Abstract
Plant extracts are rich in various bioactive compounds exerting antioxidants effects, such as phenolics, catechins, flavonoids, quercetin, anthocyanin, tocopherol, rutin, chlorogenic acid, lycopene, caffeic acid, ferulic acid, p-coumaric acid, vitamin C, protocatechuic acid, vitamin E, carotenoids, β-carotene, myricetin, kaempferol, carnosine, zeaxanthin, sesamol, rosmarinic acid, carnosic acid, and carnosol. The extraction processing protocols such as solvent, time, temperature, and plant powder should be optimized to obtain the optimum yield with the maximum concentration of active ingredients. The application of novel green extraction technologies has improved extraction yields with a high concentration of active compounds, heat-labile compounds at a lower environmental cost, in a short duration, and with efficient utilization of the solvent. The application of various combinations of extraction technologies has proved to exert a synergistic effect or to act as an adjunct. There is a need for proper identification, segregation, and purification of the active ingredients in plant extracts for their efficient utilization in the meat industry, as natural antioxidants. The present review has critically analyzed the conventional and green extraction technologies in extracting bioactive compounds from plant biomass and their utilization in meat as natural antioxidants.
Keywords: plant extracts, conventional and green extraction, bioactive compounds, antioxidant effect
1. Introduction
The meat industry’s focus is shifting towards antioxidants that are novel, efficient, economical, green, or natural alternatives to potentially harmful synthetic preservatives. There has been an ever-increasing effort by the meat industry to explore novel, effective and edible antioxidants and antimicrobials compounds obtained from natural sources. Recently, there have been renewed interests in applying natural compounds (plant extracts, herbs, spices) in food preservation. Since ancient times, these additives have been added to foods as flavorings/seasonings, folk medicine, and food preservatives owing to antioxidant, bacteriostatic or bactericidal properties of polyphenolic compounds, and essential oils. The consumption of these compounds is known to exert beneficial effects on consumer’s health in addition to their preservative role. These herbs, spices, essential oils, or plant extracts exhibit various protective actions against the occurrence of diseases, and thus are widely used as potential green alternatives to food additives, preservatives, and dietary supplements.
Natural antioxidants are safe to consume, available in large quantities at a relatively lower price than their synthetic counterparts, and can be extracted and applied in the meat industry [1]. These compounds are abundantly present in the vegetable kingdom, such as in spices, herbs, and essential oils, with their concentration varying in throughout a plant, for example bark in the cinnamon and arjuna trees, the roots of liquorice, rosemary, cloves, and grape seeds, and the leaves of oregano and tea. Natural antioxidants are recognized as nutraceutical ingredients or supplements that can be used for raw meat and/or meat products [2]. The commonly used spices and herbs (such as oregano, rosemary, cinnamon, aniseed, fennel, basil, garlic, and ginger) are rich in phenolic compounds like phenolic acids, phenolic diterpenes, flavonoids, volatile oils, carnosic, caffeic, and chlorogenic acid. In their B rings, these polyphenols have 30–40 dihydroxy groups, and galloyl ester in the C rings of flavonoids can bind iron, making these compounds very potent antioxidants [3]. Phenolic compounds are lipid-soluble, owing to the hydroxyl group present in their chemical structure; they react with microbes’ cellular membranes, leading to loss of cell membrane integrity and, thus, antimicrobial effect. Fruits such as apple, plum, grape, pomegranate, and several types of berries, such as blueberries, cranberries, and bearberries, which are all good sources of antioxidants, owing to their high concentrations of flavonoids.
2. Plant Extracts as Natural Antioxidants
At present, various plant extracts are increasingly being explored in meat products as potential natural antioxidants for improving oxidative stability due to the potent antioxidant potential of these compounds in the meat matrix. The antioxidant attributes of these plant extracts are due to flavonoids (flavanol, flavones, anthocyanins), phenolic acid (hydroxybenzoic, hydroxycinnamic acids), diterpenes tannins (hydrolyzable and condensed tannins), stilbenes, coumarins, lignans, quinones, curcuminoids, and others including phenolic alkaloids, phenolic glycosides, phenolic terpenoids, and essential oil) [4,5]. Tea phenolics, such as epigallocatechin gallate (EGCG), have antioxidant potential in terms of their having electron reduction (550 mV) potential comparable to alpha-tocopherols or vitamin E (480 mV) [6].
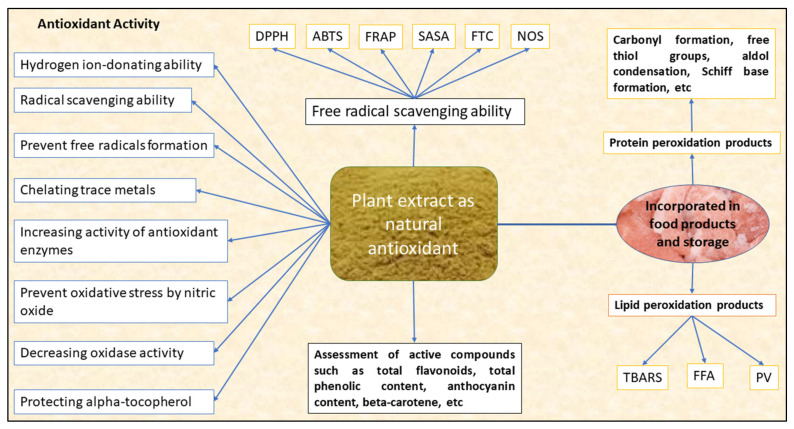
The antioxidant potential of these plant extracts can be measured by assessing their free radical-scavenging ability or by measuring the compounds associated with lipid and protein peroxidation. The free radical-scavenging ability of the plant extract is measured spectrophotometrically by using stable radicals, such as 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity or percent inhibition or 2-2-azinobis-3ethylbenthiazoline-6-sulphonic acid (ABTS) radical cation activity or percent inhibition, by measuring the ability of antioxidants to quench stable DPPH+ or ABTS+ radical cations, respectively, in ferric reducing antioxidant power (FRAP) by assessing the reduction of ferric-TPTZ (2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ) into ferrous from ferric thiocyanate assay (FTC), nitric oxide scavenging (NOS), aldehyde/carboxylic acid assay (ACA), superoxide scavenging, oxygen radical absorbance capacity (ORAC) and total antioxidant capacity (TAC). The antioxidant potential of the plant extract can also be measured by various compounds associated with lipid peroxidation (malonaldehyde formation, thiobarbituric acid reacting substances [TBARS], peroxide value [PV], free fatty acids [FFA]), protein oxidation (carbonyl formation, free thiols, conjugated diene) and beta carotene bleaching assay. Figure 1 depicts the antioxidant activity of the bioactive compounds extracted from plant biomass.
Figure 1.
Overview of the antioxidant activity of the bioactive compounds extracted from a plant biomass source [7,8]. DPPH-1,1-diphenyl-2-picrylhydrazyl radical-scavenging activity, ABTS-2-2-azinobis-3ethylbenthiazoline-6-sulphonic acid radical cation activity, FRAP—ferric reducing antioxidant power, SASA-superoxide anion-scavenging ability, FTC—ferric thiocyanate assay, NOS—nitric oxide scavenging, TBARS-thiobarbituric acid-reacting substances, FFA—free fatty acids, PV—peroxide value.
3. Extraction Protocols
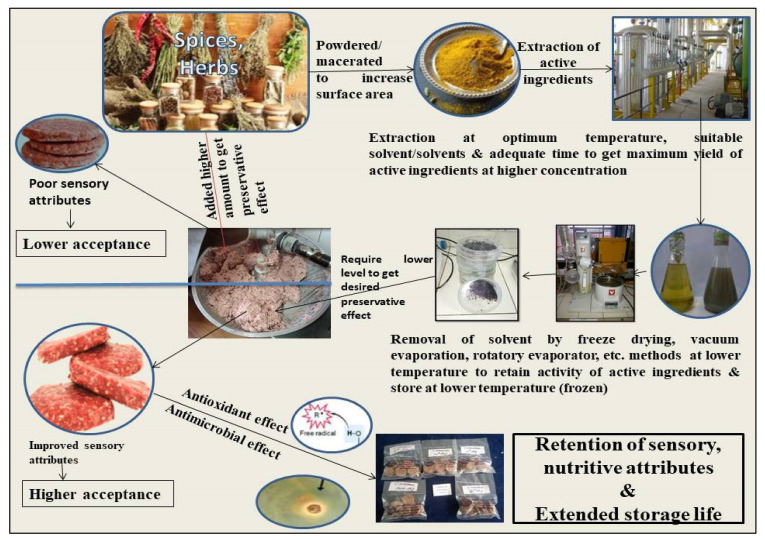
The concentration of recovered bioactive molecules in extracts and their yield largely depend upon the various processing parameters. It is desirable to have a higher yield along with a significant concentration of active compounds. A proper extraction protocol is a primary step in ensuring the proper availability and utilization of these natural extracts, with higher preservative potential, and at an economical rate to industry [9].The overall composition of the extract obtained varies with the extraction protocols, plant species, part of the plant used (root, stem, leaves, root), geographical origin, harvesting time, type of storage, drying methods in case of use of powder and developmental stage of plant [10,11,12]. The use of extraction solvents, method of sample preparation, extraction duration, ratio of sample to extraction solvent, and temperature affect the composition and concentration of active compounds in an extract, thereby affecting its antioxidant efficacy [13,14,15,16]. Figure 2 depicts the various technical aspects of using plant extracts as a natural antioxidant in meat.
Figure 2.
Plant extract as natural preservatives in meat.
Green Solvents for Extraction
Vegetable matter, such as specific parts of plants or as such a whole plant, when properly harvested for maximum yield, is cleaned and mostly dried at a low temperature to preserve the maximum activity of heat-labile active compounds. This dried material is ground into powder to increase its surface area for better segregation of bioactive compounds from the plant matrix. The finely ground powder is dissolved in either a suitable solvent or a combination of solvents (depending upon the suitability and efficiency of these solvents) and dissolves bioactive compounds from plant powders. The yield and concentration of active principles increased in the extracts upon increasing the total surface or contact area between the powders and solvents. In the plant kingdom, a huge variety of bioactive active ingredients showing antioxidant potential exist. These compounds have different chemical and physiological attributes that affect their solubility. Hence, these bioactive compounds are soluble in different solvents. It is practically not feasible to recommend a single universal extraction solvent to extract active ingredients from all plant biomass.
The commonly utilized solvents for extraction are ethanol, methanol, ethyl acetate, acetone, heptane, dimethyl sulfoxide (DMSO), and water. The food industry prefers to use solvents that are non-toxic and easy to handle. DMSO and methanol solvents for extraction have food safety issues due to their toxic nature; hence, mostly preferred as solvent of choice for various non-food-related uses. DMSO can be used for dissolving both polar and non-polar compounds, as well as its ability to mix in organic solutions, including water, completely. Methanol was also reported suitable for lower molecular weight polyphenols extraction whereas water has been recommended to extract polyphenols with larger flavanols [17]. Thus, a combination of these two has been recommended. Methanol retards polyphenol oxidase, and hence is recommended for phenolic-compounds extraction from the raw plant matrix [18]. Water is very efficient in extracting non-polar compounds, whereas the use of organic solvents proves efficient in extracting organic compounds from plant biomass. The hydrophilic compounds present in plants are the most active compounds that can be efficiently extracted by using water as a solvent. Water was also reported as a suitable extraction medium for tea catechins, as compared with combinations of methanol (80%) and ethanol (70%) [19]. Do et al. [20] reported significantly higher antioxidant activity in Limnopholia aromatic extracts obtained by absolute ethanol with maximum yield by the combination of 50% acetone and 50% water. They recommended using combinations of aqueous and organic solvents in suitable concentrations for the efficient extraction of active principles that are soluble in water and the organic solvent.
Organic solvents are commonly used to efficiently extract phenolic compounds, but their left-over residues may be potentially harmful to consumers’ health even in traces. Thus, while using organic solvents as an extraction medium, it is imperative to take utmost care to remove all extracting solvents from the filtrate. The application of aqueous media in extraction has several inherent advantages. Water is considered a cheap and the safest solvent but is not as efficient as organic solvents, especially in extracting active compounds possessing antioxidant potential [21]. Turkmen et al. [22] utilized water, acetone, N, N dimethylformamide, ethanol/methanol as extraction solvents for obtaining green tea and mate tea compounds and reported the use of 50% aqueous ethanol and 50% aqueous acetone to get the maximum yield and so antioxidant potential.
The food industry increasingly prefers green solvents for extraction due to their non-toxic nature, food safety, and recycling, such as water, ethanol, deep eutectic solvents, synthetic ionic liquids, and carbon dioxide, and water again, as a supercritical fluid in extraction [23,24,25]. The ionic liquids are salt mixtures in the liquid phase at room temperature, comprising ionic components that bind ionically [25]. These solvents have very low vapor pressure, high conductivity, are very stable at high temperature, and have a wide range of polarity, but their food-safety risk, high cost, and poor environmental degradability hampers their proper utilization in the extraction sector [26].
4. Extraction Methodology
Extraction conditions have a critical role in determining the overall efficacy of extracts. Generally, it is recommended to perform extraction at lower temperature and avoid prolonged exposure to high temperature, as the latter could lead to significant loss of active principle of the extract. Based on the type of plant biomass used, suitable solvent and extraction techniques must be employed to get the maximum concentration and yield of the active compounds in the extract. After extraction in solvents for a sufficient time, the solution is filtered. The resultant filtrate is dried at low temperature using advanced technologies such as vacuum (vacuum oven/rotator evaporator/rotavapor) or freeze-drying (for aqueous solvent mostly). Extracts should be stored under frozen conditions, in suitable, inert packaging material, such as glass bottles/trays, to prevent reaction between the active ingredients and packaging material. A solvent used in the extraction of bioactive compounds or essential oils should have low toxicity, a high mass transfer rate, a low boiling points, preserve the active ingredients, and should not interact with the organic compounds in the plant biomass or food [27].
4.1. Traditional Extraction Methods
Water or alcohol-based extraction methods, steam distillation, or Soxhlet extraction are widely used methods for extracting bioactive compounds from plant biomass. These methods are simple, low cost, and fast, but have some practical problems, such as difficult and complex operations, lower yield/inefficient methods, energy-intensiveness, and extract at high temperatures, leading to the degradation of heat-sensitive active compounds, problems in the purification of extracts, and removal of solvents—especially, poor recovery of solvents and the generation of organic wastes, a requirement of specific organic and inorganic solvents [28]. Under solvent extraction or portioning, active ingredients from plant biomass (solid or liquid) are separated by dissolving them into a suitable solvent. This is one of the most commonly used traditional methods by the food industry. The active ingredients (solutes/extracts) are extracted from one solid or liquid phase to another liquid i.e., solvent. This extraction process is generally completed at a higher temperature for long exposure, resulting in the possible degradation of the active principle in the extract and high energy consumption.
Under Soxhlet extraction, a small, dried sample is placed in an extractor and placed in direct contact with a suitable solvent (water, petroleum ether, and hexane) repeatedly until the extraction is complete. Its higher solvent consumption, energy requirement, and long extraction time are important issues with the Soxhlet extraction technology [29].
Maceration is the process of breaking/subdividing or softening matter into pieces of plant biomass in a suitable solvent. This method is recommended to preserve the typical essence of extracts of some valuable herbs that contain very delicate, heat sensitive, and volatile compounds. It is used for the preparation of traditional food products with specific flavors and organoleptic attributes.
Hydrodistillation is applied to extract volatile compounds from food/plants by using distilled water. This process, of extracting volatile organic compounds by azeotropic distillation and non-volatile organic compounds by boiling water, takes 6–8 h. It comprises three processes viz. penetration of water into solutes (hydrodiffusion), hydrolysis, and some level of degradation of heat-labile compounds due to high temperature [30]. Figure 3 provides a comparative description of conventional and green extraction technologies.
Figure 3.
Comparative analysis of conventional and green extraction technologies.
4.2. Greener/Advanced Extraction Methods
The high energy and solvent consumption and lower solvent recovery in traditional extraction methods has forced various researchers and biochemists to explore a more efficient method utilizing environmentally friendly and less harmful solvents. These technologies, such as supercritical fluid extraction (SFE), ultrasound-assisted extraction (UAE), subcritical water extraction (SWE), microwave-assisted extraction, instant controlled-pressure drop extraction (French-DIC détente instantanée contrôlée) are widely referred to as green extraction technologies [23,31]. These technologies require a lower amount of organic solvent or utilizeg less harmful or environmentally friendly solvents, show better reuse of solvents, use solvents from sustainable sources with reduced or no toxicity, consume less energy, have a lower environmental impact/footprint, better worker safety; that is, they are highly efficient extraction methods with higher yields, containing the maximum percentage of desirable bioactive ingredients in active form [23,32].
Various operational aspects of various green extraction technologies are described in Table 1.
Table 1.
Technological aspects of various green extraction technologies.
| Parameter | MAE | SFE | UAE | DIC |
|---|---|---|---|---|
| Process | Used along with traditional extraction methods to improve the extraction process | Very fast process | Used along with traditional extraction methods to improve extraction process | Rapid extraction |
| Solvent consumption | A small amount of solvent required | Very little amount of organic solvent or no solvent due to re-use | A small amount of solvent | Steam-driven progress with rapid depressurization |
| Solvent residue | Less solvent residue | No solvent residue due to phase separation on depressurization | Less solvent residue | Very low |
| Suitability/Applicability | Applicable for limited samples | Minimal application for a selected compound | High versatility and suitability | Used for sample pre-treatment process |
| Selectivity | Non-selectivity, extraction of a range of compounds | High selective for extraction of a small number of compounds | Non-selective extracts a range of compound | Non-selective extracts a range of compound |
| Processing conditions | High temperature and pressure | Not harsh conditions for SC CO2 | Not harsh conditions | High temperature |
| Suitability for heat-labile compounds | Not suitable | Suitable to preserve the activity of heat-labile compounds | Suitable | Not suitable for heat-labile compounds |
| Energy consumption | High | Low due to re-use of solvent | Relatively low | High |
| Capital cost | Low initial capital cost | Very high | Lower capital cost | Very high |
| Technical workforce | Simple process | Needs very high technical workforce | Simple and easier operations | Needs high technical workforce |
MAE—microwave-assisted extraction, SFE–supercritical fluid extraction, UAE–ultrasound assisted extraction, DIC—détente instantanée controlee, SC CO2—Carbon dioxide as supercritical fluid; source [33].
5. Supercritical Fluid Extraction
A supercritical fluid is a dense gas or compressed liquid lacking any molecular interactions. These gases, in a supercritical fluid state, are compressed, exhibiting properties of both gas-like (mass transfer, diffusion, lower viscosity, higher penetration power) and liquid-like (high density, solvent power, reduced surface tension, and viscosity) attributes (mesophase) at temperatures and pressures above critical points, with even minor change/adjustment in these parameters resulting in a significant change in density (tunable). Active principle or bioactive compounds are separated from the plant matrix (solid or liquid) by utilizing supercritical fluids to extract solvents. Carbon dioxide and water are the two commonly used supercritical fluids in the industry on a large scale.
Recently, there is an increasing focus on extracting bioactive compounds from the plant by using various solvents such as carbon dioxide, propane, ethylene, and butane in the supercritical fluid stage (SCF), due to their use in efficient and environmentally friendly technology. This supercritical fluid technology has resulted in the development of several other subsidiary technologies, used in the pharmaceutical and food sectors, such as supercritical fluid extraction (SFE), gas antisolvent process (GAS), supercritical solutions (RESS), supercritical antisolvent process (SAS), and their suitable modifications, such as solvent-extraction systems (ASES), supercritical solvent impregnation (SSI), and supercritical assisted atomization (SAA) [34]. Supercritical fluid extraction (SCFE) is a fast, efficient, economical, selective, practically solvent-free method, having a simple procedure for sample preparations’ extraction of bioactive compounds from plant biomass [35].
