Abstract
Several studies have investigated the beneficial effects of vitamin D on survival of cancer patients. Overall evidence has been accumulating with contrasting results. This paper aims at narratively reviewing the existing articles examining the link between vitamin D supplementation and cancer mortality. We performed two distinct searches to identify observational (ObS) studies and randomized clinical trials (RCTs) of vitamin D supplementation (VDS) in cancer patients and cohorts of general population, which included cancer mortality as an outcome. Published reports were gathered until March 2021. We identified 25 papers published between 2003 and 2020, including n. 8 RCTs on cancer patients, n. 8 population RCTs and n. 9 ObS studies. There was some evidence that the use of VDS in cancer patients could improve cancer survival, but no significant effect was found in population RCTs. Some ObS studies reported evidence that VDS was associated with a longer survival among cancer patients, and only one study found an opposite effect. The findings do not allow conclusive answers. VDS may have the potential as treatment to improve survival in cancer patients, but further investigations are warranted. We strongly support investment in well-designed and sufficiently powered RCTs to fully evaluate this association.
Keywords: cancer, vitamin D, supplementation, mortality, survival
1. Introduction
Vitamin D is highly important for bone health and mineral metabolism, but more recently interest has been driven by its non-musculoskeletal functions. Vitamin D deficiency is linked with numerous illnesses, including osteoporosis and osteomalacia, autoimmune disorders, infectious diseases, muscle weakness and falls, cardiovascular diseases (CVDs), cancers, and neurological disorders [1]. The vitamin D status in the body is mainly dependent on the exposure to ultraviolet (UV) light. It is well established that the main source of vitamin D3 occurs via skin exposure to UV light from the sun rather than by food intake. Vitamin D production is initiated in the skin by UVB with the conversion of 7-dehydrocholesterol into pre-vitamin D3 in the skin, followed by two successive hydroxylations in the liver, to produce 25-hydroxycholecalciferol [25(OH)D] and then in the kidneys to produce the active metabolite, 1,25-dihydroxycholecalciferol [1,25(OH)2D]. Dietary intake of vitamin D, mainly from fish oils, fish, egg yolks, and to a lesser extent mushrooms, and from dietary supplements, accounts for a small amount of the daily vitamin D requirement. Inadequate exposure to sunlight and dietary intake of vitamin D may affect cancer incidence and mortality [2,3].
Evidence from observational studies indicates that low vitamin D status is associated with higher mortality for cancer and cardiovascular disease [4,5]. Several epidemiological studies, including both ObS studies and RCTs, have investigated the alleged beneficial effects of vitamin D on survival and mortality of patients with cancer. Vitamin D acts as an effective regulator of cell growth and differentiation in several different cell types, including cancer cells [6]. Findings from ObS studies constitute evidence suggestive of a relationship between Vitamin D and cancer survival and mortality, but they are insufficient to establish causality. A recent critical appraisal of meta-analyses found that vitamin D supplementation did not affect cancer incidence, but a weak reduced total cancer mortality risk emerged from the analyses’ results. Specifically, five out of six meta-analyses reported a risk reduction up to 16%, due to trials with small sample size and follow-up and not adequately powered to detect cancer outcomes [7].
Numerous trials assessing the effect of vitamin D supplementation on different outcomes are currently available. Among these, the VITAL, RECORD and ViDA trials are the largest in terms of number of participants. The VITAL (Vitamin D and Omega 3 Trial) randomized more than 25,000 participants for the prevention of cancer and cardiovascular disease among men 50 years of age or older, and women 55 years of age or older in the United States [8]. The RECORD Trial recruited more than 5000 participants aged at least 70 years reporting fragility fracture within the last 10 years from 21 orthopedic centers in the United Kingdom (https://www.thelancet.com/protocol-reviews/02PRT-35 accessed on 19 July 2021). Participants were randomly allocated to daily vitamin D3 (800 IU), calcium (1000 mg), both or placebo for 24–62 months, with a follow-up of 3 years after intervention. The Vitamin D Assessment (ViDA) study was a RCT carried out in New Zealand to examine whether high-dose vitamin D supplementation received monthly, without calcium, was associated with a reduction in cancer incidence and cancer mortality in community adults (in post hoc analysis). None of these trials confirmed the benefit of vitamin D3 supplementation on overall mortality [9,10,11].
Our group previously examined the mortality in subjects who participated in RCTs, testing the impact of vitamin D supplementation (i.e., vitamin D2 or vitamin D3) on any health condition [12]. This meta-analysis pooled 18 trials among various study populations vitamin D concentrations, followed for a mean 5.7 years. We found that ordinary doses of vitamin D supplements (300 to 2000 IU) were associated with a significant decrease in total mortality rates and no between-study heterogeneity.
The past two decades have witnessed a vigorous increase in interest in vitamin D from both the lay and biomedical worlds. Much of the growing interest is powered by new data available and the interest in repurposing drugs as anticancer therapeutics that could shorten the conventional investigational pathway and open multiple new avenues of investigation [13]. Because of the conflicting evidence, limitations of previous reviews and availability of new data, we here present a narrative review of RCTs and ObS studies examining the impact of vitamin D supplementation on cancer mortality.
2. Materials and Methods
A systematic literature search was conducted. We performed two distinct searches to identify RCTs and ObS studies. RCTs were proposed in cancer patients or in cohorts of general population in which vitamin D supplementation was provided and cancer mortality was reported as a trial outcome. We searched for ObS studies with different study designs investigating the association between previous vitamin D supplementation and reporting cancer mortality estimates.
Published reports were gathered from the following databases: PUBMED, EMBASE and ISI Web of Knowledge up until March 2021.
We searched the following MeSH terms and keywords: “supplementation”, “Vitamin D” or “cholecalciferol”, “RCT” or “epidemiologic studies” or “cohort”, “cancer”, “neoplasm” or “tumor”, and “mortality”, “survival” and “outcome”, without any restriction. We also performed manual search of references cited in the retrieved articles and preceding reviews on the topic.
Titles and abstracts were screened by two researchers (P.G. and V.M.), who then assessed full texts for eligibility.
The inclusion criteria were based on the PICO’S framework [14]. Regarding participants, we considered all the individuals over the age of 18 years. All the selected studies present an intervention with vitamin D supplementation or a reported past use of vitamin D supplementation. The supplemented groups were compared with those treated with a placebo, a lower dose of vitamin D or no use of vitamin D supplementation. The main outcome was cancer mortality or all measures of survival, progression-free survival (PFS) and overall survival (OS) in cancer patients. According to the study design, both RTC and ObS were included.
Data Extraction
A standardized data-collection protocol was used to gather the relevant data from each selected article. The data from eligible studies were extracted into a designed database, including the following information from each publication: authors, journal and year of publication, country, study population, type of study and cohort/trial name, sample size, sex distribution, mean age and standard deviation (SD) or range, inclusion criteria, primary and secondary outcomes. Concerning vitamin D supplementation, we recorded number of study arms, type of supplementation, daily or weekly dose and duration of use, comparators and time of follow-up. When available, we reported the measured outcome fully adjusted (e.g., Hazard Ratio (HR) for OS, disease free-survival (DFS) or Relative Risk (RR)). Due to the relevance of possible confounders in the studied association, only adjusted estimates were considered. We considered the placebo arm group as reference group in our analyses for RCT and vitamin D, no users in ObS studies. Because of the heterogeneity of studies and the lack of appropriate control of major confounding factors, we decided that it was not appropriate to perform a meta-analysis of their results.
Articles were reviewed and data were extracted and crosschecked independently by two investigators (V.M. and P.G.). Any disagreement at any stage was resolved by consensus among the two or within the working group.
