Abstract
Irresponsible human interventions, encroachment of natural habitats, and climate change negatively affect wildlife. In this study, the effects of human influence on Wadi Hagul, an unprotected area in the north of the Egyptian Eastern Desert that has recently been subjected to blatant encroachments of vegetation, were studied. The most important of these threats is the construction of the new road Al-Galala–Wadi Hagul–Zafarana. In Wadi Hagul, 80 species are reported in this study; the most represented plant families are Asteraceae (15 species) and Brassicaceae (6 species). Perennial, chamaephyte and Saharo-Arabian species were recorded in the highest percentage. Detrended canonical correspondence analysis showed that latitude, longitude, altitude, silt, sand contents, pH, and CO32− content are the factors that have the highest effect on vegetation distribution in the studied stands. Several invasive and alien species such as Euphorbia prostrata have been listed; these species typically have a negative effect on native species. The Soil Adjusted Vegetation Index (SAVI) indicated a decrease in plant cover during the study period, as compared to previous years. In 2013 and 2020, SAVI ranged from −0.02 to 0.42 and from −0.18 to 0.28, respectively. Recently, the violation and destruction of wildlife have increased, therefore, preserving it along with general biodiversity has become an urgent necessity.
Keywords: plant diversity, anthropogenic impacts, vegetation dynamics, wildlife, Wadi Hagul, DCCA, SAVI
1. Introduction
Land degradation and vegetation reduction caused by external stresses, affect biodiversity and natural ecosystems and the numerous services they provide [1]. Environmental deterioration, habitat changes, inappropriate vegetation management, translocation, fragmentation, and deforestation modify biotic and abiotic ecosystem components, resulting in changes in ecological processes such as vegetation dynamics [2,3,4]. Overgrazing, road building, overharvesting, solid wastes, salinization, industrialization, urbanization, and military activities are considered to be the main anthropogenic activities that lead to changes and transformation of vegetation and natural habitat loss in arid and semi-arid environments [5,6,7].
In terrestrial ecosystems, climate and land cover changes such as cover, height, biomass, relative humidity, soil temperature, moisture, fertility, and erosion affect the structural properties of vegetation [8]. Biodiversity decline is influenced by various types of human activities, including land cover changes, the introduction of invasive species, overexploitation, and pollution [9,10,11]. Alteration of vegetation growth is the result of climate variation, and human activities can modify atmosphere–biosphere interaction, causing changes in the hydrological cycle, either directly or indirectly [12,13,14].
Environmental issues, including soil fertility decline, heavy winds, increase in the evaporation rate, high temperature, and heavy rainfall, lead to dramatic changes in plant species community structure [7,15,16]. Ecosystem services and biodiversity contribute approximately 57% of gross domestic production [17], therefore, there is a pressing need to save biodiversity, especially endangered species through convert these locations to protected areas [18]. There are a variety of approaches that are able to reverse biodiversity loss, ranging from economic, through ecological, to ethical [19].
Human pressures in Africa, include agricultural and pasture activities, illegal timber harvesting, and bush fires. All of these stresses have a negative impact on the plant ecosystem [20]. From the middle of the last century, the human population of the earth doubled [21]. Population growth and urbanization are the most important causes of ecosystems collapse [22]. Rapid population growth leads to many problems such as fire prevalence, air pollution, light pollution, loss of genetic diversity, the prevalence of invasive species, and wildlife destruction [23]. Human activities, through the civilizational and agricultural expansion and by the destruction of natural habitats, have increased extinction rates up to 500 times [24] (Baillie et al., 2004). Over the ages, human activities have caused three powerful waves of extinction [25]. It is estimated that nearly 8390 plant species are listed as endangered [26]. Approximately 32% of the existing plant species are classified as either critically endangered or extinct [27], nearly 20% being extinct because of human activities [28]. In China, nearly 11% of the plant species that were evaluated are extinct [29].
Species with a narrow distribution range are more likely to be lost, while widespread species are more likely to survive [30]. Some wild plants have adapted to the conditions and survived in their natural habitats [31]. However, invasive species spread causes disturbance in communities and may lead to the extinction of endemic and native species [32].
Anthropogenic activities are causing changes in natural plant communities in Egypt from ancient times through draining of lakes and marshes and reduction of species number in aquatic communities [33]. Remote sensing is one of the most unique techniques used for estimating environmental changes [34]. Some characteristics of vegetation detected using remote sensing techniques are photosynthetic activity, chlorophyll content, plant density, green leaf biomass, the leaf area index, and plant health [35]. The second factor that includes climatic measures includes air temperature and solar radiation [36,37,38]. SAVI is one of the most common vegetation indices used as a tool to discriminate vegetation covers [39]. SAVI can be used efficiently in arid regions [40]. In areas where vegetative cover is low (i.e., <40%) and the soil surface is exposed, the reflectance of light in the red and near-infrared spectra can influence vegetation index values. This is especially problematic when comparisons are being made across different soil types that may reflect different amounts of light in the red and near-infrared wavelengths (i.e., soils with different brightness values). The soil-adjusted vegetation index was developed as a modification of the Normalized Difference Vegetation Index (NDVI) to correct for the influence of soil brightness when vegetative cover is low [41]. The output of SAVI is a new image layer with values ranging from −1 to 1. The lower the value, the lower the amount/cover of green vegetation [42].
A wadi is a natural depression on the earth’s surface. Wadi Hagul is a morphotectonic depression located between Gebel Ataqa’s southern scarps in the north and El-Galala El-Bahariya Plateau’s northern scarp in the south. Wadi Hagul is one of the unprotected wadies in Egypt and is one of the most anticipated project areas in Egypt to alleviate congestion. As a result, several integrated development projects are being worked on that implement many critical infrastructure areas such as quarries, roads, power plants, mines, landfills, and resorts. The most important national project is the development of the northern part of the Gulf of Suez pipeline surcharge, implemented through the main track of the study area and its environment [43]. Three main sectors can be recognized based on the vegetation and geological aspects of Wadi Hagul: upstream, middle, and downstream. Two distinct plant communities can be found in the upstream section of Wadi Hagul’s main channel: one is dominated by Zilla spinosa on elevated terraces of mixed deposits. The other is dominated by Launaea spinosa and represents a further stage in the building up of the wadi bed. In the middle section, Leptadenia pyrotechnica community occurs. In the downstream section, the vegetation is dominated by Hammada elegans with individuals of Launaea spinosa and Lygos raetam [44].
This paper focuses on Wadi Hagul, which has neither attained sufficient interest from intended responsible parts nor ecological studies, although it is considered an environment rich in various plants including a number of endangered plants, whereas other Eastern desert wadis of Egypt have received both interest and studies convenient for them. Therefore, the objective of this study is the estimation of vegetation cover in Wadi Hagul through the illustration of the relationship between environmental factors in the studied localities and the distribution of plant communities. The study also aimed at comparing vegetation changes and at testing new methods to record the reasons for changes in vegetation dynamics by calculating the temporal patterns of SAVI during 2013, 2015, and 2020 in Wadi Hagul, Eastern Egyptian Desert.
2. Materials and Methods
2.1. Study Area
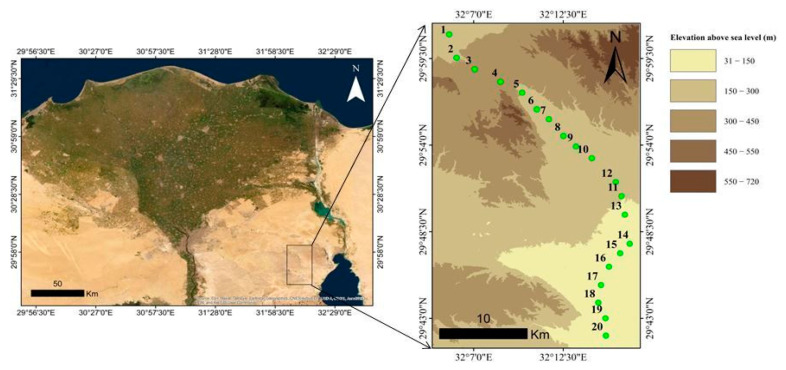
Wadi Hagul lies between 32°09′32″ E and 32°17′27″ E and between 29°48′28″ N and 29°57′43″ N, with an area of 350 km2 (0.16% of the Eastern Desert total area). The main canal of Wadi Hagul extends until it reaches the Gulf of Suez, with a width ranging from 6 to 10 km and a length of approximately 36 km. Wadi Hagul is considered to be one of 15 wadies located on the Red Sea coast. It is situated 112 km west of Cairo, between Gebel Ataqa and the Kahaliya ridge [44]. Wadi Hagul is characterized by a dry desert climate with little rain, high temperatures, and a high evaporation rate. The three sections represent dry natural habitats inhabited mainly by xerophytic plants. The dominant species are Fagonia mollis, Echinops spinosus, and other xerophytic plants [45].
2.2. Climate Data
Climate data for the past 30 years (1990–2020) were extracted from www.meteoblue.com; accessed on 15 April 2021. They include mean maximum temperature (°C), mean minimum temperature (°C), precipitation (mm), hot days (°C), and cold nights (°C) (T S1). Wind direction and velocity (km/h) are provided in Figure S2.
2.3. Floristic Study
To estimate the vegetation cover in Wadi Hagul, 20 stands were chosen to represent different plant habitats in Wadi Hagul. A total of 20 stands were selected for the study of the vegetation of Wadi Hagul during the spring and summer seasons of 2019 and 2020. In each stand, four quadrates were chosen (quadrate area = 10 m × 10 m = 100 m2) (Table 1 and Figure 1 and Figure 2). Vegetation cover was measured using visual estimation. Recorded plant species in all localities were identified, and life span was documented according to Boulos [46,47,48,49,50]. Life form and floristic category were recognized after Raunchier [51], Tutin et al. [52], and Davis [53].
