Abstract
Objective.
Racial/ethnic minorities experience more severe outcomes of coronavirus disease 2019 (COVID-19) in the general US population. This study was undertaken to examine the association between race/ethnicity and COVID-19 hospitalization, ventilation status, and mortality in people with rheumatic disease.
Methods.
US patients with rheumatic disease and COVID-19 were entered into the COVID-19 Global Rheumatology Alliance physician registry between March 24, 2020 and August 26, 2020 were included. Race/ethnicity was defined as White, African American, Latinx, Asian, or other/mixed race. Outcome measures included hospitalization, requirement for ventilatory support, and death. Multivariable regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) adjusted for age, sex, smoking status, rheumatic disease diagnosis, comorbidities, medication use prior to infection, and rheumatic disease activity.
Results.
A total of 1,324 patients were included, of whom 36% were hospitalized and 6% died; 26% of hospitalized patients required mechanical ventilation. In multivariable models, African American patients (OR 2.74 [95% CI 1.90–3.95]), Latinx patients (OR 1.71 [95% CI 1.18–2.49]), and Asian patients (OR 2.69 [95% CI 1.16–6.24]) had higher odds of hospitalization compared to White patients. Latinx patients also had 3-fold increased odds of requiring ventilatory support (OR 3.25 [95% CI 1.75–6.05]). No differences in mortality based on race/ethnicity were found, though power to detect associations may have been limited.
Conclusion.
Similar to findings in the general US population, racial/ethnic minorities with rheumatic disease and COVID-19 had increased odds of hospitalization and ventilatory support. These results illustrate significant health disparities related to COVID-19 in people with rheumatic diseases. The rheumatology community should proactively address the needs of patients currently experiencing inequitable health outcomes during the pandemic.
INTRODUCTION
People with rheumatic disease, particularly those receiving immunosuppressive medications, have a higher risk of developing severe infections. Therefore, there have been several reports examining the prevalence of coronavirus disease 2019 (COVID-19) infection in patients with rheumatic diseases (1). While most literature suggests a similar prevalence of COVID-19 in the rheumatic disease population as in the general population, one study indicated a slightly higher risk of mortality in individuals with rheumatic diseases (2), and some immunosuppressive medications may place patients at higher risk of hospitalization (3). Further, similar to studies conducted in the general population, rheumatic disease patients with comorbidities also have higher odds of poor outcomes (3).
Growing research has illustrated the disproportionate burden of COVID-19 in racial/ethnic minority populations. COVID-19–related deaths are significantly higher in communities with higher proportions of African American, Latinx, Asian American, or other racial/ethnic minorities (4–8). Additionally, in multivariable analyses at the patient level, a number of studies have shown increased risk of hospitalization in racial/ethnic minorities (9–11). A recent study of patients with systemic lupus erythematosus (SLE) in New York City showed that non-White and Hispanic patients diagnosed as having COVID-19 were more likely to be hospitalized (12). However, no studies have yet examined disparities in COVID-19 health outcomes among people with different rheumatic diseases. Given that racial/ethnic minority patients with rheumatic conditions tend to experience a higher burden of disease activity and severity compared to White patients (13–16), disproportionate adverse outcomes of COVID-19 could have a substantial long-term impact on patients’ health and quality of life.
The aim of this study was to examine the association between race/ethnicity and COVID-19 hospitalization, ventilation status, and mortality in people with rheumatic diseases in the US using data from a large COVID-19 rheumatology registry.
PATIENTS AND METHODS
Study population and design.
We performed a cross-sectional analysis of the COVID-19 Global Rheumatology Alliance (C19-GRA) registry to investigate the association of race/ethnicity with outcomes. Details of the registry design have been described previously (17–20) (see Appendix A for a list of the members of the COVID-19 Global Rheumatology Alliance). Briefly, C19-GRA data regarding individuals with rheumatic diseases diagnosed as having COVID-19 are captured from rheumatology physicians via a data entry portal. Provider-level information includes physician city, state, and country. This analysis was limited to cases entered by physicians for their patients in the US from March 24, 2020 to August 26, 2020. Data from a total of 1,380 patients were collected during this time period.
COVID-19 diagnosis.
Physicians indicated whether the diagnosis of COVID-19 was based on polymerase chain reaction, antibody testing, metagenomic testing, computed tomography scan, laboratory assay, or a presumptive diagnosis based only on characteristic symptoms.
Racial/ethnic categorization.
