Abstract
Simple Summary
Chemotherapeutic DNA-damaging agents targeting replication are widely used but predictive rationales for drug combinations and patient selection still need clinical definition. Here, we review cancer-associated replication stress (RepStress) and its genomic signature, and propose how to utilize RepStress-targeted therapies in the context of ATR inhibitors and Schlafen 11 (SLFN11).
Abstract
Precision medicine aims to implement strategies based on the molecular features of tumors and optimized drug delivery to improve cancer diagnosis and treatment. DNA replication is a logical approach because it can be targeted by a broad range of anticancer drugs that are both clinically approved and in development. These drugs increase deleterious replication stress (RepStress); however, how to selectively target and identify the tumors with specific molecular characteristics are unmet clinical needs. Here, we provide background information on the molecular processes of DNA replication and its checkpoints, and discuss how to target replication, checkpoint, and repair pathways with ATR inhibitors and exploit Schlafen 11 (SLFN11) as a predictive biomarker.
Keywords: replication stress, ATR, schlafen 11, berzosertib, Camptothecin, cisplatin, Ceralasertib, Adavoserib, PARP, olaparib, niraparib, talazoparib, Rucaparib, temozolomide
1. Introduction
Targeting the genome and DNA replication were the first approaches to the chemical treatment of cancers. Most of the drugs were discovered empirically and later found to have highly specific targets and molecular mechanisms of action [1]. For example, DNA topoisomerase (TOP) inhibitors were discovered as potent anticancer agents prior to the elucidation of their molecular mechanism of action by selectively trapping of the TOP cleavage complexes (TOPcc) by interfacial inhibition [1,2,3]. DNA replication is widely targeted in the anticancer armamentarium by various replication stress (RepStress) inducers including TOP inhibitors, alkylating agents, platinum compounds, and poly (ADPribose) polymerase (PARP) inhibitors [4,5,6]. While RepStress inducers cause DNA damage directly, additional targeting of the replicative stress response signaling pathways is emerging as a potential combination strategy by which detrimental RepStress and irreparable DNA breakage accumulate [7,8]. For the clinical success of these RepStress-targeted therapies, identification of reliable biomarkers is essential. For instance, the selection of patients according to the expression of Schlafen 11 (SLFN11) has recently been reported to be correlated with improved response in several human cancer types [9,10,11].
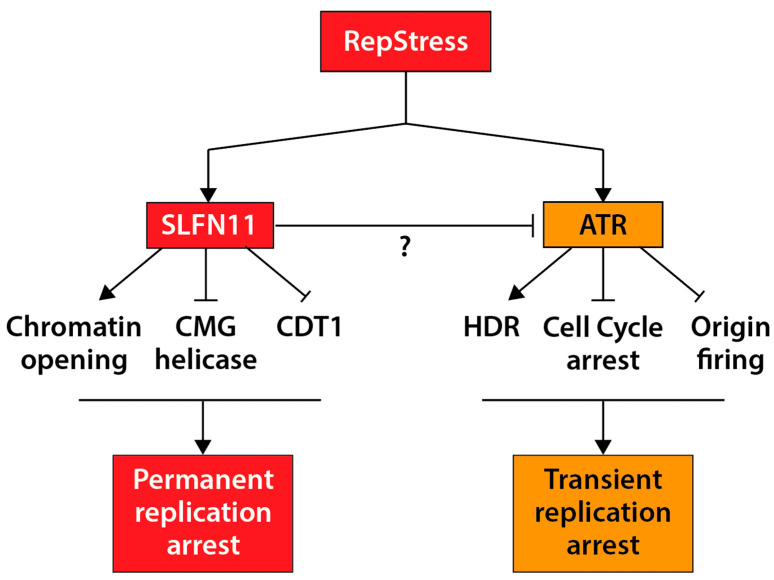
Here, we review precision medicine in the context of RepStress-targeted agents, which primarily act by interfering with DNA replication. We summarize key molecular features of DNA replication and discuss how to exploit the RepStress, ATR (ataxia telangiectasia mutated and Rad 3-related) protein kinase inhibitors and SLFN11 for cancer therapy. Figure 1 outlines the molecular mechanisms of action of SLFN11 and how they differ from ATR; they are detailed in Section 5.1.
Figure 1.
The SLFN11 and ATR pathways in response to RepStress. SLFN11 irreversibly blocks DNA replication under RepStress by promoting chromatin opening, blocking the CMG helicase complex, and promoting the degradation of CDT1 (see Section 5.1 for details). In contrast, ATR transiently halts DNA replication by arresting cell cycle and prohibiting origin firing, thereby enabling homology-directed repair (HDR).
2. Precision Medicine
Precision medicine is rapidly expanding for cancer diagnosis, treatment, and prognosis [12] providing a roadmap to classify cancer patients into sub-groups that differ in their susceptibility to a particular tumor incidence and evolutive outcome, or in their response to anticancer therapies (Figure 2). Knowledge of this framework should enable cancer patients to receive the most effective and safest treatments, while avoiding the use of ineffective therapies with toxic side effects.
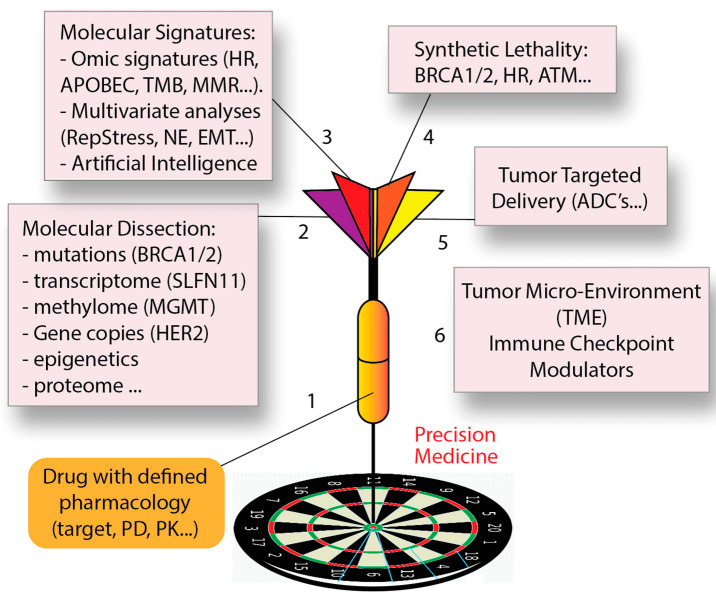
Figure 2.
Precision medicine in the context of DNA-targeted therapies. Critical steps are: (1) Defining the molecular and clinical pharmacology of the drugs; (2) molecular dissection of tumors by multi-omics approaches; (3) identification of molecular signatures combining omic parameters; (4) determination of synthetic lethal interactions; (5) Targeted-delivery of drugs to cancer cells; and (6) adjuvant therapy by targeting the tumor microenvironment and immune checkpoints.
The paradigm for precision medicine is exemplified by the MATCH (molecular analysis for therapy choice) clinical trials with emphasis on kinase inhibitors and phase 0 trials [13,14,15,16,17,18,19]. The identification of predictive and pharmacodynamic biomarkers has also been a recent focus for the development of immune checkpoint inhibitors [20] as they yield durable clinical benefit in relatively small numbers of patients, while producing potentially severe toxicity and high financial burden for a large number of non-responding patients [21].
Extending precision medicine to the large number of widely used agents targeting the genome and DNA replication remains a mostly unmet need. Completion of the Human Genome Project has paved the way for groundbreaking advances in sequencing technologies that have profoundly impacted precision medicine by providing access to the underlying genetic codes of cancer development and progression [22]. The combination of next-generation sequencing with high-throughput analyses is also providing massive information to elucidate the cancer genomes. In parallel, new drugs have been developed for specific molecular targets. Trastuzumab and imatinib have opened the gate for targeted cancer therapy based on tumor genetic characteristics [23]. They are routinely used to treat patients with HER2-amplified metastatic breast cancer and BCR-ABL fusion-positive chronic myelogenous leukemia, respectively. However, omics analyses remain technically complex and require a bioinformatic infrastructure limited to only a small number of cancer centers. In addition, genetic mutations provide a limited spectrum of therapeutic options, and it is increasingly clear that transcriptome analyses based on whole genome RNA sequencing, gene copy number determinations, epigenetic and proteomic analyses need to be developed and included (part 2 in Figure 2). For instance, lack of methylguanine methyltransferase (MGMT) expression predicts potential response to temozolomide [24,25] and high Schlafen 11 (SLFN11) expression potential response to DNA replication-targeted therapies (Table 1) [11].
Table 1.
Pharmacological targets and drugs inducing replication stress.
| Primary Target | Clinically Approved Drugs | Drugs in Development or Preclinical | How They Target Replication | Predictive Biomarker |
|---|---|---|---|---|
| Nucleoside analogs | Cytarabine Gemcitabine 5-azacytidine Decitabine | Incorporation into newly synthesized DNA blocking polymerases | SLFN11 RepStress | |
| Ribonucleotide reductase (RNR) | Hydroxyurea Gemcitabine | Depletion of deoxyribonucleotides (dNTPs) | SLFN11 RepStress | |
| Thymidylate synthetase (TS) | Methotrexate Pemetrexed | Thymidine depletion | SLFN11 RepStress | |
| Dihydrofolate reductase (DHFR) | Methotrexate Pemetrexed | Thymidine depletion | SLFN11 RepStress | |
| POLA, POLE, POLD | Aphidicolin | Polymerase arrest with chain termination | SLFN11 RepStress | |
| POLA1 | CD437 | SLFN11 | ||
| POLQ | Blocks MHEJ | |||
| TLS polymerases | Blocks POL H | |||
| DNA template MGMT | Temozolomide | 06-methyl-guanine-SSB | MGMT, MMR, SLFN11, RepStress | |
| DNA template | Nitrosoureas | DNA adducts-SSBs | ||
| DNA template | Cyclophosphamide | DNA-DNA crosslinks | ||
| DNA template | Cisplatin; Carboplatin Oxaliplatin | DNA-DNA crosslinks & DPC | SLFN11 HR | |
| TOP1 trapping | Camptothecins (topotecan, irinotecan, belotecan) TTT is (Enhertu, Trodelvy | Indenoisoquinolines (LMP400, LMP776, LMP744) PLX038 CBX-12 | Replication blocks (3′-DPC, SSBs) ≥ Replication run-off | SLFN11 HR RepStress |
| TOP2 trapping | Doxorubicin Epirubicin Etoposide mitoxantrone | Replication blocks (5′-DPC, SSBs & DSBs) | SLFN11 HR RepStress | |
| PARP trapping (catalytic inhibition) | Talazoparib Olaparib Niraparib Rucaparib | Veliparib | Replication blocks Defective DSB repair | SLFN11 HR RepStress |
| ATR | Berzosertib (M6620) Ceralasertib (AZD6738)BAY1895344 M4344, M1774 | Abrogate the cell cycle checkpoints as well as CHK1 and WEE1 | ATM, TP53, APOBEC3B, ARID1A, MYC RepStress | |
| CHK1 | Prexasertib SRA737 | Abrogates cell cycle checkpoint-activates CDKs | ||
| WEE1 | Adavosertib (AZD1775) | Abrogates cell cycle checkpoint-activates CDKs | ||
| NEDD8 (Cullin neddylation) | Pevonedistat (TAK-924/MLN4924) TAS4464 | CDT1 stabilization ≥ unscheduled origin firing | SLFN11 RepStress |
Yet, single gene features (mutations and expression) are generally insufficient for predicting drug response. Multivariate analyses and artificial intelligence (AI) approaches are likely to increase the predictive value of omics analyses. For instance, cancer cells expressing high SLFN11 but with high expression of the drug efflux genes ABCB1 (encoding P-glycoprotein) are unlikely to respond to doxorubicin [26]. Multivariate analyses can be combined to provide molecular signatures such as mismatch repair (MMR), homologous recombination (HR), neuroendocrine (NE), epithelial mesenchymal (EMT) signatures (part 3 in Figure 2). Moreover, molecular analyses have led to the concept of synthetic lethality with the use of poly (ADP ribose) polymerase (PARP) inhibitors for BRCA- and HR-deficient tumors [27,28] (part 4 in Figure 2).
