Abstract
Tissue-resident memory T cells (TRM) comprise an important memory T cell subset that mediates local protection upon pathogen re-encounter. TRM populations preferentially localize at entry sites of pathogens, including epithelia of the skin, lungs and intestine, but have also been observed in secondary lymphoid tissue, brain, liver and kidney. More recently, memory T cells characterized as TRM have also been identified in tumors, including but not limited to melanoma, lung carcinoma, cervical carcinoma, gastric carcinoma and ovarian carcinoma. The presence of these memory T cells has been strongly associated with favorable clinical outcomes, which has generated an interest in targeting TRM cells to improve immunotherapy of cancer patients. Nevertheless, intratumoral TRM have also been found to express checkpoint inhibitory receptors, such as PD-1 and LAG-3. Triggering of such inhibitory receptors could induce dysfunction, often referred to as exhaustion, which may limit the effectiveness of TRM in countering tumor growth. A better understanding of the differentiation and function of TRM in tumor settings is crucial to deploy these memory T cells in future treatment options of cancer patients. The purpose of this review is to provide the current status of an important cancer immunotherapy known as TIL therapy, insight into the role of TRM in the context of antitumor immunity, and the challenges and opportunities to exploit these cells for TIL therapy to ultimately improve cancer treatment.
Keywords: adoptive cell therapy, CD8+ memory T cells, Cytotoxic T cells, immunotherapy, tissue-resident memory T cells, T cell exhaustion
1. Introduction
The potency of the immune system to combat malignancies has been of great interest for the development of novel therapies for cancer patients [1]. Of particular interest are cytotoxic CD8+ T lymphocytes that mediate antitumor immunity through recognition of peptide-bound major histocompatibility complex (MHC) class I molecules on the surface of malignant cells. Peptide epitopes for CD8+ T cells can arise from neoantigens formed by genome instability of tumor cells [2,3,4,5]. Upon antigen recognition, tumor-specific CD8+ T cells have an unsurpassed capacity to eliminate tumor cells through the release of proinflammatory cytokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α, and cytotoxic molecules including granzyme B and perforin [6]. However, malignant cells are able to utilize various mechanisms to evade elimination by CD8+ T cells. These immune evasion mechanisms include the loss of MHC class I molecule expression on the surface of tumor cells by downmodulating antigen processing and the presentation of peptide antigens on MHC molecules, thereby directly preventing recognition by CD8+ T cells [7,8,9]. Another strategy of malignant cells to cripple the immune system is to induce an anti-inflammatory tumor micro-environment (TME). The TME includes a large repertoire of immune cells with immunosuppressive activity, such as tumor-associated macrophages, myeloid-derived suppressor cells and regulatory T (TREG) cells. These immune cells are able to dampen effector responses of CD8+ T cells through the secretion of anti-inflammatory cytokines, such as IL-4, IL-10 and TGF-β [3,7]. Effector functions and the proliferative capacity of CD8+ T cells can also be impaired by the high glycolytic activity of rapidly growing tumor cells resulting in limited availability of glucose for tumor-infiltrating CD8+ T cells [10]. The lack of glucose impairs the glycolytic activity in CD8+ T cells, which is required for the upregulation of effector functions such as the production of proinflammatory IFN-γ [11]. Moreover, malignant cells can upregulate the metabolic enzyme indoleamine-2,3-dioxygenase (IDO) to limit T cell function via deprivation of the essential amino acids arginine and tryptophan from the TME [12]. Finally, malignant cells and immune cells in the TME upregulate ligands that interact with inhibitory receptors on CD8+ T cells to promote immunosuppression and to favor the outgrowth of the tumor [13]. The best characterized inhibitory receptors on tumor-infiltrating lymphocytes (TILs) are programmed cell death protein 1 (PD-1), cytotoxic T lymphocyte associated-antigen 4 (CTLA-4), lymphocyte-activation gene 3 (LAG-3) and T cell immunoglobulin and mucin-domain containing 3 (TIM-3) [14,15,16,17]. Triggering of these receptors induces a state of exhaustion in CD8+ T cells resulting in the impaired ability of CD8+ T cells to release proinflammatory cytokines [18,19]. The challenge of cancer immunotherapy is to counteract the manipulative strategies that malignant cells utilize to evade elimination through CD8+ T cells and other immune cells.
Promising strategies that employ CD8+ T cells to fight tumor growth include immune checkpoint blockade therapy and TIL therapy. These therapies reinvigorate antitumor responses of CD8 T cells through direct suppression of inhibitory pathways or through the introduction of greatly expanded numbers of CD8+ T cells. However, these therapies currently do not take into account the heterogeneity of the tumor-infiltrating CD8+ T cell population. Distinct subsets of CD8+ T cells have been identified in in vivo tumor models and in cancer patients. Recently, it has become clear that a large TIL fraction consists of tissue-resident memory T cells (TRM). Intratumoral TRM share characteristics with previously identified pathogen-specific TRM. These CD8+ T cells express adhesion receptors such as CD103 that provide interactions with surrounding tumor cells and downregulate migratory pathways that facilitate entry into the circulation. These characteristics enable TRM to maintain themselves at the tumor site, where they can exert antitumor activities such as the production of proinflammatory cytokines to attract other immune cells or cytotoxic mediators to eliminate tumor cells. Importantly, the presence of intratumoral TRM has been associated with favorable clinical outcomes in various solid cancers [20,21,22,23,24], suggesting that intratumoral TRM may form powerful immunological weapons against tumor growth. Nevertheless, similar to other TILs, intratumoral TRM are exposed to an anti-inflammatory TME and have upregulated expression of inhibitory receptors, which may compromise their ability to clear tumor cells. Therefore, the focal points of our discussion are the challenges and opportunities to apply TRM for immunotherapy. We will focus our discussion on the relevance of TRM for immunotherapy on one important strategy known as TIL therapy that employs TILs to target solid cancers.
2. TIL Therapy Is an Important Cancer Immunotherapy
Conventional cancer therapies including surgical resection, radiation therapy, endocrine therapy and chemotherapy have been the standard of care for many decades. These therapies have limitations and are currently insufficient to cure the majority of cancers [7]. A proportion of tumors commonly referred to as ‘hot’ tumors, have a high degree of lymphocyte infiltration and appear to be immunogenic. Therefore, deployment of the host immune system may be a promising strategy to target these hot tumors. Indeed, more recently, several immunotherapies such as chimeric antigen receptor (CAR)-T cell therapy [1,12,25,26], T cell receptor (TCR) gene transfer therapy [1,12,26] and immune checkpoint inhibition [13,27,28,29,30,31] have emerged as successful treatment strategies for cancer patients. In addition to these immunotherapies, TIL therapy has currently also achieved substantial success in the treatment of cancer patients with solid tumors.
TIL therapy utilizes in vitro expanded TILs from resected tumor material for the treatment of cancer patients. TIL therapy was developed based on in vivo experiments showing the antitumor reactivity of in vitro expanded TILs [32,33]. The total lymphocyte fraction at the tumor site was isolated to include tumor-specific T cells in the cultures. After in vitro expansion via anti-CD3-mediated T cell activation in the presence of high doses of IL-2 and reinfusion in tumor-bearing mice, these TILs demonstrated a 50- to 100-fold higher therapeutic potency compared with lymphocyte cultures that were not derived from the tumor [32]. Translation of these mouse studies to human patients have led to promising clinical results in the treatment of metastatic melanoma [34,35,36,37]. Current TIL therapy for melanoma patients employs in vitro expanded TILs originating from resected tumor material for reinfusion into the patient. Using a two-step ex vivo expansion protocol, TILs are initially cultured in the presence of IL-2, before subsequent culture using anti-CD3 and IL-2 in the presence of irradiated allogeneic feeder cells [38,39,40]. Exogenous IL-2 is supplied during these cultures to reinvigorate exhausted T cells that were extracted from the tumor tissue [41,42]. Clinical studies have shown that TIL therapy is highly effective and results in objective response rates of up to 50%, and complete remission in 10–20% of patients with metastatic melanoma [35,43,44,45]. The success in the treatment of end stage melanoma patients has opened doors for adoptive cell therapy employing TILs in the fight against several other types of cancers, such as cervical carcinoma [46], breast carcinoma [47] and non-small-cell lung carcinoma [48,49].
Despite these clinical successes, improvements of TIL therapy are required to further optimize the treatment options of cancer patients. TIL therapy is a personalized therapy that employs expanded T cells from resected tumor material from the patient. The strict dependence on T cells of the patient results from their HLA restrictions. T cells recognize antigens in the context of HLA molecules, which are highly polymorphic, limiting the utility of T cells between different individuals. The importance of limiting patient material for TIL therapy suggests that strategies that reduce cell number requirements will benefit therapeutic options. Currently, TIL therapy requires large numerical expansion to generate the more than 1 × 1010 TILs required for reinfusion into the patient to counter tumor growth [38]. TIL expansion protocols are lengthy and laborious to achieve these cell numbers. Moreover, the introduction of a large number of donor T cells in TIL therapy also present challenges for the availability of homeostatic cytokines, which are crucial for the persistence of T cells under steady state conditions. The homeostatic cytokines IL-7 and IL-15 in recipients of adoptive T cell therapy are essential to support the survival of donor T cells after reinfusion. However, donor T cells have to compete with host cells for the limited availability of these homeostatic cytokines. Lymphodepletion prior to TIL infusion maximizes the potential of the adoptively transferred cells through removal of competing host T cells [50]. Lymphodepletion also augments TIL efficacy through transient elimination of suppressive CD4+CD25+ TREGS and increased activity of antigen-presenting cells to stimulate donor T cells [40,51]. However, lymphodepletion protocols have disadvantages such as collateral damage to the recipient tissues. Therefore, more sophisticated strategies to improve the efficiency of TIL therapy are required.
