Abstract
We report a case of varicella zoster virus (VZV) meningitis following BNT162b2 mRNA COVID-19 vaccination in an immunocompetent patient. A final diagnosis was made based on identification of VZV via positive polymerase chain reaction of cerebrospinal fluid along with characteristic symptoms such as fever, headache, and stiff neck. This phenomenon has been reported elsewhere; this is the 13th such case reported worldwide and the 7th case in immunocompetent patients, indicating the need for careful monitoring after COVID-19 vaccines.
Keywords: COVID-19, SARS-CoV-2, BNT162b2 mRNA COVID-19 vaccination, Varicella zoster virus
Introduction
Since December 2019, COVID-19, caused by SARS-CoV-2 infection, has been a major health threat worldwide (Hayakawa et al., 2020). The mRNA-based BNT162b2 mRNA COVID-19 vaccine has demonstrated a high efficacy rate with an acceptable safety profile (Polack et al., 2020). In Japan, a nationwide mass BNT162b2 mRNA vaccination campaign was launched and rolled out at an exceptionally rapid pace with high vaccine acceptance. As of June 13, 2021, 14.0% (n=17 580 587) and 4.9% (n=6 104 732) of the population had received their first and second vaccine dose, respectively (Ministry of Health, Labour and Welfare, Japan, 2021).
Although vaccination is expected to be one of the most important COVID-19 infection prevention and control measures (Polack et al., 2020), several adverse reactions have been reported (Chen et al., 2021), including a few cases of varicella zoster virus (VZV) reactivation in Israel, Finland and Spain (Furer et al., 2021; Rodríguez-Jiménez et al., 2021; Tessas et al., 2021). Herein, we report a case of VZV meningitis following BNT162b2 mRNA COVID-19 vaccination and discuss the potential mechanisms.
Case Report
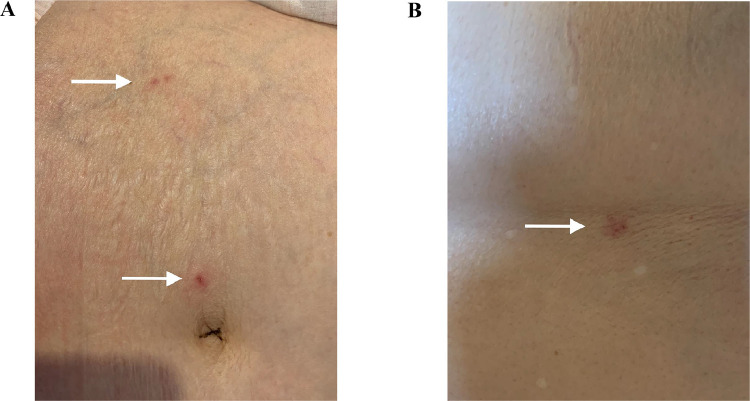
A 71-year-old woman with immunoglobulin A (IgA) nephritis presented to the hospital with fever and headache, which had persisted for 1 week since the day after her first BNT162b2 mRNA COVID-19 vaccination. She had regular visits to the hospital for IgA nephritis but had never used immunosuppressive drugs. She had a history of chickenpox in childhood but no history of vaccination for herpes zoster virus. On admission, she was conscious, and her temperature, blood pressure, heart rate, respiratory rate, and percutaneous arterial oxygen saturation were 38.1°C, 122/78 mmHg, 91 bpm, 16 breaths/min, and 98% (while breathing ambient air), respectively. Her physical examination tests revealed stiff neck, jolt accentuation and Kernig's sign. However, there were no obvious neurodegenerative finding. Five days post-vaccination, she developed painful vesicles and erythematous patches on the right side of her umbilicus and on her back (Figure 1 ). Blood test results showed a normal level of C-reactive protein (0.04 mg/dL) and white blood cell count (4860 cells/μL) with no lymphopenia (23%). The patient had 1.92 IU/mL and ≥128 IU/mL of VZV IgM and IgG antibodies, respectively. Notably, the patient was HIV-negative. Cerebrospinal fluid (CSF) examination revealed a normal opening pressure level (160 mmH2O), a high leukocyte count (289 cells/μL) with lymphocytic predominance (95.8%), a high protein level (295 mg/dL), and a normal glucose level (60 mg/dL).
Figure 1.
Vesicles and erythematous patches of varicella zoster virus infection.
Skin lesions with crusting and vesiculation, and surrounding erythema (white arrow) affecting the abdomen (A) and back (B) in the Th10 dermatome.
A final diagnosis of VZV meningitis was made based on positive rapid immunochromatography (abdominal vesicles tested) and CSF polymerase chain reaction (PCR) results (1.86 × 106 /μL). The patient's skin rash was confined to a single location, so she did not meet the diagnostic criteria for disseminated herpes zoster. However, because of the presence of meningitis and the skin rash, standard, contact, and airborne infection control measures using a private room were conducted based on the hospital infection prevention and control manual. These infection control measures were continued until her skin rash crusted over. Her SARS-CoV-2 PCR results were negative for both nasal swab and CSF samples. She was started on intravenous acyclovir treatment; as of hospital day 3, she became afebrile, and her headache improved. On hospital day 14, she was discharged without any complications. Because the first vaccination did not cause potentially fatal allergic symptoms, we recommended the second vaccination after discharge based on our policy. The patient provided written informed consent to have her clinical details presented in this report.
