Abstract
Background: As life expectancy increases, cognitive performance decline in the elderly has become one of the major global challenges. We aimed to evaluate the association of dietary vitamin D (VD), serum 25-hydroxyvitamin D3 (25(OH)D3), 25-hydroxyvitamin D2 (25(OH)D2), and total 25-hydroxyvitamin (25(OH)D) concentration with cognitive performance in older Americans. Methods: The data from the National Health and Nutrition Examination Survey (NHANES), 2011–2014 was used. The cognitive performance was assessed by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word Learning sub-test, Animal Fluency test, and Digit Symbol Substitution Test (DSST). A binary logistic regression model was applied to evaluate the association between VD and cognitive performance, and restricted cubic spline model was adopted to evaluate the dose–response relationship. Results: While comparing to the lowest dietary VD intake group, the multivariate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of the highest dietary VD intake group were 0.51 (0.36–0.72) for the Animal Fluency test score and 0.45 (0.31–0.66) for DSST score, respectively; and those of serum total 25(OH)D and 25(OH)D3 concentration were 0.68 (0.47–0.97) and 0.62 (0.44–0.86) for DSST score. L-shaped relationships were identified for dietary VD intake, serum total 25(OH)D and 25(OH)D3 concentration with cognition performance. The associations between dietary VD intake, serum total 25(OH)D and cognitive performance were non-significant when stratified by gender. Conclusions: The study indicates that dietary VD intake, serum total 25(OH)D and 25(OH)D3 concentration were positively associated with cognitive performance. Further studies are needed to clarify the possible effects of dietary VD intake and serum 25(OH)D2, 25(OH)D3 on cognitive performance.
Keywords: cognitive performance, dietary vitamin D, 25-hydroxyvitamin D3, 25-hydroxyvitamin D2, does–response
1. Introduction
As life expectancy increases, cognitive performance decline in the elderly has become one of the major global challenges [1]. According to projections, the number of people worldwide living with dementia will rise to an estimated 152 million by 2050 [2]. Considering the social and economic burden of cognitive decline especially dementia, it is particularly important to control, delay, and prevent cognitive decline. Genetic factors, the history of illness, and life stress may increase the risk of cognitive performance decline. Additionally, healthy dietary habits, such as proper intake of vitamin C, vitamin E, and polyunsaturated fatty acids, may have a protective effect on cognitive performance [3,4,5].
Vitamin D (VD), as one of the common fat-soluble vitamins, plays an important role not only in bone growth and development, but also in cell differentiation and the immune system [6]. VD is neuroprotective, anti-inflammatory, and antioxidant, so it is considered to be one of the protective factors for cognitive performance [7]. To assess the vitamin D status, the dietary VD intake and serum 25-hydroxyvitamin D (25(OH)D) concentration were commonly used [8]. Dietary intake is a source of VD [9], but the association between dietary VD intake and cognition remains controversial [8,10,11,12]. A cross-sectional study in Dutch and a follow-up study in men showed that dietary VD intake was not associated with cognitive performance [8,11]. Other studies suggested an association between dietary VD intake and cognitive performance [10,12]. Moreover, to our knowledge, the dose–response relationship between intake of dietary VD and cognitive performance has not yet been explored.
In addition, the effect of serum 25(OH)D on cognitive performance is still controversial [8,13,14,15,16,17,18]. A recent cohort in Boston-area Puerto Ricans showed that the association of serum 25(OH)D concentration with individual cognitive test scores was not statistically significant [19]. However, another recent study in women suggested that higher 25(OH)D concentrations might have negative cognitive effects [20]. Additionally, currently, no study investigated the dose–response relationships between them. Due to different chemical construction and physiological activities of 25-hydroxyvitamin D3 (25(OH)D3), D2 (25(OH)D2) in serum [21,22,23], the effect of serum 25(OH)D2, 25(OH)D3 on cognition might differ. However, these associations have not been extensively explored up until now.
Therefore, we aimed to investigate the association and dose–response relationships of dietary VD intake, serum total 25(OH)D, serum 25(OH)D2, and serum 25(OH)D3 with cognitive performance in older Americans based on the data from the National Health and Nutrition Examination Survey (NHANES).
