Abstract
We evaluated the role of the G alpha-q (Gαq) subunit of heterotrimeric G proteins in the insulin signaling pathway leading to GLUT4 translocation. We inhibited endogenous Gαq function by single cell microinjection of anti-Gαq/11 antibody or RGS2 protein (a GAP protein for Gαq), followed by immunostaining to assess GLUT4 translocation in 3T3-L1 adipocytes. Gαq/11 antibody and RGS2 inhibited insulin-induced GLUT4 translocation by 60 or 75%, respectively, indicating that activated Gαq is important for insulin-induced glucose transport. We then assessed the effect of overexpressing wild-type Gαq (WT-Gαq) or a constitutively active Gαq mutant (Q209L-Gαq) by using an adenovirus expression vector. In the basal state, Q209L-Gαq expression stimulated 2-deoxy-d-glucose uptake and GLUT4 translocation to 70% of the maximal insulin effect. This effect of Q209L-Gαq was inhibited by wortmannin, suggesting that it is phosphatidylinositol 3-kinase (PI3-kinase) dependent. We further show that Q209L-Gαq stimulates PI3-kinase activity in p110α and p110γ immunoprecipitates by 3- and 8-fold, respectively, whereas insulin stimulates this activity mostly in p110α by 10-fold. Nevertheless, only microinjection of anti-p110α (and not p110γ) antibody inhibited both insulin- and Q209L-Gαq-induced GLUT4 translocation, suggesting that the metabolic effects induced by Q209L-Gαq are dependent on the p110α subunit of PI3-kinase. In summary, (i) Gαq appears to play a necessary role in insulin-stimulated glucose transport, (ii) Gαq action in the insulin signaling pathway is upstream of and dependent upon PI3-kinase, and (iii) Gαq can transmit signals from the insulin receptor to the p110α subunit of PI3-kinase, which leads to GLUT4 translocation.
G-protein-coupled receptors (GPCRs) are seven-transmembrane-domain-containing cell surface proteins which activate heterotrimeric G proteins consisting of α and βγ subunits. Ligand binding to GPCRs induces GDP-GTP exchange on the Gα subunit, causing dissociation from the Gβγ subunits (18). Gα, as well as the Gβγ subunit complex, can independently propagate a cascade of intracellular signaling events, and it is clear that heterotrimeric G proteins have numerous biological functions (4). Since there are several classes of Gα, Gβ, and Gγ subunits, it is still not clearly understood how the different subunits mediate the large variety of biological effects of GPCRs.
Although heterotrimeric G proteins typically respond to GPCRs, cross talk between receptor tyrosine kinases (RTKs) and GPCR signaling pathways has recently been reported (27). Thus, insulin-like growth factor I mediates mitogen-activated protein kinase (MAPK) activation through a pathway that is, at least in part, Gαi dependent. GPCR stimulation of G proteins can also lead to mitogenic signaling through MAPK activation (25). For example, angiotensin II, which signals through Gαq, stimulates MAPK activity following Ras activation (21). On the other hand, there is only limited information on any potential role for G proteins in metabolic signaling, such as stimulation of glucose transport. It has been reported that after bradykinin receptor overexpression, bradykinin can stimulate glucose uptake into cells in a Gαq-dependent manner (12). Interestingly, Gαq also mediates the α1-adrenergic effects of catecholamines, which are counterregulatory to the effects of insulin, in part due to inhibition of glucose uptake (13). These findings raise the possibility that Gαq modulates insulin signaling in target tissues.
Therefore, we have directly studies the involvement of Gαq/11 in insulin-stimulated glucose transport and insulin-responsive glucose transporter (GLUT4) translocation. We show that in 3T3-L1 adipocytes, endogenous Gαq function is necessary for insulin-induced GLUT4 translocation and that a constitutively active Gαq (Q209L-Gαq) stimulates 2-deoxy-d-glucose (2-DOG) uptake and GLUT4 translocation in a phosphatidylinositol 3-kinase (PI3-kinase)-dependent mechanism. Taken together, our results suggest a necessary role for Gαq in the metabolic signaling cascade leading from the insulin receptor to PI3-kinase and glucose uptake.
MATERIALS AND METHODS
Materials.
Anti-IRS-1 and anti-phospho-specific Akt antibodies were purchased from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Mouse monoclonal anti-GLUT4 antibody (1F8) was from Biogenesis Inc. (Brentwood, N.H.), and rabbit polyclonal anti-GLUT4 antibody (F349) was kindly provided by Michael Mueckler (Washington University, St. Louis, Mo.). Sodium azide-free monoclonal antiphosphotyrosine (PY-20) and protein kinase C-λ (PKC-λ) antibodies, and horseradish peroxidase-conjugated antiphosphotyrosine antibody (RC-20) were from Transduction Laboratories (Lexington, Ky.). Horseradish peroxidase-linked anti-rabbit, anti-mouse, and anti-goat antibodies and anti-Akt1 and Gαq/11, antibodies were from Santa Cruz Biotechnology (Santa Cruz, Calif.). Sheep immunoglobulin G (IgG) and fluorescein isothiocyanate (FITC)-, tetramethylrhodamine isothiocyanate (TRITC)- and aminomethylcoumarin acetate-conjugated anti-rabbit, anti-mouse, anti-goat, and anti-sheep IgG antibodies were from Jackson Immunoresearch Laboratories Inc. (West Grove, Pa.). Wild-type and GTPase-deficient (activated) Q209L mutant Gαq expression vector and recombinant adenoviruses have been described elsewhere (1). The RGS2 expression vector was kindly provided by John R. Hepler (Washington University). Dulbecco’s modified Eagle’s medium (DMEM) and fetal calf serum (FCS) were purchased from Life Technologies (Grand Island, N.Y.). All radioisotopes were from ICN (Costa Mesa, Calif.). All other reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Cell culture and microinjection.
3T3-L1 cells were cultured and differentiated as previously described (8). Microinjection of the various reagents was carried out with a semiautomatic Eppendorf microinjection system. All reagents for microinjection were dissolved in microinjection buffer consisting of 5 mM sodium phosphate (pH 7.2) and 100 mM KCl. Antibodies were coinjected into the cytoplasm of the cell with 5 mg of sheep IgG per ml to allow identification of injected cells. Expression vectors for the various Gαq proteins were directly injected into the nuclei of living cells, and protein expression was allowed to continue for 24 h. Cytomegalovirus-green fluorescent protein (GFP) expression vector was coinjected for identification of injected cells in the nuclear microinjection studies.
Immunostaining and immunofluorescence microscopy.
Immunostaining of GLUT4 was performed essentially as described previously (26). The cells were fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature. After being washed, the cells were permeabilized and blocked with 0.1% Triton X-100–2% FCS in PBS for 10 min. The cells were then incubated with anti-GLUT4 antibody (1F8 or F349) in PBS–2% FCS overnight at 4°C. After the cells were washed, anti-GLUT4 and injected IgG were detected by incubation with TRITC-conjugated donkey anti-mouse or anti-rabbit IgG antibody and AMCA-conjugated donkey anti-sheep antibody, respectively, followed by observation under an immunofluorescence microscope.
For the membrane-ruffling staining, fixed cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min. After being washed with PBS, the cells were incubated with TRITC-conjugated phalloidin to visualize the membrane ruffles and with FITC-conjugated donkey anti-rabbit antibody to detect the injected IgG, as described previously (17).
