Abstract
Simple Summary
There is limited information on the safety and efficacy of approved SARS-CoV-2 vaccines in cancer patients, as they were excluded from registration vaccine trials. We investigated the humoral immunity post SARS-CoV-2 vaccination in cancer patients compared to healthy volunteers. In this prospective cohort study, the seropositivity rate after two doses of vaccine was high in cancer patients despite active antineoplastic treatment, but their antibody titers were significantly lower than in healthy control subjects. Factors affecting immunogenicity in cancer patients, included older age, poor PS, active treatment, certain cancer types, i.e., pancreatic cancer and SCLC, male gender, and, interestingly, smoking status. Our results suggest that, given the lower immunogenicity, adjustments in vaccination strategies for more vulnerable subgroups of cancer patients may be required. Monitoring of antibody responses and elucidation of the clinical factors that influence immunity could guide future vaccination policies.
Abstract
Data on the effectiveness and safety of approved SARS-CoV-2 vaccines in cancer patients are limited. This observational, prospective cohort study investigated the humoral immune response to SARS-CoV-2 vaccination in 232 cancer patients from 12 HeCOG-affiliated oncology departments compared to 100 healthcare volunteers without known active cancer. The seropositivity rate was measured 2–4 weeks after two vaccine doses, by evaluating neutralising antibodies against the SARS-CoV-2 spike protein using a commercially available immunoassay. Seropositivity was defined as ≥33.8 Binding-Antibody-Units (BAU)/mL. A total of 189 patients and 99 controls were eligible for this analysis. Among patients, 171 (90.5%) were seropositive after two vaccine doses, compared to 98% of controls (p = 0.015). Most seronegative patients were males (66.7%), >70-years-old (55.5%), with comorbidities (61.1%), and on active treatment (88.9%). The median antibody titers among patients were significantly lower than those of the controls (523 vs. 2050 BAU/mL; p < 0.001). The rate of protective titers was 54.5% in patients vs. 97% in controls (p < 0.001). Seropositivity rates and IgG titers in controls did not differ for any studied factor. In cancer patients, higher antibody titers were observed in never-smokers (p = 0.006), women (p = 0.022), <50-year-olds (p = 0.004), PS 0 (p = 0.029), and in breast or ovarian vs. other cancers. Adverse events were comparable to registration trials. In this cohort study, although the seropositivity rate after two vaccine doses in cancer patients seemed satisfactory, their antibody titers were significantly lower than in controls. Monitoring of responses and further elucidation of the clinical factors that affect immunity could guide adaptations of vaccine strategies for vulnerable subgroups.
Keywords: SARS-CoV-2 vaccine, cancer patient, antibody response, neutralizing IgG, anti-spike
1. Introduction
Cancer patients are at increased risk of severe Coronavirus disease 2019 (COVID-19) disease but also have significantly higher mortality rates and are, therefore, highly prioritized for vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1,2,3]. In a recent meta-analysis of 52 studies and 18,650 cancer patients with COVID-19, the mortality rate was 25.6% [4]. Patients with hematological and lung malignancies, as well as those with active and/or advanced disease, have a persistently increased risk [5,6,7]. In Greece, vaccination of vulnerable groups such as elderly citizens and cancer patients of all ages was prioritized in mid-March 2021.
There are limited data on the effectiveness of vaccination in cancer patients overall, with most existing information relating to influenza vaccine [8,9,10]. Observational clinical studies suggest lower influenza mortality and morbidity in cancer patients who have been vaccinated, suggesting an effective immune response [11,12], even when undergoing systemic chemotherapy [13]. Based on extrapolation from other vaccines, the efficacy and safety of vaccination against SARS-CoV-2 in cancer patients may be similar to that in patients without cancer, although it may be generally lower in certain severely immunosuppressed subgroups. Data from clinical trials are not available because pivotal trials of the SARS-CoV-2 vaccine have excluded immunosuppressed patients. Cancer patients have a varying state of immunosuppression either due to cancer itself or treatments and complications. Therefore, cancer patients were generally excluded from the placebo-controlled randomized vaccine trials, and recommendations for them have been extrapolated from the general population. Only two vaccine trials have enrolled cancer patients, but in very small numbers: 4% cancer patients were enrolled in the Pfizer vaccine trial, and only 0.5% in the Janssen vaccine trial. However, even these patients were not analysed separately to provide information on the safety and efficacy of the vaccines [14,15,16]. A lower response to vaccination was observed in solid organ transplant recipients and, more recently, in patients with hematologic malignancies [17,18,19].
Recent reports focused on cancer patients examined antibody responses following infection with COVID-19 [19,20], while data on immune responses elicited following SARS-CoV-2 vaccination in cancer patients have been published since April 2021 [21,22,23,24,25,26,27]. From these reports, it is evident that not all cancer patients are the same. The risk of severe COVID-19 disease may be related to various cancer-related factors affecting immunocompetence [28,29], demographic or clinical parameters, leading to different levels of protection in vaccinated cancer patients. For all these reasons, international professional societies, such as ESMO, have made early calls for vaccination, but also for surveillance and monitoring of cancer patients [30].
This observational cohort study was designed to prospectively record and monitor responses of cancer patients following SARS-CoV-2 vaccination. Clinical outcomes were described, such as adverse events or COVID-19 infections after vaccination. Serological responses were monitored at three time points: before vaccination, 2–4 weeks and three months after two vaccine doses. In the present analysis, the seropositivity of cancer patients compared to control volunteers 2–4 weeks after the two doses of vaccine is presented. Possible factors that influence immunity were recorded and analysed.
2. Results
2.1. Participants’ Characteristics
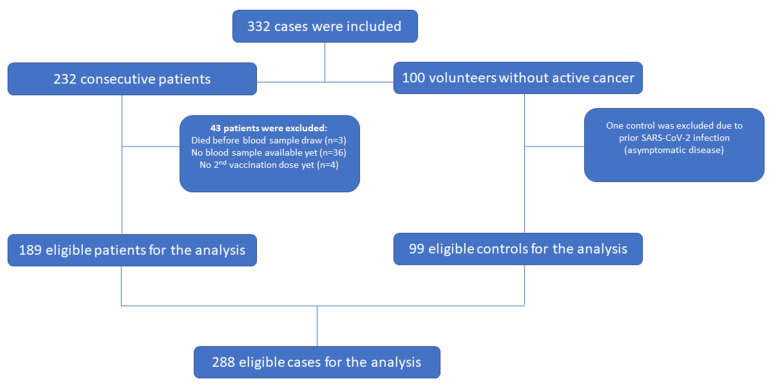
A total of 232 consecutive cancer patients vaccinated against SARS-CoV-2 with the BNT162b2 (Pfizer-BioNTech, Marburg, Germany), mRNA-1273 (Moderna Biotech, Madrid, Spain S.L.) or the AZD1222 (Astra Zeneca, Leiden, the Netherlands) vaccine, and 100 hospital-personnel volunteers without active cancer at the time of vaccination who received the BNT162b2 mRNA vaccine, were enrolled. Forty-three patients and one control did not meet the criteria for the current analysis (completion of two doses and at least 2 weeks post-second dose, no documented COVID-19 infection prior to vaccination), and were excluded, leading to a total of 189 cancer patients and 99 controls (Figure 1). More than half of the patients and most of the controls were females (54% and 62.6%, respectively), with most patients being older than 60 years (64%). Volunteer healthcare workers were mostly 30–59 years old (82.8%). Three patients (1.6%) and 14.1% of the controls reported a history of autoimmune disease. Among patients, the most common cancer type was breast cancer (27%), followed by non-small cell lung cancer (NSCLC) (19.6%) and colorectal cancer (14.3%). Overall, 162 patients (85.7%) were on active antineoplastic treatment at time of vaccination with most receiving chemotherapy alone (54.9%). The baseline characteristics for the total cohort and separately for the group of patients and controls are depicted in Table 1. Most of the patients received the BNT162b2 vaccine (163 patients, 86.2%), 19 the mRNA-1273 vaccine (10.1%) and only 7 (3.7%) the AZD1222 vaccine. The median time from administration of the second vaccine dose to the date of blood sampling was 30 days (range 14–62) (32 days for patients versus 28 days for controls). In total, 120 cases (41.7%), including 98 cancer patients (51.9%) and 22 controls (22.2%), reported comorbidities at the time of first vaccine dose. Hypertension was the most reported comorbidity among both patients and controls (Supplementary Materials, Table S1).
