Abstract
Meliaceae are widely distributed across the world in tropical or subtropical climates and are of considerable ethnobotanical importance as sources of traditional medicine and cosmetics. This comprehensive review summarizes the ethnobotanical uses and chemistry of 12 South African species, belonging to six genera: Ekebergia, Nymania, Entandrophragma, Pseudobersama, Trichilia, and Turraea. Eight of the species have ethnomedicinal records, classified into 17 major disease categories. The ethnomedicinal uses comprise 85 ailments dominated by gastrointestinal complaints, followed by gynaecological and obstetrics related problems. Chemical records were found for 10 species, which describe nine classes of compounds. In nearly all South African Meliaceae, limonoids are the predominant constituents while triterpenes, sterols, and coumarins are also common. The widest range of use-records and medicinal applications are found with the two most chemically diverse species, Ekebergia capensis and Trichilia emetica. Of the chemical compounds identified in the various plant organs of the 10 species of South African Meliaceae for which data are available, 42% was found in bark and 17% in seeds. Roots represent 35% and bark 33% of the organs that are used medicinally, and they are typically prepared as decoctions or infusions. Root and bark harvesting are destructive so that it may be important to examine the chemistry of plant parts such as wild-crafted leaves and fruits.
Keywords: South African Meliaceae, ethnomedicinal importance, functional uses, chemistry, limonoids
1. Introduction
Ethnobotany is the cultural study of the practical uses of a region’s plants by the local people. It is interdisciplinary and can often progress into a lab-based collaborative project with the vision of benefiting modern society in the form of wild food crops, pharmaceuticals, nutraceuticals, or cosmetics [1]. Furthermore, by recording traditional plant use, indigenous knowledge and belief systems are conserved [2] and incentives for biodiversity conservation are realized. Unfortunately, the floras that are used traditionally are the most likely to be destroyed or threatened by over-exploitation. The management of plants that are utilised by local people or small grass-roots level industries may be facilitated by a more complete understanding of the dynamics of people–plant interactions [2,3,4].
The culture of plant-based subsistence is rapidly becoming a rarity in the modern world. Hence, the cultures of the African people represent a minority that have continued practicing holistic environmentalism that utilises raw plant-based materials for food, medicines, as pesticides or tools and in spiritual pursuits including rituals [5]. In the modern day, most South Africans rely on traditional medicine as a first line of treatment. This is chiefly due to its affordability, accessibility, and the high level of knowledge by local traditional healers [6,7]. In this regard, about 3000 out of over 20,000 species of higher plants in South Africa are used in traditional medicine [8]. The botanical prescriptions made by the archetypical traditional healers in South Africa are collectively called ‘muthi’ and are generally distributed out of informal markets.
Since the turn of the 21st century, there has been a renewal of interest in the use of medicinal plants and herbal remedies for the treatment of health afflictions or in nutritional support [9,10]. While traditional healing systems in Africa have become a rich source of information on plant-based health, there are minimal written records to draw upon to guide the integration of natural products into developed societies. This is due to the fact that medicinal plant-use knowledge of traditional healers is passed on from generation to generation via word of mouth [6]. Hence, the importance of creating written records of traditional knowledge cannot be over emphasized, particularly in South Africa [2] or in highly remote locations such as Ethiopia [5]. Pappe [11] published the first synthesis of South African medicinal plants, then nearly a century later, Watt and Breyer-Brandwijk [12] provided a more comprehensive report on medicinal plants used in the same country. Hutchings et al. [13] created a focused account of medicinal plants commonly used in the Zulu nation, which was further elaborated by Van Wyk et al. [6] and especially by Mhlongo and Van Wyk [14].
The chemistry of taxa from South African Meliaceae is highly diverse. Many sesquiterpenes, sterols, coumarins, flavonoids, and other phenolics have been reported. Species in Meliaceae are well known for their bitter and biologically active nortriterpenoids, known as limonoids or meliacins [15,16]. Over 300 limonoids have been isolated from the world’s flora and their production is confined to the order Rutales, of which they are more diverse and abundant in Meliaceae than in any other family [15,16,17]. They are derivatives of 4,4,8-trimethyl-17-furanylsteroid. These compounds have aroused considerable commercial interest due to their molluscicidal, antifungal, bactericidal, insect-antifeedant, insect-repellent, insecticidal, and plant antiviral activities, as well as their numerous medicinal effects in humans and animals [16,18,19,20]. Hence, limonoids have attracted significant interest within biological and chemical research disciplines.
Several researchers have reported the chemistry, biosynthesis, and biological activities of meliaceous limonoids [21,22,23,24,25]. Azadirachta indica L. is known as a famous limonoid producing plant as well as a source of environmentally friendly biopesticide of commercial importance in the agricultural sector. Products of A. indica (such as align, azitin, margosan-O, and turplex) were recognized and approved as pest control agents in the United States [26]. In China, three commercial limonoid products (from A. indica, Melia azedarach L., and Melia toosendan Siebold & Zucc.) were granted approval for insect controls on organic vegetable plantings.
The ethnobotanical uses of taxa in South African Meliaceae are well documented [6,12,13,27,28,29,30]. Several limonoids and other secondary metabolites with appreciable biological activities have also been reported in South African species of Meliaceae [31,32,33,34,35]. Hence, the aim of this study is to present a detailed and comprehensive review of the ethnobotanical uses and compounds that have been previously isolated from South African indigenous Meliaceae, which can be used for comparisons at a continental and global level. Additionally, to identify knowledge gaps in terms of the ethnobotany and chemistry that can be used as a guide for future research work.
2. The Ethnobotany and Chemistry of South African Meliaceae
2.1. Ethnobotanical Uses
Although there are many uses in woodwork, staining, and construction [36], the traditional and contemporary uses as medicines are also well documented [6,12,13,29,30,37,38,39]. Furthermore, the plants find a place in horticulture for ornamental purposes and shade, as food, as anti-feedants, and for ritual purposes [36]. The most frequently cited medicinal uses of species in Meliaceae are as anthelmintics and antimicrobials. However, they are included in a wide range of applications including as therapeutic interventions in cardiovascular function, and respiratory, urinary, gastrointestinal, dermatological, and oral infections [12,30].
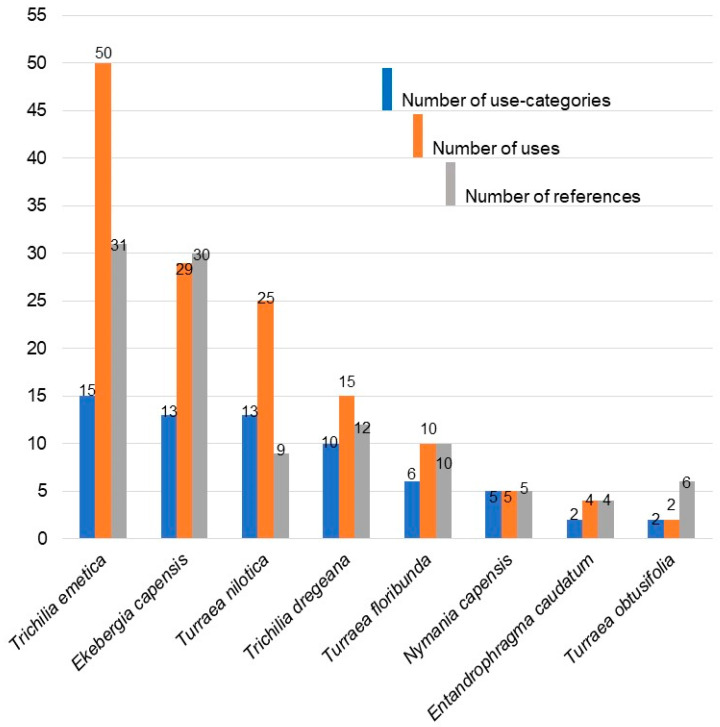
Ethnobotanical uses and prescriptions of South African Meliaceae are reported in Table 1. Although six genera and twelve species are recognised in South Africa, only eight species from five genera have recorded ethnomedicinal uses, giving a total of 85 different ethnomedicinal uses summarised from published records. Out of the functional uses, Trichilia emetica Vahl demonstrated the highest utility, followed by Ekebergia capensis Sparrm (Figure 1). Both were frequently cited as being used as shade trees and for ornamental purposes, as well as for furniture, timber, and cosmetics (Section 2.1.5).
Table 1.
The traditional uses of South African Meliaceae. The categories are according to Moffett’s (2010) classification. *NR: Not recorded; A: Afrikaans; E: English; N: Ndebele; NS: Northern Sotho; S: Sotho; Sh: Shona; T: Tsonga; Ts: Tswana; V: Vhavenda; X: Xhoza; Z: Zulu.
| Taxa | Local Names | Traditional Use | References | ||
|---|---|---|---|---|---|
| Medicinal Use | Part Use | Preparation and Administration | |||
| Ekebergia capensis Sparrm. | Esseboom, essenhout (A); dogplum, cape ash (E); munyonga, mmidibidi (NS); umNyamatsi (S); mumbafwe (T); nyamaru (Ts); mudouma, muṱobvuma, muzhouzhou (V); umgwenyezinja (X); imanaya, isimanaye, mahlunzidintaba, ronyamati, simanaya, umathunzi wentaba, umathunzini, umgwenyana wezinja, umnyamathi, umthoma, usimanaye, uvungu, (Z) | Analgesic | |||
| Headache | Root | Powdered, charred pulverized roots are sniffed | [12,41,42] | ||
| Leaf | NR | [43] | |||
| Malaria | Root and leaf | Extracts from maceration of crushed roots and leaves are drunk | [44] | ||
| Bark | inner bark is boiled and drunk | [45] | |||
| Anthelmintic | |||||
| Worms | Bark and leaf | Bark powder is added to leaf decoction and drunk | [6,46] | ||
| Antimicrobial | |||||
| Anthrax | Leaf | Crushed leaf is boiled and drunk | [47,48] | ||
| Venereal diseases | Bark and root | Freshly collected bark and roots are boiled in water and the extract is drunk three times daily | [49] | ||
| Cardio-vascular | |||||
| Blood purifier and blood pressure | Leaf and inner bark | Leaf or inner bark is boiled and drunk | [13,45,47] | ||
| Heart ailment | Bark | NR | [13] | ||
| Cytological | |||||
| Cancers | Fruits | Fruits are crushed, sieved, and drunk | [50] | ||
| Dermatological | |||||
| Abscess, scabies, and acne | Bark | Infusion or maceration of the bark powder is applied | [6,39,51,52] | ||
| Scabies | Root and leaf | NR | [12,41] | ||
| Abscess and boil | Bark | Crushed bark added to flour and water poultices is applied | [13,51] | ||
| Pimples | Bark | Crushed bark in hot water infusion is drunk and used as a wash | [13,51] | ||
| Skin ailments | Leaf | NR | [37] | ||
| Gastro-Intestinal | |||||
| Bloody stool | Bark | Bark is macerated with bark of Diospyros lycioides Desf. and extract is drunk | [53] | ||
| Emetic and heartburn | Bark and root | Bark or root decoctions are taken as emetics | [6,43,54] | ||
| Gastritis, dysentery, and heartburn | Bark and root | One teaspoon of bark and root powder in a half cup of hot water is taken as tea | [6,12,41,44,55] | ||
| Purgative | Leaf | A cup of leaf infusion is drunk | [13] | ||
| Stomachache | Fruits | Fruits are masticated and swallowed | [50] | ||
| Gynaecological and Obstetrics; Genital system | |||||
| Dystocia | Leaf and twig | Leafy twigs mixed with Indigofera oubanguiensis Tisser. are taken orally and used as a wash to treat dystocia | [30,56] | ||
| Infertility | Bark | NR | [13] | ||
| Nervous system | |||||
| Epilepsy | Bark | Bark infusion is drunk | [57] | ||
| Stress relief | NR | NR | [14] | ||
| Respiratory system | |||||
| Chest complaints and coughs | Bark and root | Bark or root decoctions are taken orally | [54] | ||
| Chronic cough | Leaf | NR | [43] | ||
| Cough and respiratory complaints | Bark | Bark decoction with root of Euclea natalensis A.DC. is drunk | [54] | ||
| Trauma | |||||
| Snakebite | Root and leaf | Extracts from maceration of crushed roots and leaves are drunk | [44] | ||
| Ethnoveterinary | |||||
| Tuberculosis | Bark | Crushed bark is boiled and administered orally | [48,58] | ||
| Abortion | Galls | Galls on plant are boiled and administered orally | [59] | ||
| Magic | |||||
| Protection | Bark | Bark is used to protect chiefs against witchcraft | [13,60] | ||
| Love | Bark | Bark decoction is drunk as love charm emetics | [13,60] | ||
| Ekebergia pterophylla (C.DC.) Hofmeyr | Rotsessenhout (A); rock ash (E); maGwedla (S) | No Ethnomedicinal Records | |||
| Entandrophragma caudatum (Sprague) Sprague | Bergmahonie (A); mountain mahogany, wooden-banana (E); mophumêna (Ts); munzhounzhou (V) | Analgesic | |||
| Malaria | Bark | NR | [61] | ||
| Antimicrobial | |||||
| Gonorrhoea | Root | Root decoction is drunk | [62] | ||
| Genital warts | Fruit | Burnt fruit peels mixed with Vaseline are applied topically | [62] | ||
| Nymania capensis (Thunb.) Lindb. | Kankerbos, kiepkiepies, klapperbos, klapperbossie, lanternbos, oumeidsbos, oumeidebos, stuipebos, stuipebossie, stinkbossie, ystervarkbos (A); chinese lantern tree, kipkippers, klapper (E) | Ear, Nose, and Throat | |||
| Influenza | Leaf | Leaf infusion is taken | [63] | ||
| Gastro-Intestinal | |||||
| Stomach complaints and nausea | Root | Root decoction is taken | [64] | ||
| Nervous system | |||||
| Convulsion | Leaf | Leaf decoction | [12,53,63] | ||
| Trauma | |||||
| Wound healing | Root | Roasted, pulverized roots are sprinkled on the affected part and can also be mixed with fat into an ointment and applied as a salve | [64] | ||
| Urinary system | |||||
| Kidney problem | Root | Root decoction is taken | [64] | ||
| Pseudobersama mossam bicensis (Sim) Verdc. | Valswitessenhout (A); umopho (Z) | No Ethnomedicinal Records | |||
| Trichilia dregeana Sond. | Bos Rooi-essenhout, bosrooiessenhout (A); cape mahogany, forest mahogany, forest natal mahogany, white mahogany (E); mutshikili, mutuhu, muuhu (V); umhlakele, umkhuhlu, umkhuhlwa (X), ixolo, umathunzi, umathunzini, umkhuhlu (Z) | Analgesic | |||
| Back pain | Bark | A teaspoon of pulverised bark boiled in a cup of milk is allowed to cool and strained, then half a cup of the extract is taken as an enema in the early morning | [12,13] | ||
| Fever | Root | Root decoction is taken orally | [12,65] | ||
| Toothache | NR | NR | [14] | ||
| Antimicrobial | |||||
| Gonorrhoea and Syphilis | Leaf | Handful of leaves is boiled with a handful of Albizia adianthifolia leaves in 2 L water and half a cup of the decoction is taken daily | [27,43,53,66] | ||
| Leprosy | NR | NR | [65] | ||
| Cardio-Vascular | |||||
| Blood purifier | Bark | Bark is taken as an enema for men | [43] | ||
| Dermatological | |||||
| Bruises and eczema | Leaf or fruit | Leaf or fruit poultice is applied topically | [65] | ||
| Gastro-Intestinal | |||||
| Bloody diarrhoea | Bark | Bark decoction is taken daily | [53,65] | ||
| Purgative and stomach complaints | Bark or root | Bark infusion is taken as an enema or root decoction is taken orally | [13,65,67] | ||
| Gynaecological and Obstetrics; Genital system | |||||
| Abortifacient | Bark | Bark infusion or decoction is taken orally or used as an enema | [65,67] | ||
| Musculo-Skeletal | |||||
| Lumbago | NR | NR | [65] | ||
| Rheumatism | Seed | Oil from seed is used for massage | [65] | ||
| Trauma | |||||
| Fractures | Seed | Oil from seed is rubbed into scarifications made on fractured limb | [65] | ||
| Urinary system | |||||
| Kidney problem | Bark | Bark maceration is taken as an enema | [13,43,53] | ||
| Ethnoveterinary | |||||
| Fishing poison | Bark | NR | [8,65,67] | ||
| Trichilia emetica Vahl | Rooiessenhout (A); natal-mahogany (E); mamba (NS); umkuhlu (Si); ankulu, nkulu (T); mutshikili, mutuhu (V); umkhuhlu (X); umathunzini (Z) | Analgesic | |||
| Back pain | Bark or leaf | Bark or leaf maceration is taken as an enema | [12,13,68] | ||
| Dental care | Twig, trunk, wood, root or flower | Twig, trunk, wood or root is chewed and the crushed flowers is used as a toothpaste | [69] | ||
| Headache | Leaf | Leaf infusion is used to wash the head | [69] | ||