In all counting experiments, the observer was blinded to the experimental condition of each coverslip. For the nuclear microinjection study, FITC-positive (GFP protein-expressing) cells were evaluated for the presence of plasma membrane-associated GLUT4 staining.
Cell treatments.
3T3-L1 adipocytes were incubated with 300 nM wortmannin or 0.1% dimethyl sulfoxide vehicle for 1 h at 37°C after starvation.
For adenovirus infection, 3T3-L1 adipocytes were transduced at a multiplicity of infection (MOI) of 10 PFU/cell for 16 h with either a control recombinant adenovirus containing a lacZ gene or the recombinant adenoviruses wild-type Gαq, Q209L mutant Gαq, or p110caax. Transduced cells were incubated for 60 h at 37°C under 10% CO2 in high-glucose DMEM with 2% heat-inactivated FCS, followed by incubation in the starvation media required for the assay. The efficiency of adenovirus-mediated gene transfer was above 90% as measured by histocytochemical staining of lacZ-infected cells with β-galactosidase, as we reported previously (22). The survival of the differentiated 3T3-L1 adipocytes was unaffected by incubation of cells with the different adenovirus constructs, since the total amount of cell protein remained the same in infected and uninfected cells.
Western blotting.
3T3-L1 adipocytes were starved for 12 hrs in regular glucose DMEM plus with 0.1% bovine serum albumin. The cells were stimulated with 100 ng of insulin per ml for 10 or 30 min at 37°C and lysed for 30 min at 4°C in a solubilizing buffer containing 20 mM Tris, 1 mM EDTA, 140 mM NaCl, 1% Nonidet P-40, 50 U of aprotinin/ml, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and 1 mM NaF (pH 7.5). The cell lysates were centrifuged to remove insoluble materials. For Western blot analysis, whole-cell lysates (30 to 80 μg of protein per lane) were denatured by boiling in Laemmli sample buffer containing 100 mM dithiothreitol and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Gels were transferred to polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, Mass.) by using a Transblot apparatus (Bio-Rad, Hercules, Calif.). For immunoblotting, the membranes were blocked and probed with specified antibodies. The blots were then incubated with horseradish peroxidase-linked second antibody followed by chemiluminescence detection, as specified by the manufacturer (Pierce Chemical Co., Rockford, Ill.).
PI3-kinase assay.
After 60 h of adenovirus infections, serum-starved (for 12 h) 3T3-L1 adipocytes were incubated in the absence (basal) or presence of insulin (100 ng/ml) for 10 min or platelet-derived growth factor (PDGF) (100 ng/ml) for 2 min, washed once with ice-cold PBS, lysed, and subjected to immunoprecipitation (300 to 500 mg of total protein) with anti-p110α or anti-p110γ antibody (2 μg) for 4 h at 4°C. Immunocomplexes were precipitated from the supernatant with protein G plus agarose (Santa Cruz Biotechnology) and washed as described previously (23). The washed immunocomplexes were incubated with phosphatidylinositol plus [γ-32P]ATP (3,000 Ci/mmol) for 10 min at room temperature. The reactions were stopped with 20 μl of 8 N HCl, the reaction mixtures were mixed with 160 μl of CHCl3-methanol (1:1) and centrifuged, and the lower (organic) phase was removed and applied to a silica gel thin-layer chromatography plate which had been coated with 1% potassium oxalate. The thin-layer chromatography plates were developed in CHCl3-CH3OH-H2O-NH4OH (60:47:11.3:2), dried, and visualized, and quantitated on a PhosphorImager (Molecular Dynamics).
2-Deoxyglucose uptake.
The procedure for glucose uptake was a modification of the methods described by Klip et al. (14). After 60 h of adenovirus infections, serum- and glucose-deprived 3T3-L1 adipocytes were incubated in alpha MEM in the absence (basal) or presence of 100 ng of insulin per ml for 1 h at 37°C. Glucose uptake was determined in duplicate or triplicate at each point after the addition of 10 μl of substrate (0.1 μCi of 2-[3H]deoxyglucose or l-[3H]glucose; final concentration, 0.01 mmol/liter) to provide a concentration at which cell membrane transport was rate limiting. The value for l-glucose was subtracted to correct each sample for the contributions of diffusion and trapping.
RESULTS
Effect of endogenous Gαq on insulin-induced GLUT4 translocation in 3T3-L1 adipocytes.
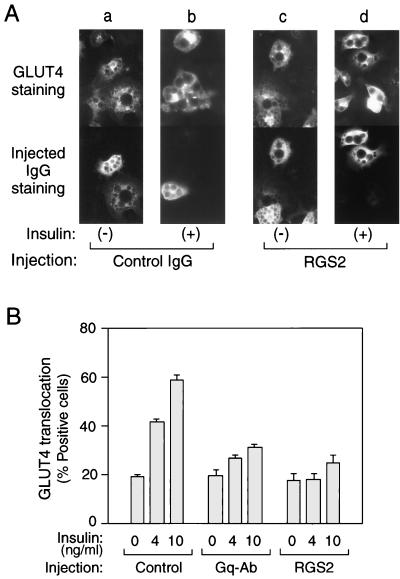
Gαq protein is distributed in many tissues, including 3T3-L1 adipocytes (see Fig. 2A, lanes a to d). Insulin induces glucose uptake by promoting the translocation of GLUT4 proteins from an intracellular pool to the cell surface in adipocytes and myocytes (20). We have assessed the role of endogenous Gαq on insulin stimulated-GLUT4 translocation in 3T3-L1 adipocytes by using microinjection of anti-Gαq/11 antibody or purified RGS2 protein, which is a specific Gαq GAP protein (10). For the quantitative detection of GLUT4 translocation, we used immunofluorescent staining of GLUT4 with specific antibodies (8). In the basal state, cells display GLUT4 staining mostly in a perinuclear localization, with some staining distributed in the cytoplasm (Fig. 1A, panel a); after insulin stimulation, GLUT4 staining is seen at the plasma membrane as a circumferential ring, with a concomitant decrease in intracellular distribution (Fig. 1A, panel b), as previously described (26). As shown in Fig. 1B, single-cell microinjection of Gαq/11 antibody or RGS2 protein into 3T3-L1 adipocytes markedly inhibited (70 and 81%, respectively) insulin induced-GLUT4 translocation. These findings suggest that activated Gαq plays a necessary role in the insulin signaling cascade leading to GLUT4 translocation.
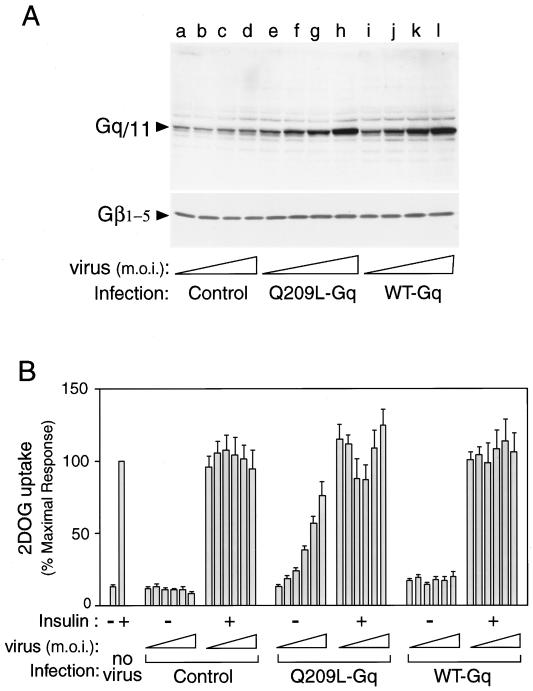
FIG. 2.
