Abstract
Purpose:
Consolidative thoracic radiation therapy (TRT) has been shown to improve outcomes for patients with extensive stage small cell lung cancer. We hypothesized that the addition of ipilimumab (IPI) and nivolumab (NIVO) after TRT would improve outcomes for patients with extensive stage small cell lung cancer.
Methods and Materials:
Eligibility required stable disease or better after platinum doublet chemotherapy. Study therapy included consolidative TRT to 30 Gy in 10 fractions, targeting residual primary tumor and initially involved regional lymph nodes. Two weeks after TRT, patients received concurrent IPI (3 mg/kg) and NIVO (1 mg/kg) every 3 weeks for 4 doses followed by NIVO monotherapy (480 mg) every 4 weeks until progression or up to 1 year.
Results:
The study enrolled 21 patients, with 6-month progression-free survival (PFS) of 24% (90% confidence interval [CI], 11%–40%) and a median PFS of 4.5 months (95% CI, 2.7%–4.6%). The 12-month overall survival (OS) was 48% (95% CI, 29%–64%) with a median OS of 11.7 months (95% CI, 4.7%–16.0%). Fifty-two percent of patients had ≥1 possibly related grade 3 to 4 immune-related adverse event. Grade 3 pulmonary and gastrointestinal immune-related adverse events were recorded in 19% and 24% of patients, respectively. Exploratory analysis showed increased cytotoxic T cell (CD3+CD8+) tumor infiltration was associated with favorable PFS (P = .01) and OS (P = .02). Reduction in peripheral blood CD3+CD8+ from baseline to after first dose of IPI/NIVO was associated with improved PFS (P = .02) and OS (P = .02).
Conclusions:
Consolidative IPI and NIVO after platinum-based chemotherapy and TRT demonstrated a toxicity profile consistent with the known adverse events attributable to IPI and NIVO. Although the study regimen did not significantly improve PFS, the OS was higher than historic expectations. CD3+CD8+ tumor infiltration and migration may identify patients most likely to have improved outcomes in small cell lung cancer.
Introduction
Small cell lung cancer (SCLC) accounts for 10% to 15% of new lung cancer cases1 and is associated with poor outcomes.2 Most patients at diagnosis have extensive stage SCLC (ES-SCLC: TNM stage IV with distant metastases [M1], including malignant pleural effusions). Patients with ES-SCLC have historically been treated with 4 to 6 cycles of etoposide plus platinum-based therapy (EP). Overall survival (OS) remains poor, with median survival for ES-SCLC in the range of 9 to 12 months from initial diagnosis.3–6 Recent studies published after initiation of our study have demonstrated a survival benefit with added anti–PD-L1–directed therapy, including atezolizumab7 and durvalumab.8 Despite high initial response rates, the disease often recurs rapidly after completion of chemotherapy, with median progression-free survival (PFS) of only 2 to 3 months even with consolidative immunotherapy.7,8 A Dutch randomized phase 3 trial of patients with ES-SCLC treated with thoracic radiation therapy (TRT) after EP reported a significant improvement in PFS at 6 months (24% vs 7%; hazard ratio [HR] 0.73) and a significant improvement in 2-year OS (13% vs 3%, P = .004) with the addition of TRT.4 Aside from TRT, no standard-of-care treatments have been established for patients with ES-SCLC who complete first-line therapy with EP and have achieved stable disease or response. In a phase 3 trial of topotecan versus observation, topotecan did not show an OS prolongation for SCLC patients after completion of EP.9
Proof of an active immune environment in SCLC has been described in a few analyses of patient samples. First, analysis of 64 SCLC tumors demonstrated that a wide range of CD45+ cells infiltrated the tumor, an average of 40 immune cells/field, and that high CD45+ counts were associated with a better prognosis.10 Evaluation of peripheral blood cells in 35 SCLC patients demonstrated a high CD4+ effector T cell to regulatory T cell ratio in patients with limited stage SCLC (LS-SCLC) versus ES-SCLC.11
Furthermore, SCLC is often linked with high pack-year smoking history,12 which has been shown to be associated with improved response to immunotherapy compared with never smokers.13 Near universal genetic aberration of RB1 and p53 in SCLC facilitates poor genomic stability,14 which has also been associated with response to anti-CTLA4 immunotherapy.
These data support the notion that there is an active immune microenvironment within primary and metastatic SCLC lesions. A phase 1/2 nonrandomized trial (Checkmate 032) evaluated nivolumab with or without ipilimumab in LS-SCLC/ES-SCLC patients who failed platinum-based therapy and demonstrated a response rate of 23% at the most efficacious dose level of ipilimumab 3 mg/kg and nivolumab 1 mg/kg.15 Considering the immune response associated with improved prognosis in SCLC patients and the results of early phase studies using checkpoint inhibitors nivolumab and ipilimumab in SCLC patients in later treatment lines, we hypothesized that radiation therapy (RT) with nivolumab and ipilimumab combination therapy would be safe to administer and possibly offer maintenance treatment benefit in SCLC. Accumulating evidence indicates that RT can trigger immunogenic cell death,16 which we anticipated could amplify the likelihood of antitumor systemic immune response with combined checkpoint blockade and induce an in situ vaccine of the targeted tumor to aid in overcoming the immunosuppressive environment.17
Methods and Materials
Study design and participants
This was an investigator initiated, phase 1/2, single-arm study performed at H Lee Moffitt Cancer Center and Research Institute (clinicaltrials.gov identifier: NCT03043599). Participants with metastatic SCLC that had not progressed on first-line platinum doublet chemotherapy were enrolled from February 2017 until February 2018. A 6-participant phase 1 safety lead-in was followed by enrollment in a phase 2 study. Participants received consolidative thoracic RT (30 Gy in 10 fractions) targeting initially involved thoracic sites. Two weeks after completion of RT, nivolumab (1 mg/kg) and ipilimumab (3 mg/kg) were administered intravenously every 3 weeks for 4 doses followed by nivolumab 480 mg every 4 weeks for up to 1 year or until disease progression. The full protocol is available in the Appendix. Appropriate regulatory approval, including ethics institutional review board approval, was obtained before enrollment of any participants.