5.1. Selection of SCF
5.1.1. Carbon Dioxide as a Supercritical Fluid (SC CO2)
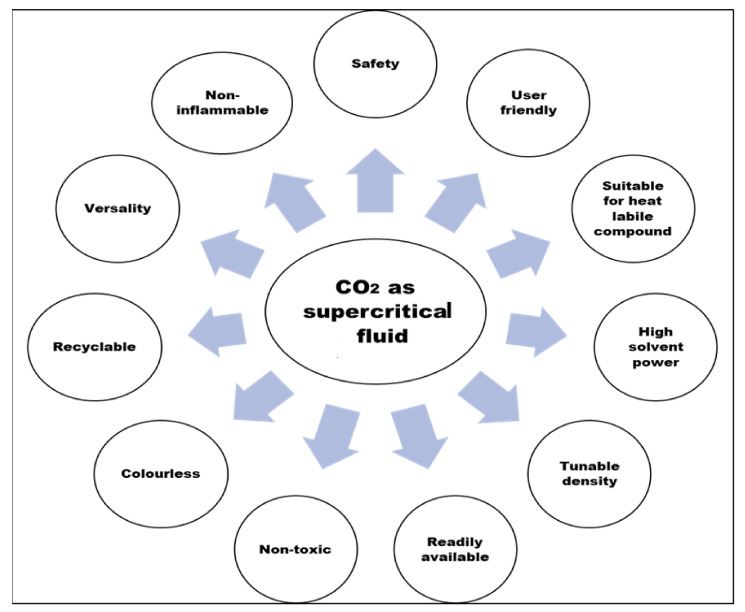
Carbon dioxide is the most commonly applied gas in SCFE. Supercritical fluid extraction with carbon dioxide is performed at 31 °C and 74-bar pressures. These processing conditions viz. temperature and pressure depend on the nature and type of the extracts to be obtained; for example, low pressure (100 bar) is used to extract volatile oils/essential oils, polyphenols, and unsaturated fatty acids [36]. SC CO2 is the most common (about more than 90% of total SCFs used) used supercritical fluid (SC CO2) used in the extraction of flavor and fragrance compounds. The SC CO2 has the following operational advantages:
-
(a)
Safe and user-friendly critical temperature (Tc-31.2 °C) and operates at low pressure (critical pressure Pc-7.3 Mpa) [34,37];
-
(b)
Low critical temperature, suitable for the extraction of heat-labile compounds;
-
(c)
© High density (467.6 kg/m3), at a critical point, leading to higher dissolving power;
-
(d)
Easily adjustable/tunable density, such as, at 42 °C, 766.5 kg/m3 density at 150 bar, 950 kg/m3 at 400 bar and 1075 kg/m3 (near to liquid CO2 i.e., 1256.7 kg/m3) at 750 bar. It allows the collecting of every compound present in the plant biomass by suitable processing conditions, such as [33]:
-
(e)
Readily available in the environment and economy;
-
(f)
Non-toxic, colorless, odorless, and non-inflammable gas;
-
(g)
Purity and recyclability;
-
(h)
Wide versatility during fractionalization and extraction.
Various parameters that make carbon dioxide as most common gas used for SCFE are presented in Figure 4.
Figure 4.
Suitability of CO2 in supercritical fluid extraction.
The carbon dioxide gas in the supercritical fluid stage exhibits properties of a lipophilic solvent, with greater and easier manipulation of its solvent between its use as gas solvent versus a liquid one. SC CO2 has a low polarity index, and thus is useful in dissolving lipophilic compounds such as lipids and essential oils, but unsuitable for polar compounds. To overcome this challenge, SC CO2 is used with other co-solvents to increase its polarity index [38,39], or an anti-solvent with plant material is injected into SC CO2.
For the extraction of polar compounds, supercritical nitrous oxide (SC N2O) fluid (7.1 MPa Pc and 36.7 °C Tc) is utilized. Still, it has safety issues regarding its high concentration in organic compounds [40]. Water exhibits a supercritical fluid state at a high critical pressure (Pc 22.1 MPa) and critical temperature (374 °C Tc). Still, its corrosive nature has limited its use in the food and pharmaceutical industry [41]. The pre-treatment of plant biomass with water before extraction with SC CO2 or used as a co-solvent with carbon dioxide, in a subcritical or supercritical state, for the extraction of polar compounds from aromatic plants significantly improves the composition of its extracts [42,43]. Carbon dioxide can segregate flavor and aroma compounds from complex mixtures in various herbs and spices, such as ginger, garlic, and pepper.
Besides CO2, ethane (4.8 MPa Pc and 32.4 °C Tc), dimethyl ether, and propane (4.2 MPa Pc and 96.6 °C Tc) are also used in extracting bioactive compounds from plant biomass, due to their critical temperature and pressure being close to CO2 and better extraction efficiency in extracting polar compounds due to their higher polarizability as compared with CO2 [44]. There is a range of solvents that have the potential to be used in supercritical fluid extraction, such as n-butane, nitrous oxide, freon, propane, methoxymethane and water, but various food-safety matters and operational hazards/harsh handling should be given due consideration before their, application such as the very high critical temperature of water (400 °C), which poses serious handling challenges during its application in SFE.
5.1.2. Propane as a Supercritical Fluid
Propane’s application in supercritical fluid extraction processes has certain inherent merits over carbon dioxide, such as higher yield, an improved solubility of non-polar compounds, and a fast and efficient process requiring less time with better specificity, and less solvent consumption [45]. Liquified petroleum gas (LPG) is a mixture of propane and butane used in domestic food preparation and heating. In extraction under high pressure (245 g/kg), it improves the operation speed. It improves the catalytic power of enzymes, such as increasing enzymes’ activity, leading to improved enzymatic hydrolysis of lignocellulosic material of sugarcane bagasse, for example [46]. The uses of propane and LPG as supercritical fluids in the extraction of bioactive compounds are presented in Table 2.
Table 2.
Use of propane and LPG as supercritical fluid in the extraction of bioactive compounds.
| Plant Material | Processing Protocol (Temp, Pressure, Flow Rate) |
Remark | Reference |
|---|---|---|---|
| Propane as supercritical fluid | |||
| Flaxseed | 30–45 °C, 80–120 bar | 28% higher yield of flaxseed oil with better composition, purity, and oxidative stability as compared with convention chloroform-methanol-water Soxhlet extraction | [47] |
| Perilla | 40–80 °C, 80–160 bar, 1.0 cm3/min flow rate | Higher extraction yield of perilla oil with oxidative stability | [48] |
| Crambe seed | 79.85 °C, 160 bar | The temperature has a vital role in affecting yield, less than 2% free fatty acids in the extract | [49] |
| Pequi pulp | 30–40 °C, 50–150 | 43% higher yield of oil at 15 MPa | [50] |
| Canola seed | 30–60 °C, 80–120 bar along with SC CO2 (40–60 °C and 200–250 bar) | Propane SFE faster than CO2 SFE | [51] |
| Sesame seed | 30–40 °C, 20–120 bar along with SC CO2 (30–40 °C, 190–250 bar) | Extract quality same with both solvents, and temperature and pressure have an important role. | [52] |
| Sunflower seed | Propane and CO2 | High concentration of tocopherol in the oil | [53] |
| LPG as supercritical fluid | |||
| Rice bran | Compressed LPG and SC CO2 | LPG decreased extraction time and save energy of re-compression | [54] |
| Elaeis guineensis | Compressed LPG | Advantages in terpene extraction with improve speed and reducing cost | [55] |
SC CO2–supercritical carbon dioxide, LPG–liquified petroleum gas, SFE—supercritical fluid extraction.
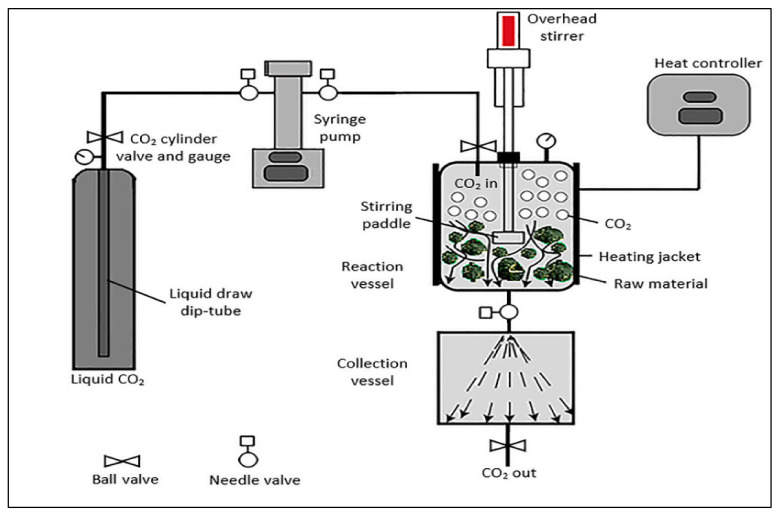
5.2. SCF Extraction Process
The SFE unit has the following essential components viz. a carbon dioxide compressor or pump, along with a modifier pump in a co-solvent, such as water or an organic solvent, an extraction reactor, and a collection or fractionation vessels. To improve the selectivity and sequential separation of active compounds from plant biomass in SFE operations, a series of reactors are applied, or a various combination of temperature, pressure, and solvent flow rate is applied [56]. Other processing variables that affect extraction in SFE are flow rate, extractor diameter, particle size (larger particles require more time), diameter-to-length ratio, the application of extractors, bed porosity, and stirring diameter [57]. Samples are pre-treated by various processes, such as microwaves, ultrasound, irradiation and enzymatic treatment, to increase the efficiency of the process and improve the recovery of bioactive compounds. The preparation of raw samples has an impact on the recovery of bioactive compounds, such as how air-drying may lead to a loss of heat-labile compounds, whereas freeze-drying helps in retaining the activity of these heat-labile compounds.
The optimization of processing conditions is critical for obtaining the desired outcome in supercritical fluid extraction. In general, an increase in pressure above certain points results in increasing fluid density and improving solute solubility, leading to higher yield. In some cases, pressure increase also proves disadvantageous, by decreasing the diffusivity of the supercritical solvents, thereby decreasing contact/interactions and dissolving solutes or even in some cases, cause the formation of void fractions and undesirable outcomes due to a compact solid matrix [58,59]. Temperature exhibits a crossover effect in SFE, where high-temperatures decreases yield by reducing density, increasing diffusivity, and imparting high energy to the system, whereas low temperature causes increasing extract yield by increasing density and reducing vapor-pressure solutes [33]. However, in essential oils, the diffusion has a greater impact than density, thus higher temperature result in higher yields [60].
Water is soluble in SC CO2 at 0.3% v/v [35], and this was observed to affect the extraction process, depending upon the nature of plant biomass. Paprika, for example, with 85% moisture recorded a marginal increase in yield [61], while no effect was observed on extract yield from Nannochloropsis sps containing 5–20% moisture [62], though extract yield increased (40%) and CO2 consumption was reduced by 25% by soaking the flowers of Helichrysum italicum prior to processing [63].
Overall, the SFE is completed in five basic steps:
-
(a)
Penetration of the matrix;
-
(b)
Supercritical solvent solubilizes the solutes/plant compounds inside the pores;
-
(c)
Internal diffusion of the solute until it has reached the external surface;
-
(d)
External diffusion of solutes from the solid–fluid interface of the supercritical fluid;
-
(e)
Precipitation of compounds/solutes by suitable pressure and temperature modifications [34,40].
The various components of an SFE unit are depicted in Figure 5.
Figure 5.
Supercritical fluid-extraction process. Adopted from [33].
5.3. Other Extraction Methods as SFE-Adjunct
The application of various other extraction methods in combination with SFE has been reported to improve the efficiency and recovery of active compounds in the SFE process. Porto et al. [64] observed an 11%-higher yield (up to 34%) than controls (23%) upon microwave pre-treatment followed by CO2 SFE at 40 °C and 300 bar with Moringa oleifera seeds at 100 W for 30 s.
5.3.1. Enzyme-Assisted SFE
The enzymatic treatment of plant materials, such as cellulase, alpha-amylase, hemicellulose, and pectinase, also improves the extraction efficiency and yield by enzymatic degradation of the structural integrity of plant’s cell wall, thereby increasing the interactions between solvent and solute. Black pepper, treated with alpha-amylase obtained from Bacillus licheniformis and followed by CO2 supercritical fluid extraction (60 °C, 300 bar at 2 L/min flow) increased black pepper oleoresin yield by 53%, with a 46% more piperine-enriched extract and higher enzymatic activity, noticed in continuous flow (2.13%), than the batch process (1.5%) [65].
Pre-treating pomegranate peel with recombinant enzyme mixtures containing cellulase, pectinase, and protease (2:1:1) followed by SFE (using SC CO2 and ethanol as co-solvent) resulted in a two-fold increasing crude extract yield with higher levels of total phenolics as compared with extracts obtained by only the SFE process. Vanillic acid (108.36 μg/g), ferulic acid (75.19 μg/g), and syringic acid (88.24 μg/g) were reported as principal phenolic compounds in the pomegranate peel extract [66]. The authors recommended using enzyme-assisted supercritical fluid extraction (EASFE) as a state-of-the-art green technology for extracting bioactive compounds from plant biomass. The pre-treatment of black tea leftovers with 2.9% kemzyme (2.8% w/w at 45 °C, pH 5.4 for 98 min) followed by SFE using SC CO2, along with ethanol as a co-solvent, increased extract yield by five-fold, displaying a higher amount of caffeic and para-coumaric acid as compared with controls. The enzymatic treatment hydrolyzed the cellulose and hemicellulose of the black tea leftovers, thus releasing the non-extractable polyphenols [67]. The application of plant cell-wall glycosidase to freeze-dried tomato matrix, prior to SC CO2 (500 bar, 86 °C, 4 mL/min, SC CO2 flow) extraction, was observed to increase lycopene content three fold [68].
5.3.2. Ultrasound-Assisted SFE
Ultrasound application at 10–20 kHz creates a mechanical breakdown of plant biomass and improves the extraction yield with less solvent and energy consumption. A longer ultrasound treatment, above permissible limits, causes undesirable outcomes due to non-enzymatic browning reactions and oxidative degradation, for example, a 200 W and 10 min ultrasonic treatment followed by SC CO2 extraction process increased oil yields by 3.3%, whereas further increasing the duration of ultrasonic treatment lead to an undesirable outcome [69]. Ultrasonic pre-treatment of ginger rhizome (40 kHz, 40 °C, 1 h), followed by SC CO2 extraction (250 bar, 40 °C and 15 g/min flow rate), resulted in a 110% improvement in extract yield as compared with controls [70]. The application of various extraction processes as SFE-adjuncts are presented in Table 3.
Table 3.
Application of various extraction processes as SFE-adjuncts.
| Plant Source | Pre-Treatment | SC CO2 Protocols | Extract Yield | References |
|---|---|---|---|---|
| Microwave-assisted SFE (MASFE) | ||||
| Moringa oleifera seeds | 100 W for 30 s | 40 °C, 300 bar | 11% higher yield | [64] |
| Enzymatic-assisted SFE (EASFE) | ||||
| Black pepper | amylase | 60 °C, 300 bar, 2 L/min flow | 53% increase yield with 46% higher piperine-enriched extract | [65] |
| Pomegranate peel | cellulase, pectinase and protease (2:1:1) | 55 °C, 33 bar, 30–120 min, 2 g/min flow rate | vanillic acid (108.36 mu g/g), ferulic acid (75.19 mu g/g), and syringic acid (88.24 mu g/g) content in the extract | [66] |
| Black tea leftover | kemzyme (2.8% w/w at 45 °C, pH 5.4 for 98 min) | 55 °C, 300 bar, 0.2–2 g/min flow rate, 30–120 min, ethanol as co-solvent | five-fold increase in extract yield | [67] |
| Tomato peel | glycosidase | 500 bar, 86 °C, 4 mL/min | three-fold increase in lycopene yield | [68] |
| Ultrasound-assisted SFE (UASFE) | ||||
| Zinger | 300 W, 20 kHz | 40 °C, 160 bar, 4–8 mm particle size, | 30% higher yield | [71] |
| Clove | 185 W | 32 °C, 95 bar, 0.233 × 10−4 flow rate, 115 min | 11% higher clove oil with 1.2 times higher α-humelene | [72] |
| Korean perilla | 750 W, 25 kHz for 125 s | 25 °C, 100 bar, 1 h; ethanol as co-solvent | 53% increase of luteolin and 144% increase of apigenin | [73] |
| Capsicumbaccatum | 600 W | 40 °C, 250 bar 1.7569 × 10−4 kg/s flow rate, 80 s | 45% higher yield with 12% increase in capsaicinoid | [74] |
| Capsicumfrutescens | 360 W | 40 °C, 150 bar, 1.673 × 10−4 flow rate, 1 h | 77% higher yield | [75] |
|
Hedyotis
diffusa |
185 W | 55 °C, 245 bar, 95 s | 11–14% higher yield | [76] |
| Almond oil |
20 kHz | 55 °C, 280 bar, 55.6 × 10−4 flow rate, 510 s | 24% higher yield | [77] |
5.4. SFE of Bioactive Compounds
Various essential oils present in these aromatic herbs and spices are the principal compounds affecting the flavor and fragrance of food and pharmaceutical products, owing to the presence of oxygenated compounds such as phenols, ketones, aldehydes, acids, alcohols, ethers, acids, and esters, along with mono and sesquiterpenes hydrocarbons [34]. The flavor and aroma of food products has significant impact on its sensory attributes and acceptance by consumers. Various aromatic herbs or spices are added during their preparation to improve their flavor and aroma. These compounds also form a significant part of the global market of natural products. SFE is increasingly being applied in the food, pharmaceutical and cosmetic sectors as suitable alternatives to traditional solvent or steam distillation [35], the latter possessing lower selectivity, is energy-intensive/expansive, and exhibits a loss of volatile compounds during the extraction process [78]. The extraction of various bioactive compounds by using SFE technology are presented in Table 4.
Table 4.
Extraction of bioactive compounds from plant biomass by using supercritical fluids.