Race/ethnicity was reported by the physician entering the case, and multiple categories could be selected among the following: Arab, African American, East Asian, South Asian, West Asian/Middle Eastern, Pacific Islander, Latin American, White, Native American/Aboriginal/First Nations, other, unknown, or prefer not to answer. Physicians recorded race/ethnicity with the data available to them, which typically includes data available in the electronic health record (EHR), patient-reported race/ethnicity, or data derived from inference. In this study, race/ethnicity was categorized as either White (reference group), African American, Latinx (Latin American), Asian (East, South, or Southeast), or other/mixed race. Patients classified as White and Latinx (n = 5) or African American and Latinx (n = 2) were categorized as Latinx. For all other race/ethnicity combinations, patients were categorized as “other/mixed race” (n = 6).
Outcome measures.
This study examined 3 separate, non–mutually exclusive outcome measures: hospitalization status, ventilatory support requirement, and death. The variable for ventilatory support was limited to patients who were hospitalized and categorized as receiving one of the following: no supplementary oxygen, supplementary oxygen or noninvasive ventilation, or mechanical ventilation/extracorporeal membrane oxygenation (ECMO).
Covariates.
Case information, including age, sex, smoking status, rheumatic disease diagnosis, rheumatic disease activity (physician global assessment), and comorbidities, was collected by physician report. Medications used prior to COVID-19 were categorized as conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) including antimalarials (hydroxychloroquine, chloroquine), azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine, and tacrolimus; biologic DMARDs including abatacept, belimumab, CD20 inhibitors, interleukin-1 (IL-1) inhibitors, IL-6 inhibitors, IL-12/IL-23 inhibitors, IL-17 inhibitors, and tumor necrosis factor inhibitors; and targeted synthetic DMARDs, namely JAK inhibitors.
Statistical analysis.
Categorical variables were reported as the number and percentage. In univariate analyses, differences in demographic and rheumatic disease–specific features according to race/ethnicity were compared using chi-square test.
Multivariable logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) of hospitalization and mortality; ordinal logistic regression was used to estimate ORs and 95% CIs of ventilatory support among hospitalized patients (no supplementary oxygen [reference category], supplementary oxygen or noninvasive ventilation, and mechanical ventilation/ECMO). Two models were explored: one model included adjustments for sex and age (model 1), and a second model included adjustments for sex, age, rheumatic diseases (rheumatoid arthritis [RA], SLE, psoriatic arthritis [PsA], axial spondyloarthritis [SpA] or other spondyloarthritis, vasculitis, and other), most common comorbidities (hypertension, lung disease, diabetes, cardiovascular disease, and chronic renal insufficiency/end-stage renal disease), smoking status (ever versus never), rheumatic disease activity (dichotomized as remission or low disease activity versus moderate or high disease activity), rheumatic disease medication use prior to infection (csDMARD monotherapy, biologic and targeted small molecule DMARD monotherapy [biologic/targeted sDMARD], and csDMARD + biologic/targeted sDMARD combination therapy), and prednisone-equivalent glucocorticoid use (0 mg/day, 1–9 mg/day, or ≥10 mg/day) (model 2).
For univariate and multivariable models, patients with >1 of the following rheumatic diseases recorded were classified using the indicated hierarchy: SLE > RA > PsA > vasculitis > axal SpA/other spondyloarthritis > other. Cardiovascular disease and hypertension were collapsed as a single comorbidity in the regression model due to significant collinearity between the 2 variables. A complete case analysis was conducted for each outcome measure separately (i.e., analysis limited to observations with all covariates and specific outcome measure present). Exposure categorization (race/ethnicity) was missing for 56 patients (4%). Outcome status was unknown for 89 hospitalization cases (7%), 105 ventilation status cases (24% among hospitalized cases), and 1 death case (<1%). Some data were missing for the following covariates: 5% for disease activity, 5% for smoking status, and 2% for glucocorticoid use. Differences in demographic and disease characteristics by missing outcome status were examined using chi-square tests.
To assess the robustness of the results, several sensitivity analyses were performed. First, we repeated the above analyses after excluding patients deemed a “presumptive case,” meaning that the physician reported that the COVID-19 diagnosis was based on characteristic symptoms only, and the patient was not reported as having 1) a confirmatory COVID-19 test, 2) documentation of chest imaging showing bilateral infiltrates consistent with COVID-19–associated pneumonia, or 3) close contact with a known COVID-19–positive patient (n = 135). Second, we limited the analyses to patients whose COVID-19 outcome was resolved (n = 1,062). Resolution was defined as a case marked by the provider as either deceased, resolved at the time of data entry, not hospitalized >30 days after initial diagnosis date, hospitalized and discharged, or not at risk of further interventions/death. Due to a relatively low number of deaths in each race/ethnicity subgroup and potential for overfitting of the mortality model, we also explored a reduced model in which only covariates with significant values (P < 0.05) remained. Finally, a model adjusted for region defined by the US Census Bureau (Northeast, Midwest, South, and West) was used to account for regional variation. The parallel regression assumption was examined for ordinal logistic regression models and successfully met (P > 0.05). P values less than 0.05 (2-sided) were considered significant. All analyses were conducted using Stata version 16.0.
