Abstract
Colorectal cancer is one of the most prevalent tumours, but with improved treatment and early detection, its prognosis has greatly improved in recent years. However, when the tumour is locally advanced at diagnosis or if there is local recurrence, it is more difficult to perform a complete tumour resection, and there may be a residual macroscopic tumour. In this paper, we review the literature on residual macroscopic tumour resections, concerning both locally advanced primary tumours and recurrences, evaluating the main problems encountered, the treatments applied, the prognosis and future perspectives in this field.
Keywords: Rectal cancer, Surgery, Treatment, Incomplete resection, Local recurrence
Core Tip: We review various series of rectal cancer with incomplete macroscopic resection, commenting on the problems addressed, the approaches taken, the oncological results obtained and future perspectives.
INTRODUCTION
The treatment of colorectal cancer is multidisciplinary, requiring the contributions of specialists in diverse medical and surgical areas (digestive system, medical oncology, radiotherapeutic oncology, radiodiagnosis, etc.) to achieve effective control of the disease. The choice of treatment depends on many factors, depending on the patient (age, comorbidities, will/preference, etc.), the tumour (anatomical location, histology, stage of the disease, etc.) and treatment characteristics (tolerance, efficacy, security, etc.).
Due to the increased risk of local (pelvic) recurrence and a poorer overall prognosis, treatment for rectal cancer differs from that usually provided for colon cancer, in areas such as the surgical technique, the use of radiation therapy and the delivery of chemotherapy. As well as determining the intention of the surgery performed (curative or palliative), aspects related to the maintenance or restoration of the anal sphincter and of normal genitourinary and sexual functions must be considered.
However, a special case arises when the patient presents a locally advanced primary or recurrent tumour with invasion of neighbouring organs, and when after surgery a macroscopically visible tumour remains, because resection was not feasible. These patients are considered R2, and their situation is discussed in the present article, which addresses questions such as the problems that arise, how they should be approached, the main treatment modes available, the results to be expected and the odds of survival.
The situation of these patients is described by the R (residual tumour) classification. This system was specifically devised for patients who have received surgical treatment for cancer[1] and refers exclusively to the extension of the tumour at the time of treatment, or of diagnosis if the case is inoperable[2]. In 1978, the American Joint Committee on Cancer[3] recommended the use of the R classification as an adjunct to staging. In 1987, the Union for International Cancer Control (UICC)[4,5] published an expanded residual tumour classification system that considered both distant and locoregional residual tumours and was applicable to all patients regardless of the form of primary treatment. Under this classification, a completely resected tumour with negative margin is classed as R0, a microscopic residual tumour as R1 and a macroscopic residual tumour as R2.
To date, the most important predictor of survival is a negative resection margin (R0)[6,7]. Overall, R1 is associated with better survival than R2, within any given stage group.
Incomplete R1 and R2 resections usually occur in locally advanced rectal tumours or in recurrences.
In the modern era, 6%-10% of rectal cancer patients have locally advanced disease without metastasis at the time of diagnosis[8] and may be eligible for exenterative surgery[9,10]. However, the research findings supporting this option are limited and diverse[8,11]. Advances in vascular and reconstructive surgical techniques have facilitated an increase in resection rates[12,13], and ever-more radical resections are now being performed to achieve negative resection margins[14]. However, despite the considerable experience that has been acquired by specialists in exenterative surgery, resection of the pelvic lateral wall remains challenging[15,16], especially in patients who have received neoadjuvant radiation therapy[17].
Despite the above, R2 resections do not commonly occur in primary tumours, since the majority of these tumours do not invade neighbouring organs and will be resected with margins. Accordingly, most R2 resections are observed in local recurrences of a previously operated rectal cancer.
In recent decades, the reported incidence of local recurrence of rectal adenocarcinoma after surgical treatment ranged from 5% to 50%[18,19]. This considerable variability was due to the heterogeneity of the series and to the quality of the surgery performed. Since the introduction of total mesorectum excision and the routine use of preoperative radiochemotherapy, rates of local recurrence have fallen significantly, and now range from 2.6% to 12%[20-24]. In most cases, recurrence occurs in the first 2 years after surgery for the primary tumour[25], although later occurrence has been reported in up to 36% of cases[26].
Prevention, treatment and results of R2 resections will be reviewed in this article. We used Scopus, Embase and PubMed for extracting data. Key words such as incomplete resection, R2, survival, recidivism and surgery were used.
PREVENTION AND TREATMENT
The locoregional recurrence of rectal cancer after curative treatment of the primary tumour presents a major challenge for colorectal surgeons. In these cases, the only means of avoiding non-curative resection is to perform a radical excision[27]. This drastic approach must be coordinated by a multidisciplinary team, employing a multimodal treatment strategy including neoadjuvant radiochemotherapy, radical (salvage) surgery, intraoperative radiation therapy and adjuvant chemotherapy. With the experience acquired and as surgical procedures have been refined, together with appropriate support from intensive care units, operative mortality is now reasonably well controlled. For this reason, the current general consensus is that in cases of rectal cancer, radical resection is the only curative alternative for a patient with a local recurrence, despite the still inevitably high rate of perioperative morbidity. Even when carefully selected patients with local recurrence are treated by expert teams, complete resection is achieved in less than 50% of the cases[28].
In the surgical treatment of primary or recurrent tumours with invasion of neighbouring organs, the difficulty is intensified with complete tumour resection. Depending on the area affected, modifications to the intervention technique may be necessary to avoid an R1 or R2 outcome.
In colorectal cancer affecting the bladder with no distal metastasis, good clinical results have been obtained by en bloc resection of the full thickness of the adherent bladder wall, with a margin of at least 2-3 cm. When the colorectal cancer has invaded the bladder, prostate or ureter, a urologist must be consulted to determine whether the operation can be reconstructive. Indications for cystectomy include invasion of the trigone or extension to the prostate.
