Abstract
Metastatic cancer is a persistent clinical enigma, which requires combination of several treatment modules. Here, we developed an all-in-one nanomedicine strategy to systemically co-deliver photosensitive, chemotherapeutic, and immunomodulating agents for effective immunochemo-photothermal therapy (PTT) to inhibit both primary tumor and distal metastatic tumor. Two types of polydopamine (dp)-coated nanoparticles (NPs) (N/PGEM/dp-5 and N/PGEM/dp-16) co-loaded with gemcitabine (GEM) and NLG919, a potent indoleamine-2, 3-dioxygenase (IDO) inhibitor, were prepared. N/PGEM/dp-16 NPs with a thicker dp coating layer showed higher photothermal conversion ability, more favorable biodistribution profile and better tumor inhibition effect compared to N/PGEM/dp-5 NPs with a thinner coating layer. Combination with laser irradiation further enhanced the tumor inhibition effect of N/PGEM/dp-16 NPs. In an “early metastatic” pancreatic cancer PANC02 model with small distal tumors, introduction of NLG and dp coating improved the inhibition effect on both primary and distal tumors. Compared to N/PGEM/dp-16, N/PGEM/dp-16 plus laser irradiation further enhanced the inhibition effect on primary tumor, but didn’t improve the abscopal antitumor effect. When the initial volume of distal tumor was sufficiently large in a “late metastasis” model, a more dramatic abscopal antitumor effect was achieved, resulting in a significant growth inhibition of both primary tumor and the unirradiated distal tumor. Furthermore, laser irradiation can amplify the immunochemo-NPs-mediated innate and adaptive immune responses in both tumors. This work demonstrated a distal tumor-size dependent abscopal effect, and provided a perspective for future design of more effective immunochemo-PTT nano-formulations for early- and late-stage metastatic tumors.
Keywords: PTT, polydopamine, abscopal effect, immunochemotherapy, metastatic tumor
1. Introduction
Metastasis is the major cause of cancer mortality, and about 90% of cancer deaths are largely attributed to tumor metastasis [1, 2]. Immune checkpoint blockade (ICB) that can stimulate systemic immune response is one of the most attractive strategies to treat primary and metastatic tumors [3, 4]. However, the clinical application of ICB alone is impeded by the relatively low response rates among the patients because the majority of tumors are immunologically inactive “cold” [5, 6]. Thus, combination of ICB with chemotherapy is the major approach to treat cancer metastasis in clinical trials, although it only improves the therapeutic effect and survival rate to a very limited extent [7]. To further boost the therapeutic outcomes, combination with other approaches that can convert “cold” non-immunoresponsive tumors and metastases into “hot” immunoresponsive tumors could be a promising strategy.
Photothermal therapy (PTT) is a highly selective and minimally invasive approach for local cancer treatment with limited therapeutic resistance and low systemic toxicity [8–10]. In PTT, photothermal agents are employed to absorb and convert near-infrared (NIR) light into heat, which irreversibly kills tumor cells [11]. Although PTT itself is only suitable for local cancer treatment, combinations of PTT with other therapies demonstrated a benefit of inducing abscopal effect, in which the localized treatment not only suppresses the treated tumor, but also inhibits the distal metastatic tumors. For example, some immunosuppressive proteins such as PD-L1, indoleamine 2,3-dioxygenase (IDO), and heat shock protein (HSP), are induced by PTT in the tumors for self-protection [12], which provides a good rationale for combination of PTT with immunotherapy to increase the tumor immunogenicity and responses to ICB. Huang et al reported that mild PTT could reprogram the “cold” tumor microenvironment (TME) by upregulating the expression of PD-L1 on the surface of tumor cells and sensitize tumors to anti-PD therapy, leading to remarkable abscopal effect [13]. Recent study has also found that PTT combined with chemotherapeutic agent doxorubicin (DOX) can trigger robust anti-tumor immune responses and exert an abscopal effect against untreated distant tumors in addition to eliminating locally treated tumors in mice bearing CT26 colon carcinoma [14]. However, significant abscopal effect has only been demonstrated in limited PTT studies and the efficacy is affected by the treatment regimen, the dosage, and the type of combination. The underlying mechanism is still unclear and needs to be further explored to benefit more patients [15]. Moreover, the limited efficiency of delivery of the therapeutic agents to the primary tumors as well as metastatic tumors is another important issue for achieving the ideal abscopal effect. Most reported regimens use intratumoral injection to enhance synergistic effect of PTT and chemo/immunotherapy, which is limited to certain tumor types [16–18]. To prime abscopal response, it is needed to develop an all-in-one intravenously injectable nanomedicine strategy to achieve effective systemic co-delivery of various agents to tumor sites.
Current nanocarriers of relatively large sizes may offer limited help because many types of solid tumors are poorly vascularized with small fenestrae and are comprised of dense stroma [19]. To overcome these obstacles, we have designed an ultra-small nanocarrier based on gemcitabine (GEM)-conjugated polymer (PGEM) for effective co-delivery of various agents into tumors. Compared to larger NPs, the small-sized NPs showed enhanced accumulation and penetration in tumors [20]. In this work, a new polydopamine (dp)-coated PGEM nanocarrier was developed for co-delivery of water-soluble chemotherapy drug GEM and water-insoluble immunomodulatory agent NLG919 (IDO inhibitor). IDO is an important immunosuppressive enzyme involved in tryptophan (Trp) depletion, resulting in the apoptosis of effector T cells and the production of regulatory T (Treg) cells [21]. Unlike strategies targeting other immune checkpoint molecules, the IDO-targeted ICB could affect “up-stream” immune responses, including activation of antigen-presenting cells and initial cross presentation of tumor antigens [22]. NLG919 (denoted as NLG) is a potent IDO inhibitor. Combination of NLG and GEM could significantly inhibit tumor growth through both directly killing tumors and enhancing anti-tumor immune response [23]. The facile dp coating strategy could cross-link the NLG-loaded PGEM NPs (N/PGEM) to further improve the stability of the formulation. Furthermore, dp is a bioinspired coating material with excellent biocompatibility and photothermal conversion ability. Combination of dp-mediated PTT with immunochemo-NPs is expected to enhance the tumor suppression effect in both primary and metastasis tumors via abscopal effect (Scheme 1). The physicochemical properties, PTT effect, biodistribution, as well as antitumor and anti-metastatic activity were evaluated in vitro and in vivo. The abscopal antitumor effect and underlying mechanism were also investigated in various bilateral tumor models.
Scheme 1.
Schematic illustration of polydopamine coated N/PGEM NPs for immunochemo-PTT to inhibit both primary and distal metastatic tumor. Systemic administration of N/PGEM/dp NPs could efficiently deliver chemotherapeutic GEM and IDO inhibitor NLG into both primary and distal tumors, and induced the infiltration of tumor antigen-specific CTLs, the recruitment of NK cells and the production of IFN-γ and granzyme B by CD8+ T and NK cells in both tumors. The laser irradiation on the primary tumors could further reinforce the immune response of N/PGEM/dp by increasing more production of IFN-γ and GZB by CTLs and NK cells, leading to enhanced inhibition of both primary and distal tumors.
2. Materials and Methods
2.1. Materials and animals
PGEM polymer was synthesized as described previously [20]. Dopamine hydrochloride (99%) was purchased from Alfa Aesar. Dulbecco’s Modified Eagle’s Medium (DMEM), trypsin-EDTA solution, and 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were all bought from Sigma-Aldrich (MO, U. S. A.). Fetal bovine serum (FBS) and penicillin-streptomycin solution were purchased from Invitrogen (NY, U. S. A.). Antibodies used for flow cytometry were purchased from reputable vendors such as BioLegend and BD Biosciences as detailed in Table S1, Supporting Information. Female C57BL/6 and BALB/c mice (4–6 weeks) were purchased from Charles River Laboratories. The mouse-related experiments were performed in full compliance with institutional guidelines and approved by the Animal Use and Care Administrative Advisory Committee at the University of Pittsburgh.
2.2. Fabrication of polydopamine (dp) coated NLG-loaded PGEM NPs
Film hydration method was used to prepare the NLG-loaded PGEM (N/PGEM) NPs. In brief, PGEM polymer and NLG were mixed in dichloromethane at a ratio of 10:1 (w/w). After evaporating the solvent, nano water was added to hydrate the film to obtain the N/PGEM NPs. The dp-coated NPs were prepared by adding dopamine hydrochloride into the Tris buffer solution (pH=8.5) containing N/PGEM NPs. After stirring at room temperature for 5 h or 16 h, the solution was dialyzed against PBS solution for 24 h to give the N/PGEM/dp-5 and N/PGEM/dp-16 NPs. The drug loading capacity (DLC) and drug loading efficiency (DLE) of NLG were determined by high-performance liquid chromatography (HPLC). The particle size and zeta potential were measured by dynamic light scattering (DLS). The morphologies of the NPs were evaluated by transmission electron microscopy (TEM) with uranyl acetate negative staining.
