Abstract
Purpose
To evaluate the occurrence of retinal microvasculopathy in patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and who developed coronavirus disease (COVID-19).
Design
Systematic review and meta-analysis.
Methods
The Pubmed and Embase databases were comprehensively searched to identify studies that reported retina vascular changes in eyes with COVID-19. Two independent reviewers selected papers and extracted data for analysis. Data of interest were extracted and analyzed in RevMan Web versions 3.3. Quality of evidence was assessed using the National Institute of Health quality assessment tool for a case-control study.
Results
Thirty-one studies reporting on 1373 subjects (972 COVID-19 and 401 controls) were included. Only case-control studies were included in the pooled analysis. There was a significantly higher likelihood of retinal microvasculopathy in subjects with COVID-19 compared to controls (odds ratio [95% confidence interval], 8.86 [2.54-27.53], P < .01). Optical coherence tomography angiography (OCTA) revealed reduced vessel density and enlarged foveal avascular zone in subjects with COVID-19 compared to controls.
Conclusions
The results suggested that COVID-19-related retinal microvasculopathy is a significant ocular manifestation of COVID-19 and may herald future retinal complications. These microvascular impairments might have occurred antecedent to clinically visible changes and could be detected earlier by OCTA. These findings are significant, due to the large numbers with COVID-19, and need to be recognized by ophthalmologists as a potential long-term sequalae of the disease.
Keywords: COVID-19, SARS-CoV-2, retina microvasculopathy, retinopathy, cotton wool spots, retina haemorrhage, OCTA, vessel density, FAZ, review
INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a pandemic in March 2020.1 To date, there have been more than 170 million confirmed COVID-19 cases globally. The virus primarily affects the respiratory system and varies from mild constitutional symptoms to pneumonia, sepsis, and sometimes severe acute respiratory distress syndrome (ARDS) necessitating hospitalization and intensive care unit (ICU) admission.2 There is increasing evidence of the virus also causing thrombo-embolic complications, with rates reported to be as high as 30%.3, 4, 5 This thrombo-embolic sequalae is linked to thrombo-inflammation and endothelial cell injury thought to be mediated by the over production of inflammatory cytokines.6
The eyes are not spared by COVID-19. In fact, anterior segment involvement has been described since early case series, manifesting as conjunctivitis.7, 8, 9, 10 SARS-CoV-2 RNA has been detected in infected patients’ tears as well as in conjunctival tissue.11 , 12 In contrast, posterior segment manifestations are less well understood. Marinho and associates13 first reported 12 cases of patients with mild-to-moderate COVID-19 infection with retinal signs, including retinal hemorrhages, cotton wool spots (CWS), and optical coherence tomography (OCT) abnormalities.13 However, several authors have demonstrated how the OCT abnormalities initially reported were a misinterpretation and could be explained by variation in normal retinal vasculature on OCT b-scans.14, 15, 16 In addition, without a comparative control group, the retinal findings such as CWS could have been non-specific and incidental. However, since the initial report by Marinho, several case-control studies have reported retinal features in patients with COVID-19 infection with various degrees of severity.17, 18, 19, 20, 21, 22, 23, 24, 25
In addition to clinical features, several OCT angiography (OCTA) studies have demonstrated a reduction in vessel density (VD) in COVID-19 patients, which corroborated with the possibility of COVID-19-related retinal microvasculopathy.17, 18, 19, 20 , 23, 24, 25, 26 Importantly, these perfusion deficits have been detected in asymptomatic individuals with no significant medical comorbidities who have recovered from seemingly mild COVID-19 infections. Therefore, understanding of the prevalence and progression pattern of such perfusion deficits may have significant public health implications in the long term.
This review aimed to summarize the evidence and critique the evidence related to COVID-19-related retinal microvasculopathy and examine the potential underlying biological mechanisms.
METHODS
All eligible studies were identified using a two-level search strategy. First, MEDLINE and EMBASE were searched on May 26, 2021 using PubMed. The search terms that were used were ‘retina’ OR ‘retinal vasculature’ AND ‘COVID’ OR ‘SARS-CoV’. Next, relevant studies were identified by two authors, who reviewed the abstract of each article.
DATA EXTRACTION
Potentially relevant studies describing retinal findings, retinal pathology, and retinal imaging were retained. Each article was individually screened for reports on the prevalence of retinal signs. Excluded studies were those investigating other ocular associations with COVID-19 infection, including uveitis, ocular inflammation, and other anterior segment changes.
Data were separately summarized for 1) case-control studies and 2) case series. For case-control studies, it was planned to analyze data for the prevalence of retinal microangiopathy and quantitative changes indicative of retinal microangiopathy on OCTA. Retinal microangiopathy was defined by the presence of retinal hemorrhages, CWS, and/or vascular tortuosity on clinical examination or fundus imaging. Parafoveal VD and foveal avascular zone (FAZ) size were assessed as quantitative parameters on OCTA. These data were extracted from each study where available.
The number of eyes with retinal hemorrhages or CWS in cases and controls (where available) was collated. Data regarding the prevalence of retinal microangiographic signs (retinal hemorrhages and/or CWS) were pooled in the case-control studies and a subject was defined to have retinal microvasculopathy if both or either retinal hemorrhages or CWS were detected. These data regarding retinal microvasculopathy prevalence in both the group with COVID-19 infection and non-infected controls were used to generate pooled odds ratios (ORs) and 95% confidence intervals (CIs) of presence for retinal microvasculopathy versus no retinal microvasculopathy.
In addition, data regarding the mean parafoveal VD and FAZ size (when available) on OCTA were used to generate pooled means and 95% CIs of each measure, respectively. Sensitivity analysis was used to confirm each difference by sequentially omitting each of the included studies and recalculating the summary difference and 95% CI. The I2 statistic was used to determine heterogeneity across studies, such that heterogeneity was quantified irrespective of the number of studies. A P-value of < .05 was considered to be statistically significant. Forest plots were used to summarize pooled results. Risk of bias assessment was performed on the case-control studies using the National Institute of Health quality assessment tool for a case-control study. Pooled analysis was performed with RevMan Web versions 3.3. This review has not been registered.
