Abstract
The exploration of new green, ecofriendly bioactive compounds has attracted the attention of researchers and scientists worldwide to avoid the harmful effects of chemically synthesized compounds. Persicaria lapathifolia has been reported to have various bioactive compounds, while its essential oil (EO) has not been determined yet. The current work dealt with the first description of the chemical composition of the EO from the aerial parts of P. lapathifolia, along with studying its free radical scavenging activity and herbicidal effect on the weed Echinochloa colona. Twenty-one volatile compounds were identified via GC–MS analysis. Nonterpenoids were the main components, with a relative concentration of 58.69%, in addition to terpenoids (37.86%) and carotenoid-derived compounds (1.75%). n-dodecanal (22.61%), α-humulene (11.29%), 2,4-dimethylicosane (8.97%), 2E-hexenoic acid (8.04%), γ-nonalactone (3.51%), and limonene (3.09%) were characterized as main compounds. The extracted EO exhibited substantial allelopathic activity against the germination, seedling root, and shoot growth of the weed E. colona in a dose-dependent manner, showing IC50 values of 77.27, 60.84, and 33.80 mg L−1, respectively. In addition, the P. lapathifolia EO showed substantial antioxidant activity compared to ascorbic acid as a standard antioxidant. The EO attained IC50 values of 159.69 and 230.43 mg L−1, for DPPH and ABTS, respectively, while ascorbic acid exhibited IC50 values 47.49 and 56.68 mg L−1, respectively. The present results showed that the emergent leafy stems of aquatic plants such as P. lapathifolia have considerably low content of the EO, which exhibited substantial activities such as antioxidant and allelopathic activities. Further study is recommended to evaluate the effects of various environmental and climatic conditions on the production and composition of the EOs of P. lapathifolia.
Keywords: pale smartweed, essential oil, phytotoxicity, green chemistry, herbicides
1. Introduction
Essential oils (EOs) are complex mixtures of small compounds, and they have been documented to have persuasive medicinal potentialities including antioxidant [1,2], antibacterial [3,4], anti-inflammatory [5], anticancer [6], antiaging [2] antipyretic [5], and other activities. Because of these activities, EOs have been integrated into the food and cosmetics industries [7]. In the agriculture sector, EOs can be integrated as green, ecofriendly bioherbicides, where they can substitute the synthetic and chemical herbicides that are responsible for environmental pollution and affect human health [8].
Allelopathy is defined as the chemical interference between plants [9]. Understanding this biological phenomenon aids the development of applications for natural and agricultural systems [10]. Allelopathic interactions are considered to be crucial for the success of many invasive plants and are a key element in determining species distribution and abundance within plant ecosystems. Temperature, water content, salinity, nutrition availability, competitive stress, and microbiota are among the most important variables influencing allelopathy [11,12,13,14]. EOs from various wild plants have been reported as allelochemicals that interfere with the germination and growth of weeds [6,12,14,15]. These EOs have promising characteristics against weeds, as they are ecofriendly, have low resistance from weeds, and possess a wide allelopathic spectrum [8]. Over 3000 plant species have been studied for their EO composition, and hundreds of these EOs have been produced commercially [16]. In consequence, the exploration of new EOs from various wild plants with biological activities has attracted the attention of researchers and scientists worldwide.
Polygonaceae is an important edible families of flowering plants and has a worldwide distribution with 46 genera and 1100 species [17,18]. In the flora of Egypt, there are 28 species of Polygonaceae, belonging to eight genera [17]. Persicaria is commonly known as smartweeds or knotweeds, and this genus comprises about 150 herbaceous cosmopolitan species. Most species are found in temperate regions, with a few others are found in tropical and subtropical regions from sea level to a range of different altitudes [19]. Seven Persicaria species have been recorded in the flora of Egypt. Persicaria lapathifolia (L.) Delarbre (synonym Polygonum lapathifolium) is commonly known as pale persicaria, pale smartweed, or curlytop knotweed (Figure 1).
Figure 1.
Persicaria lapathifolia (L.) Delarbre: (a) overview of the aerial parts growing on the canal bank habitat, (b) close view of the aerial parts, and (c) close view of the inflorescence.
Persicaria lapathifolia is an annual herb that grows up to 80 cm and has reddish stems and alternate leaves [17]. This plant has grown worldwide (cosmopolitan) and inhabits various moist habitats and in agricultural fields. This species is considered a troublesome weed. Persicaria lapathifolia has many uses in traditional medicine such as antibacterial, antiviral, anti-inflammatory, astringent, antiseptic, anti-stomach complaint, hepatoprotective, and antifungal uses in addition to its use for the treatment of dysentery, burns, and fevers [20]. Several studies have revealed that different extracts and isolated metabolites of this plant have antimicrobial [21], anthelmintic and antiemetic [20], anticancer [22], antioxidant, α/β-glucosidase inhibitory, and anticholinesterase activity [23]. These activities were ascribed to the numerous phytoconstituents in this plant such as flavonoids, chalcones, acylated flavonoids, ferulate esters, and phenolic acids [21,22,23,24,25]. However, to our knowledge, the EO of P. lapathifolia has not been described yet, and in consequence, its EO’s allelopathic effect against weeds has not been studied. Echinochloa colona (L.) Link is commonly recognized as jungle rice because of its excellent competition with rice [26]. It is a noxious worldwide distributed weed that infests many crops (rice, maize, sugarcane, and cotton) as well as other habitats such as roadsides, gardens, disturbed sites, fallow lands, and pastures [27]. This weed is hardly controlled in the agroecosystem because of its diverse ecotypes, vast production of seeds, short seed dormancy, fast growth rate, high competitive perspective, allelopathic interference, and herbicide resistance [26].