Ultimately, precision medicine can be achieved by concentrating the therapeutic delivery to tumors while sparing normal tissues, as is the case for surgery and radiotherapy. This goal is now achievable with tumor-targeted antibody drug conjugates (ADC) that use DNA-targeted payloads such as the DNA alkylating agent PBD (pyrrolobenzodiazepine dimer) [29] or the TOP1 inhibitors, SN-38 and deruxtecan [30] (part 5 in Figure 2). Finally, targeting the tumor microenvironment (TME) with immune checkpoint inhibitors that block the programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA4) pathways is becoming routine and can complement the above approaches for precision medicine (part 6 in Figure 2).
3. DNA Replication, Replication Stress (RepStress), and Maintenance of Genome Stability
Genome instability is a hallmark of cancer [31,32]. It is not only a driving force for tumorigenesis but also a key target of anti-cancer agents. These include not only the recently developed agents targeting Poly (ADP-ribose) polymerase (PARP) and ataxia telangiectasia and Rad3-related protein (ATR), but also the classical DNA-targeted chemotherapeutic agents such as antimetabolites, alkylating agents, platinum derivatives, and TOP inhibitors (Table 1). Among the various cellular processes, DNA replication is essential for preserving genome integrity. Its deregulation caused by exogenous or endogenous stresses results in replication fork collapse and DNA damage, frequently driving cancer development [4].
Many clinical anticancer drugs inhibit DNA replication by generating replicative stress through a range of mechanisms, ultimately inducing genomic damage and killing the cancer cells [33] (Table 1). However, molecular surveillance and repair machineries in cancer cells hinder the therapeutic effects of RepStress-targeted anticancer agents through DNA lesion detection, repair, and cell cycle control [34]. The replicative DNA damage response (DDR) pathways are among the well-defined cellular processes in initiation, sensing, and correction of DNA lesions. Thus, understanding the DNA replication-targeted agents and DDR is required for providing biomarkers (Figure 2) that can be utilized for selecting patients who should benefit from targeted therapies.
3.1. Overview of DNA Replication
The coordination of DNA replication is highly regulated in a timely manner. DNA replication is also an orchestrated and dynamic process consisting of multi-subunit protein complexes for the accurate timing of replication initiation, elongation, and termination, chronologically encompassing the G1, S, and G2 phases of the cell cycle (Figure 3).
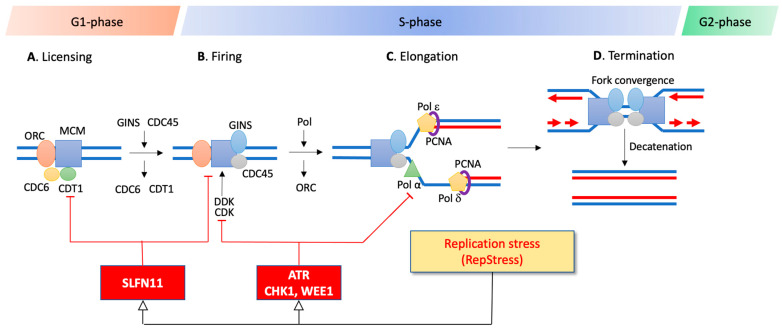
Figure 3.
Schematic representation of the DNA replication steps in the context of the cell cycle and of the ATR and SLFN11 replication checkpoints. DNA replication is initiated by recognition of replication origins scattered along the genome. A, In G1, active origins of replication are licensed by ORC, CDC6, CDT1, and MCM2-7, forming the pre-replication complex (pre-RC). B, At the G1-S transition, the pre-RC complex is fired by activation of kinases (DDK and CDK) and loading GINS and CDC45, thereby unwinding the DNA duplex and initiating the replication by DNA polymerases. C, Active replication forks are elongated by the replication machinery including helicases, PCNA and DNA polymerases throughout S-phase. D, The replication process is terminated when the bidirectional replication forks merge. The RepStress is monitored and modulated by replication checkpoints, ATR and SLFN11, which are both recruited to RPA filaments. While ATR produces a transient cell cycle arrest allowing DNA repair, SLFN11 produces an irreversible replication arrest.
In G1 where chromosomes are relaxed, replication is initiated by the assembly of replication initiation factors on replication origins scattered along chromosomes [4,35]. Remarkably, only a small subset of replication origins is licensed by the loading of the six-subunit origin recognition complex (ORC), the cell division cycle 6 (CDC6), the chromatin licensing, and DNA replication factor 1 (CDT1). This follows the recruitment of the replicative DNA helicase minichromosome maintenance complex 2-7 (MCM2-7) as the pre-replicative complex (pre-RC) that remains in an inactive state prior to S-phase. Subsequentially, CDC6 and CDT1 are released from the initiated replicons to prevent the re-licensing of same replication origins [36,37]. Indeed, the licensing of replication origins at pre-RC needs to occur restrictively in the G1-phase [38]. Otherwise, it can cause replication restart from already fired replication origins during S-phase, which leads to replication catastrophe and DNA breakage [39]. Cyclin-dependent kinases (CDKs) are key regulators controlling new origin firing from late G1 phase to mitosis to ensure the duplication of the entire genome only once per cell division.
In S-phase where most of the genome is duplicated, the MCM helicases trigger origin firings by their phosphorylation mediated by the CDKs and the Dbf4-dependent kinase (DDK), subsequentially recruiting CDC45 and GINS within CMG active helicase complexes [40]. At the same time, some of the MCM2-7 complex on replication origins remain dormant. Yet, these dormant origins can be activated under RepStress for compensating main origins have given rise to aborted replicons [36,41]. The activated CMG helicase complex unwinds and separates the DNA double-helix into single-stranded DNA, forming the classical Y-shaped replication forks (Figure 3). Once the DNA double-strands are separated, replication forks spatially recruit a large set of proteins to form replisomes with DNA polymerases, helicases, PCNA (proliferating cell nuclear antigen), primase, RNase H, ligases, and TOPs [42]. DNA synthesis is initiated by DNA polymerase α from RNA primers that are replaced with DNA at the end of DNA replication. DNA polymerases ε and δ extend the leading and lagging strands, respectively (Figure 3). PCNA plays an essential role in replication as processivity factor for the polymerases and for switching the DNA polymerases upon RepStress and for translesion synthesis (TLS) [43].
DNA replication is terminated in G2-M by the convergence of replication forks [44]. At the end of the S- and in G2-phases, all remaining damage in the newly replicated DNA caused by RepStress must be resolved to ensure that the whole genome information is completely duplicated before the chromosomes are segregated during mitosis.
3.2. Replication Stress (RepStress)
Because of their molecular complexity and dynamism, replication machineries are prone to encounter various stress conditions derived from spatial and temporal obstacles including dNTP pool depletion, collisions between replication forks and/or transcription, DNA single- and double-strand breaks (SSBs and DSBs), unscheduled firing of dormant origins, topological stress resulting from DNA torsional tensions and condensed heterochromatin regions [4]. Additionally, replication can be hindered by DNA secondary structures that interfere with the loading of replication proteins and/or opening of the double helix, including mismatched DNA bases, misincorporated ribonucleotides, abnormal DNA structures such as guanosine quartets, palindromic cruciforms and knots, as well as DNA-DNA and DNA-protein crosslinks, which can all impair replication fork progression. Furthermore, shortage of deoxyribonucleotides (dNTPs), core proteins comprising the replication machinery, and the uncoupling of regulatory factors from replisomes can also interfere with replication. They strongly disturb DNA synthesis and replication progression, resulting in RepStress with fork stalling and collapse, single-ended DNA double-strand breaks (seDSBs), and unscheduled termination of replication [33].
Once replication forks are stalled, replication protein A (RPA) complexes accumulate on long stretches of single-stranded DNA (ssDNA), eventually causing global RPA exhaustion that leads to cellular catastrophe and DNA breakage [33,45]. These RPA filaments are key for the activation of the ATR and the SLFN11 response pathways (Figure 3).
3.3. Endogenous RepStress
“Sustaining proliferative signaling” is one of the six primary hallmarks of cancers highlighted by Weinberg and Hanahan [46]. To induce sustained proliferation, cancer cells rely on three main genetic drivers: (i) activation of oncogenes such as MYC, (ii) inactivation of tumor suppressors such as TP53 and RB1, and (iii) a mutator phenotype related to reduced replication fidelity such as DNA polymerase alterations [47]. Additionally, oncogene-induced unscheduled firing of dormant origins and failure in sustaining DNA damage response and DNA repair lead to genomic instability including DNA hypermutations, gene deletions and amplifications, loss of heterozygosity, chromosomal rearrangements, and chromosome gain or loss (aneuploidy), which all contribute further to cancer progression and chemoresistance.
3.4. RepStress Induced by Clinically Approved Chemotherapeutic Agents
Many conventional chemotherapeutic agents are RepStress inducers (Table 1). They act by multiple mechanisms including depletion of dNTP pools or generation of cytotoxic DNA obstacles such as mismatches, abasic sites, breaks, DNA-protein crosslinks (DPCs) and DNA-DNA crosslinks that cause replication fork stalling. Direct inhibition of the replicative polymerases by chain terminating drugs such as cytarabine is another mechanism for stalling replication forks.
The various DNA polymerases carry out DNA synthesis with high proofreading ability and participate in replication-coupled repair processes [48]. Thus, blocking polymerase activity can be a pivotal therapeutic strategy for cancer treatment. In general, the activity of the DNA polymerases (Pol α, Pol δ, and pol ε) that carry out DNA synthesis is blocked by the incorporation of nucleoside analogs [49] (Table 1). Deoxycytidine nucleoside analogs target DNA polymerases by competing with dCTP, thereby resulting in failure of elongation of the nascent DNA strands [50]. Cytarabine (cytosine arabinoside [Ara-C]) specifically shows enhanced cytotoxicity in the BER enzyme 8-oxoguanine DNA glycosylase-1 (OGG1)-deficient acute myeloid leukemia cells [51]. A functional genomic screen in mesothelioma uncovered that loss of function of BRCA1-associated protein 1 (BAP1) is associated with vulnerability to ribonucleotide reductase (RNR) inhibitors such as gemcitabine [52].
Alkylating agents and platinum-based compounds used to treat a wide range of solid tumors directly attacking the DNA bases forming covalent DNA adducts that interfere with the progression of DNA polymerases. Expression of O-6-methylguanine-DNA methyltransferase (MGMT) is a commonly used biomarker for the alkylating agent temozolomide in glioma and glioblastoma patients [25], and temozolomide has recently shown to be effective in combination with the base excision repair inhibitor TRC102 (methoxamine) in patients with relapsed solid tumors and lymphomas [53] (Table 1). The sensitivity of platinum compounds including cisplatin and carboplatin is increased in triple-negative breast and serous ovarian cancers with overexpression of the Bloom (BLM) helicase [54]. Besides, BRCA-deficient cells are highly vulnerable to treatment of platinum-based compounds [55,56] and the TOP inhibitors [57,58].
During DNA replication, TOPs transiently cut DNA strands to resolve topological problems ahead of and behind replicating forks [59]. TOP inhibitors trap TOP cleavage complexes (TOPccs), leading to RepStress and DNA damage [30,59]. Recent studies found that homologous recombination defects (HRD and BRCAness) and SLFN11 expression increase susceptibility to TOP1 inhibitors [10,11,30] (Table 1). Additionally, the TOP1 inhibitors are currently being exploited as toxic payloads in tumor-targeted drug delivery by using liposomes, PEGylation, and antibody drug conjugates (ADC) to limit their toxic side-effects by selectively targeting tumor cells (part 4 in Figure 2) [30].
In a similar manner as TOP trapping [2,28], the most potent PARP inhibitors such as talazoparib and niraparib trap PARP1 on pre-existing DNA lesions along with their inhibitory activity of PARP, making them potent RepStress inducers [28,60]. The therapeutic benefits of PARP inhibitors are significantly associated with HRD cancers and expression status of SLFN11 (Table 1) [61,62].
Enhancing replicative damage by blunting the RepStress response pathways with ATR inhibitors is discussed below [8,63,64,65,66,67]. Synthetic lethality for the PARP inhibitors and platinum derivatives in HRD cancers is being applied for cancer treatment [68,69] and recent studies in animal models suggest such synthetic lethality for TOP1 inhibitors [10,58]. The PARP inhibitors, the TOP1 inhibitors, and platinum derivatives are also highly synergistic in combination with ATR and CHK1 inhibitors.
