Abstract
The emergence of multidrug-resistant H. pylori poses a public healthcare threat, particularly in low- and middle-income countries. Recently, the World Health Organization has classified clarithromycin-resistant H. pylori as high priority in the research and discovery of novel antibiotics. This study was aimed to systematically review the prevalence of primary antibiotic resistance in H. pylori in Southeast Asian countries (SEAC) and to review current studies of antimicrobial peptides against H. pylori. We systematically searched through electronic databases of studies conducted on antimicrobial resistance of H. pylori in SEA countries. Furthermore, we searched articles that conducted studies on antimicrobial peptides, naturally occurring host’s defense molecules, against H. pylori. After a series of screening processes, 15 studies were included in our systematic review. Our analysis revealed that primary resistance of H. pylori to metronidazole, clarithromycin, and levofloxacin were high in SEAC, although the primary resistance to amoxicillin and tetracycline remains low. Multidrug-resistant H. pylori are emerging in SE Asian countries. The antimicrobial peptides show promising antibacterial and antibiofilm activity against drug-resistant H. pylori. The research and discovery of antimicrobial peptides against H. pylori in SEAC will help in limiting the spread of antimicrobial resistance of H. pylori.
Keywords: Helicobacter pylori, low and middle-income countries, antimicrobial resistance, antimicrobial peptides, multidrug resistance, clarithromycin, metronidazole
1. Introduction
Helicobacter pylori is one of the most common infectious disease agents worldwide, with more than 50% of the world’s population being infected with this pathogen, mostly in developing countries [1]. Transmission of H. pylori is still uncertain, but several studies have shown that close contact of a mother with a child during childhood is the main transmission route, apart from drinking contaminated water [2]. Since its discovery by Warren and Marshall almost three decades ago, it is now established that this bacterium orchestrates gastric carcinogenesis by producing multiple virulence factors that lead to peptic ulcer and gastric cancer [3]. Gastric cancer is still one of the most common cancers globally, with more than 1 million new cases reported yearly, leading to 768,793 deaths in 2020 alone [4]. Furthermore, gastric cancer patients are mainly detected at the advanced stage of cancer, where cancer prognosis is worse than that of detection at an early stage [5]. A high economic and mortality burden is associated with gastric cancer, with most cases occurring in developing countries where medical resources for early screening and patient management are limited [4]. Therefore, early screening of gastric cancer for detecting precancerous lesions for better prognosis and eradicating H. pylori are two essential strategies to prevent gastric cancer development.
Treatment of H. pylori includes administering proton pump inhibitors (PPI) coupled with multiple antibiotics through several treatment regimens. In the first-line triple therapy, PPI is administered with amoxicillin and clarithromycin. However, in areas where the clarithromycin resistance rate is higher than 15%, triple therapy containing metronidazole or bismuth-containing quadruple therapy is recommended [6]. Nevertheless, the increasing rate of multiple-drug-resistant H. pylori has implications for the eradication of bacteria to prevent gastric diseases, including gastric cancer and ulcers [7]. The World Health Organization (WHO) has declared clarithromycin-resistant H. pylori a high priority in the research and development of novel antimicrobial discovery [8]. As such, an alarming concern of the increasing rate of antibiotic resistance presents urgency for the discovery of novel or alternative therapies for H. pylori. SEAC are home to more than 650 million people with diverse ethnicities and cultures. Most of these countries are mainly low-and middle-income countries, where medical resources are limited [9]. However, it is also home to diverse natural flora and fauna, with more than 20% of global plant and animal species, and where four out of twenty-five global biodiversity hotspots are found [10]. Antimicrobial peptides (AMP) are a small class of peptides that are part of an organism’s innate immunity with an inhibitory effect against pathogens such as the inhibition of nucleotide and protein synthesis of pathogens, formation of the pore at the cell membrane, and the host’s immunomodulation [11]. AMPs are good candidates for alternative therapies for the treatment of antibiotic-resistant infections and are broad-spectrum antimicrobial agents to which most bacteria evolution is slow. AMP has been isolated from multiple flora and fauna such as amphibians, fish, and plants [12]. Given that SEA has one of the world’s greatest biodiversity hotspots, this region can be one in which AMP research and discovery can be conducted rapidly. The objective of this review was to systematically study antibiotic resistant in H. pylori in SEA and to summarize our current understanding of AMP and its role as an alternative therapeutic option for H. pylori infections.
2. Results
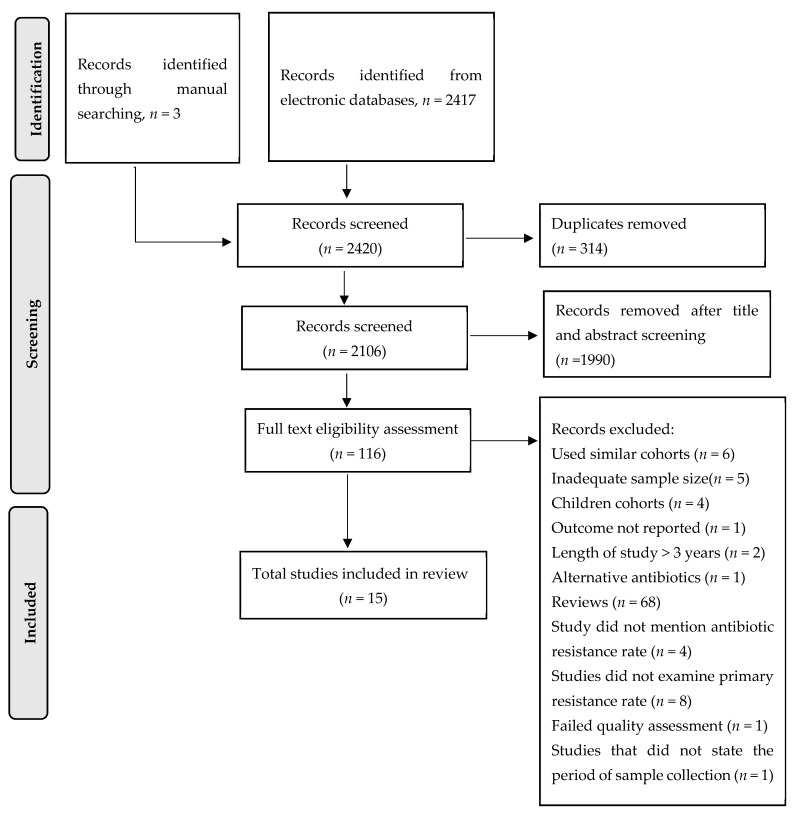
The systematic search through electronic databases resulted in 2417 articles, and an additional three articles were found by checking references of a previous systematic review. After title and abstract screening, 116 articles underwent full-text assessment (Figure 1). By applying inclusion and exclusion criteria, only 16 articles from 7 out of 10 SEA countries met the eligibility criteria for inclusion in this systematic review. After quality assessment, one article was excluded due to low quality (quality score = 3), and the final number of articles included in this systematic review was 15. The articles from countries that were included in this systematic review were as follows: Cambodia (n = 1), Indonesia (n = 1), Laos (n = 1), Malaysia (n = 3), Singapore (n = 1), Thailand (n = 5), and Vietnam (n = 3) and were published between 2011 to 2020. The patients recruited in the studies were diagnosed with symptoms of dyspepsia. The mean patient age in the included studies ranged from 38 to 55 years old. The methods employed to diagnose H. pylori infection in the included studies were bacterial culture, rapid urease test, molecular technique of polymerase chain reaction (PCR), and histopathological examination. For antibiotic resistance testing, a molecular technique and a culture-based method, namely the E-test and the agar dilution assay, were used (Table 1).