Patients were required to be aged 18 years or older and willing to provide informed consent, with good performance status (Eastern Cooperative Oncology Group score 0–1) and with stage TX, NX, M1b histologically proven ED-SCLC (as per the eighth edition of the American Joint Committee on Cancer staging manual). Patients were required to have adequate bone marrow and hepatic and renal function, defined as hemoglobin higher than 80 g/L; absolute neutrophil count >1.0×109 cells/L; platelet count >100×109/L; bilirubin concentration ≤1.5 times the upper limit of normal; aspartate aminotransferase or alanine aminotransferase ≤2.5 times the upper limit of normal; and lipase and amylase <1.5 times the upper limit of normal. Baseline imaging evaluation included computed tomography (CT) of the chest, abdomen, and pelvis with intravenous contrast before thoracic RT and again before initiation of immunotherapy. For radiographic response assessment, only tumors outside the radiation field were included in the analysis. Participants also underwent magnetic resonance imaging of the brain with gadolinium contrast at baseline.
The exclusion criteria included serious medical comorbidities precluding RT or immunotherapy, suspected or known autoimmune disorder, previous invasive malignancy within 2 years (except nonmelanoma skin cancer), oxygen dependence, and pregnancy or lactation.
Procedures
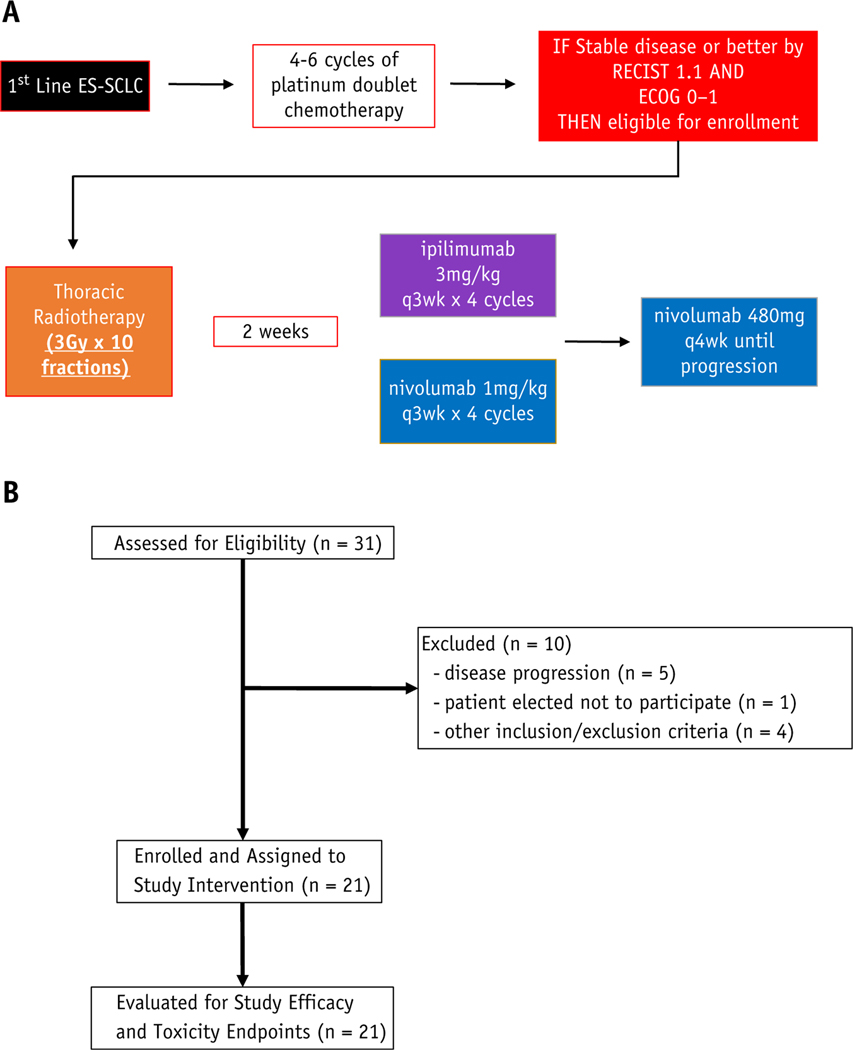
The study schematic is outlined in Figure 1A. Radiation was delivered by a board-certified radiation oncologist using 3-dimensional conformal or intensity modulated RT techniques, including volumetric modulated arc therapy. Full details of the radiation treatment design are available in the protocol, which is included in Appendix E1. Briefly, the prescribed consolidative thoracic RT dose was 30 Gy in 10 fractions over 2 weeks to areas of initially involved disease before first-line platinum doublet chemotherapy in the thorax. Ipilimumab (3 mg/kg) and nivolumab (1 mg/kg) were prescribed by a board-certified medical oncologist and commenced 2 weeks after completion of TRT. They were delivered every 3 weeks for 4 doses. Nivolumab maintenance therapy was delivered every 4 weeks for up to 1 year after commencing study therapy. Treatment response was evaluated every 6 weeks during the first 4 cycles and then every 12 weeks with follow-up visits according to the study schedule. Radiographic surveillance used RECIST version 1.1 criteria. Follow-up included patient history and physical examination (all visits) and adverse event monitoring (all visits). No incentives to increase compliance or adherence were offered to study participants. Participants could voluntarily discontinue participation in the study at any time.
Fig. 1.
(A) Trial profile. (B) CONSORT diagram. Abbreviations: ECOG = Eastern Cooperative Oncology Group; ES-SCLC = extensive stage small cell lung cancer.
Outcomes
The primary objective for the safety run in phase 1 was to confirm the recommended phase 2 dose of ipilimumab and nivolumab. The phase 1 primary endpoint was unacceptable toxicity status at the end of a 13-week safety observation period. Unacceptable toxicity was defined as any related grade ≥3 non–immune-related adverse event (irAE) except for grade 3 fatigue lasting ≤7 days, grade 3 asymptomatic endocrine disorder, grade 3 infusion-related reaction that resolves within 6 hours, grade 3 or 4 nonfebrile neutropenia that improves by at least 1 grade within 3 days, or any grade 3 or 4 irAE that does not downgrade to grade 2 within 7 days. See Appendix E1 for full details.
The phase 2 primary endpoint was 6-month PFS status. PFS was defined as the duration from date of registration to date of first documentation of progression or symptomatic deterioration or death due to any cause. Patients last known to be alive without report of progression are censored at date of last disease assessment. OS was defined as the duration from date of registration to date of death due to any cause. Patients last known to be alive are censored at date of last contact. Exploratory objectives included documenting patterns of radiographic response, in addition to evaluation of archival pretreatment tumor biopsy specimens and serially collected peripheral blood specimens.