2.3. Measurement of photothermal performance
The absorption spectra of N/PGEM, N/PGEM/dp-5 and N/PGEM/dp-16 NPs solution (at 2 mg/mL of equivalent dopamine concentration) were recorded at the range of 400~860 nm using a UV-vis spectrophotometer.
N/PGEM, N/PGEM/dp-5 and N/PGEM/dp-16 NPs solution were irradiated for 10 min using a near-infrared laser at 808 nm at a power density of 1.18 W/cm2, with pure water as a negative control. The temperature of solutions was measured every 60 s using a digital thermometer with a thermocouple probe.
2.4. Cytotoxicity assay
PANC02 pancreatic carcinoma cells were seeded into a 96-well plate at a density of 5000 cells/well and incubated in 100 μL of DMEM containing 10% FBS overnight. Afterwards, the cells were treated with PGEM/dp-16 NPs at an equivalent PGEM concentration of 200 μg/mL for 1h, and then irradiated or not for 5 min with a near-infrared laser (808 nm, 1.18 W/cm2). The cells that received laser irradiation but no other treatment were used as controls. Then, the cells were co-stained with fluorescein diacetate (FDA) and propidium iodide (PI), and observed using KEYENCE Fluorescence Microscope BZ-X700.
In a separate study, the PANC02 cells were incubated with PGEM, PGEM/dp-16, N/PGEM and N/PGEM/dp-16 NPs at an equivalent PGEM concentration of 200 μg/mL, irradiated or not for 5 min, and then incubated for another 2 h and 48 h, respectively. Cell viability was evaluated by MTT assay.
2.5. NIR imaging
C57BL/6 mice were each inoculated with 2×105 PANC02 cells at the flank. When the tumors grew up to ~500 mm3, the mice were i.v. administered with DiR-loaded PGEM and PGEM/dp NPs at a DiR concentration of 0.2 mg/mL. At indicated time points, the mice were imaged by IVIS 200 system (Perkin Elmer, USA) at a 60 s exposure time with excitation at 730 nm and emission at 835 nm. The tumors and major organs were also collected for ex vivo imaging.
2.6. In vivo tumor penetration
Fluorophore rhodamine-loaded PGEM and PGEM/dp-16 NPs were intravenously injected into C57BL/6 mice bearing PANC02 tumors, respectively. At 24 h after injection, tumors were excised and frozen sectioned at 10-μm thickness. The sections were fixed in 4% paraformaldehyde prior to immunofluorescence staining. The primary and secondary antibodies used were rat antimouse CD31 (diluted 1:250; catalog no. 553370) and Alexa Fluor 488-conjugated goat anti-rat IgG (diluted 1:500; catalog no. A11006). After washing with PBS, the cell nucleus was stained with DAPI for fluorescence imaging.
2.7. Western blot analysis
PANC02 tumor-bearing mice were intravenously administered with PGEM and PGEM/dp (PGEM dosage: 200 mg/kg) twice at an interval of 2 days, followed by laser irradiation for 5 min (1.18 W/cm2) at 24 h after the second injection. Afterwards, the mice received another two injections of the NPs at an interval of four days. The tumor volumes and body weights were monitored at specific days. At the end of the experiment, tumor tissues were collected and homogenized in RIPA lysis buffer. After lysis at 4 °C for 10 min and then centrifugation (10,000 g) for another 10 min, the supernatant was mixed with loading buffer, and electrophoresed on 10% SDS-PAGE gels, followed by transferring to polyvinylidene difluoride (PVDF) membrane. The membranes were then blocked with 5% non-fat dry milk in Tris-buffer containing 0.05% Tween-20 (TBST) for1 h and incubated with primary antibody rabbit anti-IDO1 (#51851S, Cell Signaling Technology) overnight at 4 °C. After washing three times for 5 min, the membranes were incubated in secondary antibody goat anti-rabbit IgG-HRP at room temperature for 1 h. After washing with TBST three times, the blot was exposed to the SuperSignal West Dura Extended Duration substrate.
2.8. In vivo anti-tumor study
The antitumor effect was evaluated in mice bearing PANC02 tumors, which was established by inoculating 2×105 PANC02 cells into the right flank. When the tumor volume reached ~70 mm3, the mice were intravenously administered with N/PGEM, N/PGEM/dp and PGEM/dp NPs (NLG dosage: 10 mg/kg) twice at an interval of 2 days, followed by laser irradiation for 5 min (1.18 W/cm2) at 24 h after the second injection. Afterwards, the mice received another two injections of the NPs at an interval of four days. The tumor volumes and body weights were monitored at specific days. After treatments, the tumor tissues were fixed in 10% formalin and dehydrated. Then they were embedded in paraffin and sectioned at 5 μm, followed by staining with Ki67-specific antibody for histopathological examination. The biochemical parameters, including AST, ALT, and creatinine, were evaluated by a standard spectrophotometric method.
2.9. In vivo anti-metastasis study
The anti-metastasis activity was first evaluated in an early metastatic PANC02 tumor model. The primary tumor was established by inoculating 2×105 PANC02 cells into the right flank, and when the primary tumor volume reached ~150 mm3, 2×105 PANC02 cells were inoculated into the left flank to establish the secondary tumors. Then, the mice were intravenously administered with N/PGEM, N/PGEM/dp-16 and PGEM/dp-16 NPs (NLG dosage: 20 mg/kg) twice at an interval of 2 days, followed by laser irradiation on the primary tumors for 5 min (1.18 W/cm2) at 24 h after the second injection. Afterwards, the mice received another two injections of the NPs at an interval of four days. The tumor volumes of both flanks and body weights were monitored at specific days. Both tumors were weighed at the end of the experiment. The anti-metastasis activity was also evaluated in early metastatic 4T1.2 breast cancer model, which was established by inoculating 2×105 4T1.2 cells into mammary fat pad of female BALB/c mice. Tumor establishment and treatment regimen were similar to those described for PANC02 model.
In addition, the anti-metastasis activity was evaluated in a late-stage metastatic PANC02 tumor model. The model was established by inoculating 2×105 PANC02 cells into both flanks of the C57BL/6 mice at same time and mice were similarly treated as described for the early-stage metastatic PANC02 model.
2.10. Quantification of tumor-infiltrating lymphocytes by flow cytometry
C57BL/6 mice bearing primary and (I think you later use small distal tumors as well) distal PANC02 tumors received various treatments via i.v. administration every 3 days for 3 times. Tumors and spleen were collected at 24 h following the last treatment. Single cell suspensions were prepared and red blood cells were lysed. Then the cells were stained with various antibodies for flow cytometry analysis with FlowJo software (Tree Star Inc.) [24].