Figure 1.
Flow chart of study selection. Exclusion criteria were: (1) Studies that measured the prevalence of alternative antibiotics for H. pylori treatment, (2) studies that reported prevalence results using < 50 isolates for culture method or <50 H. pylori positive gastric biopsies for nucleic acid-based method, (3) studies that only reported percentage of resistance rates with no description of the number of isolates used, (4) studies with no time frame of sample collection, (5) studies that clustered prevalence rates data more than three years, (6) studies that failed to state the percentage of primary antibiotic resistance in H. pylori isolates examined, and (7) studies that only examined the prevalence of resistance rate of secondary isolates.
Table 1.
List of studies included in the systematic review.
| Author | Year Published |
Patient’s Age (Mean in Years) | Country | No. ofPatients | HP Diagnostic Test | Patients’ Diseases | Antibiotic Resistance Method |
Ref. |
|---|---|---|---|---|---|---|---|---|
| Auttajaroon et al. | 2019 | 54.5 | Thailand | 93 | RUT and culture | Functional dyspepsia | E-test | [13] |
| Vilaichone et al. | 2017 | 55.9 | Thailand | 148 | RUT and culture | Dyspepsia | E-test | [14] |
| Vilaichone et al. | 2016 | 46.7 | Thailand | 291 | RUT and culture | Dyspepsia | E-test | [15] |
| Vilaichone et al. | 2011 | 49.1 | Thailand | 412 | Culture and CLO test | Dyspepsia | E-test | [16] |
| Tongtawee et al. | 2015 | 45.2 | Thailand | 300 | HPE and RUT | Dyspepsia | Molecular technique a | [17] |
| Tuan et al. | 2019 | 45.3 | Cambodia | 206 | Culture | NA | Agar dilution assay | [18] |
| Miftahussurur et al. | 2016 | 49.6 (male) and 48.9 (female) | Indonesia | 849 | Culture and HPE | Dyspepsia | E-test | [19] |
| Vannarath et al. | 2016 | 46 | Laos | 329 | CLO and HPE | Dyspepsia | Molecular technique a | [20] |
| Hanafiah et al. | 2019 | 52.41 | Malaysia | 288 | Culture, HPE and RUT | Chronic dyspepsia | E-test | [21] |
| Goh et al. | 2011 | 50.5 | Malaysia | 90 | Culture | NA | E-test | [22] |
| Ahmad et al. | 2011 | NA | Malaysia | 777 | Culture, RUT and HPE | Gastritis, peptic ulcer and gastric cancer | E-test | [23] |
| Ang et al. | 2015 | 48 | Singapore | 462 | UBT, RUT and HPE | NA | Agar dilution method | [24] |
| Dang et al. | 2020 | 38.3 | Vietnam | 153 | Culture and RUT | Chronic gastritis | E-test | [25] |
| Binh et al. | 2013 | 47.3 (male) and 42.3 (female) | Vietnam | NA | Culture | Dyspepsia | E-test | [26] |
| Phan et al. | 2015 | 44.1 | Vietnam | 92 | Culture | Dyspepsia | E-test | [27] |
a Point mutation of A2143/2142CG in 23S rRNA to detect clarithromycin resistance; Abbreviations HP: H. pylori; RUT: Rapid urease test; HPE: Histopathological examination; CLO: Campylobacter-like organism; E-test: Epsilometer test; UBT: Urea breath test; CLA: Clarithromycin; MET: Metronidazole; LEVO: Levofloxacin; AMX: Amoxicillin; TET: Tetracycline; NA: Not available.
2.1. Summary of Primary Antibiotic Resistance Rate in H. pylori
2.1.1. Clarithromycin Resistance
From our systematic search, 15 studies from SEA countries examined primary clarithromycin resistance in H. pylori (Table 2). In Thailand, clarithromycin resistance ranged from as low as 5.6% to as high as 76.2% for four studies conducted from 2013 to 2017. Of the four studies, the trend of clarithromycin resistance in Thailand was 5.6% in 2013 [15], 76.2% in 2014 to 2015 [17], 2% in 2016 [14], and 12.9% in 2017 [13]. The variability of clarithromycin resistance in H. pylori isolated in Thailand can be attributed to locations where the sampling was conducted and the type of diagnostic test used to assess antibiotic resistance. The prevalence of clarithromycin resistance was as high as 76.2% from the samples collected in the Northeast region of the country and molecular detection was used instead of culture-based detection [17], while the prevalence was less than 13% from the samples collected from the Mountain people [15], namely Karen, Thai, and Hmong people, the Pathumthani region [13], and the Chiangrai province [14] using culture-based diagnosis. In Cambodia, one study conducted in the country revealed primary clarithromycin resistance of H. pylori as 25.5% in 2015 [18] while in Indonesia, the clarithromycin resistance rate was 9.1% from 2012 to 2015 [19]. In Laos, the clarithromycin resistance rate was 12.6% from 2010 to 2012 [20]. Three studies examined primary clarithromycin resistance in H. pylori in Malaysia, in which the resistance rate showed an increasing trend from 2004 to 2015. From 2004 to 2009, the clarithromycin resistance rate was <2.1% [22,23]. However, the resistance rate increased to 12.2% from 2014 to 2015 [21]. Meanwhile, in Singapore, a study published in 2015 revealed the primary clarithromycin resistance was 17.9% [24]. In Vietnam, the primary resistance rate of clarithromycin increased from 33% in 2008 [26] to 34.2% from 2012 to 2014 [27] and 66.1% from 2014 to 2016 [25].
Table 2.