Data quality was assessed by a data quality team who also confirmed there were no duplicate entries. The C19-GRA physician registry was determined “not human subjects research” under US Federal Guidelines assessed by the University of California, San Francisco, and patient consent was not required.
RESULTS
As of August 26, 2020, a total of 1,380 US COVID-19 cases were entered in the registry, of which 1,324 cases had race/ethnicity information documented. Case numbers by state are shown in Supplementary Figure 1 (available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41567/abstract). Overall, 36% of the patients were hospitalized (445 of 1,235), and 6% died (85 of 1,323). Of the patients who were hospitalized, 26% required mechanical ventilation/ECMO (90 of 340). Demographic and disease characteristics by race/ethnicity are shown in Table 1. Significant differences by race/ethnicity were found with respect to sex, age, rheumatic disease, hypertension/cardiovascular disease, lung disease, diabetes, chronic renal insufficiency/end-stage renal disease, ever smoking, DMARD medication category, glucocorticoid use, and rheumatic disease activity (P < 0.05).
Table 1.
Demographic and disease characteristics of US patients entered into the COVID-19 Global Rheumatology Alliance Registry, by race/ethnicity (n = 1,324)*
| White (n = 690) | American (n = 273) | Latinx (n = 295) | Asian (n = 39) | Other/mixed race (n = 27) | |
|---|---|---|---|---|---|
| Sex, female† | 497 (72) | 227 (83) | 232 (79) | 29 (74) | 20 (74) |
| Age, years† | |||||
| 18–29 | 35 (5) | 11 (4) | 19 (6) | 2 (5) | 6 (22) |
| 30–49 | 155 (22) | 77 (28) | 126 (43) | 16 (41) | 5 (19) |
| 50–65 | 288 (42) | 114 (42) | 97 (33) | 15 (38) | 10 (37) |
| >65 | 212 (31) | 71 (26) | 53 (18) | 6 (15) | 6 (22) |
| Rheumatic disease†‡ | |||||
| Rheumatoid arthritis | 272 (39) | 96 (35) | 112 (38) | 11 (28) | 13 (48) |
| Systemic lupus erythematosus | 56 (8) | 89 (33) | 63 (21) | 7 (18) | 3 (11) |
| Psoriatic arthritis | 94 (14) | 5 (2) | 18 (6) | 7 (18) | 0 (0) |
| Axial spondyloarthritis | 42 (6) | 3 (1) | 9 (3) | 1 (3) | 4 (15) |
| Vasculitis | 61 (9) | 6 (2) | 26 (9) | 4 (10) | 2 (7) |
| Other | 165 (24) | 74 (27) | 67 (23) | 9 (23) | 5 (19) |
| Hypertension/cardiovascular† | 264 (38) | 169 (62) | 15 (39) | 11 (28) | 7 (26) |
| Lung disease† | 146 (21) | 78 (29) | 60 (20) | 5 (13) | 3 (11) |
| Diabetes† | 88 (13) | 57 (21) | 74 (25) | 5 (13) | 3 (11) |
| Renal† | 41 (6) | 39 (14) | 28 (9) | 5 (13) | 1 (4) |
| Ever smoker (n = 1,256)† | 218 (33) | 68 (26) | 57 (21) | 4 (11) | 6 (23) |
| Medication pre-COVID-19† | |||||
| DMARDs§ | |||||
| No DMARDs | 142 (21) | 52 (19) | 61 (21) | 8 (21) | 5 (19) |
| csDMARDs only | 234 (34) | 135 (49) | 115 (39) | 14 (36) | 8 (30) |
| Biologic/targeted synthetic DMARDs only | 173 (25) | 32 (12) | 51 (17) | 7 (18) | 6 (22) |
| csDMARDs + biologic/targeted synthetic DMARDs | 141 (20) | 54 (20) | 68 (23) | 10 (26) | 8 (30) |
| Prednisone-equivalent glucocorticoids (n = 1,301)† | |||||
| None | 523 (77) | 180 (67) | 173 (60) | 30 (79) | 16 (59) |
| 1–9 mg/day | 98 (14) | 64 (24) | 74 (26) | 3 (8) | 6 (22) |
| 10 mg/day | 59 (9) | 25 (9) | 40 (14) | 5 (13) | 5 (19) |
| Rheumatic disease activity (n = 1,254)† | |||||
| Remission or low | 535 (81) | 197 (78) | 198 (71) | 25 (68) | 16 (70) |
| Moderate or high | 125 (19) | 57 (22) | 82 (29) | 12 (32) | 7 (30) |
Values are the number (%). COVID-19 = coronavirus disease 2019.
P < 0.05 across racial groups, by chi-square test.