RESULTS
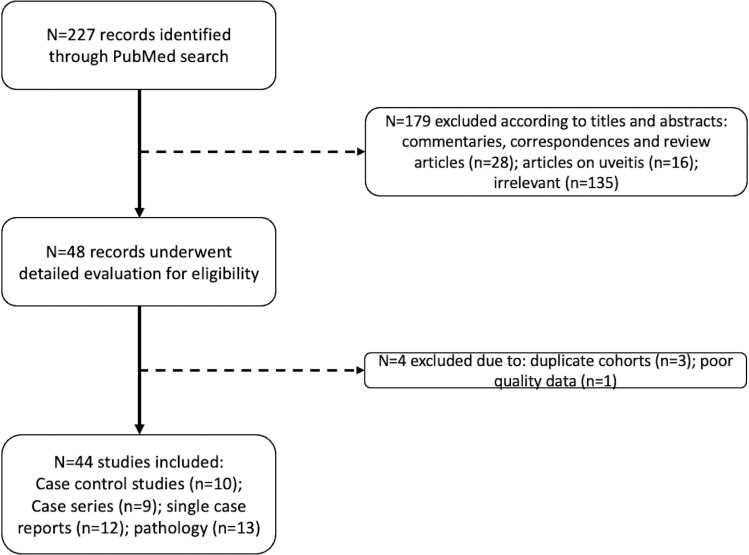
This study identified 227 articles from the initial search (Figure 1 ). After reviewing the abstracts, 179 articles were excluded. The remaining 48 articles were further reviewed with full-text examination. This analysis included articles that described retinal signs related to microvasculopathy or evaluated retinal vasculature through imaging. Cases describing retinal abnormalities secondary to endophthalmitis, opportunistic infections, and uveitis were not included. There were three publications by Guemes-Villahoz;19 , 26 , 27 due to the overlapping recruitment period and to avoid duplication, only the case-control study was included in this analysis. A follow-up article by Costa and associates28 was also excluded, which outlined the longitudinal outcomes of a similar cohort by Pereira and associates.29 A total of 10 case-control studies (Table 1 ), nine case series (Table 2 ), and 12 single case reports (Supplementary Table 1) were included, comprising 944 cases and 401 controls. In summary, all case-control studies were assessed to be of moderate quality when assessed by the National Institute of Health quality assessment tool for case-control studies. Despite the inclusion of control groups in case-control studies, it was observed that there was still significant heterogeneity in preexisting comorbidities, which may have led to potential selection bias. Reporting bias may affect the quality of conclusions from case series; therefore, pooled analysis was only performed on data from cases-control studies.
FIGURE 1.
Search strategy resulting in 226 records from PubMed.
TABLE 1.
Case-control Studies of COVID-19 Subjects and Associated Retinal Findings.
| Study | Cohort Size | Age (years) ± SD | COVID Confirmation Method and Severity | Examination Time Frame | Retinal Signs | Detailed Findings | OCT Abnormalities | OCTA Protocol andFindings | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Zapata et al.25 | Cases | 96 | 41-44* | RT-PCR, mild (n = 24), moderate (n = 24), severe (n = 21) | 60 days after diagnosis36 days after discharge | 0.01% | Retinal hemorrhage (n = 1) | No abnormalities described | Triton, Topcon, 4.5 × 4.5 mmVD reduction | |
| Controls | 27 | 39 | 0% | NA | No abnormalities described | |||||
| Savastano et al.23 | Cases | 70 | 53.7 | ± 14 | RT-PCR, moderate to severe | 36.1 days after discharge | 13% | CWS (n = 9) | x | Cirrus 5000, Zeiss, 3 × 3 mmVD not altered |
| Controls | 22 | 44.7 | ± 11 | 0% | NA | x | ||||
| Guemes-Villahoz et al.19 | Cases | 66 | 57.2 | ± 7 | RT-PCRcase divided intothrombotic event (n = 19), no thrombotic event (n = 47) | 88 days after diagnosis | 0% | NA | x | Cirrus 5000, Zeiss, 6 × 6 mmVD reduction more in the thrombotic event group |
| Controls | 29 | 51 | ± 11.9 | 0% | NA | x | ||||
| Invernizzi et al.21 | Cases | 54 | 49.9 | ± 15.6 | RT-PCR, mild (74%), severe (26%) | 30 days from onset of symptoms | 31% | hemorrhages (n = 5)CWS (n = 4)dilated veins (n = 15)tortuous vessels (n = 7) | x | x |
| Controls | 133 | 44.2 | ± 12.8 | 7% | hemorrhages (n = 2)CWS (n = 0)dilated veins (n = 4)tortuous vessels (n = 9) | x | x | |||
| Gonzalez-Zamora et al.18 | Cases | 25 | 61.3 | ± 2.4 | RT-PCR, moderate to severe | 14 days after discharge | 20% | CWS (n = 5) | VCIPL thinning | Triton, Topcon, 4.5 × 4.5 mmVD reduction |
| Controls | 25 | 60 | ± 2.3 | 0% | NA | |||||
| Hazar et al.20 | Cases | 50 | 37 | ± 5.9 | RT-PCR, mild to moderate | 30 days after discharge | x | x | x | Angiovue, Optovue, 3 × 3 mmVD reduction |
| Controls | 50 | 35.2 | ± 6.9 | x | x | x | ||||
| Oren et al.20 | Cases | 35 | 48.9 | ± 14.7 | RT-PCR, mild | 14 to 30 days after onset of symptoms | x | x | Increased CMT,reduced GCIPL | x |
| Controls | 25 | 51.2 | ± 7.6 | x | x | x | ||||
| Abrishami et al.17 | Cases | 31 | 40.4 | ± 9.2 | RT-PCR, mild (71%), severe (29%) | 14 days from last symptoms | x | x | x | Angiovue, Optovue, 3 × 3 mmVD reduction |
| Controls | 23 | 36.6 | ± 7.1 | x | x | x | ||||
| Turker et al.17 | Cases | 27 | 38.7 | ± 10.7 | RT-PCR, moderate | Within 1 week of discharge | x | x | x | Angiovue, Optovue, 6 × 6 mmVD reduction |
| Controls | 27 | 37.4 | ±10 | x | x | x | ||||
| Cennamo et al.30 | Cases | 40 | 49.7 | ±12.6 | RT-PCR, moderate | 4.1 months | 0% | NA | No abnormalities described | Angiovue, Optovue, 6 × 6 mmVD reduction |
| Controls | 40 | 48.6 | ±12.2 | 0% | NA | No abnormalities described | ||||
| x finding not part of study protocol | ||||||||||
| * only age range of recruited patients presented | ||||||||||
Abbreviations: SD = standard deviation, OCT = optical coherence tomography, OCTA = optical coherence tomography angiography, NA = not applicable, VD = vessel density, RT-PCR = reverse transcriptase polymerase chain reaction, CWS = cotton wool spot, GCIPL = ganglion cell- inner plexiform layer, CMT = central macular thickness
TABLE 2.