Figure 1.
REMARK diagram.
Table 1.
Baseline characteristics for patients and controls.
| Parameter | Total (n = 288) |
Patients (n = 189) |
Controls (n = 99) |
|---|---|---|---|
| BMI, Median (min, max) | 25.2 (17.6, 51.3) | 24.9 (17.6, 51.3) | 25.4 (17.9, 45.7) |
| N of comorbidities, Median (min, max) | 0.00 (0.00, 4.0) | 1.00 (0.00, 4.0) | 0.00 (0.00, 2.0) |
| n (%) | n (%) | n (%) | |
| Gender | |||
| Male | 124 (43.1) | 87 (46.0) | 37 (37.4) |
| Female | 164 (56.9) | 102 (54.0) | 62 (62.6) |
| Age | |||
| 18–29 | 5 (1.7) | 1 (0.53) | 4 (4.0) |
| 30–39 | 28 (9.7) | 3 (1.6) | 25 (25.3) |
| 40–49 | 57 (19.8) | 25 (13.2) | 32 (32.3) |
| 50–59 | 64 (22.2) | 39 (20.6) | 25 (25.3) |
| 60–69 | 60 (20.8) | 51 (27.0) | 9 (9.1) |
| 70–79 | 56 (19.4) | 52 (27.5) | 4 (4.0) |
| 80–85 | 15 (5.2) | 15 (7.9) | 0 (0.0) |
| >85 | 3 (1.0) | 3 (1.6) | 0 (0.0) |
| BMI | |||
| Underweight | 7 (2.4) | 5 (2.6) | 2 (2.0) |
| Normal | 134 (46.5) | 91 (48.1) | 43 (43.4) |
| Obese | 53 (18.4) | 38 (20.1) | 15 (15.2) |
| Overweight | 94 (32.6) | 55 (29.1) | 39 (39.4) |
| Smoking Status | |||
| Never smoker | 124 (43.1) | 82 (43.4) | 42 (42.4) |
| Current smoker | 88 (30.6) | 50 (26.5) | 38 (38.4) |
| Previous smoker—stopped within the last 10 years | 71 (24.7) | 53 (28.0) | 18 (18.2) |
| Not Reported | 5 (1.7) | 4 (2.1) | 1 (1.0) |
| Comorbidities | |||
| No | 168 (58.3) | 91 (48.1) | 77 (77.8) |
| Yes | 120 (41.7) | 98 (51.9) | 22 (22.2) |
| Vaccine administered | |||
| BNT162b2 (Pfizer) | 262 (91.0) | 163 (86.2) | 99 (100.0) |
| mRNA-1273 (Moderna) | 19 (6.6) | 19 (10.1) | 0 (0.0) |
| AZD1222 (Astra Zeneca) | 7 (2.4) | 7 (3.7) | 0 (0.0) |
| Autoimmune disease | |||
| No | 266 (92.4) | 184 (97.4) | 82 (82.8) |
| Active autoimmune disease | 17 (5.9) | 3 (1.6) | 14 (14.1) |
| Inactive autoimmune disease | 4 (1.4) | 1 (0.53) | 3 (3.0) |
| Not Reported | 1 (0.35) | 1 (0.53) | 0 (0.0) |
| Cancer type (primary cancer diagnosis) | |||
| Breast cancer | 51 (27.0) | 51 (27.0) | ---- |
| NSCLC | 37 (19.6) | 37 (19.6) | ---- |
| SCLC | 20 (10.6) | 20 (10.6) | ---- |
| Mesothelioma | 1 (0.53) | 1 (0.53) | ---- |
| Head and Neck cancer | 1 (0.53) | 1 (0.53) | ---- |
| Stomach cancer | 6 (3.2) | 6 (3.2) | ---- |
| Pancreatic cancer | 11 (5.8) | 11 (5.8) | ---- |
| Colorectal cancer | 27 (14.3) | 27 (14.3) | ---- |
| Ovarian cancer | 11 (5.8) | 11 (5.8) | ---- |
| Other Gynecological cancer | 1 (0.53) | 1 (0.53) | ---- |
| Bladder cancer | 4 (2.1) | 4 (2.1) | ---- |
| Prostate cancer | 4 (2.1) | 4 (2.1) | ---- |
| Kidney cancer | 4 (2.1) | 4 (2.1) | ---- |
| Testicular cancer | 1 (0.53) | 1 (0.53) | ---- |
| Melanoma | 3 (1.6) | 3 (1.6) | ---- |
| Other * | 7 (3.7) | 7 (3.7) | ---- |
| Performance Status | |||
| 0 | 157 (83.1) | 157 (83.1) | ---- |
| 1 | 32 (16.9) | 32 (16.9) | ---- |
| Concomitant Corticosteroid use | |||
| No | 179 (94.7) | 179 (94.7) | ---- |
| Yes | 10 (5.3) | 10 (5.3) | ---- |
| Active antineoplastic treatment | |||
| No | 27 (14.3) | 27 (14.3) | ---- |
| Yes | 162 (85.7) | 162 (85.7) | ---- |
| Type of antineoplastic treatment | |||
| Chemotherapy | 89 (54.9) | 89 (54.9) | ---- |
| Immunotherapy only | 32 (19.8) | 32 (19.8) | ---- |
| Chemo-immunotherapy combination | 12 (7.4) | 12 (7.4) | ---- |
| Targeted agent, TKI only | 4 (2.5) | 4 (2.5) | ---- |
| Other biologic therapy only | 21 (13.0) | 21 (13.0) | ---- |
| Endocrine therapy only | 2 (1.2) | 2 (1.2) | ---- |
| Radiotherapy only | 2 (1.2) | 2 (1.2) | ---- |
| Cancer status at vaccination | |||
| Primary recently operated | 45 (23.8) | 45 (23.8) | ---- |
| Recurrent | 49 (25.9) | 49 (25.9) | ---- |
| Metastatic | 89 (47.1) | 89 (47.1) | ---- |
| Other | 6 (3.2) | 6 (3.2) | ---- |
n, number; BMI, body mass index; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer. * Neuroendocrine lung cancer (n = 1), Cancer of Unknown Primary (n = 2), Anal Cancer (n = 1), GIST small intestine (n = 1), GIST large intestine (n = 2).
