Abstract
Colorectal carcinoma (CRC) is one of the leading causes of cancer-related deaths worldwide, and up to 50% of patients with CRC develop colorectal liver metastases (CRLM). For these patients, surgical resection remains the only opportunity for cure and long-term survival. Over the past few decades, outcomes of patients with metastatic CRC have improved significantly due to advances in systemic therapy, as well as improvements in operative technique and perioperative care. Chemotherapy in the modern era of oxaliplatin- and irinotecan-containing regimens has been augmented by the introduction of targeted biologics and immunotherapeutic agents. The increasing efficacy of contemporary systemic therapies has led to an expansion in the proportion of patients eligible for curative-intent surgery. Consequently, the use of neoadjuvant strategies is becoming progressively more established. For patients with CRLM, the primary advantage of neoadjuvant chemotherapy (NCT) is the potential to down-stage metastatic disease in order to facilitate hepatic resection. On the other hand, the routine use of NCT for patients with resectable metastases remains controversial, especially given the potential risk of inducing chemotherapy-associated liver injury prior to hepatectomy. Current guidelines recommend upfront surgery in patients with initially resectable disease and low operative risk, reserving NCT for patients with borderline resectable or unresectable disease and high operative risk. Patients undergoing NCT require close monitoring for tumor response and conversion of CRLM to resectability. In light of the growing number of treatment options available to patients with metastatic CRC, it is generally agreed that these patients are best served at tertiary centers with an expert multidisciplinary team.
Keywords: Colorectal liver metastases, Neoadjuvant chemotherapy, Hepatic resection, Conversion therapy, Chemotherapy-associated liver injury, Disappearing liver metastases, Future liver remnant, Immunotherapy
Core Tip: Colorectal carcinoma is one of the leading causes of cancer-related deaths worldwide. Neoadjuvant chemotherapy is an important treatment strategy for patients with colorectal liver metastases that can downstage tumors and facilitate hepatic resection. Although its use in patients with initially resectable disease is controversial, it is currently recommended for those with unresectable to borderline resectable metastases.
INTRODUCTION
Colorectal carcinoma (CRC) is a leading cause of cancer-related death among Western populations and the second most common malignancy worldwide[1,2]. In the United States, around 148000 new cases are diagnosed each year. Between 40%-50% of patients with CRC develop colorectal liver metastases (CRLM) during the course of their disease[3-5]. Up to 70% of these patients have synchronous CRLM at their initial presentation[4-7]. Historically, metastatic CRC has been associated with poor survival. However, outcomes of patients with CRLM have improved significantly over the past few decades due to advances in systemic therapy and locoregional treatment, each of which have contributed to the expansion of safe hepatic resection. As a result of these advances, the median five-year survival rate of patients with metastatic CRC has risen from < 10% to 35%-40%, while median overall survival (OS) has increased from < 12 mo to approximately 42 mo[7-9].
Surgical resection of CRLM is generally considered necessary for potential long-term survival and has been shown to improve OS[9-11]. Fortunately, advances in operative technique, perioperative care, and effective chemotherapy have increased the proportion of patients eligible for curative-intent surgery[12]. Indeed, the growing efficacy of systemic treatments has accentuated the role of surgery for CRLM by improving patient selection for liver-directed therapies and downstaging initially unresectable tumors. Nevertheless, the routine delivery of chemotherapy before surgery remains controversial given the lack of consistent evidence demonstrating an associated survival benefit, as well as the risk for chemotherapy-associated liver injury (CALI). In this review, we present the most recent literature on neoadjuvant chemotherapy (NCT) for CRLM and discuss the rationale, supporting evidence, technical considerations, and current indications for its use.
RATIONALE FOR NCT
Several empirical and theoretical benefits are associated with NCT, and in turn, it is increasingly utilized in the treatment of many solid-organ cancers[13]. With the improved availability and effectiveness of modern systemic therapies, neoadjuvant strategies have become progressively more established in the management of advanced malignancies requiring a multimodal approach. For patients with CRLM, the primary advantage of NCT is the potential to render previously unresectable tumors resectable in what is often termed “conversion therapy.” NCT can down-stage metastatic disease to resectability in up to 35% of these patients[14-17]. Given the importance of surgical resection to patient prognosis, current guidelines recommend the initiation of systemic therapy in those who have unresectable CRLM, coupled with close monitoring and continued re-evaluation for operative candidacy by an experienced multidisciplinary team[18].
Even among patients with initially resectable disease, downstaging with NCT could have certain advantages. Preoperative chemotherapy may facilitate a parenchymal-sparing or minimally invasive surgical approach, as well as increase the likelihood of obtaining negative margins. In addition, the use of NCT could lead to better selection of surgical candidates by allowing time to identify those who have rapidly progressive metastatic disease and therefore would not benefit from a potentially morbid operation. In contrast, postoperative complications following major liver surgery can prohibit some patients from receiving adjuvant chemotherapy, and the administration of chemotherapy preoperatively can increase the probability of completing all intended treatment by those who would benefit the most from a multimodal approach. Moreover, it is thought that the delivery of early chemotherapy may reduce the risk of recurrence by prioritizing the treatment of micro-metastatic disease. Lastly, monitoring of the radiographic, biochemical, and histological response to NCT provides important prognostic information that may guide future management.
Despite these potential advantages, enthusiasm for NCT has been tempered by reports of hepatotoxicity associated with common chemotherapeutic agents used to treat CRC, especially given the concern that the resulting liver injury may preclude hepatic resection. 5-Fluorouracil can cause steatosis, although this is generally considered clinically insignificant. Oxaliplatin can lead to sinusoidal injury, which, in the absence of severe injury resulting in portal hypertension, does not usually contribute to increased postoperative mortality[19]. Irinotecan, however, has been linked with clinically significant steatohepatitis that, in retrospective studies, has been associated with increased rates of postoperative liver insufficiency and mortality[20-22]. However, evidence is mixed on the association of NCT with increased risk for postoperative complications. Although higher rates of complication have been reported in patients who received NCT vs individuals who underwent immediate hepatectomy for CRLM (25% vs 16%, P = 0.04) in the European Organization for Research and Treatment of Cancer (EORTC) intergroup trial 40983[23], a population-based study using the American College of Surgeons National Surgical Quality Improvement Program found no significant difference in the rates of postoperative morbidity or mortality[24].
Other commonly cited disadvantages of NCT include failure to proceed with potentially curative resection due to local disease progression, toxicity of systemic therapy, challenges in identifying sites of metastases experiencing complete macroscopic response (disappearing liver metastases, DLM), and inconsistent data related to survival benefits associated with its routine use (Table 1).
Table 1.
Potential advantages and disadvantages of neoadjuvant chemotherapy for colorectal liver metastases
|
Advantages
|
Disadvantages
|
| (1) Conversion to resectability | (1) Chemotherapy-associated liver injury |
| (2) Facilitate parenchymal-sparing or minimally invasive approach | (2) Local progression that precludes hepatic resection (rare) |
| (3) Higher rates of negative margins | (3) Disappearing liver metastases |
| (4) Improved patient selection for surgery | (4) Toxicity from chemotherapy |
| (5) Improved rates of multimodality therapy | |
| (6) Early treatment of micro-metastases | |
| (7) Ability to assess response to therapy |
NCT
Chemotherapy regimens
First-line chemotherapy for metastatic CRC primarily consists of fluorouracil-based regimens containing oxaliplatin and/or irinotecan. Current NCCN guidelines list FOLFOX (fluorouracil, leucovorin, and oxaliplatin), FOLFIRI (fluorouracil, leucovorin, and irinotecan), XELOX (capecitabine and oxaliplatin), and FOLFOXIRI (fluorouracil, leucovorin, oxaliplatin, and irinotecan) as recommended courses for systemic therapy[18]. The choice of regimen largely depends on the patient’s performance status and the presence of RAS or BRAF mutation[25]. Among patients who can tolerate a more intensive regimen, triplet therapy with FOLFOXIRI is an option that may lead to higher response rates[17,26].
Biological agents
The recent development of targeted biologic agents has further increased the number of treatment options available to patients with metastatic disease and become the focus of newer studies investigating perioperative systemic therapy. The COIN trial was a randomized trial examining the effects of adding cetuximab, a monoclonal antibody that binds EGFR, to standard oxaliplatin-based chemotherapy regimens as first-line treatment for patients with advanced CRC. Although the addition of cetuximab did not affect overall or progression-free survival (PFS), higher rates of tumor response were reported in patients with wild-type KRAS genotype[27]. Based on these results, the New EPOC (eloxatin perioperative chemotherapy) randomized controlled trial was conducted to evaluate the outcomes of adding cetuximab to oxaliplatin- or irinotecan-based perioperative chemotherapy in patients with potentially resectable CRLM. With an overall median follow-up of 66.7 mo, both PFS (15.5 mo vs 22.2 mo, P = 0.304) and median OS (55.4 mo vs 81.0 mo, P = 0.036) were shorter in the chemotherapy plus cetuximab group than in the chemotherapy alone group[28,29]. The results of this study were unexpected, and it was theorized that molecular modifications in the KRAS pathway rendered the tumors more resistant to treatment. Indeed, significantly higher rates of response, PFS, and OS had previously been observed in patients with KRAS wild-type disease treated with cetuximab in addition to FOLFIRI compared with patients receiving FOLFIRI alone[30,31]. Similarly, the addition of panitumumab, another anti-EGFR monoclonal antibody, to FOLFOX was shown to improve PFS in patients with KRAS wild-type CRC[32]. While the addition of EGFR inhibitors to chemotherapy regimens continues to be recommended for patients with advanced metastatic CRC, it should not be used in those with initially resectable CRLM.