In lower rectal cancer, in particular, partial or complete resection of the perineum and/or vagina may be necessary, resulting in large defects requiring reconstruction, to facilitate healing and preserve sexual function, among other goals. In the case of partial posterior vaginal defects, vaginal reconstruction is most commonly undertaken by the creation of a rectus abdominis myocutaneous flap. With complete vaginal excision, a bilateral gracilis myocutaneous flap is normally preferred[29].
Lateral pelvic invasion is associated with a poor prognosis and is a relative contraindication for surgical intervention[13] due to possible vascular involvement, for example in the superior mesenteric or common iliac artery[30]. However, these contraindications are relative, and vascular reconstruction, including interposition grafts, femoral-femoral bypass and/or primary anastomosis, may be an option[31]. No single, exclusive surgical procedure for the treatment of laterally invasive colorectal cancer has been identified in the literature. The type of operation performed depends on the site of the tumour, its size and the number of organs affected. Ideally, the tumour should be approached laterally to the internal iliac vessels, which would require en bloc resection of all or some of these structures. However, in the presence of lateral compartment involvement, R0 resection is achieved significantly less frequently than with axial, anterior or posterior recurrences[13].
In cases of posterior invasion, extended radical sacral resection is the treatment of choice. This can be performed either as a two-part procedure with an abdominal phase and a prone phase or as an abdominolithotomy to complete the sacral resection. The proximal level of the sacral resection (high vs low) does not significantly influence the chance of achieving negative margins. If R0 resection is achievable, this is of significant benefit for disease-free survival vs R1 and R2 resections (median 45 mo vs 19 and 8 mo, respectively, P = 0.045)[32].
When considering such an aggressive surgical option, the patient’s functional state must be taken into account. In this respect, Vieira et al[33] evaluated prognostic factors in 95 patients who had undergone extended resections for locally advanced colon cancer. In this study, multivariate analysis showed that Karnofsky performance status was closely related to the risk of postoperative complications (P = 0.01). Moreover, postoperative deaths were associated with the type of surgery and with Karnofsky performance status at the time of admission (P = 0.001). These authors conclude that curative radical resection is the best treatment for patients with locally advanced colon cancer, and that while some patients may benefit from palliative intent procedures, they should be indicated with caution in patients with low Karnofsky performance status due to high postoperative mortality rates and poor survival.
In general, the rate of incomplete resections is low in primary rectal tumours because this outcome mainly occurs with T4 tumours after invasion of neighbouring organs, and this circumstance is statistically uncommon. However, in interventions for pelvic recurrence of rectal cancer, due to the (more frequent) infiltration of neighbouring structures, more incomplete resections (R2) are observed, in 10%-30% of cases.
In patients with rectal cancer, the main objective of radiotherapy is to control the microscopic foci of disease and thus reduce local recurrences. Used as a preoperative treatment, radiotherapy also enhances the possibility of R0 resections and the probability of preserving the anal sphincter in tumours of the lower third of the rectum.
With conventional fractionated radiotherapy, the preoperative doses most frequently used to control microscopic disease within the pelvis are in the range of 45-50.4 Gy.
In the event of local or regional recurrence of rectal cancer or in response to R2 resection, the treatment strategy must be individualised, depending on the site of the local recurrence and on the type of treatment previously received.
After an incomplete surgical outcome (R1-R2), radiotherapy doses of > 60 Gy would be necessary. However, the risk tolerance of the abdominopelvic organs limits the degree of external radiotherapy that can be administered, and so the results obtained are normally only palliative.
In general, the treatment of choice is surgical resection with curative intent associated with preoperative combination therapy (around 50 Gy for 5-6 wk plus concomitant chemotherapy)[34-36] if the patient has not previously received radiotherapy. For patients who have previously received pelvic radiation, limited re-irradiation, with or without adjuvant chemotherapy, would be considered, using techniques such as intensity modulated radiotherapy or volumetric modulated arc therapy, seeking to administer higher doses without damaging previously irradiated tissues.
Intraoperative radiation therapy is another treatment option for patients with locally recurrent rectal cancer, including those who have previously received external pelvic radiation[37]. However, very little is known about the effectiveness and safety of this technique, and the available data have been obtained from observational and descriptive studies of limited methodological quality, reporting uneven results.
After R2 surgery, patients may be administered radiotherapy with concurrent capecitabine, at a dose of 45 Gy on pelvic lymph nodes and 54-60 Gy overimpression of the remainder of the tumour, according to the tolerance of organs at risk, with 2 Gy fractionations.
After the incomplete resection of locally advanced rectal cancer, the addition of postoperative (adjuvant) RT improves local control and survival in patients with R1 (microscopic residual disease) but is less effective in those with R2 (macroscopic residual disease)[38-40].
When this pelvic radiotherapy is administered, over 20% of patients are reported to experience gastrointestinal side effects (radiation enteritis)[41].
Radiation enteritis can be acute or chronic. Acute gastrointestinal side effects include bloating, crampy abdominal pain, loss of appetite, nausea and diarrhoea. These usually occur during the second week of treatment, peak in the fourth or fifth week of exposure, and resolve within 3 mo. Chronic radioenteritis usually manifests 1-2 years after therapy, but in some cases it may not become apparent for several decades[42,43].
The acute and chronic effects of radiation therapy can be devastating, and therefore serious consideration should be given to protecting/excluding the pelvic intestine from this treatment. Furthermore, at the above doses, radiotherapy does not compensate for an inadequate surgical procedure; therefore, if greater doses could be applied, solely to the affected area, better results might be obtained.