Primary antibiotic resistance rates of H. pylori.
| Author | Period of Sample Collection | Country | No. of HP Isolates | Primary Antibiotic Resistance Rate, % | MDR Rate, % (No. of Isolates) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CLA | MET | LEVO | AMX | TET | ||||||
| Auttajaroon et al. | 2017 | Thailand | 93 a | 12.9 | 62.8 | - | - | - | - | [13] |
| Vilaichone et al. | 2016 | Thailand | 50 | 2 | 26 | 22 | 0 | 0 | 4% (2/50) | [14] |
| Vilaichone et al. | 2013 | Thailand | 124 | 5.6 | 71.8 | 19.4 | 0.8 | 0 | 21.8% (27/124) | [15] |
| Vilaichone et al. | 2008–2010 | Thailand | 100 | - | 30 | - | - | - | - | [16] |
| Tongtawee et al. | 2014–2015 | Thailand | 300 b,* | 76.2 c | - | - | - | - | - | [17] |
| Tuan et al. | 2015 | Cambodia | 55 | 25.5 | 96.4 | 67.3 | 9.1 | 0 | 76.4% (42/55) | [18] |
| Miftahussurur et al. | 2012–2015 | Indonesia | 77 | 9.1 | 46.8 | 31.2 | 5.2 | 2.6 | - | [19] |
| Vannarath et al. | 2010–2012 | Laos | 119 b,** | 12.6 | - | - | - | - | - | [20] |
| Hanafiah et al. | 2014–2015 | Malaysia | 59 d | 12.2 | 56.1 | 17.1 | 0 | 0 | 7.4% (2/27) | [21] |
| Goh et al. | 2009 | Malaysia | 90 | 0 | 75.5 | 0 | 0 | - | - | [22] |
| Ahmad et al. | 2004–2007 | Malaysia | 187 | 2.1 | 36.9 | - | 0 | 0 | 2.1% (4/187) | [23] |
| Ang et al. | 2011–2014 | Singapore | 106 | 17.9 | 48.1 | - | 4.7 | - | 7.5% (8/106) | [24] |
| Dang et al. | 2014–2016 | Vietnam | 153 d | 66.1 | - | 38.1 | - | - | 30.7% e (47/153) |
[25] |
| Binh et al. | 2008 | Vietnam | 103 | 33 | 69.9 | 18.4 | 0 | 5.8 | 24.3% (25/103) | [26] |
| Phan et al. | 2012–2014 | Vietnam | 92 d | 34.2 | 75.3 | 35.6 | 0 | - | 50.7% (37/73) for primary resistance and 78.9% (15/19) for secondary resistance | [27] |
a Metronidazole resistance was performed in 43 isolates while clarithromycin resistance was performed in 70 isolates; b indicates antibiotic susceptibility test was conducted directly on gastric biopsies using molecular technique without isolation of the bacteria; c 76.2% was derived from the number of isolates that harbored both mutation and wildtype variants in 23S rRNA sequence; d Total number of isolates were derived from primary and secondary isolates. The primary resistance rates were recalculated using total primary isolates as denominator; e Multidrug resistance from both primary and secondary isolates; * DNA was extracted from all 300 gastric biopsies for molecular detection of antibiotic resistance, ** DNA was extracted from 119/329 patients for molecular detection of antibiotic resistance. Abbreviations: HP: H. pylori; CLA: Clarithromycin; MET: Metronidazole; LEVO: Levofloxacin; AMX: Amoxicillin; TET: Tetracycline; -: Not available.
2.1.2. Metronidazole Resistance
Fifteen studies examined the primary resistance rate of metronidazole resistance in H. pylori, of which all studies found the resistance rate was high (>15% resistance rate). In Thailand, the metronidazole resistance rate showed an increasing trend from 30% between 2008 to 2010 [16] to 71.8% in 2013 [18] and 62.8% in 2017 [13]. A similar trend was also observed in Malaysia, in which the metronidazole resistance rate ranged from 36.9% [23] to 75.5% [22] from 2004 to 2009 and 56.1% between 2014 to 2015 [21]. In Vietnam, the trend of the metronidazole resistance rate was 69.9% in 2008 [26] and increased to 75.3% from 2012 to 2014 [27]. In Singapore, the metronidazole resistance rate observed was 48.1% from 2011 to 2014 [24]. The resistance rate was alarmingly high in Laos, in which a recent observation in 2015 revealed the rate was 96.4% [18]. Meanwhile, in Indonesia, the resistance rate of clarithromycin resistance in H. pylori was 46.8% from 2012 to 2015 [19].
2.1.3. Levofloxacin Resistance
The primary levofloxacin resistance rate was observed to increase from year to year in Thailand, in which the resistance rate increased from 19.4% [15] in 2013 and 22% in 2016 [14]. Meanwhile, high resistance rate of levofloxacin was observed in Cambodia [18] and Indonesia [19] (67.3% and 31.2%, respectively). In Malaysia, the primary resistance rate of levofloxacin was high at 17.1% from 2014 to 2015 [21]. The trend of levofloxacin resistance rate increased in Vietnam from 18.4% in 2008 [26], 35.6% from 2012 to 2014 [27] to 38.1% from 2014 to 2016 [25].
2.1.4. Amoxicillin Resistance
Our analysis revealed that the primary amoxicillin resistance rate of H. pylori in SEAC is still low. No H. pylori isolate was reported to be resistant to amoxicillin in Malaysia [21,22,23] while in Thailand, the resistance rate of H. pylori to amoxicillin was reported to be 0.8% in a study conducted from samples collected in 2013 [15] while no amoxicillin resistance isolate was observed in another study [14]. In Cambodia and Indonesia, rates of amoxicillin resistant H. pylori were 9.1% [18] and 5.2% [19], respectively. In Singapore, the resistance rate was 4.7% from 2011 to 2014 [24] while in Vietnam, the resistance rate was not observed in the study conducted from 2012 to 2014 [27].
2.1.5. Tetracycline Resistance
Similar to the amoxicillin resistance rate, the tetracycline resistance rate in most SEACs is low. No reports of tetracycline-resistant H. pylori were observed in Thailand and Malaysia, while in Indonesia, the resistance rate was 2.8% [19]. However, in Vietnam, the tetracycline resistance rate was reported to be 5.8% in 2008 [26].
2.1.6. Multidrug Resistance Rate of H. pylori
The multidrug resistance rate of H. pylori reported in SEA countries varied across all countries examined. In Malaysia, the reported multidrug resistance was still low, but than increasing resistance rate was observed. Of the two studies that reported a multidrug resistance rate in Malaysia, the resistance rates were 2.1% from 2004 to 2007 [23] but increased to 7.4% from 2014 to 2015 [21]. This finding suggests that there is an increase in multidrug-resistant H. pylori in Malaysia. Similarly, Singapore, the neighboring country of Malaysia, had a multidrug resistance rate of 7.5% between 2011 to 2014 [24]. Of the two studies that reported multidrug resistance rate in Thailand, one study found that the rate was 4% in 2016 [14] and 21.8% in 2013 [15]. Contrary to Malaysia, Thailand, and Singapore, the multidrug resistance rates of H. pylori in Vietnam and Cambodia were high. The rate of multidrug-resistant H. pylori in Vietnam increased from 24.3% in 2008 [26] to 30.7% from 2014 to 2016 [25]. Meanwhile, the multidrug resistance rate of H. pylori strains in Cambodia was extremely high at 76.4% [18], of which 40% were resistant to levofloxacin and metronidazole, 7.3% were resistant to clarithromycin and metronidazole, 9.1% were resistant to metronidazole, levofloxacin, and amoxicillin, and 18.2% were resistant to clarithromycin, levofloxacin, and metronidazole.