Thereby, the present study aimed to analyze for the first time the chemical composition of the volatile oil extracted from the aerial parts of P. lapathifolia, evaluate its allelopathic activity of the EO on the weed E. colona, and determine its antioxidant activity.
2. Results and Discussion
2.1. Chemical Composition of P. lapathifolia EO
The hydrodistillation of the aerial parts of P. lapathifolia via the Clevenger apparatus produced 0.18% (v/w) of a dark yellow oil accompanied by a slight scent. This yield was lower than those reported from other Persicaria species such as Vietnamese P. odorata (0.41%) [28], Malaysian P. odorata (0.64%) [29], and P. hydropiper (0.70%) [15]. Overall, twenty-one volatile components were identified via GC–MS analysis, representing 98.3% of the total mass of oil (Table 1).
Table 1.
Essential oil chemical composition from aerial parts of Persicaria lapathifolia.
| No | Rt a | Relative Conc. (%) b | Compound Name | Type | Identification c | |
|---|---|---|---|---|---|---|
| KIObserved d | KIPublished e | |||||
| 1 | 3.23 | 1.74 ± 0.02 | α-Pinene | MH | 932 | 935 |
| 2 | 4.14 | 1.97 ± 0.05 | Sabinene | MH | 969 | 966 |
| 3 | 5.43 | 8.04 ± 0.06 | 2E-Hexenoic acid | Others | 1007 | 1009 |
| 4 | 13.84 | 3.09 ± 0.05 | Limonene | MH | 1024 | 1028 |
| 5 | 14.54 | 10.43 ± 0.14 | 2-Methyl butyl isovalerate | OM | 1104 | 1104 |
| 6 | 18.26 | 1.98 ± 0.03 | Hydrocinnamyl alcohol | Others | 1227 | 1232 |
| 7 | 20.12 | 3.51 ± 0.06 | γ-Nonalactone | Others | 1361 | 1367 |
| 8 | 21.67 | 1.29 ± 0.02 | trans-Caryophyllene | SH | 1408 | 1403 |
| 9 | 22.64 | 22.61 ± 0.16 | n-Dodecanal | Others | 1408 | 1415 |
| 10 | 23.07 | 11.29 ± 0.13 | α-Humulene | SH | 1452 | 1459 |
| 11 | 32.46 | 2.69 ± 0.04 | 2-Ethyl chromone | Others | 1614 | 1618 |
| 12 | 32.84 | 3.78 ± 0.04 | Phytane | DH | 1810 | 1818 |
| 13 | 36.88 | 1.75 ± 0.02 | Hexahydrofarnesyl acetone | Car | 1845 | 1847 |
| 14 | 38.16 | 3.19 ± 0.03 | Carissone | OS | 1927 | 1934 |
| 15 | 39.42 | 8.97 ± 0.08 | 2,4-Dimethylicosane | Others | 2080 | 2087 |
| 16 | 40.02 | 1.08 ± 0.04 | Abienol | OD | 2150 | 2156 |
| 17 | 42.56 | 2.27 ± 0.06 | n-Docosane | Others | 2200 | 2202 |
| 18 | 46.02 | 1.18 ± 0.03 | n-Tricosane | Others | 2300 | 2300 |
| 19 | 46.2 | 2.72 ± 0.05 | n-Nonacosane | Others | 2900 | 2905 |
| 20 | 49.22 | 3.01 ± 0.04 | n-Hentriacontane | Others | 3100 | 3101 |
| 21 | 51.14 | 1.71 ± 0.03 | n-Dotriacontane | Others | 3200 | 3203 |
| 6.80 ± 0.07 | Monoterpene Hydrocarbons (MH) | |||||
| 10.43 ± 0.14 | Oxygenated Monoterpenes (OM) | |||||
| 12.58 ± 0.12 | Sesquiterpene Hydrocarbons (SH) | |||||
| 3.19 ± 0.03 | Oxygenated Sesquiterpenes (OS) | |||||
| 3.78 ± 0.04 | Diterpene Hydrocarbons (DH) | |||||
| 1.08 ± 0.04 | Oxygenated Diterpenes (OD) | |||||
| 1.75 ± 0.02 | Carotenoid derived compounds (Car) | |||||
| 58.69 ± 0.13 | Other compounds (Others) | |||||
| 98.3 | Total | |||||
a Rt: retention time, b average value ± standard deviation, c the identification of EO constituents was based on the comparison of mass spectral data and Kovats indices (KI) with those of the NIST Mass Spectral Library (2011) and Wiley Registry of Mass Spectral Data 8th edition and literature, d KIpublished: reported Kovats retention indices; e KIObserved: experimentally calculated Kovats index relative to C8–C28 n-alkanes.
Eight classes of the compounds were characterized for the EO, including monoterpenes (hydrocarbons and oxygenated), sesquiterpenes (hydrocarbons and oxygenated), diterpenes (hydrocarbons and oxygenated), carotenoid-derived compounds, and other nonterpenoid compounds (Table 1). Among these components, nonterpenoid constituents represented the predominant type of compounds, with a relative concentration of 58.69%. This result is consistent with the previously described essential oils from Vietnamese [28] and Australian [30] P. odorata. In contrast, Dũng, et al. [31] described that sesquiterpene hydrocarbons were the main compounds of the Vietnamese P. odorata along with the oxygenated constituents. Herein, 11 nonterpenoids were identified, comprising n-dodecanal (22.61%), 2,4-dimethylicosane (8.97%), 2E-hexenoic acid (8.04%), and γ-nonalactone (3.51%) as the abundant compounds, while n-tricosane (1.18%) was determined as a minor compound. The preponderance of the alkyl aldehyde, n-dodecanal, in the EO of P. lapathifolia is in harmony with the published data on the EOs of Vietnamese and Australian P. odorata [28,30].
