Abstract
Cycle threshold (CT) values are correlated with the amount of viral nucleic acid in a sample and may be obtained from some qualitative real-time polymerase chain reaction tests used for diagnosis of most patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, CT values cannot be directly compared across assays, and they must be interpreted with caution as they are influenced by sample type, timing of sample collection, and assay design. Presently, the correlation between CT values and clinical outcomes is not well understood. We conducted a systematic review and meta-analysis of published studies through April 19, 2021, that reported an association between CT values and hospitalization, disease severity, and mortality in patients ≥18 years old with SARS-CoV-2. A meta-analysis of 7 studies showed no significant difference in mean CT values between hospitalized and nonhospitalized patients. Among hospitalized patients, those with CT values <25 had a high risk of more severe disease and mortality than patients with CT values >30 (odds ratio [OR], 2.31; 95% CI, 1.70 to 3.13; and OR, 2.95; 95% CI, 2.19 to 3.96; respectively). The odds of increased disease severity and mortality were less pronounced in patients with CT values of 25–30 compared with >30.
Keywords: clinical outcomes, COVID-19, cycle threshold, meta-analysis, prognosis, SARS-CoV-2
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may lead to a spectrum of disease, ranging from asymptomatic infection to severe symptomatic coronavirus disease 2019 (COVID-19). As of May 5, 2021, there have been over 32 million confirmed cases of COVID-19 in the United States, resulting in >5 million hospitalizations [1, 2]. Among hospitalized patients with COVID-19, roughly one-third of patients have required intensive care unit (ICU) admission, and 1 in 9 patients have died [3–6].
Current known host risk factors for progression to severe COVID-19 include advanced age, male sex, and certain comorbidities including obesity and heart failure [7–9]. Laboratory values such as interleukin-6 level, C-reactive protein level, and peripheral blood lymphocyte count have also been correlated with disease severity [10–12].
There has also been interest in assessing the impact of viral load on clinical outcomes. Most patients with COVID-19 are diagnosed with real-time polymerase chain reaction (rtPCR) assays, which are most commonly qualitative tests (ie, providing a positive or negative result). Many rtPCR assays can provide a cycle threshold (CT) value, which refers to the number of PCR cycles required to generate target amplification (as measured by fluorescence) that is distinguishable from baseline fluorescence [13]. Using a standard curve correlating CT values to different known concentrations of SARS-CoV-2 virions, a quantitative viral load can be determined in a given clinical specimen.
While CT value is inversely proportional to viral load, this correlation is nonlinear, and many factors influence this association, including sample collection and rtPCR assay [14]. Additional limitations in the use of CT values in patients with SARS-CoV-2 include the impact of the timing of sample collection, as generally earlier in the disease course individuals will have a higher viral load. Despite these limitations, there is widespread interest among clinicians in how the CT value can be used to better manage patients infected with SARS-CoV-2.
However, a gap remains in the knowledge of the clinical utility of CT values to aid in prognostication of patients with COVID-19. An early systematic review evaluated the clinical utility of CT values in patients with COVID-19, but this analysis only included 1 study on disease progression and another study on patient mortality [15]. Several studies have reported noncorrelative results between clinical outcomes in patients with COVID-19 and both SARS-CoV-2 viral load and CT values [16–21]. These discrepancies may be due, in part, to different technologies used, timing of testing, and differing criteria for assessing clinical outcomes at varying institutions across the globe. Given this uncertainty, we conducted a systematic review and meta-analysis to assess the association between CT values and clinical outcomes, including the risk of hospitalization among patients with COVID-19 and the risk of disease severity and death in such patients.
METHODS
This study was registered in PROSPERO (CRD42021235617), and findings were reported according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guideline (Supplementary Data).
Outcomes of Interest
We sought to identify published studies evaluating the association between CT values and 3 distinct outcomes among patients with a confirmed SARS-CoV-2 infection: (1) need for hospitalization; and among hospitalized patients, (2) disease severity (WHO Severity scale grade 5 or higher, specifically invasive or noninvasive ventilation and/or ICU need); (3) in-hospital and 30-day mortality.
Data Sources and Search Strategies
A comprehensive search of several databases from January 1, 2019, through January 28, 2021, limited to the English language and excluding animal studies, was conducted. Given the rapid pace of publications, the search was repeated on April 19, 2021. The databases included Ovid MEDLINE and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus. The search strategy was designed and conducted by a medical reference librarian (L.C.H.) with input from the study investigators. Controlled vocabulary supplemented with keywords was used to search for studies describing the association between SARS-CoV-2 CT values and clinical outcomes. The actual strategy listing all search terms used and how they are combined is described in the Appendix (Supplementary Data).
Eligibility Criteria and Study Selection
We included cohort studies, randomized controlled trials, and case reports and series that met the following criteria: (1) adults ≥18 years, (2) publication in English, (3) reported CT value data, (4) specified sample source (eg, nasopharyngeal swab), (5) specified rtPCR assay, (6) minimum 5 study subjects with specified outcome of interest, and (7) full manuscript available.
Each study was assessed for inclusion by 2 independent reviewers, first by screening the publication title and abstract and subsequently by analyzing content in the full-text articles (V.P.S., W.H.F., or J.C.H.). Discordance of study data was resolved by evaluation by a third reviewer or discussion on eligibility and consensus agreement.
Data Collection
Two reviewers abstracted data from each included study (V.P.S. and W.H.F.). Disagreements were resolved by discussion. When multiple studies from the same data set were reported, we included only the largest data set. If a study reported the use of multiple rtPCR assays, data were abstracted and synthesized separately for each assay.
For each outcome of interest, adjusted odds ratios (ORs) for low (CT <25) and medium (CT 25–30) compared with high (CT >30) CT values were collected. If unavailable or not reported, data from tables were abstracted and unadjusted odds ratios were calculated. If data were reported but were insufficient for meta-analysis (eg, graphic data), authors were contacted for more details.
Additionally, the mean CT value and SD for each outcome were collected if available (eg, survivor vs nonsurvivor mean CT values). If mean CT values and SD data were not available, information was imputed from interquartile ranges. Sample population, sample source, and rtPCR platform data were also collected.
