Abstract
Chronic exercise is widely recognized as an important contributor to healthspan in humans and in diverse animal models. Recently, we have demonstrated that Sestrins, a family of evolutionarily conserved exercise-inducible proteins, are critical mediators of exercise benefits in flies and mice. Knockout of Sestrins prevents exercise adaptations to endurance and flight in Drosophila, and similarly prevents benefits to endurance and metabolism in exercising mice. In contrast, overexpression of dSestrin in muscle mimics several of the molecular and physiological adaptations characteristic of endurance exercise. Here, we extend those observations to examine the impact of dSestrin on preserving speed and increasing lysosomal activity. We find that dSestrin is a critical factor driving exercise adaptations to climbing speed, but is not absolutely required for exercise to increase lysosomal activity in Drosophila. The role of Sestrin in increasing speed during chronic exercise requires both the TORC2/AKT axis and the PGC1α homolog spargel, while dSestrin requires interactions with TORC1 to cell-autonomously increase lysosomal activity. These results highlight the conserved role of Sestrins as key factors that drive diverse physiological adaptations conferred by chronic exercise.
Keywords: Sestrin, exercise, Drosophila
1. Introduction
As global populations age, the burden of caring for a disproportionately older demographic and their rising medical costs has become a major healthcare problem [1,2]. Interventions that can preserve healthy function at advanced ages are thus of substantial importance. Preservation of mobility is a major concern in aging populations, and declining mobility is associated with reduced independence [3]. Individuals with healthy mobility tend to experience fewer falls and retain the ability to travel to essential healthcare visits. Furthermore, preservation of mobility allows individuals to continue social interactions, increasing personal satisfaction and morale into advanced age [4,5]. Thus, identification of easily accessible, low-cost interventions that preserve healthy lifespan and mobility in the elderly would have widespread benefits, reducing healthcare costs and enhancing quality of life for aging individuals and those responsible for their care.
One effective intervention that reduces age-related functional decline in humans and animal models is endurance exercise [6]. Endurance exercise induces remodeling in muscle tissue, which triggers cell non-autonomous effects that improve physiological and metabolic health in multiple organ systems [7,8,9]. Studies in both invertebrate and vertebrate models show that endurance exercise promotes increased mitochondrial biogenesis/efficiency [10,11], decreased triglyceride storage [12,13], improved insulin sensitivity [14,15], and protection of both muscle and neural functions [16,17,18,19]. However, widespread application of endurance exercise as an anti-aging therapeutic is limited by a number of barriers. Many elderly individuals are unable to reach the level of training necessary to generate these adaptations because of advanced age, injury, illness, or lifestyle factors leading to long sedentary periods [20,21]. Therefore, the identification of conserved factors that induce the benefits of exercise, even without training, would have enormous therapeutic potential. One such conserved factor has been recently identified in the Sestrin family of proteins.
Sestrins are small stress-activated proteins conserved from invertebrates to humans [22]. Three Sestrins are tissue-specifically expressed in mammals (mSesn1–3), while Drosophila and C. elegans express single Sestrin orthologues (dSesn and cSesn, respectively) [23]. Drosophila Sestrin (dSesn) and mammalian SESN1 are highly expressed in skeletal and cardiac muscle [23,24,25]. Sestrins have intrinsic antioxidant function [22], which can contribute to attenuation of aging [26]. However, independent of their antioxidant activity, Sestrins can modulate mTOR signaling, in part through AMPK activation [27]. Sestrin inhibits TORC1 and enhances TORC2 activity [23,28] coordinating metabolism and healthspan. Inhibition of TORC1 by Sestrins can also induce autophagy [29], essential for elimination and recycling of damaged macromolecules and organelles from cells. Studies in Drosophila have established dSestrin (dSesn) as a critical negative feedback regulator of mTOR, whose absence results in underactivation of AMPK and overactivation of mTOR. Loss of dSesn leads to accelerated development of several age-related pathologies including lipid accumulation, mitochondrial dysfunction, accumulation of protein aggregates, cardiac arrhythmia and muscle degeneration [23]. These pathologies, which are strikingly similar to age- and obesity-associated human diseases, were partially prevented by mTOR inhibition through AICAR, metformin or rapamycin [23].
We have recently found that Sestrins are necessary and sufficient for beneficial adaptations to muscle function and metabolism in flies and mice [30]. In Drosophila, dSesn is required for exercise-induced improvements in climbing endurance and flight performance [30]. Here, we extended our invertebrate studies to examine exercise- and dSesn-dependent changes to climbing speed and lysosomal activity. We found that in Drosophila, dSesn mediates exercise-induced improvements in climbing speed, and that this effect requires both the TORC-2/AKT axis and PGC1α. In contrast, dSesn is not required for chronic exercise to simulate lysosomal activity in adipose tissue. However, when dSesn is overexpressed in muscle, it can act additively with exercise, in a TORC1-dependent way, to further increase adipose lysosomal activity during chronic exercise. Adding further complexity, dSesn overexpression in the heart induced lysosomal activity cell-autonomously, whether flies were exercised or not.
2. Materials and Methods
2.1. Fly Stocks and Maintenance
Wild-type, dSesnXP4 (UAS-dSesn) and dSesn8A11 (dSesn−/−) were previously produced in Exelixis w1118 background. Mef2-Gal4 (#27390), MHC-GS-Gal4 (#43641), ap-Gal4 (#3041), UAS-dAKT (#8191), UAS-dAKTRNAi (#33615), UAS-dSin1RNAi (#32371), UAS-4E-BPRNAi (#36667), UAS-dTSC2RNAi (#34737), UAS-dPGC1RNAi (#33915), UAS-dSnf1RNAi (#32371) and fly lines with an attP landing platform were obtained from the Bloomington Drosophila Stock Center (BDSC, Bloomington, IN, USA). UAS-dSesnRNAi-A (#38481) and UAS-dSesnRNAi-B (#104365) were obtained from the Vienna Drosophila RNAi Center (VDRC, Vienna, Austria). UAS-dPGC1 is a kind gift from David Walker. dSesn cDNA14 of wild-type (dSesnWT) and C86S(dSesnCS), D424A (dSesnDA) or D423A/D424A (dSesnDDAA) mutated forms were previously produced and are described in Kim et al., [30]. C86, D423 and D424 in dSesn correspond to C125, D406 and D407 in mammalian SESN2.
Flies were grown and aged on 10% sugar-yeast medium at 25 °C, 50% humidity, and 12/12 h light/dark cycle. Control flies for all non-gene-switch UAS Gal4 experiments consisted of both the UAS and Gal4 lines into Attp or w1118, as appropriate. For experiments in which both the Gal4 control and UAS control flies were found to be statistically identical, results were pooled and graphed as a single datum. In gene-switch experiments, RU-flies of the same genotype served as the negative control.
2.2. Exercise Training
Cohorts of at least 800 flies were collected under light CO2 anesthesia within 2 h of eclosion and separated into vials of 20. Flies were then further separated into 2 large cohorts of at least 400 flies, which served as exercised and unexercised groups. Exercised groups received three weeks of ramped exercise as described [31]. The unexercised groups were placed on the exercise training device at the same time as the exercised groups, but were prevented from running by the placement of a foam stopper low in the vial. Both cohorts were housed in the same incubator with normal foam stopper placement at all times other than during an exercise bout.
2.3. Genetic Controls
dSesn8A11/8A11 (dSesn−/−) flies were compared to their w1118 background [23]. For dSesn RNAi experiments, adult progeny were age-matched and randomly split into control (OFF) and experimental (ON) groups. Flies in this study were longitudinally assessed from the same cohorts as Kim et al. [30]. The ON group received 100 μM mifepristone (Cayman Chemical, Ann Arbor, MI), which activates the gene switch (GS) driver, while the OFF group received the same volume of vehicle solution (70% ethanol). dSesn RNAi efficacy was verified in the initial study, as flies originate from the same replicate biological cohorts [30]. For dSesn overexpression experiments, the control attP (#24486 from BDSC) strain, the background for UAS-dSesnWT, was crossed to Mef2-Gal4.