2.2. Serological Outcomes
Overall, 268 cases (93.1%) had positive anti-SARS-CoV-2 anti-spike IgG antibodies: 171 patients (90.5%) and 97 (98%) controls (Fisher’s p = 0.015).
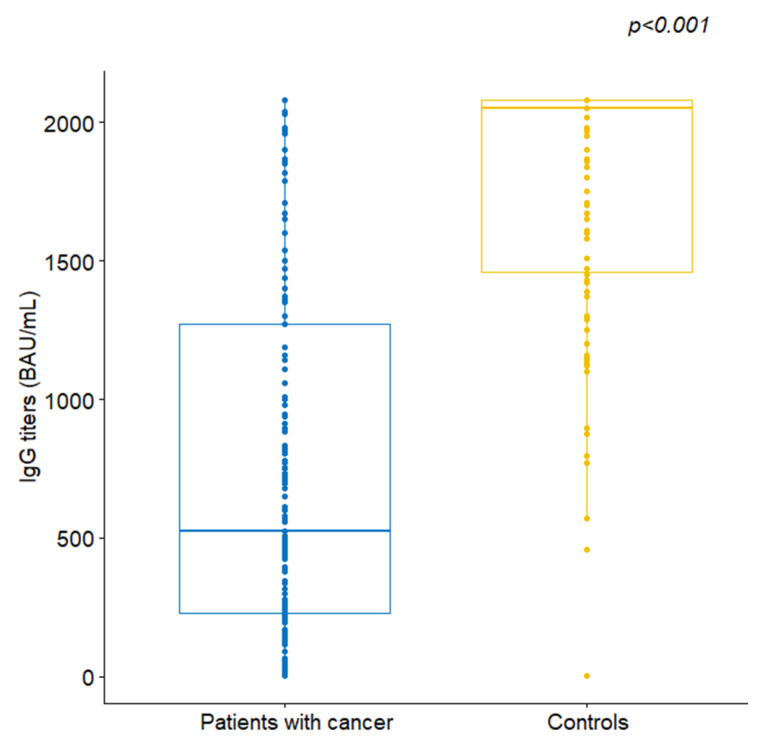
The median IgG titer for the patient group was 523 BAU/mL, ranging from 4.81 to 2080, and was significantly lower compared to a median of 2050 BAU/mL (range 4.81–2080) observed in the control group (Wilcoxon rank-sum p < 0.001, Figure 2). The rate of persons with protective titers (>488.8 BAU/mL) was 54.5% (n = 103) in cancer patients, as opposed to 97% in the controls (Fisher’s p < 0.001). The seropositivity rates and IgG titers did not differ based on gender, age, BMI, smoking status and the presence of comorbidities or autoimmune disease among controls.
Figure 2.
Comparison of anti-SARS-CoV-2 spike IgG antibodies between patients and controls.
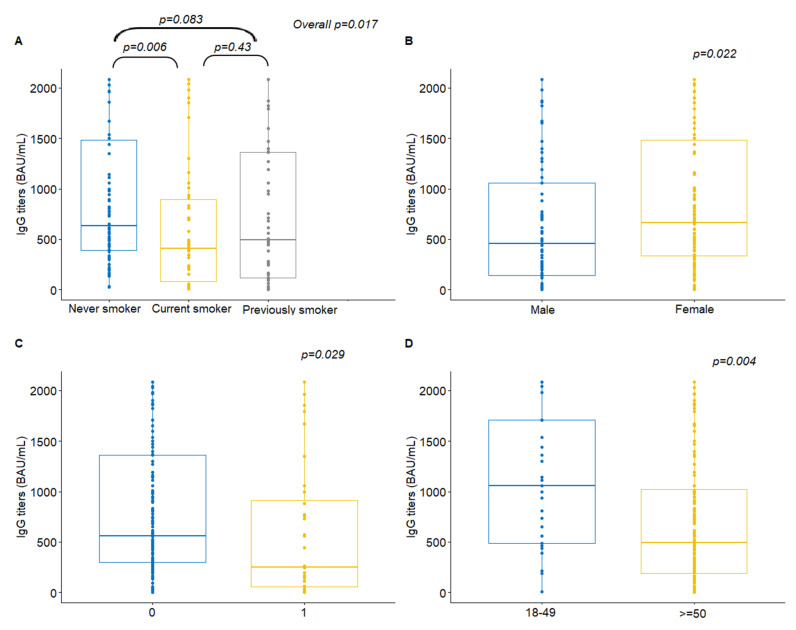
Among cancer patients, a significant association was identified between IgG titers and smoking status (Kruskal–Wallis p = 0.017). Post-hoc analysis revealed that never smokers had significantly higher antibody titers compared to current smokers (median value 632 versus 409.5, Wilcoxon rank-sum p = 0.006, Figure 3A). Additionally, female patients presented with higher antibody titers compared to males (median value 665 versus 456, p = 0.022, Figure 3B). Higher antibody titers were also observed for PS 0 patients compared to those with PS 1 (median value 560 versus 253.5, p = 0.029, Figure 3C).
Figure 3.
Anti-SARS-CoV-2 spike IgG antibodies by (A) smoking status, (B) gender, (C) performance status and (D) age among patients with cancer.
Regarding cancer type, patients with small-cell lung cancer (SCLC), colorectal and pancreatic cancer had considerably lower antibody titers as opposed to patients with breast or ovarian cancer (Supplementary Materials, Figure S1). Comparison of IgG antibodies in the subgroup of patients with breast, NSCLC, SCLC, pancreatic, colorectal and ovarian cancer showed a significant difference among them, with ovarian cancer patients achieving the highest median number of antibodies among all, and those with pancreatic cancer the lowest (p = 0.041).
Regarding antineoplastic treatment, patients treated with endocrine therapy only and radiotherapy alone had considerably higher antibody titers; however, due to the small number of cases in these treatment groups, no formal comparisons were performed.
Further categorization of age showed that patients aged 18–49 had higher antibody rates compared to those aged 50 and over (median value 1060 versus 491.5, p = 0.004, Figure 3D) (Table 2). No correlation was found among patients and controls between the number of antibody titers and the number of comorbidities (Spearman rho = −0.13, p = 0.076 and rho = −0.03, p = 0.79, respectively) or the time interval between the serum sample and the date of second vaccination (rho = −0.02, p = 0.74 and rho = −0.13, p = 0.21, respectively) (Supplementary Materials, Figure S1).
Table 2.