Effective TIL therapy is dependent on the presence of endogenous tumor-specific T cells in the tumor. However, recent studies have shown that the majority of tumor-infiltrating T cells are bystanders that do not recognize tumor antigens [52,53]. Therefore, improvement of TIL therapy may be achieved through selection of tumor-specific T cells within the donor T cell pool [40,50]. An alternative strategy to improve TIL therapy may be through selection of tumor-specific T cells with optimal capacity to counter tumor growth. The exhausted phenotype of a large proportion of TILs suggests that room for improvement may exist in the selection of functional T cells at the tumor site. The strong association of TRM with increased survival of cancer patients suggests that these T cells are prime candidates for selection into TIL therapy. We will next discuss the differentiation pathways of T cells after tumor development. This information is essential to address a major future challenge of TIL therapy on how to achieve the selective expansion of tumor-specific T cells and of T cells with optimal ability to counter tumor growth.
3. Development of T Cell Exhaustion in the Tumor Microenvironment
Efforts to improve T cell-dependent immunotherapies against cancer start with a better understanding of T cell differentiation in a tumor setting. Tumors create an environment in which T cells are persistently activated with antigens, thereby triggering these T cells to enter a distinct differentiation pathway resulting in T cell exhaustion [54]. Exhausted T (TEX) cells have been described in melanoma [18,55], ovarian carcinoma [16], hepatocellular carcinoma [56], urothelial carcinoma [57], pancreatic carcinoma [58], and non-small-cell lung carcinoma [59]. TEX cells form a lineage with a unique epigenetic and transcriptional profile distinct from that of memory T cells arising after acute infection [54]. In contrast to these memory T cells that survive independent of cognate antigen and undergo self-renewal driven by the homeostatic cytokines IL-7 and IL-15, TEX cells require persistent antigenic stimulation [60]. Therefore, it is not unexpected that antitumor T cells exhibit similar characteristics to virus-specific T cells in chronic infections [61,62,63]. In fact, TEX cells have first been described in the lymphocytic choriomeningitis virus (LCMV) Clone 13 infection model, which similarly to tumors, induces persistent antigenic stimulation [64,65]. More recently, TEX cells have been observed in human infections, including human immunodeficiency virus (HIV) [66,67,68], hepatitis B and C viruses (HBV/HCV) [69,70].
T cell exhaustion is identified by the progressive loss of effector functions, in particular, the production of proinflammatory cytokines and by the sustained expression of inhibitory receptors that suppress T cell activity [41,54]. T cell exhaustion is a differentiation process under the control of transcription factors including TOX, BLIMP-1, EOMES and NR4A that regulate their effector function and the expression of inhibitory receptors [71]. Persistent antigen stimulation and inflammation are thought to drive the sequential loss of effector functions. Loss of IL-2 production is the earliest sign of exhaustion [72,73]. Next, TNF-α production can become compromised [72,73]. IFN-γ production has shown to be more resistant to exhaustion, but is ultimately lost after chronic inflammation [72,73]. TEX cells may undergo these adaptations to reduce immunopathology, as they potentially cause major tissue damage by secreting proinflammatory cytokines [74,75]. While the production of cytokines is sequentially lost, TEX cells appear to maintain the expression of chemokines including CCL3 (MIP-1α), CCL4 (MIP-1β) and CXCL10 (IP-10) [76]. Exhausted CD8+ T cells may also maintain cytotoxic function, given that they have been shown to constitutively produce high levels of granzyme B [63]. The persistence of partial effector function in TEX appears to be functionally relevant in combatting tumor growth.
TEX upregulate inhibitory receptors, which function as immune checkpoints that limit immune activation and prevent autoimmunity [77,78]. Inhibitory receptors that have been associated with T cell exhaustion include PD-1, CTLA-4, LAG-3, TIM-3, CD38, CD39, CD160, 2B4 and TIGIT [79]. PD-1 is the most prominent inhibitory receptor associated with T cell exhaustion [41]. PD-1 is readily upregulated upon T cell activation and its expression persists on TEX [80]. PD-1 recognizes its ligand PD-L1, which is often expressed on tumor cells, and PD-L2, which is present on dendritic cells and macrophages, allowing these cells to employ interactions with inhibitory PD-1 to dampen T cell responses [81]. PD-1 carries an intracellular tail containing an immunotyrosine inhibitory motif (ITIM) and an immunotyrosine switch motif (ITSM), which can recruit phosphatases that dephosphorylate key signal transducers, thereby preventing engagement of proximal signaling molecules with the TCR [82] as well as on the costimulatory molecule CD28 [83,84]. In this manner, PD-1 signaling reduces T cell activation, proliferation, and cytokine secretion of TEX [81]. Therefore, blockade of PD-1 or PD-L1 may lead to reinvigoration of TEX cells and the establishment of robust antitumor responses. Thus, blockade of PD-1 and other inhibitory receptors on tumor-specific TEX cells appears to be an effective therapeutic strategy to reinvigorate TEX cells to counter tumor growth. Taken together, although TEX cells may be interesting therapeutic targets for cancer immunotherapy, the reinvigoration of these T cells into fully functional T cells appears a necessity to boost antitumor responses.
4. Exhausted T Cell Subsets in Tumor Tissue
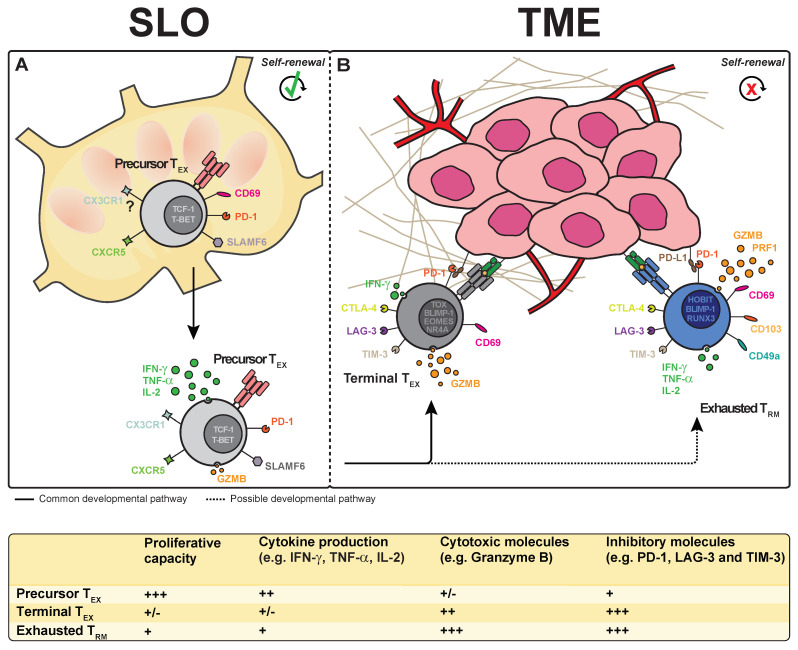
Compelling evidence shows that the TEX population is heterogeneous and consists of different subsets. The majority of TEX cells appear terminally differentiated, display a SLAMF6lowTCF-1lowCXCR5lowEOMEShighPD-1high phenotype and have low proliferative potential (Figure 1) [41]. In contrast to these terminal TEX, a numerical minority of TEX is characterized by SLAMF6highTCF-1highCXCR5highT-BEThighPD-1int expression. This subset of TEX displays high proliferative potential and predominantly localizes to lymphoid tissue rather than the tumor site, where terminal TEX mainly reside (Figure 1A). The lymphoid tissue could provide a protective niche for this minor TEX population away from the immunosuppressive environment of the tumor site [85]. In line with evidence from in vivo tumor models, it has been proposed that this fraction forms TEX precursors that can maintain the terminal TEX population (Figure 1B) [63,85]. Importantly, the increased frequency of TEX precursors is associated with an improved clinical outcome for cancer patients. Moreover, immune checkpoint blockade therapies result in an increased amount of TEX precursors that boost the T cell response against the tumor [63,71,86,87]. Thus, TEX precursors appear to be a more attractive subset for immunotherapy of cancer patients than terminal TEX.
Figure 1.
The differentiation pathway of exhausted T cells and tissue-resident memory T cells in the tumor microenvironment. (A) Upon activation, precursor exhausted T (TEX) cells expressing the surface molecules SLAMF6, CXCR5 and CD69 and the transcription factors TCF-1 and T-BET migrate from the T cell zones of the secondary lymphoid organs (SLO) towards the tumor microenvironment (TME). (B) In the TME, precursor TEX differentiate into terminal TEX, which express the transcription factors TOX, BLIMP-1, Eomes and NR4A, and have an impaired ability to produce cytokines (e.g., IFN-γ, TNF-α and IL-2), but an increased production of cytotoxic molecules (e.g., granzyme B). Terminal TEX also upregulate the expression of inhibitory receptors, such as PD-1, CTLA-4, LAG-3 and TIM-3. Precursor TEX may also give rise to intratumoral tissue-resident memory T (TRM) cells expressing the transcription factors BLIMP-1, HOBIT and RUNX3 and the extracellular molecules CD69, CD103 and CD49a. Similar to terminal TEX, TRM upregulate inhibitory receptors and downregulate cytokine responses. In contrast, TRM appear to maintain expression of cytotoxic molecules. Abbreviations: BLIMP-1, B lymphocyte-induced maturation protein 1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CX3CR1, CX3C chemokine receptor 1; CXCR5, C-X-C chemokine receptor type 5; GZMB, granzyme B; HOBIT, homolog of Blimp-1 in T cells; IFN-γ, interferon gamma; IL-2, interleukin 2; LAG-3, lymphocyte-activation gene 3; NR4A, nuclear hormone receptor 4A; PD-1, programmed cell death protein 1; PRF1, perforin 1; RUNX3, runt-related transcription factor 3; SLAMF6, SLAM family member 6; T-BET, T-box transcription factor 21; TCF-1, T-cell factor 1; TIM-3, T-cell immunoglobulin and mucin domain 3; TNF-α, tumor necrosis factor alpha; TOX, thymocyte selection-associated high-mobility group box protein.
Recent studies also suggest the presence of T cells displaying a phenotype resembling that of TRM in tumor tissues of cancer patients. Tumor infiltrating T cells with TRM-like characteristics have been described in several human cancers, including melanoma [20,88], endometrial adenocarcinoma [23], lung cancer [49,89,90,91,92], bladder cancer [93,94], ovarian cancer [21,95,96], cervical cancer [97], breast cancer [22,98] and colorectal cancer [99]. TRM have initially been identified in acute infection models as a lineage that is distinct from circulating memory subsets, including central memory T (TCM) cells and effector memory T (TEM) cells. Under steady state conditions, TRM cells are permanently maintained in peripheral tissues without accessing the bloodstream, in contrast to circulating TCM and TEM that patrol secondary lymphoid organs and peripheral tissues, respectively [100,101]. Although TRM persist in the peripheral tissues during homeostasis, they are able to exit these tissues after antigenic or inflammatory stimulation, such as occurs during reinfection [102,103]. Thus, it is not inconceivable that tumor TRM may have access to the bloodstream in the presence of persistent antigens, such as occurs in a tumor setting.
