Abstract
Both the detrimental effect of prenatal exposure to di-(2-ethylhexyl)-phthalate (DEHP) and the beneficial effects of physical exercise on brain functions have been reported. The oxytocin pathway has been implicated in the onset of maternal behaviors. Epigenetic modification of the oxytocin receptor gene (OXTR) through DNA methylation has been associated with the pathogenesis of neuropsychiatric disorders. The purpose of this study was to investigate the effects of prenatal DEHP exposure on oxytocin-regulated maternal behaviors and to examine the protective effect of exercise. Pregnant rats (F0) were fed with vehicle or DEHP during gestation and the offspring females (F1) were assessed for their maternal behaviors by pup retrieval test at postpartum. The results showed that reduced pup retrieval activities without significant alteration of stress responses were observed in the prenatally DEHP-exposed females. Prenatal DEHP exposure decreased the expressions of oxytocin, Oxtr mRNA, and oxytocin receptor, and increased Oxtr methylation in the hypothalamus of postpartum female rats. There were no significant effects of exercise on behavioral, biochemical, and epigenetic measurements. These results suggest that prenatal DEHP exposure has a long-term adverse effect on maternal behaviors; Oxtr hyper-methylation may be a potential epigenetic mechanism for this alteration, which cannot be prevented by physical exercise during childhood.
Keywords: maternal behaviors, oxytocin, DNA methylation, di-(2-ethylhexyl)-phthalate, exercise
1. Introduction
The quality of maternal care has a significant influence on the offspring’s physiological and psychological development across many mammalian species [1]. The initiation of maternal behaviors relies on the functions of neurotransmitters and neuromodulators acting during postpartum. The neuropeptide oxytocin, produced in the hypothalamus, is implicated as a system supporting neurobehavioral adaptation during pregnancy, childbirth, and postpartum caregiving in several species [2]. In humans, oxytocin levels are related to a set of maternal bonding behaviors, including gaze, vocalization, positive affect, affectionate touch, and frequent checking of the infant [3,4]. In rodents, oxytocin administration induces maternal behaviors such as more efficient pup retrieval and pup grouping in the nest [5,6], whereas oxytocin antagonist treatment impairs maternal behaviors in postpartum rats [7]. There is a concurrent expression of the oxytocin receptor (OTR) in the hypothalamus [8]. Rat dams with higher levels of OTR expression display enhanced maternal behaviors [9,10], while blockade of OTR by antagonist and gene knockout reduces the frequency of pup retrieval and licking/grooming [11,12,13]. Collectively, these studies provide strong evidence that the central oxytocin pathway plays an important role in activating and coordinating maternal behaviors.
The expression of OTR is determined by not only heritable genetic variation but also by epigenetic modification of the oxytocin receptor gene (human: OXTR; rodent: Oxtr) [14,15]. One of the epigenetic biomarkers is DNA methylation, which refers to the covalent binding of a methyl group to a cytosine nucleotide in the DNA sequence. In mammals, DNA methylation mainly modifies the cytosine and guanine dinucleotides (CpG sites) and generally alters the transcriptional activity [16]. Hyper-methylation within the promoter of OXTR appears to be responsible for the majority of epigenetic silencing of OXTR transcription [17]. An animal study shows that Oxtr methylation level is negatively correlated with OTR expression in the hypothalamus [18]. In humans, hyper-methylation of OXTR is associated with decreased OTR expression in the temporal cortex of autism patients, implicating the epigenetic regulation of OXTR in the pathogenesis of neuropsychiatric disorders [19]. Similar correlations between OXTR methylation and behavior are also reported in a broad range of socioemotional dysfunctions in humans, such as schizoaffective disorders, attachment anxiety, and depression [20,21,22]. Therefore, the methylation level of OXTR has been suggested as a prediction of phenotypic variability of the oxytocin pathway as well as of general impairments of social behaviors [23].
In addition to oxytocin, brain-derived neurotrophic factor (BDNF) has been implicated in the regulation of maternal behaviors. BDNF is a member of the neurotrophin family and plays a significant role in neuronal survival, synaptic plasticity, learning, and memory [24]. Hypothalamic BDNF deficiency may decrease the expression of oxytocin mRNA and reduce the pup retrieval, suggesting that coordination of BDNF and oxytocin in the hypothalamic circuit is involved in the regulation of maternal behaviors [25]. Rat dams with a higher level of BDNF in the brain exhibit an increased frequency of maternal licking/grooming behavior, which is mediated by the mechanism of oxytocin-induced BDNF expression [26]. These studies imply the synergistic interaction of oxytocin and BDNF in the establishment and maintenance of maternal behaviors.
The attempt to modulate oxytocin and BDNF to improve social behavior has been achieved by physiological or pharmacological intervention. Physical exercise has been shown to significantly stimulate the release of oxytocin and BDNF in humans [27,28]. In rodents, physical exercise induces the secretions of oxytocin and BDNF in the brain, implying a mechanism that involves the modulation of behavioral outcomes induced by exercise [29,30]. Physical exercise has been identified as an effective method to improve the symptoms of anxiety, depression, and cognitive deficits in humans and animals [31,32]. Epigenetic studies suggest that physical exercise can reduce the methylation levels within the promotors of some exercise-induced genes in human skeletal muscle and rodent brain [33,34,35]. Overall, these results suggest that physical exercise can differentially modulate the expression of gene transcripts through epigenetic mechanisms.
Early-life exposure to endocrine-disrupting chemicals may exert latent and profound consequences for the organization and regulation of the hypothalamic neuroendocrine systems [36]. Plasticizer di-(2-ethylhexyl) phthalate (DEHP), used to soften polyvinyl chloride plastics in many commercial items, has been identified as an endocrine-disrupting chemical [37]. The routes of DEHP exposure are through inhalation, ingestion, and dermal contact in children and adults, as well as through placental and nursing transfers in developing fetuses and newborns [38,39]. The fetal developing hypothalamus is vulnerable to DEHP exposure. For example, perinatal DEHP exposure may alter hypothalamic–pituitary regulation, resulting in reproductive dysfunction and precocious puberty in young rats [40,41]. The relationship between early-life DEHP exposure and socioemotional disorders has been established in humans and rodents. Perinatal DEHP exposure may increase anxiety-like behaviors in adult rats by dysregulating the feedback mechanism in the hypothalamic–pituitary–adrenal axis, such as increased adrenocorticotropic hormone (ACTH) levels and decreased corticosterone levels under stressed conditions [42,43]. Perinatal exposure to phthalates exerts an impact on hypothalamic gene expressions and social interactions in rodents [44,45]. In humans, higher serum DEHP levels are found in the autism spectrum disorders, hyperactivity, and inattention groups compared to healthy children, suggesting that endocrine disruptors may have a role in the pathogenesis of autism and attention-deficit hyperactivity disorder (ADHD) [46,47]. Importantly, altered OXTR methylation levels have been found in autism and ADHD patients [19,48]. This suggests that OXTR methylation patterns are altered across neurodevelopmental disorders and may be correlated with common clinical outcomes.
In our previous study, both the detrimental effect of early-life DEHP exposure and the beneficial effect of physical exercise on anxiety-like behaviors were reported [43]. Because maternal exposure to early-life stress during development can impair maternal care later in life [49], it is very likely that environmental factors, such as DEHP and exercise, may exert opposing effects on maternal behaviors by their counteraction of anxiety-like behaviors. Therefore, prenatal DEHP exposure with subsequent childhood exercise presents health concerns and the effect of combining these factors on maternal behaviors should be determined. Considering the critical role of oxytocin in the regulation of maternal behaviors, the current study aimed to investigate the effects of prenatal DEHP exposure and/or childhood exercise on hypothalamic oxytocin functions. To examine this hypothesis, prenatally DEHP-exposed female rats were trained to exercise during childhood–adolescence followed by a pup retrieval test at postpartum. Finally, the functions of hypothalamic oxytocin were evaluated to reveal the possible mechanisms underlying the effects of DEHP exposure and/or exercise on maternal behaviors.
2. Results
2.1. Effects of Prenatal DEHP Exposure and Childhood Exercise on Anxiety-like Behaviors in Postpartum Dams
The elevated plus-maze was performed on postpartum day 8 to examine anxiety-like behaviors (Table 1). There were no effects of DEHP and exercise on the number of open arms entries, the number of close arms entries, and the proportion of open arms entries. Meanwhile, there were no effects of DEHP and exercise on the exploration time in the open arms and close arms, and the percent of the time in open arms exploration. These results suggest that stress responses were not affected by either prenatal DEHP exposure or childhood exercise in postpartum dams.
Table 1.
Effects of prenatal DEHP exposure and childhood exercise on anxiety-like behaviors.
| Observed Categories | Groups (Mean ± SEM) | Effect (F Value) | ||||
|---|---|---|---|---|---|---|
| C | Cex | D | Dex | DEHP | Exercise | |
| Number of open arms entries (counts) | 9.42 ± 1.47 | 9.92 ± 1.82 | 8.17 ± 1.81 | 8.83 ± 1.87 | 0.444 | 0.111 |
| Number of close arms entries (counts) | 15.33 ± 2.06 | 15.35 ± 1.91 | 16.17 ± 2.39 | 17.25 ± 2.34 | 0.145 | 0.002 |
| Number of open arms entries (%) | 38.47 ± 3.68 | 37.83 ± 2.34 | 31.77 ± 1.88 | 32.06 ± 2.39 | 2.336 | 0.077 |
| Time of open arms entries (s) | 67.00 ± 8.03 | 66.33 ± 9.29 | 63.25 ± 9.66 | 64.92 ± 9.22 | 0.081 | 0.003 |
| Time of close arms entries (s) | 184.50 ± 7.84 | 190.25 ± 6.38 | 193.75 ± 8.05 | 194.50 ± 8.53 | 0.760 | 0.176 |
| Time of open arms entries (%) | 22.33 ± 2.68 | 22.11 ± 3.10 | 21.08 ± 3.22 | 21.64 ± 3.07 | 0.081 | 0.003 |
C: vehicle control; Cex: exercised vehicle; D: DEHP exposure; Dx: exercised DEHP.