| Plant Source | Extraction Medium | Extraction Protocols | Bioactive Compounds in the Extract | Remarks | Reference |
|---|---|---|---|---|---|
| Roasted peanuts | SC CO2 | 96 bar, 50 °C, fluid density-0.35 g/mL | 74 flavor compounds (8–86 µg/kg) as hexanol, benzene acetaldehyde, methyl and ethyl pyrazines, methyl pyrrole, ethyl pyrazine, methyl pyrazines identified in the extract | Increasing roasting temperature and time significantly improved flavor compounds, with carboxylic acid becoming the most prominent | [38,39,79,80,81] |
| Coffee beans | SC CO2, 9.5% ethanol | 200 bar, 100 °C | 79% efficiency for acrylamide without affecting caffeine content in coffee | Temperature variation affected the extraction efficiency | [82] |
| Cumin | SC CO2, toluene as static modifier | 550 bar, 100 °C | Cumin essential oil concentration ranging from 1.74 to 3.51% (v/w) | Significantly decreased the extraction time from 8 h to 2 h | [83] |
| Turmeric | SC CO2, ethanol, isopropyl alcohol | In a fixed-bed extractor 300 bar, 30 °C | Increased curcuminoid content to 0.72% at 50% co-solvent without compromising extract yield percent (10%) | The best solvent mixture was 50% with 1.8 bed height/diameter ratio | [84] |
| Hyssop | Methanol (1.5% v/v) | 101.3 bar, 55 °C, 30 min dynamic and 35 static time for sabinene | Sabinene (4.2–17.1%, w/w), iso-pinocamphone (0.9–16.5%), and pinocamphone (0.7–13.6%) | Composition of essential oil varies with extraction protocols | [85] |
| Black pepper | SC CO2 | 75–150 bar, 30–50 °C, particle size (0.5, 0.75 mm and whole berries) | Smaller particle size increase yield, Higher sesquiterpene concentration in SFE | Increase pressure and decrease temperature increase extract yield | [86] |
| Long pepper | SC CO2 with 10% ethanol, 10% methanol | 400 bar, 40–70 °C | Piperovatine (0.93%) > palmitic acid > pentadecane > pipercallosidine | Drying leaves reduced amide concentration, the highest yield of piperovaltine by taking fresh leaves | [87] |
| Orange oil | SC CO2 | 131 bar, 35 °C, 2 kg/h flow rate | Increase concentration of oxygenated flavoring compounds (20 times more decanal) | Low temperature and flow rate improve fractionalization | [88] |
| Hop | CO2, ethanol, and water | 111. 4 bar, 50 °C, 0.5 g/mL density | Highly concentrated oxygenated sesquiterpenoids | Reducing bitterness by decreasing lupulone and humulone | [89] |
| Eucalyptus globus | SC CO2, ethanol (0–0.5%) | 200 bar, 40 °C | 1.2% extraction yield, 50% concentration of triterpenic acid (5.1 g/kg of bark) with methyl 3-hydroxyolean-18-en-28-oate most abundant | About 80% more yield than conventional Soxhlet extraction method | [90] |
| Polygala senega and Acorus tatarinowii | SC CO2 | 450 bar, 35 °C, 2 h | 24 compounds with 6 compounds (eugenol, beta-asarone, ethyl oleate, 1,2,3-trimethoxy-5(2-propenyl)-benzene, 6-octadecenoic acid, and 9–12-octadecadienoic acid) had more than 1.0% | Herb combinations increase the bioactive compounds with less compounds with one benzene ring compounds | [91,92] |
| Frankincense (Boswellia carterii) | SC CO2 | 200 bar, 55 °C, 94 min | The volatile oil contains 80% octyl acetate | SC CO2 extraction as the optimum extraction method | [93] |
|
Cannabis sativa var. indica |
Ultrasound extraction with cyclohexane and isopropanol solvent | 100 bar, 35 °C, 1 mL/min | Isopropanol/cyclohexane 1:1 mixture, cycles 3 s, amplitude (80%) and sonication time (5 min) at 100 bar, 35 °C, 1 mL/min, no co-solvent for the terpenes and 20% of ethanol for the cannabinoids | Three monoterpenes and three cannabinoids were quantified in the ranges of 0.006–6.2 μg/g and 0.96–324 mg/g | [94] |
| Croton zehntneri | SFE CO2 | 66.7 bar, 15 °C | €-anethole α-muurolene, methyl chavicol or estragole, germacrene | Maximum solubility and yield at 20 °C | [95,96] |
| Bay laurel Laurus nobilis berries | SC CO2 | 90–250 bar 40 °C | €-β-ocimene (20.9%), α-pinene (4.2%),1,8-cineole (8.8%), β-longipinene (7.1%), α-bulnesene (3.5%) | 15% yield, extraction at 250 bar produced an odorless liquid fraction with dominant triacylglycerols | [97] |
| Spearmint (Mentha spicata) | SC CO2, ethanol and ethyl acetate | 50 °C and 300 bar | Carvone, 1,8-cineole, pulegone | Ethanol co-solvent has a maximum yield | [98] |
| SC CO2 | 90 bar, 45 °C, 5 mL/s flow rate, 120 min dynamic time; 90 bar, 35 °C, 250 μm, 1 mL s−1, and 30 min | 500 μm particle size has highest yield | 2.03% extract yield and CO2 concentration 0.033 mg/mL | [99] | |
| Ocimum basilicum (sweet basil) | Hydrodistillation and SC CO2 | 100–120 bar, 40–50 °C | Four times higher percentage of 1,8-cineole, 5–8 times, linalool, 1-2-fold eugenol, 28-fold germacrene | Higher t-cadinol and sesquiterpenes in essential oil | [100] |
| Clove basil (O. gratissimum |
SC CO2 | 90–128 bar, 25–50 °C; 0.05–0.35 g/min flow rate | Eugenol (35–60%) and β-selinene (11.5–14.1%) | Solvent-to-feed ratio, 16:21; finely ground particle improves yield | [101] |
| Tomato skin and seed | SC CO2 | 300 bar, 60 °C, 0.16, 0.27, 3–8 h, 0.41 g/min flow rate | 86% recovery of E-lycopen | Solvent to solid ratio 220 g CO2/g | [102] |
| Passion fruit bagasse | SC CO2 | 50–60 °C, 170–260 bar, 20.64 g/min flow rate | 1.5- and 5.8-times higher tocopherols and carotenoids | SFE applied in the second stage improved the efficiency | [103] |
| Winter melon | SC CO2, ethanol | 244 bar, 46 °C, 10 g/min flow rate, 97 min, 0.5 L extractor dimension | 176 mg extract/g dried sample | Antioxidant activity of extract higher than obtained by ultrasound-assisted extraction or Soxhlet extraction | [104] |
| Cannabis sativa | SC CO2 | 300–400 bar, 40–60 °C, 1.94 kg/h flow rate | 125.37 µg/g tocopherol in extract | 2–3 times higher gamma-tocopherol content and higher alpha-tocopherol | [105] |
| Carica papaya fruit | SC CO2 | 200 bar, 80 °C, 16.45 mL/min flow rate, 3 h | Benzyl isothiocyanate (anthelmetic), carpaine and pseudocarpaine | Solvent/solid ratio 1180.4 g CO2/g | [106] |
| Camelina sativa | SC CO2 | 450 bar, 70 °C, 1 L/m flow rate, 510 min | Alpha-linoleic, oleic, eicosaenoic and erusic acids, higher phytosterol content | Solvent/Solid Ratio (gCO2/g)-16.14 | [107] |
| Quinoa seed | SC CO2 | 185 bar, 130 °C, 0.175–0.45 g/min flow rate, 3 h, 1.2 mL extractor size | Four-fold increase in tocopherol content (336 mg/100 g oil) with SFE as compared with extraction with hexane | Solvent/Solid Ratio (gCO2/g)-8.02 to 67.5 | [108] |
| Crocus sativus | SC CO2 | 349 bar, 44.9 °C, 10.1 L/h flow rate, 150.2 min | Extraction yield-10.94 g/kg with large amount of unsaturated fatty acid | Solvent/solid Ratio (gCO2/g) 1377.27 | [109] |
| Eugenia uniflora | SC CO2 ethanol (polarity 5.2) and water (polarity-9.0) | 400 bar, 60 °C, 2.4 g/min flow rate, 6 h | Trans-caryophyllene (14.18%), germacrenos bicyclogermacrene (40.75%), Selina epoxide (27.7%) | Solvent/solid Ratio (gCO2/g) 20.09 sequential extraction process most effective |
[110] |
| Moringa oleifera | SC CO2 | 500 bar, 60 °C, 2 mL/min flow rate, 2 h | Selective extraction of 12 bioactive compounds in SFE | Solvent/Solid Ratio (gCO2/g) 37.85 | [111] |
| 350 bar, 30 °C, 20 kg/h flow rate, 5 h, 2 L extractor diameter | Oleic acid (72.26–74.72%), sterol and tocopherol rich extract | Solvent/solid Ratio (gCO2/g) 1329.77 | [112] | ||
| Microwave pre-treatment (100 W, 30 s) followed by SC CO2 | 300 bar, 40 °C, 166.7 flow rate, 210 min, extractor dimensional 1 L | Microwave pre-treatment improves the extraction yield, polyunsaturated fatty acids, oil yield-35.28% w/w | Solvent/Solid Ratio (gCO2/g) 921.23–1000.2 | [64] | |
| Pleurotus ostreatus | SC CO2 | 210 bar, 48 °C, 333.33 g/min flow rate, 1.5 h, extractor dimension-100 mL | Phenol content: 5.48 mg GAE/g (dry weight) with 0.135 g dry weight content |
Solvent/Solid Ratio (gCO2/g) 222,220 | [113] |
| Vine/Humulus lupulus | SC CO2, ethanol, ethyl acetate and compressed propane | 250 bar, 80 °C, compressed propane at 100 bar, 20 °C |
Yield increases to 2.7% in compressed propane and 10.1% in SC CO2-ethyl acetate | Ethyl acetate as a co-solvent improve extraction yield and increases the concentration of bioactive compounds | [114] |
| Catharanthus roseus | SC CO2, ethanol | 159 bar, Flow rate-0.3 mL/min, 8 min | Vincristine (size 5–200 nm) rich extract | Improve bioavailability | [115] |
| Cacao pod husk | SC CO2, ethanol (13.7%) | 299 bar, 60 °C | 0.52% extract yield having 12.97 mg GAE/g extract phenolic contents | Extract enriched in phenolic compounds, green technology | [116] |
| Yacon leaves | SC CO2, ethanol | 250 bar, 70 °C, ethanol to solid ratio-3:1 | High amount of total phenolic compounds and highest ω-6/ω-3 fatty acids ratios | Major unsaturated fatty acid in extract-gamma-linolenic acid, eicosapentaenoic acid and linoleic acid | [117] |
| Tilia flower | SC CO2, ethanol (5–10%) | 220 bar, 65 °C, 15 min | Tiliroside as main flavonoids in the extract | Increase in temperature and pressure increase deficiency | [118] |
| Odontonema strictum leaves | SC CO2, ethanol | 200 bar, 270 min | Three-fold increase in total flavonoid recovery containing 5 major flavonoids | The temperature does not affect extraction | [119] |
| S age leaves | SC CO2 | 150–200 bar, 25 °C, 90 min | High content of α-humulene, viridiflorol, and manool at low pressure (0.24–0.73%) | Pressure as the most critical parameter | [120] |
| Piper leaves | SC CO2, 5% methanol | 220 bar, 80 °C | Germacrene D, pipercallosidine, 14-oxy-α-muuroleno, bicyclogermacrene and (E)-caryophyllene | 40% more yield (1.36% to 2.18%) by using methanol co-solvent | [121] |
| 15 vegetable waste matrices | SC CO2, 15.5% ethanol | 350 bar, 59 °C, 15 g/min flow rate, 30 min | Total carotenoid recovery more than 90% with beta carotene dominant compound (88–100%) | SC CO2 valuable method for carotenoid extraction from vegetable waste | [122] |
| Carrot peel | SC CO2, 14.3% ethanol | 58.5 °C, 306 bar, 30 min | 5.31% yield having 96.2% higher carotenoid recovery | More manageable scale-up of the extraction process | [123] |
| Rosemary | SC CO2 | 3.4–172.4 bar, 40–50 °C, 600 μm particle size | Eucalyptol, camphor, and beta-caryophyllene as principal compounds | Essential oils yield—1.4–2.5 g/100g (w/w), with a higher yield than hydrodistillation | [124] |
| Clove leaves | SC CO2 | 220 bar, 40 °C | Eugenol (30%), chavicol (13%), n-pentacosane (12%), hexacosanal (11%), and vitamin E (9%) | High yield (1.8%) with eugenol as most prominent compound | [125] |
| Radish leaves | SC CO2, ethanol | 400 bar, 35–40 °C | Total phenolic contents-1375–1455 mg GAE/100 g | Extracts exhibiting anti-inflammatory effects | [126] |
SC CO2—supercritical carbon dioxide, GAE-gallic acid equivalent.
6. Pressurized Liquid Extraction (PLE)
Under pressurized liquid extraction (PLE) or accelerated solvent extraction (ASE), solvents at high temperature (25–200 °C) and pressure (up to 200 bar or 20 MPa) are used for the extraction of bioactive compounds. The high temperature and pressure decrease the solvent’s surface tension, which facilitates penetration into the pores of the plant matrix, thereby improving the mass transfer of the active compounds to the solvent [127]. The solvent under pressure remains in a liquid state, even at its boiling point, and facilitates extraction at a higher temperature. Under these conditions, solvents that are not efficient in extracting analytes such as phenolic compounds or anthocyanins under normal conditions may be used for the same extraction. Pressurized solvents have improved, featuring desirable physicochemical properties, such as increased diffusivity, solubility, viscosity and dielectric constant, and these can be further modified by changing temperature and pressure. This is a rapid (completed within 30 min) and efficient process with reduced solvent consumption, but higher temperature-induced damage to heat-labile active compounds [128]. The requirement of large and sophisticated equipment and extraction at higher temperatures are drawbacks to this method.
The use of pressurized extraction technology for extracting bioactive compounds from plant biomass is presented Table 5.
Table 5.
Pressurized liquid extraction of bioactive compounds from plant biomass.
| Plant Source | Solvent | Extraction Protocol | Extract Attributes | Reference |
|---|---|---|---|---|
| Jabuticaba skins | Ethanol | 50 bar, 280 °C, 9 min | 40-fold lower price, 2.15 times higher anthocyanin, and 1.66-fold higher phenolic content | [128] |
| Cranberry waste | Water, acidified water, ethanol, ethanol-water (50% v/v) | Ethanol and water at 100 °C | Total phenolic-7.36 mgGAE/g | [129] |
| Gooseberry | Water | 16 bar, 52 °C, 51 min | 11.68% yield of polysaccharides with high content of arabinose and glucose | [130] |
| Pepper | Water | 200 bar, 120–240 °C, 10–20 min |
113% higher extract yield as compared with conventional Soxhlet extraction | [131] |
7. Ultrasound-Assisted Extraction (UAE)
Plant biomass, exposed to high-intensity ultrasonic waves (20 kHz–100 MHz), results in tiny cavitations/bubbles around the cells. These bubbles suddenly collapse during the procedure, producing shockwaves that disintegrate the cell wall and release its intracellular contents, thereby improving the release of target compounds. This technology is simple to operate, has relatively inexpensive extraction systems, increases mass transfer, increases kinetics, yields bioactive compounds, and is suitable for a wide range of solvents for industrial applications [132]. There are two main physical phenomena in the UAE viz. diffusion of solvent through the cell wall and dissolving cell content after breaking the cell wall.
Ultrasound-treated plant biomass increases the extraction yield by fragmentation or reduction of particle size; erosion and improved accessibility to the solvent; sonocapillary effects, by increasing the velocity and depth of penetration of the solvent through pores and canals; sonoporation, by increasing cell membrane pore size; local shear stress by friction between the liquid molecules; detexturization; and combinations of these methods [133]. UAE efficiency is affected by the temperature of solvent, pressure, the frequency of ultrasound waves/energy, and the sonication time [134]. This method is relatively easy to use and can be suitable/applied for/to a range of plant matrices, features tunability, and requires low capital cost compared with other methods.
Ultrasound can be applied directly to the extraction medium by a probe, which amplifies the intensity of the ultrasound waves (up to 100 times), whereas indirect ultrasound treatment is given by using an ultrasound water bath, wherein the water acts as a transport medium for the ultrasound waves [135]. Ultrasound-assisted extractions of bioactive compounds from plant biomass are presented in Table 6.
Table 6.
Ultrasound-assisted extraction (UAE) of bioactive compounds from plant biomass.
| Plant Source | Solvent | Extraction Protocol | Remark | Reference |
|---|---|---|---|---|
| Eucommia oliver | Hot water extraction followed by UAE | 1:20 solid to liquid ratio, room temperature, 1 h | High yield with a higher concentration of natural antioxidants | [136] |
| Black chokeberry fruit | Ethanol (0–50%)-water | 20–70 °C, 0–100 W, up to 4 h | High temperature and ethanol increased yield | [137] |
| Avaram shell | Distilled water with ultrasound probe | 100 W with magnetic stirring (85 rpm), 5 h | 1.6 times higher extraction of condensed tannin | [138] |
| Orange peel | 4:1 ethanol-water | 150 W, 40 °C | Increase yield of extract (11%) with higher polyphenols | [139] |
| Moringa oleifera | Ethanol: water (1:1) | 40 °C, 15 min | Phenolic acids most prominent in extract | [140] |
| Artichoke residues | 50% Ethanol | 240 W, 10 min | 95% higher retention of chlorogenic ac | [141] |
| Pine waste | Water | 40 °C, 0.67 W/cm2 sonication intensity, 43 min | Pine sawdust as potential source of polyphenols (40% higher) | [142] |
| Pine seeds | Water with 0.2 N NaOH | 25 °C, 30 KHz, 1 h | 30% higher phenolic compounds | [143] |
| Flax seed | 70–80% ethanol | 30 °C, Material-solvent ratio of 0.55 g/mL |
Higher content of azadirachtin | [144] |
| Piteguo fruit | Water | 70 °C, 230 W, 13:1 mL/g solvent solute ratio | 5.16% higher yield of extract | [145] |
| Zizyphus lotus fruit | 50% ethanol | 63 °C, 25 min, 67 mL/g solvent-solute ratio | High phenolic compounds (40.782 mg gallic acid equivalents/g dry matter) with higher antioxidant activity | [146] |
| Black mulberry fruit |
Water | 69 °C, 190 W, 40:25 solvent-solute ratio | Higher yield (3.13%) | [30] |
8. Microwave-Assisted Extraction (MAE)
Microwaves (300 MHz–300 GHz frequency, 1 mm–1 m wavelength) penetrates food materials, agitating water molecules and charging ions, leading to non-contact heating. It enables faster heat transfer by converting electromagnetic energy to thermal energy through ionic conduction and dipole rotation [147]. This technique is commonly used to extract active ingredients, by combining microwave heating and traditional solvent-extraction techniques. This combination of microwave heating and conventional solvent extraction has several merits over conventional extraction methods, such as less use of solvents with better yields; it is afast process; it’s economics are better as it is a fast and efficient process with less energy consumption and carbon dioxide emissions [148]. Due to these procedural and technological advantages, this technology has become a popular method for extracting substances from plant materials.
At present, several advanced technological innovations have been incorporated in this method, such as pressurized microwave-assisted extraction (PMAE), and solvent-free microwave-assisted extraction (SFMAE). The microwave-assisted extraction resulted in a significant increase in apple-pomace-extract yield with active ingredients (55%), as compared with other extraction methods, such as ultrasound-assisted extraction (33%), pressurized liquid extraction (33%), and maceration (43%) [149]. Microwave-assisted extraction of bioactive compounds from plant biomass are presented in Table 7.
Table 7.
Microwave-assisted extraction of bioactive compounds from plant biomass.
| Plant Source | MAE Protocols | Remark | Reference |
|---|---|---|---|
| Terminalia bellerica | 100 °C, 40 mL/g, solvent-solid ratio in water | Maximum flavonoid yield (25.21 mg/g) with water with 82.74% recovery as compared with 63.75% in conventional methods | [150] |
| Citrus unshiu fruit peel | 140 °C, 1 kW, 2.45 GHz, 8 min in 70% ethanol | 47.7 mg/g hesperidin (86.8% higher yield) | [151] |
| Dragon fruit peel | 100 W, 35 °C, 8 min | 9 mg/L betalains (food additive, stable at broad pH and stable at low acidic food) | [152] |
| Chokeberries | 300 W, 53.6% ethanol, 5 min | The highest yield of phenolic compounds (420.1 mg GAE/100 g) | [153] |
| Passion fruit skin peel | 628 W, 9 min | Tartaric acid as best extracting agent for pectin, acetic acid, and nitric acid as agents for pectin extraction with better properties | [154] |
| Citrullus lanatus fruit rind | 477 W, 128 s, solvent-solute ratio 1:20, 20.3 g/mL | Highest pectin yield (25.79%), hydrodiffusion microwave as the green and efficient extraction process | [155] |
| Boldo leaves | 200 W, 56 min, 7.5% solid-solvent ratio | Efficient extraction of volatile and non-volatile organic compounds | [156] |
9. Pulsed Electric Field Assisted Extraction
Pulse electric field (PEF) is a novel, environment friendly, non-thermal and green technology. Its application increases the cell membrane permeability and mass-transfer rate. It has wide application in the meat industry, such as accelerated curing, drying and freezing, meat tenderization, novel product development, and restructured meat products. The application of PEF is performed by placing the food items between or passing it through two electrodes while applying a very high-voltage electric field for a very short duration [157]. To achieve a visible PEF effect on preservation and quality of food—the purpose here underconsideration—electric pulses of 20–1000 µs (several nanoseconds to several milliseconds) are required in an electric field several orders of magnitude strong (0.1–80 kV/cm) [158]. The overall efficiency of PEF depends upon the applied field strength, temperature, and energy-delivery efficiency. During the application of PEF, irreversible structural changes occur in cells’ membranes, leading to a marked increase in cell permeability and an enhanced mass-transfer rate across the membrane. These two factors ultimately result in the breakdown of cellular tissue [159].
This technology could be very useful in recovering bioactive compounds of more specificity from plant matrices more economically and with a low environmental impact, by softening and disrupting the cell membrane, resulting in the release of intracellular compounds [160]. Pulse electric field-assisted extraction can utilize renewable, alternative, efficient, and less toxic solvents obtained from plant resources, such as water and agri-solvents, which exhibit lower energy consumption, and produce higher quality and purity yields. Parniakov et al. [161] applied pulse field-assisted extraction of bioactive compounds from the Agaricus bisporus mushroom and obtained mushroom extracts with higher polysaccharide content and high purity with less energy. Boussetta et al. [162] also noted increased yield and improved extraction efficiency, even at low temperatures and with less solvent, in this method. It facilitates easier removal of bioactive compounds from the plant matrix without damaging it and facilitates segregation and purification later. Table 8 depicts the application of pulse electric field extraction of bioactive compounds from plant biomass.
Table 8.
Pulse electric field (PEF)-assisted extraction of bioactive compounds from plant biomass.
| Plant Source | PEF Extraction Protocol | Remark | Reference |
|---|---|---|---|
| Orange peel | 60 μs (20 pulses of 3μs), 7 kV/cm | Improved naringin and hesperidin, total phenolic compounds increased up to 192% | [163] |
| Button mushroom | 85 °C, 38.4 kV/cm | Increased yield of polysaccharides, phenolic compounds, and protein, a synergistic effect of temperature and electric pulses | [164] |
| Grape juices | 1.5 kV/cm, electric conductivity-20 mS/cm, 50 Hz | Increasing anthocyanin, Vitamin C, and bioactive compounds having higher antioxidant potential | [165] |
| Borago leaves | 300 Hz, 30 kV, 200 A current, | Polyphenol and antioxidant potential increased between 1.3 and 6.6-fold and from 2.0 to 13.7 fold, respectively as compared with conventional methods | [166] |
| Apple juice | 3 μs, 3 kV/cm, electric conductivity-2.3 mS/cm | Higher polyphenols content and reduced processing time as compared with conventional methods | [167] |
Although PEF systems have low maintenance costs, high efficiency, and are fast, their exorbitant initial capital cost is still prohibitory. With the introduction of new technologies and the scaling-up of production, its cost is decreasing steadily and finding wider acceptance by the food industry.
