber regression and the random sample consensus (RANSAC) algorithm [46,47]. The Huber regression is a robust technique that uses a Huber loss function instead of the standard least squares in order to penalize the error depending on their magnitude [47]. RANSAC is an iterative estimation algorithm which fits several iterative models on subsets of data, and then selects the subset with the least average error that, by assumption, is the subset with no outlier points [46]. The value of the slope coefficient corresponding to each of the three models was eventually compared as an evaluation metric about the influence of outliers in the coefficient estimation carried out by OLS. All three models were fitted using Python module Scikit-learn [48]; the rest of the processing was performed in Python programming language [49].
3. Results
The characteristics of the studies included in this work are provided in Table 1.
Table 1.
Descriptive data of the studies that met the inclusion criteria. CP, cisplatin; i.p., intraperitoneal; i.v., intravenous; NPT, nephroprotectant; p.o., per os (i.e., oral administration).
| Reference | Animal Species | Study Length |
Cisplatin | NPT Daily Dose, Administration Route, Dose |
CP + NPT (n) |
|
|---|---|---|---|---|---|---|
| Dose and Route | n | |||||
| Abdel-Aziz et al., 2018 [50] | Rat | 15 days | 5 mg/kg, i.p. | 8 | Diacerein 50 mg/kg, p.o. 14 doses 100 mg/kg, p.o. 14 doses |
8 8 |
| Abdel Moneim et al., 2014 [51] | Rat | 10 days | 5 mg/kg, i.p. | 7 |
Azadirachta indica leaf methanolic extract 500 mg/kg, p.o. 5 doses before CP 500 mg/kg, p.o. 5 doses after CP |
7 7 |
| Abdel-Wahab et al., 2017 [52] | Rat | 4 weeks | 6 mg/kg, i.p. | 6 | N-acetylcisteine 50 mg/kg, i.p. 12 doses Taurine 50 mg/kg, i.p. 12 doses N-acetylcisteine + Taurine 50 mg/kg, i.p. + 50 mg/kg, i.p. 12 doses |
6 6 6 |
| Alibakhshi et al., 2018 [53] | Rat | 7 days | 7.5 mg/kg, i.p. | 5 | Zingerone 50 mg/kg, p.o. 7 doses |
5 |
| Al-Husseiny et al. 2016 [54] | Rat | 30 days | 5 mg/kg, i.p. | 20 | Human amniotic fluid stem cells (5 × 106) | 20 |
| Amirshahrokhi and Khalili, 2015 [55] | Mouse | 4 days | 15 mg/kg, i.p. | 8 | Thalidomide 100 mg/kg, p.o. 4 doses. |
8 |
| An et al., 2011 [56] | Mouse | 5 days | 5 mg/kg, i.p. once daily for 5 days | 9 | Pravastatin 80 mg/kg, p.o. 5 doses |
9 |
| Badawy et al., 2019 [57] | Rat | 12 days | 7 mg/kg, i.p. | 10 | Wogonin 40 mg/kg, i.p. 12 days |
10 |
| Bami et al., 2017 [58] | Rat | 5 days | 10 mg/kg, i.p. | 6 | Ferulic acid 50 mg/kg, p.o. 5 doses |
6 |
| Bayomi et al., 2013 [59] | Rat | 7 days | 10 mg/kg, i.p. | 10 | SB-4315421 mg/kg, i.p. 3 doses |
10 |
| Bazmandegan et al., 2019 [60] | Mouse | 4 days | 20 mg/kg, i.p | 7 | Sumatriptan 0.3 mg/kg, i.p. 3 doses |
7 |
| Chen et al., 2019 [61] | Mouse | 3 days | 20 mg/kg, i.p | 5 | Hesperetin 50 mg/kg, i.p. 3 doses |
5 |
| Chirino et al., 2008 [62] | Rat | 10 days | 7.5 mg/kg, i.p. | 10 | Apocynin 2 g/L in drinking water, p.o. for 10 days |
10 |
| Darwish et al., 2017 [63] | Rat | 14 days | 6 mg/kg, i.p. | 6 | Vitamin E 75 mg/kg, i.p. 14 doses |
6 |
| Dehnamaki et al., 2019 [64] | Mouse | 5 days | 20 mg/kg, i.p | 7 | Troxerutin 150 mg/kg |
7 |
| Divya et al., 2016 [65] | Rat | 6 days | 16 mg/kg, i.p. | 6 | Silymarin 100 mg/kg, p.o. 5 doses Apodytes dimidiata leaf methanolic extract 250 mg/kg, p.o. 5 doses before CP 250 mg/kg, p.o. 5 doses after CP |
6 6 6 |
| Elhusseini et al., 2016 [66] | Rat | 30 days | 10 mg/kg, i.p. | 20 | Human adipose-derived mesenchymal stem cells (5 × 106), i.v. | 20 |
| El-Naga, 2014 [67] | Rat | 2 weeks | 7 mg/kg, i.p. | 10 | Cardamonin 10 mg/kg, p.o. 14 doses 30 mg/kg, p.o. 14 doses |
10 10 |
| El-Naga and Mahran, 2016 [7] | Rat | 2 weeks | 7 mg/kg, i.p. | 10 | Indole-3-carbinol 20 mg/kg, p.o. 14 doses |
10 |
| Elsherbiny et al., 2016 [68] | Rat | 10 days | 10 mg/kg, i.p. | 10 | Arjunolic acid 100 mg/kg, p.o. 3 doses 250 mg/kg, p.o. 3 doses |
10 10 |
| Fatima et al., 2016 [69] | Rat | 6 days | 7 mg/kg, i.p. | 8 | Epigallocatechin gallate + coenzyme Q10 15 mg/kg, i.p. + 5 mg/kg, i.p. 6 doses |
8 |
| Fernández-Rojas et al., 2014 [70] | Mouse | 4 days | 18 mg/kg, i.p. | 4 | C-phycocyanin 10 mg/kg, i.p. 1 dose 30 mg/kg, i.p. 1 dose |
4 4 |
| Hassan et al., 2014 [71] | Rat | 6 weeks | 7.5 mg/kg, i.p. | 8 | Grape seed proanthocyanidin extract 100 mg/kg, p.o. 42 doses |
8 |
| Helmy et al., 2014 [72] | Rat | 4 days | 6 mg/kg, i.p. | 7–8 | BQ-123 1 mg/kg, i.p. 2 doses |
7–8 |
| Hosseini et al., 2018 [73] | Rat | 3 days | 8 mg/kg, i.p. | 6 |
Rheum turkestanicum root extract 100 mg/kg, i.p. 1 dose 200 mg/kg, i.p. 1 dose |
6 6 |
| Y.C. Huang et al., 2017 [74] | Mouse | 3 days | 20 mg/kg, i.p. | 5 | Galangin 75 mg/kg, p.o. 3 doses |
5 |
| H. Huang et al., 2017 [75] | Rat | 12 days | 8 mg/kg, i.p. | 6 |
Schisandra chinensis bee pollen extract 400 mg/kg, p.o. 12 doses starting 7 days prior to CP 800 mg/kg, p.o. 12 doses starting 7 days prior to CP 1200 mg/kg, p.o. 12 doses starting 7 days prior to CP after |
6 6 6 |
| Huang et al., 2019 [76] | Mouse | 4 days | 22 mg/kg, i.p. | 8 | N-Acetylcysteine 50 mg/kg, i.p. 3 doses |
8 |
| Kandemir et al., 2019 [77] | Rat | 8 days | 7 mg/kg, i.p. | 8 | Zingerone 25 mg/kg, p.o. 7 doses 50 mg/kg, p.o. 7 doses |
8 8 |
| Kang et al., 2016 [78] | Mouse | 7 days | 20 mg/kg, i.p. | 12 | Sappanone A 10 mg/kg, i.p. 3 doses 20 mg/kg, i.p. 3 doses 40 mg/kg, i.p. 3 doses |
12 12 12 |
| Kenza et al., 2017 [79] | Mouse | 11 days | 8 mg/kg, i.p. | 6 | Vitamin E 100 mg/kg, p.o. 10 doses Centaurea choulettiana Pomel leaf butanolic extract 150 mg/kg, p.o. 10 doses |
6 6 |
| Khairnar et al., 2020 [80] | Rat | 6 days | 5 mg/kg, i.p. | 6 | Disulfiram 50 mM/kg, p.o. 5 doses Disulfiram + CuCl2 50 mM/kg + 50 mM/kg, p.o. 5 doses Disulfiram cooper chelate (Cu-DEDC) 50 mM/kg, p.o. 5 doses Amifostine 100 mg/kg i.v. 1 dose |
6 6 6 6 |
| Kim et al., 2018 [81] | Mouse | 6 days | 15 mg/kg, i.p. | 8 | Ac-YVAD-cmk 10 mg/kg, i.p. 3 doses |
8 |
| F. Li et al., 2018 [82] | Mouse | 4 days | 20 mg/kg, i.p. | 12 | Xanthohumol 12.5 mg/kg, i.p. 3 doses 25 mg/kg, i.p. 3 doses 50 mg/kg, i.p. 3 doses |
12 12 12 |
| Y.Z. Li et al., 2018 [83] | Mouse | 10 days | 20 mg/kg, i.p. | 8 |
Schisandra chinensis extract 300 mg/kg, p.o. 10 doses 600 mg/kg, p.o. 10 doses |
8 8 |
| Li et al., 2019 [84] | Mouse | 11 days | 20 mg/kg, i.p. | 10 | Arginyl-fructosyl-glucose 40 mg/kg, p.o. 10 doses starting 3 days prior to CP 80 mg/kg, p.o. 10 doses starting 3 days prior to CP |
10 10 |
| Ma et al., 2015 [85] | Mouse | 7 days | 15 mg/kg, i.p. | 10 | Icariin 30 mg/kg, p.o. 6 doses 60 mg/kg, p.o. 6 doses |
10 10 |
| Ma et al., 2017 [86] | Rat | 5 days | 7 mg/kg, i.p. | 7 | Puerarin 3 days before CP and 5 days after CP 30 mg/kg i.v. 50 mg/kg i.v. |
7 7 |
| Malik et al., 2015 [87] | Rat | 10 days | 8 mg/kg, i.p. | 6 | Nobiletin 5 mg/kg, i.p. 10 doses |
6 |
| Mi et al., 2018 [88] | Mouse | 10 | 25 mg/kg i.p. | 8 | Maltol 100 mg/kg p.o. 10 doses starting 7 days prior to CP |