Monoterpenes were determined with a relative concentration of 17.23% of the EO mass. They can be divided into monoterpene hydrocarbons (6.80%) and oxygenated monoterpenes (10.43%). Three monoterpene hydrocarbon compounds were assigned, of which limonene (3.09%) was the major and α-pinene was a minor compound. Limonene is not a widely distributed compound in the EOs of Persicaria plants, although it has been reported as a major constituent of the EOs of several species such as Schinus terebinthifolius [32], Callistemon viminalis [33], Artemisia scoparia [34], Heterothalamus psiadioides [35], and Carum carvi [36].
On the other side, 2-methyl butyl isovalerate was the only identified oxygenated monoterpene, with a relative concentration of 10.43%. Isovalerate derivatives have been described in the EOs of several plants such as Eucalyptus brockwayii [37], Algerian Daucus gracilis [38], and Chamaemelum fuscatum [39].
Sesquiterpenes made up a relative concentration of 15.77% of the total oil mass. They consisted of sesquiterpene hydrocarbons (12.58%) and oxygenated sesquiterpene (3.19%). α-Humulene (11.29%), and trans-caryophyllene (1.29%) were the only identified sesquiterpene hydrocarbons. The two compounds are popular in the EOs of the plants belonging to Persicaria such as Vietnamese P. odorata [28,31] and Australian P. odorata [30]. In the EO of P. lapathifolia, diterpenes represented 4.86% of the total oil mass, including one diterpene hydrocarbon, phytane (3.78%), and one oxygenated compound, abienol (1.08%). Diterpenes have been recorded in the EOs of Persicaria species as traces [28,30,31]. The scarcity of diterpenoids in EOs derived from plants is a common phenomenon with a few exceptions such as Araucaria heterophylla [5] and Calotropis procera [14].
Carotenoid-derived components were represented by only the common compound, hexahydrofarnesyl acetone (1.75%), which has been characterized in the EOs of many plants such as Launaea mucronata, Launaea nudicaulis [40], and Heliotropium curassavicum [13].
2.2. Allelopathic Activity of P. lapathifolia EO
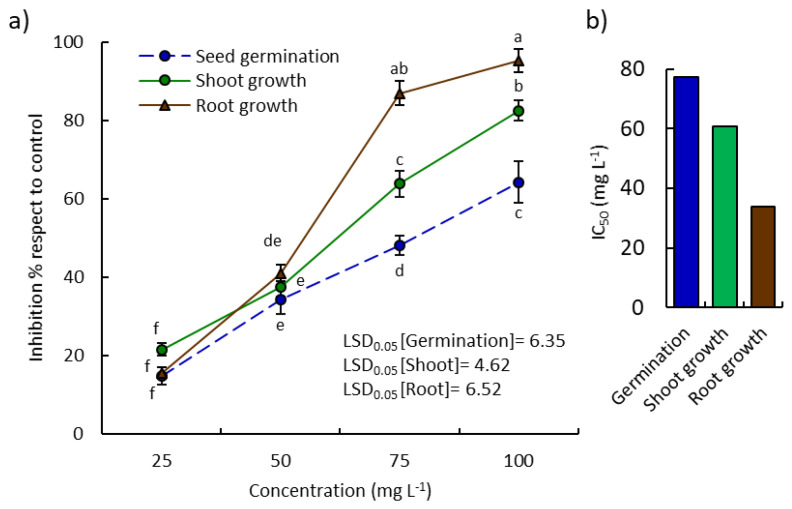
The extracted EO of P. lapathifolia exhibited substantial allelopathic activity against the germination, seedling root, and shoot growth of the weed E. colona in a dose-dependent manner (Figure 2). At the highest concentration (100 mg L−1), the germination, seedling root, and shoot growth were inhibited by 64.25, 82.48, and 95.25%, respectively. Additionally, the EO showed IC50 values of 77.27, 60.84, and 33.80 mg L−1, respectively (Figure 2). It is clear that the seedling growth was more inhibited than germination. However, the root growth of E. colona was more sensitive to the EO than the shoots, and this observation was in harmony with other studies [13,41]. This could be attributed to the permeability of the root cells and the direct contact with the medium that contained the EO [14,41,42].
Figure 2.
Allelopathic activity of the Persicaria lapathifolia essential oil. (a) Inhibitory effect on the germination, seedling root, and shoot growth of the weed Echinochloa colona; (b) IC50 values. Different letters mean a significant difference in values after Tukey’s HSD test (p < 0.05).
To our knowledge, the allelopathic activity of P. lapathifolia EO has not been described yet. Herein, the EO of P. lapathifolia was found to exhibit allelopathic effects on E. colona. Many published data have revealed the principal and direct relationship between the allelopathic properties of EOs derived from plants and their chemical compositions [4,14,40,41,42]. Therefore, the observed allelopathic activity of P. lapathifolia EO might be ascribed to its chemical constituents, especially the main compounds n-dodecanal, α-humulene, 2,4-dimethylicosane, and 2E-hexenoic acid, γ-nonalactone, and limonene. These compounds could act either singularly or in a synergistic manner as allelochemicals that inhibit germination and seedling growth [41]. The allelopathic effect of allelochemicals may occur by inhibition of cell division, reduction of respiration, affecting photosynthesis, inhibition of enzymatic systems, affecting nucleic acid, or induction of reactive oxygen species in plant cells [43,44,45]. Humulene has been reported as a major compound in the EO of Symphyotrichum squamatum, which exhibited significant allelopathic activity on the weed Bidens pilosa [12]. Also, limonene has been reported as a major compound in the EOs of various plants that exhibited considerable allelopathic activity such as Heterothalamus psiadioides [35], Agastache rugosa [46], Schinus terebinthifolius [32], Callistemon viminalis [33], Artemisia scoparia [34], Pinus pinea [47], and Carum carvi [36].