Case Series of COVID-19 Subjects and Associated Retinal Findings.
| Study | Cohort Size | Mean Age (years) | Medical Status | COVID Confirmation Method and Severity | Retina Signs | OCT Abnormalities | Detailed Findings |
|---|---|---|---|---|---|---|---|
| Sim et al.35 | 108 | 36.4 | Not hospitalized | RT-PCR, mild | 11.6% combined | Microhemorrhages (n = 6)Vascular tortuosity (n = 3)Cotton wool spots (n = 1)GC-IPL hyperreflective plaques (n = 10) | |
| Pirraglia et al.34 | 46 | 70 | Hospitalized | RT-PCR, mild to severe | 0% | x | No fundus abnormalities |
| Landecho et al.32 | 27 | 56 | Discharged | Positive antibodies, moderate (n = 26)severe (n = 1) | 22% combined | CWS (n = 6) | |
| Lani-Louzada et al.33 | 25 | 51.2 | Hospitalized | RT-PCR, moderate to severe | 12% | x | CWS and microhemorrhages (n = 1)Flame hemorrhages (n = 1)Microhemorrhages (n = 1) |
| Pereira et al.29 | 18 | 62.5 | Hospitalized | RT-PCR, severe | 55.60% | x | Flame hemorrhages (n = 4)CWS (n = 4)Flame hemorrhages and CWS (n = 1)Peripheral hemorrhages (n = 2) |
| Marinho et al.13 | 12 | 25-69 | Not hospitalized | RT-PCR (n = 9), antibodies (n = 2), mild | 100% combined | GCIPL plaques (n = 12) CWS (n = 4)Microhemorrhages (n = 4) | |
| Costa et al. 28 | 64 | 46.7-55.6 | Hospitalized | RT-PCR, mild to moderate (n = 7), severe (n = 33), critical (n = 24) | 17.2% | x | Vascular tortuosity (n = 11) |
| Bypareddy et al. 31 | 138 | 38.5 | Hospitalized | RT-PCR, mild to moderate | 0.72% | x | Flame hemorrhage (n = 1) |
| Caporossi et al.36 | 28 | 39 to 82 | Hospitalized | Not defined, ICU ARDS COVID-19, severe | 28.6% | x | Intraretinal microvascular abnormalities, arterial saccular dilatation, CWS, and microhemorrhages (n = 8) |
Abbreviations: OCT = optical coherence tomography, RT-PCR = reverse transcriptase polymerase chain reaction, GC-IPL = ganglion cell-inner plexiform layer, CWS = cotton wool spots, ARDS = adult respiratory distress syndrome
SUMMARY FINDINGS OF RETINAL FEATURES RELATED TO MICRO-VASCULOPATHY
Among case-control studies and case series, 18 publications (10 case-control studies17, 18, 19, 20, 21, 22, 23, 24, 25 , 30 and nine case series13 , 28 , 29 , 31, 32, 33, 34, 35, 36) reported on the presence or absence of retinal signs related to microvasculopathy. Among the 10 case-control studies, retinal signs were reported in six, with frequencies ranging from 0.01% to 20%.
RETINAL FEATURES RELATED TO MICRO-VASCULOPATHY IN CASE-CONTROL STUDIES
The largest COVID-19 population was described by Zapata and associates, in a case-control study including 96 cases and 27 controls. Among the cases, a range of severity was included: 24 with mild disease, 24 with moderate disease requiring hospital admission but with no acute respiratory distress, and 21 with severe disease who developed ARDS and were admitted to the ICU. Age distribution was similar between cases (mean 41 to 44 years) and controls (mean 39 years). Eye examination was performed after patients had recovered and been discharged. The authors reported presence of a small posterior pole retinal hemorrhage in one patient who was under treatment with heparin for presenting a thromboembolic episode during his stay in ICU. In view of the concurrent medical issues, the authors did not attribute the retinal hemorrhage to SARS-CoV-2. However, in the same series, the authors reported that cases with moderate or severe infection had significantly reduced VD compared with control and patients with mild infection.25
Guemes-Villahoz19 evaluated macular vessel density in 66 cases with confirmed diagnosis of COVID-19 (mean age 57.1 to 57.3 years) and 29 controls (mean age 51.0 years). The cases were further sub-divided according to presence or absence of thrombotic events. Eye examinations were performed 88 days (86 to 90) after diagnosis of the infection. Fundus examination of the 66 patients revealed no retinal vascular changes. However, significantly lower VD was detected in COVID-19 patients compared to controls. Within the COVID-19 group, no differences in OCTA parameters were found when considering the history of thrombotic events.19
Invernizzi and associates21 included 54 cases and 133 controls. The majority of the cases had mild infection (74%), while 26% had severe infection, but no cases requiring previous ICU were included. Fundus examination was performed within 30 days of symptom onset. The cases were older (49.9 vs 44.2 years, P = .01) and had higher BMI (25.8 vs 24.5 kg/m2, P = .03) but did not differ in other medical history compared to controls. Significantly higher frequencies of retinal hemorrhages (9.25% vs 1.5%, P = .01) and CWS (7.4% vs 0, P = .006) were noted in COVID-19 patients compared to controls. In addition, dilated veins were more frequently observed in COVID-19 patients than in controls (27.7% vs 3.0%, P = .0001). Semi -automatic quantitative measures of mean vein diameter and mean artery diameter further confirmed that COVID-19 patients had more dilated retinal vessels. In addition, in multiple regression, mean vein diameter was negatively correlated with the time from symptom onset and positively correlated with disease severity.21
Gonzalez-Zamora and associates compared the prevalence of retinal microvascular signs in 25 COVID-19 patients (mean age 61.35 years) requiring hospitalization, with 25 age-matched and gender-matched controls (mean age 60.03 years). Eye examination was performed 14 days after hospital discharge. CWS were observed in 20% of the COVID-19 group and none of the controls, and lower VD and larger FAZ were detected on OCTA in the COVID-19 group compared to controls. It was noted that prevalence of hypertension and dyslipidemia was numerically higher in the COVID-19 group. Based on these results, the authors suggested that thrombotic and inflammatory phenomena could be happening in the retina of COVID-19 patients.18
After pooling of all reported data from case-control studies that evaluated retinal signs, comprising 494 cases and 401 controls, the frequency of CWS was significantly higher in COVID-19 patients compared with subjects not exposed to the virus (1.8% vs 0.0%, P < .01). The frequency of retinal hemorrhages was not significantly different between the two populations (1.2% vs 0.5%, P = .25).
