Abstract
This review summarizes the definition and surgical methods of oncometabolic surgery according to previous studies. Then, the authors discuss the beneficial effects observed after gastrectomy in gastric cancer (GC) patients with concurrent hypertension or type 2 diabetes mellitus (T2DM). The authors summarize the current studies analyzing the remission rate and the hypotheses of the mechanisms underlying these effects. The remission rate ranged from 42.5%-65.4% in T2DM patients and from 11.1%-57.6% among those with hypertension. Furthermore, the remission of T2DM could have an impact on overall survival rates as well. The mechanisms underlying the remission of hypertension and T2DM is unclear in current studies, but oncometabolic surgery is expected to be applied in clinical practice. In addition, the effect of oncometabolic surgery on other chronic metabolic comorbidities is expected to be proven in further studies. Therefore, the purpose of this review is to discuss the effects of oncometabolic surgery reported in current studies with a primary focus on the remission of hypertension and T2DM after gastrectomy in GC patients. The possibility of the remission of other metabolic comorbidities in GC patients who undergo oncometabolic surgery is also discussed.
Keywords: Gastric cancer, Type 2 diabetes mellitus, Hypertension, Remission, Oncometabolic surgery
Core Tip: The purpose of this review is to discuss the effects of oncometabolic surgery observed in current studies, mainly including the remission of hypertension and type 2 diabetes mellitus after gastrectomy in gastric cancer (GC) patients, and to evaluate the possibility of the remission of other metabolic comorbidities in GC patients who undergo oncometabolic surgery.
INTRODUCTION
Gastric cancer (GC) is the second most common digestive system cancer. As estimated by GLOBOCAN in 2018, the incidence of GC ranks fifth among all cancers, with 1 million new cases and 780 thousand deaths worldwide[1]. Radical resection plus standardized D2 Lymphadenectomy is the standard method for curative intention[2]. The 5-year survival rate of early GC patients has reached 90%, causing concern about these patients’ quality of life after gastrectomy[3,4]. With the number of cancer-related deaths decreasing among postoperative GC patients, the control of chronic diseases has become another medical concern.
The initial and obvious purpose of bariatric surgery is to achieve weight loss and maintenance[5]. However, morbid obesity drastically elevates the risk of some chronic metabolic comorbidities, especially the most common diseases including hypertension, type 2 diabetes mellitus (T2DM), dyslipidemia, sleep apnea, and so on[6]. It has rapidly come under observation that bariatric surgery is effective in treating metabolic comorbidities in obese patients[7,8]. A meta-analysis including eleven randomized controlled trials reported by Cummings and Cohen provided class 1A evidence demonstrating the effect of bariatric surgery on T2DM remission[9]. Thus, the concept of metabolic bariatric surgery is gradually being developed.
To our knowledge, the extent of gastrectomy and reconstruction methods in bariatric surgery has a strong level of similarity with that in gastrectomy of GC patients. Therefore, surgeons began to pay close attention to the remission of hypertension or T2DM after gastrectomy in patients who suffer from concurrent GC and metabolic comorbidities[10,11]. Fortunately, this hypothesis was proposed in some previous studies of oncometabolic surgery[12,13]. It has been reported that insulin resistance improves and fasting blood glucose returns to normal after gastrectomy, although controversy regarding the mechanism underlying this remission still exists[14]. There was a retrospective study of 143 patients in which the hypertension remission rate after gastrectomy was evaluated[15], and it was relatively less than the T2DM remission rate reported in previous studies.
Although limited studies exist, the potential of oncometabolic surgery is nonnegligible. Therefore, the purpose of this review is to discuss the effect of oncometabolic surgery based on current studies, mainly including the remission of hypertension and T2DM after gastrectomy in GC patients, and to evaluate the possibility of the remission of other metabolic comorbidities in patients who undergo oncometabolic surgery.