3. Results
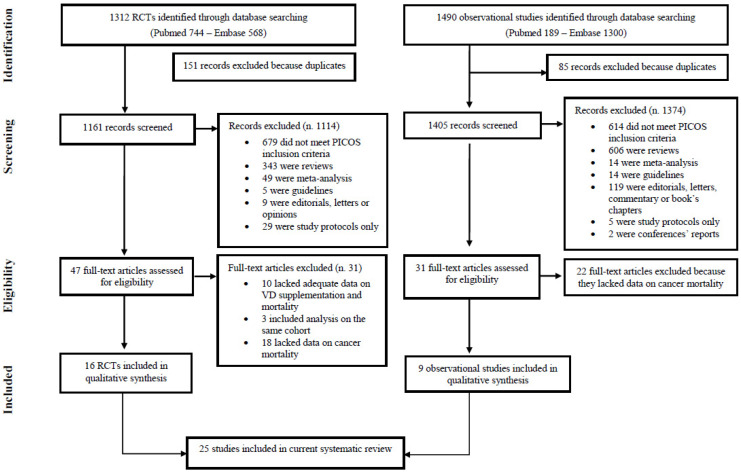
The literature search yielded a total of 1312 RCTs and 1490 ObS studies, according to the two searching strategies. Figure 1 represents the double flowchart of the selection process. Regarding the RCTs, after eliminating the duplicates, we found 1161 publications, of which 47 were assessed for eligibility. Upon subsequent review of the full-text articles identified, we excluded further 31 studies either because they lacked adequate data on the association between vitamin D supplementation and mortality, were not independent, or in which data on cancer mortality were missing. As a result, this review covers 16 RCTs. We performed the same selection process for observational studies. Likewise, after eliminating the duplicates, we collected 1405 records, of which 31 were assessed for eligibility. The assessment of the full text articles led us to exclude additional 22 studies which lacked data on cancer mortality. Finally, nine ObS studies were included in the review.
Figure 1.
Flowchart of study selection.
General Characteristics of Studies
The main characteristics of the included studies are summarized in Table 1a–c. Among the 16 identified RCTs, eight were carried out on cancer patients [15,16,17,18,19,20,21,22] (Table 1a) and eight were population trials [9,10,11,23,24,25,26,27] (Table 1b); as previously reported, the ObS studies were 9 [28,29,30,31,32,33,34,35] (Table 1c).
Table 1.
Main characteristics of included studies (n. 25 articles: n. 8 RCT on cancer patients, n. 8 population RCT, n. 9 observational studies).
| (a) Main Characteristics of RCTs in Cancer Patients | |||||||
|---|---|---|---|---|---|---|---|
| First Author, Publication Year, Study Name | Cancer Site | Country | Participants | Sex | Age | Inclusion Criteria | Primary and Secondary Outcomes |
| Beer, 2007 [15] ASCENT | Prostate | US | 250 | Males | Range 45–92 | Progressive metastatic androgen-independent prostate cancer—serum PSA 5.0 ng/mL, serum testosterone level 50 ng/dL, Eastern Cooperative Oncology Group performance status 2, life expectancy 3 months, age 18 yrs. | PSA reduction, OS, PSA, tumour, and clinical PFS, TRR measurable disease, skeletal morbidity-free survival, as well as safety and tolerability of the study treatment. |
| Attia, 2008 [16] | Prostate | US | 70 | Males | Range 52–85 | >18 yrs of age, histologic diagnosis of prostate adenocarcinoma, radiographic evidence of metastasis, chemotherapy naive | PSA reduction, PFS, OS, ORR and toxicity |
| Scher, 2011 [17] ASCENT | Prostate | US | 953 | Males | 70.9 VD and 70.4 control | Pathologically or cytologically proven adenocarcinoma of the prostate, metastatic disease and disease progression after medical or surgical castration (CRPC) | OS, thromboembolic event rates |
| Akiba, 2018 [18] | NSCLC | Japan | 155 | Males and females | 68 (SD 9) | NSCLC (stage IA to IIIA), aged 20 to 75 yrs at entry; diagnosed and operated at one of four Jikei University Hospitals; tumour totally resected; no major complications; followed-up for as long as possible | RFS, OS |
| Golubic, 2018 [19] | CRC metastatic | Croatia | 72 | Males and females | 69 (range 24–79) | CRC metastatic and 25(OH)D levels <75 nmol/L | OS, PFS |
| Ng, 2019 [20] SUNSHINE | CRC metastatic | US | 139 | Males and females | 56 (Range 47–65) | Pathologically confirmed, unresectable locally advanced or metastatic CRC, no prior treatment, no previous VD supplementation | PFS, ORR, OS and change in plasma 25(OH)D level |
| Urashima, 2019 [21] AMATERASU | Digestive tract | Japan | 417 | Males and females | 66 (Range 30–90) | Post-operative digestive tract cancer from the esophagus to the rectum, stages I to III, taking VD supplements or active VD; no history of urinary tract stones | RFS, OS, relapse, cancer-specific death, and no cancer death |
| Yonaga, 2019 [22] AMATERASU | Digestive tract | Japan | 400 | Males and females | Range 35–90 | Post-operative digestive tract cancer from the esophagus to the rectum, stages I to III, taking VD supplements; no history of urinary tract stones | RFS, OS, relapse, cancer-specific death, and no cancer death |
| (b) Main Characteristics of Population RCTs | |||||||
| First Author, Publication Year, Study Name | Health Status | Country | Participants | Sex | Age | Inclusion Criteria | Primary and Secondary Outcomes |
| Trivedi, 2003 [23] | General population | UK | 2686 | Males and females | VD group 74.8 (SD 4.6) and Placebo group 74.7 (SD 4.6) (range 65-85) | Fracture incidence | Fracture incidence and total mortality by cause. |
| Wactawski-Wende, 2006 [24] WHI |
Postmenopausal women | US | 36,282 | Female | Range 50–79 | Postmenopausal women 50 to 79 yrs enrolled in the WHI randomized trials | Prevent hip fracture, CRC |
| Chlebowski, 2008 [25] WHI | Postmenopausal women | US | 36,282 | Female | Range 50–79 | Postmenopausal women 50 to 79 yrs enrolled in the WHI randomized trials | Hip fracture, breast and CRC |
| Brunner, 2011 [26] WHI | Postmenopausal women | US | 36,282 | Female | Range 50–79 | Postmenopausal women 50 to 79 yrs enrolled in the WHI randomized trials | Prevent other fractures, or CRC |
| Avenell, 2012 [9] RECORD | Elderly | UK | 5292 | Males and females | 77 (SD 6) | Fragility fracture within the last 10 yrs and aged at least 70 yrs | All-cause mortality, CVD mortality, cancer mortality, and cancer incidence |
| Ammann,2017 [27] WHI | Postmenopausal women |
US | 34,763 | Female | Range 58-69 | Postmenopausal women 50 to 79 yrs enrolled in the WHI randomized trials | Hip fracture, breast and CRC |
| Scragg, 2018 [11] VIDA | Community adults | NZ | 5108 | Males and females | 65.9 (SD 8.3) (range 50–84) | 50 to 84 yrs; resident of Auckland, New Zealand, at the time of recruitment; and anticipated residence in New Zealand for the 4-yrs study period | Cancer incidence and cancer mortality (Primary aim: assess the effect of VD suppl. on incidence of CVD) |
| Manson, 2019 [10] VITAL | General population | US | 25,871 | Males and females | 67.1 (SD 7.1) | Men 50 yrs of age or older and women 55 yrs of age or older in the US | Cancer of any type and major CVE, site-specific cancers, cancer mortality, and additional CVE |
| (c) Main Characteristics of Observational Studies | |||||||
| First Author, Publication Year, Study Name | Health Status | Country | Participants | Sex | Age | Inclusion Criteria | Primary and Secondary Outcomes |
| Poole, 2013 [35] (ABCPP) |
Breast | US and China | 12019 | Female | 58.0 (10.0) * | Breast cancer survivors, stage I–IV stage | BC recurrence, BC specific mortality, and all-cause mortality |
| Holm,2014 [28] (cohort study) |
Breast | DK | 1064 | Female | 62 (range 50–64) | Breast cancer diagnosis | BC specific mortality |