8 |
| Mohamed et al., 2013 [89] | Rat | 2 weeks | 9 mg/kg, i.p. divided in two doses once a week for two weeks. | 20 | Recombinant human erythropoietin 100 IU/kg, i.p. 14 doses |
20 |
| Morsy and Heeba, 2016 [90] | Rat | 7 days | 6 mg/kg, i.p. | 6–8 | Nebivolol 10 mg/kg, p.o. 7 doses |
6–8 |
| Mundhe et al., 2015 [91] | Rat | 5 days | 7.5 mg/kg, i.p. | 6 | Nordihydroguaiaretic acid 10 mg/kg, i.p. 5 doses |
6 |
| Mundhe et al., 2019 [92] | Rat | 10 days | 7.5 mg/kg i.p. | 8 | Nordihydroguaiarectic acid 10 mg/kg i.p. 5 doses before CP 10 mg/kg i.p. 5 doses after CP |
8 8 |
| Nazari Soltan Ahmad et al., 2018 [93] | Rat | 10 days | 8 mg/kg i.p. | 6 | Tangeretin 2.5 mg/kg i.p. 7 doses before CP and 3 after 5 mg/kg i.p. 7 doses before CP and 3 after |
6 6 |
| Nazari Soltan Ahmad et al., 2018 [94] | Rat | 4 days | 20 mg/kg i.p. | 5 | Dunnione 10 mg/kg p.o. 4 doses starting 12 h prior to CP 20 mg/kg p.o. 4 doses starting 12 h prior to CP |
5 5 |
| Neamatallah et al., 2018 [95] | Rat | 11 days | 7.5 mg/kg, i.p. | 6 | Talh honey 2.5 g/kg, p.o. 10 doses |
6 |
| Purena et al., 2018 [96] | Rat | 14 days | 12 mg/kg, i.p. | 5 |
Emblica officinalis leaf ethanolic extract 100 mg/kg, p.o. 14 doses 200 mg/kg, p.o. 14 doses |
5 5 |
| Qi et al., 2018 [97] | Mouse | 10 days | 20 mg/kg i.p. | 8 | Pseudoginsengenin DQ 30 mg/kg p.o. 10 doses starting 7 days prior to CP 60 mg/kg p.o. 10 doses starting 7 days prior to CP |
8 8 |
| Radwan et al., 2017 [98] | Rat | 10 days | 7.5 mg/kg, i.p. | 6 | Rutin 200 mg/kg p.o. Low dose gamma radiation (LDR) Rutin 200 mg/kg p.o. + LDR |
6 6 6 |
| Rana et al., 2016 [99] | Rat | 10 days | 6 mg/kg, i.p. | 6 |
Bauhinia purpurea bark ethanolic extract 400 mg/kg, p.o. 9 doses Bauhinia purpurea unripe pod extract 400 mg/kg, p.o. 9 doses |
6 6 |
| Sahin et al., 2014 [100] | Rat | 12 days | 7 mg/kg, i.p. | 7 | Curcumin difluorinated 50 mg, p.o. 12 doses Curcumin 50 mg, p.o. 12 doses |
7 7 |
| Saifi et al., 2019 [101] | Mouse | 14 days 28 days |
10 mg/kg i.p. 5 mg/kg i.p. CP every week for 3 consecutive weeks |
6 6 |
Nanoceria 2 mg/kg i.p. 14 doses starting 5 days prior to CP Nanoceria 0.2 mg/kg i.p. 28 doses starting 5 days prior to 1st dose of CP 2 mg/kg i.p. 28 doses after 1st dose of CP |
6 6 6 |
| Sen et al., 2018 [102] | Rat | 25 days | 5 mg/kg, i.p. every five days (four injections) for 25 days | 6 |
Dillenia indica fruit methanolic extract 300 mg/kg, p.o. 25 doses Dillenia indica fruit ethanolic extract 300 mg/kg, p.o. 25 doses |
6 6 |
| Sener et al., 2012 [103] | Rat | 14 days | 10 mg/kg, i.p. 14 days | 7 | Mirtazapine 15 mg/kg, p.o. 14 doses 30 mg/kg, p.o. 14 doses |
7 7 |
| Sharma and Goyal, 2012 [104] | Mouse | 7 days | 16 mg/kg, i.p. | 6 |
Heliotropium eichwaldii root methanolic extract 400 mg/kg, p.o. 7 doses |
6 |
| Sherif, 2015 [105] | Rat | 10 days | 7 mg/kg, i.p. | 10 | Arjunolic acid 20 mg/kg, p.o. 10 doses |
10 |
| Shi et al., 2019 [106] | Mouse | 7 days | 20 mg/kg i.p. | 8 | Docosahexaenoic acid (DHA) 12.5 mg per day p.o. for 4 days prior to CP Docosahexaenoic acid-phosphatidylcholine (DHA-PC) 12.5 mg per day p.o. for 4 days prior to CP |
8 8 |
| Topcu-Tarladacalisir et al., 2016 [107] | Rat | 10 days | 7.5 mg/kg, i.p. | 6 | Curcumin 200 mg/kg, p.o. 4 doses |
6 |
| Z. Wang et al., 2018 [108] | Mouse | 10 days | 25 mg/kg i.p. | 8 | Ginsenoside-Re (G-Re) 25 mg/kg p.o. 10 doses starting 7 days prior to CP |
8 |
| Yang et al., 2017 [109] | Mouse | 10 days | 25 mg/kg, i.p. | 8 | Sika deer antler protein powder 10 mg/kg, p.o. 10 doses 20 mg/kg, p.o. 10 doses |
8 8 |
| Yu et al., 2018 [110] | Mouse | 96 h | 20 mg/kg i.p. | 8 | Celastrol 1 mg/kg i.p. 24 h before CP 2 mg/kg i.p. 24 h before CP |
8 8 |
| L. Zhang et al., 2018 [111] | Mouse | 7 days | 20 mg/kg i.p. | 3 | Daphnetin 2.5 mg/kg i.p. 3 doses before CP 5 mg/kg i.p. 3 doses before CP 10 mg/kg i.p. 3 doses before CP Pyrrolidine dithiocarbamate (PDTC) |
3 3 3 3 |
The Begg–Mazumdar test applied to assess potential publication bias yielded a Kendall’s tau of 0.74 (p < 0.001). Similarly, the Egger test provided a bias of 10.49 (95% CI = 9.56, 11.43; p < 0.001). Both tests showed the presence of asymmetry. However, in our study, this result was expected and is not necessarily reflective of publication bias. In fact, pursuant of our objective, only studies reporting a statistically significant nephroprotective effect were included, as stated in the Methods.
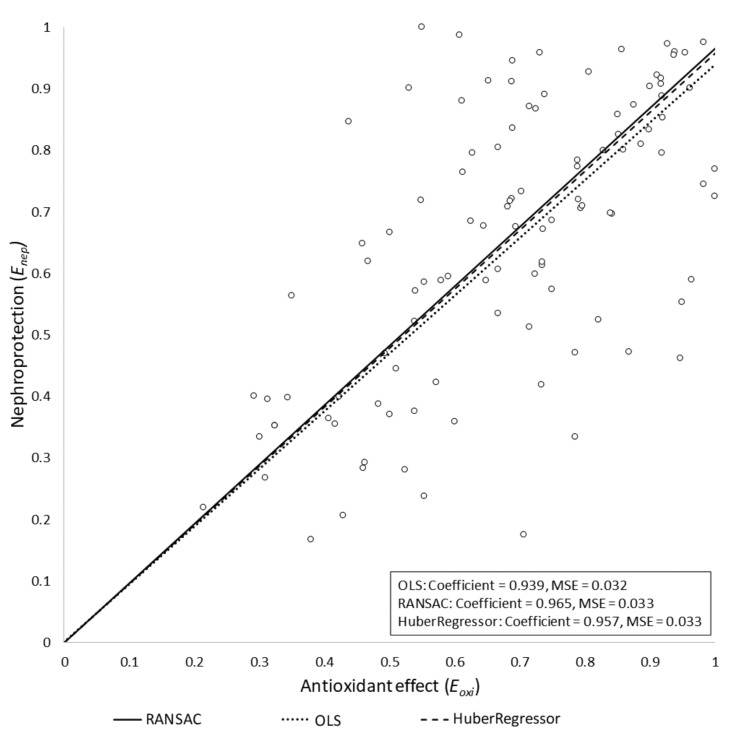
The OLS model was evaluated by checking the statistical significance of the coefficients with an alpha error threshold of 0.01. We obtained the following results: w = 0.938 (95% CI = (0.89, 0.987), p < 0.0001). We used the Akaike information criterion (AIC) [112] to assess the choice of including or excluding the bias term from the final model. We obtained an AICintercept = −66.32 for the linear model with bias term, and AICbase = −61.90 for the model without bias term. Therefore, based on this result, we only kept the slope term in the resulting model, R2 = 0.932, meaning that 93.2% of the variability of could be explained by .
The potential influence of outliers in the final model was also assessed. In particular, Huber and RANSAC regressions were obtained, assuming the same model as for the OLS case (i.e., without intercept term). Both algorithms yielded similar slope values to that obtained by the OLS regression model: wHuber = 0.957 and wRANSAC = 0.965. We concluded that, under our assumptions, the outliers had no significant influence on the final model. The three models are depicted in Figure 2. Studies in which Eoxi > 1 were removed from the models. In these studies, the antioxidant reduced oxidative stress beyond the basal level (i.e., the level of oxidative stress in the control group), which had a negative impact on nephroprotection. Specifically, Enep showed a negative slope beyond Eoxi = 1 (data not shown). This is because normal (i.e., basal) production of reactive oxygen species (ROS) has been shown to have homeostatic signaling roles [113,114,115]. As a corollary, inhibition of basal ROS production may reasonably result in deleterious effects for cell and organ function [116].
Figure 2.
Regression results for the three tested models. The nephroprotection index (Enep) is represented versus the antioxidant index (Eoxi) for the OLS, Huber regression, and RANSAC regression models, all of them yielding similar results. The RANSAC model provided the most robust fit, as it empirically ignored some outlier points, giving them a zero weight in the final adjustment. MSE, mean squared error.
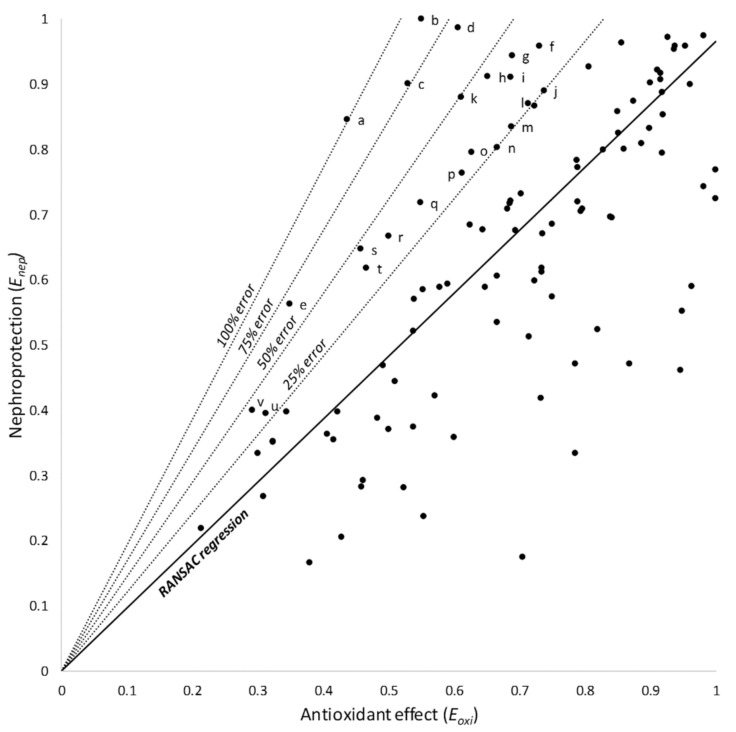
Products located over the model provided more nephroprotection than expected from their antioxidant effect. Based on the RANSAC regression (the model with the most robust fit), these products were subclassified as showing 25, 50, 75, or 100% of additional nephroprotection (Figure 3); they are identified and listed in Table 2.