In addition to the reduction in VD on OCTA reported by Zapata and associates, Guemes-Villahoz and associates, Gonzalez-Zamora and associates, and Cennamo and associates,18 , 19 , 25 , 30 three other case-control studies compared OCTA findings between COVID-19 patients to controls but did not evaluate fundus microvascular signs.17 , 20 , 24 Hazar and associates compared 50 COVID-19 patients with mild-to-moderate pneumonia with 50 controls (age 37.00 vs 35.14 years, P = .147, respectively). Eye examination was performed one month after discharge and recovery.20 Abrshami and associates compared 31 COVID-19 patients with 23 controls; the patients had a mean age of 40.4 years and were free of past medical history, except two patients who disclosed hypertension. The majority of patients (71%) had mild infection and did not require hospitalization. None of the hospitalized patients required invasive ventilation.17 Turker and associates compared OCTA in 27 COVID-19 patients with moderate pneumonia (hospitalized but not admitted to ICU) and 27 controls. Eye examination was performed within 1 week of discharge. The age distribution of the groups was similar (37.44 years vs 38.74 years, P = .648).24 All three studies reported significantly lower VD in the COVID-19 cases compared to controls. In total, seven case-control studies evaluated retinal microvasculature perfusion using OCTA, comprising 454 cases and 361 controls. A reduction in vessel density in cases was reported in six of seven studies.17, 18, 19, 20 , 23, 24, 25 Oren and associates compared retinal changes using OCT quantitative assessment in 35 cases with mild pneumonia not requiring hospitalization and 25 age-matched and sex-matched controls. Examinations were performed 14 to 30 days after symptom onset. The authors reported higher central macular thickness and thinner ganglion cell layer and inner nuclear layer in the COVID-19 group compared to controls. The authors hypothesized that the ganglion cell-inner plexiform layer (GC-IPL) changes may represent virus involvement of the retina and/or endothelial cells within the retinal plexus.22
POOLED ANALYSIS OF THE PRESENCE OF COVID-19-RELATED RETINAL MICRO-VASCULOPATHY
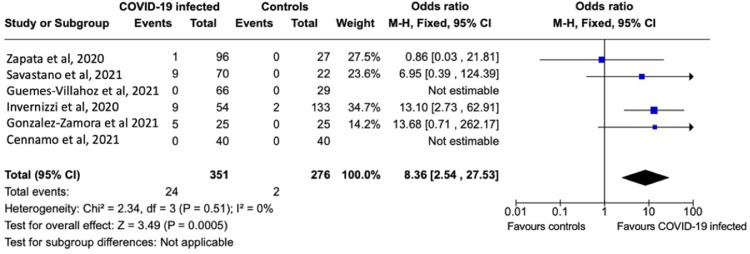
Of the 10 case control studies, six reported the presence of retinal microvasculopathy signs as an outcome. These six studies were included in the meta-analysis providing data on a total of 627 subjects (351 COVID-19-infected subjects versus 276 non-infected controls). Pooled analyses of these studies demonstrated a statistically significant 8.86-fold increase (OR, 8.86; 95% CI, 2.54-27.53; P < .01) in retinal microvasculopathy prevalence in COVID-19-infected patients relative to non-infected controls (Figure 2 ). Sensitivity analysis performed by randomly excluding each study in a sequential manner showed a persistently significant difference between COVID-19 and control subjects regarding the presence of retinal microvasculopathy (P < .01 for all).
FIGURE 2.
Forest plot of odds ratios for retina microvasculopathy in patients with COVID-19 infection versus non-infected controls.
POOLED ANALYSIS OF VESSEL DENSITY AND FOVEAL AVASCULAR ZONE SIZE IN SUBJECTS WITH THE COVID-19 INFECTION
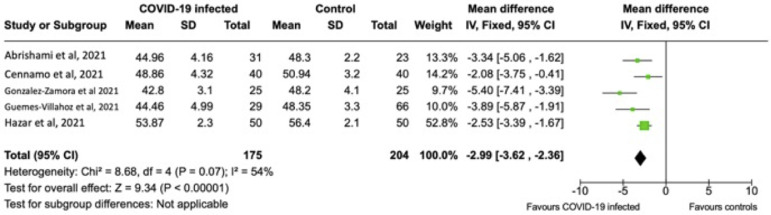
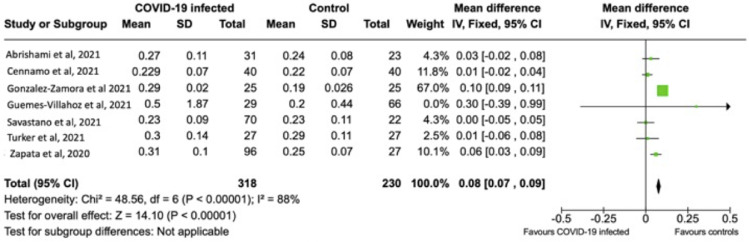
Of the 10 case-control studies, eight measured VD and FAZ on OCTA. Mean parafoveal VD was pooled in five of eight studies. The remaining three studies did not measure parafoveal VD and were therefore excluded. The results of a total of 379 subjects (175 COVID-infected versus 204 non-infected controls) were pooled for the measurement of parafoveal VD. The pooled mean parafoveal VD (95% CI) was 2.99% (–3.63 to –1.67) less in the COVID-19-infected subjects compared to non-infected controls (P < .01) (Figure 3 ). Mean FAZ size was pooled in seven of the eight studies. Hazar and associates did not performed this measurement in their study cohort.20 The results of a total of 548 subjects (318 COVID-infected versus 230 non-infected controls) were pooled for the measurement of FAZ size. The pooled mean FAZ (95% CI) was 0.08 mm2 (0.07-0.09) larger in COVID-19 infected patients compared to non-infected controls (P < .01) (Figure 4 ). Sensitivity analysis performed by randomly excluding each study in a sequential manner showed a persistently significant difference between COVID-19 and control subjects regarding VD and FAZ size (P < .05 for all). Reasons for high heterogeneity in the pooled data for VD and FAZ are likely due to the differences in OCTA instruments and measurements methodology. From these pooled analyses, the prevalence of retinal microvasculopathy was significantly associated with COVID-19 compared to controls. There was a probable association between lower VD and larger FAZ with COVID-19 subjects compared to controls.