| Malaria | Leaf, bark, and root | Leaf decoction mixed with lemon is drunk or used as a bath for 3–7 days. Bark decoction mixed with honey is also taken orally. Root, stem, and leaf decoction is taken 2–3 times daily for 3 days. Root maceration of T. emetica, Pseudocedrala kotschii (Schweinf.) Harms and Nauclea latifolia Sm. mixed with honey can also be drunk for 10 days | [38] | ||
| Anthelmintic | |||||
| Teniasis | Bark | Crushed bark mixed with root of Securidaca longependonculata Fresen. is taken orally for 3 days | [38] | ||
| Worm | Bark or root | Bark or root decoction is taken daily for 3 days | [53,70] | ||
| Antimicrobial | |||||
| Dysentery | Bark or leaf | Bark or leaf maceration is taken as an enema | [12,13,71] | ||
| Gonorrhoea and syphilis | Bark and leaf | Bark and leaf decoction is drunk | [43,62] | ||
| Leprosy | Root | Root maceration is drunk or used as a bath | [38,42,71] | ||
| Cardio-vascular | |||||
| Blood and digestive tract cleanser | Bark | Bark infusion is applied as an enema | [13,43] | ||
| Blood pressure | Leaf | A teaspoon of 1 h decoction of T. emetica leaf, Aloe. marlothii leaf, and Hyphaene coriaceae root is taken orally three times daily | [27] | ||
| Dermatological | |||||
| Burns and bruises | Leaf | Hot leaf infusion is applied to the affected part | [69] | ||
| Dermatitis | Leaf and bark | Leaf decoction is used in a steam bath or crushed leaves are applied on the affected part while the powdered bark can also be used for cleansing | [38,72] | ||
| Eczema | Fruit or leaf | Fruit or leaf poultice is applied topically | [12,13,73,74] | ||
| Ear, Nose, and Throat | |||||
| Colds and bronchial inflammation | Root | Root decoction is taken orally | [37] | ||
| Gastro-Intestinal | |||||
| Abdominal pain | Leaf or root | Crushed leaf or root decoction is used as a bath and taken orally with salt and lemon twice daily | [38] | ||
| Constipation | Bark | 50 g of chopped bark is boiled with 50 g of chopped bark of Spirostachys africana Sond. in 5 L of water and taken orally | [68] | ||
| Diarrhoea | Bark | Bark infusion is administered anally twice a day |
[27] | ||
| Digestive infections | Root | Root decoction mixed with coffee is taken orally for 3 days or decoction mixed with Cassia sieberiana DC. root and honey drunk in the morning for 5 days | [38] | ||
| Emetic | Bark or root | Bark maceration or pulverized bark in hot water is taken, root extract can also be taken orally | [12,13,37,46,72,75,76,77] | ||
| Flatulence | Root | Powdered root infusion added to Acacia nilotica seed powder is taken | [38] | ||
| Hemorrhoids | Root | Crushed root bark mixed with black pepper and crushed fruit of Xylopia aethiopica (Dunal) A. Rich. is taken daily, while the powdered root in salt water is used as an enema | [38] | ||
| Gastric ulcer | Bark | Crushed bark mixed with salt and ginger is added to porridge and taken twice daily | [38] | ||
| Hernia | Root | Crushed root is added to porridge and taken orally | [38] | ||
| Inflamed anus | Bark | Pulverized bark puffed into the anus | [30] | ||
| Jaundice | Root | Root decoction is taken daily for 3 days, chopped roots are also mixed with honey and used as a bath | [38,70] | ||
| Laxative | Bark | Bark is mixed with eggs and taken orally to clean the stomach | [78] | ||
| Purgative | Bark | Bark infusion or decoction is used as an enema or taken orally | [53,67,76] | ||
| Stomach complaints | Bark | Bark infusion or decoction is taken orally or administered as an enema | [43,53,54,67] | ||
| Gynaecological and Obstetrics; Genital system | |||||
| Abortifacient | Bark | Bark infusion is taken | [67,71] | ||
| Bark and root | One handful of crushed bark and root in a litre of milk and Coca Cola mixture is boiled for 15 min and taken orally | [27] | |||
| Breast pain | Leaf | Leaf decoction is used to bath | [38] | ||
| Dysmenorrhoea | Leaf | Leaf decoction added to Tamarindus indica L. or lemon is taken orally for 7 days | [38,79] | ||
| Fertility | Leaf | Leaf decoction with Combretum molle R. Br. ex G. Don is taken orally | [38] | ||
| Bark | Crushed bark mixed with the same amount of Gymnosporia senegalensisa root is boiled and decoction is used as an enema | [27] | |||
| Labour pain | Leaf | Hot leaf decoction is used to massage the belly of a woman during labour to ease pain until she delivers | [27] | ||
| Sterility | Root | Powdered root with ginger and salt is taken as a porridge | [38] | ||
| Musculo-Skeletal | |||||
| Lumbago | Bark or leaf | Bark or leaf maceration is taken as an enema | [12,30] | ||
| Paralyses | Root | Crushed root is mixed with porridge and taken orally | [38] | ||
| Rheumatism | Seed | Oil from boiled seeds is taken orally and rubbed topically | [8,12,13,46,75] | ||
| Opthalmic | |||||
| Eye infection | Leaf and bark | Leaf and bark decoction is used for eye cleansing for 2 days | [38] | ||
| Respiratory system | |||||
| Cardiac problems | Leaf | Leaf decoction is taken orally | [80] | ||
| Chest pain | Leaf | Leaf decoction is used in a steam bath or rubbed on the chest | [38] | ||
| Cough | Bark and root | Bark and root decoction is drunk | [81] | ||
| Pneumonia | Root or leaf | Root or leaf decoction is taken orally or used as a bath for 12 days | [37,38] | ||
| Trauma | |||||
| Fracture | Seed | Oil from the seed is rubbed into incisions on broken limbs and baked pulverized root of Sideroxylon inerme L. is applied | [8,12,13,82] | ||
| Stiffness or sprains | Bark | Bark extract is applied topically | [69] | ||
| Wound | Seed | Oil from the seed is applied to prevent infection from maggots | [37] | ||
| Urinary system | |||||
| Kidney problem | Bark | Bark decoction is administered as an enema | [43] | ||
| Renal ailments | Bark | Bark decoction is taken | [13] | ||
| Ethnoveterinary | |||||
| Fishing poison | Bark | NR | [67] | ||
| Magic | |||||
| Burial rituals | Leaf | Leaves are worn during burial rituals | [54] | ||
| Turraea floribunda Hochst. | Kanferfoelieboom (A); honeysuckle-tree, wild honeysuckle-tree (E); umdlozana, inkunzane (Si); umhlatholana, umlahlana (X); umadlozane, umlulama, ubhukulo (Z) | Dermatological | |||
| Abscesses | Root | Root decoction is taken orally | [30] | ||
| Gastro-Intestinal | |||||
| Ascites | Root | Root maceration is taken orally | [12,13] | ||
| Emetic | Bark | Bark extract is taken orally | [8,37] | ||
| Purgative | Bark and root | Bark and root decoction are taken orally | [37] | ||
| Musculo-Skeletal | |||||
| Rheumatism | Root | Root maceration is taken orally | [8,12,13,75,76] | ||
| Respiratory system | |||||
| Cardiac problems | Root | Root maceration is taken orally | [12,13,47,75,76] | ||
| Cough | Root | Root decoction is taken orally | [37] | ||
| Urinary system | |||||
| Urethral infection | Bark | Bark decoction is taken orally 1-3 times daily | [83] | ||
| Magic | |||||
| To induce a state of trance | Bark | Bark infusion is taken orally | [30,75,76] | ||
| Protection from bad dreams | Bark | NR | [76] | ||
| Turraea nilotica Kotschy and Peyr. | Bushveld honeysuckle-tree, lowveld honeysuckle-tree, miombo honeysuckle-tree, small mahogany (E) isidlamvundala (N); chipindura, chirambagavakava, chitsvimbovarisa, chitunguru, mudyakuwe, mukondanyoka, muzaramhanga (Sh) | Analgesic | |||
| Headache | Root or leaf | Root decoction or leaf infusion is taken orally | [46,67] | ||
| Toothache | Root | Root decoction is used as a mouthwash | [76,84] | ||
| Anthelmintic | |||||
| Ascariasis | Root | Root decoction is taken orally | [30] | ||
| Antimicrobial | |||||
| Gonorrhoea | Root | Root decoction is taken orally | [81] | ||
| Venereal diseases | Root | Root infusion is taken orally | [8,67] | ||
| Dermatological | |||||
| Abscesses | Root | Root decoction is taken orally and applied as a compress | [30] | ||
| Gastro-Intestinal | |||||
| Abdominal pain | Leaf or root | Leaf decoction or root infusion is taken orally | [8,67,85] | ||
| Constipation | Root | Root bark decoction is taken orally | [8,81] | ||
| Diarrhoea | Leaf or root | Leaf or root decoction is taken orally or pulverized root is added to porridge | [8,67,85] | ||
| Indigestion | Root | Root decoction is taken orally | [37] | ||
| Schistosomiasis or hernia | Root | Root infusion mixed with honey is taken orally | [81] | ||
| Gynaecological and Obstetrics; Genital system | |||||
| Aphrodisiac | Root | Powdered root mixed with beer or porridge is taken | [67] | ||
| Dysmenorrhoea | Root | Powdered root mixed with porridge is taken | [8,67] | ||
| Prevent abortion | Root | Root infusion is taken orally | [67] | ||
| Sterility | Root | Root decoction is taken orally | [81] | ||
| Nervous system | |||||
| Dizziness | Leaf | Leaf infusion is taken orally | [67] | ||
| Epilepsy | Root | Powdered root mixed with porridge is taken | [8,67] | ||
| Opthalmic | |||||
| Eye problems | Leaf | Leaf paste is applied to the eyelids | [67] | ||
| Respiratory system | |||||
| Dyspnea | Root | Powdered root mixed with porridge is taken orally | [67] | ||
| Pneumonia | Root | Powdered root is rubbed into scarification on the painful area, root infusion is taken orally, and smoke from the burnt roots is inhaled | [8,67] | ||
| Trauma | |||||
| Snakebite antidote | Root | Burnt root ashes are applied to the bite | [67] | ||
| Wound | Root | Root scrapings are applied topically | [30] | ||
| Urinary system | |||||
| Dysuria or rectal prolapse | Root | Root bark decoction is taken orally | [67] | ||
| Ethnoveterinary | |||||
| Anthelminthic for dogs | Root | Root infusion is administered orally | [67] | ||
| Magic | |||||
| To calm the insane | Leaf | Leaf infusion is administered orally and smoke from burnt leaves is inhaled | [67] | ||
| Turraea obtusifolia Hochst. | Kleinkanferfoelieboom (A); small honeysuckle tree, lesser honeysuckle tree, (E); amzulu, ikhambi-lomsinga, ikunzi, inkunzi, inkunzi-embomvana, umhlatholana, uswazi (Z) | Gastro-Intestinal | |||
| Stomach and intestinal complaints | Leaf, bark and root | Hot water maceration of leaf, bark or root is given as an enema and also mixed with porridge to be taken orally | [13,54,75,76,86] | ||
| Ethnoveterinary | |||||
| Wounds in livestock | Leaf | Crushed leaves are applied topically on the affected part | [87] | ||
| Turraea pulchella (Harms) T.D.Penn. | No Ethnobotanical Record | ||||
| Turraea streyi F. White and Styles | No Ethnobotanical Record | ||||
Figure 1.
Number of use-categories, uses, and references for each species of South African Meliaceae.
In the context of strictly therapeutic applications, E. capensis represents an important complement to South African materia medica [6]. The parts of E. capensis used traditionally vary according to end-use, but range from bark, leaf, fruit, root, wood to twig. Such applications include the treatment of epilepsy, malaria, pain, skin ailments, and gastrointestinal, respiratory, cardiovascular, and reproductive problems (Table 1).
The most commonly used organs are the bark or root bark, administered as a decoction that is boiled in about 2 L of water and taken as an emetic for coughs, heartburn, and respiratory chest complaints [6]. When a poultice is made from the crushed bark, it is combined with flour and water as a caking agent and applied as a skin scrub for use as a topical blood purifying agent for abscesses, boils, and in hot water infusions for pimples [13]. Different parts of the plant could either be used alone or in combination with other species. The bark powder and leaf decoctions are used in the treatment of intestinal worms and epilepsy [13,30,40]. In this regard, approximately 200 mL of the aqueous leaf infusion is drunk as a purgative parasiticide. Furthermore, the bark and root are combined to treat gastritis, dysentery, heartburn, and as an expectorant [6,8,12]. The bark is also used in rituals to guard tribal chiefs against witchcraft and taken orally as a love charm emetic [13].
2.1.1. Categories of Medicinal Uses
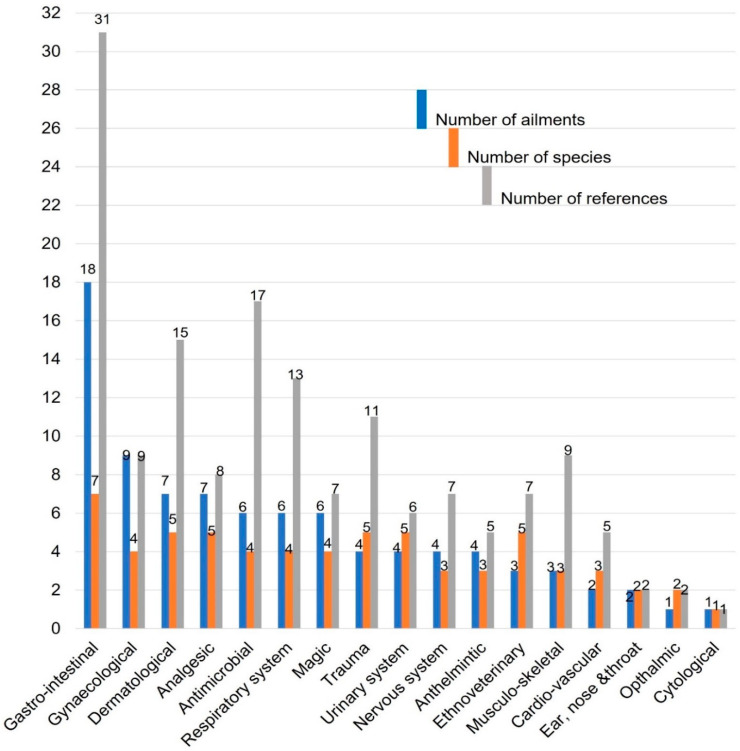
Remedies made from the South African Meliaceae are used to treat a wide variety of medical conditions in humans, as well as for ritual purposes. They are also used in ethnoveterinary treatments. Most of the species had more than one therapeutic use, with T. emetica having the highest number of uses and categories (50 and 15, respectively), followed by E. capensis (29 and 13, respectively). The lowest number of uses and categories was recorded against T. obtusifolia (Figure 1). The highest number of citations for ethnomedicinal uses was recorded against T. emetica (31) followed by E. capensis (30), T. dregeana (12), T. nilotica (9), T. obtusifolia (6), N. capensis (5), and E. caudatum (2) (Figure 1). Eighty-seven different ailments grouped into 17 major categories which are gastro-intestinal; gynaecological and obstetrics; dermatological; analgesic; antimicrobial; respiratory system; magic; trauma; urinary system; nervous system; anthelmintic; ethnoveterinary; cardio-vascular; ear, nose and throat; ophthalmic; and cytological were recorded in this study (Figure 2). Most of the species of South African Meliaceae are mostly used in the treatment of gastro-intestinal ailments followed by gynaecological and obstetrics related ailments (Figure 2). However, there was no ethnomedicinal record found for four of the species (E. pterophylla, P. mossambicensis, T. pulchella, and T. streyi).
Figure 2.
Number of ailments in each category, species used in the treatment of each category of ailment, and references for South African Meliaceae.
An example of the use of E. capensis in multi-therapeutic combinations with other species is the decoction that is made from a combination of E. capensis leafy twigs and I. oubanguiensis, which is taken orally and used as a wash to treat dystocia [30]. Bryant [54], also described how the bark decoction of E. capensis is mixed with roots of E. natalensis, to be taken orally to treat respiratory problems. More examples of species combinations in multi-therapeutics with E. capensis are provided in Table 1. Preparation of herbal remedies using more than one plant species can be attributed to the synergistic or additive effects that could occur during the treatment [88].
In contrast to E. capensis, E. caudatum has minimal presence in South African materia medica. The root decoction is being used as a remedy for gonorrhoea, while the burnt fruit is mixed with Vaseline and applied topically to treat genital warts [62]. This is not the only record of dry heating/burning as a materia medica modality.
In some applications, N. capensis processing involves a roasting step, i.e., according to Von Koenen [64] the root is roasted, pulverized, and applied topically to treat wounds and relieve knee pain. However, medical uses of N. capensis broaden to include a root decoction taken orally to treat kidney and stomach complaints, as well as nausea. The leaf decoction is also taken orally as a herbal remedy for convulsion [63,89].
Trichilia dregeana is an important medicinal plant with all of the parts used traditionally [55]. It is used as a herbal remedy for the treatment of syphilis, bloody diarrhoea, skin diseases, rheumatism, as an abortifacient, blood cleanser, and as fish poison (Table 1). The bark infusion or maceration is used as an enema for the treatment of kidney problems, bronchial inflammation, skin diseases, as well as general cleaning [13,43,52]. The bark is eaten as a purgative or for procuring abortion and also as a fish poison [67]. The leaf decoction is taken orally as a herbal remedy for syphilis [13,66].