3. Results
3.1. Preparation and characterization of N/PGEM/dp NPs
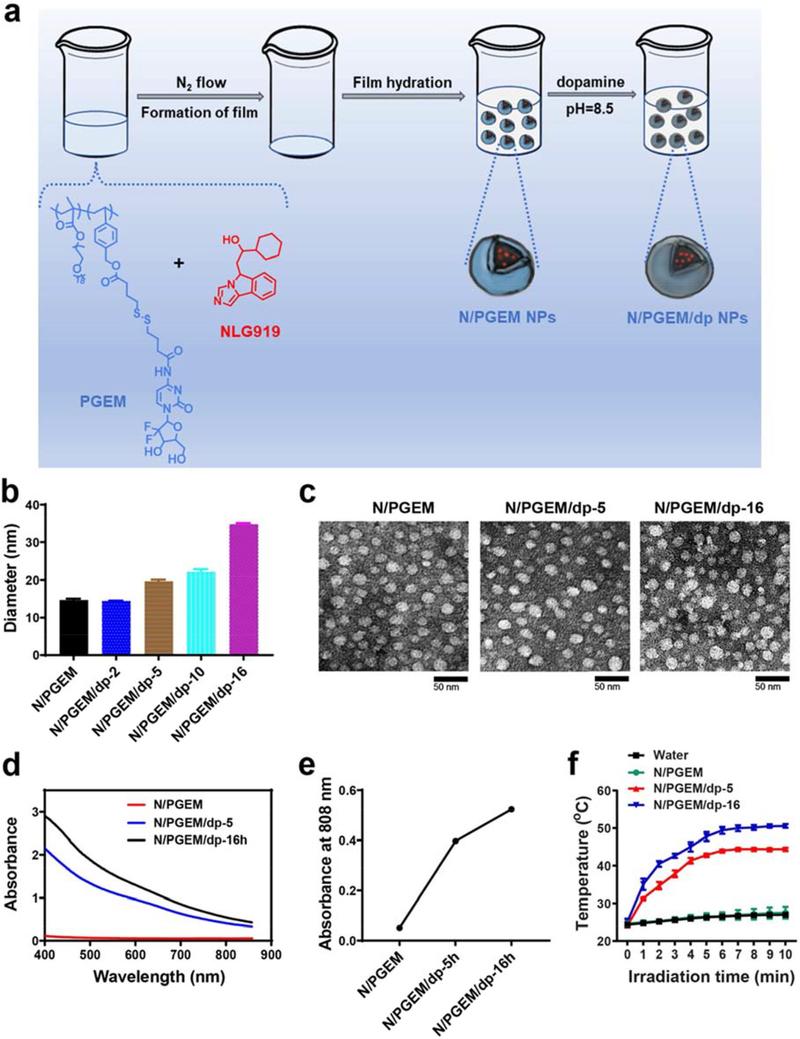
N/PGEM NPs were prepared by film hydration method at a PGEM carrier/NLG ratio of 10:1 (w/w, Figure 1a). The particle size determined by DLS was 14.6 ± 0.39 nm (Table 1 and Figure 1b). Several N/PGEM/dp NPs were prepared by incubating N/PGEM NPs with dopamine for different time periods (2 h, 5 h, 10 h, 16 h) under an oxidative condition (pH 8.5). The particle sizes were determined by DLS (Figure 1b). The color of solution became brown after 2 h, but there was no obvious change in size. After 5 h or 10 h coating, the particle size increased to ~20 nm. After 16 h incubation, the obtained NPs turned black in color (Figure S1, Supporting Information), and the particle size increased to ~30 nm. To investigate the effect of the thickness of dp coating layer on the biophysical and biological properties of NPs, N/PGEM/dp-5 (incubation time: 5 h) and N/PGEM/dp-16 (incubation time: 16 h) NPs with respective particle sizes of 20.03 ± 0.27 nm and 34.77 ± 0.33 nm (Figure S2, Supporting Information) were used in the following studies. To confirm that the increased size comes from the dp coating layer instead of the polydopamine NPs itself, we examined the sizes after a same amount of dopamine was added into the solution without PGEM carrier. As shown in Figure S3, without PGEM micelles as the substrate, dopamine self-polymerized into large aggregates, which were precipitated in the solution, while no large particles were observed in the solution of N/PGEM/dp NPs, further confirming that there are no self-polymerized polydopamine NPs and the increased particle size is attributed to the dp coating layer. In addition, the surface charge of the NPs was slightly changed after dp coating (Table 1). The morphologies of N/PGEM/dp NPs were examined by transmission electron microscopy (TEM, Figure 1c). All the NPs showed spherical morphologies.
Figure 1.
(a) Schematic illustration of preparation process of N/PGEM/dp NPs. (b) Particle sizes and (c) morphologies of N/PGEM, N/PGEM/dp-5 and N/PGEM/dp-16 NPs by DLS and TEM (Scale bar: 50 nm). (d) The UV-Vis spectra of different NPs. (e) The absorbance at 808 nm of different NPs. (f) Temperature curves versus irradiation time with 808 nm laser (1.18 W/cm2).
Table 1.
Particle size and zeta potential of N/PGEM and N/PGEM/dp NPs.
| NPs | PDI | Size | Zeta potential |
|---|---|---|---|
| N/PGEM | 0.108±0.031 | 14.6 ± 0.39 nm | 1.66±0.69 mV |
| N/PGEM/dp-5 | 0.210±0.027 | 20.03 ± 0.27 nm | −3.19±0.15 mV |
| N/PGEM/dp-16 | 0.179±0.016 | 34.77±0.33 nm | −4.46±1.26 mV |
|
| |||
Then the NIR absorption of N/PGEM/dp NPs was evaluated. A NIR absorption was observed for N/PGEM/dp NPs in a broad range of 400–860 nm, whilst the N/PGEM NPs showed very weak absorption (Figure 1d). Particularly, the absorption at 808 nm (the wavelength of the laser for PTT) was much stronger for N/PGEM/dp NPs with a thicker polydopamine coating, and N/PGEM/dp-16 NPs showed a higher level of absorption at 808 nm than N/PGEM/dp-5 NPs (Figure 1e). The photothermal conversion properties of N/PGEM/dp NPs were then determined by using an 808 nm NIR laser (Figure 1f). After 10 min of laser irradiation, PBS and N/PGEM NPs solution only showed slight temperature changes, while the temperatures of N/PGEM/dp-5 NPs and N/PGEM/dp-16 NPs solutions were significantly increased to 43 °C and 50 °C, respectively (Figure 1f). The higher temperature change of N/PGEM/dp-16 NPs was mainly due to the stronger NIR absorption. The infrared thermal images of N/PGEM and N/PGEM/dp-16 NPs solutions following 5 min of laser irradiation further confirmed the remarkable photothermal conversion ability of polydopamine coated N/PGEM NPs (Figure S4, Supporting Information).
3.2. Effects of NPs on cell viability
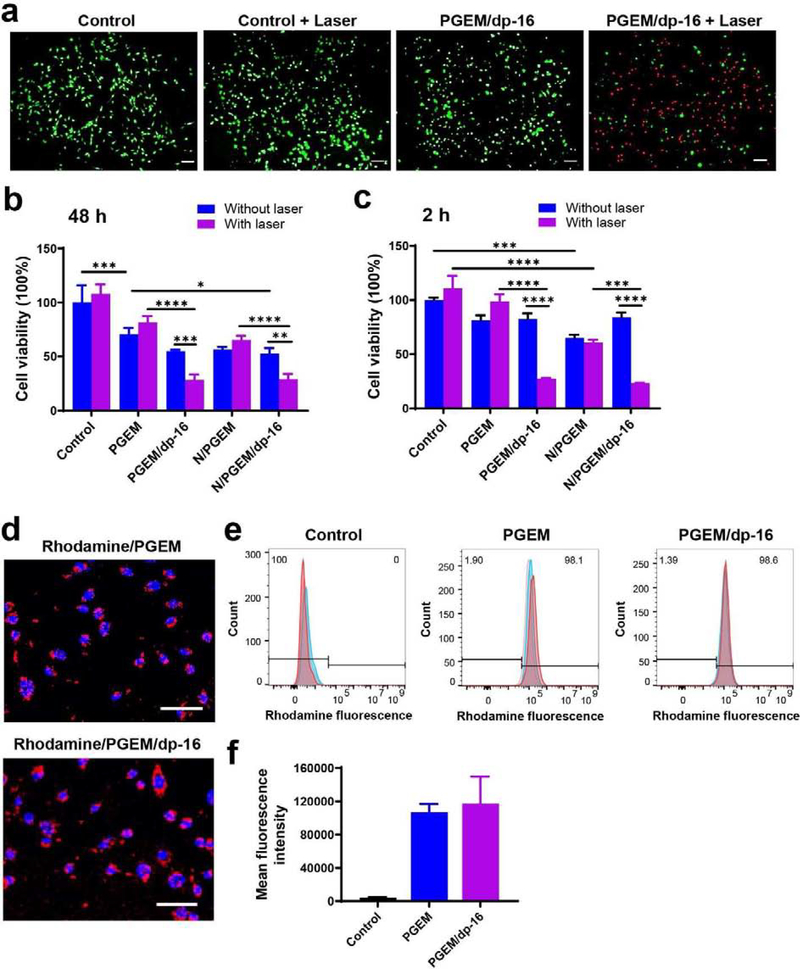
The remarkable ability of dp-coated NPs to convert NIR light into heat suggests a potential killing effect on tumor cells. Thus, the effect of dp-coated NPs on cancer cell viability was investigated by LIVE/DEAD assay in the presence/absence of laser irradiation. Figure 2a shows the fluorescence images of PANC02 cells co-stained with fluorescein diacetate (FDA) and propidium iodide (PI) after various treatments. The control cells showed widespread green fluorescence, suggesting negligible cell death after laser irradiation. In comparison, most of the cells treated with PGEM/dp-16 NPs showed red fluorescence after laser irradiation, suggesting PGEM/dp-16 NPs-mediated PTT could efficiently kill cancer cells within 5 min.
Figure 2.
(a) Fluorescence images of PANC02 cells treated with laser irradiation alone, PGEM/dp-16 NPs and PGEM/dp-16 plus laser irradiation, followed by being stained with fluorescein diacetate (Green) and propidium iodide (red). Green and red indicate live and dead cells, respectively. Scale bar: 100 μm. (b-c) Relative viabilities of PANC02 cells after various treatments at (b) 48 h and (c) 2h. (d) Fluorescence images of PANC02 cells treated with rhodamine-loaded PGEM and PGEM/dp-16 NPs at 4 h. Scale bar: 100 μm. (e) Flow cytometry histogram and (f) mean fluorescence intensity of PANC02 cells treated with rhodamine-loaded PGEM and PGEM/dp-16 NPs at 4 h.