Expression and effects of Gαq on glucose uptake into the 3T3-L1 adipocytes. (A) Differentiated 3T3-L1 adipocytes were infected with various concentrations (MOI = 1, 5, 10, and 20) of adenovirus expressing WT Gαq, Q209L-Gαq, or mock-infected control. After 60 h of infection, these cells were lysed, and total-cell lysates were analyzed by Western blotting with anti-Gαq/11 C-terminal antibody (top) or anti-Gβ1–5 antibody (bottom), as described in Materials and Methods. These experiments were repeated twice. (B) 3T3-L1 adipocytes were infected with various concentrations (MOI = 1, 5, 10, 20, 30, and 40) of adenovirus expressing WT Gαq, Q209L-Gαq, or mock control. After 60 h of infection, these cells were stimulated with 100 ng of insulin per ml for 1 h and 2-[3H]deoxyglucose uptake was measured as described in Materials and Methods. The data are the means and standard errors from four independent experiments.
FIG. 1.
Effects of anti-Gαq antibody or RGS2 protein microinjection on insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Serum-starved 3T3-L1 adipocytes on coverslips were incubated with or without insulin (4 and 10 ng/ml) for 20 min after the microinjection of sheep IgG, anti-Gαq/11 antibody, or RGS2 protein, mixed with sheep IgG as a marker, as described in Materials and Methods. (A) Representative cells of GLUT4 staining are shown in the top panels (a to d), and staining of injected RGS2/sheep IgG (c and d) or sheep IgG alone (a and b) is shown in the bottom panels. (B) Percentage of cells positive for GLUT4 translocation, calculated by counting at least 100 cells at each point. Injected materials are sheep IgG for Control, anti-Gαq/11 C-terminal antibody for Gq-Ab, and RGS2 protein mixed with sheep IgG for RGS2. The data are means and standard errors from four independent experiments.
Effect of a constitutively active Gαq mutant on glucose transport in 3T3-L1 adipocytes.
To further explore the functional importance of activated Gαq in insulin induced-glucose transport in 3T3-L1 adipocytes, we used recombinant adenovirus vectors containing either wild-type (WT) or a constitutively active mutant (Q209L) Gαq cDNA. To demonstrate Gαq protein expression in 3T3-L1 adipocytes after infection with these adenovirus vectors, we performed Western blotting on whole-cell lysates with an anti-Gαq/11 antibody directed against the C terminus of the protein. As shown in Fig. 2A, Gαq protein expression was proportional to the Gαq adenovirus concentration (MOI). Infection with both WT and mutant Q209L-Gαq adenoviruses at an MOI of 10 (Fig. 2A, top, lanes g and k) increased the intensity of the 42-kDa band (corresponding to Gαq protein) 10-fold compared with infection by mock control adenovirus at an MOI of 10 (lane c). On the other hand, there was almost no change in the amount of Gβ subunit (β1–5) expression after 60 h of Gαq adenovirus infection (Fig. 2A, bottom). These results show that Gαq adenovirus transfection results in increased Gαq protein levels that are not accompanied by increased Gβγ-subunit expression.
We next assessed the effects of WT and Q209L-Gαq expression on insulin-induced 2-DOG uptake (Fig. 2B). Increasing concentrations of control- or WT-expressing adenovirus had no effect on basal or insulin-stimulated 2-DOG uptake compared to that in mock-transfected cells (Fig. 2B). In contrast, Q209L-Gαq increased basal 2-DOG uptake to 80% of the effect of insulin in a dose-dependent manner. At intermediate MOIs of 20 and 30, which led to substantial stimulation of glucose transport, the effects of subsequent addition of insulin were somewhat reduced.
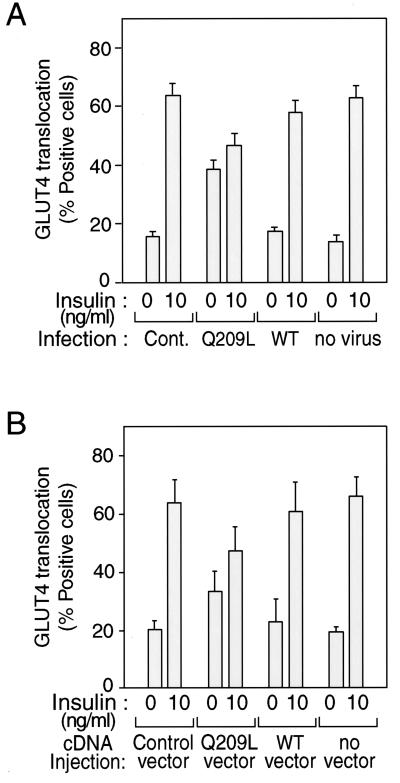
The effects of constitutively active Gαq on GLUT4 translocation were comparable to its effects on glucose transport. Thus, as shown in Fig. 3A, insulin (10 ng/ml) led to a fourfold stimulation of GLUT4 translocation in control cells. WT Gαq expression had no effect on either basal or insulin-induced GLUT4 translocation. On the other hand, Q209L-Gαq expression increased basal GLUT4 translocation by 2.2-fold to 39%. Addition of insulin to Q209L-Gαq-expressing cells led to minimal further stimulation.
FIG. 3.
Effects of Gαq expression on insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Serum-starved 3T3-L1 adipocytes on coverslips were incubated with or without insulin (10 ng/ml) for 20 min after 60 h of Gαq-expressing adenovirus infection (MOI = 10) (A) or after 24 h of nuclear microinjection with Gαq expression vector and with GFP expression vector as a marker (B). Fixed cells were stained with rabbit anti-GLUT4 antibody and incubated with TRITC-conjugated anti-rabbit IgG antibody, as described in Materials and Methods. The percentage of cells positive for GLUT4 translocation was calculated by counting at least 100 cells at each point. The data are means and standard errors from three independent experiments. (A) Cont., mock control adenovirus; Q209L, Q209L-Gαq-expressing adenovirus; WT, wild-type-Gαq-expressing adenovirus. (B) Control vector, GFP-expressing vector only.
To confirm the effects of overexpressed Gαq protein independent of adenovirus infection, we microinjected the expression vectors containing the Gαq cDNA directly into the nucleus of living 3T3-L1 adipocytes and then assessed their acute effects on GLUT4 translocation by using previously described methods (26). Vectors for WT and Q209L-Gαq (0.1 mg/ml) were microinjected into the nucleus of 3T3-L1 adipocytes, along with a vector for GFP, to allow the detection of injected cells. Cells positive for GFP expression (detected by FITC fluorescence) were analyzed for GLUT4 translocation. As shown in Fig. 3B, expression of WT Gαq had no effect on basal or insulin-induced GLUT4 translocation compared to the control (GFP expression alone). Expression of Q209L-Gαq increased basal GLUT4 translocation by 1.7-fold and inhibited insulin-induced (10 ng/ml) GLUT4 translocation. These results are comparable to those observed after adenovirus-mediated WT and Q209L-Gαq expression (Fig. 3A).
Mechanisms of Gαq signaling to glucose transport.