Study statistical analysis
The statistical goal of this study was to confirm safety and evaluate efficacy compared with historical control. Slotman et al4 reported a 6-month PFS of 24% with platinum-based chemotherapy and TRT. We proposed to pursue additional randomized study if there was a 20% improvement in 6-month PFS with the addition of combined immune checkpoint blockade after RT. The trial used a Simon’s 2-stage design18 with 1-sided 0.05 level type I error and 90% power. The total target sample size was 52 participants. During the first stage, 18 participants were accrued, with 3 additional participants accrued on the phase 2 study while awaiting 6-month PFS data for the first 18 enrolled participants. For exploratory analyses, Mann-Whitney exact tests and Kaplan-Meier log-rank tests are reported to compare outcomes. Cox proportional hazards models were developed to evaluate the relationship between change in peripheral T cell populations with the primary and secondary study endpoints. Correction for multiple hypothesis testing was not performed.
Baseline tumor tissue multiplex immunofluorescence analysis
Initial evaluation of histomorphologic features on hematoxylin and eosin (H&E) was performed to designate regions of interest for further analysis using the VECTRA method described in the following. Formalin fixed paraffin embedded tissue samples were immunostained using the PerkinElmer OPAL 7-Color Automation IHC kit (Waltham, MA) on the BOND RX auto-stainer (Leica Biosystems, Vista, CA). The OPAL 7-color kit uses tyramide signal amplification conjugated to individual fluorophores to detect various targets within the multiplex assay. Sections were baked at 65°C for 1 hour and transferred to the BOND RX (Leica Biosystems). All subsequent steps (eg, deparaffinization, antigen retrieval) were performed using the automated OPAL IHC procedure (PerkinElmer). OPAL staining of each antigen occurred by using a PerkinElmer blocking buffer for 10 minutes, incubating with a primary antibody, OPAL HRP polymer, and one of the OPAL fluorophores. Individual antibody complexes were stripped after each round of antigen detection. A DAPI counterstain was applied to the multiplexed slide and removed from BOND RX for cover-slipping. Auto-fluorescence slides with primary and secondary antibodies omitting the OPAL fluorophores and DAPI were used as a negative control. Subsequent imaging was performed with the Vectra3 Automated Quantitative Pathology Imaging System.
Multilayer TIFF images were exported from InForm (PerkinElmer) into HALO (Indica Labs) for quantitative analysis. The tissue was then classified as regions of tumor, stroma, or nontissue using a combination of DAPI and pan-cytokeratin. The cells were segmented by DAPI and specific fluorophore staining (pan-cytokeratin, CD3, CD4, PD-L1, FOXP3, CD11b, CD8, CD19, CD69, and CD103) and were quantitatively analyzed via thresholds set per marker based on staining patterns and intensities. Quantifications were evaluated as percent of total cells within evaluated regions, and all analyses were performed on a per-patient basis.
Serial peripheral blood immune cell analysis
For analysis of the immune phenotype of the peripheral blood mononuclear cells, cryopreserved human peripheral blood mononuclear cell samples collected at baseline and after the first dose of ipilimumab/nivolumab were thawed in media and subsequently stained in phosphate-buffered saline containing 5% fetal bovine serum (vol/vol, FACS buffer) with CD3 (BUV496), CD4 (BUV737), CD8 (BUV395), CD14 (BV605), and CD19 (BV605) from BD Biosciences. Dead cells were excluded using the Zombie NIR Fixable Viability Kit from Biolegend, incubated at 4°C for 1 hour, washed twice with FACS buffer, and finally fixed in phosphate-buffered saline containing 1% paraformaldehyde before undergoing flow cytometry. Cells were acquired on a BD FACSymphony A5, and data were analyzed with FlowJo Version 10.0 software. All cell gates were drawn uniformly for analysis across patients and time points.
Serial radiographic image analysis
We evaluated an irradiated region of interest (ROI) within the lung and outside the planning target volume receiving >20 Gy. The ROI was defined using the 20 Gy isodose line and subtracting the planning target volume. Within the ROI, we calculated the Hounsfield unit (HU) mean for each patient before therapy and at subsequent follow-up CT of the thorax at least 60 days and closest to 120 days after commencing TRT. To quantify CT density change, we measured the difference in HU mean within the irradiated ROI before and after treatment.
Results
Twenty-one patients with ES-SCLC that had not progressed with initial platinum doublet chemotherapy were enrolled in this phase 1/2 study. We used Simon 2 stage statistical design, and the study was discontinued at the time of interim analysis. The CONSORT diagram is outlined in Figure 1B. The patient characteristics are outlined in Table 1. The median participant age was 66 years (range, 45–77). Six of 21 (28.6%) patients had brain metastasis (BM) at baseline. Four patients were treated with whole brain RT before enrolling on the study, and 2 patients were enrolled with asymptomatic, subcentimeter brain metastases. The median time from last cycle of platinum doublet chemotherapy to start of RT was 6 weeks (range, 4–8). Patients received a median of 2 ipilimumab and nivolumab doses.
Table 1.
Baseline and treatment characteristics
| Characteristic | n (%) or median (range) N = 21 |
|---|---|
| Age, y | 66 (45–77) |
| Sex | |
| Female | 8 (38%) |
| Male | 13 (62%) |
| Race | |
| White | 19 (90%) |
| Black | 0 (0%) |
| Other | 2 (10%) |
| ECOG performance status at study enrollment | |
| ECOG 0 | 5 (24%) |
| ECOG 1 | 15 (76%) |
| Prior BM treated with WBRT | 4 (19%) |
| BM <1 cm at enrollment | 2 (10%) |
| No BM treated with PCI | 0 (0%) |
| Platinum doublet chemotherapy cycles | |
| 4 | 14 (66%) |
| 5–6 | 7 (33%) |
| Time to RT, wk | 6 (4–8) |
Abbreviations: BM = brain metastases; ECOG = Eastern Cooperative Oncology Group; PCI = prophylactic cranial irradiation; RT = radiation therapy; WBRT = whole brain radiation therapy.