2. Materials and Methods
2.1. Study Population
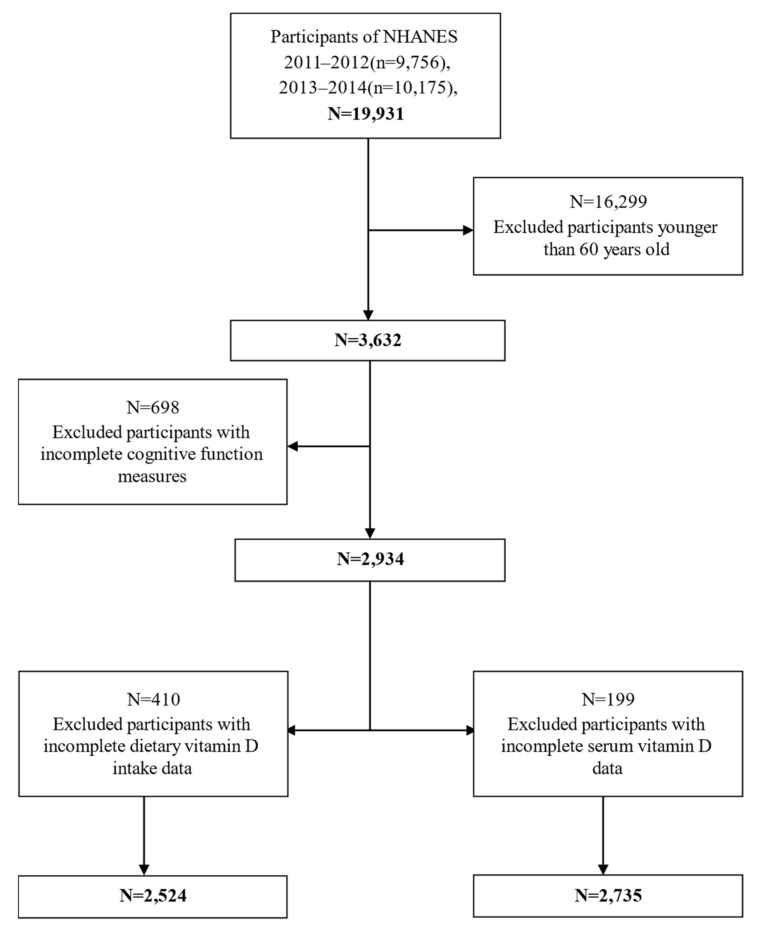
The data on dietary VD intake, serum 25(OH)D concentration, and cognitive performance test scores were obtained from the two cycles of NHANES: 2011–2012 and 2013–2014. Since only one-third of NHANES samples were serologically tested each year, we will waste a deal of valuable information when excluding the participants with both dietary VD intake and serum 25(OH)D simultaneously. Thus, we chose two different samples to study the association between dietary VD intake, serum 25(OH)D and cognition, respectively. A total of 19,931 Americans were included in the study, leaving 3632 older participants after excluding those under 60 years old. Among them, we retained 2934 participants with complete cognitive assessment test scores. Additionally, we separately excluded participants with incomplete dietary VD intake data (n = 410) and serum VD data (n = 199). Finally, a total of 2425 survey participants for dietary VD intake and a total of 2735 examination participants for serum 25(OH)D were included in this study (Figure 1).
Figure 1.
Flow chart of the screening process for the selection of eligible dietary VD intake and serum 25(OH)D participants.
2.2. Cognitive Performance
The NHANES database contained cognitive performance data that were obtained through the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word Learning sub-test (assessing the ability to learn new verbal information), the Animal Fluency test (examining categorical verbal fluency in executive function) and the Digit Symbol Substitution Test (DSST) (assessing processing speed, sustained attention and working memory).
The CERAD test included three immediate recall tests and one delayed recall test. In three immediate recall tests, participants read 10 unrelated words and then recalled as many words as possible immediately. Delayed recall test was completed after Animal Fluency test and DSST. The maximum score of each test was 10. The total score of CERAD test was the sum of three immediate recall tests and one delayed recall test. The Animal Fluency test participants were renamed as many animals as possible within one minute. The score was the sum of the correct answers. The DSST asked participants to copy the corresponding symbols from 133 boxes within two minutes. The score ranges from 0 to 133, which was the sum of the number of correct matches [24]. Higher scores of three tests indicated better cognitive performance.
As the CERAD test, Animal Fluency test, and DSST lacked recognized standards for defining low cognitive performance, the study referred to the processing methods in the relevant published research [25]. Additionally, the minimum quartile of these three test scores was used as the cutoff point. Furthermore, considering that the participants were aged over 60 years old and the effect of age on cognitive performance was even more significant, the three test scores were further adjusted according to age (≥60 years and ≥70 years) [26], and these cutoff points are shown in Table 1. Participants with scores lower than or equal to cutoff points were considered to have low cognitive performance, while those with scores greater than cutoff points were considered to have normal cognitive performance.
Table 1.