The in vitro antitumor effect of dp-coated NPs combined with PTT was further investigated by MTT assay. As shown in Figure 2b, laser irradiation alone showed no effect on PANC02 cells. Cells treated for 48 h with PGEM or N/PGEM NPs showed a decreased viability of 70% and 57%, respectively. Addition of laser irradiation to these treatments led to minimal changes in cell viability. For the cells treated with dp-coated PGEM (PGEM/dp-16) and NLG/PGEM (N/PGEM/dp-16) NPs, laser irradiation remarkably improved the cytotoxicity, and the cell viability decreased from 52~54% to 28% after laser irradiation. To better evaluate the contribution of PTT to the overall cytotoxicity, we reduced the drug exposure time to 2h prior to PTT to minimize the impact from PGEM (Figure 2c). With such a short time treatment, PGEM and N/PGEM NPs showed very limited cytotoxic effects with more than 80% cell viability. Again, dp coating didn’t affect the cytotoxic effect of PGEM and N/PGEM NPs, and laser irradiation caused minimal cytotoxic effects on cells treated with PGEM and N/PGEM NPs. In contrast, the cell viability drastically decreased to 23~27% by combination of dp coating and laser irradiation, clearly demonstrating the PTT effect with the dp-coated PEGM NPs.
The cellular uptake of PGEM and PGEM/dp-16 NPs was also evaluated by using rhodamine as a fluorescence probe. Fluorescence imaging (Figure 2d) and flow cytometry (Figure 2e&f) demonstrated that both PGEM and PGEM/dp-16 NPs were efficiently taken up by PANC02 cells at 4 h. There is no significant difference in the mean fluorescence intensity between the two groups.
3.3. In vivo biodistribution and penetration
In vivo distribution of dp coated NPs was examined in PANC02 tumor bearing mice using near-infrared imaging system. A fluorescence dye DiR was loaded into the PGEM, PGEM/dp-5, and PGEM/dp-16 NPs, which were then i.v. injected into the mice for NIR imaging with free DiR as the control. At 1 h after administration, no obvious fluorescence signals were observed in the tumor treated with free DiR, and the signals were mainly accumulated in the lung, liver, and spleen (Figure S5, Supporting Information). In contrast, mice treated with DiR/PGEM and DiR/PGEM/dp-5 showed stronger signals in the tumors. Interestingly, there were no obvious signals in all the organs treated with DiR/PGEM/dp-16 NPs. At 15 and 24 h after administration, PGEM-treated mice showed high accumulation in the tumor in addition to spleen and lung (Figure 3a). In comparison, mice treated with PGEM/dp-5 and PGEM/dp-16 showed lower fluorescence signals in liver, spleen, and lung, indicating that dp coating could decrease the non-specific uptake by these organs. Compared to early time points, the signals in tumors were decreased at 48 h in all treatment groups. Nonetheless, there were still significant amounts of signals in the tumor treated with PGEM/dp-16 NPs, significantly higher than those in tumors treated with PGEM and PGEM/dp-5 NPs (Figure S6, Supporting Information), suggesting that a thick dp coating could improve the retention time of NPs in the tumor.
Figure 3.
(a) In vivo and ex vivo NIR images of PANC02 tumor-bearing mice treated with free DiR, DiR/PGEM, DiR/PGEM/dp-5 and DiR/PGEM/dp-16 NPs at different time points (15, 24 and 48 h). (b) Tumor penetration profiles of rhodamine-loaded PGEM and PGEM/dp-16 NPs with CD-31 antibody to stain blood vessels (green). Scale bar: 100 μm.
It has been reported that small-sized NPs have an advantage in deep penetration into tumors [25]. In our previous study, we found PGEM NPs with small particle size of ~13 nm showed better tumor penetration compared to larger NPs [20]. After dp coating, the particle size increased to ~30 nm. To investigate if dp coating will affect the penetration capability of small-sized PGEM NPs, we evaluated the tumor penetration capability of the PGEM/dp-16 NPs with PGEM NPs as a control. Fluorescence probe rhodamine was loaded into the NPs. At 24 h post-injection, the tumor sections were prepared and co-stained with Ab specific for blood vessel marker CD31. As shown in Figure 3b, both PGEM NPs and PGEM/dp-16 NPs showed widespread red signals outside of the blood vessels, suggesting that dp coating didn’t affect the excellent tumor penetration capability of PGEM NPs.
3.4. In vivo antitumor activity
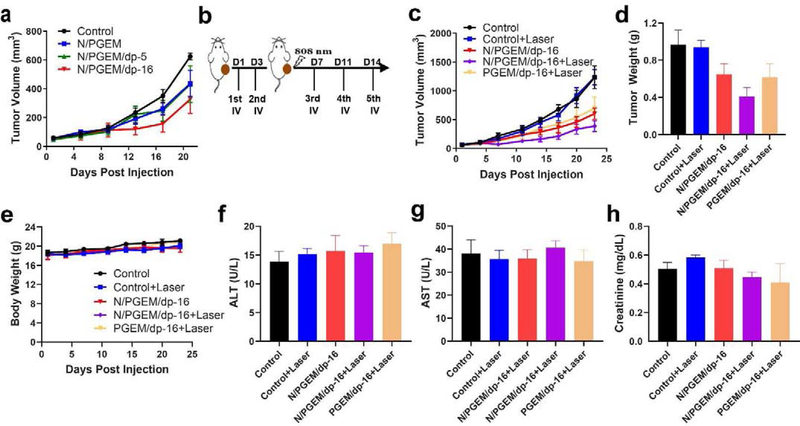
We first evaluated the effect of dp coating on the antitumor activity of the NPs in PANC02 tumor model (Figure 4a) using relatively low doses of PGEM (100 mg/kg) and NLG (10 mg/kg). N/PGEM and N/PGEM/dp-5 NPs showed similar tumor inhibition effect. In comparison, N/PGEM/dp-16 NPs showed higher tumor inhibition effect, likely due to the improved accumulation of the formulation in the tumors (Figure 3). All of the micellar formulations were well tolerated, and no significant changes were found in mouse body weights (Figure S7, Supporting Information).
Figure 4.
In vivo therapeutic effect and safety profiles in PANC02 model. (a) Tumor volume changes of the mice treated with various formulations at NLG dosages of 10 mg/kg (PGEM carrier: 100 mg/kg). (b) The administration route of PTT combined with NPs. (c) Tumor volume, (d) tumor weight and (e) body weights of the mice after various treatments. Serum levels of alanine aminotransferase (ALT, f), aspartate aminotransferase (AST, g), and creatinine (h) in mice after various treatments.
Then we chose the N/PGEM/dp-16 NPs to evaluate the combination effect of dp coating-mediated PTT immunochemotherapy. When the tumor volumes reached ~80 mm3, the mice were administered with PGEM/dp-16 and N/PGEM/dp-16 NPs (Figure 4b). Two doses were given to mice to increase the amounts of dp-coated NPs delivered to tumors for effective PPT. Mice were irradiated one day after the 2nd treatment and then received another 3 treatments of NPs on day 7, 11, and 14. Figure 4c showed that laser irradiation alone had no effect on tumor growth. Significant tumor growth inhibition was observed in mice treated with PGEM/dp-16 NPs in combination with laser irradiation. N/PGEM/dp-16 NPs showed similar tumor inhibition effect as PGEM/dp-16 NPs plus laser combination. Addition of laser irradiation to N/PGEM/dp-16 NPs led to a further improvement in antitumor activity. Figure 4d showed the weights of tumors collected from different groups at the end of experiment. N/PGEM/dp-16 NPs plus laser irradiation led to the lowest tumor weights, which further confirmed the best tumor inhibition effect of this combination treatment, indicating that incorporation of immunomodulatory agent NLG and laser irradiation are both important for enhancing the therapeutic efficacy. In addition, there were no obvious changes in the mouse body weights following various treatments (Figure 4e). The serum levels of Alanine aminotransferase (ALT), Aspartate aminotransferase (AST) and creatinine in the treatment groups were also similar to those in the control group, suggesting that the treatments showed negligible toxicity to liver and kidney (Figure 4f–h).