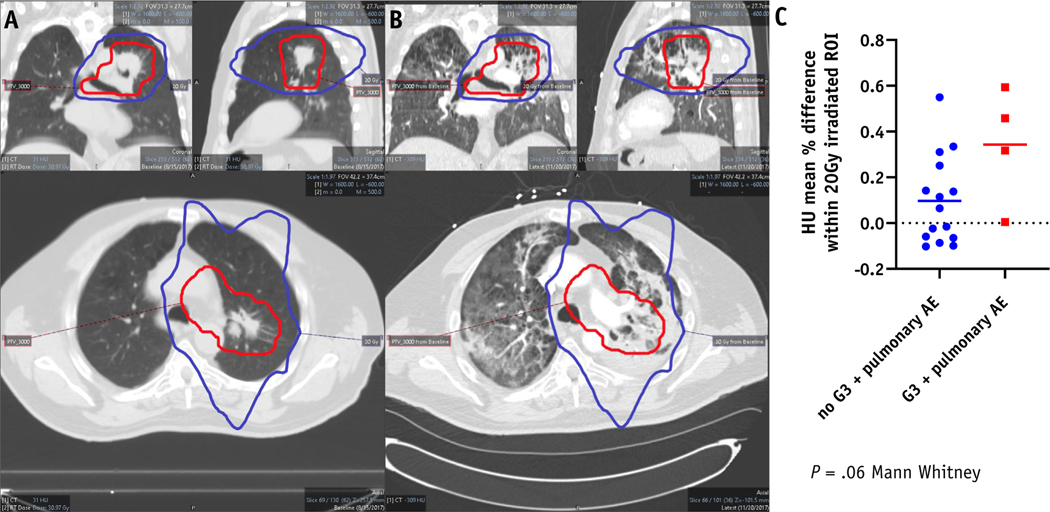
The study adverse event profile is outlined in Table 2. The toxicity profile is similar to previous reported studies of ipilimumab and nivolumab including recently reported Checkmate 451 (Owonikoko et al, ELCC, LBA1, 2019). Thirteen of 21 (61.9%) patients had treatment-related grade 3 or higher adverse events. Eleven (52.4%) patients had possibly immune-related grade 3 or higher possible adverse events (iRAE). The most common grade 3 or higher iRAEs included diarrhea (19%), pneumonitis (9.5%), and thrombocytopenia (9.5%). No grade 3 or higher skin toxicity was reported (Table 2). Grade 3+ pulmonary toxicity was predicted by radiographic changes in the lung after treatment. The mean HU difference within the 20 Gy irradiated ROI demonstrates a trend toward predicting for grade 3+ pulmonary toxicity (P = .06, Fig. 2). There was one grade 4 toxicity, thrombocytopenia, possibly related to immunotherapy. One patient without progression at 4 months developed grade 3 colitis after treatment, which was attributed to ipilimumab, and discontinued study therapy. This patient required treatment with anti–TNF-alpha therapy and subsequently died of fungal pneumonia. There were no treatment-related deaths. Five of 21 patients (24%) discontinued study treatment due to treatment-related adverse events.
Table 2.
Summary of adverse events
| Any grade |
Grade 3–5 |
|
|---|---|---|
| (N = 21) | (N = 21) | |
| Any adverse event | 21 (100.0%) | 16 (76.2%) |
| Treatment-related adverse event | 20 (95.2%) | 13 (61.9%) |
| iRAE | 16 (76.2%) | 11 (52.4%) |
| GI tract | ||
| Diarrhea | 5 (23.8%) | 4 (19.0%) |
| Colitis | 2 (9.5%) | 1 (4.8%) |
| Lungs | ||
| Pneumonitis | 5 (23.8%) | 2 (9.5%) |
| Lung infection | 1 (4.8%) | 1 (4.8%)* |
| COPD exacerbation | 1 (4.8%) | 1 (4.8%) |
| Cough | 2 (9.5%) | 0 (0.0%) |
| Dyspnea | 2 (9.5%) | 0 (0.0%) |
| Wheezing | 1 (4.8%) | 0 (0.0%) |
| Skin | ||
| Pruritus | 2 (9.5%) | 0 (0.0%) |
| Rash | 2 (9.5%) | 0 (0.0%) |
| Liver | ||
| Blood bilirubin increased | 1 (4.8%) | 1 (4.8%) |
| Endocrine system | ||
| Thyroiditis | 1 (4.8%) | 0 (0.0%) |
| Hyperthyroidism | 1 (4.8%) | 0 (0.0%) |
| Hypothyroidism | 2 (9.5%) | 0 (0.0%) |
| Neurologic | ||
| Peripheral sensory neuropathy | 1 (4.8%) | 0 (0.0%) |
| Other | ||
| Thrombocytopenia | 2 (9.5%) | 2 (9.5%) |
| Arthralgia | 2 (9.5%) | 0 (0.0%) |
| Arthritis | 1 (4.8%) | 0 (0.0%) |
| Fever | 1 (4.8%) | 0 (0.0%) |
Abbreviations: COPD = chronic obstructive pulmonary disease; GI = gastrointestinal; iRAE = immune-related adverse event.
Grade 5 lung infection possibly related to complications from study therapy.
Fig. 2.
Hounsfield unit change within the 20 Gy isodose volume but outside the radiation planning target volume (PTV_30 Gy) may predict for grade 3+ possible ipilimumab/nivolumab (IPI/NIVO) related radiation therapy (RT) pulmonary toxicity. (A) Treatment planning computed tomography (CT) thorax scan demonstrating isodose line PTV_30 Gy (red color) and 20 Gy (blue color). (B) Post RT followed by IPI/NIVO CT thorax scan demonstrating RT isodose line PTV_30 Gy (red color) and 20 Gy (blue color). (C) Dot plot comparing Mean Hounsfield unit (HU) percent difference from baseline to post treatment scan at least 60 days after thoracic RT among patients with possible ipi/nivo/RT related grade 3+ pulmonary adverse events (N = 4) and no IPI/NIVO/RT related grade 3+ pulmonary adverse (N = 15) events.