It is worth mentioning here that E. colona has been reported to have allelopathic activity against various crops and weeds such as rice, soybean [48], and Avena fatua [49]. Also, the allelopathic activity of various plant extracts, such as those of Sorghum bicolor, Helianthus annuus, Brassica campestris [50], Mikania micrantha, Clidemia hirta, Dicranopteris linearis, and Ageratum conyzoides [51], has been studied against E. colona. However, no study has revealed the activity of the essential oil of P. lapathifolia against this weed. In this line, the present study showed that the EO of the aerial parts of P. lapathifolia could be used in the management of weeds as a green, ecofriendly herbicide, particularly against species of Echinochloa, including E. colona, which has been reported to be resistant against herbicides [52].
2.3. Antioxidant Activity of P. lapathifolia EO
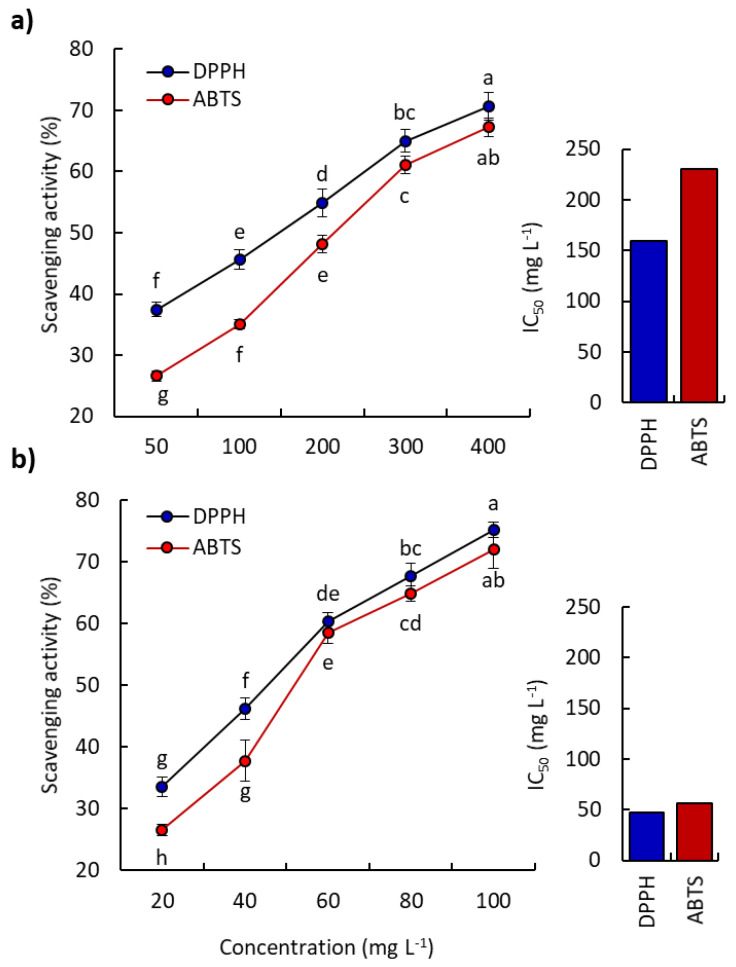
The EO of P. lapathifolia was tested for antioxidant activity via the reduction of the free radicals DPPH and ABTS, and it showed a substantial antioxidant activity compared to ascorbic acid as a standard antioxidant (Figure 3). By increasing the concentration of the EO, the reduction of radicals was increased. At the highest concentration of the EO (400 mg L−1), the DPPH and ABTS were reduced by 70.68 and 67.23%, respectively. The EO showed IC50 values of 159.69 and 230.43 mg L−1, respectively (Figure 3a), while ascorbic acid exhibited IC50 values 47.49 and 56.68 mg L−1, respectively (Figure 3b).
Figure 3.
Antioxidant activity of Persicaria lapathifolia essential oil (a) and ascorbic acid as a standard antioxidant (b). Different letters mean a significant difference in values after Tukey’s HSD test (p < 0.05).
The antioxidant activity of the EO is usually correlated to the oxygenated compounds in the EO profile [8,12,41]. Thereby, the substantial antioxidant activity of EO of P. lapathifolia could be attributed to its high content of oxygenated components (> 70%), especially oxygenated hydrocarbons. Additionally, terpenoid compounds have been stated to have important functions as free radical scavengers, especially the oxygenated derivatives [12,40]. In the present study, about 38% of the EO mass consisted of terpenoids, including 15% oxygenated derivatives that could be responsible for the scavenging of free radicals. Many EOs of plants have been proven to exhibit significant antioxidant effects via different methods because of their high percentages of terpenoids, such as those of Euphorbia mauritanica [1], Deverra tortuosa [3], and Launaea species [40].
3. Materials and Methods
3.1. Plant Materials Collected and Preparation
The aerial parts of Persicaria lapathifolia were collected in May from a population growing on the canal bank habitat of an irrigation canal near the city of Mansoura, Egypt (31.0708553N 31.4394701E). The collected aerial parts were air dried at room temperature (25 ± 3 °C), crushed gently by hand, and packed in a paper bag till further analyses. A plant voucher specimen was deposited in the herbarium of the Department of Botany, Faculty of Science, Mansoura University, Egypt.