Bevacizumab, a monoclonal antibody that binds VEGF, is a newer biologic agent that is theorized to increase response rates and median OS among patients with metastatic CRC when added to standard chemotherapy regimens[33]. Currently, there are two ongoing clinical trials investigating its role as an adjunct to perioperative chemotherapy for CRLM. The goal of the PERIMAX trial will be to compare resection and adjuvant FOLFOX to perioperative FOLFOXIRI with bevacizumab in patients with resectable CRLM, while the CHARTA trial intends to evaluate the effect of adding bevacizumab to either FOLFOX or FOLFOXIRI in the treatment of patients with unresectable CRLM[34]. Initially, there was concern that major complications associated with bevacizumab, such as thromboembolism, bowel perforation, bleeding, and impaired wound healing, could interfere with surgical resection. However, numerous studies have since demonstrated the safety of its use in the preoperative setting when administered 5 or more weeks prior to surgery[35-41]. In the BECOME trial examining the use of bevacizumab in patients with RAS mutant, unresectable CRLM, significantly improved outcomes were noted among individuals treated with chemotherapy plus bevacizumab compared to those treated with chemotherapy alone. With a median follow-up time of 37.0 mo, patients who received mFOLFOX6 plus bevacizumab had higher rates of R0 resection (22.3% vs 5.8%, P < 0.01), response rates (54.5% vs 36.7%, P < 0.01), median PFS (9.5 vs 5.6 mo, P < 0.01), and median OS (25.7 vs 20.5 mo, P = 0.03) vs their counterparts who received mFOLFOX6 alone[42]. In practice, bevacizumab is frequently omitted from the final cycle of NCT prior to liver resection in order to limit safety concerns at the time of surgery.
INDICATIONS FOR NCT
Patients with initially resectable disease
For patients with resectable CRLM at the time of presentation, the routine use of NCT over upfront surgery is controversial. For some malignancies, such as pancreatic adenocarcinoma, all patients have been found to benefit from adjuvant chemotherapy. Therefore, strategies involving the delivery of chemotherapy before surgery for these patients is rational as it ensures the receipt of all intended treatment[43]. In contrast, it is not clear that all patients with resectable CRLM would benefit from the receipt of systemic therapy at all, whether in a neoadjuvant or adjuvant setting.
The use of fluorouracil-based chemotherapy as an adjuvant treatment strategy following resection of CRLM was investigated in two multicenter phase III trials, the Federation Francophone de Cancerologie trial 9002[44] and the EORTC trial 40923[45]. Although preliminary results demonstrated a trend toward improved disease-free survival (DFS) and OS in patients who received chemotherapy after hepatic resection, both were closed prematurely due to slow accrual. In a pooled analysis of both trials (n = 278), longer median PFS (27.9 mo vs 18.8 mo, P = 0.058) and OS (62 mo vs 47 mo, P = 0.095) were associated with adjuvant chemotherapy vs surgery alone[46]. In a recent study conducted by the Japan Clinical Oncology Group, 300 patients were randomized to either hepatectomy alone or hepatectomy with adjuvant mFOLFOX. While DFS was improved in the adjuvant chemotherapy group, OS was worse, potentially due to an increased number of deaths following disease recurrence[47].
Only one randomized controlled trial has been conducted on the use of chemotherapy prior to surgery in patients with resectable CRLM. In the EORTC intergroup trial 40983[48], 364 patients were randomized to receive either perioperative chemotherapy (with six cycles of FOLFOX4 before and after surgery) or surgery alone. Resectable disease was defined as £4 Liver lesions, absence of extrahepatic metastases, and a primary tumor that had been or could be completely resected; the primary endpoint was PFS. The administration of perioperative chemotherapy resulted in a 9.2% increase in 3-year PFS from 33.2% to 42.4% (P = 0.025), but no significant difference in OS. Although patients receiving perioperative chemotherapy experienced higher rates of hepatic failure (7% vs 5%), biliary fistulas (8% vs 4%), and intraabdominal infection (7% vs 2%), operative mortality was < 1% in both groups. Overall, these early results demonstrated a modest but statistically significant benefit for perioperative administration of FOLFOX without significant added morbidity or mortality. Long-term results of the EORTC 40983 trial were published five years later focusing on OS. After a median follow-up of 8.5 years, no significant difference in OS was noted between the two groups (52.4% of chemotherapy patients vs 48.3% of surgery only patients, P = 0.34)[23]. Median OS was 63.7 mo among individuals who received perioperative chemotherapy and 55.0 mo among those who underwent surgery alone.
Interestingly, the EORTC 40983 trial continues to be cited in support of both NCT and upfront surgery. Proponents of NCT cite the nearly 10% improvement in PFS, while those who support upfront surgery point to the lack of difference in OS. It is important to note that this trial was largely comprised of metachronous and oligometastatic CRLM, which typically represent patients with more favorable prognoses. Focusing solely on patients with synchronous liver metastases, a large retrospective study was published reporting no difference in OS between patients who received NCT vs those underwent surgery alone. However, patients who received NCT had worse DFS in the setting of multi-centric metastatic disease[49]. Of note, the same study reported that postoperative chemotherapy was associated with improved OS and DFS. In contrast, the use of NCT was associated with decreased OS and DFS in a meta-analysis including 17 cohort studies including the EORTC 40983 trial[50]. However, these results appeared to be confounded by the fact that patients undergoing NCT typically presented with a larger disease burden. When only studies with patients at high risk for disease recurrence were included, NCT was associated with improved survival (pooled HR for 5-year OS = 0.69). CHARISMA is a currently ongoing multicenter phase III clinical trial that aims to determine whether the addition of NCT to surgery will improve OS in a well-defined high-risk patient group with CRLM[51].
Patients with initially unresectable disease
For patients with unresectable disease at the time of diagnosis, the decision to proceed with chemotherapy is more straightforward. Multiple studies have demonstrated the ability to down-stage initially inoperable CRLM with preoperative chemotherapy to facilitate secondary hepatic resection. Between 12%-35% of patients with unresectable CRLM experience a sufficient response to chemotherapy and are subsequently able to undergo surgery with curative intent[14-17]. Five- and ten-year survival following NCT and resection in these patients have been reported at 33% and 23%, respectively[14,52]. Some of the variability in survival between different studies can be attributed to the use of different chemotherapeutic regimens. Indeed, one study reported that rates of R0 resection and OS were higher among patients who were treated with FOLFOXIRI compared with individuals receiving FOLFIRI before surgery[17]. In addition, the definition of “unresectable” disease varied depending on study criteria, surgeon opinion, and quality of cross-sectional imaging. Nevertheless, it is clear that some patients with primarily unresectable CRLM can experience successful downstaging prior to hepatic resection which, when feasible, is associated with improved OS.
Current guidelines
At this time, NCT is not recommended for all patients with CRLM. Recommendations from multiple consensus statements generally advise upfront resection followed by adjuvant chemotherapy in patients with resectable disease and low operative risk (medically fit with four or fewer lesions), and reserve NCT for patients at higher operative risk with borderline resectable or unresectable disease (Table 2). Overall, guidelines on the delivery of perioperative chemotherapy, either pre- or post-hepatectomy, in patients with resectable CRLM are not well established as more conclusive data on the optimal regimen and timing of chemotherapy is necessary.
Table 2.
Consensus statements and guidelines for the use of neoadjuvant chemotherapy in patients with colorectal liver metastases
|
Organization
|
Year
|
Recommendation
|
| National Cancer Comprehensive Network[18] | 2020 | For resectable synchronous CRLM, can consider neoadjuvant therapy for 2-3 mo with FOLFOX, CAPOX, FOLFIRI, or FOLFOXIRI followed by synchronous or staged colectomy and hepatic resection. For unresectable synchronous CRLM, give systemic therapy with FOLFIRI/FOLFOX/CAPOX/FOLFOXIRI ± bevacizumab, FOLFIRI/FOLFOX/FOLFOXIRI ± panitumumab or cetuximab (wild-type KRAS/NRAS/BRAF), or pembrolizumab (dMMR/MSI-H only). Re-evaluate for conversion to resectable every 2 mo. For resectable metachronous metastases, resection and/or local therapy is preferred, though neoadjuvant chemotherapy for 2-3 mo with FOLFOX, CAPOX, capecitabine, or 5-FU/leucovorin can be given |
| American Society of Clinical Oncology[108] | 2020 | It is critical for a multidisciplinary team to evaluate the patient and determine eligibility for curative-intent treatment and best treatment sequence. The choice of chemotherapy depends on the resources available, toxicity concerns, whether the patient had prior chemotherapy, and the patient’s clinical performance status |
| SEOM, AEC, SEOR, SERVEI, and SEMNIM[109] | 2020 | Neoadjuvant chemotherapy might provide benefit in high-risk patients with resectable liver metastases. Neoadjuvant chemotherapy may initially turn unresectable liver metastases into resectable liver metastases with good long-term results |
| European Society of Medical Oncology[110] | 2016 | In patients with clearly resectable disease and favorable prognostic criteria, perioperative treatment may not be necessary and upfront resection is justified. In patients with technically resectable disease where the prognosis is unclear or probably unfavorable, perioperative complication chemotherapy (FOLFOX or CAPOX) should be administered. In situations where the criteria for prognosis and resectability are not sharply defined, perioperative therapy should be considered. Patients with synchronous onset of metastases should be allocated to this group and therapeutic pathway |
| Expert Group on OncoSurgery Management of Liver Metastases[111] | 2015 | For patients with resectable CRLM, chemotherapy should be given preoperatively unless resection of the primary tumor and liver metastases is considered easy. A total of 6 mo of chemotherapy is recommended, independent of whether it is given pre- or postoperatively. For unresectable CRLM, chemotherapy should be administered first with the aim of achieving resectability |
| International Hepato-Pancreato-Biliary Association[112] | 2013 | For resectable CRLM, perioperative chemotherapy with resection has shown progression-free benefits compared with resection alone. The use of an oxaliplatin-containing regimen is the reference treatment for this approach. For downsizing of unresectable CRLM, FOLFOX and FOLFIRI represent chemotherapy backbones of similar efficacy, though FOLFOXIRI may provide a higher likelihood of response |
| Americas Hepato-Pancreato-Biliary Association[112] | 2013 | For resectable CRLM, perioperative chemotherapy with resection has been shown to have improved PFS compared with resection alone. For patients with unresectable CRLM, oxaliplatin- or irinotecan-containing chemotherapeutic regimens represent the standard options, although the addition of bevacizumab carries the potential for a greater response and reduced risk for CALI. In tumors without KRAS mutations, anti-EGFR agents are a reasonable choice. In general, preoperative chemotherapy should not extend beyond 3 mo |
NCT: Neoadjuvant chemotherapy; CRLM: Colorectal liver metastases; FOLFOX: Fluorouracil/leucovorin/oxaliplatin; CAPOX: Capecitabine, oxaliplatin; FOLFIRI: Fluorouracil/leucovorin/irinotecan; FOLFOXIRI: Fluorouracil/leucovorin/oxaliplatin/irinotecan; dMMR: Deficient mismatch repair; MSI-H: Microsatellite instability high; SEOM: Spanish Society of Medical Oncology; AEC: Spanish Association of Surgeons; SEOR: Spanish Society of Radiation Oncology; SERVEI: Spanish Society of Vascular and Interventional Radiology; SEMNIM: Spanish Society of Nuclear Medicine and Molecular Imaging; PFS: Progression-free survival; CALI: Chemotherapy-associated liver injury; 5-FU: 5-Fluorouracil.