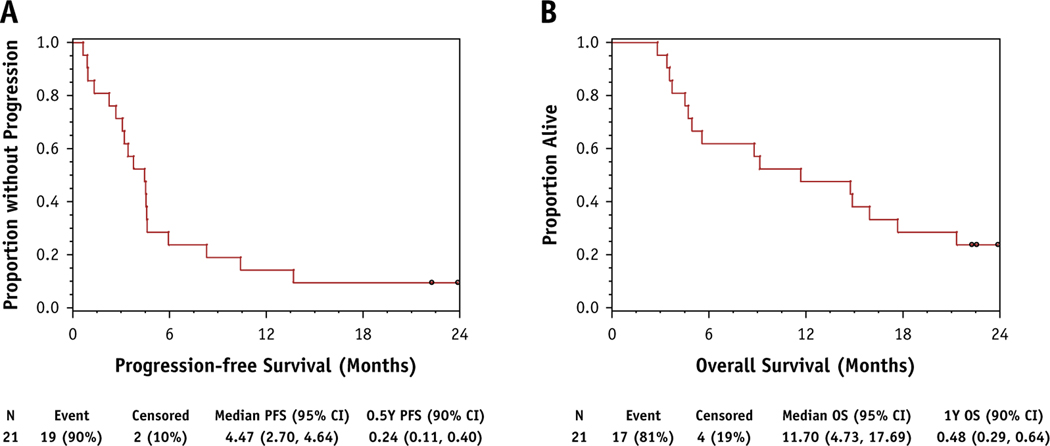
Among 21 evaluable patients who received RT, the primary endpoint of 6-month PFS was 24% (90% CI, 11%–40%). The median PFS was 4.47 months (95% CI, 2.7%–4.64%; Fig. 3A). The extracranial 6-month PFS was 29% (95% CI, 14%–45%; Fig. E1). Five out of 21 (24%) patients had BM as first site of progression. Although PFS was not improved compared with historical estimates, the 12-month OS was 48% (90% CI, 29%–64%). The median OS was 11.7 months (95% CI, 4.73%–17.69%), which is improved by >3 months compared with historical estimates (Fig. 3B). Some patients with at least 12-month survival showed an initial increase in target lesion size followed by tumor shrinkage (Fig. E2).
Fig. 3.
Kaplan-Meier curves for phase 2 study. (A) Primary endpoint of progression-free survival (PFS). (B) Secondary endpoint of overall survival (OS).
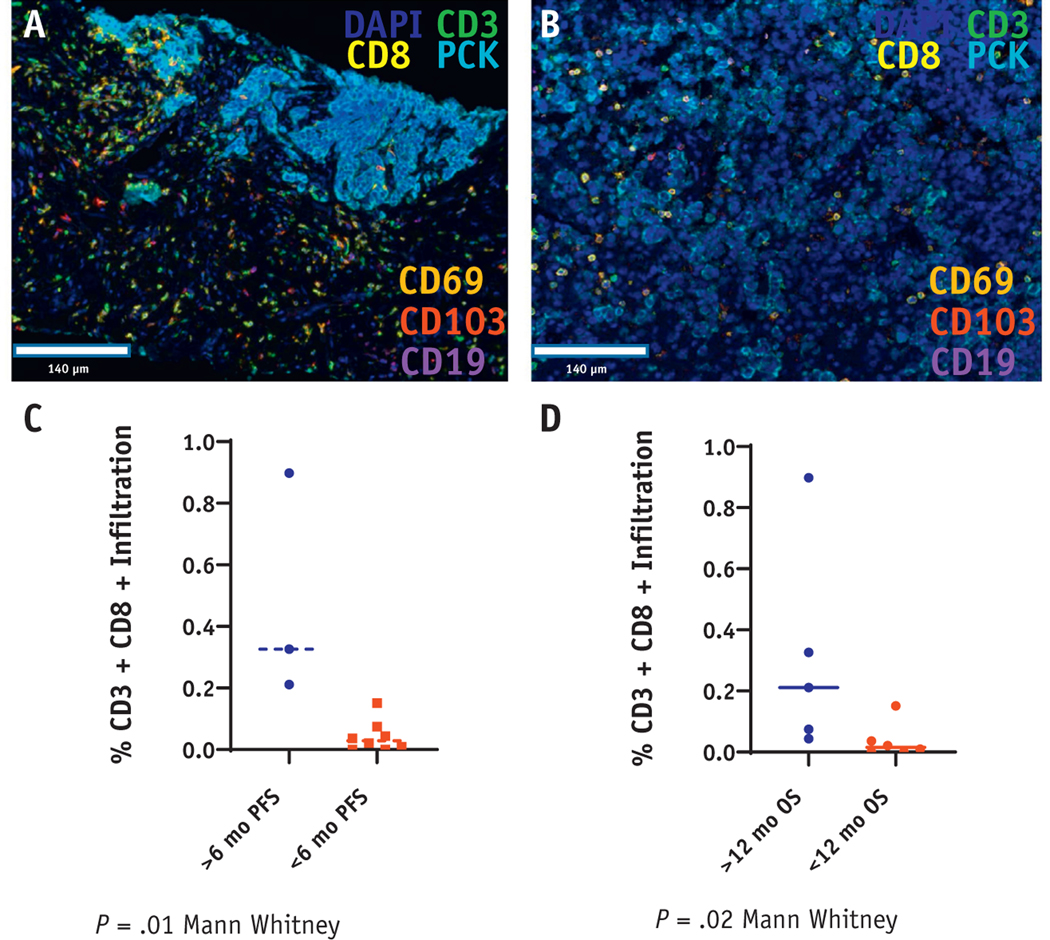
Eleven patients had baseline tumor tissue available for evaluation by multiplex immunofluorescent staining. Pancytokeratin and DAPI expression identified THE intraepithelial cell region, which was evaluated for immune cell infiltration. The baseline tumor tissue was assessed for CD3+CD8+ infiltration, PDL1, and other immune markers, including CD3+CD4+, CD3+CD4+FOXP3+, CD3+CD69+, CD3+CD69+CD103+, CD19+, and CD11b+. Elevated baseline intraepithelial (intratumoral) CD3+CD8+ infiltration predicted achieving the primary endpoint of 6-month PFS (P = .01, P = .07 Bonferroni corrected for multiple comparisons; Fig. 4C). More importantly, patients who survived for at least 12 months after treatment showed denser CD3+CD8+ infiltration compared with patients with <12-month survival (P = .02, P = .14 corrected for multiple comparisons; Fig. 4D).
Fig. 4.
Baseline tumor CD3+CD8+ infiltration predicts response to treatment. (A and B) Representative multiplex immunofluorescence staining for CD3, CD8, CD69, CD103, CD19, and pancytokeratin regions from 2 unique patients. (C) Percent CD3+CD8+ infiltration comparing 11 patients with available data achieving 6-month progression-free survival (PFS; primary endpoint) with patients who progressed sooner than 6 months. (D) %CD3+CD8+ infiltration comparing 11 patients with available data comparing patients achieving 12-month overall survival (OS; secondary endpoint) with patients who died less than 12 months after enrollment.