The main phenotypic characteristics to distinguish TRM cells from their circulating counterparts include the expression of extracellular markers, such as the C-type lectin CD69, the αE integrin CD103 and the VLA-1 subunit CD49a [104,105]. These molecules provide essential contributions for the persistence of TRM in the tissues. CD69 captures TRM in the peripheral tissues through suppression of S1PR1-driven tissue exit in response to the chemoattractant S1P in blood and lymph [106,107]. CD103 is an integral component of the αEβ7 integrin that mediates adhesion to E-cadherin on epithelial cells [108,109]. In addition, CD49a is an integrin component that allows TRM to anchor into the extracellular matrix through binding of collagens [110]. TRM also express and utilize a distinct set of transcription factors including RUNX3, HOBIT, BLIMP-1 and NOTCH that regulate their tissue residence and effector functions [104,105,111]. These characteristics distinguish TRM from circulating memory T cells that develop in acute infection. Tumor TRM share many surface molecules including CD69, CD103 and CD49a with pathogen-specific TRM, although their expression may vary between TRM in different tumor types [24]. It is less clear how well these molecules identify TRM from other T cell subsets arising in a tumor setting. The definitions of tumor TRM have not yet been clearly demarcated to separate them from populations of TEX. For example, tumor TRM may share expression of CD69 with subsets of precursor and terminal TEX [112]. It is of importance to note that tumor-resident TRM can be clearly identified as a separate population from other T cell subsets based on their CD103 expression [89,90,96,99,113,114]. Transcriptional analysis of CD103+ T cells in lung carcinoma and in head and neck squamous carcinoma have shown that these cells appear to genuinely represent TRM, based on other characteristics such as lack of tissue exit receptors such as S1PR1 [113]. However, the overlay of the current classifications of circulating T cells versus TRM and precursor versus terminal TEX requires further research.
TRM in skin, lungs, female reproductive tract and at other sites have been established as essential immune cells in the protection against reinfection in different experimental infection models [115,116,117,118,119]. Their strategic location at prime entry sites of pathogens in the epithelial and mucosal tissues as well as their potential to immediately respond with the production of proinflammatory cytokines may contribute to the superior potential of TRM in protection against reinvading pathogens [100,120,121]. Moreover, the persistence of TRM in the peripheral tissues, which may depend on the presence of homeostatic cytokines such as IL-7 and IL-15, ensures long-term protection against reinfection [122,123]. The importance of TRM cells in protection against secondary infection with acute viruses [102,124] has sparked the interest for their role in tumor control. Underlining a protective role of TRM against tumor growth, the prevalence of these T cells in tumor tissue has been associated with favorable clinical outcomes in several cancer types, among which are breast cancer, bladder cancer, lung cancer, cervical cancer, colorectal cancer, gastric cancer, ovarian cancer, melanoma and endometrial adenocarcinoma [20,21,22,23,89,97,98,125,126,127,128]. The frequency of TRM appears to outperform the total T cell count in the tumor as a prognostic marker in these cancer patients [89,126,129]. However, the frequency of TRM cells was not able to predict survival of patients suffering from pancreatic cancer [130], suggesting that TRM may not be protective against all cancer types. Nevertheless, these findings highlight the presence and relevance of CD103+ TRM in tumors for the majority of cancer types.
Tumor-infiltrating TRM do not appear to control tumor growth through the production of proinflammatory cytokines. IFN-γ, TNF-α and IL-2 expression were relatively decreased in tumor-derived TRM cells compared with circulating T cells in melanoma patients [88]. Similarly, IFN-γ and TNF-α production were decreased in CD103+ tumor-infiltrating T cells compared with other T cell subsets in head and neck squamous cell carcinoma [131]. In contrast, TRM-like cells found in endometrial and breast cancer retained equal capacities to produce IFN-γ, TNF-α and IL-2, compared with tumor-infiltrating T cells that did not display a TRM phenotype [126,132]. Relative to other tumor-infiltrating T cells, CD103+ TRM also displayed upregulation of immune checkpoint receptors such as PD-1, LAG-3, CTLA-4 and TIM-3 (Figure 1B) [20,88,90,133]. Differences may exist between TRM populations, given that TRM extracted from NSCLC and melanoma do not express CTLA-4 [20,89] and TRM originating from ovarian cancer only weakly express CTLA-4, TIM-3 and LAG-3 [127]. The expression of these inhibitory receptors suggests that the majority of tumor TRM display an exhausted phenotype and that these memory T cells may be reinvigorated using immune checkpoint inhibition therapies. In fact, immune checkpoint blockade of PD-1 and TIM-3 appears to enhance TRM-driven cytokine production [89,134,135]. Furthermore, anti-PD-1 blockade in both a melanoma mouse model, as well as in patients receiving anti-PD-1 therapy, increased the numbers of intratumoral TRM cells [88,136]. These findings imply that inhibitory receptors on TRM may restrain TRM-driven antitumor responses and that relief of their suppression may enhance the therapeutic potential of TRM. Their decreased cytokine production and increased expression of inhibitory receptors also indicate that T cells defined as TRM in these tumors overlap with a fraction of the TEX population.
The compromised cytokine responses of tumor-infiltrating TRM suggest that these cells employ different effector pathways to counter tumor growth. Indeed, tumor-resident T cells appear well-equipped to eliminate tumor cells through the release of cytotoxic molecules. Transcripts of cytotoxic effector molecules granzyme A and B were found to be upregulated in CD103+ T cells that exerted a TRM phenotype in lung carcinoma patients [90]. Moreover, CD103+ TRM-like T cells expressed perforin and granzymes A and B at protein level, in contrast to the CD103− fraction of tumor-infiltrating T cells [22,89,90,94]. CD103+ T cells were also more efficient in killing autologous tumor cells, compared with their CD103− counterparts, as was demonstrated using in vitro co-cultures [89,131]. These studies suggest that the enhanced expression of cytotoxic molecules endows tumor TRM with superior killing abilities to maintain control of tumor growth. Taken together, tumor-associated TRM appear to maintain high expression of cytotoxic molecules, whereas their ability to produce proinflammatory cytokines to counter tumor growth might be restrained through the expression of inhibitory receptors. These findings indicate that, similar to precursor TEX cells, TRM cells are an attractive target for cancer immunotherapy.
5. T Cell Subsets in TIL Therapy
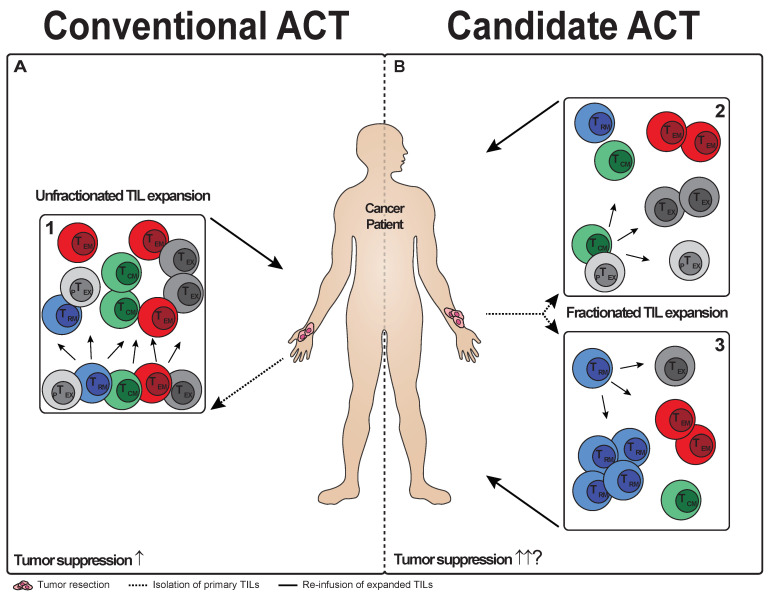
Adoptive cellular therapies such as TIL exploit the antitumor potential of the immune system and have shown promising clinical results in the regression of various tumors. However, despite the substantial progress that has been made, only a fraction of treated patients achieves durable responses, suggesting room for improvement of these cellular therapies. Current TIL therapies make use of unfractionated T cell populations for in vitro expansion to generate large quantities of T cells with antitumor potential. These unfractionated T cells extracted from the tumor are heterogeneous in respect to their specificity and their differentiation stage (Figure 2A). In fact, the majority of CD8+ tumor-infiltrating T cells do not recognize tumor antigens and are considered bystander cells without an apparent direct role in establishing tumor clearance. To a large degree, such bystander T cells in tumors have the phenotype of TRM, as indicated by co-expression of CD69 and CD103 [137]. A proportion of these intratumoral, but non-tumor-responsive T cells have been shown to recognize viruses [52,138]. Interestingly, reactivation of these intratumoral virus-specific CD8+ T cells via local injection of viral peptides induced an immunostimulatory environment within the tumor, resulting in delay of tumor growth [137]. This supports the notion that tumor-residing TRM can contribute to tumor clearance upon adequate stimulation with cognate antigen.
Figure 2.
Strategies of conventional and candidate adoptive T cell therapy. (A) TIL therapy involves the isolation and expansion of tumor-infiltrating lymphocytes (TILs) from tumor tissue for reinfusion into the cancer patient. The current strategy employs unfractionated TILs that may include central memory T (TCM) cells, effector memory T (TEM) cells, tissue-resident memory T (TRM) cells and precursor and terminal exhausted T (TEX) cells (panel 1). (B) A potential novel strategy of TIL therapy is to select TCM or precursor TEX, which have high potential to form the complete spectrum of T cell subsets. However, these precursor cells may have limited potential to form TRM (panel 2). Therefore, another approach to establish improved TIL therapy may be to select TRM cells from tumor tissue, which have intrinsic capacity to reform TRM (panel 3). Both strategies may have the potential to improve the efficacy of TIL therapy to counter tumor growth.