The cognitive performance cutoff points of CERAD test, Animal Fluency test, and DSST score adjusted according to age (≥60 years and ≥70 years).
| CERAD Test Score | Animal Fluency Test Score |
Digit Symbol Test Score |
|
|---|---|---|---|
| Dietary VD intake (μg/d) | |||
| ≥60 years | 23 | 14 | 38 |
| ≥70 years | 19 | 12 | 31 |
| Serum 25-hydroxyvitamin D (nmol/L) | |||
| ≥60 years | 22 | 14 | 37 |
| ≥70 years | 19 | 12 | 30 |
2.3. The Intake of Dietary VD
The intake of dietary VD was assessed by the two 24 h diet recall interviews in NHANES. The details of NHANES dietary survey were described elsewhere [27]. The collected data of dietary VD intake was further processed and analyzed. Referred to dietary VD Recommended Nutrient Intakes (RNIs) from the Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO) (5.00 μg/d) [28], participants with dietary VD intake lower than RNIs were classified as a reference group (Q1: ≤5.00 μg/d). For participants with dietary VD intake higher than RNIs, we used two methods to bring them into the model: (a) participants being evenly divided into two groups (Q2: ≤20.75 μg/d; Q3: >20.75 μg/d); (b) participants being classified as one group (Q2: >5.00 μg/d).
2.4. Laboratory Measurement of 25(OH)D
Participants had to fast for nine hours before drawing blood, which was then processed into vials and stored in the Mobile Examination Center (MEC). The vials were then refrigerated or frozen and shipped to laboratories around the United States of America [29,30]. High-performance liquid chromatography (HPLC) tandem mass spectrometry was used to analyzed serum 25(OH)D3 and 25(OH)D2 concentration (nmol/L), and the sum of them was the serum total 25(OH)D concentration (nmol/L). NHANES proposed that when the concentrations of 25(OH)D2 were lower than the limit of detection (LOD), the 25(OH)D2 concentration was shown as the imputed value (1.45 nmol/L). [31]. The optimal level of 25(OH)D has been debated [32,33,34]; thus, participants were divided into three groups according to the tertiles of serum total 25(OH)D and 25(OH)D3 concentration (nmol/L). Additionally, participants were divided into two groups based on the 25(OH)D2 concentration imputed value (Q1: ≤1.45 nmol/L and Q2: >1.45 nmol/L).
2.5. Covariates
When referring to previous research [4,35,36], we included a number of factors (age, gender, race, marital status, educational level, poverty-income ratio, body mass index, smoking and drinking status, hypertension, diabetes, and stroke) as covariates. Given the influence of seasons and physical activities on dietary VD intake and serum 25(OH)D concentration, we also included the seasons of exam and physical activity level as covariates. In addition, the total energy intake was also taken as a covariate for dietary VD intake. Hypertension and diabetes were defined by a combination of the physician’s history of diagnosis in the questionnaire and laboratory measurements of systolic/diastolic blood pressure and glycated hemoglobin.
2.6. Statistical Analysis
We referred to the NHANES weight analysis guide [37] to process the new sample weights after combining two cycles. Kolmogorov–Smirnov normality test was used to test the normality of continuous variables. We used the mean ± standard deviation (SD) to describe normally distributed variables and the median (standard error) to describe non-normally distributed variables. If the variable was normally distributed, the Student t-test was used to compare the mean levels between the low cognitive performance group and the normal cognitive performance group. If the variable was not normally distributed, Mann–Whitney U test was used. Chi-square test was selected to compare the percentage of categorical variables between different groups.
Binary logistic regression analyses were used to explore whether there were associations between dietary VD intake, serum total 25(OH)D, 25(OH)D2, and 25(OH)D3 and cognitive performance. Age and gender were adjusted for in model 1, and the other covariates were further adjusted for in model 2. We also conducted a gender stratified analysis. In addition, we used restricted cubic spline to further explore the dose–response relationships between serum 25(OH)D, dietary VD intake and different cognitive performance test scores, which located 5 percent, 50 percent, and 95 percent of the exposure distribution in the logistic regression after adjusting all covariates. The size of a test was 0.05, and the results were considered statistically significant when the bilateral p-value ≤ 0.05.
3. Results
3.1. Sample Characteristics
The characteristics of participants for dietary VD intake survey by cognitive performance are shown in Table 2. We could see that people who were non-Hispanic white, with lower educational level, lower income, higher prevalence of diabetes, hypertension, and stroke, often alcohol drinkers, with lower physical activity level, and higher total energy intake had lower cognitive performance. Additionally, the characteristics of participants for serum 25(OH)D by cognitive performance are shown in Table 3. We could see that people who were non-Hispanic white, with lower educational level, often drink alcohol, have lower physical activity level, higher prevalence of diabetes, hypertension, and stroke had lower cognitive performance. We included the interaction term of exam season and serum 25(OH)D multiplication in the multi-factor logistics regression model, and interaction terms were not statistical significance in the cognitive tests of CERAD, Animal Fluency test, and DSST.
