Abstract
Simple Summary
Gastric resection with D2 lymphadenectomy is considered the gold standard for the treatment of both advanced and early gastric cancer with lymph node metastasis. The performance of D2 lymphadenectomy is technically challenging and represents a key factor in improving patients’ survival. For these reasons, the execution of gastrectomy with D2 lymphadenectomy using the traditional open surgical technique still represents the most widespread approach and, based on current international guidelines, the indication for laparoscopic surgery is limited to early gastric cancer that does not require a D2 lymphadenectomy. The present study aimed to investigate the use of laparoscopic versus open surgical approaches in performing gastrectomy with D2 lymphadenectomy for cancer in terms of intraoperative and postoperative outcomes and long-term survival. The study was conducted using the data collected in the International study group on Minimally Invasive surgery for Gastric Cancer (IMIGASTRIC) international database.
Abstract
Background: The laparoscopic approach in gastric cancer surgery is being increasingly adopted worldwide. However, studies focusing specifically on laparoscopic gastrectomy with D2 lymphadenectomy are still lacking in the literature. This retrospective study aimed to compare the short-term and long-term outcomes of laparoscopic versus open gastrectomy with D2 lymphadenectomy for gastric cancer. Methods: The protocol-based, international IMIGASTRIC (International study group on Minimally Invasive surgery for Gastric Cancer) registry was queried to retrieve data on patients undergoing laparoscopic or open gastrectomy with D2 lymphadenectomy for gastric cancer with curative intent from January 2000 to December 2014. Eleven predefined, demographical, clinical, and pathological variables were used to conduct a 1:1 propensity score matching (PSM) analysis to investigate intraoperative and recovery outcomes, complications, pathological findings, and survival data between the two groups. Predictive factors of long-term survival were also assessed. Results: A total of 3033 patients from 14 participating institutions were selected from the IMIGASTRIC database. After 1:1 PSM, a total of 1248 patients, 624 in the laparoscopic group and 624 in the open group, were matched and included in the final analysis. The total operative time (median 180 versus 240 min, p < 0.0001) and the length of the postoperative hospital stay (median 10 versus 14.8 days, p < 0.0001) were longer in the open group than in the laparoscopic group. The conversion to open rate was 1.9%. The proportion of patients with in-hospital complications was higher in the open group (21.3% versus 15.1%, p = 0.004). The median number of harvested lymph nodes was higher in the laparoscopic approach (median 32 versus 28, p < 0.0001), and the proportion of positive resection margins was higher (p = 0.021) in the open group (5.9%) than in the laparoscopic group (3.2%). There was no significant difference between the groups in five-year overall survival rates (77.4% laparoscopic versus 75.2% open, p = 0.229). Conclusion: The adoption of the laparoscopic approach for gastric resection with D2 lymphadenectomy shortened the length of hospital stay and reduced postoperative complications with respect to the open approach. The five-year overall survival rate after laparoscopy was comparable to that for patients who underwent open D2 resection. The types of surgical approaches are not independent predictive factors for five-year overall survival.
Keywords: gastric cancer, laparoscopy, minimally invasive surgery
1. Introduction
Gastric cancer is the fifth most common malignancy worldwide and the third leading cause of cancer death globally [1].
Gastrectomy with adequate lymphadenectomy still represents the treatment of choice to obtain radical resection and achieve better survival outcomes in the case of resectable gastric cancer.
Based on the current international guidelines, D2 lymphadenectomy should be performed in the event of T2-4aN0-3M0 stage gastric cancer [2].
The laparoscopic approach in gastric cancer surgery was first described in 1994 to perform distal gastrectomy for early gastric cancer [3].
The current Japanese guidelines for gastric cancer treatments consider laparoscopic surgery as an option to treat cStage I cancer that is resectable with a distal gastrectomy and indicate that, for advanced gastric cancer, some concern still exists based on the available evidence in the literature, mainly concerning the survival endpoints with respect to the open approach, particularly for total gastrectomy [2].
However, primarily thanks to the well-established benefits of laparoscopy in early postoperative outcomes, the laparoscopic approach is also being increasingly adopted for the performance of gastrectomy with D2 lymphadenectomy [4].
Randomized controlled trials (RCTs) represent the gold standard of medical evidence for assessing the efficacy and safety of therapeutic interventions. To date, many published RCTs [5,6,7,8,9,10,11,12] have specifically investigated the laparoscopic versus open D2 lymphadenectomy, but only two of these studies reported data on five-year survival; yet, these studies included only distal gastrectomies [7] or a small proportion of total gastrectomies [10]. The other RCTs on laparoscopic D2 lymphadenectomy, which did not assess five-year survival data, focused only on distal gastrectomy [6,12] or included only a small or very small proportion of total gastrectomy procedures in their analysis [5,8,9,11].
Observational studies based on large databases may notably represent an acceptable methodological alternative to RCTs if propensity score matching is applied to reduce inference biases and distortions potentially introduced by uncontrolled confounders. Some observational large database studies have already been conducted to investigate laparoscopic D2 lymphadenectomy with respect to the open approach [13,14,15,16,17], but these studies were all single-institution studies conducted in eastern countries. Some of these studies also included a proportion of D1 lymphadenectomy procedures and/or a smaller proportion of total gastrectomies or did not perform a case-matched analysis.
Based on this background, the present study aimed to investigate the short-term intraoperative surgical and pathological endpoints as well as postoperative recovery and long-term (five-year) survival outcomes of laparoscopic total and distal gastrectomy with D2 lymphadenectomy for gastric cancer in comparison to the open approach by analyzing data from a large international database (western and eastern centers), the International study group on Minimally Invasive surgery for Gastric Cancer (IMIGASTRIC) database, with the adoption of propensity score matching analysis.
2. Materials and Methods
2.1. Overall Objective and Type of Study
The objective of this retrospective case-matched study was to compare laparoscopic versus open gastrectomy with D2 lymphadenectomy in terms of clinical, surgical, and long-term oncological outcomes using the retrospective data stored in the IMIGASTRIC database (www.imigastric.com (accessed on 20 December 2020)). IMIGASTRIC is a protocol-based, international, multi-institutional registry aimed at retrieving clinical, surgical, and oncological variables of patients undergoing laparoscopic, robotic, or open surgery for gastric cancer [18]. The retrospective data extracted for the present study were shared by a total of 14 institutions worldwide (Chile, China, Germany, Italy, Luxembourg, Turkey, and the USA) that participated in the IMIGASTRIC registry at the time the data extraction was performed (January 2018). The surgical procedures were carried out in the participating institutions from January 2000 to August 2018. During this period, all institutions treated patients with gastric cancer according to international guidelines. The present study was conducted after approval by the institutional review board of each participating institution.
2.2. Inclusion and Exclusion Criteria
Eligible patients were identified from the IMIGASTRIC database based on the following inclusion and exclusion criteria:
Inclusion criteria: (1) histologically proven gastric adenocarcinoma, (2) preoperative staging workup performed by upper endoscopy and/or endoscopic ultrasound, and CT scan, (3) total or distal gastrectomy with D2 lymphadenectomy using the laparoscopic or open surgical approach, (4) treatment with a curative intent in accordance with international guidelines, (5) availability of follow-up data (alive, died, or lost to follow-up), (6) surgery performed in the participating institutions from January 2000 to December 2014.
Exclusion criteria: (1) evidence of metastatic tumor (peritoneal carcinomatosis, liver metastasis, distant lymph node metastasis, Krukenberg tumors, involvement of other organs), (2) American Society of Anesthesiologists (ASA) score ≥4, (3) history of previous abdominal surgery for gastric cancer, (4) synchronous malignancy in other organs, (5) palliative surgery, (6) in situ neoplasms.
2.3. Data Collection and Outcomes
The following demographic, clinical, and pathological variables were recorded for each selected patient: age, sex, body mass index (BMI, kg/m2), ASA score, comorbidities (present/absent), geographic area (east/west), type of gastric resection (total or distal gastrectomy), year of surgery, tumor location (upper, middle, or distal third of the stomach), tumor histology based on the Japanese classification of gastric carcinoma, 3rd English edition [19] (differentiated, poorly differentiated/undifferentiated, and subtypes tubular well-differentiated, tubular moderately differentiated, papillary, mucinous, signet ring cell, poorly differentiated solid/non-solid type, undifferentiated), and tumor pathological TNM (pTNM) stage. Cancer stage was determined according to the AJCC/UICC TNM staging system, 8th edition [20].
The following intraoperative and postoperative findings were considered as the study endpoints: total operative time (minutes), conversion to open surgery rate, number of harvested lymph nodes, R factors (R0, R1, R2), number of metastatic lymph nodes, detailed histological types (tubular well-differentiated, tubular moderately differentiated, papillary, mucinous, signet ring cell, poorly differentiated solid/non-solid type, undifferentiated), postoperative hospital stay (days), number of patients with in-hospital complications, grade of in-hospital complications according to Clavien-Dindo classification [21], the proportion of patients undergoing neoadjuvant chemo or radiotherapy, proportion of in-hospital complications with a severity of grade 3 or more according to the Clavien-Dindo classification (severe complications), number of each in-hospital complication, reoperation for complications, and in-hospital mortality rates. The five-year overall survival was investigated, also stratifying patients according to the AJCC/UICC TNM staging system, 8th edition [20], and regression analysis was carried out to identify relevant predictors for overall survival.