Table 1.
Global Positioning System coordinates of the studied localities in Wadi Hagul.
| Stands | Latitude (N) | Longitude (E) | Altitude above Sea Level (m) | Stands | Latitude (N) | Longitude (E) | Altitude above Sea Level (m) |
|---|---|---|---|---|---|---|---|
| 1 | 30°1′2.58345″ | 32°5′29.5121″ | 277 | 11 | 29°50′45.68″ | 32°16′4.86″ | 152 |
| 2 | 29°59′33.13075″ | 32°5′56.79499″ | 295 | 12 | 29°51′39.19″ | 32°15′43.54135″ | 163 |
| 3 | 29°58′49.0674″ | 32°7′3.39269″ | 307 | 13 | 29°49′35.08406″ | 32°16′17.25956″ | 131 |
| 4 | 29°58′1.74144″ | 32°8′38.88139″ | 327 | 14 | 29°47′44.49253″ | 32°16′35.06138″ | 109 |
| 5 | 29°57′19.91″ | 32°9′58.45″ | 286 | 15 | 29°47′7.80″ | 32°15′59.88″ | 132 |
| 6 | 29°56′16.55599″ | 32°10′52.53848″ | 258 | 16 | 29°46′16.54″ | 32°15′18.87″ | 113 |
| 7 | 29°55′38.91″ | 32°11′37.12″ | 243 | 17 | 29°45′6.97″ | 32°14′49.35″ | 89 |
| 8 | 29°54′34.95031″ | 32°12′30.83965″ | 223 | 18 | 29°43′59.88″ | 32°14′39.51″ | 78 |
| 9 | 29°53′55.03″ | 32°13′17.35″ | 213 | 19 | 29°43′0.09″ | 32°15′5.75″ | 65 |
| 10 | 29°53′10.26654″ | 32°14′15.37418″ | 190 | 20 | 29°41′54.08515″ | 32°15′7.61404″ | 66 |
Figure 1.
Location on the map showing the digital elevation model of Wadi Hagul.
Figure 2.
A quadrate with an area of 10 m × 10 m in.
2.4. Soil Analyses
Many soil factors were examined in order to determine the characteristics of the soil in the study area. From each stand, four soil samples were collected and merged to form a homogeneous and representative sample of a single locality. Fifteen soil factors were analyzed: soil texture (sand, silt, and clay), pH, total dissolved salts, electrical conductivity, magnesium, calcium, sodium, potassium, chloride, bicarbonate, carbonate, sulfate, and organic carbon content. Soil texture was determined according to Estefan et al. [54] using a pipette method. Digital portable pH, TDS, and EC meters (Adwa®, Adwa Instruments, Szeged, Hungry) were used to calculate pH, total dissolved salts (T.D.S.), and electrical conductivity (E.C.). Magnesium and calcium were determined using a titration method according to Page et al. [55]. A flame photometer at wavelengths of 589 and 767 nm was used to determine sodium and potassium, respectively [54]. Chlorides, bicarbonates, and carbonates were determined using a titration method [56]. Sulfates were determined after Estefan et al. [54] using the turbid metric method. Organic carbon was determined using a titration method [57].
2.5. Statistical Analyses
Descriptive statistical measures (mean, median, standard deviation, range, minimum, maximum, and interquartile range) for different soil factors were determined using Sigmaplot ver. 12.5.
Several biodiversity indicators were measured such as Shannon′s and Simpson′s diversity indices, species richness, and evenness were extracted using PC-ORD ver. 5 software [58]. Hills numbers were calculated using the R program.
To clarify the relationship between environmental factors, especially soil factors, the study area was divided into three areas: the beginning of the Wadi (stands 1 to 7), the middle (stands 8 to 14), and the end of the Wadi (stands 15 to 20). A detrended canonical correspondence analysis (DCCA) was performed using CANOCO ver. 4.5 and CanoDraw ver. 4.1 [59].
2.6. Vegetation Change
To confirm the negative impact of human interventions on the vegetation cover in Wadi Hagul, SAVI was calculated in the study area during different years. Satellite images for 2013, 2015, and 2020 were obtained from the Landsat 8 satellite (Band 4 and Band 5) during the spring season using the website https://earthexplorer.usgs.gov/; accessed on 15 April 2021. Due to the position of the satellite in its orbit, haze, dust, and sun angle, satellite images have some defects which should be processed. Satellite images should be geometrically, atmospherically, and radiometrically corrected to become ready for extracting the required data. For that, satellite image processing software ENVI 5.3 and Arc GIS 10.5 were used to correct the collected satellite imageries. Soil Adjusted Vegetation Index (SAVI) was calculated according to the following equation:
| SAVI = ((Band 5 − Band 4)/(Band 5 + Band 4 + L)) × (1 + L)). |
where, L = The amount of green vegetation cover (For example, 0.5).
Then the study area (Hagul) was extracted from the satellite images.
3. Results
3.1. Floristic Composition
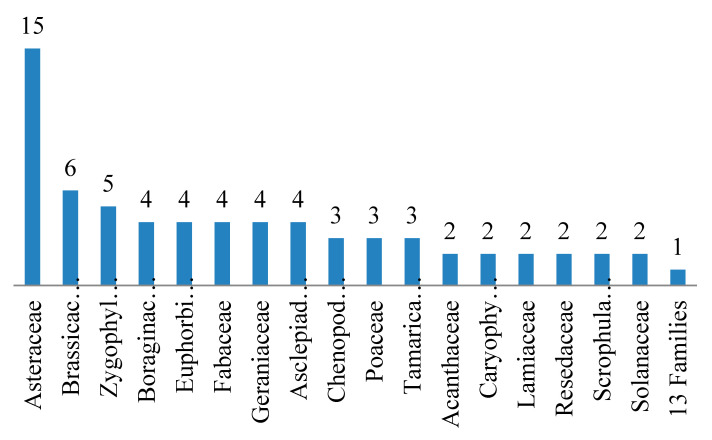
A collection of 80 plant taxa, belonging to 30 families, was recorded in Wadi Hagul. The most represented plant families were Asteraceae, Brassicaceae, and Zygophyllaceae with 15 (18.8%), 6 (7.5%), and 5 species (6.3%), respectively. Thirteen families (Amaranthaceae, Apiaceae, Capparaceae, Convolvulaceae, Cucurbitaceae, Ephedraceae, Malvaceae, Nitrariaceae, Orobanchaceae, Fabaceae, Plantaginaceae, Polygonaceae, and Rutaceae) were represented by one species (Table 2 and Figure 3).
Table 2.
The list of species recorded in Wadi Hagul with their plant families, life span, life form, floristic category, and abbreviations.
| Species | Family | Life Span | Life Form | Floristic Category | Record | Abbreviation |
|---|---|---|---|---|---|---|
| Blepharis attenuata Napper | Acanthaceae | Pe. | Cha. | IR and SA | B | Ble att |
| Blepharis edulis (Forssk.) Pers. | Pe. | Cha. | SA and SZ | B | Ble edu | |
| Aizoon canariensis L. | Aizoaceae | An. | The. | SA and SZ | A | Aiz can |
| Aerva javanica (Burm.f.) Juss. ex Schult. | Amaranthaceae | Pe. | Cha. | SA and SZ | B | Aer jav |
| Deverra tortuosa (Desf.) DC. | Apiaceae | Pe. | Cha. | SA | A,B | Dev tor |
| Calotropis procera (Aiton) W.T. Aiton | Asclepiadaceae | Pe. | Pha. | SA and SZ | A,B | Cal pro |
| Cynanchum acutum L. | Pe. | Hem. | Med, R, and ER | A,B | Cyn acu | |
| Leptadeniapyrotechnica (Forrsk.) Decne. | Pe. | Pha. | SA | A,B | Lep pyr | |
| Pergularia tomentosa L. | Pe. | Cha. | SA | A,B | Per tom | |
| Achilleafragrantissima (Forssk.) Sch. Bip. | Asteraceae | Pe. | Cha. | SA and IR | A,B | Ach fra |
| Artemisia judaica L. | Pe. | Cha. | SA | A | Art jud | |
| Atractylis carduus (Forssk.) C.Chr. | Pe. | Hem. | ME and SA | A | Atr car | |
| Bidens pilosa L. | An. | The. | PAN | A | Bid pil | |
| Centaurea aegyptiaca L. | Bi. | The. | SA | A,B | Cen aeg | |
| Conyza aegyptiaca (L.) Dryand. | An. | The. | Med | A,B | Con aeg | |
| Conyza bonariensis (L.) Cronquist | An. | The. | Med | A,B | Con bon | |
| Cotula cinerea Del. | An. | The. | SA | A | Cot cin | |
| Echinops spinosus L. | Pe. | Hem. | Med and SA | A,B | Ech spi | |
| Ifloga spicata (Forssk.) Sch. Bip. | An. | The. | SA | A | Ifl spi | |
| Iphiona mucronata (Forssk.)Asch. | Pe. | Cha. | SA | A,B | Iph muc | |
| Launaea capitata (Spreng)Dandy | Bi. | The. | SA and SZ | A | Lau cap | |
| Launea nudicalus (L.) Hook. f. | Pe. | Hem. | SA | A,B | Lau nud | |
| Launea spinosa (Forssk.) Sch. Bip. ex Kuntze. | Pe. | Cha. | SA | A,B | Lau spi | |
| Nauplius graveolens (Forssk.)Wilklund | Pe. | Cha. | SA | A,B | Nau gra | |
| Pluchea dioscoridis (L.) DC. | Pe. | Pha. | SA and SZ | A,B | Plu dio | |
| Pulicaria undulata (L.) C.A.Mey. | Pe. | Cha. | SA nd SZ | A,B | Pul und | |
| Reichardia tingitana (L.) Roth | An. | The. | Med and IR | B | Rei tin | |
| Senecio glaucus L. | An. | The. | Med, IR, andSA | A,B | Sen gla | |