2.2. Effects of Prenatal DEHP Exposure and Childhood Exercise on Levels of BDNF, ACTH, and Corticosterone in Postpartum Dams
BDNF and stress-related hormones in the plasma and hypothalamus were analyzed by ELISA (Table 2). The results showed that there were no effects of DEHP and exercise on the levels of BDNF in plasma and the hypothalamus. Regarding the stress-related hormones, the results showed an effect of DEHP on the enhancement of plasma ACTH levels [F(1, 44) = 5.011, p < 0.05, η2 = 0.102], while no effect of exercise was found. Additionally, there were no effects of DEHP and exercise on plasma corticosterone levels in postpartum dams. These results suggest that secretions of BDNF and corticosterone were not affected by prenatal DEHP exposure and childhood exercise in postpartum dams.
Table 2.
Effects of prenatal DEHP exposure and childhood exercise on expressions of BDNF and stress-related hormones.
| Measured Concentrations | Groups (Mean ± SEM) | Effect (F Value) | ||||
|---|---|---|---|---|---|---|
| C | Cex | D | Dex | DEHP | Exercise | |
| Plasma BDNF (pg/mL) | 348.72 ± 31.10 | 375.33 ± 27.92 | 308.97 ± 24.52 | 330.01 ± 24.02 | 2.475 | 0.777 |
| Hypothalamic BDNF (pg/100 μg protein) | 128.26 ± 12.46 | 131.88 ± 9.27 | 121.60 ± 9.17 | 117.63 ± 7.65 | 1.138 | 0.000 |
| Plasma ACTH (pg/mL) | 115.65 ± 5.11 | 112.67 ± 4.92 | 128.96 ± 4.96 | 121.50 ± 4.79 | 5.011 * | 1.115 |
| Plasma corticosterone (ng/mL) | 239.43 ± 22.42 | 241.72 ± 18.74 | 303.83 ± 27.73 | 263.02 ± 28.59 | 3.009 | 0.608 |
C: vehicle control; Cex: exercised vehicle; D: DEHP exposure; Dx: exercised DEHP. *: p < 0.05.
2.3. Effects of Prenatal DEHP Exposure and Childhood Exercise on Maternal Behaviors in Postpartum Dams
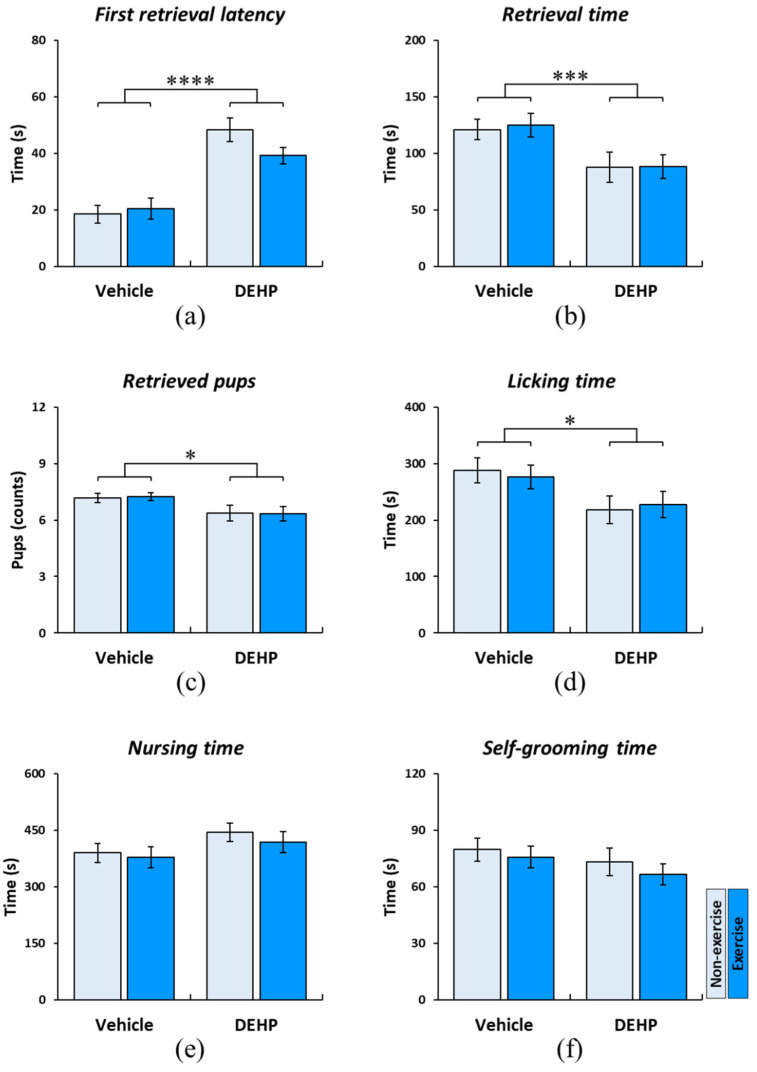
There was a significant effect of DEHP on the first retrieval latency (F(1, 44) = 48.607, p < 0.001, η2 = 0.525), while no effect of exercise was found (Figure 1a). This result revealed that DEHP-exposed dams began to retrieve their pups later than non-exposed females and exercise failed to ameliorate this impairment. A significant effect of DEHP was found on reducing the retrieval time in DEHP-exposed dams (F(1, 44) = 10.255, p < 0.005, η2 = 0.189), suggesting that DEHP-exposed dams spent less time retrieving their pups. There was no effect of exercise on improving this impairment (Figure 1b). The number of retrieved pups was reduced by the effect of DEHP (F(1, 44) = 7.111, p < 0.05, η2 = 0.139), while no effect of exercise was found (Figure 1c). There was a significant effect of DEHP on reducing the licking time (F(1, 44) = 6.719, p < 0.05, η2 = 0.132), while exercise had no effect on ameliorating this reduction (Figure 1d). There were no effects of DEHP and exercise on nursing time and self-grooming time, suggesting that not only nursing behavior but also a few non-maternal behaviors were affected by DEHP and exercise (Figure 1e,f).
Figure 1.
Effects of prenatal DEHP exposure and childhood exercise on maternal behaviors in postpartum dams. The pup retrieval test was performed postpartum, and the mean values of each responsive behavior were shown. There was a significant effect of DEHP on the first retrieval latency (a), the total retrieval time (b), the total number of retrieved pups (c), and the total licking time (d). No significant effects of DEHP and exercise on the total nursing time (e), and the total self-grooming time (f) were found in the present study. Data are presented in mean ± SEM (n = 12 in each group). *: p < 0.05; ***: p < 0.005; ****: p < 0.001.
2.4. Effects of Prenatal DEHP Exposure and Childhood Exercise on the Oxytocin Pathway in Postpartum Dams
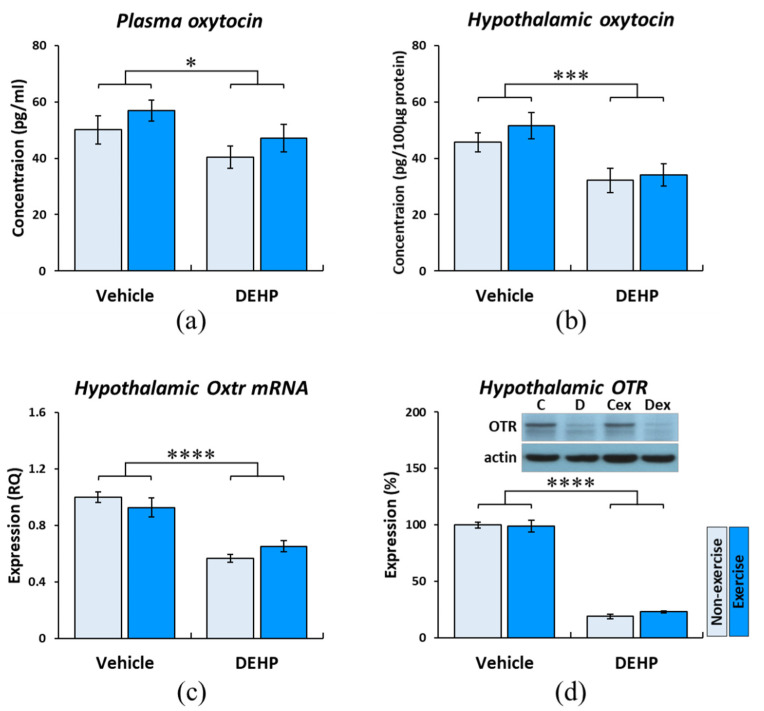
The results of ELISA showed that oxytocin levels in the plasma and hypothalamus were reduced by the effect of DEHP (plasma: F(1, 44) = 4.759, p < 0.05, η2 = 0.098; hypothalamus: F(1, 20) = 13.998, p < 0.005, η2 = 0.412), while no effect of exercise was found (Figure 2a,b). These results suggest that secretions of oxytocin were significantly reduced in the plasma and hypothalamus by prenatal DEHP exposure, and childhood exercise provided few effects on ameliorating this reduction in postpartum dams.
Figure 2.
Effects of prenatal DEHP exposure and childhood exercise on the oxytocin pathway in postpartum dams. The results of ELISA showed that oxytocin levels in the plasma (a) and hypothalamus (b) were reduced by the effect of DEHP. (c) The result of qPCR showed a reduction of hypothalamic Oxtr mRNA influenced by the effect of DEHP exposure. (d) The result of the Western blot showed there was a significant effect of DEHP on reducing the hypothalamic OTR levels. Data are presented in mean ± SEM (n = 12 per group for plasma; n = 6 per group for hypothalamus). *: p < 0.05; ***: p < 0.005; ****: p < 0.001.