CONCEPT OF ONCOMETABOLIC SURGERY
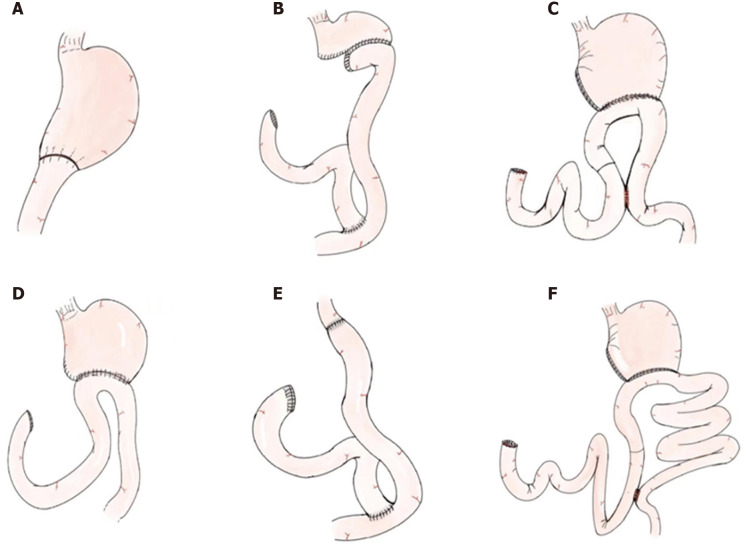
Oncometabolic surgery is a single operation that not only achieves tumor radical resection but also controls metabolic diseases, including hypertension and T2DM[10,16]. The extent of gastrectomy includes total gastrectomy and subtotal gastrectomy, and the reconstruction methods consist of Billroth I (B-I), Billroth II (B-II) and Roux-en-Y (RY) reconstruction (Figure 1). In a previous study, it was demonstrated that total gastrectomy with RY reconstruction had the highest remission rate[17]. In addition, Peng et al[18] considered that only the extent of gastrectomy influenced T2DM remission[18]. Similarly, Wang et al[19] found that total gastrectomy itself was closely related to hypertension remission[19]. Park et al[20] considered that long-limb RY reconstruction, which is closely associated with RY gastric bypass, was oncometabolic surgery[20]. However, we considered total gastrectomy and subtotal gastrectomy with all reconstruction methods, including B-I reconstruction, B-II reconstruction, RY reconstruction, conventional uncut RY gastrojejunostomy, and long-limb uncut RY gastrojejunostomy, as oncometabolic surgeries.
Figure 1.
Schematic illustration of gastrectomy and reconstruction methods. A: Distal gastrectomy with Billroth I reconstruction; B: Distal gastrectomy with Roux-en-Y reconstruction; C: Distal gastrectomy with conventional uncut Roux-en-Y gastrojejunostomy; D: Distal gastrectomy with Billroth II reconstruction; E: Total gastrectomy with Roux-en-Y reconstruction; F: Distal gastrectomy with long-limb uncut Roux-en-Y gastrojejunostomy.
T2DM REMISSION
GC patients can experience T2DM remission after gastrectomy, and the remission rate ranges from 42.5%-65.4% (Table 1)[13,14,17,21-26]. However, the factors predicting T2DM remission remain unclear. An et al[13] suggested that the degree of T2DM control was related to its duration[13]. Kim et al[17] analyzed the records of 385 patients and concluded that body mass index reduction was significantly correlated with T2DM remission. Total gastrectomy with RY reconstruction was reported to lead to higher remission rates than other surgical methods, however, whether the extent of gastrectomy or the reconstruction method played an important role in T2DM remission remains controversial[17]. Wang et al[21] reported that the extent of gastrectomy rather than the reconstruction method was the key factor affecting T2DM remission[21], but Choi et al[22] suggested that RY reconstruction played an important role in T2DM remission[22]. Peng et al[18] conducted a meta-analysis and reported that the extent of gastrectomy rather than the reconstruction method might play an important role in T2DM remission after gastrectomy in patients with GC[18]. However, there were only three studies in the subgroup analysis about the extent of gastrectomy on T2DM remission; therefore, the results might not be robust[17,18,21].
Table 1.