2.4. Climbing Speed
Negative geotaxis was assessed by Rapid Negative Geotaxis (RING) assays in groups of 100 flies as described [32]. Briefly, vials of 20 flies were tapped down 4 times each, then measured for climbing distance 2 s after the final tap. For each group of vials, an average of 4 consecutive trials was calculated and analyzed using ImageJ (Bethesda, MD). Flies were longitudinally tested 5 times per week for 4–5 weeks. Between assessments, flies were returned to food vials and housed normally as described above. Negative geotaxis results were analyzed using two-way ANOVA analysis with post hoc Tukey multiple comparison tests in GraphPad Prism (San Diego, CA, USA). All negative geotaxis experiments were performed in duplicate or triplicate, with one complete trial shown in each graph.
2.5. qRT PCR
cDNA was prepared from 20 whole flies. Three independent RNA extractions were prepared for each sample. Differences between genotypes were assessed by ANOVA. Primer sequences are listed below.
Atg8a 5′-: GCGCATCGGTGATTTGGACAAGAA
Atg8a 3′-: TGCGCTTGCGAATGAGGAAGTAGA
rp49 5′-: ACTCAATGGATACTGCCCAAGAA
rp49 3′-: CAAGGTGTCCCACTAATGCATAAT
Relative message abundance was determined by amplification and staining with SYBR Green I using an ABI 7300 Real Time PCR System (Applied Biosystems, Waltham, MA, USA). Expression of Rp49 and corresponding control (gal4/+, UAS-RNAi/+) flies were used for normalization.
2.6. Lysotracker
Lysotracker staining of adult fat bodies and myocardium was performed as in [33]. Adult flies were partially dissected, ventral side up, in room temperature PBS to expose hearts and fat bodies, then rinsed once in fresh PBS. Preps were exposed to 0.01 µM of Lysotracker green (Molecular Probes, Eugene, OR, USA) in PBS for 30 s, then washed 3 times in fresh PBS. Stained tissue was mounted in Vectashield reagent (Vector Laboratories, Burlingame, CA, USA) and imaged in the Department of Physiology Confocal Microscopy Core at Wayne State University School of Medicine on a Leica DMI 6000 with a Crest X-light spinning disc confocal using a 63X oil immersion objective (W. Nuhsbaum, Inc., McHenry, IL, USA). Images were analyzed using ImageJ (NIH, Bethesda, MD, USA). A minimum of 5 samples were analyzed for each genotype and duplicate or triplicate biological cohorts were assessed for each group. Significance was assessed by ANOVA with Tukey post-hoc comparison.
3. Results
3.1. Sestrin Drives Exercise-Induced Increases to Climbing Speed and Atg8a Expression
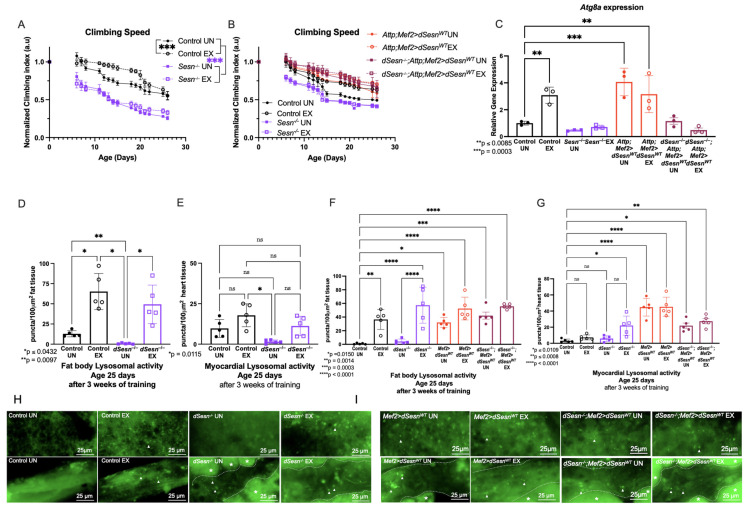
We have established an endurance exercise paradigm for Drosophila that induces several conserved adaptations after 3 weeks of training [31]. Control flies retain faster climbing speed than age-matched unexercised siblings as described previously (Figure 1A). Sesn−/− flies have lower climbing speed than control flies across ages, and do not increase climbing speed with exercise (Figure 1A). In contrast, muscle specific dSesn expression is sufficient to increase climbing speed in both a wild-type and dSesn-deficient background (Attp;Mef2 > dSesnWT, Sesn−/−;Mef2 > dSesnWT), whether exercised or not (Figure 1B).
Figure 1.
Sestrin drives exercise adaptations to climbing speed and lysosomal activity. (A) dSesn−/− flies have lower climbing speed than control flies whether exercised or not (2-way ANOVA, genotype effect, p ≤ 0.019 beginning at day 4). Exercise increases climbing speed in control flies (2-way ANOVA, exercise effect, p ≤ 0.0465, days 8–22). (B) Flies expressing dSesn in muscles in either a wild-type or dSesn null background (Attp;Mef2 > dSesnWT, dSesn−/−; Attp;Mef2>dSesnWT, respectively) have high climbing speed whether exercised or not (2-way ANOVA, genotype effect, p ≥ 0.1032). Climbing speed experiments performed in triplicate, n ≥ 100 flies. (C) Atg8 gene expression is elevated in exercised background controls (ANOVA, p = 0.0085) and Attp;Mef2 > dSesnWT flies, whether exercised or not (ANOVA, p = 0.0003, EX p = 0.0064, UN). Data are presented as ΔΔCT normalized to Atg8a gene expression in unexercised Control flies. N = 20 flies per cDNA extraction, performed in triplicate. (D) Exercise increases fat body Lysotracker in control flies (ANOVA, p = 0.0284). Unexercised dSesn−/− flies have low fat body Lysotracker (ANOVA, p = 0.0097) but staining increases with exercise (ANOVA, p = 0.0432). (E) Myocardial Lysotracker staining is low in unexercised dSesn−/− flies (ANOVA, p = 0.0115). All other genotypes have similar myocardial Lysotracker staining. (F) Exercised dSesn−/−; Attp;Mef2 > dSesnWT flies have high fat body Lysotracker whether exercised or not (ANOVA, p ≤ 0.0014). (G) Myocardial Lysotracker staining is high in both Attp;Mef2 > dSesnWT and dSesn−/−; Attp;Mef2 > dSesnWT flies whether exercised or not (ANOVA, p ≤ 0.0109). (H) Representative 63× images of Lysotracker staining in fat body (upper panels) and myocardium (lower panels) from control and dSesn−/− flies, quantified in (D,E). (I) Representative 63× images of Lysotracker staining in fat body (upper panels) and myocardium (lower panels) from Attp;Mef2 > dSesnWT and dSesn−/−; Attp;Mef2 > dSesnWT flies, quantified in (F,G). Lysotracker staining n = 5, performed in duplicate or triplicate. Dotted lines in myocardial images delineate heart tissue. White triangles point to example regions of puncta. White asterisks indicate pericardial cells. Brackets represent pairwise post-hoc comparisons from ANOVA. ns or lack of brackets indicates no significant difference. Error bars depict ± SD. EX = cohort after 3 weeks of ramped, daily endurance training. UN = control unexercised flies exposed to identical training environment without running.