| Sonchus oleraceus L. | An. | The. | COSM | A,B | Son ole | |
| Volutaria lippii (L.) Cass. Ex Maire. | An. | The. | SA | A,B | Vol lip | |
| Anchusa humilis (Desf.) I. M. Johnst. | Boraginaceae | An. | The. | Med and SA | B | Anc hum |
| Heliotropiumarabinense Fresen. | Pe. | Cha. | SA | B | Hel ara | |
| Heliotropium digynum (Forssk.) Christens | Pe. | Cha. | SA | A,B | Hel dig | |
| Trichodesmaafricanum (L.) R. Br. | Pe. | Cha. | SA and SZ | A,B | Tri afr | |
| Anastaticahierochuntica L. | Brassicaceae | An. | The. | SA | A,B | Ana hie |
| Brassica tournefortii Gouan. | An. | The. | Med, IR, and SA | A | Bra tou | |
| Diplotaxis acris (Forssk.) Boiss. | An. | The. | SA | A,B | Dip acr | |
| Diplotaxis harra (Forssk.) Boiss. | Pe. | Hem. | Med and SA | A,B | Dip har | |
| Eruca sativa Mill. | An. | The. | Med, IR, R, and SA | B | Eru sat | |
| Farsetia aegyptia Turra. | Pe. | Cha. | SA and SZ | A,B | Far aeg | |
| Matthiola longipetala (Vent.) DC. | An. | The. | Med and IR | A | Mat lon | |
| Zilla spinosa (L.) Prant. | Pe. | Cha. | SA | A,B | Zil spi | |
| Cleome droserifolia (Forssk.) Delile | Capparaceae | Pe. | Cha. | SA and IR | A,B | Cle dro |
| Gymnocarpos decandrus Forssk. | Caryophyllaceae | Pe. | Cha. | SA | A,B | Gym dec |
| Gypsophila capillaris (Forssk.) C. Chr. | Pe. | Hem. | SA and IR | A,B | Gyp cap | |
| Herniaria hemistemon J. Gay | Pe. | Hem. | Med and SA | A | Her hem | |
| Polycarpaea repens (Forssk.) Asch. | Pe. | Cha. | SA | A | Pol rep | |
| Silene viviani Steud. | An. | The. | Med and SA | A | Sil viv | |
| Sphaerocoma hookeri T. Anderson | Pe. | Cha. | SA | A | Sph hoo | |
| Anabasis setifera Moq. | Chenopodiaceae | Pe. | Cha. | SA | B | Ana Moq |
| Bassia indica (Wight) Scott. | An. | The. | IR and SZ | A | Bas ind | |
| Bassia muricata (L.) Asch. | An. | The. | IR and SA | A | Bas mur | |
| Chenopodium murale L. | An. | The. | COSM | A,B | Che mur | |
| Haloxylonsalicornicum (Moq.) Bunge | Pe. | Cha. | SA | A,B | Hal sal | |
| Helianthemum lippi (L.) Dum. Cours. | Cistaceae | Pe. | Cha. | SA and SZ | A | Hel lip |
| Cleome amblyocarpa Barratte and Murb. | Cleomaceae | An. | The. | IR and SA | A | Cle amb |
| Convolvulus hystrix Vahl | Convolvulaceae | Pe. | Cha. | SA and SZ | B | Con hys |
| Citrullus colocynthis (L.) Schrad. | Cucurbitaceae | Pe. | Hem. | SA | B | Cit col |
| Ephedra alata Decne. | Ephedraceae | Pe. | Cha. | SA | B | Eph ala |
| Chrozophora plicata (Vahl) A. Juss. ex Spreng. | Euphorbiaceae | An. | The. | SZ | B | Chr pli |
| Euphorbia peplus L. | An. | The. | Med, IR, and ER | A,B | Eup pep | |
| Euphorbia prostrata Aiton | An. | The. | PAN | A | Eup pro | |
| Euphorbia retusa Forssk. | Pe. | Hem. | SA | A,B | Eup ret | |
| Euphorbia exigua L. | An. | The. | Med and IR | B | Eup | |
| Acacia tortilis (Forssk.) Hayne | Fabaceae | Pe. | Pha. | SA and SZ | A,B | Aca tor |
| Astraglus spinosus (Forssk.) Muschl. | Pe. | Cha. | Med, IR, and SA | A | Ast spi | |
| Crotalaria aegyptiaca Benth. | Pe. | Cha. | SA | A,B | Cro aeg | |
| Lotus glinoides Delile | An. | The. | SZ | A | Lot gli | |
| Melilotus indicus (L.) All. | An. | The. | Med, IR, and SA | A | Mel ind | |
| Retama raetam (Forssk.) Webb and Berthel. | Pe. | Pha. | SA | A,B | Ret rae | |
| Senna alexandrina Mill. | Pe. | Cha. | SA and SZ | A | Sen ale | |
| Trigonella stellata Forssk. | An. | The. | SA and IR | A,B | Tri ste | |
| Erodium crassifolium L′Her. | Geraniaceae | Pe. | Hem. | SA | B | Ero cra |
| Erodium glaucophyllum (L.) L′Hér. | Pe. | Hem. | SA | B | Ero gla | |
| Erodium laciniatum (Cav.) Wild. subsp. Laciniatum | An. | The. | Med | A,B | Ero lac | |
| Erodium laciniatum (Cav.) Wild. subsp. Pulverulentum | An. | The. | Med | A,B | Ero lac | |
| Lavandula coronopifolia Poir. | Lamiaceae | Pe. | Cha. | SA | A,B | Lav cor |
| Salvia aegyptiaca L. | Pe. | Cha. | IR and SA | A | Sal aeg | |
| Salvia deserti Decne. | Pe. | Cha. | SA and IR | A,B | Sal des | |
| Asphodelus fistulosus L. | Liliaceae | An. | The. | Med, IR, and SA | A | Asp fis |
| Malva parvifolra L. | Malvaceae | An. | The. | Med and IR | A,B | Mal par |
| Neurada procumbens L. | Neuradaceae | An. | The. | IR and SA | A | Neu pro |
| Nitraria retusa (Forssk.) Asch. | Nitrariaceae | Pe. | Pha. | SA | B | Nit ret |
| Orobanche crenata Forssk. | Orobanchaceae | An. | Parasite | Med and IR | B | Oro cre |
| Medicago laciniata (L.) Mill. | Fabaceae | An. | The. | SA | B | Med (L. |
| Plantago ovata Forssk. | Plantaginaceae | An. | The. | Med, IR, and SA | A,B | Pla ova |
| Avena fatua L. | Poaceae | An. | The. | PAL | A | Ave fat |
| Imperata cylindrica (L.) Raeusch | Pe. | Hem. | Med, IR, and SA | A,B | Imp cyl | |
| Lasiurus scindicus Henrard. | Pe. | Hem. | SA and SZ | A | Las sci | |
| Lolium multiflorum Lam. | An. | The. | Med, IR, and ER | A | Lol mul | |
| Panicum turgidum Forssk. | Pe. | Hem. | SA | A,B | Pan tur | |
| Pennisetum divisum Forssk. Ex J. F. Gmel | Pe. | Hem. | SA | A | Pen div | |
| Phalaris minor Retz. | An. | The. | Med and IR | A | Pha min | |
| Phragmites australis (Cav.) Trin. ex Steud. | Pe. | Geophyte | COSM | A,B | Phr aus | |
| Poa annua L. | An. | The. | COSM | A | Poa ann | |
| Schismus barbatus (L.) Thell. | An. | The. | Med, IR, and SA | A | Sch bar | |
| Trisetaria linearis Forssk. | An. | The. | Med and IR | A | Tri lin | |
| Emex spinosa (L.) Campd. | Polygonaceae | An. | The. | Med and SA | A | Eme spi |
| Rumex vesicarius L. | Polygonaceae | An. | The. | Med, SA, and SZ | A,B | Rum ves |
| Caylusea hexagyna (Forssk.) M. L. Green | Resedaceae | An. | The. | SA and SZ | A | Cay hex |
| Ochradenus baccatus Delile. | Pe. | Pha. | SA | A,B | Och bac | |
| Reseda decursiva Forssk. | An. | The. | SA | A,B | Res dec | |
| Haplophyllum tuberculatum (Forssk.) A. Juss. | Rutaceae | Pe. | Hem. | SA | A,B | Hap tub |
| Kickxia aegyptiaca (L.) Nabelek | Scrophulariaceae | Pe. | Cha. | Med and SA | A,B | Kic aeg |
| Scrophularia deserti Delile | Pe. | Cha. | SA | A,B | Scr des | |
| Hyoscyamus muticus L. | Solanaceae | Pe. | Cha. | IR and SA | A | Hyo mut |
| Lycium shawii Roem. and Schult. | Pe. | Pha. | SA and SZ | A,B | Lyc sha | |
| Solanum nigrum L. | An. | The. | COSM | B | Sol nig | |
| Reaumuria hirtella Jaub. and Spach. | Tamaricaceae | Pe. | Cha. | IR and SA | A,B | Rea hir |
| Tamarix aphylla (L.) H. Karst. | Pe. | Cha. | SA, IR, and SZ | A,B | Tam aph | |
| Tamarix nilotica(Ehrenb.) Bunge | Pe. | Pha. | SA and SZ | A,B | Tam nil | |
| Forsskaolea tenacissima L. | Urticaceae | Pe. | Hem. | SA and SZ | A | For ten |
| Verbena officinalis L. | Verbenaceae | Pe. | Hem. | Med and SA | A | Ver off |
| Fagonia arabica L. | Zygophyllaceae | Pe. | Cha. | SA | A,B | Fag ara |
| Fagonia mollis Delile | Pe. | Cha. | SA | A,B | Fag mol | |
| Zygophyllumcoccineum L. | Pe. | Cha. | SA and SZ | A,B | Zyg coc | |
| Zygophyllumdecumbens Delile | Pe. | Cha. | SA | A,B | Zyg dec | |
| Zygophyllum simplex L. | An. | The. | PAL | A,B | Zyg sim |
An. = Annual, Bi. = Biennial, Pe. = Perennial, The. = Therophyte, Hem. = Hemicryptophyte, Cha. = Chamaephyte, Pha. = Phanerophyte, SA = Saharo-Sindian, Med = Mediterranean, SZ = Sudano-Zambesian, IR = Irano-Turanian, ER = Euro-Siberian, A = a species recorded only by Abdelaal [60], B = a species recorded only in the current study, and A,B = a species shared between the current study and Abdelaal [60].