Figure 3.
RANSAC linear regression and 25, 50, 75, and 100% relative error areas. Products over the model afford a greater nephroprotection than expected from their antioxidant effect. Products within these areas are identified and shown in Table 2. Letters (a through v) identify individual products, as listed in Table 2.
Table 2.
Products providing higher nephroprotection than expected from their antioxidant effect, according to their relative position with respect to the RANSAC linear regression model.
| ID | Product | Relative Error (%) |
|---|---|---|
| a | Nanoceria 0.2 mg/kg (before CP) (28 doses) | >100% |
| b | Nanoceria 2 mg/kg (after CP) (28 doses) | >75% |
| c | RH Erythropoietin 100 IU/kg | >75% |
| d | Maltol 100 mg/kg | >50% |
| e | Centaurea choulettiana Pomel leaf butanolic extract 150 mg/kg | >50% |
| f | Wogonin 40 mg/kg | >25% |
| g | Rutin 200 mg/kg | >25% |
| h | Celastrol 1 mg/kg | >25% |
| i | SB-431542 1 mg/kg | >25% |
| j | Arjunolic acid 100 mg/kg | >25% |
| k | Troxerutin 150 mg/kg | >25% |
| l | Sika deer antler protein powder 10 mg/kg | >25% |
| m | Puerarin 50 mg/kg | >25% |
| n | N-acetylcisteine 50 mg/kg | >25% |
| o | Mesenchymal stem cells 5 × 106 cells | >25% |
| p | Human amniotic fluid stem cells 5 × 106 cells | >25% |
| q | Vitamin E 100 mg/kg | >25% |
| r | Sumatriptan 0.3 mg/kg | >25% |
| s | Celastrol 2 mg/kg | >25% |
| t | Taurine 50 mg/kg | >25% |
| u | Daphnetin 2.5 mg/kg | >25% |
| v | Sappanone A 10 mg/kg | >25% |
4. Discussion
The regression model best adjusting our experimental data shows a linear relationship between inhibition of oxidative stress and amelioration of cisplatin nephrotoxicity (Figure 2). This relation intercepts the nephroprotection axis (i.e., the y-axis) very near the Enep = 1 value at the maximal antioxidant point (i.e., Eoxi = 1 in the x-axis). This indicates that a complete abrogation of oxidative stress apparently leads to a complete prevention of nephrotoxicity. Thus, oxidative stress might not only be a contributing, but a pivotal mechanism of cisplatin nephrotoxicity. Cisplatin nephrotoxicity is a tubulopathy, in which all pathophysiological and clinical manifestations derive from cytotoxic tubular injury as the primary event (Figure 1) [2,13]. Consequently, oxidative stress must also be in the core of cisplatin cytotoxicity.
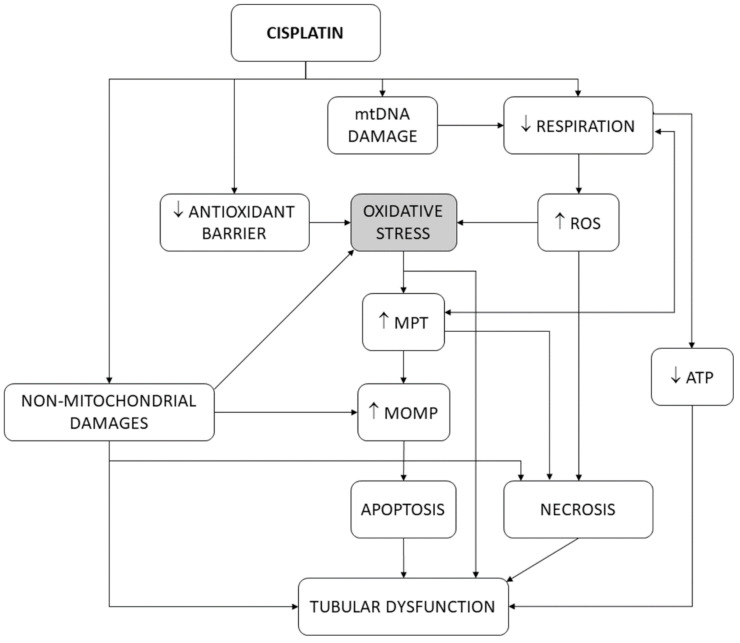
Mitochondria are the main intracellular site of cell life/death decision [117,118,119]. Mitochondria funnel and integrate stress signals arising from damaged subcellular structures and organelles, including themselves, and activate apoptotic and necrotic death programs that mostly pose no-return points for cell demise [120]. One of these signals is oxidative stress. Extramitochondrial sources of ROS exist (e.g., the cytosol and the endoplasmic reticulum) [121], but mitochondria are the main source of ROS production and overproduction [115]. Mitochondrial outer membrane permeabilization (MOMP) is a mandatory event for the release of proapoptotic factors (e.g., cytochrome c and AIF), apoptosome formation in the cytosol, and initiation of intrinsic apoptosis [119]. Intracellular death signals regulate MOMP by targeting the outer membrane through pro- and anti-apoptotic Bcl-2 family members, which directly modulate its permeability [118,122]. Inner membrane permeabilization (i.e., mitochondrial permeability transition, MPT) is also intimately related to cell death. MPT is bidirectionally linked to transmembrane mitochondrial potential (∆Ψ) dissipation, and causes intermembrane swelling, outer membrane disruption, and MOMP. MPT is mediated by a multiprotein complex, the permeability transition pore (PT pore or PTP). PTP is located at sites of inner–outer membrane connections (where Bcl-2 family members accumulate), is inhibited by anti-apoptotic Bcl-2 members, is critical for apoptosis, and participates in MOMP [123,124,125].
In isolated mitochondria [126], cisplatin interferes with the respiratory chain, produces oxidative stress [127] and rapid cytochrome c release [128], and causes calcium-dependent mitochondrial swelling and mitochondrial depolarization, as a consequence of PT pore opening [129]. In this scenario, oxidative stress may be the cause or the consequence of the other events. In fact, decoupling or inhibition of mitochondrial respiration induces both PT pore opening and oxidative stress [117,130,131]. PT pore opening (and, thus, MPT) is triggered by mitochondrial Ca2+ and potentiated by oxidative stress [125,132,133], suggesting that alterations in respiration induce oxidative stress, and this, in turn, contributes to the opening of the PT pore. In agreement, antioxidants inhibit MPT [134]. However, vice versa is also possible: PT pore opening produces ∆Ψ dissipation, respiratory uncoupling, and oxidative stress [124,132,135]. As such, oxidative stress and mitochondrial dysfunction induce one another [136,137], and so a causality dilemma existed for cisplatin cytotoxicity [16]. Cisplatin also causes oxidative stress by directly damaging mitochondrial DNA (mtDNA) [128,138,139,140], which impairs appropriate expression of mitochondrial enzymes forming the respiratory chain, and thus induces oxidative stress. Finally, cisplatin abates the antioxidant barrier by inhibiting superoxide dismutase (SOD), catalase, glutathione peroxidase, glutathione S-transferase [141,142,143], and glutathione reductase [144] in kidney tissues. Figure 4 summarizes the participation of oxidative stress in the tubular pathophysiological scenario. The results of the present study are more congruent with oxidative stress being mainly upstream of MPT and MOMP, because, after these mitochondrial events have occurred, the cell is irreversibly committed to dying [120].
Figure 4.
Schematic depiction of the pivotal role of oxidative stress in the tubular dysfunction induced by cisplatin. ATP, adenosine triphosphate. MOMP, mitochondrial outer membrane permeability. MPT, mitochondrial permeability transition. mtDNA, mitochondrial DNA. ROS, reactive oxygen species.
Our study closely relates oxidative stress to the reduction in glomerular filtration (using pUrea as a proxy). GFR reduction is a pivotal alteration in cisplatin nephrotoxicity, derived mostly from tubular cytotoxicity (as shown in Figure 1) [2], and an internationally recognized hallmark of AKI, regardless of etiology [145]. However, tubular damage and GFR decline are not directly proportional. In fact, an undetermined degree of tubular damage may occur without affecting GFR [146,147], as undamaged nephrons may, to a certain extent, sustain (total) GFR by increasing their single-nephron GFR (SNGFR) [148]. This implies that additional injury mechanisms (unrelated to oxidative stress) might remain under maximal antioxidant circumstances. Potential oxidative stress-independent mechanisms are known (see Figure 1 and Figure 4, and [2,16,21]), but their weight in cisplatin toxicity is unknown. They would pose potential targets for pharmacological intervention in combination with antioxidants to optimize cisplatin nephrotoxicity prophylaxis. As previously reported [42], nephroprotectants whose effect lies above the model line are products showing greater protective effect than expected from their antioxidant effect. This suggests that additional protection mechanisms are involved, which makes them especially interesting candidates for clinical application. The most effective candidates include nanoceria, recombinant human erythropoietin, maltol, and the butanolic extract of Centaurea choulettiana Pomel (Table 2). On the contrary, those compounds lying below the model line are less effective than expected, implying that they also activate counteracting mechanisms, and are thus less interesting.
Along with its antioxidant effect, nanoceria (cerium oxide nanoparticles) also shows anti-inflammatory [101] and antiapoptotic properties [149]. Its anti-inflammatory effect has been shown to derive from the inhibition of inducible nitric oxide synthase (iNOS) expression [150] and of the NF-κB signaling pathway [151]. With regard to erythropoietin, multiple additional mechanisms have been invoked, including (i) the promotion of tubular cell regeneration, (ii) the reduction in vascular endothelial growth factor (VEGF), hemeoxygenase-1 (HO-1) and iNOS expression [89], (iii) the inactivation of macrophages via NF-κB [152], (iv) the inhibition of TGF-β1 expression [153], and (v) the reduction in polymorphonuclear cell infiltration [154]. Anti-inflammatory and antiapoptotic properties with involvement of the AMPK/PI3K/Akt pathway have also been attributed to maltol, an ingredient in the food industry [88]. Finally, traditional medicine has attributed anti-inflammatory properties to Centaurea choulettiana [155]. However, oxidative stress is known to be involved in the development and perpetuation of inflammation [156,157], and in the activation of apoptosis [158]. Accordingly, their anti-inflammatory and antiapoptotic properties might be the consequence of their antioxidant capacity, and would thus not explain their additional properties, which need to be further explored. Because drug discovery from plant extracts is a complicated and long process, nanoceria, erythropoietin, and maltol hoard readier potential to become clinical applications, and should thus be further explored.