| Zeichner, 2015 [30](retrospective study) | Breast | US | 246 | Female | >50 years (users 53.0 SD 12.1) | VD with trastuzumab-based chemotherapy for HER2-positive (HER2D) nonmetastatic breast cancer | DFS, OS |
| Jeffreys, 2015 [33] (cancer register) | Breast, CRC, lung, ovarian or uterine | UK | 21565 | Female | >55 years | First diagnosis of breast, colorectal, lung, ovarian or uterine cancer in postmenopausal women identified at least 5 years of CPRD data prior to diagnosis and 3+ to 1–2 (but no more) VD prescriptions | Cancer survival |
| Wang, 2016 [32] (Longitudinal study) | Esophageal | China | 303 | Males and females | non-users 64.9 (SD 7.6) users 61.7 (SD 7.6) | Esophageal cancer patients undergoing esophagectomy post-surgery | QoL and survival |
| Lewis, 2016 [36] (cancer register) | CRC | US | 453 | Males and females | 63.3 (SD 10.4) | Stage II CRC | VD supplementation and QoL |
| Mulpur, 2016 [31] (case-control) | Glioblastoma | US | 470 | Males and females | 59 (median) (range 18-89) | >18 age, recent diagnosis of primary (nonrecurrent) GBM and undergoing treatment at participating medical and oncology centers in the South Eastern US | Associations of CAM use and GBM outcome/mortality |
| Madden, 2018 [35] (cancer register) | Breast | Ireland | 5417 | Female | non-users 68 (59–74) users 66 (59–73) (range 50–80) | Aged 50-80 yrs, stage I-II breast cancer diagnosis and no VD use in yr prior to diagnosis | BC specific mortality |
| Yokosawa, 2018 [29] (cohort study) | HNC | US | 434 | Males and females | NR | HNC diagnosis, >18 yrs | Death from any cause, HNC-specific death and recurrence of disease. |
25(OH)D: 25 hydroxyvitamin D; ASCENT: Androgen Independent Prostate Cancer Study of Calcitriol Enhancing Taxotere; CAM: Complementary and Alternative Medicine; CPRD: Clinical Practice Research Datalink, UK; CRPC: Metastatic Castration-Resistant Prostate Cancer; CRC: Colon Rectal Cancer; CVE: Cardiovascular Event; CVD: Cardiovascular Disease; DFS: disease-free survival; GBM: Glioblastoma; GP: General Practice; HNC: Head and Neck Cancer; NSCLC: Non-Small-Cell Lung Cancer; ORR: Overall Response Rate; OS: Overall Survival; PFS: Progression-Free Survival; PSA: Prostate-Specific Antigen; QoL: Quality Of Life; RECORD: Randomized Evaluation of Calcium Or vitamin D; RFS: Relapse-Free Survival; TRR: Tumor Regression Rates; VD: Vitamin D; VIDA: the VItamin D Assessment; VITAL: The VITamin D and OmegA-3 TriaL; WHI: Women’s Health Initiative; Yrs: years; ABCPP: After Breast Cancer Pooling Project, consortium of four prospective cohorts. * age at diagnosis for users of single supplement (n. 5279).
Regarding RCTs on cancer patients, we included three publications on prostate, two of which were on the Androgen-Independent Prostate Cancer Study of Calcitriol Enhancing Taxotere (ASCENT) study [15,17], and two on digestive tract cancers (from esophagus to rectum), both of the AMATERASU trial, a randomized, double-blind, placebo-controlled trial conducted in Japan [21,22]. Two trials were conducted on metastatic colorectal cancer (CRC) [19,20], and one study on Non-Small-Cell Lung Cancer [18]. Altogether, four trials were conducted in North America [15,16,17,20], two in Japan [21,22], and one in Croatia [19], while publication year ranged from 2007 to 2019. In total, 1806 cancer survivors were randomly assigned to dietary supplements or placebo, ranging from 24 to 92 years of age (Table 1a).
Most of the population trials were conducted in the United States (five studies) [10,24,25,26,27], two were conducted in the United Kingdom [9,23], and only one in New Zealand [11]. We included four reports on the Women’s Health Initiative, which had the largest size (36,282 participants) among all included trials. This is a randomized controlled trial of Calcium/Vitamin D supplementation compared with placebo in postmenopausal women [24,25,26,27]. The remaining trials enrolled individuals of both sexes and the total sample size was 75,239 persons, ranging from 50 to 84 years of age (Table 1b).
Out of nine ObS studies included, two were cohort studies [28,29], one had a retrospective study design [30], one was a case–control study [31], one was a longitudinal study [32], three used data from the National/Local Cancer Registry [33,34,36] and one reported data of a consortium of four prospective cohorts from the United States and China including 12,000 breast cancer patients [35] (Table 1c). All studies were published between 2013 to 2018. The total sample size was 41,971 cancer survivors, ranging from 18 to 89 years of age at cancer diagnosis. Five studies included only women [28,30,33,34,35] and four both sexes [29,31,32,36]. The average follow-up time ranged from 1.8 to 13.1 years. In Table 1a–c are reported the inclusion criteria and primary and secondary outcomes.
Table 2a–c summarizes the main characteristics of the selected studies and the HR with corresponding 95% CI, adjusted for the maximum number of confounding variables for OS, cancer mortality, and cancer-specific mortality.
Table 2.
Details of the included studies (statistically significant estimates are in bold).
| (a) Details of RCTs in Cancer Patients (Statistically Significant Estimates Are in Bold) | ||||||||
|---|---|---|---|---|---|---|---|---|
| First Author, Publication Year, Study Name | Arms | Intervention Dose/Day | Comparator | Duration of Treatment/ Follow-Up |
Cancer Deaths/ Treatment |
Cancer Deaths/ Controls |
Contrast | Estimates |
| Beer, 2007 [15] ASCENT | 2 | 45mcg DN-101 on day 1, 8 and 15 + therapy * | Placebo | Every 3 weeks/18.3 mths | NR | NR | VDS vs. placebo | OS HR = 0.67 (95% CI = 0.45–0.97) |
| Attia, 2008 [16] | 2 | 10 mcg Doxercalciferol Orally/days 1–28 ** |
Placebo | Every 28 days/17.6 mths | 31 | 25 | VDS vs. placebo | OS Median 17.8 mths (95% CI = 14.9–23.6) vs. 16.4 mths (95% CI = 11.9-23.8) (P = 0.383) |
| Scher, 2011 [17] ASCENT | 2 | 45mcg DN-101 on day 1, 8 and 15 + therapy * | Placebo | Every 3 weeks/11.7 mths | 108 | 142 | VDS vs. placebo | OS Median 17.8 mths (95% CI = 16.0–19.5) vs. 20.2 mths (95% CI = 18.8–23.0) (log-rank P = 0.002). |
| Akiba, 2018 [18] | 2 | 1200 IU VD3/d | Placebo | 12 months/3.3 yrs | 40 | 24 | VDS vs. placebo | OS HR = 1.22 (95% CI = 0.54–2.79) |
| Golubic, 2018 [19] | 2 | 2000 IU/d + Standard chemotherapy | Placebo | 2 years/46 mths | NR | NR | VDS vs. placebo | OS HR = 1.01 (95% CI = 0.39–2.61) |
| Ng, 2019 [20] SUNSHINE | 2 | 8000 IU VD3/d followed by 4000 IU VD3/d *** | 400 IU/d Standard dose |
14 cycle/22.9 mths | 45 | 54 | High VDS vs. standard dose | OS Median 24.3 mths (95% CI = 19.0–33.2) vs. 24.3 mths (95% CI = 20.3–32.4) (log rank P = 0.43) OS HR = 0.64 (95% CI = 0–0.90) |