Despite high phenotypic overlap with tumor-specific T cells, bystander T cells lack surface expression of CD39 and 4-1BB. These receptors have been identified as TCR-induced molecules that are preferentially expressed on tumor-reactive T cells in several solid cancers [52,139]. These surface molecules may enable selection of tumor-reactive TILs to improve the response rate of donor T cells in adoptive T cell therapy [52,53,139,140]. Therefore, improvement of TIL therapy may be achieved through selection of tumor-specific CD8+ T cells with optimal capacity to counter tumor growth.
Deletion of undesirable T cell subsets or selection of desirable T cell subsets for in vitro expansion may also maximize the therapeutic potential of adoptive TIL therapy (Figure 2B). Regulatory T cells have been found to accumulate in tumor tissue relative to peripheral blood [141]. These cells have the ability to suppress antitumor responses of T cells and therefore constitute an undesirable T cell subset in the TIL product. Therefore, the selective removal of CD4+ T cells that includes the complete fraction of regulatory T cells may improve the effectiveness of the TIL product. Not only deletion of counter-effective T cells from the TIL product, but also selection to allow the specific outgrowth of T cell subsets with an optimal ability to counter tumor growth may improve TIL therapy. The capacity of specific memory CD8+ T cell subsets to eliminate tumor cells has been addressed in experimental settings of adoptive cellular therapy. Adoptively transferred populations of tumor-specific TCM and TEM have been shown to give rise to effector responses that suppressed tumor growth in tumor-bearing mice. However, responses originating from TCM demonstrated superior antitumor activity compared with those originating from TEM [142]. The underlying reason for the efficacy of TCM cells in countering tumor growth may relate to their superior in vivo proliferative capacity and their ability to induce recall responses [143,144,145,146,147]. TCM are able to generate secondary TCM ensuring self-renewal and persistence of the adoptively transferred memory cells. They are also able to differentiate into TEM and effector T cells, which have robust abilities to eliminate tumor cells [148]. In contrast, TEM are restricted in their potential to form secondary responses of effector T cells [149,150]. These findings designate TCM as superior candidates for fractionated adoptive cell therapies compared with TEM. Characterization of TILs in solid tumors, such as prostate carcinoma, lung carcinoma and melanoma has shown that they are dominated by subsets of exhausted T cells distinct from TCM and TEM [47,151]. Intratumoral TEX are maintained by precursor TEX, which similar to TCM, have high self-renewal and repopulation potential, suggesting that these TEX are superior in countering tumor growth upon adoptive transfer. Since the majority of precursor TEX reside in lymphoid tissue, only a minor fraction of these cells will be retrieved from the tumor in the TIL product for adoptive transfer into cancer patients. Nevertheless, exploring selective employment of precursor TEX appears to be relevant for tumor immunotherapy.
Tumor-infiltrating TRM are associated with improved tumor growth control, suggesting that the successful implementation of these T cells in cellular therapy may benefit treatment of cancer patients. However, the current nonselective culture protocols position TRM at a disadvantage relative to other tumor-derived T cells for inclusion in the TIL product. The antigen non-specific expansion of unfractionated TILs using anti-CD3 antibodies and IL-2 suggests that fast growing subsets can outcompete slow growing subsets in the culture. These differential growth rates of distinct T cell subsets in the tumor may result in the omission of TRM from the final TIL product. Indeed, it appears that CD103+ TRM-like TILs underwent fewer rounds of proliferation compared with their CD103− counterparts upon culture in IL-2 [133]. These findings suggest that current regimens for TIL expansion may result in a substantial reduction of CD103+ TRM-like cells in the final TIL product. Despite their competitive disadvantage in current TIL cultures, TRM possess a considerable proliferative capacity. In response to antigenic challenge, TRM have been shown to substantially contribute to both local and systemic secondary T cell responses [102,124]. Importantly, TRM can achieve durable repopulation of local TRM pools after restimulation [102,124,149,152,153,154]. Previous reports have also demonstrated that TRM from various tissues can be expanded in culture [133,155]. It is possible that current culture protocols are not yet optimized for the expansion of TRM. Standardized culture regimens provide glucose-rich media to expand T cells. However, evidence suggests that TRM mainly rely on mitochondrial β-oxidation of exogenous free fatty acids (FFA) to persist long-term in the peripheral tissues [156]. FFA uptake is regulated by the increased expression of fatty acid binding protein (FABP)4 and FABP5 on TRM relative to circulating memory T cells [156]. Although it is currently unclear how these findings apply to intratumoral settings, they suggest an opportunity to improve expansion of TRM in culture for the purpose of immunotherapy.
The inclusion of TRM in the TIL product may even require selective outgrowth of TRM, given that strong evidence suggests that other T cells have an impaired potential to induce CD103+ TRM [149]. Recent studies have shown that in contrast to naïve T cells, TCM are compromised in their potency to develop into TRM in the skin upon restimulation [136]. Similarly, we and others have reported that TCM were unable to give rise to CD103+ TRM cells at mucosal sites including the skin and small intestine [149,157]. In contrast to naïve T cells, TCM and TEM are unable to robustly upregulate CD103 expression upon stimulation with TGF-β. The inability of circulating T cell subsets to upregulate CD103 in response to TGF-β signaling may be attributed to differential epigenetic imprinting of the Itgae locus [149]. Chromatin accessibility of the Itgae locus encoding CD103 was found to be higher in naïve T cells compared with circulating memory T cells [149,158,159]. In particular, the accessibility of binding regions for RUNX and SMAD transcription factors, which are key targets of TGF-β signaling, was higher in naïve T cells compared with circulating memory T cells [160,161]. Given that circulating memory T cells are unable to induce CD103+ TRM, strategies selectively employing TRM for expansion seem relevant to develop these memory T cells for immunotherapy.
Challenges remain in the development of TRM for cellular adoptive therapies, such as their relocation into tumor tissue following reinfusion in the bloodstream. TRM take permanent residence in the tissues and do not access the bloodstream. Therefore, it is uncertain whether TRM maintain the machinery that is required to access the tumor site after injection into the bloodstream. Reports showing that TRM cells are predisposed to home to their original tissue sites upon transfer suggest that TRM maintain the ability to relocate from the bloodstream into the tissues [124,162,163]. Additionally, intratumoral delivery of expanded TRM cells may be an alternative approach to reinfuse these cells. The injection of DCs into the tumor site has previously been proven effective [164,165,166], but it is unclear whether this strategy is feasible for TRM. Taken together, despite these hurdles, TRM cells appear promising candidates for employment in tumor eradication. TRM cells are able to undergo multiple rounds of proliferation after restimulation and exert robust effector responsiveness [167]. These characteristics of TRM may be highly beneficial for persistence at sites where chronic stimulation might occur, such as in tumor settings. However, further investigation is crucial to elucidate the full potential of TRM for adoptive transfer therapy to eradicate solid tumors.
6. Concluding Remarks
The deployment of immune cells in the fight against cancer has become of great interest in the past years. TIL therapy has shown promise in the treatment of different cancer types. However, durable responses are not achieved in a large fraction of cancer patients, indicating that further improvement of this T cell-driven therapy is required. An area of intense investigation is the differentiation pathway of T cells in a tumor setting. Distinct subsets of precursor TEX, terminal TEX and TRM have been characterized from resected tumor material and in in vivo tumor models. In particular, precursor TEX and TRM have been strongly associated with improved survival of cancer patients [20,23,89,90,94,97]. Thus, the fractionation of T cells into subsets, in particular the enrichment of T cell preparations for precursor TEX or TRM, may boost the potential of current TIL-centered therapies.
Acknowledgments
The authors thank R.A.W. van Lier and M.D. Hazenberg for critical reading of the manuscript.