2.2. A Review on Intervention Strategy Using AMP
Given the high resistance rates of H. pylori to critically important antibiotics in this region, we searched the database on intervention strategies of H. pylori treatment using AMP. AMPs are small peptides that have been demonstrated to possess broad-spectrum activity against bacteria. They are usually isolated from natural sources such as the skin of amphibians and venoms. The SEACs are a region with high biological diversity and lush tropical forests where the sources for discovery and research on novel antimicrobial drugs against H. pylori can be further explored. As such, we aimed to summarize the studies conducted on AMPs against H. pylori for understanding the current progress in research. Table 3 summarizes the studies conducted on AMPs against H. pylori. We found that all the studies conducted demonstrated antibacterial activity of AMPs against H. pylori and all studies employed synthetically synthesized AMPs (Table 3). Five AMPs, namely cathelicidin [28], bicarinalin [29], odorranain-HP [30], tilapia Piscidin 4 [31], and pleurain-A [32], were initially identified from natural sources and then synthesized synthetically in the laboratory. Notably, some AMPs, including cathelicidin-like AMP [33], bicarinalin [29], fusion human neutrophil peptide 1 [34], and epinecidin 1 [35], showed bactericidal activity against drug-resistant H. pylori. The mechanism of bactericidal and bacteriostatic AMPs includes the perturbation, permeabilization, and rupture of the bacterial cell membrane (Table 3). In addition, cathelicidin from humans and mice exhibited antibiofilm activity against H. pylori and protected the animal model from inflammation induced by H. pylori [28]. A study conducted by Jiang et al. [33] found that a cathelicidin-like peptide, namely Cbf-K16, reduced intercellular and intracellular activity of H. pylori and decreased H. pylori colonization in animal model. Furthermore, helix-coil conformation transitional antimicrobial polypeptides demonstrated bactericidal activity against H. pylori at low pH, suggesting its potential for use in human. Besides that, the peptide demonstrated low toxicity in animal study and an examined stomach, suggesting that the peptide is worthy to be evaluated in clinical trials [36].
Table 3.
Summary of studies conducted on antimicrobial peptides against H. pylori.
| Author | Year | Name of AMP | Source | Finding on Antibacterial Activity against H. pylori | Reference |
|---|---|---|---|---|---|
| Zhang et al. | 2016 | Cathelicidin | Mouse and human | Bactericidal activity against clarithromycin-resistant H. pylori; anti-biofilm activity against H. pylori SS1 strain; protected mouse from H. pylori orchestrated inflammation and reduced H. pylori colonization | [28] |
| Guzman et al. | 2018 | Bicarinalin | Synthetically synthesized in lab (anti venom Tetramorium bicarinatum) | Perturbation of membrane permeability against drug-resistant H. pylori | [29] |
| Chen et al. | 2007 | Odorranain-HP | Synthetically synthesized in lab (Diskless odorous frog, Odorrana graham) | Showed antimicrobial activity against H. pylori (MIC of 20 µg/mL) | [30] |
| Narayana et al. | 2015 | Tilapia Piscidin 4 (TP4) | Synthetically synthesized in lab (Nile tilapia, Oreochromis niloticus) | Demonstrated potential lytic activity against H. pylori surface membrane; disrupted the bacterial cell membrane | [31] |
| Wang et al. | 2007 | Pleurain-A | Synthetically synthesized in lab (Yunnan frog, Rana pleuraden) | Inhibited growth of H. pylori in vitro (30 µg/mL) | [32] |
| Jiang et al. | 2020 | Cbf-K16(cathelicidin-like AMP) | Synthetically synthesized | Demonstrated bactericidal activity against clarithromycin- and amoxicillin-resistant H. pylori; reduced intercellular and intracellular drug-resistant H. pylori in cell culture; showed increased membrane permeation in drug-resistant H. pylori | [33] |
| Zhang et al. | 2018 | Fusion human neutrophil peptide 1 | Expression system in yeast | Eradication of wild type and drug-resistant H. pylori in animal model | [34] |
| Narayana et al. | 2015 | Epinecidin-1 | Synthetically synthesized in lab | Showed bactericidal activity against drug-resistant H. pylori and modulated immune response in mouse-infected H. pylori for bacterial clearance | [35] |
| Xiong et al. | 2017 | Helix–coil conformation transitional antimicrobial polypeptides |
Synthetically synthesized in lab | Displayed bactericidal activity against H. pylori at low pH, both in vitro and in vivo | [36] |
| Zhang et al. | 2015 | Pexiganan | Synthetically synthesized in lab | Inhibited the growth of H. pylori (MIC = 4 µg/mL); decreased H. pylori colonization in animal model | [37] |
| Zhang et al. | 2017 | Fusion PGLa-AM1 | Synthetically synthesized in lab | Showed bactericidal activity against H. pylori in vitro and clearance of the bacteria in vivo | [38] |
| Makobongo et al. | 2012 | C12K-2β12 | Synthetically synthesized in lab | Ruptured H. pylori surface membrane for bactericidal effect; reduced colonization of H. pylori in gerbil model | [39] |
| Rigano et al. | 2012 | Tomato defensin | Synthetically synthesized in lab | Showed antibacterial activity against H. pylori at MIC: 15 µg/mL | [40] |
| Iwahori et al. | 1997 | Magainin 2 analog | Synthetically synthesized in lab | Inhibited growth of H. pylori in vitro | [41] |
Abbreviations: AMP: Antimicrobial peptide; MIC: Minimum inhibitory concentration.