2.4. Statistical Analysis
Categorical variables were summarized using frequencies and percentage values, while continuous variables were summarized using mean values and their relative standard deviation (SD) and median with the relative interquartile range (IQR).
To control for potential confounders, propensity score matching (PSM) was performed to create two treatment groups with a balanced distribution of baseline demographic, clinical, and pathological features. A total of 11 variables were used in a logistic regression model to calculate the propensity score: age, sex, BMI, ASA score, year of surgery (2000–2007/2008–2014), comorbidities (present/absent), geographic area (east/west), type of gastric resection (total or distal gastrectomy), tumor location (upper, middle, or distal third), tumor histology (differentiated or poorly differentiated/undifferentiated), and tumor pathological TNM (pTNM) stage. The proportion of patients who underwent laparoscopic or open surgery in each participating institution was highly variable, and the use of PSM for this specific parameter was considered to be difficult. Patients were matched with a 1:1 ratio using the nearest-neighbor method without replacement and with a caliper of 0.05 of the SD of the logit of the estimated propensity score. Patients who were found to be outside of the caliper were excluded from the analysis. After matching, the balance within groups was evaluated using the overall balance test by Hansen and Bowers and the relative multivariate imbalance measure, L1, proposed by Iacus, King, and Porro.
The chi-square test or Fisher’s exact test, when appropriate, was used to compare categorical variables, and the student’s t-test or the Mann–Whitney non-parametric U test was applied to compare continuous variables.
Overall survival was calculated using the Kaplan–Meier method, and the log-rank test (Mantel–Hanszel) was used to assess differences in five-year overall survival between study groups. Survival analyses were performed for the entire study cohort and for the propensity score-matched cohorts to adjust for significant differences in the clinical and pathological characteristics. Overall survival was calculated from the date of surgery until the date of death or until the date of the last contact. The follow-up and the survival time were reported as the median and interquartile range (IQR, 25–75° percentiles).
To investigate potential predictors of overall survival, the Hazard ratio and its relative 95% confidence interval (95% CI) were calculated using the Cox proportional univariate regression model. Then, a multivariate Cox proportional hazard model was generated using stepwise regression (forward selection with the entry limit of p = 0.05 and the remove limit of p = 0.10).
All analyses were two-tailed, and p-values < 0.05 were considered to be statistically significant. Additionally, all analyses were conducted with the “intention to treat” method. All statistical analyses were performed using SPSS software version 23.0 (SPSS Inc., Chicago, IL, USA) and GraphPad software version 6.01 (La Jolla, CA, USA).
3. Results
3.1. Patient Selection
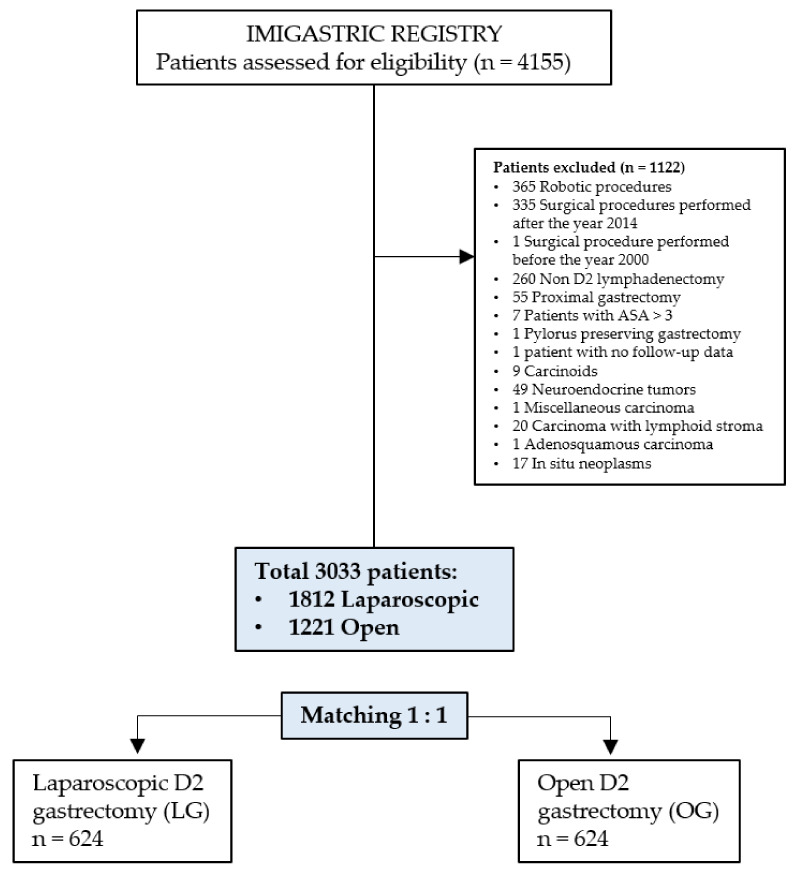
As indicated in Figure 1, a total of 4155 patients affected by gastric adenocarcinoma and registered in the IMIGASTRIC registry underwent total or distal gastrectomy and were therefore considered for inclusion in the study. After the application of the predefined inclusion and exclusion criteria, a total of 1122 patients were excluded (reasons for exclusion are indicated in Figure 1). Finally, a total of 3033 patients were included in the study, of whom 1812 underwent laparoscopic D2 gastric resection and 1221 underwent open D2 gastric resection for gastric adenocarcinoma. After 1:1 matching using the propensity score, two well-balanced groups, one composed of 624 patients undergoing laparoscopic D2 gastrectomy and one 624 patients undergoing open D2 gastrectomy, were selected for data analysis.
Figure 1.
Study flow-chart.
3.2. Patient Characteristics
Considering the entire patient cohort before the matching procedure and comparing the two study groups, a statistically significant difference was found in the distribution of gender, geographic area, year of surgery, ASA score, comorbidities, tumor location, pTNM stage, and histology, and in terms of average BMI between the laparoscopic and open approach groups (Supplementary Table S1). After statistical matching, the two study groups were found to be well balanced for all considered clinical, surgical, and demographic variables, as indicated in Table 1.
Table 1.
Clinical, surgical, and demographic patient characteristics by surgical technique compared in the matched cohort.
| Matched Cohort | |||
|---|---|---|---|
| Laparoscopic n = 624 |
Open n = 624 |
p-Value | |
| Age, years | 0.985 # | ||
| median (IQR) | 63 (6–72) | 64 (56–72) | |
| mean (SD) | 63.2 (11.3) | 63 (11.9) | |
| Gender, n (%) | 0.849 | ||
| Male | 466 (73.1) | 453 (72.6) | |
| Female | 168 (26.9) | 171 (27.4) | |
| Geographic area, n (%) | 0.829 | ||
| East | 504 (80.8) | 507 (81.3) | |
| West | 120 (19.2) | 117 (18.8) | |
| Year of surgery, n (%) | 1.000 | ||
| 2000–2007 | 16 (2.6) | 16 (2.6) | |
| 2008–2014 | 608 (97.4) | 608 (97.4) | |
| Body Mass Index (BMI) | 0.350 # | ||
| median (IQR) | 22 (20–24) | 22 (20–24) | |
| mean (SD) | 22.3 (3.1) | 22.1 (3.3) | |
| ASA score, n (%) | 1.000 | ||
| I | 295 (47.3) | 295 (47.3) | |
| II | 245 (39.3) | 245 (39.3) | |
| III | 84 (13.5) | 84 (13.5) | |
| Comorbidities, n (%) | 0.816 | ||
| No | 388 (62.2) | 384 (61.5) | |
| Yes | 236 (37.8) | 240 (38.5) | |
| Type of resection, n (%) | 0.955 | ||
| Distal gastrectomy | 293 (47) | 294 (47.1) | |
| Total gastrectomy | 331 (53) | 330 (52.9) | |
| Tumor location, n (%) | 0.731 | ||
| Distal third | 290 (46.5) | 284 (45.5) | |
| Middle third | 149 (23.9) | 161 (25.8) | |
| Upper third | 185 (29.6) | 179 (28.7) | |
| pTNM AJCC stage, 8th edition, n (%) | 0.860 | ||
| IA | 101 (16.2) | 91 (14.6) | |
| IB | 49 (7.9) | 43 (6.9) | |
| IIA | 67 (10.7) | 69 (11.1) | |
| IIB | 73 (11.7) | 68 (10.9) | |
| IIIA | 112 (17.9) | 117 (18.8) | |
| IIIB | 120 (19.2) | 117 (18.8) | |
| IIIC | 102 (16.3) | 119 (19.1) | |
| Histology, n (%) | 0.767 | ||
| Differentiated | 405 (64.9) | 400 (64.1) | |
| Poorly differentiated/undifferentiated | 219 (35.1) | 224 (35.9) | |
LG: laparoscopic gastrectomy; GO: open gastrectomy; n = number; # = Mann–Whitney U test; SD: standard deviation; IQR: interquartile range.
