Abstract
Background: The human gut microbiota is defined as the microorganisms that collectively inhabit the intestinal tract. Its composition is relatively stable; however, an imbalance can be precipitated by various factors and is known to be associated with various diseases. Humans are daily exposed to ionizing radiation from ambient and medical procedures, and gastrointestinal side effects are not rare. Methods: A systematic search of PubMed, EMBASE, and Cochrane Library databases was conducted. Primary outcomes were changes in composition, richness, and diversity of the gut microbiota after ionizing radiation exposure. Standard methodological procedures expected by Cochrane were used. Results: A total of 2929 nonduplicated records were identified, and based on the inclusion criteria, 11 studies were considered. Studies were heterogeneous, with differences in population and outcomes. Overall, we found evidence for an association between ionizing radiation exposure and dysbiosis: reduction in microbiota diversity and richness, increase in pathogenic bacteria abundance (Proteobacteria and Fusobacteria), and decrease in beneficial bacteria (Faecalibacterium and Bifidobacterium). Conclusions: This review highlights the importance of considering the influence of ionizing radiation exposure on gut microbiota, especially when considering the side effects of abdominal and pelvic radiotherapy. Better knowledge of these effects, with larger population studies, is needed.
Keywords: microbiome, microbiota, intestinal microbiome, gut microbiota, microflora, ionizing radiation, radiotherapy, radiation effects
1. Introduction
The human gut microbiota is defined as the microorganisms (bacteria, viruses, archaea, and protists) that collectively inhabit the lumen and the mucosal surface of the intestinal tract. The collection of all genomes of those microorganisms constitutes the intestinal microbiome [1,2].
Each individual gut microbiota composition is established early in life and is relatively stable over time. However, an imbalance of its composition (dysbiosis) can be precipitated by various exogenous and endogenous factors, such as significant changes in diet, infections, the use of antibiotics, or abdominal surgery [2,3]. Dysbiosis has been associated with a wide variety of pathologies, including gastrointestinal and nongastrointestinal diseases [1,2,4].
Ionizing radiation consists of energy capable of detaching electrons from atoms or molecules, thus ionizing them. It results from the decay of radionuclides (unstable atoms) and may take the form of electromagnetic waves (gamma (γ) or X-rays) or particles (alpha, beta, or neutrons) [5,6,7,8].
Interaction of radiation with matter results in indirect and direct effects, ranging from creating free radicals (mainly from water molecules) to altering the DNA molecule and its actual destruction [5,6,7,8].
As such, depending on its type, energy, and penetration, ionizing radiation may temporarily affect the function of molecules and atoms, lead to mutations that may be transmitted to the following generations, and, ultimately, lead to the destruction of cells [8,9].
Following the exposure, possible damage to the tissues depends on the radiation dose and tissue’s radiosensitivity. The most radiosensitive cells are those rapidly dividing, well-nourished, and with high metabolic activity [1,10,11]. Nevertheless, beyond certain thresholds, there are known expectable acute effects that will occur independently of the tissue [8].
It should be noted that some microorganisms are resistant to higher levels of ionizing radiation. Bacterial survival and adaptation to stressors include a complex network of regulation, including post-transcriptional regulators, such as small RNAs, that when adequately combined may enhance bacterial resistance to ionizing radiation. A better understanding of these mechanisms and which bacteria are more prone to be affected by ionizing radiation may prove helpful to predict and prevent dysbiosis [12,13].
Exposure may be natural or human-made. Daily, global natural exposure derives from naturally occurring radioactive materials and cosmic rays. Human-made sources of exposure result from nuclear power generation or, more frequently, from medical procedures, namely in radiology and nuclear medicine procedures and in radiotherapy treatments.
Despite its vast benefits, radiation from medical procedures can cause adverse effects [8,14,15], including frequent gastrointestinal toxicity during abdominal and pelvic radiotherapy [5,6,7]. Current evidence suggests that the gut microbiome influences radiotherapy efficacy [16,17] and radiation-induced gastrointestinal toxicity [5,7,18,19].
The relation between the gut microbiota and the pathogenesis of radiation-induced gastrointestinal toxicity is believed to be mediated through inflammatory processes, disruption of the epithelial barrier and intestinal permeability, epithelial repair and expression, and release of immune molecules in the intestine. Dysbiosis, whether caused by radiation or other factors, can influence both local and systemic immune responses. Research suggests that gut microbiota composition and diversity could be used as predictive biomarkers for radiotherapy outcomes, so further investigation is essential. Hence, we sought to systematically review the existing evidence of the effects of ionizing radiation on gut microbiota [20,21,22].
The aim was to conduct a systematic literature review of all studies involving human subjects that reported effects of ionizing radiation on gut microbiota, either performed in vivo or in vitro. Key outcomes were radiation-induced changes in the gut microbiota, namely in its composition, diversity, or richness/abundance.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
A systematic search was carried out using the following electronic databases: PubMed/MedLine (23/03/2021), EMBASE (16/08/2021), and Cochrane Library (17/08/2021). Additional articles were identified through the reference list from the included articles and relevant reviews. To ensure that studies had not been missed or wrongly excluded and the search was comprehensive, we searched gray literature, general search engines, and reference lists of included papers.
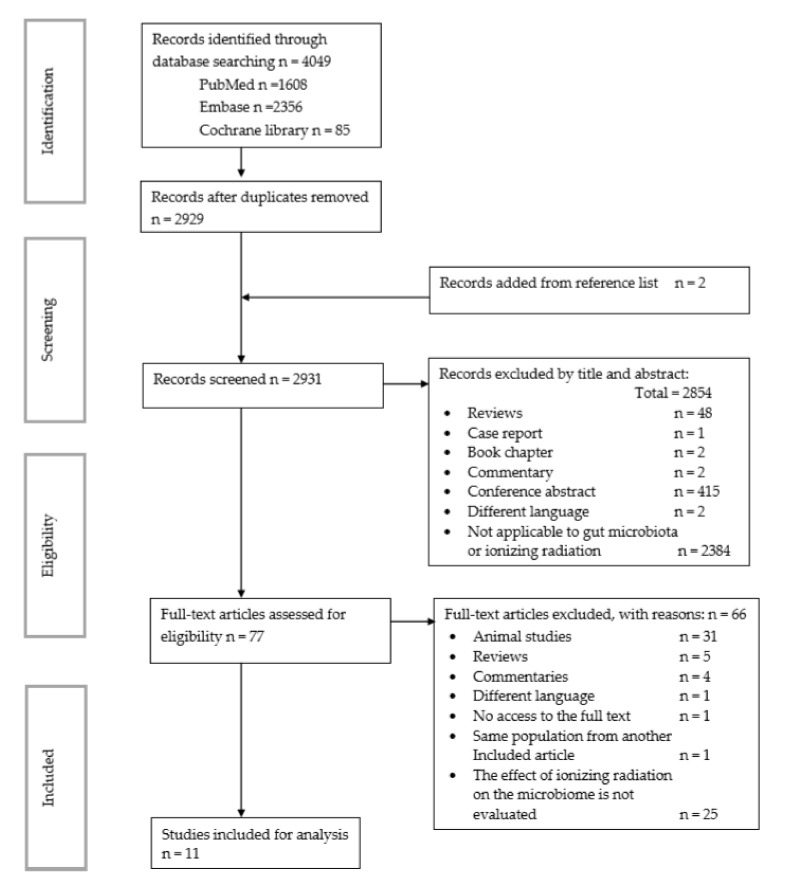
This review was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines checklist (see Table S1). In addition, the review protocol was registered on the International PROSPERO review database on 5 November 2020: PROSPERO 2020: CRD42020210951 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020210951 (5 November 2020) (see Figure 1 for PRISMA diagram and Table 1, Table 2 and Table 3 for search terms).
Figure 1.
PRISMA flow chart search strategy.
Table 1.
Literature search algorithm—PubMed.
| Search Number | Search Terms |
|---|---|
| Search #1 | “microbiota” OR “gastrointestinal microbiome” OR “microbiome” OR “16s rRNA” |
| Search #2 | “radiation” OR “radiotherapy” |
| Search #3 | Search #1 AND Search #2 |
Table 2.