Risk of Bias Assessment
Risk of Bias assessment was performed using a modified Newcastle-Ottawa scale by 2 independent reviewers (V.P.S. and W.H.F.) (Supplementary Data). Disagreements were resolved by discussion and consensus. We assessed the representativeness of the study population, selection of the nonexposed cohort, comparability, and outcome assessment. A quantitative score for risk of bias was not used, but we focused on the most critical element of bias in this specific context, which was adjustment for confounders [22].
Data Synthesis
Studies that reported ORs or reported data from which odds ratios could be calculated were analyzed separately from studies that reported CT values as continuous variables for outcomes of interest. If studies reported CT values both as categorical and continuous variables, the study was evaluated as part of the synthesis that had the larger data set.
Because of heterogeneity across study settings and populations, the DerSimonian-Laird random effect model as implemented in the OpenMeta Analyst software package was used [23]. Heterogeneity was assessed using the I2 statistic, with low heterogeneity being <50%, moderate 50% to 75%, and high >75%. Heterogeneity was explored using subgroup analyses by sample source, rtPCR assay, use of adjusted vs unadjusted ORs, and risk of bias. We were unable to statistically evaluate the presence of publication bias due to the small number of studies included per analysis.
RESULTS
Study Selection and Characteristics
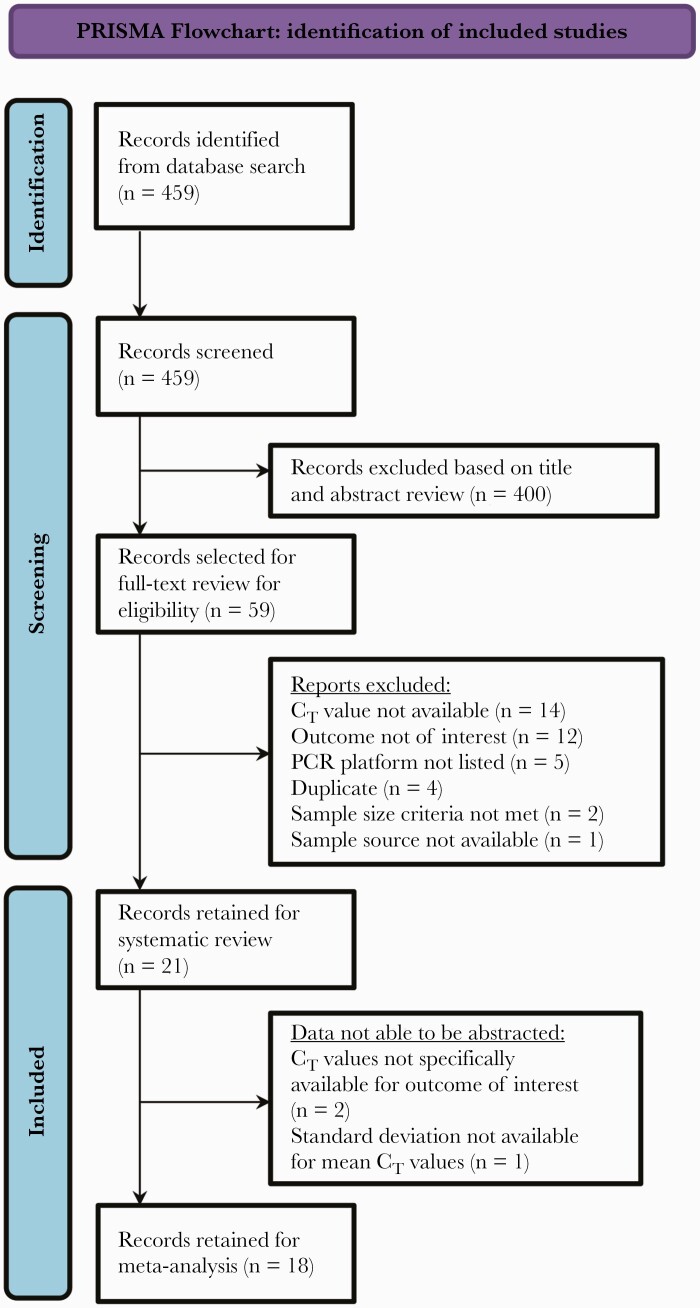
The search yielded 459 potentially relevant articles, of which 21 studies met inclusion criteria (Figure 1). Study characteristics are listed in Tables 1–3 for each outcome. A total of 18 studies contributed data to the meta-analysis. Overall, 8 and 10 studies reported CT values as categorical and continuous variables, respectively, in relation to outcomes of interest and were synthesized collectively for each outcome.
Figure 1.
PRISMA flowchart. Abbreviations: CT, cycle threshold; PCR, polymerase chain reaction.
Table 1.