5. Conclusions
Our results have revealed that oxidative stress is not a contributing, but a central mediator of cisplatin nephrotoxicity in preclinical models. In agreement, a recent meta-analysis identified antioxidants as the most effective protectants of cisplatin nephrotoxicity in clinical studies [159]. Interestingly, several antioxidants have shown, in animal models, nephroprotective properties without interfering with the antitumor effect of cisplatin [160,161,162,163,164], a critical issue for clinical application. This might be attributed to cisplatin genotoxicity mostly impacting on proliferating cells, such as tumor cells. In perspective, this study provides a rationale for further clinical development of preventive strategies based on single or combined therapies containing antioxidants.
Author Contributions
Conceptualization, L.V.-V., A.G.C., A.I.M. and F.J.L.-H.; methodology, M.H., Ó.J.P.-V. and J.D.M.-G.; validation, M.H. and A.G.C.; formal analysis, all authors.; data curation, A.G.C., A.I.M. and L.V.-V.; writing—original draft preparation, A.G.C., L.V.-V., F.J.L.-H. and J.D.M.-G.; writing—review and editing, all authors; supervision, F.J.L.-H. and J.D.M.-G.; funding acquisition, A.I.M., F.J.L.-H. and J.D.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto de Salud Carlos III (Madrid, Spain), grant number PI20/01351 and PI18/00996 and FEDER funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Florea A.M., Büsselberg D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers. 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sánchez-González P.D., López-Hernández F.J., López-Novoa J.M., Morales A.I. An Integrative View of the Pathophysiological Events Leading to Cisplatin Nephrotoxicity. Crit. Rev. Toxicol. 2011;41:803–821. doi: 10.3109/10408444.2011.602662. [DOI] [PubMed] [Google Scholar]
- 3.Sato K., Watanabe S., Ohtsubo A., Shoji S., Ishikawa D., Tanaka T., Nozaki K., Kondo R., Okajima M., Miura S., et al. Nephrotoxicity of Cisplatin Combination Chemotherapy in Thoracic Malignancy Patients with CKD Risk Factors. BMC Cancer. 2016;16:222. doi: 10.1186/s12885-016-2271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkel M., Goldstein A., Steinberg Y., Granowetter L., Trachtman H. Cisplatinum Nephrotoxicity in Oncology Therapeutics: Retrospective Review of Patients Treated between 2005 and 2012. Pediatr. Nephrol. 2014;29:2421–2424. doi: 10.1007/s00467-014-2935-z. [DOI] [PubMed] [Google Scholar]
- 5.Sharbaf F., Farhangi H., Assadi F. Prevention of Chemotherapy-Induced Nephrotoxicity in Children with Cancer. Int. J. Prev. Med. 2017;8:76. doi: 10.4103/ijpvm.IJPVM_40_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoek J., Bloemendal K.M., van der Velden L.A.A., van Diessen J.N.A., van Werkhoven E., Klop W.M.C., Tesselaar M.E.T. Nephrotoxicity as a Dose-Limiting Factor in a High-Dose Cisplatin-Based Chemoradiotherapy Regimen for Head and Neck Carcinomas. Cancers. 2016;8:21. doi: 10.3390/cancers8020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Naga R.N., Mahran Y.F. Indole-3-Carbinol Protects against Cisplatin-Induced Acute Nephrotoxicity: Role of Calcitonin Gene-Related Peptide and Insulin-like Growth Factor-1. Sci. Rep. 2016;6 doi: 10.1038/srep29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pabla N., Dong Z. Cisplatin Nephrotoxicity: Mechanisms and Renoprotective Strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 9.Landau S.I., Guo X., Velazquez H., Torres R., Olson E., Garcia-Milian R., Moeckel G.W., Desir G.V., Safirstein R. Regulated Necrosis and Failed Repair in Cisplatin-Induced Chronic Kidney Disease. Kidney Int. 2019;95:797–814. doi: 10.1016/j.kint.2018.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Novoa J.M., Quiros Y., Vicente L., Morales A.I., Lopez-Hernandez F.J. New Insights into the Mechanism of Aminoglycoside Nephrotoxicity: An Integrative Point of View. Kidney Int. 2011;79:33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 11.Kuhlmann M.K., Burkhardt G., Köhler H. Insights into Potential Cellular Mechanisms of Cisplatin Nephrotoxicity and Their Clinical Application. Nephrol. Dial. Transpl. 1997;12:2478–2480. doi: 10.1093/ndt/12.12.2478. [DOI] [PubMed] [Google Scholar]
- 12.Price P.M., Safirstein R.L., Megyesi J. Protection of Renal Cells from Cisplatin Toxicity by Cell Cycle Inhibitors. Am. J. Physiol. Ren. Physiol. 2004;286 doi: 10.1152/ajprenal.00192.2003. [DOI] [PubMed] [Google Scholar]
- 13.Karasawa T., Steyger P.S. An Integrated View of Cisplatin-Induced Nephrotoxicity and Ototoxicity. Toxicol. Lett. 2015;237:219–227. doi: 10.1016/j.toxlet.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casanova A.G., Fuentes-Calvo I., Hernández-Sánchez M.T., Quintero M., Toral P., Caballero M.T., Martínez-Salgado C., Morales A.I., Layton A.T., Eleno N., et al. The Furosemide Stress Test and Computational Modeling Identify Renal Damage Sites Associated with Predisposition to Acute Kidney Injury in Rats. Transl. Res. 2021;231:76–91. doi: 10.1016/j.trsl.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Macciò A., Madeddu C. Cisplatin: An Old Drug with a Newfound Efficacy-from Mechanisms of Action to Cytotoxicity. Expert Opin. Pharm. 2013;14:1839–1857. doi: 10.1517/14656566.2013.813934. [DOI] [PubMed] [Google Scholar]
- 16.Sancho-Martínez S.M., Prieto-García L., Prieto M., López-Novoa J.M., López-Hernández F.J. Subcellular Targets of Cisplatin Cytotoxicity: An Integrated View. Pharmacol. Ther. 2012;136:35–55. doi: 10.1016/j.pharmthera.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Volarevic V., Djokovic B., Jankovic M.G., Harrell C.R., Fellabaum C., Djonov V., Arsenijevic N. Molecular Mechanisms of Cisplatin-Induced Nephrotoxicity: A Balance on the Knife Edge between Renoprotection and Tumor Toxicity. J. Biomed. Sci. 2019;26:1–14. doi: 10.1186/s12929-019-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sancho-Martínez S.M., Prieto-García L., Prieto M., Fuentes-Calvo I., López-Novoa J.M., Morales A.I., Martínez-Salgado C., López-Hernández F.J. N-Acetylcysteine Transforms Necrosis into Apoptosis and Affords Tailored Protection from Cisplatin Cytotoxicity. Toxicol. Appl. Pharmacol. 2018;349:83–93. doi: 10.1016/j.taap.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Sancho-Martínez S.M., Piedrafita F.J., Cannata-Andía J.B., López-Novoa J.M., López-Hernández F.J. Necrotic Concentrations of Cisplatin Activate the Apoptotic Machinery but Inhibit Effector Caspases and Interfere with the Execution of Apoptosis. Toxicol. Sci. 2011;122:73–85. doi: 10.1093/toxsci/kfr098. [DOI] [PubMed] [Google Scholar]
- 20.Kartalou M., Essigmann J.M. Mechanisms of Resistance to Cisplatin. Mutat. Res. Fundam. Mol. Mech. Mutagenesis. 2001;478:23–43. doi: 10.1016/S0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 21.Chirino Y.I., Pedraza-Chaverri J. Role of Oxidative and Nitrosative Stress in Cisplatin-Induced Nephrotoxicity. Exp. Toxicol. Pathol. 2009;61:223–242. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Vogetseder A., Picard N., Gaspert A., Walch M., Kaissling B., Le Hir M. Proliferation Capacity of the Renal Proximal Tubule Involves the Bulk of Differentiated Epithelial Cells. Am. J. Physiol. Cell Physiol. 2008;294:C22–C28. doi: 10.1152/ajpcell.00227.2007. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Y., Wang H., Wu Y., Zhang B., Wang N., Mao H., Xing C. P53 Contributes to Cisplatin Induced Renal Oxidative Damage via Regulating P66shc and MnSOD. Cell. Physiol. Biochem. 2015;37:1240–1256. doi: 10.1159/000430247. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa M., Nagatomi H., Nishijima M., Ohira G., Chang B.J., Sato E., Inoue M. Targeting Superoxide Dismutase to Renal Proximal Tubule Cells Inhibits Nephrotoxicity of Cisplatin and Increases the Survival of Cancer-Bearing Mice. Cancer Lett. 2001;171:133–138. doi: 10.1016/S0304-3835(01)00591-2. [DOI] [PubMed] [Google Scholar]
- 25.Kong M.J., Han S.J., Kim J.I., Park J.W., Park K.M. Mitochondrial NADP+-Dependent Isocitrate Dehydrogenase Deficiency Increases Cisplatin-Induced Oxidative Damage in the Kidney Tubule Cells Article. Cell Death Dis. 2018;9:488. doi: 10.1038/s41419-018-0537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott M., Gogvadze V., Orrenius S., Zhivotovsky B. Mitochondria, Oxidative Stress and Cell Death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 27.Ryter S.W., Hong P.K., Hoetzel A., Park J.W., Nakahira K., Wang X., Choi A.M.K. Mechanisms of Cell Death in Oxidative Stress. Antioxid. Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 28.Manohar S., Leung N. Cisplatin Nephrotoxicity: A Review of the Literature. J. Nephrol. 2018;31:15–25. doi: 10.1007/s40620-017-0392-z. [DOI] [PubMed] [Google Scholar]
- 29.Xiao T., Choudhary S., Zhang W., Ansari N.H., Salahudeen A. Possible Involvement of Oxidative Stress in Cisplatin-Induced Apoptosis in LLC-PK1 Cells. J. Toxicol. Environ. Health Part A. 2003;66:469–479. doi: 10.1080/15287390306449. [DOI] [PubMed] [Google Scholar]