3.5. Anti-metastatic tumor activity in early-stage metastatic tumor model
We evaluated the anti-metastasis activity of these treatments in an “early metastatic tumor model” with a primary tumor that receives irradiation and another small tumor that is located at a distal site and does not receive irradiation. The primary tumor was established by subcutaneously inoculating PANC02 cells to the right flank of the mice. When the primary tumor volumes reached 150 mm3, PANC02 cells were similarly injected to the left flank of the mice as the early metastatic tumors. One day later, the mice received the first two injections (IV) and subsequent laser irradiation on the primary tumors, followed by another three injections (Figure 5a). To achieve a better PTT effect and higher efficacy of immunochemotherapy, the dosage of these formulations was doubled compared to an earlier study (Figure 4).
Figure 5.
In vivo therapeutic effect of NPs at the NLG dosage of 20 mg/kg (PGEM carrier: 200 mg/kg) in bilateral tumor model with small distal tumor as early metastasis. (a) The administration route in primary and distal PANC02 tumor model. The tumor received laser irradiation was designated as primary tumors. (b) Primary PANC02 tumor volume and (c) distal tumor volume of the mice after various treatments. (d) Primary PANC02 tumor weight and (e) distal tumor weight at day 19 after various treatments. (f) The body weights of the mice after various treatments. (g) H&E staining of the primary and (h) distal PANC02 tumors after various treatments. Scale bar: 100 μm. (*P<0.05, **P<0.01, ***P<0.001)
First, we evaluated IDO expression levels after treatment with PGEM, PGEM/dp-16 and PGEM/dp-16+Laser by Western blot. We found that that PGEM treatment and PGEM/dp-16 plus laser irradiation could upregulate the IDO expression (Figure S8, Supporting Information), providing a good rationale for including NLG in the combination therapy. It is interesting to note that compared to PGEM group, PGEM/dp-16NPs group was associated with lower expression levels of IDO, suggesting that dp itself may play a role in inhibiting IDO expression, which warrants future studies to better understand the underlying mechanism.
Then, we compared the therapeutic efficacy of PGEM/dp-16 vs PGEM, PGEM/dp-16 vs PGEM/dp-16+Laser, and PGEM/dp-16 vs N/PGEM/dp-16 to investigate the effect of dp coating, laser irradiation and NLG on the overall therapeutic effect (Figure S9, Supporting Information). PGEM/dp-16 NPs showed a little higher tumor inhibition effect than PGEM NPs on both primary and distal tumors. Compared to PGEM/dp-16 NPs, PGEM/dp-16+Laser treatment showed significantly higher inhibition effect on primary tumors, but not on the distal tumors. In addition, we found that the tumor inhibition effect of N/PGEM/dp-16 NPs was much higher than that of PGEM/dp-16 NPs on both tumors, suggesting incorporation of NLG into PGEM/dp-16 NPs significantly improved the anti-tumor and anti-metastasis activity.
The PTT effect of N/PGEM/dp-16 NPs was evaluated by visual assessment of tumor temperature of mice using an infrared thermal camera (Figure S10, Supporting Information). The temperature of the tumor treated with N/PGEM/dp-16 NPs (~47 °C) was higher than the one treated with N/PGEM NPs (~35°C) after 5 min of laser irradiation. Then, we evaluated the combination effect of PTT with N/PGEM/dp-16 NPs mediated immunochemotherapy in early-stage metastatic tumor model (Figure 5b&c). For the primary tumors (Figure 5b), N/PGEM/dp-16 NPs showed higher tumor inhibition effect than N/PGEM NPs, which further confirmed the benefit of dp coating. Combination with irradiation led to an improvement in the antitumor activity for either dp-coated NPs, N/PGEM/dp-16 or PGEM/dp-16 NPs. Yet, N/PGEM/dp-16+Laser treatment showed better tumor inhibition effect than PGEM/dp-16+Laser treatment. All tumors completely regressed at day 16 following the 5th treatment. These data suggest that introduction of NLG could further improve the efficacy of PGEM/dp-16-mediated PTT-chemotherapy. Tumor weights measurements (Figure 5d) and H&E staining analysis (Figure 5g) of the primary tumors further confirmed the results.
Similar to the data in primary tumors, N/PGEM NPs showed significant activity in inhibiting the growth of the distal tumors (Figure 5c). Coating of N/PGEM NPs with dp led to a further improvement in antitumor activity. However, irradiation of the primary tumor had no impact on the antitumor activity of N/PGEM/dp-16 NPs on the distal tumors. Little control of tumor growth was seen in mice subjected to treatment with PGEM/dp-16 plus irradiation of primary tumors.
Figure 5e shows the weights of distal tumors collected at the end of the experiment, which was consistent with the results of tumor size measurements. H&E staining of tumor tissues further demonstrated that both N/PGEM/dp-16 NPs and N/PGEM/dp-16+Laser were effective in inhibiting the growth of the small distal tumors (Figure 5h). In addition, there were only slight changes in the body weights during the entire experiment, suggesting a low systemic toxicity of the treatments (Figure 5f). Similar results were observed in another 4T1.2 breast cancer model (Figures S11~13, Supporting Information). In addition, we noticed that the control mice with/without laser irradiation showed obvious metastases in the lung (Figure S14, Supporting Information), while the mice treated with N/PGEM/dp-16 NPs with/without laser irradiation showed no noticeable lung metastases, indicating both N/PGEM/dp-16 NPs and N/PGEM/dp-16+Laser were effective in inhibiting the formation of lung metastases in 4T1.2 model.
3.6. Anti-metastatic tumor activity and the underlying mechanism in late-stage metastatic tumor model
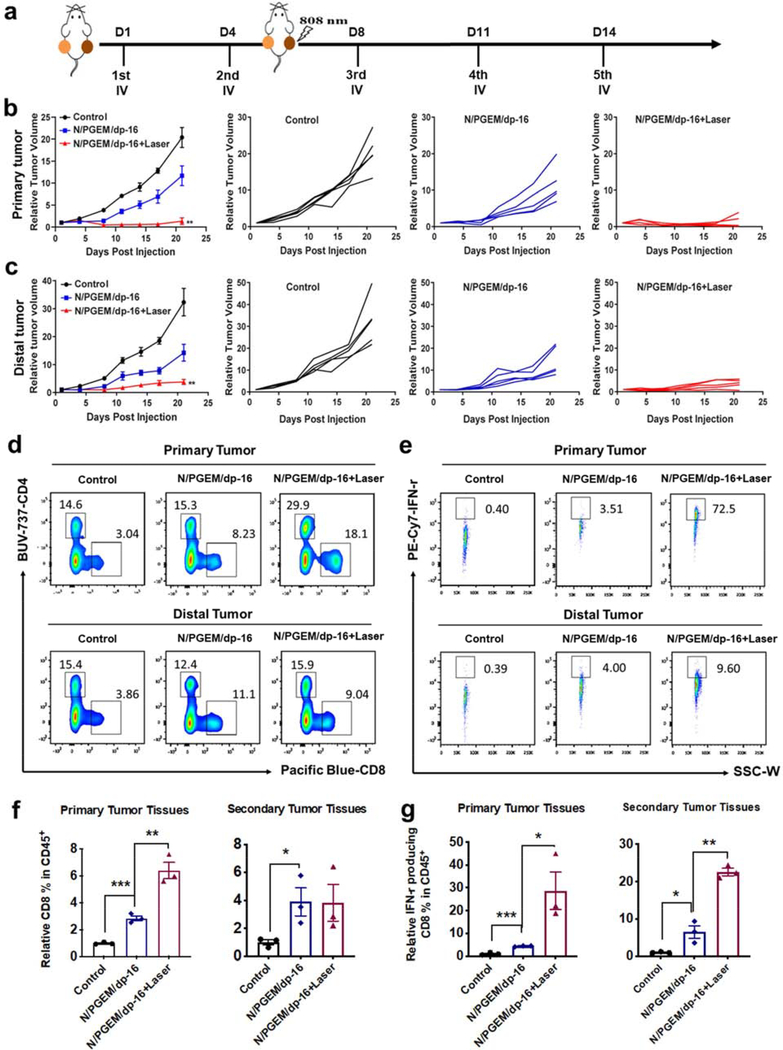
To investigate whether N/PGEM/dp-16 NPs can inhibit the late metastatic tumor, a bilateral PANC02 model with two tumors of similar sizes at both flanks was employed (Figure 6a). N/PGEM/dp-16 NPs or N/PGEM/dp-16 NPs plus laser irradiation could inhibit the growth of both primary and distal tumors. Compared to N/PGEM/dp-16 NPs, N/PGEM/dp-16 NPs plus laser irradiation not only dramatically suppressed the growth of primary tumors (Figure 6b), but also significantly inhibited the growth of unirradiated distal tumors (Figure 6c). Although the distal tumors didn’t receive the laser irradiation, N/PGEM/dp-16 NPs plus laser irradiation on the primary tumors led to significant growth inhibition of distal tumors, which was much more dramatic than that by N/PGEM/dp-16 NPs treatment alone, suggesting a strong abscopal antitumor effect of N/PGEM/dp-16 NPs combined with PTT. Due to the larger size of distal tumors in the late metastasis model, the effect of N/PGEM/dp-16 NPs alone was not as effective as their impact on a smaller distant tumor (Figure 5a).