Summary of Studies Evaluating Association Between CT Values and Hospitalization
| Author | Year | City, State, Country | Study Enrollment Period | Sample Size | Sample Source | rtPCR Assay | Comparator | Reference Group | Lower CT Values Associated With Hospitalization | Summary |
|---|---|---|---|---|---|---|---|---|---|---|
| Ade et al. [37] | 2021 | Western Germany | March 15–September 2020 | 1077 | NP | Qiagen QIAgility | Hospitalized patients | Nonhospitalized patients | No | There was no significant difference in mean CT values between hospitalized and nonhospitalized patients with SARS-CoV-2. |
| Amodio et al. [38] | 2020 | Palermo, Italy | February 24–April 8, 2020 | 381 | Nasal, pharyngeal, or NP | Thermo Fisher Scientific TaqMan (custom) | Hospitalized patients | Nonhospitalized patients | Yes | After adjusting for age and sex, authors found a lower risk of hospitalization with higher CT values (aOR, 0.95; 95% CI, 0.91 to 0.99). |
| Faico-Filho et al. [39] | 2020 | São Paulo, Brazil | March 17–June 17, 2020 | 875 | NP | Thermo Fisher Scientific AgPath-ID | Hospitalized patients | Nonhospitalized patients and health care workers | No | Study participants who did not require hospitalization had lower mean CT values than patients who required hospitalization. |
| Koureas et al. [40] | 2021 | Thessaly, Greece | April 8–June 4, 2020 | 142 | OP | AriaMx Real-time PCR System; Roche Cobas; Qiagen Rotor-Gene Q analyzer | Hospitalized patients | Nonhospitalized patients | Yes | During an outbreak investigation, index cases were evaluated for their CT values and followed up for need for hospitalization. Cases who required hospitalization had lower mean CT values. |
| Maltezou et al. [21] | 2021 | Athens and Thessaloniki, Greece | February 26–May 3, 2020 | 518 | NP or OP | Genesig COVID-19 CE-IVD real-time RT-PCR kit or Thermo Fischer Scientific TaqMan | Patients with CT <25 and 25–30 | Patients with CT >30 | No | In univariate analyses, compared with patients with CT >30 or 25–30, patients with CT <25 were older, more often had comorbidities, developed symptomatic COVID-19, were intubated, and died. In multivariate analysis controlling for age, sex, and comorbidities, patients with CT <25 had a higher likelihood of having symptomatic disease, but did not have increased odds of hospitalization, ICU admission, or mortality. |
| McEllistrem et al. [41] | 2021 | Pennsylvania, USA | June 24–August 23, 2020 | 53 | NP | Cepheid Xpert Xpress | Hospitalized patients | Nonhospitalized patients and health care workers | No | At a Veterans Affairs Hospital, patients who were hospitalized had a higher CT value than both outpatients and nonhospitalized health care workers. |
| Seeni et al. [42] | 2021 | Nevada, USA | April 1–October 30, 2020 | 134 | NP | Cepheid Xpert Xpress | CT <34 | CT >34 | NR | Adjusting for age, diabetes, heart disease, kidney disease, and lung disease, patients with a CT <34 had a higher odds of a composite of hospitalization requiring supplemental oxygen, ICU need, mechanical ventilation, and death compared with patients with a CT value >34 (aOR, 4.0; 97.5% CI, 1.69 to 10.10). |
| Yagci et al. [43] | 2020 | Istanbul, Turkey | March 22–May 20, 2020 | 730 | NP | Biospeedy COVID-19 qPCR detection kit, version 2 | Hospitalized patients | Nonhospitalized patients | No | Among patients with a positive SARS-CoV-2 rtPCR and a chest CT performed, nonhospitalized patients had a lower mean CT value compared with hospitalized patients. |
Abbreviations: aOR, adjusted odds ratio; COVID-19, coronavirus disease 2019; CT, cycle threshold; CT, computed tomography; ICU, intensive care unit; NP, nasopharyngeal; NR, not reported; OP, oropharyngeal; PCR, polymerase chain reaction; qPCR, quantitative polymerase chain reaction; rtPCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 3.
Summary of Studies Evaluating the Association Between CT Values and Mortality Among Hospitalized Patients
| Author | Year | City, State, Country | Study Enrollment Period | Sample Size | Sample Source | rtPCR Assay | Comparator | Reference Group | Lower CT Values Associated With Mortality | Summary |
|---|---|---|---|---|---|---|---|---|---|---|
| Bryan et al. [48] | 2020 | Washington, USA | NR | 109 | NP | Hologic Panther Fusion; LDT | Patients with CT <22 | Patients with CT >22 | Yes | After adjusting for SARS-CoV-2 antibody status, age, and sex, hospitalized patients with CT <22 had higher odds of 30-day mortality compared with patients with CT >22 (aOR, 4.20; 95% CI, 1.62 to 10.86). |
| Choudhuri et al. [49] | 2020 | New York, USA | March 26–August 5, 2020 | 1044 | NP | Hologic Panther Fusion | Patients with CT <22.9 and 23–27.9 | Patients with CT >32.9 | Yes | After adjusting for age, sex, BMI, hypertension, and diabetes, patients with CT <22.9 or 23–27.9 had higher odds of mortality compared with patients with CT >32.9 (aOR, 3.85; 95% CI, 2.3 to 6.2; and aOR, 2.63; 95% CI, 1.6 to 4.2; respectively). |
| de la Calle et al. [44] | 2021 | Madrid, Spain | March 1–18, 2020 | 455 | NP | Thermo Fisher Scientific TaqMan | Patients with CT <25 and 25–30 | Patients with CT >30 | Yes (No for CT 25–30 compared with >30) | Adjusting for age, cardiovascular disease, chronic renal disease, smoking history, tachypnea, LDH, CRP, patients with CT <25 had higher odds of mortality compared with patients with CT >30 (aOR, 2.04; 95% CI, 1.44–4.00). These increased odds of mortality were not found in patients with CT 25–30 compared with >30. |
| Faico-Filjo et al. [39] | 2020 | São Paulo, Brazil | March 17–June 17, 2020 | 376 | NP | Thermo Fisher Scientific AgPath-ID | Nonsurvivors | Survivors | Yes | Among hospitalized patients with SARS-CoV-2, patients who did not survive had lower mean CT values at the time of diagnosis compared with survivors. Compared with patients with CT >24, hospitalized patients with a CT <25 had increased odds of mortality (OR, 2.93; 95% CI, 1.87 to 4.60). |
| Maltezou et al. [21] | 2021 | Athens and Thessaloniki, Greece | February 26–May 3, 2020 | 518 | NP or OP | Genesig COVID-19 CE-IVD real-time RT-PCR kit OR Thermo Fischer Scientific TaqMan | Patients with CT <25 and 25–30 | Patients with CT >30 | No | In univariate analyses, compared with patients with CT >30 or 25–30, patients with CT <25 were older, more often had comorbidities, developed symptomatic COVID-19, were intubated, and died. In multivariate analysis controlling for age, sex, and comorbidities, patients with CT <25 had higher likelihood of having symptomatic disease, but without increased odds of hospitalization, ICU admission, or mortality. Multivariate analysis described in the article included hospitalized and nonhospitalized patients. Data were abstracted to evaluate mortality among hospitalized patients. |
| Seeni et al. [42] | 2021 | Nevada, USA | April 1—October 30, 2020 | 88 | NP | Cepheid Xpert Xpress | Patients with CT <34 | Patients with CT >34 | NR | Adjusting for age, diabetes, heart disease, kidney disease, and lung disease, patients with CT <34 had higher odds of a composite of hospitalization requiring supplemental oxygen, ICU need, mechanical ventilation, and death, compared with patients with CT >34 (aOR, 4.0; 97.5% CI, 1.69 to 10.10). Authors were contacted and provided data on mortality outcomes among hospitalized patients. |