Literature search algorithm—EMBASE (via OVID).
| Search Number | Search Terms |
|---|---|
| Search #1 | “microbiota” OR “gastrointestinal microbiome” OR “microbiome” OR “16s rRNA” OR “microflora” |
| Search #2 | “radiation” OR “radiotherapy” |
| Search #3 | English OR Spanish OR Portuguese |
| Search #4 | Search #1 AND Search #2 AND Search #3 |
Table 3.
Literature search algorithm—Cochrane Library.
| Search Number | Search Terms |
|---|---|
| Search #1 | “microbiota” OR “gastrointestinal microbiome” OR “microbiome” OR “16s rRNA” OR “microflora” |
| Search #2 | “radiation” OR “radiotherapy” |
| Search #3 | Search #1 AND Search #2 |
The PROSPERO database and Cochrane Library revealed no similar systematic reviews. All selected citations were exported from the databases to the reference management software EndNote X20 (Thompson Reuters, New York, New York, USA), and duplicates were excluded.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria were defined using the following components: patient population (P): humans exposed to radiation; exposure of interest (I): ionizing radiation; comparator (C): before and after exposure of the same subject or with controls; outcome (O): the changes in the gut microbiome following exposure to radiation; study design (S) of interest: randomized controlled trials and prospective and retrospective observational cohort studies. Initially, the authors searched for studies in human and animal subjects, but after analyzing the significant differences between the studies, the authors decided to separate the review. Therefore, this review is focused on human studies.
2.3. Study Selection and Data Extraction
All relevant peer-reviewed journal articles in English, Portuguese, and Spanish indexed until March 2021 were identified. A combination of search terms was used: microbiome, gut microbiota, radiotherapy, ionizing radiation, 16S rRNA, and microflora (Table 1, Table 2 and Table 3). The final search was performed on 17 August 2021 by two authors (AF and PB).
According to the defined inclusion and exclusion criteria, relevant studies were independently screened by two reviewers (AF and PB) based on title and abstract. All decisions were recorded on a spreadsheet.
All studies that did not fulfill the defined PICOS characteristics, conference papers, abstracts, and articles for which we could not obtain the full text were excluded.
Full-text papers of all available eligible studies were obtained, and the two reviewers independently screened and selected papers a second time.
A tabular summary with the following variables that were extracted from each eligible study was developed for this review: first author name; date of publication; study design; number of patients and controls; radiation exposure characteristics; type, number, and time point of samples; and the most relevant findings (Table 4).
Table 4.
Summary of study characteristics, demographics, radiation type, sample collection and analysis, and main findings of the eligible studies included.
| Author, Year / DOI |
Study Design | Participant Demographics: N; Sex (M:F); Age; Type of Cancer/Other |
Type of Radiation | Microbiome Assessment Method / Software for Sequencing and For Identification |
Type of Sample / Number of Samples |
Main Findings | Antibiotic Used as Exclusion? / Comments |
|---|---|---|---|---|---|---|---|
|
Cuzzolin et al., 1992 [23] / 10.1080/1120009x.1992.11739160 |
Prospective cohort | N = 15 (0:15) / 45–79 years / Gynecological cancer |
Pelvic RT / 4000 cGy in 4 to 5 weeks overall 175–200 cGy daily 5 days per week |
Culture counts / Agar-based methods |
Fecal / 5 samples: - 1 before - 4 after irradiation fractions |
|
Yes / Other exclusion criteria: cytotoxic chemotherapy |
|
Sajjadieh et al., 2012 [24] / PMID: 23400266; PMCID: PMC3564093 |
Prospective cohort | N = 75 Control group n = 20 / 4–18 years / Living in a contaminated area near Chernobyl |
Ambient radiation / Internal whole-body radioactivity Cs-137 measured by γ-ray spectrometry |
Bacterial culture; colony-forming units / CPLX agar Bifidobacterium; LBS agar Lactobacillus; COBA agar Enterococcus; DHL agar Enterobacter |
Fecal / 1 sample |
|
Yes / - |
|
García-Peris et al., 2012 [25] / 10.3305/nh.2012.27.6.5992 |
RCT | N = 31 (0:31) / 36–77 years (median 59) / Gynecological cancer |
Pelvic RT / 52.2 Gy 1.8 Gy/day 5 times a week 29 sessions |
Culture counts / Fluorescent in situ hybridization, genus-specific probes (Bifidobacterium: Bif164 and Lactobacillus: LAC158) |
Fecal / 4 samples: - 7 days before RT - 15 days after RT - At the end of the treatment - 3 weeks after RT |
|
Yes / Other exclusion criteria: previous RT; previous or adjuvant QT; immunosuppressive |
|
Nam et al., 2013 [5] / 10.1371/journal.pone.0082659 |
Prospective cohort | N = 9 (0:9) Control N = 6 / 35–63 years / Gynecologic cancer (cervix and endometrium) |
Pelvic RT / 50.4 Gy 5 times a week 5 week period 25 fractions |
16S rRNA V1/V2 / QIIME MOTHUR UPARSE / Ribosomal and SILVA databases |
Fecal / 4 samples:- 1 week before - After the first RT - At the end of the fifth RT - 1–3 months after final RT |
|
Yes / QT (two individuals did not take QT during radiotherapy) |
|
Wang A et al., 2015 [7] / 10.1371/journal.pone.0126312 |
Prospective cohort | N = 11 (2:9) Control: N = 4 / 41–65 years (median 51) / Cervical, anal, and colorectal cancer |
Pelvic RT / 44–50 Gy 1.8–2.0 Gy/day 5 times a week 5 week period 25 fractions |
16s rRNA V3 region / SILVA ribosomal RNA database/MOTHUR |
Fecal 2 samples: - Immediately before - Just after RT |
|
Yes / Other exclusion criteria: chemotherapy, steroid, immunosuppressor 1 month before / Comparison between patients that developed diarrhea and those who did not |
|
Yi et al., 2021 [17] / 10.1158/1078-0432.CCR-20-3445 |
Prospective cohort | N = 84 (58:26) Control N = 31 / Nonresponder group 56.46 ± 9.47 years Responder 56.64 ± 10.43 / Locally advanced rectal cancer |
Pelvic RT / 45–50 Gy daily fraction 1.8–2 Gy |
16S rRNA gene V3–V4 region / Ribosomal Database Project classifier /Illumina Miseq / VSearch; USearch STAMP |
Fecal / 2 samples :- Initial day (n = 84) - Within three days upon completion of (n = 83) nCRT treatment |
|
No / Exclusion: exposure to prebiotics, probiotics, steroids, or immunosuppressants / QTconcurrent |
|
Wang Z et al., 2019 [26] / 10.1111/jcmm.14289 |
Prospective cohort | N = 18 (0:18) / 30–67 years (median 57) / Cervical cancer |
Pelvic RT / 50.4 Gy 180cGy/fraction |
16s rRNA / Illumina Hiseq / QIIME / UPARSE / Greengene database |
Fecal / 2 samples: - One day before - First day after the treatment |
|
Yes / Other exclusion criteria:recent use of probiotics; proton pump inhibitors; other morbidities such as enteritis or autoimmune condition |
|
Sahly et al., 2019 [27] / 10.7717/peerj.7683 |
Prospective cohort | N = 3 (3:0) Control N = 2 / 3.5–7 years / Rhabdomyosarcoma near pelvic region |
Pelvic RT / 50.4 Gy 180 cGy / fraction 28 fractions |
16s rRNA V3–V5 / Illumina Miseq / QIIME 2 / SILVA database |