Both Sestrin and endurance exercise are known to enhance autophagy [13,29], so we next examined autophagy-specific gene 8a (Atg8a) expression, a factor that controls the rate of autophagy [34], in these same flies. Exercised background control flies have higher Atg8a expression than age-matched, unexercised siblings (Figure 1C). dSesn−/− flies do not increase Atg8a expression after exercise, but flies overexpressing dSesn in muscle have high Atg8a expression whether exercised or not (Figure 1C). Taken together, these results indicate that dSesn is required for exercise to induce increases in speed and Atg8a expression, and that overexpression of dSesn can mimic the effect of exercise on speed and Atg8a expression.
3.2. Dsesn Induces Lysosomal Activity in Parallel with Exercise
In order to examine tissue-specific lysosomal activity, we performed Lysotracker staining in the fat body and myocardium (Figure 1F,I). Unexercised dSesn−/− flies have low Lysotracker staining in the fat body and myocardium (Figure 1D,E), but increased fat body Lysotracker staining with exercise (Figure 1D). On the other hand, muscle-specific dSesn overexpression increases Lysotracker in both exercised and unexercised fat bodies and myocardium. Endurance exercise and Sestrin have been observed to enhance lysosomal activity, including autophagy, in multiple muscle tissues in flies and mammals [17,29,35,36,37,38,39], and we have previously shown that exercise increases Lysotracker staining in Drosophila fat body [13,17,34]. Background control flies increase fat body Lysotracker staining with exercise (Figure 1H upper panels, quantification in Figure 1D), but not myocardial Lysotracker staining (Figure 1H lower panels, quantification in E). Sestrin-deficient flies have significantly reduced Lysotracker staining at baseline in both the fat body and myocardium (Figure 1D,E,H). Exercise increases lysotracker levels in dSesn mutants, but only to the level of wild type unexercised, not to the level of wild type exercised flies (Figure 1D,E,H). Muscle specific dSesn overexpression increases fat body Lysotracker staining in unexercised flies, and exercise further increases it, indicating an additive effect (Figure 1F,I). Muscle-specific dSesn expression has similar effects in a dSesn−/− background, meaning expression of Sestrin in muscle is sufficient to increase fat body Lysotracker, even in flies where dSesn is deleted genetically in all other tissues (Figure 1G,I). This tissue-autonomous effect of dSesn overexpression is independent of exercise, as exercise does not significantly modify Lysotracker staining in the myocardium (Figure 1E,G,H,I).
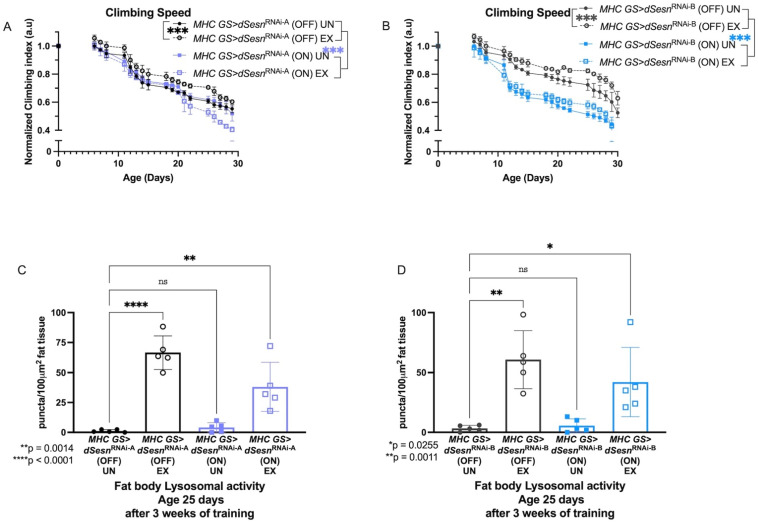
To address muscle-specific requirements for dSesn, we expressed two RNAi constructs against dSesn specifically in adult skeletal muscles (MHC GS > dSesnRNAi-A, MHC GS > dSesnRNAi-B) and assessed climbing speed and lysosomal activity with exercise. We have previously shown that dSesn expression in muscle is high and induced by endurance exercise [30]. Muscle-specific expression of both RNAi constructs abolished climbing speed improvement with exercise, and muscle-specific RNAi-B expression reduced baseline climbing speed across ages (Figure 2A,B). Similarly, both MHC GS > dSesnRNAi-A and MHC GS > dSesnRNAi-B flies have low fat body (Figure 2C,D) lysosomal activity at baseline. However, when exercised, muscle-specific dSesn RNAi flies increase fat body Lysotracker, but not as much as exercised wild-type flies, again consistent with an additive effect between exercise and dSesn in this context (Figure 2C,D, myocardial Lysotracker: see Supplementary Figure S1). Together with the results from Figure 1, these results indicate that dSesn is required in muscle to improve climbing speed during chronic exercise, and that muscle-specific dSesn expression is sufficient to drive increased speed across ages. Further, dSesn acts in muscle to cell-autonomously drive Lysotracker staining in myocardium, but acts additively with exercise to cell-non-autonomously drive Lysotracker staining in the fat body.
Figure 2.
Sestrin RNAi in adult muscles abrogates exercise-dependent increases in climbing speed and lysosomal activity. (A) RNAi against Sestrin in adult muscles (MHC GS > dSesnRNA-A (ON) UN) reduces climbing speed (2-way ANOVA, p ≥ 0.0489), and exercise further reduces climbing speed, particularly at later timepoints (2-way ANOVA, p ≤ 0.0184 after adult day 22). Control flies respond to exercise as normal. (B) A separate RNAi construct against Sestrin in adult muscles (MHC GS > dSesnRNA-B (ON) UN) also reduces climbing speed (2-way ANOVA, genotype effect, p ≤ 0.0046 after adult day 8). Control flies respond to exercise as normal. Climbing experiments performed in duplicate, n ≥ 100 flies. (C) RNAi against dSesn in muscle reduces exercise-induced increase in lysosomal activity in fat body (ANOVA, p = 0.0014), but MHC GS > dSesnRNA-A (ON) flies still increase fat body lysosomal activity compared to unexercised siblings (ANOVA, p = 0.0030). (D) MHC GS > dSesnRNA-B (ON) flies also increase fat body Lysotracker staining after exercise (ANOVA, p = 0.0372). EX = cohort after 3 weeks of ramped, daily endurance training. UN = control unexercised flies exposed to identical training environment without running. ns or lack of brackets indicates no significant difference.
3.3. Exercise-Induced Adaptations to Climbing Speed and Lysosomal Activity Require both Oxidoreductase and TOR-Modulating Functions of Sestrin
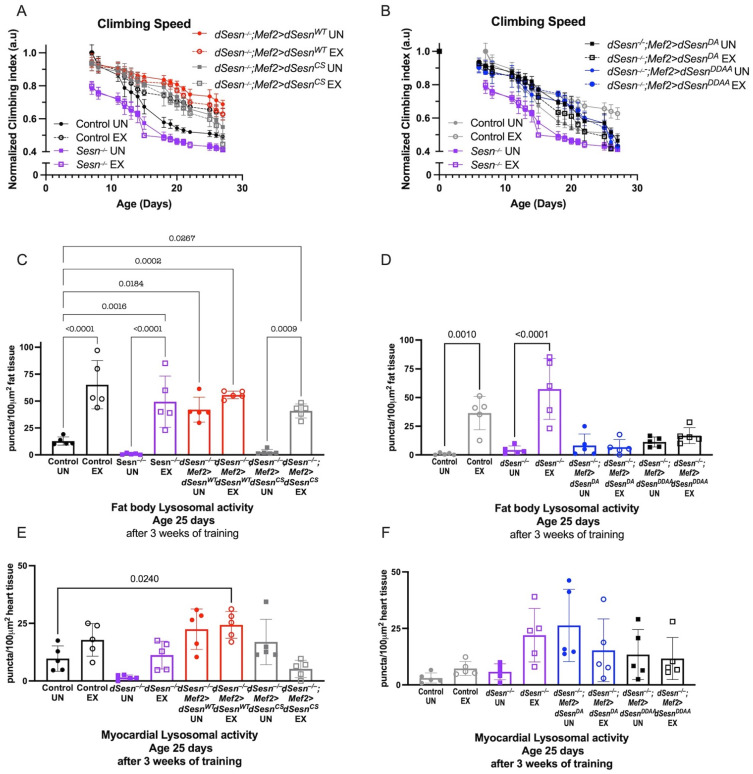
We next expressed dSesn constructs containing specific functional mutations in a dSesn-deficient background in order to determine the mechanisms by which dSesn improves climbing speed and modifies lysosomal activity. The C86S mutation in Sestrin disrupts oxidoreductase function, and the D423A and D424A substitutions eliminate TORC1 inhibition and TORC2/AKT activation [28]. Wild-type dSesn expression in muscle rescued climbing speed in dSesn-deficient flies (Figure 3A), but expressing dSesn with the C86S mutation or with D423A and D424A substitutions did not (Figure 3A,B, compare to red dSesn−/−;Mef2 > dSesnWT flies).