| Shah et al. [29] | 2021 | Mumbai, India | March 23–June 30, 2020 | 111 | NP | Altona Diagnostics Real Star SARS-CoV-2 kit | CT of 11–20 | CT of 31–40 | Yes | Among hospitalized patients with a pulse oximetry ≤93%, patients with CT 11–20 had a higher percentage of inpatient mortality (66.7%) compared with patients with CT 31–40 (39.1%). Notably, the duration of illness before testing in patients who died was shorter (3 days) than in those who survived (5 days). |
| Westblade et al. [24] | 2020 | New York, USA | March 15–May 14, 2020 | 3914 | NP | Roche Cobas and Cepheid Xpert Xpress | Patients with CT <25 and 25–30 | Patients with CT >30 | Yes | Among patients with cancer, after adjusting for age and need for supplemental O2 within 3 hours of ED presentation, a CT value <25 compared with a CT value >30 was associated with inpatient mortality (aOR, 4.71; 95% CI, 1.44 to 15.44), but not for patients with a CT value 25–30 compared with >30. These results were similar to a comparative cohort without cancer. |
| Yagci et al. [43] | 2020 | Istanbul, Turkey | March 22–May 20, 2020 | 284 | NP | Biospeedy COVID-19 qPCR detection kit, version 2 | Nonsurvivors | Survivors | No | Among patients with a positive SARS-CoV-2 RT-PCR and a chest CT performed on hospital admission, mean CT values did not significantly differ between survivors and nonsurvivors. Mortality was not associated with CT value, but was associated with older age, CRP positivity, and worsened CT chest total severity score. |
| Zhao et al. [50] | 2021 | New Jersey, USA | March 12–April 8, 2020 | 722 | NPa | LDT | Nonsurvivors | Survivors | Yes | Among hospitalized patients with SARS-CoV-2, patients who did not survive had lower mean CT values at the time of diagnosis compared with survivors for both the E and N2 gene targets. |
Abbreviations: aOR, adjusted odds ratio; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, cycle threshold; CT, computed tomography; ICU, intensive care unit; LDH, lactate dehydrogenase; LDT, laboratory-developed test; NP, nasopharyngeal; NR, not reported; OP, oropharyngeal; OR, odds ratio; PCR, polymerase chain reaction; qPCR, quantitative polymerase chain reaction; rtPCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
a99.6% of samples were NP, and 0.42% were OP.
Table 2.
Summary of Studies Evaluating the Association Between CT Values and Disease Severity Among Hospitalized Patients
| Author | Year | City, State, Country | Study Enrollment Period | Sample Size | Sample Source | rtPCR Assay | Comparator | Reference Group | Lower CT Values Associated With Disease Severity | Summary |
|---|---|---|---|---|---|---|---|---|---|---|
| de la Calle et al. [44] | 2021 | Madrid, Spain | March 1–March 18, 2020 | 455 | NP | Thermo Fisher Scientific TaqMan | Patients with CT <25 and 25–30 | Patients with CT >30 | Yes | In a multivariate analysis adjusted for age, sex, cardiovascular disease, chronic lung disease, immunosuppression, smoking status, presence of dyspnea, abnormal chest x-ray findings on admission, severe lymphopenia (≤0.7 × 103 cells/μL), LDH ≥350 U/L, and C-reactive protein ≥6 mg/dL, CT value <25 was independently associated with increased risk of respiratory failure (aOR, 2.99; 95% CI, 1.57 to 5.69) compared with CT >30 among hospitalized patients. Similarly, this association was significant for patients with CT 25–30 compared with >30 (aOR, 1.81; 95% CI, 1.02 to 3.22). |
| Faico-Filjo et al. [39] | 2020 | São Paulo, Brazil | March 17–June 17, 2020 | 376 | NP | Thermo Fisher Scientific AgPath-ID | Patients requiring the ICU | Hospitalized patients | Yes | Among hospitalized patients with SARS-CoV-2, patients who required ICU-level care had lower mean CT values compared with other hospitalized patients. |
| Fukushima et al. [45] | 2021 | Tokyo, Japan | March 24–May 14, 2020 | 19 | NP | LDT | Patients who required the ICU, mechanical ventilation, or died | Hospitalized patients | Yes | Among hospitalized patients with a chest CT confirming COVID pneumonia who were initially hospitalized in a non–intensive care unit, patients who subsequently died or needed mechanical ventilation or ICU care had lower mean CT values compared with other hospitalized patients. |
| Gaston et al. [46] | 2020 | Connecticut, USA | March 1–May 25, 2020 | 25 | NP or OP + NP | LDT; Cepheid Xpert Xpress | Patients who required noninvasive positive pressure ventilation, mechanical ventilation, ICU, or who died | Hospitalized patients | No | Among a cohort of patients with solid organ transplant, mean CT scores did not significantly differ between patients who required ICU-level care and other hospitalized patients. |
| Guo et al. [47] | 2020 | Guangdong Province, China | January 13–February 28, 2020 | 195 | NP | LDT | Patients requiring mechanical ventilation or ICU, or with shock | Hospitalized patients | Yes | Patient requiring ICU-level care had lower mean CT values compared with other hospitalized patients. |
| Magleby et al. [20] | 2020 | New York, USA | March 30–April 30, 2020 | 678 | NP | Roche Cobas | Patients with CT <25 and 25–30 | Patients with CT >30 | Yes | After adjusting for BMI, use of steroids as an outpatient, fever, dyspnea, infiltrates on chest x-ray, patients with CT <25 had a higher odds of intubation compared with other hospitalized patients with CT >30 (aOR, 2.73; 95% CI, 1.68 to 4.44). This finding did not reach significance for patients with CT 25–30 compared with >30 (aOR, 1.59; 95% CI, 0.96–2.63). |
| Maltezou et al. [21] | 2021 | Athens and Thessaloniki, Greece | February 26–May 3, 2020 | 518 | NP or OP | Genesig COVID-19 CE-IVD real-time RT-PCR kit; Thermo Fischer Scientific TaqMan | Patients with CT <25 and 25–30 | Patients with CT >30 | No | In univariate analyses, compared with patients with CT >30 or 25–30, patients with CT value <25 were older, more often had comorbidities, developed symptomatic COVID-19, were intubated, and died. In multivariate analysis controlling for age, sex, and comorbidities, patients with CT <25 had a higher likelihood of having symptomatic disease, but not increased odds of hospitalization, ICU admission, or mortality. Multivariate analysis described in the article included hospitalized and nonhospitalized patients. Data were abstracted to evaluate disease severity outcomes among hospitalized patients. |
| Seeni et al. [42] | 2021 | Nevada, USA | April 1–October 30, 2020 | 88 | NP | Cepheid Xpert Xpress | CT <34 | CT >34 | NR | Adjusting for age, diabetes, heart disease, kidney disease, and lung disease, patients with CT <34 had a higher odds of a composite of hospitalization requiring supplemental oxygen, ICU need, mechanical ventilation, and death compared with patients with CT >34 (aOR, 4.0; 97.5% CI, 1.69 to 10.10). |
Abbreviations: aOR, adjusted odds ratio; COVID-19, coronavirus disease 2019; CT, cycle threshold; CT, computed tomography; ICU, intensive care unit; NP, nasopharyngeal; NR, not reported; OP, oropharyngeal; PCR, polymerase chain reaction; qPCR, quantitative polymerase chain reaction; rtPCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Risk of Bias Assessment
Overall, 15 studies had a high risk of bias (Supplementary Data). Three studies were deemed to have a moderate risk of bias.