Fecal / 3 samples: - Before radiotherapy - 12–16 days after - 26–28 days after |
|
No / QT weeks before RT |
|
Shi et al., 2020 [16] / 10.3389/fcimb.2020.562463 |
Prospective cohort | N = 22 (16:6) / 45–72 years (median 61) / Rectal cancer |
Pelvic RT / 50Gy 2Gy daily fractions |
16s rRNA V3–4 region / MOTHUR / SILVA database / Ribosomal Database project |
Fecal samples / 2 samples: - At treatment initiation - Just after nCRT |
|
Yes / concurrent chemotherapy / Exclusion criteria: steroids and immunosuppressants within the previous 6 months |
|
Mitra et al., 2020[18] / 10.1016/j.ijrobp.2019.12.040 |
Prospective cohort | N = 35 (0:35) / 35–72 years (median 47) / Cervical cancer |
Pelvic RT / No information found about doses |
16s rRNA V4 region / Illumina MiSeq / SILVA database / UPARSE |
Fecal / 4 samples: - Before RT - During radiation therapy (weeks 1, 3, and 5) |
|
No / QT Weekly cisplatin |
|
El Alam et al., 2021[19] / 10.1371/journal.pone.0247905 |
Prospective cohort | N = 58 (50:8) / Mean 49.36 ± 10.52 years / Gynecologic cancer patients (55 cervical, 2 vulvar, and 1 with vaginal cancer) |
45 Gy (minimum radiation dose) 5 weeks 25 fractions / Either 2 or 5 pulsed dose brachytherapy |
16S rRNA V4 region / Alkek Center for Metagenomics and Microbiome Research at Baylor College of Medicine using a methodology from the Human Microbiome Project |
Rectal swabs / 5 samples: - Immediately before treatment - 1, 3, 5, and 12 weeks after treatment initiation |
|
No / QTcisplatin and brachytherapy / 53 patients did not provide samples at all time points |
|
Cuzzolin et al., 1992 [23] / 10.1080/1120009x.1992.11739160 |
Prospective cohort | N = 15 (0:15) / 45–79 years / Gynecological cancer |
Pelvic RT / 4000 cGy in 4 to 5 weeks overall 175–200 c Gy daily 5 days per week |
Culture counts / Agar-based methods |
Fecal / 5 samples: - 1 before - 4 after irradiation fractions |
|
Yes / Other exclusion criteria: cytotoxic chemotherapy |
|
Sajjadieh et al., 2012 [24] / PMID: 23400266; PMCID: PMC3564093 |
Prospective cohort | N = 75 Control group n = 20 / 4–18 years / Living in a contaminated area near Chernobyl |
Ambient radiation / Internal whole-body radioactivity Cs-137 measured by γ-ray spectrometry |
Bacterial culture; colony-forming units / CPLX agar Bifidobacterium; LBS agar Lactobacillus; COBA agar Enterococcus; DHL agar Enterobacter |
Fecal / 1 sample |
|
Yes / - |
|
García-Peris et al., 2012 [25] / 10.3305/nh.2012.27.6.5992 |
RCT | N = 31 (0:31) / 36–77 years (median 59) / Gynecological cancer |
Pelvic RT / 52.2 Gy 1.8 Gy/day 5 times a week 29 sessions |
Culture counts / Fluorescent in situ hybridization, genus-specific probes (Bifidobacterium: Bif164 and Lactobacillus: LAC158) |
Fecal / 4 samples: - 7 days before RT - 15 days after RT - At the end of the treatment - 3 weeks after RT |
|
Yes / Other exclusion criteria: previous RT; previous or adjuvant QT; immunosuppressive |
|
Nam et al., 2013 [5] / 10.1371/journal.pone.0082659 |
Prospective cohort | N = 9 (0:9) Control N = 6 / 35–63 years / Gynecologic cancer (cervix and endometrium) |
Pelvic RT / 50.4 Gy 5 times a week 5 week period 25 fractions |
16S rRNA V1/V2 / QIIME MOTHUR UPARSE / Ribosomal and SILVA databases |
Fecal / 4 samples: - 1 week before - After the first RT - At the end of the fifth RT - 1–3 months after final RT |
|
Yes / QT (two individuals did not take QT during radiotherapy) |
|
Wang A et al., 2015 [7] / 10.1371/journal.pone.0126312 |
Prospective cohort | N = 11 (2:9) Control: N = 4 / 41–65 years (median 51) / Cervical, anal, and colorectal cancer |
Pelvic RT / 44–50 Gy 1.8–2.0 Gy/day 5 times a week 5 week period 25 fractions |
16s rRNA V3 region / SILVA ribosomal RNA database / MOTHUR |
Fecal 2 samples: - Immediately before - Just after RT |
|
Yes / Other exclusion criteria: chemotherapy, steroid, immunosuppressor 1 month before / Comparison between patients that developed diarrhea and those who did not |
|
Yi et al., 2021 [17] / 10.1158/1078-0432.CCR-20-3445 |
Prospective cohort | N = 84 (58:26) Control N = 31 / Nonresponder group 56.46 ± 9.47 years Responder 56.64 ± 10.43 / Locally advanced rectal cancer |
Pelvic RT / 45–50 Gy daily fraction 1.8–2 Gy |
16S rRNA gene V3–V4 region / Ribosomal Database Project classifier / Illumina Miseq / VSearch; USearch STAMP |
Fecal / 2 samples: - Initial day (n = 84) - Within three days upon completion of (n = 83) nCRT treatment |
|
No / Exclusion: exposure to prebiotics, probiotics, steroids, or immunosuppressants /QT concurrent |
|
Wang Z et al., 2019 [26] / 10.1111/jcmm.14289 |
Prospective cohort | N = 18 (0:18) / 30–67 years (median 57) / Cervical cancer |
Pelvic RT / 50.4 Gy 180cGy/fraction |
16s rRNA / Illumina Hiseq / QIIME / UPARSE / Greengene database |
Fecal / 2 samples:- One day before - First day after the treatment |
|
Yes / Other exclusion criteria: recent use of probiotics; proton pump inhibitors; other morbidities such as enteritis or autoimmune condition |
|
Sahly et al., 2019 [27] / 10.7717/peerj.7683 |
Prospective cohort | N = 3 (3:0) Control N = 2 /3.5–7 years / Rhabdomyosarcoma near pelvic region |
Pelvic RT / 50.4 Gy 180 cGy/fraction 28 fractions |
16s rRNA V3–V5 / Illumina Miseq / QIIME 2 / SILVA database |
Fecal / 3 samples: - Before radiotherapy - 12–16 days after - 26–28 days after |
|
No / QT weeks before RT |
|
Shi et al., 2020 [16] / 10.3389/fcimb.2020.562463 |
Prospective cohort | N = 22 (16:6) / 45–72 years (median 61) / Rectal cancer |
Pelvic RT / 50Gy 2Gy daily fractions |
16s rRNA V3–4 region / MOTHUR / SILVA database / Ribosomal Database project |
Fecal samples / 2 samples: - At treatment initiation - Just after nCRT |
|
Yes / concurrent chemotherapy / Exclusion criteria: steroids and immunosuppressants within the previous 6 months |
|
Mitra et al., 2020[18] / 10.1016/j.ijrobp.2019.12.040 |
Prospective cohort | N = 35 (0:35) / 35–72 years (median 47) / Cervical cancer |
Pelvic RT / No information found about doses |
16s rRNA V4 region / Illumina MiSeq / SILVA database / UPARSE |
Fecal / 4 samples: - Before RT - During radiation therapy (weeks 1, 3, and 5) |
|
No / QT Weekly cisplatin |
|
El Alam et al., 2021[19] / 10.1371/journal.pone.0247905 |
Prospective cohort | N = 58 (50:8) / Mean 49.36 ± 10.52 years / Gynecologic cancer patients (55 cervical, 2 vulvar, and 1 with vaginal cancer) |
45 Gy (minimum radiation dose) 5 weeks 25 fractions / Either 2 or 5 pulsed dose brachytherapy |
16S rRNA V4 region / Alkek Center for Metagenomics and Microbiome Research at Baylor College of Medicine using a methodology from the Human Microbiome Project |
Rectal swabs / 5 samples: - Immediately before treatment - 1, 3, 5, and 12 weeks after treatment initiation |
|
No / QT cisplatin and brachytherapy / 53 patients did not provide samples at all time points |
2.4. Risk of Bias in Individual Studies
Two reviewers (AF and PB) assessed the risk of bias of each study independently, with disagreements resolved by consensus. The risk of bias was assessed as described in the Cochrane Handbook [28] by recording the methodology used.
