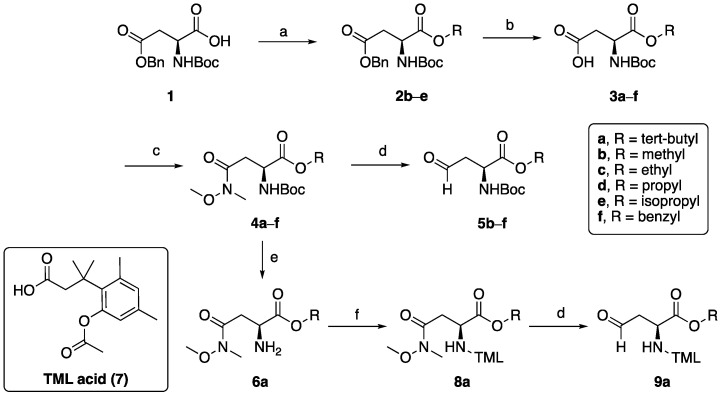
Scheme 1.
Synthesis of the prodrug forms of amino acid building blocks 5b–f and 9a. Reagents and conditions: (a) RI, DMF, K2CO3, rt, overnight (65–79%); (b) 10% Pd/C, MeOH, overnight (82–90%); (c) CH3NHOCH3·HCl, BOP, Et3N, CH2Cl2, rt, 2 h (77–83%); (d) DIBAL-H (1 M in hexanes), THF, −78 °C, assumed quant; (e) HCl (4N in dioxanes), 0 °C to rt, 2.25 h; (f) TML acid 7, BOP, Et3N, CH2Cl2, rt, overnight, 88% over two steps.