| Urashima, 2019 [21] AMATERASU | 2 | 2000 IU VD/d | Placebo | 3.5 yrs (median) | 27 | 16 | VDS vs. placebo | Cancer-specific death HR = 1.09 (95% CI = 0.58–2.01) |
| Yonaga, 2019 [22] AMATERASU | 2 | 2000 IU VD/d |
Placebo | 3.5 years (median) | Well D AC: 19 Moderately D AC: 15 Poorly D AC: 3 Signet-ring CC: 1 SCC: 7 |
Well D AC: 13 Moderately D AC: 9 Poorly D AC: 8 Signet-ring CC: 4 SCC: 3 |
VDS vs. placebo | Well D AC OS HR = 0.82 (95%CI = 0.40–1.65) Moderately D AC OS HR = 1.31 (95%CI = 0.57–2.99) Poorly D AC OS HR = 0.25 (95%CI = 0.07–0.94) Signet-ring CC OS HR = 0.30 (95%CI = 0.03–2.65) SCC - OS HR = 1.39 (95%CI = 0.35–5.49) |
| (b) Details of Population RCTs (In Bold Statistical Significant Estimates) | ||||||||
| First Author, Publication Year, Study Name | Arms | Intervention Dose/Day | Comparator |
Duration of Treatment/
Follow-Up |
Cancer Deaths/
Treatment |
Cancer Deaths/
Controls |
Contrast | Estimates |
| Trivedi, 2003 [23] | 2 | 100 000 IU cholecalciferol | Placebo | Every 4 months for 5 yrs/5yrs | 63 | 72 | VDS vs. placebo | Cancer mortality RR = 0.86 (95% CI = 0.61–1.20) |
| Wactawski-Wende, 2006 [24] WHI |
2 | Calcium elemental 1000 mg + 400 IU VD3/d (two doses) | Placebo | 7.0 ± 1.4 yrs | 34 | 41 | VDS vs. no VDS | CRC mortality HR = 0.82 (95% CI = 0.52–1.29) |
| Chlebowski, 2008 [25] WHI | 2 | Calcium elemental 1000 mg + 400 IU VD3/d (two doses) | Placebo | 7 yrs | 23 | 23 | VDS vs. no VDS | Breast cancer mortality HR = 0.99 (95% CI = 0.55–1.76) |
| Brunner, 2011 [26] WHI | 2 | Calcium elemental 1000 mg + 400 IU VD3/d (two doses) | Placebo | 7.0 ± 1.4 yrs | 315 | 347 | VDS vs. no VDS | Cancer mortality HR = 0.90 (95% CI = 0.77–1.05) |
| Avenell, 2012 [9] RECORD | 4 | 800 IU VD3/day + 1000 mg Calcium/d | Placebo | 3 yrs/ 6.2 yrs (median) |
151 | 178 | VDS vs. no VDS | Cancer mortality HR = 0.85 (95% CI= 0.68–1.06) |
| Ammann,2017 [27] WHI | 2 | Calcium elemental 1000 mg + 400 IU VD3/d (two doses) | Placebo | 7 yrs | NR | NR | Calcium/VDS vs. placebo | Hematologic cancer-specific mortality HR = 0.77 (95% CI = 0.53–1.11) |
| Scragg, 2018 [11] VIDA | 2 | 200,000 IU VD3 (initial bolus) followed by 100 000 IU/mths | Placebo | 3 yrs/ 3.3 yrs (median) |
44 | 45 | NR | Cancer mortality HR = 0.97 (95% CI = 0.64–1.47) |
| Manson, 2019 [10] VITAL | 2 | 2000 IU VD3 + ω3 = 1 g/d |
Placebo | 5 yrs/5.3 yrs (median) | 154 | 187 | NR | Cancer mortality HR = 0.83 (95% CI = 0.67–1.02) |
| (c) Details of Observational Studies in Cancer Patients (In Bold Statistical Significant Estimates) | ||||||||
| First Author, Publication Year, Study Name | Arms | Intervention Dose/Day | Comparator |
Duration of Treatment/
Follow-Up |
Cancer Deaths/
Treatment |
Cancer Deaths/
Controls |
Contrast | Estimates |
| Poole, 2013 [35] (ABCPP) |
2 | Regular VD use at least 1-yr post diagnosis | No VDS | 2.2 yrs (0.7) ^ | 41 | 808 | Users vs. non-users | BC mortality HR = 0.97 (95% CI = 0.68–1.38) |
| Holm,2014 [28] (cohort study) |
2 | VD use previous 12 months: low mcg (200 IU)/d); medium (5–10 mcg (400 IU)/d); high (>10 mcg (400 IU)/d). | No VDS | 6.3 yrs | 60 | 45 | for an increase in one category of the variable | BC mortality HR = 1.47 (95% CI = 1.07–2.00) |
| Zeichner, 2015 [30](retrospective study) | 2 | VD use during chemotherapy: <10,000 UI/week or >10,000 UI/week | No VD users | 29.5 mths | NR | NR | Users vs. non-users | OS HR = 0.31 (95% CI = 0.11–0.89) |
| Jeffreys, 2015 [33] (cancer register) | 2 | Any suppl 5 yrs prior to cancer diagnosis | No VDS | 30.4 mths | 314 | 1789 | Any vs. No suppl | BC survival HR = 0.78 (95% CI = 0.70–0.88) |
| 252 | 1474 | CRC survival HR = 0.90 (95% CI = 0.78–1.04) | ||||||
| 443 | 2313 | LC survival HR = 1.06 (95% CI = 0.96–1.17) | ||||||
| 134 | 1017 | GC survival HR = 0.89 (95% CI = 0.73–1.07) | ||||||
| ≥3 prescriptions 5 yrs prior to cancer diagnois | 1–2 prescriptions | 228 | 86 | ≥3 prescriptions vs. 1–2 prescriptions | BC survival HR = 1.02 (95% CI = 0.79–1.32) | |||
| 191 | 61 | CRC survival HR = 0.81 (95% CI = 0.59–1.11) | ||||||
| 323 | 120 | LC survival HR = 0.86 (95% CI = 0.70–1.07) | ||||||
| 98 | 36 | GC survival HR = 0.84 (95% CI = 0.59–1.30) | ||||||
| Wang, 2016 [32] (Longitudinal study) | 2 | Regular VD use after esophagectomy, during treatment and recovery phases: 200-400 IU/day | No VD users | 24-mths after surgery | NR | NR | Users vs. non-users | OS HR = 0.80 (95% CI = 0.51–1.24) |
| Lewis, 2016 [36] (cancer register) | 2 | Regular VD use 12 months prior to cancer diagnosis | No VD users | 24 months | NR | NR | Users vs. non-users | OS HR = 0.77 (95% CI = 0.37, 1.58) |
| Mulpur, 2016 [31] (case-control) | 2 | Regular VD use 5 yrs prior to cancer diagnosis | No VD users | 1.3 wks to 5.3 yrs | 373 | NR | Users vs. non-users | OS HR = 0.72 (95% CI = 0.52–0.99) |
| Madden, 2018 [35] (cancer register) | 2 | De novo VD use post diagnosis | No VD users | NR | 208 | 598 | Users vs. non-users Users (initiation < 180d) vs. non-users Users (initiation ≥ 180d) vs. non-users |
BC survival HR = 0.80 (95% CI = 0.64–0.99) BC survival HR = 0.51 (95% CI = 0.34–0.74) BC survival HR = 0.91 (95% CI = 0.70–1.18) |
| Yokosawa, 2018 [29] (cohort study) | 3 | Past use of VD: 0; 0-400 UI/day; ≥400 UI/day |
Level of VDS | NR | 32 | 28 | Use of ≥400 UI vs. 0 UI |
HNC survival HR = 1.11 (95% CI = 0.65–1.90) |
25(OH)D: 25 hydroxyvitamin D; AC: Adenocarcinoma; ASCENT: Androgen Independent Prostate Cancer Study of Calcitriol Enhancing Taxotere; BC: Breast Cancer; CAM: Complementary and Alternative Medicine; CRC: Colon Rectum Cancer; D: differentiated; GC: Gynecologic Cancer; HNC: Head and Neck Cancer; LC: Lung Cancer; Mths: months; NR: not reported; ORR: Overall Response Rate; OS: Overall Survival; PFS: Progression-Free Survival; RECORD: Randomized Evaluation of Calcium Or vitamin D; SCC,: squamous cell carcinoma; Signet-ring: signet-ring cell carcinoma; suppl: supplementation; VD: Vitamin D; VDS: Vitamin D supplementation; VIDA: the VItamin D Assessment; VITAL: The VITamin D and OmegA-3 TriaL; WHI: Women’s Health Initiative; wks: weeks; yrs: years. HR = hazard ratio; RR = relative risk. * Weekly dose of 36 mg/m2 of docetaxel and 24 mg of dexamethasone for 3 weeks of a 4-week cycle. ** In addition to a 4-week cycle of docetaxel (35 mg/m2 i.v., days 1, 8, and 15), daily oral doxercalciferol were 10 mcg (initial), 7.5 mcg (dose level-1), 5.0 mcg (dose level-2), and 2.5 mcg (dose level-3). *** A continuous infusion of 2400 mg/m2 of 5-fluorouracil (5-FU) over 46 to 48 h, a bolus of 400 mg/m2 of 5-FU, 400 mg/m2 of leucovorin, and 85 mg/m2 of oxaliplatin (mFOLFOX6) plus 5 mg/kg of bevacizumab administered intravenously every 14 days (cycle). ^ years between diagnosis and study entry for users of single supplement (n. 5279).