3.2. Extraction of EO, GC-MS Analysis, and Identification of Chemical Constituents
About 250 g of the air-dried powder aerial parts of P. lapathifolia was subjected to hydrodistillation over Clevenger-type apparatus for three hours, and the dark yellow, oily layer was separated by n-hexane via a separating funnel. The EO chemical composition was analyzed and identified based on gas chromatography–mass spectrometry (GC–MS) using the instrument at the Medicinal and Aromatic Plants Research Dept., National Research Center, Egypt. The instrument comprises a TRACE GC Ultra Gas Chromatographs (THERMO Scientific Corp., Miami, CA, USA) and a thermo mass spectrometer detector (ISQ Single Quadrupole Mass Spectrometer; Model ISQ spectrometer, THERMO Scientific Corp., Miami, CA, USA). The system was equipped with a TR-5 MS column with specifications of 30 m × 0.32 mm i.d. and 0.25 µm film thickness. Helium was used as a carrier gas at a flow rate of 1.0 mL min−1 and a split ratio of 1:10. The temperature program started at 60 °C for one minute and was then raised 4.0 °C every minute to 240 °C, then held for one minute. Electron ionization (EI) at 70 eV with a spectral range of m/z 40–450 was used for performing mass spectra. The chemical constituents of the EO were tentatively identified by their retention indices (relative to n-alkanes C8-C22) and mass spectrum matching to the Wiley spectral library collection and the NSIT library database [41].
3.3. Allelopathic Bioassay
The extracted EO from the aerial parts of P. lapathifolia was tested in vitro for its allelopathic activity against the germination and seedling growth of the weed E. colona. The ripened seeds of E. colona were collected from a cultivated field near the city of Manzala, Al-Dakahlya Governorate, Egypt (31.1691466N 31.896379E). The homogenized seeds in size and color were sterilized with 0.3% NaClO and dried in a sterilized condition. The viability of seeds was preliminarily tested and found to be 94.56% ± 3.25. For bioassay, different concentrations (0, 25, 50, 75, and 100 mg L−1) of the extracted EO were prepared in 1% Tween® 80 (Sigma-Aldrich, Darmstadt, Germany) as an emulsifier. Twenty sterilized seeds were arranged over a filter paper (Whatman No. 1) in Petri dishes. About four mL of each concentration and Tween® 80 (as control) were poured over the filter paper, and the dishes were sealed with Parafilm® tape (Sigma, St. Louis, MO, USA) to avoid the loss of EO [41]. For each concentration, five dishes were tested, and the experiment was repeated three times. A total of 75 dishes (5 treatments (4 concentrations + control) × 5 dishes (replications) × 3 times) were prepared and incubated at 27 °C in a growth chamber with adjusted light conditions of 16 h light and 8 h dark. After ten days of incubation, the number of germinated E. colona seeds was counted, and the lengths of the seedling root and shoot of the weed were measured. The inhibition of germination and growth was calculated with respect to control according to the following equation:
where N is the number of germinated seeds and L is the length of the seedling root or shoot.
3.4. Antioxidant Activity Estimation
The antioxidant activity of the extracted EO was assessed by its ability to reduce the free radicals 2,2-diphenyl-1-picrylhydrazyl (DPPH, Sigma-Aldrich, Germany) and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS, Sigma-Aldrich, Germany). According to Miguel [53], a range of concentrations of the EO (50–400 mg L−1) were prepared in MeOH. This range was selected based on the observed scavenging percentage, i.e., to be suitable to determine the IC50 (the concentration of EO necessary to scavenge the radical by 50%) [3]. In glass tubes, 2 mL of each concentration was mixed vigorously with 2 mL of freshly prepared 0.3 mM DPPH. Negative control was performed using MeOH treated with DPPH-like treatment. The reaction mixtures were kept in dark conditions at room temperature for 30 min, and the absorbance was measured immediately at 517 nm by spectrophotometer (Spectronic 21D model).
For confirmation, the scavenging of ABTS was performed following the method of Re et al. [54]. Briefly, the ABTS radical was prepared by mixing about 7 mM ABTS (1/1, v/v) with 2.45 mM potassium persulfate, and this mixture was stored in dark conditions at 25 ± 2 °C for 16 h. In glass tubes, 2 mL of the prepared ABTS radical and 0.2 mL of each EO concentration (50–400 mg L−1) were mixed well and kept for 6 min at room temperature. The absorbance was measured by a spectrophotometer at 734 nm. Moreover, the antioxidant activity of ascorbic acid as a standard antioxidant was determined following the same procedures for DPPH and ABTS using a range of 20–100 mg L−1. The scavenging activity was calculated according to the following equation:
3.5. Statistical Analysis
The experiment of allelopathic activity was performed three times with five replicas per treatment. The mean values and standard error were calculated, while the raw data were subjected to one-way ANOVA followed by Tukey’s HSD test. Moreover, the data of antioxidant activity of the EO and ascorbic acid with three replicates were subjected to one-way ANOVA followed by Tukey’s HSD test as well. The analysis was accomplished in the CoStat program (version 6.311, CoHort Software, Monterey, CA, USA). The IC50 for allelopathic and antioxidant assays were calculated graphically using MS Excel.
4. Conclusions
The current study showed for the first time the chemical composition of the EO from the aerial parts of P. lapathifolia. The EO had 21 compounds, with 58.69% as nonterpenoids and 37.86% as terpenoids. The main compounds were n-dodecanal, α-humulene, 2,4-dimethylicosane, 2E-hexenoic acid, γ-nonalactone, and limonene. The EO of P. lapathifolia showed substantial herbicidal activity against the weed E. colona; 100 mg L−1 of this EO inhibited the germination, seedling root, and shoot growth by 64.25, 82.48, and 95.25%, respectively. Also, the EO exhibited considerable antioxidant activity compared to ascorbic acid as a standard. The present results showed that the emergent leafy stems of aquatic plants such as P. lapathifolia have considerably low content of the EO, which exhibited substantial activities such as antioxidant and allelopathic activities. Further study is recommended to evaluate the effects of various environmental and climatic conditions on the production and composition of the EOs of P. lapathifolia.