Association of antibody titers with baseline characteristics in patients and controls.
| Patients (n = 189) | Controls (n = 99) | |||||
|---|---|---|---|---|---|---|
| Parameter | n | IgG Median (Min, Max) | p-Value | n | IgG Median (Min, Max) | |
| Cancer Type (Primary Diagnosis) |
0.041 * | |||||
| Breast cancer | 51 | 698 (7.15–2080) | ||||
| Lung cancer NSCLC | 37 | 580 (10.50–2080) | ||||
| Lung cancer SCLC | 20 | 343.5 (18–2080) | ||||
| Mesothelioma | 1 | 2080 (----) | ||||
| Head and Neck cancer | 1 | 735 (----) | ||||
| Stomach cancer | 6 | 340.50 (18.30–2080) | ||||
| Pancreatic cancer | 11 | 238 (8.39–2080) | ||||
| Colorectal cancer | 27 | 491.50 (35.70–2080) | ||||
| Ovarian cancer | 11 | 939 (25.10–2080) | ||||
| Other Gynecological cancer | 1 | 509 (----) | ||||
| Bladder cancer | 4 | 447 (23.6–509) | ||||
| Prostate cancer | 4 | 671.5 (4.81–883) | ||||
| Kidney cancer | 4 | 330 (66.3–897) | ||||
| Testicular cancer | 1 | 1980 (----) | ||||
| Melanoma | 3 | 482 (164.00–730) | ||||
| Other | 7 | 476 (4.81–2080) | ||||
| Cancer status at vaccination | 0.27 | |||||
| Primary recently operated | 45 | 650 (4.81–2080) | ||||
| Recurrent | 49 | 556 (4.81–2080) | ||||
| Metastatic | 89 | 482 (8.07–2080) | ||||
| Other | 6 | 729.50 (193.00–1870) | ||||
| Active antineoplastic treatment | 0.28 | |||||
| No | 27 | 696.00 (18–2080) | ||||
| Yes | 162 | 492.50 (4.81–2080) | ||||
| Type of antineoplastic treatment | ---- | |||||
| Chemotherapy | 89 | 478 (4.81–2080) | ||||
| Immunotherapy only | 32 | 469 (25.10–2080) | ||||
| Chemo-immunotherapy combination | 12 | 536.50 (20.90–1500) | ||||
| Targeted agent, TKI only | 4 | 378.50 (168.00–2080) | ||||
| Other biologic therapy only | 21 | 698 (4.81–2080) | ||||
| Endocrine therapy only | 2 | 1425.50 (771–2080) | ||||
| Radiotherapy only | 2 | 1185 (1000–1370) | ||||
| Concomitant corticosteroid use | 0.83 | |||||
| No | 179 | 509 (4.81–2080) | ||||
| Yes | 10 | 817 (4.81–2080) | ||||
| PS at vaccination | 0.029 | |||||
| 0 | 157 | 560 (4.81–2080) | ||||
| 1 | 32 | 253.5 (4.81–2080) | ||||
| Smoking status | 0.017 | 0.81 | ||||
| Never smoker | 82 | 632 (25.00–2080) | 42 | 1885 (4.81-2080) | ||
| Current smoker | 50 | 409.50 (8.07–2080) | 38 | 2065 (770-2080) | ||
| Previous smoker—stopped within the last 10 years | 53 | 491 (4.81–2080) | 18 | 1970 (4.81–2080) | ||
| Gender | 0.022 | 0.30 | ||||
| Male | 87 | 456 (4.81–2080) | 37 | 1800 (4.81–2080) | ||
| Female | 102 | 665 (4.81–2080) | 62 | 2065 (770–2080) | ||
| Age | ---- | ---- | ||||
| 18–29 | 1 | 1110 (----) | 4 | 2025 (1700–2080) | ||
| 30–39 | 3 | 563 (215–2080) | 25 | 2080 (770–2080) | ||
| 40–49 | 25 | 1060 (7.15–2080) | 32 | 1905 (1100–2080) | ||
| 50–59 | 39 | 604 (20.90–2080) | 25 | 2080 (4.81–2080) | ||
| 60–69 | 51 | 452 (4.81–2080) | 9 | 1370 (4.81–2080) | ||
| 70–79 | 52 | 491.5 (4.81–2080) | 4 | 1325 (4.81–2080) | ||
| 80–85 | 15 | 347 (8.07–2080) | 0 | ---- | ||
| >85 | 3 | 730 (509–771) | 0 | ---- | ||
| 18–49 | 29 | 1060 (7.15–2080) | 0.004 | 61 | 2080 (770–2080) | 0.26 |
| ≥50 | 160 | 491.5 (4.81–2080) | 38 | 1885 (4.81–2080) | ||
| Comorbidities | 0.051 | 0.72 | ||||
| No | 91 | 650 (4.81–2080) | 77 | 2020 (457–2080) | ||
| Yes | 98 | 491.5 (4.81–2080) | 22 | 2080 (4.81–2080) | ||
| Hypertension | 0.76 | 0.077 | ||||
| No | 120 | 543 (4.81–2080) | 88 | 2080 (4.81–2080) | ||
| Yes | 69 | 509 (8.39–2080) | 11 | 1510 (4.81–2080) | ||
| BMI | 0.80 | ---- | ||||
| Underweight | 5 | 680 (10.5–1500) | 2 | 1835 (1700–1970) | ||
| Normal weight | 91 | 509 (7.15–2080) | 43 | 1950 (570–2080) | ||
| Obese | 38 | 606 (4.81–2080) | 15 | 2080 (4.81–2080) | ||
| Overweight | 55 | 509 (4.81–2080) | 39 | 1860 (4.81–2080) | ||
* Comparisons were performed among patients with breast, NSCLC, SCLC, pancreatic, colorectal and ovarian cancer due to the small number of cases in the rest of the patient groups.
2.3. Seronegative Volunteers and Cancer Patients
Of the two seronegative controls, one had a previous renal transplant, and the other a history of autoimmune disease. More than half of the seronegative cancer patients were males (66.7%), older than 70 years (55.5%), with comorbidities (61.1%), and on active antineoplastic treatment (88.9%). Half of the seronegative patients had metastatic disease at the time of vaccination, 3 (16.7%) and had received concomitant corticosteroids, and one had an active autoimmune disease. Of note, all seronegative cancer patients were vaccinated with mRNA vaccines (BNT162b2 (n = 15) or mRNA-1273 (n = 3)).
2.4. Adverse Events Post Vaccination
Among healthy volunteers, the majority (74.7%, n = 74) reported at least one adverse event following the first vaccine dose and 68.7% (n = 68) following the second dose. Most of the reported events after the first dose (68/130) included topical reactions at the site of vaccination (pain, redness or local inflammation; 64.6% of controls), while fatigue/sleepiness, myalgias/arthralgias and headache were more commonly reported after the second dose (31.3%, 24.2% and 21.2%, respectively). Rarer events included tachycardia in two cases after the first and in three after the second dose, lymphadenitis and bruising in one case after the second dose and two allergic reactions requiring intravenous medication, one after the first and one after the second dose.