Cases could have more than one disease diagnosis. The “other” rheumatic disease category included Sjögren’s syndrome, other inflammatory arthritis, inflammatory myopathy, gout, systemic sclerosis, polymyalgia rheumatica, sarcoidosis, undifferentiated connective tissue disease, ocular inflammation, autoinflammatory syndrome, mixed connective tissue disease, antiphospholipid syndrome, calcium pyrophosphate deposition disease, systemic juvenile idiopathic arthritis, juvenile idiopathic arthritis (not systemic), and IgG4-related disease.
Disease-modifying antirheumatic drugs (DMARDs) included conventional synthetic DMARDs (csDMARDs), biologic DMARDs, and targeted synthetic DMARDs.
Differences in COVID-19 outcomes by race/ethnicity are shown in Figure 1 and Supplementary Table 1 (http://onlinelibrary.wiley.com/doi/10.1002/art.41567/abstract). White patients were less likely to be hospitalized (29%) compared to African Americans (51%), Latinx (37%), Asians (43%), and other/mixed races (35%) (P < 0.01). However, no significant differences in ventilation status among patients who were hospitalized or those who died were found by race/ethnicity.
Figure 1.
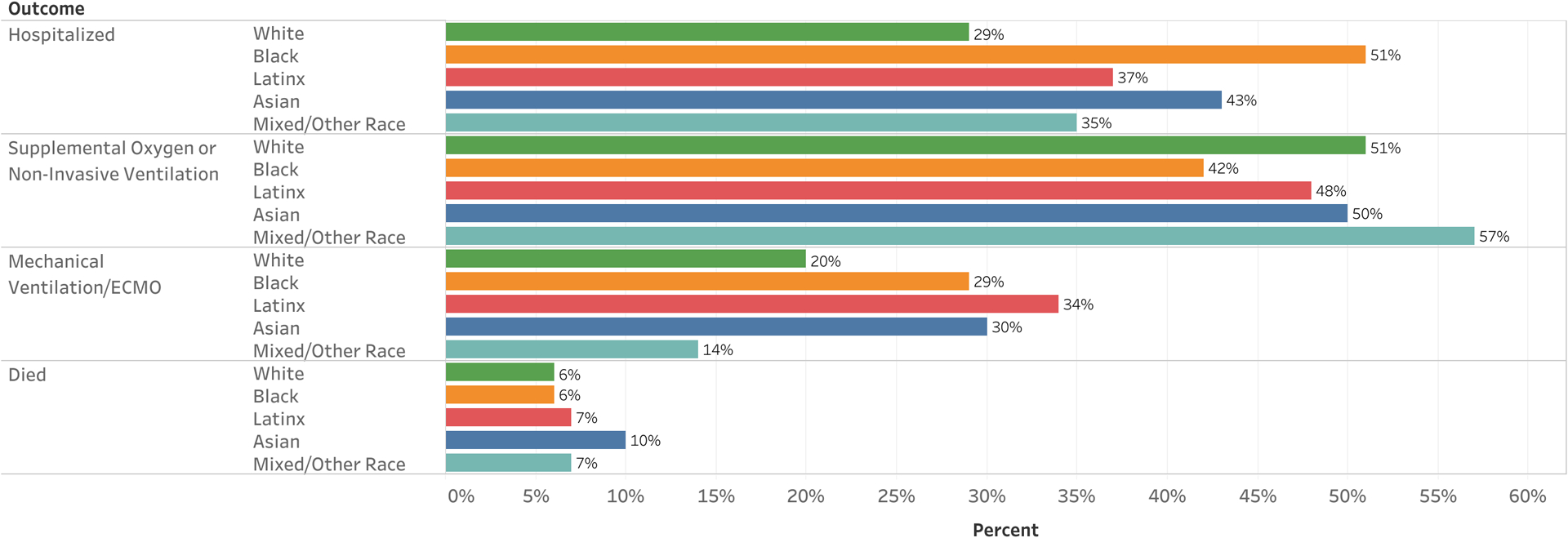
Coronavirus disease 2019 (COVID-19) outcomes in patients with rheumatic disease, by race/ethnicity. Numbers displayed represent the percentage of individuals with COVID-19 in each category who experienced the outcome listed. Outcome categories are not mutually exclusive. Ventilatory support was determined among hospitalized patients only. ECMO = extracorporeal membrane oxygenation.