Figure 3.
Number of species distributed across different families recorded in Wadi Hagul.
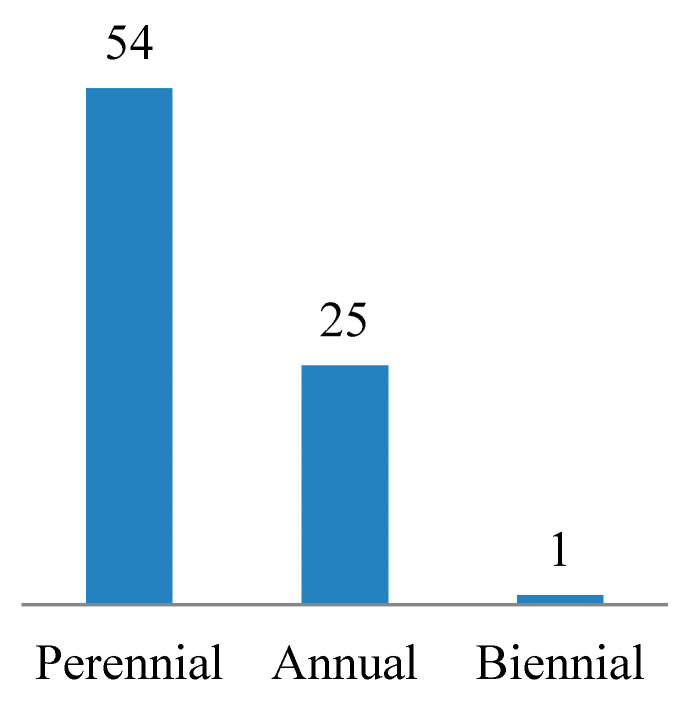
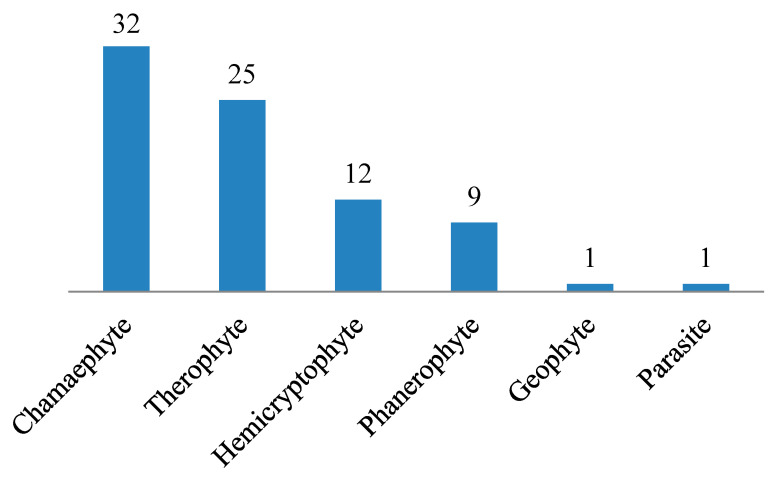
Regarding the life span of the listed species, most of them are perennials (54 species, 67.5%), followed by annuals (25 species, 31.3%). A single biennial species was Centaurea aegyptiaca (Table 2 and Figure 4). Comparing species according to their life form, we found approximately 40% chamaephytes, 31% therophytes, 15% hemicryptophytes, and 11% phanerophytes. Parasite and geophyte species were represented by a single species each (Table 2 and Figure 5).
Figure 4.
Life span of the species recorded in Wadi Hagul.
Figure 5.
Life forms of species recorded in Wadi Hagul.
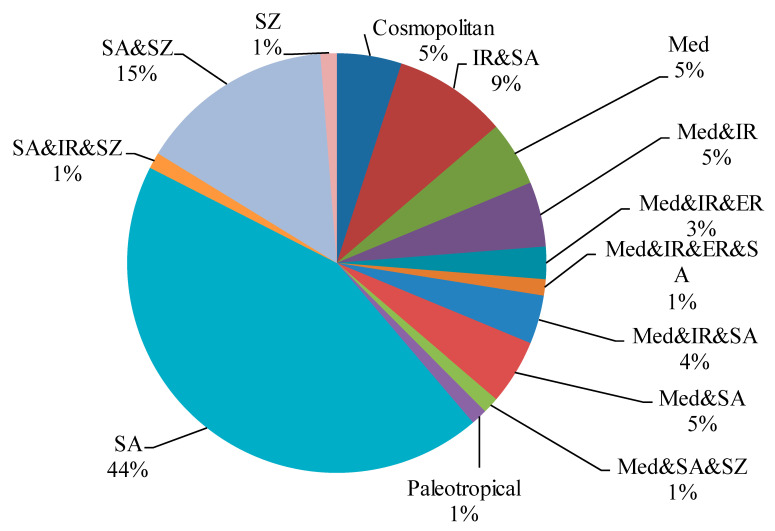
With respect to a phytogeographical region, monoregional geoelements were the most common floristic category (40 species, 50%), followed by biregionals (27 species, 33.8%), and pleuriregionals (8 species, 10%). Cosmopolitan and palaeotropical species were represented by one and one species, respectively (Table 2 and Figure 6).
Figure 6.
Chorotypes of the species recorded in Wadi Hagul.
In the current study, 80 taxa were recorded, whereas Abdelaal [60] listed 98 taxa. The number of species recorded in this study was 19 (not recorded in the previous study), whereas 38 species were recorded by Abdelaal [60]. A total of 61 plant species were shared between the two studies (Table 2).
The averages of species richness, evenness, and Shannon‘s and Simpson‘s indices were 3, 0.556, 0.722, and 0.3755, respectively. Hill′s number when Q = zero, gamma, alpha and beta diversity were 63, 9.7 and 6.5, respectively. Hill′s number when Q = one, gamma, alpha and beta diversity were 8.2, 4.3 and 1.9, respectively. Hill′s number when Q = two, gamma, alpha and beta diversity were 5, 3.3 and 1.5, respectively.
3.2. Soil Characteristics
Soil samples were taken at a depth of 0 to 30 cm in the studied localities, and 15 soil factors were analyzed. Soil pH, TDS, and EC values ranged 7.4 to 8.5, 82 to 9840 ppm, and 0.128 to 15.375 dS/m, respectively. Calcium, magnesium, sodium, and potassium contents ranged 0.7 to 49 meq/L, 0.22 to 28 meq/L, 0.21 to 63 meq/L, and 0.104 to 11.2 meq/L, respectively. Carbonate, bicarbonate, chloride, and sulfate contents ranged between 0.15 and 0.45%, 0.305 and 0.61%, 0.5 and 43 meq/L, and 1.2 to 76 meq/L, respectively. Organic carbon, clay, and silt contents in all soil samples were less than 1.4%, 5%, and 15%, respectively. In addition, sand contribution in all soil samples was more than 81% (Table 3).
Table 3.
Descriptive statistical analysis of soil factors.
| Soil Factor | Mean ± Standard Error | Range (Max–Min) | Standard Deviation | Median | Interquartile Range (IQR) |
|---|---|---|---|---|---|
| pH | 8 ± 0.0736 | 1.1 (8.5–7.4) | 0.329 | 8.1 | 0.55 |
| T.D.S. (ppm) | 1077.65 ± 508.726 | 9,758 (9840–82) | 2,275.094 | 360.5 | 533 |
| E.C. (dS/m) | 1.684 ± 0.795 | 15.247 (15.375–0.128) | 3.555 | 0.563 | 0.832 |
| Ca+2 (meq/L) | 6.353 ± 2.537 | 48.3 (49–0.7) | 11.346 | 2.65 | 2.952 |
| Mg+2 (meq/L) | 3.053 ± 1.366 | 27.78 (28–0.22) | 6.108 | 1.27 | 1.37 |
| Na+ (meq/L) | 6.025 ± 3.421 | 62.79 (63–0.21) | 15.301 | 1.15 | 2.205 |
| K+ (meq/L) | 1.18 ± 0.565 | 11.096 (11.2–0.104) | 2.528 | 0.165 | 1.288 |
| CO3−2 (%) | 0.225 ± 0.0231 | 0.3 (0.45–0.15) | 0.103 | 0.15 | 0.15 |
| HCO3− (%) | 0.32 ± 0.0153 | 0.305 (0.61–0.305) | 0.0682 | 0.305 | 0 |
| Cl− (meq/L) | 4.532 ± 2.278 | 42.5 (43–0.5) | 10.189 | 1.235 | 1.37 |
| SO4−2 (meq/L) | 9.57 ± 3.961 | 74.8 (76–1.2) | 17.714 | 3.9 | 4.75 |
| Organic carbon (%) | 0.888 ± 0.079 | 1.2 (1.32–0.12) | 0.353 | 0.84 | 0.66 |
| Sand (%) | 83.333 ± 0.19 | 3.05 (84.7–81.65) | 0.851 | 83.4 | 1.428 |
| Silt (%) | 13.31 ± 0.137 | 1.9 (14.3–12.4) | 0.613 | 13.4 | 1.15 |
| Clay (%) | 3.357 ± 0.165 | 2.64 (4.7–2.06) | 0.739 | 3.325 | 1.087 |
pH = soil reaction, T.D.S. = total dissolved salts, E.C. = electrical conductivity, Ca2+ = calcium, Mg2+ = magnesium, Na+ = sodium, K+ = potassium, CO32− = carbonates, HCO3− = bicarbonates, Cl− = chlorides, SO42− = sulfates, ppm = parts per million, dS/m = deciSiemens per meter and meq/L = milliequivalents per liter.