Among the many interventions that have been proposed to prevent damage to the pelvic organs, some are technical strategies, attempting to divert physically the radiation dose from normal tissues, while others are medical, to modulate the cellular and tissue response to ionising radiation. In this respect, various surgical methods for excluding the small intestine have been described. Cole et al[44] surgically implanted a Tenckhoff catheter within the peritoneal cavity and achieved small bowel exclusion by daily insufflation with nitrous oxide. In another approach, Hoffman et al[45] employed saline-filled tissue expanders. These authors concluded that the expanders had a useful duration 4 mo longer than that obtained by the pelvic mesh method, while surgical placement and removal were less complex. The main disadvantage was the higher cost of the procedure. Burnett et al[46] used a silicone plastic prosthetic device filled with saline and renografin to exclude the small intestine from the radiation field. The device remained in place during radiation therapy and was then removed through a small incision after draining the contents of the prosthesis[47,48]. Finally, pelvic mesh placement methods have been reported by Sener et al[49] and by Joyce et al[50].
Sener et al[49] first proposed the pelvic mesh technique to be applied after open colorectal or urological resection, while Joyce et al[50] presented the first series of cases with laparoscopic placement of pelvic mesh using a stapler for mesh fixation. The main aim of this approach is to enable the use of radiotherapy without affecting the intestine[47,48,50,51]. Accordingly, this technique is an excellent option in many cases, enabling patients to avoid the harmful side effects of radiotherapy on the intestine. Moreover, it allows higher doses to be used in the target area, which is expected to improve the prognosis of incompletely resected rectal tumours (R2). Several studies have documented the efficacy of the technique[51-54].
For the surgeon, Vicryl mesh placement is feasible, has a low learning curve, and effectively displaces the small intestine from the target area for pelvic radiotherapy, while for the radiation oncologist, this displacement may decrease radiation damage to the small intestine during treatment for pelvic tumours.
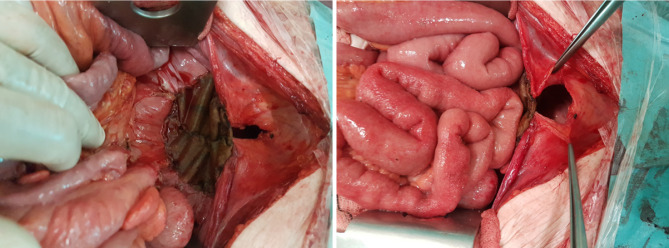
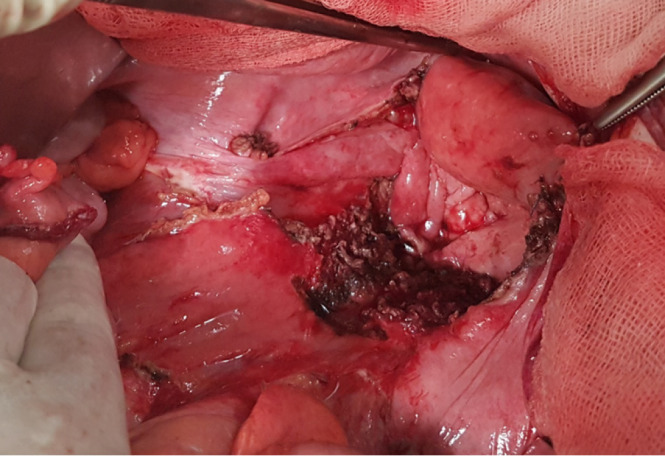
In our hospital, a new treatment approach has been adopted in recent years for patients with locally advanced rectal cancer or pelvic recurrence. When, following resection, a residual macroscopic unresectable tumour persists (verified with intraoperative biopsy, see Figure 1), pelvic exclusion is achieved using native tissue (with an omental pelvic plug as the first choice). When the patient does not have sufficient tissue, due to prior surgical intervention or radiotherapy, we recommend the intraoperative insertion of an open or laparoscopic mesh (depending on the type of intervention), sutured to the pelvic edge, avoiding the middle sacral and iliac vessels (Figure 2) to leave the entire small intestine outside the pelvis. In Figure 3, post-operative CT control where the pelvis is free of intestinal loops is shown. In our experience, insertion of the mesh takes about 20 min, after which full-dose irradiation (56 Gy) can be applied with minimal risk of radioenteritis. Since this treatment protocol was first devised, it has been applied to 3 patients, producing very good oncological results and no intestinal complications.
Figure 1.
Residual macroscopic tumour in the sacral promontory, confirmed R2 by anatomo-pathological examination.
Figure 2.
Insertion of mesh prosthesis to exclude the small intestine from the pelvic space.
Figure 3.
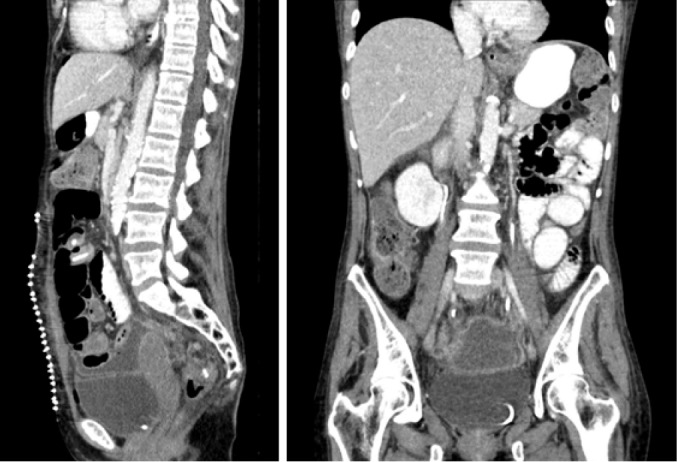
Post-operative computed tomography control where the pelvis is free of intestinal loops.
RESULTS
According to previous studies, oncological outcomes for patients with incomplete R2 resections vary considerably (Table 1)[21,55-65]. Moreover, although many papers have reported results in this area, most are based on relatively small series. Therefore, a comprehensive review is needed to obtain an overview of the prognosis for this condition.
Table 1.