Similar to E. capensis, species in Trichilia are also strongly represented in the ethnobotanical tradition of South African people. Trichilia emetica is a multipurpose tree that is widely distributed throughout Africa, meaning it is not exclusively a South African medicine [38]. All plant parts, the leaf, twig, bark, flower, wood, root, and fruit of T. emetica are used (Table 1). It is used as a purgative, an antipyretic, antiepileptic, and antimalarial agent [72]. The twig, trunk, wood, and root are chewed as herbal remedy for dental care [30]. The powdered bark is taken orally as a remedy for infertility and also to ease labour pain [27,38]. The bark decoction or infusion is also used to treat various ailments including dysentery, gastrointestinal problems, breast pains, back pains fever, malaria, and as a purgative (Table 1). The bark decoction is used by the Xhosa tribe as an enema to treat kidney problems [12].
In other applications, a hot leaf infusion of T. emetica is rubbed on the affected part to treat burns while the leaf decoction is taken orally as a remedy for dysmenorrhoea and syphilis [13,38]. The leaves are also used as a poultice for wound healing, skin problems, and contusions [29,52]. The leaves can also induce drowsiness or sleep at night when placed in the bed [38]. The root decoction is used as a herbal remedy for colds and bronchial inflammation, chest pain, fever, pneumonia, jaundice, gastrointestinal infections, and sexually transmitted diseases (Table 1). The fruit is used as a herbal remedy for eczema [13,82]. Pulverized seeds of T. emetica are boiled and the oil is rubbed on the affected part to treat rheumatism and leprosy [46]. However, the combination of two or more organs of T. emetica can also be used as a herbal remedy (Table 1) that is allegedly more potent than the individual parts. The decoction of stem, root, and bark is taken orally as a herbal remedy for whooping cough and ulcers [38,81]. The leaf and bark decoction of T. emetica is rubbed on the eye to treat an eye infection [38].
Similar to other taxa in Meliaceae, the bark and root of T. floribunda is used as a remedy for a broad range of ailments (Table 1). It is boiled in water and taken orally as an emetic and herbal remedy for urethral infection [37,54,83]. A bark infusion of T. floribunda is also taken orally to induce a state of trance prior to rituals [75]. The root decoction is taken orally as a remedy for cough and hardened abscess [37], while the root maceration is taken orally to treat rheumatism, cardiac problems, ascites, and dropsy [8,13]. The root and bark decoction are taken orally as a purgative [12,37].
The root decoction of T. nilotica is taken orally as a remedy for headaches, hardened abscess, indigestion, gonorrhoea, sterility, dysuria, jaundice, as a poison antidote, and against intestinal worms [30,67,70,81]. The root decoction is used as a mouth wash for toothache and the ash of the burnt root is applied topically as a snakebite antidote [30,84]. The root infusion is taken orally to treat venereal diseases, abdominal pain, constipation, inflammation of navel cord, and to prevent abortion [8,30,67]. The root infusion is taken orally with honey to treat schistosomiasis, hernia, and bilharziasis, while in ethnoveterinary medicine, the root infusion is used as an anthelmintic for dogs [8,81]. The root powder is taken orally in beer or porridge as an aphrodisiac, it could also be used as a remedy for dysmenorrhea, epilepsy, and dyspnoea [67]. The leaf decoction is taken orally as a remedy for abdominal pain and diarrhoea, while the leaf infusion is taken orally as a remedy for dizziness [30,37,85]. The leaf infusion of T. nilotica is taken orally and the smoke from burnt leaves is inhaled to calm an ‘insane person’, while the leaf paste is applied to the eyelid to treat eye problems [67].
The leaves, bark, and root bark of T. obtusifolia are macerated together in hot water and taken with porridge as a remedy for stomach and intestinal complaints [86]. Due to the diverse use of E. capensis, T. dregeana, T. emetica, T. floribunda, and T. obtusifolia as herbal remedies, the bark is sold in informal herbal medicine markets as traditional medicines in Gauteng and KwaZulu-Natal provinces in South Africa [90].
2.1.2. Categories of Uses
Remedies made from South African Meliaceae are used to treat a wide variety of medical conditions in humans as well as for ritual purposes. They are also used in ethnoveterinary treatments. All of the species had more than one therapeutic use, with T. emetica having the highest number of uses and categories (50 and 15, respectively), followed by E. capensis (29 and 13, respectively) and the lowest number of uses and categories was recorded against T. obtusifolia (Figure 1). The highest number of citations for ethnomedicinal uses was recorded against T. emetica (31) followed by E. capensis (30), T. dregeana (12), T. nilotica (9), T. obtusifolia (6), N. capensis (5), and E. caudatum (2) (Figure 1). Eighty-seven different ailments were grouped into 17 major categories including gastro-intestinal; gynaecological and obstetrics; dermatological; analgesic; antimicrobial; respiratory system; magic; trauma; urinary system; nervous system; anthelmintic; ethnoveterinary; cardio-vascular; ear, nose, and throat; opthalmic; and cytological were recorded in this study (Figure 2). South African Meliaceae are mostly used in the treatment of gastro-intestinal ailments followed by gynaecological and obstetrics related ailments (Figure 2). However, there was no ethnomedicinal record found for four of the species (E. pterophylla, P. mossambicensis, T. pulchella, and T. streyi).
2.1.3. Plant Parts Used
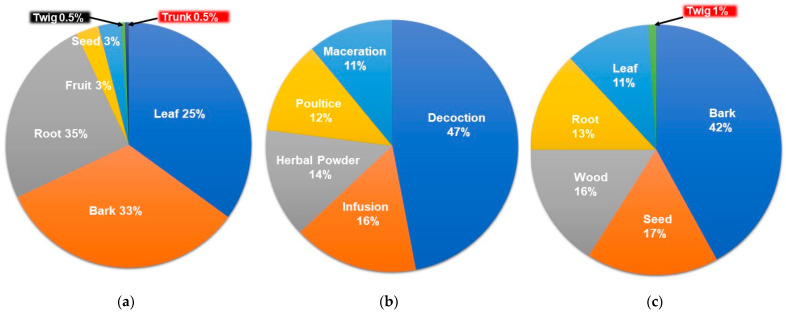
The plant parts of South African Meliaceae used in making herbal remedies were the root, bark, leaf, fruit, seed, twig, and trunk. Roots (35%), followed by barks (33%) and leaves (25%), were the most frequently used plant parts in preparation of the recorded herbal remedies (Figure 3a). Several studies reported roots to be more effective than other herbal plant parts. Hence, these were most frequently sourced [5,91,92,93]. This practice can also be linked to the scientific reasoning that roots and other underground parts contain high concentrations of bioactive compounds [94]. However, harvesting of roots for medicinal purposes is not sustainable as it threatens the existence of many medicinal plants which could lead to depletion of the plants. It is well documented by conservationists that medicinal plants mostly sourced for their root parts and bark are likely to be the most threatened by over-exploitation [95].
Figure 3.
(a) Percentage of different plant parts of South African Meliaceae reported to be used in ethnomedicine; (b) different dosage forms of herbal remedies of South African Meliaceae reported in the literature; (c) plant parts of South African Meliaceae from which compounds were reported to be extracted.
2.1.4. Mode of Herbal Preparation
Decoction (47%) was the most common mode of preparation recorded followed by infusion (16%), direct use as herbal powder (14%), poultice (12%), and maceration (11%) (Figure 3b). Decoction (boiling of the plant material) has been reported to be the most commonly used method of preparation in herbal medicine as it is believed that boiling extracts all of the potential bioactive compounds from the plant [96,97,98,99,100]. Moreover, decoction was reported to be the most common method of herbal preparations in South Africa [6]. Most of the remedies were administered orally followed by topically in the case of wound and skin infections, while some are sniffed into the nose (Table 1).
2.1.5. Other Uses
South African Meliaceae have also been reported to be useful for other purposes apart from medicinal uses. Ekebergia capensis, E. pterophylla, Entandrophragma caudatum, T. dregeana, T. emetica, T. floribunda, and T. obtusifolia are used as ornamental plants in gardens and on roadsides to create shade, wind breaks, and soil conservation (Table 2). The timber of E. capensis, E. caudatum, P. mossambicensis, and T. dregeana are mostly sought after by the furniture industry since they are soft, easy to work with, and durable (Table 2).
Table 2.
The functional uses of South African Meliaceae.
| Taxa | Traditional Use | References | ||
|---|---|---|---|---|
| Functional Use | Part Use | Method of Use | ||
| Ekebergia capensis | Tanning, furniture, brush, broom heads and handle, beams, planks, wagon, ship and boat building, light construction, poles and tool handles, light flooring, joinery, interior trim, vehicle bodies, sporting goods, toys, novelties | Wood | Wood is used in furniture industry | [12,40] |
| Firewood and charcoal production | Wood | Wood is used in cooking | [12,40] | |
| Animal feed | Fruit and leaf | Birds feed on fleshy parts of the fruit, while the leaf is used as a fodder | [12,40] | |
| Shades and wind break | Whole plant | It serves as an ornamental tree planted in gardens and roadsides for shades, as well as for wind break and soil conservation | [40,43] | |
| Edible caterpillar | Whole plant | Caterpillars are gathered from the plant and prepared as food | [43,101] | |
| Ekebergia pterophylla | Garden tree | Whole plant | Whole plant is used as an ornamental garden tree as well as a bonsai | [76] |
| Entandrophragma caudatum | Furniture, cabinet making, carving canoes | Wood | Wood is light and durable, hence high demand by the furniture industry | [8] |
| Tanning | Wood | Wood sap is used for tanning | [43] | |
| Toy | Fruit | Fruit pericarp is used to make ‘zwihwilili’ with which children like to play | [43,101] | |
| Shade | Whole plant | Whole plant is favoured for shade | [43] | |
| Animal feed | Seed | Seed is eaten by antelope | [75] | |
| Nymania capensis | Forage | Leaf | Source of forage for goats | [89] |
| Pseudobersa mamossam bicensis | Buildings and charcoal | Wood | Wood is used in making poles in local house buildings, as well as firewood and making charcoal | [102] |
| Trichilia dregeana | Furniture and carving | Wood | Wood is used for carving, repair of ships, and for making household furniture | [8,55,75] |
| Craftwork | Wood | NR | [101] | |
| Food condiments | Fruit | Fruit content is cooked with vegetables, fruit pulp is eaten as sour milk, while the oil made from the fruit pulp is used in cooking vegetables and other relishes | [43,65] | |
| Polish | Fruit and seed | Oil made from seed and fruit pulp is used to polish women’s clothes made from leather, furniture, and other household implements made from wood | [43] | |
| Soap and cosmetics | Seed | Oil from seeds is used to make soap, cosmetics, and candles | [43,75,103] | |
| Forage or fertilizer | Seed | Residue from seeds after oil extraction is used as a fertilizer or animal feed | [65] | |
| Fruit | Fruits are eaten by birds and bats | [75] | ||
| Drink | Seed aril | Seed aril is pounded and made into a sauce or sweet drink | [65] | |
| Shade | Whole plant | Whole plant is used to create shady avenue and as an ornamental tree | [65,75] | |
| Fishing | Seed | Bright-coloured seed is used as bait for fishing | [65] | |
| Trichilia emetica | Shade | Whole plant | Whole plant is used for shade | [43,75] |
| Soap and cosmetics | Seed | Oil from seed is used to make soap, body ointment, and candle | [8,71,76,103,104] | |
| Fertilizer | Seed | Residue from seed after oil extraction is used as a fertilizer | [71] | |
| Kola | Seed aril | Seed aril is eaten as a substitute for kola | [71] | |
| Forage | Leaf | Leaf is eaten by cattle and goats | [71] | |
| Fruit | Fruit is eaten by baboons, antelopes, and monkeys | [75] | ||
| Seed | Seed is eaten by birds | [75,76] | ||
| Carvings | Wood | Wood is used in carving furniture, household implements, musical instruments, canoes, and as chew-stick | [8,71,75,76,105] | |
| Dyeing | Bark | Pinkish or light red-brown dye is obtained from the beaten boiled bark | [4,71,75] | |
| Cooking | Seed aril | Seed aril is soaked and cooked together with squash or sweet potatoes | [8,75,101] | |
| Multivitamin | Seed | Juice is made from the seeds and other edible plants to control malnutrition | [106] | |
| Beverage | Fruit | NR | [101] | |
| Turraea floribunda | Traps | Wood | Wood is used for making traps | [107] |
| Ornamental | Whole plant | Tree is used as an ornamental plant in humid, frost-free subtropical and tropical gardens, and as a greenhouse plant in temperate countries | [76,82] | |
| Turraea nilotica | Handicrafts | Stem/branches | Stems/branches are used for handicrafts and domestics purposes | [107] |
| Firewood | Branches | Branches are sorted for firewood | [107] | |
| Turraea obtusifolia | Ornamental | Whole plant | Plant is used as an ornamental container plant in landscape designs, as well as an attractive garden plant | |
| Insect repellent | Leaf | Insect repellent | [87,101] | |
The wood of E. capensis, P. mossambicensis, and T. nilotica are used for charcoal and firewood for cooking (Table 2). Birds feed on the fleshy part of the fruit of E. capensis and the leaves are used as a fodder for domestic stock and game [12,40]. Edible caterpillars gathered from E. capensis are eaten as food by the Vhavenda [43,101]. Nymania capensis is used as a garden plant and also as a source of forage for goats [89]. The seeds of T. dregeana and T. emetica are known for their high fat content, hence the fat is used in soap making, as a body ointment, as polish, hair oil, and in cooking [43,75,103]. The seed arils of T. dregeana and T. emetica are cooked as vegetables or crushed for the milky juice which is taken with side dishes or as a drink [8,101]. In northern KwaZulu-Natal, the wood of T. dregeana is used to carve birds and animals which are sold along roadsides [8].
The wood of T. emetica is used to carve meat dishes, bowls, spoons, head rests, and animal carvings in Maputaland [4]. The leaves of T. emetica are eaten by wild animals and also worn in burial rituals by the Zulu [13,82]. The VaVhenda people in South Africa use the wood to construct the frame of an African traditional musical instrument (‘mbila’), while oil from T. emetica is applied on the instrument to soften the animal skin used for the instrument [82]. Saka and Msonthi [106] reported the juice from T. emetica seeds mixed with other edible plants to be used as multivitamins in cases of malnutrition.
The wood of T. floribunda is used for making traps, while stems of T. nilotica are used for handcrafts [107]. Turraea obtusifolia can also be used as a container plant in landscape design [76].
2.2. Reported Active Compounds
Several limonoids and other secondary metabolites of appreciable biological activities have been reported in South African species of the Meliaceae. The extracted compounds and parts extracted are represented in Table 3. Chemical studies for 10 out of the 12 South African Meliaceae were found. The two species that had no records are T. streyi and T. pulchella.
Table 3.