Author Contributions
A.B.-C., R.L.R.E.T., T.A.N. and K.P.J.M.v.G. drafted the manuscript. A.B.-C. and K.P.J.M.v.G. edited the manuscript. A.B.-C. drafted and edited the figures and figure legends. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a fellowship from the Landsteiner Foundation for Blood Transfusion Research (LSBR, project number 1629) awarded to K.P.J.M.v.G.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors declare no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rohaan M.W., Wilgenhof S., Haanen J.B.A.G. Adoptive cellular therapies: The current landscape. Virchows. 2018;474:449–461. doi: 10.1007/s00428-018-2484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen D., Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Beatty G.L., Gladney W.L. Immune Escape Mechanisms as a Guide for Cancer Immunotherapy. Clin. Cancer Res. 2014;21:687–692. doi: 10.1158/1078-0432.CCR-14-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schumacher T.N., Scheper W., Kvistborg P. Cancer Neoantigens. Annu. Rev. Immunol. 2019;37:173–200. doi: 10.1146/annurev-immunol-042617-053402. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 6.Pennock N., White J.T., Cross E.W., Cheney E.E., Tamburini B.A., Kedl R.M. T cell responses: Naïve to memory and everything in between. Adv. Physiol. Educ. 2013;37:273–283. doi: 10.1152/advan.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinay D.S., Ryan E.P., Pawelec G., Talib W., Stagg J., Elkord E., Lichtor T., Decker W.K., Whelan R.L., Kumara H.S., et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015;35:S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Restifo N.P., Esquivel F., Kawakami Y., Yewdell J.W., Mulé J.J., Rosenberg A.S., Bennink J.R. Identification of human cancers deficient in antigen processing. J. Exp. Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnsen A.K., Templeton D.J., Sy M., Harding C. V Deficiency of transporter for antigen presentation (TAP) in tumor cells allows evasion of immune surveillance and increases tumorigenesis. J. Immunol. 1999;163:4224–4231. [PubMed] [Google Scholar]
- 10.Chang C.-H., Curtis J.D., Maggi L.B., Faubert B., Villarino A., O’Sullivan D., Huang S.C.-C., van der Windt G.J., Blagih J., Qiu J., et al. Posttranscriptional Control of T Cell Effector Function by Aerobic Glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearce E.L., Poffenberger M.C., Chang C.-H., Jones R.G. Fueling Immunity: Insights into Metabolism and Lymphocyte Function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye B., Stary C.M., Gao Q., Wang Q., Zeng Z., Jian Z., Gu L., Xiong X. Genetically Modified T-Cell-Based Adoptive Immunotherapy in Hematological Malignancies. J. Immunol. Res. 2017;2017:1–13. doi: 10.1155/2017/5210459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayor M., Yang N., Sterman D., Jones D.R., Adusumilli P.S. Immunotherapy for non-small cell lung cancer: Current concepts and clinical trials. Eur. J. Cardio-Thoracic Surg. 2015;49:1324–1333. doi: 10.1093/ejcts/ezv371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keenan T., Burke K.P., Van Allen E.M. Genomic correlates of response to immune checkpoint blockade. Nat. Med. 2019;25:389–402. doi: 10.1038/s41591-019-0382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walunas T.L., Lenschow D.J., Bakker C.Y., Linsley P.S., Freeman G.J., Green J.M., Thompson C.B., Bluestone J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-X. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzaki J., Gnjatic S., Mhawech-Fauceglia P., Beck A., Miller A., Tsuji T., Eppolito C., Qian F., Lele S., Shrikant P., et al. Tumor-infiltrating NY-ESO-1–specific CD8+T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegde P.S., Karanikas V., Evers S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin. Cancer Res. 2016;22:1865–1874. doi: 10.1158/1078-0432.CCR-15-1507. [DOI] [PubMed] [Google Scholar]
- 18.Baitsch L., Baumgaertner P., Devêvre E., Raghav S.K., Legat A., Barba L., Wieckowski S., Bouzourene H., Deplancke B., Romero P., et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Investig. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakuishi K., Apetoh L., Sullivan J.M., Blazar B.R., Kuchroo V.K., Anderson A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore antitumor immunity. J. Exp. Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards J., Wilmott J., Madore J., Gide T., Quek C., Tasker A., Ferguson A., Chen J., Hewavisenti R., Hersey P., et al. CD103+ Tumor-Resident CD8+ T Cells Are Associated with Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly During Anti–PD-1 Treatment. Clin. Cancer Res. 2018;24:3036–3045. doi: 10.1158/1078-0432.CCR-17-2257. [DOI] [PubMed] [Google Scholar]
- 21.Bösmüller H.-C., Wagner P., Peper J.K., Schuster H., Pham D.L., Greif K., Beschorner C., Rammensee H.-G., Stevanović S., Fend F., et al. Combined Immunoscore of CD103 and CD3 Identifies Long-Term Survivors in High-Grade Serous Ovarian Cancer. Int. J. Gynecol. Cancer. 2016;26:671–679. doi: 10.1097/IGC.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 22.Savas P., Kathleen Cuningham Foundation Consortium for research into Familial Breast cancer (kConFab) Virassamy B., Ye C., Salim A., Mintoff C.P., Caramia F., Salgado R., Byrne D.J., Teo Z.L., et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018;24:986–993. doi: 10.1038/s41591-018-0078-7. [DOI] [PubMed] [Google Scholar]
- 23.Workel H.H., Komdeur F.L., Wouters M.C., Plat A., Klip H.G., Eggink F., Wisman G.B.A., Arts H.J., Oonk M.H., Mourits M.J., et al. CD103 defines intraepithelial CD8+ PD1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma. Eur. J. Cancer. 2016;60:1–11. doi: 10.1016/j.ejca.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Okła K., Farber D.L., Zou W. Tissue-resident memory T cells in tumor immunity and immunotherapy. J. Exp. Med. 2021;218:e20201605. doi: 10.1084/jem.20201605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golubovskaya V., Wu L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers. 2016;8:36. doi: 10.3390/cancers8030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.June C.H., Riddell S.R., Schumacher T.N. Adoptive cellular therapy: A race to the finish line. Sci. Transl. Med. 2015;7:280. doi: 10.1126/scitranslmed.aaa3643. [DOI] [PubMed] [Google Scholar]
- 27.Linette G.P., Carreno B.M. Tumor-Infiltrating Lymphocytes in the Checkpoint Inhibitor Era. Curr. Hematol. Malign- Rep. 2019;14:286–291. doi: 10.1007/s11899-019-00523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin S., Xu L., Yi M., Yu S., Wu K., Luo S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer. 2019;18:1–14. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korman A.J., Peggs K.S., Allison J.P. Checkpoint Blockade in Cancer Immunotherapy. Adv. Immunol. 2006;90:297–339. doi: 10.1016/s0065-2776(06)90008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma P., Allison J.P. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies with Curative Potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei S.C., Duffy C.R., Allison J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg S., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg S.A., Yannelli J.R., Yang J.C., Topalian S.L., Schwartzentruber D.J., Weber J.S., Parkinson D.R., Seipp C.A., Einhorn J.H., White D.E. Treatment of Patients With Metastatic Melanoma With Autologous Tumor-Infiltrating Lymphocytes and Interleukin 2. J. Natl. Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 34.Dudley M.E., Wunderlich J.R., Robbins P.F., Yang J.C., Hwu P., Schwartzentruber D.J., Topalian S.L., Sherry R., Restifo N.P., Hubicki A.M., et al. Cancer Regression and Autoimmunity in Patients after Clonal Repopulation with Antitumor Lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg S.A., Yang J.C., Sherry R.M., Kammula U.S., Hughes M.S., Phan G.Q., Citrin D., Restifo N.P., Robbins P.F., Wunderlich J.R., et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T-Cell Transfer Immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudley M.E., Wunderlich J.R., Yang J.C., Sherry R.M., Topalian S.L., Restifo N.P., Royal R.E., Kammula U., White D.E., Mavroukakis S.A., et al. Adoptive Cell Transfer Therapy Following Non-Myeloablative but Lymphodepleting Chemotherapy for the Treatment of Patients with Refractory Metastatic Melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Besser M.J., Shapira-Frommer R., Treves A.J., Zippel D., Itzhaki O., Hershkovitz L., Levy D., Kubi A., Hovav E., Chermoshniuk N., et al. Clinical Responses in a Phase II Study Using Adoptive Transfer of Short-term Cultured Tumor Infiltration Lymphocytes in Metastatic Melanoma Patients. Clin. Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 38.Dudley M.E., Wunderlich J.R., Shelton T.E., Even J., Rosenberg S.A. Generation of Tumor-Infiltrating Lymphocyte Cultures for Use in Adoptive Transfer Therapy for Melanoma Patients. J. Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riddell S.R., Watanabe K., Goodrich J., Li C., Agha M., Greenberg P. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 40.Gattinoni L., Powell D.J., Rosenberg S.A., Restifo N.P. Adoptive immunotherapy for cancer: Building on success. Nat. Rev. Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blattman J.N., Grayson J.M., Wherry E.J., Kaech S.M., Smith K.A., Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 43.Besser M.J., Shapira-Frommer R., Itzhaki O., Treves A.J., Zippel D.B., Levy D., Kubi A., Shoshani N., Zikich D., Ohayon Y., et al. Adoptive Transfer of Tumor-Infiltrating Lymphocytes in Patients with Metastatic Melanoma: Intent-to-Treat Analysis and Efficacy after Failure to Prior Immunotherapies. Clin. Cancer Res. 2013;19:4792–4800. doi: 10.1158/1078-0432.CCR-13-0380. [DOI] [PubMed] [Google Scholar]