Meta-analysis
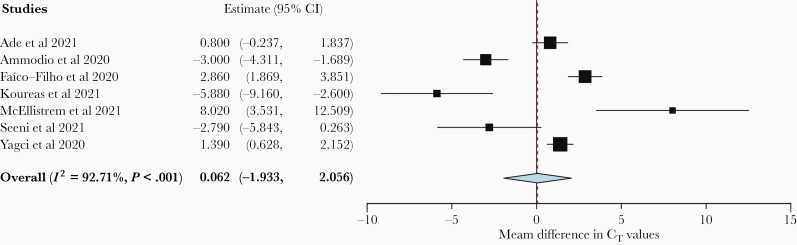
For the outcome of hospitalization, 1 study reported only categorical CT values, and thus we were not able to perform an analysis [21]. Seven studies (n = 3291 patients) were analyzed, and 4 studies reported higher CT values in hospitalized patients, 1 of which did not reach statistical significance. Three studies reported lower mean CT values among hospitalized patients, 1 of which did not reach statistical significance. Meta-analysis found no difference in the mean CT value between hospitalized and nonhospitalized patients with SARS-CoV-2 with high heterogeneity (0.062; 95% CI, –1.933 to 2.056; I2 = 92.71%) (Figure 2).
Figure 2.
Forest plot of mean CT value difference between hospitalized and nonhospitalized patients. Abbreviation: CT, cycle threshold.
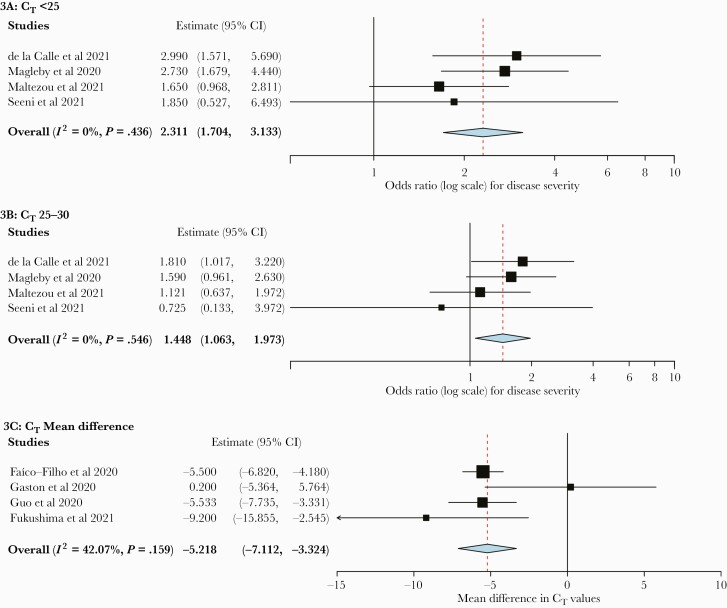
For disease severity among hospitalized patients, 4 studies (n = 2347 patients) reported categorical CT values. Hospitalized patients with CT values <25 or 25–30 had an increased risk of more severe disease compared with patients with CT values >30 (OR, 2.31; 95% CI, 1.70 to 3.13; and OR, 1.45; 95% CI, 1.06 to 1.97, respectively) (Figure 3). There was low heterogeneity for these outcomes (I2 = 0%). Analysis of 4 studies (n = 675 patients) found a mean CT difference of –5.22 (95% CI, –7.11 to –3.32) in patients with severe disease compared with nonsevere disease among hospitalized patients, also with low heterogeneity (I2 = 42.07%) (Figure 3C).
Figure 3.
Forest plots of disease severity outcome among hospitalized patients. A, CT value <25 vs >30. B, CT value 25–30 vs >30. C, Mean CT value difference between patients with severe and nonsevere disease in subgroup analysis by sample source. Abbreviation: CT, cycle threshold.