3.5. RepStress Induced by Non-FDA-Approved Drugs Targeting Replication
Aphidicolin, a natural tetracyclic alkaloid widely used in preclinical models, and which acts by blocking the incorporation of dCTP through its binding at the interface of the Pol α active site and rotating the template guanine [70], is not used clinically because of its poor pharmacokinetics [70]. Recently, a small molecule inhibitor of Pol δ (POLD) (Zelpolib) has been found to bind to the active site of the polymerase, inhibiting DNA replication and demonstrating synergy with the PARP inhibitors [71]. A small molecule inhibitor (PNR-7-02) of Pol η (POLH) that functions in DNA (TLS) also shows synergistic effects with cisplatin in HAP1 chronic myelogenous haploid leukemia cells [72] and inhibitors of the microhomology end-joining (MHEJ) polymerase, pol θ (POL Q) are being developed to overcome resistance to the PARP inhibitors [73,74]. Additionally, the retinoic-acid-derived inhibitor of pol α (POLA1), CD437 (AHPN), and ST1926 were recently reported to exhibit anticancer activity [75].
Given that the replicative MCM2-7 helicase complex plays a critical role by unwinding the DNA helix in the replisome during replication initiation and elongation (Figure 3), efforts to develop anticancer agents targeting the MCM helicase protein complex are ongoing [76,77,78]. Post-translational modifications of PCNA and inhibitory interactions with key regulators through their PCNA interacting protein box (PIP-Box) motif are being pursued to design anticancer drugs [79,80]. A small molecule inhibitor (T2AA: T2 amino alcohol) targeting monoubiquinated PCNA and inhibiting DNA replication and TLS significantly increases cisplatin-induced DSBs [81]. Another PCNA inhibitor PCNA-I1S was found to selectively bind PCNA, suppress its chromatin association, and to suppress cancer cell growth [82,83]. Furthermore, PCNA is functionally engaged with the stability of replication modulator proteins for DNA synthesis and replicative DNA damage response.
During DNA replication, the assembly and disassembly of replication complexes and their regulators are critically regulated by Cullin-RING (Really Interesting New Gene) E3 ubiquitin ligases (CRL) [84]. The licensing factor CDT1 (Figure 3) must be degraded by CRL1SKP2 (at the G1/S transition) and CRL4CDT2 (during S-phase) through the ubiquitin proteasome pathways (UPP) driven by PCNA interactions. Dysregulation of CDT1 stability causes replication reactivation and polyploidy, resulting in cancer cell death and potential chemoresistance [85,86,87]. The degradation of CDK inhibitors such as p27, p21, and p57 during cell cycle transition is also controlled by CRL E3 ubiquitin ligase complexes, suggesting that the ubiquitin pathways can be a promising target for cancer treatment [88]. Two small molecules, pevonedistat (TAK-924/MLN4924) and TAS4464, are in clinical trials [89,90]. They suppress the activity of the NEDD8-activating enzyme, interfering with NEDD8 conjugation (neddylation) that activates CRLs as a key post-translational modification [91]. Thus, deliberately forcing RepStress in cancer cells can be a promising strategy for chemotherapy because it can be combined with the pre-existing RepStress (see Section 3.1) and the defective repair pathways of the cancer cells [5].
3.6. Detecting RepStress in Cancers
The RepStress leads to excessive RPA accumulation, which is recognized by ATR-interacting protein (ATRIP), leading to the recruitment of ATR. ATR belongs to the phosphatidylinositol 3-kinase (PI3K) like kinase (PIKK) family along with ATM (ataxia telangiectasia mutated) and DNA-PK (DNA-dependent protein kinase), which are also involved in the DDR and phosphorylate a number of overlapping proteins [92]. Localization of ATRIP and ATR to RPA filaments is insufficient for the activation of ATR. It requires the recruitment of the ATR activators TOPBP1 or ETAA1. While TOPBP1 is recruited by the 9-1-1 (RAD9-RAD1-HUS1) complex in response to RepStress, ETAA1 (Ewing tumor associated antigen 1) is recruited by RPA during unperturbed S-phase [93]. ATR subsequentially activates the downstream effector protein checkpoint kinase 1 (CHK1) to arrest cell cycle and stabilize replication forks [94,95]. CHK1 negatively regulates CDK activity through phosphorylation and inactivation of CDC25, while WEE1 directly inactivates CDKs by phosphorylation, and acts as a G2/M checkpoint to prevent mitotic entry of incompletely replicated DNA [96]. The DSBs generated in the context of RepStress are sensed by the Mre11-Rad50-Nbs1 (MRN) complex [33], which triggers the activation of ATM [92,97]. ATR and ATM phosphorylate the histone variant H2AX (at Ser139), which is referred to as γH2AX, and whose detection is a sensitive biomarker for DSBs and RepStress [98,99].
Detecting RepStress in tumor samples remains a challenge. Measures of RepStress including ssDNA, RPA levels bound to ssDNA, and γH2AX are widely used in experimental settings but are not optimized for use in large cohorts of clinical biopsy samples. Proteomic biomarkers to be considered for reliable immunohistochemistry include RPA1 (#IHC-00409, Bethyl labs, Montgomery, TX, USA), RPA2 (#IHC-00417, Bethyl labs), hyperphosphorylated RPA2 (S4/S8, #A300-245A, Bethyl labs), and γH2AX (S139, #07-627, Millipore, Burlington, MA, USA).
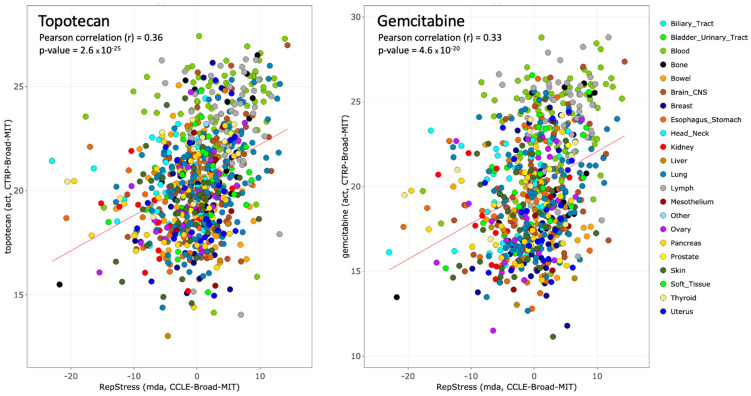
We recently developed a transcriptional profiling-based approach—the RepStress gene signature—that characterizes the cellular response to RepStress at a functional network level. The RepStress gene signature is a weighted expression signature encompassing 18 genes—SRSF1, SUV39H1, GINS1, PRPS1, KPNA2, AURKB, TNPO2, ORC6, CCNA2, LIG3, MTF2, GADD45G, POLA1, POLD4, POLE4, RFC5, RMI1, RRM1—derived by leveraging established cellular characteristics that portend high RepStress including amplification of MYC and its paralogs (MYCN and MYCL), expression of phosphorylated CHK1 (p-CHK1), and sensitivity to the CHK1 and WEE1 cell cycle checkpoint inhibitors [100]. The RepStress gene signature was recently validated as a response predictor for ATR inhibitors across a set of 14 cell lines from different tissues of origin [64]. Figure 4 also shows its predictive value also for topotecan and gemcitabine in the Broad Institute database.
Figure 4.
Predictive value of the RepStress genomic signature in the CCLE-CTRP Broad Institute cancer cell line databases for the TOP1 inhibitor topotecan (left) and the replication inhibitor gemcitabine (right). Data were generated from CellMinerCDB (https://discover.nci.nih.gov/cellminercdb/ (accessed on 9 February 2021)).
4. Targeting the RepStress Response with Replication Checkpoint Inhibitors
Although biallelic loss of ATR is early embryonic lethal [101] and hypomorphic ATR mutations produce Seckel syndrome with severe microcephaly and growth retardation [102]; ATR inhibitors are surprisingly well-tolerated in patients [8]. Moreover, mice with Seckel syndrome crossed with p53-deficient mice are not cancer-prone [103] and ATR has been shown to support homologous recombination repair (HRR) [63,104], which provides the rationale for developing ATR inhibitor as anticancer treatments. Most relevant to the development of clinical ATR inhibitors are the findings that genetic inactivation of ATR and ATM produce a strong synergy with DNA-targeted agents such as TOP1 inhibitors [57,85,105,106] and PARP inhibitors [60,107]. The rationale for targeting ATR is that cancer cells are under RepStress, and that their survival require active replication checkpoints, namely ATR, CHK1, and WEE1, especially when the RepStress is exacerbated by DNA-targeted agents [7] (see Section 3 and Table 1). Moreover, ATR inactivation has been proposed to be synthetic lethal with ATM inactivation because of the overlapping substrates and functions of ATR and ATM, and because ATM appears inactivated in a significant proportion of cancers (10–16% of colorectal, gastric, lung and prostate cancers) [92].
Three ATR inhibitors are in advanced clinical development: Berzosertib (M6620, alias VX-970), Ceralasertib (AZD6738), and Elimusertib (BAY1895344) [8,65,66,67,108]. Additional drugs are in early development comprising M4344 and M1774 (EMD-Serono Merck, Darmstadt, Germany), ART0380 (Artios Pharma, New York, NY, USA) and RP-3500 (Repare Therapeutics, Quebec, QC, Canada) [64]. Currently, over 30 clinical trials are ongoing with ATR inhibitors alone or in combination with DNA damaging agents including nucleoside analogues, platinum-based agents, TOP and PARP inhibitors [5,6,63,64,66,109,110,111]. Broadly, these clinical trials aim to exploit: (i) the synthetic lethal interactions of the ATR-CHK1 pathway with the overexpression of oncogenes (RAS, APOBEC3A, and c-MYC), deficiency of ATM and ARID1A (AT-rich interaction domain 1A) and SLFN11 [6,63,65,85,92] using ATR inhibitor monotherapy or (ii) the dependence of high RepStress tumors on ATR, generally in combination with RepStress-inducing agents (described in Section 3.4 and Section 3.5).
The phase I trials of Elimusertib and Berzosertib provided the clinical evidence of durable single-agent antitumor activity of an ATR inhibitor in patients with advanced cancers with ATM aberrations (ATM protein expression loss and/or ATM deleterious mutation), supporting a synthetically lethal interaction between ATM deficiency and ATR inhibition [66,67]. We recently reported that targeting RepStress provides clinical benefit in cancer patients [8]. Durable regressions were observed in response to a combination of Berzosertib and Topotecan in patients with chemotherapy-resistant small cell neuroendocrine cancers (SCNCs), tumors with high endogenous RepStress [8]. Notably, tumors responding to the combination displayed marked enrichment for pathways associated with cell-cycle progression and DNA repair, including E2F target genes, the G2-M checkpoint, ATM, ATR, and Fanconi DNA repair pathways, consistent with responding tumors harboring a RepStress phenotype. Adding Berzosertib to gemcitabine improved progression-free survival compared with gemcitabine alone in platinum-resistant high-grade serous ovarian cancer, which like SCNCs exhibit high RepStress [111]. Additional promising combination trials are ongoing with platinum derivatives [66,67].
Several questions need to be considered for the further clinical development and rational use of ATR inhibitors. First, how do the ATR inhibitors differ from each other. Our recent preclinical studies showed a broad range of potency among the ATR inhibitors with M4344 being the most potent followed by Elimusertib (BAY1895344), Berzosertib (M6620), and Ceralasertib (AZD6738) [64]. Elimusertib and Ceralasertib are given orally while Berzosertib is given intravenously. The oral route is advantageous for avoiding infusions and repeated administrations. Yet, the intestinal absorption may vary from patient to patient and compliance with treatment can be an issue. The differential pharmacokinetics of ATR inhibitors is another important consideration. Although their plasma elimination half-life are comparable (approximately 12 h) [65,66,67,108], serum protein binding and thus free drug availability, tumor penetration/retention, and blood-brain barrier penetration may be different.