Studies that have presented survival data for patients with R2 resections
|
Ref.
|
n
|
Survival
|
| Hermanek et al[56], 2000 | 86 | MS 4.7 mo (1.8-12.2) |
| Wiig et al[57], 2002 | 24 | OS 5 yr 0% |
| Treiber et al[58], 2004 | 20 | CL 3 yr 29% |
| Landmann et al[59], 2005 | 19 | MS < 12 mo OS 5 yr 0% |
| Dresen et al[60], 2008 | 36 | OS 3 yr 24.1% |
| Rahbari et al[21], 2011 | 13 | MS 23 mo 23.1% |
| Bhangu et al[61], 2012 | 163 | MS 17 mo |
| Roeder et al[62], 2012 | 29 | OS 3 yr 35%, LC 3 yr 18% |
| Haddock et al[63], 2017 | 158 | OS 5 yr 16% |
| Holman et al[64], 2017 | 125 | OS 3 and 5 yr 37% and 17% |
| PelvEx Collaborative et al[65], 2019 | 26 | OS 5 yr 8.1% |
| Westberg et al[66], 2019 | 50 | OS 3 yr 8%, OS 5 yr 4% |
LC: Local control; MS: Median survival; OS: Overall survival.
The 2002 Mayo Clinic series[66] included 73 patients who underwent surgical exploration for locoregional recurrence of rectal cancer. Complete resection was achieved in 52% of these patients. Incomplete resection was observed in 26% (R1) and 22% (R2), respectively. All patients received intraoperative radiotherapy or radiation in the course of their treatment. For the entire cohort, 5-year survival was 25%, and the median survival was 33 mo (from the time of diagnosis of recurrent disease). The 5-year survival in the R1 cohort was 25%, and there were no 5-year survivors in the R2 group.
In 2007, Andreoni et al[67] reported the results of a prospective study in 902 patients operated on for colorectal cancer. With respect to overall survival at 5 years, there were marked differences between the patients with R0 or R1/R2 resection and the unresected patients, with rates of 82%, 35% and 0%, respectively. Moreover, a significant difference was observed between R0 vs R1 + R2 (P < 0.0001) and between R1 + R2 vs unresected cases (P = 0.0009). Significant factors in the univariate analysis were matched in a multivariate analysis of overall survival, evaluating UICC tumour stage, the radicality of the surgical procedure and the tumour site. According to this analysis, only surgical radicality and UICC tumour stage were associated with the outcome.
In 2009, a prospective evaluation was conducted of 100 patients with stage T4 N0 extraperitoneal rectal cancer treated with radiochemotherapy and surgery[68]. In this case, the overall 5-year survival was 59%, with higher rates after R0 resection vs R1 or R2 resection (68% vs 22%, respectively).
Another series was published in 2011[21] of 92 patients undergoing surgical treatment for recurrent rectal cancer. In this multivariate analysis, surgical morbidity (P = 0.001), the presence of extrapelvic disease (P = 0.006) and non-curative resection (R1; R2) (P < 0.0001) were all identified as independent adverse predictors of disease-specific survival.
A paper published in 2012[60] described 22 studies, with the results for 1460 patients operated on for local recurrence of rectal cancer. In this population, the patients with non-complete resection (R1 and R2) had a two to three-fold smaller chance of survival than those with complete resection (R0). The patients with R0 and R1 resections had mean survival rates 4.5 years and 1 year longer, respectively, than those with R2 resections.
An important aspect of the latter study with respect to prognosis is the conclusion drawn that there is no benefit from R2 resection over palliative treatment in terms of survival. However, it is unclear whether incomplete resection provides benefits in terms of quality of life.
A 2014 literature review[69] of studies published in the PubMed and EMBASE databases and in the Cochrane Library of English-language publications, from January 1993 to July 2013, using the medical subject headings search terms “Locally advanced colorectal cancer”, “Recurrent colorectal cancer” and “Surgical treatment” found that the overall 5-year median survival for R0 resection was 50%, vs 12% and < 5% after survival after R1 or R2, respectively.
In 2015, Yang et al[70] presented the clinical results of pelvic exenteration in 40 patients with locally advanced colorectal cancer or locally recurrent colorectal cancer. Multivariate analysis showed that radicality (R0 vs R1/R2) was an independent prognostic factor for overall survival (P = 0.020).
In 2018, Westberg et al[71] published data on 426 patients with rectal cancer recurrence, resected with curative intent. The 5-year survival rates were 43% after R0 resection, 14% after R1 resection and 4% after R2 resection. The most common type of first failure was distal metastasis, which affected 30% of the R0 patients and 42% of those with R1. Local recurrence was significantly more frequent in the R1 patients than in those with R0 (29% vs 20%, respectively, P = 0.044).
The risk of any failure, including recurrence, distal metastasis or death, was significantly influenced by the state of the margins. In an adjusted analysis, the patients resected with R1 had a two-fold risk of failure compared with those resected with R0 (HR 2.04, 95%CI 1.22-3.40). No significant associations were found with age, sex, time to locally recurrent rectal cancer or location of the latter.
In an anonymous data study[64] conducted in 14 countries on 1291 patients with locally advanced rectal cancer between 2004 and 2014, multivariate analysis showed that the status of the margins and of the nodes was significant. Compared with patients with R0 resection, those with R1 margin status had 1.80 times (95%CI 1.43-2.25) higher risk of death during the study period (P < 0.001), while those with R2 resection had 3.01 times (95% confidence interval 1.97–4.87) higher risk of death (P < 0.001).
CONCLUSION
In summary, patients with colorectal cancer who undergo tumour resection have the best chance of survival when the residual tumour is R0. Moreover, survival is greater for R1 resection than for R2, and according to multivariate analysis, the radicality of the resection is an independent factor of survival. Therefore, extended curative resection appears to be the best treatment for patients with locally advanced colon cancer. However, this treatment option should be indicated with caution in patients with low Karnofsky status, due to their high postoperative mortality rates and poor survival.
For patients with R2, resection treatment should be individualised but generally consists of radiochemotherapy. For R2 resections due to pelvic recurrence, intraoperative radiotherapy may be required. The difficulty encountered in postoperative pelvic irradiation due to the content of the intestinal loops and the consequent risk of complications means that radiography doses are necessarily limited.