10. Miscellaneous
The other extraction methods are high-voltage electric discharges and high hydrostatic pressure. The high-voltage electric current is applied through an aqueous solution through electrodes, inducing an avalanche of electrons, bubble cavitation, and pressure shock waves, causing cell damage and resulting in the release of bioactive compounds in a process similar to ultrasound [134,160]. The electric energy leads to chemical electrolysis and free radical formation, which may react with bioactive compounds, leading to reduced antioxidant activity of the extracts [161].
The high-hydrostatic-pressure method is seen as an alternative to thermal processes in improving the microbiological quality of food products. It is a green technology, involving thermal treatment and increased mass transfer rates and metabolites movement [168]. High-pressure treatment leads to protein denaturation by deprotonation and breakage of hydrophobic linkages and salt bonds, improving permeability and diffusion of the solvent, resulting in a higher yield of bioactive compounds [169].
Thus, the selectivity and yield of traditional and novel green extraction techniques depend on the proper selection of extraction protocols, such as critical input parameters, the nature and state of the plant biomass, the structure and heat sensitivity of its bioactive compounds, the yield of its extracts, and the equipment needed. However, these technologies should be commercialized and developed on a large scale, with suitable technological advancements and better, safer, and greener solvents to maximize benefits for the food, pharmaceutical, and nutraceutical sectors. Higher capital costs or larger initial investments are still prohibitive and must be reduced to facilitate their large-scale adoption.
11. Plant Extracts as Natural Antioxidants in Meat
The most common method of applying these extracts in meat processing is mixing them with water (mostly for water-soluble extracts; for organic solvent-assisted extracts, it is advised to mix in vegetable oil or fat) during preparation. It ensures the homogenous distribution of extracts in the product. The overall oxidative stability of products depends upon the concentration of the extracts; the higher the extract concentration, the greater the antioxidant effect. Phenolics, catechins, flavonoids, quercetin, anthocyanin, tocopherol, rutin, chlorogenic acid, lycopene, caffeic acid, ferulic acid, p-coumaric acid, vitamin C, protocatechuic acid, vitamin E, carotenoids, β-carotene, myricetin, kaempferol, chrysin, carnosine, zeaxanthin, sesamol, rosmarinic acid, chlorophyll, carnosic acid, carnosol, and gallic acid are compounds, present in plants, possessing antioxidant potential [170].
The important bioactive compounds present in various plant biomass and their meat application as natural antioxidants are described next.
Moringa oleifera a common vegetable in South Asian and African countries, is widely explored for its use as natural preservatives, owing to its various bioactive compounds viz. rhamnetin, isoquercitrin, kaempferol, kaempferitrin, saponins, triterpenoids, tannins, anthraquinones, alkaloids, and terpenoids [171], with concentration varying with the maturity of the plant and climatic and geographical conditions. M. oleifera is a rich source of protein, provitamins, vitamin C, A and E, zinc, calcium, iron, and potassium along with anti-cancerous agents such as glycerol-1-9-octadecanoate, glucosinolates, isothiocyanates, and glycoside compounds.
Rosemary contains a high amount of rosmarinic acid, carnosol, and carnosic acid in extract, and eucalyptol, α-pinene-bornyl acetate and camphor in rosemary essential oil [172,173]. Rosemary leaves were reported as a rich source of vitamin C, as much as as 18.51 g/100 g raw materials and extracts (0.26 mg/100 mL aqueous, 0.34 mg/100 mL alcoholic, and 0.36 mg/100 mL acetonic) have been reportedly obtained [10]. Monoterpenes hydrocarbons, esters, oxygenated sesquiterpenes, phenol, sesquiterpene hydrocarbons, oxygenated monoterpenes, ketones, and alcohol are the primary flavoring compounds of rosemary [173]. Generally, camphor, caryophyllene, borneol, bornyl acetate, and verbenone are chief compounds present in rosemary extracts. Peng et al. [174] advocated the application of supercritical fluids for the extraction of rosemary extracts, as it is associated with saving time, maximizing yield, and inhibiting the conversion of carnosic acid into carnosol. For rosemary stems, flowers, and leaves extracts, a suitable extraction methodology must be adopted, as lipophillic solvents and water (in the case of fresh samples were reported to result in a significant loss of phenols due to the action of phenoloxidase enzyme [175,176].
Arjuna or Arjun tree (Terminalia arjuna) bark, stem, leaves, roots, and fruits are a rich source of various beneficial bioactive compounds (polyphenols, flavonoids, triterpenoids, tannins, glycosides, sitosterol) and minerals. The Arjuna tree bark was reported to have the highest concentration of flavonoids such as arjunolone, quercetin, flavones, kaempferol, baicalein, and pelargonidin. Ethanolic and aqueous arjuna fruit extracts have been reported to contain a good amount of total phenolics (11.04–16.53 mg gallic acid equivalents/g), exerting significant scavenging activity (50.02–58.62%) [177].
Cinnamon bark is considered a promising antioxidant source, even exhibiting antioxidant potential comparable to synthetic antioxidants [178,179]. Most of the bark available in the market is Ceylon bark; dried bark (cork and cortex) of shoots of tree Cinnamomum zeylanicum F. lauraceae, containing approximately 0.5–1.0 % of volatile oil made up of 50.5% cinnamaldehyde, 8.7% cinnamic acid, methoxycinnamaldehyde and cinnamyl acetate, and 4.7% eugenol [178]. Chan et al. [180] reported ahigher antioxidant efficacy of deodorized cinnamon in meatballs, and noted significantly reduced lipid oxidation without causing any detrimental effects to its sensory attributes. Spices in the Lamiaceae family contain high rosmarinic acid, the major phenol (ranging from 1086–2563 mg/100 g of dry-weight) [181]. Rosmarinic acid possesses strong antioxidant activity due to two ortho-dihydroxy groups in its structure. This compound can act as an antioxidant and control the oxidation of low-density lipoproteins (LDLs). Rosmarinic acid also possesses anti-inflammatory activity by stimulating interleukin-10 (IL-10) secretion [182].
Several studies have confirmed the anti-inflammatory activity of oregano extracts, which are aqueous extracts observed to inhibit cyclooxygenase-2 (COX-2) secretion in epithelial carcinoma cells [183], and to exhibit anti-inflammatory properties by controlling stress-induced gastritis and hypersensitivity [184]. Oxidative stress leads to incorrect protein folding in the endoplasmic reticulum. Kaempferol, an aglycone flavonoid widely present in aloe vera, ivy gourd, saffron coccus and Peking spurge, is known to prevent hepatocellular carcinoma by controlling oxidative stress caused by reactive oxygen species [185]. Five bioflavonoids obtained from moss fern (Selaginella doederleinii) were observed to inhibit non-small cell lung cancer cells by suppressing XIAP and survivin expression, increasing the upregulation of caspase-3/cleaved-caspase-3, inducing cell apoptosis in A549 cells with low toxicity to non-cancer cells MRC-5 cells [186]. The Kalmia angustifolia extract exerted antioxidant, anti-inflammatory and anti-aging effects, at concentrations up to 200 μg/mL, by enhancing the expression of elastin and collagen-1 [187]. Flavonoids, such as apigenin, myricetin, and luteolin, were observed to exert anti-cancer effects against a range of human epithelial cancers by selectively reducing the viability of cancer cells, the alteration of ROS signaling, and the arrest of cell multiplication [188]. The extract of Polyalthia spp. is known to exert antioxidant, anti-ulcer, anti-plasmodial, anti-cancer, anti-microbial and anti-inflammatory effects due to the inhibition of COX-2 activity, inhibiting downstream prostaglandin E2 (PGE2) production, and inhibiting focal adhesion kinase, phosphoinositide 3-kinase, 3-hydroxy-3-methylglutaryl co-enzyme A reductase, and dipeptidyl peptidase 4 [189].
Clove exerts the most potent antioxidant activity among all spices and condiments commonly used in the food industry. Carnosic acid and carnosol, tocopherols, carotenoids, and sterols are important active ingredients present in herbs. Clove extracts mainly contain eugenol, caryophyllene, and eugenyl acetate. These two compounds have strong antioxidant potential and are considered the main bioactive compounds responsible for the increased antioxidant activity of clove, equivalent to vitamin E [190]. While processing, n-hexane solvent was recorded to produce a better yield, with high antioxidant activity (total flavonoid content- 15.54 mg GAE/g, total polyphenol content-54.05 mg GAE/g, FRAP = 0.69 mg/mL, DPPH = 0.29 mg/mL), and antimicrobial activity as compared with extracts obtained with other solvents (alcohol, water, and petroleum ether) [191]. Clove extract, at 0.25%, reported exerting antimicrobial activity with an 8.8 mm-to-9.27 mm minimum inhibitory concentration, as seen in S. typhimurium and E. coli, respectively [191].
Citrus byproducts/coproducts viz. peel and pulp are a rich source of various active ingredients, such as dietary fiber, minerals, vitamins, organic acids, flavonoids (ferulic, sinapic acids, and chlorogenic), phenolics (hesperetin, hesperidium, diosmin, and narirutin), carotenoids (carotene, zeaxanthin, lutein) [192,193,194,195]. These compounds are applied in the meat industry due to their associated health effects, such as antimicrobial, antioxidant, anticancer, anti-allergic, and antihypertensive effects [196]. The antioxidant activity of different citrus extracts varies with the methodology of their extraction, their fruit type, their environmental conditions, such as soil type and climate, their fruit-ripening stage, and their harvesting time [197,198]. Nayak et al. [199] reported the high antioxidant potential of Citrus sinensis peel extracts obtained by using microwave and ultrasound-assisted accelerated extraction technology at 337.16–433.09 mL/L DPPH (50% inhibition). Aqueous and methanolic extracts of lemon pomace had high antioxidant potential, wherein the methanolic extract exhibited higher antioxidant potential as compared with the aqueous extract, as measured in terms of DPPH assay (0.17, 0.13 mg Trolox equivalents/g, respectively) and ABTS (0.403, 0.458 mg Trolox equivalents/g, respectively) [200].
Catechin, present in green tea, is a group of flavonoids (flavan-3-ols), especially epicatechin, epicatechin-3-gallate, epigallocatechin (EGC), and epigallocatechin-3-gallate (EGCG), with EGC and EGCG regarded as the main active principle in green tea, and which is studied for its various antioxidant effects in food processing. The incorporation of tea catechins at 300 ppm in beef, duck, ostrich, pork, and chicken markedly reduced their TBARS values during refrigerated storage and was noted to exert 2–4 times higher antioxidant potential as compared with vitamin E [201]. Catechins reduced the production of putrescine and tyramine in dry, fermented pork sausage. Green tea catechins (GTC) and green coffee antioxidants (GCA) in linseed oil and fish oil, added in pork sausage at 200 mg/kg, decreased the lipid peroxidation of the sausages during seven days refridgerated storage. A significantly decreased lipid oxidation rate and higher organoleptic attributes were recorded in the fish- oil-substituted sausages [202].
Grape seed extract has a larege amount of polyphenols (such as epicatechin, gallic acid, resveratrol, and procyanidin dimers) and was reported to exert high antioxidant efficacy [203]. The antioxidant activity of grape seed is reported to be 20–50 times greater vitamins E and C [204], due to its possessing proanthocyanidins and oligomers of flavan-3-ol units. Grape seed extract (GSE) is the richest known source of polyphenolic compounds (catechins, flavanols, phenolic acids, anthocyanins, and proanthocyanidins) and has 20–50 times more antioxidant potential as compared with vitamins E and C, respectively [205,206]. Libera et al. [207] prepared grape seed extract by heating a grape seed slurry in water at 50–60 °C under high pressure (100–175 hPa or 10000–17500 bar). The authors evaluated the antioxidant potential of grape seed extract by incorporating it into pork neck containing Lactobacillus rhamnosus LOCK900 and reported that the lipid oxidation (PV-1% oleic acid and TBARS-0.46 mg MDA/kg) in the extract-incorporated samples was comparable to samples containing sodium ascorbate (PV-0.9% oleic acid and TBARS-0.53 mg MDA/kg).
The aqueous extract of Cantharellus cibarius hads high antioxidant potential due to its high concentrations of polyphenols, beta-carotene, and ascorbic acid, but has lower ABTS radical scavenging potency than vitamin C in cases where the extract is prepared by the water-decoction method i.e., first smashing and grinding, followed by boiling in water to obtain the extract [208,209]. The incorporation of Cantharellus cibarius water decoctions during the preparation of frankfurters, at 0.75- and 1.5% levels, resulted in significantly reducing the total plate counts with potent inhibitory action against Candida albicans, and improved the sensory attributes of the frankfurters during 60 days of storage under refrigerated conditions [209].
The ethanolic extract of mesquite leaf of Proposis was reported to exert high antioxidant potential due to its total phenolic content (278.5 mg GAE/g) and total flavonoid content (226.8 mg RE/g). The incorporation of the extract (0.05–0.10%) during the preparation of pork patties resulted in significantly improved oxidative stability of the treated patties as measured by theie marked reduction in TBARS value (90%) and conjugated dienes (40%) as compared with the positive control, i.e., patties with BHT [210]. The Carob (Ceratonia siliqua L.) tree is an underutilized tree of the Mediterranean region. Carob has high dietary fiber content and has 1.2–7.0% polyphenolic compounds, especially catechins, myricetin, rutin, and gallic acid [211].
The various plant extracts utilized in meat as natural antioxidants are presented in Table 9.
Table 9.
Plant extracts as a natural antioxidant in meat.
| Plant Source | Extraction Protocol | Experimental Design (Level, Meat Product, Storage Temp, Days) | Significant Outcome (Extract Quality and Its Antioxidant Effect on Incorporation in Meat) |
Reference |
|---|---|---|---|---|
| Cinnamon barks | Ethanol (90%), 60 °C, 9 h | 0.25%, chevon rolls, 4 ± 1 °C, 35 days | Overall acceptability of treated rolls was higher than control, significantly (p < 0.05) lower TBARS, FFA, PV, SPC, and psychrophilic count | [13,14,212] |
| Papaya leaves | Ethanol (60%), 65 °C, 15 min | 0.5%, chevon emulsion, 4 ± 1 °C, 9 days | TBARS, FFA and PV (p < 0.05) higher in control than treatments | [213,214] |
| Terminalia arjuna bark | Ethanol (60%), 10 min at 75 °C | 1.0%, pork emulsion, 4 ± 1 °C, 9 days |
2.5-fold reduction in TBARS value than control (0.79 from 1.75 mg malonaldehyde/kg), better colour stability (L *, a *, b * values) |
[215,216] |
| Terminalia arjuna fruit | Ethanol-water (60:40), 27 °C ± 1 °C, overnight, vortex shaking at 400 rpm for 8 h | 1.0%, ground pork, 4 ± 1 °C, 9 days | Higher total phenolics (16.53 mg GAE/g), DPPH IC50—10.37 μg/mL, FRAP-1.33, Metmyoglobin content comparable to BHT added sample and significantly lower than control | [177] |
| Oregano vulgare leaves | Ethanol (60%), 80 °C, 10 min | 1.0%, chevon emulsion, 4 ± 1 °C, 9 days | Total phenolic content-328.71 mg GAE/100 g, SASA-44.49%, DPPH activity-30.72%, improving oxidative and microbial quality of chevon meat | [213] |
| Clove buds | Ethanol (95%), 12 h at 100 rpm, residue again re-extracted | 0.25%, 0.5%, 1.0%, 2.0%, Chinese-style sausage, 4 °C, 21 days | Concentration dependence effectiveness in controlling lipid and protein oxidation, better retention of textural and sensory attributes during storage | [217] |
| Watermelon rind | Ethanol (95%) 25 °C, 24 h at 200 rpm | 0.10%, pork patties, 4 ± 1 °C, 28 days | DPPH (% inhibition)-77.46, ABTS (% inhibition)-75.57, FRAP (mM of Fe++ equivalent/mL)-77.5 and SASA (% inhibition)-47.5; zone of inhibition for S. aureus-5.68 mm | [218] |
| Sea buckthorn seeds | Methanol (60%), 55 °C, 20 min | 0.30%, ground pork, 4 ± 1 °C, 9 days | TPC-128.23 mgGAE/g, DPPH-66.11% inhibition, ABTS-87.13% inhibition, significantly lower TBARS, FFA and PV in treated samples | [219] |
| Moringa oleifera leaves | Water for 18–20 h at 40–50 °C | 0.10%, goat meat patties, 4 ± 1 °C, 15 days | TPC-48.36 mgGAE/g, TFC-31.42 mg/g, Lower TBARS value on 15 th day of storage in treated sample-0.53 mg malonaldehyde/kg | [220] |
| Boiled distilled water, 5 min | 450–600 ppm, raw and cooked patties | TPC-60.78–70.27 mg/g, non-significant reduction in metmyoglobin formation in control and treated samples during storage | [220,221] | |
| Ginger rhizomes, potato peel, seeds of fenugreek | Ethanol (90%), room temperature, 1 h at freeze dried −60 °C | 500–1000 ppm, ground beef patties, 5, 25 & 37 °C, 12 days | Ginger rhizome extract has the highest antioxidant (% inhibition)-(77.4) followed by fenugreek seeds (71.4) and potato peel (59.5) | [222] |
| Garlic ginger and onion | Water, 40 °C,30 min, Ultrasonic extractor (200 W, 40 kHz) | 5–10% ginger-garlic-onion, stewed pork, 4 °C, 12 days | Synergistic effect of combinations of extracts, storage life extended to 5–6 days | [223] |
| Leaves of hyssop and rosemary | Dimethyl sulfoxide for 5 h at ambient temperature | Solution with 5.8 pH, cooked pork meat, 4 °C, 8 days | Hyssop and rosemary extract inhibit lipid oxidation and metmyoglobin formation | [224] |
| Leaves of myrtle, lemon balm, rosemary and nettle | De-ionized water ambient temperature, 15 min | 10% each extract, ground beef, 20 ± 2 °C, 120 days | Inhibited lipid oxidation (lemon and nettle-23–24% lower peroxide value; myrtle and rosemary-33–41%) and protected colour | [225] |
| Green tea and grape seed | Boiling water, 10 min | 500, 3000, 6000 ppm, Baladi goat meat, 5 °C, 9 days | Lower antioxidant capacity of green tea extract (7.5 h) than grape seed extract (9.4 h), plant extract increased the induction time | [226] |
| Red grape pomace | Methanol ambient temp, 10 min, sudden pressure changes to 5 × 103 Pa (N/m2), rotatory evaporator at 200 rpm at 50 °C | 0.06 g/100 g, pork burger, 4 °C, 6 days | TPC-546.0, total anthocyanins-1783.5 mg/L, antioxidant capacity-141.8 mmol/L Trolox, the application of instantaneous high-low pressure increased the extract yield | [227] |
| Wine residues | Aqueous acetone (50%), ambient temperature | 7–15 g/100 g, dried minced pork slice, room temperature, 21 days | Decreased hexanal, TBARS (up to 108%), carbonyls, sulfhydryl loss | [179] |
| Mustard leave kimchi | Ethanol (70%), room temperature, overnight | 0.05%, 0.1% & 0.2%, ground pork, 4 °C, 14 days | Extract at 0.1% and 0.2% having antioxidant effect equal to 0.02% ascorbic acid. MDA concentration below 0.5 mg/kg at the end of storage | [228] |
| Lotus rhizome knot (LRK) and leaf (LL) | Aqueous, room temperature, overnight | 3%, bovine and porcine meat, 4 °C, 10 days | TPC-(LRK-17.0 gGAE/100 g, LL-34.9 g GAE/100 g), TTC-(LRK-13.02 gGAE/100 g, LL-6.02 gGAE/100 g), TFC-(LRK-7.96 g rutin euivalent/100 g, LL-33.0 g rutin equivalent/100 g) | [229] |
| Curry berry | Boiled water for 2 h followed by centrifuge at 5000 rpm for 10 min | 2.5–5.0%, raw chicken meat homogenate, 4 ± 1 °C, 48 days | TPC-9.5 mg TAE/gdw, TFC-11.9 mgCE/gdw; the extract incorporation inhibited oxidative changes in meat | [230] |
| Lychee fruit pericarp | Boiled distilled water, 1 h | 0.50, 1.0 and 1.5%, sheep meat nuggets, 4 ± 1 °C, 12 days | TPC-18.36 mgGAE/g, high anthocyanins content, the extract has good antioxidant potential. | [231] |
| Byproducts of olive, pomegranate, tomato and grape | Water, 60 °C, 2.5 h | 0.1%, lamb patties, 4 ± 1 °C, 7 days | Water extracts exhibited antimicrobial and antioxidant potential, red grape and olive extract (1000 mg/kg) in patties reduced microbial counts | [232] |
| Bamboo shoot | Boiled water with 1% NaCl, 10 min | 6% kordoi juice and 4% aqueous extract, pork nuggets, 4 ± 1 °C, 35 days | TPC-246 mg GAC/100 g, Ascorbic acid-4.1 mg AAE/100 g, The incorporation of extract and kordoi juice extended storage life from 21 days to 35 days |
[233] |
| Colombian berry | Ethanol-water (50:50 v/v), solvent-solute ratio (5:1), 4 °C, lyophilized (0.18 bar, −50 °C) | 250, 500 and 750 ppm, pork patties, 2 ± 1 °C, 9 days, 15–20 lux value | TPC-83976 mg/kg, total anthocyanin content-29077.5 mg/kg, making upto 35%. Extract improved colour stability and oxidative stability in dose dependent manner. | [234] |
| Petals blue pea flower | Spray-dried, vacuum packaged | 0.02–0.16% w/w, pork patties, 4 ± 1 °C, 12 days | TPC-28.8 mgGAE/g, TEAC value of cooked patties-0.10–0.167 mg TE/g; Addition of 0.16% extract protect lipid and protein oxidation during storage | [235] |
| Bee pollen | Ethanol, 40 °C, 1 h, 150 rpm, lyophilized | 0.02%, pork sausage, 4 ± 1 °C, 30 days | TPC-19.69 mgGAE/g, 10 mg/mL can neutralise 91.93% of beta carotene. | [236] |
| Monkfruit | Water, 200 W ultrasound power, 80 °C, 2 h | 7–15 g/100 g, dried minced pork | 98.51% DPPH inhibition at 200 g/L, 34.93% mongroside in extract. Extract delayed hexanal formation, TBARS, carbonyls and sulphydryl loss | [179] |
| Jabuticaba | Water, 60 °C, 6 h, microencapsulated | 2–4%, fresh pork sausage | TPC-15.63 mg GAE/mg, FRAP-20.51μmol equivalent Trolox/g, Extract added fresh sausage as natural colorant had an antimicrobial and antioxidant effect |
[237] |
| Peanut skin |
Ethanol (80%), 60 °C, 50 min; followed by 15 min sonication at ambient temperature | 3.0%, chicken patties 1 ± 1 °C, 15 days | TPC-32.6 mg GAE/g, FRAP-of 26.5 μmol Trolox equivalent/g. Decreased a * values (p < 0.05) and reduced lipid oxidation, with 0.97 malondialdehyde (MDA)/kg as compared with 19 mg MDA/kg |
[238] |
TBARS-thiobarbituric acid reactive substances, FFA-free fatty acid, PV-peroxide vale, DPPH—1,1-diphenyl-2-picrylhydrazyl, ABTS-2-2-azinobis-3ethylbenthiazoline-6-sulphonic acid, GAE–gallic acid equivalent, SPC–standard plate count, MDA–malondialdehyde, FRAP—ferric reducing antioxidant power, SASA–superoxide anion radical scavenging assay, TPC–total flavonoid content, TFC–total flavonoid content, TTC–total tannin content, TAE—tannin acid equivalent, AAE—ascorbic acid equivalent, TEAC value–Trolox equivalence antioxidant capacity, * p < 0.05.