A second question is the selection of patients who are likely to respond to ATR inhibitors. In our recent preclinical study, we found that the transcriptomic RepStress signature could predict drug response. Whether the RepStress signature will closely predict clinical response to ATR inhibitors needs to be further tested. An additional established predictor is ATM deficiency, which can be viewed as synthetic lethality as ATM and ATR exert overlapping functions and share many cellular targets, thereby creating an overreliance on the ATR pathway for DNA repair and replication checkpoints [63,92,104]. Here the challenge is to confirm loss or reduction of ATM in tumor samples. Immunohistochemistry remains to be routinely applied in the clinic and scoring ATM mutations is made difficult by the large size of the ATM gene and difficulties of predicting whether a certain mutation is deleterious or a variant without functional significance [67,92,112].
A third consideration regarding the clinical use of ATR inhibitors is how to limit the toxicity to normal tissues. Although quiescent normal cells are likely to be spared by ATR inhibitors, effects on replicating cells will be dose-limiting. This practical challenge is being addressed by adjusting doses and schedules in the combinations of ATR inhibitors and DNA-targeted chemotherapies [8,65,66]. Another approach being explored in our NCI clinic is to combine ATR inhibitors with tumor-targeted DNA damaging agents, such as tumor-targeted TOP1 inhibitors (TTT is). This approach is based on our “Gap-schedule” protocol with tumor-targeted delivery of DNA damaging agents [30] (see part 4 in Figure 2). In short, the ATR inhibitor is given during the interval between the administrations of the TTT is when the normal tissues have cleared the TOP1 inhibitors, while the tumors remain loaded with the TOP1 inhibitor [30].
The clinically developed CHK1 inhibitor (SRA737) also confirmed synthetic lethal interaction with the B-family of DNA polymerases (POLA1, POLE, and POLE2) [113]. Another CHK1 inhibitor (LY2606368/prexasertib) synergistically suppressed tumor burden with DNA-damaging agents in subtypes of medulloblastoma [114]. Furthermore, prexasertib led to HRD deficiency in triple-negative breast cancer cells, thereby promoting sensitivity to the PARP inhibitor olaparib [115]. As for the ATR and CHK1 inhibitors, the clinical WEE1 inhibitor Adavosertib (AZD1775) increases unscheduled origin firings that lead to replication fork stalling, implying it could be synergistic with replicative DNA damaging agents [116]. Adavosertib also enhances the anticancer efficacy of carboplatin in patients with TP53-mutated ovarian cancer [117].
5. Exploiting SLFN11 as a Therapeutic Biomarker
In 2012, we discovered that SLFN11 is a dominant predictor of response to replication damaging agents widely used in cancer chemotherapy including TOP inhibitors, nucleoside analogues, platinum-based, and alkylating agents [118] (Table 1). This observation was simultaneously validated by the Broad Institute team for the TOP1 inhibitors [119], and further studies have extended the causality of SLFN11 expression to PARP inhibitors regardless of BRCA1/2 mutations [62,120].
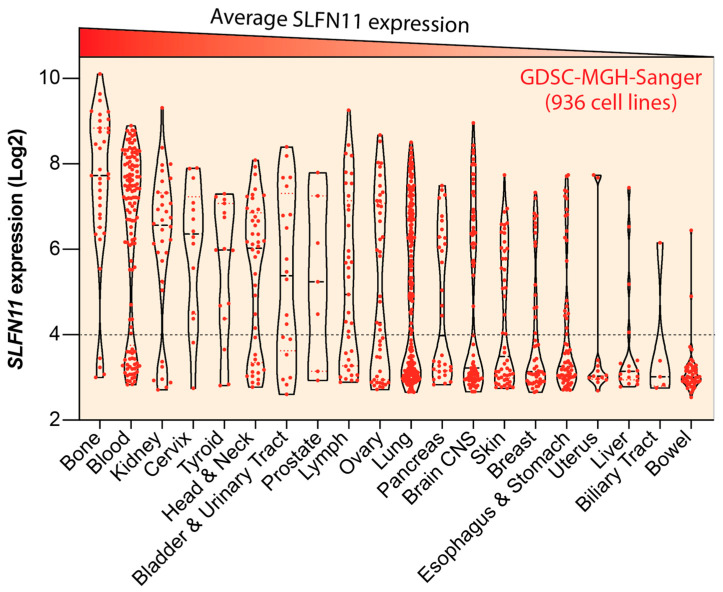
Remarkably, SLFN11 expression is bimodal in cancer cell lines with ~50% of the NCI, CCLE and GDSC cancer cell lines expressing high SLFN11 and the other ~50% cell lines not expressing SLFN11 (Figure 5) [11,15,119]. A similar broad range of expression is observed in human cancers [9,11,121]. The cancers with consistently high SLFN11 expression are Ewing’s sarcoma and hematological malignancies [11,122,123]. This observation is related to the transcriptional regulation of SLFN11 by the ETS (erythroblast transformation specificity) transcription factors, and most notably FLI1 [11,122].
Figure 5.
Expression status of SLFN11 across cancer cell types. Violin plot of SLFN11 expression for the GDSC-MGH-Sanger cancer cell line dataset (https://discover.nci.nih.gov/cellminercdb/ (accessed on 9 February 2021)) (total of 936 cancer cell lines). Individual cell lines are represented as red dots. The cell lines are grouped by cancer type in decreasing order (from left to right) of SLFN11 expression. Note the bimodal expression in most tissue types. Value below 4 (dotted line) indicate background (lack of) SLFN11 expression.
Epigenetic modifications, which are frequent in tumorigenesis and chemoresistance, are responsible for the lack of SLFN11 expression in many cancer cells, resulting in chemoresistance to widely used clinical agents that target DNA replication [11,15,121,124,125,126]. Accordingly, reactivation of SLFN11 expression by epigenetic drugs targeting DNA methylation, histone deacetylase (HDAC), and histone methyltransferase EZH2 (Enhancer of zeste homolog 2) can reverse the chemoresistance of cancer cell lines that do not express SLFN11 (Figure 6A) [121,125,127]. Thus, based on the high dynamic range of SLFN11 expression and its epigenetic regulation, profiling SLFN11 status can be utilized to inform therapeutic options for precision medicine with epigenetic modulators (Figure 6A) [9,11,128].
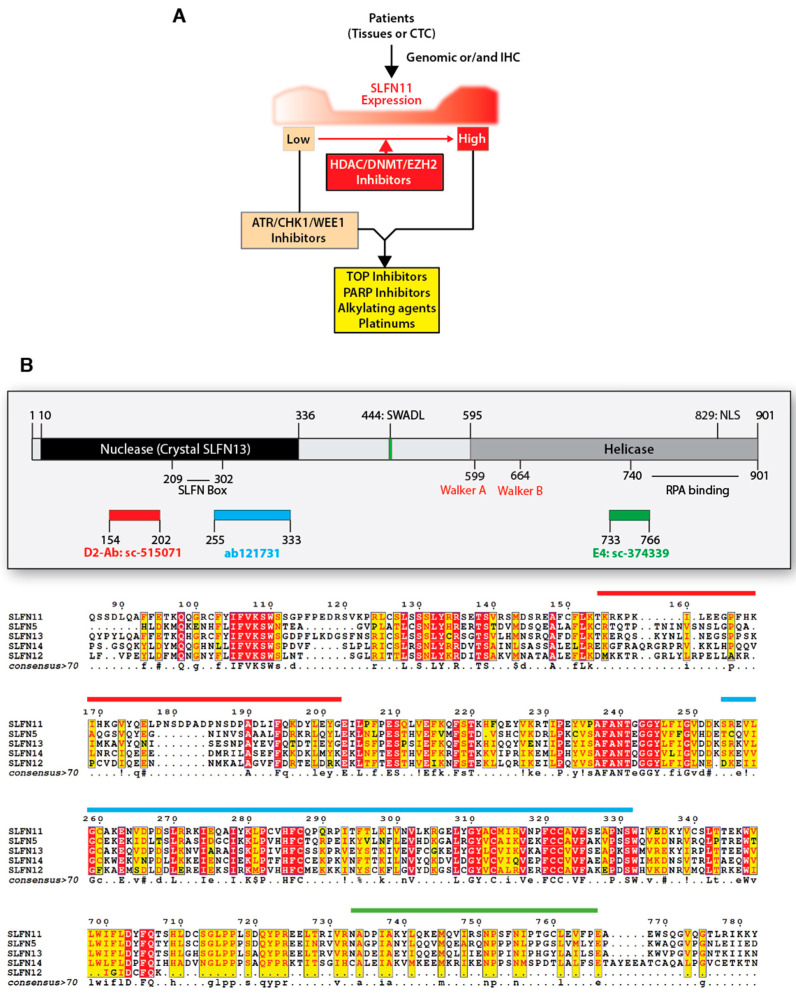
Figure 6.
Translating SLFN11 to the clinic. (A) Guidance of SLFN11-coupled cancer therapy. Profiling SLFN11 expression levels in tumor tissues and circulating tumor cells (CTC) provides a predictive biomarker. SLFN11 expression can be reactivated by the inhibitors of epigenetic modulators (HDAC, DNMT, or EZH2). Low SLFN11 tumors are generally resistant to DNA damaging agents. Resistance of low SLFN11 tumors can be overcome synergistically by combination with replication checkpoint inhibitors (ATR/CHK1/WEE1 inhibitors). (B) Schematics of the SLFN11 polypeptide with its two main functional domains, its nuclear localization signal (NLS), its putative helicase Walker domains, and the epitopes for the commonly used antibodies. The bottom part shows the sequence alignment of human SLFNs with the targeted sequences for the available SLFN11 antibodies used for IHC detection. (D-2 and E-4: Santa Cruz Biotechnology, Dallas, TX, USA, ab121731: Abcam, Cambridge, UK).
5.1. Molecular Activities of SLFN11
Although SLFN11 is not activated by ATR, it negatively regulates replication fork progression in response to RepStress induced by replicative DNA-damaging agents [129]. SLFN11 is recruited by RPA on stalled forks and damaged replication sites [129,130] to hinder the CMG helicase, chromatin remodeling, and induce the degradation of CDT1 (Figure 1) [85,129,130,131]. These findings indicate that SLFN11 irreversibly blocks stressed replication forks and the firing of distant origins, which is different from the classical ATR-CHK1-mediated checkpoint pathway that transiently arrests replication [11] (Figure 1). The irreversible pathway of SLFN11 in RepStress is supported by recent findings that SLFN11 degrades stalled replication forks induced by interstrand crosslink inducers in Fanconi anemia cells, disrupts codon-specific translation process in response to DNA damaging agents, and stimulates CUL4-DDB1CDT2 E3 ubiquitin ligase to remove replication-related factors [85,132,133]. These molecular effects of SLFN11 have been linked to its putative helicase activity encoded in the C-terminal domain of SLFN11 (Figure 6B) [85,118,129,131]. In addition, SLFN11 has been found to act outside of the nucleus by degrading type II tRNAs and blocking the translation of ATR and ATM [133,134,135]. This ribonuclease function of SLFN11 is likely related to its N-terminal domain, which is conserved in all of human SLFN genes (Figure 6B) [133,136]. Recently we also reported that SLFN11 regulates proteotoxic stress and the UPR (Unfolded Protein Response) pathway outside of the nucleus [137].
5.2. SLFN11 as a Predictive Biomarker
Based on the findings in cancer cells-based models, the clinical exploitation of SLFN11 expression as a treatment biomarker is being actively explored. Cancer patients whose tumors show high SLFN11 expression show better chemotherapy response to PARP inhibitors, irinotecan, and temozolomide in recurrent or refractory solid tumors such as Ewing sarcoma and small cell lung cancers (SCLCs) [138,139,140]. SLFN11 is positively linked to stromal signatures of basal-like phenotype and estrogen receptor negative (ER-) type in breast cancer [141]. SLFN11 expression as a prognostic biomarker has also been confirmed in esophageal and gastric cancers after chemoradiotherapy, showing a positive correlation. Esophageal cancer patients who receive chemotherapy with nedaplatin and 5-fluorouracil and have high SLFN11 expression show a significantly longer overall survival than patients whose tumors express low SLFN11 [142]. Gastric cancer patients with lower tumor classification and stage show high SLFN11 expression, exhibiting better survival rate after platinum-based chemotherapy [143]. Moreover, chemoresistance to TOP1 or PARP inhibitors due to loss of SLFN11 can be overcome with ATR inhibitors [62,64,65] (Figure 1 and Figure 6A). By RNA sequencing of metastatic biopsy tissue, SLFN11 expression has been reported as a better prognostic marker in platinum chemotherapy than histology and genomic alterations in patients with metastatic castration-resistant prostate cancers [144]. Notably, promoter hypermethylation of SLFN11, which is a key source for SLFN11 inactivation, also predicts worse drug-response in platinum-based chemotherapy in ovarian or lung cancer patients, as well as a poor clinical outcome biomarker [125]. Yet, prospective clinical trials are warranted to establish SLFN11 status as a practical biomarker for patient selection.