The expressions of hypothalamic Oxtr mRNA were quantified by qPCR. The result showed a significant reduction of Oxtr mRNA acting by the effect of DEHP exposure (F(1, 20) = 59.145, p < 0.001, η2 = 0.747). However, no effect of exercise was obtained (Figure 2c). A significant effect of DEHP on reducing the expression of hypothalamic OTR was obtained by Western blot (F(1, 20) = 643.977, p < 0.001, η2 = 0.970); there was also no effect of exercise on improving the expression of hypothalamic OTR (Figure 2d).
2.5. Effects of Prenatal DEHP Exposure and Childhood Exercise on Oxtr Methylation Levels in Postpartum Dams
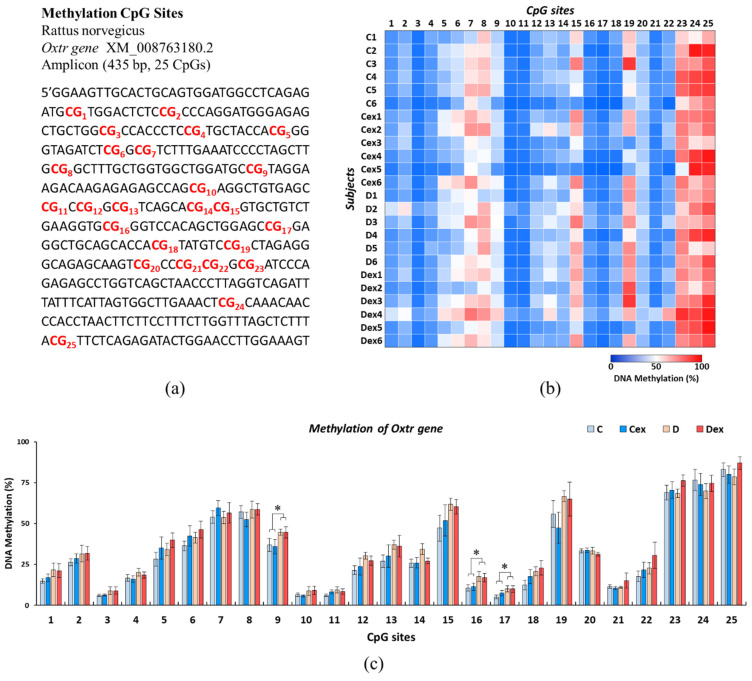
DNA methylation was measured across a 435 base pair region of the Oxtr promoter spanning 25 CpG sites (Figure 3a). The methylation of each CpG site was analyzed by pyrosequencing. The overall methylation of each CpG site within the Oxtr gene was illustrated in Figure 3b.
Figure 3.
Effects of prenatal DEHP exposure and childhood exercise on Oxtr methylation levels in postpartum dams. (a) Epigram of 25 CpG sites (red-coded) within the Oxtr promoter region. (b) Heatmap of methylation levels of observed animals. (c) Mean methylation levels across 25 CpG sites in the hypothalamic Oxtr gene. The analyzed results revealed that 3 out of 25 CpG sites were significantly hyper-methylated by the effect of DEHP, including CpG9, CpG16, and CpG17. Data are presented in mean ± SEM (n = 6 in each group). *: p < 0.05.
Mean methylation levels across 25 CpG sites in the hypothalamic Oxtr gene were shown in Figure 3c. The analyzed results revealed that 3 out of 25 CpG sites were significantly hyper-methylated by the effect of DEHP, including CpG9 (F(1, 20) = 5.559, p < 0.05, η2 = 0.217), CpG16 (F(1, 20) = 6.118, p < 0.05, η2 = 0.234), and CpG17 (F(1, 20) = 4.693, p < 0.05, η2 = 0.190), while there was no effect of exercise on Oxtr methylation across 25 CpG sites. This result suggested that methylation of the Oxtr gene was increased by prenatal DEHP exposure, and exercise during childhood had few effects on ameliorating this Oxtr hyper-methylation in postpartum dams.
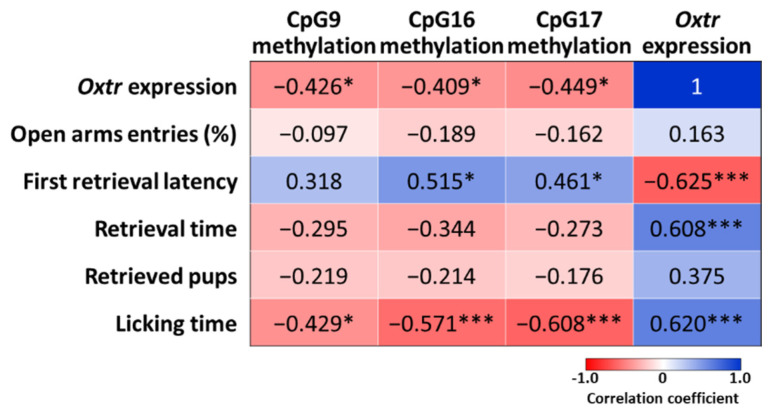
2.6. Correlations between Oxtr Methylation, Oxtr Expression, and Maternal Behaviors
Pearson’s correlation was used to assess the relationships between Oxtr methylation, Oxtr mRNA, and maternal behaviors. The correlation coefficients across 25 CpG sites are shown in Supplementary Materials Table S1, and data from CpG9, CpG16, and CpG17 are shown in Figure 4 for their significant vulnerability to prenatal DEHP exposure. The analysis showed that the Oxtr expressions were negatively correlated with Oxtr methylation levels in CpG9 (r = −0.426, p < 0.05), CpG16 (r = −0.409, p < 0.05), and CpG17 (r = −0.449, p < 0.05). Regarding maternal behaviors, the expressions of Oxtr mRNA were negatively correlated with the first retrieval latency (r = −0.625, p < 0.005), and positively correlated with retrieval time (r = 0.608, p < 0.005) and licking time (r = 0.620, p < 0.005). Furthermore, these three CpG sites were also significantly correlated with maternal behaviors, such as licking time, were negatively correlated with methylation levels in CpG9 (r = −0.429, p < 0.05), CpG16 (r = −0.571, p < 0.005), and CpG17 (r = −0.608, p < 0.005).
Figure 4.
Correlations between Oxtr methylation, Oxtr expression, and maternal behaviors. Pearson’s correlation was used to assess the relationships between Oxtr methylation, Oxtr mRNA, and maternal behaviors. Data are presented in the correlation coefficient. *: p < 0.05; ***: p < 0.005.
3. Discussion
The major finding of the present study is that prenatally DEHP-exposed female rats exhibited impaired maternal behaviors in some categories, such as longer latency to retrieve the first pup, lower number of retrieved pups, and less time spent in pup retrieval and licking. These results suggest that prenatal DEHP exposure had long-term effects on maternal behaviors in adulthood. However, a previous study reports that there is little influence of prenatal DEHP exposure on maternal behaviors in mice [45]. This disagreement may be caused by methodological differences, such as the administered dose and timing, as well as the duration of exposure. In Quinnies’ article, DEHP at doses of 5, 40, and 400 μg/kg/day were treated throughout pregnancy and during the first ten days of lactation, while we fed pregnant rats with DEHP (10 mg/kg/day) from gestational days 14 to 21. The effect of prenatal DEHP exposure on hypothalamic neuroendocrine function has been suggested as a non-monotonic dose-response profile with a J-shaped curve [50]. Prenatal DEHP exposure at higher doses (10–50 mg/kg/day) significantly interferes with the secretion of hypothalamic neurotransmitters and the expression of genes related to social behaviors [51,52]. This evidence suggested that the effect of prenatal DEHP exposure was dependent on the administered dose and the developmental stage of the hypothalamus.
The alteration in the caregiving environment produces long-term changes in anxiety-related and social behaviors. Female rats who experienced low maternal care in their early life may demonstrate less maternal care for their offspring [53]. It is possible that gestational DEHP treatment might affect the quality of maternal caregiving in the F0 dams and transmit this dysregulation to the F1 offspring. Although the performance of maternal behaviors in the F0 dams is absent in the present study, previous evidence shows that gestational DEHP exposure has few effects on maternal behaviors in the DEHP-exposed F0 dams [44,45]. The poor maternal care experience may not or partially contribute to the disrupted maternal behaviors obtained in the prenatally DEHP-exposed female rats.
The hypothalamus begins to release oxytocin prohormone at embryonic day 14 in rodents. It is very likely to be the critical stage for the vulnerability of endocrine-disrupting effects [54]. This notion was supported by a reduction in hypothalamic gene expression in adult rats after DEHP exposure from gestational day 14 to 19 [52]. In the present study, the reductions in plasma and hypothalamic oxytocin, as well as hypothalamic Oxtr mRNA, and OTR, were found in the DEHP-exposed rats at postpartum, suggesting a long-term interference in oxytocin pathways caused by prenatal DEHP exposure. The oxytocin pathways play important roles in maternal behaviors; for example, oxytocin or OTR knockout animals exhibit a lower frequency of pup retrieval and pup licking [11,12]. Therefore, we showed that the reduction of the oxytocin pathway in the hypothalamus might underlie the impairment of maternal behaviors after prenatal DEHP exposure. To our knowledge, there is no information available on the disrupting activity of DEHP affecting oxytocin pathways linked to maternal behaviors.