Isolated compounds extracted from various parts of South African Meliaceae.
| Plant. | Compound | Part Extracted | References |
|---|---|---|---|
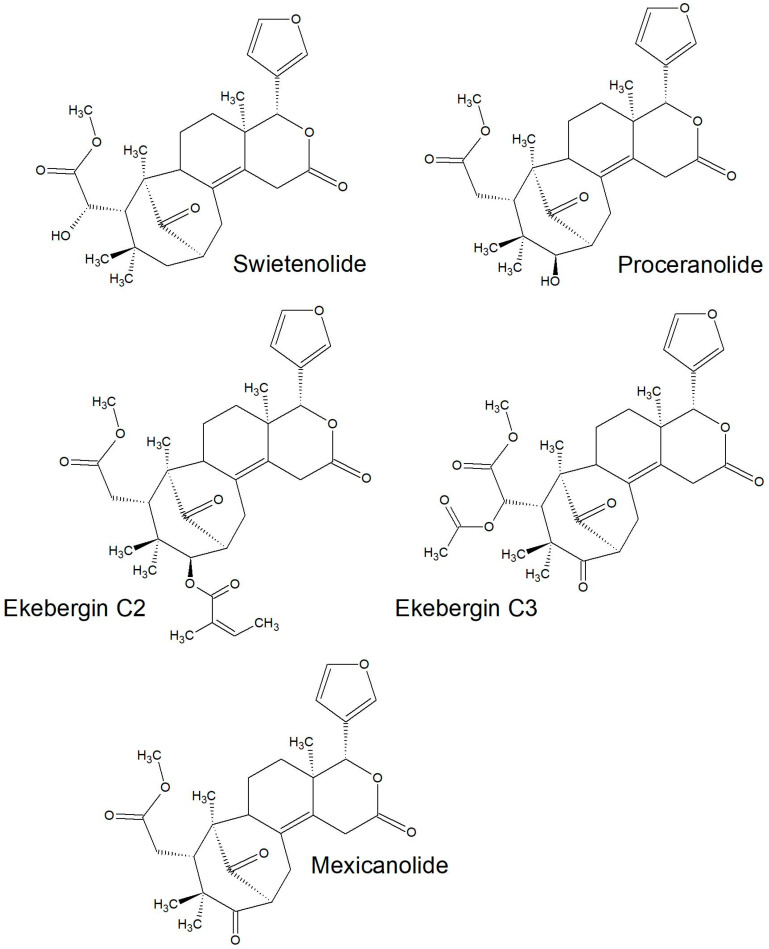
| Ekebergia capensis | Limonoids | ||
| Capensolactones 1-3 | Seed | [108] | |
| Methyl 3α-hydroxy-3-deoxy angolensate | Seed | [108] | |
| Ekebergin | Seed | [109] | |
| Ekebergins C1-C3 | Bark | [33] | |
| 7-Deacetoxy-7-oxogedunin | Bark | [33] | |
| Methylangolensate | Bark | [33] | |
| Mexicanolide | Bark | [33] | |
| Proceranolide | Leaf and bark | [31,33] | |
| Swietenolide | Bark | [33] | |
| Triterpenes | |||
| 3,11-Dioxoolean-12-en-28-oic acid | Bark | [33] | |
| Ekebergin A | Bark and root | [31,33] | |
| Ekebergins D1-D5 | Bark | [33] | |
| Melliferone | Bark | [33] | |
| 7-Acetylneotrichilenone | Bark | [33] | |
| Lupeol | Bark | [32] | |
| 2-hydroxymethyl-2,3,22,23-tetrahydroxy-6,10,15,19,23-penta methyl-6,10,14,18-tetra cosatetraene | Bark | [31,33,34] | |
| 2,3,22,23-tetrahydroxy- 2,6,10,15,19,23-hexamethyl-6,10,14,18-tetracosatetraene | Bark and wood | [31,33,34,35] | |
| 3-Epi-oleanolic acid | Bark, root, and wood | [31,32,33,34,35] | |
| 3-Oxo-12β-hydroxy-oleanan-28,13β-olide | Bark and root | [33] | |
| Oleanolic acid | Bark, root, and wood | [31,32,33,34,35] | |
| Coumarins | |||
| Ekersenin | Bark | [33,135] | |
| 4,6-Dimethoxy-5-methylcoumarin | Bark | [33] | |
| 7-Hydroxy-6-methoxycoumarin | Wood | [35] | |
| Glycoflavonoids | |||
| kaempferol-3-O-β-D-glucopyranoside; quercetin-3-O-β-D-glucopyranoside | Leaf | [31] | |
| Phenolics | |||
| Atraric acid | Bark | [32] | |
| Sterols | |||
| β-sitosterol | Bark and wood | [32,35] | |
| β-sitosterol oleate; β-sitosterol palmate | Bark | [32] | |
| Protolimonoid | |||
| Ekebergin B | Bark | [33] | |
| Pregnane | |||
| (Z)-volkendousin | Bark | [33] | |
| Ekebergia pterophylla | Limonoids | ||
| Ekebergin | Seed | [110] | |
| Ekebergolactones and prieurianin | Seed | [110] | |
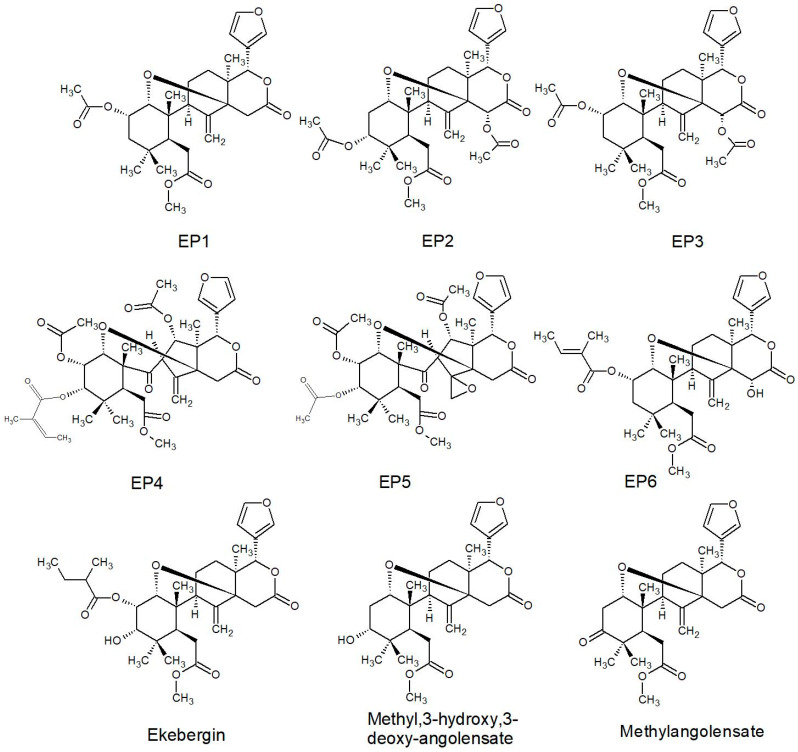
| EP1-EP6 | Seed | [111] | |
| Coumarins | |||
| Pterophyllins 1 and 2 | Bark | [108] | |
| Pterophyllins 3-5 | Wood | [108] | |
| Triterpenes | |||
| Lupeol | Leaf | [108] | |
| Oleanonic acid; β-amyrin; and β-amyrone | Bark | [108] | |
| Sterols | |||
| β-sitosterol | Bark | [108] | |
| β-sitosteryl acetate | Bark | [108] | |
| Phenolics | |||
| Atraric acid | Bark | [108] | |
| Entandrophragma caudatum | Limonoids | ||
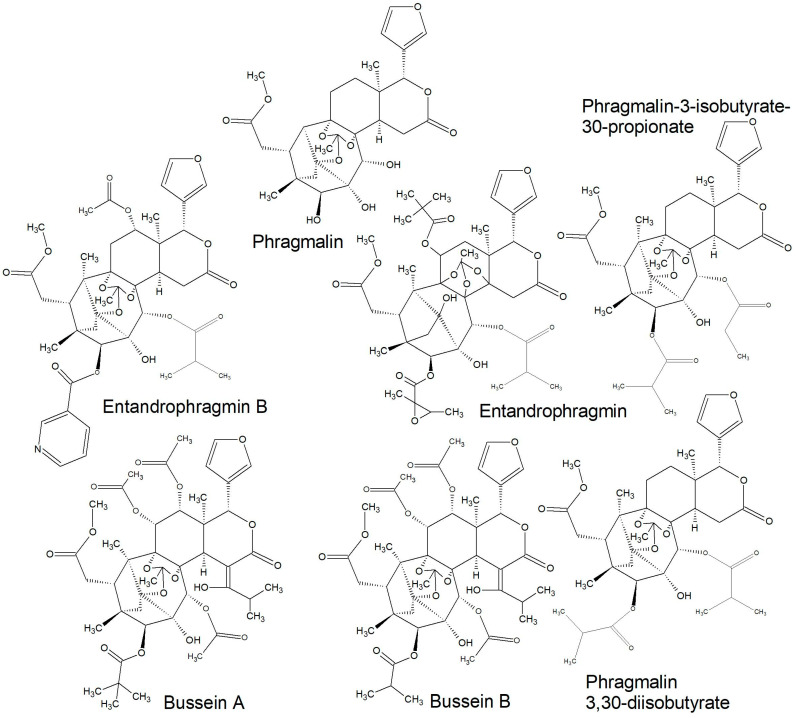
| Phragmalin; phragmalin 3,30-diisobutyrate; phragmalin 3-isobutyrate-30-propionate; Entandrophragmin B (12α-acetoxyphragmalin 3-nicotinate-30-isobutyrate) | Seed | [116] | |
| Bussein A and B; entandrophragmin | Wood | [113] | |
| Protolimonoids | |||
| 3α–turreanthin; melianone | Wood | [114] | |
| Nymania capensis | Limonoids | ||
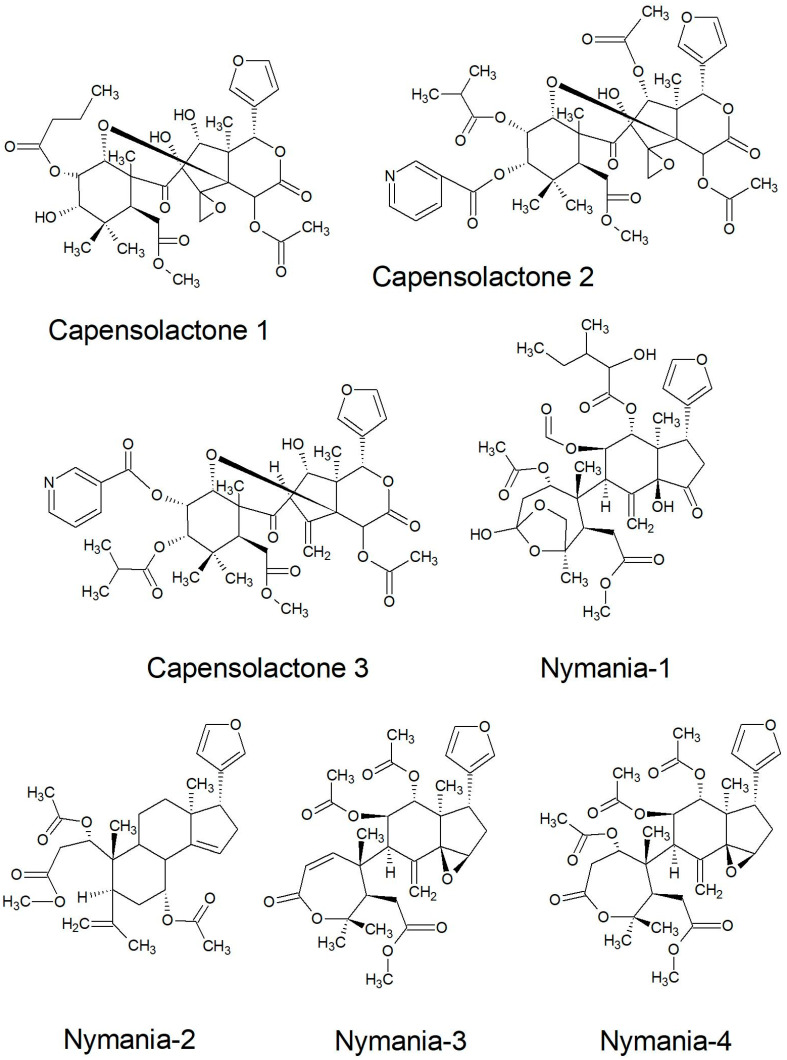
| Nymania 1-4; Prieurianin | Bark and Wood | [112] | |
| Pseudobersama mossambicensis | Sterols | ||
| Ergosta-5,24(28)-diene-3β, 7α-diol; 24,28-epoxyergost-5-ene-3β, 7α-diol; and ergost-5-ene-3β,7 α,24,28-tetraol | Twig and leaf | [129] | |
| Trichilia dregeana | Limonoids | ||
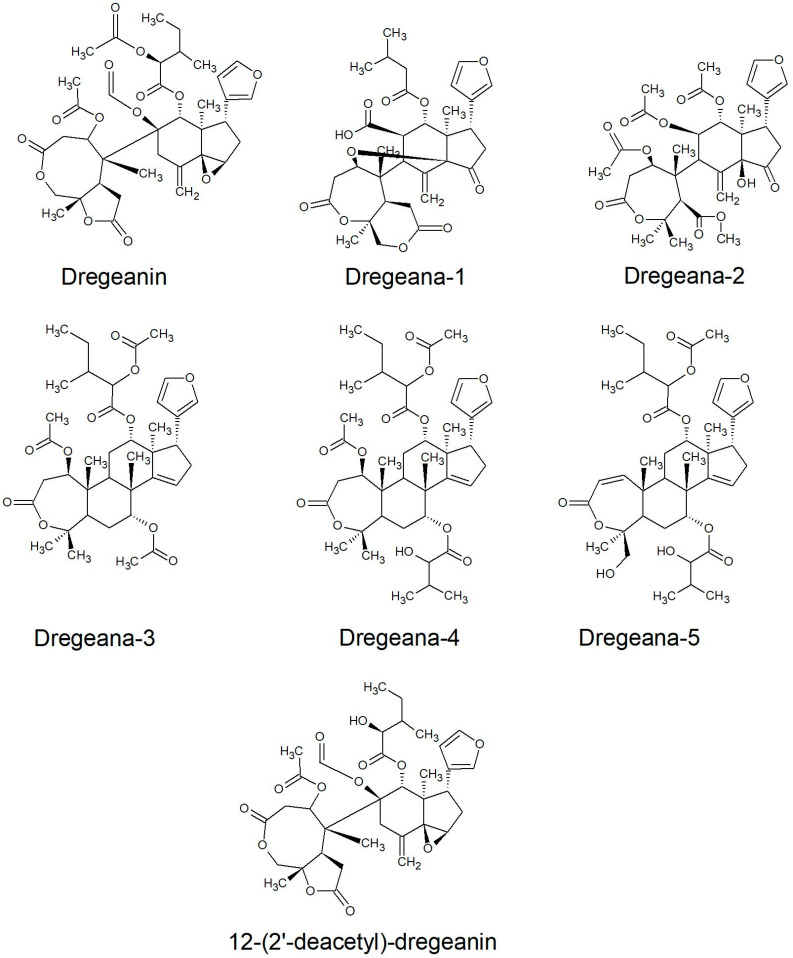
| Dregeana-5; dregeanin; and 12-(2′-deacetyl)-dregeanin | Stem | [136] | |
| Dregeana 1-4; hispidin C | Seed | [117] | |
| Sterol | |||
| Cycloart-23-ene-3β,25-diol | Leaf | [130] | |
| Trichilia emetica | Limonoids | ||
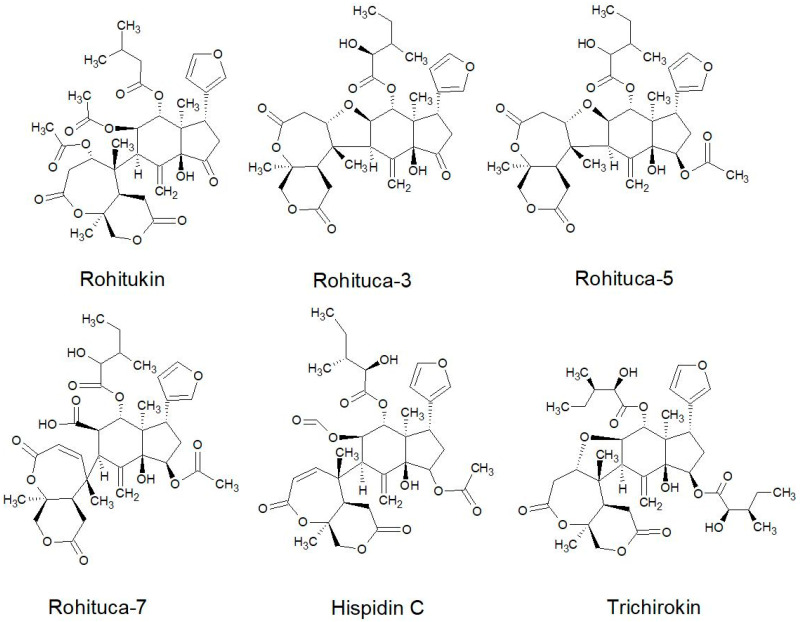
| Trichirokin | Stem | [24] | |
| Rohituka | Stem | [24,118] | |
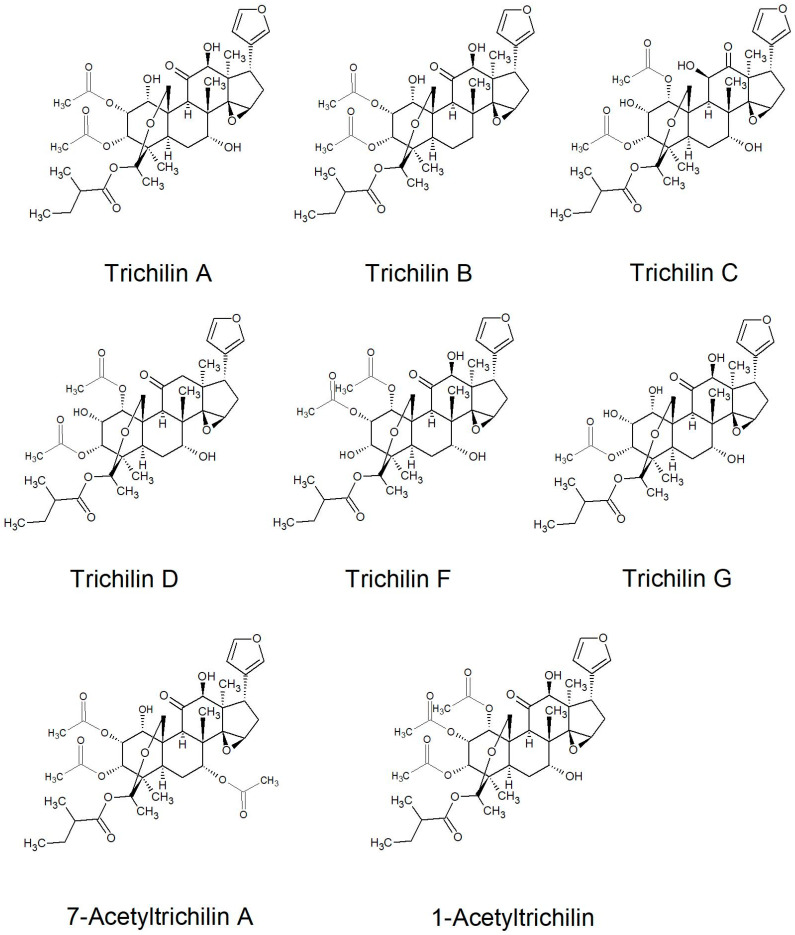
| Trichilin A; trichilin B; and 7-acetyltrichilin A | Stem | [137] | |
| Trichilin C; trichilin D; and trichilin G | Stem | [138] | |
| Trichilin F and trichilin G | Stem | [139] | |
| 1-acetyltrichilin; Tr-A; Tr-B; Tr-C | Stem | [140] | |
| Dregeana-4; rohituca-3; rohituca-5; rohituca-7; and Nymania-1 | Stem | [118] | |
| Sesquiterpenes | |||
| Kurubasch aldehyde | Leaf | [131] | |
| Triterpenes | |||
| Methyl-1(S),23(R)-diacetoxy-7(R),24,25-trihydroxy-20(S)-21,24-epoxy-3,4-seco-apotirucall-4(28), 14(15)-dien-3-oate | Stem | [118] | |
| Pregnane | |||
| 1-methoxy-pregnan-17(R)-1,4-dien-3,16-dione; 1-methoxy-pregnan-17(S)-1,4-dien-3,16-dione; 2,3-seco-pregnan-17(S)-2,3-dioic acid-16-oxo-dimethyl ester; 2,3,16-trihydroxy-5-pregnan-17(R)-20-yl acetate; 1-methoxy-androstan-1,4-dien-3,16-dione; 2,3-seco-androstan-2,3-dioic acid-16-oxo-dimethyl ester; 3-methoxycarbonyl-2,3-seco-androstan-3-oic acid-16-oxo-2,19-lactone; 2,3,16,20-tetrahydroxy-5-pregnane; 2,3-dihydroxypregnan-16-one | Root | [126] | |
| Phenolics | |||
| Benzoic acid; protocatechuic acid | Stem | [24] | |
| Coumarin | |||
| Scopoletin | Stem | [24] | |
| Sterols | |||
| Ergosta-5,24(28)-diene-3S,16S,20S-triol; β-sitosterol; stigmasterol; and β-sitosterol-3-O-β-D-glucopyranoside | Stem | [24] | |
| Turraea floribunda | Limonoids | ||
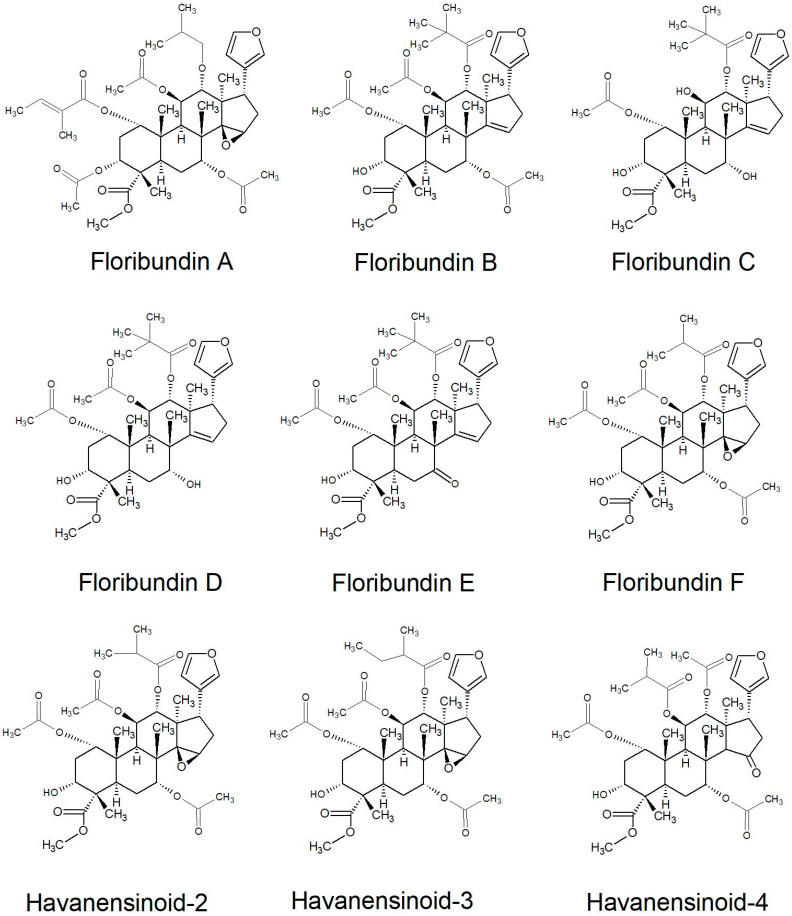
| Floribundin A (11β-acetoxy-3,7-diacetyl-4α-carbomethoxy- 12α-isobutyryloxy-28-nor-1-tigloyl-havanensin); |
Root | [141] | |
| Floribundin B (28-nor-4α-carbomethoxy-11β-acetoxy-12α-(2-methylbutanoyloxy)-14,15-deoxyhavanensin-1,7-diacetate); Floribundin C (28-nor-4α-carbomethoxy-11β-hydroxy-12α-(2-methylbutanoyloxy)-14,15-deoxyhavanensin-1,7-diacetate); Floribundin D (2218-nor-4α-carbomethoxy-11β-acetoxy-12α-(2-methylbutanoyloxy)-14,15-deoxyhavanensin-1-acetate); Floribundin E (28-nor-4α-carbomethoxy-7-deoxy-7-oxo-11β-acetoxy-12α-(2-methylbutanoyloxy)-14,15-deoxyhavanensin-1-acetate) | Root | [142] | |
| Havanensinoids 2-4 | Root | [143] | |
| Floribundin F (1α,7α-12α -triacetoxy-4α -carbomethoxy-11β-(2-methylpropanoyloxy)-14β,15β -epoxyhavanensin) | Bark | [121] | |
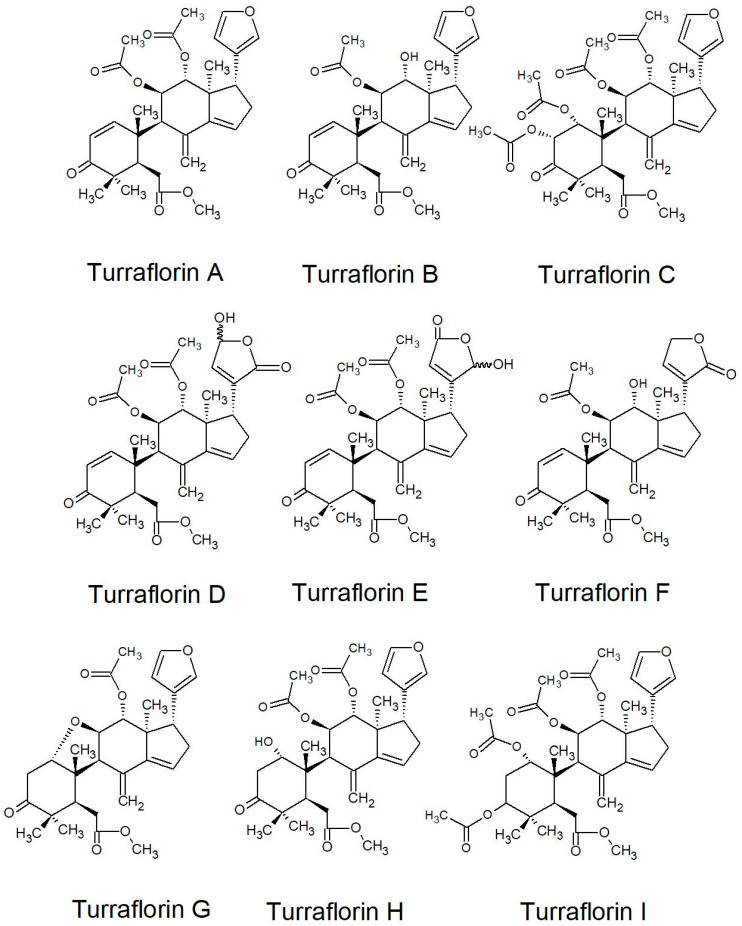
| Turraflorins A, B, and C | Seed | [144] | |
| Turraflorins A and B; turraflorins D-I | Seed | [112] | |
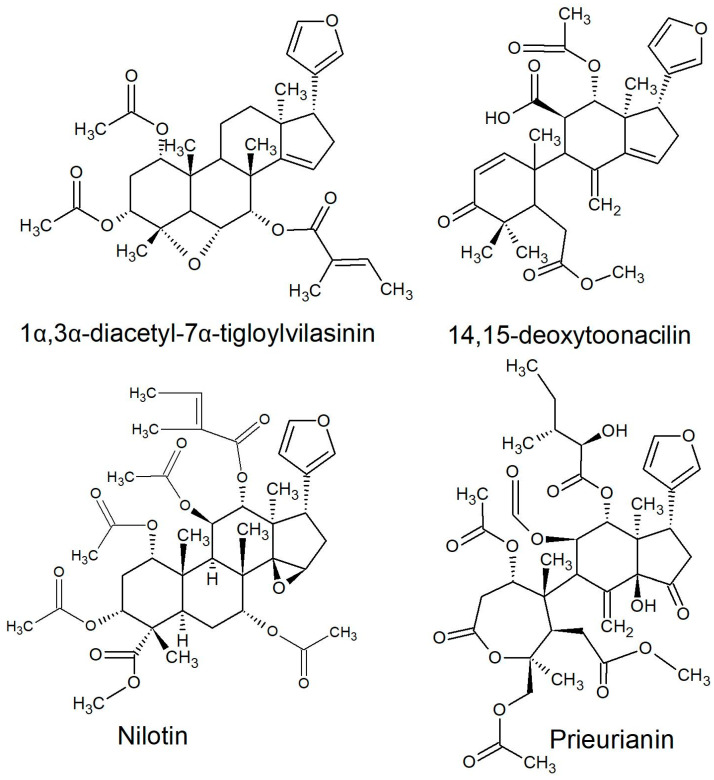