FIGURE 3.
Forest plot of means of parafoveal vessel density (%) measured on optical coherence tomography angiography in subjects with COVID-19 infection versus non-infected controls.
FIGURE 4.
Forest plot of means area of foveal avascular zone (mm2) measured on optical coherence tomography angiography in subjects with COVID-19 infection versus non-infected controls.
RETINAL FEATURES RELATED TO MICRO-VASCULOPATHY IN CASE SERIES
Nine case series reported retinal features in COVID-19 patients. Among these, the two largest series comprised 138 cases (mean age 38.5 years)31 and 108 cases (36.4 years)35 with mild-to-moderate infection. Examination in the largest series revealed one patient with a flame hemorrhage (0.72%). Patients in this series were examined at the bedside, with a non-mydriatic handheld fundus camera, a mean of 6 days after diagnosis of COVID-19.31 In contrast, retinal signs observed in the second largest series included microhemorrhages (3.7%), CWS (0.93%), and vascular tortuosity (2.8%). Hyper-reflective plaques in the GC-IPL layer were noted on OCT in 5.1%. Fundus imaging and OCT were performed on a Topcon 3D OCT-1 Maestro System (Topcon, Japan). There was no significant difference in the prevalence of retinal signs in symptomatic versus asymptomatic patients. The examination was performed about 16 days after diagnosis of COVID-19 with polypoidal choroidal vasculopathy (PCV).35
The highest prevalence of retinal microvascular abnormalities was reported by Pereira and associates in a series comprising 18 COVID-19 patients with severe infection. Seventeen patients (94.4%) required ICU stay and 77.8% received invasive mechanical ventilation. Dilated eye examination was performed at a median of 11.5 days after COVID-19 diagnosis: 55.6% of patients had abnormalities detected, including flame-shaped hemorrhages (22.2%) or CWS (22.2%). Lani-Louzada and associates also evaluated 25 hospitalized patients with severe or critical COVID-19 infection (mean age 51.2 years). ICU monitoring was required in 12 patients and eight required mechanical ventilation. Ocular examination was performed at the bedside using a handheld digital retinal camera connected to a smartphone (mean 23.9 days after symptom onset). Retinal changes were observed in 12%, including nerve fiber layer infarcts, microhemorrhages, and flame-shaped hemorrhage. Landecho and associates evaluated a series of 27 patients with retinal fundoscopy, OCT, and OCTA, with moderate COVID-19 infection, at 14 days after hospital discharge and reported the presence of CWS in 22%.29
Pirraglia and associates examined 46 patients with severe pneumonia (mean age 70 years) during the acute phase. Eye examination was performed after a median of 21.5 days from hospitalization, with bedside evaluation using direct and indirect ophthalmoscopy and a handheld fundus camera. The reported ocular findings included one case of chorioretinitis thought to be due to fungal infection. There were other eyes with retinal findings but the authors had ascribed these to hypertensive retinopathy (9.3%) and diabetic retinopathy (2.3%), but concluded an absence of retinal involvement in SARS-CoV-2.34 Marihno and associates reported presence of CWS and microhemorrhages detected on color fundus photography and red free imaging in four of 12 patients with mild COVID-19 infection examined 11 to 33 days after symptom onset.13 Caporossi and associates described the retinal findings in a cohort of patients (n = 28 eyes) with ARDS managed in an ICU, and found that 28.6% had a variety of retinal findings including intra-retinal microvascular abnormalities, arterial saccular dilatation, CWS, and microhemorrhages.36
INDIVIDUAL CASE REPORTS
Twelve published case reports evaluated fundus and/or OCT findings and reported abnormalities in 10 patients.37, 38, 39, 40, 41, 42, 43, 44, 45, 46 The majority of these cases were symptomatic and presented with blurred vision or scotoma. In four cases, the COVID-19 diagnosis was made as a result of systemic investigation for ocular abnormality.39 , 42 , 45 , 46 There were four cases with retinal venous occlusion.38 , 41 , 45 , 46 Bilateral CWS and corresponding neurofilament light chain (NfL) infarct on OCT were reported in two cases receiving oxygen therapy.37 , 40 A further case presenting with sudden-onset scotoma was found to have CWS and evidence of paracentral acute middle maculopathy (PAMM) on OCT.39 In the remaining four cases, OCT showed evidence of PAMM (three cases), and acute macular neuroretinopathy (one case) .43 , 44
DISCUSSION
Ocular involvement associated with SARS-CoV-2 infection in the anterior segment has been well recognized. However, evidence regarding posterior segment involvement has been inconsistently reported. Marinho and associates first reported retinal microhemorrhages and CWS and hyper-reflective plaques in the GC-ILP layers in a series of 12 healthcare workers with COVID-19 infection.13 However, multiple authors have highlighted how the OCT features initially described as COVID-19-related were simply reflections from normal retinal vasculature.14, 15, 16 Nonetheless, a number of subsequent reports of retinal microvasculopathy have been published and are summarized in this review. The most common retinal features described include CWS, microhemorrhages, and venous tortuosity (Figure 5 ). The pooled analysis of the literature to date shows that COVID-19 infection is associated with an 8.86-fold retinal microvasculopathy prevalence. This is further supported by the pooled analysis showing reduced parafoveal VD and increased FAZ in eyes of subjects with COVID-19 infection compared to non-infected controls. Taken together, the current findings suggest that COVID-19 infection appears to result in sub-clinical microvascular damage, which manifests in the retinal microcirculation (detected on OCTA) and is a continuation of a spectrum that can result in clinically detectable features of microvasculopathy (as seen as retinal hemorrhages and CWS).