5.3. Clinical Evaluation of SLFN11
With regards to translating SLFN11 to the clinic, reliable approaches are required to evaluate the SLFN11 status of tumor tissues and circulating tumor cells (CTCs) (Figure 6A). Studies performed at different cancer research centers have established the feasibility of immunohistochemistry (IHC)-based detection of SLFN11 in malignant and adjacent normal tissues [9,11,100,120,138,140,141,142,143,145]. SLFN11 expression by IHC is also under-investigation in CTCs from advance prostate cancer and SCLC patients [144,146].
It is important to note that SLFN11 expression in tumors is not limited to the cancer cells but can also be detected in stromal cells (see Figure 6 in [11]). IHC assessment may therefore need to specify whether the SLFN11-positive cells are cancer cells and/or stromal cells. Indeed, SLFN11 is normally expressed in lymphocytes [11,126]. Moreover, SLFN11 activity is not limited to the nucleus [133,134,135,137,147] and it remains to be determined whether cytosolic IHC staining of SLFN11 should be taken into consideration [141,148]. This is particularly relevant to the fact that human SLFN proteins including SLFN5, 11, 12, 12L, 13, and 14 have strong similarity in their amino acid sequence (Figure 6B) [11,149] and that some of these SLFN proteins only act in the cytosol [149,150]. Hence, careful examination of the epitopes is important for assessing the specificity of SLFN11 antibodies. The above consideration implies that IHC reports need to be carefully assessed to properly develop SLFN11 as a tumor biomarker (Figure 6B).
Given the highly significant correlation between SLFN11 mRNA expression and SLFN11 protein expression [118,119,121,128], RNA sequencing of patient tumor cells can be readily applied to determine the levels of SLFN11 expression. In contrast to IHC methods, profiling SLFN11 mRNA expression needs to consider the infiltrating immune cells in tumor tissues that may influence SLFN11 positiveness (see the above paragraph). Lastly, measurement of promoter hypermethylation of SLFN11 by using DNA samples can be useful for predicting chemosensitivity and prognosis. SLFN11 inactivation in cancer cells is primarily driven by epigenetic modifications. Yet, exploitation of SLFN11 promoter methylation needs to be interpreted with caution as a large number of cancer cells also suppress SLFN11 expression by histone acetylation, which escapes from promoter methylation evaluations [15,121,151]. Nevertheless, SLFN11 promoter methylation can be used to assist the accuracy and reliability of SLFN11 inactivation determined by IHC and RNA sequencing.
6. Conclusions and Perspectives
Targeting DNA replication, checkpoints, and repair pathways are promising approaches for cancer treatment. A rich portfolio of drugs is in development, especially targeting ATR. Although the CHK1 inhibitors UCN-01 (7-hydroxystaurosprine), SRA737, and prexasertib preceded the ATR inhibitors, their development is lagging. It is plausible that the successful development of the ATR and WEE1 inhibitors will generate a renewed interest in CHK1 inhibitors.
The successful development of the ATR inhibitors and the use of SLFN11 as predictive biomarkers are likely to benefit from the rapid progress in precision medicine as better technologies are becoming available to map the genome and the pathways selective to cancer cells. The development of multivariate analyses appears promising and the RepStress signature is a step in that direction. Solid molecular data and detailed patient annotations should form the basis for artificial intelligence approaches that ultimately will generate therapeutic options based on individual patient characteristics.
Although we did not discuss the tumor microenvironment (TME) here, its contribution to the activity of drugs targeting the RepStress is an active area of investigation. Further investigations are warranted to elucidate the potential roles of SLFN11 in regulating and executing the immune responses. SLFN family genes have been discovered in the context of the immune system and they are commonly referred to as interferon-inducible genes. Therefore, elucidating the molecular connections between the immune system and the RepStress can be viewed as opening new avenues for cancer treatments.
Acknowledgments
Our studies are supported by the Center for Cancer Research (CCR), the Intramural Program of the US National Cancer Institute (NCI), NIH (BC-006150). We wish to thank the prior and current members of the Developmental Therapeutics Branch and Laboratory of Molecular Pharmacology, CCR-NCI for their contributions.
Author Contributions
Conceptualization, U.J. and Y.P.; writing original draft preparation, U.J., Y.M., N.T., A.T. and Y.P.; writing review and editing, U.J. and Y.P.; project administration and supervision, Y.P.; funding acquisition, Y.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kohn K.W. Beyond DNA cross-linking: History and prospects of DNA-targeted cancer treatment—Fifteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1996;56:5533–5546. [PubMed] [Google Scholar]
- 2.Pommier Y., Leo E., Zhang H., Marchand C. DNA Topoisomerases and Their Poisoning by Anticancer and Antibacterial Drugs. Chem. Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pommier Y., Marchand C. Interfacial inhibitors: Targeting macromolecular complexes. Nat. Rev. Drug Discov. 2011;11:25–36. doi: 10.1038/nrd3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaillard H., Garcia-Muse T., Aguilera A. Replication stress and cancer. Nat. Rev. Cancer. 2015;15:276–289. doi: 10.1038/nrc3916. [DOI] [PubMed] [Google Scholar]
- 5.Ubhi T., Brown G.W. Exploiting DNA Replication Stress for Cancer Treatment. Cancer Res. 2019;79:1730–1739. doi: 10.1158/0008-5472.CAN-18-3631. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H., Swami U., Preet R., Zhang J. Harnessing DNA Replication Stress for Novel Cancer Therapy. Genes. 2020;11:990. doi: 10.3390/genes11090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas A., Pommier Y. Small cell lung cancer: Time to revisit DNA-damaging chemotherapy. Sci. Transl. Med. 2016;8:346fs12. doi: 10.1126/scitranslmed.aaf6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas A., Takahashi N., Rajapakse V.N., Zhang X., Sun Y., Ceribelli M., Wilson K.M., Zhang Y., Beck E., Sciuto L., et al. Therapeutic targeting of ATR yields durable regressions in small cell lung cancers with high replication stress. Cancer Cell. 2021;39:566–579.e7. doi: 10.1016/j.ccell.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B., Ramkumar K., Cardnell R.J., Gay C.M., Stewart C.A., Wang W.-L., Fujimoto J., I Wistuba I., Byers L.A. A wake-up call for cancer DNA damage: The role of Schlafen 11 (SLFN11) across multiple cancers. Br. J. Cancer. 2021 doi: 10.1038/s41416-021-01476-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coussy F., El-Botty R., Château-Joubert S., Dahmani A., Montaudon E., Leboucher S., Morisset L., Painsec P., Sourd L., Huguet L., et al. BRCAness, SLFN11, and RB1 loss predict response to topoisomerase I inhibitors in triple-negative breast cancers. Sci. Transl. Med. 2020;12:eaax2625. doi: 10.1126/scitranslmed.aax2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murai J., Thomas A., Miettinen M., Pommier Y. Schlafen 11 (SLFN11), a restriction factor for replicative stress induced by DNA-targeting anti-cancer therapies. Pharmacol. Ther. 2019;201:94–102. doi: 10.1016/j.pharmthera.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsimberidou A.M., Fountzilas E., Nikanjam M., Kurzrock R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020;86:102019. doi: 10.1016/j.ctrv.2020.102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conley B.A., Doroshow J.H. Molecular Analysis for Therapy Choice: NCI MATCH. Semin. Oncol. 2014;41:297–299. doi: 10.1053/j.seminoncol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Kinders R.J., Hollingshead M., Khin S., Rubinstein L., Tomaszewski J.E., Doroshow J.H., Parchment R.E., The National Cancer Institute Phase 0 Clinical Trials Team Preclinical Modeling of a Phase 0 Clinical Trial: Qualification of a Pharmacodynamic Assay of Poly (ADP-Ribose) Polymerase in Tumor Biopsies of Mouse Xenografts. Clin. Cancer Res. 2008;14:6877–6885. doi: 10.1158/1078-0432.CCR-08-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinhold W.C., Thomas A., Pommier Y. DNA-Targeted Precision Medicine; Have we Been Caught Sleeping? Trends Cancer. 2016;3:2–6. doi: 10.1016/j.trecan.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaherty K.T., Gray R., Chen A., Li S., Patton D., Hamilton S.R., Williams P.M., Mitchell E.P., Iafrate A.J., Sklar J., et al. The Molecular Analysis for Therapy Choice (NCI-MATCH) Trial: Lessons for Genomic Trial Design. J. Natl. Cancer Inst. 2020;112:1021–1029. doi: 10.1093/jnci/djz245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Chavez A., Thomas A., Rajan A., Raffeld M., Morrow B., Kelly R., Carter C.A., Guha U., Killian K., Lau C.C., et al. Molecular Profiling and Targeted Therapy for Advanced Thoracic Malignancies: A Biomarker-Derived, Multiarm, Multihistology Phase II Basket Trial. J. Clin. Oncol. 2015;33:1000–1007. doi: 10.1200/JCO.2014.58.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim E.S., Herbst R.S., Wistuba I.I., Lee J.J., Blumenschein G.R., Tsao A., Stewart D.J., Hicks M.E., Erasmus J., Gupta S., et al. The BATTLE Trial: Personalizing Therapy for Lung Cancer. Cancer Discov. 2011;1:44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Hoff D.D., Stephenson J.J., Jr., Rosen P., Loesch D.M., Borad M.J., Anthony S., Jameson G., Brown S., Cantafio N., Richards D.A., et al. Pilot Study Using Molecular Profiling of Patients’ Tumors to Find Potential Targets and Select Treatments for Their Refractory Cancers. J. Clin. Oncol. 2010;28:4877–4883. doi: 10.1200/JCO.2009.26.5983. [DOI] [PubMed] [Google Scholar]
- 20.Keenan T., Burke K.P., Van Allen E.M. Genomic correlates of response to immune checkpoint blockade. Nat. Med. 2019;25:389–402. doi: 10.1038/s41591-019-0382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X., Luo X., Jiang C., Zhao H. Difficulties and challenges in the development of precision medicine. Clin. Genet. 2019;95:569–574. doi: 10.1111/cge.13511. [DOI] [PubMed] [Google Scholar]
- 22.Berger M.F., Mardis E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018;15:353–365. doi: 10.1038/s41571-018-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afghahi A., Sledge G.W. Targeted Therapy for Cancer in the Genomic Era. Cancer J. 2015;21:294–298. doi: 10.1097/PPO.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 24.Butler M., Pongor L., Su Y.-T., Xi L., Raffeld M., Quezado M., Trepel J., Aldape K., Pommier Y., Wu J. MGMT Status as a Clinical Biomarker in Glioblastoma. Trends Cancer. 2020;6:380–391. doi: 10.1016/j.trecan.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas A., Tanaka M., Trepel J., Reinhold W., Rajapakse V.N., Pommier Y. Temozolomide in the Era of Precision Medicine. Cancer Res. 2017;77:823–826. doi: 10.1158/0008-5472.CAN-16-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robey R., Pluchino K.M., Hall M.D., Fojo A.T., Bates S.E., Gottesman M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer. 2018;18:452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lord C.J., Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pommier Y., O’Connor M.J., De Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci. Transl. Med. 2016;8:362ps17. doi: 10.1126/scitranslmed.aaf9246. [DOI] [PubMed] [Google Scholar]