Several studies suggest that prenatal exposure to DEHP might induce developmental toxicity and endocrine disruption by altering DNA methylation in several tissues, such as the liver, blood cells, heart, and brain [55,56,57]. However, few studies have reported the epigenetic modification of prenatal DEHP exposure on the hypothalamic Oxtr gene. In the present study, increased hypothalamic Oxtr methylation in CpG9, CpG16, and CpG17 sites was found in prenatally DEHP-exposed female rats. There was a negative correlation between Oxtr methylation and Oxtr mRNA expression, suggesting hyper-methylation of the Oxtr gene might underlie the down-regulation of Oxtr mRNA in DEHP-exposed female rats. Evidence shows that Oxtr hyper-methylation in CpG5, CpG14, CpG15, and CpG25 sites are found in the peripheral blood mononuclear cells of female rats performing high-licking behaviors. However, Oxtr hyper-methylation in CpG6 and CpG7 sites is obtained in the hippocampus of low-licking dams [18]. In prairie voles, early life experience such as less parental care can increase Oxtr methylation in CpG18, CpG19, and CpG20 sites in the nucleus accumbens [58]. Interestingly, specific CpG sites are differentially methylated between distinct brain regions expressing different levels of Oxtr mRNA in mice brains [59]. This evidence suggests that brain region-specific methylation of the Oxtr gene may represent the effects of different environmental factors on epigenetic modification. In rodents, hyper-methylation of the Oxtr gene in the promoter region is associated with reduced Oxtr gene expression [18,58]. Some CpG sites within transcription factor estrogen receptor (ER) binding sites are sufficient to predict Oxtr mRNA expression [59]. Interestingly, perinatal DEHP exposure has been shown to reduce ER expression in adult rats’ hypothalamus and pituitary glands [52,60]. These studies suggest that prenatal DEHP exposure may impair the expression of Oxtr mRNA by concurrent alterations of Oxtr methylation and ERα functions.
The relationship between the environment, epigenetic modification, and behavior contributes novel findings of physical interaction of the environment with genes, leading to changes in behavior and health. Early-life adverse experiences, such as maltreatment, stress, and environmental toxicants, have been identified to affect gene expression by epigenetic modification [61]. The methylation of the OXTR gene has attracted considerable attention in the research of inter-individual differences in maternal behavior and social cognition [17,20]. In the present study, the Oxtr methylation levels were positively correlated with first retrieval latency but negatively correlated with licking time in prenatally DEHP-exposed female rats. This new evidence provides the causal link between prenatal DEHP exposure, Oxtr methylation, and maternal behaviors. Environmental factors, such as early-life stress and poor maternal care, have been identified to decrease Oxtr mRNA expression in the amygdala and hypothalamus of female rats [62,63]. In humans and rodents, higher levels of maternal care during childhood are associated with increased OXTR methylation within the promoter region in females but not in males [58,64,65]. Regarding OXTR methylation and behavior, lower levels of OXTR methylation and higher plasma oxytocin levels are associated with less socioemotional anxiety in young adults [21]. Interestingly, early-life stressed female rats give less care to their offspring. However, the administration of an epigenetic inhibitor can reduce the levels of adverse care toward their offspring by normalizing gene expressions [66]. Taken together, we investigated the relationship between Oxtr methylation, Oxtr mRNA expression, and maternal behaviors. The findings suggest that the alteration of Oxtr methylation may be an important mediator for down-regulation of the oxytocin pathway and subsequent maternal behavior deficiencies in prenatally DEHP-exposed female rats.
In the present study, prenatal DEHP exposure had few effects on anxiety-like behaviors in postpartum dams. We also reported that plasma ACTH levels were increased in the DEHP-exposed rats, while corticosterone levels showed no significant difference. Previous studies have shown that prenatal DEHP exposure may increase anxiety-like behaviors in adolescent animals [42,43]. Our current findings provide new evidence suggesting that emotional disturbance during adolescence might recover postpartum. The postpartum anxiolytic effect of oxytocin may have mediated the recovery of corticosterone levels and stress response in prenatally DEHP-exposed rats. The oxytocin system is functionally connected to the hypothalamic–pituitary–adrenal (HPA) axis interactively, such that the release of hypothalamic oxytocin during lactation provides an anxiolytic effect on stress-induced responses [67,68]. This buffering effect of oxytocin on stress response is important for the suppression of HPA reactivity to protect the fetus from adverse programming by maternal stress [69,70]. Importantly, evidence demonstrates that female rats with variations in maternal behavior, such as high-licking and low-licking rates, do not show behavioral differences in the elevated plus maze, the forced swimming test, or the open field test [71]. Therefore, it is very possible that enhanced oxytocin release at postpartum might provide an anxiolytic effect to buffer the stress response in the DEHP-exposed female rats.
The present study showed that there was no effect of prenatal DEHP exposure on plasma and hypothalamic BDNF levels in adult female rats at postpartum. Our result was in agreement with the effects of DEHP exposure on BDNF levels in a sex-specific manner, namely a reduction in males and preservation of BDNF in females [72,73]. The relationship between BDNF and oxytocin has been noticed; lower BDNF and oxytocin levels are correlated to the impairment of maternal behaviors and the symptoms of postpartum depression, suggesting coordination of BDNF and oxytocin in the regulation of behavioral outcomes at postpartum [25,26,74,75]. Early life stress strongly modulates BDNF and OTR expressions by the hyper-methylation of BDNF and OXTR genes in adult animals and humans, suggesting a synergism of BDNF and oxytocin in response to environmental insults [65,76]. The synergistic effect of BDNF and oxytocin was not observed in the present study, which was in agreement with the finding showing that both oxytocin and BDNF levels were higher in pregnant women. However, not oxytocin but BDNF levels were markedly decreased before and after childbirth [77,78].
In the present study, childhood exercise failed to recover the impaired oxytocin pathway and maternal behaviors in prenatally DEHP-exposed female rats at postpartum. We are concerned about the efficacy of the exercise regimen used in the present study. In male rats, prolonged voluntary wheel running results in a decrease in pituitary oxytocin content without evident changes in hormone concentrations in peripheral blood [79]. Forced swimming-induced oxytocin release is found in the hypothalamus without significant changes in plasma [30]. Although there were no significant changes in plasma and hypothalamic oxytocin in the exercised female rats of the present study, high levels of oxytocin release in the lactating dams may mask the effect of exercise-induced responses. The same exercise protocol has been identified to effectively ameliorate the cognitive and emotional deficits in DEHP-exposed adolescent rats [43,80,81]. Therefore, the efficacy of the exercise regimen may not be the factor interfering with the outcomes. Another noticed effect is the sex-specific manner of the exercise-induced BDNF response. The enhancement of BDNF is the most important mechanism underlying the improvement of cognitive function after exercise. However, evidence also shows that exercise-induced BDNF release is lower in females relative to males [82,83]. It is unlikely that sex difference is a key modulator of exercise effect, as our previous report indicates that exercise provides an anxiolytic effect on DEHP-exposed adolescent female rats [43]. It is possible that tissue or neuronal specific responses to exercise might affect the outcomes. Treatment of androgenic steroids may increase anxiety-like behaviors in female mice, while exercise does not ameliorate steroid-induced anxiety or alter amygdalar stress reactivity in females [84]. Evidence also shows that androgenic steroids impair hippocampal spatial learning and memory, and this effect is not rescued by exercise in male rats [85]. These results suggest that exercise is unable to improve the disruption of cognitive and emotional functions by androgenic steroid treatment depending on the investigated tissue-specific behaviors.
In conclusion, the present study provides evidence showing that prenatal DEHP exposure has a long-term adverse effect on maternal behaviors in adulthood. Some of these changes in behaviors may be associated with epigenetic modification of the Oxtr gene. Physical exercise during childhood provides a few effects on ameliorating epigenetic modification. Future work should combine detailed behavioral measures with analyses of epigenetic markers to establish a direct link between maternal emotions and caregiving following prenatal DEHP exposure, as well as to reveal the effects of exercise on ameliorating the socioemotional problem. As more research aims to understand the role of epigenetic markers on a range of human outcomes, it will be vital to have a comparable animal model. Our findings suggest that early-life exposure to endocrine disruptors will be useful in this regard, allowing for an expanded understanding of the role of epigenetic markers in controlling oxytocin pathways and impacting complex socioemotional behavior that can then inform and guide work on human conditions.
4. Materials and Methods
4.1. Animals
Sprague Dawley rats (BioLasco, Taipei, Taiwan) were used in the experiment. This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All the experimental procedures were approved by the Animal Care and Use Committee of Kaohsiung Medical University (IACUC Approval Number: 104160 approved on 4 May 2016), and all efforts were made to minimize the suffering and number of animals used.
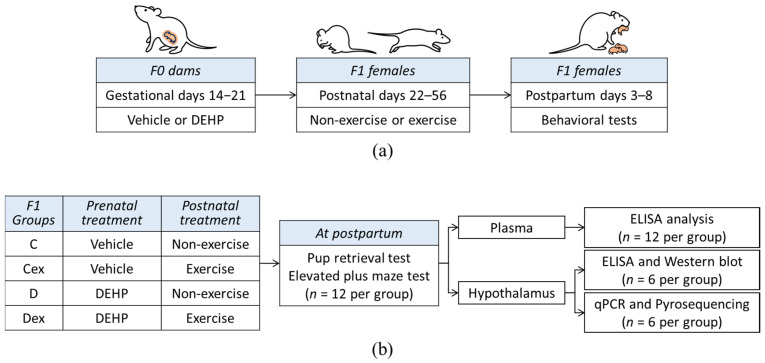
4.2. Experimental Design
The experimental design is shown in Figure 5. Female rats of first-generation (F0) were mated with age-matched male rats (female:male = 2:1) for five days. The vaginal plug was checked every morning, and the day of obtaining a vaginal plug was regarded as gestational day 1. Each male sired 2 litters, which were subjected to either the vehicle or DEHP group. Pregnant rats were housed individually and administered daily with vehicle (n = 8) or DEHP (n = 8) by oral gavage from gestational days 14 to 21. Second-generation (F1) pups were examined with anogenital distance on postnatal day 1 (PND1) and then were culled to four females and four males in one cage. The F1 female rats were weaned on PND 21 and two female siblings were housed in a cage. The F1 female rats were divided into four groups: two non-exercised groups, including vehicle (C) and DEHP (D), and two exercised groups, including exercised vehicle (Cex) and exercised DEHP (Dex). In the exercised groups, rats were trained to run on a treadmill for 5 weeks. The development of F1 female rats is shown in Supplementary Materials Table S2. The changes in body weight during development showed that there was an effect of DEHP on body weight at 3 weeks of age (F(1, 44) = 5.007, p < 0.05, η2 = 0.102) and at 4 weeks of age (F(1, 44) = 4.677, p < 0.05, η2 = 0.096). Additionally, there was an effect of exercise on body weight at 7 weeks of age (F(1, 44) = 6.740, p < 0.05, η2 = 0.133) and at 8 weeks of age (F(1, 44) = 6.722, p < 0.05, η2 = 0.133). At 12 weeks of age, F1 female rats were allowed to produce the F2 generation, and the pups were culled to four females and four males in one cage on PND1 as previously mentioned. The F1 female rats that failed to be impregnated during the first mating week were excluded from this study. There was no significant difference in the litter size or sex ratio among groups in F2 offspring, as shown in Supplementary Materials Table S3.