After ligand binding to GPCRs, Gβγ subunits dissociate from the Gα subunit and can mediate biologic signals (4). To determine whether Gβγ subunits were involved in glucose transport signaling, we used a glutathione S-transferase (GST) fusion protein containing the C-terminal portion of the β-adrenergic receptor kinase (GST-BARK). This protein binds to Gβγ subunits and behaves as a dominant negative inhibitor of Gβγ signaling (7). We therefore microinjected GST-BARK into the cytoplasm of cells infected with WT or Q209L-Gαq adenovirus and measured insulin-stimulated GLUT4 translocation. As shown in Fig. 4A, GST-BARK had no effect on basal, insulin-stimulated, or Q209L-Gαq-stimulated GLUT4 translocation. As a control, we demonstrated the functional integrity of GST-BARK, by showing that it inhibited lysophosphatidic acid- and thrombin-stimulated DNA synthesis when microinjected into HIRc cells (data not shown).
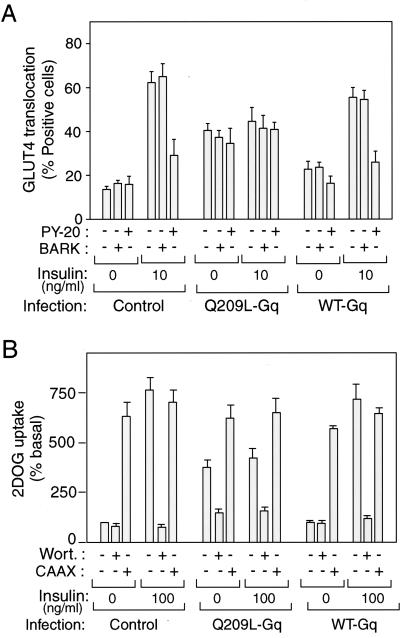
FIG. 4.
Microinjection and treatments of inhibitors and p110caax expression on Gαq expressed 3T3-L1 adipocytes. (A) After 60 h of Gαq-expressing adenovirus infection (MOI = 10), 3T3-L1 cells were injected with antiphosphotyrosine antibody (PY-20), GST-BARK, or sheep IgG as a control, prior to 3 h of insulin stimulation. Fixed cells were stained with rabbit anti-GLUT4 antibody and incubated with TRITC-conjugated anti-rabbit IgG antibody and AMCA-conjugated anti-sheep IgG antibody, as described in Materials and Methods. (B) After 60 h of Gαq-expressing adenovirus infection (MOI = 10) with or without p110caax-expressing adenovirus coinfection (CAAX), 3T3-L1 adipocytes were incubated with 300 nM wortmannin (Wort) or 0.1% DMSO vehicle for 60 min and with insulin (100 ng/ml) for 50 min or left untreated. 2-[3H]deoxyglucose uptake was measured as described in Materials and Methods. The data are means and standard errors from three independent experiments.
To determine whether the effect of Q209L-Gαq on GLUT4 translocation was dependent on downstream tyrosine phosphorylation, we microinjected antiphosphotyrosine antibodies (PY-20) into control and WT- and Q209L-Gαq-infected cells. Although PY-20 antibody inhibited insulin stimulated GLUT4 translocation, it had no effect on the action of Q209L-Gαq. Interestingly, neither BARK nor PY-20 prevented the effect of Q209L-Gαq from inhibiting the effects of insulin on GLUT4 translocation (Fig. 4A).
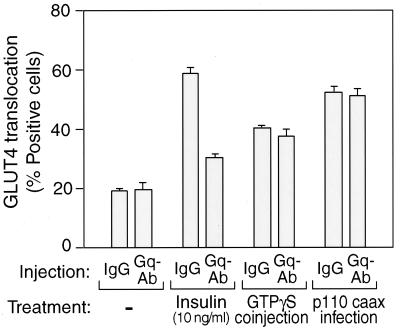
Insulin-stimulated glucose transport is PI3-kinase dependent, and therefore, to evaluate the role of PI3-kinase in the action of Gαq on glucose transport, we used several approaches. We found that the PI3-kinase inhibitor wortmannin blocked insulin- and Q209L-Gαq-stimulated glucose transport and GLUT4 translocation (Fig. 4B). p110caax is a membrane-targeted, constitutively active form of the p110α subunit of PI3-kinase, and we have previously shown that adenovirus-mediated expression of this protein in 3T3-L1 adipocytes is sufficient to stimulate GLUT4 translocation and glucose transport (6). As seen in Fig. 4B and 5, p110caax stimulates GLUT4 translocation and glucose transport to approximately the same magnitude, as does insulin. Importantly, however, while microinjection of the anti-Gαq/11 antibody blocks insulin action, it is without effect on the stimulatory effects of p110caax (Fig. 5). Taken together, these findings demonstrate that the effects of Q209L-Gαq are PI3-kinase dependent, indicating that Gαq lies upstream of PI3-kinase in the insulin signaling pathway.
FIG. 5.
Effect of Gαq antibody microinjection on GTPγS and p110caax signaling to GLUT4 translocation. 3T3-L1 cells infected with or without p110caax-expressing adenovirus were injected with anti-Gαq/11 antibody or sheep IgG with or without GTPγS. After 1 h, the cells were stimulated with insulin or left unstimulated, and then fixed. Fixed cells were stained with rabbit anti-GLUT4 antibody and incubated with TRITC-conjugated anti-rabbit IgG antibody and AMCA-conjugated anti-sheep IgG antibody as described in Materials and Methods.
We have previously shown that when microinjected or electroporated into 3T3-L1 adipocytes, GTPγS stimulates GLUT4 translocation in a PI3-kinase independent manner (9). Along these lines, Fig. 5 demonstrates that microinjection of anti-Gαq/11 antibody does not inhibit GTPγS-stimulated GLUT4 translocation, and this is consistent with the view that the locus of Gαq in the insulin signaling cascade is upstream of PI3-kinase.
The effect of Q209L-Gαq on glucose transport depends on the p110α subunit of PI3-kinase.
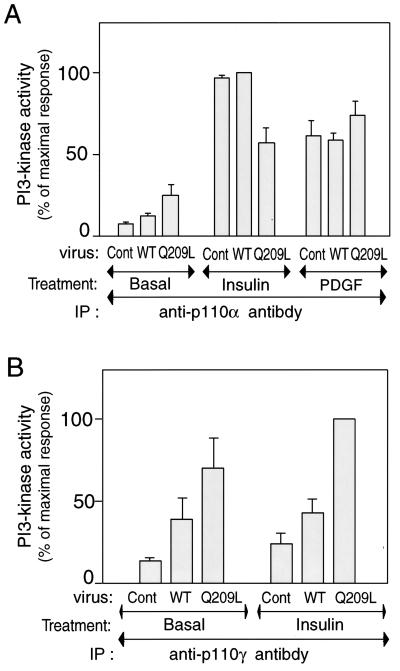
To further explore the role of PI3-kinase in Gαq action, we assessed PI3-kinase activity in anti-p110α or anti-p110γ antibody immunoprecipitates. As shown in Fig. 6A, PI3-kinase activity in anti-p110α immunoprecipitates was modestly increased (threefold) in Q209L-Gαq expressing cells compared to controls. In contrast, insulin led to a much larger stimulation of p110α activity in both control and WT-Gαq-expressing cells. Interestingly, the effect of insulin on increasing p110α activity was modestly blunted in the Q209L-Gαq expressing cells, although the effect of PDGF on p110α activity was unimpaired. In contrast to the p110α results, p110γ activity was markedly stimulated (fivefold) by Q209L-Gαq whereas insulin had only a small effect on p110γ activity (Fig. 6B).
FIG. 6.