- 30.Jiang M., Wei Q., Pabla N., Dong G., Wang C.Y., Yang T., Smith S.B., Dong Z. Effects of Hydroxyl Radical Scavenging on Cisplatin-Induced P53 Activation, Tubular Cell Apoptosis and Nephrotoxicity. Biochem. Pharmacol. 2007;73:1499–1510. doi: 10.1016/j.bcp.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araujo M., Wilcox C.S. Oxidative Stress in Hypertension: Role of the Kidney. Antioxid. Redox Signal. 2014;20:74–101. doi: 10.1089/ars.2013.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilcox C.S. Redox Regulation of the Afferent Arteriole and Tubuloglomerular Feedback. Acta Physiol. Scand. 2003;179:217–223. doi: 10.1046/j.0001-6772.2003.01205.x. [DOI] [PubMed] [Google Scholar]
- 33.Schnackenberg C.G. Physiological and Pathophysiological Roles of Oxygen Radicals in the Renal Microvasculature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R335–R342. doi: 10.1152/ajpregu.00605.2001. [DOI] [PubMed] [Google Scholar]
- 34.Wang D., Chabrashvili T., Wilcox C.S. Enhanced Contractility of Renal Afferent Arterioles from Angiotensin-Infused Rabbits: Roles of Oxidative Stress, Thromboxane Prostanoid Receptors, and Endothelium. Circ. Res. 2004;94:1436–1442. doi: 10.1161/01.RES.0000129578.76799.75. [DOI] [PubMed] [Google Scholar]
- 35.Dunlop M.E., Muggli E.E. Small Heat Shock Protein Alteration Provides a Mechanism to Reduce Mesangial Cell Contractility in Diabetes and Oxidative Stress. Kidney Int. 2000;57:464–475. doi: 10.1046/j.1523-1755.2000.00866.x. [DOI] [PubMed] [Google Scholar]
- 36.Stenvinkel P. Interactions between Inflammation, Oxidative Stress, and Endothelial Dysfunction in End-Stage Renal Disease. J. Ren. Nutr. 2003;13:144–148. doi: 10.1053/jren.2003.50018. [DOI] [PubMed] [Google Scholar]
- 37.Matsuoka H. Endothelial Dysfunction Associated with Oxidative Stress in Human. Diabetes Res. Clin. Pract. 2001;54:S65–S72. doi: 10.1016/S0168-8227(01)00337-0. [DOI] [PubMed] [Google Scholar]
- 38.Jha J.C., Ho F., Dan C., Jandeleit-Dahm K. A Causal Link between Oxidative Stress and Inflammation in Cardiovascular and Renal Complications of Diabetes. Clin. Sci. 2018;132:1811–1836. doi: 10.1042/CS20171459. [DOI] [PubMed] [Google Scholar]
- 39.Tucker P.S., Scanlan A.T., Dalbo V.J. Chronic Kidney Disease Influences Multiple Systems: Describing the Relationship between Oxidative Stress, Inflammation, Kidney Damage, and Concomitant Disease. Oxidative Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/806358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Himmelfarb J. Linking Oxidative Stress and Inflammation in Kidney Disease: Which Is the Chicken and Which Is the Egg? Semin. Dial. 2004;17:449–454. doi: 10.1111/j.0894-0959.2004.17605.x. [DOI] [PubMed] [Google Scholar]
- 41.Casanova A.G., Hernández-Sánchez M.T., Martínez-Salgado C., Morales A.I., Vicente-Vicente L., López-Hernández F.J. A Meta-Analysis of Preclinical Studies Using Antioxidants for the Prevention of Cisplatin Nephrotoxicity: Implications for Clinical Application. Crit. Rev. Toxicol. 2020:1–21. doi: 10.1080/10408444.2020.1837070. [DOI] [PubMed] [Google Scholar]
- 42.Casanova A.G., Vicente-Vicente L., Hernández-Sánchez M.T., Pescador M., Prieto M., Martínez-Salgado C., Morales A.I., López-Hernández F.J. Key Role of Oxidative Stress in Animal Models of Aminoglycoside Nephrotoxicity Revealed by a Systematic Analysis of the Antioxidant-to-Nephroprotective Correlation. Toxicology. 2017;385:10–17. doi: 10.1016/j.tox.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Begg C.B., Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 44.Egger M., Smith G.D., Schneider M., Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kutner M.H., Nachtsheim C.J., Neter J., Li W. Applied Linear Statistical Models. Volume 5 McGraw-Hill Irwin; New York, NY, USA: 2005. [Google Scholar]
- 46.Fischler M.A., Bolles R.C. Random Sample Consensus: A Paradigm for Model Fitting with Applications to Image Analysis and Automated Cartography. Commun. ACM. 1981;24:381–395. doi: 10.1145/358669.358692. [DOI] [Google Scholar]
- 47.Huber P.J. Breakthroughs in Statistics. Springer; New York, NY, USA: 1992. Robust estimation of a location parameter; pp. 492–518. [Google Scholar]
- 48.Pedregosa F., Varoquaux G., Gramfort A., Michel V., Thirion B., Grisel O., Blondel M., Prettenhofer P., Weiss R., Dubourg V. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011;12:2825–2830. [Google Scholar]
- 49.Python Language Reference. Python Software Foundation; Wilmington, DE, USA: 2013. [Google Scholar]
- 50.Abdel-Aziz A.M., Ibrahim M.A., El-Shiekh A.A., Osman N.A.T., Geddawy A., Abdelrahman A. Prophylactic Effect of Diacerein against Cisplatin-Induced Nephrotoxicity in Rats. Int. J. Pharmacol. 2018;14:384–390. doi: 10.3923/ijp.2018.384.390. [DOI] [Google Scholar]
- 51.Abdel Moneim A.E., Othman M.S., Aref A.M. Azadirachta Indica Attenuates Cisplatin-Induced Nephrotoxicity and Oxidative Stress. Biomed. Res. Int. 2014;2014:647131. doi: 10.1155/2014/647131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdel-Wahab W.M., Moussa F.I., Saad N.A. Synergistic Protective Effect of N-Acetylcysteine and Taurine against Cisplatin-Induced Nephrotoxicity in Rats. Drug Des. Dev. 2017;11:901–908. doi: 10.2147/DDDT.S131316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alibakhshi T., Khodayar M.J., Khorsandi L., Rashno M., Zeidooni L. Protective Effects of Zingerone on Oxidative Stress and Inflammation in Cisplatin-Induced Rat Nephrotoxicity. Biomed. Pharm. 2018;105:225–232. doi: 10.1016/j.biopha.2018.05.085. [DOI] [PubMed] [Google Scholar]
- 54.Al-Husseiny F., Sobh M.A., Ashour R.H., Foud S., Medhat T., El-Gilany A.-H., Elghannam D., Abdel-Ghaffar H., Saad M.-A., Sobh M. Amniotic Fluid-Derived Mesenchymal Stem Cells Cut Short the Acuteness of Cisplatin-Induced Nephrotoxicity in Sprague-Dawley Rats. Int. J. Stem Cells. 2016;9:70–78. doi: 10.15283/ijsc.2016.9.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amirshahrokhi K., Khalili A.-R. Thalidomide Ameliorates Cisplatin-Induced Nephrotoxicity by Inhibiting Renal Inflammation in an Experimental Model. Inflammation. 2015;38:476–484. doi: 10.1007/s10753-014-9953-7. [DOI] [PubMed] [Google Scholar]
- 56.An Y., Xin H., Yan W., Zhou X. Amelioration of Cisplatin-Induced Nephrotoxicity by Pravastatin in Mice. Exp. Toxicol. Pathol. 2011;63:215–219. doi: 10.1016/j.etp.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Badawy A.M., El-Naga R.N., Gad A.M., Tadros M.G., Fawzy H.M. Wogonin Pre-Treatment Attenuates Cisplatin-Induced Nephrotoxicity in Rats: Impact on PPAR-γ, Inflammation, Apoptosis and Wnt/β-Catenin Pathway. Chem. Biol. Interact. 2019;308:137–146. doi: 10.1016/j.cbi.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 58.Bami E., Ozakpınar O.B., Ozdemir-Kumral Z.N., Köroglu K., Ercan F., Cirakli Z., Sekerler T., Izzettin F.V., Sancar M., Okuyan B. Protective Effect of Ferulic Acid on Cisplatin Induced Nephrotoxicity in Rats. Environ. Toxicol Pharm. 2017;54:105–111. doi: 10.1016/j.etap.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Bayomi H.S., Elsherbiny N.M., El-Gayar A.M., Al-Gayyar M.M.H. Evaluation of Renal Protective Effects of Inhibiting TGF-β Type I Receptor in a Cisplatin-Induced Nephrotoxicity Model. Eur. Cytokine Netw. 2013;24:139–147. doi: 10.1684/ecn.2014.0344. [DOI] [PubMed] [Google Scholar]
- 60.Bazmandegan G., Amirteimoury M., Kaeidi A., Shamsizadeh A., Khademalhosseini M., Nematollahi M.H., Hassanipour M., Fatemi I. Sumatriptan Ameliorates Renal Injury Induced by Cisplatin in Mice. Iran. J. Basic Med. Sci. 2019;22:563–567. doi: 10.22038/ijbms.2019.33620.8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X., Wei W., Li Y., Huang J., Ci X. Hesperetin Relieves Cisplatin-Induced Acute Kidney Injury by Mitigating Oxidative Stress, Inflammation and Apoptosis. Chem. Biol. Interact. 2019;308:269–278. doi: 10.1016/j.cbi.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 62.Chirino Y.I., Sánchez-González D.J., Martínez-Martínez C.M., Cruz C., Pedraza-Chaverri J. Protective Effects of Apocynin against Cisplatin-Induced Oxidative Stress and Nephrotoxicity. Toxicology. 2008;245:18–23. doi: 10.1016/j.tox.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 63.Darwish M.A., Abo-Youssef A.M., Khalaf M.M., Abo-Saif A.A., Saleh I.G., Abdelghany T.M. Vitamin E Mitigates Cisplatin-Induced Nephrotoxicity Due to Reversal of Oxidative/Nitrosative Stress, Suppression of Inflammation and Reduction of Total Renal Platinum Accumulation. J. Biochem. Mol. Toxicol. 2017;31:1–9. doi: 10.1002/jbt.21833. [DOI] [PubMed] [Google Scholar]
- 64.Dehnamaki F., Karimi A., Pilevarian A.A., Fatemi I., Hakimizadeh E., Kaeidi A., Allahtavakoli M., Rahmani M.R., Khademalhosseini M., Bazmandegan G. Treatment with Troxerutin Protects against Cisplatin-Induced Kidney Injury in Mice. Acta Chir. Belg. 2019;119:31–37. doi: 10.1080/00015458.2018.1455418. [DOI] [PubMed] [Google Scholar]