Figure 5.
Schematic representation of the experimental design. (a) The experimental processes in F0 and F1 generations of dams. (b) Second-generation (F1) female rats were divided into four groups: vehicle control (c), exercised vehicle (Cex), DEHP exposure (d), and exercised DEHP exposure (Dex). The performances of maternal behavior and stress response in F1 dams were assessed at the first postpartum week. Biochemical analyses and pyrosequencing were used to evaluate the functions of the oxytocin pathway.
During the first postpartum week, the F1 females were assessed for their maternal behaviors by a pup retrieval test followed by an elevated plus maze to examine their stress responses. The blood and hypothalamus samples from F1 female rats were used for analysis. The levels of oxytocin, BDNF, adrenocorticotropic hormone (ACTH), and glucocorticoid were analyzed by enzyme-linked immunosorbent assay (ELISA). The expression of OTR was analyzed by Western blotting. The levels of hypothalamic Oxtr mRNA were analyzed by quantitative real-time polymerase chain reaction (qPCR) and the methylations of the hypothalamic Oxtr gene were analyzed by pyrosequencing.
4.3. Gestational Administration of DEHP
DEHP (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in corn oil (Sigma-Aldrich, St. Louis, MO, USA) which was prepared fresh every day and treated by oral gavage. The dose of DEHP exposure was 10 mg/kg/day. The control group was supplied with corn oil, and the DEHP group was fed with the same volume of DEHP/corn oil mixture. The estimated DEHP exposure for the adult human population is 1 to 30 µg/kg/day [86]. According to the conversion coefficient, humans are exposed to DEHP doses corresponding to 0.18–2.5 mg/kg/day for exposure in rats [87,88]. The no-observed-adverse-effect level (NOAEL) of DEHP for humans is 48 mg/kg/day, which is converted to an equivalent dose corresponding to 300 mg/kg/day for rats [88]. Therefore, prenatal exposure to DEHP at the dose of 10 mg/kg/day is lower than NOAEL and is considered human-friendly with no known adverse effects.
4.4. Treadmill Running
Rats were trained to run on a treadmill at night (19:00–21:00, when the light was off). Initially, rats in the exercised groups were allowed to run on a motor-driven horizontal treadmill (Model Exer 3/6, Columbus Instruments, Columbus, OH, USA), starting at a very low speed and gradually increasing to 8 m/min for 30 min each day for 7 days. Then, the animals were trained for 40 min/day (8 m/min warm-up for 10 min), 7 days/week for 4 weeks. The running speed started at 12 m/min, increased by 3 m/min every week, and reached up to 21 m/min at the end of the training period [43]. The rats were trained on a treadmill without an electric foot shock to reduce stress during treadmill running [89]. In contrast, animals in the non-exercising group were placed on the treadmill without running for 10 min each day for 5 weeks.
4.5. Pup Retrieval Test
The pup retrieval test was conducted between 17:00 and 19:00 on postpartum days 3, 5, and 7 according to the reported observational methods [6,90]. Postpartum F1 dams and their pups were brought to the test room in the home cage for 20 min, then the pups were separated from the dam and placed on a heating pad to maintain their body temperature at 37 °C for 30 min. After separation, 8 pups were returned to their home cage in the manner of one male and one female pup placed in each corner. A 30-min video recording began immediately following the return of the pups. During each observation, the following categories of maternal behaviors were recorded by an observer blind to the experimental treatments: the latency to retrieve the first pup (first retrieval latency), the time spent in pup retrieval (retrieval time), the number of pups retrieved (retrieved pups), the time spent on pup licking (licking time), the time spent on arched-back nursing (nursing time), and the time spent in self-grooming of the dam (self-grooming time). The average value from the 3-day observation of the same rat was used for comparison.
4.6. Elevated plus Maze
The elevated plus maze was conducted between 17:00 and 19:00 on postpartum day 8. The apparatus consisted of four arms, two opposing open arms (60 cm length × 10 cm width) and two opposite black plastic closed arms (60 cm length × 10 cm width × 40 cm height), joined by a central platform (10 × 10 cm). The four arms were equally illuminated under red light so that the animals did not perceive lighting differences. Each rat was first placed on the central platform facing an open arm, and then its behavior was video recorded for 5 min. All trials were conducted between 17:00 and 19:00, and each rat was tested only once. Ethanol (40% v/v) was used to clean each arm of the maze between trials to remove odor cues. The number and time of entering into each arm were recorded by an observer blind to the experimental treatments. All four paws inside the arm determined the successful entries. The percentage of open arms entries was calculated by: number of open arms entries (%) = ((number of open arms entries) ÷ (total number of arms entries) × 100%). The following equation calculates the percentage of time spent in open arms: time spent in open arms (%) = ((time spent in open arms) ÷ (exploration time 300 s) × 100%).
4.7. Blood and Tissue Sample Collection
After exploration of the elevated plus maze, rats were returned to their home cages and stayed there for 30 min and were sacrificed by 1 min inhalation of CO2. The blood samples were collected from the right atria and centrifuged at 1500 rpm for 30 min, then the supernatants were collected and stored at −80 °C. After blood collection, the brain was removed and soaked in ice-cold phosphate-buffered saline (0.05 M Na2HPO4 and 0.137 M NaCl, pH 7.4) to remove the residual blood, and then the whole hypothalamus was isolated under microscopic observation by dissecting ventral to the thalamus, posterior to the optic chiasma, anterior to the mammillary bodies, and demarcated laterally by the optic tracts. The hypothalamic samples soaked in ice-cold lysis buffer (n = 6) were used for Western blot and ELISA. The rest of the hypothalamus was soaked in RNAlater solution (ThermoFisher, Waltham, MA, USA) (n = 6) and was used for qPCR and pyrosequencing.
4.8. Western Blot
The tissue was homogenized in ice-cold lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1% deoxycholate, 1 mM sodium fluoride, and 2 mM sodium orthovanadate) and centrifuged at 13,000× g for 20 min at 4 °C. Protein in the supernatant was quantified using a BCA Protein Assay kit (ThermoFisher, Waltham, MA, USA) according to the manufacturer’s instructions. Thirty micrograms of protein were mixed with NuPage LDS sample buffer (Invitrogen, Carlsbad, CA, USA) and separated by pre-cast 10% Bis-Tris gel (Invitrogen, Carlsbad, CA, USA) in MOPS running buffer (Invitrogen, Carlsbad, CA, USA) for 50 min at 120 mA and 200 V. Proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Burlington, MA, USA) in NuPage transfer buffer (Invitrogen, Carlsbad, CA, USA) for 60 min at 170 mA and 30 V. After blocking with 5% nonfat milk in TTBS buffer (10 mM Tris, pH 7.5, 150 mM NaCl, and 0.1% Tween 20), the membrane was incubated with primary antibodies specific for each protein for 24 h at 4 °C: rabbit anti-OTR antibody (1:1000, ab217212, Abcam, USA) and mouse anti-actin antibody (1:5000, A2228, Sigma-Aldrich, St. Louis, MO, USA). After washing, the blot was incubated with horseradish peroxidase-conjugated goat secondary antibodies (1:2000, ab97051 and ab97023, Abcam, Eugene, OR, USA) for 60 min at room temperature. The expression of the protein was detected by the enhanced chemiluminescence kit (Invitrogen, Carlsbad, CA, USA) according to the recommended conditions. Digital images of the blots were created by scanning the blots and the optical densities were determined with the Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA). Each protein level was normalized to the control level from the same membrane and presented as the percent of expression (%). Western blot analysis was performed in duplicate, and the average from the same rat was used for comparison.
4.9. Enzyme-Linked Immunosorbent Assay
The levels of plasma and hypothalamic oxytocin and BDNF, as well as plasma ACTH and corticosterone, were determined by commercially available assay kits optimized for small volumes, according to the manufacturer’s instructions. The detection limit of each kit for corresponding hormones is 15 pg/mL for oxytocin detection kit (ADI-901-153, Enzo, Farmingdale, NY, USA), 12 pg/mL for BDNF detection kit (ERBDNF, Invitrogen, USA), 6 pg/mL for ACTH detection kit (ab263880, Abcam, USA), and 8.2 pg/mL for corticosterone detection kit (501320, Cayman, USA).
4.10. Quantitative Real-Time Polymerase Chain Reaction
RNA was extracted from the hypothalamus using the AllPrep DNA/RNA kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. One microgram of RNA was processed for cDNA synthesis following the protocol provided in the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA, USA) and cDNA was then amplified using a 7500 Real-Time PCR System (ThermoFisher, Waltham, MA, USA) and Power SYBR Green Master Mix (ThermoFisher, Waltham, MA, USA). The amplification was performed under the following cycling conditions: (1) 10 min of denaturation at 95 °C; (2) 35 cycles of 15 s at 95 °C and 60 s at 63 °C. All reactions were run in triplicate and their specificity was verified by melting curve analysis. The primer sequences designed for qPCR are shown in the Supplementary Materials Table S4. The comparative Ct measures were used to obtain fold changes (RQ) for the Cex, D, and Dex groups relative to the C group.