TECHNICAL CONSIDERATIONS
Timing and duration
The optimal timing and duration of chemotherapy in relation to surgical resection for CRLM is not definitively known. The duration of NCT has varied widely among studies, though in most studies examining perioperative chemotherapy for CRLM, patients have undergone between 6 cycles to 10 cycles preoperatively, usually followed by 6 cycles to 8 cycles postoperatively[14,15,17,48,52]. In one report, administration of more than 12 wk of chemotherapy and/or an interval of less than four weeks between chemotherapy and surgery was associated with increased postoperative complications, rates of reoperation, and longer hospital stay[53]. Another study found that NCT consisting of FOLFOX with or without bevacizumab was not associated with an increase in complete or major pathologic response after 9 cycles, though it was associated with higher incidences of liver insufficiency and sinusoidal injury[54]. Patients receiving extensive chemotherapy are also at risk for experiencing hepatic atrophy, which is associated with higher rates of postoperative mortality related to liver failure[55]. Thus, avoiding unnecessarily lengthy courses of systemic therapy should be a priority before planned hepatectomy. For patients with initially unresectable disease, radiographic assessment should be performed every 2-3 mo and patients should proceed to surgery as soon as they achieve resectability. For individuals with resectable disease, 2-3 mo of chemotherapy (as conducted in the EORTC 40983 trial) before and after liver resection is often preferred.
No prospective randomized trials have been performed to compare neoadjuvant (or perioperative) to adjuvant chemotherapy. This topic was evaluated in a retrospective multi-institutional study comparing the outcomes of different chemotherapy strategies, including pre-hepatectomy alone, post-hepatectomy alone, perioperative, and no chemotherapy, used to treat synchronous CRLM. Multivariate analysis revealed that timing of chemotherapy relative to hepatic resection did not impact recurrence-free survival (RFS). However, post-hepatectomy chemotherapy was associated with increased OS. Median OS for patients treated with no chemotherapy, pre-hepatectomy chemotherapy, post-hepatectomy chemotherapy, and perioperative chemotherapy were 36, 53, 76, and 67 mo, respectively (P < 0.001). When narrowing the patient population to individuals with low-risk disease, patients receiving post-hepatectomy and perioperative chemotherapy experienced even higher rates of survival relative to other treatment groups: median OS among patients receiving no chemotherapy, pre-hepatectomy chemotherapy, post-hepatectomy chemotherapy, and perioperative chemotherapy were 39, 56, 99, and 97 mo, respectively (P < 0.001)[56].
Clinical risk scoring
The Fong clinical risk score is arguably the most well-known algorithm to assess prognosis in patients with CRLM being considered for resection. It assigns a point for each of the following variables: positive margin, extrahepatic disease, node-positive primary, disease-free interval from primary to metastases < 12 mo, number of hepatic tumors > 1, largest hepatic tumor > 5 cm, and carcinoembryonic antigen (CEA) level > 200 ng/mL, as these factors were significant and independent predictors of poor long-term outcomes[57]. A variety of other models for clinical risk stratification have since been developed. Of note, in a study evaluating the accuracy of eight recognized scoring systems, including the Fong clinical risk score, only the Rees postoperative index was a significant predictor of disease-free and disease-specific survival at 1, 3, 5, and 10 years[58]. Overall, the clinical relevance of risk scores has been challenged. In particular, while these risk scores may function relatively well in predicting survival outcomes, their use in guiding decisions in the management of CRLM has not been validated.
Newer scoring systems that include RAS mutational status as a prognostic factor have outperformed traditional scoring systems[59]. KRAS mutation is present in approximately 15%-36% of patients with CRLM and has been found to be an independent predictor of decreased RFS [hazard ratio (HR) = 1.89] and OS (HR = 2.24)[60]. In patients treated with FOLFOX4 plus bevacizumab, radiographic and pathologic rates of response were worse in KRAS mutants (10.5% and 36.8%) compared to wild-type (32.9% and 58.9%)[61]. Among patients treated surgically for CRLM, those with KRAS mutation had lower five-year disease-specific survival than their wild-type counterparts (29% vs 21%, P = 0.024)[62]. BRAF is another commonly implicated gene in the pathogenesis of CRC, with the V600E mutation occurring in approximately 9% of patients with CRC[63]. RFS was 5.7 mo in patients with BRAF mutation, compared to 11 mo in those with KRAS mutation and 14.7 mo in those with wild-type KRAS and BRAF[64]. Although the presence of BRAF mutation is now considered when choosing a chemotherapy regimen, it is not currently factored into any clinical risk algorithms. With an increasing understanding of cancer biology and ability to detect molecular markers, future scoring systems will likely evolve to incorporate individual tumor mutational status and may be able to better select which patients stand to benefit from NCT.
Monitoring response to therapy
The Response Evaluation Criteria in Solid Tumors (RECIST) is used to objectively measure radiographic response to treatment for most solid organ cancers. However, conventional size-based RECIST criteria have been poor to predict pathologic response for CRLM[65]. Modified criteria based on morphologic changes (mRECIST) has been superior to RECIST in assessing response to NCT, though neither were predictive of residual tumor burden[66]. Optimal morphologic response to chemotherapy is defined as a change toward homogenous, hypoattenuating lesions with thin, sharply defined tumor-liver interface (Figure 1). While morphologic response correlates with pathologic response, the majority of treated CRLM (83%) that appear to have complete radiographic response to chemotherapy are subsequently found to have viable tumor on pathologic review[65,67,68].
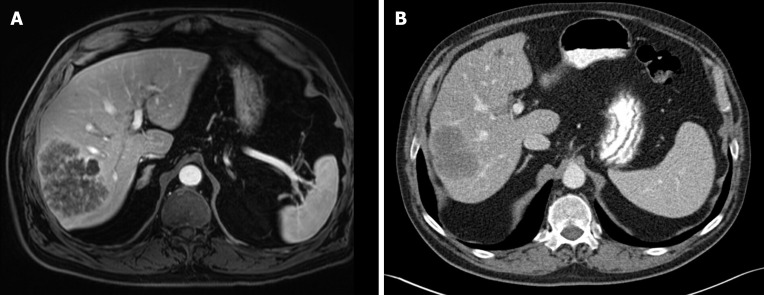
Figure 1.
Radiographic response to neoadjuvant chemotherapy. A: A 70-year-old man presented with a T2-hyperintense metachronous rectal liver metastasis, seen on magnetic resonance imaging; B: Computed tomography scan performed after 6 cycles of FOLFOX plus bevacizumab showed partial Response Evaluation Criteria in Solid Tumors response with decrease in maximum diameter from 9.6 cm to 7.0 cm, as well as optimal morphologic response. The section planes displayed above show the tumor in maximum diameter. The patient subsequently underwent right posterior sectionectomy with no evidence of disease 3 yr after surgery.
Computed tomography (CT) scan remains the most common imaging study used in the initial diagnosis of CRLM. The PROMETEO-01 study demonstrated, however, that magnetic resonance imaging (MRI) was more sensitive than CT scan (91% vs 82%, P = 0.002), and that after NCT, the sensitivity of CT for detection of CRLM dropped to 71%[69]. The use of positron emission tomography (PET) scan is not recommended as a tool for routine staging or surveillance, though it may have a role in the detection of occult extrahepatic disease[70]. A meta-analysis comparing multiple imaging modalities in the preoperative detection of CRLM reported pooled sensitivity estimates of MRI, CT, PET, and PET-CT at 85.7%, 69.9%, 54.5%, and 51.7%, respectively[71]. The investigators noted that, in the neoadjuvant setting, MRI was the most appropriate imaging modality for preoperative assessment of CRLM. In addition, they found that the diagnostic accuracy of PET and PET-CT scans were strongly affected by the use of chemotherapy.
Among laboratory work-up for CRC, CEA is commonly used for prognostication before resection, as well as for postoperative surveillance for recurrent disease. The use of CEA levels has also been studied in conjunction with imaging to monitor response to systemic therapy in metastatic CRC and can accurately predict disease non-progression[72-76]. In one large analysis of 2643 patients with metastatic CRC, a reduction in CEA levels by at least 7.5% from baseline differentiated non-progressive from progressive disease with a sensitivity of 71.6% and specificity of 76.2%. Using this cutoff, it was concluded that progressive disease could be excluded in 97.3% of patients and 72.8% of initial restaging CT scans obtained 3 wk after initiation of systemic chemotherapy could be avoided[77]. It was therefore concluded that CEA levels, which are inexpensive and easy to obtain, are a useful adjunct to imaging for initial monitoring of response to systemic therapy in patients with metastatic CRC.
DLM
With the development of increasingly effective chemotherapeutic agents, dramatic tumor reduction has been observed following initiation of therapy in many cases of CRLM. The phenomenon in which there is a complete radiologic response in hepatic tumors on cross-sectional imaging is referred to as DLM. DLM can represent a unique problem in patients treated with NCT because although the tumor may become undetectable radiologically, this does not necessarily equate complete pathologic response (Figure 2). A number of imaging modalities can be used to detect CRLM both prior to and during surgery. In the preoperative setting, MRI, CT, and PET had sensitivity estimates of 85.7%, 69.9%, and 54.5%, respectively[71]. Meanwhile, the use of contrast-enhanced intraoperative ultrasound (IOUS) had the highest sensitivity (99%) compared with IOUS without contrast (88%), contrast-enhanced MRI (83%), and contrast-enhanced CT (81%)[78]. While DLM has been described in 7%-37% of patients undergoing preoperative systemic therapy, residual disease, either macroscopic microscopic, or early disease recurrence in situ has been observed in 61%-83% of CRLM that appear to have a complete response on imaging[67,79-84]. For patients with DLM left in situ, the intrahepatic recurrence rate is significantly higher. Patients with untreated DLM had one- and three-year intrahepatic RFS of 40.2% and 16.1% compared to 68.8% and 35.1% for those whose original disease sites were resected (P = 0.04)[80]. Lesions at greatest risk for disappearance are those that are < 2 cm in diameter or deeper than 1 cm into the liver parenchyma, while the likelihood of developing DLM increases with longer duration of NCT[80,85]. In these situations, the placement of a fiducial marker can facilitate the localization of metastatic disease that has otherwise become undetectable on imaging[84,85]. Alternatively, patients at highest risk for DLM may benefit from upfront surgery rather than NCT if clinically feasible.