- 29.Hartley J.A. Antibody-drug conjugates (ADCs) delivering pyrrolobenzodiazepine (PBD) dimers for cancer therapy. Expert Opin. Biol. Ther. 2020;21:931–943. doi: 10.1080/14712598.2020.1776255. [DOI] [PubMed] [Google Scholar]
- 30.Thomas A., Pommier Y. Targeting Topoisomerase I in the Era of Precision Medicine. Clin. Cancer Res. 2019;25:6581–6589. doi: 10.1158/1078-0432.CCR-19-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negrini S., Gorgoulis V.G., Halazonetis T.D. Genomic instability—An evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 32.Malone E.R., Oliva M., Sabatini P.J.B., Stockley T., Siu L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020;12:1–19. doi: 10.1186/s13073-019-0703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berti M., Cortez D., Lopes M. The plasticity of DNA replication forks in response to clinically relevant genotoxic stress. Nat. Rev. Mol. Cell Biol. 2020;21:633–651. doi: 10.1038/s41580-020-0257-5. [DOI] [PubMed] [Google Scholar]
- 34.Cortez D. Replication-Coupled DNA Repair. Mol. Cell. 2019;74:866–876. doi: 10.1016/j.molcel.2019.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limas J.C., Cook J.G. Preparation for DNA replication: The key to a successful S phase. FEBS Lett. 2019;593:2853–2867. doi: 10.1002/1873-3468.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aladjem M.I., Redon M.I.A.C.E. Order from clutter: Selective interactions at mammalian replication origins. Nat. Rev. Genet. 2016;18:101–116. doi: 10.1038/nrg.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H. Regulation of DNA Replication Licensing and Re-Replication by Cdt1. Int. J. Mol. Sci. 2021;22:5195. doi: 10.3390/ijms22105195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker M.W., Botchan M.R., Berger J.M. Mechanisms and regulation of DNA replication initiation in eukaryotes. Crit. Rev. Biochem. Mol. Biol. 2017;52:107–144. doi: 10.1080/10409238.2016.1274717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blow J.J., Gillespie P.J. Replication licensing and cancer—A fatal entanglement? Nat. Rev. Cancer. 2008;8:799–806. doi: 10.1038/nrc2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boos D., Ferreira P. Origin Firing Regulations to Control Genome Replication Timing. Genes. 2019;10:199. doi: 10.3390/genes10030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moiseeva T.N., Bakkenist C.J. Dormant origin signaling during unperturbed replication. DNA Repair. 2019;81:102655. doi: 10.1016/j.dnarep.2019.102655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgers P.M., Kunkel T.A. Eukaryotic DNA Replication Fork. Annu. Rev. Biochem. 2017;86:417–438. doi: 10.1146/annurev-biochem-061516-044709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magaña A.G., Blanco F.J. Human PCNA Structure, Function and Interactions. Biomolecules. 2020;10:570. doi: 10.3390/biom10040570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dewar J., Walter J.C. Mechanisms of DNA replication termination. Nat. Rev. Mol. Cell Biol. 2017;18:507–516. doi: 10.1038/nrm.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toledo L., Neelsen K.J., Lukas J. Replication Catastrophe: When a Checkpoint Fails because of Exhaustion. Mol. Cell. 2017;66:735–749. doi: 10.1016/j.molcel.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Loeb L.A. Human cancers express mutator phenotypes: Origin, consequences and targeting. Nat. Rev. Cancer. 2011;11:450–457. doi: 10.1038/nrc3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lujan S.A., Williams J.S., Kunkel T.A. DNA Polymerases Divide the Labor of Genome Replication. Trends Cell Biol. 2016;26:640–654. doi: 10.1016/j.tcb.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berdis A.J. Inhibiting DNA Polymerases as a Therapeutic Intervention against Cancer. Front. Mol. Biosci. 2017;4:78. doi: 10.3389/fmolb.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ewald B., Sampath D., Plunkett W. Nucleoside analogs: Molecular mechanisms signaling cell death. Oncogene. 2008;27:6522–6537. doi: 10.1038/onc.2008.316. [DOI] [PubMed] [Google Scholar]
- 51.Owen N., Minko I.G., Moellmer S.A., Cammann S.K., Lloyd R.S., McCullough A.K. Enhanced cytarabine-induced killing in OGG1-deficient acute myeloid leukemia cells. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2016833118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okonska A., Bühler S., Rao V., Ronner M., Blijlevens M., van der Meulen-Muileman I.H., de Menezes R.X., Wipplinger M., Oehl K., Smit E.F., et al. Functional Genomic Screen in Mesothelioma Reveals that Loss of Function of BRCA1-Associated Protein 1 Induces Chemoresistance to Ribonucleotide Reductase Inhibition. Mol. Cancer Ther. 2019;19:552–563. doi: 10.1158/1535-7163.MCT-19-0356. [DOI] [PubMed] [Google Scholar]
- 53.Coyne G.O., Kummar S., Meehan R.S., Do K., Collins J.M., Anderson L., Ishii K., Takebe N., Zlott J., Juwara L., et al. Phase I trial of TRC102 (methoxyamine HCl) in combination with temozolomide in patients with relapsed solid tumors and lymphomas. Oncotarget. 2020;11:3959–3971. doi: 10.18632/oncotarget.27784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birkbak N., Li Y., Pathania S., Greene-Colozzi A., Dreze M., Bowman-Colin C., Sztupinszki Z., Krzystanek M., Diossy M., Tung N., et al. Overexpression of BLM promotes DNA damage and increased sensitivity to platinum salts in triple-negative breast and serous ovarian cancers. Ann. Oncol. 2018;29:903–909. doi: 10.1093/annonc/mdy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalachand R.D., Stordal B., Madden S., Chandler B., Cunningham J., Goode E.L., Ruscito I., I Braicu E., Sehouli J., Ignatov A., et al. BRCA1 Promoter Methylation and Clinical Outcomes in Ovarian Cancer: An Individual Patient Data Meta-Analysis. J. Natl. Cancer Inst. 2020;112:1190–1203. doi: 10.1093/jnci/djaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krais J.J., Johnson N. BRCA1 Mutations in Cancer: Coordinating Deficiencies in Homologous Recombination with Tumorigenesis. Cancer Res. 2020;80:4601–4609. doi: 10.1158/0008-5472.CAN-20-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maede Y., Shimizu H., Fukushima T., Kogame T., Nakamura T., Miki T., Takeda S., Pommier Y., Murai J. Differential and Common DNA Repair Pathways for Topoisomerase I- and II-Targeted Drugs in a Genetic DT40 Repair Cell Screen Panel. Mol. Cancer Ther. 2013;13:214–220. doi: 10.1158/1535-7163.MCT-13-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marzi L., Szabova L., Gordon M., Ohler Z.W., Sharan S.K., Beshiri M., Etemadi M., Murai J., Kelly K., Pommier Y. The Indenoisoquinoline TOP1 Inhibitors Selectively Target Homologous Recombination-Deficient and Schlafen 11-Positive Cancer Cells and Synergize with Olaparib. Clin. Cancer Res. 2019;25:6206–6216. doi: 10.1158/1078-0432.CCR-19-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pommier Y., Sun Y., Huang S.-Y.N., Nitiss J. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016;17:703–721. doi: 10.1038/nrm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murai J., Huang S.-Y.N., DAS B.B., Renaud A., Zhang Y., Doroshow J.H., Ji J., Takeda S., Pommier Y. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rose M., Burgess J.T., O’Byrne K., Richard D.J., Bolderson E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020;8:564601. doi: 10.3389/fcell.2020.564601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murai J., Feng Y., Yu G.K., Ru Y., Tang S.-W., Shen Y., Pommier Y. Resistance to PARP inhibitors by SLFN11 inactivation can be overcome by ATR inhibition. Oncotarget. 2016;7:76534–76550. doi: 10.18632/oncotarget.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bradbury A., Hall S., Curtin N., Drew Y. Targeting ATR as Cancer Therapy: A new era for synthetic lethality and synergistic combinations? Pharmacol. Ther. 2019;207:107450. doi: 10.1016/j.pharmthera.2019.107450. [DOI] [PubMed] [Google Scholar]
- 64.Jo U., Senatorov I.S., Zimmermann A., Saha L.K., Murai Y., Kim S.H., Rajapakse V.N., Elloumi F., Takahashi N., Schultz C.W., et al. Novel and highly potent ATR inhibitor M4344 kills cancer cells with replication stress and enhances the chemotherapeutic activity of widely used DNA damaging agents. Mol. Cancer Ther. 2021;20:1431–1441. doi: 10.1158/1535-7163.MCT-20-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yap T.A., Krebs M.G., Postel-Vinay S., El-Khouiery A., Soria J.-C., Lopez J., Berges A., Cheung S.A., Irurzun-Arana I., Goldwin A., et al. Ceralasertib (AZD6738), an Oral ATR Kinase Inhibitor, in Combination with Carboplatin in Patients with Advanced Solid Tumors: A Phase I Study. Clin. Cancer Res. 2021 doi: 10.1158/1078-0432.CCR-21-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yap T.A., O’Carrigan B., Penney M.S., Lim J.S., Brown J.S., Luken M.J.D.M., Tunariu N., Perez-Lopez R., Rodrigues D.N., Riisnaes R., et al. Phase I Trial of First-in-Class ATR Inhibitor M6620 (VX-970) as Monotherapy or in Combination with Carboplatin in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2020;38:3195–3204. doi: 10.1200/JCO.19.02404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yap T.A., Tan D.S., Terbuch A., Caldwell R., Guo C., Goh B.C., Heong V., Haris N.R.M., Bashir S., Drew Y., et al. First-in-Human Trial of the Oral Ataxia Telangiectasia and RAD3-Related (ATR) Inhibitor BAY 1895344 in Patients with Advanced Solid Tumors. Cancer Discov. 2020;11:80–91. doi: 10.1158/2159-8290.CD-20-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farmer H., McCabe N., Lord C., Tutt A.N.J., Johnson D.A., Richardson T.B., Santarosa M., Dillon K.J., Hickson I., Knights C., et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 69.Bryant H.E., Schultz N., Thomas H.D., Parker K.M., Flower D., Lopez E., Kyle S., Meuth M., Curtin N., Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 70.Baranovskiy A.G., Babayeva N.D., Suwa Y., Gu J., Pavlov Y., Tahirov T.H. Structural basis for inhibition of DNA replication by aphidicolin. Nucleic Acids Res. 2014;42:14013–14021. doi: 10.1093/nar/gku1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mishra B., Zhang S., Zhao H., Darzynkiewicz Z., Lee E.Y., Lee M.Y., Zhang Z. Discovery of a novel DNA polymerase inhibitor and characterization of its antiproliferative properties. Cancer Biol. Ther. 2018;20:474–486. doi: 10.1080/15384047.2018.1529126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zafar M.K., Maddukuri L., Ketkar A., Penthala N.R., Reed M.R., Eddy S.D., Crooks P.A., Eoff R.L. A Small-Molecule Inhibitor of Human DNA Polymerase η Potentiates the Effects of Cisplatin in Tumor Cells. Biochemistry. 2018;57:1262–1273. doi: 10.1021/acs.biochem.7b01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou J., Gelot C., Pantelidou C., Li A., Yücel H., Davis R.E., Färkkilä A., Kochupurakkal B., Syed A., Shapiro G.I., et al. A first-in-class polymerase theta inhibitor selectively targets homologous-recombination-deficient tumors. Nat. Rev. Cancer. 2021;2:598–610. doi: 10.1038/s43018-021-00203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zatreanu D., Robinson H.M.R., Alkhatib O., Boursier M., Finch H., Geo L., Grande D., Grinkevich V., Heald R.A., Langdon S., et al. Polθ inhibitors elicit BRCA-gene synthetic lethality and target PARP inhibitor resistance. Nat. Commun. 2021;12:1–15. doi: 10.1038/s41467-021-23463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdel-Samad R., Aouad P., Gali-Muhtasib H., Sweidan Z., Hmadi R., Kadara H., D’Andrea E.L., Fucci A., Pisano C., Darwiche N. Mechanism of action of the atypical retinoid ST1926 in colorectal cancer: DNA damage and DNA polymerase α. Am. J. Cancer Res. 2018;8:39–55. [PMC free article] [PubMed] [Google Scholar]