In model 1, adjusted for sex and age, racial/ethnic minorities were more likely to experience poor outcomes, including hospitalization and requirement for ventilatory support, compared to White patients (Table 2). After adjustment for additional covariates (model 2), all associations remained. African American and Asian patients had nearly 3-fold higher odds of being hospitalized compared to White patients (OR 2.74 [95% CI 1.90–3.95] and OR 2.69 [95% CI 1.16–6.24], respectively). Latinx patients also had elevated odds of hospitalization compared to White patients (OR 1.71 [95% CI 1.18–2.49]). Among hospitalized patients, Latinx patients had 3-fold odds of requiring increased ventilatory support compared to White patients (OR 3.25 [95% CI 1.75–6.05], P < 0.01). African American, Asian, and other/mixed race patients also had elevated odds of requiring increased ventilatory support compared to White patients, but results were not statistically significant. Latinx patients demonstrated higher odds of mortality compared to White patients in the model adjusted for sex and age (model 1); however, after additional adjustment for factors such as rheumatic disease, medication use prior to COVID-19 diagnosis, comorbidities, and others (model 2), the association did not remain statistically significant. No other associations between race/ethnicity and mortality were found.
Table 2.
Multivariable models examining the association between race/ethnicity and COVID-19 outcomes in patients with rheumatic disease*
| Hospitalization | Ventilatory support† | Death | ||||
|---|---|---|---|---|---|---|
| Race/ethnicity | Model 1 (n = 1,235) | Model 2 (n = 1,103) | Model 1 (n = 340) | Model 2 (n = 303) | Model 1 (n = 1,323) | Model 2 (n = 1,172) |
| White | Referent | Referent | Referent | Referent | Referent | Referent |
| African American | 3.18 (2.31–4.36) | 2.74 (1.90–3.95) | 1.65 (0.99–2.73) | 1.54 (0.89–2.68) | 1.36 (0.74–2.50) | 1.39 (0.69–2.79) |
| P | <0.01 | <0.01 | 0.05 | 0.13 | 0.33 | 0.35 |
| Latinx | 2.00 (1.46–2.75) | 1.71 (1.18–2.49) | 2.79 (1.61–4.83) | 3.25 (1.75–6.05) | 1.93 (1.07–3.49) | 1.67 (0.81–3.41) |
| P | <0.01 | <0.01 | <0.01 | <0.01 | 0.03 | 0.16 |
| Asian | 2.50 (1.21–5.15) | 2.69 (1.16–6.24) | 2.05 (0.61–6.85) | 1.73 (0.45–6.63) | 3.01 (0.93–9.77) | 2.67 (0.58–12.16) |
| P | 0.01 | 0.02 | 0.24 | 0.43 | 0.07 | 0.21 |
| Other/mixed race | 1.44 (0.55–3.81) | 2.59 (0.97–6.90) | 1.53 (0.36–6.52) | 1.43 (0.33–6.15) | 1.91 (0.41–8.96) | 2.49 (0.49–12.65) |
| P | 0.46 | 0.06 | 0.57 | 0.63 | 0.41 | 0.27 |
Values are the odds ratio (95% confidence interval). Model 1 was adjusted for sex and age. Model 2 was adjusted for sex, age, rheumatic disease, comorbidities, ever smoking, medication use prior to coronavirus disease 2019 (COVID-19), glucocorticoid use, and disease activity; sample size varies due to missing data on outcomes and certain covariates.
Determined among hospitalized patients only.
When comparing demographic and disease characteristics between patients with and those without missing outcome data, we did not find large differences between groups. The only significant finding was between race/ethnicity and missing ventilation status among patients who were hospitalized. Patients missing ventilation status were more likely to be White compared to patients without missing data (P < 0.05) (Supplementary Table 2, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41567/abstract).
Findings remained consistent in an analysis excluding unresolved cases (n = 238) (Supplementary Table 3, http://onlinelibrary.wiley.com/doi/10.1002/art.41567/abstract), as well as an analysis excluding presumptive cases (n = 135) (data not shown). Due to potential for overfitting of the mortality model, we explored a reduced model in which only covariates with significant values (P < 0.05) remained. The reduced model also showed no significant differences in mortality by race/ethnicity (Supplementary Table 4, http://onlinelibrary.wiley.com/doi/10.1002/art.41567/abstract). Lastly, models accounting for regional variation across all 3 outcome measures also produced similar results (data not shown).
DISCUSSION
To our knowledge, this is the first study to examine racial/ethnic differences in US COVID-19 outcomes among people with rheumatic diseases. Similar to findings in the general population, we found that racial/ethnic minority patients with rheumatic disease had increased odds of hospitalization and ventilatory support, even after adjustment for demographic characteristics, disease-specific features, and comorbidities. Our data also mirror recent results from a study examining SLE patients diagnosed as having COVID-19, which found higher odds of hospitalization among non-White patients (12). These data add to a growing body of literature describing racial/ethnic health disparities related to COVID-19 and suggest that extra attention should be focused on addressing disparities in patients with rheumatic disease during public health emergencies.