| 14,15-deoxytoonacilin | Seed | [145] | |
| Toonafolin A and B | Seed | [146] | |
| Sterols | |||
| Stigmasterol and sitosterol | Wood | [146] | |
| Turraea nilotica | Limonoids | ||
| Nilotin | Root | [119] | |
| Mzikonone; azadirone; 12α-acetoxy-7-deacetylazadirone; 1α,3α-diacety-7α-tigloyvilasinin | Root | [147] | |
| Protolimonoids | |||
| Niloticin; hispidol B; piscidinol A; toonapubesin F | Bark | [147] | |
| Niloticin; dihydroniloticin; and piscidinol | Bark | [120] | |
| Sterols | |||
| Sitosterol-3-O-β-D-glucopyranoside acetate; stigmasterol-3-O-βD-glucopyranoside acetate; and sitosterol-3-O-β-D-glucopyranoside | Leaf | [147] | |
| Turraea obtusifolia | Limonoids | ||
| Nymania-1 | Seed | [122,148] | |
| Prieurianin and rohitukin | Seed | [123] | |
| Prieurianin | Whole plant | [121] | |
| Protolimonoids | |||
| 7-deacetylglabretal-3-acetate | Wood | [149] | |
| Melianone; Turraeanthin | Seed | [148] | |
| Melianodiol; melianotriol; and 7,8-dihydroTurraeanthin 3-acetate | Wood | [146] | |
| Melianone; sepalin-F | Leaf | [146] |
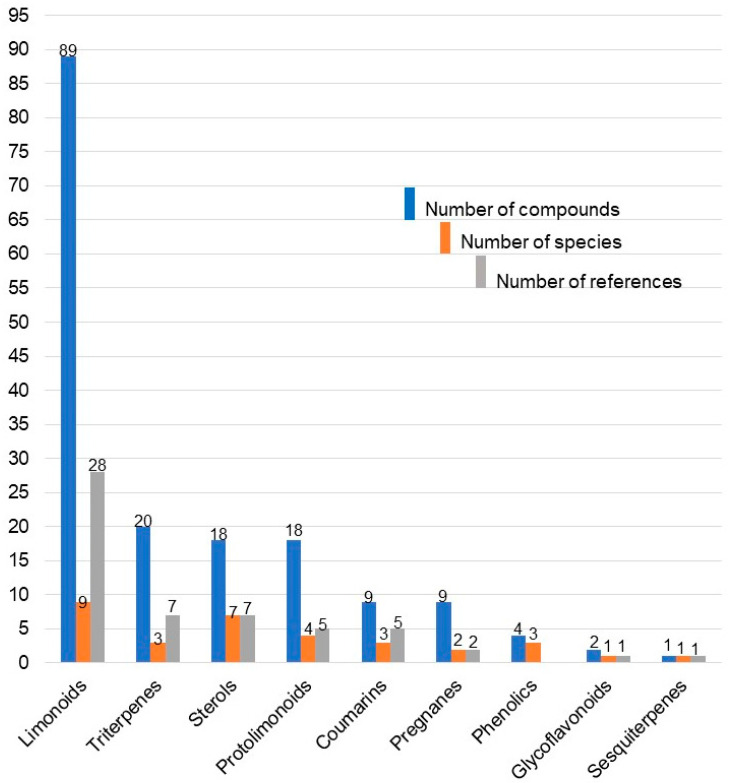
The compounds are classified according to nine categories of chemical class which are: (1) Limonoids, (2) triterpene, (3) coumarin, (4) glycoflavonoid (glycoside), (5) phenolic aglycone, (6) sterol (phytosterol), (7) pregnane, (8) protolimonoid, and (9) sesquiterpene. The highest reported chemical classes were limonoids followed by triterpenes and sterols. However, sterols are common in higher plants. On the other hand, a significant number of triterpenes, coumarins, and limonoids are described in South African species for the first time and their naming is etymologically related to the genus or species. Unsurprisingly, the richest diversity of new metabolites include a heterocyclic moiety in the form of a lactone, coumarin or furan.
Novel types of limonoid of the ekebergolactone class were first described from a species of Ekebergia and received the name capensolactones, which were isolated among others (Table 3) from the leaves, seeds, and stem bark of E. capensis [31,33,108,109]. From E. pterophylla, more new limonoids were described and given initials taken from the genus and species name (EP1-EP6), which are closely related to methyl angolensate and similar to those of other Ekebergia species [110,111]. Furthermore, complex limonoids referred to as Nymania 1-4 were isolated from the bark of N. capensis [112], reiterating the etymological genesis.
All of the species in the current study, except one, were rich sources of new limonoids, often described nowhere else in the world. While no limonoids were reported from P. mossambicensis, the nine other species investigated so far constitute valuable sources of these compounds [113,114,115]. The limonoids of E. caudatum were named phragmalins after the genus [116]. From seeds of T. dregeana, the limonoids include dregeana 1–4 [117]. Several limonoids from other South African Meliaceae were assigned in the stem bark of T. emetica, and previously undescribed compounds including trichirokin and trichilins A-G [24,118]. Then, in the genus Turraea several limonoids were also reported, such as turraflorins A–I and the floribundins A–F (names assigned in the current review, Table 3) from the root of T. floribunda, nilotin from the root and stem bark of T. nilotica [119,120] or prieurianins from seeds of T. obtusifolia [121,122,123].
The limonoid class, per se, was first isolated from citrus, typically from leaves, fruit, and peel of lemons, limes, oranges, pomelos, grapefruits, bergamots, and mandarins (Manners 2007; Hamdan 2011; Wang 2016). Citrus limonoids represent the traditional limonoid that is known as limonin or its derivatives, which occur in aglycone and glycoside forms [124]. They are distinctly different from the ekebergolactone class of limonoid (that is a pentaneotriterpenoid) represented strongly in South African Meliaceae. These include Nymania limonoids and capensolactones. Therefore, it is expected that a functional overlap is minimal in the context of biology.
A third category of limonoids, ekebergins 1–10 from E. capensis are triterpenes and have demonstrated anti-plasmodial activity in an in vivo mouse model, giving moderate parasitemia suppression [33]. However, the ekebergins have a structural feature that classifies them as nortriterpenes, but conveys a structure that is between the traditional limonoid and a triterpene. In this regard, some researchers classify them as limonoids, whilst others call them nortriterpenes [125].
The protolimonoids are also of triterpenoid origin but are classed as steroids. Protolimonoids were isolated and assigned as new compounds from the root and stem bark of T. nilotica and named according to the species as niloticin and dihydroniloticin [119,120]. Melianone type protolimonoids were isolated and identified from E. caudatum [113,114,115] and T. obtusifolia, which also had turraeanthin [121,122,123].
The species that expressed the highest steroid composition was T. emetica, which produced an extract comprising C21 steroids known as 17β-ethylandrostane derivatives or simply pregnanes. Nine pregnanes are known thus far from T. emetica [126] and one from E. capensis [33]. The pregnanes are agonists of the nuclear pregnane x receptor, which controls the elimination of toxins from the body by xenobiotic monooxygenase metabolism (cytochrome P450 3A) [127]. Activation can cause a variety of effects that include promotion of elimination of toxins that have similar structures. However, drug-drug interactions can also result, preventing co-administered therapies from being metabolised. The net outcome is an increase in drug half-life for some types of xenobiotics, which can be positive in terms of prolonging efficacy effects, but negative by augmenting the risk of toxicity [128].