Oncometabolic surgery on type 2 diabetes
|
Ref.
|
Surgery
|
Sample size
|
CR + PR
|
CR
|
PR
|
Follow-up (mo)
|
| Lee et al[14], 2012 | RYTG, BI, BII, RYGJ | 229 | 56.8% | 19.7% | 37.1% | NA |
| An et al[13], 2013 | RYTG, BI, BII | 64 | 57.8% | 3.1% | 54.7% | 12 |
| Wang et al[19], 2020 | RYTG, BI, BII, RYGJ | 69 | 43.5% | 13.0% | 30.5% | NA |
| Wei et al[23], 2014 | RYTG, BII | 67 | 59.7% | 26.9% | 32.8% | 57.4 |
| Kim et al[17], 2012 | RYTG, BI, BII | 385 | 45.5% | 15.1% | 30.4% | 33.7 |
| Zhu et al[24], 2015 | RYTG, BI, BII | 292 | 65.4% | 55.5% | 9.9% | 24 |
| Choi et al[22], 2017 | RYTG, BI | 40 | 42.5% | 2.5% | 40.0% | 12 |
| Kim et al[25], 2020 | LLBR, BII | 226 | 52.2% | 4.9% | 47.3% | 12 |
| Park et al[20], 2020 | RYTG, BI, BII, RYGJ | 52 | 63.5% | NA | NA | 12 |
CR: Complete remission; PR: Partial remission; RYTG: Roux-en-Y total gastrectomy; BI: Billroth I reconstruction; BII: Billroth II reconstruction; RYGJ: Subtotal gastrectomy with Roux-en-Y gastrojejunostomy reconstruction; LLBR: Long-limb bypass reconstruction; NA: Not available.
The remission of T2DM could also have an impact on overall survival. Wei et al[23] compared a remission group with a non-remission group and found that recovery from pre-existing T2DM after radical gastrectomy was associated with better overall survival; however, the study included only 67 patients, and larger studies are needed in the future[23].
The mechanism underlying T2DM remission after gastrectomy is unclear, but bariatric surgery might contribute to T2DM remission in a similar way. T2DM remission was related to lifestyle changes, including decreased food intake, reduced body weight and intestinal malabsorption. Several theories explaining T2DM remission beyond lifestyle changes have been proposed. The foregut theory states that patients undergoing duodenal bypass experience anti-diabetic effects, and the hindgut theory states that early contact of unabsorbed nutrients with the distal intestine improves T2DM. Furthermore, hormones, including ghrelin, glucagon-like peptide (GLP)-1 and GLP-2, can influence T2DM remission.
HYPERTENSION REMISSION
GC patients also experience hypertension remission after gastrectomy. Peng et al[15] conducted a retrospective study of 143 patients who underwent gastrectomy and the resulting effects on hypertension and found that the hypertension complete remission rate was 55.3%. In addition, age and total gastrectomy were predictors of hypertension remission 6 mo after gastrectomy[15]. Wang et al[19] compared hypertension remission between total gastrectomy and subtotal gastrectomy in non-obese non-diabetic GC patients and found that total gastrectomy itself, beyond weight loss, was associated with hypertension remission[19]. Another study included 33 patients and found that 14 patients experienced hypertension remission 1 year after gastrectomy[20]. Kim et al[27] reported on 66 early GC patients undergoing gastrectomy and endoscopic submucosal dissection and found that the hypertension remission rate was 57.6%, and gastrectomy was a predictor of hypertension remission 1 year after surgery[27]. Lee et al[12] reported the largest number of patients (n = 351) from nationwide data, and after a follow-up of 36.7 mo, only 11.1% of patients experienced hypertension remission; however, the results analyzed were based on nationwide data, and limited baseline information was available[12]. Therefore, the hypertension remission rate after gastrectomy was 11.1%-57.6% (Table 2), and Peng et al[15] proposed that total gastrectomy is an oncometabolic surgery that can cure younger patients with concurrent GC and hypertension[15].
Table 2.