3.3. Operative Outcomes
The operating findings, postoperative complications, and pathology data analyzed comparing the two study groups and considering both the matched cohort and the entire patient cohort are summarized in Table 2 and in Supplementary Table S2, respectively. In this section, only the results of the comparison of the two study groups for the matched cohort of patients are reported.
Table 2.
Operating findings, postoperative complications, and pathology by surgical technique compared in the matched cohort.
| Matched Cohort | |||
|---|---|---|---|
| LG n = 624 |
OG n = 624 |
p-Value | |
| Total operative time (minutes) | <0.0001 # | ||
| Median (IQR) | 180 (150–210) | 240 (180–300) | |
| Mean (SD) | 192.7 (72.6) | 243.7 (86.5) | |
| Conversion to open surgery, n (%) | 12 (1.9) | N/A | N/A |
| N. harvested lymph nodes | <0.0001 # | ||
| Median (IQR) | 32 (24–40) | 28 (22–38) | |
| Mean (SD) | 33.2 (12.6) | 31.3 (14.1) | |
| N. metastatic lymph nodes | 0.400 # | ||
| Median (IQR) | 3 (0–11) | 3 (0–11) | |
| Mean (SD) | 7 (9.1) | 7.6 (10.8) | |
| R factors | 0.021 | ||
| R0 | 604 (96.8) | 587 (94.1) | |
| R + (R1 − R2) | 20 (3.2) | 37 (5.9) | |
| Neoadjuvant chemotherapy, n (%) | 0.493 | ||
| Yes | 20 (3.2) | 15 (2.4) | |
| No | 604 (96.8) | 609 (97.6) | |
| Neoadjuvant radiotherapy, n (%) | 1.000 | ||
| Yes | 1 (0.2) | 0 (0) | |
| No | 623 (99.8) | 624 (100) | |
| Histology types in detail, n (%) | 0.767 | ||
| Differentiated (total) | 405 (64.9) | 400 (64.1) | |
| Tubular well-differentiated | 358 (57.4) | 301 (48.2) | |
| Tubular moderately differentiated | 40 (6.4) | 91 (14.6) | |
| Papillary | 7 (1.1) | 8 (1.3) | |
| Poorly differentiated/Undifferentiated (total) | 219 (35.1) | 224 (35.9) | |
| Mucinous | 69 (11.1) | 50 (8) | |
| Signet ring cell | 97 (15.5) | 96 (15.4) | |
| Poorly differentiated solid/non-solid type * | 43 (6.9) | 76 (12.2) | |
| Undifferentiated | 10 (1.6) | 2 (0.3) | |
| Postoperative hospital stay (days) | <0.0001 # | ||
| Median (IQR) | 10 (9–13) | 14.8 (10–17) | |
| Mean (SD) | 12.2 (8.2) | 13 (7.6) | |
| Patients with complications, n (%) | 94 (15.1) | 133 (21.3) | 0.004 |
| Clavien-Dindo complications, n (%) | 0.433 | ||
| I | 9 (6.4) | 16 (9.3) | |
| II | 106 (74.6) | 121 (70) | |
| IIIa | 8 (5.6) | 7 (4) | |
| IIIb | 8 (5.6) | 17 (9.8) | |
| IVa | 8 (5.6) | 10 (5.8) | |
| IVb | 2 (1.4) | 0 (0) | |
| V | 1 (0.8) | 2 (1.1) | |
| Total | 142 (100) | 173 (100) | |
| Severe in-hospital complications, n (%) | 0.689 | ||
| Clavien-Dindo ≥ 3 | 27 (19) | 36 (20.8) | |
| Reoperation, n of patients (%) | 10 (1.6) | 17 (2.7) | 0.392 |
| In-hospital mortality, n (%) | 1 (0.2) | 2 (0.3) | 0.563 |
OG: open gastrectomy; LG: laparoscopic gastrectomy; n = number; # = Mann–Whitney U test; ml: milliliter; N/A: not applicable; IQR: interquartile range; SD: standard deviation; * only 2 patients with poorly differentiated: non-solid type (por2).
In the matched cohort, the total operative time was significantly longer in the open group than in the laparoscopic group (median 180 versus 240 min, p < 0.0001). The laparoscopic to open conversion rate was 1.9% (total of 12 procedures converted to open surgery).
The median number of harvested lymph nodes was significantly higher in the laparoscopic group than in the open group (median 32 versus 28 harvested lymph nodes, p < 0.0001) but the median number of metastatic lymph nodes was similar in the two groups (median 3 metastatic lymph nodes in both study groups, p = 0.400).
The proportion of positive resections (R1 or R2) was significantly higher (p = 0.021) in the open group (5.9%) than in the laparoscopic group (3.2%).
No significant differences in terms of the proportion of patients undergoing neoadjuvant chemotherapy (p = 0.493) or neoadjuvant radiotherapy (p = 1.000) were found comparing the two study groups.
3.4. Histology
The distribution of the tumor histology types between the two study groups was similar (p = 0.767). In both study groups, the tubular well-differentiated type represented the most common histotype among the differentiated tumors, and the signet ring cell histotype was the most common histotype among the poorly differentiated/undifferentiated tumors.
3.5. Post-Operative Outcomes
In the laparoscopic group, a significantly shorter length of postoperative hospital stay was found than in the open group (median 10 versus 14.8 days, p < 0.0001).
The proportion of patients with at least one in-hospital complication was significantly higher in the open group than in the laparoscopic group (21.3% versus 15.1%, p = 0.004). The distribution of in-hospital complications classified with the Clavien–Dindo score was similar between the laparoscopic and open groups (p = 0.433), as was the proportion of severe (Clavien–Dindo grade ≥3) in-hospital complications (19% versus 20.8%, p = 0.686).
No significant differences were found when comparing the laparoscopic and open approaches in terms of the proportion of reoperation rates for in-hospital complications (1.6% versus 2.7%, p = 0.392) and in terms of in-hospital mortality rates (0.2% versus 0.3%, p = 0.563).
In Table 3, the number of in-hospital complications are reported in detail for the matched study cohort. In the matched cohort, a statistically significant difference was found in the proportion of postoperative in-hospital complications between the laparoscopic and open surgical approaches only in terms of pneumonia (p = 0.003), which was higher in the open group (9.8%) than in the laparoscopic group (5.3%). No significant differences were found for the remaining postoperative complications when comparing the two study groups.
Table 3.
In-hospital complications in the matched cohort.
| Matched Cohort | |||
|---|---|---|---|
| Laparoscopic n = 624 |
Open n = 624 |
p-Value | |
| Acute pancreatitis | 0 | 0 | 1.000 |
| Acute renal failure | 0 | 0 | 1.000 |
| Adhesive ileus | 0 | 0 | 1.000 |
| Anastomotic stenosis | 2 | 0 | 0.499 |
| Anostomosis leakage | 12 | 12 | 1.000 |
| Arrhythmias | 4 | 4 | 1.000 |
| Atelectasia | 1 | 0 | 1.000 |
| Bleeding (intra/extraluminal) | 9 | 10 | 1.000 |
| Cholecystitis | 0 | 0 | 1.000 |
| Chylous leakage | 6 | 8 | 0.789 |
| Congestive heart failure | 3 | 1 | 0.624 |
| Cerebrovascular accident | 0 | 0 | 1.000 |
| Deep vein thrombosis | 2 | 0 | 0.499 |
| Delayed gastric emptying | 8 | 7 | 1.000 |
| Delirium | 0 | 0 | 1.000 |
| Disseminated intravascular coagul. | 1 | 0 | 1.000 |
| Dizziness | 0 | 0 | 1.000 |
| Dumping syndrome | 0 | 0 | 1.000 |
| Intra-abdominal fluid collection | 14 | 9 | 0.400 |
| Incisional hernia | 1 | 2 | 1.000 |
| Liver failure | 0 | 0 | 1.000 |
| Myocardial infarction | 1 | 0 | 1.000 |
| Omental infarction | 0 | 1 | 1.000 |
| Pancreatic fistula | 2 | 6 | 0.287 |
| Pleural effusion | 3 | 2 | 1.000 |
| Pneumonia | 33 | 61 | 0.003 |
| Prolonged postoperative ileus | 1 | 1 | 1.000 |
| Pseudomembranous colitis | 2 | 2 | 1.000 |
| Pulmonary edema | 0 | 1 | 1.000 |
| Pulmonary embolism | 1 | 0 | 1.000 |
| Remnant stomach necrosis | 1 | 0 | 1.000 |
| Sepsis | 1 | 0 | 1.000 |
| Small bowel infarction | 0 | 2 | 0.499 |
| Small bowel perforation | 0 | 3 | 0.249 |
| Unexplained postoperative fever | 2 | 1 | 1.000 |
| Wound infection | 9 | 15 | 0.302 |
| Wound seroma | 0 | 1 | 1.000 |
| Other complications | 23 | 24 | 1.000 |
| Total | 142 | 173 | 0.043 |
p-Values in bold are statistically significant.
3.6. Survival Analysis
In Table 4, the results of the survival analysis in terms of five-year overall survival rates using the Kaplan–Meier method in both the entire and matched study cohort are reported.
Table 4.