PI3-kinase activities of p110α and p110γ subunit in 3T3-L1 adipocytes after Gαq expression. Serum-starved 3T3-L1 adipocytes were incubated in the absence or presence of insulin (100 ng/ml) for 10 min or PDGF (50 nM) for 2 min, after 60 h of Gαq-expressing adenovirus infection. Cell lysates were immunoprecipitated (IP) with anti-p110α or anti-p110γ antibody, and immune complexes were assayed for their ability to phosphorylate phosphatidylinositol. Reaction products (phosphatidylinositol-3 phosphate) were analyzed by thin-layer chromatography, and signals were quantitated on a PhosphorImager, as described in Materials and Methods. The data are means and standard errors from three independent experiments. (A) p110α PI3-kinase activity; (B) p110γ PI3-kinase activity. Cont., control.
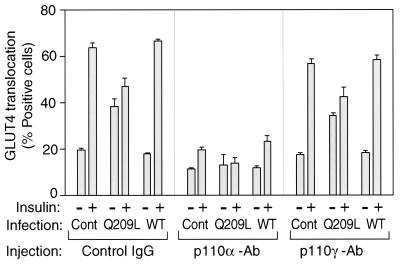
To determine the functional role of the different p110 subunits in insulin- and Q209L-Gαq-induced signaling to glucose transport, we examined GLUT4 translocation following microinjection of antibodies against the C terminus of the p110α or p110γ catalytic domains. As seen in Fig. 7A, the anti-p110α antibody blocked both Q209L-Gαq- and insulin-stimulated GLUT4 translocation whereas anti-p110γ antibody had no inhibitory effects.
FIG. 7.
Role of PI3-kinase in insulin-induced GLUT4 translocation in Gαq expressed 3T3-L1 adipocytes. After 60 h of Gαq-expressing adenovirus infection (MOI = 10), 3T3-L1 cells were injected with anti-p110α, anti-p110γ, or sheep IgG as a control (Cont). After insulin stimulation, fixed cells were stained with rabbit anti-GLUT4 antibody and incubated with TRITC-conjugated anti-rabbit IgG antibody and FITC-conjugated anti-goat or anti-sheep IgG antibody, as described in Materials and Methods. The data are means and standard errors from three independent experiments.
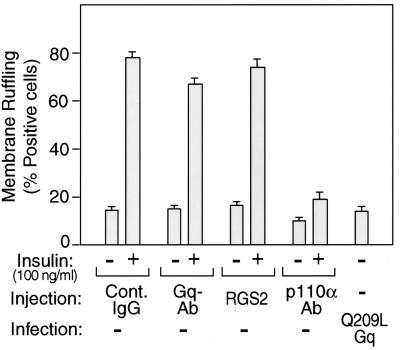
We have previously demonstrated that insulin has a robust effect on the stimulation of membrane ruffling in 3T3-L1 adipocytes and that this effect is dependent on PI3-kinase activation, since it is inhibitable by wortmannin (17). In the present studies, we have examined the role of Gαq in the insulin signaling cascade leading to membrane ruffling. As seen in Fig. 8, microinjection of the anti-p110α antibody inhibited insulin-induced membrane ruffling, consistent with the PI3-kinase dependency of this biological effect. Importantly, however, microinjection of anti-Gαq/11 antibody or the RGS2 protein did not affect insulin-stimulated membrane ruffling, although injection of these reagents inhibited insulin-stimulated GLUT4 translocation (Fig. 8) and the effect of Q209L-Gαq was dependent on p110α. Taken together, these results indicate that insulin-stimulated GLUT4 translocation and membrane ruffling are both dependent on PI3-kinase activity but that insulin-directed Gαq stimulation of PI3-kinase is specifically targeted to the glucose transport stimulatory pathway.
FIG. 8.
Effects of Gαq on insulin-induced membrane ruffling in 3T3-L1 adipocytes. 3T3-L1 cells were microinjected with anti-Gαq/11, anti-p110α antibody, RGS2 protein with sheep IgG, or sheep IgG as a control. After insulin stimulation, fixed cells were stained with rhodamine-phalloidin and FITC-conjugated anti-goat, anti-sheep, or anti-rabbit IgG antibody, as described in Materials and Methods. The data are means and standard errors from three independent experiments.
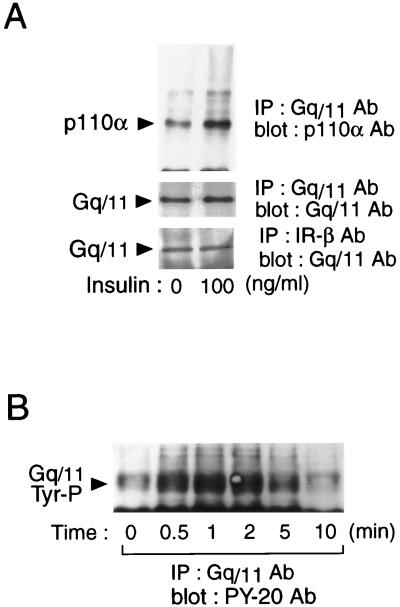
To further assess the mechanisms of Gαq stimulation of PI3-kinase, we investigated whether endogenous Gαq directly interacts with the p110α subunit of PI3-kinase. p110α was observed in the anti-Gαq/11 C-terminal antibody immunoprecipitates from basal cells, and the amount of coprecipitated p110α increased markedly after insulin stimulation (Fig. 9A). IRS-1 was not detected in the immunoprecipitates with anti-Gαq antibody (data not shown). Importantly, Gαq/11 protein was immunoprecipitated with anti-insulin receptor β-subunit antibody, and the intensity of the bands was not changed by insulin stimulation (Fig. 9A).
FIG. 9.
Gαq activity and association of Gαq with insulin receptor β-subunit and p110α subunit of PI3-kinase. 3T3-L1 adipocytes were lysed and immunoprecipitated (IP) with anti-Gαq/11 or IR-β antibodies. Immunoprecipitates were analyzed by Western blotting with anti-p110α antibody (A, top), anti-Gαq/11 antibody (A, middle and bottom panel), or PY-20 antibody (B). These experiments were repeated twice.
Tyrosine phosphorylation of Gαq/11.
Since it has been reported that a tyrosine residue in the C terminus of Gαq/11 becomes phosphorylated after GPCR stimulation, we determined whether Gαq/11 tyrosine phosphorylation was enhanced by insulin treatment (24). Cells were stimulated with insulin for various periods and then subjected to followed by phosphotyrosine blotting of Gαq/11 immunoprecipitates. As can be seen in Fig. 9B, insulin treatment led to a rapid, large, and transient increase in tyrosine phosphorylation of Gαq/11. Since earlier studies have shown that activation of Gαq/11 correlates with tyrosine phosphorylation, these data suggest that insulin can lead to Gαq/11 activation.
Specificity of Gαq for insulin-stimulated glucose transport.