The quality of nonrandomized studies was assessed by using the Newcastle–Ottawa Scale. The quality of the studies was examined for (a) selection, (b) comparability, and (c) outcome (Table 5) [29].
Table 5.
Risk of bias—prospective cohorts.
| Author, Year | Selection | Comparability | Outcome | Score |
|---|---|---|---|---|
| Cuzzolin et al., 1992 [23] | *0** | *0 | *0* | 5/9 |
| Sajjadieh et al., 2012 [24] | *00* | *0 | *0* | 5/9 |
| Nam et al., 2013 [5] | **** | *0 | *** | 8/9 |
| Wang A et al., 2015 [7] | *0** | *0 | *0* | 6/9 |
| Yi et al., 2021 [17] | **** | *0 | *** | 8/9 |
| Wang Z et al., 2019 [26] | **** | *0 | *0* | 7/9 |
| Sahly et al., 2019 [27] | **** | *0 | *0* | 7/9 |
| Shi et al., 2020 [16] | **** | ** | *0* | 8/9 |
| Mitra et al., 2020 [18] | **** | ** | *0* | 8/9 |
| El Alam et al., 2021 [19] | **** | *0 | **0 | 7/9 |
Note: *, yes; 0, no.
The quality of the randomized controlled study was analyzed as recommended by the Cochrane Collaboration [28], using the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias (Table 6).
Table 6.
Risk of bias of the randomized controlled trial—García-Peres et al., 2012 [25].
| Domain | Risk of Bias | Comments |
|---|---|---|
| Sequence generation | High | No information regarding the sequence generation. “patients were randomised to receive...” |
| Allocation concealment | Low | “coded sachets” |
| Blinding of participants, personnel, and outcome assessors | Low | Outcome assessors and participants blinded |
| Incomplete outcome data | Low | “Nine patients were excluded from the study: four because they were prescribed antibiotics, three for personal reasons, and two due to lack of adherence” |
| Selection outcome reporting | Low | Study protocol available and all of study’s pre-specified outcomes have been reported |
| Other sources of bias | Low | Study appears to be free of other sources of bias |
No formal statistical analysis was undertaken due to the small number of eligible studies and the heterogeneity of the data and outcomes presented.
3. Results
3.1. Search Results
The database search resulted in 4049 titles (August 2021): 1608 relevant abstracts were identified through PubMed, 2356 through EMBASE, and 85 through Cochrane Library (Figure 1). After removing duplicates and including two papers identified from the reference list, 2929 papers were screened for inclusion based on title and abstract. Of these, 2854 were excluded based on title and abstract (415 were conference abstracts, 2 were book chapters, 48 were reviews, 2 were commentaries, 2 were articles in different languages unreadable by authors, 1 was a case report, and 2384 were not focused on gut microbiota and/or ionizing radiation). Full texts of the remaining 77 studies were carefully assessed, and further 66 were excluded (31 concerned animal studies, 25 did not report the effect of ionizing radiation in microbiota [30,31,32], 5 were reviews [33], 4 were commentaries [34], 1 was in a different language unreadable by authors [35], and the authors could not access to the full text of 1 article). Thus, finally, a total of 11 studies were included in this review.
3.2. Study Characteristics
Eleven analytic studies were included, 1 randomized control trial and 10 prospective cohort studies. A summary of the study characteristics and key findings is presented in Table 4. The analyzed studies were quite heterogeneous regarding patient characteristics, study methodology, and evaluated outcomes. The 11 studies recruited 424 participants, of which 361 were exposed to ionizing radiation and 63 were controls. The median number of participants exposed to ionizing radiation was 22 (range 5–115). Healthy controls were enrolled in five studies, ranging from 2 to 31 participants [5,7,17,24,27]. The patients’ demographics of each study are summarized in Table 4. The age range of participants was 3–79 years. Two studies included 98 participants younger than 18 years old [24,27], including the study published by Sahly et al. that analyzed the gut microbiota of only three children with rhabdomyosarcoma and two controls [27]. One study did not provide complete information regarding the participants’ demographics besides their age range [24]. Of the remaining 10 studies, five only included women [5,18,23,25,26], and one included only male participants [27].
Most studies evaluated the effect of ionizing radiation from a medical exposure (pelvic radiotherapy) [5,7,16,17,18,19,23,26], except the study of Sajjadieh et al., who conducted a study to evaluate the gut microbiota changes in 75 rural patients aged between 3 and 18 who lived in a contaminated area at a distance of 60 to 90 km from the Chernobyl Nuclear Power Plant and were exposed to natural environmental radiation and presented abdominal/gastrointestinal symptoms. Additionally, an older method of microbiome analysis, relying on bacterial culture colony-forming units, was used [24].
García-Peris et al. and Cuzzolin et al. also used older microbiota detection methods for taxa identification: fluorescent in situ hybridization using genus-specific probes for Bifidobacterium and Lactobacillus (Bif164 and LAC158, respectively) and agar-based culture for enteric bacteria, respectively [23,25].
The remaining seven studies were found to be more homogeneous. These prospective cohort studies were conducted in 237 patients, 128 gynecological cancer patients, and 109 colorectal cancer patients treated with pelvic radiotherapy, with doses ranging from 44.0 to 50.4 Gy, mostly five times a week for five weeks and used 16s rRNA for taxa identification [5,7,16,17,18,19,26]. Nevertheless, one of the studies did not report the doses to which the patients were exposed [18].
The number of obtained samples per patient was very heterogeneous between studies: one sample [24], two samples [7,16,17,26], three samples [27], four samples [5,18,25], and five samples [19,23].
The time points of the sampling collections within the studies were also found to be quite heterogeneous. Ten studies collected samples before and after exposure. The first samples were collected a week before, the previous days, or immediately before treatment initiation. The collection of samples after exposure varied from immediately after exposure to three months after.
In six studies, patients were concomitantly treated with chemotherapy [5,16,17,18,19,27], and in four of those studies, patients were also concomitantly treated with antibiotics [17,18,19,27]. Only five studies were conducted without using concomitant chemotherapy or antibiotic therapy [7,23,24,25,26]. Most studies considered treatments with immunosuppressor drugs, prebiotics, or probiotics but failed to mention other concomitant medications or comorbidities.
3.3. Sampling and Microbiota Analysis
Overall, the studies included in this review characterized the gut microbiota through fecal samples, except for the study of El Alam et al., which used rectal swabs [19]. Fecal samples are considered the most convenient and the most frequently used collection method in large-scale studies. They are noninvasive and have long been considered as an accurate representation of the distal gut microbiota. Fecal samples have the disadvantages that they might contain inactive bacteria, bacteria from other gastrointestinal tract compartments, and less controlled sampling variables, compared with biopsy [36]. Rectal swabs, despite being easier to sample, have the disadvantages of no visual aid to pinpoint areas of interest, limited biomass for host studies, more discomfort than fecal sampling, and potential contamination with skin bacteria [36]. The methods used to characterize the microbiota also varied throughout the studies. Most of the reviewed studies opted for 16S rRNA-based sequencing; however, the studies performed before 2013 used culture-based microbiota assessment techniques [23,24,25]. Even though there are several software packages available for microbiome data analysis, most of the reviewed studies chose to use QIIME [5,26,27] and/or MOTHUR [5,7,16] and a reference database for taxonomic classification, such as SILVA [7,16,18], Greengenes [26], Ribosomal Database Project [5,17], or UNITE [36]. Richness (number of OTUs/species) and diversity (alpha diversity (within a single sample) and/or beta diversity (between two samples)) were parameters assessed in most of the reviewed studies. There are various methods available for calculating alpha diversity, which considers the richness of the sample and/or the evenness (relative abundance of different OTUs/species and their even distribution). Commonly used methods included in our studies were the number of observed species, Chao1 index (estimates the richness), Shannon’s index, and Simpson’s index (richness and evenness). For beta diversity, Bray–Curtis, unweighted UniFrac, and weighted UniFrac were used [36,37,38].
3.4. Findings
The analyzed studies suggest that ionizing radiation causes significant changes in the composition, diversity, and richness of the gut microbiota. Key findings of the studies are organized in Table 7.
Table 7.