The best option appears to be the application of radiotherapy after excluding the intestinal loops from the pelvic space, an approach that makes it possible to irradiate the tumour at full dose with minimal side effects on the intestine, which is very beneficial to the patient’s prognosis. In our hospital, good results have been obtained with this technique, although the number of patients is very low, given the infrequent nature of the condition in question. To validate our findings, further research in this field is necessary, based on multicentre studies with a significant number of patients.
In this area, several questions remain to be addressed. Does the survival pattern of incomplete R2 resections of tumour recurrence differ from that for incomplete R2 resections of primary tumours? Do irradiated patients fitted with a pelvic displacement prosthesis have fewer side effects? Is survival greater among the latter patients, when full-dose irradiation is applied? To draw meaningful conclusions, these questions should be considered in future multicentre studies based on large numbers of patients.
Footnotes
Conflict-of-interest statement: No conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: February 9, 2021
First decision: April 19, 2021
Article in press: July 23, 2021
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carannante F, Sugoor P S-Editor: Zhang H L-Editor: Filipodia P-Editor: Yuan YY
Contributor Information
Francisco Javier Pérez Lara, Department of Surgery, Antequera Hospital, Málaga 29200, Spain. javinewyork@hotmail.com.
Maria Luisa Hebrero Jimenez, Department of Oncologic Radiotherapy, Hopital Regional de Málaga, Málaga 29010, Spain.
Francisco Javier Moya Donoso, Department of Surgery, Antequera Hospital, Málaga 29200, Spain.
Jose Manuel Hernández Gonzalez, Department of Surgery, Antequera Hospital, Málaga 29200, Spain.
Maria Pitarch Martinez, Department of Surgery, Antequera Hospital, Málaga 29200, Spain.
Tatiana Prieto-Puga Arjona, Department of Surgery, Antequera Hospital, Málaga 29200, Spain.
References
- 1. American Joint Committee on Cancer: Manual for staging of cancer. 2nd ed. Philadelphia: J.B. Lippincott, 1983. [Google Scholar]
- 2. American Joint Committee on Cancer: Manual for staging of cancer. 3rd ed. Philadelphia: J.B. Lippincott, 1988. [Google Scholar]
- 3. American Joint Committee on Cancer. Staging and End Results Reporting. In: Manual for staging of cancer. AJCC, 1978. [Google Scholar]
- 4.Hermanek P, Sobin LH. TNM classification of malignant tumours. 4th ed. Berlin: Springer, 1987. [Google Scholar]
- 5.Hermanek P, Sobin LH. TNM classification of malignant tumours. 4th ed. Berlin: Springer, 1992. [Google Scholar]
- 6.Kusters M, Austin KK, Solomon MJ, Lee PJ, Nieuwenhuijzen GA, Rutten HJ. Survival after pelvic exenteration for T4 rectal cancer. Br J Surg. 2015;102:125–131. doi: 10.1002/bjs.9683. [DOI] [PubMed] [Google Scholar]
- 7.Radwan RW, Jones HG, Rawat N, Davies M, Evans MD, Harris DA, Beynon J Swansea Pelvic Oncology Group. Determinants of survival following pelvic exenteration for primary rectal cancer. Br J Surg. 2015;102:1278–1284. doi: 10.1002/bjs.9841. [DOI] [PubMed] [Google Scholar]
- 8.Yang TX, Morris DL, Chua TC. Pelvic exenteration for rectal cancer: a systematic review. Dis Colon Rectum. 2013;56:519–531. doi: 10.1097/DCR.0b013e31827a7868. [DOI] [PubMed] [Google Scholar]
- 9.Kokelaar RF, Evans MD, Davies M, Harris DA, Beynon J. Locally advanced rectal cancer: management challenges. Onco Targets Ther. 2016;9:6265–6272. doi: 10.2147/OTT.S100806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domes TS, Colquhoun PH, Taylor B, Izawa JI, House AA, Luke PP. Total pelvic exenteration for rectal cancer: outcomes and prognostic factors. Can J Surg. 2011;54:387–393. doi: 10.1503/cjs.014010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyond TME Collaborative. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg. 2013;100:E1–33. doi: 10.1002/bjs.9192_1. [DOI] [PubMed] [Google Scholar]
- 12.Brown KG, Koh CE, Solomon MJ, Qasabian R, Robinson D, Dubenec S. Outcomes After En Bloc Iliac Vessel Excision and Reconstruction During Pelvic Exenteration. Dis Colon Rectum. 2015;58:850–856. doi: 10.1097/DCR.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 13.Austin KK, Solomon MJ. Pelvic exenteration with en bloc iliac vessel resection for lateral pelvic wall involvement. Dis Colon Rectum. 2009;52:1223–1233. doi: 10.1007/DCR.0b013e3181a73f48. [DOI] [PubMed] [Google Scholar]
- 14.Harris DA, Davies M, Lucas MG, Drew P, Carr ND, Beynon J Swansea Pelvic Oncology Group. Multivisceral resection for primary locally advanced rectal carcinoma. Br J Surg. 2011;98:582–588. doi: 10.1002/bjs.7373. [DOI] [PubMed] [Google Scholar]