3.3. Effect of Environmental Factors on the Distribution of Plant Communities in the Studied Localities
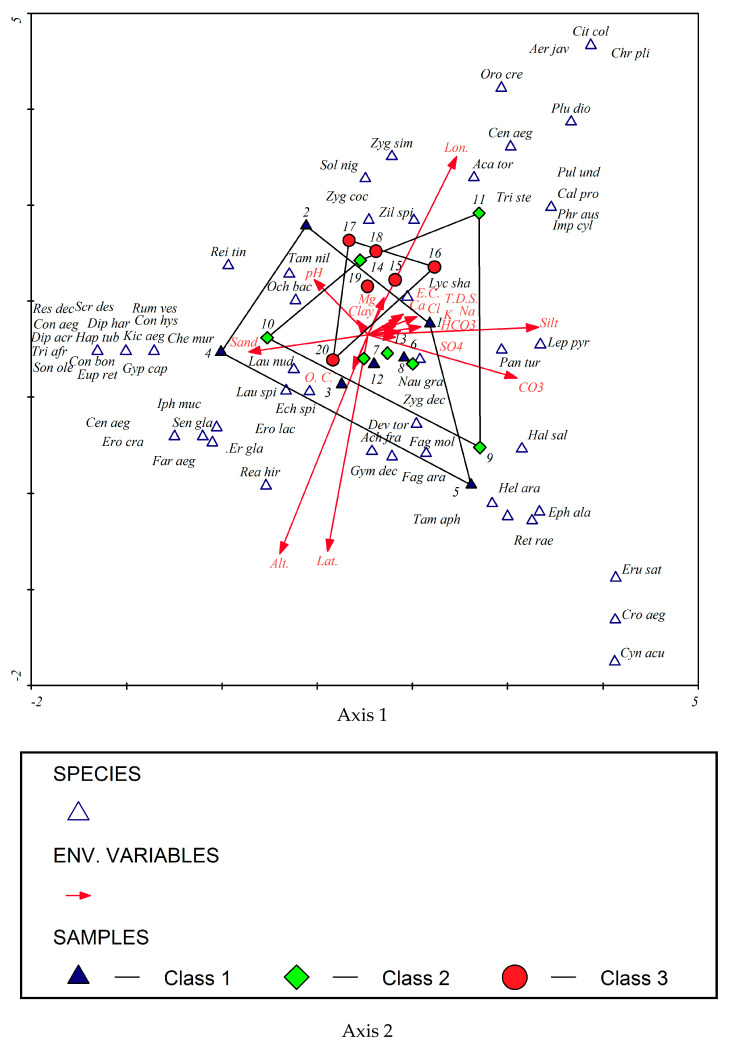
The distribution of the studied localities and recorded plant species and their relationships to environmental factors are illustrated in Figure 7. DCCA showed that altitude, latitude, organic carbon content, Tamarix aphylla, Gymnocarpos decandrus, Fagonia Arabica, Reaumuria hirtella, and Erodium laciniatum positively correlated with axis 1. Longitude, Mg2+, clay content, Citrullus colocynthis, Chrozophora plicata, Aerva javanica, and Orobanche crenata are negatively correlated with axis 1. Sand content, pH, Reseda decursiva, Scrophularia deserti, Rumex vesicarius, Conyza aegyptiaca, Diplotaxis acris, Diplotaxis harra, Haplophyllum tuberculatum, Trichodesma africanum, Sonchus oleraceus, Conyza bonariensis, Euphorbia retusa, Gypsophila capillaris, Chenopodium murale, and Kickxia aegyptiaca positively correlated with axis 2, whereas T.D.S., E.C., Cl−, Ca2+, K+, Na+, SO42−, CO3−, HCO32−, Pulicaria undulata, Calotropis procera, Phragmites australis, and Imperata cylindrica negatively correlated with axis 2.
Figure 7.
DCCA triplot showing the distribution of the studied localities and the recorded plant species as well as their relationships to environmental factors. The triangles represent species, and the arrows represent environmental variables. Arrow length expresses relative importance of an environmental variable. Dropping perpendicular to the arrows from each of the “species″ indicates the species′ relative position along the ecological gradient represented by an arrow.
3.4. Vegetation Change
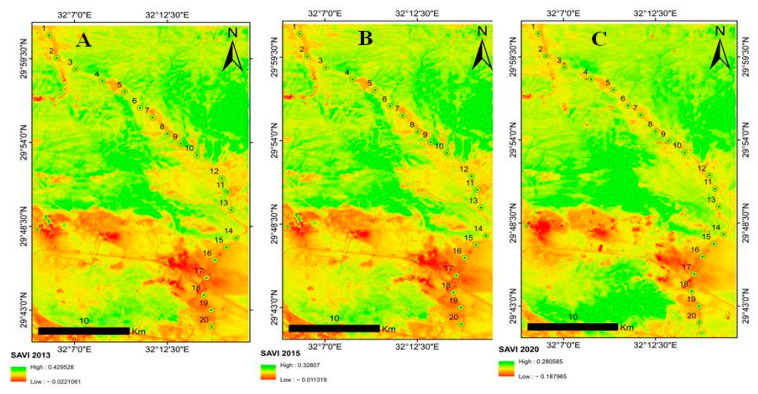
SAVI for 2013 ranged from −0.02 to 0.42; for 2015, it ranged from −0.011 to 0.32; and for 2020, it ranged from −0.18 to 0.28 (Figure 8).
Figure 8.
SAVI maps for 2013 (A), 2015 (B), and 2020 (C).
4. Discussion
In Wadi Hagul, 80 plant species belonging to 30 families were recorded. Among them, Asteraceae, Brassicaceae, and Zygophyllaceae were the most frequent families. These results are very similar to those reported in 2009 by Zahran and Willis [44] (31 species recorded) and in 2000 by Marie [61] (37 species recorded), who found that Asteraceae and Zygophyllaceae were the most common plant families in Wadi Hagul. By contrast, in 2016, Abdelaal [60] recorded 98 species, where Asteraceae and Poaceae were the most frequent families. Eight families (Aizoaceae, Cistaceae, Cleomaceae, Liliaceae, Neuradaceae, Polygonaceae, Urticaceae and Verbenaceae), each represented by only one plant species, were recorded in Abdelaal [60], and none of them were recorded in this study. Asteraceae were reported as the most common family in other Eastern desert wadies (Wadi Asyouti and Wadi Habib) [62]. Asteraceae was known for having a proportion of salt-tolerant and xerophytic species [63]. Asteraceae makes up the bulk of floristic composition in Egypt. It is represented by 98 genera, and 234 species [48,64]. Mashaly [65] reported a list of 62 species, with no alien species found.
The perennial plant group was most represented (67.5%) in the current study; this is consistent with the results of Zahran and Willis [44], Marie [61], and Abdelaal [60], who studied vegetation in the same study area during previous years. Regarding the number of annual species in Wadi Hagul, it has changed over the years of the study. This may be due to variation in the total rainfall during the studied years.
Life forms of species depend mainly on adaptation to the environment, particularly climate [66,67,68,69]. Life forms of desert plants are closely related to precipitation [70,71] and are correlated with both landform and topography [72,73,74]. In the present study, the chamaephyte life form was most represented (40%). Therophyte was the second most common life form (31.3%). These results are in accordance with the results of Abd El-Galil [75], who studied the floristic composition of Wadi Al-Assiuty, Eastern Desert, Egypt. In arid and semi-arid regions, chamaephyte and therophyte were found to be the most common life-forms [76,77,78].
Saharo-Arabian species captured the highest percentage in the floristic categories (43.75%). This result is concordant with Zahran and Willis [44], Marie [61], and Abdelaal [60]. It is worth noting that Saharo-Arabian species are good indicators for desert environmental conditions [79,80,81].
Species richness, which refers to the number of various plant species in the stands, is one of the most important indices of species diversity. In the studied stands, the average of species richness recorded three species, which is a low number. This could be due to a variety of factors, including the severe environment and climate that characterize the study area, which may be an obstacle to the growth of some plant species. Species evenness is a description of the distribution of species abundance in a community. The average of species evenness was 0.7. Species evenness is measured on a scale of 0 to 1, with 0 representing the lowest evenness (one species has 100% coverage) and 1 representing the highest evenness (coverage is evenly spread among a number of species). This may be due to the presence of a very dominant species in a community causes the less competitive species to be suppressed [82]. Shannon index depends strongly on species richness [83]. Simpson index is not a very intuitive measure of diversity since higher values indicate lower diversity [84].