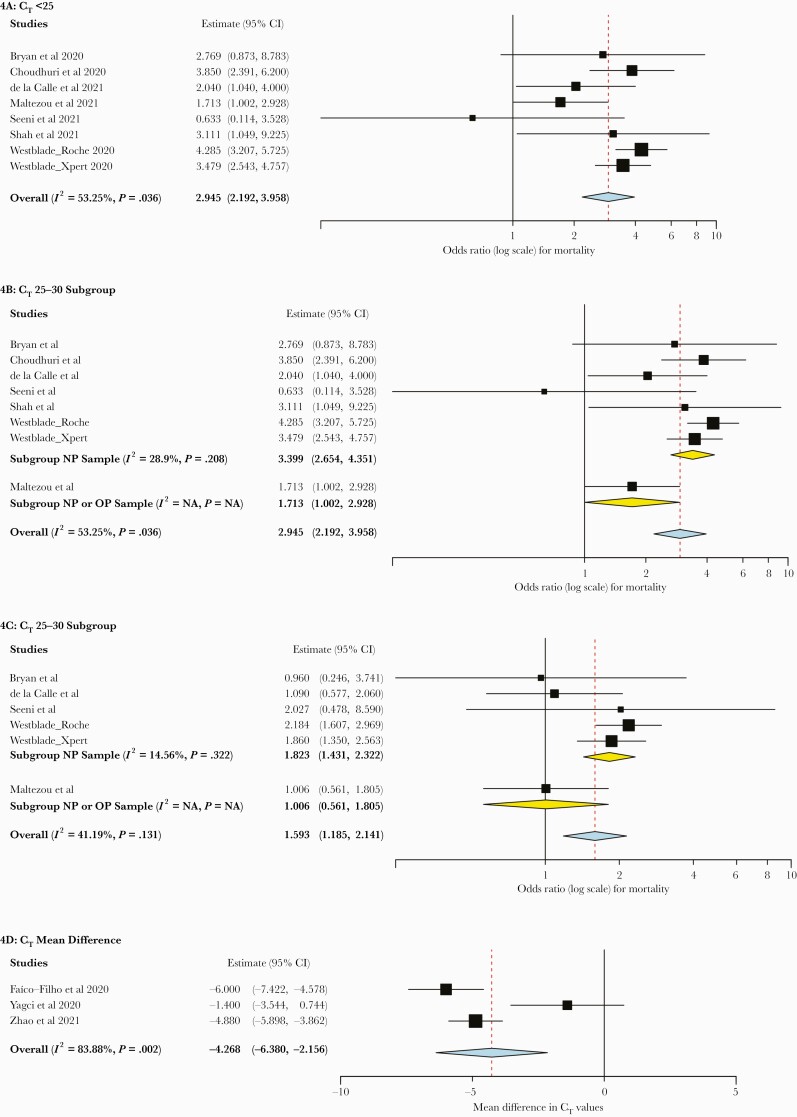
For the outcome of mortality, 7 studies (n = 6053 patients) reported categorical CT values. While Magleby et al. reported on the relationship between CT values and mortality, this data set was also included in the report by Westblade et al., which was a larger data set [20, 24]. Thus, the synthesis did not include data from Magleby et al. for the mortality outcome to avoid duplication of results. Hospitalized patients with CT values <25 had an increased risk of mortality compared with those with CT values >30 (OR, 2.95; 95% CI, 2.19 to 3.96) (Figure 4A). There was moderate heterogeneity (I2 = 53.25%), which did not change significantly during a subgroup analysis by risk of bias or rtPCR assay (data not shown). In subgroup analysis by sample source, the 6 studies that utilized only nasopharyngeal swab had low heterogeneity (I2 = 28.9%) (Figure 4B).
Figure 4.
Forest plots of mortality outcome among hospitalized patients. A, CT value <25 vs >30. B, CT <25 vs >30, subgroup analysis by sample source. C, CT value 25–30 vs >30. D, Mean CT value difference between survivors and nonsurvivors. Abbreviations: CT, cycle threshold; NP, nasopharyngeal.
Hospitalized patients with CT values of 25–30 compared with >30 also had an increased mortality risk (OR, 1.59; 95% CI, 1.19 to 2.14) with low heterogeneity (I2 = 41.19%), though this finding was driven by a single large study (Figure 4C) [24]. Three additional studies (n = 1382 patients) reported on the relationship between mean CT values and mortality in hospitalized patients and found a lower mean CT value among nonsurvivors than survivors (OR, –4.27; 95% CI, –6.38 to –2.16) with high heterogeneity (I2 = 83.88%).
Three studies did not provide sufficient data for meta-analysis and are summarized narratively. Piubelli et al. reported 373 patients from a single center in Italy and reported CT values by month. CT values decreased from March 2020 through April 2020 with decreased ICU need, consistent with a waning epidemic trajectory, but the CT values for patients who required ICU-level care did not change [25, 26]. Young et al. reported a prospective observational study of 100 patients from Singapore in which 20 patients had pneumonia and hypoxia and found no difference in CT values compared with patients without pneumonia [27]. However, there was no separate analysis for the 12 patients who required ICU care. Yu et al. reported a study from China of 92 patients comparing baseline CT values in patients with severe disease with CT values in those with mild or moderate disease on admission [28]. They found that patients with more severe disease on admission, as well as patients who went on to have severe disease during their hospitalization, had lower admission CT values compared with those with mild or moderate disease. However, disease severity was not defined.
DISCUSSION
This systematic review and meta-analysis did not find an association between CT values and hospitalization of persons with SARS-CoV-2. Four studies reported higher CT values in hospitalized patients, while 3 studies reported lower CT values. The single study that reported only OR for the outcome of hospitalization also found no association between low CT value and risk of hospitalization [21]. There was high heterogeneity in the data, which did not significantly decrease in subgroup analysis by sample source (data not shown). These 7 studies from 6 different countries utilized 6 different rtPCR assays, which may account for the difference in results. Additionally, the different study periods and local disease dynamics may contribute to the heterogeneity in the reported data. If testing was limited or delayed, this could also have an impact on the comparator group and may, in part, account for some of the observed heterogeneity. The certainty of a lack of association is also limited by different standards for hospitalization globally, particularly early in the COVID-19 pandemic when many institutions were admitting all patients with SARS-CoV-2 infection regardless of symptoms.
For the disease severity and mortality outcomes, CT value data were evaluated both as a numerical difference between outcomes and as a categorical variable depending upon how individual studies reported data. Comparing outcomes across studies using categorical CT values is challenging due to variations in sample collection and the rtPCR platform utilized between studies. Evaluating mean differences in CT values has the advantage of canceling out systematic differences within studies such as testing availability and the rtPCR platform, which allows for more robust comparisons between studies.
Among patients hospitalized with COVID-19, those with lower CT values had more severe disease necessitating noninvasive ventilation, mechanical ventilation, or ICU admission. This association was most notable when comparing patients with CT values <25 with patients with CT values >30 and was also noted among patients with CT values of 25–30. Consistent with this finding, our analyses also revealed a lower mean CT value among those with more severe disease (Figure 3C). Contrary to the other 3 studies, Gaston et al. found no mean difference in those with more severe outcomes, though confidence intervals overlapped with other studies. However, this study was in patients with a solid organ transplant, representing a unique patient population. Overall, we observed low heterogeneity in the data.
Among patients hospitalized with COVID-19, lower CT values, particularly CT values <25, were associated with higher mortality compared with those with CT values >30. This analysis included the study by Shah et al., which evaluated mortality among patients with severe disease, defined as having pulse oximetry readings of <93% on room air [29]. This cohort is similar to other hospitalized patients and was thus included in the pooled analysis. There was moderate heterogeneity that decreased during subgroup analysis by sample source (Figure 4B). The association between CT values and mortality was less pronounced when comparing hospitalized patients with CT values of 25–30 with patients with CT values of >30, driven largely by a single study (Figure 4C). Our analysis also revealed higher mean CT values among survivors compared with nonsurvivors.