Table 2.
Characteristics of the dietary VD and cognition study population, National Health and Nutrition Examination Survey (NHANES) 2011–2014 (N = 2524).
| CERAD Test | Animal Fluency Test | Digit Symbol Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Subjects (N) | Normal Cognitive Performance | Low Cognitive Performance | p-Value | Normal Cognitive Performance | Low Cognitive Performance | p-Value | Normal Cognitive Performance | Low Cognitive Performance | p-Value | |
| Number of subjects (%) | 1806(71.6) | 718(28.4) | 1792(71.0) | 731(29.0) | 1867(74.0) | 647(26.0) | ||||
| Age(%) 1 | 2524 | 0.028 | 0.415 | 0.993 | ||||||
| ≥60 years | 954(52.8) | 414(57.7) | 962(53.7) | 406(55.5) | 1012(54.2) | 356(54.2) | ||||
| ≥70 years | 852(47.2) | 304(42.3) | 830(46.3) | 326(44.5) | 855(45.8) | 301(45.8) | ||||
| Gender(%) 1 | 2524 | <0.01 | 0.664 | <0.01 | ||||||
| Male | 785(43.5) | 432(60.2) | 869(48.5) | 348(47.5) | 847(45.4) | 370(56.3) | ||||
| Female | 1021(56.5) | 286(39.8) | 923(51.5) | 384(52.5) | 1020(54.6) | 287(43.7) | ||||
| Race(%) 1 | 2524 | <0.01 | <0.01 | <0.01 | ||||||
| Mexican American | 129(7.1) | 82(11.4) | 149(8.3) | 62(8.5) | 119(6.4) | 92(14.0) | ||||
| Other Hispanic | 141(7.8) | 103(14.3) | 151(8.4) | 93(12.7) | 120(6.4) | 124(18.9) | ||||
| Non-Hispanic White | 971(53.8) | 298(41.5) | 1033(57.6) | 236(32.2) | 1094(58.6) | 175(26.6) | ||||
| Non-Hispanic Black | 401(22.3) | 192(26.7) | 334(18.6) | 260(35.5) | 350(18.7) | 244(37.1) | ||||
| Other races | 163(9.0) | 43(6.0) | 125(7.0) | 81(11.1) | 184(9.9) | 22(3.3) | ||||
| Educational level (%) 1 | 2522 | <0.01 | <0.01 | <0.01 | ||||||
| Below high school | 382(19.0) | 304(42.2) | 369(19.3) | 317(38.6) | 276(13.7) | 410(56.9) | ||||
| High school | 475(23.6) | 175(24.3) | 435(22.7) | 215(26.2) | 489(24.3) | 161(22.3) | ||||
| Above high school | 1158(57.5) | 241(33.5) | 1110(58.0) | 289(35.2) | 1249(62.0) | 150(20.8) | ||||
| Marital status (%) 1 | 2521 | 0.883 | 0.027 | <0.01 | ||||||
| Married/living with partner | 1060(58.8) | 419(58.4) | 1075(60.1) | 404(55.3) | 1150(61.7) | 329(50.2) | ||||
| Widowers/divorced/separated/never married | 744(41.2) | 298(42.6) | 715(39.9) | 317(44.7) | 715(38.3) | 327(49.8) | ||||
| Poverty-income ratio (%) 1 | 2333 | <0.01 | <0.01 | <0.01 | ||||||
| ≤1.00 | 228(13.6) | 145(22.0) | 219(13.1) | 154(23.2) | 192(11.1) | 181(30.4) | ||||
| ≥1.00 | 1446(86.4) | 514(78.0) | 1450(86.9) | 510(76.8) | 1545(88.9) | 415(69.6) | ||||
| Body mass index (%) 1 | 2492 | 0.163 | 0.662 | 0.886 | ||||||
| <25 kg/m2 | 466(26.1) | 195(27.6) | 465(26.6) | 196(27.5) | 491(26.5) | 170(26.7) | ||||
| <30 kg/m2 | 607(34.0) | 259(36.6) | 627(35.3) | 239(33.5) | 650(35.0) | 216(34.0) | ||||
| ≥30 kg/m2 | 712(39.9) | 253(35.8) | 686(38.6) | 279(39.1) | 715(38.5) | 250(39.3) | ||||
| Physical activity level(%) 1 | 2524 | <0.01 | <0.01 | <0.01 | ||||||
| Moderate and high | 803(44.5) | 264(36.8) | 825(46.0) | 242(33.1) | 875(46.9) | 192(29.2) | ||||
| Low | 1003(55.5) | 454(63.2) | 968(54.0) | 490(66.9) | 992(53.1) | 465(70.8) | ||||
| Season of exam (%) 1 | 2524 | 0.545 | 0.832 | 0.023 | ||||||