Oncometabolic surgery on hypertension
|
Ref.
|
Country
|
Surgery
|
Sample size
|
CR + PR
|
CR
|
PR
|
Follow-up (mo)
|
| Peng et al[15], 2020 | China | RYTG, BI, BII, RYGJ | 143 | 55.3% | 46.9% | 8.4% | 6 |
| Wang et al[21], 2014 | China | RYTG, RYGJ | 16 | 93.8% | 100% | 87.5% | 12 |
| Park et al[26], 2017 | Korea | uRYGJ, LLBR | 33 | 42.4% | NA | NA | 12 |
| Kim et al[27], 2019 | Korea | RYTG, BI, BII, RYGJ | 66 | 57.6% | 45.5% | 12.1% | 12 |
| Lee et al[28], 2017 | Korea | RYTG, BI, BII, RYGJ | 351 | 11.1% | NA | NA | 36.7 |
CR: Complete remission; PR: Partial remission; RYTG: Roux-en-Y total gastrectomy; BII: Billroth II reconstruction; BI: Billroth I reconstruction; RYGJ: Subtotal gastrectomy with Roux-en-Y gastrojejunostomy reconstruction; uRYGJ: Subtotal gastrectomy with uncut Roux-en-Y gastrojejunostomy reconstruction; LLBR: Long-limb bypass reconstruction; NA: Not available.
There were some limitations because of the limited number of studies. Previous studies reported on the remission of hypertension; however, the follow-up time was short, and hypertension might reoccur in long term follow-up. In addition, the improvement of sodium absorption dysfunction, instead of weight loss, might be the factor leading to better management of blood pressure. However, no studies have reported the lifestyle factors of patients after gastrectomy, including physical exercise, drinking and smoking, and these lifestyle factors might also play an important role in hypertension remission.
Furthermore, the mechanism underlying hypertension remission is still unknown. It is hypothesized that the levels of some hormones, such as renin, angiotensin II, aldosterone, adiponectin, GLP-1, and tumor necrosis factor, might cause blood pressure fluctuations. However, a previous study found no difference in serum levels of these indicators before and after surgery. Thus, more experiments need to be carried out in the future.
FUTURE PERSPECTIVE
Evidence for the effects of bariatric surgery (also referred to as metabolic surgery) on T2DM remission has been demonstrated in previous studies. Metabolic surgery is expected to become an alternative for the treatment of T2DM in the future, instead being used only for weight loss[28]. Similarly, it is notable that gastrectomy has the same metabolic effect as bariatric surgery on patients with concurrent GC and metabolic comorbidities. Thus, oncometabolic surgery, which has been considered a developing concept in recent years, has the ability to improve the quality of life of patients with metabolic comorbidities[10,16]. Surgeons continuously improve traditional gastrectomy techniques to benefit patients. T2DM remission after gastrectomy has been found to be associated with better overall survival outcomes[23], but there is no previous evidence demonstrating a relationship between hypertension remission and overall survival outcomes. Short follow-up times are the current obstacle to the observation of the remission of comorbidities that may influence long-term survival rates after gastrectomy.
In addition, the focus of attention in oncometabolic surgery research has been T2DM rather than hypertension or other metabolic comorbidities. The effects of oncometabolic surgery on other metabolic comorbidities, such as fatty liver disease, hyperglycemia and hyperosmotic syndrome, leukodystrophy, hyperlipidemia, gout, and osteoporosis, are expected to be evaluated in the future. With the maturation of the concept of oncometabolic surgery, the opportunity to treat both GC and metabolic diseases through a single operation is possible. Therefore, a larger sample size and basic medical experiments are needed in future research.
CONCLUSION
With the maturation of the concept of oncometabolic surgery, the opportunity to treat both GC and metabolic diseases through a single operation is possible.
ACKNOWLEDGEMENTS
We acknowledge all the authors whose publications are referred in our article.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing interests.
Manuscript source: Invited manuscript
Peer-review started: May 27, 2021
First decision: June 16, 2021
Article in press: July 29, 2021
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Papazafiropoulou A, Salman A S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
Contributor Information
Yu-Xi Cheng, Department of Gastrointestinal Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China.