- 44.Radvanyi L.G., Bernatchez C., Zhang M., Fox P.S., Miller P., Chacon J., Wu R., Lizee G., Mahoney S., Alvarado G., et al. Specific Lymphocyte Subsets Predict Response to Adoptive Cell Therapy Using Expanded Autologous Tumor-Infiltrating Lymphocytes in Metastatic Melanoma Patients. Clin. Cancer Res. 2012;18:6758–6770. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pilon-Thomas S., Kuhn L., Ellwanger S., Janssen W., Royster E., Marzban S., Kudchadkar R., Zager J., Gibney G., Sondak V.K., et al. Efficacy of Adoptive Cell Transfer of Tumor-infiltrating Lymphocytes After Lymphopenia Induction for Metastatic Melanoma. J. Immunother. 2012;35:615–620. doi: 10.1097/CJI.0b013e31826e8f5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevanovic S., Draper L.M., Langhan M.M., Campbell T.E., Kwong M.L., Wunderlich J.R., Dudley M.E., Yang J.C., Sherry R.M., Kammula U.S., et al. Complete Regression of Metastatic Cervical Cancer After Treatment With Human Papillomavirus–Targeted Tumor-Infiltrating T Cells. J. Clin. Oncol. 2015;33:1543–1550. doi: 10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee H.J., Kim Y.-A., Sim C.K., Heo S.-H., Song I.H., Park H.S., Park S.Y., Bang W.S., Park I.A., Lee M., et al. Expansion of tumor-infiltrating lymphocytes and their potential for application as adoptive cell transfer therapy in human breast cancer. Oncotarget. 2017;8:113345–113359. doi: 10.18632/oncotarget.23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thommen D.S., Koelzer V., Herzig P., Roller A., Trefny M., Dimeloe S., Kiialainen A., Hanhart J., Schill C., Hess C., et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 2018;24:994–1004. doi: 10.1038/s41591-018-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Groot R., Van Loenen M.M., Guislain A., Nicolet B.P., Heeren J.J.F.-V., Verhagen O.J., Heuvel M.M.V.D., De Jong J., Burger P., Van Der Schoot C., et al. Polyfunctional tumor-reactive T cells are effectively expanded from non-small cell lung cancers, and correlate with an immune-engaged T cell profile. OncoImmunology. 2019;8:e1648170. doi: 10.1080/2162402X.2019.1648170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klebanoff C., Khong H.T., Antony P.A., Palmer D.C., Restifo N.P. Sinks, suppressors and antigen presenters: How lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antony P.A., Piccirillo C.A., Akpinarli A., Finkelstein S.E., Speiss P.J., Surman D.R., Palmer D., Chan C.-C., Klebanoff C., Overwijk W.W., et al. CD8+T Cell Immunity Against a Tumor/Self-Antigen Is Augmented by CD4+T Helper Cells and Hindered by Naturally Occurring T Regulatory Cells. J. Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simoni Y., Becht E., Fehlings M., Loh C.Y., Koo S.-L., Teng K.W.W., Yeong J., Nahar R., Zhang T., Kared H., et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 53.Li H., Van Der Leun A.M., Yofe I., Lubling Y., Gelbard-Solodkin D., van Akkooi A., Braber M.V.D., Rozeman E.A., Haanen J.B., Blank C.U., et al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell. 2018;176:775–789.e18. doi: 10.1016/j.cell.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McLane L.M., Hakeem M.A., Wherry E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 55.Fourcade J., Sun Z., Benallaoua M., Guillaume P., Luescher I.F., Sander C., Kirkwood J.M., Kuchroo V., Zarour H.M. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen–specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi F., Shi M., Zeng Z., Qi R.-Z., Liu Z.-W., Zhang J.-Y., Yang Y.-P., Tien P., Wang F.-S. PD-1 and PD-L1 upregulation promotes CD8+ T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int. J. Cancer. 2010;128:887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 57.Nakanishi J., Wada Y., Matsumoto K., Azuma M., Kikuchi K., Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 2006;56:1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nomi T., Sho M., Akahori T., Hamada K., Kubo A., Kanehiro H., Nakamura S., Enomoto K., Yagita H., Azuma M., et al. Clinical Significance and Therapeutic Potential of the Programmed Death-1 Ligand/Programmed Death-1 Pathway in Human Pancreatic Cancer. Clin. Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y., Huang S., Gong D., Qin Y., Shen Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell. Mol. Immunol. 2010;7:389–395. doi: 10.1038/cmi.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cieri N., Camisa B., Cocchiarella F., Forcato M., Oliveira G., Provasi E., Bondanza A., Bordignon C., Peccatori I., Ciceri F., et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121:573–584. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 61.Philip M., Schietinger A. Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr. Opin. Immunol. 2019;58:98–103. doi: 10.1016/j.coi.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Q., Huang F., Goncalves C., Del Rincón S.V., Miller Jr W.H. Translation of cancer immunotherapy from the bench to the bedside. Adv Cancer Res. 2019;143:1–62. doi: 10.1016/bs.acr.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Miller B.C., Sen D.R., Al Abosy R., Bi K., Virkud Y.V., LaFleur M.W., Yates K.B., Lako A., Felt K., Naik G.S., et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019;20:326–336. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallimore A., Glithero A., Godkin A., Tissot A.C., Plückthun A., Elliott T., Hengartner H., Zinkernagel R. Induction and Exhaustion of Lymphocytic Choriomeningitis Virus–specific Cytotoxic T Lymphocytes Visualized Using Soluble Tetrameric Major Histocompatibility Complex Class I–Peptide Complexes. J. Exp. Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zajac A.J., Blattman J.N., Murali-Krishna K., Sourdive D.J., Suresh M., Altman J.D., Ahmed R. Viral Immune Evasion Due to Persistence of Activated T Cells Without Effector Function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shankar P., Russo M., Harnisch B., Patterson M., Skolnik P., Lieberman J. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 2000;96:3094–3101. doi: 10.1182/blood.V96.9.3094.h8003094_3094_3101. [DOI] [PubMed] [Google Scholar]
- 67.Kostense S., Ogg G.S., Manting E.H., Gillespie G., Joling J., Vandenberghe K., Veenhof E.Z., Van Baarle D., Jurriaans S., Klein M.R., et al. High viral burden in the presence of major HIV-specific CD8+ T cell expansions: Evidence for impaired CTL effector function. Eur. J. Immunol. 2001;31:677–686. doi: 10.1002/1521-4141(200103)31:3<677::AID-IMMU677>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 68.Day C.L., Kaufmann D.E., Kiepiela P., Brown J.A., Moodley E.S., Reddy S., Mackey E.W., Miller J.D., Leslie A., DePierres C., et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 69.Gruener N.H., Lechner F., Jung M.-C., Diepolder H., Gerlach T., Lauer G., Walker B., Sullivan J., Phillips R., Pape G.R., et al. Sustained Dysfunction of Antiviral CD8 + T Lymphocytes after Infection with Hepatitis C Virus. J. Virol. 2001;75:5550–5558. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ye B., Liu X., Li X., Kong H., Tian L., Chen Y. T-cell exhaustion in chronic hepatitis B infection: Current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. doi: 10.1038/cddis.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kallies A., Zehn D., Utzschneider D. Precursor exhausted T cells: Key to successful immunotherapy? Nat. Rev. Immunol. 2019;20:128–136. doi: 10.1038/s41577-019-0223-7. [DOI] [PubMed] [Google Scholar]
- 72.Fuller M.J., Zajac A.J. Ablation of CD8 and CD4 T Cell Responses by High Viral Loads. J. Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 73.Wherry E.J., Blattman J.N., Murali-Krishna K., van der Most R., Ahmed R. Viral Persistence Alters CD8 T-Cell Immunodominance and Tissue Distribution and Results in Distinct Stages of Functional Impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Speiser D., Utzschneider D., Oberle S.G., Münz C., Romero P., Zehn D. T cell differentiation in chronic infection and cancer: Functional adaptation or exhaustion? Nat. Rev. Immunol. 2014;14:768–774. doi: 10.1038/nri3740. [DOI] [PubMed] [Google Scholar]
- 75.Blank C.U., Haining W.N., Held W., Hogan P.G., Kallies A., Lugli E., Lynn R.C., Philip M., Rao A., Restifo N.P., et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 2019;19:665–674. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wherry E.J., Ha S.-J., Kaech S.M., Haining W.N., Sarkar S., Kalia V., Subramaniam S., Blattman J.N., Barber D.L., Ahmed R. Molecular Signature of CD8+ T Cell Exhaustion during Chronic Viral Infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Thommen D.S., Schumacher T. T Cell Dysfunction in Cancer. Cancer Cell. 2018;33:547–562. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seidel J., Otsuka A., Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blackburn S.D., Shin H., Haining W.N., Zou T., Workman C.J., Polley A., Betts M.R., Freeman G.J., Vignali A.A.D., Wherry E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2008;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duraiswamy J., Ibegbu C.C., Masopust D., Miller J.D., Araki K., Doho G.H., Tata P., Gupta S., Zilliox M.J., Nakaya H., et al. Phenotype, Function, and Gene Expression Profiles of Programmed Death-1hi CD8 T Cells in Healthy Human Adults. J. Immunol. 2011;186:4200–4212. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharpe A.H., Pauken K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2017;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 82.Bengsch B., Johnson A.L., Kurachi M., Odorizzi P.M., Pauken K.E., Attanasio J., Stelekati E., McLane L.M., Paley M.A., Delgoffe G.M., et al. Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8 + T Cell Exhaustion. Immunity. 2016;45:358–373. doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hui E., Cheung J., Zhu J., Su X., Taylor M.J., Wallweber H.A., Sasmal D.K., Huang J., Kim J.M., Mellman I. T cell costimulatory receptor CD28 is a primary target for PD-1–mediated inhibition. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parry R.V., Chemnitz J.M., Frauwirth K.A., Lanfranco A.R., Braunstein I., Kobayashi S.V., Linsley P.S., Thompson C.B., Riley J.L. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol. Cell. Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Im S.J., Hashimoto M., Gerner M.Y., Lee J., Kissick H.T., Burger M.C., Shan Q., Hale J.S., Lee J., Nasti T.H., et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siddiqui I., Schaeuble K., Chennupati V., Marraco S.A.F., Calderon-Copete S., Ferreira D.P., Carmona S.J., Scarpellino L., Gfeller D., Pradervand S., et al. Intratumoral Tcf1+PD-1+CD8+ T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity. 2019;50:195–211.e10. doi: 10.1016/j.immuni.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 87.Sade-Feldman M., Yizhak K., Bjorgaard S.L., Ray J.P., de Boer C., Jenkins R.W., Lieb D.J., Chen J.H., Frederick D.T., Barzily-Rokni M., et al. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell. 2018;175:998–1013.e20. doi: 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boddupalli C.S., Bar N., Kadaveru K., Krauthammer M., Pornputtapong N., Mai Z., Ariyan S., Narayan D., Kluger H., Deng Y., et al. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight. 2016;1:e88955. doi: 10.1172/jci.insight.88955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Djenidi F., Adam J., Goubar A., Durgeau A., Meurice G., de Montpréville V., Validire P., Besse B., Mami-Chouaib F. CD8+CD103+ Tumor–Infiltrating Lymphocytes Are Tumor-Specific Tissue-Resident Memory T Cells and a Prognostic Factor for Survival in Lung Cancer Patients. J. Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 90.Ganesan A.-P., Clarke J., Wood O., Garrido-Martin E.M., Chee S.J., Mellows T., Samaniego-Castruita D., Singh D., Seumois G., Alzetani A., et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol. 2017;18:940–950. doi: 10.1038/ni.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nizard M., Roussel H., Diniz M.O., Karaki S., Tran T., Voron T., Dransart E., Sandoval F., Riquet M., Rance B., et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat. Commun. 2017;8:15221. doi: 10.1038/ncomms15221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oja A.E., Piet B., Van Der Zwan D., Blaauwgeers H., Mensink M., de Kivit S., Borst J., Nolte M.A., Van Lier R.A.W., Stark R., et al. Functional Heterogeneity of CD4+ Tumor-Infiltrating Lymphocytes With a Resident Memory Phenotype in NSCLC. Front. Immunol. 2018;9:2654. doi: 10.3389/fimmu.2018.02654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang B., Wu S., Zeng H., Liu Z., Dong W., He W., Chen X., Dong X., Zheng L., Lin T., et al. CD103 + Tumor Infiltrating Lymphocytes Predict a Favorable Prognosis in Urothelial Cell Carcinoma of the Bladder. J. Urol. 2015;194:556–562. doi: 10.1016/j.juro.2015.02.2941. [DOI] [PubMed] [Google Scholar]