The results of DCCA analysis indicated that latitude, longitude, altitude, silt and sand contents, pH, and CO32− content are the most important factors affecting the distribution of vegetation in Wadi Hagul. These results are somewhat consistent with those of Mashaly [62], who stated that the most influential soil factors for the distribution of vegetation in Wadi Hagul are soil texture, Na+, pH, and organic matter. Abdelaal [60] mentioned that K+, Na+, organic matter, moisture content, pH, E.C., and Cl− were the most affecting soil parameters for the distribution of vegetation in Wadi Hagul. Plant species associated with the increase in the proportion of sand in the soil and soil pH were Tamarix nilotica, Ochradenus baccatus, Launea nudicalus, Launea nudicalus and Rumex vesicarius. The longitude and the amount of Mg2+ in the soil were the most important factors associated with many plant species such as; Zilla spinosa, Zygophyllum simplex and Zygophyllum coccineum. Most of the salinity factors (E.C., T.D.S., Ca2+, Cl− , Na+ and K+), CO32− , HCO3− and the percentage of silt in soil were associated with some species such as; Lycium shawii, Leptadenia pyrotechnica, Panicum turgidum and Haloxylon salicornicum. Latitude, altitude and the amount of organic carbon in soil were important factors in the distribution of some plant species such as Echinops spinosus, Erodium laciniatum, Erodium glaucophyllum and Reaumuria hirtella.
Many of the threatened plant species recorded in previous studies were not recorded in this study, including Aizoon canariensis, Artemisia judaica, Ifloga spicata, Silene viviani, Sphaerocoma hookeri, Helianthemum lippi, Astraglus spinosus, Senna alexandrina, Salvia aegyptiaca, Schismus barbatus, Hyoscyamus muticus, and Verbena officinalis. As a result, action must be taken to safeguard threatened species by a variety of measures, including the establishment of protected areas, criminalizing exposure to endangered plants, creating a gene bank for these plants, and attempting to increase their numbers in practice.
In this study, many alien and invasive species, such as Euphorbia prostrate, were recorded [85]. The introduction of invasive and alien species into natural habitats represents a threat to existing species. Assaeed et al. [86] indicated that invasive and exotic plants may pose a threat to natural resources and biodiversity, especially in arid habitats. Successful invaders often exhibit great degrees of adaptability, allowing them to thrive in a variety of environments [87,88]. Plant shoot and root system features are thought to be good morphological criteria for predicting successful invasion in many habitat types [89]. Many invasive species contain allelopathic chemicals that enable them to invade and control plant communities [90].
Climate changes, in addition to human encroachments such as the construction of roads and the establishment of new cities, pose the main pressure on vegetation. Climate changes and human impact negatively affect biodiversity in several Wadies in the Egyptian Eastern Desert [91].
In this study, SAVI decreased during 2013 (from −0.02 to 0.42), 2015 (from −0.011 to 0.32), and 2020 (from −0.18 to 0.28). This result could be due to various human impacts in Wadi Hagul such as the construction of the new road Al-Galala–Hagul–Zafarana, which is 84 km long and 24 m wide and crosses Wadi Hagul, in addition to many other threats such as overgrazing, plant collection, and increasing demand for energy, which have led to exploration for oil and natural gas near Wadi Hagul. Large population growth in recent times has also led to increasing demand for building materials and opening quarries inside Wadi Hagul, which resulted in a local increase in transport and pollution. Finally, it was discovered that if environmental conditions (such as human interventions and climate changes) alter and become unsuitable for plant growth, they have a negative impact on vegetation cover, thus lowering the SAVI values.
In recent times, the preservation and protection of wildlife have become an urgent necessity, especially in the light of misuse of natural resources and encroachment of wildlife. Economic development and wildlife conservation can be simultaneously achieved by following the principles, rules, and requirements of sustainable development for balanced usage of available resources.
5. Conclusions
This study was conducted in an unprotected area, Wadi Hagul, Eastern desert, Egypt, to evaluate the relationship between environmental factors and the distribution of plant species, as well as evaluate the negative effects of uncontrolled human activities on both floristic composition and vegetation structure. Climate change, reflected in high temperatures and a lack of rain, also has a negative impact on vegetation cover in the study area, which is classified as a semi-arid desert. Ecosystems in general, especially deserts, are greatly affected by irresponsible human interventions. Hence, plans and strategies should be developed to conserve biodiversity. Within this context, the outcomes of this study, as well as those of other similar studies, will aid in the implementation of necessary environmental protection measures. Our study clearly showed a decreasing trend of SAVI across Wadi Hagul during 2013, 2015, and 2020. Physical factors, rather than anthropogenic, were the primary driving force for vegetation dynamics, whereas the effects of anthropogenic factors may be magnified when physical environmental factors provide unsuitable ambiance for vegetation growth. Other indexes must be measured to estimate vegetation cover, especially in unprotected areas subject to major human interventions, as can be carried out in future studies.
Acknowledgments
Authors thank Abdo Marei Hamed for his keen help during field trips, Ibrahim A. Mashaly for his keen help in identification of plant samples, Om Mohamed A. Khafagi for her help in paper reviewing and Al-Baraa S. M. El-Saied for his help in statistical analysis.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants10091906/s1, Figure S1: Climate diagram of Wadi Hagul (1990–2020): mean maximum temperature (°C), mean minimum temperature (°C), precipitation (mm), hot days (°C), and cold nights, Figure S2: Wind direction and velocity in Wadi Hagul (1990–2020).
Author Contributions
Conceptualization, A.A.I., R.B. and S.R.; software, R.B. and A.A.I.; validation, A.A.I., S.A. and A.A.A.; formal analysis, R.B., A.A.I., S.A., A.A.A. investigation, A.A.I., R.B. and S.R.; resources, A.A.I., S.R. and R.B.; data curation, A.A.I., S.R. and R.B.; writing—original draft preparation, R.B., A.A.I., S.R.; writing—review and editing, A.A.I., R.B. and S.R.; visualization, A.A.I., R.B., S.A. and A.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Relevant data applicable to this research are within the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Millennium Ecosystem Assessment . Ecosystems and Human Well-Being: Desertification Synthesis. World Resources Institute; Washington, DC, USA: 2005. [Google Scholar]
- 2.Referowska-Chodak E. Pressures and threats to nature related to human activities in European urban and suburban forests. Forests. 2019;10:765. doi: 10.3390/f10090765. [DOI] [Google Scholar]
- 3.Newton A.C., Cayuela C., Echeverría C., Armesto J.J., Del Castillo R.F., Golicher D., Geneletti D., Gonzalez-Espinosa M., Huth A., López-Barrera F., et al. Toward integrated analysis of human impacts on forest biodiversity: Lessons from Latin America. Ecol. Soc. 2009;14:2. doi: 10.5751/ES-02847-140202. [DOI] [Google Scholar]
- 4.Kapos V., Lysenko I., Lesslie R. Assessing Forest Integrity and Naturalness in Relation to Biodiversity. Forestry Department FAO; Rome, Italy: 2002. Forest Resources Assessment Programme, Working Paper 54. [Google Scholar]
- 5.Moustafa A.A., Abd El-Wahab R.H., Zaghloul M.S. Conservation and Sustainable Use of Medicinal Plants in Arid and Semi-Arid Ecosystems of Egypt. Egyptian Environmental Affairs Agency (EEAA), United Nations Development Programme (UNDP) and Global Environmental Facilities (GEF); Giza, Egypt: 1999. Final Report. [Google Scholar]
- 6.Nakahama N., Hirasawa Y., Minato T., Hasegawa M., Isagi Y., Shiga T. Recovery of genetic diversity in threatened plants through use of germinated seeds from herbarium specimens. Plant Ecol. 2015;216:1635–1647. doi: 10.1007/s11258-015-0547-8. [DOI] [Google Scholar]
- 7.Hussein E.A., Abd El-Ghani M.M., Hamdy R.S., Shalabi L.F. Do anthropogenic activities affect floristic diversity and vegetation structure more than natural soil properties in hyper-arid desert environments? Diversity. 2021;13:157. doi: 10.3390/d13040157. [DOI] [Google Scholar]
- 8.Sebastian O., Sibyll S., Wolfgang L., Dieter G. Three centuries of dual pressure from land use and climate change on the biosphere. Environ. Res. Lett. 2015;10:044011. [Google Scholar]