4.11. Oxtr Methylation Pyrosequencing
Methylation analysis was performed with next generation sequencing (NGS). Genomic DNA was extracted from the hypothalamus by using the AllPrep DNA/RNA kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Cell-free DNA (160 ng) was subjected to the bisulfite DNA conversion process using the EZ DNA Methylation-Gold kit (Zymo Research, Freiburg, Germany) according to manufacturer instructions. A region of the Oxtr gene corresponding to nucleotide positions 07717398 to 207717832 on chromosome 4 was amplified by PCR using bisulfite-treated DNA as the template [18]. The target Oxtr gene was amplified according to the general guidelines of pyrosequencing under the following cycling conditions: (1) denaturation at 95 °C for 10 min; (2) 45 cycles at 95 °C, 54 °C, and 72 °C (each for 30 s); and (3) a final extension cycle at 72 °C for 10 min. The bisulfite specific primer sequences are shown in the Supplementary Materials Table S4. The PCR product with a length of ~400 bp was examined by 2% agarose gel electrophoresis. The NGS library preparation was performed by using Collibri NGS Whole-Genome Library Prep kits (ThermoFisher, Waltham, MA, USA) and VAHTS Multiplex Oligo set 5 Adapters for Illumina (Vazyme, Nanjing, China). The amplicons were purified with VAHTS DNA Clean Magnetic Beads (Vazyme, Nanjing, China) and the size distribution of the amplicons of the adapter-ligated library was checked by the MultiNA MCE-202 with DNA-2500 Kit (Shimadzu, Kyoto, Japan). Paired sequencing was performed by MiSeq sequencer (Illumina, San Diego, CA, USA) with paired 150 bp sequencing reads. The bioinformatics analysis was applied with FASTX-toolkit on Galaxy (www.usegalaxy.org (accessed on 15 October 2018)) and MethTargetedNGS (www.bioconductor.org (accessed 20 October 2018)) version 1.14.0 package with RStudio (Boston, MA, USA).
4.12. Statistical Analysis
The statistical analysis was performed with SPSS Statistics (v. 25.0, IBM, Armonk, NY, USA). The two-way ANOVA was used to analyze the data, with DEHP (vehicle and DEHP) and exercise (non-exercise and exercise) as between-subjects factors, and normality was determined using the Shapiro–Wilk normality test. The effect size was shown by partial eta squares (η2) as having small (η2 = 0.01), medium (η2 = 0.06), or large (η2 = 0.14) effects. The correlation was used to examine the relationship between Oxtr methylation of each CpG site, Oxtr mRNA, and maternal behaviors. Normality was assessed by the Kolmogorov–Smirnov test. Spearman’s rank correlation was used when the data were not normally distributed, whereas Pearson’s correlation was used when the data were normally distributed. All values were expressed as mean ± standard error of the mean (SEM) in the figures. Significance was assumed as p < 0.05.
Acknowledgments
The authors acknowledge the contributions of the students who assisted in this research. The NGS procedures were conducted according to the technical support from Topgen Biotechnology (Kaohsiung, Taiwan).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22189847/s1.
Author Contributions
Conceptualization, D.-C.W. and Y.-J.L.; formal analysis, D.-C.W., Y.-J.L., and H.-T.L.; funding acquisition, D.-C.W. and H.-T.L.; investigation, D.-C.W., Y.-J.L., M.A.C., and Y.-C.L.; methodology, D.-C.W. and Y.-J.L.; supervision, D.-C.W. and H.-T.L.; visualization, D.-C.W., Y.-J.L., and Y.-C.L.; writing—original draft: D.-C.W. and Y.-J.L.; writing—review and editing, D.-C.W. and M.A.C. All authors reviewed the final manuscript and approved the presentation. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Ministry of Science and Technology of Taiwan (MOST 105-2410-H-037-005).
Institutional Review Board Statement
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All the experimental procedures were approved by the Animal Care and Use Committee of Kaohsiung Medical University (IACUC Approval Number: 104160 approved on 4 May 2016).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there are no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rilling J.K., Young L.J. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S., Strathearn L. Oxytocin and maternal brain plasticity. New Dir. Child Adolesc. Dev. 2016;2016:59–72. doi: 10.1002/cad.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman R., Weller A., Zagoory-Sharon O., Levine A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol. Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim S., Fonagy P., Koos O., Dorsett K., Strathearn L. Maternal oxytocin response predicts mother-to-infant gaze. Brain Res. 2014;1580:133–142. doi: 10.1016/j.brainres.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guoynes C.D., Marler C.A. An acute dose of intranasal oxytocin rapidly increases maternal communication and maintains maternal care in primiparous postpartum California mice. PLoS ONE. 2021;16:e0244033. doi: 10.1371/journal.pone.0244033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen C.A., Prange A.J., Jr. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl. Acad. Sci. USA. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Leengoed E., Kerker E., Swanson H.H. Inhibition of post-partum maternal behaviour in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J. Endocrinol. 1987;112:275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- 8.Breton C., Zingg H.H. Expression and region-specific regulation of the oxytocin receptor gene in rat brain. Endocrinology. 1997;138:1857–1862. doi: 10.1210/endo.138.5.5127. [DOI] [PubMed] [Google Scholar]
- 9.Champagne F., Diorio J., Sharma S., Meaney M.J. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Sci. USA. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis D.D., Champagne F.C., Meaney M.J. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen C.A., Vadlamudi S.V., Boccia M.L., Amico J.A. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes. Brain Behav. 2006;5:274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- 12.Rich M.E., deCárdenas E.J., Lee H.J., Caldwell H.K. Impairments in the initiation of maternal behavior in oxytocin receptor knockout mice. PLoS ONE. 2014;9:e98839. doi: 10.1371/journal.pone.0098839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takayanagi Y., Yoshida M., Bielsky I.F., Ross H.E., Kawamata M., Onaka T., Yanagisawa T., Kimura T., Matzuk M.M., Young L.J., et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc. Natl. Acad. Sci. USA. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King L.B., Walum H., Inoue K., Eyrich N.W., Young L.J. Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biol. Psychiatry. 2016;80:160–169. doi: 10.1016/j.biopsych.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkeybile A.M., Carter C.S., Wroblewski K.L., Puglia M.H., Kenkel W.M., Lillard T.S., Karaoli T., Gregory S.G., Mohammadi N., Epstein L., et al. Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology. 2019;99:128–136. doi: 10.1016/j.psyneuen.2018.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore L.D., Le T., Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maud C., Ryan J., McIntosh J.E., Olsson C.A. The role of oxytocin receptor gene (OXTR) DNA methylation (DNAm) in human social and emotional functioning: A systematic narrative review. BMC Psychiatry. 2018;18:154. doi: 10.1186/s12888-018-1740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beery A.K., McEwen L.M., MacIsaac J.L., Francis D.D., Kobor M.S. Natural variation in maternal care and cross-tissue patterns of oxytocin receptor gene methylation in rats. Horm. Behav. 2016;77:42–52. doi: 10.1016/j.yhbeh.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory S.G., Connelly J.J., Towers A.J., Johnson J., Biscocho D., Markunas C.A., Lintas C., Abramson R.K., Wright H.H., Ellis P., et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell A.F., Carter C.S., Steer C.D., Golding J., Davis J.M., Steffen A.D., Rubin L.H., Lillard T.S., Gregory S.P., Harris J.C., et al. Interaction between oxytocin receptor DNA methylation and genotype is associated with risk of postpartum depression in women without depression in pregnancy. Front. Genet. 2015;6:243. doi: 10.3389/fgene.2015.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebner N.C., Lin T., Muradoglu M., Weir D.H., Plasencia G.M., Lillard T.S., Pournajafi-Nazarloo H., Cohen R.A., Sue Carter C., Connelly J.J. Associations between oxytocin receptor gene (OXTR) methylation, plasma oxytocin, and attachment across adulthood. Int. J. Psychophysiol. 2019;136:22–32. doi: 10.1016/j.ijpsycho.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin L.H., Connelly J.J., Reilly J.L., Carter C.S., Drogos L.L., Pournajafi-Nazarloo H., Ruocco A.C., Keedy S.K., Matthew I., Tandon N., et al. Sex and diagnosis specific associations between DNA methylation of the oxytocin receptor gene with emotion processing and temporal-limbic and prefrontal brain volumes in psychotic disorders. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1:141–151. doi: 10.1016/j.bpsc.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berdasco M., Esteller M. Clinical epigenetics: Seizing opportunities for translation. Nat. Rev. Genet. 2019;20:109–127. doi: 10.1038/s41576-018-0074-2. [DOI] [PubMed] [Google Scholar]
- 24.Kowiański P., Lietzau G., Czuba E., Waśkow M., Steliga A., Moryś J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018;38:579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maynard K.R., Hobbs J.W., Phan B.N., Gupta A., Rajpurohit S., Williams C., Rajpurohit A., Shin J.H., Jaffe A.E., Martinowich K. BDNF-TrkB signaling in oxytocin neurons contributes to maternal behavior. Elife. 2018;7:e33676. doi: 10.7554/eLife.33676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang T.Y., Shahrokh D., Hellstrom I.C., Wen X., Diorio J., Breuillaud L., Caldji C., Meaney M.J. Brain-derived neurotrophic factor in the nucleus accumbens mediates individual differences in behavioral responses to a natural, social reward. Mol. Neurobiol. 2020;57:290–301. doi: 10.1007/s12035-019-01699-2. [DOI] [PubMed] [Google Scholar]