Figure 3.
Wild-type dSesn expressed in muscle of dSesn−/− flies improves climbing speed and increases fat body lysosomal activity. Wild-type, C86S, D423A, D423A/D424A dSesn transgenes were expressed in muscle of dSesn−/− flies. (A,B) dSesn−/−;Mef2 > dSesnWT climb faster than dSesn−/−;Mef2 > dSesnCS (shown in (A)), dSesn−/−;Mef2 > dSesnDA and dSesn−/−;Mef2 > dSesnDDAA flies (shown in (B)) whether exercised or not (2-way ANOVA, genotype effect, dSesn−/−;Mef2 > dSesnWT compared to unexercised and exercised Mef2 > dSesnCS, dSesn−/−;Mef2 > dSesnDA and dSesn−/−;Mef2>dSesnDDAA f lies: p ≤ 0.0335, d15+). Climbing speed experiments performed in triplicate, n ≥ 100 flies. (C,D) dSesn−/−;Mef2>dSesnWT flies have higher Lysotracker staining in fat bodies than dSesn−/−;Mef2 > dSesnCS (shown in (C), compare to red bars), dSesn−/−;Mef2 > dSesnDA and dSesn−/−;Mef2 > dSesnDDAA flies (shown in (D), compare to red bars in (C)) (ANOVA, p ≤ 0.0319). Exercise increases fat body Lysotracker in dSesn−/−;Mef2 > dSesnCS (shown in C) (ANOVA, p < 0.0001) (E) Exercised dSesn−/−;Mef2 > dSesnCSflies have lower myocardial Lysotracker than dSesn−/−;Mef2 > dSesnWT flies (ANOVA, p ≤ 0.0102). (F) Myocardial Lysotracker staining in dSesn−/−;Mef2 > dSesnDA and dSesn−/−;Mef2 > dSesnDDAA flies is similar to flies expressing Wild-type dSesn (Compare to red bars in (E)) whether exercised or not (ANOVA, p ≥ 0.3075). Lysotracker staining n = 5, performed in duplicate or triplicate. Brackets represent pairwise post-hoc comparisons from ANOVA. Error bars depict ± SD. EX = cohort after 3 weeks of ramped, daily endurance training. UN = control unexercised flies exposed to identical training environment without running. ns or lack of brackets indicates no significant difference.
Wild-type dSesn rescued fat body Lysotracker staining to normal levels in dSesn−/− flies, and exercise again increased Lysotracker additively (Figure 3C). dSesn−/− flies expressing a rescue construct containing the C86S mutation had low baseline Lysotracker in the fat body, but were able to increase Lysotracker in response to chronic exercise (Figure 3C). When a rescue construct containing the D423A and D424A substitutions was expressed instead, both the effects of dSestrin and the effects of exercise on Lysotracker were blocked. Neither mutant construct significantly altered the cell-autonomous increase of Lysotracker when dSesn is expressed in myocardium (Figure 1E,F). Taken together, these results indicate that both the oxidoreductase and the TOR-modulating activities of dSesn are required for dSesn to tissue-non-autonomously increase Lysotracker in adipose tissue. However, the oxidoreductase activity is not required for exercise to increase Lysotracker in parallel with dSestrin.
3.4. AKT Is Critical for Sestrin to Improve Climbing Speed and Enhance Lysosomal Activity
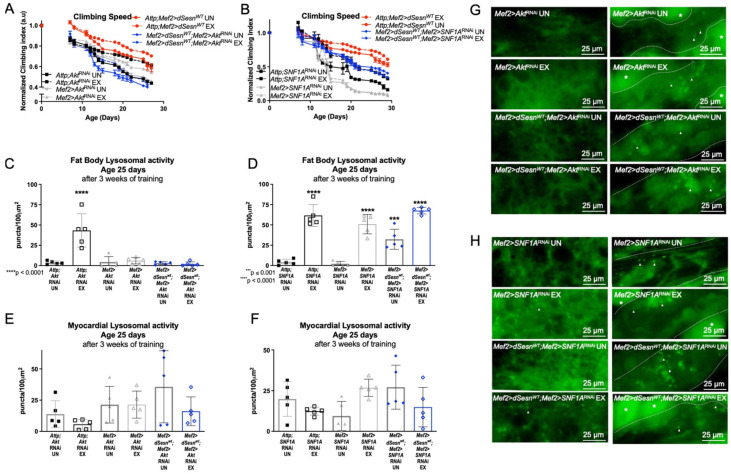
We have previously shown that both Sestrin and exercise upregulate TORC2-mediated AKT phosphorylation [30]. RNAi against Akt in muscles (Mef2 > dSesnWT; Mef2 > AktRNAi) completely abolished the climbing speed improvement conferred by dSesn overexpression (Figure 4A). In contrast, RNAi against an essential subunit of AMPK (Mef2 > dSesnWT;Mef2 > SNF1ARNAi) reduced, but did not completely abolish, the climbing speed improvement conferred by dSesn overexpression (Figure 4B). Likewise, RNAi against Akt abolished the effect of dSesn on fat body lysosomal activity, but knockdown of AMPK did not block the effects of dSesn overexpression (Figure 4C,D,G,H). Myocardial Lysotracker was not induced by dSesn in the presence of RNAi against Akt (Figure 4E,F, rightmost panels in G,H). These results indicate that AMPKα in muscles is only partially required for the climbing speed enhancing effects of Sestrin, while Akt is absolutely necessary for adaptations to both climbing speed and lysosomal activity.
Figure 4.