South African Meliaceae also express several common triterpenes of the oleanane type and common sterols such as β-sitosterol. For example, the type, as well as common triterpenes β-amyrin, β-amyrone, oleanonic acid, lupeol, and other common sterols were reported from the wood and bark of E. pterophylla [108]. Ergosterols were isolated from twigs and leaves of P. mossambicensis [129], cycloarten-diol triterpenes from the leaves of T. dregeana [130], and sterols from the stem bark of T. emetica [24]. The common triterpene oleanolic acid and its derivatives from E. capensis demonstrated cytotoxicity against cancer cell lines and moderate antiplasmodial activity that may be related to the cytotoxic activity [31]. However, novel acyclic triterpene derivatives of cosatetraene or squalene are also reported in E. capensis. These hydroxylated structures are atypical in that they are tail-to-tail sesquiterpenes that demonstrate noteworthy antiplasmodial activity comparable to the ekebergin limonoid mentioned previously [33]. Some sesquiterpenes have also demonstrated these biological effects. Kurubasch aldehyde is another antiplasmodial terpene isolated from T. emetica, which is a hydroxylated humulene that is a potent inhibitor (IC50 7.4 μM) of the S180 cancer cell line and demonstrates a modest anti-protozoal effect [131].
South African Meliaceae are also a reservoir of rare coumarins. The pterophyllin 1-5 series was isolated from the wood and bark of E. pterophylla [132]. These chemical species belong to the group of furocoumarins. The pterophyllins are moderate antifungal compounds which are active against fruit pathogens [133]. Coumarins were also reported in E. capensis, one of which is ekersenin. This compound was first described in E. senegalensis but is now known to be widespread in African Meliaceae. Several derivatives of ekersenin were isolated from the stem bark, wood, and root of E. capensis [31,32,33,34,35]. While the cytotoxic effects against cancer cell lines have not yet been tested, it will be a worthy undertaking since a related limonoid demonstrated potent inhibition (IC50 6.8 μM) against the A2780 cell line [134], although the authors did not specify the mechanism nor did they screen against healthy cells, hence toxicity was not determined.
2.2.1. Class of Isolated Compounds and Parts Extracted
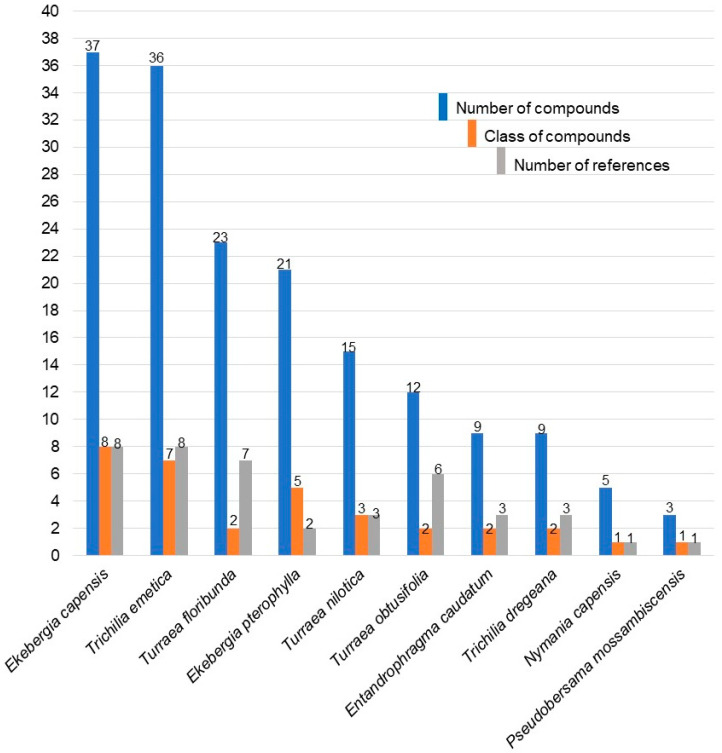
The isolated compounds are categorised into limonoids, triterpenes, sterols, protolimonoids, coumarins, pregnanes, phenolics, glycoflavonoids, and sesquiterpenes. The highest number of compounds reported in the studied species are limonoids (89 compounds) followed by triterpenes (20 compounds) (Figure 4). Limonoids were identified in all of the species except P. mossambicensis where only sterols have been reported until now (Table 3). Out of the various compounds isolated from South African Meliaceae, the highest number were from E. capensis (37) followed by T. emetica (36), T. floribunda (23), E. pterophylla (21), T. nilotica (15), T. obtusifolia (12), E. caudatum (9), T. dregeana (9), N. capensis (5), and P.a mossambicensis (3) (Figure 5). Most of the compounds were isolated from the bark (42%), followed by seed (17%), wood (16%), root (13%), leaf (11%), and twig (1 %) of the plants (Figure 3c).
Figure 4.
Number of compounds (chemical diversity), classes of compounds, and references for each species of South African Meliaceae.
Figure 5.
Number of compounds, classes of compounds and references for each species of South African Meliaceae.
2.2.2. Structures of Some of the Isolated Compounds
Most of the reported limonoids are summarised into series according to their structure (Table 4 and Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15 and Figure 16) or with common structural themes. Floribundin naming is used here for the first time.
Table 4.
Reported limonoids according to series.
| Series | Limonoids |
|---|---|
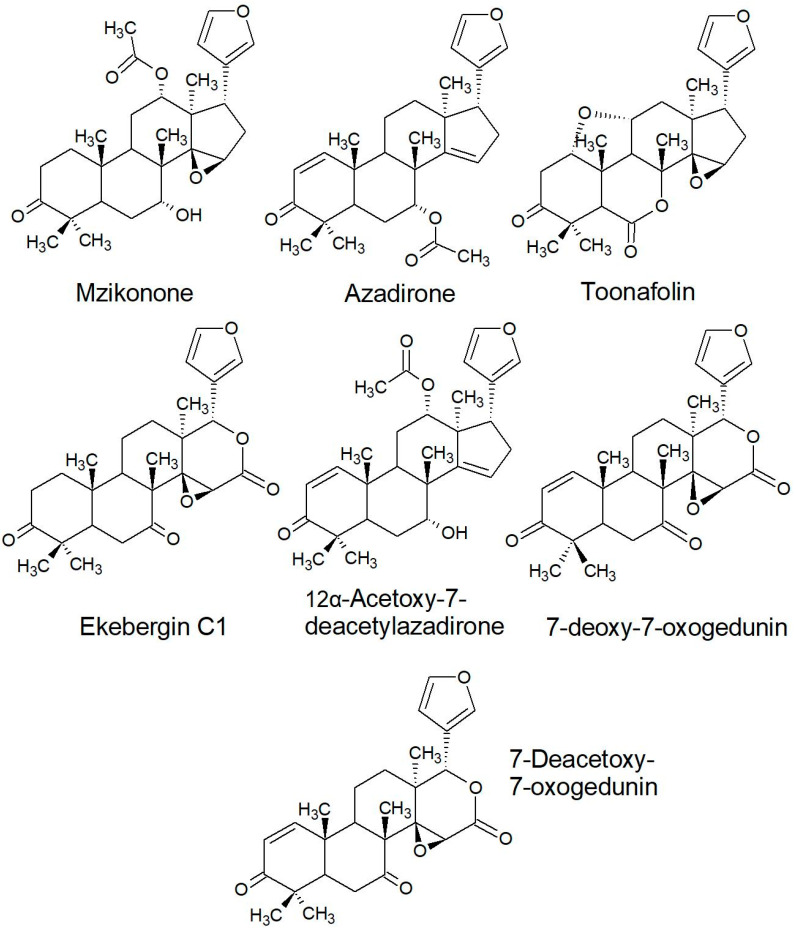
| Ketone and Azadirone | Mzikonone; Azadirone; Toonafolin; Ekebergin C1; 12α-Acetoxy-7-deacetylazadirone; 7-deoxy-7-oxogedunin; 7-deacetoxy-7-oxogedunin |
| Capensolactones and Nymania | Capensolactone 1-3; Nymania 1-4 |
| Dregeanin | Dregeanin; Dregeana 1-5; 12-(2′-deacetyl)-dregeanin |
| EP * | EP1-6; Ekebergin; Methyl,3-hydroxy,3-deoxy-angolensate; Methlyangolensate |
| Floribundin ** and Havanen | Floribundin A-F; Havanensinoid 2-4 |
| Orphan | 1α,3α-diacetyl-7α-tigloylvilasinin; 14,15-deoxytoonacilin; Nilotin; Prieurianin |
| Phragmalin and Phragmin | Entandrophragmin B; Phragmalin 3-isobutyrate-30-propionate; Entandrophragmin; Phragmalin 3,30-diisobutyrate; Phgramalin; Bussein A and B |
| Rohituka | Rohituca 3, 5 and 7; Hispidin C; Trichirokin |
| Swietenolide/Ekebergin | Swietenolide; Proceranolide; Ekebergin C2-C3; Mexicanolide |
| Trichilin | Trichilin A-G; 7-Acetyltrichilin A; 1-Acetyltrichilin |
| Turraflorin | Turraflorin A-I |
* EP is an abbreviation of Ekebergia pterophylla (Taylor and Taylor 1984; Kehrli et al., 1990; Mulholland et al., 1998; Murata et al., 2008) ** Floribundin A–F was named by the authors the since the authors did not provide a shorter name.
Figure 6.
Ketone series of limonoids reported from South African Meliaceae [33,146,147].
Figure 7.
Capensolactones and Nymania series of limonoids reported from South African Meliaceae [108,112].
Figure 8.
Dregeanin series of limonoids reported from South African Meliaceae [117,136].
Figure 9.
EP series of limonoids reported from South African Meliaceae [33,108,110,111].
Figure 10.
Floribundin and Havanen series of limonoids reported from South African Meliaceae [121,141,142,143].
Figure 11.
Orphan series of limonoids reported from South African Meliaceae [108,119,121,147].
Figure 12.
Phragmalin, phragmin, and bussein series of limonoids reported from South African Meliaceae [113,116].
Figure 13.
Rohituka series of limonoids reported from South African Meliaceae [24,117,118].
Figure 14.
Swietenolide and Ekebergin series of limonoids reported from South African Meliaceae [31,33].
Figure 15.
Trichilin series of limonoids reported from South African Meliaceae [137,138,139].
Figure 16.
Turraflorin series of limonoids reported from South African Meliaceae [112,144].
3. Materials and Methods
The recorded ethnobotanical uses and isolated compounds were based on a search of scopus and science-direct electronic databases, pubMed, reference libraries, conference papers, ethnobotanical books, dissertations, theses, and scientific articles. All of the relevant papers were included in this study except those that were not peer reviewed and those containing species that are not indigenous to South Africa. The ethnomedicinal uses are classified into 17 major categories, based on Moffett’s [150] classification, while the compounds were categorised into nine main chemical classes and the structures drawn using ACD/ChemSketch Freeware (Windows platform).
4. Conclusions
The species of South African Meliaceae have been reportedly used for a diversity of purposes from medicinal (including human and animal), to rituals, to functional uses (making of implements, furniture, oils, and dyes). A total of 85 different medicinal uses were recorded, and T. emetica is the most frequently used, followed by E. capensis. Several compounds have been isolated from South African Meliaceae. A total of 188 compounds belonging to nine classes were recorded from various plant parts. The highest number of compounds belonged to the limonoids class, followed by sterols. There was no record found for the chemistry of T. streyi and T. pulchella.
The high chemical diversity of these species may be related to the high diversity of therapeutic uses recorded. South African Meliaceae are mostly used in the treatment of gastro-intestinal ailments followed by gynaecological and obstetrics related ailments. The most common mode of herbal preparation was decoction followed by infusion. The roots followed by bark are mostly commonly used in the preparation of the remedies, whereas most of the compound isolation work focused on the bark followed by seeds – this may be a consequence of logistical difficulty in obtaining roots for chemical study.
The ethnomedicinal uses recorded in this study are of value for bioprospectors or synthetic chemists looking for chemical scaffolds as precursors to biologically enhanced derivatives. However, the current review also strengthens the call for increased conservation practice, which is due to the fact that root and bark harvesting are destructive. Hence, it may be important to examine the chemistry of plant parts such as leaves and fruits.
Author Contributions
Conceptualization, B.-E.V.W., N.J.S., and M.O.O.-A.; writing—original draft preparation, M.O.O.-A.; writing—review and editing, M.O.O.-A., N.J.S., and B.-E.V.W.; funding acquisition, B.-E.V.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of South Africa, grant number 84442 to B-EvW and MOA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Soejarto D.D., Fong H.H.S., Tan G.T., Zhang H.J., Ma C.Y., Franzblau S.G., Gyllenhaal C., Riley M.C., Kadushin M.R., Pezzuto J.M. Ethnobotany/ethnopharmacology and mass bioprospecting: Issues on intellectual property and benefit-sharing. J. Ethnopharmacol. 2005;100:15–22. doi: 10.1016/j.jep.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Grace O.M., Prendergast H.D.V., Jäger A.K., Van Staden J., Van Wyk A.E. Bark medicines used in traditional healthcare in KwaZulu-Natal, South Africa: An inventory. S. Afr. J. Bot. 2003;69:301–363. doi: 10.1016/S0254-6299(15)30318-5. [DOI] [Google Scholar]
- 3.Williams V.L., Balkwill K., Witkowski E.T.F. Unraveling the commercial market for medicinal plants and plant parts on the Witwatersrand. S. Afr. J. Bot. 2000;54:310–327. doi: 10.1007/BF02864784. [DOI] [Google Scholar]
- 4.Cunningham A.B. Review of Ethnobotanical Literature from Eastern and Southern Africa. [(accessed on 23 August 2020)]. Available online: http://www.rbgkew.org.uk/peopleplants/regions/africa/aen1/review.htm.