- 76.Banerjee T., Aggarwal M., Sommers J.A., Brosh R.M. Biochemical and cell biological assays to identify and characterize DNA helicase inhibitors. Methods. 2016;108:130–141. doi: 10.1016/j.ymeth.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simon N., Bochman M., Seguin S., Brodsky J.L., Seibel W.L., Schwacha A. Ciprofloxacin is an inhibitor of the Mcm2-7 replicative helicase. Biosci. Rep. 2013;33:783–795. doi: 10.1042/BSR20130083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toyokawa G., Masuda K., Daigo Y., Cho H.-S., Yoshimatsu M., Takawa M., Hayami S., Maejima K., Chino M., I Field H., et al. Minichromosome Maintenance Protein 7 is a potential therapeutic target in human cancer and a novel prognostic marker of non-small cell lung cancer. Mol. Cancer. 2011;10:65. doi: 10.1186/1476-4598-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartolowits M.D., Gast J.M., Hasler A.J., Cirrincione A.M., O’Connor R.J., Mahmoud A.H., Lill M.A., Davisson V.J. Discovery of Inhibitors for Proliferating Cell Nuclear Antigen Using a Computational-Based Linked-Multiple-Fragment Screen. ACS Omega. 2019;4:15181–15196. doi: 10.1021/acsomega.9b02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith S.J., Li C.M., Lingeman R.G., Hickey R.J., Liu Y., Malkas L.H., Raoof M. Molecular Targeting of Cancer-Associated PCNA Interactions in Pancreatic Ductal Adenocarcinoma Using a Cell-Penetrating Peptide. Mol. Ther. Oncolytics. 2020;17:250–256. doi: 10.1016/j.omto.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inoue A., Kikuchi S., Hishiki A., Shao Y., Heath R., Evison B.J., Actis M., Canman C.E., Hashimoto H., Fujii N. A Small Molecule Inhibitor of Monoubiquitinated Proliferating Cell Nuclear Antigen (PCNA) Inhibits Repair of Interstrand DNA Cross-link, Enhances DNA Double Strand Break, and Sensitizes Cancer Cells to Cisplatin. J. Biol. Chem. 2014;289:7109–7120. doi: 10.1074/jbc.M113.520429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan Z., Wortman M., Dillehay K.L., Seibel W.L., Evelyn C.R., Smith S.J., Malkas L.H., Zheng Y., Lu S., Dong Z. Small-Molecule Targeting of Proliferating Cell Nuclear Antigen Chromatin Association Inhibits Tumor Cell Growth. Mol. Pharmacol. 2012;81:811–819. doi: 10.1124/mol.112.077735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu S., Dong Z. Additive effects of a small molecular PCNA inhibitor PCNA-I1S and DNA damaging agents on growth inhibition and DNA damage in prostate and lung cancer cells. PLoS ONE. 2019;14:e0223894. doi: 10.1371/journal.pone.0223894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jang S.-M., Redon C.E., Aladjem M.I. Chromatin-Bound Cullin-Ring Ligases: Regulatory Roles in DNA Replication and Potential Targeting for Cancer Therapy. Front. Mol. Biosci. 2018;5:19. doi: 10.3389/fmolb.2018.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jo U., Murai Y., Chakka S., Chen L., Cheng K., Murai J., Saha L.K., Jenkins L.M.M., Pommier Y. SLFN11 promotes CDT1 degradation by CUL4 in response to replicative DNA damage, while its absence leads to synthetic lethality with ATR/CHK1 inhibitors. Proc. Natl. Acad. Sci. USA. 2021;118:e2015654118. doi: 10.1073/pnas.2015654118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liontos M., Koutsami M., Sideridou M., Evangelou K., Kletsas D., Levy B., Kotsinas A., Nahum O., Zoumpourlis V., Kouloukoussa M., et al. Deregulated Overexpression of hCdt1 and hCdc6 Promotes Malignant Behavior. Cancer Res. 2007;67:10899–10909. doi: 10.1158/0008-5472.CAN-07-2837. [DOI] [PubMed] [Google Scholar]
- 87.Fu H., Redon C.E., Thakur B.L., Utani K., Sebastian R., Jang S.-M., Gross J.M., Mosavarpour S., Marks A.B., Zhuang S.Z., et al. Dynamics of replication origin over-activation. Nat. Commun. 2021;12:2803. doi: 10.1038/s41467-021-23835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao Y. Cullin-RING Ligases as Attractive Anti-cancer Targets. Curr. Pharm. Des. 2013;19:3215–3225. doi: 10.2174/13816128113199990300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou L., Jia L. Targeting Protein Neddylation for Cancer Therapy. Adv. Exp. Med. Biol. 2020;1217:297–315. doi: 10.1007/978-981-15-1025-0_18. [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto N., Shimizu T., Yonemori K., Kitano S., Kondo S., Iwasa S., Koyama T., Sudo K., Sato J., Tamura K., et al. A first-in-human, phase 1 study of the NEDD8 activating enzyme E1 inhibitor TAS4464 in patients with advanced solid tumors. Investig. New Drugs. 2021;39:1–11. doi: 10.1007/s10637-020-01055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou L., Jiang Y., Luo Q., Li L., Jia L. Neddylation: A novel modulator of the tumor microenvironment. Mol. Cancer. 2019;18:1–11. doi: 10.1186/s12943-019-0979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jette N.R., Kumar M., Radhamani S., Arthur G., Goutam S., Yip S., Kolinsky M., Williams G.J., Bose P., Lees-Miller S.P. ATM-Deficient Cancers Provide New Opportunities for Precision Oncology. Cancers. 2020;12:687. doi: 10.3390/cancers12030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bass T.E., Luzwick J.W., Kavanaugh G., Carroll C., Dungrawala H., Glick G.G., Feldkamp M.D., Putney R., Chazin W.J., Cortez D. ETAA1 acts at stalled replication forks to maintain genome integrity. Nature. 2016;18:1185–1195. doi: 10.1038/ncb3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saldivar J., Cortez D., Cimprich K.A. The essential kinase ATR: Ensuring faithful duplication of a challenging genome. Nat. Rev. Mol. Cell Biol. 2017;18:622–636. doi: 10.1038/nrm.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shechter D., Costanzo V., Gautier J. Regulation of DNA replication by ATR: Signaling in response to DNA intermediates. DNA Repair. 2004;3:901–908. doi: 10.1016/j.dnarep.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y., Hunter T. Roles of Chk1 in cell biology and cancer therapy. Int. J. Cancer. 2013;134:1013–1023. doi: 10.1002/ijc.28226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blackford A.N., Jackson S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell. 2017;66:801–817. doi: 10.1016/j.molcel.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 98.Bonner W.M., Redon C.E., Dickey J.S., Nakamura A.J., Sedelnikova O.A., Solier S., Pommier Y. gammaH2AX and cancer. Nat. Rev. Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ji J., Zhang Y., Redon C.E., Reinhold W.C., Chen A.P., Fogli L.K., Holbeck S.L., Parchment R.E., Hollingshead M., Tomaszewski J.E., et al. Phosphorylated fraction of H2AX as a measurement for DNA damage in cancer cells and potential applications of a novel assay. PLoS ONE. 2017;12:e0171582. doi: 10.1371/journal.pone.0171582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takahashi N., Kim S., Rajapakse V., Schultz C., Zhang Y., Redon C., Fu H., Pongor L., Kumar S., Pommier Y., et al. Replication stress defines distinct molecular subtypes across cancers. Cell Rep. 2021:98. doi: 10.1158/2767-9764.CRC-22-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Klein A., Muijtjens M., van Os R., Verhoeven Y., Smit B., Carr A.M., Lehmann A., Hoeijmakers J. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr. Biol. 2000;10:479–482. doi: 10.1016/S0960-9822(00)00447-4. [DOI] [PubMed] [Google Scholar]
- 102.O’Driscoll M., Ruiz-Perez V.L., Woods C.G., Jeggo P.A., Goodship J.A. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat. Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 103.Murga M., Bunting S., Montaña M.F., Soria R., Mulero F., Cañamero M., Lee Y., McKinnon P.J., Nussenzweig A., Fernandez-Capetillo O. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat. Genet. 2009;41:891–898. doi: 10.1038/ng.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lecona E., Fernandez-Capetillo O. Targeting ATR in cancer. Nat. Rev. Cancer. 2018;18:586–595. doi: 10.1038/s41568-018-0034-3. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y., Jones T.L., Martin S.E., Caplen N., Pommier Y. Implication of Checkpoint Kinase-dependent Up-regulation of Ribonucleotide Reductase R2 in DNA Damage Response. J. Biol. Chem. 2009;284:18085–18095. doi: 10.1074/jbc.M109.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martin S.E., Wu Z.-H., Gehlhaus K., Jones T.L., Zhang Y., Guha R., Miyamoto S., Pommier Y., Caplen N.J. RNAi Screening Identifies TAK1 as a Potential Target for the Enhanced Efficacy of Topoisomerase Inhibitors. Curr. Cancer Drug Targets. 2011;11:976–986. doi: 10.2174/156800911797264734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vitiello P.P., Martini G., Mele L., Giunta E.F., De Falco V., Ciardiello D., Belli V., Cardone C., Matrone N., Poliero L., et al. Vulnerability to low-dose combination of irinotecan and niraparib in ATM-mutated colorectal cancer. J. Exp. Clin. Cancer Res. 2021;40:1–15. doi: 10.1186/s13046-020-01811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Terranova N., Jansen M., Falk M., Hendriks B.S. Population pharmacokinetics of ATR inhibitor berzosertib in phase I studies for different cancer types. Cancer Chemother. Pharmacol. 2020;87:185–196. doi: 10.1007/s00280-020-04184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nam A.-R., Yoon J., Jin M.-H., Bang J.-H., Oh K.-S., Seo H.-R., Kim J.-M., Kim T.-Y., Oh D.-Y. ATR inhibition amplifies antitumor effects of olaparib in biliary tract cancer. Cancer Lett. 2021;516:38–47. doi: 10.1016/j.canlet.2021.05.029. [DOI] [PubMed] [Google Scholar]
- 110.Wengner A.M., Siemeister G., Lücking U., Lefranc J., Wortmann L., Lienau P., Bader B., Bömer U., Moosmayer D., Eberspächer U., et al. The Novel ATR Inhibitor BAY 1895344 Is Efficacious as Monotherapy and Combined with DNA Damage–Inducing or Repair–Compromising Therapies in Preclinical Cancer Models. Mol. Cancer Ther. 2019;19:26–38. doi: 10.1158/1535-7163.MCT-19-0019. [DOI] [PubMed] [Google Scholar]
- 111.Konstantinopoulos P.A., Cheng S.-C., Hendrickson A.E.W., Penson R.T., Schumer S.T., Doyle L.A., Lee E.K., Kohn E.C., Duska L.R., A Crispens M., et al. Berzosertib plus gemcitabine versus gemcitabine alone in platinum-resistant high-grade serous ovarian cancer: A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21:957–968. doi: 10.1016/S1470-2045(20)30180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miller R.M., Nworu C., McKee L., Balcerzak D., Pham L., Pugh J., Liu Y.-Z., Gustafson H., Marwah E., Lamb T., et al. Development of an Immunohistochemical Assay to Detect the Ataxia-Telangiectasia Mutated (ATM) Protein in Gastric Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2020;28:303–310. doi: 10.1097/PAI.0000000000000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rogers R.F., Walton M.I., Cherry D.L., Collins I., Clarke P.A., Garrett M.D., Workman P. CHK1 Inhibition Is Synthetically Lethal with Loss of B-Family DNA Polymerase Function in Human Lung and Colorectal Cancer Cells. Cancer Res. 2020;80:1735–1747. doi: 10.1158/0008-5472.CAN-19-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Endersby R., Whitehouse J., Pribnow A., Kuchibhotla M., Hii H., Carline B., Gande S., Stripay J., Ancliffe M., Howlett M., et al. Small-molecule screen reveals synergy of cell cycle checkpoint kinase inhibitors with DNA-damaging chemotherapies in medulloblastoma. Sci. Transl. Med. 2021;13:eaba7401. doi: 10.1126/scitranslmed.aba7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mani C., Jonnalagadda S., Lingareddy J., Awasthi S., Gmeiner W.H., Palle K. Prexasertib treatment induces homologous recombination deficiency and synergizes with olaparib in triple-negative breast cancer cells. Breast Cancer Res. 2019;21:1–14. doi: 10.1186/s13058-019-1192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moiseeva T.N., Qian C., Sugitani N., Osmanbeyoglu H., Bakkenist C.J. WEE1 kinase inhibitor AZD1775 induces CDK1 kinase-dependent origin firing in unperturbed G1- and S-phase cells. Proc. Natl. Acad. Sci. USA. 2019;116:23891–23893. doi: 10.1073/pnas.1915108116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leijen S., Van Geel R.M.J.M., Sonke G., De Jong D., Rosenberg E., Marchetti S., Pluim D., van Werkhoven E., Rose S., Lee M.A., et al. Phase II Study of WEE1 Inhibitor AZD1775 Plus Carboplatin in Patients with TP53-Mutated Ovarian Cancer Refractory or Resistant to First-Line Therapy within 3 Months. J. Clin. Oncol. 2016;34:4354–4361. doi: 10.1200/JCO.2016.67.5942. [DOI] [PubMed] [Google Scholar]