| November-April | 796(44.1) | 326(45.4) | 799(44.6) | 323(44.1) | 805(43.1) | 317(48.2) | ||||
| May-October | 1010(55.9) | 392(54.6) | 993(55.4) | 409(55.9) | 1128(56.0) | 340(51.8) | ||||
| Smoking status (%) 1 | 2522 | 907(50.3) | 368(51.3) | 0.658 | 915(51.1) | 360(49.2) | 0.377 | 1329(71.5) | 401(62.0) | 0.796 |
| Hypertension (%) 1 | 2522 | 1248(69.1) | 514(71.7) | 0.209 | 1212(67.7) | 550(75.1) | <0.01 | 1267(67.9) | 495(75.3) | <0.01 |
| Diabetes (%) 1 | 2524 | 467(25.9) | 232(32.3) | 0.001 | 440(24.6) | 259(35.4) | <0.01 | 443(23.7) | 256(39.0) | <0.01 |
| Stroke (%) 1 | 2519 | 107(5.9) | 62(8.6) | 0.015 | 100(5.6) | 69(9.5) | <0.01 | 94(5.0) | 75(11.4) | <0.01 |
| Had at least 12 alcohol drinks/year (%) 1 | 2506 | 1244(69.1) | 486(68.7) | 0.842 | 1271(71.3) | 459(63.4) | <0.01 | 1329(71.5) | 401(62.0) | <0.01 |
| Total energy intake (kcal/day) 2 | 2524 | 1845.71(672.87) | 1761.34(685.91) | 0.001 | 1885.32(676.43) | 1666.00(655.11) | <0.01 | 1875.86(651.83) | 1667.83(724.50) | <0.01 |
| Daily dietary VD intake (μg/d) 2 | 2524 | 25.14(47.43) | 19.04(58.55) | <0.01 | 24.95(49.71) | 19.60(53.54) | <0.01 | 26.49(57.00) | 14.60(24.79) | <0.01 |
Data is number of subjects (percentage) or medians (interquartile ranges); 1 Chi-square was used to compare the percentage between participants with and without low cognitive performance; 2 Mann–Whitney U test was applied to compare the median values between participants with and without low cognitive performance.
Table 3.
Characteristics of the serum 25(OH)D and cognition study population, National Health and Nutrition Examination Survey (NHANES) 2011–2014 (N = 2735).
| CERAD Test | Animal Fluency Test | Digit Symbol Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Subjects (N) | Normal Cognitive Performance | Low Cognitive Performance | p-Value | Normal Cognitive Performance | Low Cognitive Performance | p-Value | Normal Cognitive Performance | Low Cognitive Performance | p-Value | |
| Number of subjects (%) | 2015(73.7) | 720(26.3) | 1914(70.0) | 821(30.0) | 2014(73.6) | 721(26.4) | ||||
| Age (%) 1 | 2735 | 0.258 | 0.449 | 0.963 | ||||||
| ≥60 years | 1110(55.1) | 379(52.6) | 1033(54.0) | 456(55.0) | 1097(54.5) | 392(54.4) | ||||
| ≥70 years | 905(44.9) | 341(47.4) | 881(46.0) | 365(44.5) | 917(45.5) | 329(45.6) | ||||
| Gender(%) 1 | 2735 | <0.01 | 0.460 | <0.01 | ||||||
| Male | 911(45.2) | 431(59.9) | 948(49.5) | 394(48.0) | 941(46.7) | 401(55.6) | ||||
| Female | 1104(54.8) | 289(40.1) | 966(50.5) | 427(52.0) | 1073(53.3) | 320(44.4) | ||||
| Race(%) 1 | 2735 | <0.01 | <0.01 | <0.01 | ||||||
| Mexican American | 160(7.9) | 81(11.3) | 169(8.8) | 72(8.8) | 137(6.8) | 104(14.4) | ||||
| Other Hispanic | 177(8.8) | 102(14.2) | 173(9.0) | 106(12.9) | 138(6.9) | 141(19.6) | ||||
| Non-Hispanic White | 1032(51.2) | 190(40.3) | 1057(55.2) | 265(32.3) | 1141(56.7) | 181(25.1) | ||||
| Non-Hispanic Black | 441(21.9) | 189(26.3) | 359(18.8) | 271(33.0) | 380(18.9) | 250(34.7) | ||||
| Other races | 205(10.2) | 58(8.1) | 156(8.2) | 107(13.0) | 218(10.8) | 45(6.2) | ||||
| Educational level (%) 1 | 2735 | <0.01 | <0.01 | <0.01 | ||||||