Five-year cumulative overall survival (stratified by AJCC pTNM stage, 8th edition) in laparoscopic and open groups.
| N of Patients | Five-Year Survival Rate | p-Value * | |||
|---|---|---|---|---|---|
| Laparoscopy | Open | Laparoscopy % | Open % | ||
| Entire cohort | |||||
| All patients | 1812 | 1221 | 81.1 | 66.4 | <0.0001 |
| Stage IA | 358 | 199 | 97.4 | 93.5 | 0.029 |
| Stage IB | 156 | 130 | 94.2 | 75 | <0.0001 |
| Stage IIA | 191 | 161 | 90.4 | 70.3 | <0.0001 |
| Stage IIB | 213 | 144 | 88.5 | 79 | 0.002 |
| Stage IIIA | 309 | 231 | 78.5 | 56.6 | <0.0001 |
| Stage IIIB | 352 | 195 | 69 | 51 | <0.0001 |
| Stage IIIC | 233 | 161 | 60.1 | 48.5 | 0.001 |
| Matched cohort | |||||
| All patients | 624 | 624 | 77.4 | 75.2 | 0.202 |
| Stage I | 150 | 134 | 96.4 | 94 | 0.432 |
| Stage IA | 101 | 91 | 95 | 98 | 0.189 |
| Stage IB | 49 | 43 | 100 | 82 | 0.011 |
| Stage II | 140 | 137 | 86.7 | 83.8 | 0.469 |
| Stage IIA | 67 | 69 | 87 | 77.2 | 0.146 |
| Stage IIB | 73 | 68 | 86.6 | 91 | 0.509 |
| Stage III | 334 | 353 | 67.8 | 65 | 0.264 |
| Stage IIIA | 112 | 117 | 74.8 | 74.6 | 0.679 |
| Stage IIIB | 120 | 117 | 69 | 63.7 | 0.406 |
| Stage IIIC | 102 | 119 | 59.5 | 58.2 | 0.621 |
* Log-rank test.
The follow-up duration in the entire study cohort was a median of 62 (IQR 38–78) months and a median of 60 (IQR 35–75) months in the matched study cohort. In the laparoscopic matched cohort, the median follow-up time was 63 (IQR 44–77) months, whereas in the open group the median follow-up time was 57 (IQR 24–72) months. The proportion of patients lost to follow-up was 9.2% and 14.1% in the entire and in the matched cohort, respectively. In the matched cohort, the median observation time in the population lost to follow-up was 21 (IQR 12–30) months in the laparoscopic group and 14 (IQR 2–57) months in the open group.
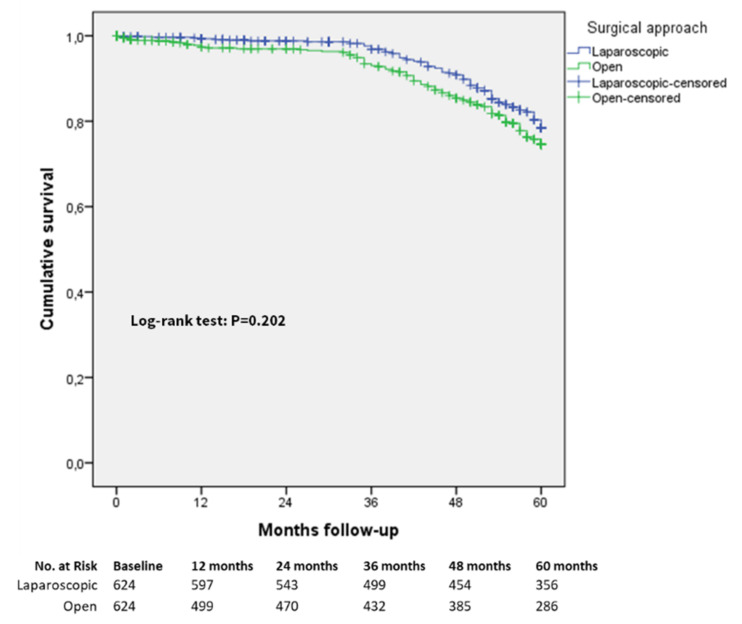
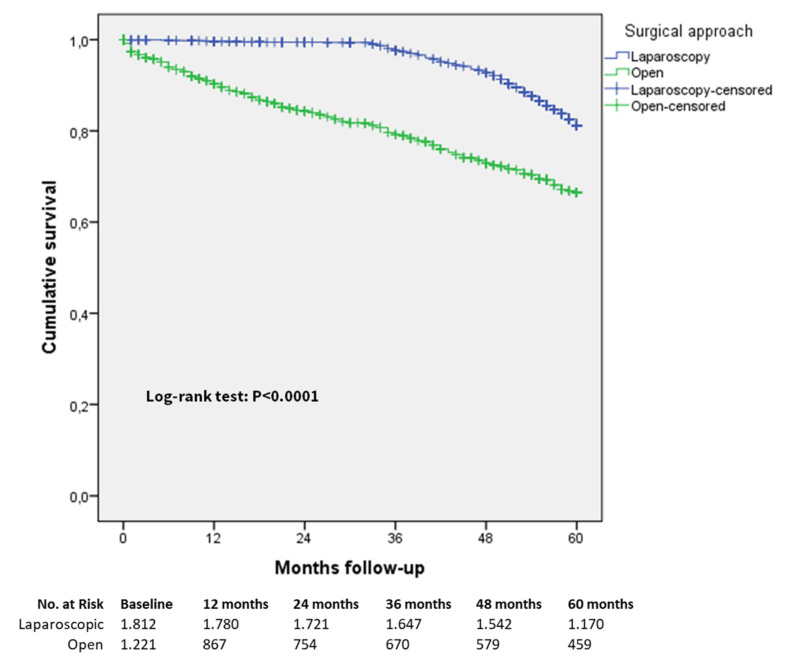
The comparison of five-year overall survival rates in the laparoscopic and open groups considering all included patients irrespective of cancer staging in the matched group did not reach a statistically significant difference, as indicated in Figure 2 (rate of 77.4% versus 75.2%, respectively, log-rank (Mantel–Cox) test p = 0.202) with a mean survival time in the laparoscopic group of 79.26 (95% CI 76.90 to 81.62) months versus 81.99 (95% CI 76.62 to 87.36) months in the open group. A total of 493 patients died during the follow-up in the matched cohort, of whom 251 were in the laparoscopic group and 242 were in the open group. In Figure 3, the comparison of five-year overall survival rates between the laparoscopic and open groups in the entire study cohort are shown, with a significant survival advantage for the laparoscopic group (rate of 81.1% versus 66.4%, log-rank (Mantel–Cox) test p < 0.0001).
Figure 2.
Five-year overall survival, matched cohort (all patients).
Figure 3.
Five-years overall survival, entire cohort (all patients).
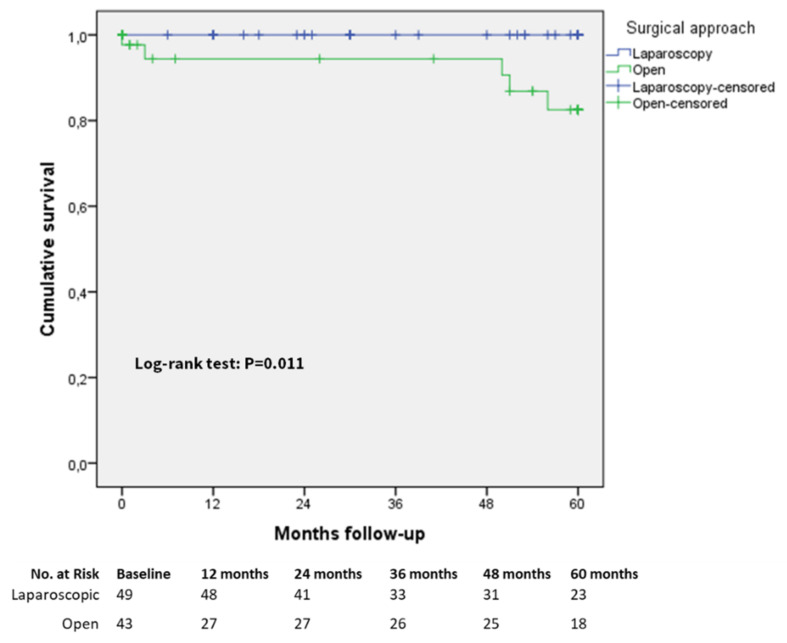
The five-year overall survival rates did not significantly differ in the matched group between the laparoscopic and open approaches, also considering separately each cancer stage as indicated in Table 4 and in Supplementary Figures S1–S9, except for the patients with cancer stage IB (Figure 4), for which five-year survival rates were 100% versus 82% in the laparoscopic and open groups, respectively (Log-rank (Mantel–Cox) test p = 0.011).
Figure 4.
Five-year overall survival matched cohort in stage IB patients.