3. Discussion
This systematic review consists of the studies conducted in the SEA region in countries with a diverse ethnic background, culture, and socioeconomic status. Our systematic review revealed that primary resistance of metronidazole in the SEA region is high with a more than 15% threshold while the primary resistance to clarithromycin varies according to the country, i.e., high in Singapore, Cambodia, and Vietnam, medium in Malaysia, and still low in Indonesia. In countries such as Thailand and Laos, resistance rates to clarithromycin are increasing and would exceed the threshold of 15% if the intervention strategy is not implemented. From the data published, resistance rates to levofloxacin also appeared to be high (>15%) in all the SEA countries, with the rate observed to be as high as >60% in Cambodia. Meanwhile, the resistance rates of H. pylori to amoxicillin and tetracycline are still low, with Malaysia reporting no cases of amoxicillin- and tetracycline-resistant H. pylori (Table 2). However, the amoxicillin- and tetracycline-resistant H. pylori have been reported in Indonesia and Cambodia, suggesting the emergence of H. pylori resistant to both antibiotics in these countries. Our findings are consistent with previous systematic reviews conducted by Savoldi et al. [7], which found that the resistance rates of H. pylori to metronidazole, clarithromycin, and levofloxacin are high in the SEA region. A similar systematic review also revealed high resistance rates of metronidazole in other parts of the world, including the Americas region, the European region, the Eastern Mediterranean region, and the Western Pacific region. Kuo et al. [42] also found an increasing rate of clarithromycin and metronidazole resistance in H. pylori in the Asian Pacific region. The rate of metronidazole resistance in SEA countries was observed to be very high (26–96.4%) in all SEA countries included in this systematic review. Metronidazole is used for treatments including parasitic infections, oral infections, gynecological infections, bone and joint infections, septicemia, and endocarditis [43], and is one of the drugs recommended as a first-line therapy in the SEA H. pylori management consensus [44]. The high resistance rate of metronidazole in this region could not be ascertained, although excessive use of the antibiotics and non-compliance among patients in treatment regimens are factors associated with the rising antibiotic resistance rate [45]. Trends of clarithromycin resistance also show a sharp increase from year to year in Malaysia, with <2.1% from 2004–2009 [22,23] to as high as 12.2% in 2014–2015 [21]. Vietnam also showed a sharp increase of clarithromycin resistance from 33% in 2008 [26] to 72.6% from 2014–2016 [25] while the trend in Thailand fluctuated from year to year. The trend of antimicrobial resistance rates in other countries could not be assessed due to a lack of studies conducted on antimicrobial resistance in H. pylori. This factor could be due to the difficulty of culturing H. pylori in the laboratory and the high cost of performing nucleic acid-based technique in the SEA region, which mostly consist of low- and middle-income countries. The Maastricht V/Florence Consensus Report suggests that primary treatment consisting of clarithromycin should be abandoned if the resistance rate to clarithromycin in the region exceeds 15%, and quadruple therapy should be administered instead for successful eradication of H. pylori [6]. In the SEA region, the ASEAN Bangkok Consensus Report in 2016 recommends triple therapy consisting of clarithromycin as a first-line regimen for 14 days, provided that the prevalence of clarithromycin resistance in the area is less than 15%. However, the triple therapy consisting of clarithromycin should be replaced with quadruple therapy when clarithromycin resistance rate exceeds 15% [44]. A systematic review and meta-analysis on the impact of eradication regimens in the areas with high clarithromycin and metronidazole resistance reveals that sequential therapy is more effective in eradicating H. pylori than that of prolonged 14-day triple therapy, while there was no difference observed between sequential and 14-day triple therapy in the areas with high metronidazole resistance [46]. While prevalence data of clarithromycin is known in some SEA countries, the prevalence in other countries is unknown. This will complicate the treatment options for H. pylori in these regions. Findings from our systematic review reveals that clarithromycin resistance in SEA countries is increasing. Taken together, these results suggest that the primary treatment consisting of quadruple therapy or sequential therapy must be administered to H. pylori-infected patients in SEA countries with high prevalence of clarithromycin resistance, provided clinical data of patients are available prior to the administration. Besides clarithromycin resistance in H. pylori, clinical factors that contribute to first-line treatment failure also include the patient’s non-compliance during therapy period, the patient’s age (eradication was more successful in older patients), and the type of ulcer, in which eradication was significantly more successful in duodenal ulcers compared to gastric ulcers, which should be taken into consideration before switching to quadruple treatment [47].
In our systematic review, we also found an increasing rate of multidrug-resistant H. pylori in the SEA countries examined. In Cambodia, the resistance rate of multidrug-resistant H. pylori was alarmingly high at 76.4% [18], suggesting the urgency of an intervention strategy in this country. The multidrug-resistance of H. pylori in Vietnam was also high and increased from year to year [25,26], while the trend was also observed in Malaysia, where the prevalence of multidrug-resistant H. pylori increased from year to year although the resistance rate was still low [21]. Similar to Malaysia, the multidrug-resistant H. pylori observed in Singapore [24] was also low while the rate in Thailand fluctuated from year to year [14,15]. Some studies in our systematic review did not report the prevalence of multidrug-resistant H. pylori; hence we were unable to conclude. However, we hypothesize that the resistance rates of H. pylori to multiple drugs are increasing in this region based on the available data from the countries that had conducted study on the surveillance rate of multidrug-resistant H. pylori in their respective countries. As such, we suggest for future studies to include the prevalence of multidrug-resistant H. pylori when reporting the surveillance of antimicrobial resistance in H. pylori.
As the emergence of antimicrobial resistance in H. pylori to important antibiotics keeps increasing, WHO has classified clarithromycin-resistant H. pylori as a high-priority pathogen in the research and discovery of novel antibiotics [8]. AMPs have emerged as a new therapeutic option for the treatment of bacterial infection as they possess antimicrobial spectrum characteristic against bacteria. Most antimicrobial peptides have been isolated from natural sources, which suggest that the SEA region can be a hotspot for the research and discovery of new AMPs [11]. As such, we narratively summarized the research and discovery of AMPs against H. pylori. While the results of AMPs against drug-resistant H. pylori are promising and effective, we found that the study of AMPs against H. pylori are still lacking globally and there was no study conducted on AMPs against H. pylori in SEAC. Furthermore, our review also found that the clinical trial of AMP against H. pylori has yet to be conducted and all studies were either limited to animal or cell culture investigations. The challenges in the application of antimicrobial peptides for treatment include the cost-effectiveness of the novel drug to the economic health and the concern of toxicity associated with AMP [12]. As the SEA region consists of mostly low- and middle-income countries, the greatest challenge is the cost of AMP synthesis and whether it is economically cost-effective for the general population in this region. However, the research on AMP in the SEA region is worth being explored further as this region is rich in tropical biodiversity, a source for new antimicrobial peptide discovery.
The strength of our systematic review on the primary antibiotic resistance of H. pylori includes the examination of the trend of resistance rate in the SEA region that consists mostly of low- and middle-income countries. Our systematic review also informs policymakers regarding the alarming rate of H. pylori resistance to essential antibiotics such as clarithromycin and metronidazole in this region, suggesting an urgency for an intervention program. Furthermore, we presented a brief narrative review on the progress of AMP against H. pylori research worldwide with paucity of the research in the SEA region where the biodiversity hotspot exists. However, our systematic review is constrained by a lack of studies conducted on the surveillance of antimicrobial resistance in H. pylori in the SEA region, suggesting the need for future surveillance programs in different countries. In conclusion, the prevalence of clarithromycin- and metronidazole-resistant H. pylori is high in most SEA countries and will continue to rise in the future if intervention strategies are not adopted. While the resistance rates to amoxicillin and tetracycline are still low, there is a need for the development of an intervention strategy by policymakers to guide clinicians and respective bodies in this region to encourage research and discovery of new antimicrobial agents for the treatment of H. pylori. More research collaboration among SEA countries should be conducted for this matter, with harmonization of data and an understanding of any successful intervention strategies in specific countries.