- 5.Teklehaymanot T., Giday M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J. Ethnobiol. Ethnomed. 2007;3:12. doi: 10.1186/1746-4269-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Wyk B.-E., Oudtshoorn B., Gericke N. Medicinal Plants of South Africa. 2nd ed. Briza Publications; Pretoria, South Africa: 2009. [Google Scholar]
- 7.Street R.A., Stirk W.A., Van Staden J. South African traditional medicinal plant trade-challenges in regulating quality, safety and efficacy. J. Ethnopharmacol. 2008;119:705–710. doi: 10.1016/j.jep.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Van Wyk B.-E., Gericke N. People’s Plants: A Guide to Useful Plants of Southern Africa. Briza Publications; Pretoria, South Africa: 2018. [Google Scholar]
- 9.Chinyama R.F. Master’s Thesis. Nelson Mandella Metropolitan University; Port Elizabeth, South Africa: 2009. Biological Activities of Medicinal Plants Traditionally Used to Treat Septicaemia in the Eastern Cape, South Africa. Unpublished. [Google Scholar]
- 10.Cieśla Ł., Waksmundzka-Hajnos M. Two-dimensional thin-layer chromatography in the analysis of secondary plant metabolites. J. Chromatogr. A. 2009;1216:1035–1052. doi: 10.1016/j.chroma.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 11.Pappe K.W.L. Florae Capensis Medicae Prodromus, Or, an Enumeration of South. African Plants Used as Remedies by the co.lonists of the Cape of Good Hope. W. Brittain; Cape Town, South Africa: 1868. [Google Scholar]
- 12.Watt J.M., Breyer-Brandwijk M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa Being an Account. of Their Medicinal and Other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal. E. & S. Livingstone; Ann Arbor, MI, USA: 1962. [Google Scholar]
- 13.Hutchings A., Scott A.H., Lewis G., Cunningham A. Zulu Medicinal Plants: An. Inventory. University Natal Press; Pietermaritzburg, South Africa: 1996. [Google Scholar]
- 14.Mhlongo L.S., Van Wyk B.-E. Zulu medicinal ethnobotany: New records from the Amandawe area of KwaZulu-Natal, South Africa. S. Afr. J. Bot. 2019;122:266–290. doi: 10.1016/j.sajb.2019.02.012. [DOI] [Google Scholar]
- 15.Taylor D.A.H. Recent developments in the biomimetic synthesis of limonoids. In: Barton D., Ollis W.D., editors. Advances in Medicinal Phytochemistry. John Libbey and Co Ltd.; Blissfield, MI, USA: 1986. pp. 179–186. [Google Scholar]
- 16.Paritala V., Chiruvella K.K., Thammineni C., Ghanta R.G., Mohammed A. Phytochemicals and antimicrobial potentials of mahogany family. Rev. Bras. Farmacogn. 2015;25:61–83. doi: 10.1016/j.bjp.2014.11.009. [DOI] [Google Scholar]
- 17.Champagne D.E., Koul O., Isman M.B., Scudder G.G.E., Towers G.H.N. Biological activity of limonoids from the Rutales. Phytochemistry. 1992;31:377–394. doi: 10.1016/0031-9422(92)90003-9. [DOI] [Google Scholar]
- 18.Abdelgaleil S.A.M., Nakatani M. Antifeeding activity of limonoids from Khaya senegalensis (Meliaceae) J. Appl. Entomol. 2003;127:236–239. doi: 10.1046/j.1439-0418.2003.00742.x. [DOI] [Google Scholar]
- 19.Greger H., Pacher T., Brem B., Bacher M., Hofer O. Insecticidal flavaglines and other compounds from Fijian Aglaia species. Phytochemistry. 2001;57:57–64. doi: 10.1016/S0031-9422(00)00471-4. [DOI] [PubMed] [Google Scholar]
- 20.Simmonds M.S.J., Stevenson P.C., Porter E.A., Veitch N.C. Insect Antifeedant Activity of Three New Tetranortriterpenoids from Trichilia Pallida. J. Nat. Prod. 2001;64:1117–1120. doi: 10.1021/np010197o. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.-E., Kim M.-R., Kim J.-H., Takeoka G.R., Kim T.-W., Park B.-S. Antimalarial activity of anthothecol derived from Khaya anthotheca (Meliaceae) Phytomedicine. 2008;15:533–535. doi: 10.1016/j.phymed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Maneerat W., Laphookhieo S., Koysomboon S., Chantrapromma K. Antimalarial, antimycobacterial and cytotoxic limonoids from Chisocheton siamensis. Phytomedicine. 2008;15:1130–1134. doi: 10.1016/j.phymed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Mulholland D.A., Parel B., Coombes P.H. The chemistry of the Meliaceae and Ptaeroxylaceae of southern and eastern Africa and Madagascar. Currr. Org. Chem. 2000;4:1011–1054. doi: 10.2174/1385272003375941. [DOI] [Google Scholar]
- 24.Tsopgni W.D.T., Happi G.M., Stammler H.-G., Neumann B., Mbobda A.S.W., Kouam S.F., Frese M., Azébazé A.G.B., Lenta B.N., Sewald N. Chemical constituents from the bark of the Cameroonian mahogany Trichilia emetica Vahl (Meliaceae) Phytochem. Lett. 2019;33:49–54. doi: 10.1016/j.phytol.2019.07.009. [DOI] [Google Scholar]
- 25.Wang X.N., Yin S., Fan C.Q., Lin L.P., Ding J., Yue J.M. Eight new limonoids from Turraea Pubescens. Tetrahedron. 2007;63:8234–8241. doi: 10.1016/j.tet.2007.05.107. [DOI] [Google Scholar]
- 26.Tan Q.-G., Luo X.-D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011;111:7437–7522. doi: 10.1021/cr9004023. [DOI] [PubMed] [Google Scholar]
- 27.De Wet H., Ngubane S.C. Traditional herbal remedies used by women in a rural community in northern Maputaland (South Africa) for the treatment of gynaecology and obstetric complaints. S. Afr. J. Bot. 2014;94:129–139. doi: 10.1016/j.sajb.2014.06.009. [DOI] [Google Scholar]
- 28.Diarra N., van’t Klooster C., Togola A., Diallo D., Willcox M., de Jong J. Ethnobotanical study of plants used against malaria in Sélingué subdistrict, Mali. J. Ethnopharmacol. 2015;166:352–360. doi: 10.1016/j.jep.2015.02.054. [DOI] [PubMed] [Google Scholar]
- 29.Mabona U., Van Vuuren S.F. Southern African medicinal plants used to treat skin diseases. S. Afr. J. Bot. 2013;87:175–193. doi: 10.1016/j.sajb.2013.04.002. [DOI] [Google Scholar]
- 30.Neuwinger H.D. African Traditional Medicine: A Dictionary of Plant Use and Applications. With Supplement: SEARCH system for Diseases. Medpharm; Stuttgart, Germany: 2000. [Google Scholar]
- 31.Irungu B.N., Orwa J.A., Gruhonjic A., Fitzpatrick P.A., Landberg G., Kimani F., Midiwo J., Erdélyi M., Yenesew A. Constituents of the roots and leaves of Ekebergia capensis and their potential antiplasmodial and cytotoxic activities. Molecules. 2014;19:14235–14246. doi: 10.3390/molecules190914235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulholland D.A., Mahomed H.A., Lourine S. A comparison of extractives from the bark of Ekebergia capensis and Ekebergia senegalensis. S. Afr. J. Bot. 1997;5:259–260. doi: 10.1016/S0254-6299(15)30763-8. [DOI] [Google Scholar]
- 33.Murata T., Miyase T., Muregi F.W., Naoshima-Ishibashi Y., Umehara K., Warashina T., Kanou S., Mkoji G.M., Terada M., Ishih A. Antiplasmodial triterpenoids from Ekebergia capensis. J. Nat. Prod. 2008;71:167–174. doi: 10.1021/np0780093. [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama Y., Moriyasu M., Ichimaru M., Tachibana Y., Kato A., Mathenge S.G., Nganga J.N., Juma F.D. Acyclic triterpenoids from Ekebergia capensis. Phytochemistry. 1996;42:803–807. doi: 10.1016/0031-9422(96)00004-0. [DOI] [Google Scholar]
- 35.Sewram V., Raynor M.W., Mulholland D.A., Raidoo D.M. The uterotonic activity of compounds isolated from the supercritical fluid extract of Ekebergia capensis. J. Pharm. Biomed. Anal. 2000;24:133–145. doi: 10.1016/S0731-7085(00)00404-0. [DOI] [PubMed] [Google Scholar]
- 36.Oyedeji-Amusa M.O., Van Vuuren S., Van Wyk B.E. Antimicrobial activity and toxicity of extracts from the bark and leaves of South African indigenous Meliaceae against selected pathogens. S. Afr. J. Bot. 2020;133:83–90. doi: 10.1016/j.sajb.2020.06.032. [DOI] [Google Scholar]
- 37.Kokwaro J.O. Medicinal Plants of East Africa. University of Nairobi Press; Nairobi, Kenya: 2009. [Google Scholar]
- 38.Komane B.M., Olivier E.I., Viljoen A.M. Trichilia emetica (Meliaceae)—A review of traditional uses, biological activities and phytochemistry. Phytochem. Lett. 2011;4:1–9. doi: 10.1016/j.phytol.2010.11.002. [DOI] [Google Scholar]
- 39.Rabe T., Van Staden J. Antibacterial activity of South African plants used for medicinal purposes. J. Ethnopharmacol. 1997;56:81–87. doi: 10.1016/S0378-8741(96)01515-2. [DOI] [PubMed] [Google Scholar]
- 40.Lovett J.C., Ruffo C.K., Gereau R.E., Taplin J.R.D. Field Guide to the Moist Forest Trees of Tanzania. Society for Environmental Exploration; London, UK: 2006. [Google Scholar]
- 41.Batten A., Bokelmann H. Wild Flowers of the Eastern Cape Province. Books Of Africa; London, UK: 1966. [Google Scholar]
- 42.Kerharo J., Bouquet A. Plantes Médicinales Et Toxiques De La Côte d’Ivoire-Haute-Volta: Mission D’étude De La Pharmacopée Indigène En A.O.F. Vigot; Paris, France: 1950. [Google Scholar]
- 43.Mabogo D.E.N. Ph.D. Thesis. Pretoria University; Pretoria, South Africa: 1990. The Ethnobotany of the Vhavenda. [Google Scholar]
- 44.Opio D.R., Andama E., Kureh G.T. Ethnobotanical survey of antimalarial plants in areas of: Abukamola, Angeta, Oculokori and Omarari of Alebtong district in Northern Uganda. Eur. J. Med. Plants. 2017;21:1–14. doi: 10.9734/EJMP/2017/38043. [DOI] [Google Scholar]
- 45.Koch A., Tamez P., Pezzuto J., Soejarto D. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J. Ethnopharmacol. 2005;101:95–99. doi: 10.1016/j.jep.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Kremnitz W.A., Knies M., Kremnitz M. Kalahari: Aus Dem Pflanzenreich: Floristische Und Ethnobotanische Betrachtungen. Ambro Lacus; München, Germany: 1988. [Google Scholar]
- 47.Duncan A.C., Jäger A.K., van Staden J. Screening of Zulu medicinal plants for angiotensin converting enzyme (ACE) inhibitors. J. Ethnopharmacol. 1999;68:63–70. doi: 10.1016/S0378-8741(99)00097-5. [DOI] [PubMed] [Google Scholar]
- 48.Tefera B.N., Kim Y.-D. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone, Southern Ethiopia. J. Ethnobiol. Ethnomed. 2019;15:25. doi: 10.1186/s13002-019-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeruto P., Too E., Mwamburi L.A., Amuka O. An inventory of medicinal plants used to treat gynaecological obstetric-urino-genital disorders in South Nandi Sub County in Kenya. J. Nat. Sci. Res. 2015;5:136–152. [Google Scholar]
- 50.Tuasha N., Petros B., Asfaw Z. Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone, Ethiopia. J. Ethnobiol. Ethnomed. 2018;14:1–21. doi: 10.1186/s13002-018-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pujol J. NaturAfrica: The Herbalist Handbook: African Flora Medicinal Plants. Natural Healers Foundation. Thorolds Contemporary Africana, Law & General Booksellers (Frank R. Thorold Pty Ltd.); Sandton, South Africa: 1990. [Google Scholar]
- 52.Van Wyk A.E., Van den Berg E., Coates Palgrave M., Jordaan M. Dictionary of Names for Southern African Trees. Scientific Names of Indigenous Trees, Shrubs and Climbers with Common Names from 30 Languages. Briza Publications; Pretoria, South Africa: 2011. [Google Scholar]
- 53.Arnold H.J., Gulumian M. Pharmacopoeia of traditional medicine in Venda. J. Ethnopharmacol. 1984;12:35–74. doi: 10.1016/0378-8741(84)90086-2. [DOI] [PubMed] [Google Scholar]
- 54.Bryant A.T. Zulu Medicine and Medicine-Men. C. Struik Publishers; Cape Town, South Africa: 1966. [Google Scholar]
- 55.Pooley E. Complete Field Guide to Trees of Natal, Zululand & Transkei. Natal Flora Publications Trust; Durban, South Africa: 1993. [Google Scholar]
- 56.Adjanohoun É.J. Contribution Aux Études Ethnobotaniques et Floristiques en République Populaire du Bénin. Agence de Coopération Culturelle et Technique; Paris, France: 1989. [Google Scholar]
- 57.Irvine F.R. Woody Plants of Ghana. Oxford University Press; Ann Arbor, MI, USA: 1961. [Google Scholar]
- 58.Kewessa G., Abebe T., Demessie A. Indigenous knowledge on the use and management of medicinal trees and shrubs in Dale District, Sidama Zone, Southern Ethiopia. Ethnobot. Res. Appl. 2015;14:171–182. doi: 10.17348/era.14.0.171-182. [DOI] [Google Scholar]
- 59.Njoroge G.N., Bussmann R.W. Herbal usage and informant consensus in ethnoveterinary management of cattle diseases among the Kikuyus (Central Kenya) J. Ethnopharmacol. 2006;108:332–339. doi: 10.1016/j.jep.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 60.Gerstner J. A preliminary check list of Zulu names of plants: With short notes. Bantu Stud. 1941;15:277–301. doi: 10.1080/02561751.1941.9676141. [DOI] [Google Scholar]
- 61.Prozesky E.A., Meyer J.J.M., Louw A.I. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected South African plants. J. Ethnopharmacol. 2001;76:239–245. doi: 10.1016/S0378-8741(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 62.Chinsembu K.C. Ethnobotanical study of medicinal flora utilised by traditional healers in the management of sexually transmitted infections in Sesheke District, Western Province, Zambia. Rev. Bras. Farmacogn. 2016;26:268–274. doi: 10.1016/j.bjp.2015.07.030. [DOI] [Google Scholar]
- 63.Archer F.M. Ph.D. Thesis. University of Cape Town; Cape Town, South Africa: 1994. Ethnobotany of Namaqualand: The Richtersveld. [Google Scholar]
- 64.von Koenen E. Medicinal, Poisonous, and Edible Plants in Namibia. Klaus Hess Publishers (Klaus Heß Verlag); Göttingen, Germany: 2001. [Google Scholar]
- 65.Maroyi A. Trichilia dregeana Sond. In: Van der Vossen H.A.M., Mkamilo G.S., editors. PROTA 14: Vegetable Oils/Oléagineux (CD-Rom) PROTA; Wageningen, The Netherlands: 2007. [Google Scholar]
- 66.Desta B. Ethiopian traditional herbal drugs. Part II: Antimicrobial activity of 63 medicinal plants. J. Ethnopharmacol. 1993;39:129–139. doi: 10.1016/0378-8741(93)90028-4. [DOI] [PubMed] [Google Scholar]
- 67.Gelfland M., Mavi S., Drummond R.B., Ndemera B. The Traditional Medical Practitioner in Zimbabwe: His Principles of Practice and Pharmacopoeia. Mambo Press; Gweru, Zimbabwe: 1985. [Google Scholar]
- 68.Amusan O.O.G., Dlamini P.S., Msonthi J.D., Makhubu L.P. Some herbal remedies from Manzini region of Swaziland. J. Ethnopharmacol. 2002;79:109–112. doi: 10.1016/S0378-8741(01)00381-6. [DOI] [PubMed] [Google Scholar]
- 69.Burkill H.M. The Useful Plants of West. Tropical Africa (New Edition of Dalziel) Royal Botanic Gardens, Kew; Richmond, GB, USA: 1997. [Google Scholar]
- 70.Assi L.A., Lazare A.A. Plants Used in Traditional Medicine in West Africa. Editions Frison Roche; Paris, France: 1991. [Google Scholar]
- 71.Mashungwa G.N., Mmolotsi R.M. Trichilia emetica Vahl. In: van der Vossen H.A.M., Mkamilo G.S., editors. Plant Resources of Tropical Africa/Ressources Végétales De l’Afrique Tropicale. PROTA; Wageningen, The Netherlands: 2007. [(accessed on 15 August 2020)]. Available online: http://www.prota4u.org/search.asp. [Google Scholar]
- 72.Iwu M.M. Handbook of African Medicinal Plants. CRC Press; New York, NY, USA: 2014. [Google Scholar]
- 73.Adeniji K.O., Amusan O.O.G., Dlamini P.S., Enow-Orock E.G., Gamedze S.T., Gbile Z.O., Langa A.D., Makhubu L.P., Mahunnah R.L.A., Mshana R.N. Traditional Medicine and Pharmacopoeia Contribution to Ethnobotanical and Floristic Studies in Swaziland. Science and Technology Research and Communications Organisation; Eswatini, Swaziland: 2000. [Google Scholar]
- 74.Germano M.P., D’angelo V., Sanogo R., Catania S., Alma R., De Pasquale R., Bisignano G. Hepatoprotective and antibacterial effects of extracts from Trichilia emetica Vahl.(Meliaceae) J. Ethnopharmacol. 2005;96:227–232. doi: 10.1016/j.jep.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 75.Boon R., Pooley E. Pooley’s Trees of Eastern South Africa: A Complete Guide. Flora and Fauna Publications Trust; Cambridge, MA, USA: 2010. [Google Scholar]
- 76.Schmidt E., Lotter M., McCleland W. Trees and Shrubs of Mpumalanga and Kruger National Park. Jacana Media; Johannesburg, South Africa: 2002. [Google Scholar]
- 77.Weiss E.A. Some indigenous plants used domestically by East African coastal fishermen. Econ. Bot. 1979;33:35–51. doi: 10.1007/BF02858210. [DOI] [Google Scholar]
- 78.Corrigan B.M., Van Wyk B.-E., Geldenhuys C.J., Jardine J.M. Ethnobotanical plant uses in the KwaNibela Peninsula, St Lucia, South Africa. S. Afr. J. Bot. 2011;77:346–359. doi: 10.1016/j.sajb.2010.09.017. [DOI] [Google Scholar]