Among cancer patients, AEs were less commonly reported and were milder overall. More than half reported an AE after the first dose (52.4%, n = 99) and slightly more after the second dose (64%, n = 121), involving topical reaction with redness or pain in 47.1% of patients after the first and/or second dose, and fatigue (28.6%, n = 54), headache (24.3%, n = 46) or myalgias/arthralgias (9.5%, n = 18) after the second dose. There were no allergic reactions.
No COVID-19 infections post-vaccination were documented in either group until data cut-off for this analysis.
3. Discussion
In this prospective cohort study, we present real-world data on the immune responses to SARS-CoV-2 vaccination among cancer patients during the COVID-19 pandemic in Greece. We found that the immunogenicity pattern was lower in the vaccinated cancer patients than in the control subjects without cancer, but most patients were seropositive after two doses (90.5%). In 189 vaccinated cancer patients and 99 healthy volunteers, there were no documented COVID-19 infections after vaccination by the time of this analysis. In the cancer patients included in our study, antibody titers were significantly lower than in the volunteer controls despite the high seropositivity rates observed one month after vaccination, and significantly fewer cancer patients had protective antibody titers compared with the controls. We found that several factors influenced immunogenicity in the cancer patients included in this study. These primarily reflect the immunosuppressed environment of cancer patients [27] and included older age, poorer PS, active treatment, certain cancer types such as pancreatic cancer and SCLC, and interestingly, smoking status. Our study supports the widespread recommendation that cancer patients should receive a full and timely SARS-CoV-2 vaccination. Nevertheless, our results suggest that adjustments in vaccination strategies may be needed for some of the more vulnerable subgroups of cancer patients given their lower immunogenicity.
Antibody response to SARS-CoV-2 vaccines is being studied as a “correlate of protection” against infection with COVID-19 and severe disease, but the evidence is not yet clear [31,32]. The CDC does not currently recommend routine antibody testing to assess immune response to SARS-CoV-2 vaccines. However, there are recently published data showing a correlation between antibody response and disease protection in healthy volunteers and patients with malignancies, suggesting the use of post-vaccination antibody titers as a “correlate of protection” for vaccines [22,33]. Antigen-specific T cells and overall cellular immunity are thought to play a central role in the protective process against COVID-19. However, their cellular mechanisms are not yet clearly defined, and the measurements involved are far more complex than serological detection of neutralizing antibodies [34,35].
To design future strategies for the protection of vulnerable populations, such as the elderly or immunocompromised, we need to create simple ways to assess who really needs further protection. In this regard, antibody measurements could be used if we can reach methodological consensus and establish comparability between tests and laboratories. Even in high-risk populations, a serologically based vaccination strategy could provide a rational pathway for targeted booster vaccinations where they are truly needed. In some countries, a third booster vaccine dose to immunocompromised and elderly citizens has already begun and is even being considered for the general population. However, in order to keep a global vaccine equity perspective, the alternative to a universal third booster dose could be a repeat dose among high-risk populations, based on a ‘serology-monitored dosing’. This could be a strategy for rational and focused booster vaccinations among cancer patients as well. Our results support this option, as do similar data and current recommendations for cancer patients [22].
Our study revealed several factors that could influence immunity and should be considered in decision making. In vaccine pivotal trials, there were no differences in efficacy depending on age, sex, or presence of comorbidities [14]. Similarly, in our study, seropositivity rates and IgG titers did not differ by sex, age, BMI, smoking status, and the presence of comorbidities or autoimmune disease in controls. However, in cancer patients, a negative association was observed between significantly lower antibody titers and male sex, poor PS (≥1 vs. 0), and age over 50 years. Lower PS and older age likely reflect lower immunocompetence [36]. Since the beginning of the pandemic, clinical outcomes have shown that the severity and mortality of COVID-19 infection is higher in men than in women [37,38]. Recent studies suggest possible mechanisms for those gender-based variations, including differences in human ACE2 (hACE2) receptor expression, behavioural differences such as smoking or prevalence of comorbidities, and gender-based differences in immunological responses [39].
Another important finding of our study is the role of smoking in the immunological response of cancer patients. We observed a significant association between IgG titers and smoking status, with never-smokers having significantly higher antibody titers than current smokers (p = 0.006). Despite previous conflicting reports on smokers’ risk, it is now apparent that smoking is a predictor of COVID-19 mortality [40]. Previous reports showed that smoking has an immunosuppressive effect [41], with direct effects on T cells [42,43], affects the dendritic-cell system, and impairs host response to vaccination [44]. Recent population-based studies during the pandemic showed that smoking is a major factor associated with lower response to the SARS-CoV-2 vaccine [45]. Other recent studies showed that smokers had lower antibody titers, suggesting an impaired humoral response [46,47,48]. Our results contribute to the above evidence and show that smoking is a significant negative factor for impaired humoral immunity in vaccinated cancer patients.
In terms of cancer type, we found that patients with SCLC, colorectal and pancreatic carcinoma had significantly lower antibody titers than patients with breast or ovarian cancer, likely reflecting differences in the degree of immunosuppression due to disease, antineoplastic treatment, or gender-related differences. Because of the small number of cases in the different treatment groups, we were unable to make formal comparisons regarding specific types of treatment, but there were no numerical differences among the groups receiving immunotherapy, chemotherapy, or their combinations. In recent studies, lower or no immune responses were observed in patients with hematologic malignancies, particularly in patients undergoing anti-CD20 therapy [23,27], whereas other treatments, including immunotherapy, had no negative impact on seropositivity [27].
Nearly 10% of our cancer patients were seronegative after two doses of vaccine, similar to the recently reported percentages of 6%, 10% and 14% [22,26,27]. Consistent with other series, most seronegative patients (88.9%) in our study were on active antineoplastic treatment, in most cases with chemotherapy alone.
The safety profile of the vaccines was consistent with previous studies, suggesting that vaccination is not associated with more adverse events in cancer patients. On the contrary, fewer adverse events occurred in our cancer cohort than in the controls.
Limitations
Regarding immunogenicity measurements, our main limitation is the lack of cellular immunity data, which would have provided a more complete picture of the vaccine’s protective mechanisms. However, our goal was to provide insight into the use of a simple, accessible, and inexpensive method to assess immunity after vaccination. In this regard, serological antibody measurements are an ideal “protective correlate”. A strength of this study is that all serological measurements were performed centrally in a reference laboratory using an approved, commercially available assay with very high clinical sensitivity and specificity. A limitation is that the upper limit of the assay is 2080 BAU/mL, so differences between groups in our study could potentially be larger.
Other limitations are that certain cancer types and treatments that could affect host immunity are poorly represented. In this regard, we were able to assess differences in specific patient subgroups but did not have sufficient power to differentiate vaccine immunogenicity in more detailed patient subgroups.
Although we did not have an age-, sex-, and comorbidity-matched control group without cancer or a control group with unvaccinated cancer patients, our results provide a clear picture of humoral immunity in real-world settings across the country. The inclusion of a large control group of hospital volunteers, who are not age-matched and thus represent a true reflection of the population, provided important comparative information. The observed differences are a strong indication that vaccination strategies against SARS-CoV-2 require tailored approaches for different populations.