4. Methods
4.1. Search Strategy and Study Selection
In the systematic review of antibiotic resistance in SEAC, we searched the potential research articles through electronic databases, namely PubMed, Scopus, Medline, and Science Direct, from 1 January 1990 to 1 May 2021. The electronic search was conducted on 6 May 2021. Additionally, we manually searched the articles by checking references from review and research articles relevant to our research questions. Details on specific keywords used for our search strategy using electronic databases are as follows:
((“Helicobacter pylori”) AND (“antibiotic” OR “antimicrobial” OR “anti-microbial” OR “antibacterial” OR “anti-bacterial” OR “drug”) AND (“resistance” OR “resistant*”) AND (“Malaysia” OR “Singapore” OR “Thailand” OR “Indonesia” OR “Brunei” OR “Myanmar” OR “Vietnam” OR “Timor Leste” OR “Laos” OR “Cambodia” OR “Philippines”)).
This systematic review was prepared according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [48]. We included studies performed on populations in SEAC as defined by the United Nations [49]. These countries include Malaysia, Thailand, Singapore, Brunei Darussalam, Indonesia, Myanmar, Cambodia, Laos, Vietnam, and Philippines. Studies performed on populations outside SEA countries or studies without a clear population description were excluded from this systematic review. We also excluded studies conducted on children.
All study types were included in our search. However, we restricted our search to publications in English only. Abstracts without full text, all types of reviews, letters to the editors, and conference posters or presentations were excluded from our study. We included studies that measured the prevalence of primary antibiotic resistance for the following antibiotics: Metronidazole, clarithromycin, amoxicillin, tetracycline, and levofloxacin. Exclusion criteria were: (1) Studies that measured the prevalence of alternative antibiotics for H. pylori treatment; (2) studies that reported prevalence results using less than 50 isolates for culture method or less than 50 H. pylori positive gastric biopsies for nucleic acid-based method; (3) studies that only reported the percentage of resistance rates with no description of the number of isolates used; (4) studies with no time frame of sample collection; (5) studies that clustered prevalence rates data more than three years; (6) studies that failed to state the percentage of primary antibiotic resistance in H. pylori isolates examined; and (7) studies that only examined the prevalence of resistance rate of secondary isolates. If duplicate studies reported prevalence data using similar H. pylori cohorts, only the first study conducted on antibiotic resistance prevalence was chosen to be included in our systematic review.
4.2. Definitions of Diagnostic Tools and Antibiotic Resistance
Patients were defined as H. pylori-infected if they tested positive using one of the following diagnostic tools: Urea breath test, histopathological examination, culture-based test, rapid urease test of biopsies, and nucleic acid-based test. Antibiotic susceptibility tests include culture-based methods (E-test and the agar dilution method), or the nucleic acid-based test performed on H. pylori isolates or directly from gastric biopsies. Primary resistance was defined as H. pylori resistance to any antibiotics prior to administration of treatment regimens. Multidrug resistance in H. pylori is defined as H. pylori resistant to two or more antimicrobial agents. The threshold of high antibiotic resistance is a rate of more than 15% [6].
4.3. Data Extraction
Two independent reviewers independently screened the eligibility of articles from our search strategy through a two-step process. First, the articles were screened for title and abstracts for relevancy to our review. The full texts were retrieved and evaluated for the articles that passed the title and abstract screening process. Disagreement on inclusion or exclusion of the articles into the review was resolved through discussion and consensus. Data extracted from included studies were authors, country, year, period of sample collection, study design, patients’ clinical information (sample size, age, sex, histological findings, diseases, and H. pylori infection diagnostic tool used), and H. pylori samples (sample size, number of resistances, type of tested samples, the method to detect antibiotic resistance, and breakpoint system used). All data were extracted and sorted into Microsoft Office Excel 2016.
4.4. Quality Assessment of Studies
The quality of included studies was assessed as previously described [50], in which the score of study quality ranged from 0 to 8. A study with a score > 5 was considered as high quality while a study with a score that ranged 4–5 was considered as medium quality. Finally, a study with a score less than 4 was considered as low quality and would be excluded from this systematic review. Two reviewers independently scored each included article and any inconsistency on score result was resolved through discussion for final consensus.
4.5. A Narrative Review on AMP Studies in H. pylori
We searched through electronic literature databases on the studies using AMP in H. pylori treatment and summarized the findings.
Author Contributions
Conceptualization, A.S., B.S.L. and A.H.; methodology, A.S. and A.H.; validation, A.S., B.S.L. and A.H.; formal analysis, A.S.; writing—original draft preparation, A.S.; writing—review and editing, A.S., B.S.L. and A.H.; supervision, A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from Ministry of Higher Education of Malaysia, grant no. FRGS/1/2019/SKK11/UKM/02/3 and Universiti Teknologi MARA (UiTM) under grant no. 600-RMC/DINAMIK-POSTDOC 5/3 (010/2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data searched and extracted from the included studies in this systematic review are available upon request to corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hooi J.K.Y., Lai W.Y., Ng W.K., Suen M.M.Y., Underwood F.E., Tanyingoh D., Malfertheiner P., Graham D.Y., Wong V.W.S., Wu J.C.Y., et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Kayali S., Manfredi M., Gaiani F., Bianchi L., Bizzarri B., Leandro G., Di Mario F., De’Angelis G.L. Helicobacter pylori, transmission routes and recurrence of infection: State of the art. Acta Biomed. 2018;89:72–76. doi: 10.23750/abm.v89i8-S.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukri A., Hanafiah A., Mohamad Zin N., Kosai N.R. Epidemiology and role of Helicobacter pylori virulence factors in gastric cancer carcinogenesis. APMIS. 2020;128:150–161. doi: 10.1111/apm.13034. [DOI] [PubMed] [Google Scholar]