- 79.Sanogo R. Medicinal plants traditionally used in Mali for dysmenorrhea. Afr. J. Tradit. Complement. Altern. Med. 2011;8:90–96. doi: 10.4314/ajtcam.v8i5S.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oliver B. Nigeria’s useful plants. Part 11. Medicinal plants. Niger. Field. 1960;25:46–48. [Google Scholar]
- 81.Chhabra S.C., Mahunnah R.L.A., Mshiu E.N. Plants used in traditional medicine in Eastern Tanzania. VI. Angiosperms (Sapotaceae to Zingiberaceae) J. Ethnopharmacol. 1993;39:83–103. doi: 10.1016/0378-8741(93)90024-Y. [DOI] [PubMed] [Google Scholar]
- 82.Palmer E., Pitman N. Trees of Southern Africa: Covering All Known Indigenous Species in the Republic of South Africa, South-West. Africa, Botswana, Lesotho & Swaziland. Volumes 1–2 A. A. Balkema; Ann Arbor, MI, USA: 1972. [Google Scholar]
- 83.Bhat R.B., Jacobs T.V. Traditional herbal medicine in Transkei. J. Ethnopharmacol. 1995;48:7–12. doi: 10.1016/0378-8741(95)01276-J. [DOI] [PubMed] [Google Scholar]
- 84.Mathias M.E. Some medicinal plants of the Hehe (Southern highlands province, Tanzania) Taxon. 1982;31:488–494. doi: 10.2307/1220678. [DOI] [Google Scholar]
- 85.Chinemana F., Drummond R.B., Mavi S., De Zoysa I. Indigenous plant remedies in Zimbabwe. J. Ethnopharmacol. 1985;14:159–172. doi: 10.1016/0378-8741(85)90084-4. [DOI] [PubMed] [Google Scholar]
- 86.Madikizela B., Ndhlala A.R., Finnie J.F., Van Staden J. Ethnopharmacological study of plants from Pondoland used against diarrhoea. J. Ethnopharmacol. 2012;141:61–71. doi: 10.1016/j.jep.2012.01.053. [DOI] [PubMed] [Google Scholar]
- 87.Luseba D., Tshisikhawe M.P. Medicinal plants used in the treatment of livestock diseases in Vhembe region, Limpopo province, South Africa. J. Med. Plants Res. 2013;7:593–601. [Google Scholar]
- 88.Bussmann R.W., Sharon D. Traditional medicinal plant use in Northern Peru: Tracking two thousand years of healing culture. J. Ethnobiol. Ethnomed. 2006;2:47. doi: 10.1186/1746-4269-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arnold T.H., Prentice C.A., Hawker L.C., Snyman E.E., Tomalin M., Crouch N.R., Pottas-Bircher C. Medicinal and Magical Plants of Southern Africa: An Annotated Checklist. Volume 13 South African National Biodiversity Institute (SANBI Publishing); Pretoria, South Africa: 2002. [Google Scholar]
- 90.Williams V.L., Balkwill K., Witkowski E.T.F. A lexicon of plants traded in the Witwatersrand umuthi shops, South Africa. Bothalia. 2001;31:71–98. doi: 10.4102/abc.v31i1.508. [DOI] [Google Scholar]
- 91.Kunwar R.M., Nepal B.K., Kshhetri H.B., Rai S.K., Bussmann R.W. Ethnomedicine in Himalaya: A case study from Dolpa, Humla, Jumla and Mustang districts of Nepal. J. Ethnobiol. Ethnomed. 2006;2:27. doi: 10.1186/1746-4269-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Semenya S.S., Potgieter M.J. Ethnobotanical survey of medicinal plants used by Bapedi traditional healers to treat erectile dysfunction in the Limpopo Province, South Africa. J. Med. Plants Res. 2013;7:349–357. [Google Scholar]
- 93.Yineger H., Kelbessa E., Bekele T., Lulekal E. Ethnoveterinary medicinal plants at Bale Mountains National Park, Ethiopia. J. Ethnopharmacol. 2007;112:55–70. doi: 10.1016/j.jep.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 94.Moore P.D. Trials in bad taste. Nature. 1994;372:410–411. doi: 10.1038/372410a0. [DOI] [Google Scholar]
- 95.Sheldon J.W., Balick M.J., Laird S.A., Milne G.M. Medicinal plants: Can utilization and conservation coexist? Adv. Econ. Bot. 1997;12:1–104. [Google Scholar]
- 96.Ahmad M., Sultana S., Fazl-i-Hadi S., Ben Hadda T., Rashid S., Zafar M., Khan M.A., Khan M.P.Z., Yaseen G. An Ethnobotanical study of Medicinal Plants in high mountainous region of Chail valley (District Swat-Pakistan) J. Ethnobiol. Ethnomed. 2014;10:36. doi: 10.1186/1746-4269-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Farooq A., Amjad M.S., Ahmad K., Altaf M., Umair M., Abbasi A.M. Ethnomedicinal knowledge of the rural communities of Dhirkot, Azad Jammu and Kashmir, Pakistan. J. Ethnobiol. Ethnomed. 2019;15:45. doi: 10.1186/s13002-019-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ojha S.N., Tiwari D., Anand A., Sundriyal R.C. Ethnomedicinal knowledge of a marginal hill community of Central Himalaya: Diversity, usage pattern, and conservation concerns. J. Ethnobiol. Ethnomed. 2020;16:29. doi: 10.1186/s13002-020-00381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tugume P., Kakudidi E.K., Buyinza M., Namaalwa J., Kamatenesi M., Mucunguzi P., Kalema J. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central Forest Reserve, Uganda. J. Ethnobiol. Ethnomed. 2016;12:5. doi: 10.1186/s13002-015-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Umair M., Altaf M., Abbasi A.M. An ethnobotanical survey of indigenous medicinal plants in Hafizabad district, Punjab-Pakistan. PLoS ONE. 2017;12:e0177912. doi: 10.1371/journal.pone.0177912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Magwede K., Van Wyk B.-E., Van Wyk A.E. An inventory of Vhavenda useful plants. S. Afr. J. Bot. 2019;122:57–89. doi: 10.1016/j.sajb.2017.12.013. [DOI] [Google Scholar]
- 102.Lemmens R.H.M.J. Dalbergia trichocarpa Baker. In: van der Vossen H.A.M., Mkamilo G.S., editors. Plant Resources of Tropical Africa/Ressources Végétales De l’Afrique Tropicale. PROTA; Wageningen, The Netherlands: 2008. [(accessed on 15 August 2020)]. Available online: http://www.prota4u.org/search.asp:PROTA. [Google Scholar]
- 103.Von Breitenbach F.W. The Indigenous Trees of Southern Africa. Volume 4 Government Printer; Pretoria, South Africa: 1965. [Google Scholar]
- 104.Jamieson J.S. Examination of the bark and seed oil of Trichilia emetica. S. Afr. J. Sci. 1916;53:496–498. [Google Scholar]
- 105.Coates Palgrave K. Trees of Southern Africa. Struik Publishers; Cape Town, South Africa: 2002. [Google Scholar]
- 106.Saka J.D.K., Msonthi J.D. Nutritional value of edible fruits of indigenous wild trees in Malawi. For. Ecol. Manag. 1994;64:245–248. doi: 10.1016/0378-1127(94)90298-4. [DOI] [Google Scholar]
- 107.Pakia M. African Traditional Plant Knowledge Today: An. Ethnobotanical Study of the Digo at the Kenya Coast. Lit Verlag Berlin; Münster, Germany: 2006. [Google Scholar]
- 108.Mulholland D.A., Lourine S.E., Taylor D.A.H., Dean F.M. Coumarins from Ekebergia pterophylla. Phytochemistry. 1998;47:1641–1644. doi: 10.1016/S0031-9422(97)00783-8. [DOI] [Google Scholar]
- 109.Taylor D.A.H. Ekebergin, a limonoid extractive from Ekebergia capensis. Phytochemistry. 1981;20:2263–2265. doi: 10.1016/0031-9422(81)80126-4. [DOI] [Google Scholar]
- 110.Kehrli A.R.H., Taylor D.A.H., Niven M. Limonoids from Ekebergia pterophylla seed. Phytochemistry. 1990;29:153–159. doi: 10.1016/0031-9422(90)89029-9. [DOI] [Google Scholar]
- 111.Taylor A.R.H., Taylor D.A.H. Limonoids from Ekebergia pterophylla. Phytochemistry. 1984;23:2676–2677. doi: 10.1016/S0031-9422(00)84126-6. [DOI] [Google Scholar]
- 112.MacLachlan L.K., Taylor D.A.H. Limonoids from Nymania capensis. Phytochemistry. 1982;21:1701–1703. doi: 10.1016/S0031-9422(82)85043-7. [DOI] [Google Scholar]
- 113.Adesida G.A., Taylor D.A.H. The chemistry of the genus Entandrophragma. Phytochemistry. 1967;6:1429–1433. doi: 10.1016/S0031-9422(00)82885-X. [DOI] [Google Scholar]
- 114.Bevan C.W.L., Ekong D.E.U., Halsall T.G., Toft P. West African timbers. Part XX. The structure of turraeanthin, an oxygenated tetracyclic triterpene monoacetate. J. Chem. Soc. C Org. 1967:820–828. doi: 10.1039/j39670000820. [DOI] [Google Scholar]
- 115.Mouthé Happi G., Tchaleu Ngadjui B., Green I.R., Fogué Kouam S. Phytochemistry and pharmacology of the genus Entandrophragma over the 50 years from 1967 to 2018: A ‘golden’ overview. J. Pharm. Pharmacol. 2018;70:1431–1460. doi: 10.1111/jphp.13005. [DOI] [PubMed] [Google Scholar]
- 116.Ansell S.M., Taylor D.A.H. Limonoids from the seed of Entandrophragma caudatum. Phytochemistry. 1988;27:1218–1220. doi: 10.1016/0031-9422(88)80311-X. [DOI] [Google Scholar]
- 117.Mulholland D.A., Taylor D.A.H. Limonoids from the seed of the natal mahogany, Trichilia dregeana. Phytochemistry. 1980;19:2421–2425. doi: 10.1016/S0031-9422(00)91040-9. [DOI] [Google Scholar]
- 118.Gunatilaka A.A.L., da S. Bolzani V., Dagne E., Hofmann G.A., Johnson R.K., McCabe F.L., Mattern M.R., Kingston D.G.I. Limonoids showing selective toxicity to DNA repair-deficient yeast and other constituents of Trichilia Emetica. J. Nat. Prod. 1998;61:179–184. doi: 10.1021/np9701687. [DOI] [PubMed] [Google Scholar]
- 119.Bentley M.D., Adul G.O., Alford A.R., Huang F.-Y., Gelbaum L., Hassanali A. An insect antifeedant limonoid from Turraea Nilotica. J. Nat. Prod. 1995;58:748–750. doi: 10.1021/np50119a015. [DOI] [Google Scholar]
- 120.Mulholland D.A., Taylor D.A.H. Protolimonoids from Turraea nilotica. Phytochemistry. 1988;27:1220–1221. doi: 10.1016/0031-9422(88)80312-1. [DOI] [Google Scholar]
- 121.Akinniyi J.A., Connolly J.D., Mulholland D.A., Rycroft D.S., Taylor D.A.H. Limonoid extractives from Turraea floribunda and T. obtusifolia. Phytochemistry. 1986;25:2187–2189. doi: 10.1016/0031-9422(86)80088-7. [DOI] [Google Scholar]
- 122.Fraser L.-A., Mulholland D.A., Taylor D.A.H. The chemotaxonomic significance of the limonoid, nymania-1, in Turraea obtusifolia. S. Afr. J. Bot. 1995;61:281–282. doi: 10.1016/S0254-6299(15)30547-0. [DOI] [Google Scholar]
- 123.Sarker S.D., Savchenko T., Whiting P., Šik V., Dinan L. Two limonoids from Turraea obtusifolia (Meliaceae), prieurianin and rohitukin, antagonise 20-hydroxyecdysone action in a Drosophila cell line. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 1997;35:211–217. doi: 10.1002/(SICI)1520-6327(1997)35:1/2<211::AID-ARCH19>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 124.Gualdani R., Cavalluzzi M.M., Lentini G., Habtemariam S. The chemistry and pharmacology of citrus limonoids. Molecules. 2016;21:1530. doi: 10.3390/molecules21111530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Obbo C.J.D., Makanga B., Mulholland D.A., Coombes P.H., Brun R. Antiprotozoal activity of Khaya anthotheca, (Welv.) C.D.C. a plant used by chimpanzees for self medication. J. Ethnopharmacol. 2013;147:220–223. doi: 10.1016/j.jep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 126.Malafronte N., Sanogo R., Vassallo A., De Tommasi N., Bifulco G., Dal Piaz F. Androstanes and pregnanes from Trichilia emetica ssp. suberosa JJ de Wilde. Phytochemistry. 2013;96:437–442. doi: 10.1016/j.phytochem.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 127.Kliewer S.A., Goodwin B., Wilson T.M. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr. Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 128.Sadgrove N.J., Jones G.L. From Petri Dish to Patient: Bioavailability Estimation and Mechanism of Action for Antimicrobial and Immunomodulatory Natural Products. Front. Microbiol. 2019;10:2470. doi: 10.3389/fmicb.2019.02470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gunatilaka A.A.L., Samaranayake G., Kingston D.G.I., Hoffmann G., Johnson R.K. Bioactive Ergost-5-ene-3β/, 7α-diol Derivatives from Pseudobersama mossambicensis. J. Nat. Prod. 1992;55:1648–1654. doi: 10.1021/np50089a014. [DOI] [PubMed] [Google Scholar]
- 130.Eldeen I.M.S., Van Heerden F.R., Van Staden J. Biological activities of cycloart-23-ene-3, 25-diol isolated from the leaves of Trichilia dregeana. S. Afr. J. Bot. 2007;73:366–371. doi: 10.1016/j.sajb.2007.02.192. [DOI] [Google Scholar]
- 131.Maminata T., Lin Z., Ming C., Olsen C.E., Nacoulma O., Guissou P.I., Quedrago Q.J., Guigemde R.T., Brogger S.C. Cytotoxic kurubasch aldehyde from Trichilia emetica. Nat. Prod. Res. 2007;21:13–17. doi: 10.1080/14786410600921698. [DOI] [PubMed] [Google Scholar]
- 132.Mulholland D.A., Lourine S.E. Limonoids from Ekebergia capensis. Phytochemistry. 1998;47:1357–1361. doi: 10.1016/S0031-9422(97)00704-8. [DOI] [Google Scholar]
- 133.Pergomet J.L., Di Liberto M.G., Derita M.G., Bracca A.B.J., Kaufman T.S. Activity of the pterophyllins 2 and 4 against postharvest fruit pathogenic fungi. Comparison with a synthetic analog and related intermediates. Fitoterapia. 2018;125:98–105. doi: 10.1016/j.fitote.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 134.Harinantenaina L., Brodie P.J., Callmander M.W., Randrianaivo R., Rakotonandrasana S., Rasamison V.E., Rakotobe E., Kingston D.G.I. Astrotricoumarin, an antiproliferative 4′-hydroxy-2′,3′-dihydroprenylated methylcoumarin from an Astrotrichilia sp. from the Madagascar Dry Forest. Nat. Prod. Commun. 2011;6:1259–1262. doi: 10.1177/1934578X1100600913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Okogun J.I., Enyenihi V.U., Ekong D.E.U. Spectral studies on coumarins and the determination of the constitution of ekersenin by total synthesis. Tetrahedron. 1978;34:1221–1224. doi: 10.1016/0040-4020(78)80149-5. [DOI] [Google Scholar]
- 136.Connolly J.D., Okorie D.A., de Wit L.D., Taylor D.A.H. Structure of dregeanin and rohitukin, limonoids from the subfamily Melioideae of the family Meliaceae. An unusually high absorption frequency for a six-membered lactone ring. J. Chem. Soc. Chem. Commun. 1976;22:909–910. doi: 10.1039/c39760000909. [DOI] [Google Scholar]
- 137.Nakatani M., James J.C., Nakanishi K. Isolation and structures of trichilins, antifeedants against the Southern army worm. J. Am. Chem. Soc. 1981;103:1228–1230. doi: 10.1021/ja00395a046. [DOI] [Google Scholar]
- 138.Nakatani M., Iwashita T., Naoki H., Hase T. Structure of a limonoid antifeedant from Trichilia roka. Phytochemistry. 1985;24:195–196. doi: 10.1016/S0031-9422(00)80842-0. [DOI] [Google Scholar]
- 139.Nakatani M., Nakanishi K. Structures of insect antifeeding limonoids, trichilins F and G, from Trichilia roka. Heterocycles. 1993;36:725–731. doi: 10.3987/COM-92-6194. [DOI] [Google Scholar]
- 140.Nakatani M., Iwashita T., Mizukawa K., Hase T. Trichilinin, a new hexacyclic limonoid from Trichilia roka. Heterocycles. 1987;26:43–46. doi: 10.3987/R-1987-01-0043. [DOI] [Google Scholar]
- 141.Torto B., Hassanali A., Nyandat E., Bentley M.D. A limonoid from Turraea floribunda. Phytochemistry. 1996;42:1235–1237. doi: 10.1016/0031-9422(95)00970-1. [DOI] [Google Scholar]
- 142.Torto B., Bentley M.D., Cole B.J.W., Hassanali A., Huang F.-Y., Gelbaum L., Vanderveer D.G. Limonoids from Turraea floribunda. Phytochemistry. 1995;40:239–243. doi: 10.1016/0031-9422(95)00198-G. [DOI] [Google Scholar]
- 143.Ndung’u M.W., Kaoneka B., Hassanali A., Lwande W., Hooper A.M., Tayman F., Zerbe O., Torto B. New mosquito larvicidal tetranortriterpenoids from Turraea wakefieldii and Turraea floribunda. J. Agric. Food Chem. 2004;52:5027–5031. doi: 10.1021/jf049474y. [DOI] [PubMed] [Google Scholar]
- 144.Fraser L.A., Mulholland D.A., Nair J.J. Limonoids from the seed of Turraea floribunda. Phytochemistry. 1994;35:455–458. doi: 10.1016/S0031-9422(00)94781-2. [DOI] [PubMed] [Google Scholar]
- 145.Mullholland D.A., Fraser L., Nair J.J. A new limonoid from the seed of Turraea floribunda. Planta Med. 1992;58:712. doi: 10.1055/s-2006-961740. [DOI] [Google Scholar]
- 146.Akerman L.A. Ph.D. Thesis. University of Natal; Durban, South Africa: 1990. Extractives from the Meliaceae and Icacinaceae. [Google Scholar]
- 147.Irungu B.N., Adipo N., Orwa J.A., Kimani F., Heydenreich M., Midiwo J.O., Björemark P.M., Håkansson M., Yenesew A., Erdélyi M. Antiplasmodial and cytotoxic activities of the constituents of Turraea robusta and Turraea nilotica. J. Ethnopharmacol. 2015;174:419–425. doi: 10.1016/j.jep.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Naidoo N. Ph.D. Thesis. University of Natal; Durban, South Africa: 1997. Extractives from the Meliaceae and Annonaceae. [Google Scholar]
- 149.Mulholland D.A., Monkhe T.V. Two glabretal-type triterpenoids from the heartwood of Aglaia ferruginaea. Phytochemistry. 1993;34:579–580. doi: 10.1016/0031-9422(93)80052-T. [DOI] [Google Scholar]
- 150.Moffett R. Sesotho Plant and Animal Names and Plants Used by the Basotho. Sun Media; Stellenbosch, South Africa: 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.