Figure 6.
In vivo therapeutic effect and FCM analysis of tumor-infiltrating T cells in the late-stage metastatic PANC02 model after various treatments (NLG dosage: 20 mg/kg). (a) The administration route in primary and distal tumor model. (b) The overall average primary tumor volume of each group and tumor growth of individual mouse. (c) The overall average distal tumor volume of each group and tumor growth of individual mouse (**P<0.01 vs NLG/PGEM/dp-16). (d) Representative flow cytometric plots of CD8+ T within the gated CD45+ population in primary and distal tumors. (e) Representative flow cytometric plots of IFN-γ + CD8+ T within the CD8+CD45+ TILs in primary and distal tumors. (f) Percentages of CD8+ T within the gated CD45+ population in primary and distal tumors. (g) Percentages of IFN-γ + T cells within CD8+CD45+ TILs in primary and distal tumors. (*P<0.05; **P<0.01; ***P<0.001)
To elucidate the underlying mechanisms in late-stage metastatic tumor model, we first examined the intratumoral infiltration of cytotoxic T lymphocytes (CTLs) in the primary and distal tumors after treatments of N/PGEM/dp-16 NPs with and without laser irradiation (Figure 6d–g). Compared to the control group, more CD8+ T cells were found in both primary and distal tumors after treatment of N/PGEM/dp-16 NPs and N/PGEM/dp-16 plus laser (Figure 6d&f). Laser irradiation efficiently increased the intratumoral infiltration of CD8+ T in the primary tumors, but showed a negligible effect on the infiltration of CD8+ T cells in distal tumor. The production of anti-tumor cytokine interferon-γ (IFN-γ) in CTLs was then evaluated to address whether the N/PGEM/dp-16 NPs + Laser treatment could further promote the activation and function of CD8+ T cells. As shown in Figure 6e&g, N/PGEM/dp treatment enhanced the production of IFN-γ by CD8+ T in the primary and distal tumors. Importantly, laser irradiation led to further increases in IFN-γ production by CD8+ T cells in both primary and distal tumors. These results demonstrated the increased antitumor immunity of the combination treatment over N/PGEM/dp-16 NPs alone.
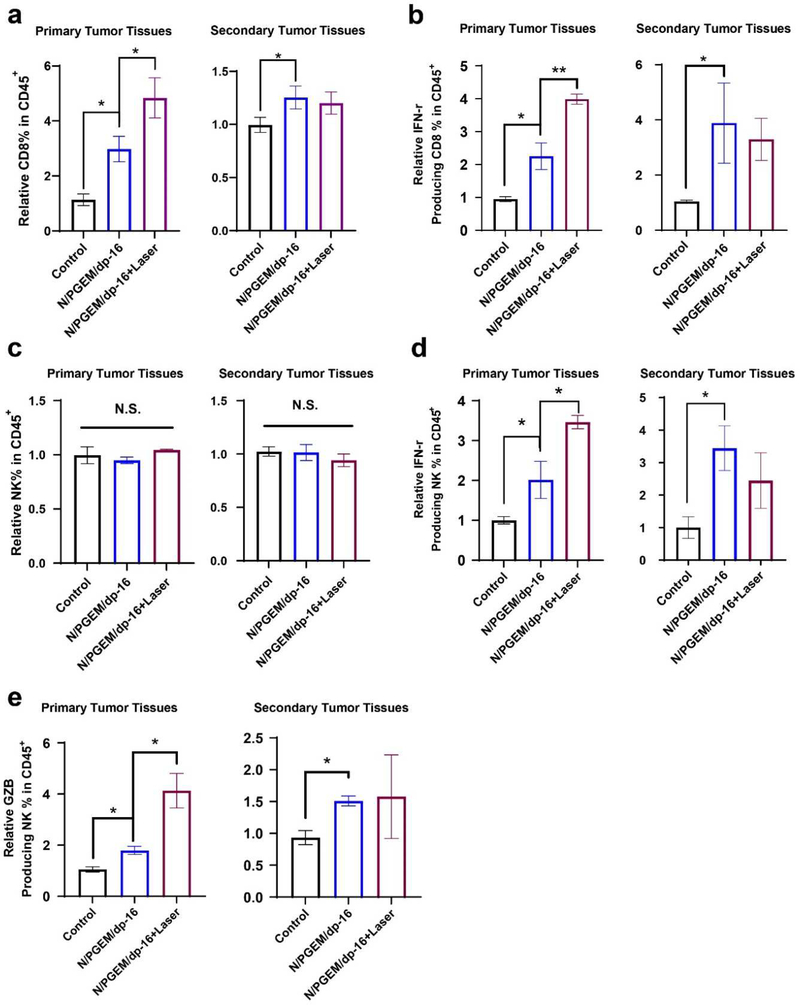
Natural Killer (NK) cells are innate lymphocytes involved in the early immune response. The percentages of NK cells in the primary and distal tumors after the two treatments were also investigated (Figures S15, Supporting Information). As shown in Figure 7a&d, N/PGEM/dp-16 treatment led to increased percentages of NK cells in both tumors. Combination with laser irradiation had no further impact on the total numbers of NK cells in both tumors. NK cells generate several chemokines and cytokines that coordinate innate and adaptive immune responses. IFN-γ is mainly produced by NK cells in the early defense against tumors before its production by antigen-specific T-cells [26]. Thus, IFN-γ production by NK cells was evaluated in primary and distal tumors after various treatments. As shown in Figure 7b&e, the percentages of IFN-γ-producing NK cells in both tumors were increased after treatment with N/PGEM/dp-16 NPs. The production of IFN-γ was further increased following combination with laser irradiation. Granzyme B (GZB) is a cytotoxic serum protease protein from NK cells that can trigger apoptosis of tumor cells. It is very important for optimal NK cell-mediated tumor control in vivo [27]. Figure 7c&f show the flow cytometric plots and percentages of GZB-positive NK cells in primary and distal tumors. Both treatment groups showed increased percentages of GZB positive NK cells. In addition, higher percentages of GZB positive NK cells were observed in both tumors in combination group compared to the group of N/PGEM/dp-16 alone. These results suggested that the addition of laser irradiation to N/PGEM/dp-16 is much more effective than N/PGEM/dp-16 alone in promoting innate and adaptive antitumor immune responses, which shall contribute significantly to a strong abscopal antitumor effect in distal tumors in the combination treatment.
Figure 7.
FCM analysis of NK cells in the late-stage metastatic PANC02 model of the mice after various treatments. (a) Representative flow cytometric plots and (d) percentages of NK cells in primary and distal tumors. (b) Representative flow cytometric plots and (e) percentages of IFN-γ+ NK cells in primary and distal tumors. (c) Representative flow cytometric plots and (f) percentages of GZB+ NK cells in primary and distal tumors. (*P<0.05; **P<0.01)
To further confirm the immune-modulating effect of NLG919 in the NPs, we also compared the tumor-infiltrating immune cells in mice treated with N/PGEM/dp-16+Laser with those in mice treated with PGEM/dp-16+Laser (Figures S16, Supporting Information). Mice treated with N/PGEM/dp plus laser showed higher percentages of CD8+ T cells, IFN-γ-positive CD8+ T cells, NK cells and IFN-γ-producing NK cells in primary tumor compared to mice treated with PGEM/dp-16 plus laser. There were no significant differences in these cells between the two groups in the distal tumors except for the higher percentages of IFN-γ-positive CD8+ T cells in mice treated with N/PGEM/dp-16 plus laser. These results indicated that incorporation of NLG in the system could enhance the anti-tumor immunity, especially for the primary tumor. Moreover, we evaluated if N/PGEM/dp-16 delivery system could promote the immune-modulating effect of NLG919 (Figures S17, Supporting Information). N/PGEM/dp-16 treatment led to more CD8+ T cells in both primary and distal tumors compared to N/PGEM treatment, suggesting dp coating could increase the number of CD8+ T cells in both tumors. However, there were no obvious differences in other tumor-infiltrating immune cells between the two groups (data not shown).