4. Methods
4.1. Patients
Eligibility criteria included age > 18 years, histologically confirmed solid cancer, life expectancy greater than 3 months, active advanced or metastatic disease, or active antineoplastic treatment. Patients were enrolled in 12 HeCOG-affiliated oncology departments prior to vaccination. To be included in the present analysis, the patients needed to have been vaccinated with two doses of SARS-CoV-2 vaccine within the Hellenic National Program and be at least two and up to four weeks after the second vaccine dose. Controls were healthcare volunteers at a participating hospital (Metropolitan Hospital, Athens, Greece) who were vaccinated in January–February 2021. Patients or volunteers with a documented by positive PCR COVID-19 infection prior to study entry were excluded. In addition, active hematological malignancy or pregnancy were also excluding factors. Volunteers receiving active immunosuppressive therapy or with active cancer of any type were also excluded.
Only patients with solid tumours were included; the diagnosis of a hematological malignancy was an exclusion criterion. The cancer types included are shown in detail in Table 1. The Categories were as follows: Breast cancer, NSCLC, SCLC, Mesothelioma, Head and Neck cancer, Stomach cancer, Pancreatic cancer, Colorectal cancer, Ovarian cancer, Other Gynecological cancer, Bladder cancer, Prostate cancer, Kidney cancer, Testicular cancer, Melanoma, and Other (Neuroendocrine lung cancer, Cancer of Unknown Primary, Anal Cancer, GIST small intestine, GIST large intestine). Smoking and type of antineoplastic treatment are also shown in detail in Table 1. The categories for smoking status are as follows: Never smoker, Current smoker, Previous smoker (who stopped within the last 10 years), Not reported. Type of antineoplastic treatment was categorised as follows: Chemotherapy, Immunotherapy only, Chemo-immunotherapy combination, Targeted agent, TKI only, Other biologic therapy only, Endocrine therapy only, Radiotherapy only. These are also given in detail in Table 1. Cancer status at vaccination included: Primary recently operated, Recurrent, Metastatic, Other. Informed consent was obtained from all participants, and the study was approved by Institutional Review Board (Metropolitan Hospital, approval no. 2975, 20 January 2021) and conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, and local ethical requirements.
Clinical Trial Registration: NCT04745377
4.2. Study Design
This was a multi-centre, prospective, observational cohort study. The primary endpoint was the rate of seropositivity measured 2–4 weeks after two doses of vaccine in cancer patients compared with controls and expressed as antibody titers using a commercially available immunoassay. A second serologic result is planned 3 months after vaccination to determine antibody levels.
Clinical co-primary endpoint was the rate of COVID-19 infections after vaccination in cancer patients compared to controls (positive PCR tests in asymptomatic or symptomatic cases within a period of up to 12 months after vaccination).
Secondary endpoints included safety and tolerability (incidence, type, and severity of Adverse Events (AEs) after vaccination) assessed according to CTCAE v4.0.
Factors that might influence the immunity of cancer patients compared to control subjects (age, sex, smoking status, BMI and comorbidities), as well as the type of cancer and the type of antineoplastic treatment in the patient group, were correlated with the level of antibody response observed after vaccination.
4.3. Sample Collection and Detection of Neutralizing Antibodies to SARS-Cov-2 Spike Protein
Participants had blood samples collected before the first vaccine dose (time 1) and 2-4 weeks (time 2) after two vaccine doses. A third sample is planned 3 months after vaccination to monitor antibody levels. Serum was collected by centrifugation of whole blood in dry tubes for each patient, frozen at −20 °C and sent to a central reference laboratory, the Immunology Department of AlfaLab Molecular Biology and Genetics Center of the Hellenic Healthcare Group (HHG), Athens, Greece. A new generation chemiluminescent immunoassay (CLIA) was used for serological testing (LIAISON© SARS-CoV-2 TrimericS IgG assay), which measures the titer of neutralizing IgG antibodies against the spike protein of the SARS-CoV-2 virus. This new recombinant Trimeric Spike glycoprotein is the stabilized native form of the SARS-CoV-2 spike protein, which is thought to result in more accurate detection of IgG neutralizing antibodies. The overall clinical sensitivity of the assay is 98.7% and the clinical specificity is 99.5% [49].
The cut-off value for classifying a sample as positive was defined by the manufacturer as ≥38.8 BAU/mL and the protective titer as a value greater than 488.8 BAU/mL. The upper limit of the assay for the quantification of IgG antibodies was 2080 BAU/mL.
4.4. Data Recording
Questionnaires were completed on the day of sampling and contained similar questions for both groups on demographic data, personal medical history, and concomitant medications, as well as information on vaccination dates and adverse events after each vaccine dose. The cancer patients’ data included additional information from their medical records on cancer type, disease stage at the time of vaccination, type of antineoplastic treatment, and date of last systemic treatment.
4.5. Statistical Analysis
Baseline characteristics for patients and controls were summarized using descriptive statistics with absolute and relative frequencies for categorical variables and medians, with the respective minimum and maximum values taken into account for continuity. Comparisons of the seropositive rates between the groups of patients and controls defined by demographic and baseline characteristics were assessed via the chi-square or Fisher’s exact test, as appropriate. The Kruskal–Wallis or the Wilcoxon rank-sum test was applied to evaluate the differences in antibody titers between groups. The association of the number of antibody titers with the BMI, number of comorbidities and the time interval from the second vaccine dose to the date of blood sampling were assessed using Spearman correlations. All tests were two-sided, and significance was set at the 5% level of significance. Analysis was performed using the SAS software (SAS for Windows, version 9.4, SAS Institute Inc., Cary, NC, USA). Boxplots were created using R studio version 1.4.1106.
5. Conclusions
In this cohort study, the seropositivity rate after two doses of a SARS-CoV-2 vaccine was high (90.5%) in cancer patients undergoing active treatment. However, they achieved significantly lower antibody titers compared to controls. Several factors that influence immunogenicity were identified, including older age, poorer PS, active treatment, certain cancer types such as pancreatic cancer and SCLC, and smoking status. Monitoring antibody response in this population and collecting clinical data to elucidate further factors that negatively influence immunity could help in tailoring vaccination strategies for the more susceptible subgroups of cancer patients.
Acknowledgments
The authors acknowledge the valuable contribution and support of the COVID-19 Vaccination Committee of Metropolitan Hospital, Athens, and especially H. Skordeli, D. Yiankoula, M. Lazarou, R. Gourvelou, S. Vourros, and P. Kanellopoulos for supervising, organizing and making the sample collection among healthy volunteers possible. We also thank all data managers from HeCOG-affiliated centres for their timely response for data collection, Kanella-Maria Gkiati for data managing and expert organization and Maria Moschoni for data coordination. We extend our gratitude to the Director of Medical Service Ch. Dervenis and the large number of healthcare workers in Metropolitan Hospital, who volunteered their time, data and serum samples for this study, and, of course, most of all, we are indebted to our patients and their families for their trust and participation in the study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13184621/s1, Table S1: Type and number of comorbidities for patients and controls reporting at least one comorbidity at the time of vaccination. Figure S1: Anti-SARS-CoV-2 spike IgG values antibodies by cancer type.