- 15.Georgiou PA, Mohammed Ali S, Brown G, Rasheed S, Tekkis PP. Extended lymphadenectomy for locally advanced and recurrent rectal cancer. Int J Colorectal Dis. 2017;32:333–340. doi: 10.1007/s00384-016-2711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaikh I, Aston W, Hellawell G, Ross D, Littler S, Burling D, Marshall M, Northover JM, Antoniou A, Jenkins JT. Extended lateral pelvic sidewall excision (ELSiE): an approach to optimize complete resection rates in locally advanced or recurrent anorectal cancer involving the pelvic sidewall. Tech Coloproctol. 2015;19:119–120. doi: 10.1007/s10151-015-1266-9. [DOI] [PubMed] [Google Scholar]
- 17.Bhangu A, Ali SM, Brown G, Nicholls RJ, Tekkis P. Indications and outcome of pelvic exenteration for locally advanced primary and recurrent rectal cancer. Ann Surg. 2014;259:315–322. doi: 10.1097/SLA.0b013e31828a0d22. [DOI] [PubMed] [Google Scholar]
- 18.Phillips RK, Hittinger R, Blesovsky L, Fry JS, Fielding LP. Local recurrence following 'curative' surgery for large bowel cancer: II. The rectum and rectosigmoid. Br J Surg. 1984;71:17–20. doi: 10.1002/bjs.1800710105. [DOI] [PubMed] [Google Scholar]
- 19.Bakx R, Visser O, Josso J, Meijer S, Slors JF, van Lanschot JJ. Management of recurrent rectal cancer: a population based study in greater Amsterdam. World J Gastroenterol. 2008;14:6018–6023. doi: 10.3748/wjg.14.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rödel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 21.Rahbari NN, Ulrich AB, Bruckner T, Münter M, Nickles A, Contin P, Löffler T, Reissfelder C, Koch M, Büchler MW, Weitz J. Surgery for locally recurrent rectal cancer in the era of total mesorectal excision: is there still a chance for cure? Ann Surg. 2011;253:522–533. doi: 10.1097/SLA.0b013e3182096d4f. [DOI] [PubMed] [Google Scholar]
- 22.Hartley JE, Lopez RA, Paty PB, Wong WD, Cohen AM, Guillem JG. Resection of locally recurrent colorectal cancer in the presence of distant metastases: can it be justified? Ann Surg Oncol. 2003;10:227–233. doi: 10.1245/aso.2003.05.039. [DOI] [PubMed] [Google Scholar]
- 23.Heriot AG, Tekkis PP, Darzi A, Mackay J. Surgery for local recurrence of rectal cancer. Colorectal Dis. 2006;8:733–747. doi: 10.1111/j.1463-1318.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- 24.Biondo S, Ortiz H, Lujan J, Codina-Cazador A, Espin E, Garcia-Granero E, Kreisler E, de Miguel M, Alos R, Echeverria A. Quality of mesorectum after laparoscopic resection for rectal cancer - results of an audited teaching programme in Spain. Colorectal Dis. 2010;12:24–31. doi: 10.1111/j.1463-1318.2008.01720.x. [DOI] [PubMed] [Google Scholar]
- 25.Heriot AG, Byrne CM, Lee P, Dobbs B, Tilney H, Solomon MJ, Mackay J, Frizelle F. Extended radical resection: the choice for locally recurrent rectal cancer. Dis Colon Rectum. 2008;51:284–291. doi: 10.1007/s10350-007-9152-9. [DOI] [PubMed] [Google Scholar]
- 26.Mirnezami AH, Sagar PM. Surgery for recurrent rectal cancer: technical notes and management of complications. Tech Coloproctol. 2010;14:209–216. doi: 10.1007/s10151-010-0585-0. [DOI] [PubMed] [Google Scholar]
- 27.Bouchard P, Efron J. Management of recurrent rectal cancer. Ann Surg Oncol. 2010;17:1343–1356. doi: 10.1245/s10434-009-0861-2. [DOI] [PubMed] [Google Scholar]
- 28.Enríquez-Navascués JM, Borda N, Lizerazu A, Placer C, Elosegui JL, Ciria JP, Lacasta A, Bujanda L. Patterns of local recurrence in rectal cancer after a multidisciplinary approach. World J Gastroenterol. 2011;17:1674–1684. doi: 10.3748/wjg.v17.i13.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McArdle A, Bischof DA, Davidge K, Swallow CJ, Winter DC. Vaginal reconstruction following radical surgery for colorectal malignancies: a systematic review of the literature. Ann Surg Oncol. 2012;19:3933–3942. doi: 10.1245/s10434-012-2503-3. [DOI] [PubMed] [Google Scholar]
- 30.Shibata D, Paty PB, Guillem JG, Wong WD, Cohen AM. Surgical management of isolated retroperitoneal recurrences of colorectal carcinoma. Dis Colon Rectum. 2002;45:795–801. doi: 10.1007/s10350-004-6300-3. [DOI] [PubMed] [Google Scholar]
- 31.Abdelsattar ZM, Mathis KL, Colibaseanu DT, Merchea A, Bower TC, Larson DW, Dozois EJ. Surgery for locally advanced recurrent colorectal cancer involving the aortoiliac axis: can we achieve R0 resection and long-term survival? Dis Colon Rectum. 2013;56:711–716. doi: 10.1097/DCR.0b013e31827dbcb0. [DOI] [PubMed] [Google Scholar]
- 32.Milne T, Solomon MJ, Lee P, Young JM, Stalley P, Harrison JD. Assessing the impact of a sacral resection on morbidity and survival after extended radical surgery for locally recurrent rectal cancer. Ann Surg. 2013;258:1007–1013. doi: 10.1097/SLA.0b013e318283a5b6. [DOI] [PubMed] [Google Scholar]
- 33.Vieira RA, Lopes A, Almeida PA, Rossi BM, Nakagawa WT, Ferreira FO, Melo CA. Prognostic factors in locally advanced colon cancer treated by extended resection. Rev Hosp Clin Fac Med Sao Paulo. 2004;59:361–368. doi: 10.1590/s0041-87812004000600009. [DOI] [PubMed] [Google Scholar]
- 34.Glimelius B, Tiret E, Cervantes A, Arnold D ESMO Guidelines Working Group. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi81–vi88. doi: 10.1093/annonc/mdt240. [DOI] [PubMed] [Google Scholar]