AKT is required for Sestrin and exercise to improve climbing speed and enhance lysosomal activity. (A) Flies overexpressing dSesn in muscles (Attp;Mef2 > dSesnWT) climb faster than controls and climb as fast as exercised controls (2-way ANOVA, p ≤ 0.0156, p ≥ 0.1300). Exercised flies expressing RNAi against AKT in muscles (Mef2 > AktRNAi UN) improve climbing speed but do not reach the speed of exercised controls (2-way ANOVA, p ≤ 0.0319). dSesn expression does not rescue climbing speed (Mef2 > dSesnWT; Mef2 > AktRNAi) (2-way ANOVA, genotype effect, Mef2 > AktRNAi UN vs. Mef2 > dSesnWT; Mef2 > AktRNAi UN, p ≤ 0.0463). (B) Flies expressing RNAi against AMPKα in muscles (Mef2 > SNF1ARNAi) have low climbing speed and do not improve with exercise (2-way ANOVA, genotype effect, p ≤ 0.0070 after day 12, exercise effect, p ≥ 0.4340). dSesn expression does not rescue climbing speed to the level of Attp;Mef2 > dSesnWT flies in flies with muscle-specific AMPKα RNAi (Mef2 > dSesnWT;Mef2 > SNF1ARNAi) (2-way ANOVA p ≤ 0.0269 after day 12). Climbing speed experiments performed in duplicate or triplicate, n ≥ 100 flies. (C) Fat body Lysotracker staining is low in both Mef2 > AktRNAi and Mef2 > dSesnWT; Mef2 > AktRNAi flies whether exercised or not (compare to Attp;AktRNAi EX, ANOVA, p < 0.0001). (D) Exercised Attp;SNF1ARNAi, Mef2 > SNF1ARNAi, and Mef2 > dSesnWT;Mef2 > SNF1ARNAi flies increase fat body Lysotracker staining with exercise (ANOVA, p < 0.0001). (E,F) Myocardial Lysotracker staining is similar in background controls and Mef2 > AktRNAi and Mef2 > SNF1ARNAi flies independent of both wild-type dSesn overexpression and exercise. Representative 63× images of Lysotracker staining in fat body (left panels) and myocardium (right panels) of exercised and control flies expressing muscle-specific RNAi against (G) Akt and (H) AMPKα, with and without muscle-specific dSesn expression. Lysotracker staining n = 5, performed in duplicate or triplicate. Dotted lines in myocardial images delineate heart tissue. White triangles point to example regions of Lysotracker puncta. White asterisks indicate pericardial cells. Asterisks in histograms represent significant post-hoc comparisons from ANOVA. Error bars depict ± SD. EX = cohort after 3 weeks of ramped, daily endurance training. UN = control unexercised flies exposed to identical training environment without running.
3.5. The TORC1 Axis Is Dispensable for Sestrin’s Effects on Climbing Speed and Lysosomal Activity
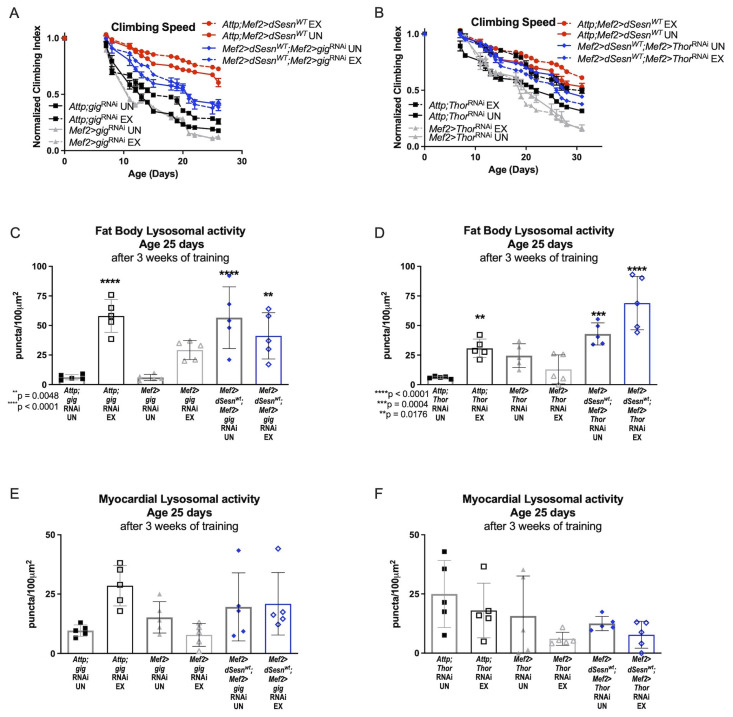
Sestrin is known to inhibit TORC1 in addition to potentiating TORC2 activity [23,28]. Muscle specific dSesn expression rescues climbing speed in flies expressing RNAi against TSC2 (Mef2 > dSesnWT;Mef2 > gigRNAi), which blocks Sestrin-mediated TORC1 inhibition [23] (Figure 5A). Similarly, dSesn expression rescues climbing speed in flies also expressing muscle-specific RNAi against d4eBP (Mef2 > dSesnWT;Mef2 > ThorRNAi), which uncouples TORC1 signaling and translational regulation [40] (Figure 5B). Fat body Lysotracker staining was low in flies expressing muscle-specific RNAi against either TSC2 (Figure 5C) or d4eBP (Figure 5D), but wild-type dSesn expression rescued fat body Lysotracker (Figure 5C,D) without increasing Lysotracker in the myocardium (Figure 5E,F). These results indicate that inhibition of TORC1 is dispensable for dSesn’s impact on climbing speed and Lysotracker.
Figure 5.
Neither TSC-2 nor 4eBP are required for climbing speed and lysosomal adaptations conferred by Sestrin. (A) Exercised flies expressing muscle specific RNAi against TSC-2 (Mef2 > gigRNAi) do not improve climbing speed (2-way ANOVA, exercise effect, p ≥ 0.6037, but dSesn expression (Mef2 > dSesnWT;Mef2 > gigRNAi flies) rescues climbing speed (2-way ANOVA, p ≤ 0.0283). (B) Flies expressing muscle specific RNAi against 4eBP (Mef2 > ThorRNAi flies) have low climbing speed and do not improve climbing speed with exercise (2-way ANOVA, exercise effect, p ≥ 0.8797, genotype effect, p ≤ 0.0219). Wild-type dSesn expression (Mef2 > dSesnWT;Mef2 > ThorRNAi flies) rescues climbing speed (2-way ANOVA, p ≥ 0.2350). Climbing speed experiments performed in triplicate, n ≥ 100 flies. (C,D) Fat body Lysotracker staining is higher than controls in both Mef2 > dSesnWT;Mef2 > ThorRNAi and Mef2 > dSesnWT;Mef2 > gigRNAi whether exercised or not (ANOVA, p ≤ 0.0048). (E,F) Myocardial Lysotracker staining is similar across genotypes independent of exercise and wild-type dSesn expression in flies with muscle specific RNAi against either TSC-2 or 4eBP. Lysotracker staining n = 5, performed in duplicate or triplicate. Asterisks represent significant post-hoc comparisons from ANOVA. Error bars depict ± SD. EX = cohort after 3 weeks of ramped, daily endurance training. UN = control unexercised flies exposed to identical training environment without running.
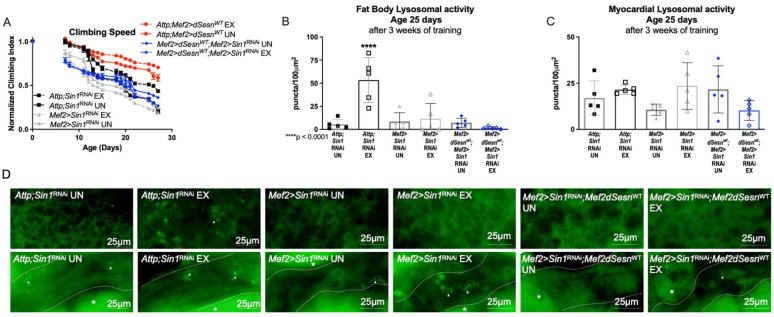
In order to further examine the role of TORC2 in this context, we expressed muscle-specific RNAi against the TORC2 complex protein-encoding Sin1 in flies also expressing wild-type dSesn in muscles [30]. Muscle-specific dSesn expression could no longer improve climbing speed when Sin1 was knocked down (Figure 6A). Similarly, neither exercise, nor dSesn overexpression could increase adipose or myocardial Lysotracker when Sin1 was knocked down (Figure 6B–D). These results underscore the importance of the TORC2-AKT axis in the mobility and lysosomal adaptations conferred by Sestrin upregulation.
Figure 6.
