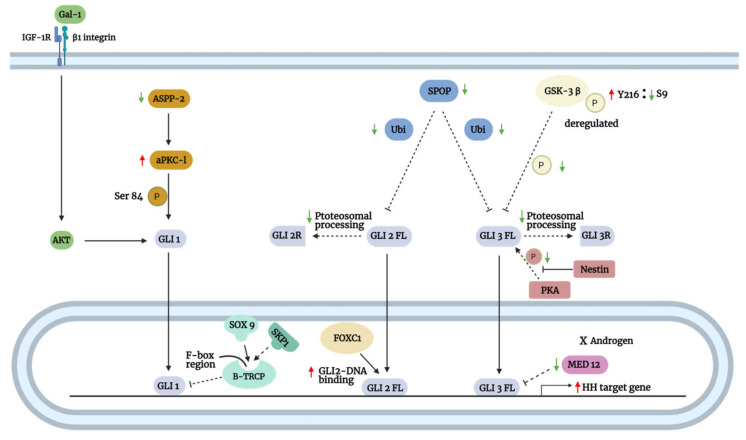
Figure 7.
A schematic representation of the Smoothened (SMO)-independent regulation of glioma-associated oncogene homolog (GLI) transcription factors by their interacting proteins. Apoptosis-stimulating of p53 protein 2 (ASPP2) deficiency enhanced the binding of atypical Protein Kinase C ι (aPKC-ι) with GLI1, which allows aPKC-ι to phosphorylate GLI1 at Ser84. The phosphorylated GLI1 is, in turn, activated, promoting its translocation into the nucleus to transcribe target genes. Galectin-1 (Gal-1) binds to β1 integrin to promote GLI1 activation. Mechanistically, activated β1 integrin forms a complex with insulin-like growth factor 1 receptor (IGF-1R) to promote protein kinase B (AKT) activation, leading to an increase in GLI1 activity. In the nucleus, SOX9 binds to the F-box region of β-TrCP, interfering with its binding to SKP1. The binding of SOX9 to β-TrCP tethers it within the nucleus, thus protecting GLI1 from degradation. Speckle-type POZ protein (SPOP) downregulation results in decreased ubiquitination of full-length GLI2/3 proteins, favoring their activation and nuclear translocation over their proteasomal processing into repressors. In the nucleus, the N-terminal domain (aa 1-68) of transcription factor forkhead box C1 (FOXC1) binds to the internal region (aa 898-1168) of GLI2, enhancing its DNA-binding and transcriptional-activating ability. The imbalance between Tyr216 and Ser9 phosphorylation of glycogen synthase kinase 3 beta (GSK3β) leads to its dysregulated function, thereby impairing its ability to phosphorylate full-length GLI3 proteins. Unphosphorylated full-length GLI3 proteins are not subjected to proteasomal processing into their repressors, allowing their translocation into the nucleus to transcribe target genes. Under androgen-deprived conditions, the downregulation of MED12 relieves its constraint on the full-length GLI3 proteins, resulting in their hyperactivation. Green downward triangle-headed arrow: downregulation; red upward triangle-headed arrow: upregulation; dotted bar-headed arrow: loss of inhibition.