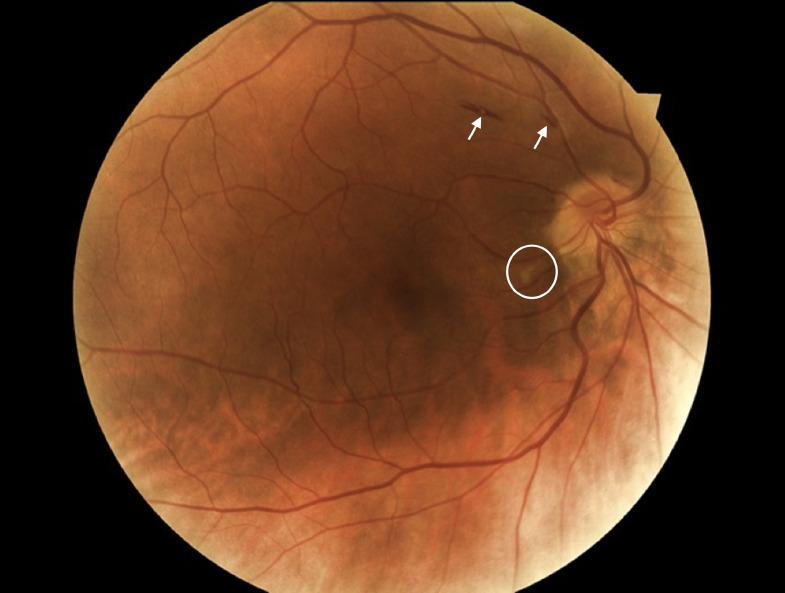
FIGURE 5.
Flame hemorrhage (white arrows) and cotton wool spots (white circle) noted on color fundus photograph of a patient with COVID-19.
A significant challenge in the interpretation of these findings is the potential imbalance between preexisting medical conditions in patients with COVID-19 infections and controls. Patients tend to be older and have significantly more comorbidities such as hypertension and diabetes. Nonetheless, the inclusion of controls has significantly improved the validity of retinal microvasculopathy as an ocular feature of COVID-19. Among the 263 controls included in published studies, none had CWS and two had microhemorrhages. In the current pooled analysis, although it was still not possible to fully adjust for differences in patient demographics, the frequency of CWS was significantly higher in cases than in controls. The frequency of microhemorrhages was numerically higher but did not achieve statistical significance.
Based on the current evidence that COVID-19 may manifest as retinal microvasculopathy in a subgroup of infected persons, it is suggested that, in addition to clinical examination, OCTA, with its ability to assess the retina microvasculature to a high degree of accuracy, be considered the imaging modality of choice when assessing the posterior segment in this condition. However, there is currently a lack of standardized OCTA-based parameters for comparative studies. It is suggested that future studies related to this condition report on two aspects of OCTA. First, perfusion characteristics such as perfused pixel area density (also known as vessel density). This metric is calculated by dividing the area of perfused pixels (often denoted as white pixels) by the area of the region of interest (ROI). Other metrics for perfusion may include perfused pixel length density (also known as skeletonized vessel density). This metric is derived by converting all vessels to a single pixel width and dividing the length of the pixels by the ROI. Non-perfusion characteristics should also be assessed. This may be an assessment of the foveal avascular zone (FAZ) in terms of its area, circularity, or diameter. In subtle cases of microvasculopathy, the FAZ may remain relatively intact, hence other metrics of non-perfusion like assessing spaces between vessels may be used. These include intercapillary areas quantified by the areas of non-perfusion (black pixels) or percent area of nonperfusion, which essentially is the ration of areas of non-perfusion to area of ROI.47 , 48
Inconsistency in reported findings among COVID-19 patients may also be related to variations in the time of eye examination, severity of infection, and method of detection.29 , 34 The timing of eye examination varied considerably between published studies, with some reporting as early as 2 weeks after diagnosis of COVID-19, while others were performed at 30 to 88 days after diagnosis. For hospitalized patients, eye examinations were carried out much later after recovery and discharge. These differences may partly explain some of the inconsistencies in the frequency of abnormal findings. The variable severity of COVID-19 is another potential confounder. Several studies have suggested that patients with moderate or severe disease have more retinal vasculature changes than controls or patients with mild infection. Patients with severe COVID-19 are known to have more marked disturbance in blood parameters and are therefore at higher risk of systemic complications, including hypertension and thrombotic events.49 These patients are also more likely to require invasive ventilation and anticoagulant therapy. The microvascular changes in the retina may partly be a result of these treatments rather than COVID-19 itself. Conversely, changes in the retinal vasculature were also seen in cases with mild symptoms who did not require hospitalization and might have been considered fully recovered in the conventional sense.17 , 21 , 5 Finally, cases who were severely ill were more likely to have been examined at the bedside with handheld devices. It is possible that the detection rate could have been affected due to suboptimal examination conditions.33 , 34
The mechanism through which SARS-CoV-2 may affect the retinal vasculature remains poorly understood. It is recognized that ACE-2 receptors, which are involved in transmission of SARS-CoV-2, are abundant in the retina and choroid.50 In keeping with the reduction in VD in in vivo studies, severely reduced microvascular density has also been reported in the retinal flat mount from donor eyes from COVID-19 subjects compared to controls, which has led some investigators proposing a direct effect of virus infection on endothelial cell damage.51 , 52 However, detection of the virus within the retina in post-mortem studies has been inconsistent. In addition to direct effects, hypercoagulation and vasculopathy may also lead to the observed retinal findings.53, 54, 55, 56 In fact, fibrin microthrombi, inflammatory cells were identified in the retinal and choroidal vessels and the choriocapillaries of eyes from patients who died from COVID-19.52
The main limitation of this analysis was the use of only case-control studies. While cohort studies would the ideal method to determine cause or effect, in the context of the COVID-19 pandemic, it would be virtually impossible for such a prospective study to be conducted. Sampling bias, confounders such as severity of the COVID-19 infection, and preexisting conditions and patient characteristics such as age, hypertension, diabetes, and hyperlipidemia may still be present despite best efforts to control them.
In summary, there is accumulating evidence suggesting possible retinal microvascular sequelae in patients infected with SARS-CoV-2. While it is important to acknowledge the potential confounders mentioned above, the scale of the potential issue warrants further investigations. At the time of writing (June 2021), there are 170 million infected individuals worldwide. Assuming retinal microvasculopathy develops in 10% of infected persons, this will translate into a substantial cohort. While the majority of infected persons with mild symptoms may appear to have fully recovered, the longitudinal course of the retinal changes is still unknown. However, if these retinal signs persist or progress, coupled with aging and co-existence of further common systemic comorbidities such as hypertension, hyperlipidemia, and diabetes, they could place these subjects at higher risk of retinal complications and visual loss. Further prospectively designed studies with large cohorts that adjust for significant confounders should be carried out to ascertain the risk of microvasculopathy and whether they are associated with longitudinal functional deficits.
This review summarises the currently available evidence on the prevalence of retinal micro-vasculopathy due to coronavirus disease (COVID-19). Due to the large numbers affected by COVID-19, it was concluded that COVID-19 retinal micro-vasculopathy is significant and needs to be recognized by ophthalmologists as a potential long-term sequalae of the disease.