Dong Peng, Department of Gastrointestinal Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China.
Wei Tao, Department of Gastrointestinal Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China.
Wei Zhang, Department of Gastrointestinal Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China. cyzhangwei@hotmail.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–248. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YI, Kim YW, Choi IJ, Kim CG, Lee JY, Cho SJ, Eom BW, Yoon HM, Ryu KW, Kook MC. Long-term survival after endoscopic resection vs surgery in early gastric cancers. Endoscopy. 2015;47:293–301. doi: 10.1055/s-0034-1391284. [DOI] [PubMed] [Google Scholar]
- 4.McCall MD, Graham PJ, Bathe OF. Quality of life: A critical outcome for all surgical treatments of gastric cancer. World J Gastroenterol. 2016;22:1101–1113. doi: 10.3748/wjg.v22.i3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scopinaro N. Bariatric metabolic surgery. Rozhl Chir. 2014;93:404–415. [PubMed] [Google Scholar]
- 6.Valentí V, Cienfuegos JA, Becerril Mañas S, Frühbeck G. Mechanism of bariatric and metabolic surgery: beyond surgeons, gastroenterologists and endocrinologists. Rev Esp Enferm Dig. 2020;112:229–233. doi: 10.17235/reed.2020.6925/2020. [DOI] [PubMed] [Google Scholar]
- 7.Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13:1–190, 215. doi: 10.3310/hta13410. [DOI] [PubMed] [Google Scholar]
- 8.Rubio-Almanza M, Cámara-Gómez R, Merino-Torres JF. Obesity and type 2 diabetes: Also linked in therapeutic options. Endocrinol Diabetes Nutr (Engl Ed) 2019;66:140–149. doi: 10.1016/j.endinu.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald H, Buchwald JN. Metabolic (Bariatric and Nonbariatric) Surgery for Type 2 Diabetes: A Personal Perspective Review. Diabetes Care. 2019;42:331–340. doi: 10.2337/dc17-2654. [DOI] [PubMed] [Google Scholar]
- 10.Lee TH, Lee CM, Park S, Jung DH, Jang YJ, Kim JH, Park SH, Mok YJ. Long-term Follow-up for Type 2 Diabetes Mellitus after Gastrectomy in Non-morbidly Obese Patients with Gastric Cancer: the Legitimacy of Onco-metabolic Surgery. J Gastric Cancer. 2017;17:283–294. doi: 10.5230/jgc.2017.17.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renehan AG, Yeh HC, Johnson JA, Wild SH, Gale EA, Møller H Diabetes and Cancer Research Consortium. Diabetes and cancer (2): evaluating the impact of diabetes on mortality in patients with cancer. Diabetologia. 2012;55:1619–1632. doi: 10.1007/s00125-012-2526-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee EK, Kim SY, Lee YJ, Kwak MH, Kim HJ, Choi IJ, Cho SJ, Kim YW, Lee JY, Kim CG, Yoon HM, Eom BW, Kong SY, Yoo MK, Park JH, Ryu KW. Improvement of diabetes and hypertension after gastrectomy: a nationwide cohort study. World J Gastroenterol. 2015;21:1173–1181. doi: 10.3748/wjg.v21.i4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An JY, Kim YM, Yun MA, Jeon BH, Noh SH. Improvement of type 2 diabetes mellitus after gastric cancer surgery: short-term outcome analysis after gastrectomy. World J Gastroenterol. 2013;19:9410–9417. doi: 10.3748/wjg.v19.i48.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee W, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK. Comparative study of diabetes mellitus resolution according to reconstruction type after gastrectomy in gastric cancer patients with diabetes mellitus. Obes Surg. 2012;22:1238–1243. doi: 10.1007/s11695-011-0580-1. [DOI] [PubMed] [Google Scholar]