- 118.Zoppoli G., Regairaz M., Leo E., Reinhold W.C., Varma S., Ballestrero A., Doroshow J.H., Pommier Y. Putative DNA/RNA helicase Schlafen-11 (SLFN11) sensitizes cancer cells to DNA-damaging agents. Proc. Natl. Acad. Sci. USA. 2012;109:15030–15035. doi: 10.1073/pnas.1205943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barretina J., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S., Wilson C.J., Lehár J., Kryukov G., Sonkin D., et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lok B., Gardner E., Schneeberger V.E., Ni A., Desmeules P., Rekhtman N., De Stanchina E., Teicher B.A., Riaz N., Powell S.N., et al. PARP Inhibitor Activity Correlates with SLFN11 Expression and Demonstrates Synergy with Temozolomide in Small Cell Lung Cancer. Clin. Cancer Res. 2016;23:523–535. doi: 10.1158/1078-0432.CCR-16-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang S.-W., Thomas A., Murai J., Trepel J.B., Bates S.E., Rajapakse V.N., Pommier Y. Overcoming Resistance to DNA-Targeted Agents by Epigenetic Activation of Schlafen 11 (SLFN11) Expression with Class I Histone Deacetylase Inhibitors. Clin. Cancer Res. 2018;24:1944–1953. doi: 10.1158/1078-0432.CCR-17-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang S.-W., Bilke S., Cao L., Murai J., Sousa F., Yamade M., Rajapakse V.N., Varma S., Helman L.J., Khan J., et al. SLFN11 Is a Transcriptional Target of EWS-FLI1 and a Determinant of Drug Response in Ewing Sarcoma. Clin. Cancer Res. 2015;21:4184–4193. doi: 10.1158/1078-0432.CCR-14-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garnett M.J., Edelman E.J., Heidorn S.J., Greenman C.D., Dastur A., Lau K.W., Greninger P., Thompson I.R., Luo X., Soares J., et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–575. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reinhold W., Varma S., Sunshine M., Rajapakse V., Luna A., Kohn K.W., Stevenson H., Wang Y., Heyn H., Nogales V., et al. The NCI-60 Methylome and Its Integration into CellMiner. Cancer Res. 2016;77:601–612. doi: 10.1158/0008-5472.CAN-16-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nogales V., Reinhold W., Varma S., Cardus A.M., Moutinho C., Moran S., Heyn H., Sebio A., Barnadas A., Pommier Y., et al. Epigenetic inactivation of the putative DNA/RNA helicase SLFN11 in human cancer confers resistance to platinum drugs. Oncotarget. 2015;7:3084–3097. doi: 10.18632/oncotarget.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moribe F., Nishikori M., Takashima T., Taniyama D., Onishi N., Arima H., Sasanuma H., Akagawa R., Elloumi F., Takeda S., et al. Epigenetic suppression of SLFN11 in germinal center B-cells during B-cell development. PLoS ONE. 2021;16:e0237554. doi: 10.1371/journal.pone.0237554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gardner E., Lok B., Schneeberger V.E., Desmeules P., Miles L., Arnold P., Ni A., Khodos I., De Stanchina E., Nguyen T., et al. Chemosensitive Relapse in Small Cell Lung Cancer Proceeds through an EZH2-SLFN11 Axis. Cancer Cell. 2017;31:286–299. doi: 10.1016/j.ccell.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Winkler C., Armenia J., Jones G.N., Tobalina L., Sale M.J., Petreus T., Baird T., Serra V., Wang A.T., Lau A., et al. SLFN11 informs on standard of care and novel treatments in a wide range of cancer models. Br. J. Cancer. 2020;124:951–962. doi: 10.1038/s41416-020-01199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Murai J., Tang S.-W., Leo E., Baechler S.A., Redon C.E., Zhang H., Al Abo M., Rajapakse V.N., Nakamura E., Jenkins L.M.M., et al. SLFN11 Blocks Stressed Replication Forks Independently of ATR. Mol. Cell. 2018;69:371–384.e6. doi: 10.1016/j.molcel.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mu Y., Lou J., Srivastava M., Zhao B., Feng X., Liu T., Chen J., Huang J. SLFN 11 inhibits checkpoint maintenance and homologous recombination repair. EMBO Rep. 2015;17:94–109. doi: 10.15252/embr.201540964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murai J., Zhang H., Pongor L., Tang S.-W., Jo U., Moribe F., Ma Y., Tomita M., Pommier Y. Chromatin Remodeling and Immediate Early Gene Activation by SLFN11 in Response to Replication Stress. Cell Rep. 2020;30:4137–4151.e6. doi: 10.1016/j.celrep.2020.02.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Okamoto Y., Abe M., Mu A., Tempaku Y., Rogers C.B., Mochizuki A.L., Katsuki Y., Kanemaki M.T., Takaori-Kondo A., Sobeck A.T., et al. SLFN11 promotes stalled fork degradation that underlies the phenotype in Fanconi anemia cells. Blood. 2021;137:336–348. doi: 10.1182/blood.2019003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li M., Kao E., Malone D., Gao X., Wang J.Y.J., David M. DNA damage-induced cell death relies on SLFN11-dependent cleavage of distinct type II tRNAs. Nat. Struct. Mol. Biol. 2018;25:1047–1058. doi: 10.1038/s41594-018-0142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li M., Kao E., Gao X., Sandig H., Limmer K., Pavon-Eternod M., Jones T.E., Landry S., Pan T., Weitzman M.D., et al. Codon-usage-based inhibition of HIV protein synthesis by human schlafen 11. Nature. 2012;491:125–128. doi: 10.1038/nature11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Malone D., Lardelli R.M., Li M., David M. Dephosphorylation activates the interferon-stimulated Schlafen family member 11 in the DNA damage response. J. Biol. Chem. 2019;294:14674–14685. doi: 10.1074/jbc.RA118.006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang J.-Y., Deng X.-Y., Li Y.-S., Ma X., Feng J.-X., Yu B., Chen Y., Luo Y.-L., Wang X., Chen M.-L., et al. Structure of Schlafen13 reveals a new class of tRNA/rRNA- targeting RNase engaged in translational control. Nat. Commun. 2018;9:1165. doi: 10.1038/s41467-018-03544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Murai Y., Jo U., Murai J., Jenkins L.M., Huang S.-Y.N., Chakka S., Chen L., Cheng K., Fukuda S., Takebe N., et al. SLFN11 Inactivation Induces Proteotoxic Stress and Sensitizes Cancer Cells to Ubiquitin Activating Enzyme Inhibitor TAK-243. Cancer Res. 2021;81:3067–3078. doi: 10.1158/0008-5472.CAN-20-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Federico S.M., Pappo A.S., Sahr N., Sykes A., Campagne O., Stewart C.F., Clay M.R., Bahrami A., McCarville M.B., Kaste S.C., et al. A phase I trial of talazoparib and irinotecan with and without temozolomide in children and young adults with recurrent or refractory solid malignancies. Eur. J. Cancer. 2020;137:204–213. doi: 10.1016/j.ejca.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 139.Pietanza M.C., Waqar S.N., Krug L.M., Dowlati A., Hann C.L., Chiappori A., Owonikoko T.K., Woo K.M., Cardnell R.J., Fujimoto J., et al. Randomized, Double-Blind, Phase II Study of Temozolomide in Combination with Either Veliparib or Placebo in Patients with Relapsed-Sensitive or Refractory Small-Cell Lung Cancer. J. Clin. Oncol. 2018;36:2386–2394. doi: 10.1200/JCO.2018.77.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Byers L.A., Bentsion D., Gans S., Penkov K., Son C., Sibille A., Owonikoko T.K., Groen H.J., Gay C.M., Fujimoto J., et al. Veliparib in Combination with Carboplatin and Etoposide in Patients with Treatment-Naïve Extensive-Stage Small Cell Lung Cancer: A Phase 2 Randomized Study. Clin. Cancer Res. 2021;14:3884. doi: 10.1158/1078-0432.CCR-20-4259. [DOI] [PubMed] [Google Scholar]
- 141.Isnaldi E., Ferraioli D., Ferrando L., Brohée S., Ferrando F., Fregatti P., Bedognetti D., Ballestrero A., Zoppoli G. Schlafen-11 expression is associated with immune signatures and basal-like phenotype in breast cancer. Breast Cancer Res. Treat. 2019;177:335–343. doi: 10.1007/s10549-019-05313-w. [DOI] [PubMed] [Google Scholar]
- 142.Kagami T., Yamade M., Suzuki T., Uotani T., Tani S., Hamaya Y., Iwaizumi M., Osawa S., Sugimoto K., Miyajima H., et al. The first evidence for SLFN11 expression as an independent prognostic factor for patients with esophageal cancer after chemoradiotherapy. BMC Cancer. 2020;20:1123. doi: 10.1186/s12885-020-07574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takashima T., Taniyama D., Sakamoto N., Yasumoto M., Asai R., Hattori T., Honma R., Thang P.Q., Ukai S., Maruyama R., et al. Schlafen 11 predicts response to platinum-based chemotherapy in gastric cancers. Br. J. Cancer. 2021;125:65–77. doi: 10.1038/s41416-021-01364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Conteduca V., Ku S.-Y., Puca L., Slade M., Fernandez L., Hess J., Bareja R., Vlachostergios P.J., Sigouros M., Mosquera J.M., et al. SLFN11 Expression in Advanced Prostate Cancer and Response to Platinum-based Chemotherapy. Mol. Cancer Ther. 2020;19:1157–1164. doi: 10.1158/1535-7163.MCT-19-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Coleman N., Zhang B., Byers L.A., Yap T.A. The role of Schlafen 11 (SLFN11) as a predictive biomarker for targeting the DNA damage response. Br. J. Cancer. 2020;124:857–859. doi: 10.1038/s41416-020-01202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Byers L.A., Stewart A., Gay C., Heymach J., Fernandez L., Lu D., Rich R., Chu L., Wang Y., Dittamore R. Abstract 2215: SLFN11 and EZH2 protein expression and localization in circulating tumor cells to predict response or resistance to DNA damaging therapies in small cell lung cancer. Cancer Res. 2019;13:2215. doi: 10.1158/1538-7445.am2019-2215. [DOI] [Google Scholar]
- 147.Stabell A.C., Hawkins J., Li M., Gao X., David M., Press W., Sawyer S.L. Non-human Primate Schlafen11 Inhibits Production of Both Host and Viral Proteins. PLOS Pathog. 2016;12:e1006066. doi: 10.1371/journal.ppat.1006066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ballestrero A., Bedognetti D., Ferraioli D., Franceschelli P., Labidi-Galy S.I., Leo E., Murai J., Pommier Y., Tsantoulis P., Vellone V.G., et al. Report on the first SLFN11 monothematic workshop: From function to role as a biomarker in cancer. J. Transl. Med. 2017;15:199. doi: 10.1186/s12967-017-1296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mavrommatis E., Fish E.N., Platanias L.C. The Schlafen Family of Proteins and Their Regulation by Interferons. J. Interf. Cytokine Res. 2013;33:206–210. doi: 10.1089/jir.2012.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Garvie C.W., Wu X., Papanastasiou M., Lee S., Fuller J., Schnitzler G.R., Horner S.W., Baker A., Zhang T., Mullahoo J.P., et al. Structure of PDE3A-SLFN12 complex reveals requirements for activation of SLFN12 RNase. Nat. Commun. 2021;12:4375. doi: 10.1038/s41467-021-24495-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tlemsani C., Pongor L., Elloumi F., Girard L., Huffman K.E., Roper N., Varma S., Luna A., Rajapakse V.N., Sebastian R., et al. SCLC-CellMiner: A Resource for Small Cell Lung Cancer Cell Line Genomics and Pharmacology Based on Genomic Signatures. Cell Rep. 2020;33:108296. doi: 10.1016/j.celrep.2020.108296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.