- 9.Titeux N., Henle K., Mihoub J.B., Regos A., Geijzendorffer I.R., Cramer W., Verburg P.H., Brotons L. Biodiversity scenarios neglect future land-use changes. Glob. Chang. Biol. 2016;22:2505–2515. doi: 10.1111/gcb.13272. [DOI] [PubMed] [Google Scholar]
- 10.Pereira H.M., Navarro L.M., Martins I.S. Global biodiversity change: The bad, the good, and the unknown. Annu. Rev. Environ. Resour. 2012;37:25–50. doi: 10.1146/annurev-environ-042911-093511. [DOI] [Google Scholar]
- 11.Zhu P., Cao W., Huang L., Xiao T., Zhai J. The impacts of human activities on ecosystems within China’s nature reserves. Sustainability. 2019;11:6629. doi: 10.3390/su11236629. [DOI] [Google Scholar]
- 12.Wu J.W., Miao C.Y., Wang Y.M., Duan Q.Y., Zhang X.M. Contribution analysis of the long-term changes in seasonal runoff on the Loess Plateau, China, using eight Budyko-based methods. J. Hydrol. 2017;545:263–275. doi: 10.1016/j.jhydrol.2016.12.050. [DOI] [Google Scholar]
- 13.Kong D., Miao C., Borthwick A.G., Lei X., Li H. Spatiotemporal variations in vegetation cover on the Loess Plateau, China, between 1982 and 2013: Possible causes and potential impacts. Environ. Sci. Pollut. Res. 2018;25:13633–13644. doi: 10.1007/s11356-018-1480-x. [DOI] [PubMed] [Google Scholar]
- 14.Enright N.J., Miller B.P., Akhter R. Desert vegetation and vegetation-environment relationships in Kirthar National Park, Sindh, Pakistan. J. Arid Environ. 2005;61:397–418. doi: 10.1016/j.jaridenv.2004.09.009. [DOI] [Google Scholar]
- 15.Abd El-Wahab R.H. Plant assemblage and diversity variation with human disturbances in coastal habitats of the western Arabian Gulf. J. Arid Land. 2016;8:787–798. doi: 10.1007/s40333-016-0084-x. [DOI] [Google Scholar]
- 16.Kotanska M., Buziak-Chmielowiec E., Dabrowska A., Gladysz M., Jakielaszek A., Wójcik T. Human impact on the plant coverof four villages in SE Poland. Steciana. 2015;19:115–121. doi: 10.12657/steciana.019.013. [DOI] [Google Scholar]
- 17.TEEB . The Economics of Ecosystems and Biodiversity: Mainstreaming the Economics of Nature: A Synthesis of the Approach, Conclusions and Recommendations of TEEB. Volume 1. United Nations Environment Programme; Geneva, Switzerland: 2010. 47p [Google Scholar]
- 18.Koagne M.D. Evaluation of Ecological Monitoring System of the Dynamics of Human Activities in the Benue National Park and Its Periphery. Engineer Memory of Water, Forests and Hunting, FASA, Dschang University; Dschang, Cameroon: 2009. 98p [Google Scholar]
- 19.Blicharska M., Grandin U. Why protect biodiversity? Perspectives of conservation professionals in Poland. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2015;11:349–362. doi: 10.1080/21513732.2015.1050969. [DOI] [Google Scholar]
- 20.Dimobe K., Wala K., Batawila K., Dourma M., Woegan Y.A. Analyse spatiale des différentes formes de pressions anthropiques dans la réserve de faune de l’Oti- Mandouri (Togo) VertigO—La Revue Électronique en Sciences de l’Environnement. 2012;14:1–22. doi: 10.4000/vertigo.12423. (In French) [DOI] [Google Scholar]
- 21.Cohen J.E. Human population: The next half century. Science. 2003;302:1172–1175. doi: 10.1126/science.1088665. [DOI] [PubMed] [Google Scholar]
- 22.United Nations . In: World Population Prospects: The Revision, Key Findings and Advance Tables. Population Division, editor. Department of Economic and Social Affairs; New York, NY, USA: 2017. 248p Working Paper No., ESA/P/WP/ [Google Scholar]
- 23.Finn H.C., Stephens N.S. The invisible harm: Land clearing is an issue of animal welfare. Wildl. Res. 2017;44:377–391. doi: 10.1071/WR17018. [DOI] [Google Scholar]
- 24.Baillie J.E., Hilton-Taylor C., Stuart S.N. IUCN Red List of Threatened Species: A Global Species Assessment. IUCN; Gland, Switzerland: Cambridge, UK: 2004. 191p [Google Scholar]
- 25.Foreman D. Rewilding North America. Island Press; Washington, DC, USA: 2004. 312p [Google Scholar]
- 26.International Union for Conservation of Nature (IUCN) Red List of Threatened Species. Gland, Switzerland: The World Conservation Union. 2006. [(accessed on 1 April 2006)]. Available online: https://www.iucnredlist.org.
- 27.International Union for Conservation of Nature (IUCN) Red List of Threatened Species (Gland, Switzerland: International Union for Conservation of Nature, 1999) 2011. [(accessed on 31 December 2011)]. Available online: http://www.iucnredlist.org.
- 28.Brummitt N.A., Bachman S.P., Griffiths-Lee J., Lutz M., Moat J.F., Farjon A., Aletrari E. Green plants in the red: A baseline global assessment for the IUCN sampled Red List Index for plants. PLoS ONE. 2015;10:e0135152. doi: 10.1371/journal.pone.0135152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin H., Yang Y., Dong S., He Q., Jia Y., Zhao L., Xiang J. Threatened species list of China’s higher plants. Biodivers. Sci. 2017;25:696. doi: 10.17520/biods.2017144. [DOI] [Google Scholar]
- 30.McKinney M.L., Lockwood J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999;14:450–453. doi: 10.1016/S0169-5347(99)01679-1. [DOI] [PubMed] [Google Scholar]
- 31.Hunter P. The human impact on biological diversity: How species adapt to urban challenges sheds light on evolution and provides clues about conservation. EMBO Rep. 2007;8:316–318. doi: 10.1038/sj.embor.7400951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mack R.N., Simberloff D., Lonsdale W.M., Evans H., Clout M., Bazzaz F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000;10:689–710. doi: 10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2. [DOI] [Google Scholar]
- 33.Kassem H.S., Bello A.R.S., Alotaibi B.M., Aldosri F.O., Straquadine G.S. Climate change adaptation in the delta Nile Region of Egypt: Implications for agricultural extension. Sustainability. 2019;11:685. doi: 10.3390/su11030685. [DOI] [Google Scholar]
- 34.Turner B.L., II, Lambin E.F., Reenberg A. From the cover land change science special feature: The emergence of land change science for global environmental change and sustainability. Proc. Natl. Acad. Sci. USA. 2007;104:20666–20671. doi: 10.1073/pnas.0704119104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benzer N. Using the geographical information system and remote sensing techniques for soil erosion assessment. Pol. J. Environ. Stud. 2010;19:881–886. [Google Scholar]
- 36.Zhang X., Hu Y., Zhuang D., Qi Y., Ma X. Ndvi spatial pattern and its differentiation on the Mongolian plateau. J. Geogr. Sci. 2009;19:403–415. doi: 10.1007/s11442-009-0403-7. [DOI] [Google Scholar]
- 37.Kaspersen P.S., Fensholt R., Huber S. A spatiotemporal analysis of climatic drivers for observed changes in Sahelian vegetation productivity (1982–2007) Int. J. Geophys. 2011;2011:715321. doi: 10.1155/2011/715321. [DOI] [Google Scholar]
- 38.Hope A.S., Boynton W.L., Stow D.A., Douglas D.C. Interannual growth dynamics of vegetation in the kuparuk river watershed, alaska based on the normalized difference vegetation index. Int. J. Remote Sens. 2003;24:3413–3425. doi: 10.1080/0143116021000021170. [DOI] [Google Scholar]
- 39.Rhyma P.P., Norizah K., Hamdan O., Faridah-Hanum I., Zulfa A.W. Integration of normalised different vegetation index and Soil-Adjusted Vegetation Index for mangrove vegetation delineation. Remote Sens. Appl. Soc. Environ. 2020;17:100280. doi: 10.1016/j.rsase.2019.100280. [DOI] [Google Scholar]
- 40.Ren H., Zhou G., Zhang F. Using negative soil adjustment factor in soil-adjusted vegetation index (SAVI) for aboveground living biomass estimation in arid grasslands. Remote Sens. Environ. 2018;209:439–445. doi: 10.1016/j.rse.2018.02.068. [DOI] [Google Scholar]
- 41.Senseman G.M., Bagley C.F., Tweddale S.A. Correlation of rangeland cover measures to satellite-imagery-derived vegetation indices. Geocarto Int. 1996;11:29–38. doi: 10.1080/10106049609354546. [DOI] [Google Scholar]
- 42.Ahmad F. Spectral vegetation indices performance evaluated for Cholistan Desert. J. Geogr. Reg. Plan. 2012;5:165–172. [Google Scholar]
- 43.Seleem T.A., Aboulela H.A. Seismicity and geologic structures indubitable in Wadi Hagul, north Eastern Desert, Egypt. Int. J. Geosci. 2011;2:55. doi: 10.4236/ijg.2011.21006. [DOI] [Google Scholar]
- 44.Zahran M.A., Willis A.J. The Vegetation of Egypt. 2nd ed. Springer; Dordrecht, The Netherlands: 2009. 437p [Google Scholar]
- 45.Abbas N.H., ELAraby M.M., Ahmad H.F., ELSherbeny E.A. Molecular insight into Wadi Hagul rare diversity: Echinops spinosus and fagonia molis, plant species. Egypt. J. Genet. Cytol. 2021;50:83–94. [Google Scholar]
- 46.Boulos L. Flora of Egypt: Azollaceae-Oxalidaceae. Volume I. Al-Hadara Publishing; Cairo, Egypt: 1999. 419p [Google Scholar]
- 47.Boulos L. Flora of Egypt: Geraniaceae-Boraginaceae. Volume II. Al-Hadara Publishing; Cairo, Egypt: 2000. 352p [Google Scholar]
- 48.Boulos L. Flora of Egypt: Verbenaceae-Compositae) Volume III. Al-Hadara Publishing; Cairo, Egypt: 2002. 373p [Google Scholar]