To investigate why the abscopal effect was more dramatic in late-stage metastatic tumor model than in early-stage metastatic tumor model, we also evaluated the intratumoral infiltration of CTLs and NK cells in the early-stage metastatic tumor model (Figure 8). Compared to the control group, more CD8+ T cells (Figure 8a) and IFN-γ positive CD8+ T cells (Figure 8b) were found in primary and distal tumors after treatment of N/PGEM/dp-16 NPs. Laser irradiation efficiently increased the intratumoral infiltration of CD8+ T and the production of IFN-γ by CD8+ T in the primary tumors, but showed a negligible effect on the infiltration of CD8+ T cells in the distal tumors. In addition, N/PGEM/dp-16 treatment with or without laser irradiation had no obvious impact on the total numbers of NK cells in both tumors (Figure 8c). However, N/PGEM/dp-16 treatment increased the percentages of IFN-γ-producing NK cells (Figure 8d) and GZB positive NK cells (Figure 8e) in both tumors. Laser irradiation further increased the production of IFN-γ and GZB in the primary tumors, but not in the distal tumors. These results demonstrated that the addition of laser irradiation to N/PGEM/dp-16 is less effective in promoting antitumor immune responses in early-stage metastatic tumors compared to late-stage metastatic tumors, which might be a possible explanation for the weak abscopal antitumor effect in early-stage metastatic tumor model.
Figure 8.
FCM analysis of (a) CD8+ T, (b) IFN-γ + CD8+ T, (c) NK cells, (d) IFN-γ+ NK and (e) GZB+ NK cells in the early-stage metastatic PANC02 model of the mice after various treatments. (*P<0.05; **P<0.01)
4. Discussion
Recently, combination of PTT with other therapies such as chemotherapy or immunotherapy has attracted increasing attention for the treatment of malignant tumors [28–30]. For example, PTT could enhance the sensitivity of tumor cells to chemotherapy and anti-PD-1/PD-L1 therapy [13, 31]. PTT has also been reported to improve the therapeutic effect of immunotherapy by facilitating the accumulation of CAR T cells in solid tumors [32]. In this work, PTT combined with IDO inhibition and GEM-based chemotherapy was achieved through an all-in-one NPs, in which an ultra-small sized PGEM prodrug carrier was used to load IDO inhibitor NLG, and then coated with photosensitive dp. Melanin-like dp is a bioinspired polymer with excellent biocompatibility [33]. It can be easily made from simple oxidization and self-polymerization of dopamine monomer under alkaline conditions. In addition, as a mimic of the adhesive proteins, dp is able to stick to different types of surfaces of inorganic and organic materials [34, 35]. Up to date, most studies were focused on using dp to coat inorganic NPs and liposome, and there are limited reported studies of using dp to coat micelles for PTT [36–40]. Our study filled the gap for dp-coated micelles for immunochemo-PTT combination therapy. In addition, the entire preparation process is simple without exhaustive steps, which is beneficial for future clinical translation.
There are several advantages for surface coating of PGEM NPs using dp. Dp-coated PGEM NPs still retained small size (<35 nm) and were as effective as PGEM NPs (~13 nm) in deep penetration into tumors (Figure 3). Meanwhile, compared to PGEM NPs, dp coated NPs showed increased retention time in the tumor, and decreased non-specific uptake by the liver, spleen and lung, which was probably due to the improved stability of dp-coated NPs and/or the changes of NPs surface chemistry [41]. In addition, dp coating endowed the NPs photothermal ability, which could efficiently kill tumor cells in vitro (Figure 2) and in vivo (Figure 5) by an NIR laser-mediated PTT effect. Furthermore, combination of dp coating-mediated PTT with immune-chemotherapeutic N/PGEM NPs could significantly inhibit the growth of primary tumor as well as early- and late-staged metastatic tumors. More importantly, PTT could promote both innate and adaptive immune responses of N/PGEM/dp in primary and late-staged metastatic tumors, leading to a strong abscopal effect.
The abscopal effect was initially used to describe the ability of localized radiation to induce an antitumor response that kills distal tumors. Abscopal effect has been extensively explored in the combination of radiation therapy (RT) and immunotherapy due to its high potential to improve the overall survival and progression-free survival of patients with malignant metastatic tumors [42]. The treatment regimen such as the dosages and the timing of the two treatment modalities significantly influences the abscopal response of the patients. However, the abscopal effect of PTT, especially combined with other therapies has not been fully investigated. The released tumor antigens from the dying primary tumor cells following PTT have been reported to elicit systemic anti-tumor response, which shows potential abscopal effect to eliminate metastatic tumors [43–45]. However, PTT alone often leads to very weak and not durable abscopal effect [46, 47]. Here, we found that PTT combined with N/PGEM immunochemotherapy showed potent abscopal effect, and the abscopal effect is tumor size dependent. In a late-stage metastasis model with large distal tumors, treatment of primary tumors with N/PGEM/dp-16 plus laser irradiation showed significant inhibition of primary and distal tumors, compared to N/PGEM/dp treatment alone, demonstrating a drastic abscopal effect (Figure 6). However, in an early-stage metastasis model with small distal tumors, very limited abscopal effect was observed: comparable growth inhibition was seen in distal tumors treated with N/PGEM/dp-16 treatment alone and in combination with laser irradiation (Figure 5). There are two possible reasons for the difference between the two models. One is that substantial tumor growth inhibition of the small metastatic tumors was already achieved with N/PGEM/dp-16 treatment alone. Further inhibition of the residual tumors may require a more drastic immune response or a different strategy such as the one that is targeted to cancer stem cells. On the other hand, N/PGEM/dp-16 treatment alone only showed limited impact on the large distal tumors, which may benefit more from the PTT-mediated abscopal effect. We are currently testing whether addition of PD-L1 antibody may improve the abscopal effect on small distal tumors. Another reason could be that the distal tumors with different sizes showed different uptake of N/PGEM/dp-16 NPs and different infiltration of immune cells, which resulted in different levels of anti-tumor immune response (Figure 6~8).
The underlying mechanism of N/PGEM/dp-16 mediated PTT-immunochemotherapy in late-stage metastasis model is illustrated in Scheme 1. Systemic administration of N/PGEM/dp-16 NPs could efficiently deliver chemotherapeutic GEM and IDO inhibitor NLG into both primary and distal tumors. This immunochemotherapy induced the infiltration of tumor antigen-specific CTLs and the production of IFN-γ by CD8+ T in both tumors. It also induced the recruitment of NK cells and increased cytotoxic function (granzyme B expression) and cytokine production (IFN-γ) of NK cells in both tumors. Both innate and adaptive immune pathways were activated to destroy the primary and distal tumors. The irradiation on the primary tumors could further reinforce the immune response of N/PGEM/dp-16 by increasing more production of IFN-γ and GZB by CTLs and NK cells, leading to enhanced inhibition of both primary and distal tumors. We hypothesize that a strong immune response was initially induced in primary tumors following PTT. Factors that are produced in the primary tumors and are effective in promoting IFN-γ and GZB production are subsequently transported to distal tumors through mechanisms like exosomes and amplify the immune response in distal tumors [48]. We are surprised to notice that increased infiltration of CD8+ T cells following laser irradiation was only seen in primary but not distal tumors, which is different from other reported studies related to abscopal effect. It is unclear whether it is due to the tumor type, the specific immunochemotherapy, or the dose of laser irradiation used in our study. More studies are needed to better understand the underlying mechanisms.
5. Conclusion
In summary, we have developed a biocompatible dp-coated PGEM NPs loaded with IDO inhibitor NLG919 for cancer immunochemo-PTT. N/PGEM/dp NPs showed excellent photothermal conversion ability, and laser irradiation efficiently inhibited the tumor cell growth in vitro and in vivo. Besides, dp coating increased the retention time in the tumor and decreased the non-specific uptake by liver, spleen and lung, yet without affecting the tumor penetration capability of PGEM, which resulted in an improved antitumor activity compared to the N/PGEM NPs without coating. In early metastasis model with small distal tumors, introduction of NLG and dp coating improved the inhibition effect on both primary and distal tumors. Combination with laser irradiation further enhanced the inhibition effect on primary tumor, but didn’t show obvious abscopal antitumor effect. When the initial volume of distal tumor was sufficiently large, a more dramatic abscopal antitumor effect was achieved, resulting in a significant growth inhibition of both primary tumor and the unirradiated distal tumor. Furthermore, laser irradiation can amplify the immunochemo-NPs-mediated immune responses in late-stage metastasis model, leading to more production of IFN-γ and GZB by CD8+ T and NK cells in both tumors. These results underscore that introduction of NLG and dp coating play an important role in improving the anti-metastatic activity for early metastatic tumors, and combination with PTT could further improve the abscopal effect for late metastatic tumor.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health grants R01CA174305, R01CA219399, R01CA223788 and a grant from Shear Family Foundation.
Footnotes
Declaration of competing interest
There are no real or potential conflicts of interest associated with the present manuscript.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information files. Correspondence and requests for materials should be addressed to J.S. or S.L.