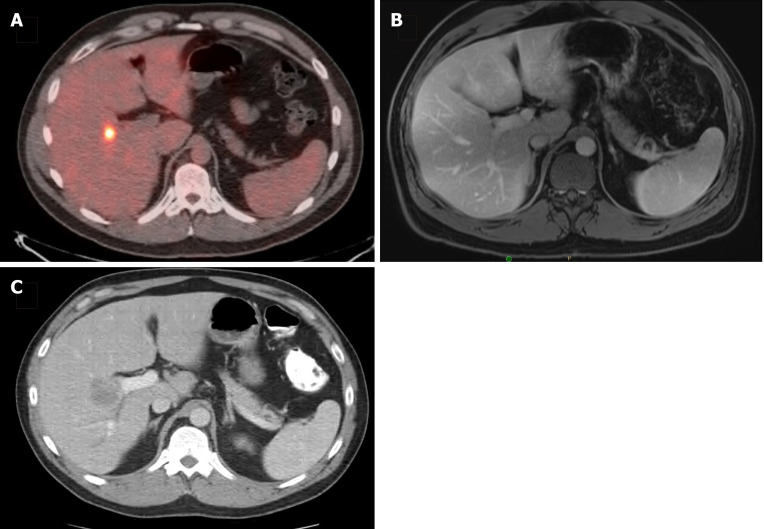
Figure 2.
Disappearing liver metastasis. A: A 36-year-old man presented with a solitary synchronous colorectal liver metastasis to segment 5, seen on staging positron emission tomography-computed tomography scan; B: After 6 cycles of CAPOX plus bevacizumab, followed by pelvic radiation (5040 cGy in 28 fractions), subsequent magnetic resonance imaging (shown) and intraoperative ultrasound were unable to localize the lesion; C: He was then lost to follow-up and returned 18 mo later with an intrahepatic recurrence, seen on surveillance computed tomography scan and confirmed with tissue biopsy.
SURGICAL CONSIDERATIONS
Resection margins
Achieving negative margins at the time of resection remains an important determinant of survival for patients undergoing hepatectomy for CRLM. Data on the minimum margin width needed to optimize survival and avoid disease recurrence have been controversial. In one large meta-analysis, resection margins > 10 mm were associated with significant improvement in OS at 3 years [relative risk (RR) = 0.86], 5 years (RR = 0.91), and 10 years (RR = 0.94), as well as in DFS at 3 years (RR = 0.93) and 5 years (RR = 0.88) after surgery[86]. However, multiple large retrospective studies have failed to demonstrate a survival benefit in patients who underwent resection with margin width between 1-10 mm vs > 10 mm[87-89]. Nonetheless, the need for margins at least 1-mm wide has been well-established as significantly decreased OS has been associated with submillimeter margins (36 mo vs 65 mo, P = 0.03)[90]. Notably, the negative impact of positive margins was most pronounced in patients with a suboptimal response to NCT[91].
Future liver remnant
Currently, CRLM are defined as resectable if it is anticipated that the disease can be completely resected, two adjacent liver segments can be spared, adequate vascular inflow and outflow and biliary drainage can be preserved, and the functional capacity/volume of the future liver remnant (FLR) will be sufficient[92]. The FLR is most commonly measured via CT volumetry. Importantly, given the impact of chemotherapy on liver function, the threshold FLR volume may be higher in patients who have received NCT. For example, while a FLR ≥ 20% of total liver volume (TLV) has been proposed for patients with normal liver function, the presence of pre-existing liver disease or liver injury from systemic therapy necessitates increased preservation or augmentation of liver tissue. For patients with marked steatosis or patients who have received extensive chemotherapy prior to hepatic resection, a FLR volume > 30% of TLV is recommended, while patients with frank cirrhosis require FLR volume > 40% of TLV[93].
For patients projected to have insufficient FLR volume post-hepatectomy, preoperative portal vein embolization (PVE) can be pursued to induce hypertrophy of the non-involved portion of the liver. PVE has also been found to reduce the incidence of postoperative liver insufficiency among patients who experienced hepatic atrophy secondary to prolonged NCT[94]. In patients with extensive bilateral liver metastases, the use of NCT to decrease tumor burden in combination with PVE and two-stage hepatic resection has been shown to improve survival. After a median follow-up of 50 mo, patients with advanced bilateral CRLM who completed two-stage hepatic resection following initial treatment with chemotherapy were found to have significantly increased OS compared with individuals who received chemotherapy only (64% vs 15% at 5 years, P < 0.001)[95].
ALTERNATIVE APPROACHES
Immunotherapy
Immune checkpoint inhibition directed against programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4) has revolutionized the treatment of CRC with microsatellite instability. Immunotherapeutic agents such as pembrolizumab and nivolumab (monoclonal antibodies targeting PD-1), and ipilimumab (a monoclonal targeting CTLA-4), have been introduced as alternative treatment options to chemotherapy for patients with metastatic CRC and high microsatellite instability (MSI-H) or mismatch repair deficiency (dMMR). Due to deficits in mismatch repair, these tumors have a high mutation burden that create abundant tumor-specific neoantigens, drawing T-cell infiltrates to the tumor microenvironment. In a phase III clinical trial, pembrolizumab monotherapy was found to be superior to chemotherapy when given to patients who had tumors with MSI-H and dMMR. Patients who were treated with pembrolizumab experienced longer PFS (16.5 mo vs 8.2 mo, P < 0.01) and higher rates of partial or complete response (43.8% vs 33.1%) compared to those treated with established chemotherapeutic regimens, including mFOLFOX6 ± bevacizumab or cetuximab and FOLFIRI ± bevacizumab or cetuximab[96]. Although the use of immunotherapy has not been well studied in the neoadjuvant setting, early investigations indicate that neoadjuvant immunotherapy either alone or with chemotherapy can be a viable treatment strategy for CRC with dMMR[97,98]. Furthermore, several trials are currently underway to evaluate the safety and efficacy of combined immuno- and chemotherapy regimens[99]. It is likely that in the future, immunotherapy may have a significant role in the neoadjuvant management of CRLM with dMMR.
Intra-arterial therapies
Strategies including hepatic arterial infusion (HAI) and trans-arterial chemoembolization (TACE) allow for the delivery of chemotherapy to a more targeted distribution within the liver while minimizing systemic effects. These locoregional treatment methods take advantage of the fact that liver metastases derive their blood supply predominantly from the hepatic artery circulation and allow for direct infusion of chemotherapeutic drugs at much higher concentrations than could be administered with systemic therapy. The perioperative use of HAI in patients with CRLM has been associated with increased rates of complete pathologic response and conversion to resectability, as well as improved OS[81,100-102]. Despite these advantages, the practice of HAI carries a relatively high rate of technical failure. TACE, which arises from the same basic principles as HAI, does not have the same level of technical difficulty. The use of irinotecan-loaded drug-eluting beads (DEBIRI) has emerged as a safe and effective technique for the delivery of TACE to hepatic metastases. Patients treated with DEBIRI experienced improved survival and tumor response, as well as higher rates of conversion to resectability, compared with individuals treated with systemic therapy alone[103-105]. The ability to deliver locoregional chemotherapy to hepatic metastases continues to be a topic of high interest given the potential to limit adverse systemic effects and preserve patient functional status prior to surgical resection, as well as administer more potent and targeted doses of cytotoxic agents. Comprehensive reviews of perioperative trans-arterial therapies have been recently published[106,107].
CONCLUSION
The treatment of CRC is unique from most other cancers in that surgery for curative intent is routinely performed for stage IV disease. Over the last two to three decades, innovations in medical therapies and surgical techniques, based on an increasingly individualized approach to oncological care, have dramatically expanded the number of treatment options for patients with metastatic CRC. For patients with CRLM, surgical resection remains the only chance for cure, though optimization of patients preoperatively and the prevention of disease recurrence postoperatively requires a multidisciplinary approach. The initial evaluation of patients with CRLM involves determining the resectability of their disease. For those with borderline resectable or unresectable CRLM, systemic chemotherapy is recommended as conversion to resectability can occur in a substantial proportion of these patients. In contrast, the routine use of NCT remains controversial for patients with resectable CRLM given the lack of existing data showing definitive benefit in these cases, while hepatotoxicity is a known risk of current standard chemotherapeutic agents. Future endeavors should seek to determine the optimal selection criteria for patients with high-risk resectable CRLM, as well as standardize the definition of resectability, establish universal criteria for conversion to resectability, and examine outcomes regarding quality of life in patients receiving NCT.
Overall, the landscape of oncological treatment continues to trend toward more personalized and targeted therapy, and efforts in characterizing the biochemical profile of individual tumors are underway with the goal of discovering novel chemo- or immunotherapeutic agents with higher potency and lower risk for adverse effects. Advancements in recent years have expanded the opportunity for curative-intent treatment to an increasing number of patients, as well as resulted in significantly improved survival rates for those with CRLM. In face of the continuously growing number of possible therapies available for metastatic CRC, consensus statements have come to agree that these patients are best served at tertiary centers where a multimodal treatment strategy can be facilitated by an expert multidisciplinary team. Ultimately, outcomes for patients with metastatic CRC are expected to continually improve moving forward.
Footnotes
Conflict-of-interest statement: Authors report no conflicts of interest for this article.
Manuscript source: Invited manuscript
Peer-review started: March 16, 2021
First decision: May 3, 2021
Article in press: August 6, 2021
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cerwenka H, Wang ZJ S-Editor: Gao CC L-Editor: A P-Editor: Xing YX
Contributor Information
Marissa Guo, Department of Surgery, The Ohio State University Medical Center, Columbus, OH 43210, United States.
Ning Jin, Department of Internal Medicine, Division of Medical Oncology, The Ohio State University Medical Center, Columbus, OH 43210, United States.
Timothy Pawlik, Department of Surgery, The Ohio State University, Columbus, OH 43210, United States.