- 49.Boulos L. Flora of Egypt: Monocotyledons (Alismataceae-Orchidaceae) Volume IV. Al-Hadara Publishing; Cairo, Egypt: 2005. 617p [Google Scholar]
- 50.Boulos L. Flora of Egypt Checklist. Revised Annotated ed. Al-Hadara Publishing; Cairo, Egypt: 2009. 410p [Google Scholar]
- 51.Raunkiaer C. The Plant Life Forms and Statistical Plant Geography. Clarendon Press; Oxford, UK: 1934. 632p [Google Scholar]
- 52.Tutin T.G., Heywood V.H., Burges N.A., Moore M.D., Valentine D.H., Walters S.M., Webb D.A. Flora Europaea. Volumes 1–5 Cambridge University Press; Cambridge, UK: 1964. [Google Scholar]
- 53.Davis P.H. Flora of Turkey and the East Aegean Islands. Volumes 1–9 Edinburgh University Press; Edinburgh, UK: 1965–1985. [Google Scholar]
- 54.Estefan G., Sommer R., Ryan J. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region. International Center for Agricultural Research in the Dry Areas; Beirut, Lebanon: 2013. 243p [Google Scholar]
- 55.Page A.L., Miller R.H., Keeney D.R. Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties. American Society of Agronomy; Madison, WI, USA: 1982. 1159p [Google Scholar]
- 56.Jackson M.L. Soil Chemicals Analysis. Prentice-Hall of India Private; New Delhi, India: 1967. 498p [Google Scholar]
- 57.Piper C.S. Soil and Plant Analysis. University of Adelaide; Adelaide, Australia: 1950. 368p [Google Scholar]
- 58.McCune B., Mefford M.J. PCORD for Windows: Multivariate Analysis of Ecological Data. MjM Software; Gleneden Beach, OR, USA: 1999. 47p Version 4.14. User’s Guide. [Google Scholar]
- 59.ter Braak C.J.F. CANOCO-an extension of DECORANA to analyze species-environment relationships. Plant Ecol. 1988;75:159–160. doi: 10.1007/BF02392953. [DOI] [Google Scholar]
- 60.Abdelaal M. Current status of the floristic composition in Wadi Hagul, northwest Suez gulf, Egypt. Rendiconti Lincei. 2016;28:81–92. doi: 10.1007/s12210-016-0577-8. [DOI] [Google Scholar]
- 61.Marie A. Ph.D. Thesis. Faculty of Science Al-Azhar University; Cairo, Egypt: 2000. A Model for the Use of GIS in Vegetation Mapping of a Phytogeographical Region in Egypt.206p [Google Scholar]
- 62.Salama F.M., Sayed S.A., Abd Abd EL-Gelil A.A. Plant communities and floristic composition of the vegetation of Wadi Al-Assiuty and Wadi Habib in the Eastern Desert, Egypt. Not. Sci. Biol. 2014;6:196–206. doi: 10.15835/nsb629297. [DOI] [Google Scholar]
- 63.Aronson J.A. In: HALOPHA: A Data Base of Salt Tolerant Plants of the World. Whitehead E.E., editor. Office of Arid Land Studies, University of Arizona; Tuscon, AZ, USA: 1989. 77p [Google Scholar]
- 64.Täckholm V. Students’ Flora of Egypt. 2nd ed. Cooperative Printing Company; Cairo, Egypt: 1974. [Google Scholar]
- 65.Mashaly I.A. On the phytosociology of Wadi Hagul, Red Sea coast. Egypt. J. Environ. Sci. 1996;12:31–54. [Google Scholar]
- 66.Martiny N., Camberlin P., Richard Y., Philippon N. Compared regimes of NDVI and Rainfall in semi-arid regions of Africa. Int. J. Remote Sens. 2006;27:5201–5223. doi: 10.1080/01431160600567787. [DOI] [Google Scholar]
- 67.Parmesan C., Hanley M.E. Plants and climate change: Complexities and surprises. Ann. Bot. 2015;116:849–864. doi: 10.1093/aob/mcv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Irl S.D.H., Obermeier A., Beierkuhnlein C., Steinbauer M.J. Climate controls plant life-form patterns on a high-elevation oceanic island. J. Biogeogr. 2020;47:2261–2273. doi: 10.1111/jbi.13929. [DOI] [Google Scholar]
- 69.Pausata F.S.R., Gaetani M., Messori G., Berg A., Souza D.M.D., Sage R., Demenocal P. The greening of the Sahara: Past changes and future implications. One Earth. 2020;2:235–250. doi: 10.1016/j.oneear.2020.03.002. [DOI] [Google Scholar]
- 70.Nafus M.G., Tuberville T.D., Buhlmann K.A., Todd B.D. Precipitation quantity and timing affect native plant production and growth of a key herbivore, the desert tortoise, in the Mojave Desert. Clim. Chang. Responses. 2017;4:4. doi: 10.1186/s40665-017-0032-9. [DOI] [Google Scholar]
- 71.Ruhm J., Böhnert T., Weigend M., Merklinger F.F., Stoll A., Quandt D., Luebert F. Plant life at the dry limit—Spatial patterns of floristic diversity and composition around the hyperarid core of the Atacama Desert. PLoS ONE. 2020;15:e0233729. doi: 10.1371/journal.pone.0233729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Batanouny K.H. Topography and plant life. In: Batanouny K.H., editor. Plants in the Deserts of the Middle East. Springer; Berlin/Heidelberg, Germany: 2001. pp. 25–29. [Google Scholar]
- 73.Wang J., Wang H., Cao Y., Bai Z., Qin Q. Effects of soil and topographic factors on vegetation restoration in opencast coal mine dumps located in a loess area. Sci. Rep. 2016;6:22058. doi: 10.1038/srep22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baartman J.E.M., Temme A.J.A.M., Saco P.M. The effect of landform variation on vegetation patterning and related sediment dynamics. Earth Surf. Process. Landf. 2018;43:2121–2135. doi: 10.1002/esp.4377. [DOI] [Google Scholar]
- 75.Abd El-Galil A. Master’s Thesis. Faculty of Science Assiut University; Asyut, Egypt: 2014. Floristic Composition and Eco Physiological Studies on Some Xerophytic Plants in Wadi El-Assiuty, Eastern Desert, Egypt.330p [Google Scholar]
- 76.El-Amier Y.A. Vegetation structure and soil characteristics of five common geophytes in desert of Egypt. Egypt. J. Basic Appl. Sci. 2016;3:172–186. doi: 10.1016/j.ejbas.2016.03.001. [DOI] [Google Scholar]
- 77.Jafari Z., Kianian M., Aslani F., Akbarlo M. Floristic composition, life forms and geographical distribution (case study: Lashgardar rangelands of Malayer, Iran) J. Environ. Sci. Technol. 2016;9:140–148. doi: 10.3923/jest.2016.140.148. [DOI] [Google Scholar]
- 78.Al Shaye N.A., Masrahi Y.S., Thomas J. Ecological significance of floristic composition and life forms of Riyadh region, Central Saudi Arabia. Saudi J. Biol. Sci. 2020;27:35–40. doi: 10.1016/j.sjbs.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Galal T.M., Fahmy A.G. Plant diversity and community structure of Wadi Gimal protected area, Red Sea Coast of Egypt. Afr. J. Ecol. 2012;50:266–276. doi: 10.1111/j.1365-2028.2012.01320.x. [DOI] [Google Scholar]
- 80.Abbas M.S., Afefe A.A., Hatab E.-B.E., Gaber E.-S.I. Vegetation-Soil Relationships in Wadi El-Rayan Protected Area, Western Desert, Egypt. Jordan J. Biol. Sci. 2016;9:97–107. [Google Scholar]
- 81.Bouallala M.H., Neffar S., Chenchouni H. Vegetation traits are accurate indicators of how do plants beat the heat in dry lands: Diversity and functional traits of vegetation associated with water towers in the Sahara Desert. Ecol. Indic. 2020;114:106364. doi: 10.1016/j.ecolind.2020.106364. [DOI] [Google Scholar]
- 82.Mohler C.L., Liebman M. Weed productivity and competition in sole crops and intercrops of barley and field pea. J. Appl. Ecol. 1987;24:685–699. doi: 10.2307/2403903. [DOI] [Google Scholar]
- 83.Hurlbert S. The non concept of species diversity: A critique and alternative parameters. Ecology. 1971;52:577–586. doi: 10.2307/1934145. [DOI] [PubMed] [Google Scholar]
- 84.Jost L., Chao A. Diversity Analysis. Taylor & Francis; Milton Keynes, UK: 2008. [Google Scholar]
- 85.Shaltout S. Master’s Thesis. Faculty of Science Tanta University; Tanta, Egypt: 2014. Ecological Studies on the Alien Species in the Egyptian Flora.206p [Google Scholar]
- 86.Assaeed A.M., Al-Rowaily S.L., El-Bana M.I., Hegazy A.K., Dar B.A., Abd-ElGawad A.M. Functional traits plasticity of the invasive herb Argemone ochroleuca sweet in different arid habitats. Plants. 2020;9:1268. doi: 10.3390/plants9101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hegazy A.K., Soliman M.I., Mashaly I.A. Perspectives on the biology of Heliotropium curassavicum in the Deltaic Mediterranean coast of Egypt. Arab Gulf J. Sci. Res. 1994;12:525–545. [Google Scholar]
- 88.Gupta S., Narayan R. Phenotypic plasticity of Chenopodium murale across contrasting habitat conditions in peri-urban areas in Indian dry tropics: Is it indicative of its invasiveness? Plant Ecol. 2012;213:493–503. doi: 10.1007/s11258-011-9997-9. [DOI] [Google Scholar]
- 89.Herpigny B., Dassonville N., Ghysels P., Mahy G., Meerts P. Variation of growth and functional traits of invasive knotweeds (Fallopia spp.) in Belgium. Plant Ecol. 2012;213:419–430. doi: 10.1007/s11258-011-9989-9. [DOI] [Google Scholar]
- 90.Abd-ElGawad A.M., El Gendy A.E.-N.G., Assaeed A.M., Al-Rowaily S.L., Omer E.A., Dar B.A., Al-Taisan W.A.A., Elshamy A.I. Essential oil enriched with oxygenated constituents from invasive plant Argemone ochroleuca exhibited potent phytotoxic effects. Plants. 2020;9:998. doi: 10.3390/plants9080998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abd El-Ghani M.M., Salama F., Salem B., El Hadidy A., Abdel-Aleem M. Biogeographical relations of a hyper arid desert flora in Eastern Egypt. Afr. J. Ecol. 2014;52:173–191. doi: 10.1111/aje.12102. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Relevant data applicable to this research are within the paper.