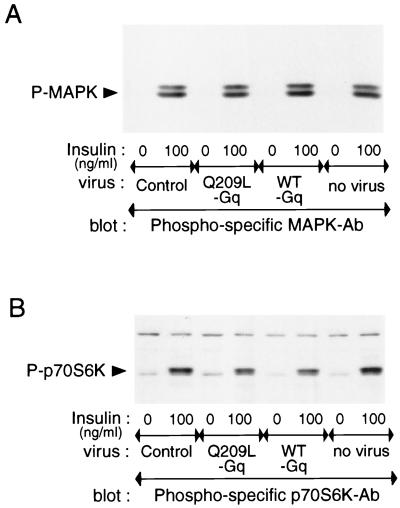
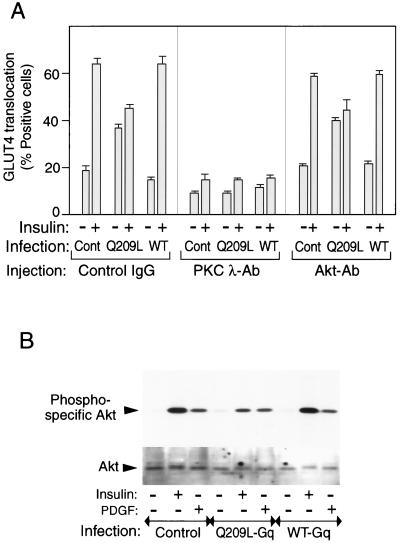
To further demonstrate the specificity of Gαq stimulation for the glucose transport pathway, we measured several other aspects of insulin action. As seen in Fig. 10, insulin stimulates MAPK and p70S6K phosphorylation whereas the effects of Q209L-Gαq expression are negligible. Akt activation involves serine/threonine phosphorylation, and this can be determined with a phospho-specific Akt antibody or by assessing the Akt gel shift with anti-Akt antibodies. As Fig. 11B demonstrates, insulin causes a robust stimulation of Akt phosphorylation and an Akt gel-shift in control and WT-Gαq-infected cells. In contrast, expression of Q209L-Gαq causes only a minimal stimulation of Akt phosphorylation, and no detectable Akt gel shift, consistent with the fact that Q209L-Gαq leads to only a modest stimulation of p110α (Fig. 6A). Interestingly, the effect of insulin on further stimulating Akt was impaired and the effect on p70S6K phosphorylation was modestly inhibited in Q209L-Gαq-expressing cells. In contrast, PDGF stimulation of Akt was unaffected by Q209L-Gαq expression.
FIG. 10.
Effects of Q209L-Gq on MAPK or p70S6-kinase activities in 3T3-L1 adipocytes. Serum-starved 3T3-L1 adipocytes were incubated in the absence or presence of insulin (100 ng/ml) for 10 min, after 60 h of Gαq-expressing adenovirus infection. Whole-cell lysates were subjected to SDS-PAGE and immunoblotted with phosphospecific MAPK antibody (A) or phosphospecific p70S6K antibody (B), as described in Materials and Methods. These experiments were repeated twice.
FIG. 11.
Effects of anti-Akt or PKC-λ antibody injection on GLUT4 translocation in 3T3-L1 adipocytes. (A) After 60 h of Gαq-expressing adenovirus infection (MOI = 10), 3T3-L1 cells were injected with anti-Akt antibody (Akt-Ab), PKC-λ antibody (PKC λ-Ab), or sheep IgG as a control. After insulin stimulation, fixed cells were stained with rabbit anti-GLUT4 antibody and incubated with TRITC-conjugated anti-rabbit IgG antibody and FITC-conjugated anti-mouse or anti-sheep IgG antibody. The data are means and standard errors from three independent experiments. (B) Serum-starved 3T3-L1 adipocytes were incubated in the absence or presence of insulin (100 ng/ml) or PDGF (50 nM) for 10 min after 60 h of Gαq-expressing adenovirus infection. Whole-cell lysates were subjected to SDS-PAGE and immunoblotted with phosphospecific Akt antibody (top) or Akt antibody (bottom) for the gel shift assay, as described in Materials and Methods. These experiments were repeated twice.
Role of PKC-λ and PKB/Akt in Gαq actions.
The serine/threonine kinase Akt, as well as atypical PKC isoforms, are activated by PI3-kinase, and evidence that either or both may be involved in insulin-stimulated glucose uptake exists (15, 16). To assess this, we microinjected anti-Akt or PKC-λ antibodies into 3T3-L1 adipocytes in the basal and insulin-stimulated states in control and Q209L-Gαq-expressing cells. Both insulin- and Q209L-Gαq-induced GLUT4 translocation were completely inhibited by anti-PKC-λ antibody injection, whereas anti-PKB/Akt antibody was without effect (Fig. 11A).
DISCUSSION
The ability of insulin to stimulate glucose transport is one of its most important physiologic actions. However, despite intense study, the signaling pathway mediating this biologic effect remains poorly understood. Although a number of molecular components have been proposed as participants in these signaling events, little consensus exists. Despite these uncertainties, there is relatively uniform agreement that activation of PI3-kinase is a necessary event, which participates in connecting the insulin receptor to stimulation of glucose transport (19). The major findings of the current studies are that heterotrimeric G proteins are important components of this insulin action pathway. Specifically, we found that Gαq/11 is necessary for insulin-stimulated glucose transport and that this molecule can be tyrosine phosphorylated by the insulin receptor and forms a molecular complex with the p110α-subunit of PI3-kinase which leads to glucose transport stimulation.
Since the initial discovery of insulin receptor substrate type 1 (IRS-1), numerous studies have demonstrated that IRS-1 and its other family members are bona fide substrates of the insulin receptor which become tyrosine phosphorylated and then bind to the p85 subunit of PI3-kinase, leading to PI3-kinase activation and downstream signaling (2, 22). Although IRS proteins are obviously candidates connecting the insulin receptor to PI3-kinase in the signaling pathway leading to glucose transport stimulation, we and others have provided previous data showing that these proteins are not necessary for insulin-stimulated glucose transport, indicating either that IRS proteins are not important components of glucose transport stimulation or that alternate pathways linking the insulin receptor to PI3-kinase exist (23). Thus, since PI3-kinase activation is an absolute requirement for glucose transport stimulation whereas IRS-1 is not, some other mechanism connecting the insulin receptor to PI3-kinase stimulation must exist.
Traditionally, GPCRs and receptor tyrosine kinase (RTK) signaling pathways have been viewed as distinct and nonoverlapping. However, recent evidence from several different systems has indicated overlap and convergence between RTK and GPCR signaling mechanisms (5, 25), since both pathways engage many of the same molecules and since GPCR stimulation can lead to tyrosine phosphorylation events and certain RTK signaling events are G-protein dependent. In the present studies, we have found that the ability of insulin to stimulate glucose transport is dependent on the function of a heterotrimeric G protein containing Gαq. Thus, microinjection of anti-Gαq/11 antibody into 3T3-L1 adipocytes markedly inhibits the ability of insulin to stimulate GLUT4 translocation. Like all G proteins, Gαq is active in the GTP-bound state and inactive when bound to GDP (25). RGS2 is a specific Gαq GAP protein which hydrolyzes Gαq bound GTP to GDP (10). Thus, the RGS2 protein will specifically inhibit normally activated Gαq, and when the RGS2 protein was microinjected into 3T3-L1 adipocytes, insulin-stimulated GLUT4 translocation was abrogated.
The inhibition of GLUT4 translocation by anti-Gαq/11 antibody and RGS2 protein was highly specific for the insulin-stimulated response, since microinjection of these inhibitory reagents had no effect on GTPγS-stimulated or p110caax-stimulated GLUT4 translocation. These results indicate that the anti-Gαq/11 antibody does not inhibit insulin-stimulated glucose transport nonspecifically and also indicate that the site of Gαq action in the insulin signaling pathway is proximal to PI3-kinase action. Furthermore, we have previously shown that in 3T3-L1 adipocytes, insulin has a robust effect on stimulation of membrane ruffling in a PI3-kinase-dependent manner. Interestingly, inhibition of Gαq function has no effect on insulin-stimulated membrane ruffling. Thus, we have measured two PI3-kinase-dependent biologic effects in 3T3-L1 adipocytes, GLUT4 translocation and membrane ruffling, and found that only GLUT4 translocation is blocked by inhibition of Gαq function. This suggests that Gαq directs the insulin signal fairly specifically to glucose transport stimulation.