- 65.Divya M.K., Lincy L., Raghavamenon A.C., Babu T.D. Ameliorative Effect of Apodytes Dimidiata on Cisplatin-Induced Nephrotoxicity in Wistar Rats. Pharm. Biol. 2016;54:2149–2157. doi: 10.3109/13880209.2016.1149494. [DOI] [PubMed] [Google Scholar]
- 66.Elhusseini F.M., Saad M.-A.A.A., Anber N., Elghannam D., Sobh M.-A., Alsayed A., El-Dusoky S., Sheashaa H., Abdel-Ghaffar H., Sobh M. Long Term Study of Protective Mechanisms of Human Adipose Derived Mesenchymal Stem Cells on Cisplatin Induced Kidney Injury in Sprague-Dawley Rats. J. Stem Cells Regen. Med. 2016;12:36–48. doi: 10.46582/jsrm.1201006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El-Naga R.N. Pre-Treatment with Cardamonin Protects against Cisplatin-Induced Nephrotoxicity in Rats: Impact on NOX-1, Inflammation and Apoptosis. Toxicol. Appl. Pharm. 2014;274:87–95. doi: 10.1016/j.taap.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 68.Elsherbiny N.M., Eladl M.A., Al-Gayyar M.M.H. Renal Protective Effects of Arjunolic Acid in a Cisplatin-Induced Nephrotoxicity Model. Cytokine. 2016;77:26–34. doi: 10.1016/j.cyto.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 69.Fatima S., Al-Mohaimeed N., Al-Shaikh Y., Tyagi P., Banu N., Hasan S., Arjumand S. Combined Treatment of Epigallocatechin Gallate and Coenzyme Q10 Attenuates Cisplatin-Induced Nephrotoxicity via Suppression of Oxidative/Nitrosative Stress, Inflammation and Cellular Damage. Food Chem. Toxicol. 2016;94:213–220. doi: 10.1016/j.fct.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 70.Fernández-Rojas B., Medina-Campos O.N., Hernández-Pando R., Negrette-Guzmán M., Huerta-Yepez S., Pedraza-Chaverri J. C-Phycocyanin Prevents Cisplatin-Induced Nephrotoxicity through Inhibition of Oxidative Stress. Food Funct. 2014;5:480–490. doi: 10.1039/C3FO60501A. [DOI] [PubMed] [Google Scholar]
- 71.Hassan H.A., Edrees G.M., El-Gamel E.M., El-Sayed E.A. Amelioration of Cisplatin-Induced Nephrotoxicity by Grape Seed Extract and Fish Oil Is Mediated by Lowering Oxidative Stress and DNA Damage. Cytotechnology. 2014;66:419–429. doi: 10.1007/s10616-013-9589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Helmy M.M., Helmy M.W., Abd Allah D.M., Abo Zaid A.M., Mohy El-Din M.M. Selective ETA Receptor Blockade Protects against Cisplatin-Induced Acute Renal Failure in Male Rats. Eur. J. Pharmacol. 2014;730:133–139. doi: 10.1016/j.ejphar.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Hosseini A., Fanoudi S., Mollazadeh H., Aghaei A., Boroushaki M.T. Protective Effect of Rheum Turkestanicum against Cisplatin by Reducing Oxidative Stress in Kidney Tissue. J. Pharm. Bioallied Sci. 2018;10:66–71. doi: 10.4103/JPBS.JPBS_9_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang Y.-C., Tsai M.-S., Hsieh P.-C., Shih J.-H., Wang T.-S., Wang Y.-C., Lin T.-H., Wang S.-H. Galangin Ameliorates Cisplatin-Induced Nephrotoxicity by Attenuating Oxidative Stress, Inflammation and Cell Death in Mice through Inhibition of ERK and NF-KappaB Signaling. Toxicol. Appl. Pharm. 2017;329:128–139. doi: 10.1016/j.taap.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 75.Huang H., Shen Z., Geng Q., Wu Z., Shi P., Miao X. Protective Effect of Schisandra Chinensis Bee Pollen Extract on Liver and Kidney Injury Induced by Cisplatin in Rats. Biomed. Pharm. 2017;95:1765–1776. doi: 10.1016/j.biopha.2017.09.083. [DOI] [PubMed] [Google Scholar]
- 76.Huang S., You J., Wang K., Li Y., Zhang Y., Wei H., Liang X., Liu Y. N-Acetylcysteine Attenuates Cisplatin-Induced Acute Kidney Injury by Inhibiting the C5a Receptor. Biomed. Res. Int. 2019;2019:4805853. doi: 10.1155/2019/4805853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kandemir F.M., Yildirim S., Caglayan C., Kucukler S., Eser G. Protective Effects of Zingerone on Cisplatin-Induced Nephrotoxicity in Female Rats. Environ. Sci. Pollut. Res. Int. 2019;26:22562–22574. doi: 10.1007/s11356-019-05505-3. [DOI] [PubMed] [Google Scholar]
- 78.Kang L., Zhao H., Chen C., Zhang X., Xu M., Duan H. Sappanone A Protects Mice against Cisplatin-Induced Kidney Injury. Int. Immunopharmacol. 2016;38:246–251. doi: 10.1016/j.intimp.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 79.Kenza B., Djihane A., Mouad B., Ratiba M., Samir B., Fadila B., Souad A. Renoprotective Effect of Centaurea Choulettiana Pomel (Asteraceae) Leaves on Cisplatin-Induced Oxidative Stress and Renal Dysfunction in Mice. J. Appl. Pharm. Sci. 2017;7:147–154. [Google Scholar]
- 80.Khairnar S.I., Mahajan U.B., Patil K.R., Patel H.M., Shinde S.D., Goyal S.N., Belemkar S., Ojha S., Patil C.R. Disulfiram and Its Copper Chelate Attenuate Cisplatin-Induced Acute Nephrotoxicity in Rats Via Reduction of Oxidative Stress and Inflammation. Biol. Trace Elem. Res. 2020;193:174–184. doi: 10.1007/s12011-019-01683-w. [DOI] [PubMed] [Google Scholar]
- 81.Kim J.-Y., Park J.-H., Kim K., Jo J., Leem J., Park K.-K. Pharmacological Inhibition of Caspase-1 Ameliorates Cisplatin-Induced Nephrotoxicity through Suppression of Apoptosis, Oxidative Stress, and Inflammation in Mice. Mediat. Inflamm. 2018;2018:e6571676. doi: 10.1155/2018/6571676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li F., Yao Y., Huang H., Hao H., Ying M. Xanthohumol Attenuates Cisplatin-Induced Nephrotoxicity through Inhibiting NF-ΚB and Activating Nrf2 Signaling Pathways. Int. Immunopharmacol. 2018;61:277–282. doi: 10.1016/j.intimp.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 83.Li Y.-Z., Ren S., Yan X.-T., Li H.-P., Li W., Zheng B., Wang Z., Liu Y.-Y. Improvement of Cisplatin-Induced Renal Dysfunction by Schisandra Chinensis Stems via Anti-Inflammation and Anti-Apoptosis Effects. J. Ethnopharmacol. 2018;217:228–237. doi: 10.1016/j.jep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 84.Li R.-Y., Zhang W.-Z., Yan X.-T., Hou J.-G., Wang Z., Ding C.-B., Liu W.-C., Zheng Y.-N., Chen C., Li Y.-R., et al. Arginyl-Fructosyl-Glucose, a Major Maillard Reaction Product of Red Ginseng, Attenuates Cisplatin-Induced Acute Kidney Injury by Regulating Nuclear Factor ΚB and Phosphatidylinositol 3-Kinase/Protein Kinase B Signaling Pathways. J. Agric. Food Chem. 2019;67:5754–5763. doi: 10.1021/acs.jafc.9b00540. [DOI] [PubMed] [Google Scholar]
- 85.Ma P., Zhang S., Su X., Qiu G., Wu Z. Protective Effects of Icariin on Cisplatin-Induced Acute Renal Injury in Mice. Am. J. Transl. Res. 2015;7:2105–2114. [PMC free article] [PubMed] [Google Scholar]
- 86.Ma X., Yan L., Zhu Q., Shao F. Puerarin Attenuates Cisplatin-Induced Rat Nephrotoxicity: The Involvement of TLR4/NF-ΚB Signaling Pathway. PLoS ONE. 2017;12:e0171612. doi: 10.1371/journal.pone.0171612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malik S., Bhatia J., Suchal K., Gamad N., Dinda A.K., Gupta Y.K., Arya D.S. Nobiletin Ameliorates Cisplatin-Induced Acute Kidney Injury Due to Its Anti-Oxidant, Anti-Inflammatory and Anti-Apoptotic Effects. Exp. Toxicol. Pathol. 2015;67:427–433. doi: 10.1016/j.etp.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 88.Mi X., Hou J., Wang Z., Han Y., Ren S., Hu J., Chen C., Li W. The Protective Effects of Maltol on Cisplatin-Induced Nephrotoxicity through the AMPK-Mediated PI3K/Akt and P53 Signaling Pathways. Sci. Rep. 2018;8:15922. doi: 10.1038/s41598-018-34156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohamed H.E., El-Swefy S.E., Mohamed R.H., Ghanim A.M.H. Effect of Erythropoietin Therapy on the Progression of Cisplatin Induced Renal Injury in Rats. Exp. Toxicol. Pathol. 2013;65:197–203. doi: 10.1016/j.etp.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 90.Morsy M.A., Heeba G.H. Nebivolol Ameliorates Cisplatin-Induced Nephrotoxicity in Rats. Basic Clin. Pharm. Toxicol. 2016;118:449–455. doi: 10.1111/bcpt.12538. [DOI] [PubMed] [Google Scholar]
- 91.Mundhe N.A., Kumar P., Ahmed S., Jamdade V., Mundhe S., Lahkar M. Nordihydroguaiaretic Acid Ameliorates Cisplatin Induced Nephrotoxicity and Potentiates Its Anti-Tumor Activity in DMBA Induced Breast Cancer in Female Sprague-Dawley Rats. Int. Immunopharmacol. 2015;28:634–642. doi: 10.1016/j.intimp.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 92.Mundhe N., Kumar P., Arora I., Ahmed S., Lahkar M. Differential Effect of NDGA on Cisplatin-Induced Nephrotoxicity in Spargue-Dawley Rats. Immunopharmacol. Immunotoxicol. 2019;41:68–75. doi: 10.1080/08923973.2018.1547741. [DOI] [PubMed] [Google Scholar]
- 93.Nazari Soltan Ahmad S., Rashtchizadeh N., Argani H., Roshangar L., Ghorbanihaghjo A., Sanajou D., Panah F., Ashrafi Jigheh Z., Dastmalchi S., Kalantary-Charvadeh A. Tangeretin Protects Renal Tubular Epithelial Cells against Experimental Cisplatin Toxicity. Iran. J. Basic Med. Sci. 2019;22:179–186. doi: 10.22038/ijbms.2018.32010.7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nazari Soltan Ahmad S., Rashtchizadeh N., Argani H., Roshangar L., Ghorbani Haghjo A., Sanajou D., Panah F., Ashrafi Jigheh Z., Dastmalchi S., Mesgari-Abbasi M. Dunnione Protects against Experimental Cisplatin-Induced Nephrotoxicity by Modulating NQO1 and NAD+ Levels. Free Radic. Res. 2018;52:808–817. doi: 10.1080/10715762.2018.1475732. [DOI] [PubMed] [Google Scholar]