As indicated in Table 5, the univariate analysis showed that the age, gender, geographic area, year of surgery, BMI, ASA score, operative time, type of surgical approach, length of hospital stay, type of resection, tumor location, number of harvested lymph nodes, margin status, TNM stage, number of metastatic lymph nodes, and histology were significantly associated with overall survival. The stepwise multivariable Cox regression analysis showed that the following variables were predictive factors for overall survival: age (HR 1.019, 95% CI 1.011 to 1.027), geographic area considering the “east” geographic area as a reference parameter (west, HR 6.482, 95% CI 5.322–7.895), ASA score, considering as a reference parameter ASA score I (ASA score II, HR 1.273, 95% CI 1.065–1.521), tumor location, considering as a reference parameter the proximal third (distal third, HR 0.772, 95% CI 0.633–0.941), TNM stage, considering as a reference parameter stage IIIC (stage IA, HR 0.057, 95% CI 0.032–0.102; stage IB, HR 0.171, 95% CI 0.102–0.288, stage IIA, HR 0.261, 95% CI 0.165–0.412; stage IIB, HR 0.282, 95% CI 0.17–0.443; stage IIIA, HR 0.645 95% CI 0.454–0.916), and the number of metastatic lymph nodes (HR 1.018 95% CI 1.006–1.030).
Table 5.
Univariate and multivariate Cox regression for five-year overall survival, entire cohort.
| Overall Cohort | Univariable Cox Regression | Multivariable Cox Regression | ||||
|---|---|---|---|---|---|---|
| N | Hazard Rate (95% CI) | p-Value | Hazard Rate (95% CI) | p-Value | ||
| Age, years | 3033 | 1.029 (1.021–1.036) | <0.0001 | 1.019 (1.011–1.027) | <0.0001 | |
| Gender | ||||||
| Male | 2165 | Reference | ||||
| Female | 868 | 1.249 (1.050–1.485) | 0.012 | |||
| Geographic area | ||||||
| East | 2187 | Reference | Reference | |||
| West | 846 | 3.718 (3.137–4.406) | <0.0001 | 6.482 (5.322–7.895) | <0.0001 | |
| Year of surgery | ||||||
| 2000–2007 | 433 | Reference | ||||
| 2008–2014 | 2600 | 0.342 (0.281–0.415) | <0.0001 | |||
| Body mass index (BMI) | 3033 | 1.027 (1.004–1.050) | 0.021 | |||
| ASA score | ||||||
| I | 1508 | Reference | Reference | |||
| II | 1211 | 1.674 (1.413–1.982) | <0.0001 | 1.273 (1.065–1.521) | 0.008 | |
| III | 314 | 2.387 (1.789–3.184) | <0.0001 | 1.338 (0.980–1.827) | 0.067 | |
| Operative time | 3033 | 1.002 (1.001–1.003) | <0.0001 | |||
| Surgical approach | ||||||
| Open | 1221 | Reference | ||||
| Laparoscopic | 1812 | 0.426 (0.363–0.500) | <0.0001 | |||
| Length of hospital stay | 3033 | 1.010 (1.001–1.018) | 0.012 | |||
| Type of resection | ||||||
| Distal gastrectomy | 1365 | Reference | ||||
| Total gastrectomy | 1668 | 2.011 (1.685–2.400) | <0.0001 | |||
| Tumor location | ||||||
| Upper third | 894 | Reference | Reference | |||
| Middle third | 819 | 0.968 (0.798–1.174) | 0.740 | 0.859 (0.707–1.045) | 0.129 | |
| Distal third | 1320 | 0.519 (0.427–0.632) | <0.0001 | 0.772 (0.633–0.941) | 0.011 | |
| N. harvested lymph nodes | 3033 | 1.009 (1.003–1.015) | 0.003 | |||
| Margin status | ||||||
| Free | 2939 | Reference | ||||
| Infiltrated | 94 | 3.072 (2.192–4.306) | <0.0001 | |||
| pTNM stage, 8th edition | ||||||
| IIIC | 394 | Reference | Reference | |||
| IIIB | 547 | 0.809 (0.651–1.006) | 0.056 | 0.810 (0.604–1.805) | 0.157 | |
| IIIA | 540 | 0.657 (0.521–0.827) | <0.0001 | 0.645 (0.454–0.916) | 0.014 | |
| IIB | 357 | 0.305 (0.217–0.429) | <0.0001 | 0.282 (0.179–0.443) | <0.0001 | |
| IIA | 352 | 0.345 (0.248–0.480) | <0.0001 | 0.261 (0.165–0.412) | <0.0001 | |
| IB | 286 | 0.258 (0.173–0.386) | <0.0001 | 0.171 (0.102–0.288) | <0.0001 | |
| IA | 557 | 0.073 (0.045–0.118) | <0.0001 | 0.057 (0.032–0.102) | 0.0004 | |
| N. of metastatic lymph nodes | 3111 | 1.040 (1.034–1.045) | 0.039 | <0.0001 | 1.018 (1.006–1.030) | 0.004 |
| Histology, n (%) | ||||||
| Tubular, Well-diff. (tub1) | 1596 | Reference | ||||
| Tubular, mod. diff. (tub2) | 368 | 3.331 (2.541–4.367) | <0.0001 | |||
| Papillary (pap) | 51 | 1.535 (0.840–2.805) | 0.163 | |||
| Mucinous (muc) | 236 | 1.422 (1.062–1.905) | 0.018 | |||
| Signet ring cell (sig) | 484 | 1.801 (1.452–2.235) | <0.0001 | |||
| Poorly diff. solid/non-solid (por1/2) * | ||||||
| Undifferentiated | 275 | 4.573 (3.481–6.008) | <0.0001 | |||
| carcinoma | 23 | 3.907 (1.933–7.897) | <0.0001 | |||
N: number of patients; CI: confidence interval; B: beta coefficient; * poorly differentiated: non-solid type (por2) only 10 patients.
4. Discussion
4.1. Study Findings (Short-Term Outcomes)
The present multicenter, registry-based study enrolled a total of 3033 patients representing, to date, the larger comparative study investigating laparoscopic versus open D2 gastrectomy for cancer.
By adopting the case-matched study design, we were able to compare clinical, pathological, and survival outcomes in a total of 1248 patients, 624 in each study group, who were selected from the IMIGASTRIC registry according to 11 predefined covariates to control for possible confounders.
Our study indicates that the laparoscopic approach for total or distal gastrectomy with D2 lymphadenectomy is capable of significantly reducing, with respect to the open approach, the total operating time (median 180 versus 240 min respectively, p < 0.0001), the length of postoperative hospital stay (median 10 versus 14.8 days, respectively, p < 0.0001) as well as the number of postoperative in-hospital complications (142 versus 173, respectively, p = 0.043) and the proportion of patients with at least one in-hospital complication (15.1% versus 21.3%, respectively, p = 0.004), despite the occurrence of postoperative severe complications, based on the Clavien–Dindo classification, with results similar between the open and laparoscopic approaches (19% versus 20.8%, respectively, p = 0.686).
No significant differences were found in terms of in-hospital mortality and reoperation rates. The rates of in-hospital mortality found in our analysis were quite low if compared to those reported in other large multicenter studies on gastric cancer surgery, such as the German Gastric Cancer Study 2 (QCGC 2) [22], in which the hospital mortality rate was overall 5.8% in the included high surgical volume centers. We can explain this wide variation by the fact that a large proportion of patients included in our analysis were treated in selected, very high volume centers dedicated to gastric cancer treatment that are mainly located in the east, particularly in China. The in-hospital mortality reported by the eastern high volume gastric cancer centers is notably very low as confirmed by the findings of a recent Cochrane review on randomized controlled trials, mainly conducted in the east, indicating an overall short-term mortality rate of about 0.5% after laparoscopic or open gastrectomy for cancer [23].
4.2. Operative Time
We can argue that the findings of a significantly shorter operative time in the laparoscopic group than in the open group in the present study could be linked with a selection bias derived from the inclusion of less technically demanding cases in the laparoscopic group despite the adoption of a case matching analysis that reduced the influence of some confounders.
Moreover, it should be underlined that in the literature, some other studies investigating open versus laparoscopic gastrectomy for advanced cancer have indicated a shorter operative time in favor of the laparoscopic approach [24,25,26], while other studies have shown no differences between these approaches in terms of operative time [17,27], confirming that the completion of an appropriate learning curve allows for a significant reduction in the operation time in the laparoscopic approach up to matching or overcoming that required for the open approach [28].
4.3. Hospital Stay and Complications
A recent systematic review with a meta-analysis on laparoscopic versus open gastrectomy for advanced cancer confirmed a significant reduction in the length of hospital stay after the laparoscopic approach with a reduction in the postoperative length of stay of about three days [29], which was comparable with our findings. The reduction in the length of hospital stay represents a notable and well-known advantage of laparoscopic surgery and is mainly linked with the minimal gastrointestinal stress and minimal abdominal incision offered by laparoscopy.
Our analysis showed a significant reduction of postoperative in-hospital complications for patients undergoing laparoscopy both in terms of the overall complication rate and the proportion of patients with a least one complication (15.1%); this is in the range of postoperative morbidity reported in the literature focusing on the laparoscopic treatment of advanced gastric cancer, which ranged between 6.3% and 24.2% [12,16,30,31,32,33,34]. By specifically analyzing the proportion of each reported postoperative complication, we found that only the occurrence of pneumonia was significantly higher in the open group. The reduction of pulmonary complications, including pneumonia, is a well-known advantage related to the adoption of laparoscopy, not only in gastric surgery [35].