Jordan M Cloyd, Department of Surgery, Division of Surgical Oncology, The Ohio State University Medical Center, Columbus, OH 43210, United States. jordan.cloyd@osumc.edu.
References
- 1.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Agius D, Agrawal S, Ahmadi A, Ahmadian E, Ahmadpour E, Ahmed MB, Akbari ME, Akinyemiju T, Al-Aly Z, AlAbdulKader AM, Alahdab F, Alam T, Alamene GM, Alemnew BTT, Alene KA, Alinia C, Alipour V, Aljunid SM, Bakeshei FA, Almadi MAH, Almasi-Hashiani A, Alsharif U, Alsowaidi S, Alvis-Guzman N, Amini E, Amini S, Amoako YA, Anbari Z, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Ansariadi A, Appiah SCY, Arab-Zozani M, Arabloo J, Arefi Z, Aremu O, Areri HA, Artaman A, Asayesh H, Asfaw ET, Ashagre AF, Assadi R, Ataeinia B, Atalay HT, Ataro Z, Atique S, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Awoke N, Ayala Quintanilla BP, Ayanore MA, Ayele HT, Babaee E, Bacha U, Badawi A, Bagherzadeh M, Bagli E, Balakrishnan S, Balouchi A, Bärnighausen TW, Battista RJ, Behzadifar M, Bekele BB, Belay YB, Belayneh YM, Berfield KKS, Berhane A, Bernabe E, Beuran M, Bhakta N, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Birungi C, Bisignano C, Bitew H, Bjørge T, Bleyer A, Bogale KA, Bojia HA, Borzì AM, Bosetti C, Bou-Orm IR, Brenner H, Brewer JD, Briko AN, Briko NI, Bustamante-Teixeira MT, Butt ZA, Carreras G, Carrero JJ, Carvalho F, Castro C, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chanie WF, Chattu VK, Chaturvedi P, Chauhan NS, Chehrazi M, Chiang PP, Chichiabellu TY, Chido-Amajuoyi OG, Chimed-Ochir O, Choi JJ, Christopher DJ, Chu DT, Constantin MM, Costa VM, Crocetti E, Crowe CS, Curado MP, Dahlawi SMA, Damiani G, Darwish AH, Daryani A, das Neves J, Demeke FM, Demis AB, Demissie BW, Demoz GT, Denova-Gutiérrez E, Derakhshani A, Deribe KS, Desai R, Desalegn BB, Desta M, Dey S, Dharmaratne SD, Dhimal M, Diaz D, Dinberu MTT, Djalalinia S, Doku DT, Drake TM, Dubey M, Dubljanin E, Duken EE, Ebrahimi H, Effiong A, Eftekhari A, El Sayed I, Zaki MES, El-Jaafary SI, El-Khatib Z, Elemineh DA, Elkout H, Ellenbogen RG, Elsharkawy A, Emamian MH, Endalew DA, Endries AY, Eshrati B, Fadhil I, Fallah Omrani V, Faramarzi M, Farhangi MA, Farioli A, Farzadfar F, Fentahun N, Fernandes E, Feyissa GT, Filip I, Fischer F, Fisher JL, Force LM, Foroutan M, Freitas M, Fukumoto T, Futran ND, Gallus S, Gankpe FG, Gayesa RT, Gebrehiwot TT, Gebremeskel GG, Gedefaw GA, Gelaw BK, Geta B, Getachew S, Gezae KE, Ghafourifard M, Ghajar A, Ghashghaee A, Gholamian A, Gill PS, Ginindza TTG, Girmay A, Gizaw M, Gomez RS, Gopalani SV, Gorini G, Goulart BNG, Grada A, Ribeiro Guerra M, Guimaraes ALS, Gupta PC, Gupta R, Hadkhale K, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hanfore LK, Haro JM, Hasankhani M, Hasanzadeh A, Hassen HY, Hay RJ, Hay SI, Henok A, Henry NJ, Herteliu C, Hidru HD, Hoang CL, Hole MK, Hoogar P, Horita N, Hosgood HD, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hussen MM, Ileanu B, Ilic MD, Innos K, Irvani SSN, Iseh KR, Islam SMS, Islami F, Jafari Balalami N, Jafarinia M, Jahangiry L, Jahani MA, Jahanmehr N, Jakovljevic M, James SL, Javanbakht M, Jayaraman S, Jee SH, Jenabi E, Jha RP, Jonas JB, Jonnagaddala J, Joo T, Jungari SB, Jürisson M, Kabir A, Kamangar F, Karch A, Karimi N, Karimian A, Kasaeian A, Kasahun GG, Kassa B, Kassa TD, Kassaw MW, Kaul A, Keiyoro PN, Kelbore AG, Kerbo AA, Khader YS, Khalilarjmandi M, Khan EA, Khan G, Khang YH, Khatab K, Khater A, Khayamzadeh M, Khazaee-Pool M, Khazaei S, Khoja AT, Khosravi MH, Khubchandani J, Kianipour N, Kim D, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Komaki H, Koyanagi A, Krohn KJ, Bicer BK, Kugbey N, Kumar V, Kuupiel D, La Vecchia C, Lad DP, Lake EA, Lakew AM, Lal DK, Lami FH, Lan Q, Lasrado S, Lauriola P, Lazarus JV, Leigh J, Leshargie CT, Liao Y, Limenih MA, Listl S, Lopez AD, Lopukhov PD, Lunevicius R, Madadin M, Magdeldin S, El Razek HMA, Majeed A, Maleki A, Malekzadeh R, Manafi A, Manafi N, Manamo WA, Mansourian M, Mansournia MA, Mantovani LG, Maroufizadeh S, Martini SMS, Mashamba-Thompson TP, Massenburg BB, Maswabi MT, Mathur MR, McAlinden C, McKee M, Meheretu HAA, Mehrotra R, Mehta V, Meier T, Melaku YA, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Mendoza W, Menezes RG, Merat S, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Mihretie KMM, Miller TR, Mills EJ, Mir SM, Mirzaei H, Mirzaei HR, Mishra R, Moazen B, Mohammad DK, Mohammad KA, Mohammad Y, Darwesh AM, Mohammadbeigi A, Mohammadi H, Mohammadi M, Mohammadian M, Mohammadian-Hafshejani A, Mohammadoo-Khorasani M, Mohammadpourhodki R, Mohammed AS, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Moodley Y, Moosazadeh M, Moossavi M, Moradi G, Moradi-Joo M, Moradi-Lakeh M, Moradpour F, Morawska L, Morgado-da-Costa J, Morisaki N, Morrison SD, Mosapour A, Mousavi SM, Muche AA, Muhammed OSS, Musa J, Nabhan AF, Naderi M, Nagarajan AJ, Nagel G, Nahvijou A, Naik G, Najafi F, Naldi L, Nam HS, Nasiri N, Nazari J, Negoi I, Neupane S, Newcomb PA, Nggada HA, Ngunjiri JW, Nguyen CT, Nikniaz L, Ningrum DNA, Nirayo YL, Nixon MR, Nnaji CA, Nojomi M, Nosratnejad S, Shiadeh MN, Obsa MS, Ofori-Asenso R, Ogbo FA, Oh IH, Olagunju AT, Olagunju TO, Oluwasanu MM, Omonisi AE, Onwujekwe OE, Oommen AM, Oren E, Ortega-Altamirano DDV, Ota E, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakhale S, Pakpour AH, Pana A, Park EK, Parsian H, Pashaei T, Patel S, Patil ST, Pennini A, Pereira DM, Piccinelli C, Pillay JD, Pirestani M, Pishgar F, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Prakash S, Prasad N, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafiei A, Rahim F, Rahimi M, Rahman MA, Rajati F, Rana SM, Raoofi S, Rath GK, Rawaf DL, Rawaf S, Reiner RC, Renzaho AMN, Rezaei N, Rezapour A, Ribeiro AI, Ribeiro D, Ronfani L, Roro EM, Roshandel G, Rostami A, Saad RS, Sabbagh P, Sabour S, Saddik B, Safiri S, Sahebkar A, Salahshoor MR, Salehi F, Salem H, Salem MR, Salimzadeh H, Salomon JA, Samy AM, Sanabria J, Santric Milicevic MM, Sartorius B, Sarveazad A, Sathian B, Satpathy M, Savic M, Sawhney M, Sayyah M, Schneider IJC, Schöttker B, Sekerija M, Sepanlou SG, Sepehrimanesh M, Seyedmousavi S, Shaahmadi F, Shabaninejad H, Shahbaz M, Shaikh MA, Shamshirian A, Shamsizadeh M, Sharafi H, Sharafi Z, Sharif M, Sharifi A, Sharifi H, Sharma R, Sheikh A, Shirkoohi R, Shukla SR, Si S, Siabani S, Silva DAS, Silveira DGA, Singh A, Singh JA, Sisay S, Sitas F, Sobngwi E, Soofi M, Soriano JB, Stathopoulou V, Sufiyan MB, Tabarés-Seisdedos R, Tabuchi T, Takahashi K, Tamtaji OR, Tarawneh MR, Tassew SG, Taymoori P, Tehrani-Banihashemi A, Temsah MH, Temsah O, Tesfay BE, Tesfay FH, Teshale MY, Tessema GA, Thapa S, Tlaye KG, Topor-Madry R, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tsadik AG, Ullah I, Uthman OA, Vacante M, Vaezi M, Varona Pérez P, Veisani Y, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vosoughi K, Vu GT, Vujcic IS, Wabinga H, Wachamo TM, Wagnew FS, Waheed Y, Weldegebreal F, Weldesamuel GT, Wijeratne T, Wondafrash DZ, Wonde TE, Wondmieneh AB, Workie HM, Yadav R, Yadegar A, Yadollahpour A, Yaseri M, Yazdi-Feyzabadi V, Yeshaneh A, Yimam MA, Yimer EM, Yisma E, Yonemoto N, Younis MZ, Yousefi B, Yousefifard M, Yu C, Zabeh E, Zadnik V, Moghadam TZ, Zaidi Z, Zamani M, Zandian H, Zangeneh A, Zaki L, Zendehdel K, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Murray CJL. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Agency for Research in Cancer. Malaysia Cancer Statistics. Global Cancer Observat. 2019;593:1–2. [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 4.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. doi: 10.1186/1471-2407-14-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465–474. doi: 10.1002/bjs.5278. [DOI] [PubMed] [Google Scholar]
- 7.Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. doi: 10.1186/s12885-017-3925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito K, Govindarajan A, Ito H, Fong Y. Surgical treatment of hepatic colorectal metastasis: evolving role in the setting of improving systemic therapies and ablative treatments in the 21st century. Cancer J. 2010;16:103–110. doi: 10.1097/PPO.0b013e3181d7e8e5. [DOI] [PubMed] [Google Scholar]
- 11.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D'Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 12.Cloyd JM, Mizuno T, Kawaguchi Y, Lillemoe HA, Karagkounis G, Omichi K, Chun YS, Conrad C, Tzeng CD, Odisio BC, Huang SY, Hicks M, Wei SH, Aloia TA, Vauthey JN. Comprehensive Complication Index Validates Improved Outcomes Over Time Despite Increased Complexity in 3707 Consecutive Hepatectomies. Ann Surg. 2020;271:724–731. doi: 10.1097/SLA.0000000000003043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aquina CT, Ejaz A, Tsung A, Pawlik TM, Cloyd JM. National Trends in the Use of Neoadjuvant Therapy Before Cancer Surgery in the US From 2004 to 2016. JAMA Netw Open. 2021;4:e211031. doi: 10.1001/jamanetworkopen.2021.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghémard O, Levi F, Bismuth H. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657; discussion 657-658. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, Levitt R, Rowland K, Nair S, Sargent DJ, Donohue JH. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243–9249. doi: 10.1200/JCO.2005.07.740. [DOI] [PubMed] [Google Scholar]