- 94.Hartana C.A., Bergman E.A., Broomé A., Berglund S., Johansson M., Alamdari F., Jakubczyk T., Huge Y., Aljabery F., Palmqvist K., et al. Tissue-resident memory T cells are epigenetically cytotoxic with signs of exhaustion in human urinary bladder cancer. Clin. Exp. Immunol. 2018;194:39–53. doi: 10.1111/cei.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Webb J.R., Milne K., Watson P., DeLeeuw R.J., Nelson B. Tumor-Infiltrating Lymphocytes Expressing the Tissue Resident Memory Marker CD103 Are Associated with Increased Survival in High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2013;20:434–444. doi: 10.1158/1078-0432.CCR-13-1877. [DOI] [PubMed] [Google Scholar]
- 96.Webb J.R., Wick D.A., Nielsen J.S., Tran E., Milne K., McMurtrie E., Nelson B. Profound elevation of CD8+ T cells expressing the intraepithelial lymphocyte marker CD103 (αE/β7 Integrin) in high-grade serous ovarian cancer. Gynecol. Oncol. 2010;118:228–236. doi: 10.1016/j.ygyno.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 97.Komdeur F.L., Prins T.M., Van De Wall S., Plat A., Wisman G.B.A., Hollema H., Daemen T., Church D., De Bruyn M., Nijman H.W. CD103+ tumor-infiltrating lymphocytes are tumor-reactive intraepithelial CD8+ T cells associated with prognostic benefit and therapy response in cervical cancer. OncoImmunology. 2017;6:e1338230. doi: 10.1080/2162402X.2017.1338230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Z.-Q., Milne K., DeRocher H., Webb J.R., Nelson B., Watson P.H. CD103 and Intratumoral Immune Response in Breast Cancer. Clin. Cancer Res. 2016;22:6290–6297. doi: 10.1158/1078-0432.CCR-16-0732. [DOI] [PubMed] [Google Scholar]
- 99.Quinn E., Hawkins N., Yip Y.L., Suter C., Ward R. CD103+ intraepithelial lymphocytes--a unique population in microsatellite unstable sporadic colorectal cancer. Eur. J. Cancer. 2003;39:469–475. doi: 10.1016/S0959-8049(02)00633-0. [DOI] [PubMed] [Google Scholar]
- 100.Behr F.M., Chuwonpad A., Stark R., Van Gisbergen K.P.J.M. Armed and Ready: Transcriptional Regulation of Tissue-Resident Memory CD8 T Cells. Front. Immunol. 2018;9:1770. doi: 10.3389/fimmu.2018.01770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jameson S.C., Masopust D. Understanding Subset Diversity in T Cell Memory. Immunity. 2018;48:214–226. doi: 10.1016/j.immuni.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Behr F.M., Parga-Vidal L., Kragten N.A.M., Van Dam T.J.P., Wesselink T.H., Sheridan B.S., Arens R., Van Lier R.A.W., Stark R., Van Gisbergen K.P.J.M. Tissue-resident memory CD8+ T cells shape local and systemic secondary T cell responses. Nat. Immunol. 2020;21:1070–1081. doi: 10.1038/s41590-020-0723-4. [DOI] [PubMed] [Google Scholar]
- 103.Beura L.K., Wijeyesinghe S., Thompson E.A., Macchietto M.G., Rosato P.C., Pierson M.J., Schenkel J., Mitchell J.S., Vezys V., Fife B., et al. T Cells in Nonlymphoid Tissues Give Rise to Lymph-Node-Resident Memory T Cells. Immunity. 2018;48:327–338.e5. doi: 10.1016/j.immuni.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Milner J.J., Toma C., Yu B., Zhang K., Omilusik K., Phan A.T., Wang D., Getzler A., Nguyen T., Crotty S., et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature. 2017;552:253–257. doi: 10.1038/nature24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mackay L.K., Minnich M., Kragten N.A.M., Liao Y., Nota B., Seillet C., Zaid A., Man K., Preston S., Freestone D., et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352:459–463. doi: 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- 106.Mackay L.K., Braun A., Macleod B.L., Collins N., Tebartz C., Bedoui S., Carbone F.R., Gebhardt T. Cutting Edge: CD69 Interference with Sphingosine-1-Phosphate Receptor Function Regulates Peripheral T Cell Retention. J. Immunol. 2015;194:2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 107.Skon C.N., Lee J.-Y., Anderson K.G., Masopust D., Hogquist K., Jameson S. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cepek K.L., Shaw S.K., Parker C.M., Russell G.J., Morrow J.S., Rimm D.L., Brenner M.B. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 109.Mueller S., Mackay L. Tissue-resident memory T cells: Local specialists in immune defence. Nat. Rev. Immunol. 2015;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 110.Cheuk S.H., Schlums H., Sérézal I.G., Martini E., Chiang S., Marquardt N., Gibbs A., Detlofsson E., Introini A., Forkel M., et al. CD49a Expression Defines Tissue-Resident CD8 + T Cells Poised for Cytotoxic Function in Human Skin. Immunity. 2017;46:287–300. doi: 10.1016/j.immuni.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hombrink P., Helbig C., Backer R.A., Piet B., Oja A.E., Stark R., Brasser G., Jongejan A., Jonkers R.E., Nota B., et al. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat. Immunol. 2016;17:1467–1478. doi: 10.1038/ni.3589. [DOI] [PubMed] [Google Scholar]
- 112.Beltra J.-C., Manne S., Abdel-Hakeem M.S., Kurachi M., Giles J.R., Chen Z., Casella V., Ngiow S.F., Khan O., Huang Y.J., et al. Developmental Relationships of Four Exhausted CD8+ T Cell Subsets Reveals Underlying Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity. 2020;52:825–841.e8. doi: 10.1016/j.immuni.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Clarke J., Panwar B., Madrigal A., Singh D., Gujar R., Wood O., Chee S.J., Eschweiler S., King E.V., Awad A.S., et al. Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J. Exp. Med. 2019;216:2128–2149. doi: 10.1084/jem.20190249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Le Floc’H A., Jalil A., Vergnon I., Chansac B.L.M., Lazar V., Bismuth G., Chouaib S., Mami-Chouaib F. αEβ7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J. Exp. Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sheridan B.S., Pham Q.-M., Lee Y.-T., Cauley L.S., Puddington L., Lefrançois L. Oral Infection Drives a Distinct Population of Intestinal Resident Memory CD8+ T Cells with Enhanced Protective Function. Immunity. 2014;40:747–757. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mackay L., Rahimpour A., Ma J., Collins N.C., Stock A.T., Hafon M.-L., Vega-Ramos J., Lauzurica P., Mueller S., Stefanovic T., et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 117.Wakim L.M., Woodward-Davis A., Bevan M.J. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reilly E.C., Emo K.L., Buckley P.M., Reilly N.S., Smith I., Chaves F.A., Yang H., Oakes P.W., Topham D.J. TRMintegrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection. Proc. Natl. Acad. Sci. USA. 2020;117:12306–12314. doi: 10.1073/pnas.1915681117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Casey K.A., Fraser K.A., Schenkel J., Moran A., Abt M.C., Beura L.K., Lucas P.J., Artis D., Wherry E.J., Hogquist K., et al. Antigen-Independent Differentiation and Maintenance of Effector-like Resident Memory T Cells in Tissues. J. Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Steinert E.M., Schenkel J., Fraser K.A., Beura L.K., Manlove L.S., Igyártó B.Z., Southern P.J., Masopust D. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ariotti S., Hogenbirk M.A., Dijkgraaf F.E., Visser L.L., Hoekstra M.E., Song J.-Y., Jacobs H., Haanen J.B., Schumacher T.N. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 122.Schenkel J.M., Fraser K.A., Casey K.A., Beura L.K., Pauken K.E., Vezys V., Masopust D. IL-15–Independent Maintenance of Tissue-Resident and Boosted Effector Memory CD8 T Cells. J. Immunol. 2016;196:3920–3926. doi: 10.4049/jimmunol.1502337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Adachi T., Kobayashi T., Sugihara E., Yamada T., Ikuta K., Pittaluga S., Saya H., Amagai M., Nagao K. Hair follicle–derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med. 2015;21:1272–1279. doi: 10.1038/nm.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fonseca R., Beura L.K., Quarnstrom C.F., Ghoneim H.E., Fan Y., Zebley C.C., Scott M.C., Fares-Frederickson N.J., Wijeyesinghe S., Thompson E.A., et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat. Immunol. 2020;21:412–421. doi: 10.1038/s41590-020-0607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin R., Zhang H., Yuan Y., He Q., Zhou J., Li S., Sun Y., Li D.Y., Qiu H.-B., Wang W., et al. Fatty acid oxidation controls CD8+ tissue-resident memory T cell survival in gastric adenocarcinoma. Cancer Immunol. Res. 2020;8:479–492. doi: 10.1158/2326-6066.CIR-19-0702. [DOI] [PubMed] [Google Scholar]
- 126.Egelston C.A., Avalos C., Tu T.Y., Rosario A., Wang R., Solomon S., Srinivasan G., Nelson M.S., Huang Y., Lim M.H., et al. Resident memory CD8+ T cells within cancer islands mediate survival in breast cancer patients. JCI Insight. 2019;4:e130000. doi: 10.1172/jci.insight.130000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Webb J.R., Milne K., Nelson B. PD-1 and CD103 Are Widely Coexpressed on Prognostically Favorable Intraepithelial CD8 T Cells in Human Ovarian Cancer. Cancer Immunol. Res. 2015;3:926–935. doi: 10.1158/2326-6066.CIR-14-0239. [DOI] [PubMed] [Google Scholar]
- 128.Huang A., Huang P., Luo Y., Wang B., Luo X., Zheng Z., Yuan K., Huang Z., Peng S., Yu H., et al. CD103 expression in normal epithelium is associated with poor prognosis of colorectal cancer patients within defined subgroups. Int. J. Clin. Exp. Pathol. 2017;10:6624–6634. [Google Scholar]