Studies have shown that racial/ethnic minorities have worse COVID-19 outcomes compared to White patients, further exacerbating health disparities that were present prior to the pandemic. For example, African American race is associated with a 2-fold higher odds of hospitalization compared to White race (9–11). Other research has demonstrated higher COVID-19 hospitalization rates among Latinx (21), Asians, and other races (10), and higher mortality among Asian patients diagnosed as having COVID-19 compared to White patients (22). We similarly observed higher odds of hospitalization among African American, Latinx, and Asian patients compared to White patients, and increased odds of ventilatory support among Latinx patients. However, we did not identify an association with other/mixed race and severe COVID-19 outcomes, possibly due to the heterogeneity of this group or limited sample size. We also did not find an association between any race/ethnicity subgroup and mortality due to COVID-19, consistent with prior studies (9,23); however, observation of a relatively small number of deaths may have contributed to a failure to detect significant differences.
Our study has certain limitations. The C19-GRA registry is voluntary, making it susceptible to selection bias (e.g., geographic location, disease severity) and does not capture all cases of COVID-19 in patients with rheumatic disease in a defined population. The approach to data collection places limitations on causal conclusions and temporal relationships, and therefore we can make only limited inferences based on our results. Missing data were present for some covariates and outcomes, including 24% for ventilation status. We found a significant difference with respect to race/ethnicity and hospitalized patients with and those without missing ventilation status; however, no differences based on any other covariates were present, nor were there differences based on any other outcomes. Results were also consistent when presumptive cases were excluded, as well as when unresolved cases were excluded. Race/ethnicity was categorized by the entering physician, which may not have been consistent with the individual’s self-reported identity; however, chart review of a subsample of patients from 2 sites (n = 273; ~21% of the total analytic sample) indicated 81% concordance between EHR- and registry-entered race/ethnicity. Misclassification largely occurred with Hispanic/Latino ethnicity being characterized as “other” in the patient’s EHR. Therefore, the current registry data collection may potentially be more accurate than standard, EHR-based assessment of race/ethnicity. Additionally, we did not collect information on insurance status or other markers of socioeconomic status, and therefore were unable to adjust for these factors. Last, there may be heterogeneous differences in outcomes within race/ethnicity categories; for example, the Asian subgroup included East, South, and Southeast Asian patients, and the other/mixed race group included various combinations of race and ethnicity.
Our results highlight the need for focused attention on non-White racial/ethnic rheumatic disease patient groups for which COVID-19 can further exacerbate existing health disparities. Racial/ethnic minority patients with rheumatic conditions tend to experience a higher burden of disease activity and severity compared to White patients, including greater disease activity, poorer functional status, and worse quality of life (13–16). Therefore, disproportionate outcomes of COVID-19 may contribute to substantial impacts on patients’ health. More research is needed to understand and address drivers of these disparities, particularly those that relate to socioeconomic status and factors of health care access.
Findings from this study show a higher burden of poor outcomes in racial/ethnic minorities compared to White patients, indicating that proactive measures to actively treat and focus attention on the care of these patients is urgently needed. Strategies, such as targeted counseling to reduce the risk of COVID-19 transmission, ensuring patients with rheumatic diseases are able to access severe acute respiratory syndrome coronavirus 2 testing, educating patients to access care early in the course of COVID-19 illness, managing immunosuppressive drugs, and controlling disease activity, should be employed (24,25). Additionally, work to mitigate factors that contribute to COVID-19 inequities at the public health, health care system, and policymaking level is critical, given structural racism in the US (8). The long-term impact of COVID-19 and other indirect consequences of the pandemic and shelter-in-place policies on patients with rheumatic diseases is unknown. Longitudinal studies examining the effect of COVID-19 in the rheumatic disease population, especially by race/ethnicity, will be essential moving forward. Immediate action to address these disparities is warranted, and physicians caring for people with rheumatic diseases should employ measures to reduce risk among the most vulnerable populations.
Supplementary Material
ACKNOWLEDGMENT
We would like to thank Dr. Charles McCulloch, University of California, San Francisco, for his expertise and feedback on the manuscript.
Dr. Gianfrancesco’s work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (grants K01-AR-070585 and K24-AR-074534). Dr. Sparks’ work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (grants K23-AR-069688, R03-AR-075886, L30-AR-066953, P30-AR-070253, and P30-AR-072577), the Rheumatology Research Foundation K Supplement and R Bridge Career Development Bridge Funding awards, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. D’Silva’s work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (grant T32-AR-007258). Dr. Serling-Boyd’s work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (grant T32-AR-007258). Dr. Hausmann’s work was supported by grants from the Rheumatology Research Foundation and the Childhood Arthritis and Rheumatology Research Alliance. Dr. Machado’s work was supported by the NIHR University College London Hospitals Biomedical Research Centre. Dr. Yazdany’s work was supported by the NIH (grant K24-AR-074534), the CDC, and the Agency for Healthcare Research and Quality.