4.4. Lymphadenectomy
The AJCC 8th TNM-staging guidelines [20] indicate a minimum of 16 lymph nodes to be assessed in radical gastric cancer surgery, with 30 lymph nodes being desirable. Some studies have demonstrated a survival benefit linked with the increased number of harvested lymph nodes, with the maximal survival advantage reached with the dissection of 29 nodes [36,37]. Although the majority of studies comparing gastrectomy with laparoscopic versus open D2 lymphadenectomy found no significant difference in the number of retrieved lymph nodes [4], some studies have indicated significantly more harvested lymph nodes with laparoscopy [24,27,32,38,39,40]. Also in the present study, we found that patients in the laparoscopic group had a significantly higher median number of harvested lymph nodes than in the open group (median of 32 versus 28 harvested lymph nodes, p < 0.0001). This finding could be partly explained by the technical advantages offered by laparoscopy in terms of a magnified view of the surgical field, allowing for finer and deeper tissue dissection, in turn facilitating a more extensive lymphadenectomy than open surgery, in adequately experienced hands [41]. The same technical advantages of laparoscopy could explain the findings of a significantly higher p = 0.021) proportion of positive surgical resection margins in the open (5.9%) versus laparoscopic (3.2%) approaches in the present analysis.
4.5. Long-Term Survival and Related Factors
As previously underlined, only two RCTs on laparoscopic versus open D2 lymphadenectomy reported data on five-year survival, but these studies included only distal gastrectomies [7] or a small proportion of total gastrectomies [10]. The five-year overall survival rates calculated in our study for each cancer stage in the laparoscopic (stage I: 96.4%, stage II: 86.7%, stage III: 67.8%) and open (stage I: 94%, stage II: 83.8%, stage III: 65%) groups, which included well-balanced groups of total and distal gastrectomies, are in line with the survival rate reported in previous observational studies [31,42,43]. In our analysis, only the five-year overall survival rate in the stage IIB patients was significantly higher in the laparoscopic group (100%) than in the open group (82%). However, this finding is probably linked with a type 2 statistical error for this specific subgroup of patients due to the small sample size of the stage IIB patients in the laparoscopic (n = 49) and open (n = 43) groups in the matched study cohort. Overall, our data confirm that the adoption of the laparoscopic approach in gastric cancer resection with D2 lymphadenectomy is capable of providing pathological and long-term survival endpoints comparable to those of the open approach.
This evidence is also supported by the findings of the present study indicating that the surgical approach (laparoscopic or open) was not an independent prognostic factor for five-year overall survival after multivariable regression analysis. Conversely, age, geographic area (west versus east), ASA score, tumor location (distal), pTNM stage, and the number of metastatic lymph nodes were identified as independent prognostic factors for five-year overall survival after univariate and multivariate analysis of the entire cohort of patients. Interestingly, our multivariable regression analysis showed the five-year risk of death increased 6.4 times more in patients treated in the west geographic area than in the east area. A recent survival analysis conducted in the German Gastric Cancer Study 2 (QCGC 2) [22] showed a five-year overall survival rate of 38.7%, calculated on the included high surgical volume centers, which is about half of that obtained in the present study, which included a large proportion of patients treated in very high volume eastern centers. Although the observed differences in five-year survival between eastern and western cohorts can be largely explained by differences in baseline demographic, clinical, and pathological characteristics [44], some studies have indicated the number of lymph nodes examined to be a key contributing factor to the observed survival difference, which was also related to the well-known phenomenon of “stage migration” [45,46]. On the other hand, the number of examined lymph nodes is dependent on some factors, such as the extent of the surgery, the examiner’s technique, fat volume in the specimen, or the innate number of the lymph nodes. Some of these factors, such as the extent of surgery and the pathological examiner’s technique, are correlated with the institution’s surgical case volume and to the consequent surgical expertise that is, in general, greater in eastern centers due to both the higher incidence of gastric cancer and the earlier and longer adoption of the surgical principles of oncological radicality, especially inherent to D2 lymphadenectomy, in eastern rather than in western countries.
In the present study, the multivariable analysis showed that the location of the tumor in the distal portion of the stomach was linked with an improved five-year overall survival of about 23% with respect to the proximal tumor location despite the performance of distal versus total gastrectomy not being an independent predictive factor for overall survival. Similar to our findings, a recent systematic review investigating the prognostic role of primary tumor location in non-metastatic gastric cancer [47] found a 25% increased risk of mortality of proximal compared with distal gastric cancers assuming that this evidence is related to more aggressive biological characteristics of the proximal tumors.
4.6. Strengths and Limitations of the Study
Although this registry-based analysis represents, to date, the study with the largest sample size investigating laparoscopic versus open D2 lymphadenectomy for the treatment of gastric cancer, including a well-represented end-balanced subgroup of patients undergoing total and distal gastrectomy, it has some limitations. First of all, this was a retrospective study and, despite the adoption of the case matching analysis, the risk of unmeasurable bias in the analysis persisted, especially of selection bias in favor of the laparoscopic approach. The patient study cohort was retrieved from the IMIGASTRIC database, which is an international, multi-institutional registry with participating centers located in the east and west geographic areas of the world, with the risk of substantial general heterogeneity in some aspects of gastric cancer treatments. Despite the analyzed data coming from centers located on three different continents (Europe, America, Asia), it should be acknowledged that most of the analyzed surgical procedures were performed in eastern countries and in particular in China; therefore, the results cannot be extrapolated to all gastric cancer patients, in particular from western countries.
It should also be pointed out that the IMIGASTRIC database was established and is maintained based on a dedicated protocol [18] regulating the collection, management, and analysis of data that was shared, between the participating centers, at the beginning of the data collection. The definitions of the clinical, pathological, and surgical parameters selected for storage in the IMIGASTRIC database are specified in the study protocol, allowing for a reduction in the heterogeneity and inconsistency of data analysis. These aspects markedly differentiate the IMIGASTRIC database from the databases created to store administrative data, which are to date increasingly used in clinical research. Studies conducted on these large administrative databases, which are mostly based in the USA (such as the Nationwide Inpatient Sample (NIS) and Medicare database) are burdened by the peculiar bias defined as “coding bias” [48,49].
The assessment of disease-free survival and disease recurrence and patterns would certainly increase the completeness of the survival analysis in the present study. Unfortunately, the data needed to perform these specific survival analyses are not yet available in the IMIGASTRIC registry. However, it should be noted that to date only two RCTs have reported data on five-year survival, but these RCTs included only distal gastrectomy [7] or a small proportion of total gastrectomies [10], and observational studies analyzing five-year overall survival assessed samples of patient smaller than that in our study and/or did not adopt the case-matched design or included only small subgroups of patients undergoing total gastrectomy [14,15,16,41,50].
The patients enrolled in the present study were treated over a rather long time, from January 2000 until December 2014. This could theoretically pose the risk of patients treated in different historical periods having been subjected to different surgical and neoadjuvant/adjuvant treatments. This choice was based on the necessity to collect a sample of patients as large as possible to conduct analysis with satisfactory statistical power and based on the evidence that the guidelines on the surgical and chemo-radiotherapy treatment of gastric cancer, in particular regarding the procedures for performing D2 lymphadenectomy, were published in 1998 [51].
Moreover, the adjuvant chemotherapy treatments did not present, in the time frame identified for the collection of these retrospective data, substantial changes in terms of indications, types of drugs, and the chemo-radiotherapy schemes used. Furthermore, adjuvant treatments (chemo and radiotherapy) for advanced gastric cancer showed only a modest effect in improving the survival of patients (about 7 months in a meta-analysis of 64 randomized studies) [52]. Even the efficacy and indications for the use of neoadjuvant (preoperative) chemotherapy treatment remains highly controversial and is therefore not routinely performed worldwide [53].
5. Conclusions
Our study, despite the aforementioned limits, mainly related to its retrospective nature, suggests that laparoscopic gastric resection with D2 lymphadenectomy for gastric cancer allows the retrieval of better recovery outcomes with respect to the open approach by reducing the hospital stay and the postoperative overall morbidity with no significant differences in terms of short-term pathological outcomes and long-term survival, after both total and distal gastrectomy. The findings of the present study, which contribute to enriching the existing literature on minimally invasive D2 lymphadenectomy, could also be useful for planning further RCTs, which are still strongly needed to definitively assess the benefits and possible harms of laparoscopic gastrectomy with D2 lymphadenectomy with respect to the open approach, particularly in western countries.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13184526/s1, Figure S1: Five-years overall survival, matched cohort in stage I (IA-IB) patients, Figure S2: Five-years overall survival, matched cohort in stage II (IIA-IIB) patients, Figure S3: Five-years overall survival, matched cohort in stage III (IIIA-IIIB-IIIC) patients, Figure S4: Five-years overall survival, matched cohort in Stage IA patients, Figure S5: Five-years overall survival, matched cohort in stage IIA patients, Figure S6: Five-years overall survival, matched cohort in stage IIB patients, Figure S7: Five-years overall survival, matched cohort in stage IIIA patients, Figure S8: Five-years overall survival, matched cohort in stage IIIB patients, Figure S9: Five-years overall survival, matched cohort in stage IIIC patients, Table S1: Clinical, surgical and demographic patients’ characteristics by surgical technique compared in the entire cohort, Table S2: Operating findings and postoperative complications and pathology by surgical technique compared in the entire cohort, Table S3: In hospital complications in the entire cohort.