12. Current Scenario
The studied plant extracts showed strong antioxidant activity in meat products, owing to strong H°-donating activity, high radical-scavenging capacity or the ability to sequester metal catalysts. Various studies had been undertaken to standardize the extraction protocols and incorporation of extracts in meat products, and to document the preservative effect of plant extracts, as indicated, by reduced oxidation (in terms of lower TBARS value, PV, FFA value, carbonyl content, and free thiols) and microbial growth inhibition (in term of total plate count, coliform count, psychrophilic count, and food pathogens), inhibiting various carcinogens, reducing levels of heterocyclic aromatic amines, and better preservation/maintenance of sensory attributes (such as freshness, color, juiciness, flavor, and overall acceptability). At present, advanced technologies are being applied (such as supercritical water extraction, microwave-assisted extraction, ultrasonication, vacuum drying, and freeze-drying) to produce high-quality extracts with higher concentrations of active principle, on an industrial scale, for food application.
Various commercial preparations of some commonly used plant extracts are now readily available for application in food processing. The active ingredients or bioactive compounds from plant extracts are concentrated or purified using various ion-exchange adsorptions, followed by elution or chromatography technology. These are prepared using these improved and advanced technologies to obtain the maximum concentration of active ingredients in the extracts and protect any loss or degradation of active ingredients due to undesirable liquids, enzymes, and fermentation leading to alcohol production due to vinification. Angeletti and Sparapani [239] patented a process for the preparation of grape seed extract, containing a lower concentration of monomeric polyphenols and a higher concentration of procyanidin oligomers, by using a combination of acetone and methyl alcohol as a primary solvent in addition to ethyl acetate, methylene chloride, and dichloroethane.
The purified preparations of these plant extracts are now readily available in the market as premier products/dietary supplements/nutraceuticals. These are recommended for various health benefits, such as reducing oxidative stress, maintaining cardiovascular health, lowering LDLs and proper blood pressure, improving memory, controlling atherosclerosis, and anticancer effect. Natural Products Insiders Inc. California, USA had commercial preparation of grape seed extracts with the trade name ORAC-15 M (80% polyphenols and 15000 oxygen radical absorbing capacity) and recommended its use in alleviating the oxidative stress of the body. Other commercial preparations of Natural Products Insiders Inc. are MegaNatural, Enovita, and Leucoselect. Activin, Gravinol-S, Activin, Gravinol Super are some commercial preparations of grape seed extract produced and marketed by Inter Health Nutraceuticals Inc. (Broadfield Park, Crawley RH11 9RT, United Kingdom). Pycnogenol, an extract obtained from pine bark is commercialized by Natural health Science Inc. New York, USA and Horphag Research, Cointrin, Switzerland. It has a higher concentration of procyanidins, phenolic acids, and bioflavonoids, and is recommended for cardiovascular health, skin and eye care, proper brain functioning, and improving respiratory and women’s health. Applied Food Science Inc. has a wide range of commercial preparations of plant extracts, such as green coffee extract (GCE-50% chlorogenic acids from raw coffee beans), green coffee caffeine extract (Java G with 45% chlorogenic acid, 60% polyphenols, and 40% caffeine), organic ginger powder (PureGinger with 2% gingerol), cascara fruit extract (CoffeeNectar from coffee cherry), caffeine from green tea (Purtea), etc. Herbalox Seasoning HT-25 and Herbalox HT-25 are trade names of rosemary extracts manufactured by Kalsec Inc. Kalamazoo, Michigan, USA. Kemin Americas Inc., Iowa, USA, manufactures, who markets the rosemary extract as Fortium R 20.
The consumer preference towards healthier and greener alternatives to synthetic preservatives has been the main driving force of the rapid growth of plant extracts. The global market value of plant extracts was reported to be 43.32 billion USD in 2019 and is forecasted to grow with a compound annual growth rate (CAGR) of 6.0%. The market value of plant extract is expected to reach 66.81 billion USD by 2027 [240]. With the increasing preference of consumers towards green alternatives and minimally processed or natural foods, this sector is expected to maintain high growth in the near future.
13. Prospects and Challenges
As plant extracts are very potent antioxidants and rich in phenolic compounds, there is a high probability that, at higher concentrations or incorporations, these will lead to bitterness or aftertaste and darkened color of the developed product. It is always desirable to explore and use plant extracts, owing to their higher antioxidant ability with minimum effect on the sensory attributes of meat products. Alternatively, combinations of various plant extracts are also used to achieve desired effects without modifying/altering sensory attributes. As flavor and taste are crucial in determining the overall acceptability and acceptance of food products by consumers, these extracts should be added to meat products, if well within the recommended limit/optimum level. Another challenge before the meat industry is the differing outcomes of plant phenolics in in-vitro systems and in the food matrix. It has been noticed that these compounds exert a potent antioxidant effect, even at small concentrations, in in-vitro conditions, but need comparatively higher concentrations in meat model systems, which may result in bitterness and off-flavor. This could be due to interactions between these phenolic compounds with other meat components, such as muscle protein, lipid and minerals [241,242].
The application of plant extracts in the meat industry shows promising results in extending storage life and preserving product quality by significantly inhibiting the oxidation of meat lipids and protein and microbial growth. A proper extraction protocol is critical for obtaining the desired yield percentages and activities of plant extracts that determine their overall effectiveness and success. The high energy and solvent consumption, associated food-safety hazards due to organic solvent residue and waste, are major concerns among food technologists, to the extent that even brand extracts are being prepared from such green alternative methods [241]. The remnants of toxic solvent in plant extracts make them unsuitable for use in the food industry, so to for the loss of active ingredients due to improper high temperature and storage, change in color and flavor, bitterness, increasing cost of production are some important considerations and various food technologists, and researchers are studying these aspects. Further, the current focus of the food industry is on the proper segregation and identification of active principles and assessing their safety levels, toxicity, if any, and optimum levels of incorporation of these active principles in food.
Mbah et al. [243] and Porkorny [244] noted the economical cost, better safety, and easier application of synthetic antioxidants, compared with natural antioxidants, as major factors leading to the higher utilization of these synthetic antioxidants in the food industry. Consumer perceptions about stored products as stale, their preferences towards the use of meat products with shorter shelf lives, without preservatives and minimally processed are some factors limiting the use of these plant extracts in the meat industry. There is a need for exploring a better suitable, efficient, readily available, safer, easy-to-use application; proper labeling; and cost-effective materials for application in the meat industry. Proper and uniform legislation is required for extracts, as some extracts have been classified as safe for consumption since ancient times but nonetheless produce allergic reactions in some consumers upon consumption.
14. Conclusions
Natural extracts are extracted from various plant sources and are rich in several bioactive compounds possessing potent antioxidants. Some of these plant extracts exhibit comparable or even better antioxidant properties than commonly used synthetic antioxidants in the meat industry. Plant extracts should be utilized by following proper extraction protocols to obtain higher yields with higher concentrations of bioactive compounds exerting desired antioxidant effects. Green extraction technologies are proving to be a better and more efficient method for extracting bioactive compounds from plant biomass. However, the high initial cost and lack of scaling-up have proven to be a significant challenge for food technologists and biochemists. The application of combinations of extraction technologies is recommended, such as using conventional means with supercritical fluid extraction, and Soxhlet extraction paired with microwave-assisted extraction due to their synergistic effects. There is also a need for further identification and separation of the active ingredients in plant extracts and their potential toxicological effects and permissible limits in meat products.
Authors Contribution
Conceptualization, A.Q.S., M.F.A.A., S.J., Writing original draft, A.M.A., P.K., Writing-review and editing, A.M.A., P.K., I.F.M.R., S.J., M.F.A.A., A.Q.S. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors would like to acknowledge the Geran Putra—IPS (Vote No.: 9687700) funded by the Universiti Putra Malaysia (UPM) as the financial grant for the research work of Alzaidi Mohammed Awad.
Funding
This research was funded by Universiti Putra Malaysia, through grant number Geran Putra-IPS (Vote No.: 9687700).
Conflicts of Interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumar P., Chatli M.K., Mehta N., Malav O.P., Verma A.K., Kumar D., Rathour M. Antioxidant and antimicrobial efficacy of sapota powder in pork patties stored under different packaging conditions. Korean J. Food Sci. Anim. Resour. 2018;38:593. doi: 10.5851/kosfa.2018.38.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang J., Xiong Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016;120:107–117. doi: 10.1016/j.meatsci.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Chu S.-C., Chen C. Effects of origins and fermentation time on the antioxidant activities of kombucha. Food Chem. 2006;98:502–507. doi: 10.1016/j.foodchem.2005.05.080. [DOI] [Google Scholar]
- 4.Kumar P., Verma A.K., Umaraw P., Mehta N., Rajeev R. Natural extracts are very promising: They are a novel green alternative to synthetic preservatives for the meat industry. Fleischwirtsch. Int. J. Meat Prod. Meat Process. 2020;3:48–57. [Google Scholar]
- 5.Efenberger-Szmechtyk M., Nowak A., Czyzowska A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021;61:149–178. doi: 10.1080/10408398.2020.1722060. [DOI] [PubMed] [Google Scholar]
- 6.Dai F., Chen W.-F., Zhou B. Antioxidant synergism of green tea polyphenols with alpha-tocopherol and L-ascorbic acid in SDS micelles. Biochimie. 2008;90:1499–1505. doi: 10.1016/j.biochi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Moon J.-K., Shibamoto T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009;57:1655–1666. doi: 10.1021/jf803537k. [DOI] [PubMed] [Google Scholar]
- 8.Kumar P., Verma A.K., Umaraw P., Mehta N., Malav O.P. Plant phenolics as natural preservatives in food system. In: Lone R., Shuab R., Kamili A.N., editors. Plant Phenolics in Sustainable Agriculture. Springer; Berlin/Heidelberg, Germany: 2020. pp. 367–406. [Google Scholar]
- 9.Vuong Q.V., Stathopoulos C.E., Nguyen M.H., Golding J.B., Roach P.D. Isolation of green tea catechins and their utilization in the food industry. Food Rev. Int. 2011;27:227–247. doi: 10.1080/87559129.2011.563397. [DOI] [Google Scholar]
- 10.Dumbrava D.G., Moldovan C., Raba D.-N., Popa M.-V. Vitamin C, chlorophylls, carotenoids and xanthophylls content in some basil (Ocimum basilicum L.) and rosemary (Rosmarinus officinalis L.) leaves extracts. J. Agroaliment. Process. Technol. 2012;18:253–258. [Google Scholar]
- 11.Negi P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012;156:7–17. doi: 10.1016/j.ijfoodmicro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Riahi L., Chograni H., Elferchichi M., Zaouali Y., Zoghlami N., Mliki A. Variations in Tunisian wormwood essential oil profiles and phenolic contents between leaves and flowers and their effects on antioxidant activities. Ind. Crops Prod. 2013;46:290–296. doi: 10.1016/j.indcrop.2013.01.036. [DOI] [Google Scholar]
- 13.Rathour M., Malav O.P., Kumar P., Chatli M.K., Mehta N. Storage stability of chevon rolls incorporated with ethanolic extracts of aloe vera and cinnamon bark at refrigeration temperature (4 ± 1 °C) J. Anim. Res. 2017;7:183–190. doi: 10.5958/2277-940X.2017.00025.0. [DOI] [Google Scholar]
- 14.Rathour M., Malav O.P., Kumar P., Chatli M.K., Mehta N. Standardization of protocols for extraction of aloe vera and cinnamon bark extracts. J. Anim. Res. 2017;7:175–182. doi: 10.5958/2277-940X.2017.00024.9. [DOI] [Google Scholar]
- 15.Casazza A.A., Aliakbarian B., Perego P. Recovery of phenolic compounds from grape seeds: Effect of extraction time and solid–liquid ratio. Nat. Prod. Res. 2011;25:1751–1761. doi: 10.1080/14786419.2010.524889. [DOI] [PubMed] [Google Scholar]
- 16.Yeh H.-y., Chuang C.-h., Chen H.-c., Wan C.-j., Chen T.-l., Lin L. Bioactive components analysis of two various gingers (Zingiber officinale Roscoe) and antioxidant effect of ginger extracts. LWT Food Sci. Technol. 2014;55:329–334. doi: 10.1016/j.lwt.2013.08.003. [DOI] [Google Scholar]
- 17.Dai J., Mumper R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao L., Jiang Y., Datta N., Singanusong R., Liu X., Duan J., Raymont K., Lisle A., Xu Y. HPLC analyses of flavanols and phenolic acids in the fresh young shoots of tea (Camellia sinensis) grown in Australia. Food Chem. 2004;84:253–263. doi: 10.1016/S0308-8146(03)00209-7. [DOI] [Google Scholar]
- 19.Khokhar S., Magnusdottir S.G.M. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J. Agric. Food Chem. 2002;50:565–570. doi: 10.1021/jf010153l. [DOI] [PubMed] [Google Scholar]
- 20.Do Q.D., Angkawijaya A.E., Tran-Nguyen P.L., Huynh L.H., Soetaredjo F.E., Ismadji S., Ju Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah M.A., Bosco S.J.D., Mir S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014;98:21–33. doi: 10.1016/j.meatsci.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Turkmen N., Sari F., Velioglu Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006;99:835–841. doi: 10.1016/j.foodchem.2005.08.034. [DOI] [Google Scholar]
- 23.Pateiro M., Gómez-Salazar J.A., Jaime-Patlán M., Sosa-Morales M.E., Lorenzo J.M. Plant extracts obtained with green solvents as natural antioxidants in fresh meat products. Antioxidants. 2021;10:181. doi: 10.3390/antiox10020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chemat F., Abert Vian M., Ravi H.K., Khadhraoui B., Hilali S., Perino S., Fabiano Tixier A.-S. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules. 2019;24:3007. doi: 10.3390/molecules24163007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi Y.H., Verpoorte R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019;26:87–93. doi: 10.1016/j.cofs.2019.04.003. [DOI] [Google Scholar]
- 26.Vekariya R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017;227:44–60. doi: 10.1016/j.molliq.2016.11.123. [DOI] [Google Scholar]
- 27.Da Silva R.P.F.F., Rocha-Santos T.A.P., Duarte A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016;76:40–51. doi: 10.1016/j.trac.2015.11.013. [DOI] [Google Scholar]
- 28.Harbourne N., Marete E., Jacquier J., O’Riordan D. Handbook of Plant Food Phytochemicals. John Wiley & Sons Ltd.; Hoboken, NJ, USA: 2013. Conventional extraction techniques for phytochemicals; pp. 399–411. [Google Scholar]
- 29.Heleno S.A., Diz P., Prieto M.A., Barros L., Rodrigues A., Barreiro M.F., Ferreira I.C.F.R. Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Pt BFood Chem. 2016;197:1054–1063. doi: 10.1016/j.foodchem.2015.11.108. [DOI] [PubMed] [Google Scholar]
- 30.Wu C., Wang F., Liu J., Zou Y., Chen X. A comparison of volatile fractions obtained from Lonicera macranthoides via different extraction processes: Ultrasound, microwave, Soxhlet extraction, hydrodistillation, and cold maceration. Integr. Med. Res. 2015;4:171–177. doi: 10.1016/j.imr.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aminzare M., Hashemi M., Ansarian E., Bimkar M., Azar H.H., Mehrasbi M.R., Daneshamooz S., Raeisi M., Jannat B., Afshari A. Using natural antioxidants in meat and meat products as preservatives: A review. Adv. Anim. Vet. Sci. 2019;7:417–426. doi: 10.17582/journal.aavs/2019/7.5.417.426. [DOI] [Google Scholar]
- 32.Rombaut N., Tixier A.-S., Bily A., Chemat F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefin. 2014;8:530–544. doi: 10.1002/bbb.1486. [DOI] [Google Scholar]
- 33.Khaw K.Y., Parat M.O., Shaw P.N., Falconer J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules. 2017;22:1186. doi: 10.3390/molecules22071186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capuzzo A., Maffei M.E., Occhipinti A. Supercritical fluid extraction of plant flavors and fragrances. Molecules. 2013;18:7194–7238. doi: 10.3390/molecules18067194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pourmortazavi S.M., Hajimirsadeghi S.S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A. 2007;1163:2–24. doi: 10.1016/j.chroma.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Hamburger M., Baumann D., Adler S. Supercritical carbon dioxide extraction of selected medicinal plants-effects of high pressure and added ethanol on yield of extracted substances. Phytochem. Anal. 2004;15:46–54. doi: 10.1002/pca.743. [DOI] [PubMed] [Google Scholar]
- 37.Hamdan S., Daood H.G., Toth-Markus M., Illés V. Extraction of cardamom oil by supercritical carbon dioxide and sub-critical propane. J. Supercrit. Fluids. 2008;44:25–30. doi: 10.1016/j.supflu.2007.08.009. [DOI] [Google Scholar]