Dr. Sparks has received consulting fees from Bristol Myers Squibb, Gilead, Inova, Janssen, and Optum (less than $10,000 each) and research support from Amgen and Bristol Myers Squibb. Dr. Bhana has received consulting fees, speaking fees, and/or honoraria from Novartis (less than $10,000). Dr. Grainger has received consulting fees, speaking fees, and/or honoraria from AbbVie, Janssen, Novartis, Pfizer, and Cornerstones (less than $10,000 each). Dr. Hausmann has received consulting fees from Novartis (less than $10,000). Dr. Liew has received research support from Pfizer. Ms Sirotich is a Board Member of the Canadian Arthritis Patient Alliance, a patient run, volunteer-based organization whose activities are largely supported by independent grants from pharmaceutical companies. Dr. Wallace has received consulting fees from Viela Bio (less than $10,000) and research grants from Bristol Myers Squibb. Dr. Machado has received consulting fees, speaking fees, and/or honoraria from AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, MSD, Novartis, Pfizer, Roche, and UCB (less than $10,000 each). Dr. Robinson has received consulting fees, speaking fees, and/or honoraria from AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, UCB, and Roche (less than $10,000 each). Dr. Yazdany has received consulting fees from Eli Lilly and AstraZeneca (less than $10,000 each). No other disclosures relevant to this article were reported.
APPENDIX A: MEMBERS OF THE COVID-19 GLOBAL RHEUMATOLOGY ALLIANCE
Members of the COVID-19 Global Rheumatology Alliance, in addition to the authors, are as follows: Khurram Abbass (Private Practice, San Jose, CA), Christopher Adams (East Alabama Medical Center, Opelika, AL), Kathleen Anthony (Rheumatology and Osteoporosis Specialists, Shreveport, LA), Byung Ban (Georgetown University, Washington, DC), Alison Bays (University of Washington, Seattle), Cassandra Calabrese (Cleveland Clinic, Cleveland, OH), Eduardo Cepeda (Austin Diagnostic Clinic, Austin, TX), Kathryn Dao (UT Southwestern Medical Center, Dallas, TX), Nicole Daver (Institute of Rheumatic and Autoimmune Diseases, Summit, NJ), Theodore Fields (Hospital for Special Surgery, New York City, NY), Michael Guma (Riverside Medical Group, North Arlington, NJ), Ammar Haikal (Riverside Medical Group, North Arlington, NJ), Denise Hare (Capital Health Rheumatology Specialists, Pennington, NJ), Melissa Harvey (Institute of Rheumatic and Autoimmune Diseases, Summit, NJ), Suneya Hogarty (Integrative Arthritis and Pain Consultants, Goldsboro, NC), Shraddha Jatwani (Albert Einstein Medical Center, Philadelphia, PA), Arundathi Jayatilleke (Temple University Hospital, Philadelphia, PA), Gilbert Kepecs (Private Practice, Hackensack, NJ), Arezou Khosroshahi (Emory University, Atlanta, GA), Adam Kilian (George Washington University, Washington, DC), Neil Kramer (Institute of Rheumatic and Autoimmune Diseases, Summit, NJ), Concetta Lamore (Institute of Rheumatic and Autoimmune Diseases, Summit, NJ), Lilliam Miranda (Rheumatology Center Inc., Pembroke Pines, FL), Sushama Mody (Riverside Medical Group, North Arlington, NJ), Daric Mueller (Shores Rheumatology PC, Saint Clair Shores, MI), Deborah Parks (Washington University Division of Rheumatology, Saint Louis, MO), Elliot Rosenstein (Atlantic Health System, Summit, NJ), Eric Ruderman (Northwestern Memorial, Chicago, IL), Faizah Siddique (Loyola University Medical Center, Chicago, IL), Caroline Siegel (Hospital for Special Surgery, New York City, NY), Tamara Tanner (Montefiore Medical Center, Bronx, NY), Tamika Webb-Detiege (Ochsner Department of Rheumatology, New Orleans, LA), Leanna Wise (Los Angeles County and USC Medical Center, Los Angeles, CA), Karen Yeter (Kaiser Permanente, Long Beach, CA), Kristen Young (Parkland Hospital, Dallas, TX), JoAnn Zell (University of Colorado, Denver).
Footnotes
The views expressed here are those of the authors and participating members of the COVID-19 Global Rheumatology Alliance, and do not necessarily represent the views of the American College of Rheumatology, the NIH, the NHS, the NIHR, or the UK Department of Health.