Author Contributions
Conceptualization, S.T., J.D. and J.-X.L.; methodology, S.T., J.D. and I.T.; software, S.T. and J.D.; validation, I.T., V.D., S.B., C.-M.H. and A.P.; formal analysis, S.T., J.D. and I.T.; investigation, S.T., J.D., J.-X.L., D.R., C.-H.Z., F.B. (Felice Borghi), F.C., E.N., N.T.N., F.Q., A.C., M.C., F.B. (Francesca Bazzocchi), O.A., S.T.B., G.P., S.D., J.-S.A., Y.-B.Z., S.-G.C., E.G., C.M., G.A., F.G., T.L., P.G., F.S., A.M., V.D., S.B., C.-M.H. and A.P.; data curation, S.T., J.D., I.T., J.-X.L., D.R., C.-H.Z., F.B. (Felice Borghi), F.C., E.N., N.T.N., F.Q., A.C., M.C., F.B. (Francesca Bazzocchi), O.A., S.T.B., G.P., S.D., J.-S.A., Y.-B.Z., S.-G.C., E.G., C.M., G.A., F.G., T.L., P.G., F.S., A.M., V.D., S.B., C.-M.H. and A.P.; writing—original draft preparation, S.T. and J.D.; writing—review and editing, S.T., J.D., I.T., J.-X.L., D.R., C.-H.Z., F.B. (Felice Borghi), F.C., E.N., N.T.N., F.Q., A.C., M.C., F.B. (Francesca Bazzocchi), O.A., S.T.B., G.P., S.D., J.-S.A., Y.-B.Z., S.-G.C., E.G., C.M., G.A., F.G., T.L., P.G., F.S., A.M., V.D., S.B., C.-M.H. and A.P.; project administration, A.P. and J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) CEAS Umbria: Prot. No. 4310/14/ON dated 12 November 2014.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
Further information is available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2018 (5th edition) Gastric Cancer. 2021;24:1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitano S., Iso Y., Moriyama M., Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg. Laparosc. Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 4.Zhu Z., Li L., Xu J., Ye W., Zeng J., Chen B., Huang Z. Laparoscopic versus open approach in gastrectomy for advanced gastric cancer: A systematic review. World J. Surg. Oncol. 2020;18:126. doi: 10.1186/s12957-020-01888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inaki N., Etoh T., Ohyama T., Uchiyama K., Katada N., Koeda K., Yoshida K., Takagane A., Kojima K., Sakuramoto S., et al. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901) World J. Surg. 2015;39:2734–2741. doi: 10.1007/s00268-015-3160-z. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z., Xing J., Cai J., Zhang Z., Li F., Zhang N., Wu J., Cui M., Liu Y., Chen L., et al. Short-term surgical outcomes of laparoscopy-assisted versus open D2 distal gastrectomy for locally advanced gastric cancer in North China: A multicenter randomized controlled trial. Surg. Endosc. 2019;33:33–45. doi: 10.1007/s00464-018-6391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park Y.K., Yoon H.M., Kim Y.W., Park J.Y., Ryu K.W., Lee Y.J., Jeong O., Yoon K.Y., Lee J.H., Lee S.E., et al. Laparoscopy-assisted versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: Results From a Randomized Phase II Multicenter Clinical Trial (COACT 1001) Ann. Surg. 2018;267:638–645. doi: 10.1097/SLA.0000000000002168. [DOI] [PubMed] [Google Scholar]
- 8.Cai J., Wei D., Gao C.F., Zhang C.S., Zhang H., Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig. Surg. 2011;28:331–337. doi: 10.1159/000330782. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y., Huang C., Sun Y., Su X., Cao H., Hu J., Xue Y., Suo J., Tao K., He X., et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016;34:1350–1357. doi: 10.1200/JCO.2015.63.7215. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y., Xu X., Zhao Y., Qian F., Tang B., Hao Y., Luo H., Chen J., Yu P. Long-term oncologic outcomes of a randomized controlled trial comparing laparoscopic versus open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surgery. 2019;165:1211–1216. doi: 10.1016/j.surg.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Cui M., Li Z., Xing J., Yao Z., Liu M., Chen L., Zhang C., Yang H., Zhang N., Tan F., et al. A prospective randomized clinical trial comparing D2 dissection in laparoscopic and open gastrectomy for gastric cancer. Med. Oncol. 2015;32:241. doi: 10.1007/s12032-015-0680-1. [DOI] [PubMed] [Google Scholar]
- 12.Lee H.J., Hyung W.J., Yang H.K., Han S.U., Park Y.K., An J.Y., Kim W., Kim H.I., Kim H.H., Ryu S.W., et al. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT) Ann. Surg. 2019;270:983–991. doi: 10.1097/SLA.0000000000003217. [DOI] [PubMed] [Google Scholar]
- 13.Li Q., Wang J., Zhang G., Wang J., Yang B., Zhang Z. Feasibility and safety comparison of laparoscopy-assisted versus open gastrectomy for advanced gastric carcinoma with D2 lymphadenectomy. Jpn. J. Clin. Oncol. 2016;46:323–328. doi: 10.1093/jjco/hyw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao Y., Yu P., Qian F., Zhao Y., Shi Y., Tang B., Zeng D., Zhang C. Comparison of laparoscopy-assisted and open radical gastrectomy for advanced gastric cancer: A retrospective study in a single minimally invasive surgery center. Medicine. 2016;95:e3936. doi: 10.1097/MD.0000000000003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z., Li B., Bai B., Yu P., Lian B., Zhao Q. Long-term outcomes of laparoscopic versus open D2 gastrectomy for advanced gastric cancer. Surg. Oncol. 2018;27:441–448. doi: 10.1016/j.suronc.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y., Hua J., Li J., Shi L., Xue H., Shuang J., Du J. Long-term outcomes of laparoscopic versus open gastrectomy for advanced gastric cancer: A large cohort study. Am. J. Surg. 2019;217:750–756. doi: 10.1016/j.amjsurg.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y., Yu P., Hao Y., Qian F., Tang B., Shi Y., Luo H., Zhang Y. Comparison of outcomes for laparoscopically assisted and open radical distal gastrectomy with lymphadenectomy for advanced gastric cancer. Surg. Endosc. 2011;25:2960–2966. doi: 10.1007/s00464-011-1652-y. [DOI] [PubMed] [Google Scholar]
- 18.Desiderio J., Jiang Z.W., Nguyen N.T., Zhang S., Reim D., Alimoglu O., Azagra J.S., Yu P.W., Coburn N.G., Qi F., et al. Robotic, laparoscopic and open surgery for gastric cancer compared on surgical, clinical and oncological outcomes: A multi-institutional chart review. A study protocol of the International study group on Minimally Invasive surgery for GASTRIc Cancer-IMIGASTRIC. BMJ Open. 2015;5:e008198. doi: 10.1136/bmjopen-2015-008198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancerc. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 20.AJCC. Brierley J., Gospodarowicz M., Wittekind C. In: TNM Classification of Malignant Tumours. 8th ed. Brierley J., Gospodarowicz M., Wittekind C., editors. Wiley-Blackwell; Hoboken, NJ, USA: 2016. [Google Scholar]
- 21.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ptok H., Gastinger I., Meyer F., Ilsemann A., Lippert H., Bruns C. Hospital volume effects in surgical treatment of gastric cancer: Results of a prospective multicenter observational study. Der Chir. Z. Fur Alle Geb. Der Oper. Medizen. 2017;88:328–338. doi: 10.1007/s00104-016-0292-0. [DOI] [PubMed] [Google Scholar]
- 23.Best L.M., Mughal M., Gurusamy K.S. Laparoscopic versus open gastrectomy for gastric cancer. Cochrane Database Syst. Rev. 2016;3:CD011389. doi: 10.1002/14651858.CD011389.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan B.Y.O., Yau K.K.W., Chan C.K.O. Totally laparoscopic versus open gastrectomy for advanced gastric cancer: A matched retrospective cohort study. Hong Kong Med. J. Xianggang Yi Xue Za Zhi. 2019;25:30–37. doi: 10.12809/hkmj177150. [DOI] [PubMed] [Google Scholar]
- 25.Lin J.X., Huang C.M., Zheng C.H., Li P., Xie J.W., Wang J.B., Lu J. Laparoscopy-assisted gastrectomy with D2 lymph node dissection for advanced gastric cancer without serosa invasion: A matched cohort study from South China. World J. Surg. Oncol. 2013;11:4. doi: 10.1186/1477-7819-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Q.Y., Huang C.M., Lin J.X., Zheng C.H., Li P., Xie J.W., Wang J.B., Lu J. Laparoscopy-assisted versus open D2 radical gastrectomy for advanced gastric cancer without serosal invasion: A case control study. World J. Surg. Oncol. 2012;10:248. doi: 10.1186/1477-7819-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang F., Lan Y., Tang B., Hao Y., Shi Y., Yu P. Comparative study of laparoscopy-assisted and open radical gastrectomy for stage T4a gastric cancer. Int. J. Surg. 2017;41:23–27. doi: 10.1016/j.ijsu.2017.01.116. [DOI] [PubMed] [Google Scholar]