- 129.Amsen D., van Gisbergen K., Hombrink P., Van Lier R.A.W. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat. Immunol. 2018;19:538–546. doi: 10.1038/s41590-018-0114-2. [DOI] [PubMed] [Google Scholar]
- 130.Lohneis P., Sinn M., Bischoff S., Jühling A., Pelzer U., Wislocka L., Bahra M., Sinn B.V., Denkert C., Oettle H., et al. Cytotoxic tumour-infiltrating T lymphocytes influence outcome in resected pancreatic ductal adenocarcinoma. Eur. J. Cancer. 2017;83:290–301. doi: 10.1016/j.ejca.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 131.Duhen T., Duhen R., Montler R., Moses J., Moudgil T., de Miranda N., Goodall C.P., Blair T.C., Fox B.A., McDermott J.E., et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 2018;9:1–13. doi: 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Workel H.H., Van Rooij N., Plat A., Spierings D.C., Fehrmann R.S.N., Nijman H.W., De Bruyn M. Transcriptional Activity and Stability of CD39+CD103+CD8+ T Cells in Human High-Grade Endometrial Cancer. Int. J. Mol. Sci. 2020;21:3770. doi: 10.3390/ijms21113770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Komdeur F.L., Wouters M., Workel H.H., Tijans A.M., Terwindt A.L., Brunekreeft K.L., Plat A., Klip H.G., Eggink F., Leffers N., et al. CD103+ intraepithelial T cells in high-grade serous ovarian cancer are phenotypically diverse TCRαβ+ CD8αβ+ T cells that can be targeted for cancer immunotherapy. Oncotarget. 2016;7:75130–75144. doi: 10.18632/oncotarget.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gamradt P., Laoubi L., Nosbaum A., Mutez V., Lenief V., Grande S., Redoulès D., Schmitt A.-M., Nicolas J.F., Vocanson M. Inhibitory checkpoint receptors control CD8+ resident memory T cells to prevent skin allergy. J. Allergy Clin. Immunol. 2019;143:2147–2157.e9. doi: 10.1016/j.jaci.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 135.Shwetank A., Abdelsamed H., Frost E.L., Schmitz H.M., Mockus T.E., Youngblood B.A., Lukacher A.E. Maintenance of PD-1 on brain-resident memory CD8 T cells is antigen independent. Immunol. Cell Biol. 2017;95:953–959. doi: 10.1038/icb.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Enamorado M., Iborra S., Priego E., Cueto F.J., Quintana J.A., Martínez-Cano S., Mejías-Pérez E., Esteban M., Melero I., Hidalgo A., et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat. Commun. 2017;8:16073. doi: 10.1038/ncomms16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rosato P.C., Wijeyesinghe S., Stolley J.M., Nelson C.E., Davis R.L., Manlove L.S., Pennell C.A., Blazar B.R., Chen C.C., Geller M.A., et al. Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy. Nat. Commun. 2019;10:1–9. doi: 10.1038/s41467-019-08534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Farber D., Yudanin N., Restifo N.P. Human memory T cells: Generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2013;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ye Q., Song D.-G., Poussin M., Yamamoto T., Best A., Li C., Coukos G., Powell D.J. CD137 Accurately Identifies and Enriches for Naturally Occurring Tumor-Reactive T Cells in Tumor. Clin. Cancer Res. 2013;20:44–55. doi: 10.1158/1078-0432.CCR-13-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scheper W., Kelderman S., Fanchi L.F., Linnemann C., Bendle G., De Rooij M.A.J., Hirt C., Mezzadra R., Slagter M., Dijkstra K., et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med. 2018;25:89–94. doi: 10.1038/s41591-018-0266-5. [DOI] [PubMed] [Google Scholar]
- 141.Curiel T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., Evdemon-Hogan M., Conejo-Garcia J., Zhang L., Burow M., et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 142.Hinrichs C.S., Borman Z.A., Cassard L., Gattinoni L., Spolski R., Yu Z., Sanchez-Perez L., Muranski P., Kern S.J., Logun C., et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl. Acad. Sci. USA. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Klebanoff C.A., Gattinoni L., Torabi-Parizi P., Kerstann K., Cardones A.R., Finkelstein S.E., Palmer D.C., Antony P.A., Hwang S.T., Rosenberg S.A., et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wherry E.J., Teichgräber V., Becker T.C., Masopust D., Kaech S.M., Antia R., von Andrian U.H., Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 145.Bouneaud C., Garcia Z., Kourilsky P., Pannetier C. Lineage relationships, homeostasis, and recall capacities of central– and effector–memory CD8 T cells in vivo. J. Exp. Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ahlers J.D., Belyakov I.M. Memories that last forever: Strategies for optimizing vaccine T-cell memory. Blood. 2010;115:1678–1689. doi: 10.1182/blood-2009-06-227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kaech S.M., Tan J.T., Wherry E.J., Konieczny B.T., Surh C.D., Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 148.Huster K.M., Koffler M., Stemberger C., Schiemann M., Wagner H., Busch D.H. Unidirectional development of CD8+ central memory T cells into protectiveListeria-specific effector memory T cells. Eur. J. Immunol. 2006;36:1453–1464. doi: 10.1002/eji.200635874. [DOI] [PubMed] [Google Scholar]
- 149.Behr F.M., Beumer-Chuwonpad A., Kragten N.A.M., Wesselink T.H., Stark R., Van Gisbergen K.P. Circulating memory CD8 + T cells are limited in forming CD103 + tissue-resident memory T cells at mucosal sites after reinfection. Eur. J. Immunol. 2020;51:151–166. doi: 10.1002/eji.202048737. [DOI] [PubMed] [Google Scholar]
- 150.Graef P., Buchholz V.R., Stemberger C., Flossdorf M., Henkel L., Schiemann M., Drexler I., Höfer T., Riddell S.R., Busch D.H. Serial Transfer of Single-Cell-Derived Immunocompetence Reveals Stemness of CD8+ Central Memory T Cells. Immunity. 2014;41:116–126. doi: 10.1016/j.immuni.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 151.Yunger S., Bar El A., Zeltzer L.-A., Fridman E., Raviv G., Laufer M., Schachter J., Markel G., Itzhaki O., Besser M.J. Tumor-infiltrating lymphocytes from human prostate tumors reveal antitumor reactivity and potential for adoptive cell therapy. OncoImmunology. 2019;8:e1672494. doi: 10.1080/2162402X.2019.1672494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Beura L.K., Mitchell J.S., Thompson E.A., Schenkel J., Mohammed J., Wijeyesinghe S., Fonseca R., Burbach B.J., Hickman H., Vezys V., et al. Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol. 2018;19:173–182. doi: 10.1038/s41590-017-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wakim L.M., Waithman J., van Rooijen N., Heath W.R., Carbone F.R. Dendritic Cell-Induced Memory T Cell Activation in Nonlymphoid Tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 154.Park S., Zaid A., Hor J.L., Christo S.N., Prier J., Davies B., Alexandre Y.O., Gregory J.L., Russell T., Gebhardt T., et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 2018;19:183–191. doi: 10.1038/s41590-017-0027-5. [DOI] [PubMed] [Google Scholar]
- 155.Kumar B.V., Kratchmarov R., Miron M., Carpenter D.J., Senda T., Lerner H., Friedman A., Reiner S.L., Farber D. Functional heterogeneity of human tissue-resident memory T cells based on dye efflux capacities. JCI Insight. 2018;3:e123568. doi: 10.1172/jci.insight.123568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pan Y., Tian T., Park C.O., Lofftus S.Y., Mei S., Liu X., Luo C., O’Malley J.T., Gehad A., Teague J.E., et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 2017;543:252–256. doi: 10.1038/nature21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Osborn J.F., Hobbs S.J., Mooster J.L., Khan T.N., Kilgore A.M., Harbour J., Nolz J.C. Central memory CD8+ T cells become CD69+ tissue-residents during viral skin infection independent of CD62L-mediated lymph node surveillance. PLOS Pathog. 2019;15:e1007633. doi: 10.1371/journal.ppat.1007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lau C.M., Adams N.M., Geary C.D., Weizman O.-E., Rapp M., Pritykin Y., Leslie C.S., Sun J.C. Epigenetic control of innate and adaptive immune memory. Nat. Immunol. 2018;19:963–972. doi: 10.1038/s41590-018-0176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yu B., Zhang K., Milner J.J., Toma C., Chen R., Scott-Browne J.P., Pereira R., Crotty R.C.S., Chang J., Pipkin M., et al. Epigenetic landscapes reveal transcription factors that regulate CD8+ T cell differentiation. Nat. Immunol. 2017;18:573–582. doi: 10.1038/ni.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ito Y., Miyazono K. RUNX transcription factors as key targets of TGF-β superfamily signaling. Curr. Opin. Genet. Dev. 2003;13:43–47. doi: 10.1016/S0959-437X(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 161.Hata A., Chen Y.-G. TGF-β Signaling from Receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016;8:a022061. doi: 10.1101/cshperspect.a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Masopust D., Vezys V., Usherwood E.J., Cauley L.S., Olson S., Marzo A.L., Ward R.L., Woodland D.L., Lefrançois L. Activated Primary and Memory CD8 T Cells Migrate to Nonlymphoid Tissues Regardless of Site of Activation or Tissue of Origin. J. Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 163.Hofmann M., Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc. Natl. Acad. Sci. USA. 2011;108:16741–16746. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mazzolini G., Alfaro C., Sangro B., Feijoó E., Ruiz J., Benito A., Tirapu I., Arina A., Sola J., Herraiz M., et al. Intratumoral Injection of Dendritic Cells Engineered to Secrete Interleukin-12 by Recombinant Adenovirus in Patients with Metastatic Gastrointestinal Carcinomas. J. Clin. Oncol. 2005;23:999–1010. doi: 10.1200/JCO.2005.00.463. [DOI] [PubMed] [Google Scholar]
- 165.Yao W., Li Y., Zeng L., Zhang X., Zhou Z., Zheng M., Wan H. Intratumoral injection of dendritic cells overexpressing interleukin-12 inhibits melanoma growth. Oncol. Rep. 2019;42:370–376. doi: 10.3892/or.2019.7165. [DOI] [PubMed] [Google Scholar]
- 166.Kobayashi M., Sakabe T., Chiba A., Nakajima A., Okamoto M., Shimodaira S., Yonemitsu Y., Shibamoto Y., Suzuki N., Nagaya M., et al. Therapeutic effect of intratumoral injections of dendritic cells for locally recurrent gastric cancer: A case report. World J. Surg. Oncol. 2014;12:390. doi: 10.1186/1477-7819-12-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mackay L., Wakim L., Van Vliet C.J., Jones C., Mueller S., Bannard O., Fearon D.T., Heath W.R., Carbone F.R. Maintenance of T Cell Function in the Face of Chronic Antigen Stimulation and Repeated Reactivation for a Latent Virus Infection. J. Immunol. 2012;188:2173–2178. doi: 10.4049/jimmunol.1102719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.