Key findings from selected studies.
| Dysbiosis | |
|---|---|
| |
| Diversity | |
| Alpha diversity | |
| Alpha diversity |
|
| Shannon index |
|
| Simpson index | |
| Beta diversity | |
| |
| Richness | |
| Richness index |
|
| Chao1 index |
|
| OTUs |
|
| Composition | |
| Phylum level | |
| Firmicutes/Bacteroidetes ratio |
|
| Unclassified bacteria | |
| Actinobacteria | |
| Bacteroidetes | |
| Firmicutes | |
| Fusobacteria |
|
| Proteobacteria | |
| Class level | |
| Gammaproteobacteria |
|
| Bacilli |
|
| Clostridia |
|
| Order level | |
| Clostridiales | |
| Lactobacillales |
|
| Fusobacteriales |
|
| Pasteurellales | |
| Family level | |
| Defluviitaleaceae |
|
| Eubacteriaceae |
|
| Fusobacteriaceae |
|
| Lachnospiracea |
|
| Streptococcaceae |
|
| Veillonellaceae |
|
| Enterococcaceae |
|
| Pasteurellaceae |
|
| Ruminococcaceae |
|
| Genus level | |
| Bacteroides | |
| Bifidobacterium | |
| Citrobacter |
|
| Clostridium_XIVa |
|
| Clostridium XI and XVIII and unclassified (others) |
|
| Coprococcus |
|
| Dorea |
|
| Enterobacter |
|
| Enterococcus |
|
| Escherichia–Shigella |
|
| Ezakiella |
|
| Fusobacterium |
|
| Faecalibacterium | |
| Haemophilus |
|
| Lactobacillus | |
| Megamonas |
|
| Oscillibacter | |
| Parvimonas |
|
| Peptostreptococcus |
|
| Porphyromonas |
|
| Roseburia | |
| Ruminococcus |
|
| Serratia |
|
| Streptococcus | |
| Subdoligranulum |
|
| Sutterella |
|
| Veilonella |
|
| Prevotella_2 |
|
| Prevotella_9 |
|
| Species level | |
| Actinomyces odontolyticus |
|
| Adlercreutzia equolifaciens |
|
| Aeromonas hydrophila |
|
| Amphibacillus sp. YIM-kkny6 |
|
| Bacteroides sp. CCUG 39913 |
|
| Butyrate-producing bacterium T1–815 |
|
| Butyrate-producing bacterium |
|
| Butyrate-producing bacterium SS2/1 |
|
| Candidatus Bacilloplasma |
|
| Coriobacterium sp. CCUG 33918 |
|
| Clostridium methylpentosum |
|
| Clostridiales bacterium DJF CP67 |
|
| Clostridium leptum |
|
| Clostridiales bacterium A2–162 |
|
| Clostridium sp. BGC36 |
|
| Clostridium spp. (Cl. histolyticum, Cl. bifermentans, Cl. sporogenes) |
|
| Dialister sp. E2 20 |
|
| Escherichia coli |
|
| Eubacterium eligens |
|
| Eubacterium hallii |
|
| Enterococcus faecium 1 |
|
| Enterobacter sp. mcp11b |
|
| Fusobacterium nucleatum |
|
| Faecalibacterium Prausnitzii |
|
| Faecalibacterium sp. DJF VR20 |
|
| Human intestinal firmicute CB47 |
|
| Klebsiella pneumonia |
|
| Lactobacillus murinus |
|
| Lachnospiraceae bacterium DJF RP14 |
|
| Lachnospira pectinoschiza |
|
| Lactobacillales bacterium |
|
| Lactobacilli aerobi spp. |
|
| Lactobacilli anaerobi spp. |
|
| Oscillospira sp. BA04013493 |
|
| Prevotella stercorea |
|
| Prevotella copri |
|
| Peptococcus and Peptostreptococcus spp. |
|
| Roseburia inulinivorans |
|
| Ruminococcus sp. DJF VR52 |
|
| Ruminococcus sp. CO28 |
|
| Roseburia sp. DJFVR77 |
|
| Ruminococcus sp. CO41 |
|
| Ruminococcus callidus |
|
| Ruminococcus sp. CO28 |
|
| Ruminococcus sp. CS1 |
|
| Swine fecal bacterium FPC110 |
|
| Weissella confuse |
|
3.4.1. Diversity and Richness Analysis
Seven studies demonstrated that ionizing radiation decreases richness, as measured by the number of OTUs, Chao1 index, and richness index [5,7,17,18,19,26,27]. Only one study reported that the richness and diversity remained unchanged [16]. The three studies that used cultured-based methods could not assess these parameters [23,24,25]. Other parameters such as α-diversity, as measured by Shannon index and Simpson index, also decreased after ionizing radiation exposure in most studies [5,7,18,19,26,27]. The only exception was observed by Yi Y et al.; despite decreases in other parameters (Chao1 index and richness index), they reported an increase in Simpson index, even without statistical significance (p=0.32) [17].
3.4.2. Gut Microbial Composition
All studies reported changes in the microbiota composition after exposure to ionizing radiation, but the methodology of reporting of results was highly variable among them; some only analyzed alterations at phylum or genus level. In addition, only three studies analyzed species level [5,16,23]. The culture-based studies had limited results of the specific bacteria taxa analyzed.
Regarding the composition of the gut microbiota, one of the most consistent findings was an increase in the relative abundance of the bacteria from the Proteobacteria phylum following radiation exposure. Sahly et al. and El Alam et al. reported an increase in the Proteobacteria [19,27], and there was a fluctuating pattern in the findings of Nam et al., initially increasing after the first session and then decreasing after the fifth and in follow-up samples [5]. Other taxonomic levels from the Proteobacteria phylum also increased after exposure: Gammaproteobacteria class [19], the order Pasteurellales [18,19], the family Pasteurellaceae and Haemophilus genera [19], and the genera Serratia [26].
One study showed an increase in the Actinobacteria phylum [27], and Nam et al. found a fluctuating pattern, increasing after the first radiotherapy session and decreasing after the fifth session and in the follow-up sample [5]. Bifidocaterium, the most important genus from the Actinobacteria phylum, decreased in three studies [24,25].
The abundance of the Fusobacteria phylum significantly increased in one study [5]. The relative abundance of the order Fusobacteriales increased in one study [18], and the family Fusobacteriacea was also significantly increased in one study; conversely, the genus Fusobacterium showed a significant decrease reported only in one study [5,17].
The relative abundance of unclassified bacteria showed significant differences after IR exposure in two studies, increasing following radiotherapy treatments [5,7].
Three studies showed a decrease in the Firmicutes/Bacteroidetes ratio (F/B ratio) (decrease in the Firmicutes phylum and increase in the Bacteroidetes phylum) [5,7,27].
Results observed for the phylum Bacteroidetes were mixed. The relative abundance decreased in one study [19]. Conversely, Nam et al. reported decreases during radiation therapy but large increases in the follow-up samples, while two other studies reported relative abundance increases [5,7,27]. The genus Bacteroides, from the Bacteroidetes phylum, also showed mixed outcomes, increasing in two studies [7,27] and decreasing in two other studies [17,26].
Nam et al. reported a decrease of 10.1% in the Firmicutes phylum [5]. Regarding the taxa from the Firmicutes phylum, the order Lactobacillales increased in one study [18]. The genus Lactobacillus decreased in two studies [24,25] and increased in one study [17]. The Lactobacillus murinus species decreased in one study [5] and the Lactobacilli aerobi and anaerobi species both decreased in one study [23]. Oscillibacter significantly decreased in two studies [7,17]. The relative abundance of the Faecalibacterium genus decreased in three studies [7,17,27], and Faecalibacterium prausnitzii was reported to decrease in another study [16].
4. Discussion
This review provides a detailed overview of the clinical studies describing the effect of ionizing radiation on gut microbiota composition.
Nevertheless, there were several limitations of the study. Most studies had a reduced number of participants, and in some of the larger trials, not all participants provided all the samples. For example, El Alam et al. recruited 58 participants, but only 5 provided samples at every time point [19].