- 35.Engstrom PF, Arnoletti JP, Benson AB 3rd, Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS, Enzinger PC, Fakih MG, Fleshman J Jr, Fuchs C, Grem JL, Kiel K, Knol JA, Leong LA, Lin E, Mulcahy MF, Rao S, Ryan DP, Saltz L, Shibata D, Skibber JM, Sofocleous C, Thomas J, Venook AP, Willett C National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: rectal cancer. J Natl Compr Canc Netw. 2009;7:838–881. doi: 10.6004/jnccn.2009.0057. [DOI] [PubMed] [Google Scholar]
- 36.Willet CG, Rodriguez-B , Willetigas MA, Ryan DP. Treatment of locally recurrent rectal adenocarcinoma. Apr 04, 2016. Uptodate Waltham, Massachusetts. Available from: http://www.uptodate.com/
- 37.Konski AA, Suh WW, Herman JM, Blackstock AW Jr, Hong TS, Poggi MM, Rodriguez-Bigas M, Small W Jr, Thomas CR Jr, Zook J. ACR Appropriateness Criteria®-Recurrent Rectal Cancer. Gastrointest Cancer Res. 2012;5:3–12. [PMC free article] [PubMed] [Google Scholar]
- 38.Allee PE, Tepper JE, Gunderson LL, Munzenrider JE. Postoperative radiation therapy for incompletely resected colorectal carcinoma. Int J Radiat Oncol Biol Phys. 1989;17:1171–1176. doi: 10.1016/0360-3016(89)90522-1. [DOI] [PubMed] [Google Scholar]
- 39.Schild SE, Martenson JA Jr, Gunderson LL, Dozois RR. Long-term survival and patterns of failure after postoperative radiation therapy for subtotally resected rectal adenocarcinoma. Int J Radiat Oncol Biol Phys. 1989;16:459–463. doi: 10.1016/0360-3016(89)90342-8. [DOI] [PubMed] [Google Scholar]
- 40.Ghossein NA, Samala EC, Alpert S, DeLuca FR, Ragins H, Turner SS, Stacey P, Flax H. Elective postoperative radiotherapy after incomplete resection of colorectal cancer. Dis Colon Rectum. 1981;24:252–256. doi: 10.1007/BF02641870. [DOI] [PubMed] [Google Scholar]
- 41.Theis VS, Sripadam R, Ramani V, Lal S. Chronic radiation enteritis. Clin Oncol (R Coll Radiol) 2010;22:70–83. doi: 10.1016/j.clon.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Deveney CW, Lewis FR Jr, Schrock TR. Surgical management of radiation injury of the small and large intestine. Dis Colon Rectum. 1976;19:25–29. doi: 10.1007/BF02590847. [DOI] [PubMed] [Google Scholar]
- 43.Mann WJ. Surgical management of radiation enteropathy. Surg Clin North Am. 1991;71:977–990. doi: 10.1016/s0039-6109(16)45529-5. [DOI] [PubMed] [Google Scholar]
- 44.Cole H. Displacement of small bowel from pelvic radiation field. Lancet. 1988;2:1341–1342. doi: 10.1016/s0140-6736(88)90872-0. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman JP, Sigurdson ER, Eisenberg BL. Use of saline-filled tissue expanders to protect the small bowel from radiation. Oncology (Williston Park) 1998;12:51–54; discussion 54, 60, 62, passim. [PubMed] [Google Scholar]
- 46.Burnett AF, Coe FL, Klement V, O'Meara AT, Muderspach LI, Roman LD, Morrow CP. The use of a pelvic displacement prosthesis to exclude the small intestine from the radiation field following radical hysterectomy. Gynecol Oncol. 2000;79:438–443. doi: 10.1006/gyno.2000.5965. [DOI] [PubMed] [Google Scholar]
- 47.Valle M, Federici O, Ialongo P, Graziano F, Garofalo A. Prevention of complications following pelvic exenteration with the use of mammary implants in the pelvic cavity: Technique and results of 28 cases. J Surg Oncol. 2011;103:34–38. doi: 10.1002/jso.21716. [DOI] [PubMed] [Google Scholar]
- 48.Tuech JJ, Chaudron V, Thoma V, Ollier JC, Tassetti V, Duval D, Rodier JF. Prevention of radiation enteritis by intrapelvic breast prosthesis. Eur J Surg Oncol. 2004;30:900–904. doi: 10.1016/j.ejso.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 49.Sener SF, Imperato JP, Blum MD, Ignatoff JM, Soper TG, Winchester DP, Meiselman M. Technique and complications of reconstruction of the pelvic floor with polyglactin mesh. Surg Gynecol Obstet. 1989;168:475–480. [PubMed] [Google Scholar]
- 50.Joyce M, Thirion P, Kiernan F, Byrnes C, Kelly P, Keane F, Neary P. Laparoscopic pelvic sling placement facilitates optimum therapeutic radiotherapy delivery in the management of pelvic malignancy. Eur J Surg Oncol. 2009;35:348–351. doi: 10.1016/j.ejso.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 51.Devereux DF, Feldman MI, McIntosh TK, Palter D, Kavanah MT, Deckers PJ, Williams LF Jr. Efficacy of polyglycolic acid mesh sling in keeping the small bowel in the upper abdomen after abdominal surgery: a 12-month study in baboons. J Surg Oncol. 1986;31:204–209. doi: 10.1002/jso.2930310314. [DOI] [PubMed] [Google Scholar]
- 52.Devereux DF, Chandler JJ, Eisenstat T, Zinkin L. Efficacy of an absorbable mesh in keeping the small bowel out of the human pelvis following surgery. Dis Colon Rectum. 1988;31:17–21. doi: 10.1007/BF02552563. [DOI] [PubMed] [Google Scholar]
- 53.Dasmahapatra KS, Swaminathan AP. The use of a biodegradable mesh to prevent radiation-associated small-bowel injury. Arch Surg. 1991;126:366–369. doi: 10.1001/archsurg.1991.01410270114018. [DOI] [PubMed] [Google Scholar]