Regarding the RCTs on cancer patients, all the included studies were two-arms [15,16,17,18,19,20,21,22]. Four studies were placebo-controlled trials [18,19,21,22] consisting of a prescription of vitamin D, in doses of UI (International Units) compared to placebo. Only in the SUNSHINE trial [20] did the control group receive a standard dose of vitamin D (400 UI/day). The supplementation ranged from a minimum of 1200 UI to a maximum of 8000 UI, with a single dose a day. In two studies, the vitamin D supplementation was provided in association with prescribed therapy. In the ASCENT study, they administered 45 mcg of DN-101 (high dose of calcitriol) in association with 36 mg/m2 docetaxel, and 24 mg dexamethasone weekly for 3 weeks of a 4-week cycle [15,17], and in Attia [16] they administered a 4-week cycle of docetaxel (35 mg/m2 i.v., days 1, 8, and 15) with or without doxercalciferol (10 mcg orally, days 1–28) (Table 2a).
Among population RCTs, seven were two-arm trials [10,11,23,24,25,26,27] and only one was a four-arm trial [9]. Considering vitamin D supplementation, the intervention varied among the studies. In almost all the studies, the intervention was characterized by a daily dose of vitamin D with the exception of VIDA trial, in which a first load of 200,000 UI was followed by a monthly supplementation of 100,000 UI [11] (Table 2b).
In two of the analyzed cohorts, WHI and RECORD trial, the Vitamin D supplementation was associated with 1000 mg of calcium [9,24,25,26,27]. In the VITAL trial, 2000 UI of vitamin D were supplemented with 1 g of omega 3 fatty acids [10]. All the RCTs on population followed the intervention from a minimum of 3 years to a maximum of 7 years. None of the included studies reported a statistically significant effect of VDS compared to placebo group in term of overall cancer mortality or specific-cancer mortality.
In the included studies, OS was expressed as HR and in some cases as median in months [16,17,20] comparing VDS versus placebo. VDS was associated with a significant increased OS rate in three studies [15,20,22]. The strongest effect was found in the AMATERASU trial on cancer digestive tract [20]. In a post hoc analysis among patients with poorly differentiated adenocarcinoma, they found a 5-year OS rate of 92% in the vitamin D group compared with 72% in the placebo group (HR = 0.25; 95% CI, 0.07–0.94; P = 0.04). Considering the median OS expressed in months, Attia [16], Ng [20] and Scher [17] reported no significant difference between the treatment and the placebo or standard vitamin D dose groups (Table 2c).
Regarding observational studies, the included populations were stratified for different levels of supplementation, or for past vitamin D use. Jeffreys and colleagues analyzed the pre-diagnostic different VDS (three or more versus one to two prescriptions of vitamin D and any vitamin D prescription compared to no past use of vitamin D supplements) in a group of women with a first diagnosis of breast, CRC, lung, ovarian or uterine cancer between 2002 and 2009 [33]. Yokosawa considered three different levels of vitamin D supplementation (from 0 UI to 400 UI and >400 UI/die) [29]. The follow up ranged from 1.3 weeks to 7.0 ± 1.4 years (Table 2c).
The included studies reported the OS rate expressed as HR or cancer-specific survival rate. Four studies reported a statistically significant improved survival [30,31,33,34]. Mulpur et al. found that the use of vitamin D in GBM patients was associated with a reduced mortality, adjusting for age and other covariates (HR = 0.72; 95% CI 0.52–0.99) [31]. The remaining studies found a beneficial effect of VDS in breast cancer patients. Zeichner [30] found that VDS use was associated with an improved OS (HR, 0.31; 95% CI, 0.11–0.89) in women who received VD supplementation during neoadjuvant chemotherapy. Madden [34] found a 20% reduction in breast cancer-specific mortality in de novo vitamin D users compared to non-users (HR 0.80; 95% CI 0.64–0.99), analyzing records of invasive breast cancer patients identified on the National Cancer Registry Ireland database. The reduction was greater (HR 0.51; 95% CI 0.34–0.74) when vitamin D was initiated after the breast cancer diagnosis (within 6 months). Jeffreys [33] found that exposure to three or more versus one to two prescriptions of VD was not associated with survival from any of the four cancers studied (CRC, lung, gynecological, breast), but they found that any VD prescription, compared with no past prescriptions, was associated with a better survival from breast cancer (HR 0.78, 95% CI 0.70–0.88). Only Holm [28] reported a higher BC specific mortality (HR: 1.47; 95% CI, 1.07–2.00) in women with high pre-diagnostic intake of vitamin D supplements associated with hormone replacement therapy (HRT) (Table 2c).
4. Discussion
We conducted a narrative review to extensively address the effect of VDS use on overall cancer mortality or cancer-specific mortality, in RCTs conducted among cancer patients or the general population and in observational studies. This is the first narrative review to simultaneously and critically evaluate the scientific evidence of the effect of Vitamin D supplementation on cancer survival and mortality. Because of the heterogeneity of studies, we decided that it was not appropriate to perform a meta-analysis of their results. We identified 25 papers published between 2003 and 2020. There was limited evidence that the use of VDS could reduce cancer-related mortality among cancer patients, but no effect on mortality was found in population trials. Some observational studies reported evidence that VDS was associated with a longer survival among cancer patients, and only one study [28] found an opposite effect.
The reason for the divergent findings for cancer mortality is not clear. There are plausible mechanisms for the operation of vitamin D in decreasing tumor invasiveness and propensity to metastasize, and influencing immunomodulatory properties [37] that may contribute to reduced metastatic disease and fatal cancer [2]. Vitamin D deficiency prevalence is high in cancer patients [38,39,40] and some studies report vitamin D deficiency in more than 70% of cancer patients. According to Alkan, risk factors linked with vitamin D deficiency include female sex, low sunlight exposure, being under palliative care or adjuvant chemotherapy or history of gastrointestinal surgery [40]. Moreover, the VDS dose may have been inadequate to sufficiently increase vitamin D levels. In the AMATERASU trial, Urashima found the 5-year relapse-free survival was higher than placebo (85% vs. 71%) in the subgroup of patients with 25(OH)D between 20 and 40 ng/mL, but not in patients with level <20 ng/mL [21]. Meanwhile, Ng found a greater effect of high-dose vitamin D3 (8000 IU VD3/day followed by 4000 IU VD3/day during chemotherapy) on PFS among patients with a lower BMI (P = 0.04 for interaction), more metastatic sites (P = 0.02 for interaction), and KRAS wild-type tumors (P = 0.04 for interaction) in the SUNSHINE trial [20]. In a recent meta-analysis of Vitamin D supplementation in RCTs [41], authors found a statistically significantly reduced 15% risk of cancer death (RR = 0.85,CI = 0.74–0.97) and subgroup analyses suggest that all-cause mortality may be significantly lower in trials with vitamin D3 supplementation than in trials with vitamin D2 supplementation (P for interaction = 0.04) [41]. In fact, Vitamin D3 seems to be more efficient at raising serum 25(OH)D concentrations than vitamin D2, and thus vitamin D3 could potentially become the preferred choice for supplementation [42].