- 95.Neamatallah T., El-Shitany N.A., Abbas A.T., Ali S.S., Eid B.G. Honey Protects against Cisplatin-Induced Hepatic and Renal Toxicity through Inhibition of NF-ΚB-Mediated COX-2 Expression and the Oxidative Stress Dependent BAX/Bcl-2/Caspase-3 Apoptotic Pathway. Food Funct. 2018;9:3743–3754. doi: 10.1039/C8FO00653A. [DOI] [PubMed] [Google Scholar]
- 96.Purena R., Seth R., Bhatt R. Protective Role of Emblica Officinalis Hydro-Ethanolic Leaf Extract in Cisplatin Induced Nephrotoxicity in Rats. Toxicol. Rep. 2018;5:270–277. doi: 10.1016/j.toxrep.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qi Z., Li Z., Li W., Liu Y., Wang C., Lin H., Liu J., Li P. Pseudoginsengenin DQ Exhibits Therapeutic Effects in Cisplatin-Induced Acute Kidney Injury via Sirt1/NF-ΚB and Caspase Signaling Pathway without Compromising Its Antitumor Activity in Mice. Molecules. 2018;23:3038. doi: 10.3390/molecules23113038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Radwan R.R., Abdel Fattah S.M. Mechanisms Involved in the Possible Nephroprotective Effect of Rutin and Low Dose γ Irradiation against Cisplatin-Induced Nephropathy in Rats. J. Photochem. Photobiol. B. 2017;169:56–62. doi: 10.1016/j.jphotobiol.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 99.Rana M.A., Khan R.A., Nasiruddin M., Khan A.A. Amelioration of Cisplatin-Induced Nephrotoxicity by Ethanolic Extract of Bauhinia Purpurea: An in Vivo Study in Rats. Saudi J. Kidney Dis. Transpl. 2016;27:41–48. doi: 10.4103/1319-2442.174068. [DOI] [PubMed] [Google Scholar]
- 100.Sahin K., Orhan C., Tuzcu M., Muqbil I., Sahin N., Gencoglu H., Guler O., Padhye S.B., Sarkar F.H., Mohammad R.M. Comparative in Vivo Evaluations of Curcumin and Its Analog Difluorinated Curcumin against Cisplatin-Induced Nephrotoxicity. Biol. Trace Elem. Res. 2014;157:156–163. doi: 10.1007/s12011-014-9886-x. [DOI] [PubMed] [Google Scholar]
- 101.Saifi M.A., Sangomla S., Khurana A., Godugu C. Protective Effect of Nanoceria on Cisplatin-Induced Nephrotoxicity by Amelioration of Oxidative Stress and Pro-Inflammatory Mechanisms. Biol. Trace Elem. Res. 2019;189:145–156. doi: 10.1007/s12011-018-1457-0. [DOI] [PubMed] [Google Scholar]
- 102.Sen S., Chakraborty R., Kalita P. Dillenia Indica Fruit Prevents Cisplatin-Induced Kidney Injury in Experimental Rats through Modulation of Oxidative Stress, Marker Enzyme, and Biochemical Changes. Nutrire. 2018;43:15. doi: 10.1186/s41110-018-0074-1. [DOI] [Google Scholar]
- 103.Sener M.T., Sener E., Tok A., Polat B., Cinar I., Polat H., Akcay F., Suleyman H. Biochemical and Histologic Study of Lethal Cisplatin Nephrotoxicity Prevention by Mirtazapine. Pharm. Rep. 2012;64:594–602. doi: 10.1016/S1734-1140(12)70855-1. [DOI] [PubMed] [Google Scholar]
- 104.Sharma S.K., Goyal N. Protective Effect of Heliotropium Eichwaldi against Cisplatin-Induced Nephrotoxicity in Mice. Zhong Xi Yi Jie He Xue Bao. 2012;10:555–560. doi: 10.3736/jcim20120511. [DOI] [PubMed] [Google Scholar]
- 105.Sherif I.O. Amelioration of Cisplatin-Induced Nephrotoxicity in Rats by Triterpenoid Saponin of Terminalia Arjuna. Clin. Exp. Nephrol. 2015;19:591–597. doi: 10.1007/s10157-014-1056-0. [DOI] [PubMed] [Google Scholar]
- 106.Shi H.-H., Wang C.-C., Guo Y., Xue C.-H., Zhang T.-T., Wang Y.-M. DHA-PC Protects Kidneys against Cisplatin-Induced Toxicity and Its Underlying Mechanisms in Mice. Food Funct. 2019;10:1571–1581. doi: 10.1039/C8FO02386G. [DOI] [PubMed] [Google Scholar]
- 107.Topcu-Tarladacalisir Y., Sapmaz-Metin M., Karaca T. Curcumin Counteracts Cisplatin-Induced Nephrotoxicity by Preventing Renal Tubular Cell Apoptosis. Ren. Fail. 2016;38:1741–1748. doi: 10.1080/0886022X.2016.1229996. [DOI] [PubMed] [Google Scholar]
- 108.Wang Z., Li Y.-F., Han X.-Y., Sun Y.-S., Zhang L.-X., Liu W., Liu X.-X., Li W., Liu Y.-Y. Kidney Protection Effect of Ginsenoside Re and Its Underlying Mechanisms on Cisplatin-Induced Kidney Injury. Cell. Physiol. Biochem. 2018;48:2219–2229. doi: 10.1159/000492562. [DOI] [PubMed] [Google Scholar]
- 109.Yang H., Li W., Wang L., Li W., Sun H., He X., Zhang J. The Protective Effects of Sika Deer Antler Protein on Cisplatin-Induced Nephrotoxicity. Cell. Physiol. Biochem. 2017;43:395–404. doi: 10.1159/000480418. [DOI] [PubMed] [Google Scholar]
- 110.Yu X., Meng X., Xu M., Zhang X., Zhang Y., Ding G., Huang S., Zhang A., Jia Z. Celastrol Ameliorates Cisplatin Nephrotoxicity by Inhibiting NF-ΚB and Improving Mitochondrial Function. EBioMedicine. 2018;36:266–280. doi: 10.1016/j.ebiom.2018.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang L., Gu Y., Li H., Cao H., Liu B., Zhang H., Shao F. Daphnetin Protects against Cisplatin-Induced Nephrotoxicity by Inhibiting Inflammatory and Oxidative Response. Int. Immunopharmacol. 2018;65:402–407. doi: 10.1016/j.intimp.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 112.Burnham K., Anderson D. Model Selection and Multi-Model Inference. 2nd ed. Volume 63. Springer; New York, NY, USA: 2002. [Google Scholar]
- 113.Nolfi-Donegan D., Braganza A., Shiva S. Mitochondrial Electron Transport Chain: Oxidative Phosphorylation, Oxidant Production, and Methods of Measurement. Redox Biol. 2020;37:101674. doi: 10.1016/j.redox.2020.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murphy M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dan Dunn J., Alvarez L.A.J., Zhang X., Soldati T. Reactive Oxygen Species and Mitochondria: A Nexus of Cellular Homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Galluzzi L., Kepp O., Trojel-Hansen C., Kroemer G. Mitochondrial Control of Cellular Life, Stress, and Death. Circ. Res. 2012;111:1198–1207. doi: 10.1161/CIRCRESAHA.112.268946. [DOI] [PubMed] [Google Scholar]
- 118.Tait S.W.G., Green D.R. Mitochondria and Cell Death: Outer Membrane Permeabilization and Beyond. Nat. Rev. Mol. Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 119.Armstrong J.S. Mitochondrial Membrane Permeabilization: The Sine qua Non for Cell Death. BioEssays. 2006;28:253–260. doi: 10.1002/bies.20370. [DOI] [PubMed] [Google Scholar]
- 120.Green D.R., Amarante-Mendes G.P. The Point of No Return: Mitochondria, Caspases, and the Commitment to Cell Death. Results Probl. Cell Differ. 1998;24:45–61. doi: 10.1007/978-3-540-69185-3_3. [DOI] [PubMed] [Google Scholar]
- 121.Cao S.S., Kaufman R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Green D.R., Kroemer G. The Pathophysiology of Mitochondrial Cell Death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 123.Marzo I., Brenner C., Kroemer G. The Central Role of the Mitochondrial Megachannel in Apoptosis: Evidence Obtained with Intact Cells, Isolated Mitochondria, and Purified Protein Complexes. Biomed. Pharmacother. 1998;52:248–251. doi: 10.1016/S0753-3322(98)80009-7. [DOI] [PubMed] [Google Scholar]
- 124.Bernardi P., Rasola A., Forte M., Lippe G. The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol. Rev. 2015;95:1111–1155. doi: 10.1152/physrev.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Crompton M. The Mitochondrial Permeabitity Transition Pore and Its Role in Cell Death. Biochem. J. 1999;341:233–249. doi: 10.1042/bj3410233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cullen K.J., Yang Z., Schumaker L., Guo Z. Mitochondria as a Critical Target of the Chemotheraputic Agent Cisplatin in Head and Neck Cancer. J. Bioenerg. Biomembr. 2007;39:43–50. doi: 10.1007/s10863-006-9059-5. [DOI] [PubMed] [Google Scholar]
- 127.Santandreu F.M., Roca P., Oliver J. Uncoupling Protein-2 Knockdown Mediates the Cytotoxic Effects of Cisplatin. Free Radic. Biol. Med. 2010;49:658–666. doi: 10.1016/j.freeradbiomed.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 128.Yang Z., Schumaker L.M., Egorin M.J., Zuhowski E.G., Quo Z., Cullen K.J. Cisplatin Preferentially Binds Mitochondrial DNA and Voltage-Dependent Anion Channel Protein in the Mitochondrial Membrane of Head and Neck Squamous Cell Carcinoma: Possible Role in Apoptosis. Clin. Cancer Res. 2006;12:5817–5825. doi: 10.1158/1078-0432.CCR-06-1037. [DOI] [PubMed] [Google Scholar]
- 129.Custódio J.B.A., Cardoso C.M.P., Santos M.S., Almeida L.M., Vicente J.A.F., Fernandes M.A.S. Cisplatin Impairs Rat Liver Mitochondrial Functions by Inducing Changes on Membrane Ion Permeability: Prevention by Thiol Group Protecting Agents. Toxicology. 2009;259:18–24. doi: 10.1016/j.tox.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 130.Larosa V., Remacle C. Insights into the Respiratory Chain and Oxidative Stress. Biosci. Rep. 2018;38:BSR20171492. doi: 10.1042/BSR20171492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stöckl P., Zankl C., Hütter E., Unterluggauer H., Laun P., Heeren G., Bogengruber E., Herndler-Brandstetter D., Breitenbach M., Jansen-Dürr P. Partial Uncoupling of Oxidative Phosphorylation Induces Premature Senescence in Human Fibroblasts and Yeast Mother Cells. Free Radic. Biol. Med. 2007;43:947–958. doi: 10.1016/j.freeradbiomed.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 132.Šileikytė J., Forte M. The Mitochondrial Permeability Transition in Mitochondrial Disorders. Oxidative Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/3403075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chernyak B.V. Redox Regulation of the Mitochondrial Permeability Transition Pore. Biosci. Rep. 1997;17:293–302. doi: 10.1023/A:1027384628678. [DOI] [PubMed] [Google Scholar]