In complementary experiments, we have used adenovirus gene transfer to introduce a constitutively active form of Gαq (Q209L-Gαq) into 3T3-L1 adipocytes. The results demonstrate that Q209L-Gαq leads to GLUT4 translocation and stimulation of glucose transport with 60 to 70% effectiveness compared to insulin. The effects of Q209L-Gαq on stimulation of glucose transport are also specific, in that they do not lead to global activation of all the insulin action pathways. For example, Q209L-Gαq does not stimulate membrane ruffling and has a weak or undetectable effect on PKB/Akt, p70-S6 kinase, or MAPK activation. The stimulatory effects of Q209L-Gαq were strongly blocked by coincubation with the PI3-kinase inhibitor wortmannin, indicating that the action of Gαq on stimulating glucose transport is proximal to and dependent on PI3-kinase. Thus, consistent with the microinjection studies, constitutively active Gαq displays specificity for stimulation of the glucose transport-stimulatory pathway. Microinjection of an antiphosphotyrosine antibody (PY-20) inhibits insulin-stimulated but not Q209L-Gαq-stimulated GLUT4 translocation, indicating that tyrosine phosphorylation events do not lie downstream of the site of action of Gαq, and this is also consistent with the findings that expression of Q209L-Gαq did not lead to tyrosine phosphorylation of either the insulin receptor or IRS-1 (data not shown). With respect to PI3-kinase, our results by no means exclude the possibility that PI3-kinase plays a role in facilitating downstream events in GLUT4 vesicle recruitment, such as vesicle cycling and fusion. In this event, PI3-kinase could also be parallel to Gαq/11 in the insulin signaling pathway.
An interesting observation in our studies is that some of the measured biologic effects of insulin were blunted in Q209L-Gαq-expressing cells. Thus, the effect of insulin on stimulation of PI3-kinase and Akt activity was reduced in Q209L-Gq-expressing cells, and these additive effects of insulin on stimulation of glucose transport were slightly and variably decreased. Since, in the adenovirus system, cells are exposed to constitutively active Gαq for 60 h prior to the assay, our results are consistent with other models of cellular insulin resistance. Thus, osmotic shock (3) and chronic exposure to constitutively active PI3-kinase (6) both cause glucose transport stimulation and both render cells relatively refractory to subsequent insulin stimulation. Indeed, it is well recognized that chronic insulin treatment will induce a state of cellular insulin resistance. This is consistent with the view that chronically stimulating the insulin action pathway will lead to desensitization of the pathway at one or more steps. Although our results do not identify the exact site of desensitization in Q209L-Gαq-expressing cells, our results do show that the insulin resistance is not global. Thus, the ability of insulin to stimulate PI3-kinase and Akt activity were inhibited whereas the effects of PDGF on these activities were unchanged. Furthermore, not all of the actions of insulin were inhibited, since stimulation of MAPK was normal. Clearly, further studies to precisely elucidate the molecular cause of the insulin resistance in this system should be performed.
The above-described studies clearly demonstrate the necessity for Gαq in the signaling pathway leading to insulin-stimulated glucose transport and show that the effects of Gαq are proximal to, and dependent on, PI3-kinase. Additionally, our results have further explored the mechanisms whereby Gαq interacts with PI3-kinase. Thus, we show that Gαq has a large effect on stimulation of the PI3-kinase activity of the p110γ subunit but only a modest effect on stimulation of p110α. On the other hand, insulin has a large effect on stimulation of p110α and only a small effect on stimulation of p110γ. However, upon microinjecting both p110α and p110γ antibodies into 3T3-L1 adipocytes, we found that the p110α antibody blocked the effects of both insulin and Q209L-Gαq on stimulation of GLUT4 translocation whereas the p110γ antibody was without effect. These results indicate that under the direction of insulin, Gαq stimulates the p110α subunit of PI3-kinase, which then leads to glucose transport stimulation. It is interesting that Q209L-Gαq has only a modest effect on p110α activity, compared to its robust effect on p110γ. This suggests that only a small degree of p110α stimulation is sufficient for transport activation and that proper subcellular localization of the p110α activity is more important than the total intracellular levels of this activated enzyme. From these findings, we suggest that Gαq is an insulin receptor-directed targeting molecule which localizes active p110α to the correct intracellular compartment, where the distal events of transport signaling occur.
Our studies also provide insight as to how Gαq is engaged by the insulin signaling pathway. Thus, we find that Gαq/11 coprecipitates with the p110α subunit and that following insulin stimulation, more activated p110α is associated with Gαq/11. Gαq/11 also physically associates with the insulin receptor, since it can be identified in a molecular complex with the insulin receptor in immunoprecipitation experiments. Interestingly, stimulation with insulin did not alter the amount of insulin receptor-associated Gαq/11. Previous reports have demonstrated a tyrosine phosphorylation site in the Gαq/11 C terminus, and activation of Gαq/11 is associated with phosphorylarion of this residue (24). Importantly, we found that insulin stimulation leads to a marked increase in tyrosine phosphorylation of Gαq/11, consistent with the view that insulin leads to tyrosine phosphorylation and activation of Gαq/11, which then signals downstream through the p110α subunit of PI3-kinase. This conclusion is fortified by the fact that microinjection of the RGS2 protein, which would only inhibit activated Gαq/11, blocks insulin-stimulated GLUT4 translocation.
The molecular events downstream of PI3-kinase, which cause glucose transport stimulation, have also been extensively studied. Akt and PKC-λ are serine kinases activated through the action of PI3-kinase, and both have been suggested as mediators of glucose transport stimulation (15, 16). To assess this in our system, we microinjected anti-PKC-λ and Akt antibodies into insulin-stimulated, as well as Q209L-Gαq-expressing, 3T3-L1 adipocytes. The PKC-λ antibody inhibited both insulin- and Q209L-Gαq stimulated GLUT4 translocation, whereas the Akt antibody had no inhibitory effect. These results certainly implicate PKC-λ as a downstream mediator of glucose transport stimulatory events, consistent with the recent work of Kotani et al. (16). With respect to PKB/Akt, there are a number of papers reporting both positive and negative evidence for its role in glucose transport stimulation (11, 15). Our Akt antibody injection results would argue against a role for Akt in this pathway; however, these data are by no means conclusive, since they are negative, and a positive effect of the antibody is not measurable in a single cell microinjection system. Nevertheless, it is interesting that although Q209L-Gαq has a substantial effect on the stimulation of GLUT4 translocation, it has a very negligible effect on the stimulation of PKB/Akt phosphorylation. This is consistent with the antibody injection findings, and, taken together, these results suggest that Akt may not be a major downstream effecter of glucose transport stimulation in our model system.
ACKNOWLEDGMENTS
This work was supported in part by NIH grant DK-33651 and the V.A. Medical Research Service. Takeshi Imamura is supported through an ADA Mentor Board Fellowship Award. Peter Vollenweider was supported by a grant from the Schweizerische Stiftung fur Medizinisch-Biologische Stipendien, and Martin Clodi was supported by grants J01287-Med and J1584-Med from the Ewin Schrödinger Stipendium by the Austrian Fonds zur Förderany der Wissenschaftlichen Forschung.