- 38.Herrero M., Castro-Puyana M., Mendiola J., Ibáñez E. Compressed fluids for the extraction of bioactive compounds. TrAC Trends Anal. Chem. 2013;43:67–83. doi: 10.1016/j.trac.2012.12.008. [DOI] [Google Scholar]
- 39.Herrero M., Cifuentes A., Ibañez E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006;98:136–148. doi: 10.1016/j.foodchem.2005.05.058. [DOI] [Google Scholar]
- 40.Pereira C.G., Meireles M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010;3:340–372. doi: 10.1007/s11947-009-0263-2. [DOI] [Google Scholar]
- 41.Ong E.S., Cheong J.S.H., Goh D. Pressurized hot water extraction of bioactive or marker compounds in botanicals and medicinal plant materials. J. Chromatogr. A. 2006;1112:92–102. doi: 10.1016/j.chroma.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 42.Leal P.F., Maia N.B., Carmello Q.A.C., Catharino R.R., Eberlin M.N., Meireles M.A.A. Sweet basil (Ocimum basilicum) extracts obtained by supercritical fluid extraction (SFE): Global yields, chemical composition, antioxidant activity, and estimation of the cost of manufacturing. Food Bioprocess Technol. 2008;1:326–338. doi: 10.1007/s11947-007-0030-1. [DOI] [Google Scholar]
- 43.Gámiz-Gracia L., Luque de Castro M.D. Continuous subcritical water extraction of medicinal plant essential oil: Comparison with conventional techniques. Talanta. 2000;51:1179–1185. doi: 10.1016/S0039-9140(00)00294-0. [DOI] [PubMed] [Google Scholar]
- 44.Catchpole O.J., Grey J.B., Perry N.B., Burgess E.J., Redmond W.A., Porter N.G. Extraction of chili, black pepper, and ginger with near-critical CO2, propane, and dimethyl ether: Analysis of the extracts by quantitative nuclear magnetic resonance. J. Agric. Food Chem. 2003;51:4853–4860. doi: 10.1021/jf0301246. [DOI] [PubMed] [Google Scholar]
- 45.Correa M., Mesomo M.C., Pianoski K.E., Torres Y.R., Corazza M.L. Extraction of inflorescences of Musa paradisiaca L. using supercritical CO2 and compressed propane. J. Supercrit. Fluids. 2016;113:128–135. doi: 10.1016/j.supflu.2016.03.016. [DOI] [Google Scholar]
- 46.Silva J.R.F., Cantelli K.C., Soares M.B.A., Tres M.V., Oliveira D., Meireles M.A.A., Oliveira J.V., Treichel H., Mazutti M.A. Enzymatic hydrolysis of non-treated sugarcane bagasse using pressurized liquefied petroleum gas with and without ultrasound assistance. Renew. Energy. 2015;83:674–679. doi: 10.1016/j.renene.2015.04.065. [DOI] [Google Scholar]
- 47.Zanqui A.B., De Morais D.R., Da Silva C.M., Santos J.M., Gomes S.T.M., Visentainer J.V., Eberlin M.N., Cardozo-Filho L., Matsushita M. Subcritical extraction of flaxseed oil with n-propane: Composition and purity. Food Chem. 2015;188:452–458. doi: 10.1016/j.foodchem.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 48.Da Silva C.M., Zanqui A.B., Gohara A.K., De Souza A.H.P., Cardozo-Filho L., Visentainer J.V., Rovigatti Chiavelli L.U., Bittencourt P.R.S., Da Silva E.A., Matsushita M. Compressed n-propane extraction of lipids and bioactive compounds from Perilla (Perilla frutescens) J. Supercrit. Fluids. 2015;102:1–8. doi: 10.1016/j.supflu.2015.03.016. [DOI] [Google Scholar]
- 49.Santos K.A., Bariccatti R.A., Cardozo-Filho L., Schneider R., Palú F., Da Silva C., Da Silva E.A. Extraction of crambe seed oil using subcritical propane: Kinetics, characterization and modeling. J. Supercrit. Fluids. 2015;104:54–61. doi: 10.1016/j.supflu.2015.05.026. [DOI] [Google Scholar]
- 50.Pessoa A.S., Podestá R., Block J.M., Franceschi E., Dariva C., Lanza M. Extraction of pequi (Caryocar coriaceum) pulp oil using subcritical propane: Determination of process yield and fatty acid profile. J. Supercrit. Fluids. 2015;101:95–103. doi: 10.1016/j.supflu.2015.03.006. [DOI] [Google Scholar]
- 51.Pederssetti M.M., Palú F., Da Silva E.A., Rohling J.H., Cardozo-Filho L., Dariva C. Extraction of canola seed (Brassica napus) oil using compressed propane and supercritical carbon dioxide. J. Food Eng. 2011;102:189–196. doi: 10.1016/j.jfoodeng.2010.08.018. [DOI] [Google Scholar]
- 52.Corso M.P., Fagundes-Klen M.R., Silva E.A., Cardozo Filho L., Santos J.N., Freitas L.S., Dariva C. Extraction of sesame seed (Sesamun indicum L.) oil using compressed propane and supercritical carbon dioxide. J. Supercrit. Fluids. 2010;52:56–61. doi: 10.1016/j.supflu.2009.11.012. [DOI] [Google Scholar]
- 53.Nimet G., da Silva E.A., Palú F., Dariva C., dos Santos Freitas L., Neto A.M., Filho L.C. Extraction of sunflower (Heliantus annuus L.) oil with supercritical CO2 and subcritical propane: Experimental and modeling. Chem. Eng. J. 2011;168:262–268. doi: 10.1016/j.cej.2010.12.088. [DOI] [Google Scholar]
- 54.Soares J.F., Dal Prá V., de Souza M., Lunelli F.C., Abaide E., da Silva J.R.F., Kuhn R.C., Martinez J., Mazutti M.A. Extraction of rice bran oil using supercritical CO2 and compressed liquefied petroleum gas. J. Food Eng. 2016;170:58–63. doi: 10.1016/j.jfoodeng.2015.09.016. [DOI] [Google Scholar]
- 55.Dal Prá V., Soares J.F., Monego D.L., Vendruscolo R.G., Freire D.M.G., Alexandri M., Koutinas A., Wagner R., Mazutti M.A., Da Rosa M.B. Extraction of bioactive compounds from palm (Elaeis guineensis) pressed fiber using different compressed fluids. J. Supercrit. Fluids. 2016;112:51–56. doi: 10.1016/j.supflu.2016.02.011. [DOI] [Google Scholar]
- 56.Reverchon E., De Marco I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids. 2006;38:146–166. doi: 10.1016/j.supflu.2006.03.020. [DOI] [Google Scholar]
- 57.Duba K.S., Fiori L. Supercritical CO2 extraction of grape seed oil: Effect of process parameters on the extraction kinetics. J. Supercrit. Fluids. 2015;98:33–43. doi: 10.1016/j.supflu.2014.12.021. [DOI] [Google Scholar]
- 58.Belwal T., Dhyani P., Bhatt I.D., Rawal R.S., Pande V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM) Food Chem. 2016;207:115–124. doi: 10.1016/j.foodchem.2016.03.081. [DOI] [PubMed] [Google Scholar]
- 59.Bimakr M., Rahman R.A., Ganjloo A., Taip F.S., Salleh L.M., Sarker M.Z.I. Optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves by using response surface methodology. Food Bioprocess Technol. 2012;5:912–920. doi: 10.1007/s11947-010-0504-4. [DOI] [Google Scholar]
- 60.Nejad-Sadeghi M., Taji S., Goodarznia I. Optimization of supercritical carbon dioxide extraction of essential oil from Dracocephalum kotschyi Boiss: An endangered medicinal plant in Iran. J. Chromatogr. A. 2015;1422:73–81. doi: 10.1016/j.chroma.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 61.Nagy B., Simándi B. Effects of particle size distribution, moisture content, and initial oil content on the supercritical fluid extraction of paprika. J. Supercrit. Fluids. 2008;46:293–298. doi: 10.1016/j.supflu.2008.04.009. [DOI] [Google Scholar]
- 62.Crampon C., Mouahid A., Toudji S.A.A., Leṕine O., Badens E. Influence of pretreatment on supercritical CO2 extraction from Nannochloropsis oculata. J. Supercrit. Fluids. 2013;79:337–344. doi: 10.1016/j.supflu.2012.12.022. [DOI] [Google Scholar]
- 63.Ivanovic J., Ristic M., Skala D. Supercritical CO2 extraction of Helichrysum italicum: Influence of CO2 density and moisture content of plant material. J. Supercrit. Fluids. 2011;57:129–136. doi: 10.1016/j.supflu.2011.02.013. [DOI] [Google Scholar]
- 64.Porto C., Decorti D., Natolino A. Microwave pretreatment of Moringa oleifera seed: Effect on oil obtained by pilot-scale supercritical carbon dioxide extraction and Soxhlet apparatus. J. Supercrit. Fluids. 2016;107:38–43. doi: 10.1016/j.supflu.2015.08.006. [DOI] [Google Scholar]
- 65.Dutta S., Bhattacharjee P. Enzyme-assisted supercritical carbon dioxide extraction of black pepper oleoresin for enhanced yield of piperine-rich extract. J. Biosci. Bioeng. 2015;120:17–23. doi: 10.1016/j.jbiosc.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Mushtaq M., Sultana B., Anwar F., Adnan A., SSH R. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids. 2015;104:122–131. doi: 10.1016/j.supflu.2015.05.020. [DOI] [Google Scholar]
- 67.Mushtaq M., Sultana B., Akram S., Anwar F., Adnan A., Rizvi S.S.H. Enzyme-assisted supercritical fluid extraction: An alternative and green technology for non-extractable polyphenols. Anal. Bioanal. Chem. 2017;409:3645–3655. doi: 10.1007/s00216-017-0309-7. [DOI] [PubMed] [Google Scholar]
- 68.Lenucci M.S., De Caroli M., Marrese P.P., Iurlaro A., Rescio L., Böhm V., Dalessandro G., Piro G. Enzyme-aided extraction of lycopene from high-pigment tomato cultivars by supercritical carbon dioxide. Food Chem. 2015;170:193–202. doi: 10.1016/j.foodchem.2014.08.081. [DOI] [PubMed] [Google Scholar]
- 69.Da Porto C., Natolino A., Decorti D. Effect of ultrasound pre-treatment of hemp (Cannabis sativa L.) seed on supercritical CO2 extraction of oil. J. Food Sci. Technol. 2015;52:1748–1753. doi: 10.1007/s13197-013-1143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Said P.P., Arya O.P., Pradhan R.C., Singh R.S., Rai B.N. Separation of oleoresin from ginger rhizome powder using green processing technologies. J. Food Process Eng. 2015;38:107–114. doi: 10.1111/jfpe.12127. [DOI] [Google Scholar]
- 71.Balachandran S., Kentish S.E., Mawson R., Ashokkumar M. Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason. Sonochem. 2006;13:471–479. doi: 10.1016/j.ultsonch.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Wei M.-C., Xiao J., Yang Y.-C. Extraction of α-humulene-enriched oil from clove using ultrasound-assisted supercritical carbon dioxide extraction and studies of its fictitious solubility. Food Chem. 2016;210:172–181. doi: 10.1016/j.foodchem.2016.04.076. [DOI] [PubMed] [Google Scholar]
- 73.Kawamura H., Mishima K., Sharmin T., Ito S., Kawakami R., Kato T., Misumi M., Suetsugu T., Orii H., Kawano H., et al. Ultrasonically enhanced extraction of luteolin and apigenin from the leaves of Perilla frutescens (L.) Britt. using liquid carbon dioxide and ethanol. Ultrason. Sonochem. 2016;29:19–26. doi: 10.1016/j.ultsonch.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 74.Dias A.L.B., Arroio Sergio C.S., Santos P., Barbero G.F., Rezende C.A., Martínez J. Effect of ultrasound on the supercritical CO2 extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L. var. pendulum) Ultrason. Sonochem. 2016;31:284–294. doi: 10.1016/j.ultsonch.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Santos P., Aguiar A.C., Barbero G.F., Rezende C.A., Martínez J. Supercritical carbon dioxide extraction of capsaicinoids from malagueta pepper (Capsicum frutescens L.) assisted by ultrasound. Ultrason. Sonochem. 2015;22:78–88. doi: 10.1016/j.ultsonch.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 76.Wei M.-C., Yang Y.-C. Kinetic studies for ultrasound-assisted supercritical carbon dioxide extraction of triterpenic acids from healthy tea ingredient Hedyotis diffusa and Hedyotis corymbosa. Sep. Purif. Technol. 2015;142:316–325. doi: 10.1016/j.seppur.2015.01.008. [DOI] [Google Scholar]
- 77.Riera E., Golás Y., Blanco A., Gallego J.A., Blasco M., Mulet A. Mass transfer enhancement in supercritical fluids extraction by means of power ultrasound. Ultrason. Sonochem. 2004;11:241–244. doi: 10.1016/j.ultsonch.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 78.Malaman F.S., Moraes L.A.B., West C., Ferreira N.J., Oliveira A.L. Supercritical fluid extracts from the Brazilian cherry (Eugenia uniflora L.): Relationship between the extracted compounds and the characteristic flavour intensity of the fruit. Food Chem. 2011;124:85–92. doi: 10.1016/j.foodchem.2010.05.109. [DOI] [Google Scholar]
- 79.Girotra P., Singh S.K., Nagpal K. Supercritical fluid technology: A promising approach in pharmaceutical research. Pharm. Dev. Technol. 2013;18:22–38. doi: 10.3109/10837450.2012.726998. [DOI] [PubMed] [Google Scholar]
- 80.Díaz-Reinoso B., Moure A., Domínguez H., Parajó J.C. Supercritical CO2 extraction and purification of compounds with antioxidant activity. J. Agric. Food Chem. 2006;54:2441–2469. doi: 10.1021/jf052858j. [DOI] [PubMed] [Google Scholar]
- 81.Guelph L., Davidson V.J., Kakuda Y. Analysis of volatile flavor components in roasted peanuts using supercritical fluid extraction and gas chromatography-mass spectrometry. J. Agric. food Chem. 1996;44:2694–2699. [Google Scholar]
- 82.Banchero M., Pellegrino G., Manna L. Supercritical fluid extraction as a potential mitigation strategy for the reduction of acrylamide level in coffee. J. Food Eng. 2013;115:292–297. doi: 10.1016/j.jfoodeng.2012.10.045. [DOI] [Google Scholar]
- 83.Heikes D.L., Scott B., Gorzovalitis N.A. Quantitation of volatile oils in ground cumin by supercritical fluid extraction and gas chromatography with flame ionization detection. J. AOAC Int. 2001;84:1130–1134. doi: 10.1093/jaoac/84.4.1130. [DOI] [PubMed] [Google Scholar]
- 84.Braga M.E.M., Meireles M.A.A. Accelerated solvent extraction and fractioned extraction to obtain the curcuma longa volatile oil and oleoresin. J. Food Process Eng. 2007;30:501–521. doi: 10.1111/j.1745-4530.2007.00133.x. [DOI] [Google Scholar]
- 85.Kazazi H., Rezaei K., Ghotb-Sharif S.J., Emam-Djomeh Z., Yamini Y. Analytical, nutritional and clinical methods. Food Chem. 2007;105:805–811. doi: 10.1016/j.foodchem.2007.01.059. [DOI] [Google Scholar]
- 86.Kumoro A., Singh H. Extraction of Sarawak black pepper essential oil using supercritical carbon dioxide. Arab. J. Sci. Eng. 2010;35:7–16. [Google Scholar]
- 87.Pimentel F.A., das Cardoso M.G., Guimarães L.G.L., Queiroz F., Barbosa L.C.A., Morais A.R., Nelson D.L., Andrade M.A., Zacaroni L.M., Pimentel S.M.N.P. Extracts from the leaves of Piper piscatorum (Trel. Yunc.) obtained by supercritical extraction of with CO2, employing ethanol and methanol as co-solvents. Ind. Crops Prod. 2013;43:490–495. doi: 10.1016/j.indcrop.2012.07.067. [DOI] [Google Scholar]
- 88.Shen Z., Mishra V., Imison B., Palmer M., Fairclough R. Use of adsorbent and supercritical carbon dioxide to concentrate flavor compounds from orange oil. J. Agric. Food Chem. 2002;50:154–160. doi: 10.1021/jf010582j. [DOI] [PubMed] [Google Scholar]
- 89.Van Opstaele F., Goiris K., De Rouck G., Aerts G., De Cooman L. Production of novel varietal hop aromas by supercritical fluid extraction of hop pellets. Part 1: Preparation of single variety total hop essential oils and polar hop essences. Cerevisia. 2013;37:97–108. doi: 10.1016/j.cervis.2012.12.002. [DOI] [Google Scholar]
- 90.Domingues R.M.A., Oliveira E.L.G., Freire C.S.R., Couto R.M., Simões P.C., Neto C.P., Silvestre A.J.D., Silva C.M. Supercritical Fluid Extraction of Eucalyptus globulus Bark—A Promising Approach for Triterpenoid Production. Int. J. Mol. Sci. 2012;13:7648–7662. doi: 10.3390/ijms13067648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y., Chang L., Zhao X., Meng X., Liu Y. Gas chromatography-mass spectrometry analysis on compounds in volatile oils extracted from yuan zhi (Radix polygalae) and shi chang pu (Acorus tatarinowii) by supercritical CO2. J. Tradit. Chin. Med. 2012;32:459–464. doi: 10.1016/S0254-6272(13)60055-2. [DOI] [PubMed] [Google Scholar]
- 92.Opstaele F., Goiris K., De Rouck G., Aerts G., Cooman L. Production of novel varietal hop aromas by supercritical fluid extraction of hop pellets—Part 2: Preparation of single variety floral, citrus, and spicy hop oil essences by density programmed supercritical fluid extraction. J. Supercrit. Fluids. 2012;71:147–161. doi: 10.1016/j.supflu.2012.06.004. [DOI] [Google Scholar]
- 93.Zhou J., Ma X., Qiu B.-H., Chen J., Bian L., Pan L. Parameters optimization of supercritical fluid-CO2 extracts of Frankincense using response surface methodology and its pharmacodynamics effects. J. Sep. Sci. 2013;36:383–390. doi: 10.1002/jssc.201200647. [DOI] [PubMed] [Google Scholar]
- 94.Omar J., Olivares M., Alzaga M., Etxebarria N. Optimisation and characterisation of marihuana extracts obtained by supercritical fluid extraction and focused ultrasound extraction and retention time locking GC-MS. J. Sep. Sci. 2013;36:1397–1404. doi: 10.1002/jssc.201201103. [DOI] [PubMed] [Google Scholar]
- 95.Lee S., Park M.K., Kim K.H., Kim Y.-S. Effect of supercritical carbon dioxide decaffeination on volatile components of green teas. J. Food Sci. 2007;72:S497–S502. doi: 10.1111/j.1750-3841.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 96.Sousa E.M.B.D., Martínez J., Chiavone-Filho O., Rosa P.T.V., Domingos T., Meireles M.A.A. Extraction of volatile oil from Croton zehntneri Pax et Hoff with pressurized CO2: Solubility, composition and kinetics. J. Food Eng. 2005;69:325–333. doi: 10.1016/j.jfoodeng.2004.08.023. [DOI] [Google Scholar]
- 97.Marzouki H., Piras A., Marongiu B., Rosa A., Dessì M.A. Extraction and separation of volatile and fixed oils from berries of Laurus nobilis L. by Supercritical CO2. Molecules. 2008;13:1702–1711. doi: 10.3390/molecules13081702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Almeida P., Mezzomo N., Ferreira S. Extraction of Mentha spicata L. volatile compounds: Evaluation of process parameters and extract composition. Food Bioprocess Technol. 2012;5:548–559. doi: 10.1007/s11947-010-0356-y. [DOI] [Google Scholar]
- 99.Ansari K., Goodarznia I. Optimization of supercritical carbon dioxide extraction of essential oil from spearmint (Mentha spicata L.) leaves by using Taguchi methodology. J. Supercrit. Fluids. 2012;67:123–130. doi: 10.1016/j.supflu.2012.03.011. [DOI] [Google Scholar]
- 100.El-Ahmady S.H., Ashour M.L., Wink M. Chemical composition and anti-inflammatory activity of the essential oils of Psidium guajava fruits and leaves. J. Essent. Oil Res. 2013;25:475–481. doi: 10.1080/10412905.2013.796498. [DOI] [Google Scholar]
- 101.Leal P.F., Chaves F.C.M., Ming L.I.N.C., Petenate A.J., Meireles M.A.A. Global yields, chemical compositions and antioxidant activities of clove basil (Ocimum gratissimum L.) extracts obtained by supercritical fluid extraction. J. Food Process Eng. 2006;29:547–559. doi: 10.1111/j.1745-4530.2006.00082.x. [DOI] [Google Scholar]
- 102.Nobre B.P., Gouveia L., Matos P.G.S., Cristino A.F., Palavra A.F., Mendes R.L. Supercritical extraction of lycopene from tomato industrial wastes with ethane. Molecules. 2012;17:8397–8407. doi: 10.3390/molecules17078397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vigano J., Coutinho J.P., Souza D.N., Baroni N.A.F., Godoy H.T., Macedo J.A., Martinez J. Exploring the selectivity of supercritical CO2 to obtain nonpolar fractions of passion fruit bagasse extracts. J. Supercrit. Fluids. 2016;110:1–10. doi: 10.1016/j.supflu.2015.12.001. [DOI] [Google Scholar]