- 15.Peng D, Cheng YX, Tao W, Zou YY, Qian K, Zhang W. Onco-Metabolic Surgery: A Combined Approach to Gastric Cancer and Hypertension. Cancer Manag Res. 2020;12:7867–7873. doi: 10.2147/CMAR.S260147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim WJ, Kwon Y, Lee CM, Lim SH, Li Y, Wang J, Hu W, Zheng J, Zhao G, Zhu C, Wang W, Xiong W, Wang Q, Xia M, Park S. Oncometabolic surgery: Emergence and legitimacy for investigation. Chin J Cancer Res. 2020;32:252–262. doi: 10.21147/j.issn.1000-9604.2020.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JW, Cheong JH, Hyung WJ, Choi SH, Noh SH. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol. 2012;18:49–54. doi: 10.3748/wjg.v18.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng D, Cheng YX, Zhang W. Does Roux-en-Y Construction Really Bring Benefit of Type 2 Diabetes Mellitus Remission After Gastrectomy in Patients with Gastric Cancer? Diabetes Ther. 2020;11:2863–2872. doi: 10.1007/s13300-020-00934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Yang W, Zhu Y, Jin N, Wu W, Zheng F. Decreased hypertension in non-obese non-diabetic gastric cancer patients after gastrectomy. Asian J Surg. 2020;43:926–929. doi: 10.1016/j.asjsur.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Park YS, Park DJ, Kim KH, Lee Y, Park KB, Min SH, Ahn SH, Kim HH. Nutritional safety of oncometabolic surgery for early gastric cancer patients: a prospective single-arm pilot study using a historical control group for comparison. Surg Endosc. 2020;34:275–283. doi: 10.1007/s00464-019-06763-5. [DOI] [PubMed] [Google Scholar]
- 21.Wang KC, Huang KH, Lan YT, Fang WL, Lo SS, Li AF, Wu CW. Outcome after curative surgery for gastric cancer patients with type 2 diabetes. World J Surg. 2014;38:431–438. doi: 10.1007/s00268-013-2291-3. [DOI] [PubMed] [Google Scholar]
- 22.Choi YY, Noh SH, An JY. A randomized controlled trial of Roux-en-Y gastrojejunostomy vs. gastroduodenostomy with respect to the improvement of type 2 diabetes mellitus after distal gastrectomy in gastric cancer patients. PLoS One. 2017;12:e0188904. doi: 10.1371/journal.pone.0188904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei ZW, Li JL, Wu Y, Xia GK, Schwarz RE, He YL, Zhang CH. Impact of pre-existing type-2 diabetes on patient outcomes after radical resection for gastric cancer: a retrospective cohort study. Dig Dis Sci. 2014;59:1017–1024. doi: 10.1007/s10620-013-2965-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Z, Shan X, Cheng Y, Xu J, Fu H, Wang W, Yan R, Cai Q. Clinical course of diabetes after gastrectomy according to type of reconstruction in patients with concurrent gastric cancer and type 2 diabetes. Obes Surg. 2015;25:673–679. doi: 10.1007/s11695-014-1426-4. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Huh YJ, Park S, Park YS, Park DJ, Kwon JW, Lee JH, Heo YS, Choi SH. Multicenter results of long-limb bypass reconstruction after gastrectomy in patients with gastric cancer and type II diabetes. Asian J Surg. 2020;43:297–303. doi: 10.1016/j.asjsur.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Park MJ, Kim DH, Park BJ, Kim S, Park S, Rosenthal RJ. Impact of preoperative visceral fat proportion on type 2 diabetes in patients with low body mass index after gastrectomy. Surg Obes Relat Dis. 2017;13:1361–1368. doi: 10.1016/j.soard.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Cho EJ, Kwak MH, Eom BW, Yoon HM, Cho SJ, Lee JY, Kim CG, Ryu KW, Kim YW, Choi IJ. Effect of gastrectomy on blood pressure in early gastric cancer survivors with hypertension. Support Care Cancer. 2019;27:2237–2245. doi: 10.1007/s00520-018-4491-8. [DOI] [PubMed] [Google Scholar]
- 28.Lee WJ, Almalki O. Recent advancements in bariatric/metabolic surgery. Ann Gastroenterol Surg. 2017;1:171–179. doi: 10.1002/ags3.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]