Acknowledgments
The authors extend their appreciation to The Researchers Supporting Project number (RSP-2021/200) King Saud University, Riyadh, Saudi Arabia.
Author Contributions
Conceptualization, A.M.A.-E. and A.I.E.; validation, A.M.A.-E., G.B., and A.I.E.; formal analysis, A.M.A.-E. and A.I.E.; investigation, A.M.A.-E., G.B., and A.I.E.; writing—original draft preparation, A.M.A.-E., G.B., and A.I.E.; writing—review and editing, A.M.A.-E., G.B., S.A.A.-R., and A.I.E. All authors have read and agreed to the published version of the manuscript.
Funding
The Researchers Supporting Project number (RSP-2021/200) King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Essa A.F., El-Hawary S.S., Abd-ElGawad A.M., Kubacy T.M., El-Khrisy E.E.-D.A., Elshamy A., Younis I.Y. Prevalence of diterpenes in essential oil of Euphorbia mauritanica L.: Detailed chemical profile, antioxidant, cytotoxic and phytotoxic activities. Chem. Biodivers. 2021;18:e2100238. doi: 10.1002/cbdv.202100238. [DOI] [PubMed] [Google Scholar]
- 2.Elgamal A.M., Ahmed R.F., Abd-ElGawad A.M., El Gendy A.E.-N.G., Elshamy A.I., Nassar M.I. Chemical profiles, anticancer, and anti-aging activities of essential oils of Pluchea dioscoridis (L.) DC. and Erigeron bonariensis L. Plants. 2021;10:667. doi: 10.3390/plants10040667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fayed E.M., Abd-EIGawad A.M., Elshamy A.I., El-Halawany E.S.F., EI-Amier Y.A. Essential oil of Deverra tortuosa aerial parts: Detailed chemical profile, allelopathic, antimicrobial, and antioxidant activities. Chem. Biodivers. 2021;18:e2000914. doi: 10.1002/cbdv.202000914. [DOI] [PubMed] [Google Scholar]
- 4.Saleh I., Abd-ElGawad A., El Gendy A.E.-N., Abd El Aty A., Mohamed T., Kassem H., Aldosri F., Elshamy A., Hegazy M.-E.F. Phytotoxic and antimicrobial activities of Teucrium polium and Thymus decussatus essential oils extracted using hydrodistillation and microwave-assisted techniques. Plants. 2020;9:716. doi: 10.3390/plants9060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elshamy A.I., Ammar N.M., Hassan H.A., Al-Rowaily S.L., Ragab T.I., El Gendy A.E.-N.G., Abd-ElGawad A.M. Essential oil and its nanoemulsion of Araucaria heterophylla resin: Chemical characterization, anti-inflammatory, and antipyretic activities. Ind. Crops Prod. 2020;148:112272. doi: 10.1016/j.indcrop.2020.112272. [DOI] [Google Scholar]
- 6.Ali H., Al-Khalifa A.R., Aouf A., Boukhebti H., Farouk A. Effect of nanoencapsulation on volatile constituents, and antioxidant and anticancer activities of Algerian Origanum glandulosum Desf. essential oil. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-59686-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvestre W., Livinalli N., Baldasso C., Tessaro I. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019;93:42–52. doi: 10.1016/j.tifs.2019.09.003. [DOI] [Google Scholar]
- 8.Abd-ElGawad A.M., El Gendy A.E.-N.G., Assaeed A.M., Al-Rowaily S.L., Alharthi A.S., Mohamed T.A., Nassar M.I., Dewir Y.H., Elshamy A.I. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants. 2021;10:36. doi: 10.3390/plants10010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum U. Plant-Plant Allelopathic Interactions. Springer; Dordrecht, The Netherlands: 2011. Plant–plant allelopathic interactions; pp. 1–7. [Google Scholar]
- 10.Serra S.N., Shanmuganathan R., Becker C. Allelopathy in rice: A story of momilactones, kin recognition, and weed management. J. Exp. Bot. 2021;72:4022–4037. doi: 10.1093/jxb/erab084. [DOI] [PubMed] [Google Scholar]
- 11.Bonanomi G., Zotti M., Idbella M., Mazzoleni S., Abd-ElGawad A.M. Microbiota modulation of allelopathy depends on litter chemistry: Mitigation or exacerbation? Sci. Total Environ. 2021;776:145942. doi: 10.1016/j.scitotenv.2021.145942. [DOI] [PubMed] [Google Scholar]
- 12.Abd-ElGawad A.M., Elshamy A.I., El-Amier Y.A., El Gendy A.E.-N.G., Al-Barati S.A., Dar B.A., Al-Rowaily S.L., Assaeed A.M. Chemical composition variations, allelopathic, and antioxidant activities of Symphyotrichum squamatum (Spreng.) Nesom essential oils growing in heterogeneous habitats. Arab. J. Chem. 2020;13:4237–4245. doi: 10.1016/j.arabjc.2019.07.005. [DOI] [Google Scholar]
- 13.Abd-ElGawad A.M., Elshamy A.I., Al-Rowaily S.L., El-Amier Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants. 2019;8:482. doi: 10.3390/plants8110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Rowaily S.L., Abd-ElGawad A.M., Assaeed A.M., Elgamal A.M., Gendy A.E.-N.G.E., Mohamed T.A., Dar B.A., Mohamed T.K., Elshamy A.I. Essential oil of Calotropis procera: Comparative chemical profiles, antimicrobial activity, and allelopathic potential on weeds. Molecules. 2020;25:5203. doi: 10.3390/molecules25215203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aziman N., Abdullah N., Bujang A., Mohd Noor Z., Abdul Aziz A., Ahmad R. Phytochemicals of ethanolic extract and essential oil of Persicaria hydropiper and their potential as antibacterial agents for food packaging polylactic acid film. J. Food Saf. 2021;41:e12864. doi: 10.1111/jfs.12864. [DOI] [Google Scholar]