Sestrin-dependent adaptations in climbing speed and lysosomal activity depend on the TORC2 axis. (A) Flies expressing muscle specific RNAi against Sin1 (Mef2 > Sin1RNAi) reduce climbing speed with exercise (2-way ANOVA, exercise effect, p ≥ 0.0268). Muscle-specific wild-type dSesn expression (Mef2 > dSesnWT;Mef2 > Sin1RNAi flies) does not rescue climbing speed. Climbing speed experiments performed in triplicate, n ≥ 100 flies. (B) Mef2 > dSesnWT;Mef2 > Sin1RNAi flies have low fat body Lysotracker staining whether exercised or not. (C) Myocardial Lysotracker staining is similar across genotypes independent of exercise and wild-type dSesn expression in flies with muscle specific RNAi against Sin1. (D) Representative 63× images of Lysotracker staining from fat body (upper panels, quantified in (B)) and myocardium (lower panels, quantified in (C)). Lysotracker staining n = 5, performed in duplicate or triplicate. Dotted lines in myocardial images delineate heart tissue. White triangles point to example regions of Lysotracker puncta. White asterisks indicate pericardial cells. Asterisks in histograms indicate significant pairwise post-hoc comparisons from ANOVA. Error bars depict ± SD. EX = cohort after 3 weeks of ramped, daily endurance training. UN = control unexercised flies exposed to identical training environment without running.
3.6. PGC1α Is Essential for the Beneficial Effects of Sestrin
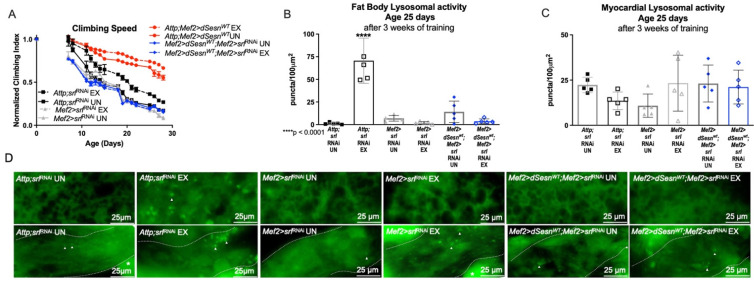
We have previously shown that the transcriptional cofactor PGC1α is upregulated during Sestrin overexpression, and that dSesn-deficient Drosophila have reduced dPGC1α (spargel, srl) expression [30]. Likewise, dPGC1α was critical for the endurance and flight performance extending effects of Sestrin [30], highlighting dPGC1α as an important downstream factor in the exercise-Sestrin pathway. Here, dSesn expression failed to improve climbing speed in flies expressing muscle specific RNAi against dPGC1α (Mef2 > dSesnWT;Mef2 > srlRNAi), (Figure 7A). dPGC1α RNAi also prevented exercise from inducing fat body lysosomal activity, even in the presence of dSesn overexpression (Figure 7B, upper panels in D) while myocardial Lysotracker staining was unaffected (Figure 7C,D). These results indicate that dPGC1α is critical for the effects of dSesn on speed and tissue-non-autonomous lysosomal activity.
Figure 7.
PGC1α is required for exercise- and Sestrin-dependent adaptations to climbing speed and lysosomal activity. (A) Flies expressing RNAi against PGC1α in muscles have a climbing speed similar to unexercised background controls independent of both dSesn expression and exercise (Mef2 > srlRNAi flies, Mef2 > dSesnWT;Mef2 > srlRNAi flies, 2-way ANOVA, p ≥ 0.2168). Climbing speed experiments performed in duplicate, n ≥ 100 flies. (B) Mef2 > dSesnWT;Mef2 > srlRNAi flies have low fat body Lysotracker staining whether exercised or not. (C) Myocardial Lysotracker staining is similar across genotypes independent of exercise and wild-type dSesn expression. (D) Representative 63× images of Lysotracker staining in fat body (upper panels) and myocardium (lower panels) of exercised and control flies expressing muscle-specific RNAi against PGC1α. Lysotracker staining n = 5, performed in triplicate. Dotted lines in myocardial images delineate heart tissue. White triangles point to example regions of Lysotracker puncta. White asterisks indicate pericardial cells. Error bars depict ± SD. EX = cohort after 3 weeks of ramped, daily endurance training. UN = control unexercised flies exposed to an identical training environment without running.
4. Discussion
We have previously shown that Sestrin is required for exercise adaptations to endurance and metabolism in flies and mice, and that dSesn overexpression is sufficient to mimic physiological improvements to endurance and flight performance in Drosophila, even without training. Our previous work also implicated Akt and PGC1α as critical factors underlying the healthspan extending effects of Sestrin in flies and mice. Here, we examine whether dSesn plays a similar role in increasing speed and lysosomal activity in response to chronic exercise in Drosophila.
Both speed and endurance decline in aging individuals, and each contributes to reduced quality of life in distinct ways [41]. Better endurance is correlated with increased independence and enhanced executive function [42], while low gait speed is a significant predictor of frailty and cognitive decline [43,44]. Furthermore, declines in gait speed are usually accompanied by cardiac and metabolic dysfunction [41]. Autophagy is one conserved process by which cells exert protein and organelle quality control, and its precise regulation ensures proper cardiac and metabolic function during aging [45,46,47]. Identifying conserved factors capable of preserving these functions in animal models has great potential to generate novel therapeutic options for the aging population.
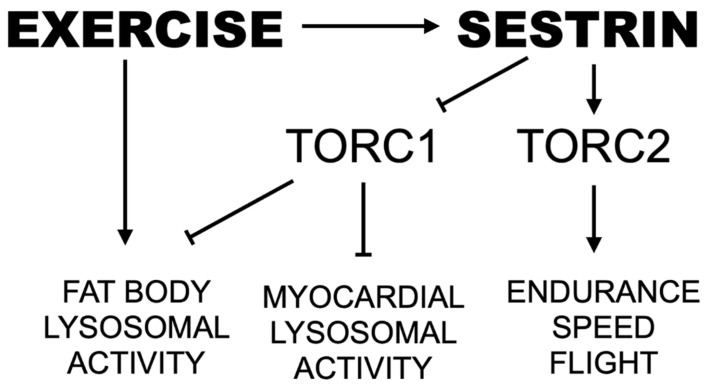
Sestrin, an evolutionarily conserved stress-inducible protein activated by endurance exercise, inhibits TORC1 while simultaneously activating TORC2 and inducing a wave of antioxidant protection [23,28,30]. mTOR is a nutrient-responsive protein kinase that regulates cell growth and metabolism in response to nutrient availability [48,49,50,51]. By activating protein and lipid anabolism and suppressing autophagic catabolism, mTOR positively regulates organ growth including muscles [49,52]. However, chronic upregulation of mTOR activity can induce excessive protein and lipid synthesis as well as chronic downregulation of autophagy [50,51,53,54,55], which can contribute to several pathologies including cancer, obesity and cardiac/skeletal muscle degeneration [53,54,55]. Over-nutrition and lack of exercise, also cause chronic mTOR activation, which can account for various age-associated pathologies, such as type II diabetes and cardiovascular diseases [50,51]. Conversely, exercise-induced energy depletion can inhibit mTOR through AMP-activated protein kinase (AMPK), attenuating age-associated pathologies and prolonging life and health span. Therefore, proper regulation of mTOR, especially the prevention of its chronic activation, is a key factor for prevention of age-associated diseases and improved quality of life in later ages. Here, we provide further support for Sestrin as a potent exercise mimetic that integrates catabolic effects from downregulation of TORC1 with maintenance of muscle and mitochondrial health driven by upregulation of Akt and PGC1α (Figure 8).
Figure 8.
Proposed pathway by which endurance exercise and Sestrin mediate speed and lysosomal activity. Arrows indicate positive regulation. Lines with perpendicular end caps indicate negative regulation.