- 27.de Poli R.A.B., Lopes V.H.F., Lira F.S., Zagatto A.M., Jimenez-Maldonado A., Antunes B.M. Peripheral BDNF and psycho-behavioral aspects are positively modulated by high-intensity intermittent exercise and fitness in healthy women. Sci. Rep. 2021;11:4113. doi: 10.1038/s41598-021-83072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jong T.R., Menon R., Bludau A., Grund T., Biermeier V., Klampfl S.M., Jurek B., Bosch O.J., Hellhammer J., Neumann I.D. Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: The Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology. 2015;62:381–388. doi: 10.1016/j.psyneuen.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Pietrelli A., Matković L., Vacotto M., Lopez-Costa J.J., Basso N., Brusco A. Aerobic exercise upregulates the BDNF-Serotonin systems and improves the cognitive function in rats. Neurobiol. Learn. Mem. 2018;155:528–542. doi: 10.1016/j.nlm.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Torner L., Plotsky P.M., Neumann I.D., de Jong T.R. Forced swimming-induced oxytocin release into blood and brain: Effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology. 2017;77:165–174. doi: 10.1016/j.psyneuen.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Ashdown-Franks G., Firth J., Carney R., Carvalho A.F., Hallgren M., Koyanagi A., Rosenbaum S., Schuch F.B., Smith L., Solmi M., et al. Exercise as medicine for mental and substance use disorders: A meta-review of the benefits for neuropsychiatric and cognitive outcomes. Sports Med. 2020;50:151–170. doi: 10.1007/s40279-019-01187-6. [DOI] [PubMed] [Google Scholar]
- 32.Erickson K.I., Hillman C., Stillman C.M., Ballard R.M., Bloodgood B., Conroy D.E., Macko R., Marquez D.X., Petruzzello S.J., Powell K.E. Physical activity, cognition, and brain outcomes: A review of the 2018 physical activity guidelines. Med. Sci. Sports Exerc. 2019;51:1242–1251. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Pinilla F., Zhuang Y., Feng J., Ying Z., Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci. 2011;33:383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seaborne R.A., Strauss J., Cocks M., Shepherd S., O’Brien T.D., van Someren K.A., Bell P.G., Murgatroyd C., Morton J.P., Stewart C.E., et al. Human skeletal muscle possesses an epigenetic memory of hypertrophy. Sci. Rep. 2018;8:1898. doi: 10.1038/s41598-018-20287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner D.C., Seaborne R.A., Sharples A.P. Comparative transcriptome and methylome analysis in human skeletal muscle anabolism, hypertrophy and epigenetic memory. Sci. Rep. 2019;9:4251. doi: 10.1038/s41598-019-40787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gore A.C., Chappell V.A., Fenton S.E., Flaws J.A., Nadal A., Prins G.S., Toppari J., Zoeller R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015;36:E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Arguelles D.B., Papadopoulos V. Prenatal phthalate exposure: Epigenetic changes leading to lifelong impact on steroid formation. Andrology. 2016;4:573–584. doi: 10.1111/andr.12175. [DOI] [PubMed] [Google Scholar]
- 38.Högberg J., Hanberg A., Berglund M., Skerfving S., Remberger M., Calafat A.M., Filipsson A.F., Jansson B., Johansson N., Appelgren M., et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ. Health Perspect. 2008;116:334–339. doi: 10.1289/ehp.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin S., Ku H.Y., Su P.H., Chen J.W., Huang P.C., Angerer J., Wang S.L. Phthalate exposure in pregnant women and their children in central Taiwan. Chemosphere. 2011;82:947–955. doi: 10.1016/j.chemosphere.2010.10.073. [DOI] [PubMed] [Google Scholar]
- 40.Carbone S., Ponzo O.J., Gobetto N., Samaniego Y.A., Reynoso R., Moguilevsky J.A., Cutrera R.A. Effect of di(2-ethylhexyl) phthalate on the neuroendocrine regulation of reproduction in adult male rats and its relationship to anxiogenic behavior: Participation of GABAergic system. Hum. Exp. Toxicol. 2019;38:25–35. doi: 10.1177/0960327118774868. [DOI] [PubMed] [Google Scholar]
- 41.Shao P., Wang Y., Zhang M., Wen X., Zhang J., Xu Z., Hu M., Jiang J., Liu T. The interference of DEHP in precocious puberty of females mediated by the hypothalamic IGF-1/PI3K/Akt/mTOR signaling pathway. Ecotoxicol. Environ. Saf. 2019;181:362–369. doi: 10.1016/j.ecoenv.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Carbone S., Ponzo O.J., Gobetto N., Samaniego Y.A., Reynoso R., Scacchi P., Moguilevsky J.A., Cutrera R. Antiandrogenic effect of perinatal exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate increases anxiety-like behavior in male rats during sexual maturation. Horm. Behav. 2013;63:692–699. doi: 10.1016/j.yhbeh.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Wang D.C., Chen T.J., Lin M.L., Jhong Y.C., Chen S.C. Exercise prevents the increased anxiety-like behavior in lactational di-(2-ethylhexyl) phthalate-exposed female rats in late adolescence by improving the regulation of hypothalamus-pituitary-adrenal axis. Horm. Behav. 2014;66:674–684. doi: 10.1016/j.yhbeh.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Kougias D.G., Cortes L.R., Moody L., Rhoads S., Pan Y.X., Juraska J.M. Effects of perinatal exposure to phthalates and a high-fat diet on maternal behavior and pup development and social play. Endocrinology. 2018;159:1088–1105. doi: 10.1210/en.2017-03047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quinnies K.M., Harris E.P., Snyder R.W., Sumner S.S., Rissman E.F. Direct and transgenerational effects of low doses of perinatal di-(2-ethylhexyl) phthalate (DEHP) on social behaviors in mice. PLoS ONE. 2017;12:e0171977. doi: 10.1371/journal.pone.0171977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kardas F., Bayram A.K., Demirci E., Akin L., Ozmen S., Kendirci M., Canpolat M., Oztop D.B., Narin F., Gumus H., et al. Increased serum phthalates (MEHP, DEHP) and bisphenol A concentrations in children with autism spectrum disorder: The role of endocrine disruptors in autism etiopathogenesis. J. Child Neurol. 2016;31:629–635. doi: 10.1177/0883073815609150. [DOI] [PubMed] [Google Scholar]
- 47.Ku H.Y., Tsai T.L., Wang P.L., Su P.H., Sun C.W., Wang C.J., Wang S.L. Prenatal and childhood phthalate exposure and attention deficit hyperactivity disorder traits in child temperament: A 12-year follow-up birth cohort study. Sci. Total. Environ. 2020;699:134053. doi: 10.1016/j.scitotenv.2019.134053. [DOI] [PubMed] [Google Scholar]
- 48.Siu M.T., Goodman S.J., Yellan I., Butcher D.T., Jangjoo M., Grafodatskaya D., Rajendram R., Lou Y., Zhang R., Zhao C., et al. DNA methylation of the oxytocin receptor across neurodevelopmental disorders. J. Autism Dev. Disord. 2021:1–14. doi: 10.1007/s10803-020-04792-x. [DOI] [PubMed] [Google Scholar]
- 49.Orso R., Creutzberg K.C., Wearick-Silva L.E., Wendt Viola T., Tractenberg S.G., Benetti F., Grassi-Oliveira R. How early life stress impact maternal care: A systematic review of rodent studies. Front. Behav. Neurosci. 2019;13:197. doi: 10.3389/fnbeh.2019.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrade A.J., Grande S.W., Talsness C.E., Grote K., Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): Non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology. 2006;227:185–192. doi: 10.1016/j.tox.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 51.Carbone S., Samaniego Y.A., Cutrera R., Reynoso R., Cardoso N., Scacchi P., Moguilevsky J.A., Ponzo O.J. Different effects by sex on hypothalamic-pituitary axis of prepubertal offspring rats produced by in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP) Neurotoxicology. 2012;33:78–84. doi: 10.1016/j.neuro.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Gao N., Hu R., Huang Y., Dao L., Zhang C., Liu Y., Wu L., Wang X., Yin W., Gore A.C., et al. Specific effects of prenatal DEHP exposure on neuroendocrine gene expression in the developing hypothalamus of male rats. Arch. Toxicol. 2018;92:501–512. doi: 10.1007/s00204-017-2049-z. [DOI] [PubMed] [Google Scholar]
- 53.Kaffman A., Meaney M.J. Neurodevelopmental sequelae of postnatal maternal care in rodents: Clinical and research implications of molecular insights. J. Child Psychol. Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- 54.Grinevich V., Desarménien M.G., Chini B., Tauber M., Muscatelli F. Ontogenesis of oxytocin pathways in the mammalian brain: Late maturation and psychosocial disorders. Front. Neuroanat. 2015;8:164. doi: 10.3389/fnana.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S., Wang K., Svoboda L.K., Rygiel C.A., Neier K., Jones T.R., Cavalcante R.G., Colacino J.A., Dolinoy D.C., Sartor M.A. Perinatal DEHP exposure induces sex- and tissue-specific DNA methylation changes in both juvenile and adult mice. Environ. Epigenet. 2021;7:dvab004. doi: 10.1093/eep/dvab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nadeem A., Al-Harbi N.O., Ahmad S.F., Alhazzani K., Attia S.M., Alsanea S., Alhoshani A., Mahmood H.M., Alfardan A.S., Bakheet S.A. Exposure to the plasticizer, Di-(2-ethylhexyl) phthalate during juvenile period exacerbates autism-like behavior in adult BTBR T + tf/J mice due to DNA hypomethylation and enhanced inflammation in brain and systemic immune cells. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;109:110249. doi: 10.1016/j.pnpbp.2021.110249. [DOI] [PubMed] [Google Scholar]