Acknowledgments
<ACK>Financial Support: National Medical Research Council Singapore Open Fund Large Collaborative Grant: NMRC/LCG/004/2018.
Conflict of interest: Dr Teo reports consultancy fees, honorarium, travel support, and speaker fees from Bayer and Novartis outside the submitted work. Dr Staurenghi reports consultation fee from Heidelberg Engineering, Centervue, Carl Zeiss Apellis, Allergan, Bayer, Boheringer, Genentech, Novartis, Roche, Chengdu Kanghong Biotechnology Co; grant support from Heidelberg Engineering, Optos, Optovue, Quantel Medical, Centervue, Carl Zeiss Meditec, Nidek, Topcon; lecture fee from Heidelberg Engineering, Carl Zeiss Meditec, Nidek, Bayer, Novartis, Roche outside the submitted work. Dr Invernizzi reports consultancy fees, grants, travel support and speaker fees from Bayer, Allergan, and Novartis outside the submitted work. Dr Cheung reports non-financial support from Bayer during the conduct of the study; grants, personal fees and non-financial support from Bayer, grants, personal fees, and non-financial support from Novartis, grants from Roche, grants from GlaxoSmith Kline, non-financial support from Allergan, non-financial support from Topcon, outside the submitted work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajo.2021.09.019.
Appendix. Supplementary materials
REFERENCES
- 1.World Health Organisation; 2019. Coronavirus disease (COVID-2019) situation reports - Situation report 51. Coronavirus disease (COVID-2019) situation reports. online. [Google Scholar]
- 2.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 3.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F, Kruip M, Van der Meer N, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS: persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 7.Latalska M, Mackiewicz J. The implication of ocular manifestation of COVID-19 for medical staff and patients-systematic review. Ann Agric Environ Med. 2020;27:165–170. doi: 10.26444/aaem/122790. [DOI] [PubMed] [Google Scholar]
- 8.Ling XC, Kang EY-C, Lin J-Y, et al. Ocular manifestation, comorbidities, and detection of severe acute respiratory syndrome-coronavirus 2 from conjunctiva in coronavirus disease 2019: A systematic review and meta-analysis. Taiwan J Ophthalmol. 2020;10(3):153–166. doi: 10.4103/tjo.tjo_53_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu C-W, Liu X-F, Jia Z-F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395:e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28:391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seah IYJ, Anderson DE, Kang AEZ, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127:977. doi: 10.1016/j.ophtha.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marinho PM, Marcos AA, Romano AC, Nascimento H, Belfort R. Retinal findings in patients with COVID-19. Lancet. 2020;395:1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouyang P, Zhang X, Peng Y, Jiang B. Seeking clarity on retinal findings in patients with COVID-19. Lancet. 2020;396:e35. doi: 10.1016/S0140-6736(20)31921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vavvas DG, Sarraf D, Sadda SR, et al. Concerns about the interpretation of OCT and fundus findings in COVID-19 patients in recent Lancet publication. Eye (Lond) 2020;34:2153–2154. doi: 10.1038/s41433-020-1084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkatesh P. Seeking clarity on retinal findings in patients with COVID-19. Lancet. 2020;396:e36. doi: 10.1016/S0140-6736(20)31922-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrishami M, Emamverdian Z, Shoeibi N, et al. Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: a case-control study. Can J Ophthalmol. 2021;56(1):24–30. doi: 10.1016/j.jcjo.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González-Zamora J, Bilbao-Malavé V, Gándara E, et al. Retinal microvascular impairment in COVID-19 bilateral pneumonia assessed by optical coherence tomography angiography. Biomedicines. 2021;9:247. doi: 10.3390/biomedicines9030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guemes-Villahoz N, Burgos-Blasco B, Vidal-Villegas B, et al. Reduced macular vessel density in COVID-19 patients with and without associated thrombotic events using optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2021;259(8):2243–2249. doi: 10.1007/s00417-021-05186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazar L, Karahan M, Vural E, et al. Macular vessel density in patients recovered from COVID 19. Photodiagnosis Photodyn Ther. 2021;34 doi: 10.1016/j.pdpdt.2021.102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Invernizzi A, Torre A, Parrulli S, et al. Retinal findings in patients with COVID-19: results from the SERPICO-19 study. E Clin Med. 2020;27 doi: 10.1016/j.eclinm.2020.100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oren B, Aksoy Aydemır G, Aydemır E, et al. Quantitative assessment of retinal changes in COVID-19 patients. Clin Exp Optom. 2021;104(6):717–722. doi: 10.1080/08164622.2021.1916389. [DOI] [PubMed] [Google Scholar]
- 23.Savastano MC, Gambini G, Cozzupoli GM, et al. Retinal capillary involvement in early post-COVID-19 patients: a healthy controlled study. Graefes Arch Clin Exp Ophthalmol. 2021:1–9. doi: 10.1007/s00417-020-05070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turker IC, Dogan CU, Guven D, Kutucu OK, Gul C. Optical coherence tomography angiography findings in patients with COVID-19. Can J Ophthalmol. 2021 doi: 10.1016/j.jcjo.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zapata MÁ, García SB, Sánchez A, et al. Retinal microvascular abnormalities in patients after COVID-19 depending on disease severity. Br J Ophthalmol. 2020 doi: 10.1136/bjophthalmol-2020-317953. bjophthalmol-2020-317953. [DOI] [PubMed] [Google Scholar]
- 26.Guemes-Villahoz N, Burgos-Blasco B, Vidal-Villegas B, et al. Reduced retinal vessel density in COVID-19 patients and elevated D-dimer levels during the acute phase of the infection. Med Clin (Barc) 2021;156(11):541–546. doi: 10.1016/j.medcli.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guemes-Villahoz N, Burgos-Blasco B, Vidal-Villegas B, et al. Reduced retinal vessel density in COVID-19 patients and elevated D-dimer levels during the acute phase of the infection. Med Clin (Engl Ed). 2021;156:541–546. doi: 10.1016/j.medcle.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa IF, Bonifacio LP, Bellissimo-Rodrigues F, et al. Ocular findings among patients surviving COVID-19. Sci Rep. 2021;11:11085. doi: 10.1038/s41598-021-90482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira LA, Soares LCM, Nascimento PA, et al. Retinal findings in hospitalised patients with severe COVID-19. Br J Ophthalmol. 2020 doi: 10.1136/bjophthalmol-2020-317576. PMID: 33067361. [DOI] [PubMed] [Google Scholar]