- 16.Barone C, Nuzzo G, Cassano A, Basso M, Schinzari G, Giuliante F, D'Argento E, Trigila N, Astone A, Pozzo C. Final analysis of colorectal cancer patients treated with irinotecan and 5-fluorouracil plus folinic acid neoadjuvant chemotherapy for unresectable liver metastases. Br J Cancer. 2007;97:1035–1039. doi: 10.1038/sj.bjc.6603988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G Gruppo Oncologico Nord Ovest. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology - Antiemesis. Nat Compre Cancer Net. 2012;1:1–43. [Google Scholar]
- 19.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, Mentha G, Terris B. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. doi: 10.1016/j.jamcollsurg.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, Risio M, Muratore A, Capussotti L, Curley SA, Abdalla EK. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 22.Robinson SM, Wilson CH, Burt AD, Manas DM, White SA. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:4287–4299. doi: 10.1245/s10434-012-2438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) Perioperative FOLFOX4 chemotherapy and surgery vs surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 24.Wiseman JT, Guzman-Pruneda F, Xourafas D, Chun YS, Ejaz A, Tsung A, Pawlik TM, Cloyd JM. Impact of Neoadjuvant Chemotherapy on the Postoperative Outcomes of Patients Undergoing Liver Resection for Colorectal Liver Metastases: A Population-Based Propensity-Matched Analysis. J Am Coll Surg. 2019;229:69–77.e2. doi: 10.1016/j.jamcollsurg.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015;33:1809–1824. doi: 10.1200/JCO.2014.59.7633. [DOI] [PubMed] [Google Scholar]
- 26.Masi G, Vasile E, Loupakis F, Cupini S, Fornaro L, Baldi G, Salvatore L, Cremolini C, Stasi I, Brunetti I, Fabbri MA, Puglisi M, Trenta P, Granetto C, Chiara S, Fioretto L, Allegrini G, Crinò L, Andreuccetti M, Falcone A. Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis. J Natl Cancer Inst. 2011;103:21–30. doi: 10.1093/jnci/djq456. [DOI] [PubMed] [Google Scholar]
- 27.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Primrose J, Falk S, Finch-Jones M, Valle J, O'Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M, Iveson T, Hickish T, Butler R, Stanton L, Dixon E, Little L, Bowers M, Pugh S, Garden OJ, Cunningham D, Maughan T, Bridgewater J. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601–611. doi: 10.1016/S1470-2045(14)70105-6. [DOI] [PubMed] [Google Scholar]
- 29.Bridgewater JA, Pugh SA, Maishman T, Eminton Z, Mellor J, Whitehead A, Stanton L, Radford M, Corkhill A, Griffiths GO, Falk S, Valle JW, O'Reilly D, Siriwardena AK, Hornbuckle J, Rees M, Iveson TJ, Hickish T, Garden OJ, Cunningham D, Maughan TS, Primrose JN New EPOC investigators. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21:398–411. doi: 10.1016/S1470-2045(19)30798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 31.Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 32.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Tian Y, Xu F, Sidhu R. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 33.Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW, Schwartzberg L, Hedrick E. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 34.Stein A, Glockzin G, Wienke A, Arnold D, Edelmann T, Hildebrandt B, Hollerbach S, Illerhaus G, Königsrainer A, Richter M, Schlitt HJ, Schmoll HJ. Treatment with bevacizumab and FOLFOXIRI in patients with advanced colorectal cancer: presentation of two novel trials (CHARTA and PERIMAX) and review of the literature. BMC Cancer. 2012;12:356. doi: 10.1186/1471-2407-12-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gruenberger B, Tamandl D, Schueller J, Scheithauer W, Zielinski C, Herbst F, Gruenberger T. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26:1830–1835. doi: 10.1200/JCO.2007.13.7679. [DOI] [PubMed] [Google Scholar]
- 36.D'Angelica M, Kornprat P, Gonen M, Chung KY, Jarnagin WR, DeMatteo RP, Fong Y, Kemeny N, Blumgart LH, Saltz LB. Lack of evidence for increased operative morbidity after hepatectomy with perioperative use of bevacizumab: a matched case-control study. Ann Surg Oncol. 2007;14:759–765. doi: 10.1245/s10434-006-9074-0. [DOI] [PubMed] [Google Scholar]
- 37.Reddy SK, Morse MA, Hurwitz HI, Bendell JC, Gan TJ, Hill SE, Clary BM. Addition of bevacizumab to irinotecan- and oxaliplatin-based preoperative chemotherapy regimens does not increase morbidity after resection of colorectal liver metastases. J Am Coll Surg. 2008;206:96–106. doi: 10.1016/j.jamcollsurg.2007.06.290. [DOI] [PubMed] [Google Scholar]
- 38.Okines A, Puerto OD, Cunningham D, Chau I, Van Cutsem E, Saltz L, Cassidy J. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br J Cancer. 2009;101:1033–1038. doi: 10.1038/sj.bjc.6605259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozloff M, Yood MU, Berlin J, Flynn PJ, Kabbinavar FF, Purdie DM, Ashby MA, Dong W, Sugrue MM, Grothey A Investigators of the BRiTE study. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the BRiTE observational cohort study. Oncologist. 2009;14:862–870. doi: 10.1634/theoncologist.2009-0071. [DOI] [PubMed] [Google Scholar]
- 40.Tamandl D, Gruenberger B, Klinger M, Herberger B, Kaczirek K, Fleischmann E, Gruenberger T. Liver resection remains a safe procedure after neoadjuvant chemotherapy including bevacizumab: a case-controlled study. Ann Surg. 2010;252:124–130. doi: 10.1097/SLA.0b013e3181deb67f. [DOI] [PubMed] [Google Scholar]
- 41.van der Pool AE, Marsman HA, Verheij J, Ten Kate FJ, Eggermont AM, Ijzermans JN, Verhoef C. Effect of bevacizumab added preoperatively to oxaliplatin on liver injury and complications after resection of colorectal liver metastases. J Surg Oncol. 2012;106:892–897. doi: 10.1002/jso.23142. [DOI] [PubMed] [Google Scholar]
- 42.Tang W, Ren L, Liu T, Ye Q, Wei Y, He G, Lin Q, Wang X, Wang M, Liang F, Cui Y, Xu J. Bevacizumab Plus mFOLFOX6 Versus mFOLFOX6 Alone as First-Line Treatment for RAS Mutant Unresectable Colorectal Liver-Limited Metastases: The BECOME Randomized Controlled Trial. J Clin Oncol. 2020;38:3175–3184. doi: 10.1200/JCO.20.00174. [DOI] [PubMed] [Google Scholar]
- 43.Cloyd JM, Heh V, Pawlik TM, Ejaz A, Dillhoff M, Tsung A, Williams T, Abushahin L, Bridges JFP, Santry H. Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials. J Clin Med. 2020;9 doi: 10.3390/jcm9041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B, Bugat R, Lazorthes F, Bedenne L. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 45.Langer B, Bleiberg H, Labianca R, Shepherd L, Nitti D, Marsoni S. Fluorouracil (FU) plus I-leucovorin (I-LV) vs observation after potentially curative resection of liver or lung metastases from colorectal cancer (CRC): results of the ENG (EORTC/NCIC CTG/GIVIO) randomized trial. Proc Am Soc Clin. 2002;21:592. [Google Scholar]
- 46.Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, Nitti D, Torri V, Elias D, O'Callaghan C, Langer B, Martignoni G, Bouché O, Lazorthes F, Van Cutsem E, Bedenne L, Moore MJ, Rougier P. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906–4911. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 47.Kanemitsu Y, Kato T, Shimizu Y, Inaba Y, Shimada Y, Nakamura K, Sato A, Moriya Y Colorectal Cancer Study Group (CCSG) of Japan Clinical Oncology Group. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone as treatment for liver metastasis from colorectal cancer: Japan Clinical Oncology Group Study JCOG0603. Jpn J Clin Oncol. 2009;39:406–409. doi: 10.1093/jjco/hyp035. [DOI] [PubMed] [Google Scholar]
- 48.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) Perioperative chemotherapy with FOLFOX4 and surgery vs surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonney GK, Coldham C, Adam R, Kaiser G, Barroso E, Capussotti L, Laurent C, Verhoef C, Nuzzo G, Elias D, Lapointe R, Hubert C, Lopez-Ben S, Krawczyk M, Mirza DF LiverMetSurvey International Registry Working Group. Role of neoadjuvant chemotherapy in resectable synchronous colorectal liver metastasis; An international multi-center data analysis using LiverMetSurvey. J Surg Oncol. 2015;111:716–724. doi: 10.1002/jso.23899. [DOI] [PubMed] [Google Scholar]
- 50.Liu W, Zhou JG, Sun Y, Zhang L, Xing BC. The role of neoadjuvant chemotherapy for resectable colorectal liver metastases: a systematic review and meta-analysis. Oncotarget. 2016;7:37277–37287. doi: 10.18632/oncotarget.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ayez N, van der Stok EP, de Wilt H, Radema SA, van Hillegersberg R, Roumen RM, Vreugdenhil G, Tanis PJ, Punt CJ, Dejong CH, Jansen RL, Verheul HM, de Jong KP, Hospers GA, Klaase JM, Legdeur MC, van Meerten E, Eskens FA, van der Meer N, van der Holt B, Verhoef C, Grünhagen DJ. Neo-adjuvant chemotherapy followed by surgery vs surgery alone in high-risk patients with resectable colorectal liver metastases: the CHARISMA randomized multicenter clinical trial. BMC Cancer. 2015;15:180. doi: 10.1186/s12885-015-1199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adam R, Wicherts DA, de Haas RJ, Ciacio O, Lévi F, Paule B, Ducreux M, Azoulay D, Bismuth H, Castaing D. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009;27:1829–1835. doi: 10.1200/JCO.2008.19.9273. [DOI] [PubMed] [Google Scholar]