- 104.Bimakr M., Rahman R.A., Taip F.S., Adzahan N.M., Sarker M.Z.I., Ganjloo A. Supercritical carbon dioxide extraction of seed oil from winter melon (Benincasa hispida) and its antioxidant activity and fatty acid composition. Molecules. 2013;18:997–1014. doi: 10.3390/molecules18010997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aladić K., Jarni K., Barbir T., Vidović S., Vladić J., Bilić M., Jokić S. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind. Crops Prod. 2015;76:472–478. doi: 10.1016/j.indcrop.2015.07.016. [DOI] [Google Scholar]
- 106.Barroso P.T.W., de Carvalho P.P., Rocha T.B., Pessoa F.L.P., Azevedo D.A., Mendes M.F. Evaluation of the composition of Carica papaya L. seed oil extracted with supercritical CO2. Biotechnol. Rep. 2016;11:110–116. doi: 10.1016/j.btre.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Belayneh H.D., Wehling R., Cahoon E., Ciftci N.O. Extraction of omega-3-rich oil from Camelina sativa seed using supercritical carbon dioxide. J. Supercrit. Fluids. 2015;104:153–159. doi: 10.1016/j.supflu.2015.06.002. [DOI] [Google Scholar]
- 108.Przygoda K., Wejnerowska G. Extraction of tocopherol-enriched oils from Quinoa seeds by supercritical fluid extraction. Ind. Crops Prod. 2015;63:41–47. doi: 10.1016/j.indcrop.2014.09.038. [DOI] [Google Scholar]
- 109.Shao Q., Huang Y., Zhou A., Guo H., Zhang A., Wang Y. Application of response surface methodology to optimise supercritical carbon dioxide extraction of volatile compounds from Crocus sativus. J. Sci. Food Agric. 2014;94:1430–1436. doi: 10.1002/jsfa.6435. [DOI] [PubMed] [Google Scholar]
- 110.Garmus T.T., Kopf S.F.M., Paula J.T., Aguiar A.C., Duarte G.H.B., Eberlin M.N., Cabral F.A. Ethanolic and hydroalcoholic extracts of pitanga leaves (Eugenia uniflora L.) and their fractionation by supercritical technology. Braz. J. Chem. Eng. 2019;36:1041–1051. doi: 10.1590/0104-6632.20190362s20180159. [DOI] [Google Scholar]
- 111.Zhao S., Zhang D. A parametric study of supercritical carbon dioxide extraction of oil from Moringa oleifera seeds using a response surface methodology. Sep. Purif. Technol. 2013;113:9–17. doi: 10.1016/j.seppur.2013.03.041. [DOI] [Google Scholar]
- 112.Ruttarattanamongkol K., Siebenhandl-Ehn S., Schreiner M., Petrasch A. Pilot-scale supercritical carbon dioxide extraction, physico-chemical properties and profile characterization of Moringa oleifera seed oil in comparison with conventional extraction methods. Ind. Crops Prod. 2014;58:68–77. doi: 10.1016/j.indcrop.2014.03.020. [DOI] [Google Scholar]
- 113.Bhattacharya M., Srivastav P., Mishra H.N. Optimization of process variables for supercritical fluid extraction of ergothioneine and polyphenols from Pleurotus ostreatus and correlation to free-radical scavenging activity. J. Supercrit. Fluids. 2014;95 doi: 10.1016/j.supflu.2014.07.031. [DOI] [Google Scholar]
- 114.Veiga B.A., Hamerski F., Clausen M.P., Errico M., de Paula Scheer A., Corazza M.L. Compressed fluids extraction methods, yields, antioxidant activities, total phenolics and flavonoids content for Brazilian Mantiqueira hops. J. Supercrit. Fluids. 2021;170:105155. doi: 10.1016/j.supflu.2020.105155. [DOI] [Google Scholar]
- 115.Karimi M., Raofie F. Micronization of vincristine extracted from Catharanthus roseus by expansion of supercritical fluid solution. J. Supercrit. Fluids. 2019;146:21. doi: 10.1016/j.supflu.2019.01.021. [DOI] [Google Scholar]
- 116.Valadez-Carmona L., Ortiz-Moreno A., Ceballos-Reyes G., Mendiola J.A., Ibáñez E. Valorization of cacao pod husk through supercritical fluid extraction of phenolic compounds. J. Supercrit. Fluids. 2018;131:99–105. doi: 10.1016/j.supflu.2017.09.011. [DOI] [Google Scholar]
- 117.Cruz P.N., Fetzer D.L., do Amaral W., de Andrade E.F., Corazza M.L., Masson M.L. Antioxidant activity and fatty acid profile of yacon leaves extracts obtained by supercritical CO2 + ethanol solvent. J. Supercrit. Fluids. 2019;146:55–64. doi: 10.1016/j.supflu.2019.01.007. [DOI] [Google Scholar]
- 118.Pieczykolan A., Pietrzak W., Rój E., Nowak R. Effects of Supercritical Carbon Dioxide Extraction (SC-CO2) on the content of tiliroside in the extracts from Tilia L. flowers. Open Chem. 2019;17:302–312. doi: 10.1515/chem-2019-0040. [DOI] [Google Scholar]
- 119.Ouédraogo J.C.W., Dicko C., Kini F.B., Bonzi-Coulibaly Y.L., Dey E.S. Enhanced extraction of flavonoids from Odontonema strictum leaves with antioxidant activity using supercritical carbon dioxide fluid combined with ethanol. J. Supercrit. Fluids. 2018;131:66–71. doi: 10.1016/j.supflu.2017.08.017. [DOI] [Google Scholar]
- 120.Jokić S., Molnar M., Jakovljević M., Aladić K., Jerković I. Optimization of supercritical CO2 extraction of Salvia officinalis L. leaves targeted on Oxygenated monoterpenes, α-humulene, viridiflorol and manool. J. Supercrit. Fluids. 2018;133:253–262. doi: 10.1016/j.supflu.2017.10.022. [DOI] [Google Scholar]
- 121.Lima R.N., Ribeiro A.S., Cardozo-Filho L., Vedoy D., Alves P.B. Extraction from leaves of Piper klotzschianum using supercritical carbon dioxide and co-solvents. J. Supercrit. Fluids. 2019;147:205–212. doi: 10.1016/j.supflu.2018.11.006. [DOI] [Google Scholar]
- 122.De Andrade Lima M., Kestekoglou I., Charalampopoulos D., Chatzifragkou A. Supercritical fluid extraction of carotenoids from vegetable waste matrices. Molecules. 2019;24:466. doi: 10.3390/molecules24030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.De Andrade Lima M., Charalampopoulos D., Chatzifragkou A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids. 2018;133:94–102. doi: 10.1016/j.supflu.2017.09.028. [DOI] [Google Scholar]
- 124.Conde-Hernández L.A., Espinosa-Victoria J.R., Trejo A., Guerrero-Beltrán J. CO2-supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary (Rosmarinus officinalis) J. Food Eng. 2017;200:81–86. doi: 10.1016/j.jfoodeng.2016.12.022. [DOI] [Google Scholar]
- 125.Frohlich P.C., Santos K.A., Palú F., Cardozo-Filho L., da Silva C., da Silva E.A. Evaluation of the effects of temperature and pressure on the extraction of eugenol from clove (Syzygium aromaticum) leaves using supercritical CO2. J. Supercrit. Fluids. 2019;143:313–320. doi: 10.1016/j.supflu.2018.09.009. [DOI] [Google Scholar]
- 126.Goyeneche R., Fanovich A., Rodriguez Rodrigues C., Nicolao M.C., Di Scala K. Supercritical CO2 extraction of bioactive compounds from radish leaves: Yield, antioxidant capacity and cytotoxicity. J. Supercrit. Fluids. 2018;135:78–83. doi: 10.1016/j.supflu.2018.01.004. [DOI] [Google Scholar]
- 127.Garcia-Mendoza M.P., Paula J.T., Paviani L.C., Cabral F.A., Martinez-Correa H.A. Extracts from mango peel by-product obtained by supercritical CO2 and pressurized solvent processes. LWT Food Sci. Technol. 2015;62:131–137. doi: 10.1016/j.lwt.2015.01.026. [DOI] [Google Scholar]
- 128.Santos D.T., Veggi P.C., Meireles M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012;108:444–452. doi: 10.1016/j.jfoodeng.2011.08.022. [DOI] [Google Scholar]
- 129.Machado A.P.D.F., Pasquel-Reátegui J.L., Barbero G.F., Martínez J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015;77:675–683. doi: 10.1016/j.foodres.2014.12.042. [DOI] [Google Scholar]
- 130.Xu Y., Cai F., Yu Z., Zhang L., Li X., Yang Y., Liu G. Optimisation of pressurised water extraction of polysaccharides from blackcurrant and its antioxidant activity. Food Chem. 2016;194:650–658. doi: 10.1016/j.foodchem.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 131.Bajer T., Bajerová P., Kremr D., Eisner A., Ventura K. Central composite design of pressurised hot water extraction process for extracting capsaicinoids from chili peppers. J. Food Compos. Anal. 2015;40:32–38. doi: 10.1016/j.jfca.2014.12.008. [DOI] [Google Scholar]
- 132.Khoddami A., Wilkes M.A., Roberts T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules. 2013;18:2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chemat F., Rombaut N., Sicaire A.G., Meullemiestre A., Fabiano-Tixier A.S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 134.Rajha H.N., Boussetta N., Louka N., Maroun R.G., Vorobiev E. Effect of alternative physical pretreatments (pulsed electric field, high voltage electrical discharges and ultrasound) on the dead-end ultrafiltration of vine-shoot extracts. Sep. Purif. Technol. 2015;146:243–251. doi: 10.1016/j.seppur.2015.03.058. [DOI] [Google Scholar]
- 135.Kek S.P., Chin N.L., Yusof Y.A. Direct and indirect power ultrasound assisted pre-osmotic treatments in convective drying of guava slices. Food Bioprod. Process. 2013;91:495–506. doi: 10.1016/j.fbp.2013.05.003. [DOI] [Google Scholar]
- 136.Xu J.K., Li M.F., Sun R.C. Identifying the impact of ultrasound-assisted extraction on polysaccharides and natural antioxidants from Eucommia ulmoides Oliver. Process Biochem. 2015;50:473–481. doi: 10.1016/j.procbio.2014.12.021. [DOI] [Google Scholar]
- 137.D’Alessandro G.L., Dimitrov K., Vauchel P., Nikov I. Kinetics of ultrasound assisted extraction of anthocyanins from Aronia melanocarpa (black chokeberry) wastes. Chem. Eng. Res. Des. 2014;92:1818–1826. doi: 10.1016/j.cherd.2013.11.020. [DOI] [Google Scholar]
- 138.Sivakumar V., Ilanhtiraiyan S., Ilayaraja K., Ashly A., Hariharan S. Influence of ultrasound on Avaram bark (Cassia auriculata) tannin extraction and tanning. Chem. Eng. Res. Des. 2014;92:1827–1833. doi: 10.1016/j.cherd.2014.04.007. [DOI] [Google Scholar]
- 139.Khan M.K., Abert-Vian M., Fabiano-Tixier A.-S., Dangles O., Chemat F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010;119:851–858. doi: 10.1016/j.foodchem.2009.08.046. [DOI] [Google Scholar]
- 140.Rodríguez-Pérez C., Quirantes-Piné R., Fernández-Gutiérrez A., Segura-Carretero A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind. Crops Prod. 2015;66:246–254. doi: 10.1016/j.indcrop.2015.01.002. [DOI] [Google Scholar]
- 141.Rabelo R.S., MacHado M.T.C., Martínez J., Hubinger M.D. Ultrasound assisted extraction and nanofiltration of phenolic compounds from artichoke solid wastes. J. Food Eng. 2016;178:170–180. doi: 10.1016/j.jfoodeng.2016.01.018. [DOI] [Google Scholar]
- 142.Meullemiestre A., Petitcolas E., Maache-Rezzoug Z., Chemat F., Rezzoug S.A. Impact of ultrasound on solid–liquid extraction of phenolic compounds from maritime pine sawdust waste. Kinetics, optimization and large scale experiments. Ultrason. Sonochem. 2016;28:230–239. doi: 10.1016/j.ultsonch.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 143.Corbin C., Fidel T., Leclerc E.A., Barakzoy E., Sagot N., Falguiéres A., Renouard S., Blondeau J.P., Ferroud C., Doussot J., et al. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015;26:176–185. doi: 10.1016/j.ultsonch.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 144.De Paula J.A.M., Brito L.F., Caetano K.L.F.N., De Morais Rodrigues M.C., Borges L.L., Da Conceição E.C. Ultrasound-assisted extraction of azadirachtin from dried entire fruits of Azadirachta indica A. Juss. (Meliaceae) and its determination by a validated HPLC-PDA method. Talanta. 2016;149:77–84. doi: 10.1016/j.talanta.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 145.Wang Y., Wang F., Ma X., Sun S., Leng F., Zhang W., Wang X. Extraction, purification, characterization and antioxidant activity of polysaccharides from Piteguo fruit. Ind. Crops Prod. 2015;77:467–475. doi: 10.1016/j.indcrop.2015.09.012. [DOI] [Google Scholar]
- 146.Hammi K.M., Jdey A., Abdelly C., Majdoub H., Ksouri R. Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Zizyphus lotus fruits using response surface methodology. Food Chem. 2015;184:80–89. doi: 10.1016/j.foodchem.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 147.Tang J., Resurreccion F.P. Electromagnetic basis of microwave heating. Dev. Packag. Prod. Use Microw. Ovens. 2009;3:38e. doi: 10.1533/9781845696573.1.3. [DOI] [Google Scholar]
- 148.Delazar A., Nahar L., Hamedeyazdan S., Sarker S.D. Microwave-assisted extraction in natural products isolation. Methods Mol. Biol. 2012;864:89–115. doi: 10.1007/978-1-61779-624-1_5. [DOI] [PubMed] [Google Scholar]
- 149.Cristina-Gabriela G., Emilie D., Gabriel L., Claire E. Bioactive compounds extraction from pomace of four apple varieties. J. Eng. Stud. Res. 2012;18:96. [Google Scholar]
- 150.Yedhu Krishnan R., Rajan K.S. Microwave assisted extraction of flavonoids from Terminalia bellerica: Study of kinetics and thermodynamics. Sep. Purif. Technol. 2016;157:169–178. doi: 10.1016/j.seppur.2015.11.035. [DOI] [Google Scholar]
- 151.Inoue T., Tsubaki S., Ogawa K., Onishi K., Azuma J. ichi Isolation of hesperidin from peels of thinned Citrus unshiu fruits by microwave-assisted extraction. Food Chem. 2010;123:542–547. doi: 10.1016/j.foodchem.2010.04.051. [DOI] [Google Scholar]
- 152.Thirugnanasambandham K., Sivakumar V. Microwave assisted extraction process of betalain from dragon fruit and its antioxidant activities. J. Saudi Soc. Agric. Sci. 2017;16:41–48. doi: 10.1016/j.jssas.2015.02.001. [DOI] [Google Scholar]
- 153.Simić V.M., Rajković K.M., Stojičević S.S., Veličković D.T., Nikolić N., Lazić M.L., Karabegović I.T. Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep. Purif. Technol. 2016;160:89–97. doi: 10.1016/j.seppur.2016.01.019. [DOI] [Google Scholar]
- 154.Seixas F.L., Fukuda D.L., Turbiani F.R.B., Garcia P.S., Petkowicz C.L.D.O., Jagadevan S., Gimenes M.L. Extraction of pectin from passion fruit peel (Passiflora edulis F. Flavicarpa) by microwave-induced heating. Food Hydrocoll. 2014;38:186–192. doi: 10.1016/j.foodhyd.2013.12.001. [DOI] [Google Scholar]
- 155.Prakash Maran J., Sivakumar V., Thirugnanasambandham K., Sridhar R. Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohydr. Polym. 2014;101:786–791. doi: 10.1016/j.carbpol.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 156.Petigny L., Périno S., Minuti M., Visinoni F., Wajsman J., Chemat F. Simultaneous microwave extraction and separation of volatile and non-volatile organic compounds of boldo leaves. From lab to industrial scale. Int. J. Mol. Sci. 2014;15:7183–7198. doi: 10.3390/ijms15057183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kumar P., Verma A.K., Umaraw P., Mehta N. Prospects in the meat industry: The new technology could be used for accelerated drying, curing, and other production steps. Fleischwirtschaft Int. J. Meat Prod. Meat Process. 2020;1:22–27. [Google Scholar]
- 158.Yogesh K. Pulsed electric field processing of egg products: A review. J. Food Sci. Technol. 2016;53:934–945. doi: 10.1007/s13197-015-2061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh P.K., Kumar S., Kumar P., Bhat Z.F. Pulsed light and pulsed electric field-emerging non thermal decontamination of meat. Am. J. Food Technol. 2012;7:506–516. doi: 10.3923/ajft.2012.506.516. [DOI] [Google Scholar]
- 160.Roselló-Soto E., Koubaa M., Moubarik A., Lopes R.P., Saraiva J.A., Boussetta N., Grimi N., Barba F.J. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015;45:296–310. doi: 10.1016/j.tifs.2015.07.003. [DOI] [Google Scholar]
- 161.Parniakov O., Lebovka N.I., Van Hecke E., Vorobiev E. Pulsed electric field assisted pressure extraction and solvent extraction from mushroom (Agaricus bisporus) Food Bioprocess Technol. 2014;7:174–183. doi: 10.1007/s11947-013-1059-y. [DOI] [Google Scholar]
- 162.Boussetta N., Vorobiev E. Extraction of valuable biocompounds assisted by high voltage electrical discharges: A review. Comptes Rendus Chim. 2014;17:197–203. doi: 10.1016/j.crci.2013.11.011. [DOI] [Google Scholar]
- 163.Luengo E., Álvarez I., Raso J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013;17:79–84. doi: 10.1016/j.ifset.2012.10.005. [DOI] [Google Scholar]
- 164.Xue D., Farid M.M. Pulsed electric field extraction of valuable compounds from white button mushroom (Agaricus bisporus) Innov. Food Sci. Emerg. Technol. 2015;29:178–186. doi: 10.1016/j.ifset.2015.03.012. [DOI] [Google Scholar]
- 165.Leong J.Y., Lam W.H., Ho K.W., Voo W.P., Lee M.F.X., Lim H.P., Lim S.L., Tey B.T., Poncelet D., Chan E.S. Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology. 2016;24:44–60. doi: 10.1016/j.partic.2015.09.004. [DOI] [Google Scholar]
- 166.Segovia F.J., Luengo E., Corral-Pérez J.J., Raso J., Almajano M.P. Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields: Pulsed electric fields (PEF) applications. Ind. Crops Prod. 2015;65:390–396. doi: 10.1016/j.indcrop.2014.11.010. [DOI] [Google Scholar]
- 167.Jaeger H., Schulz M., Lu P., Knorr D. Adjustment of milling, mash electroporation and pressing for the development of a PEF assisted juice production in industrial scale. Innov. Food Sci. Emerg. Technol. 2012;14:46–60. doi: 10.1016/j.ifset.2011.11.008. [DOI] [Google Scholar]
- 168.Oroian M., Escriche I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015;74:10–36. doi: 10.1016/j.foodres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 169.Briones-Labarca V., Plaza-Morales M., Giovagnoli-Vicuña C., Jamett F. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: Effects of extraction conditions and methods. LWT Food Sci. Technol. 2015;60:525–534. doi: 10.1016/j.lwt.2014.07.057. [DOI] [Google Scholar]
- 170.Lee S.Y., Lee D.Y., Kim O.Y., Kang H.J., Kim H.S., Hur S.J. Overview of studies on the use of natural antioxidative materials in meat products. Food Sci. Anim. Resour. 2020;40:863–880. doi: 10.5851/kosfa.2020.e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Singh T.P., Singh P., Kumar P. Drumstick (Moringa oleifera) as a food additive in livestock products. Nutr. Food Sci. 2015;45 doi: 10.1108/NFS-02-2015-0018. [DOI] [Google Scholar]
- 172.Arranz E., Mes J., Wichers H.J., Jaime L., Mendiola J.A., Reglero G., Santoyo S. Anti-inflammatory activity of the basolateral fraction of Caco-2 cells exposed to a rosemary supercritical extract. J. Funct. Foods. 2015;13:384–390. doi: 10.1016/j.jff.2015.01.015. [DOI] [Google Scholar]
- 173.Teixeira B., Marques A., Ramos C., Neng N.R., Nogueira J.M.F., Saraiva J.A., Nunes M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013;43:587–595. doi: 10.1016/j.indcrop.2012.07.069. [DOI] [Google Scholar]
- 174.Peng C.-H., Su J.-D., Chyau C.-C., Sung T.-Y., Ho S.-S., Peng C.-C., Peng R.Y. Supercritical fluid extracts of rosemary leaves exhibit potent anti-inflammation and anti-tumor effects. Biosci. Biotechnol. Biochem. 2007;71:2223–2232. doi: 10.1271/bbb.70199. [DOI] [PubMed] [Google Scholar]