In addition to CD3+CD8+ infiltration, we evaluated additional markers of immune infiltrates that we anticipated could inform a high likelihood of productive immune response (Fig. E3A and E3B). Baseline percentage of PDL1 positivity within thr tumor did not predict 6-month PFS (P = .27) or 12-month OS (P = .76); however, only 1 patient was noted to have extensive PD-L1 positive staining within the tumor (Fig. E4C). Of the additional planned evaluated biomarkers, %CD3+CD69+ infiltration, indicative of recent T cell activation (Fig. E4E), was also associated with improved PFS (P = .02) with a trend toward improvement in OS (P = .08).
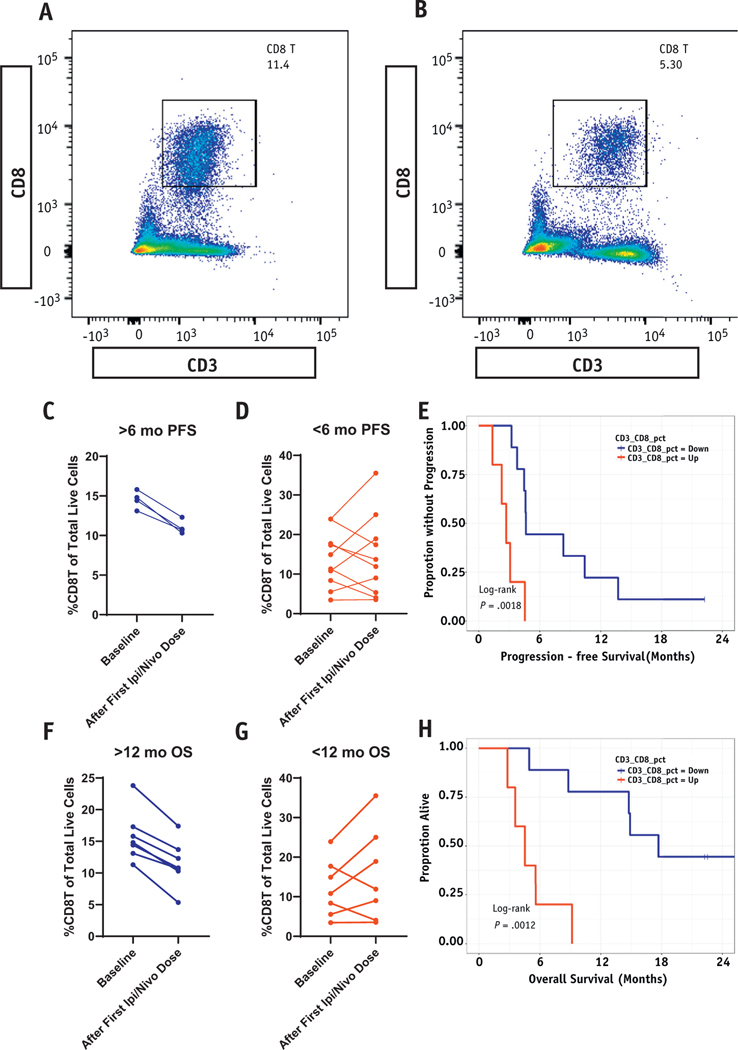
Fourteen patients had peripheral blood evaluated by flow cytometry for immune cell markers including CD4 and CD8 T cells at baseline and 3 weeks after initiating treatment with ipilimumab/nivolumab (Fig. 5A and 5B). In all 4 patients with at least 6-month PFS, the percentage of CD3+CD8+ T cells of total live cells was paradoxically decreased after treatment with ipilimumab and nivolumab (Fig. 5C). More importantly, among patients with at least 12-month OS (N = 6), the percentage of CD3+CD8+T cells was decreased in all patients after treatment with ipilimumab and nivolumab (Fig. 5D). Kaplan-Meier comparisons of PFS (Fig. 5E, P = .002) and OS (Fig. 5F, P = .001) demonstrate improved outcomes among patients with a reduction in CD3+CD8+ T cells in the periphery after treatment with ipilimumab and nivolumab. A univariable Cox proportional hazards model demonstrated that reduction in CD3+CD8+ was associated with time to progression (P = .02) and time to death (P = .02). In contrast, reduction in CD3+CD4+ T cells was not associated with time to progression (P = .11) or time to death (P = .44), according to a univariable Cox proportional hazards model for change in CD3+CD4+ cells from baseline to post iplimumab/nivolumab therapy (Fig. E4).
Fig. 5.
Reduction in peripheral CD3+CD8+ predicts for study primary and secondary endpoints. (A and B) Representative flow cytometry gating cell populations to demonstrate reduction in CD3+CD8+ from baseline to after treatment with ipilimumab/nivolumab. (C) %CD3+CD8+ at baseline and after first dose of ipilimumab and nivolumab among 4 patients achieving 6-month progression-free survival (PFS; primary endpoint). (D) %CD3+CD8+ among patients with PFS <6 months (n = 10). (E) Kaplan-Meier PFS (primary endpoint) based on whether CD3+CD8+ cells were increasing or decreasing after treatment with ipilimumab and nivolumab. (F) %CD3+CD8+ at baseline and after first dose of ipilimumab and nivolumab among 6 patients achieving 12-month overall survival (OS; secondary endpoint). (G) %CD3+CD8+ among patients with OS <12 months (n = 8). (H) Kaplan-Meier overall survival (secondary endpoint) based on whether CD3+CD8+ cells were increasing or decreasing after treatment with ipilimumab and nivolumab.
Discussion
Despite advances in immunotherapy, outcomes remain poor for patients with ES-SCLC. Our single-arm phase 1/2 study was conducted to test the hypothesis that thoracic RT (30 Gy in 10 fx) followed by ipilimumab and nivolumab would improve 6-month PFS by at least 20% compared with the 6-month historical PFS rate of 24%. The rationale for this study was that RT with ipilimumab and nivolumab may stimulate a CD8+ T cell antitumor response. Increased CD8+ infiltration at baseline was found to be associated with improved outcomes in this trial. Furthermore, we demonstrated a reduction specifically in peripheral CD8 T cells after treatment with ipilimumab in patients who met the primary and secondary study endpoints. Our study discontinued early due to low likelihood of meeting the primary endpoint (ie, 6-month PFS goal of 44%). In our study, baseline CD3+CD8+ intraepithelial infiltration was associated with improved outcomes, as was a reduction in peripheral percentage of CD3+CD8+ of total live cells. We hypothesize that the reduction in CD3+CD8+ T cells in the periphery may indicate improved T cell trafficking to the tumor and/or normal tissues.