- 57.Svoboda L.K., Wang K., Cavalcante R.G., Neier K., Colacino J.A., Sartor M.A., Dolinoy D.C. Sex-specific programming of cardiac DNA methylation by developmental phthalate exposure. Epigenet. Insights. 2020;13:1–15. doi: 10.1177/2516865720939971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danoff J.S., Wroblewski K.L., Graves A.J., Quinn G.C., Perkeybile A.M., Kenkel W.M., Lillard T.S., Parikh H.I., Golino H.F., Gregory S.G., et al. Genetic, epigenetic, and environmental factors controlling oxytocin receptor gene expression. Clin. Epigenetics. 2021;13:23. doi: 10.1186/s13148-021-01017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harony-Nicolas H., Mamrut S., Brodsky L., Shahar-Gold H., Barki-Harrington L., Wagner S. Brain region-specific methylation in the promoter of the murine oxytocin receptor gene is involved in its expression regulation. Psychoneuroendocrinology. 2014;39:121–131. doi: 10.1016/j.psyneuen.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Pérez P.A., Toledo J., Sosa L.D.V., Peinetti N., Torres A.I., De Paul A.L., Gutiérrez S. The phthalate DEHP modulates the estrogen receptors alpha and beta increasing lactotroph cell population in female pituitary glands. Chemosphere. 2020;258:127304. doi: 10.1016/j.chemosphere.2020.127304. [DOI] [PubMed] [Google Scholar]
- 61.Phillips N.L.H., Roth T.L. Animal models and their contribution to our understanding of the relationship between environments, epigenetic modifications, and behavior. Genes. 2019;10:47. doi: 10.3390/genes10010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill K.T., Warren M., Roth T.L. The influence of infant-caregiver experiences on amygdala Bdnf, OXTr, and NPY expression in developing and adult male and female rats. Behav. Brain Res. 2014;272:175–180. doi: 10.1016/j.bbr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peña C.J., Neugut Y.D., Champagne F.A. Developmental timing of the effects of maternal care on gene expression and epigenetic regulation of hormone receptor levels in female rats. Endocrinology. 2013;154:4340–4351. doi: 10.1210/en.2013-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gouin J.P., Zhou Q.Q., Booij L., Boivin M., Côté S.M., Hébert M., Ouellet-Morin I., Szyf M., Tremblay R.E., Turecki G., et al. Associations among oxytocin receptor gene (OXTR) DNA methylation in adulthood, exposure to early life adversity, and childhood trajectories of anxiousness. Sci. Rep. 2017;7:7446. doi: 10.1038/s41598-017-07950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Unternaehrer E., Meyer A.H., Burkhardt S.C., Dempster E., Staehli S., Theill N., Lieb R., Meinlschmidt G. Childhood maternal care is associated with DNA methylation of the genes for brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) in peripheral blood cells in adult men and women. Stress. 2015;18:451–461. doi: 10.3109/10253890.2015.1038992. [DOI] [PubMed] [Google Scholar]
- 66.Keller S.M., Doherty T.S., Roth T.L. Pharmacological manipulation of DNA methylation normalizes maternal behavior, DNA methylation, and gene expression in dams with a history of maltreatment. Sci. Rep. 2019;9:10253. doi: 10.1038/s41598-019-46539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith A.S., Tabbaa M., Lei K., Eastham P., Butler M.J., Linton L., Altshuler R., Liu Y., Wang Z. Local oxytocin tempers anxiety by activating GABAA receptors in the hypothalamic paraventricular nucleus. Psychoneuroendocrinology. 2016;63:50–58. doi: 10.1016/j.psyneuen.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang T., Shi C., Li X., Zhang P., Liu B., Wang H., Wang Y., Yang Y., Wu Y., Li H., et al. Injection of oxytocin into paraventricular nucleus reverses depressive-like behaviours in the postpartum depression rat model. Behav. Brain Res. 2018;336:236–243. doi: 10.1016/j.bbr.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 69.Cox E.Q., Stuebe A., Pearson B., Grewen K., Rubinow D., Meltzer-Brody S. Oxytocin and HPA stress axis reactivity in postpartum women. Psychoneuroendocrinology. 2015;55:164–172. doi: 10.1016/j.psyneuen.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sabihi S., Dong S.M., Durosko N.E., Leuner B. Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front. Behav. Neurosci. 2014;8:258. doi: 10.3389/fnbeh.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruthschilling C.A., Albiero G., Lazzari V.M., Becker R.O., de Moura A.C., Lucion A.B., Almeida S., Veiga A.B., Giovenardi M. Analysis of transcriptional levels of the oxytocin receptor in different areas of the central nervous system and behaviors in high and low licking rats. Behav. Brain Res. 2012;228:176–184. doi: 10.1016/j.bbr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 72.Roepke T.A., Yang J.A., Yasrebi A., Mamounis K.J., Oruc E., Zama A.M., Uzumcu M. Regulation of arcuate genes by developmental exposures to endocrine-disrupting compounds in female rats. Reprod. Toxicol. 2016;62:18–26. doi: 10.1016/j.reprotox.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith C.A., Holahan M.R. Reduced hippocampal dendritic spine density and BDNF expression following acute postnatal exposure to di(2-ethylhexyl) phthalate in male Long Evans rats. PLoS ONE. 2014;9:e109522. doi: 10.1371/journal.pone.0109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jobst A., Krause D., Maiwald C., Härtl K., Myint A.M., Kästner R., Obermeier M., Padberg F., Brücklmeier B., Weidinger E., et al. Oxytocin course over pregnancy and postpartum period and the association with postpartum depressive symptoms. Arch. Womens Ment. Health. 2016;19:571–579. doi: 10.1007/s00737-016-0644-2. [DOI] [PubMed] [Google Scholar]
- 75.Schechter M., Weller A., Pittel Z., Gross M., Zimmer A., Pinhasov A. Endocannabinoid receptor deficiency affects maternal care and alters the dam’s hippocampal oxytocin receptor and brain-derived neurotrophic factor expression. J. Neuroendocrinol. 2013;25:898–909. doi: 10.1111/jne.12082. [DOI] [PubMed] [Google Scholar]
- 76.Branchi I., Curley J.P., D’Andrea I., Cirulli F., Champagne F.A., Alleva E. Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology. 2013;38:522–532. doi: 10.1016/j.psyneuen.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lommatzsch M., Hornych K., Zingler C., Schuff-Werner P., Höppner J., Virchow J.C. Maternal serum concentrations of BDNF and depression in the perinatal period. Psychoneuroendocrinology. 2006;31:388–394. doi: 10.1016/j.psyneuen.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Uvnäs-Moberg K., Ekström-Bergström A., Berg M., Buckley S., Pajalic Z., Hadjigeorgiou E., Kotłowska A., Lengler L., Kielbratowska B., Leon-Larios F., et al. Maternal plasma levels of oxytocin during physiological childbirth—A systematic review with implications for uterine contractions and central actions of oxytocin. BMC Pregnancy Childbirth. 2019;19:285. doi: 10.1186/s12884-019-2365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bakos J., Hlavacova N., Makatsori A., Tybitanclova K., Zorad S., Hinghofer-Szalkay H., Johansson B.B., Jezova D. Oxytocin levels in the posterior pituitary and in the heart are modified by voluntary wheel running. Regul. Pept. 2007;139:96–101. doi: 10.1016/j.regpep.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 80.Sun G.C., Lee Y.J., Lee Y.C., Yu H.F., Wang D.C. Exercise prevents the impairment of learning and memory in prenatally phthalate-exposed male rats by improving the expression of plasticity-related proteins. Behav. Brain Res. 2021;413:113444. doi: 10.1016/j.bbr.2021.113444. [DOI] [PubMed] [Google Scholar]
- 81.Wang D.C., Lin H.T., Lee Y.J., Yu H.F., Wu S.R., Qamar M.U. Recovery of BDNF and CB1R in the prefrontal cortex underlying improvement of working memory in prenatal DEHP-exposed male rats after aerobic exercise. Int. J. Mol. Sci. 2020;21:3867. doi: 10.3390/ijms21113867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Szuhany K.L., Bugatti M., Otto M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Venezia A.C., Guth L.M., Sapp R.M., Spangenburg E.E., Roth S.M. Sex-dependent and independent effects of long-term voluntary wheel running on Bdnf mRNA and protein expression. Physiol. Behav. 2016;156:8–15. doi: 10.1016/j.physbeh.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Onakomaiya M.M., Porter D.M., Oberlander J.G., Henderson L.P. Sex and exercise interact to alter the expression of anabolic androgenic steroid-induced anxiety-like behaviors in the mouse. Horm. Behav. 2014;66:283–297. doi: 10.1016/j.yhbeh.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanehkar F., Rashidy-Pour A., Vafaei A.A., Sameni H.R., Haghighi S., Miladi-Gorji H., Motamedi F., Akhavan M.M., Bavarsad K. Voluntary exercise does not ameliorate spatial learning and memory deficits induced by chronic administration of nandrolone decanoate in rats. Horm. Behav. 2013;63:158–165. doi: 10.1016/j.yhbeh.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 86.Shelby M.D. NTP-CERHR monograph on the potential human reproductive and developmental effects of di(2-ethylhexyl) phthalate (DEHP) Ntp. Cerhr. Mon. 2006;18:v, vii-7, II-iii-xiii passim. [PubMed] [Google Scholar]
- 87.Campioli E., Martinez-Arguelles D.B., Papadopoulos V. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate promotes local adipose and systemic inflammation in adult male offspring. Nutr. Diabetes. 2014;4:e115. doi: 10.1038/nutd.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 89.Chang Y.T., Chen Y.C., Wu C.W., Yu L., Chen H.I., Jen C.J., Kuo Y.M. Glucocorticoid signaling and exercise-induced downregulation of the mineralocorticoid receptor in the induction of adult mouse dentate neurogenesis by treadmill running. Psychoneuroendocrinology. 2008;33:1173–1182. doi: 10.1016/j.psyneuen.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 90.Carini L.M., Nephew B.C. Effects of early life social stress on endocrinology, maternal behavior, and lactation in rats. Horm. Behav. 2013;64:634–641. doi: 10.1016/j.yhbeh.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.