Remarkably, the dosage and duration of radiation exposure might have a significant impact on the results. Sheikh et al. [24] analyzed the influence of ambient exposure, and the remaining studies analyzed the influence of pelvic radiotherapy treatment. Most studies used comparable doses and sessions.
Three studies used culture-based methods that limited the information to two and four genera [23,24,25]. The remaining studies used 16S rRNA sequencing to characterize the taxonomic distribution and diversity of gut microbiota. 16S rRNA is a cost-effective semiquantitative method [2].
Despite being the most commonly utilized method, 16S rRNA presents some disadvantages. For instance, the accuracy of identification depends on the extent of the reference database, the primers used for 16S rRNA amplification may lead to potential biases, and the resolution power is only at the species level, but most studies only analyzed genus level [39].
The studies that used 16S rRNA clustered reads into operational taxonomic units (OTUs), which are grouped based on 97% DNA sequence similarity [36]. The OTU clustering allows diversity analyses, taxonomic classification through databases, and a variety of statistical analyses to assess the differences in distribution and abundance between samples and groups [36].
Functional gut microbiota can be assessed using metagenomics, metatranscriptomics, metaproteomics, and metabolomics. Shotgun metagenomics is a quantitative method that provides a vast amount of functional information, identifying the strain level (low-level taxonomic rank describing genetic variants or species subtypes). However, it is very costly [2]. None of the reviewed studies in this review utilized metagenomic or metatranscriptomic shotgun sequencing.
The studies with a higher number of participants had major potential biases. Sajjadieh et al., who recruited a total of 95 participants, used culture-based techniques and only quantified the environmental radiation exposure at that moment [24]. García-Peris et al., who recruited 31 patients, did not include controls as a comparison group and used culture-based techniques to evaluate only two genera [25]. El Alam et al., who recruited 58 participants, had only five participants providing samples at all time points [19]. Finally, 115 participants were recruited by Yi Y et al.; however, samples were only collected three days after the exposure, neglecting the long-term effects. Mitra et al. (n = 35), Wang A et al. (n = 15), Cuzzolin et al. (n = 15), Wang Z et al. (n = 18), and Shi et al. (n = 22) also did not evaluate long-term effects. These are further limitations of these studies, given that the studies that had long-term evaluations reported gradual changes and significant differences in the follow-up sample [5,19,27].
Differences in diversity, richness, and taxonomic composition varied across studies, with multiple different outcome measures. Nevertheless, some concordant results emerged. Overall, gut microbiota diversity and relative abundance of individual bacterial taxa were affected after exposure to ionizing radiation. Most studies showed a decrease in diversity (especially alpha diversity), implying the development of a dysbiotic gut microbiota associated with several diseases as observed in the literature [40].
Regarding composition, the analyzed studies confirmed that the human gut microbiota is mainly composed of two bacterial phyla, Firmicutes and Bacteroidetes (usually more than 90%), and other less abundant phyla including Proteobacteria, Actinobacteria, and Verrucomicrobia [4,41,42].
Although differences in taxonomic composition varied across studies, one of the most consistent findings was the increased relative abundance of Proteobacteria following exposure to ionizing radiation. Proteobacteria phylum is composed of Gram-negative bacteria, including the well-known pathogenic genera Escherichia, Salmonella, Helicobacter, and Legionellales, and has been associated with inflammation, being a sign of dysbiosis [43,44].
Another consistent finding was the decreased relative abundance of the Faecalibacterium genus after exposure. The species Faecalibacterium prausnitzii (previously known as Fusobacterium prausnitzii) is one of the most abundant bacteria of the healthy human gut microbiota and is one of the most essential bacteria that produce butyrate and other short-chain fatty acids [45]. Its depletion has been arguably associated with inflammatory bowel disease [46].
Another noteworthy finding was an increased relative abundance of the bacteria from the Fusobacteria phylum, which are known to be associated with an extensive spectrum of infections [47].
Three studies reported a decrease in the Firmicutes-to-Bacteroidetes ratio [5,7,27]. The relationship between these two dominant phyla has been associated with several pathological conditions, including obesity [48]. However, the F/B ratio only takes into account a high-level taxonomic rank. It, therefore, is considered not reliable by more recent studies that evaluated other taxonomic levels (genus, species, or strain), suggesting that the complexity of how the gut microbiome modulates those diseases is far more complex than an imbalance of these two phyla [49].
Bifidobacterium and Lactobacillus genera are well known to exhibit probiotic effects and have shown to be beneficial for the host, being used in clinical practice for gastrointestinal diseases [24,25,50]. Two of the studies performed in culture growth-based methods reported decreases in abundance of the genera Bifidobacterium and Lactobacillus [24,25], and Cuzzolin et al., who also used a culture growth-based method, reported a decrease in Lactobacilli aerobi and anaerobi species [23]. Conversely, Yi Y et al. reported an increase in Lactobacillus [17].
Bacteroides are the most predominant anaerobes in most humans and tend to be the most abundant bacterial genus. They are known to have an essential role in the hydrolysis and fermentation of exogenous fiber and endogenous mucins, in the deconjugation of bile acids, and in the production of acetic and lactic acids [51,52]. Additionally, they have a role in stimulating the immune system, inducing the production of IL-2 by macrophages and B cells [24,53]. Generally, they tend to have a beneficial role in the gut, but when they go to another location, they can cause significant infections [53]. The analyzed studies reported mixed results: increases in relative abundance in two studies [7,27]; decreases in two other studies [17,26].
4.1. Limitations of the Studies
Overall, few high-quality studies were available, and several limitations were identified as the quality, methodology, and reporting of outcomes were highly variable among included studies. Therefore, the combined analysis of the selected studies presented conflicting results across a multitude of outcome measures.
The primary limitation is that most trials were small in sample size (mean 22; range 5–115) and single-center trials, which may condition the study results and interpretation, as a small number of patients may fail to account for interindividual differences within the study population. Besides, information regarding the number of patients treated, eligibility, selection criteria, and recruitment timescale was rarely provided. Finally, only five studies used healthy volunteers as controls, and most studies did not describe their selection methods, include demographic information, account for possible confounders, or consider possible gut microbiome variability throughout time.
The inclusion of populations with different characteristics is also noteworthy: children vs. adults, inclusion of only female or male participants, and different types of tumors. It is well established that the diversity and composition of the gut microbiota are age-related [2], and sex differences in the gut microbiota composition have been recognized [54]. The possible role of sex and age on the microbiota and its impact is not acknowledged in any included studies.
Another relevant limitation is that information regarding comorbidities, previous exposure to other sources of radiation, and concomitant medications (besides antibiotics, immunosuppressors, and prebiotics) is seldom mentioned in most studies, which makes it more complex to separate cohort effects from within-subject characteristics and could contribute to the heterogeneity of findings across the studies.
Most studies included patients with concomitant chemotherapy and/or antibiotic therapy, which are known to affect the human gut microbiota [55,56]; thus, it is not possible to isolate the unique effect of radiation.
All studies used fecal samples or rectal swabs, which may not fully represent the structure of the whole gut microbiota. Regarding detection methods, another limitation is that three studies used culture-based methods, and the remaining used 16S rRNA, so most studies only reported at the genus/phyla level; only three studies reported at the species level.
Finally, the number and time points of collection of the fecal samples also varied among the different studies, and these factors are known to affect the results regarding the gut microbiota composition.
4.2. Limitations of the Review
Due to the small number of studies found and the heterogeneity of the included study subjects, ionizing radiation exposure, and reporting methods, a meta-analysis was not performed.
One of the most significant limitations of this review was the heterogeneity among studies, especially regarding sample size, population characteristics, and sample time points.
5. Conclusions
This review highlights the importance of considering the effects of ionizing radiation exposure on the human gut microbiota, especially when abdominal and pelvic radiotherapy is being planned. The studies included herein demonstrated that dysbiosis develops after ionizing radiation exposure. It is important to note that there was high variability in the study population and in sampling time points in all included studies, which renders comparisons of the multiple findings rather tricky.
The most consistent and convincing evidence was that, after ionizing radiation exposure, diversity and richness are reduced, whereas pathogenic bacteria abundance, such as Proteobacteria and Fusobacteria, is increased. In addition, the abundance of the Faecalibacterium and Bifidobacterium, known to be beneficial bacteria, is decreased. These findings should be more explored and taken into account, especially when considering the side effects of medical treatments and further embracing prophylactic/therapeutic attitudes.