The randomized, phase 3 chest radiation in extensive disease small cell lung cancer trial showed PFS was 24% at 6 months and OS was 33% at 12 months.4 In another single-arm phase 2 study using pembrolizumab as maintenance therapy, the 6-month PFS was 24% and 12-month OS was 30%.19 A recently published phase 1 study evaluating consolidative thoracic RT followed by pembrolizumab among patients with ED-SCLC reported a 6-month PFS of 50% and 12-month OS of ∼30%.20 A phase 3 randomized study of consolidative ipilimumab with nivolumab compared with nivolumab alone or placebo reported 6-month PFS rates of 20%, 21%, and 10%, respectively.21 The 12-month OS rates were 41%, 44%, and 40% in the combined ipilimumab/nivolumab, nivolumab alone, and placebo arms, respectively. In all these studies, patients were enrolled after platinum doublet chemotherapy and 6-month PFS was comparable with our study. However, in our study, the 12-month OS rate was 48%, which is promising compared with results from the chest radiation in extensive disease small cell lung cancer trial study and recently published studies evaluating consolidative immunotherapy.
Promising results demonstrating benefits with immunotherapy have emerged in the frontline setting for patients with ES-SCLC. ImPower133 demonstrated improved OS comparing atezolizumab with platinum-etoposide chemotherapy with platinum-etoposide chemotherapy alone.7 Similarly, the recently published Durvalumab ± Tremelimumab in Combination With Platinum Based Chemotherapy in Untreated Extensive-Stage Small Cell Lung Cancer study also demonstrated a significant improvement in OS with the addition of durvalumab to platinum-etoposide chemotherapy in the first-line setting. It is difficult to compare our study results with these studies combining platinum-etoposide chemotherapy with anti–PD-L1 therapy. Even these recent successful studies do not significantly improve the long-term survival for most patients with ES-SCLC, and new studies are needed to further improve outcomes. Importantly, neither the ImPower133 nor the Durvalumab ± Tremelimumab in Combination With Platinum Based Chemotherapy in Untreated Extensive-Stage Small Cell Lung Cancer study allowed for consolidative TRT, and no biomarker selection was used to enrich enrollment for patients most likely to benefit from immunotherapy. Additionally, patients in these trials were enrolled before commencing platinum chemotherapy, whereas patients in our study were not enrolled until after completion of platinum-doublet chemotherapy, which makes any comparison difficult.
In some unique patients enrolled on our study with CD3+CD8+ infiltration, outcomes were significantly improved. This suggests that despite the aggressiveness of SCLC, some tumors retain a degree of immunogenicity even at very advanced stages. Establishing which biomarkers could be most useful to identify an immune enriched microenvironment in SCLC is challenging. It is possible that the presence of intraepithelial CD8 T cells within the baseline biopsy may help to identify patients most likely to derive significant benefit from immunotherapy in ES-SCLC.
The histomorphologic features on H&E in SCLC present unique challenges when assessing the immune tumor microenvironment. Biopsies in SCLC commonly have features such as small cancer cell size, necrosis, apoptotic bodies, crush artifact, and fragmentation that complicate the assessment of features that characterize an immunogenic response to the tumor, which include evaluation of tumor-infiltrating lymphocytes, peritumoral stromal lymphocytes, and/or tumor-adjacent lymph nodeelike structures or tertiary lymphoid structures. We used immunofluorescence markers and digital image analysis with the VECTRA/Inform platforms to circumvent these confounding features of SCLC that complicate interpretation on H&E.
Our analysis of serially collected peripheral blood samples offers an intriguing hypothesis for future validation. We selected the Cox proportional hazards model to analyze the importance of CD3+ cell migration on the study primary and secondary endpoints. Because response to immunotherapy does not follow a proportional hazards assumption, this may limit the power of our exploratory analysis. Additionally, we did not account for multiple hypothesis correction, nor did we propose all our reported exploratory analyses a priori, which limits the significance of the results. For this reason, the exploratory results are hypothesis generating. Nevertheless, it is possible that patients with improved response to immunotherapy may require the ability to traffic cytotoxic CD3+CD8+ T cells from the periphery to the tumor immune microenvironment. Amaria et al demonstrated the importance of CD8+ cell infiltration in a neoadjuvant study of melanoma patients treated with ipilimumab and nivolumab.22 Future studies should evaluate whether a reduction in the peripheral percentage of CD3+CD8+ T cells of total live cells in the blood is associated with improved T cell trafficking to the tumor and/or treatment response to immune therapies aimed at activating CD3+CD8+ T cell–driven treatment response.
Our study limitations include the small sample size and lack of a randomized comparator arm, which could confound PFS and OS estimates. There is a potential for selection bias because this was not a randomized study. We demonstrated that delivery of 30 Gy consolidative TRT with ipilimumab and nivolumab is feasible, although not without significant toxicity. Although the sample size was small, we believe that the statistical design was efficient and allowed us to test our proposed hypotheses with a relatively small number of patients. Recent practice-changing studies7,8 adding anti–PD-L1 therapy to initial platinum-etoposide chemotherapy represent a new standard of care. Future studies are required to determine whether consolidative TRT continues to offer a survival benefit in the immunotherapy era. A randomized study evaluating the role of consolidative RT to the thorax and possibly other persistent sites of disease among patients without progression on initial platinum/etoposide/anti–PD-L1 therapy is required. Because of the cumulative toxicities with chemotherapy and immunotherapy alone, special care will be required to minimize added toxicity from RT. Decreasing radiation target volumes by including only residual gross disease with limited expansion for microscopic disease extension may help to limit toxicity attributable to RT. Furthermore, advanced radiation planning techniques including magnetic resonance imaging guided therapy or particle (as opposed to photon) RT may offer opportunities to reduce toxicity from RT in patients with lung cancer.
Our study is unique because this is the first report of fractionated TRT with combined anti–PD-1 and anti-CTLA4 therapy. The high rate of grade 3 or higher immune-mediated toxicities with combined checkpoint blockade suggests that improved biomarkers are required to identify patients with SCLC most likely to benefit from immunotherapy (especially anti-CTLA4–containing regimens). Toxicity may have been increased in this study because treatment followed platinum doublet chemotherapy. Checkmate 451, which had a similar study design without TRT, showed a similar toxicity profile for the ipilimumab/nivolumab study arm.21
A neoadjuvant study of ipilimumab and nivolumab in melanoma also reported a high grade 3+ immune-mediated toxicity rate.22 However, anti-CTLA4 with anti–PD1/PD-L1 therapy may be required in some cases to derive long-term survival benefits from a stimulated immune response.