Interestingly, there have been mixed reports of the association between viral load and outcomes in patients with other respiratory illnesses. A low CT was not associated with worsened outcomes in patients with influenza [30]. Duncan et al. evaluated adults with respiratory syncytial virus and showed that higher viral loads were not independent predictors of hospitalization, but peak viral load was a predictor for mechanical ventilation [31]. Hung et al. performed a prospective study of 154 patients infected with the original SARS-CoV in 2003 and found that higher viral load later in the disease course was associated with increased rates of mechanical ventilation and death [32]. These mixed reports have been described in patients with SARS-CoV-2, and the heterogeneity in the data may be, in part, due to different sample populations, sample sources, and rtPCR assays.
The timing of clinical specimen collection is critical and may impact the CT value as well. A study by Hu et al. suggested that viral load peaks shortly after symptom onset then declines in a steady manner [33]. Early in the disease course, patients infected with SARS-CoV-2 generally have low CT values, with no discernable difference between those who require hospitalization and those who do not. However, as symptoms progress and those who require hospitalization present for medical care, patients with persistently high viral loads may have a worsened prognosis, which may be predicted using the CT value as a surrogate marker. This correlation may also be age-dependent. Faes et al. evaluated a cohort of patients in Belgium and found age to be correlated with time from symptom onset to hospitalization, with younger patients having the shortest duration [34]. The time at which patients get tested may impact the CT value, particularly for those with less severe disease.
Limitations
There are several limitations to our review. As highlighted by Rhoads et al., the use of CT values for clinical decision-making is a challenging proposal for several reasons [35]. First, sample source, collection method, volume, and storage may impact the CT value. Additionally, CT values can vary widely based on the rtPCR assay used.
This meta-analysis was limited to studies published in English. However, patients from many countries are represented in this evaluation. Due to the small number of studies per outcome, the presence of publication bias could not be evaluated; nonetheless, reporting and publication bias remain a concern as the overall large number of publications related to COVID-19 may have resulted in studies with null results that may not have been reported or published. In addition, time from symptom onset to sample collection or testing was not considered in this evaluation as such data were not widely reported in published studies. Most studies were conducted earlier in the pandemic, when rtPCR testing was more limited and individuals were immune-naive. Findings from this analysis may not be applicable to those with immunity through vaccination or prior infection. Furthermore, most studies were found to have a high risk of bias, largely due to not adjusting for potential confounding variables that are known to affect outcomes assessed in this study, such as age, gender, and use of therapeutics. Additionally, study population was mostly done by convenience sampling, which can lead to significant selection bias. Therefore, using the GRADE approach to evaluate certainty in the meta-analytic estimates, we judged this certainty to be very low due to risk of bias and heterogeneity [36].
Future Directions
Further prospective research that takes into account confounding factors such as age, gender, comorbidities, and duration from symptom onset to testing would further add to the knowledge base on the clinical utility of the CT value. A prospective serial evaluation of CT values in patients with multiple risk factors for severe disease could aid in determining whether persistently high levels of viral RNA early in the disease course are related to worse outcomes and whether patients who are able to mount an immunologic response and clear more virus have improved outcomes. Additional evaluation of viral load and CT value dynamics in emerging variants and in populations with immunity would also be valuable. Development and availability of quantitative rtPCR assays would allow for standardization and more direct comparison of the prognostic utility of viral load of SARS-CoV-2. To date, no quantitative SARS-CoV-2 assay has received Emergency Use Authorization by the US Food and Drug Administration.
Despite limitations on the interpretation of individual CT values, they may aid in prognostication of patients, along with other demographic, clinical, and laboratory findings. The CT value may allow clinicians to better triage certain patients admitted to the hospital to provide appropriate interventions in a timely manner. Another major benefit of the CT value is that it may be obtained without need for additional testing, assuming the test for SARS-CoV-2 is performed on rtPCR assays that provide this value.
CONCLUSIONS
This systematic review suggests a role for CT values in the prognostication of hospitalized individuals for the outcomes of disease severity and mortality, with lower CT values (ie, higher levels of viral RNA) correlating with increased disease severity and mortality. However, CT results must be interpreted with caution given the limitations and lack of assay standardization.
Supplementary Material
Acknowledgments
Financial support. The authors received no specific funding for this work.
Potential conflicts of interest. M.J.B. is an advisory board member for DiaSorin Molecular and Mammoth Biosciences. J.D.Y. received research grants from Roche Molecular Systems, Inc., and Abbott Molecular, Inc. All remaining authors have no reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data availability. Data abstracted from this research are available by contacting the corresponding author.
Patient consent. This systematic review and meta-analysis is not human subjects research and conforms to the ethical standards within the United States.
References
- 1. Centers for Disease Control and Prevention. COVID data tracker weekly review. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html. Accessed 10 May 2021.
- 2. Centers for Disease Control and Prevention. Estimated disease burden of COVID-19. Updated 29 April 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html. Accessed 11 May 2021.