- 28.Kunisaki C., Makino H., Yamamoto N., Sato T., Oshima T., Nagano Y., Fujii S., Akiyama H., Otsuka Y., Ono H.A., et al. Learning curve for laparoscopy-assisted distal gastrectomy with regional lymph node dissection for early gastric cancer. Surg. Laparosc. Endosc. Percutaneous Tech. 2008;18:236–241. doi: 10.1097/SLE.0b013e31816aa13f. [DOI] [PubMed] [Google Scholar]
- 29.Wei Y., Yu D., Li Y., Fan C., Li G. Laparoscopic versus open gastrectomy for advanced gastric cancer: A meta-analysis based on high-quality retrospective studies and clinical randomized trials. Clin. Res. Hepatol. Gastroenterol. 2018;42:577–590. doi: 10.1016/j.clinre.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Park D.J., Han S.U., Hyung W.J., Kim M.C., Kim W., Ryu S.Y., Ryu S.W., Song K.Y., Lee H.J., Cho G.S., et al. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: A large-scale multicenter retrospective study. Surg. Endosc. 2012;26:1548–1553. doi: 10.1007/s00464-011-2065-7. [DOI] [PubMed] [Google Scholar]
- 31.Inokuchi M., Nakagawa M., Tanioka T., Okuno K., Gokita K., Kojima K. Long- and short-term outcomes of laparoscopic gastrectomy versus open gastrectomy in patients with clinically and pathological locally advanced gastric cancer: A propensity-score matching analysis. Surg. Endosc. 2018;32:735–742. doi: 10.1007/s00464-017-5730-7. [DOI] [PubMed] [Google Scholar]
- 32.Hamabe A., Omori T., Tanaka K., Nishida T. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg. Endosc. 2012;26:1702–1709. doi: 10.1007/s00464-011-2096-0. [DOI] [PubMed] [Google Scholar]
- 33.Zemni I., Mansouri H., Ben Safta I., Ayadi M.A., Ben Dhiab T., Chargui R., Rahal K. Resectable gastric signet ring cell carcinoma: Clinicopathological characteristics and survival outcomes. J. Gastric Surg. 2020;2:71–78. doi: 10.36159/jgs.v2i3.55. [DOI] [Google Scholar]
- 34.Mansouri H., Zemni I., Ayadi M.A., Ben Safta I., Ben Dhiab T., Mahjoub N., Achouri L., Rahal K. Determinants of surgical morbidity in gastric cancer: Experience of a single center and literature review. J. Gastric Surg. 2020;2:106–117. doi: 10.36159/jgs.v2i4.71. [DOI] [Google Scholar]
- 35.Owen R.M., Perez S.D., Lytle N., Patel A., Davis S.S., Lin E., Sweeney J.F. Impact of operative duration on postoperative pulmonary complications in laparoscopic versus open colectomy. Surg. Endosc. 2013;27:3555–3563. doi: 10.1007/s00464-013-2949-9. [DOI] [PubMed] [Google Scholar]
- 36.Naffouje S.A., Salti G.I. Extensive Lymph Node Dissection Improves Survival among American Patients with Gastric Adenocarcinoma Treated Surgically: Analysis of the National Cancer Database. J. Gastric Cancer. 2017;17:319–330. doi: 10.5230/jgc.2017.17.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo Y., Goldner B., Ituarte P., Lee B., Melstrom L., Son T., Noh S.H., Fong Y., Hyung W.J. Lymphadenectomy with Optimum of 29 Lymph Nodes Retrieved Associated with Improved Survival in Advanced Gastric Cancer: A 25,000-Patient International Database Study. J. Am. Coll. Surg. 2017;224:546–555. doi: 10.1016/j.jamcollsurg.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiki N., Honda M., Etoh T., Yoshida K., Kodera Y., Kakeji Y., Kumamaru H., Miyata H., Yamashita Y., Inomata M., et al. Higher incidence of pancreatic fistula in laparoscopic gastrectomy. Real-world evidence from a nationwide prospective cohort study. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2018;21:162–170. doi: 10.1007/s10120-017-0764-z. [DOI] [PubMed] [Google Scholar]
- 39.Zhong Q., Huang C.-M., Chen Q.-Y., Lin J.-X., Xie J.-W., Li P., Zheng C.-H. Current Status of Indocyanine Green Tracer-Guided Lymph Node Dissection in Minimally Invasive Surgery for Gastric Cancer. J. Gastric Surg. 2021;3 doi: 10.36159/jgs.v3i1.76. [DOI] [Google Scholar]
- 40.Coco D., Leanza S. Assessment of the Completeness of Lymph Node Dissection Using Indocyanine Green in Laparoscopic and Robotic Gastrectomy for Gastric Cancer—A Review. J. Gastric Surg. 2021;3 doi: 10.36159/jgs.v3i1.78. [DOI] [Google Scholar]
- 41.Kinoshita T., Uyama I., Terashima M., Noshiro H., Nagai E., Obama K., Tamamori Y., Nabae T., Honda M., Abe T., et al. Long-term Outcomes of Laparoscopic Versus Open Surgery for Clinical Stage II/III Gastric Cancer: A Multicenter Cohort Study in Japan (LOC-A Study) Ann. Surg. 2019;269:887–894. doi: 10.1097/SLA.0000000000002768. [DOI] [PubMed] [Google Scholar]
- 42.Gordon A.C., Kojima K., Inokuchi M., Kato K., Sugihara K. Long-term comparison of laparoscopy-assisted distal gastrectomy and open distal gastrectomy in advanced gastric cancer. Surg. Endosc. 2013;27:462–470. doi: 10.1007/s00464-012-2459-1. [DOI] [PubMed] [Google Scholar]
- 43.Shinohara T., Satoh S., Kanaya S., Ishida Y., Taniguchi K., Isogaki J., Inaba K., Yanaga K., Uyama I. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: A retrospective cohort study. Surg. Endosc. 2013;27:286–294. doi: 10.1007/s00464-012-2442-x. [DOI] [PubMed] [Google Scholar]
- 44.Lin J.X., Lin J.P., Desiderio J., Xie J.W., Gemini A., Wang J.B., Lu J., Chen Q.Y., Cao L.L., Lin M., et al. Difference in the short-term outcomes of laparoscopic gastrectomy for gastric carcinoma between the east and west: A retrospective study from the IMIGASTRIC trial. J. Cancer. 2019;10:4106–4113. doi: 10.7150/jca.31192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kong S.H., Lee H.J., Ahn H.S., Kim J.W., Kim W.H., Lee K.U., Yang H.K. Stage migration effect on survival in gastric cancer surgery with extended lymphadenectomy: The reappraisal of positive lymph node ratio as a proper N-staging. Ann. Surg. 2012;255:50–58. doi: 10.1097/SLA.0b013e31821d4d75. [DOI] [PubMed] [Google Scholar]
- 46.Ito Y., Miyashiro I., Ishikawa T., Akazawa K., Fukui K., Katai H., Nunobe S., Oda I., Isobe Y., Tsujitani S., et al. Determinant Factors on Differences in Survival for Gastric Cancer Between the United States and Japan Using Nationwide Databases. J. Epidemiol. 2021;31:241–248. doi: 10.2188/jea.JE20190351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrelli F., Ghidini M., Barni S., Steccanella F., Sgroi G., Passalacqua R., Tomasello G. Prognostic Role of Primary Tumor Location in Non-Metastatic Gastric Cancer: A Systematic Review and Meta-Analysis of 50 Studies. Ann. Surg. Oncol. 2017;24:2655–2668. doi: 10.1245/s10434-017-5832-4. [DOI] [PubMed] [Google Scholar]
- 48.Groth S.S., Habermann E.B., Massarweh N.N. United States Administrative Databases and Cancer Registries for Thoracic Surgery Health Services Research. Ann. Thorac. Surg. 2020;109:636–644. doi: 10.1016/j.athoracsur.2019.08.067. [DOI] [PubMed] [Google Scholar]
- 49.Sarrazin M.S., Rosenthal G.E. Finding pure and simple truths with administrative data. JAMA. 2012;307:1433–1435. doi: 10.1001/jama.2012.404. [DOI] [PubMed] [Google Scholar]
- 50.Fang C., Hua J., Li J., Zhen J., Wang F., Zhao Q., Shuang J., Du J. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymphadenectomy for advanced gastric cancer. Am. J. Surg. 2014;208:391–396. doi: 10.1016/j.amjsurg.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 51.Japanese Gastric Cancer Association Japanese Classification of Gastric Carcinoma—2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/PL00011681. [DOI] [PubMed] [Google Scholar]
- 52.Wagner A.D., Syn N.L., Moehler M., Grothe W., Yong W.P., Tai B.C., Ho J., Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017;8:CD004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reddavid R., Sofia S., Chiaro P., Colli F., Trapani R., Esposito L., Solej M., Degiuli M. Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake? World J. Gastroenterol. 2018;24:274–289. doi: 10.3748/wjg.v24.i2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Further information is available from the corresponding author on reasonable request.