| Below high school | 382(19.0) | 304(42.2) | 369(19.3) | 317(38.6) | 276(13.7) | 410(56.9) | ||||
| High school | 475(23.6) | 175(24.3) | 435(22.7) | 215(26.2) | 489(24.3) | 161(22.3) | ||||
| Above high school | 1158(57.5) | 241(33.5) | 1110(58.0) | 289(35.2) | 1249(62.0) | 150(20.8) | ||||
| Marital status (%) 1 | 2733 | 0.089 | 0.020 | <0.01 | ||||||
| Married/living with partner | 1189(59.1) | 399(55.4) | 11139(59.5) | 449(54.8) | 1226(60.9) | 392(50.3) | ||||
| widowers/divorced/separated/never married | 824(40.9) | 321(44.6) | 774(40.5) | 371(45.2) | 78(39.1) | 358(49.7) | ||||
| Poverty-income ratio (%) 1 | 2509 | 0.199 | 0.928 | 0.572 | ||||||
| ≤1.00 | 318(17.1) | 96(14.9) | 292(16.5) | 122(16.4) | 312(16.7) | 102(15.8) | ||||
| ≥1.00 | 1546(82.9) | 549(85.1) | 1473(83.5) | 622(83.6) | 1551(83.3) | 544(84.2) | ||||
| Body mass index (%) 1 | 2697 | 0.178 | 0.449 | 0.675 | ||||||
| <25 kg/m2 | 535(26.9) | 205(29.0) | 508(26.8) | 232(29.0) | 543(27.2) | 197(28.2) | ||||
| <30 kg/m2 | 699(35.1) | 360(36.8) | 685(36.1) | 274(31.1) | 706(35.3) | 253(36.2) | ||||
| ≥30 kg/m2 | 757(38.0) | 241(34.1) | 705(37.1) | 293(36.7) | 749(37.5) | 249(35.6) | ||||
| Physical activity level (%) 1 | 2735 | <0.01 | <0.01 | <0.01 | ||||||
| Moderate and high | 892(44.3) | 258(35.8) | 889(46.4) | 261(31.8) | 946(47.0) | 204(28.3) | ||||
| Low | 1123(55.7) | 462(64.2) | 1025(53.6) | 560(68.2) | 1068(53.0) | 517(71.7) | ||||
| Season of exam (%) 1 | 2735 | 0.877 | 0.859 | 0.011 | ||||||
| November-April | 914(45.4) | 329(45.7) | 872(45.6) | 371(45.2) | 886(44.0) | 357(49.5) | ||||
| May-October | 1101(54.6) | 391(54.3) | 1042(54.4) | 450(54.8) | 1128(56.0) | 364(50.5) | ||||
| Smoking status (%) 1 | 2733 | 1013(50.3) | 369(51.3) | 0.669 | 969(50.7) | 413(50.3) | 0.857 | 1005(50.0) | 377(52.3) | 0.281 |
| Hypertension (%)1 | 2733 | 1381(68.6) | 514(71.5) | 0.145 | 1283(67.1) | 612(74.5) | <0.01 | 1357(67.4) | 538(74.6) | <0.01 |
| Diabetes (%) 1 | 2735 | 529(26.3) | 230(31.9) | 0.003 | 473(24.7) | 286(34.8) | <0.01 | 480(23.8) | 279(38.7) | <0.01 |
| Stroke (%) | 2730 | 115(5.7) | 72(10.0) | <0.01 | 104(5.4) | 83(10.1) | <0.01 | 105(5.2) | 82(11.4) | <0.01 |
| Had at least 12 alcohol drinks/year (%) 1 | 2689 | 1376(69.2) | 466(66.6) | 0.201 | 1339(70.9) | 503(63.8) | <0.01 | 1413(70.9) | 429(61.5) | <0.01 |
| Serum total 25(OH)D (nmol/L) 2 | 2735 | 78.17(32.02) | 72.55(30.20) | <0.01 | 78.3(31.1) | 72.68(32.84) | <0.01 | 79.06(31.33) | 69.72(31.78) | <0.01 |
| Serum 25(OH)D3 (nmol/L) 2 | 2735 | 71.38(32.31) | 65.32(29.33) | <0.01 | 71.90(31.55) | 64.91(31.37) | <0.01 | 72.43(31.62) | 62.04(30.73) | <0.01 |
| Serum 25(OH)D2 (nmol/L) 1 | 2735 | 0.076 | 0.159 | 0.45 | ||||||
| ≤1.45 | 1512(75.0) | 516(71,7) | 1434(74.9) | 594(72.4) | 1501(74.5) | 527(73.1) | ||||
| >1.45 | 503(25.0) | 204(28.3) | 480(25.1) | 227(27.6) | 513(25.5) | 194(26.9) | ||||
Data are number of subjects (percentage) or medians (interquartile ranges); 1 Chi-square was used to compare the percentage between participants with and without low cognitive performance; 2 Mann–Whitney U test was applied to compare the median values between participants with and without low cognitive performance.