- 3. Asch DA, Sheils NE, Islam MN, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med 2021; 181:471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kompaniyets L, Goodman AB, Belay B, et al. Body mass index and risk for COVID-19-related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death - United States, March-December 2020. MMWR Morb Mortal Wkly Rep 2021; 70:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US medical centers. JAMA Netw Open 2021; 4:e210417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roth GA, Emmons-Bell S, Alger HM, et al. Trends in patient characteristics and COVID-19 in-hospital mortality in the United States during the COVID-19 pandemic. JAMA Netw Open 2021; 4:e218828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open 2020; 3:e2022310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020; 11:6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med 2020; 58:1021–8. [DOI] [PubMed] [Google Scholar]
- 11. Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care 2020; 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tom MR, Mina MJ. To Interpret the SARS-CoV-2 test, consider the cycle threshold value. Clin Infect Dis 2020; 71:2252–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dahdouh E, Lázaro-Perona F, Romero-Gómez MP, et al. Ct values from SARS-CoV-2 diagnostic PCR assays should not be used as direct estimates of viral load. J Infect 2021; 82:414–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rao SN, Manissero D, Steele VR, Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther 2020; 9:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Argyropoulos KV, Serrano A, Hu J, et al. Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with outcome and symptoms. Am J Pathol 2020; 190:1881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Le Borgne P, Solis M, Severac F, et al. SARS-CoV-2 viral load in nasopharyngeal swabs in the emergency department does not predict COVID-19 severity and mortality. Acad Emerg Med 2021; 28:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pujadas E, Chaudhry F, McBride R, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med 2020; 8:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsukagoshi H, Shinoda D, Saito M, et al. Relationships between viral load and the clinical course of COVID-19. Viruses 2021; 13:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Magleby R, Westblade LF, Trzebucki A, et al. Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis 2020; 30:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maltezou HC, Raftopoulos V, Vorou R, et al. Association between upper respiratory tract viral load, comorbidities, disease severity and outcome of patients with SARS-CoV-2 infection. J Infect Dis 2021; 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viswanathan M, Patnode CD, Berkman ND, et al. Recommendations for assessing the risk of bias in systematic reviews of health-care interventions. J Clin Epidemiol 2018; 97:26–34. [DOI] [PubMed] [Google Scholar]
- 23. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH.. Closing the Gap between methodologists and end-users: R as a computational back-end. J Stat Softw 2012; 49:15. [Google Scholar]
- 24. Westblade LF, Brar G, Pinheiro LC, et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell 2020; 38:661–71.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piubelli C, Deiana M, Pomari E, et al. Overall decrease in SARS-CoV-2 viral load and reduction in clinical burden: the experience of a hospital in Northern Italy. Clin Microbiol Infect 2021; 27:131.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hay JA, Kennedy-Shaffer L, Kanjilal S, et al. Estimating epidemiologic dynamics from cross-sectional viral load distributions. Science 2021; 373:eabh0635.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Young BE, Ong SWX, Ng LFP, et al. Viral dynamics and immune correlates of COVID-19 disease severity. Clin Infect Dis 2020; 28:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu X, Sun S, Shi Y, et al. SARS-CoV-2 viral load in sputum correlates with risk of COVID-19 progression. Crit Care 2020; 24:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shah S, Singhal T, Davar N, Thakkar P. No correlation between Ct values and severity of disease or mortality in patients with COVID 19 disease. Indian J Med Microbiol 2021; 39:116–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lalueza A, Folgueira D, Muñoz-Gallego I, et al. Influence of viral load in the outcome of hospitalized patients with influenza virus infection. Eur J Clin Microbiol Infect Dis 2019; 38:667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duncan CB, Walsh EE, Peterson DR, et al. Risk factors for respiratory failure associated with respiratory syncytial virus infection in adults. J Infect Dis 2009; 200:1242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hung IF, Cheng VC, Wu AK, et al. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis 2004; 10:1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–5. [DOI] [PubMed] [Google Scholar]
- 34. Faes C, Abrams S, Van Beckhoven D, et al. Time between symptom onset, hospitalisation and recovery or death: statistical analysis of Belgian COVID-19 patients. Int J Environ Res Public Health 2020; 17:7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rhoads D, Peaper DR, She RC, et al. College of American Pathologists (CAP) Microbiology Committee perspective: caution must be used in interpreting the cycle threshold (Ct) value. Clin Infect Dis 2021; 72:e685–6.. [DOI] [PubMed] [Google Scholar]
- 36. Murad MH. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc 2017; 92:423–33. [DOI] [PubMed] [Google Scholar]
- 37. Ade C, Pum J, Abele I, et al. Analysis of cycle threshold values in SARS-CoV-2-PCR in a long-term study. J Clin Virol 2021; 138:104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Amodio E, Pipitone RM, Grimaudo S, et al. SARS-CoV-2 viral load, ifnlambda polymorphisms and the course of COVID-19: an observational study. J Clin Med 2020; 9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Faíco-Filho KS, Passarelli VC, Bellei N. Is higher viral load in SARS-CoV-2 associated with death? Am J Trop Med Hyg 2020; 103:2019–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koureas M, Speletas M, Bogogiannidou Z, et al. Transmission dynamics of SARS-CoV-2 during an outbreak in a Roma Community in Thessaly, Greece—control measures and lessons learned. Int J Environ Res Public Health 2021; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McEllistrem MC, Clancy CJ, Buehrle DJ, et al. SARS-CoV-2 is associated with high viral loads in asymptomatic and recently symptomatic healthcare workers. PLoS One 2021; 16:e0248347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seeni R, Firzli T, Riddle MS, et al. Using COVID-19 cycle threshold and other lab values as predictors of hospitalization need. J Med Virol 2021; 93:3007–14. [DOI] [PubMed] [Google Scholar]
- 43. Karahasan Yagci A, Sarinoglu RC, Bilgin H, et al. Relationship of the cycle threshold values of SARS-CoV-2 polymerase chain reaction and total severity score of computerized tomography in patients with COVID 19. Int J Infect Dis 2020; 101:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de la Calle C, Lalueza A, Mancheno-Losa M, et al. Impact of viral load at admission on the development of respiratory failure in hospitalized patients with SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis 2021; 7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fukushima T, Kabata H, Yamamoto R, et al. ; Keio Donner Project Team. The real-time reverse transcription-polymerase chain reaction threshold cycle values for severe acute respiratory syndrome coronavirus 2 predict the prognosis of coronavirus disease 2019 pneumonia. Respir Investig 2021; 59:360–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gaston DC, Malinis M, Osborn R, et al. Clinical implications of SARS-CoV-2 cycle threshold values in solid organ transplant recipients. Am J Transplant 2020; 12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guo X, Jie Y, Ye Y, et al. Upper respiratory tract viral ribonucleic acid load at hospital admission is associated with coronavirus disease 2019 disease severity. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bryan A, Fink SL, Gattuso MA, et al. SARS-CoV-2 viral load on admission is associated with 30-day mortality. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Choudhuri J, Carter J, Nelson R, et al. SARS-CoV-2 PCR cycle threshold at hospital admission associated with patient mortality. PLoS One 2020; 15:e0244777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao Y, Cunningham MH, Mediavilla JR, et al. Diagnosis, clinical characteristics, and outcomes of COVID-19 patients from a large healthcare system in Northern New Jersey. Sci Rep 2021; 11:4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.