The need for a long follow-up period is necessary to evaluate the possible effect of VDS. Most of the included trials on cancer patients had follow-up periods of no more than 4 years [19], while population trials have a longer follow-up, up to 7 years. Among the largest of these trials is the Women’s Health Initiative (WHI). The WHI randomized >36,000 US postmenopausal women to 7 years of daily calcium and vitamin D3 or to placebo and found a suggestion of a protective effect against overall cancer mortality [26], CRC [24], breast [25] and hematologic cancer mortality [27], but the dose was below the Recommended Dietary Allowance (RDA, 600 UI) (https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ accessed on 19 July 2021) and the VDS proposed in the other trials.
A recent systematic overview of pertinent meta-analyses [7] found that VDS reduced total cancer mortality risk in population clinical trials, with five out of six meta-analyses reporting a relative risk (RR) reduction of up to 16% (RR 0.84, 95% CI = 0.74–0.95). We did not perform a meta-analysis of the included studies due to the great heterogeneity in reported estimates. Most published trials did not present adjusted estimates for general or specific cancer mortality, but they all showed a promising indication of reduction in total cancer mortality. In VITAL, VDS did not significantly reduce total cancer mortality (HR = 0.83, 95% CI = 0.67–1.02), but accounting for latency by excluding the first year or first two years of follow-up, they found a statistically significant cancer mortality reduction (HR = 0.79; 95% CI = 0.63–0.99 and HR = 0.75; 95% CI = 0.59–0.96 respectively).
Regarding RCT on cancer patients, two trials (ASCENT and SUNSHINE) evaluated the effect of VDS in patients with progressive metastatic prostate cancer and metastatic CRC, respectively. The ASCENT interventions proposed different VDS [15]. They evaluated the safety and activity of DN-101, a new high-dose oral formulation of calcitriol designed for cancer therapy, and docetaxel compared with placebo and docetaxel (chemotherapy). They found a trend favoring DN-101 over placebo with regard to skeletal morbidity-free survival. According to the authors, the observed trends could reflect a more effective anti-neoplastic therapy (with DN-101) resulting in delay of skeletal-related events (bone metastases). The SUNSHINE trial provided mFOLFOX6 plus bevacizumab chemotherapy every 2 weeks and either high-dose vitamin D3 (8000–4000 UI/day) or standard-dose vitamin D3 (400 UI/day) daily until disease progression or intolerable toxicity. Several potential mechanisms of action may explain the activity of vitamin D in CRC, but the hypothesis-generating finding could be that high-dose vitamin D3 supplementation is responsible in maintenance of gut mucosal barrier integrity [43]
In the AMATERASU trial, the authors found a significant effect of VDS in the subgroup of patients with digestive tract cancer with poorly differentiated adenocarcinoma, but not in any other subgroup based on histopathological characteristics [22]. The authors concluded that VDS could induce/increase differentiation of undifferentiated cancer cells as reported in in vitro experiments [44,45,46] and in a clinical pilot trial [47].
Regarding observational studies, four studies have reported a statistically significant improved survival [30,31,33,34], and only one study [28] found a contrasting result. The “Danish Diet, Cancer and Health” found that the use of VDS was significantly associated with a 47% increase in breast cancer mortality in women reporting a pre-diagnostic use of HRT. This association could be due to the fact that previous HRT users could carry the worst prognosis compared to both never and current users as reported in WHI trial (more advanced tumors and higher frequency of lymph node positive tumors) [48].
Madden found that de novo post-diagnostic VDS was associated with a 20% reduction in breast cancer-specific mortality in a large cohort study [34]. In additional analysis, they also found a greater reduction in breast cancer-specific mortality (49%) when VDS was initiated soon after the breast cancer diagnosis (within 6 months), similar to Jeffreys [33], who examined over 11,000 breast cancer patients from the largest prospective cancer cohort included in this review. They found that any vitamin D prescription, compared to never receiving it, in the 5 years prior to diagnosis was associated with improved survival from breast cancer, but not for the other cancer types (CRC, lung, ovarian and uterine). Time of initiation of VDS could have significant clinical implications, particularly for breast cancer patients, but further studies are needed to clarify the association.
Moreover, another aspect to be considered is vitamin D receptor polymorphism, which could explain the different activity of VDS. There are several reports linking vitamin D receptor genotype with cancer risk and mortality, which could explain the variability in the effect of VDS [49]. Therefore, further studies should aim at evaluating not only the effect of vitamin D, but its activity in cancer risk reduction according to the patient’s receptors polymorphisms.
Vitamin D has also been proposed for COVID-19 cancer patients who were observed to have a higher risk of severe COVID-19 events. The immunomodulatory role of Vitamin D has long been known, and its antagonistic effect on viral replication in the respiratory tract, enteric infections, otitis media, Clostridium infections, vaginosis, urinary tract infections, sepsis, flu, dengue, hepatitis to be attributed to ability of vitamin D to increase antimicrobial peptides with antiviral and immunomodulatory activity [50]. A meta-analysis including 25 RCTs showed that supplementation with Vitamin D reduced by the incidence of acute respiratory infections two-thirds in subjects with levels of 25 (OH) D lower than 16 ng [51].
This is the first narrative review to simultaneously evaluate the effect of VDS in RCTs and observational studies, thus making it possible to obtain a comprehensive picture of all the existing scientific literature on the topic. Despite this, the present work is not without limitations, such as the limited number of papers eligible, the high degree of heterogeneity in several study characteristics in terms of vitamin D supplementation dose, concomitant therapies or supplementation, the length of treatments and follow-up, and the possibility of having missed some eligible studies. We did not evaluate the level of circulating 25(OH)D, because not all studies reported this information. Whether total 25(OH)D is the best indicator of vitamin D status is still a controversial issue, since there are actually divergent opinions for defining vitamin D status [52]. We were not able to take into account other factors, such as BMI, dietary intake, sun exposure, and all cancer sites and stages due to the limited data published in the original articles.
5. Conclusions
Evidence from RTCs and ObS studies does not presently make it possible to provide definitive answers on whether VDS has a beneficial impact on cancer survival/mortality. VDS has potential as treatment to improve survival in cancer patients, but none of the RCTs were conclusive due to limitations in the study designs. Only by critically reviewing the available evidence can we overcome those limitations to obtain a properly designed clinical trial. This evaluation could help researchers to plan well-designed and sufficiently powered RCTs to fully evaluate this association. Considering the uncertainty of the above results, we strongly recommend stratifying patients according to their baseline vitamin D status, preferring vitamin D3 supplementation, and diversifying the dose proposed according to the patients’ characteristics (e.g., BMI, cancer stage, patient’s receptor polymorphisms), as well as planning a proper duration of the supplementation and length of the follow-up according to outcomes.
Acknowledgments
The authors thank William Russel-Edu for help with the literature search.