Future Directions
Given the small sample sizes, the results are exploratory and should be interpreted cautiously. Only 11 studies were included in this review; the evidence regarding the effects of ionizing radiation on human gut microbiota calls for further studies, and the interpretation of the results should consider the several limitations listed above.
High-quality, large-scale trials should be carefully designed to determine the role of ionizing radiation in dysbiosis. More extensive studies, better-designed studies, and longer follow-up periods are needed to understand the process better.
Current evidence suggests that the gut microbiota is directly related to ionizing radiation-induced gastrointestinal toxicity and radiotherapy efficacy. A better understanding of the systemic effects of ionizing radiation and their relation to the gut microbiota is essential. Baseline gut microbial characteristics may serve as predictive tools to identify patients more likely to benefit from cancer treatments.
Future prospective longitudinal studies with larger samples will allow more complex models that account for important factors such as demographics, chronic medications, exercise, diet, and biological factors that might impact the gut microbiota composition.
Acknowledgments
We appreciate Jorge Pereira for his valuable support during the elaboration of this systematic review.
Supplementary Materials
The following are available online at www.mdpi.com/article/10.3390/nu13093025/s1, Table S1: PRISMA Checklist for Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Author Contributions
Conceptualization, A.F, R.S., and P.B.; methodology, A.F. and P.B.; writing—original draft preparation, A.F.; writing—review and editing, A.F., A.O., R.S., and P.B.; supervision, R.S. and P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumagai T., Rahman F., Smith A.M. The Microbiome and Radiation Induced-Bowel Injury: Evidence for Potential Mechanistic Role in Disease Pathogenesis. Nutrients. 2018;10:1405. doi: 10.3390/nu10101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch S.V., Pedersen O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 3.Zhernakova A., Kurilshikov A., Bonder M.J., Tigchelaar E.F., Schirmer M., Vatanen T., Mujagic Z., Vila A.V., Falony G., Vieira-Silva S., et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchesi J.R., Adams D.H., Fava F., Hermes G.D., Hirschfield G.M., Hold G., Quraishi M.N., Kinross J., Smidt H., Tuohy K.M., et al. The gut microbiota and host health: A new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nam Y.D., Kim H.J., Seo J.G., Kang S.W., Bae J.-W. Impact of Pelvic Radiotherapy on Gut Microbiota of Gynecological Cancer Patients Revealed by Massive Pyrosequencing. PLoS ONE. 2013;8:e82659. doi: 10.1371/journal.pone.0082659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford P.A., Gordon J.I. Microbial regulation of intestinal radiosensitivity. Proc. Natl. Acad. Sci. USA. 2005;102:13254–13259. doi: 10.1073/pnas.0504830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang A., Ling Z., Yang Z., Kiela P.R., Wang T., Wang C., Cao L., Geng F., Shen M., Ran X., et al. Gut Microbial Dysbiosis May Predict Diarrhea and Fatigue in Patients Undergoing Pelvic Cancer Radiotherapy: A Pilot Study. PLoS ONE. 2015;10:e0126312. doi: 10.1371/journal.pone.0126312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desouky O., Ding N., Zhou G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015;8:247–254. doi: 10.1016/j.jrras.2015.03.003. [DOI] [Google Scholar]
- 9.Mollà M., Panes J. Radiation-induced intestinal inflammation. World J. Gastroenterol. 2007;13:3043–3046. doi: 10.3748/wjg.v13.i22.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leibowitz B.J., Wei L., Zhang L., Ping X., Epperly M., Greenberger J., Cheng T., Yu J. Ionizing irradiation induces acute haematopoietic syndrome and gastrointestinal syndrome independently in mice. Nat. Commun. 2014;5:3494. doi: 10.1038/ncomms4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth C., Tudor G., Tudor J., Katz B.P., MacVittie T.J. Acute Gastrointestinal Syndrome in High-Dose Irradiated Mice. Health Phys. 2012;103:383–399. doi: 10.1097/HP.0b013e318266ee13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villa J.K., Han R., Tsai C.-H., Chen A., Sweet P., Franco G., Vaezian R., Tkavc R., Daly M.J., Contreras L.M. A small RNA regulates pprM, a modulator of pleiotropic proteins promoting DNA repair, in Deinococcus radiodurans under ionizing radiation. Sci. Rep. 2021;11:12949. doi: 10.1038/s41598-021-91335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Šiková M., Janoušková M., Ramaniuk O., Páleníková P., Pospisil J., Bartl P., Suder A., Pajer P., Kubičková P., Pavliš O., et al. Ms1 RNA increases the amount of RNA polymerase inMycobacterium smegmatis. Mol. Microbiol. 2019;111:354–372. doi: 10.1111/mmi.14159. [DOI] [PubMed] [Google Scholar]
- 14.Andreyev J. Gastrointestinal complications of pelvic radiotherapy: Are they of any importance? Gut. 2005;54:1051–1054. doi: 10.1136/gut.2004.062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauer-Jensen M., Wang J., Boerma M., Fu Q., Denham J.W. Radiation damage to the gastrointestinal tract: Mechanisms, diagnosis, and management. Curr. Opin. Support. Palliat. Care. 2007;1:23–29. doi: 10.1097/SPC.0b013e3281108014. [DOI] [PubMed] [Google Scholar]
- 16.Shi W., Shen L., Zou W., Wang J., Yang J., Wang Y., Liu B., Xie L., Zhu J., Zhang Z. The Gut Microbiome Is Associated With Therapeutic Responses and Toxicities of Neoadjuvant Chemoradiotherapy in Rectal Cancer Patients—A Pilot Study. Front. Cell. Infect. Microbiol. 2020;10:562463. doi: 10.3389/fcimb.2020.562463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi Y., Shen L., Shi W., Xia F., Zhang H., Wang Y., Zhang J., Wang Y., Sun X., Zhang Z., et al. Gut Microbiome Components Predict Response to Neoadjuvant Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer: A Prospective, Longitudinal Study. Off. J. Am. Assoc. Cancer Res. 2021;27:1329–1340. doi: 10.1158/1078-0432.CCR-20-3445. [DOI] [PubMed] [Google Scholar]
- 18.Mitra A., Grossman Biegert G.W., Delgado A.Y., Karpinets T.V., Solley T.N., Mezzari M.P., Yoshida-Court K., Petrosino J.F., Mikkelson M.D., Lin L., et al. Microbial Diversity and Composition Is Associated with Patient-Reported Toxicity during Chemoradiation Therapy for Cervical Cancer. Int. J. Radiat. Oncol. 2020;107:163–171. doi: 10.1016/j.ijrobp.2019.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Alam M.B., Sims T.T., Kouzy R., Biegert G.W.G., Jaoude J., Karpinets T.V., Yoshida-Court K., Wu X., Delgado-Medrano A.Y., Mezzari M.P., et al. A prospective study of the adaptive changes in the gut microbiome during standard-of-care chemoradiotherapy for gynecologic cancers. PLoS ONE. 2021;16:e0247905. doi: 10.1371/journal.pone.0247905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulluwishewa D., Anderson R.C., McNabb W.C., Moughan P.J., Wells J.M., Roy N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 21.Stringer A., Gibson R., Yeoh A., Bowen J., Keefe D. Chemotherapy-induced diarrhoea is associated with a modified intestinal microbiome and intestinal inflammation. Support. Care Cancer. 2011;19:S153. [Google Scholar]
- 22.Stringer A.M. Interaction between Host Cells and Microbes in Chemotherapy-Induced Mucositis. Nutrients. 2013;5:1488–1499. doi: 10.3390/nu5051488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuzzolin L., Zambreri D., Donini M., Griso C., Benoni G. Influence of Radiotherapy on Intestinal Microflora in Cancer Patients. J. Chemother. 1992;4:176–179. doi: 10.1080/1120009X.1992.11739160. [DOI] [PubMed] [Google Scholar]
- 24.Sheikh Sajjadieh M.R., Kuznetsova L.V., Bojenko V.B. Dysbiosis in Ukrainian Children with Irritable Bowel Syndrome Affected by Natural Radiation. Iran. J. Pediatr. 2012;22:364–368. [PMC free article] [PubMed] [Google Scholar]
- 25.García-Peris P., Velasco C., Lozano M.A., Moreno Y., Paron L., de la Cuerda C., Bretón I., Camblor M., García-Hernández J., Guarner F., et al. Effect of a mixture of inulin and fructo-oligosaccharide on Lactobacillus and Bifidobacterium intestinal microbiota of patients receiving radiotherapy: A randomised, double-blind, placebo-controlled trial. Nutr. Hosp. 2012;27:1908–1915. doi: 10.3305/nh.2012.27.6.5992. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z., Wang Q., Wang X., Zhu L., Chen J., Zhang B., Chen Y., Yuan Z. Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy. J. Cell. Mol. Med. 2019;23:3747–3756. doi: 10.1111/jcmm.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahly N., Moustafa A., Zaghloul M., Salem T.Z. Effect of radiotherapy on the gut microbiome in pediatric cancer patients: A pilot study. PeerJ. 2019;7:e7683. doi: 10.7717/peerj.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane. 2021. [(accessed on 5 November 2020)]. Available online: www.training.cochrane.org/handbook.