- 4.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.Rugge M., Fassan M., Graham D. Epidemiology of Gastric Cancer. In: Strong V.E., editor. Gastric Cancer. Springer; Cham, Switzerland: 2015. pp. 23–34. [Google Scholar]
- 6.Malfertheiner P., Megraud F., O’Morain C.A., Gisbert J.P., Kuipers E.J., Axon A.T., Bazzoli F., Gasbarrini A., Atherton J., Graham D.Y., et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 7.Savoldi A., Carrara E., Graham D.Y., Conti M., Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155:1372–1382.e17. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 9.The World Bank. [(accessed on 29 June 2021)]. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 10.Hughes A. Understanding the drivers of Southeast Asian biodiversity loss. Ecosphere. 2017;8:e01624. doi: 10.1002/ecs2.1624. [DOI] [Google Scholar]
- 11.Chen C.H., Lu T.K. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics. 2020;9:24. doi: 10.3390/antibiotics9010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswaro L.S., da Costa Sousa M.G., Rezende T.M.B., Dias S.C., Franco O.L. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front. Microbiol. 2018;9:855. doi: 10.3389/fmicb.2018.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auttajaroon J., Chotivitayatarakorn P., Yamaoka Y., Vilaichone R.K. CYP2C19 Genotype, CagA genotype and antibiotic resistant strain of Helicobacter pylori infection. Asian Pac. J. Cancer Prev. 2019;20:1243–1247. doi: 10.31557/APJCP.2019.20.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilaichone R.K., Ratanachu ek T., Gamnarai P., Subsomwong P., Uchida T., Yamaoka Y., Mahachai V. High fluoroquinolone resistant strains of Helicobacter pylori in the Golden triangle. Asian Pac. J. Cancer Prev. 2017;18:455–458. doi: 10.22034/APJCP.2017.18.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilaichone R.K., Ratanachu ek T., Gamnarai P., Chaithongrat S., Uchida T., Yamaoka Y., Mahachai V. Extremely high prevalence of metronidazole-resistant Helicobacter pylori strains in mountain people (Karen and Hmong) in Thailand. Am. J. Trop Med. Hyg. 2016;94:717–720. doi: 10.4269/ajtmh.15-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vilaichone R.K., Mahacahai V., Tumwasorn S., Kachintorn U. CagA genotype and metronidazole resistant strain of Helicobacter pylori in functional dyspepsia in Thailand. J. Gastroenterol. Hepatol. 2011;26((Suppl. S3)):46–48. doi: 10.1111/j.1440-1746.2011.06652.x. [DOI] [PubMed] [Google Scholar]
- 17.Tongtawee T., Dechsukhum C., Matrakool L., Panpimanmas S., Loyd R.A., Kaewpitoon S.J., Kaewpitoon N. High Prevalence of Helicobacter pylori resistance to clarithromycin: A hospital-based cross-sectional study in Nakhon Ratchasima Province, Northeast of Thailand. Asian Pac. J. Cancer Prev. 2015;16:8281–8285. doi: 10.7314/APJCP.2015.16.18.8281. [DOI] [PubMed] [Google Scholar]
- 18.Tuan V.P., Narith D., Tshibangu-Kabamba E., Dung H.D.Q., Viet P.T., Sokomoth S., Binh T.T., Sokhem S., Tri T.D., Ngov S., et al. A next-generation sequencing-based approach to identify genetic determinants of antibiotic resistance in Cambodian Helicobacter pylori clinical isolates. J. Clin. Med. 2019;8:858. doi: 10.3390/jcm8060858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miftahussurur M., Syam A.F., Nusi I.A., Makmun D., Waskito L.A., Zein L.H., Akil F., Uwan W.B., Simanjuntak D., Wibawa I.D., et al. Surveillance of Helicobacter pylori antibiotic susceptibility in Indonesia: Different resistance types among regions and with novel genetic mutations. PLoS ONE. 2016;11:e0166199. doi: 10.1371/journal.pone.0166199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vannarath S., Vilaichone R.K., Rasachak B., Mairiang P., Yamaoka Y., Mahachai V. Antibiotic resistant pattern of Helicobacter pylori infection based on molecular tests in Laos. Asian Pac. J. Cancer Prev. 2016;17:285–287. doi: 10.7314/APJCP.2016.17.1.285. [DOI] [PubMed] [Google Scholar]
- 21.Hanafiah A., Binmaeil H., Raja Ali R.A., Mohamed Rose I., Lopes B.S. Molecular characterization and prevalence of antibiotic resistance in Helicobacter pylori isolates in Kuala Lumpur, Malaysia. Infect. Drug Resist. 2019;12:3051–3061. doi: 10.2147/IDR.S219069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goh K.L., Navaratnam P. High Helicobacter pylori resistance to metronidazole but zero or low resistance to clarithromycin, levofloxacin, and other antibiotics in Malaysia. Helicobacter. 2011;16:241–245. doi: 10.1111/j.1523-5378.2011.00841.x. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad N., Zakaria W.R., Mohamed R. Analysis of antibiotic susceptibility patterns of Helicobacter pylori isolates from Malaysia. Helicobacter. 2011;16:47–51. doi: 10.1111/j.1523-5378.2010.00816.x. [DOI] [PubMed] [Google Scholar]
- 24.Ang T.L., Fock K.M., Song M., Ang D., Kwek A.B., Ong J., Tan J., Teo E.K., Dhamodaran S. Ten-day triple therapy versus sequential therapy versus concomitant therapy as first-line treatment for Helicobacter pylori infection. J. Gastroenterol. Hepatol. 2015;30:1134–1139. doi: 10.1111/jgh.12892. [DOI] [PubMed] [Google Scholar]
- 25.Dang N.Q.H., Ha T.M.T., Nguyen S.T., Le N.D.K., Nguyen T.M.T., Nguyen T.H., Pham T.T.H., Tran V.H. High rates of clarithromycin and levofloxacin resistance of Helicobacter pylori in patients with chronic gastritis in the south east area of Vietnam. J. Glob. Antimicrob. Resist. 2020;22:620–624. doi: 10.1016/j.jgar.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Binh T.T., Shiota S., Nguyen L.T., Ho D.D., Hoang H.H., Ta L., Trinh D.T., Fujioka T., Yamaoka Y. The incidence of primary antibiotic resistance of Helicobacter pylori in Vietnam. J. Clin. Gastroenterol. 2013;47:233–238. doi: 10.1097/MCG.0b013e3182676e2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phan T.N., Santona A., Tran V.H., Tran T.N., Le V.A., Cappuccinelli P., Rubino S., Paglietti B. High rate of levofloxacin resistance in a background of clarithromycin- and metronidazole-resistant Helicobacter pylori in Vietnam. Int. J. Antimicrob. Agents. 2015;45:244–248. doi: 10.1016/j.ijantimicag.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L., Wu W.K., Gallo R.L., Fang E.F., Hu W., Ling T.K., Shen J., Chan R.L., Lu L., Luo X.M., et al. Critical role of antimicrobial peptide cathelicidin for controlling Helicobacter pylori survival and infection. J. Immunol. 2016;196:1799–1809. doi: 10.4049/jimmunol.1500021. [DOI] [PubMed] [Google Scholar]