REFERENCES
- 1.Gianfrancesco M, Yazdany J, Robinson PC. Epidemiology and outcomes of novel coronavirus 2019 in patients with immune-mediated inflammatory diseases. Curr Opin Rheumatol 2020;32:434–40. [DOI] [PubMed] [Google Scholar]
- 2.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadhera RK, Wadhera P, Gaba P, Figueroa JF, Maddox KE, Yeh RW, et al. Variation in COVID-19 hospitalizations and deaths across New York City boroughs [letter]. JAMA 2020;323:2192–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millett GA, Jones AT, Benkeser D, Baral S, Mercer L, Beyrer C, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol 2020;47:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahajan UV, Larkins-Pettigrew M. Racial demographics and COVID-19 confirmed cases and deaths: a correlational analysis of 2886 US counties. J Public Health (Oxf) 2020;42:445–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SJ, Bostwick W. Social vulnerability and racial inequality in COVID-19 deaths in Chicago. Health Educ Behav 2020;47:509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross CP, Essien UR, Pasha S, Gross JR, Wang SY, Nunez-Smith M. Racial and ethnic disparities in population-level COVID-19 mortality. J Gen Intern Med 2020;35:3097–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with COVID-19. N Engl J Med 2020;382:2534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lassale C, Gaye B, Hamer M, Gale CR, Batty GD. Ethnic disparities in hospitalisation for COVID-19 in England: the role of socioeconomic factors, mental health, and inflammatory and pro-inflammatory factors in a community-based cohort study. Brain Behav Immun 2020;88:44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azar KM, Shen Z, Romanelli RJ, Lockhart SH, Smits K, Robinson S, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood) 2020;39: 1253–62. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Ruiz R, Masson M, Kim MY, Myers B, Haberman RH, Castillo R, et al. Leveraging the United States epicenter to provide insights on COVID-19 in patients with systemic lupus erythematosus. Arthritis Rheumatol 2020;72:1971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg JD, Spruill TM, Shan Y, Reed G, Kremer JM, Potter J, et al. Racial and ethnic disparities in disease activity in patients with rheumatoid arthritis. Am J Med 2013;126:1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dall’Era M, Cisternas MG, Snipes K, Herrinton LJ, Gordon C, Helmick CG. The incidence and prevalence of systemic lupus erythematosus in San Francisco county, California: the California Lupus Surveillance Project. Arthritis Rheumatol 2017;69:1996–2005. [DOI] [PubMed] [Google Scholar]
- 15.Bruce B, Fries JF, Murtagh KN. Health status disparities in ethnic minority patients with rheumatoid arthritis: a cross-sectional study. J Rheumatol 2007;34:1475–9. [PubMed] [Google Scholar]
- 16.Barton JL, Trupin L, Schillinger D, Gansky SA, Tonner C, Margaretten M, et al. Racial and ethnic disparities in disease activity and function among persons with rheumatoid arthritis from university-affiliated clinics. Arthritis Care Res (Hoboken) 2011;63:1238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace ZS, Bhana S, Hausmann JS, Robinson PC, Sufka P, Sirotich E, et al. The Rheumatology Community responds to the COVID-19 pandemic: the establishment of the COVID-19 Global Rheumatology Alliance [editorial]. Rheumatology (Oxford) 2020;59:1204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson PC, Yazdany J. The COVID-19 Global Rheumatology Alliance: collecting data in a pandemic [review]. Nat Rev Rheumatol 2020;16:293–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianfrancesco MA, Hyrich KL, Gossec L, Strangfeld A, Carmona L, Mateus EF, et al. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol 2020;2:e250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liew JW, Bhana S, Costello W, Hausmann JS, Machado PM, Robinson PC, et al. The COVID-19 Global Rheumatology Alliance: evaluating the rapid design and implementation of an international registry against best practice. Rheumatology (Oxford) 2021;60: 353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu HE, Ashe EM, Silverstein M, Hofman M, Lange SJ, Razzaghi H, et al. Race/ethnicity, underlying medical conditions, homelessness, and hospitalization status of adult patients with COVID-19 at an urban safety-net medical center: Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep 2020;69:864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan B, Ng F, Nguyen T. High Mortality from COVID-19 among Asian Americans in San Francisco and California. URL: https://asianarch.org/press_releases/Asian%20COVID-19%20Mortality%20Final.pdf.Asian American Research Center on Health. 2020. [Google Scholar]
- 23.Tartof SY, Qian L, Hong V, Wei R, Nadjafi RF, Fischer H, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Int Med 2020. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikuls TR, Johnson SR, Fraenkel L, Arasaratnam RJ, Baden LR, Bermas BL, et al. American College of Rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 1. Arthritis Rheumatol 2020;72:1241–51. [DOI] [PubMed] [Google Scholar]
- 25.Mikuls TR, Johnson SR, Fraenkel L, Arasaratnam RJ, Baden LR, Bermas BL, et al. American College of Rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 2. Arthritis Rheumatol 2020;72:e1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