References
- [1].Guan X Cancer metastases: challenges and opportunities. Acta pharmaceutica sinica B. 2015;5:402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jiang K, Song X, Yang L, Li L, Wan Z, Sun X, et al. Enhanced antitumor and anti-metastasis efficacy against aggressive breast cancer with a fibronectin-targeting liposomal doxorubicin. J Control Release. 2018;271:21–30. [DOI] [PubMed] [Google Scholar]
- [3].Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. [DOI] [PubMed] [Google Scholar]
- [4].Auslander N, Zhang G, Lee JS, Frederick DT, Miao B, Moll T, et al. Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nat Med. 2018;24:1545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discovery. 2019;18:197–218. [DOI] [PubMed] [Google Scholar]
- [6].Vareki SM. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. Journal for immunotherapy of cancer. 2018;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang C, Wang J, Zhang X, Yu S, Wen D, Hu Q, et al. In situ formed reactive oxygen species–responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci Transl Med. 2018;10:eaan3682. [DOI] [PubMed] [Google Scholar]
- [8].McGrath AJ, Chien Y-H, Cheong S, Herman DA, Watt J, Henning AM, et al. Gold over branched palladium nanostructures for photothermal cancer therapy. ACS Nano. 2015;9:12283–91. [DOI] [PubMed] [Google Scholar]
- [9].Zhou F, Wu S, Song S, Chen WR, Resasco DE, Xing D. Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials. 2012;33:3235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhao J, Wan Z, Zhou C, Yang Q, Dong J, Song X, et al. Hyaluronic acid Layer-By-Layer (LbL) nanoparticles for synergistic chemo-phototherapy. Pharm Res. 2018;35:196. [DOI] [PubMed] [Google Scholar]
- [11].Liu R, Hu C, Yang Y, Zhang J, Gao H. Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharmaceutica Sinica B. 2019;9:410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Peng J, Xiao Y, Li W, Yang Q, Tan L, Jia Y, et al. Photosensitizer micelles together with IDO inhibitor enhance cancer photothermal therapy and immunotherapy. Advanced science. 2018;5:1700891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang L, Li Y, Du Y, Zhang Y, Wang X, Ding Y, et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat Commun. 2019;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun. 2018;9:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nature communications. 2018;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Han Y, Pan H, Li W, Chen Z, Ma A, Yin T, et al. T cell membrane mimicking nanoparticles with bioorthogonal targeting and immune recognition for enhanced photothermal therapy. Advanced Science. 2019;6:1900251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jia YP, Shi K, Yang F, Liao JF, Han RX, Yuan LP, et al. Multifunctional Nanoparticle Loaded Injectable Thermoresponsive Hydrogel as NIR Controlled Release Platform for Local Photothermal Immunotherapy to Prevent Breast Cancer Postoperative Recurrence and Metastases. Advanced Functional Materials. 2020:2001059. [Google Scholar]
- [19].Adiseshaiah PP, Crist RM, Hook SS, McNeil SE. Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nature reviews Clinical oncology. 2016;13:750. [DOI] [PubMed] [Google Scholar]
- [20].Sun J, Chen Y, Xu J, Song X, Wan Z, Du Y, et al. High Loading of Hydrophobic and Hydrophilic Agents via Small Immunostimulatory Carrier for Enhanced Tumor Penetration and Combinational Therapy. Theranostics. 2020;10:1136–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wan Z, Sun J, Xu J, Moharil P, Chen J, Xu J, et al. Dual functional immunostimulatory polymeric prodrug carrier with pendent indoximod for enhanced cancer immunochemotherapy. Acta Biomater. 2019;90:300–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sun J, Wan Z, Chen Y, Xu J, Luo Z, Parise RA, et al. Triple Drugs Co-Delivered by a Small Gemcitabine-Based Carrier for Pancreatic Cancer Immunochemotherapy. Acta Biomater. 2020;106:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen Y, Xia R, Huang Y, Zhao W, Li J, Zhang X, et al. An immunostimulatory dual-functional nanocarrier that improves cancer immunochemotherapy. Nat Commun. 2016;7:13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chauhan VP, Stylianopoulos T, Martin JD, Popović Z, Chen O, Kamoun WS, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nature nanotechnology. 2012;7:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].French AR, Yokoyama WM. Natural killer cells and viral infections. Current opinion in immunology. 2003;15:45–51. [DOI] [PubMed] [Google Scholar]
- [27].Pardo J, Balkow S, Anel A, Simon MM. Granzymes are essential for natural killer cell mediated and perf facilitated tumor control. European journal of immunology. 2002;32:2881–6. [DOI] [PubMed] [Google Scholar]
- [28].Guo Q, Wang D, Yang G. Photoacoustic Imaging Guided Photothermal and Chemodynamic Combined Therapy for Cancer Using. Journal of biomedical nanotechnology. 2019;15:2090–9. [DOI] [PubMed] [Google Scholar]
- [29].Yang S, Zhou L, Su Y, Zhang R, Dong C-M. One-pot photoreduction to prepare NIR-absorbing plasmonic gold nanoparticles tethered by amphiphilic polypeptide copolymer for synergistic photothermal-chemotherapy. Chinese Chemical Letters. 2019;30:187–91. [Google Scholar]
- [30].Yu Z, Guo Y, Dai H, Zeng B, Zheng X, Yi C, et al. On-demand drug release and re-absorption from pirarubicin loaded Fe3O4@ ZnO core–shell nanoparticles for targeting infusion chemotherapy for urethral carcinoma. Materials Express. 2019;9:467–74. [Google Scholar]
- [31].Zhao R, Han X, Li Y, Wang H, Ji T, Zhao Y, et al. Photothermal effect enhanced cascade-targeting strategy for improved pancreatic cancer therapy by gold nanoshell@ mesoporous silica nanorod. ACS Nano. 2017;11:8103–13. [DOI] [PubMed] [Google Scholar]
- [32].Chen Q, Hu Q, Dukhovlinova E, Chen G, Ahn S, Wang C, et al. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv Mater. 2019:1900192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu Y, Ai K, Liu J, Deng M, He Y, Lu L. Dopamine melanin colloidal nanospheres: an efficient near infrared photothermal therapeutic agent for in vivo cancer therapy. Adv Mater. 2013;25:1353–9. [DOI] [PubMed] [Google Scholar]
- [34].Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jiang J, Zhu L, Zhu L, Zhu B, Xu Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir. 2011;27:14180–7. [DOI] [PubMed] [Google Scholar]
- [36].Chen W, Qin M, Chen X, Wang Q, Zhang Z, Sun X. Combining photothermal therapy and immunotherapy against melanoma by polydopamine-coated Al2O3 nanoparticles. Theranostics. 2018;8:2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Park J, Brust TF, Lee HJ, Lee SC, Watts VJ, Yeo Y. Polydopamine-based simple and versatile surface modification of polymeric nano drug carriers. ACS nano. 2014;8:3347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hu D, Zhang J, Gao G, Sheng Z, Cui H, Cai L. Indocyanine green-loaded polydopamine-reduced graphene oxide nanocomposites with amplifying photoacoustic and photothermal effects for cancer theranostics. Theranostics. 2016;6:1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang S, Zhao X, Wang S, Qian J, He S. Biologically inspired polydopamine capped gold nanorods for drug delivery and light-mediated cancer therapy. ACS Appl Mater Interfaces. 2016;8:24368–84. [DOI] [PubMed] [Google Scholar]
- [40].Jin A, Wang Y, Lin K, Jiang L. Nanoparticles modified by polydopamine: Working as “drug” carriers. Bioactive Materials. 2020;5:522–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Poinard B, Kamaluddin S, Tan AQQ, Neoh KG, Kah JCY. Polydopamine Coating Enhances Mucopenetration and Cell Uptake of Nanoparticles. ACS applied materials & interfaces. 2019;11:4777–89. [DOI] [PubMed] [Google Scholar]
- [42].Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. Journal of hematology & oncology. 2018;11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Guo L, Yan DD, Yang D, Li Y, Wang X, Zalewski O, et al. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS nano. 2014;8:5670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tao Y, Ju E, Ren J, Qu X. Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials. 2014;35:9963–71. [DOI] [PubMed] [Google Scholar]
- [45].Li X, Naylor MF, Le H, Nordquist RE, Teague TK, Howard CA, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer biology & therapy. 2010;10:1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nature communications. 2016;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Slovak R, Ludwig JM, Gettinger SN, Herbst RS, Kim HS. Immuno-thermal ablations–boosting the anticancer immune response. Journal for immunotherapy of cancer. 2017;5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cho J-a, Lee Y-S, Kim S-H, Ko J-K, Kim C-W. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer letters. 2009;275:256–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information files. Correspondence and requests for materials should be addressed to J.S. or S.L.