- 53.Welsh FK, Tilney HS, Tekkis PP, John TG, Rees M. Safe liver resection following chemotherapy for colorectal metastases is a matter of timing. Br J Cancer. 2007;96:1037–1042. doi: 10.1038/sj.bjc.6603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kishi Y, Zorzi D, Contreras CM, Maru DM, Kopetz S, Ribero D, Motta M, Ravarino N, Risio M, Curley SA, Abdalla EK, Capussotti L, Vauthey JN. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2010;17:2870–2876. doi: 10.1245/s10434-010-1166-1. [DOI] [PubMed] [Google Scholar]
- 55.Yamashita S, Shindoh J, Mizuno T, Chun YS, Conrad C, Aloia TA, Vauthey JN. Hepatic atrophy following preoperative chemotherapy predicts hepatic insufficiency after resection of colorectal liver metastases. J Hepatol. 2017;67:56–64. doi: 10.1016/j.jhep.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 56.Reddy SK, Zorzi D, Lum YW, Barbas AS, Pawlik TM, Ribero D, Abdalla EK, Choti MA, Kemp C, Vauthey JN, Morse MA, White RR, Clary BM. Timing of multimodality therapy for resectable synchronous colorectal liver metastases: a retrospective multi-institutional analysis. Ann Surg Oncol. 2009;16:1809–1819. doi: 10.1245/s10434-008-0181-y. [DOI] [PubMed] [Google Scholar]
- 57.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318; discussion 318-321. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts KJ, White A, Cockbain A, Hodson J, Hidalgo E, Toogood GJ, Lodge JP. Performance of prognostic scores in predicting long-term outcome following resection of colorectal liver metastases. Br J Surg. 2014;101:856–866. doi: 10.1002/bjs.9471. [DOI] [PubMed] [Google Scholar]
- 59.Brudvik KW, Jones RP, Giuliante F, Shindoh J, Passot G, Chung MH, Song J, Li L, Dagenborg VJ, Fretland ÅA, Røsok B, De Rose AM, Ardito F, Edwin B, Panettieri E, Larocca LM, Yamashita S, Conrad C, Aloia TA, Poston GJ, Bjørnbeth BA, Vauthey JN. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann Surg. 2019;269:120–126. doi: 10.1097/SLA.0000000000002319. [DOI] [PubMed] [Google Scholar]
- 60.Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015;102:1175–1183. doi: 10.1002/bjs.9870. [DOI] [PubMed] [Google Scholar]
- 61.Zimmitti G, Shindoh J, Mise Y, Kopetz S, Loyer EM, Andreou A, Cooper AB, Kaur H, Aloia TA, Maru DM, Vauthey JN. RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann Surg Oncol. 2015;22:834–842. doi: 10.1245/s10434-014-4042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allievi N, Goffredo P, Utria AF, Pisano M, Poiasina E, Lucianetti A, Zhou P, Hassan I. The association of KRAS mutation with primary tumor location and survival in patients undergoing resection of colorectal cancers and synchronous liver metastases. Chin Clin Oncol. 2019;8:46. doi: 10.21037/cco.2019.08.10. [DOI] [PubMed] [Google Scholar]
- 63.Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, Taylor G, Barrett JH, Quirke P. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27:5931–5937. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 64.Schirripa M, Bergamo F, Cremolini C, Casagrande M, Lonardi S, Aprile G, Yang D, Marmorino F, Pasquini G, Sensi E, Lupi C, De Maglio G, Borrelli N, Pizzolitto S, Fasola G, Bertorelle R, Rugge M, Fontanini G, Zagonel V, Loupakis F, Falcone A. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br J Cancer. 2015;112:1921–1928. doi: 10.1038/bjc.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shindoh J, Loyer EM, Kopetz S, Boonsirikamchai P, Maru DM, Chun YS, Zimmitti G, Curley SA, Charnsangavej C, Aloia TA, Vauthey JN. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30:4566–4572. doi: 10.1200/JCO.2012.45.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Egger ME, Cannon RM, Metzger TL, Nowacki M, Kelly L, Tatum C, Scoggins CR, Callender GG, McMasters KM, Martin RC 2nd. Assessment of chemotherapy response in colorectal liver metastases in patients undergoing hepatic resection and the correlation to pathologic residual viable tumor. J Am Coll Surg. 2013;216:845–856; discussion 856-857. doi: 10.1016/j.jamcollsurg.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 67.Benoist S, Brouquet A, Penna C, Julié C, El Hajjam M, Chagnon S, Mitry E, Rougier P, Nordlinger B. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24:3939–3945. doi: 10.1200/JCO.2006.05.8727. [DOI] [PubMed] [Google Scholar]
- 68.Chun YS, Vauthey JN, Boonsirikamchai P, Maru DM, Kopetz S, Palavecino M, Curley SA, Abdalla EK, Kaur H, Charnsangavej C, Loyer EM. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rojas Llimpe FL, Di Fabio F, Ercolani G, Giampalma E, Cappelli A, Serra C, Castellucci P, D'Errico A, Golfieri R, Pinna AD, Pinto C. Imaging in resectable colorectal liver metastasis patients with or without preoperative chemotherapy: results of the PROMETEO-01 study. Br J Cancer. 2014;111:667–673. doi: 10.1038/bjc.2014.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lake ES, Wadhwani S, Subar D, Kauser A, Harris C, Chang D, Lapsia S. The influence of FDG PET-CT on the detection of extrahepatic disease in patients being considered for resection of colorectal liver metastasis. Ann R Coll Surg Engl. 2014;96:211–215. doi: 10.1308/003588414X13814021679195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Kessel CS, Buckens CF, van den Bosch MA, van Leeuwen MS, van Hillegersberg R, Verkooijen HM. Preoperative imaging of colorectal liver metastases after neoadjuvant chemotherapy: a meta-analysis. Ann Surg Oncol. 2012;19:2805–2813. doi: 10.1245/s10434-012-2300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ward U, Primrose JN, Finan PJ, Perren TJ, Selby P, Purves DA, Cooper EH. The use of tumour markers CEA, CA-195 and CA-242 in evaluating the response to chemotherapy in patients with advanced colorectal cancer. Br J Cancer. 1993;67:1132–1135. doi: 10.1038/bjc.1993.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grem JL, Steinberg SM, Chen AP, McAtee N, Cullen E, Hamilton JM, Allegra CJ. The utility of monitoring carcinoembyronic antigen during systemic therapy for advanced colorectal cancer. Oncol Rep. 1998;5:559–567. doi: 10.3892/or.5.3.559. [DOI] [PubMed] [Google Scholar]
- 74.Wang WS, Lin JK, Lin TC, Chiou TJ, Liu JH, Fan FS, Yen CC, Chen WS, Jiang JK, Yang SH, Wang HS, Chen PM. Carcinoembryonic antigen in monitoring of response to systemic chemotherapy in patients with metastatic colorectal cancer. Int J Colorectal Dis. 2001;16:96–101. doi: 10.1007/s003840000266. [DOI] [PubMed] [Google Scholar]
- 75.Trillet-Lenoir V, Chapuis F, Touzet S, Barbier JY, Freyer G, Gaudin JL, Lombard-Bohas C, Valette PJ, Lledo G, Gouttebel MC, Boyer JD, Chassignol L, Hamon H, Claudel-Bonvoisin S, Leprince E, Amoyal P, Glehen O, Darnand P, Heilmann MO, Bleuse JP. Any clinical benefit from the use of oncofoetal markers in the management of chemotherapy for patients with metastatic colorectal carcinomas? Clin Oncol (R Coll Radiol) 2004;16:196–203. doi: 10.1016/j.clon.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 76.de Haas RJ, Wicherts DA, Flores E, Ducreux M, Lévi F, Paule B, Azoulay D, Castaing D, Lemoine A, Adam R. Tumor marker evolution: comparison with imaging for assessment of response to chemotherapy in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17:1010–1023. doi: 10.1245/s10434-009-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gulhati P, Yin J, Pederson L, Schmoll HJ, Hoff P, Douillard JY, Hecht JR, Tournigand C, Tebbut N, Chibaudel B, Gramont A, Shi Q, Overman MJ. Threshold Change in CEA as a Predictor of Non-Progression to First-Line Systemic Therapy in Metastatic Colorectal Cancer Patients With Elevated CEA. J Natl Cancer Inst. 2020;112:1127–1136. doi: 10.1093/jnci/djaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arita J, Ono Y, Takahashi M, Inoue Y, Takahashi Y, Matsueda K, Saiura A. Routine Preoperative Liver-specific Magnetic Resonance Imaging Does Not Exclude the Necessity of Contrast-enhanced Intraoperative Ultrasound in Hepatic Resection for Colorectal Liver Metastasis. Ann Surg. 2015;262:1086–1091. doi: 10.1097/SLA.0000000000001085. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka K, Takakura H, Takeda K, Matsuo K, Nagano Y, Endo I. Importance of complete pathologic response to prehepatectomy chemotherapy in treating colorectal cancer metastases. Ann Surg. 2009;250:935–942. doi: 10.1097/sla.0b013e3181b0c6e4. [DOI] [PubMed] [Google Scholar]
- 80.van Vledder MG, de Jong MC, Pawlik TM, Schulick RD, Diaz LA, Choti MA. Disappearing colorectal liver metastases after chemotherapy: should we be concerned? J Gastrointest Surg. 2010;14:1691–1700. doi: 10.1007/s11605-010-1348-y. [DOI] [PubMed] [Google Scholar]
