Figure 9.
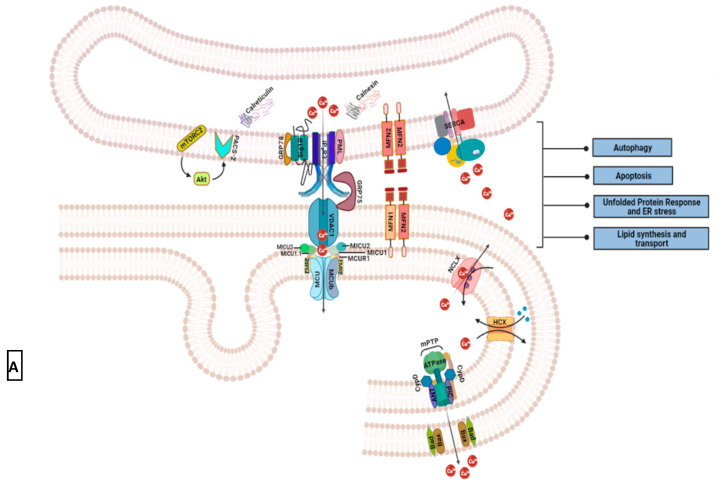
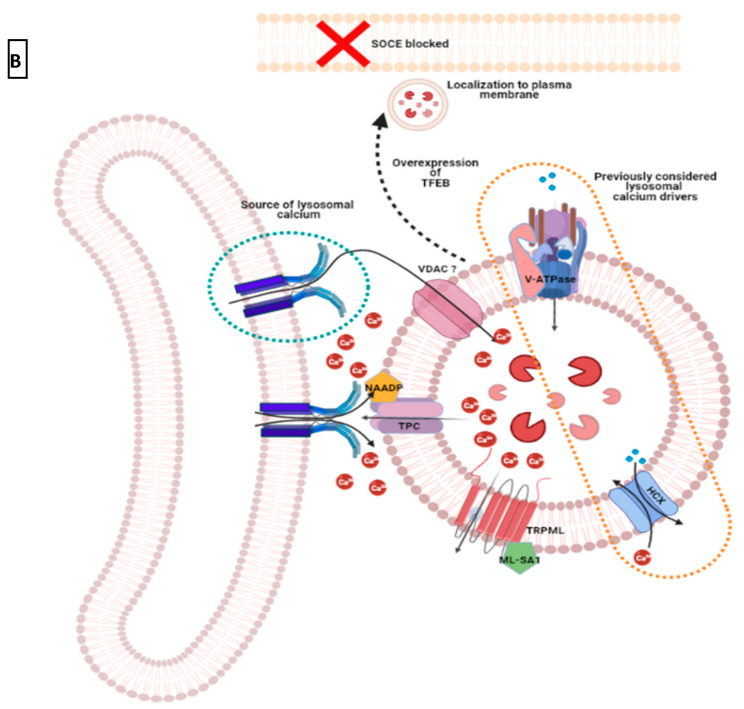
Mitochondrial and lysosomal impact on intracellular Ca2+ signal. (A) Primary components of Ca2+ signaling at the mitochondrial associated membranes (MAMs) include IP3R3 on the endoplasmic reticulum, VDAC1 on the outer mitochondrial membrane, and MCU complex on the inner mitochondrial membrane [151,154,156,161]. Transport of Ca2+ ions from ER to mitochondria plays a crucial role in cellular metabolism (autophagy), cell survival (during unfolded protein response and impinging cell death signals), lipid production, and distribution [162,163,164,165,166]. For these cellular processes to proceed normally, the integrity of MAMs is essential. Various tethering proteins regulate MAM structural integrity. Such secondary MAM components include GRP75, PML, GRP78 (or BiP), mitofusins (MFN1 and 2), and phosphofurin acidic cluster sorting protein (PACS2) [158,159,160,164,167,168,169]. Localized to the ER side of MAMs, growth factor-activated mTORC2 utilizes phosphorylated PACS2 (via Akt) to help avoid structural disruption of this sub-compartment [170]. It also phosphorylates IP3R3 to promote Ca2+ release at MAM sites. Other key MAM localized proteins maintain Ca2+ homeostasis in the region. These are calreticulin and calmodulin in the ER lumen, and SERCA on the ER membrane (184). Excessive Ca2+ ions in the mitochondrial matrix are extruded mainly by a permeability transition pore (mPTP). This Ca2+ efflux pore is situated on the inner mitochondrial membrane and is comprised of F1F0 ATPase with cyclophilin D (CyPD), adenine nucleotide translocase (ANT), and mitochondrial phosphate carrier (PiC) as its regulators [171,172]. Proapoptotic molecules Bax and Bak influence mPTP opening by controlling outer mitochondrial membrane permeability [173]. Ca2+ exchangers such as NCLX and HCX are less understood efflux mechanisms that may prevent overload of these cations in the organelle [151,174]; (B) Lysosomal Ca2+ promotes IP3R3 mediated Ca2+ release from the endoplasmic reticulum [175,176]. Activated by NAADP, two-pore channels (TPCs) and TRPMLs are the main mode of Ca2+ extrusion from lysosomes. Multi-subunit V-ATPases actively transport hydrogen ions into the lysosomal lumen to maintain acidic pH that has been previously considered as the driving force for Ca2+ entry into lysosomes via HCX. Although any direct association between acidic lumen and Ca2+ store maintenance is now considered controversial, any indirect impact on lysosomal Ca2+ stores has not been ruled out. Parallel to this, the dependence of lysosomal Ca2+ uptake on ER Ca2+ stores is speculated [177]. Lysosomes can also play a more active role in intracellular Ca2+ homeostasis as indicated by their inhibition of SOCE [178]. Akt, a serine-threonine kinase; BiP, binding immunoglobulin protein; CyPD, cyclophilin D or peptidyl-prolyl cis-trans isomerase D; EMRE, essential mitochondria regulator; GRP75, glucose-regulated protein 75; HCX, hydrogen-Ca2+ exchanger; MCU; mitochondrial Ca2+ uniporter; MCUR1, mitochondrial Ca2+ uniporter regulator 1; MICU1/2, mitochondrial Ca2+ uptake 1 or 2; MFN1/2, mitofusin 1 or 2; mPTP, mitochondrial permeability transition pore; ML-SA1, mucolipin synthetic agonist 1; mTORC2, mTOR complex 2; NAADP, nicotinic acid adenine dinucleotide phosphate; NCLX, mitochondrial sodium Ca2+ exchanger; PML, promyeloctic leukemia protein; PACS2, phosphofurin acidic cluster sorting protein 2; SERCA, sarcoendoplasmic reticulum Ca2+ ATPase; Sig1R, sigma 1 receptor; TFEB, transcription factor EB; TPC, two-pore channel; TRPML, transient receptor potential mucolipin channel; V-ATPase, vacuolar ATPase; VDAC, voltage-dependent anion channel.