- 16.Sankarikutty B., Narayanan C.S. Essential oils isolation and production. In: Caballero B., editor. Encyclopedia of Food Sciences and Nutrition. 2nd ed. Academic Press; Oxford, UK: 2003. pp. 2185–2189. [Google Scholar]
- 17.Boulos L. Flora of Egypt. Volume 1 All Hadara Publishing; Cairo, Egypt: 1999. [Google Scholar]
- 18.Tackholm V. Students’ Flora of Egypt. 2nd ed. Cairo University Press; Cairo, Egypt: 1974. [Google Scholar]
- 19.Heywood V.H., Brummitt R., Culham A., Seberg O. Flowering Plant Families of the World. Volume 88 Kew Publishing; London, UK: 2007. [Google Scholar]
- 20.Bulbul L., Sushanta S.M., Uddin M.J., Tanni S. Phytochemical and pharmacological evaluations of Polygonum lapathifolium stem extract for anthelmintic and antiemetic activity. Int. Curr. Pharm. J. 2013;2:57–62. doi: 10.3329/icpj.v2i3.13582. [DOI] [Google Scholar]
- 21.Hailemariam A., Feyera M., Deyou T., Abdissa N. Antimicrobial Chalcones from the Seeds of Persicaria lapathifolia. Biochem. Pharmacol. 2018;7:2167-0501. doi: 10.4172/2167-0501.1000237. [DOI] [Google Scholar]
- 22.Takasaki M., Konoshima T., Kuroki S., Tokuda H., Nishino H. Cancer chemopreventive activity of phenylpropanoid esters of sucrose, vanicoside B and lapathoside A, from Polygonum lapathifolium. Cancer Lett. 2001;173:133–138. doi: 10.1016/S0304-3835(01)00670-X. [DOI] [PubMed] [Google Scholar]
- 23.Kubínová R., Pořízková R., Bartl T., Navrátilová A., Čížek A., Valentová M. Biological activities of polyphenols from Polygonum lapathifolium. B. Latinoam. Caribe Plantas Med. Aromát. 2014;13:506–516. [Google Scholar]
- 24.Park S.-H., Oh S.R., Jung K.Y., Lee I.S., Ahn K.S., Kim J.H., Kim Y.S., Lee J.J., Lee H.-K. Acylated flavonol glycosides with anti-complement activity from Persicaria lapathifolia. Chem. Pharm. Bull. 1999;47:1484–1486. doi: 10.1248/cpb.47.1484. [DOI] [PubMed] [Google Scholar]
- 25.Smolarz H.D. Flavonoids from Polygonum lapathifolium ssp. tomentosum. Pharm. Biol. 2002;40:390–394. doi: 10.1076/phbi.40.5.390.8455. [DOI] [Google Scholar]
- 26.Peerzada A.M., Bajwa A.A., Ali H.H., Chauhan B.S. Biology, impact, and management of Echinochloa colona (L.) Link. Crop Prot. 2016;83:56–66. doi: 10.1016/j.cropro.2016.01.011. [DOI] [Google Scholar]
- 27.Holm L.G., Plucknett D.L., Pancho P.V., Herberger J.P. The World’s Worst Weeds: Distribution and Biology. University Press of Hawaii; Honolulu, HI, USA: 1991. [Google Scholar]
- 28.Řebíčková K., Bajer T., Šilha D., Houdková M., Ventura K., Bajerová P. Chemical composition and determination of the antibacterial activity of essential oils in liquid and vapor phases extracted from two different southeast Asian herbs—Houttuynia cordata (saururaceae) and Persicaria odorata (polygonaceae) Molecules. 2020;25:2432. doi: 10.3390/molecules25102432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almarie A., Mamat A., Wahab Z., Rukunudin I. Chemical composition and phytotoxicity of essential oils isolated from Malaysian plants. Allelopathy J. 2016;37:55–70. [Google Scholar]
- 30.Hunter M.V., Brophy J.J., Ralph B.J., Bienvenu F.E. Composition of Polygonum odoratum Lour. from southern Australia. J. Essent. Oil Res. 1997;9:603–604. doi: 10.1080/10412905.1997.9700789. [DOI] [Google Scholar]
- 31.Dũng N.X., Van Hac L., Leclercq P.A. Volatile constituents of the aerial parts of Vietnamese Polygonum odoratum L. J. Essent. Oil Res. 1995;7:339–340. doi: 10.1080/10412905.1995.9698534. [DOI] [Google Scholar]
- 32.Pinheiro P.F., Costa A.V., Tomaz M.A., Rodrigues W.N., Fialho Silva W.P., Moreira Valente V.M. Characterization of the Essential Oil of Mastic Tree from Different Biomes and its Phytotoxic Potential on Cobbler’s Pegs. J. Essent. Oil-Bear. Plants. 2016;19:972–979. doi: 10.1080/0972060X.2016.1197799. [DOI] [Google Scholar]
- 33.Bali A.S., Batish D.R., Singh H.P., Kaur S., Kohli R.K. Chemical characterization and phytotoxicity of foliar volatiles and essential oil of Callistemon viminalis. J. Essent. Oil-Bear. Plants. 2017;20:535–545. doi: 10.1080/0972060X.2017.1313708. [DOI] [Google Scholar]
- 34.Kaur S., Singh H.P., Mittal S., Batish D.R., Kohli R.K. Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as a bioherbicide. Ind. Crops Prod. 2010;32:54–61. doi: 10.1016/j.indcrop.2010.03.007. [DOI] [Google Scholar]