3.2. Association between Dietary VD and Cognition
While comparing to the lowest group of dietary VD intake, the multivariate adjusted ORs (95% CIs) of the highest group of dietary VD intake was 0.51 (0.36–0.72) for the Animal Fluency test score and 0.45 (0.31–0.66) for the DSST score, respectively. After only adjusting for age and gender, dietary VD intake higher than 20.75 μg/d was associated with a reduced low cognitive performance risk assessed by CERAD. When the dietary VD intake was divided into two groups by RNIs, the associations persisted even after adjusting all covariates with ORs (95% CIs) being 0.60 (0.41–0.89) for the Animal Fluency test score and 0.59 (0.40–0.86) for DSST score (Table 4).
Table 4.
Weighted ORs (95%CI) for scores on the Consortium to CERAD test, Animal Fluency test, DSST across dietary VD intake, NHANES 2011–2014 (N = 2524).
| Dietary VD Intake (μg/d) | Dietary VD Intake (μg/d) | |||||
| ≤5.00 | ≤20.75 | >20.75 | ≤5.00 | >5.00 | ||
| CREAD Test | Case/Participants | 265/798 | 268/888 | 185/838 | 265/798 | 453/1726 |
| Crude | 1.00 (Ref.) | 1.02 (0.80–1.30) | 0.69 (0.51–0.92) * | 1.00 (Ref.) | 0.84 (0.67–1.04) | |
| Model 1 | 1.00 (Ref.) | 0.96 (0.75–1.24) | 0.70 (0.51–0.96) * | 1.00 (Ref.) | 0.82 (0.65–1.05) | |
| Model 2 | 1.00 (Ref.) | 0.97 (0.72–1.29) | 0.83 (0.58–1.19) | 1.00 (Ref.) | 0.90 (0.69–1.17) | |
| Animal Fluency Test | Case/Participants | 284/798 | 260/888 | 188/838 | 284/798 | 448/1726 |
| Crude | 1.00 (Ref.) | 0.69 (0.49–0.97) * | 0.43 (0.34–0.55) * | 1.00 (Ref.) | 0.55 (0.43–0.70) * | |
| Model 1 | 1.00 (Ref.) | 0.67 (0.48–0.93) * | 0.41 (0.31–0.53) * | 1.00 (Ref.) | 0.53 (0.41–0.67) * | |
| Model 2 | 1.00 (Ref.) | 0.70 (0.43–1.13) | 0.51 (0.36–0.72) * | 1.00 (Ref.) | 0.60 (0.41–0.89) * | |
| Digit Symbol Test | Case/Participants | 277/798 | 247/888 | 133/838 | 277/798 | 380/1726 |
| Crude | 1.00 (Ref.) | 0.82 (0.55–1.20) | 0.36 (0.26–0.51) * | 1.00 (Ref.) | 0.56 (0.41–0.77) * | |
| Model 1 | 1.00 (Ref.) | 0.77 (0.53–1.14) | 0.34 (0.25–0.46) * | 1.00 (Ref.) | 0.53 (0.39–0.73) * | |
| Model 2 | 1.00 (Ref.) | 0.72 (0.45–1.15) | 0.45 (0.31–0.66) * | 1.00 (Ref.) | 0.59 (0.40–0.86) * | |
Binary logistic regression analyses were used to calculate weighted OR values. Reference (Ref.); model 1 adjusted for age and gender; model 2 adjusted for age and gender, race, educational level, marital status, income, body mass index (BMI), season of exam, physical activity level, drinking status, smoking status, hypertension, diabetes, and stroke. Energy was adjusted, when we studied the intake of dietary VD. * p-value ≤ 0.05.
Model 2 showed that when the dietary VD intake was higher than 20.75 μg/d, it had statistically significant effects on cognitive performance assessed by DSST in both males (0.46 (0.26–0.82)) and females (0.50 (0.30–0.83)). In terms of the Animal Fluence test, the fully adjusted model was statistically significant (0.38 (0.22–0.67)) when the dietary VD intake higher than 20.75 μg/d in males only. Moreover, when the dietary VD intake was divided into two groups by RNIs, the associations between the VD and Animal Fluency test score (0.68 (0.47–1.00)) and DSST score (0.53 (0.31–0.93)) were more pronounced in female whose dietary VD intake higher than 5.00 μg/d (Table 5).
Table 5.