Author Contributions
Conceptualization: H.L., G.F. and G.S.; Formal analysis: G.-A.K.; Investigation: N.S. and D.C.; Resources: H.L., A.C., N.A., S.S., S.K., A.V., N.T., E.R. (Evangelia Razisand), J.S., E.F., E.R. (Eleni Res), S.L., Z.S., G.M., G.S. and G.F.; Writing—original draft preparation: H.L., G.-A.K., G.F., N.S. and G.S.; Writing—review and editing: All authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Metropolitan Hospital, Athens (approval no. 2975, approval date 20 January 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the article and supplementary material while further details can be obtained on request from the corresponding author.
Conflicts of Interest
Helena Linardou reports Advisory/Consultation Fees from Roche, Astra Zeneca, Novartis, BMS, MSD, Pfizer, Amgen and Merck outside of the submitted work. Elena Fountzilas reports an Advisory Role at LEO Pharma; Speaker Fees from Roche, Pfizer, AstraZeneca; Stock Ownership of GENPREX INC, Deciphera Pharmaceuticals, Inc; Travel Grants from Merck, Pfizer, K.A.M Oncology/Hematology and DEMO. Evangelia Razis reports Honoraria from BMS; Consulting/advisory roles with Astra Zeneca, BMS and Pfizer; Research Funding from Novartis, Parexel and Tesaro; Travel/Accommodation/Expenses from Genesis, Leo Pharma, Roche, Genekor, Merck, Ipsen, Sanofi, Novartis, Pfizer and BMS. Giannis Mountzios reports Advisory/Consultation Fees from Roche, Astra Zeneca, Novartis, BMS, MSD, Takeda, Pfizer, Amgen and Merck outside of the submitted work. George Fountzilas reports Advisory Board Fees from Pfizer, Novartis and Roche; Honoraria from AstraZeneca; Stock Ownership of Genprex, Daiichi Sankyo, ARIAD, RFL Holdings and FORMYCON. The remaining authors declare no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rüthrich M.M., Giessen-Jung C., Borgmann S., Classen A.Y., Dolff S., Grüner B., Hanses F., Isberner N., Köh-ler P., Lanznaster J., et al. LEOSS Study Group. COVID-19 in cancer patients: Clinical characteristics and out-come-an analysis of the LEOSS registry. Ann. Hematol. 2021;100:383–393. doi: 10.1007/s00277-020-04328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaskaran K., Bacon S., Evans S.J., Bates C.J., Rentsch C.T., MacKenna B., Tomlinson L., Walker A.J., Schultze A., Morton E.C., et al. Factors associated with deaths due to COVID-19 versus other causes: Population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet Reg. Health Eur. 2021;6:100109. doi: 10.1016/j.lanepe.2021.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., Shete S., Hsu C.Y., Desai A., de Lima Lopes G., Jr., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saini K.S., Tagliamento M., Lambertini M., McNally R., Romano M., Leone M., Curigliano G., de Azambuja E. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur. J. Cancer. 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijenthira A., Gong I.Y., Fox T.A., Booth S., Cook G., Fattizzo B., Martín-Moro F., Razanamahery J., Riches J.C., Zwicker J., et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic re-view and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang E.A.L., Chai P., Yu J., Fan X. Effects of cancer on patients with COVID-19: A systematic review and meta-analysis of 63,019 participants. Cancer Biol. Med. 2021;18:298–307. doi: 10.20892/j.issn.2095-3941.2020.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei H., Yang Y., Zhou W., Zhang M., Shen Y., Tao D., Wang L., Lei Q., Wang Y., Wu Y. Higher mortality in lung cancer patients with COVID-19? A systematic review and meta-analysis. Lung Cancer. 2021;157:60–65. doi: 10.1016/j.lungcan.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berglund Å., Willén L., Grödeberg L., Skattum L., Hagberg H., Pauksens K. The response to vaccination against influenza A(H1N1) 2009, seasonal influenza and Streptococcus pneumoniae in adult outpatients with ongoing treatment for cancer with and without rituximab. Acta Oncol. 2014;53:1212–1220. doi: 10.3109/0284186X.2014.914243. [DOI] [PubMed] [Google Scholar]
- 9.Rousseau B., Loulergue P., Mir O., Krivine A., Kotti S., Viel E., Simon T., de Gramont A., Goldwasser F., Launay O., et al. Immunogenicity and safety of the influenza A H1N1v 2009 vaccine in cancer patients treated with cytotoxic chemotherapy and/or targeted therapy: The VACANCE study. Ann. Oncol. 2012;23:450–457. doi: 10.1093/annonc/mdr141. [DOI] [PubMed] [Google Scholar]
- 10.Brydak L., Guzy J., Starzyk J., Machała M., Góźdź S. Humoral immune response after vaccination against influenza in patients with breast cancer. Support. Care Cancer. 2001;9:65–68. doi: 10.1007/s005200000186. [DOI] [PubMed] [Google Scholar]
- 11.Eliakim-Raz N., Vinograd I., Trestioreanu A.Z., Leibovici L., Paul M. Influenza vaccines in immunosuppressed adults with cancer. Cochrane Database Syst. Rev. 2013;2:CD008983. doi: 10.1002/14651858.CD008983.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanchette P.S., Chung H., Pritchard K.I., Earle C.C., Campitelli M.A., Buchan S.A., Schwartz K.L., Crowcroft N.S., Gubbay J.B., Karnauchow T., et al. Influenza Vaccine Effectiveness Among Patients With Cancer: A Population-Based Study Using Health Administrative and La-boratory Testing Data From Ontario, Canada. J. Clin. Oncol. 2019;37:2795–2804. doi: 10.1200/JCO.19.00354. [DOI] [PubMed] [Google Scholar]
- 13.Nordøy T., Aaberge I.S., Husebekk A., Samdal H.H., Steinert S., Melby H., Kolstad A. Cancer patients un-dergoing chemotherapy show adequate serological response to vaccinations against influenza virus and Streptococcus pneumoniae. Med. Oncol. 2002;19:71–78. doi: 10.1385/MO:19:2:71. [DOI] [PubMed] [Google Scholar]
- 14.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotondo J.C., Martini F., Maritati M., Mazziotta C., Di Mauro G., Lanzillotti C., Barp N., Gallerani A., Tognon M., Contini C. SARS-CoV-2 Infection: New Molecular, Phylogenetic, and Pathogenetic Insights. Efficacy of Current Vaccines and the Potential Risk of Variants. Viruses. 2021;13:1687. doi: 10.3390/v13091687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orchard K., Dignan F.L., Lee J., Pearce R., Desai M., McFarlane E., Parkin A., Shearn P., Snowden J.A. The NICE COVID-19 rapid guide-line on haematopoietic stem cell transplantation: Development, implementation and impact. Br. J. Haematol. 2021;192:467–473. doi: 10.1111/bjh.17280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzarfati K.H., Gutwein O., Apel A., Rahimi-Levene N., Sadovnik M., Harel L., Benveniste-Levkovitz P., Bar Chaim A., Koren-Michowitz M. BNT162b2 COVID -19 vaccine is significantly less effective in patients with hematologic malignancies. Am. J. Hematol. 2021;96:1195–1203. doi: 10.1002/ajh.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marra A., Generali D., Zagami P., Cervoni V., Gandini S., Venturini S., Morganti S., Passerini R., Orecchia R., Curigliano G. Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2. Ann. Oncol. 2021;32:113–119. doi: 10.1016/j.annonc.2020.10.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solodky M., Galvez C., Russias B., Detourbet P., N’Guyen-Bonin V., Herr A.-L., Zrounba P., Blay J.-Y. Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann. Oncol. 2020;31:1087–1088. doi: 10.1016/j.annonc.2020.04.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massarweh A., Eliakim-Raz N., Stemmer A., Levy-Barda A., Yust-Katz S., Zer A., Benouaich-Amiel A., Ben-Zvi H., Moskovits N., Brenner B., et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021;7:1133. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., Barrio I.D.M.D., Alaguthurai T., Domingo-Vila C., Hayday T.S., Graham C., Seow J., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jérôme B., Emmanuel C., Zoubir A., Olivier C., Abakar M., Sabine M., Emmanuel P., Axel L., Vincent R., Michel C. Impaired immunogen-icity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann. Oncol. 2021;32:1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palich R., Veyri M., Vozy A., Marot S., Gligorov J., Benderra M.-A., Maingon P., Morand-Joubert L., Adjoutah Z., Marcelin A.-G., et al. High seroconversion rate but low antibody titers after two injections of BNT162b2 (Pfizer-BioNTech) vaccine in patients treated by chemotherapy for solid cancers. Ann. Oncol. 2021 doi: 10.1016/j.annonc.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goshen-Lago T., Waldhorn I., Holland R., Szwarcwort-Cohen M., Reiner-Benaim A., Shachor-Meyouhas Y., Hussein K., Fahoum L., Baruch M., Peer A., et al. Serologic Status and Toxic Effects of the SARS-CoV-2 BNT162b2 Vaccine in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Addeo A., Shah P.K., Bordry N., Hudson R.D., Albracht B., Di Marco M., Kaklamani V., Dietrich P.Y., Taylor B.S., Simand P.F., et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39:1091–1098.e2. doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamilos G., Lionakis M.S., Kontoyiannis D.P. Are All Patients with Cancer at Heightened Risk for Severe Coronavirus Disease 2019 (COVID-19)? Clin. Infect Dis. 2021;72:351–356. doi: 10.1093/cid/ciaa1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha S., Kundu C.N. Cancer and COVID-19: Why are cancer patients more susceptible to COVID-19? Med. Oncol. 2021;38:1–7. doi: 10.1007/s12032-021-01553-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garassino M.C., Vyas M., De Vries E.G.E., Kanesvaran R., Giuliani R., Peters S. The ESMO Call to Ac-tion on COVID-19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann. Oncol. 2021;32:579–581. doi: 10.1016/j.annonc.2021.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumley S.F., O’Donnell D., Stoesser N.E., Matthews P.C., Howarth A., Hatch S.B., Marsden B.D., Cox S., James T., Warren F., et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., McMahon M., Meade P., Mendu D.R., Muellers K., et al. Robust neutralizing anti-bodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., Dull P., Plotkin S.A. Evidence for an-tibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin P., Li J., Pan H., Wu Y., Zhu F. Immunological surrogate endpoints of COVID-2019 vaccines: The evi-dence we have versus the evidence we need. Signal. Transduct Target Ther. 2021;6:48. doi: 10.1038/s41392-021-00481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 36.Giefing-Kröll C., Berger P., Lepperdinger G., Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020;11:1–13. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed S.B., Dumanski S.M. Sex, gender and COVID-19: A call to action. Can. J. Public Health. 2020;111:980–983. doi: 10.17269/s41997-020-00417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee S., Pahan K. Is COVID-19 Gender-sensitive? J. Neuroimmune Pharmacol. 2021;16:38–47. doi: 10.1007/s11481-020-09974-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garassino M.C., Whisenant J.G., Huang L.-C., Trama A., Torri V., Agustoni F., Baena J., Banna G., Berardi R., Bettini A.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sopori M.L. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez C.P., Morrow K., Velasco C., Wyczechowska D.D., Naura A.S., Rodriguez P.C. Effects of ciga-rette smoke extract on primary activated T cells. Cell Immunol. 2013;282:38–43. doi: 10.1016/j.cellimm.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piaggeschi G., Rolla S., Rossi N., Brusa D., Naccarati A., Couvreur S., Spector T.D., Roederer M., Mangino M., Cordero F., et al. Immune Trait Shifts in Associa-tion With Tobacco Smoking: A Study in Healthy Women. Front. Immunol. 2021;12:637974. doi: 10.3389/fimmu.2021.637974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nouri-Shirazi M., Guinet E. Exposure to Nicotine Adversely Affects the Dendritic Cell System and Compromises Host Response to Vaccination. J. Immunol. 2012;188:2359–2370. doi: 10.4049/jimmunol.1102552. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe M., Balena A., Tuccinardi D., Tozzi R., Risi R., Masi D., Caputi A., Rossetti R., Spoltore M.E., Filippi V., et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes/Metabolism Res. Rev. 2021:e3465. doi: 10.1002/dmrr.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobaño C., Ramírez-Morros A., Alonso S., Vidal-Alaball J., Ruiz-Olalla G., Vidal M., Rubio R., Cascant E., Parras D., Melero N.R., et al. Persistence and baseline determinants of seropositivity and reinfection rates in health care workers up to 12.5 months after COVID-19. BMC Med. 2021;19:1–6. doi: 10.1186/s12916-021-02032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jonsdottir H.R., Bielecki M., Siegrist D., Buehrer T.W., Zust R., Deuel J.W. Titers of Neutralizing Antibodies against SARS-CoV-2 Are Independent of Symptoms of Non-Severe COVID-19 in Young Adults. Viruses. 2021;13:284. doi: 10.3390/v13020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaffner A., Risch L., Aeschbacher S., Risch C., Weber M.C., Thiel S.L., Jüngert K., Pichler M., Grossmann K., Wohlwend N., et al. Characterization of a Pan-Immunoglobulin Assay Quantifying Antibodies Directed against the Receptor Binding Domain of the SARS-CoV-2 S1-Subunit of the Spike Protein: A Population-Based Study. J. Clin. Med. 2020;9:3989. doi: 10.3390/jcm9123989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonelli F., Blocki F.A., Bunnell T., Chu E., De La O.A., Grenache D.G., Marzucchi G., Montomoli E., Okoye L., Pallavicini L., et al. Evaluation of the automated LIAISON((R)) SARS-CoV-2 TrimericS IgG assay for the detection of circulating antibodies. Clin. Chem. Lab. Med. 2021;59:1463–1467. doi: 10.1515/cclm-2021-0023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the article and supplementary material while further details can be obtained on request from the corresponding author.