- 35.Silva E.R., Overbeck G.E., Soares G.L.G. Phytotoxicity of volatiles from fresh and dry leaves of two Asteraceae shrubs: Evaluation of seasonal effects. S. Afr. J. Bot. 2014;93:14–18. doi: 10.1016/j.sajb.2014.03.006. [DOI] [Google Scholar]
- 36.De Almeida L.F.R., Frei F., Mancini E., De Martino L., De Feo V. Phytotoxic activities of Mediterranean essential oils. Molecules. 2010;15:4309–4323. doi: 10.3390/molecules15064309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J., An M., Wu H., Li Liu D., Stanton R. Chemical composition of essential oils of four Eucalyptus species and their phytotoxicity on silverleaf nightshade (Solanum elaeagnifolium Cav.) in Australia. Plant Growth Regul. 2012;68:231–237. doi: 10.1007/s10725-012-9711-5. [DOI] [Google Scholar]
- 38.Benyelles B., Allali H., Dib M.E.A., Djabou N., Paolini J., Costa J. Chemical composition variability of essential oils of Daucus gracilis Steinh. from Algeria. Chem. Biodivers. 2017;14:e1600490. doi: 10.1002/cbdv.201600490. [DOI] [PubMed] [Google Scholar]
- 39.Fernández-Cervantes M., Pérez-Alonso M.J., Blanco-Salas J., Soria A.C., Ruiz-Téllez T. Analysis of the essential oils of Chamaemelum fuscatum (Brot.) Vasc. from Spain as a contribution to reinforce its ethnobotanical use. Forests. 2019;10:539. doi: 10.3390/f10070539. [DOI] [Google Scholar]
- 40.Elshamy A., Abd-ElGawad A.M., El-Amier Y.A., El Gendy A., Al-Rowaily S. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019;34:316–328. doi: 10.1002/ffj.3512. [DOI] [Google Scholar]
- 41.Abd El-Gawad A.M. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crops Prod. 2016;80:36–41. doi: 10.1016/j.indcrop.2015.10.054. [DOI] [Google Scholar]
- 42.Assaeed A., Elshamy A., El Gendy A.E.-N., Dar B., Al-Rowaily S., Abd-ElGawad A. Sesquiterpenes-rich essential oil from above ground parts of Pulicaria somalensis exhibited antioxidant activity and allelopathic effect on weeds. Agronomy. 2020;10:399. doi: 10.3390/agronomy10030399. [DOI] [Google Scholar]
- 43.El-Shora H.M., Abd El-Gawad A.M. Physiological and biochemical responses of Cucurbita pepo L. mediated by Portulaca oleracea L. allelopathy. Fresenius Environ. Bull. 2015;24:386–393. [Google Scholar]
- 44.El-Shora H.M., El-Gawad A.M.A. Response of Cicer arietinum to allelopathic effect of Portulaca oleracea root extract. Phyton-Ann. Rei Bot. 2015;55:215–232. [Google Scholar]
- 45.Reigosa M.J., Sánchez-Moreiras A., González L. Ecophysiological approach in allelopathy. Crit. Rev. Plant Sci. 1999;18:577–608. doi: 10.1080/07352689991309405. [DOI] [Google Scholar]
- 46.Kim J. Phytotoxic and antimicrobial activities and chemical analysis of leaf essential oil from Agastache rugosa. J. Plant Biol. 2008;51:276–283. doi: 10.1007/BF03036127. [DOI] [Google Scholar]
- 47.Amri I., Gargouri S., Hamrouni L., Hanana M., Fezzani T., Jamoussi B. Chemical composition, phytotoxic and antifungal activities of Pinus pinea essential oil. J. Pest Sci. 2012;85:199–207. doi: 10.1007/s10340-012-0419-0. [DOI] [Google Scholar]
- 48.Chopra N., Tewari G., Tewari L.M., Upreti B., Pandey N. Allelopathic effect of Echinochloa colona L. and Cyperus iria L. Weed extracts on the seed germination and seedling growth of rice and soyabean. Adv. Agric. 2017;2017:5748524. doi: 10.1155/2017/5748524. [DOI] [Google Scholar]
- 49.Hegab M., Abdelgawad H., Abdelhamed M., Hammouda O., Pandey R., Kumar V., Zinta G. Effects of tricin isolated from jungle rice (Echinochloa colona L.) on amylase activity and oxidative stress in wild oat (Avena fatua L.) Allelopathy J. 2013;31:345–354. [Google Scholar]
- 50.Khaliq A., Matloob A., Cheema Z.A., Farooq M. Allelopathic activity of crop residue incorporation alone or mixed against rice and its associated grass weed jungle rice (Echinochloa colona [L.] Link. Chil. J. Agric. Res. 2011;71:418–423. doi: 10.4067/S0718-58392011000300012. [DOI] [Google Scholar]
- 51.Lim C.J., Basri M., Ee G.C.L., Omar D. Phytoinhibitory activities and extraction optimization of potent invasive plants as eco-friendly weed suppressant against Echinochloa colona (L.) Link. Ind. Crops Prod. 2017;100:19–34. doi: 10.1016/j.indcrop.2017.01.025. [DOI] [Google Scholar]
- 52.Cook T., Storrie A., Moylan P., Adams B. Field testing of glyphosate-resistant awnless barnyard grass (Echinochloa colona) in northern NSW; Proceedings of the Proceedings of the 16th Australian Weeds Conference, Brisbane, QL, Australia, 2008; pp. 20–23. [Google Scholar]
- 53.Miguel M.G. Antioxidant activity of medicinal and aromatic plants. Flavour Fragr. J. 2010;25:219–312. doi: 10.1002/ffj.1961. [DOI] [Google Scholar]
- 54.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.