- 29.Guzman J., Téné N., Touchard A., Castillo D., Belkhelfa H., Haddioui-Hbabi L., Treilhou M., Sauvain M. Anti-Helicobacter pylori properties of the ant-venom peptide bicarinalin. Toxins. 2017;10:21. doi: 10.3390/toxins10010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L., Li Y., Li J., Xu X., Lai R., Zou Q. An antimicrobial peptide with antimicrobial activity against Helicobacter pylori. Peptides. 2007;28:1527–1531. doi: 10.1016/j.peptides.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Narayana J.L., Huang H.N., Wu C.J., Chen J.Y. Efficacy of the antimicrobial peptide TP4 against Helicobacter pylori infection: In vitro membrane perturbsation via micellization and in vivo suppression of host immune responses in a mouse model. Oncotarget. 2015;6:12936–12954. doi: 10.18632/oncotarget.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X., Song Y., Li J., Liu H., Xu X., Lai R., Zhang K. A new family of antimicrobial peptides from skin secretions of Rana pleuraden. Peptides. 2007;28:2069–2074. doi: 10.1016/j.peptides.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Jiang M., Ma L., Huang Y., Wu H., Dou J., Zhou C. Antimicrobial activities of peptide Cbf-K16 against drug-resistant Helicobacter pylori infection in vitro and in vivo. Microb. Pathog. 2020;138:103847. doi: 10.1016/j.micpath.2019.103847. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X.L., Jiang A.M., Ma Z.Y., Li X.B., Xiong Y.Y., Dou J.F., Wang J.F. The synthetic antimicrobial peptide pexiganan and its nanoparticles (PNPs) exhibit the anti-helicobacter pylori activity in vitro and in vivo. Molecules. 2015;20:3972–3985. doi: 10.3390/molecules20033972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayana J.L., Huang H.N., Wu C.J., Chen J.Y. Epinecidin-1 antimicrobial activity: In vitro membrane lysis and in vivo efficacy against Helicobacter pylori infection in a mouse model. Biomaterials. 2015;61:41–51. doi: 10.1016/j.biomaterials.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Xiong M., Bao Y., Xu X., Wang H., Han Z., Wang Z., Liu Y., Huang S., Song Z., Chen J., et al. Selective killing of Helicobacter pylori with pH-responsive helix-coil conformation transitionable antimicrobial polypeptides. Proc. Natl. Acad. Sci. USA. 2017;114:12675–12680. doi: 10.1073/pnas.1710408114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X., Jiang A., Qi B., Yu H., Xiong Y., Zhou G., Qin M., Dou J., Wang J. Secretion expression of human neutrophil peptide 1 (HNP1) in Pichia pastoris and its functional analysis against antibiotic-resistant Helicobacter pylori. Appl. Microbiol. Biotechnol. 2018;102:4817–4827. doi: 10.1007/s00253-018-8982-9. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X., Jiang A., Wang G., Yu H., Qi B., Xiong Y., Zhou G., Qin M., Dou J., Wang J. Fusion expression of the PGLa-AM1 with native structure and evaluation of its anti-Helicobacter pylori activity. Appl. Microbiol. Biotechnol. 2017;101:5667–5675. doi: 10.1007/s00253-017-8302-9. [DOI] [PubMed] [Google Scholar]
- 39.Makobongo M.O., Gancz H., Carpenter B.M., McDaniel D.P., Merrell D.S. The oligo-acyl lysyl antimicrobial peptide C₁₂K-2β₁₂ exhibits a dual mechanism of action and demonstrates strong in vivo efficacy against Helicobacter pylori. Antimicrob. Agents Chemother. 2012;56:378–390. doi: 10.1128/AAC.00689-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rigano M.M., Romanelli A., Fulgione A., Nocerino N., D’Agostino N., Avitabile C., Frusciante L., Barone A., Capuano F., Capparelli R. A novel synthetic peptide from a tomato defensin exhibits antibacterial activities against Helicobacter pylori. J. Pept. Sci. 2012;18:755–762. doi: 10.1002/psc.2462. [DOI] [PubMed] [Google Scholar]
- 41.Iwahori A., Hirota Y., Sampe R., Miyano S., Takahashi N., Sasatsu M., Kondo I., Numao N. On the antibacterial activity of normal and reversed magainin 2 analogs against Helicobacter pylori. Biol. Pharm. Bull. 1997;20:805–808. doi: 10.1248/bpb.20.805. [DOI] [PubMed] [Google Scholar]
- 42.Kuo Y.T., Liou J.M., El-Omar E.M., Wu J.Y., Leow A.H.R., Goh K.L., Das R., Lu H., Lin J.T., Tu Y.K., et al. Asian Pacific Alliance on Helicobacter and Microbiota. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017;2:707–715. doi: 10.1016/S2468-1253(17)30219-4. [DOI] [PubMed] [Google Scholar]
- 43.Hernández Ceruelos A., Romero-Quezada L.C., Ruvalcaba Ledezma J.C., López Contreras L. Therapeutic uses of metronidazole and its side effects: An update. Eur. Rev. Med. Pharmacol. Sci. 2019;23:397–401. doi: 10.26355/eurrev_201901_16788. [DOI] [PubMed] [Google Scholar]
- 44.Mahachai V., Vilaichone R.K., Pittayanon R., Rojborwonwitaya J., Leelakusolvong S., Maneerattanaporn M., Chotivitayatarakorn P., Treeprasertsuk S., Kositchaiwat C., Pisespongsa P., et al. Helicobacter pylori management in ASEAN: The Bangkok consensus report. J. Gastroenterol. Hepatol. 2018;33:37–56. doi: 10.1111/jgh.13911. [DOI] [PubMed] [Google Scholar]
- 45.Levy S.B. The 2000 Garrod lecture. Factors impacting on the problem of antibiotic resistance. J. Antimicrob. Chemother. 2002;49:25–30. doi: 10.1093/jac/49.1.25. [DOI] [PubMed] [Google Scholar]
- 46.Losurdo G., Leandro G., Principi M., Giorgio F., Montenegro L., Sorrentino C., Ierardi E., Di Leo A. Sequential vs. prolonged 14-day triple therapy for Helicobacter pylori eradication: The meta-analysis may be influenced by ‘geographical weighting’. Int. J. Clin. Pract. 2015;69:1112–1120. doi: 10.1111/ijcp.12687. [DOI] [PubMed] [Google Scholar]
- 47.Tang Y., Tang G., Pan L., Zhu H., Zhou S., Wei Z. Clinical factors associated with initial Helicobacter pylori eradication therapy: A retrospective study in China. Sci. Rep. 2020;10:15403. doi: 10.1038/s41598-020-72400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.PRISMA. [(accessed on 1 May 2021)]. Available online: http://www.prisma-statement.org/
- 49.United Nations. [(accessed on 29 June 2021)]. Available online: https://www.un.org/press/en/2020/sc14093.doc.htm.
- 50.Munn Z., Moola S., Riitano D., Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int. J. Health Policy Manag. 2014;3:123–128. doi: 10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data searched and extracted from the included studies in this systematic review are available upon request to corresponding author.