- 29.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 11 April 2021)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp#.YPyh9YR9Gg0.google.
- 30.Manichanh C., Varela E., Martinez C., Antolin M., Llopis M., Doré J., Giralt J., Guarner F., Malagelada J.-R. The Gut Microbiota Predispose to the Pathophysiology of Acute Postradiotherapy Diarrhea. Am. J. Gastroenterol. 2008;103:1754–1761. doi: 10.1111/j.1572-0241.2008.01868.x. [DOI] [PubMed] [Google Scholar]
- 31.Ritchie L.E., Taddeo S.S., Weeks B.R., Lima F., Bloomfield S.A., Azcarate-Peril M.A., Zwart S.R., Smith S.M., Turner N.D. Space Environmental Factor Impacts upon Murine Colon Microbiota and Mucosal Homeostasis. PLoS ONE. 2015;10:e0125792. doi: 10.1371/journal.pone.0125792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta N., Kainthola A., Tiwari M., Agrawala P.K. Gut microbiota response to ionizing radiation and its modulation by HDAC inhibitor TSA. Int. J. Radiat. Biol. 2020;96:1560–1570. doi: 10.1080/09553002.2020.1830317. [DOI] [PubMed] [Google Scholar]
- 33.Thomsen M., Clarke S., Vitetta L. The role of adjuvant probiotics to attenuate intestinal inflammatory responses due to cancer treatments. Benef. Microbes. 2018;9:899–916. doi: 10.3920/BM2017.0172. [DOI] [PubMed] [Google Scholar]
- 34.Cromer W.E., Zawieja D.C. Acute exposure to space flight results in evidence of reduced lymph Transport, tissue fluid Shifts, and immune alterations in the rat gastrointestinal system. Life Sci. Space Res. 2018;17:74–82. doi: 10.1016/j.lssr.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Kondrashova V.G., Vdovenko V.Y., Kolpakov I.E., Popova A.S., Mishchenko L.P., Gritsenko T.V., Stepanova E.I. Gut microbiota among children living in areas contaminated by radiation and having the cardiac connective tissue dysplasia syndrome. Probl. Radiatsiinoi Medytsyny Radiobiolohii. 2014;19:277–286. [PubMed] [Google Scholar]
- 36.Claesson M.J., Clooney A.G., O’Toole P.W. A clinician’s guide to microbiome analysis. Nat. Rev. Gastroenterol. Hepatol. 2017;14:585–595. doi: 10.1038/nrgastro.2017.97. [DOI] [PubMed] [Google Scholar]
- 37.Kumar R., Eipers P., Little R.B., Crowley M., Crossman D.K., Lefkowitz E.J., Morrow C.D. Getting Started with Microbiome Analysis: Sample Acquisition to Bioinformatics. Curr. Protoc. Hum. Genet. 2014;82:18.8.1–18.8.29. doi: 10.1002/0471142905.hg1808s82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jost L. Partitioning diversity into independent alpha and beta components. Ecology. 2007;88:2427–2439. doi: 10.1890/06-1736.1. [DOI] [PubMed] [Google Scholar]
- 39.Sankar S.A., Lagier J.C., Pontarotti P., Raoult D., Fournier P.E. The human gut microbiome, a taxonomic conundrum. Syst. Appl. Microbiol. 2015;38:276–286. doi: 10.1016/j.syapm.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Mosca A., Leclerc M., Hugot J.-P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016;7:455. doi: 10.3389/fmicb.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., Relman D.A. Diversity of the Human Intestinal Microbial Flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Z., Cheng W., Qu W., Shao G., Liu S. Antibiotic Alleviates Radiation-Induced Intestinal Injury by Remodeling Microbiota, Reducing Inflammation, and Inhibiting Fibrosis. ACS Omega. 2020;5:2967–2977. doi: 10.1021/acsomega.9b03906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M., Dong Y., Wu J., Li H., Zhang Y., Fan S., Li D. Baicalein ameliorates ionizing radiation-induced injuries by rebalancing gut microbiota and inhibiting apoptosis. Life Sci. 2020;261:118463. doi: 10.1016/j.lfs.2020.118463. [DOI] [PubMed] [Google Scholar]
- 45.Suau A., Rochet V., Sghir A., Gramet G., Brewaeys S., Sutren M., Rigottier-Gois L., Doré J. Fusobacterium prausnitzii and Related Species Represent a Dominant Group Within the Human Fecal Flora. Syst. Appl. Microbiol. 2001;24:139–145. doi: 10.1078/0723-2020-00015. [DOI] [PubMed] [Google Scholar]
- 46.Sokol H., Seksik P., Furet J.P., Firmesse O., Nion-Larmurier I., Beaugerie L., Cosnes J., Corthier G., Marteau P., Doré J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 47.Bennett K.W., Eley A. Fusobacteria: New taxonomy and related diseases. J. Med. Microbiol. 1993;39:246–254. doi: 10.1099/00222615-39-4-246. [DOI] [PubMed] [Google Scholar]
- 48.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12(5):1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tseng C.H., Wu C.Y. The gut microbiome in obesity. J. Formos. Med. Assoc. 2019;118:S3–S9. doi: 10.1016/j.jfma.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Yamanouchi K., Tsujiguchi T., Sakamoto Y., Ito K. Short-term follow-up of intestinal flora in radiation-exposed mice. J. Radiat. Res. 2019;60:328–332. doi: 10.1093/jrr/rrz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goudarzi M., Mak T.D., Jacobs J.P., Moon B.H., Strawn S.J., Braun J., Brenner D.J., Fornace A.J., Jr., Li H.H. An Integrated Multi-Omic Approach to Assess Radiation Injury on the Host-Microbiome Axis. Radiat. Res. 2016;186:219–234. doi: 10.1667/RR14306.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salyers A.A. Bacteroides of the Human Lower Intestinal Tract. Annu. Rev. Microbiol. 1984;38:293–313. doi: 10.1146/annurev.mi.38.100184.001453. [DOI] [PubMed] [Google Scholar]
- 53.Wexler H.M. Bacteroides: The Good, the Bad, and the Nitty-Gritty. Clin. Microbiol. Rev. 2007;20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huttenhower C., Gevers D., Knight R., Abubucker S., Badger J.H., Chinwalla A.T., Creasy H.H., Earl A.M., FitzGerald M.G., Fulton R.S., et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iizumi T., Battaglia T., Ruiz V., Perez Perez G.I. Gut Microbiome and Antibiotics. Arch. Med. Res. 2017;48:727–734. doi: 10.1016/j.arcmed.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Villéger R., Lopès A., Carrier G., Veziant J., Billard E., Barnich N., Gagnière J., Vazeille E., Bonnet M. Intestinal Microbiota: A Novel Target to Improve Anti-Tumor Treatment? Int. J. Mol. Sci. 2019;20:4584. doi: 10.3390/ijms20184584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.