Discussion
We encountered a patient who developed VZV meningitis shortly after receiving the first dose of the BNT162b2 mRNA COVID-19 vaccine. Although the association between vaccination and VZV meningitis may be coincidental, this case is noteworthy for the following three reasons.
First, several cases of VZV reactivation following BNT162b2 mRNA COVID-19 vaccination have been reported worldwide (Furer et al., 2021; Rodríguez-Jiménez et al., 2021; Tessas et al., 2021). Similar to our case, VZV reactivation occurred after vaccination in immunocompetent patients in two of the previous reports involving a total of six patients (Rodríguez-Jiménez et al., 2021; Tessas et al., 2021); the other report involved VZV reactivation in patients with autoimmune inflammatory rheumatic diseases. In these previous cases, the duration from vaccination to VZV reactivation ranged from 1 to 16 days, and VZV reactivation was reported after administration of either the first or second vaccination dose (Furer et al., 2021; Rodríguez-Jiménez et al., 2021; Tessas et al., 2021). These findings imply that VZV reactivation may develop in the BNT162b2 mRNA COVID-19 vaccinated population.
Second, patients infected with SARS-CoV-2 have been reported to naturally develop VZV reactivation (Xu et al., 2020). A cytokine storm involving release of proinflammatory cytokines, such as interleukin-6, tumor necrosis factor-alpha, and interleukin-12, disturbs the function of CD4+ T cells and promotes excessive activation, and possibly subsequent exhaustion of CD8+ T cells, may be one of the mechanisms of SARS-CoV-2-associated VZV reactivation (Zheng et al., 2020).
Third, vaccinations to prevent diseases other than COVID-19, such as hepatitis A, influenza, rabies, and Japanese encephalitis, can also result in VZV reactivation (Bostan and Yalici-Armagan, 2021). Therefore, it is conceivable that this new BNT162b2 mRNA COVID-19 vaccine may have the same effect as observed with natural infection in the long run.
Our report has several limitations. First, this is the only case report thus far. Second, VZV meningitis after BNT162b2 mRNA COVID-19 vaccination may be coincidental. However, due to supporting evidence of similar cases (Furer et al., 2021; Rodríguez-Jiménez et al., 2021; Tessas et al., 2021), BNT162b2 mRNA COVID-19 vaccine-associated VZV reactivation cannot be ruled out. Immune dysregulation by mRNA vaccines may cause VZV reactivation, even in immunocompetent patients with no lymphopenia (Rodríguez-Jiménez et al., 2021; Tessas et al., 2021).
In conclusion, we observed a case of VZV meningitis following BNT162b2 mRNA COVID-19 vaccination in an immunocompetent patient. Although there are only a few such reports to date, these cases suggest that the possibility of VZV reactivation (including meningitis) should be carefully monitored as a potential phenomenon occurring after vaccination. With the large numbers of people being vaccinated with the BNT162b2 mRNA COVID-19 vaccine, further studies on the relationship between COVID-19 vaccination and VZV reactivation after vaccination should be conducted.
Acknowledgments
Acknowledgments
We thank all the clinical staff at our hospital for their dedication to patient care.
Funding source
This research was supported by the National Center for Global Health and Medicine (NCGM) Intramural Research Fund [grant number: 21A006]. The funder played no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Ethical approval
The patient provided consent for the publication of her clinical case details.
Conflict of interest
The authors state that they have no conflict of interest.
References
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: A coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566–1567. doi: 10.1111/jocd.14035. [DOI] [PubMed] [Google Scholar]
- Chen G, Li X, Sun M, Zhou Y, Yin M, Zhao B, et al. COVID-19 mRNA vaccines are generally safe in the short term: A vaccine vigilance real-world study says. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.669010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: A case series. Rheumatology. keab345. Epub. 2021 Apr 13 doi: 10.1093/rheumatology/keab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Kutsuna S, Kawamata T, Sugiki Y, Nonaka C, Tanaka K, et al. SARS-CoV-2 infection among returnees on charter flights to Japan from Hubei, China: A report from National Center for Global Health and Medicine. Global Health Med. 2020;2(2):107–111. doi: 10.35772/ghm.2020.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Labour and Welfare, Japan. Report of suspected adverse reactions of COVID-19 vaccine [cited 2021 Jun 23]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/vaccine_hukuhannou-utagai-houkoku.html
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, Seguí M, Morales-Caballero Á, Llamas-Velasco M, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: Report of 5 cases. JAAD Case Rep. 2021;12:58–59. doi: 10.1016/j.jdcr.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessas I, Kluger N. Ipsilateral herpes zoster after the first dose of BNT162b2 mRNA COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021 doi: 10.1111/jdv.17422. Epub May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Zhou Y, Cai L, Wang L, Han J, Yang X, et al. Co-reactivation of the human herpesvirus alpha subfamily (herpes simplex virus-1 and varicella zoster virus) in a critically ill patient with COVID-19. Br J Dermatol. 2020;183(6):1145–1147. doi: 10.1111/bjd.19484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17(5):541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]