Author Contributions
Conceptualization, S.G. and P.G.; methodology, C.M. (Chiara Martinoli), S.R. and S.G.; data curation, P.G. and V.M.; investigation, P.G., V.M. and S.G.; funding acquisition, S.G. and P.G.; resources, S.C. (Stefania Canova), P.B., D.C. and F.C.; supervision, S.G.; writing—original draft, P.G. and V.M.; writing review and editing, S.C., S.R., C.M. (Chiara Martinoli), S.C. (Susanna Chiocca), C.M. (Claudia Miccolo), P.B., D.C., F.C., R.P., F.F., F.B. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
Grant Regione Lombardia. (DECRETO N. 7082 Del 17/06/2020. Identificativo Atto n. 366: COVi-taminD Trial: prevenzione di complicanze da COVID-19 in pazienti oncologici). The European Institute of Oncology, Milan, Italy is partially supported by the Italian Ministry of Health with Ricerca Corrente and 5 × 1000 funds. Federica Bellerba is a Ph.D. student within the European School of Molecular Medicine (SEMM).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang S., Miller D., Li W. Non-Musculoskeletal Benefits of Vitamin D beyond the Musculoskeletal System. Int. J. Mol. Sci. 2021;22:2128. doi: 10.3390/ijms22042128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick M.F., Vitamin D. Sunlight and Cancer Connection. Anti-Cancer Agents Med. Chem. 2013;13:70–82. doi: 10.2174/187152013804487308. [DOI] [PubMed] [Google Scholar]
- 3.Garland C.F., Garland F.C., Gorham E.D., Lipkin M., Newmark H., Mohr S.B., Holick M.F. The role of vitamin D in cancer prevention. Am. J. Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhury R., Kunutsor S., Vitezova A., Oliver-Williams C., Chowdhury S., Jong J.K.-D., Khan H., Baena C.P., Prabhakaran D., Hoshen M.B., et al. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903. doi: 10.1136/bmj.g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang T.J. Vitamin D and Cardiovascular Disease. Annu. Rev. Med. 2016;67:261–272. doi: 10.1146/annurev-med-051214-025146. [DOI] [PubMed] [Google Scholar]
- 6.Hansen C.M. Vitamin D and cancer: Effects of 1,25(OH)2D3 and its analogs on growth control and tumorigenesis. Front. Biosci. 2001;6:D820–D848. doi: 10.2741/Hansen. [DOI] [PubMed] [Google Scholar]
- 7.Sluyter J.D., E Manson J., Scragg R. Vitamin D and Clinical Cancer Outcomes: A Review of Meta-Analyses. JBMR Plus. 2021;5:e10420. doi: 10.1002/jbm4.10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manson J.E., Bassuk S.S., Lee I.-M., Cook N.R., Albert M.A., Gordon D., Zaharris E., MacFadyen J.G., Danielson E., Lin J., et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp. Clin. Trials. 2012;33:159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avenell A., MacLennan G.S., Jenkinson D.J., McPherson G.C., McDonald A.M., Pant P.R., Grant A.M., Campbell M.K., Anderson F.H., Cooper C., et al. Long-Term Follow-Up for Mortality and Cancer in a Randomized Placebo-Controlled Trial of Vitamin D3and/or Calcium (RECORD Trial) J. Clin. Endocrinol. Metab. 2012;97:614–622. doi: 10.1210/jc.2011-1309. [DOI] [PubMed] [Google Scholar]
- 10.Manson J.E., Cook N.R., Lee I.M., Christen W., Bassuk S.S., Mora S., Gibson H., Gordon D., Copeland T., D’Agostino D., et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scragg R., Khaw K.-T., Toop L., Sluyter J., Lawes C.M.M., Waayer D., Giovannucci E., Camargo C.A. Monthly High-Dose Vitamin D Supplementation and Cancer Risk: A Post Hoc Analysis of the Vitamin D Assessment Randomized Clinical Trial. JAMA Oncol. 2018;4:e182178. doi: 10.1001/jamaoncol.2018.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Autier P., Gandini S. Vitamin D supplementation and total mortality: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert D., Vale C., Haire R., Coyle C., Langley R. Repurposing Vitamin D as an Anticancer Drug. Clin. Oncol. 2016;28:36–41. doi: 10.1016/j.clon.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 14.McKenzie J.E., Brennan S.E., Ryan R.E., Thomson H.J., Johnston R.V., Thomas J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In: Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) Cochrane; London, UK: 2021. [DOI] [Google Scholar]
- 15.Beer T.M., Ryan C.W., Venner P.M., Petrylak D.P., Chatta G.S., Ruether J.D., Redfern C.H., Fehrenbacher L., Saleh M.N., Waterhouse D.M., et al. Double-Blinded Randomized Study of High-Dose Calcitriol Plus Docetaxel Compared With Placebo Plus Docetaxel in Androgen-Independent Prostate Cancer: A Report From the ASCENT Investigators. J. Clin. Oncol. 2007;25:669–674. doi: 10.1200/JCO.2006.06.8197. [DOI] [PubMed] [Google Scholar]
- 16.Attia S., Eickhoff J., Wilding G., McNeel D., Blank J., Ahuja H., Jumonville A., Eastman M., Shevrin D., Glode M., et al. Randomized, double-blinded phase II evaluation of docetaxel with or without doxercalciferol in patients with metastatic, androgen-independent prostate cancer. Clin. Cancer Res. 2008;14:2437–2443. doi: 10.1158/1078-0432.CCR-07-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scher H.I., Jia X., Chi K., De Wit R., Berry W.R., Albers P., Henick B., Waterhouse D., Ruether D.J., Rosen P.J., et al. Randomized, Open-Label Phase III Trial of Docetaxel Plus High-Dose Calcitriol Versus Docetaxel Plus Prednisone for Patients With Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2011;29:2191–2198. doi: 10.1200/JCO.2010.32.8815. [DOI] [PubMed] [Google Scholar]
- 18.Akiba T., Morikawa T., Odaka M., Nakada T., Kamiya N., Yamashita M., Yabe M., Inagaki T., Asano H., Mori S., et al. Vitamin D Supplementation and Survival of Patients with Non–small Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Cancer Res. 2018;24:4089–4097. doi: 10.1158/1078-0432.CCR-18-0483. [DOI] [PubMed] [Google Scholar]
- 19.Golubić Z.A., Barsic I., Librenjak N., Plestina S. Vitamin D Supplementation and Survival in Metastatic Colorectal Cancer. Nutr. Cancer. 2018;70:413–417. doi: 10.1080/01635581.2018.1445766. [DOI] [PubMed] [Google Scholar]
- 20.Ng K., Nimeiri H.S., McCleary N.J., Abrams T.A., Yurgelun M.B., Cleary J.M., Rubinson D.A., Schrag D., Miksad R., Bullock A.J., et al. Effect of High-Dose vs Standard-Dose Vitamin D3 Supplementation on Progression-Free Survival Among Patients With Advanced or Metastatic Colorectal Cancer: The SUNSHINE Randomized Clinical Trial. JAMA. 2019;321:1370–1379. doi: 10.1001/jama.2019.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urashima M., Ohdaira H., Akutsu T., Okada S., Yoshida M., Kitajima M., Suzuki Y. Effect of Vitamin D Supplementation on Relapse-Free Survival Among Patients With Digestive Tract Cancers: The AMATERASU Randomized Clinical Trial. JAMA. 2019;321:1361–1369. doi: 10.1001/jama.2019.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yonaga H., Okada S., Akutsu T., Ohdaira H., Suzuki Y., Urashima M. Effect Modification of Vitamin D Supplementation by Histopathological Characteristics on Survival of Patients with Digestive Tract Cancer: Post Hoc Analysis of the AMATERASU Randomized Clinical Trial. Nutrition. 2019;11:2547. doi: 10.3390/nu11102547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trivedi D.P., Doll R., Khaw K.T. Papers Effect of four monthly oral vitamin D 3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: Randomised double blind controlled trial. BMJ. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wactawski-Wende J., Kotchen J.M., Anderson G.L., Assaf A.R., Brunner R.L., O’Sullivan M.J., Margolis K., Ockene J.K., Phillips L., Pottern L., et al. Calcium plus Vitamin D Supplementation and the Risk of Colorectal Cancer. N. Engl. J. Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 25.Chlebowski R.T., Johnson K.C., Kooperberg C., Pettinger M., Wactawski-Wende J., Rohan T., Rossouw J., Lane D., O’Sullivan M.J., Yasmeen S., et al. Calcium Plus Vitamin D Supplementation and the Risk of Breast Cancer. J. Natl. Cancer Inst. 2008;100:1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunner R.L., Wactawski-Wende J., Caan B., Cochrane B.B., Chlebowski R.T., Gass M.L.S., Jacobs E.T., Lacroix A.Z., Lane D., Larson J., et al. The Effect of Calcium plus Vitamin D on Risk for Invasive Cancer: Results of the Women’s Health Initiative (WHI) Calcium Plus Vitamin D Randomized Clinical Trial. Nutr. Cancer. 2011;63:827–841. doi: 10.1080/01635581.2011.594208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ammann E.M., Drake M.T., Ms B.H., Wallace R.B., Johnson K.C., Desai P., Lin E.M., Link B. Incidence of hematologic malignancy and cause-specific mortality in the Women’s Health Initiative randomized controlled trial of calcium and vitamin D supplementation. Cancer. 2017;123:4168–4177. doi: 10.1002/cncr.30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holm M., Olsen A., Kroman N., Tjønneland A. Lifestyle influences on the association between pre-diagnostic hormone replacement therapy and breast cancer prognosis—Results from The Danish ‘Diet, Cancer and Health’ prospective cohort. Maturitas. 2014;79:442–448. doi: 10.1016/j.maturitas.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Yokosawa E.B., Arthur A.E., Mph K.M.R., Wolf G.T., Rozek L.S., Mondul A.M. Vitamin D intake and survival and recurrence in head and neck cancer patients. Laryngoscope. 2018;128:E371–E376. doi: 10.1002/lary.27256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeichner S.B., Koru-Sengul T., Shah N., Liu Q., Markward N.J., Montero A.J., Glück S., Silva O., Ahn E.R. Improved Clinical Outcomes Associated With Vitamin D Supplementation During Adjuvant Chemotherapy in Patients With HER2+ Nonmetastatic Breast Cancer. Clin. Breast Cancer. 2015;15:e1–e11. doi: 10.1016/j.clbc.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Mulpur B.H., Nabors L.B., Thompson R.C., Olson J.J., LaRocca R.V., Thompson Z., Egan K.M. Complementary therapy and survival in glioblastoma. Neuro-Oncol. Pract. 2015;2:122–126. doi: 10.1093/nop/npv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L., Wang C., Wang J., Huang X., Cheng Y. Longitudinal, observational study on associations between postoperative nutritional vitamin D supplementation and clinical outcomes in esophageal cancer patients undergoing esophagectomy. Sci. Rep. 2016;6:38962. doi: 10.1038/srep38962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeffreys M., Redaniel M.T., Martin R.M. The effect of pre-diagnostic vitamin D supplementation on cancer survival in women: A cohort study within the UK Clinical Practice Research Datalink. BMC Cancer. 2015;15:1–7. doi: 10.1186/s12885-015-1684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madden J.M., Murphy L., Zgaga L., Bennett K. De novo vitamin D supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Res. Treat. 2018;172:179–190. doi: 10.1007/s10549-018-4896-6. [DOI] [PubMed] [Google Scholar]
- 35.Poole E.M., Shu X., Caan B., Flatt S.W., Holmes M.D., Lu W., Kwan M.L., Nechuta S., Pierce J.P., Chen W.Y. Postdiagnosis supplement use and breast cancer prognosis in the After Breast Cancer Pooling Project. Breast Cancer Res. Treat. 2013;139:529–537. doi: 10.1007/s10549-013-2548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis C., Xun P., He K. Vitamin D supplementation and quality of life following diagnosis in stage II colorectal cancer patients: A 24-month prospective study. Support. Care Cancer. 2016;24:1655–1661. doi: 10.1007/s00520-015-2945-9. [DOI] [PubMed] [Google Scholar]
- 37.Feldman D., Krishnan A.V., Swami S., Giovannucci E., Feldman B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 38.Yuan C., Sato K., Hollis B.W., Zhang S., Niedzwiecki D., Ou F.-S., Chang I.-W., O’Neil B.H., Innocenti F., Lenz H.-J., et al. Plasma 25-Hydroxyvitamin D Levels and Survival in Patients with Advanced or Metastatic Colorectal Cancer: Findings from CALGB/SWOG 80405 (Alliance) Clin. Cancer Res. 2019;25:7497–7505. doi: 10.1158/1078-0432.CCR-19-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennel K.A., Drake M.T. Vitamin D in the cancer patient. Curr. Opin. Support. Palliat. Care. 2013;7:272–277. doi: 10.1097/SPC.0b013e3283640f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alkan A., Köksoy E.B. Vitamin D deficiency in cancer patients and predictors for screening (D-ONC study) Curr. Probl. Cancer. 2019;43:421–428. doi: 10.1016/j.currproblcancer.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y., Fang F., Tang J., Jia L., Feng Y., Xu P., Faramand A. Association between vitamin D supplementation and mortality: Systematic review and meta-analysis. BMJ. 2019;366:l4673. doi: 10.1136/bmj.l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tripkovic L., Lambert H., Hart K., Smith C.P., Bucca G., Penson S., Chope G., Hyppönen E., Berry J., Vieth R., et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A sys-tematic review and meta-analysis. Am. J. Clin. Nutr. 2012;95:1357–1364. doi: 10.3945/ajcn.111.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao H., Zhang H., Wu H., Li H., Liu L., Guo J., Li C., Shih D.Q., Zhang X. Protective role of 1,25(OH)2vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12:57. doi: 10.1186/1471-230X-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skrajnowska D., Bobrowska-Korczak B. Potential Molecular Mechanisms of the Anti-cancer Activity of Vitamin D. Anticancer. Res. 2019;39:3353–3363. doi: 10.21873/anticanres.13478. [DOI] [PubMed] [Google Scholar]
- 45.Shan N.L., Wahler J., Lee H.J., Bak M.J., Das Gupta S., Maehr H., Suh N. Vitamin D compounds inhibit cancer stem-like cells and induce differentiation in triple negative breast cancer. J. Steroid Biochem. Mol. Biol. 2017;173:122–129. doi: 10.1016/j.jsbmb.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Supnick H.T., Bunaciu R.P., Yen A. The c-Raf modulator RRD-251 enhances nuclear c-Raf/GSK-3/VDR axis signaling and augments 1,25-dihydroxyvitamin D3-induced differentiation of HL-60 myeloblastic leukemia cells. Oncotarget. 2018;9:9808–9824. doi: 10.18632/oncotarget.24275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao Y., Um C.Y., Fedirko V., Rutherford R.E., Seabrook M.E., Barry E.L., Baron J.A., Bostick R.M. Effects of supplemental vitamin D and calcium on markers of proliferation, differentiation, and apoptosis in the normal colorectal mucosa of colorectal adenoma patients. PLoS ONE. 2018;13:e0208762. doi: 10.1371/journal.pone.0208762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chlebowski R.T., Hendrix S.L., Langer R.D., Stefanick M.L., Gass M., Lane D., Rodabough R.J., Gilligan M.A., Cyr M.G., Thomson C.A., et al. Influence of Estrogen Plus Progestin on Breast Cancer and Mammography in Healthy Postmenopausal Women. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 49.Gnagnarella P., Raimondi S., Aristarco V., Johansson H.A., Bellerba F., Corso F., Gandini S. Vitamin D Receptor Polymorphisms and Cancer. Adv. Exp. Med. Biol. 2020;1268:53–114. doi: 10.1007/978-3-030-46227-7_4. [DOI] [PubMed] [Google Scholar]
- 50.Borella E., Nesher G., Israeli E., Shoenfeld Y. Vitamin D: A new anti-infective agent? Ann. N. Y. Acad. Sci. 2014;1317:76–83. doi: 10.1111/nyas.12321. [DOI] [PubMed] [Google Scholar]
- 51.Martineau A.R., Jolliffe D.A., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C., et al. Vitamin D supplementation to prevent acute respiratory infections: Individual participant data meta-analysis. Health Technol. Assess. 2019;23:1–44. doi: 10.3310/hta23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giustina A., Adler R.A., Binkley N., Bollerslev J., Bouillon R., Dawson-Hughes B., Ebeling P.R., Feldman D., Formenti A.M., Lazaretti-Castro M., et al. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020;21:89–116. doi: 10.1007/s11154-019-09532-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.