While some of the effects of dSesn on climbing speed parallel previous observations of Drosophila endurance, there are some important differences. For example, while AKT was essential to extend endurance and flight performance, our earlier study did not examine the impact of AMPKαon Sestrin’s mobility-enhancing effect. Sestrin inhibits TORC1 through AMPKα [27], and depletion of AMPKα in Sestrin-expressing flies only partially inhibits improvements to climbing speed. Interestingly, both Sestrin expression and exercise restore increases in lysosomal activity in AMPKα deficient flies, suggesting that Sestrin activation can increase lysosomal activity through pathways that do not require AMPK.
While this study clearly indicates that Sestrin is required for exercise to increase speed, just as we previously showed for endurance, there were some new wrinkles in the way dSesn interacts with exercise to increase Lysotracker staining. Unlike speed and endurance, dSesn is not absolutely required in any tissue for exercise to increase Lysotracker staining in the fat body. However, overexpression of dSesn in muscle can increase Lysotracker staining non-autonomously in the fat body in parallel with exercise. This indicates that exercise can increase fat body lysosomal activity through pathways that do not require dSesn. A strong candidate for such a pathway would be through upregulation of octopamine (OA) secretion, which we have shown is required for exercise adaptations [33], and requires expression of β-adrenergic receptors in the fat body [19]. Since we observed here that dSesn can act additively with exercise, it will be interesting in future work to better delineate the interactions between dSesn and OA in driving exercise adaptations.
Here, we show that Sestrin is essential for the benefits to climbing speed in Drosophila, and that muscle specific dSesn expression replicates exercise-like improvements to aging mobility. We also found that Sestrin mediates changes to lysosomal activity in multiple tissues, and that both of these adaptations depend on TORC2-Akt activity and on PGC1α. These findings reinforce the conclusion that Sestrins are critical factors modifying the physiological and subcellular benefits of endurance exercise across multiple animal models.
Acknowledgments
We would like to acknowledge our collaborators Myungjin Kim and Jun Hee Lee for the original Drosophila stocks and reagents, and Sokol V. Todi for valuable discussion.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cells10092479/s1, Supplementary Figure S1 includes myocardial Lysotracker data from Figure 2. Source data file for Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7 are included as Supplementary Materials.
Author Contributions
Conceptualization, A.S. and R.W.; methodology, A.S. and R.W.; validation, A.S.; formal analysis, A.S.; investigation, A.S. and R.W.; data curation, A.S.; writing—original draft preparation, A.S.; writing—review and editing, R.W.; supervision, R.W.; project administration, R.W.; funding acquisition, R.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIH 1RO1AG059683 to R.W.
Data Availability Statement
The datasets generated and analyzed in this study are available from the corresponding authors on request. The source data underlying Figure 1A–C,F,I, Figure 2 and Figure 3, Figure 4A–F, Figure 5A–C, Figure 6A–C and Figure 7A–C are provided in Supplementary Materials as a Source Data File.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Houde S.C., Melillo K.D. Caring for an aging population: Review of policy initiatives. J. Gerontol. Nurs. 2009;35:8–13. doi: 10.3928/00989134-20091103-04. [DOI] [PubMed] [Google Scholar]
- 2.Yazdanyar A., Newman A.B. The burden of cardiovascular disease in the elderly: Morbidity, mortality, and costs. Clin. Geriatr. Med. 2009;25:563–577. doi: 10.1016/j.cger.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartwright T. ‘Getting on with life’: The experiences of older people using complementary health care. Soc. Sci. Med. 2007;64:1692–1703. doi: 10.1016/j.socscimed.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Finlayson M.L., Peterson E.W. Falls, aging, and disability. Phys. Med. Rehabil. Clin. N. Am. 2010;21:357–373. doi: 10.1016/j.pmr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Nagatomo I., Kita K., Takigawa M., Nomaguchi M., Sameshima K. A study of the quality of life in elderly people using psychological testing. Int. J. Geriatr. Psychiatry. 1997;12:599–608. doi: 10.1002/(SICI)1099-1166(199706)12:6<599::AID-GPS505>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Cutler R.G., Mattson M.P. The adversities of aging. Ageing Res. Rev. 2006;5:221–238. doi: 10.1016/j.arr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Bo H., Zhang Y., Ji L.L. Redefining the role of mitochondria in exercise: A dynamic remodeling. Ann. N. Y. Acad. Sci. 2010;1201:121–128. doi: 10.1111/j.1749-6632.2010.05618.x. [DOI] [PubMed] [Google Scholar]
- 8.Gibala M. Molecular responses to high-intensity interval exercise. Appl. Physiol. Nutr. Metab. 2009;34:428–432. doi: 10.1139/H09-046. [DOI] [PubMed] [Google Scholar]
- 9.Ventura-Clapier R., Mettauer B., Bigard X. Beneficial effects of endurance training on cardiac and skeletal muscle energy metabolism in heart failure. Cardiovasc. Res. 2007;73:10–18. doi: 10.1016/j.cardiores.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Ascensao A., Lumini-Oliveira J., Oliveira P.J., Magalhaes J. Mitochondria as a target for exercise-induced cardioprotection. Curr. Drug Targets. 2011;12:860–871. doi: 10.2174/138945011795529001. [DOI] [PubMed] [Google Scholar]
- 11.Laker R.C., Xu P., Ryall K.A., Sujkowski A., Kenwood B.M., Chain K.H., Zhang M., Royal M.A., Hoehn K.L., Driscoll M., et al. A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J. Biol. Chem. 2014;289:12005–12015. doi: 10.1074/jbc.M113.530527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy R.L., Chokkalingham K., Srinivasan R. Obesity in the elderly: Who should we be treating, and why, and how? Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:3–9. doi: 10.1097/00075197-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Sujkowski A., Saunders S., Tinkerhess M., Piazza N., Jennens J., Healy L., Zheng L., Wessells R. dFatp regulates nutrient distribution and long-term physiology in Drosophila. Aging Cell. 2012 doi: 10.1111/j.1474-9726.2012.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittendorfer B., Horowitz J.F., Klein S. Gender differences in lipid and glucose kinetics during short-term fasting. Am. J. Physiol. Endocrinol. Metab. 2001;281:E1333–E1339. doi: 10.1152/ajpendo.2001.281.6.E1333. [DOI] [PubMed] [Google Scholar]
- 15.Corcoran M.P., Lamon-Fava S., Fielding R.A. Skeletal muscle lipid deposition and insulin resistance: Effect of dietary fatty acids and exercise. Am. J. Clin. Nutr. 2007;85:662–677. doi: 10.1093/ajcn/85.3.662. [DOI] [PubMed] [Google Scholar]
- 16.Rafalski V.A., Brunet A. Energy metabolism in adult neural stem cell fate. Prog. Neurobiol. 2011;93:182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Sujkowski A., Bazzell B., Carpenter K., Arking R., Wessells R.J. Endurance exercise and selective breeding for longevity extend Drosophila healthspan by overlapping mechanisms. Aging (Albany N. Y.) 2015;7:535–552. doi: 10.18632/aging.100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laranjeiro R., Harinath G., Hewitt J.E., Hartman J.H., Royal M.A., Meyer J.N., Vanapalli S.A., Driscoll M. Swim exercise in Caenorhabditis elegans extends neuromuscular and gut healthspan, enhances learning ability, and protects against neurodegeneration. Proc. Natl. Acad. Sci. USA. 2019;116:23829–23839. doi: 10.1073/pnas.1909210116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sujkowski A., Gretzinger A., Soave N., Todi S.V., Wessells R. Alpha- and beta-adrenergic octopamine receptors in muscle and heart are required for Drosophila exercise adaptations. PLoS Genet. 2020;16:e1008778. doi: 10.1371/journal.pgen.1008778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchner D.M., Wagner E.H. Preventing frail health. Clin. Geriatr. Med. 1992;8:1–17. doi: 10.1016/S0749-0690(18)30494-4. [DOI] [PubMed] [Google Scholar]