- 30.Cennamo G, Reibaldi M, Montorio D, D'Andrea L, Fallico M, Triassi M. Optical coherence tomography angiography features in post-COVID-19 pneumonia patients: a pilot study. Am J Ophthalmol. 2021;227:182–190. doi: 10.1016/j.ajo.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bypareddy R, Rathod BLS, Shilpa YD, et al. Fundus evaluation in COVID-19 positives with non-severe disease. Indian J Ophthalmol. 2021;69:1271–1274. doi: 10.4103/ijo.IJO_3227_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landecho M, Yuste J, Gándara E, et al. COVID-19 retinal microangiopathy as an in vivo biomarker of systemic vascular disease? J Intern Med. 2021;289(1):116–120. doi: 10.1111/joim.13156. Epub 2020 Jul 30. [DOI] [PubMed] [Google Scholar]
- 33.Lani-Louzada R, CdVF Ramos, Cordeiro RM, Sadun AA. Retinal changes in COVID-19 hospitalized cases. Plos One. 2020;15 doi: 10.1371/journal.pone.0243346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pirraglia MP, Ceccarelli G, Cerini A, et al. Retinal involvement and ocular findings in COVID-19 pneumonia patients. Sci Rep. 2020;10:1–7. doi: 10.1038/s41598-020-74446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sim R, Cheung G, Ting D, et al. Retinal microvascular signs in COVID-19. Br J Ophthalmol. 2021 doi: 10.1136/bjophthalmol-2020-318236. bjophthalmol-2020-318236. [DOI] [PubMed] [Google Scholar]
- 36.Caporossi T, Bacherini D, Tartaro R, VI G, Peris A, Giansanti F. Retinal findings in patients affected by COVID 19 intubated in an intensive care unit. Acta Ophthalmol. 2020 doi: 10.1111/aos.14734. [DOI] [PubMed] [Google Scholar]
- 37.Bottini AR, Steinmetz S, Blinder KJ, Shah GK. Purtscher-like retinopathy in a patient with COVID-19. Case Rep Ophthalmol Med. 2021;2021 doi: 10.1155/2021/6661541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finn AP, Khurana RN, Chang LK. Hemi-retinal vein occlusion in a young patient with COVID-19. Am J Ophthalmol Case Rep. 2021;22 doi: 10.1016/j.ajoc.2021.101046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gascon P, Briantais A, Bertrand E, et al. Covid-19-associated retinopathy: a case report. Ocul Immunol Inflamm. 2020;28:1293–1297. doi: 10.1080/09273948.2020.1825751. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Lopez JJ, Felix Espinar B, Ye-Zhu C. Symptomatic retinal microangiophaty in a patient with coronavirus disease 2019 (COVID-19): single case report. Ocul Immunol Inflamm. 2020:1–3. doi: 10.1080/09273948.2020.1852260. [DOI] [PubMed] [Google Scholar]
- 41.Invernizzi A, Pellegrini M, Messenio D, et al. Impending central retinal vein occlusion in a patient with coronavirus disease 2019 (COVID-19) Ocul Immunol Inflamm. 2020;28:1290–1292. doi: 10.1080/09273948.2020.1807023. [DOI] [PubMed] [Google Scholar]
- 42.Ortiz-Egea JM, Ruiz-Medrano J, Ruiz-Moreno JM. Retinal imaging study diagnoses in COVID-19: a case report. J Med Case Rep. 2021;15:1–4. doi: 10.1186/s13256-020-02620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padhy SK, Dcruz RP, Kelgaonkar A. Paracentral acute middle maculopathy following SARS-CoV-2 infection: the D-dimer hypothesis. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Virgo J, Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye. 2020;34:2352–2353. doi: 10.1038/s41433-020-1069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walinjkar JA, Makhija SC, Sharma HR, Morekar SR, Natarajan S. Central retinal vein occlusion with COVID-19 infection as the presumptive etiology. Indian J Ophthalmol. 2020;68:2572. doi: 10.4103/ijo.IJO_2575_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venkatesh R, Reddy NG, Agrawal S, Pereira A. COVID-19-associated central retinal vein occlusion treated with oral aspirin. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu Y, Simonett JM, Wang J, et al. Evaluation of automatically quantified foveal avascular zone metrics for diagnosis of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2018;59:2212–2221. doi: 10.1167/iovs.17-23498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nesper PL, Roberts PK, Onishi AC, et al. Quantifying microvascular abnormalities with increasing severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58:BIO307–BIO315. doi: 10.1167/iovs.17-21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. E Clin Med. 2020;29 doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Senanayake PD, Drazba J, Shadrach K, et al. Angiotensin II and its receptor subtypes in the human retina. Invest Ophthalmol Vis Sci. 2007;48:3301–3311. doi: 10.1167/iovs.06-1024. [DOI] [PubMed] [Google Scholar]
- 51.Jidigam VK, Singh R, Batoki JC, et al. Histopathological assessments reveal retinal vascular changes, inflammation and gliosis in patients with lethal COVID-19. medRxiv. 2021 doi: 10.1101/2021.02.25.21251531. 2021.02.25.21251531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reinhold A, Tzankov A, Matter MS, Mihic-Probst D, Scholl HP, Meyer P. Ocular pathology and occasionally detectable intraocular SARS-CoV-2 RNA in five fatal COVID-19 cases. Ophthalmic Res. 2021 Jan 20 doi: 10.1159/000514573. [DOI] [PubMed] [Google Scholar]
- 53.Bayyoud T, Iftner A, Iftner T, et al. Absence of severe acute respiratory syndrome-coronavirus-2 RNA in ocular tissues. Am J Ophthalmol Case Rep. 2020;19 doi: 10.1016/j.ajoc.2020.100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayyoud T, Iftner A, Iftner T, et al. Severe acute respiratory syndrome-Coronavirus-2: can it be detected in the retina? PloS One. 2021;16 doi: 10.1371/journal.pone.0251682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casagrande M, Fitzek A, Püschel K, et al. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm. 2020;28:721–725. doi: 10.1080/09273948.2020.1770301. [DOI] [PubMed] [Google Scholar]
- 56.Ulhaq ZS, Soraya GV. The prevalence of ophthalmic manifestations in COVID-19 and the diagnostic value of ocular tissue/fluid. Graefes Arch Clin Exp Ophthalmol. 2020;258:1351–1352. doi: 10.1007/s00417-020-04695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.