- 175.Bellumori M., Michelozzi M., Innocenti M., Congiu F., Cencetti G., Mulinacci N. An innovative approach to the recovery of phenolic compounds and volatile terpenes from the same fresh foliar sample of Rosmarinus officinalis L. Talanta. 2015;131:81–87. doi: 10.1016/j.talanta.2014.07.073. [DOI] [PubMed] [Google Scholar]
- 176.Mulinacci N., Innocenti M., Bellumori M., Giaccherini C., Martini V., Michelozzi M. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta. 2011;85:167–176. doi: 10.1016/j.talanta.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 177.Chauhan P., Pradhan S.R., Das A., Nanda P.K., Bandyopadhyay S., Das A.K. Inhibition of lipid and protein oxidation in raw ground pork by Terminalia arjuna fruit extract during refrigerated storage. Asian Australas. J. Anim. Sci. 2019;32:265–273. doi: 10.5713/ajas.17.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mathew S., Abraham T.E. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 2006;94:520–528. doi: 10.1016/j.foodchem.2004.11.043. [DOI] [Google Scholar]
- 179.Cheng J.R., Liu X.M., Zhang Y.S., Zhang Y.H., Chen Z.Y., Tang D.B., Wang J.Y. Protective effects of Momordica grosvenori extract against lipid and protein oxidation-induced damage in dried minced pork slices. Meat Sci. 2017;133:26–35. doi: 10.1016/j.meatsci.2017.04.238. [DOI] [PubMed] [Google Scholar]
- 180.Chan K.W., Khong N.M.H., Iqbal S., Ch’ng S.E., Younas U., Babji A.S. Cinnamon bark deodorised aqueous extract as potential natural antioxidant in meat emulsion system: A comparative study with synthetic and natural food antioxidants. J. Food Sci. Technol. 2014;51:3269–3276. doi: 10.1007/s13197-012-0818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shan B., Cai Y.Z., Sun M., Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005;53:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- 182.Mueller M., Hobiger S., Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010;122:987–996. doi: 10.1016/j.foodchem.2010.03.041. [DOI] [Google Scholar]
- 183.Lemay M. Anti-inflammatory phytochemicals: In vitro and ex vivo evaluation. Phytochemicals. 2006;1:41–60. [Google Scholar]
- 184.Yoshino K., Higashi N., Koga K. Antioxidant and antiinflammatory activities of oregano extract. J. Health Sci. 2006;52:169–173. doi: 10.1248/jhs.52.169. [DOI] [Google Scholar]
- 185.Sharma N., Biswas S., Al-Dayan N., Alhegaili A.S., Sarwat M. Antioxidant role of kaempferol in prevention of hepatocellular carcinoma. Antioxidants. 2021;10:1419. doi: 10.3390/antiox10091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kang F., Zhang S., Chen D., Tan J., Kuang M., Zhang J., Zeng G., Xu K., Zou Z., Tan G. Biflavonoids from Selaginella doederleinii as potential antitumor agents for intervention of non-small cell lung cancer. Molecules. 2021;26:5401. doi: 10.3390/molecules26175401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Grenier A., Legault J., Pichette A., Jean L., Bélanger A., Pouliot R. Antioxidant, anti-inflammatory, and anti-aging potential of a Kalmia angustifolia extract and identification of some major compounds. Antioxidants. 2021;10:1373. doi: 10.3390/antiox10091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tavsan Z., Kayali H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019;116:109004. doi: 10.1016/j.biopha.2019.109004. [DOI] [PubMed] [Google Scholar]
- 189.Chen Y.-C., Chia Y.-C., Huang B.-M. Phytochemicals from Polyalthia species: Potential and implication on anti-oxidant, anti-inflammatory, anti-cancer, and chemoprevention activities. Molecules. 2021;26:5369. doi: 10.3390/molecules26175369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.El-Maati M.F.A., Mahgoub S.A., Labib S.M., Al-Gaby A.M.A., Ramadan M.F. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. Eur. J. Integr. Med. 2016;8:494–504. doi: 10.1016/j.eujim.2016.02.006. [DOI] [Google Scholar]
- 191.Ishaq A., Syed Q.A., Khan M.I., Zia M.A. Characterising and optimising antioxidant and antimicrobial properties of clove extracts against food-borne pathogenic bacteria. Int. Food Res. J. 2019;26:1165–1172. [Google Scholar]
- 192.Multari S., Licciardello C., Caruso M., Martens S. Monitoring the changes in phenolic compounds and carotenoids occurring during fruit development in the tissues of four citrus fruits. Food Res. Int. 2020;134:109228. doi: 10.1016/j.foodres.2020.109228. [DOI] [PubMed] [Google Scholar]
- 193.Singh B., Singh J.P., Kaur A., Singh N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020;132:109114. doi: 10.1016/j.foodres.2020.109114. [DOI] [PubMed] [Google Scholar]
- 194.Gómez-Mejía E., Rosales-Conrado N., León-González M.E., Madrid Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019;295:289–299. doi: 10.1016/j.foodchem.2019.05.136. [DOI] [PubMed] [Google Scholar]
- 195.Czech A., Zarycka E., Yanovych D., Zasadna Z., Grzegorczyk I., Kłys S. Mineral content of the pulp and peel of various citrus fruit cultivars. Biol. Trace Elem. Res. 2020;193:555–563. doi: 10.1007/s12011-019-01727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nieto G., Fernández-López J., Pérez-Álvarez J.A., Peñalver R., Ros-Berruezo G., Viuda-Martos M. Valorization of citrus co-products: Recovery of bioactive compounds and application in meat and meat products. Plants. 2021;10:1069. doi: 10.3390/plants10061069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Radović M., Milatović D., Tešić Ž., Tosti T., Gašić U., Dojčinović B., Dabić Zagorac D. Influence of rootstocks on the chemical composition of the fruits of plum cultivars. J. Food Compos. Anal. 2020;92:103480. doi: 10.1016/j.jfca.2020.103480. [DOI] [Google Scholar]
- 198.Abeysinghe D.C., Li X., Sun C., Zhang W., Zhou C., Chen K. Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem. 2007;104:1338–1344. doi: 10.1016/j.foodchem.2007.01.047. [DOI] [Google Scholar]
- 199.Nayak B., Dahmoune F., Moussi K., Remini H., Dairi S., Aoun O., Khodir M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015;187:507–516. doi: 10.1016/j.foodchem.2015.04.081. [DOI] [PubMed] [Google Scholar]
- 200.Papoutsis K., Pristijono P., Golding J.B., Stathopoulos C.E., Bowyer M.C., Scarlett C.J., Vuong Q.V. Optimisation of aqueous extraction conditions for the recovery of phenolic compounds and antioxidants from lemon pomace. Int. J. Food Sci. Technol. 2016;51:2009–2018. doi: 10.1111/ijfs.13168. [DOI] [Google Scholar]
- 201.Tan C.Y., Zhong R.Z., Tan Z.L., Han X.F., Tang S.X., Xiao W.J., Sun Z.H., Wang M. Dietary inclusion of tea catechins changes fatty acid composition of muscle in goats. Lipids. 2011;46:239–247. doi: 10.1007/s11745-010-3477-1. [DOI] [PubMed] [Google Scholar]
- 202.Valencia I., O’Grady M.N., Ansorena D., Astiasarán I., Kerry J.P. Enhancement of the nutritional status and quality of fresh pork sausages following the addition of linseed oil, fish oil and natural antioxidants. Meat Sci. 2008;80:1046–1054. doi: 10.1016/j.meatsci.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 203.Shi C., Cui J., Yin X., Luo Y., Zhou Z. Grape seed and clove bud extracts as natural antioxidants in silver carp (Hypophthalmichthys molitrix) fillets during chilled storage: Effect on lipid and protein oxidation. Food Control. 2014;40:134–139. doi: 10.1016/j.foodcont.2013.12.001. [DOI] [Google Scholar]
- 204.Shi J., Yu J., Pohorly J.E., Kakuda Y. Polyphenolics in grape seeds—biochemistry and functionality. J. Med. Food. 2003;6:291–299. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- 205.Hygreeva D., Pandey M.C., Chauhan O.P. Effect of high-pressure processing on quality characteristics of precooked chicken patties containing wheat germ oil wheat bran and grape seed extract. J. Food Process. Preserv. 2017;41:e12980. doi: 10.1111/jfpp.12980. [DOI] [Google Scholar]
- 206.Silván J.M., Mingo E., Hidalgo M., de Pascual-Teresa S., Carrascosa A.V., Martinez-Rodriguez A.J. Antibacterial activity of a grape seed extract and its fractions against Campylobacter spp. Food Control. 2013;29:25–31. doi: 10.1016/j.foodcont.2012.05.063. [DOI] [Google Scholar]
- 207.Libera J., Latoch A., Wójciak K.M. Utilization of grape seed extract as a natural antioxidant in the technology of meat products inoculated with a probiotic strain of LAB. Foods. 2020;9:103. doi: 10.3390/foods9010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dimitrijevic M., Jovanovic V.S., Cvetkovic J., Mihajilov-Krstev T., Stojanovic G., Mitic V. Screening of antioxidant, antimicrobial and antiradical activities of twelve selected Serbian wild mushrooms. Anal. Methods. 2015;7:4181–4191. doi: 10.1039/C4AY03011G. [DOI] [Google Scholar]
- 209.Novakovic S., Djekic I., Klaus A., Vunduk J., Djordjevic V., Tomović V., Šojić B., Kocić-Tanackov S., Lorenzo J.M., Barba F.J., et al. The Effect of Cantharellus cibarius addition on quality characteristics of frankfurter during refrigerated storage. Foods. 2019;8:635. doi: 10.3390/foods8120635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ramírez-Rojo M.I., Vargas-Sánchez R.D., Torres-Martínez B., Torres-Martínez B.D.M., Torrescano-Urrutia G.R., Lorenzo J.M., Sánchez-Escalante A. Inclusion of ethanol extract of mesquite leaves to enhance the oxidative stability of pork patties. Foods. 2019;8:631. doi: 10.3390/foods8120631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Goulas V., Georgiou E. Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: Extraction optimization and application in food models. Foods. 2020;9:20. doi: 10.3390/foods9010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rathour M., Malav O.P., Kumar P., Chatli M.K., Mehta N. Functional chevon rolls fortified with cinnamon bark and Aloe-vera powder extracts. Haryana Vet. 2019;58:1–5. [Google Scholar]
- 213.Jagtap N.S., Wagh R.V., Chatli M.K., Malav O.P., Kumar P., Mehta N. Chevon meat storage stability infused with response surface methodology optimized Origanum vulgare leaf extracts. Agric. Res. 2020;9:464. doi: 10.1007/s40003-020-00464-5. [DOI] [Google Scholar]
- 214.Jagtap N.S., Wagh R.V., Chatli M.K., Kumar P., Malav O.P., Mehta N. Optimisation of extraction protocol for Carica papaya L. to obtain phenolic rich phyto-extract with prospective application in chevon emulsion system. J. Food Sci. Technol. 2019;56:3456. doi: 10.1007/s13197-018-3456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Birla R., Malav O., Wagh R., Mehta N., Kumar P., Chatli M. Storage stability of pork emulsion incorporated with Arjuna (Terminalia arjuna) tree bark extract. Int. J. Livest. Res. 2019;9:1. doi: 10.5455/ijlr.20180724064413. [DOI] [Google Scholar]
- 216.Bishnoi S., Ahlawat S.S. Development of buffalo meat rolls incorporated with aloe vera gel and arjun tree bark extract. Haryana Vet. 2015;54:174–177. [Google Scholar]
- 217.Zhang H., Peng X., Li X., Wu J., Guo X. The application of clove extract protects chinesestyle sausages against oxidation and quality deterioration. Korean J. Food Sci. Anim. Resour. 2017;37:114–122. doi: 10.5851/kosfa.2017.37.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kumar P., Mehta N., Malav O.P., Kumar Chatli M., Rathour M., Kumar Verma A. Antioxidant and antimicrobial efficacy of watermelon rind extract (WMRE) in aerobically packaged pork patties stored under refrigeration temperature (4 ± 1 °C) J. Food Process. Preserv. 2018;42:e13757. doi: 10.1111/jfpp.13757. [DOI] [Google Scholar]
- 219.Wagh R.V., Chatli M.K. Response surface optimization of extraction protocols to obtain phenolic rich antioxidant from sea buckthorn and their potential application into model meat system. J. Food Sci. Technol. 2017;54:1565–1576. doi: 10.1007/s13197-017-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Das A.K., Rajkumar V., Verma A.K., Swarup D. Moringa oleiferia leaves extract: A natural antioxidant for retarding lipid peroxidation in cooked goat meat patties. Int. J. food Sci. Technol. 2012;47:585–591. doi: 10.1111/j.1365-2621.2011.02881.x. [DOI] [Google Scholar]
- 221.Muthukumar M., Naveena B.M., Vaithiyanathan S., Sen A.R., Sureshkumar K. Effect of incorporation of Moringa oleifera leaves extract on quality of ground pork patties. J. Food Sci. Technol. 2014;51:3172–3180. doi: 10.1007/s13197-012-0831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mansour E.H., Khalil A.H. Evaluation of antioxidant activity of some plant extracts and their application to ground beef patties. Food Chem. 2000;69:135–141. doi: 10.1016/S0308-8146(99)00234-4. [DOI] [Google Scholar]
- 223.Cao Y., Gu W., Zhang J., Chu Y., Ye X., Hu Y., Chen J. Effects of chitosan, aqueous extract of ginger, onion and garlic on quality and shelf life of stewed-pork during refrigerated storage. Food Chem. 2013;141:1655–1660. doi: 10.1016/j.foodchem.2013.04.084. [DOI] [PubMed] [Google Scholar]
- 224.Fernández-López J., Sevilla L., Sayas-Barberá E., Navarro C., Marín F., Pérez-Alvarez J.A. Evaluation of the antioxidant potential of hyssop (Hyssopus officinalis L.) and Rosemary (Rosmarinus officinalis L.) extracts in cooked pork meat. J. Food Sci. 2003;68:660–664. doi: 10.1111/j.1365-2621.2003.tb05727.x. [DOI] [Google Scholar]
- 225.Akarpat A., Turhan S., Ustun N.S. Effects of hot-water extracts from myrtle, rosemary, nettle and lemon balm leaves on lipid oxidation and colour of beef patties during frozen storage. J. Food Process. Preserv. 2008;32:117–132. doi: 10.1111/j.1745-4549.2007.00169.x. [DOI] [Google Scholar]
- 226.Rababah T.M., Ereifej K.I., Alhamad M.N., Al-Qudah K.M., Rousan L.M., Al-Mahasneh M.A., Al-u’datt M.H., Yang W. Effects of green tea and grape seed and TBHQ on physicochemical properties of baladi goat meats. Int. J. Food Prop. 2011;14:1208–1216. doi: 10.1080/10942911003637327. [DOI] [Google Scholar]
- 227.Garrido M.D., Auqui M., Martí N., Linares M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT Food Sci. Technol. 2011;44:2238–2243. doi: 10.1016/j.lwt.2011.07.003. [DOI] [Google Scholar]
- 228.Lee M.-A., Choi J.-H., Choi Y.-S., Han D.-J., Kim H.-Y., Shim S.-Y., Chung H.-K., Kim C.-J. The antioxidative properties of mustard leaf (Brassica juncea) kimchi extracts on refrigerated raw ground pork meat against lipid oxidation. Meat Sci. 2010;84:498–504. doi: 10.1016/j.meatsci.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 229.Huang B., He J., Ban X., Zeng H., Yao X., Wang Y. Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat Sci. 2011;87:46–53. doi: 10.1016/j.meatsci.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 230.Yogesh K., Jha S.N., Yadav D.N. Antioxidant Activities of Murraya koenigii (L.) spreng berry extract: Application in refrigerated (4 ± 1 °C) stored meat homogenates. Agric. Res. 2012;1:183–189. doi: 10.1007/s40003-012-0018-6. [DOI] [Google Scholar]
- 231.Das A.K., Rajkumar V., Nanda P.K., Chauhan P., Pradhan S.R., Biswas S. Antioxidant efficacy of litchi (Litchi chinensis Sonn.) pericarp extract in sheep meat nuggets. Antioxidants. 2016;5:16. doi: 10.3390/antiox5020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Andrés A.I., Petrón M.J., Adámez J.D., López M., Timón M.L. Food by-products as potential antioxidant and antimicrobial additives in chill stored raw lamb patties. Meat Sci. 2017;129:62–70. doi: 10.1016/j.meatsci.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 233.Thomas R., Jebin N., Saha R., Sarma D.K. Antioxidant and antimicrobial effects of kordoi (Averrhoa carambola) fruit juice and bamboo (Bambusa polymorpha) shoot extract in pork nuggets. Food Chem. 2016;190:41–49. doi: 10.1016/j.foodchem.2015.05.070. [DOI] [PubMed] [Google Scholar]
- 234.Vargas-Ramella M., Lorenzo J.M., Zamuz S., Valdés M.E., Moreno D., Balcázar M.C.G., Fernández-Arias J.M., Reyes J.F., Franco D. The Antioxidant effect of Colombian berry (Vaccinium meridionale Sw.) extracts to prevent lipid oxidation during pork patties shelf-life. Antioxidants. 2021;10:1290. doi: 10.3390/antiox10081290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pasukamonset P., Kwon O., Adisakwattana S. Oxidative stability of cooked pork patties incorporated with clitoria ternatea extract (blue pea flower petal) during refrigerated storage. J. Food Process. Preserv. 2017;41:e12751. doi: 10.1111/jfpp.12751. [DOI] [Google Scholar]
- 236.De Florio Almeida J., dos Reis A.S., Heldt L.F.S., Pereira D., Bianchin M., de Moura C., Plata-Oviedo M.V., Haminiuk C.W.I., Ribeiro I.S., da Luz C.F.P., et al. Lyophilized bee pollen extract: A natural antioxidant source to prevent lipid oxidation in refrigerated sausages. LWT Food Sci. Technol. 2017;76:299–305. doi: 10.1016/j.lwt.2016.06.017. [DOI] [Google Scholar]
- 237.Baldin J.C., Michelin E.C., Polizer Y.J., Rodrigues I., de Godoy S.H.S., Fregonesi R.P., Pires M.A., Carvalho L.T., Fávaro-Trindade C.S., de Lima C.G., et al. Microencapsulated jabuticaba (Myrciaria cauliflora) extract added to fresh sausage as natural dye with antioxidant and antimicrobial activity. Meat Sci. 2016;118:15–21. doi: 10.1016/j.meatsci.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 238.Munekata P.E.S., Calomeni A.V., Rodrigues C.E.C., Fávaro-Trindade C.S., Alencar S.M., Trindade M.A. Peanut skin extract reduces lipid oxidation in cooked chicken patties. Poult. Sci. 2015;94:442–446. doi: 10.3382/ps/pev005. [DOI] [PubMed] [Google Scholar]
- 239.Angeletti M., Sparapani L. Process for Producing a Grape Seed Extract Having a Low Content of Monomeric Polyphenols. U.S. Patent 20,070,134,354, A1. 2006 July 12;
- 240.Plant Extracts Market Size and Forecast. [(accessed on 25 July 2021)]. Available online: https://www.verifiedmarketresearch.com/product/global-plant-extracts-market-size-and-forecast-to-2025/
- 241.Beya M.M., Netzel M.E., Sultanbawa Y., Smyth H., Hoffman L.C. Plant-based phenolic molecules as natural preservatives in comminuted meats: A review. Antioxidants. 2021;10:263. doi: 10.3390/antiox10020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Martínez-Graciá C., González-Bermúdez C.A., Cabellero-Valcárcel A.M., Santaella-Pascual M., Frontela-Saseta C. Use of herbs and spices for food preservation: Advantages and limitations. Curr. Opin. Food Sci. 2015;6:38–43. doi: 10.1016/j.cofs.2015.11.011. [DOI] [Google Scholar]
- 243.Mbah C.J., Orabueze I., Okorie N.H. Antioxidants properties of natural and synthetic chemical compounds: Therapeutic effects on biological system. Acta Sci. Pharm. Sci. 2019;3:28–42. doi: 10.31080/ASPS.2019.03.0273. [DOI] [Google Scholar]
- 244.Pokorný J. Are natural antioxidants better—And safer—Than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007;109:629–642. doi: 10.1002/ejlt.200700064. [DOI] [Google Scholar]