- 54.Rodier JF, Janser JC, Rodier D, Dauplat J, Kauffmann P, Le Bouedec G, Giraud B, Lorimier G. Prevention of radiation enteritis by an absorbable polyglycolic acid mesh sling. A 60-case multicentric study. Cancer. 1991;68:2545–2549. doi: 10.1002/1097-0142(19911215)68:12<2545::aid-cncr2820681202>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 55.Hermanek P, Mansmann U, Staimmer DS, Riedl S, Hermanek P. The German experience: the surgeon as a prognostic factor in colon and rectal cancer surgery. Surg Oncol Clin N Am. 2000;9:33–49, vi. [PubMed] [Google Scholar]
- 56.Wiig JN, Tveit KM, Poulsen JP, Olsen DR, Giercksky KE. Preoperative irradiation and surgery for recurrent rectal cancer. Will intraoperative radiotherapy (IORT) be of additional benefit? Radiother Oncol. 2002;62:207–213. doi: 10.1016/s0167-8140(01)00486-8. [DOI] [PubMed] [Google Scholar]
- 57.Treiber M, Lehnert T, Oertel S, Krempien R, Bischof M, Buechler M, Wannenmacher M, Debus J. Intraoperative radiotherapy--special focus: recurrent rectal carcinoma. Front Radiat Ther Oncol. 2004;38:52–56. doi: 10.1159/000078264. [DOI] [PubMed] [Google Scholar]
- 58.Landmann RG, Weiser MR. Surgical management of locally advanced and locally recurrent colon cancer. Clin Colon Rectal Surg. 2005;18:182–189. doi: 10.1055/s-2005-916279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dresen RC, Gosens MJ, Martijn H, Nieuwenhuijzen GA, Creemers GJ, Daniels-Gooszen AW, van den Brule AJ, van den Berg HA, Rutten HJ. Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer. Ann Surg Oncol. 2008;15:1937–1947. doi: 10.1245/s10434-008-9896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhangu A, Ali SM, Darzi A, Brown G, Tekkis P. Meta-analysis of survival based on resection margin status following surgery for recurrent rectal cancer. Colorectal Dis. 2012;14:1457–1466. doi: 10.1111/j.1463-1318.2012.03005.x. [DOI] [PubMed] [Google Scholar]
- 61.Roeder F, Goetz JM, Habl G, Bischof M, Krempien R, Buechler MW, Hensley FW, Huber PE, Weitz J, Debus J. Intraoperative Electron Radiation Therapy (IOERT) in the management of locally recurrent rectal cancer. BMC Cancer. 2012;12:592. doi: 10.1186/1471-2407-12-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haddock MG, Miller RC, Nelson H, Pemberton JH, Dozois EJ, Alberts SR, Gunderson LL. Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 2011;79:143–150. doi: 10.1016/j.ijrobp.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 63.Holman FA, Bosman SJ, Haddock MG, Gunderson LL, Kusters M, Nieuwenhuijzen GA, van den Berg H, Nelson H, Rutten HJ. Results of a pooled analysis of IOERT containing multimodality treatment for locally recurrent rectal cancer: Results of 565 patients of two major treatment centres. Eur J Surg Oncol. 2017;43:107–117. doi: 10.1016/j.ejso.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 64.PelvEx Collaborative. Surgical and Survival Outcomes Following Pelvic Exenteration for Locally Advanced Primary Rectal Cancer: Results From an International Collaboration. Ann Surg. 2019;269:315–321. doi: 10.1097/SLA.0000000000002528. [DOI] [PubMed] [Google Scholar]
- 65.Westberg K, Palmer G, Hjern F, Holm T, Martling A. Population-based study of surgical treatment with and without tumour resection in patients with locally recurrent rectal cancer. Br J Surg. 2019;106:790–798. doi: 10.1002/bjs.11098. [DOI] [PubMed] [Google Scholar]
- 66.Taylor WE, Donohue JH, Gunderson LL, Nelson H, Nagorney DM, Devine RM, Haddock MG, Larson DR, Rubin J, O'Connell MJ. The Mayo Clinic experience with multimodality treatment of locally advanced or recurrent colon cancer. Ann Surg Oncol. 2002;9:177–185. doi: 10.1007/BF02557371. [DOI] [PubMed] [Google Scholar]
- 67.Andreoni B, Chiappa A, Bertani E, Bellomi M, Orecchia R, Zampino M, Fazio N, Venturino M, Orsi F, Sonzogni A, Pace U, Monfardini L. Surgical outcomes for colon and rectal cancer over a decade: results from a consecutive monocentric experience in 902 unselected patients. World J Surg Oncol. 2007;5:73. doi: 10.1186/1477-7819-5-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valentini V, Coco C, Rizzo G, Manno A, Crucitti A, Mattana C, Ratto C, Verbo A, Vecchio FM, Barbaro B, Gambacorta MA, Montoro C, Barba MC, Sofo L, Papa V, Menghi R, D'Ugo DM, Doglietto G. Outcomes of clinical T4M0 extra-peritoneal rectal cancer treated with preoperative radiochemotherapy and surgery: a prospective evaluation of a single institutional experience. Surgery. 2009;145:486–494. doi: 10.1016/j.surg.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Courtney D, McDermott F, Heeney A, Winter DC. Clinical review: surgical management of locally advanced and recurrent colorectal cancer. Langenbecks Arch Surg. 2014;399:33–40. doi: 10.1007/s00423-013-1134-x. [DOI] [PubMed] [Google Scholar]
- 70.Yang HY, Park SC, Hyun JH, Seo HK, Oh JH. Outcomes of pelvic exenteration for recurrent or primary locally advanced colorectal cancer. Ann Surg Treat Res. 2015;89:131–137. doi: 10.4174/astr.2015.89.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Westberg K, Palmer G, Hjern F, Johansson H, Holm T, Martling A. Management and prognosis of locally recurrent rectal cancer - A national population-based study. Eur J Surg Oncol. 2018;44:100–107. doi: 10.1016/j.ejso.2017.11.013. [DOI] [PubMed] [Google Scholar]