Conclusions
Our study demonstrates that some patients treated using our design do achieve long-term survival up to 2 years. Biomarker-guided study enrollment recognizes increased toxicity exposure associated with immune checkpoint blockade, especially anti-CTLA4 therapy. More work will be needed to evaluate whether CD3+CD8+ infiltration is a predictive biomarker of immunotherapy response or simply a prognostic biomarker, as others have reported.23 Correlative analysis of randomized controlled trial data could help to identify promising predictive biomarkers of response for patients with ES-SCLC.
Supplementary Material
Acknowledgments—
The authors acknowledge the Moffitt Flow Cytometry Core and CLIA Tissue Imaging Labm including Carlos Moran Segura and Neale Lopez-Blanco who performed multiplex immune panel staining and Jonathan Nguyen for quantitative image analysis.
We would like to acknowledge grant support 3R01CA201124-02S1, K08CA231454, P30-CA076292, and Bristol-Myers Squibb CA209-840.
Disclosures: B.A.P. reports research support from BMS and personal fees from BMS and AstraZeneca outside the submitted work. S.K. reports research support from BMS. T.J.D. reports personal fees and nonfinancial support from NCCN, AstraZeneca, and Harborside Press and personal fees from AstraZeneca. A.C. reports research support from BMS. G.D.G. reports a pending patent. S.R. reports personal fees from Novocure. J.G. reports research support from Array, AstraZeneca, Boehringer Ingelheim, BMS, Merck, and Genentech and personal fees from Novartis, Inivata, and EMD Serono. A.S. reports travel support from Daiichi Sankyo and Prime Oncology. M.S. reports travel support from Janssen and personal fees from GSK. J.R.C.G. reports stock options, intellectual property, and sponsored research with Compass Therapeutics and Anixa Bioscience; consulting fees from Leidos, Compass Therapeutics, and Anixa Bioscience; and patents. S.J.A. reports a consulting or advisory role with BMS, Merck, CBMG, AstraZeneca, Memgen, RAPT, Venn, Achilles Therapeutics, Celsius, Samyang Biopharma, Glaxo Smith Klein, and Amgen; travel support from BMS, Merck, Rapt, Achilles Therapeutics, Celsius, GlaxoSmithKline, and Amgen; and research support from Novartis.
Footnotes
All other authors report nothing to disclose.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
This protocol is registered with ClinicalTrials.gov (NCT03043599).
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.09.031.
References
- 1.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the united states over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539–4544. [DOI] [PubMed] [Google Scholar]
- 2.Tai P, Tonita J, Yu E, et al. Twenty-year follow-up study of long-term survival of limited-stage small-cell lung cancer and overview of prognostic and treatment factors. Int J Radiat Oncol Biol Phys 2003; 56:626–633. [DOI] [PubMed] [Google Scholar]
- 3.Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: Perceptible progress. J Clin Oncol 1999;17:1794–1801. [DOI] [PubMed] [Google Scholar]
- 4.Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: A phase 3 randomised controlled trial. Lancet 2015;385:36–42. [DOI] [PubMed] [Google Scholar]
- 5.Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J Clin Oncol 1999;17: 2092–2099. [DOI] [PubMed] [Google Scholar]
- 6.Ready NE, Pang HH, Gu L, et al. Chemotherapy with or without maintenance sunitinib for untreated extensive-stage small-cell lung cancer: A randomized, double-blind, placebo-controlled phase II study-calgb 30504 (alliance). J Clin Oncol 2015;33:1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–2229. [DOI] [PubMed] [Google Scholar]
- 8.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929–1939. [DOI] [PubMed] [Google Scholar]
- 9.Schiller JH, Adak S, Cella D, et al. Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593dA phase III trial of the eastern cooperative oncology group. J Clin Oncol 2001;19:2114–2122. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Hodkinson P, McLaren F, et al. Histologic assessment of tumor-associated cd45(+) cell numbers is an independent predictor of prognosis in small cell lung cancer. Chest 2013;143:146–151. [DOI] [PubMed] [Google Scholar]
- 11.Koyama K, Kagamu H, Miura S, et al. Reciprocal cd4+ t-cell balance of effector cd62llow cd4+ and cd62lhighcd25+ cd4+ regulatory t cells in small cell lung cancer reflects disease stage. Clin Cancer Res 2008;14:6770–6779. [DOI] [PubMed] [Google Scholar]
- 12.Barbone F, Bovenzi M, Cavallieri F, et al. Cigarette smoking and histologic type of lung cancer in men. Chest 1997;112:1474–1479. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Kim HS, Kim BJ. Prognostic value of smoking status in non-small-cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Oncotarget 2017;8:93149–93155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanel W, Moll UM. Links between mutant p53 and genomic instability. J Cell Biochem 2012;113:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ready NE, Ott PA, Hellmann MD, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: Results from the checkmate 032 randomized cohort. J Thorac Oncol 2020;15:426–435. [DOI] [PubMed] [Google Scholar]
- 16.Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51–72. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol 2010;31:363–372. [DOI] [PubMed] [Google Scholar]
- 18.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 19.Gadgeel SM, Pennell NA, Fidler MJ, et al. Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J Thorac Oncol 2018;13:1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh JW, Heymach JV, Chen D, et al. Phase I trial of pembrolizumab and radiation therapy after induction chemotherapy for extensive-stage small cell lung cancer. J Thorac Oncol 2020;15: 266–273. [DOI] [PubMed] [Google Scholar]
- 21.Owonikoko HK, Govindan R, Ready N, et al. Nivolumab plus ipilimumab, nivolumab, or placebo as maintenance therapy in patients with extensive disease small cell lung cancer after first-line platinum-based chemotherapy: Results from the double-blind, randomized phase 3 checkmate 451 study. Geneva, Switzerland: European Lung Cancer Congress; 2019. [Google Scholar]
- 22.Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med 2018; 24:1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muppa P, Parrilha Terra SBS, Sharma A, et al. Immune cell infiltration may be a key determinant of long-term survival in small cell lung cancer. J Thorac Oncol 2019;14:1286–1295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.