We thank John R. Hepler (Washington University, St. Louis, Mo.) for providing the RGS2 expression vector, David W. Rose (University of California, San Diego, La Jolla, Calif.) for technical assistance with microinjection, and Elizabeth Hansen and Augustus P. Lestick for editorial assistance.
REFERENCES
- 1.Adams J W, Sakata Y, Davis M G, Sah V P, Wang Y, Liggett S B, Chien K R, Brown J H. Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki E, Lipes M A, Patti M E, Brüning J C, Haag B, Johnson R S, Kahn C R. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 3.Chen D, Elmendorf J S, Olson A L, Li X, Earp H S. Osmotic shock stimulates GLUT4 translocation in 3T3L1 adipocytes by a novel tyrosine kinase pathway. J Biol Chem. 1999;274:27401–27410. doi: 10.1074/jbc.272.43.27401. [DOI] [PubMed] [Google Scholar]
- 4.Clapham D E, Neer E J. G protein beta gamma subunits. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 5.Daub H, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 6.Egawa K, Sharma P M, Nakashima N, Huang Y, Huver E, Boss G R, Olefsky J M. Membrane-targeted phosphatidylinositol 3-kinase mimics insulin actions and induces a state of cellular insulin resistance. J Biol Chem. 1999;274:14306–14314. doi: 10.1074/jbc.274.20.14306. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Higuera I, Mayor F., Jr Rapid desensitization of neonatal rat liver beta-adrenergic receptors. A role for beta-adrenergic receptor kinase. J Clin Investig. 1994;93:937–943. doi: 10.1172/JCI117099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haruta T, Morris A J, Rose D W, Nelson J G, Mueckler M, Olefsky J M. Insulin-stimulated GLUT4 translocation is mediated by a divergent intracellular signaling pathway. J Biol Chem. 1995;270:27991–27994. doi: 10.1074/jbc.270.47.27991. [DOI] [PubMed] [Google Scholar]
- 9.Haruta T, Morris A J, Vollenweider P, Nelson J G, Rose D W, Mueckler M, Olefsky J M. Ligand-independent GLUT4 translocation induced by guanosine 5′-O-(3-thiotriphosphate) involves tyrosine phosphorylation. Endocrinology. 1998;139:358–364. doi: 10.1210/endo.139.1.5698. [DOI] [PubMed] [Google Scholar]
- 10.Heximer S P, Watson N, Linder M E, Blumer K J, Hepler J R. RGS2/G0S8 is a selective inhibitor of Gq alpha function. Proc Natl Acad Sci USA. 1997;94:14389–14393. doi: 10.1073/pnas.94.26.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imanaka T, Hayashi H, Kishi K, Wang L, Ishii K, Hazeki O, Katada T, Ebina Y. Reconstitution of insulin signaling pathways in rat 3Y1 cells lacking insulin receptor and insulin receptor substrate-1. Evidence that activation of Akt is insufficient for insulin-stimulated glycogen synthesis or glucose uptake in rat 3Y1 cells. J Biol Chem. 1998;273:25347–25355. doi: 10.1074/jbc.273.39.25347. [DOI] [PubMed] [Google Scholar]
- 12.Kishi K, Muromoto M, Nakaya Y, Miyata I, Hagi A, Hayashi H, Ebina I. Bradykinin directly triggers GLUT4 translocation via an insulin-independent pathway. Diabetes. 1998;47:550–558. doi: 10.2337/diabetes.47.4.550. [DOI] [PubMed] [Google Scholar]
- 13.Kishi K, Hayashi H, Wang L, Kamohara S, Tamaoka K, Shimizu T, Ushikubi F, Ebina Y. Gq-coupled receptors transmit the signal for GLUT4 translocation via an insulin-independent pathway. J Biol Chem. 1996;271:26561–26568. doi: 10.1074/jbc.271.43.26561. [DOI] [PubMed] [Google Scholar]
- 14.Klip A, Li G, Logan W J. Induction of sugar uptake response to insulin by serum depletion in fusing L6 myoblasts. Am J Physiol. 1984;247:E291–E296. doi: 10.1152/ajpendo.1984.247.3.E291. [DOI] [PubMed] [Google Scholar]
- 15.Kohn A D, Summers S A, Birnbaum M J, Roth R A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 16.Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, Kasuga M. Requirement of atypical protein kinase c-lambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1999;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakashima N, Rose D W, Xiao S, Egawa K, Martin S S, Haruta T, Saltiel A R, Olefsky J M. The functional role of CrkII in actin cytoskeleton organization and mitogenesis. J Biol Chem. 1999;274:3001–3008. doi: 10.1074/jbc.274.5.3001. [DOI] [PubMed] [Google Scholar]
- 18.Neer E J. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 19.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994;269:3563–3573. [PubMed] [Google Scholar]
- 20.Robinson L J, Pang S, Harris D S, Heuser J, James D E. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP insulin, and GTP gamma S and localization of GLUT4 to clathrin lattices. J Cell Biol. 1992;117:1181–1196. doi: 10.1083/jcb.117.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schieffer B, Paxton W G, Chai Q, Marrero M B, Bernstein K E. Angiotensin II controls p21ras activity via pp60c-src. J Biol Chem. 1996;271:10329–10333. doi: 10.1074/jbc.271.17.10329. [DOI] [PubMed] [Google Scholar]
- 22.Sharma P M, Egawa K, Gustafson T A, Martin J L, Olefsky J M. Adenovirus-mediated overexpression of IRS-1 interacting domains abolishes insulin-stimulated mitogenesis without affecting glucose transport in 3T3-L1 adipocytes. Mol Cell Biol. 1997;17:7386–7397. doi: 10.1128/mcb.17.12.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staubs P A, Nelson J G, Reichart D R, Olefsky J M. Platelet-derived growth factor inhibits insulin stimulation of insulin receptor substrate-1-associated phosphatidylinositol 3-kinase in 3T3-L1 adipocytes without affecting glucose transport. J Biol Chem. 1998;273:25139–25147. doi: 10.1074/jbc.273.39.25139. [DOI] [PubMed] [Google Scholar]
- 24.Umemori H, Inoue T, Kume S, Sekiyama N, Nagao M, Itoh H, Nakanishi S, Mikoshiba K, Yamamoto T. Activation of the G protein Gq/11 through tyrosine phosphorylation of the alpha subunit. Science. 1997;276:1878–1881. doi: 10.1126/science.276.5320.1878. [DOI] [PubMed] [Google Scholar]
- 25.van Biesen T, Hawes B E, Luttrell D K, Krueger K M, Touhara K, Porfiri E, Sakaue M, Luttrell L M, Lefkowitz R J. Receptor-tyrosine-kinase and G beta gamma-mediated MAP kinase activation by a common signalling pathway. Nature. 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- 26.Vollenweider P, Martin S S, Haruta T, Morris A J, Nelson J G, Cormont M, Marchand-Brustel Y L, Rose D W, Olefsky J M. The small guanosine triphosphate-binding protein Rab4 is involved in insulin-induced GLUT4 translocation and actin filament rearrangement in 3T3-L1 cells. Endocrinology. 1997;138:4941–4949. doi: 10.1210/endo.138.11.5493. [DOI] [PubMed] [Google Scholar]
- 27.Wan Y, Kurosaki T, Huang X Y. Tyrosine kinases in activation of the MAP kinase cascade by G-protein-coupled receptors. Nature. 1996;380:541–544. doi: 10.1038/380541a0. [DOI] [PubMed] [Google Scholar]