- 21.Phillips E.M., Schneider J.C., Mercer G.R. Motivating elders to initiate and maintain exercise. Arch. Phys. Med. Rehabil. 2004;85:S52–S57. doi: 10.1016/j.apmr.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Budanov A.V., Sablina A.A., Feinstein E., Koonin E.V., Chumakov P.M. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.H., Budanov A.V., Park E.J., Birse R., Kim T.E., Perkins G.A., Ocorr K., Ellisman M.H., Bodmer R., Bier E., et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budanov A.V., Lee J.H., Karin M. Stressin’ Sestrins take an aging fight. EMBO Mol. Med. 2010;2:388–400. doi: 10.1002/emmm.201000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peeters H., Debeer P., Bairoch A., Wilquet V., Huysmans C., Parthoens E., Fryns J.P., Gewillig M., Nakamura Y., Niikawa N., et al. PA26 is a candidate gene for heterotaxia in humans: Identification of a novel PA26-related gene family in human and mouse. Hum. Genet. 2003;112:573–580. doi: 10.1007/s00439-003-0917-5. [DOI] [PubMed] [Google Scholar]
- 26.Matheu A., Maraver A., Klatt P., Flores I., Garcia-Cao I., Borras C., Flores J.M., Vina J., Blasco M.A., Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 27.Budanov A.V., Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho A., Cho C.S., Namkoong S., Cho U.S., Lee J.H. Biochemical Basis of Sestrin Physiological Activities. Trends Biochem. Sci. 2016;41:621–632. doi: 10.1016/j.tibs.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiuri M.C., Malik S.A., Morselli E., Kepp O., Criollo A., Mouchel P.L., Carnuccio R., Kroemer G. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle. 2009;8:1571–1576. doi: 10.4161/cc.8.10.8498. [DOI] [PubMed] [Google Scholar]
- 30.Kim M., Sujkowski A., Namkoong S., Gu B., Cobb T., Kim B., Kowalsky A.H., Cho C.S., Semple I., Ro S.H., et al. Sestrins are evolutionarily conserved mediators of exercise benefits. Nat. Commun. 2020;11:190. doi: 10.1038/s41467-019-13442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piazza N., Gosangi B., Devilla S., Arking R., Wessells R. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS ONE. 2009;4:e5886. doi: 10.1371/journal.pone.0005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damschroder D., Cobb T., Sujkowski A., Wessells R. Drosophila Endurance Training and Assessment and Its Effects on Systemic Adaptations. BioProtocols. 2017 doi: 10.21769/BioProtoc.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sujkowski A., Ramesh D., Brockmann A., Wessells R. Octopamine Drives Endurance Exercise Adaptations in Drosophila. Cell Rep. 2017;21:1809–1823. doi: 10.1016/j.celrep.2017.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai H., Kang P., Hernandez A.M., Tatar M. Activin signaling targeted by insulin/dFOXO regulates aging and muscle proteostasis in Drosophila. PLoS Genet. 2013;9:e1003941. doi: 10.1371/journal.pgen.1003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji L.L., Kang C., Zhang Y. Exercise-induced hormesis and skeletal muscle health. Free. Radic. Biol. Med. 2016 doi: 10.1016/j.freeradbiomed.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 36.Lira V.A., Okutsu M., Zhang M., Greene N.P., Laker R.C., Breen D.S., Hoehn K.L., Yan Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013;27:4184–4193. doi: 10.1096/fj.13-228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogura Y., Iemitsu M., Naito H., Kakigi R., Kakehashi C., Maeda S., Akema T. Single bout of running exercise changes LC3-II expression in rat cardiac muscle. Biochem. Biophys. Res. Commun. 2011;414:756–760. doi: 10.1016/j.bbrc.2011.09.152. [DOI] [PubMed] [Google Scholar]
- 38.Kim J.S., Ro S.H., Kim M., Park H.W., Semple I.A., Park H., Cho U.S., Wang W., Guan K.L., Karin M., et al. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Sci. Rep. 2015;5:9502. doi: 10.1038/srep09502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh P., Chowdhuri D.K. Modulation of sestrin confers protection to Cr(VI) induced neuronal cell death in Drosophila melanogaster. Chemosphere. 2018;191:302–314. doi: 10.1016/j.chemosphere.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 40.Dowling R.J.O., Topisirovic I., Alain T., Bidinosti M., Fonseca B.D., Petroulakis E., Wang X.S., Larsson O., Selvaraj A., Liu Y., et al. mTORC1-Mediated Cell Proliferation, But Not Cell Growth, Controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hollmann W., Struder H.K., Tagarakis C.V., King G. Physical activity and the elderly. Eur. J. Cardiovasc. Prev. Rehabil. 2007;14:730–739. doi: 10.1097/HJR.0b013e32828622f9. [DOI] [PubMed] [Google Scholar]
- 42.Zettel-Watson L., Suen M., Wehbe L., Rutledge D.N., Cherry B.J. Aging well: Processing speed inhibition and working memory related to balance and aerobic endurance. Geriatr. Gerontol. Int. 2017;17:108–115. doi: 10.1111/ggi.12682. [DOI] [PubMed] [Google Scholar]
- 43.Liu M.A., DuMontier C., Murillo A., Hshieh T.T., Bean J.F., Soiffer R.J., Stone R.M., Abel G.A., Driver J.A. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood. 2019;134:374–382. doi: 10.1182/blood.2019000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoogendijk E.O., Rijnhart J.J.M., Skoog J., Robitaille A., van den Hout A., Ferrucci L., Huisman M., Skoog I., Piccinin A.M., Hofer S.M., et al. Gait speed as predictor of transition into cognitive impairment: Findings from three longitudinal studies on aging. Exp. Gerontol. 2020;129:110783. doi: 10.1016/j.exger.2019.110783. [DOI] [PubMed] [Google Scholar]
- 45.Shirakabe A., Ikeda Y., Sciarretta S., Zablocki D.K., Sadoshima J. Aging and Autophagy in the Heart. Circ. Res. 2016;118:1563–1576. doi: 10.1161/CIRCRESAHA.116.307474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikeda Y., Sciarretta S., Nagarajan N., Rubattu S., Volpe M., Frati G., Sadoshima J. New insights into the role of mitochondrial dynamics and autophagy during oxidative stress and aging in the heart. Oxid. Med. Cell. Longev. 2014;2014:210934. doi: 10.1155/2014/210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wohlgemuth S.E., Julian D., Akin D.E., Fried J., Toscano K., Leeuwenburgh C., Dunn W.A., Jr. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- 48.Hay N., Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 49.Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Laplante M., Sabatini D.M. An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 2009;19:R1046–R1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dann S.G., Selvaraj A., Thomas G. mTOR Complex1-S6K1 signaling: At the crossroads of obesity, diabetes and cancer. Trends Mol. Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Bentzinger C.F., Romanino K., Cloetta D., Lin S., Mascarenhas J.B., Oliveri F., Xia J., Casanova E., Costa C.F., Brink M., et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Masiero E., Agatea L., Mammucari C., Blaauw B., Loro E., Komatsu M., Metzger D., Reggiani C., Schiaffino S., Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Gottlieb R.A., Mentzer R.M. Autophagy during cardiac stress: Joys and frustrations of autophagy. Annu. Rev. Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakai A., Yamaguchi O., Takeda T., Higuchi Y., Hikoso S., Taniike M., Omiya S., Mizote I., Matsumura Y., Asahi M., et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed in this study are available from the corresponding authors on request. The source data underlying Figure 1A–C,F,I, Figure 2 and Figure 3, Figure 4A–F, Figure 5A–C, Figure 6A–C and Figure 7A–C are provided in Supplementary Materials as a Source Data File.