- 134.Kowaltowski A.J., Castilho R.F., Vercesi A.E. Mitochondrial Permeability Transition and Oxidative Stress. FEBS Lett. 2001;495:12–15. doi: 10.1016/S0014-5793(01)02316-X. [DOI] [PubMed] [Google Scholar]
- 135.Batandier C., Leverve X., Fontaine E. Opening of the Mitochondrial Permeability Transition Pore Induces Reactive Oxygen Species Production at the Level of the Respiratory Chain Complex I. J. Biol. Chem. 2004;279:17197–17204. doi: 10.1074/jbc.M310329200. [DOI] [PubMed] [Google Scholar]
- 136.Quoilin C., Mouithys-Mickalad A., Lécart S., Fontaine-Aupart M.P., Hoebeke M. Evidence of Oxidative Stress and Mitochondrial Respiratory Chain Dysfunction in an in Vitro Model of Sepsis-Induced Kidney Injury. Biochim. Biophys. Acta Bioenerg. 2014;1837:1790–1800. doi: 10.1016/j.bbabio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 137.Sastre J., Pallardö F.V., Viña J. Mitochondrial Oxidative Stress Plays a Key Role in Aging and Apoptosis. Iubmb Life. 2000;49:427–435. doi: 10.1080/152165400410281. [DOI] [PubMed] [Google Scholar]
- 138.Chang B., Nishikawa M., Sato E., Utsumi K., Inoue M. L-Carnitine Inhibits Cisplatin-Induced Injury of the Kidney and Small Intestine. Arch. Biochem. Biophys. 2002;405:55–64. doi: 10.1016/S0003-9861(02)00342-9. [DOI] [PubMed] [Google Scholar]
- 139.Murata T., Hibasami H., Maekawa S., Tagawa T., Nakashima K. Preferential Binding of Cisplatin to Mitochondrial DNA and Suppression of ATP Generation in Human Malignant Melanoma Cells. Biochem. Int. 1990;20:949–955. [PubMed] [Google Scholar]
- 140.Olivero O.A., Semino C., Kassim A., Lopez-Larraza D.M., Poirier M.C. Preferential Binding of Cisplatin to Mitochondrial DNA of Chinese Hamster Ovary Cells. Mutat. Res. Lett. 1995;346:221–230. doi: 10.1016/0165-7992(95)90039-X. [DOI] [PubMed] [Google Scholar]
- 141.Greggi Antunes L.M., D’arc C., Darin J., Bianchi M.D.L.P. Protective Effects of Vitamin C against Cisplatin-Induced Nephrotoxicity and Lipid Peroxidation in Adult Rats: A Dose-Dependent Study. Pharmacol. Res. 2000;41:405–411. doi: 10.1006/phrs.1999.0600. [DOI] [PubMed] [Google Scholar]
- 142.Hannemann J., Duwe J., Baumann K. Iron- and Ascorbic Acid-Induced Lipid Peroxidation in Renal Microsomes Isolated from Rats Treated with Platinum Compounds. Cancer Chemother. Pharmacol. 1991;28:427–433. doi: 10.1007/BF00685818. [DOI] [PubMed] [Google Scholar]
- 143.Yasuyuki S., Takahiro S., Yoshio T. Mechanism of the Increase in Lipid Peroxide Induced by Cisplatin in the Kidneys of Rats. Toxicol. Lett. 1992;62:293–300. doi: 10.1016/0378-4274(92)90033-G. [DOI] [PubMed] [Google Scholar]
- 144.Kadikoylu G., Bolaman Z., Demir S., Balkaya M., Akalin N., Enli Y. The Effects of Desferrioxamine on Cisplatin-Induced Lipid Peroxidation and the Activities of Antioxidant Enzymes in Rat Kidneys. Hum. Exp. Toxicol. 2004;23:29–34. doi: 10.1191/0960327104ht413oa. [DOI] [PubMed] [Google Scholar]
- 145.Makris K., Spanou L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016;37:85–98. [PMC free article] [PubMed] [Google Scholar]
- 146.Ronco C., Kellum J.A., Haase M. Subclinical AKI Is Still AKI. Crit. Care. 2012;16:313. doi: 10.1186/cc11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murray P.T., Mehta R.L., Shaw A., Ronco C., Endre Z., Kellum J.A., Chawla L.S., Cruz D., Ince C., Okusa M.D. Potential Use of Biomarkers in Acute Kidney Injury: Report and Summary of Recommendations from the 10th Acute Dialysis Quality Initiative Consensus Conference. Kidney Int. 2014;85:513–521. doi: 10.1038/ki.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fattah H., Layton A., Vallon V. How Do Kidneys Adapt to a Deficit or Loss in Nephron Number? Physiology. 2019;34:189–197. doi: 10.1152/physiol.00052.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hamzeh M., Amiri F.T., Beklar S.Y., Hosseinimehr S.J. Nephroprotective Effect of Cerium Oxide Nanoparticles on Cyclophosphamide-Induced Nephrotoxicity via Anti-Apoptotic and Antioxidant Properties in BALB/c Mice. Marmara Pharm. J. 2018;22 doi: 10.12991/mpj.2018.55. [DOI] [Google Scholar]
- 150.Hirst S.M., Karakoti A.S., Tyler R.D., Sriranganathan N., Seal S., Reilly C.M. Anti-Inflammatory Properties of Cerium Oxide Nanoparticles. Small. 2009;5:2848–2856. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- 151.Xu M.-X., Zhu Y.-F., Chang H.-F., Liang Y. Nanoceria Restrains PM2.5-Induced Metabolic Disorder and Hypothalamus Inflammation by Inhibition of Astrocytes Activation Related NF-ΚB Pathway in Nrf2 Deficient Mice. Free Radic. Biol. Med. 2016;99:259–272. doi: 10.1016/j.freeradbiomed.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 152.Moore E., Bellomo R. Erythropoietin (EPO) in Acute Kidney Injury. Ann. Intensive Care. 2011;1:3. doi: 10.1186/2110-5820-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Abe T., Isaka Y., Imamura R., Kakuta Y., Okumi M., Yazawa K., Ichimaru N., Tsuda H., Nonomura N., Takahara S., et al. Carbamylated Erythropoietin Ameliorates Cyclosporine Nephropathy without Stimulating Erythropoiesis. Cell Transplant. 2012;21:571–580. doi: 10.3727/096368911X605501. [DOI] [PubMed] [Google Scholar]
- 154.Pallet N., Rabant M., Legendre C., Martinez F., Choukroun G. The Nephroprotective Properties of Recombinant Human Erythropoietin in Kidney Transplantation: Experimental Facts and Clinical Proofs. Am. J. Transplant. 2012;12:3184–3190. doi: 10.1111/j.1600-6143.2012.04287.x. [DOI] [PubMed] [Google Scholar]
- 155.Koca U., Süntar I.P., Keles H., Yesilada E., Akkol E.K. In Vivo Anti-Inflammatory and Wound Healing Activities of Centaurea Iberica Trev. Ex Spreng. J. Ethnopharmacol. 2009;126:551–556. doi: 10.1016/j.jep.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 156.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C.B., Rahu N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016;2016:e7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lugrin J., Rosenblatt-Velin N., Parapanov R., Liaudet L. The Role of Oxidative Stress during Inflammatory Processes. Biol. Chem. 2014;395:203–230. doi: 10.1515/hsz-2013-0241. [DOI] [PubMed] [Google Scholar]
- 158.Kannan K., Jain S.K. K. Oxidative Stress and Apoptosis. Pathophysiology. 2000;7:153–163. doi: 10.1016/S0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 159.Casanova A.G., Hernández-Sánchez M.T., López-Hernández F.J., Martínez-Salgado C., Prieto M., Vicente-Vicente L., Morales A.I. Systematic Review and Meta-Analysis of the Efficacy of Clinically Tested Protectants of Cisplatin Nephrotoxicity. Eur. J. Clin. Pharmacol. 2020;76:23–33. doi: 10.1007/s00228-019-02771-5. [DOI] [PubMed] [Google Scholar]
- 160.Sanchez-Gonzalez P.D., Lopez-Hernandez F.J., Perez-Barriocanal F., Morales A.I., Lopez-Novoa J.M. Quercetin Reduces Cisplatin Nephrotoxicity in Rats without Compromising Its Anti-Tumour Activity. Nephrol. Dial. Transplant. 2011;26:3484–3495. doi: 10.1093/ndt/gfr195. [DOI] [PubMed] [Google Scholar]
- 161.Sánchez-González P.D., López-Hernández F.J., Dueñas M., Prieto M., Sánchez-López E., Thomale J., Ruiz-Ortega M., López-Novoa J.M., Morales A.I. Differential Effect of Quercetin on Cisplatin-Induced Toxicity in Kidney and Tumor Tissues. Food Chem. Toxicol. 2017;107:226–236. doi: 10.1016/j.fct.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 162.Muldoon L.L., Wu Y.J., Pagel M.A., Neuwelt E.A. N-Acetylcysteine Chemoprotection without Decreased Cisplatin Antitumor Efficacy in Pediatric Tumor Models. J. Neuro Oncol. 2015;121:433–440. doi: 10.1007/s11060-014-1657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hirosawa A., Niitani H., Hayashibara K., Tsuboi E. Effects of Sodium Thiosulfate in Combination Therapy of Cis-Dichlorodiammineplatinum and Vindesine. Cancer Chemother. Pharmacol. 1989;23:255–258. doi: 10.1007/BF00451652. [DOI] [PubMed] [Google Scholar]
- 164.Visacri M.B., Quintanilha J.C.F., de Sousa V.M., Amaral L.S., de F.L. Rosiane Ambrósio, Calonga L., Curi S.F.B.B., Mayra M.F., Chone C.T., Altemani J.M.C., et al. Can Acetylcysteine Ameliorate Cisplatin-Induced Toxicities and Oxidative Stress without Decreasing Antitumor Efficacy? A Randomized, Double-Blind, Placebo-Controlled Trial Involving Patients with Head and Neck Cancer. Cancer Med. 2019;8:2020–2030. doi: 10.1002/cam4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the article.