- 81.Auer RC, White RR, Kemeny NE, Schwartz LH, Shia J, Blumgart LH, Dematteo RP, Fong Y, Jarnagin WR, D'Angelica MI. Predictors of a true complete response among disappearing liver metastases from colorectal cancer after chemotherapy. Cancer. 2010;116:1502–1509. doi: 10.1002/cncr.24912. [DOI] [PubMed] [Google Scholar]
- 82.Ferrero A, Langella S, Russolillo N, Vigano' L, Lo Tesoriere R, Capussotti L. Intraoperative detection of disappearing colorectal liver metastases as a predictor of residual disease. J Gastrointest Surg. 2012;16:806–814. doi: 10.1007/s11605-011-1810-5. [DOI] [PubMed] [Google Scholar]
- 83.Tani K, Shindoh J, Akamatsu N, Arita J, Kaneko J, Sakamoto Y, Hasegawa K, Kokudo N. Management of disappearing lesions after chemotherapy for colorectal liver metastases: Relation between detectability and residual tumors. J Surg Oncol. 2018;117:191–197. doi: 10.1002/jso.24805. [DOI] [PubMed] [Google Scholar]
- 84.Vujic J, Schöllnast H, Marsoner K, Wienerroither V, Bacher H, Mischinger HJ, Kornprat P. Marking Disappearing Colorectal Liver Metastases After Complete Response to Neoadjuvant Chemotherapy via CT - A Pilot Study. Anticancer Res. 2019;39:3847–3854. doi: 10.21873/anticanres.13534. [DOI] [PubMed] [Google Scholar]
- 85.Passot G, Odisio BC, Zorzi D, Mahvash A, Gupta S, Wallace MJ, Kim BJ, Yamashita S, Conrad C, Aloia TA, Vauthey JN, Chun YS. Eradication of Missing Liver Metastases After Fiducial Placement. J Gastrointest Surg. 2016;20:1173–1178. doi: 10.1007/s11605-016-3079-1. [DOI] [PubMed] [Google Scholar]
- 86.Margonis GA, Sergentanis TN, Ntanasis-Stathopoulos I, Andreatos N, Tzanninis IG, Sasaki K, Psaltopoulou T, Wang J, Buettner S, Papalois ΑE, He J, Wolfgang CL, Pawlik TM, Weiss MJ. Impact of Surgical Margin Width on Recurrence and Overall Survival Following R0 Hepatic Resection of Colorectal Metastases: A Systematic Review and Meta-analysis. Ann Surg. 2018;267:1047–1055. doi: 10.1097/SLA.0000000000002552. [DOI] [PubMed] [Google Scholar]
- 87.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, Capussotti L, Vauthey JN. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722, discussion 722-discussion 724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sadot E, Groot Koerkamp B, Leal JN, Shia J, Gonen M, Allen PJ, DeMatteo RP, Kingham TP, Kemeny N, Blumgart LH, Jarnagin WR, DʼAngelica MI. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Ann Surg. 2015;262:476–483; discussion 483-485. doi: 10.1097/SLA.0000000000001427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller CL, Taylor MS, Qadan M, Deshpande V, Worthington S, Smalley R, Collura C, Ryan DP, Allen JN, Blaszkowsky LS, Clark JW, Murphy JE, Parikh AR, Berger D, Tanabe KK, Lillemoe KD, Ferrone CR. Prognostic Significance of Surgical Margin Size After Neoadjuvant FOLFOX and/or FOLFIRI for Colorectal Liver Metastases. J Gastrointest Surg. 2017;21:1831–1840. doi: 10.1007/s11605-017-3557-0. [DOI] [PubMed] [Google Scholar]
- 90.Wang J, Margonis GA, Amini N, Andreatos N, Yuan C, Damaskos C, Antoniou E, Garmpis N, Buettner S, Barbon C, Deshwar A, He J, Burkhart R, Pawlik TM, Wolfgang CL, Weiss MJ. The Prognostic Value of Varying Definitions of Positive Resection Margin in Patients with Colorectal Cancer Liver Metastases. J Gastrointest Surg. 2018;22:1350–1357. doi: 10.1007/s11605-018-3748-3. [DOI] [PubMed] [Google Scholar]
- 91.Andreou A, Aloia TA, Brouquet A, Dickson PV, Zimmitti G, Maru DM, Kopetz S, Loyer EM, Curley SA, Abdalla EK, Vauthey JN. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg. 2013;257:1079–1088. doi: 10.1097/SLA.0b013e318283a4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN Americas Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 2013;15:91–103. doi: 10.1111/j.1477-2574.2012.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 94.Omichi K, Yamashita S, Cloyd JM, Shindoh J, Mizuno T, Chun YS, Conrad C, Aloia TA, Vauthey JN, Tzeng CD. Portal Vein Embolization Reduces Postoperative Hepatic Insufficiency Associated with Postchemotherapy Hepatic Atrophy. J Gastrointest Surg. 2018;22:60–67. doi: 10.1007/s11605-017-3467-1. [DOI] [PubMed] [Google Scholar]
- 95.Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C, Andreou A, Loyer EM, Madoff DC, Curley SA, Vauthey JN. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083–1090. doi: 10.1200/JCO.2010.32.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 97.Demisse R, Damle N, Kim E, Gong J, Fakih M, Eng C, Oesterich L, McKenny M, Ji J, Liu J, Louie R, Tam K, Gholami S, Halabi W, Monjazeb A, Dayyani F, Cho M. Neoadjuvant Immunotherapy-Based Systemic Treatment in MMR-Deficient or MSI-High Rectal Cancer: Case Series. J Natl Compr Canc Netw. 2020;18:798–804. doi: 10.6004/jnccn.2020.7558. [DOI] [PubMed] [Google Scholar]
- 98.Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C, Beets GL, Snaebjornsson P, Maas M, Mertz M, Veninga V, Bounova G, Broeks A, Beets-Tan RG, de Wijkerslooth TR, van Lent AU, Marsman HA, Nuijten E, Kok NF, Kuiper M, Verbeek WH, Kok M, Van Leerdam ME, Schumacher TN, Voest EE, Haanen JB. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26:566–576. doi: 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 99.Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA Jr. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dizon DS, Schwartz J, Kemeny N. Regional chemotherapy: a focus on hepatic artery infusion for colorectal cancer liver metastases. Surg Oncol Clin N Am. 2008;17:759–771, viii. doi: 10.1016/j.soc.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 101.Bischof DA, Clary BM, Maithel SK, Pawlik TM. Surgical management of disappearing colorectal liver metastases. Br J Surg. 2013;100:1414–1420. doi: 10.1002/bjs.9213. [DOI] [PubMed] [Google Scholar]
- 102.Groot Koerkamp B, Sadot E, Kemeny NE, Gönen M, Leal JN, Allen PJ, Cercek A, DeMatteo RP, Kingham TP, Jarnagin WR, D'Angelica MI. Perioperative Hepatic Arterial Infusion Pump Chemotherapy Is Associated With Longer Survival After Resection of Colorectal Liver Metastases: A Propensity Score Analysis. J Clin Oncol. 2017;35:1938–1944. doi: 10.1200/JCO.2016.71.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, Mambrini A, Montagnani F, Alessandroni P, Catalano V, Coschiera P. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) vs intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 2012;32:1387–1395. [PubMed] [Google Scholar]
- 104.Martin RC 2nd, Scoggins CR, Tomalty D, Schreeder M, Metzger T, Tatum C, Sharma V. Irinotecan drug-eluting beads in the treatment of chemo-naive unresectable colorectal liver metastasis with concomitant systemic fluorouracil and oxaliplatin: results of pharmacokinetics and phase I trial. J Gastrointest Surg. 2012;16:1531–1538. doi: 10.1007/s11605-012-1892-8. [DOI] [PubMed] [Google Scholar]
- 105.Martin RC 2nd, Scoggins CR, Schreeder M, Rilling WS, Laing CJ, Tatum CM, Kelly LR, Garcia-Monaco RD, Sharma VR, Crocenzi TS, Strasberg SM. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer. 2015;121:3649–3658. doi: 10.1002/cncr.29534. [DOI] [PubMed] [Google Scholar]
- 106.Fiorentini G, Sarti D, Nani R, Aliberti C, Fiorentini C, Guadagni S. Updates of colorectal cancer liver metastases therapy: review on DEBIRI. Hepat Oncol. 2020;7:HEP16. doi: 10.2217/hep-2019-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Datta J, Narayan RR, Kemeny NE, D'Angelica MI. Role of Hepatic Artery Infusion Chemotherapy in Treatment of Initially Unresectable Colorectal Liver Metastases: A Review. JAMA Surg. 2019;154:768–776. doi: 10.1001/jamasurg.2019.1694. [DOI] [PubMed] [Google Scholar]
- 108.Chiorean EG, Nandakumar G, Fadelu T, Temin S, Alarcon-Rozas AE, Bejarano S, Croitoru AE, Grover S, Lohar PV, Odhiambo A, Park SH, Garcia ER, Teh C, Rose A, Zaki B, Chamberlin MD. Treatment of Patients With Late-Stage Colorectal Cancer: ASCO Resource-Stratified Guideline. JCO Glob Oncol. 2020;6:414–438. doi: 10.1200/JGO.19.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vera R, González-Flores E, Rubio C, Urbano J, Valero Camps M, Ciampi-Dopazo JJ, Orcajo Rincón J, Morillo Macías V, Gomez Braco MA, Suarez-Artacho G. Multidisciplinary management of liver metastases in patients with colorectal cancer: a consensus of SEOM, AEC, SEOR, SERVEI, and SEMNIM. Clin Transl Oncol. 2020;22:647–662. doi: 10.1007/s12094-019-02182-z. [DOI] [PubMed] [Google Scholar]
- 110.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 111.Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Teh C, Tejpar S, Van Cutsem E, Vauthey JN, Påhlman L of the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) group. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41:729–741. doi: 10.1016/j.ctrv.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 112.Schwarz RE, Berlin JD, Lenz HJ, Nordlinger B, Rubbia-Brandt L, Choti MA. Systemic cytotoxic and biological therapies of colorectal liver metastases: expert consensus statement. HPB (Oxford) 2013;15:106–115. doi: 10.1111/j.1477-2574.2012.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]