Weighted ORs (95% CI) for score on CERAD test, Animal Fluency test and DSST across dietary VD intake and serum total 25(OH)D, stratified by gender, NHANES 2011–2014.
| CREAD Test | Animal Fluency Test | Digit Symbol Test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case/Participants | Crude | Model 1 | Model 2 | Case/Participants | Crude | Model 1 | Model 2 | Case/Participants | Crude | Model 1 | Model 2 | |
| Dietary VD intake (μg/d) | ||||||||||||
| male | 432/1217 | 348/1217 | 370/1217 | |||||||||
| ≤5.00 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | |||
| ≤20.75 | 1.04 (0.66–1.64) | 1.04 (0.66–1.64) | 1.09 (0.68–1.75) | 0.65 (0.35–1.20) | 0.64 (0.35–1.17) | 0.73 (0.37–1.44) | 0.95 (0.58–1.54) | 0.93 (0.56–1.53) | 0.90 (0.55–1.47) | |||
| >20.75 | 0.79 (0.46–1.36) | 0.79 (0.45–1.40) | 0.86 (0.44–1.68) | 0.34 (0.20–0.57) | 0.31 (0.19–0.55) | 0.38 (0.22–0.67) | 0.36 (0.23–0.58) | 0.34 (0.22–0.55) | 0.46 (0.26–0.82) | |||
| female | 286/1217 | 184/1217 | 287/1217 | |||||||||
| ≤5.00 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | |||
| ≤20.75 | 0.90 (0.61–1.33) | 0.88 (0.60–1.27) | 0.80 (0.49–1.32) | 0.73 (0.50–1.06) | 0.70 (0.47–1.03) | 0.69 (0.46–1.05) | 0.66 (0.41–1.07) | 0.62 (0.39–1.00) | 0.57 (0.26–1.25) | |||
| >20.75 | 0.66 (0.42–1.02) * | 0.62 (0.42–0.93) * | 0.81 (0.53–1.23) | 0.51 (0.34–0.77) * | 0.48 (0.31–0.72) * | 0.68 (0.43–1.07) | 0.36 (0.23–0.57) * | 0.33 (0.21–0.50) * | 0.50 (0.30–0.83) * | |||
| Dietary VD intake (μg/d) | ||||||||||||
| male | 432/1217 | 348/1217 | 370/1217 | |||||||||
| ≤5.00 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | |||
| >5.00 | 0.93 (0.62–1.38) | 0.93 (0.62–1.40) | 1.00 (0.64–1.56) | 0.51 (0.30–0.85) * | 0.49 (0.30–0.83) * | 0.58 (0.33–1.04) | 0.68 (0.44–1.03) | 0.65 (0.42–1.02) | 0.72 (0.47–1.10) | |||
| female | 286/1217 | 184/1217 | 287/1217 | |||||||||
| ≤5.00 | 432/1217 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | ||
| >5.00 | 0.75 (0.52–1.07) | 0.72 (0.52–0.99) * | 0.81 (0.54–1.19) | 0.59 (0.41–0.85) * | 0.56 (0.38–0.81) * | 0.68 (0.47–1.00) * | 0.47 (0.32–0.69) * | 0.44 (0.30–0.63) * | 0.53 (0.31–0.93) * | |||
| Total 25-Hydroxyvitamin D (nmol/L) | ||||||||||||
| male | 431/1342 | 394/1342 | 401/1342 | |||||||||
| ≤61.41 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | |||
| ≤86.30 | 0.83 (0.59–1.17) | 0.84 (0.60–1.18) | 0.99 (0.65–1.51) | 0.56 (0.39–0.85) * | 0.57 (0.38–0.85) * | 0.84 (0.54–1.30) | 0.44 (0.28–0.68) * | 0.44 (0.29–0.68) * | 0.84 (0.44–1.61) | |||
| >86.30 | 0.63 (0.43–0.93) * | 0.61 (0.41–0.89) * | 0.77 (0.46–1.31) | 0.46 (0.25–0.84) * | 0.44 (0.25–0.79) * | 0.65 (0.35–1.23) | 0.35 (0.20–0.63) * | 0.35 (0.20–0.61) * | 0.67 (0.30–1.48) | |||
| female | 289/1393 | 427/1393 | 320/1393 | |||||||||
| ≤61.41 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | |||
| ≤86.30 | 1.09 (0.75–1.58) | 1.04 (0.70–1.53) | 1.01 (0.67–1.54) | 1.03 (0.69–1.55) | 0.99 (0.65–1.51) | 1.49 (0.93–2.41) | 0.84 (0.55–1.29) | 0.78 (0.50–1.23) | 1.04 (0.61–1.78) | |||
| >86.30 | 0.82 (0.58–1.15) | 0.73 (0.50–1.06) | 0.75 (0.47–1.20) | 0.83 (0.58–1.19) | 0.76 (0.53–1.07) | 1.30 (0.92–1.83) | 0.56 (0.41–0.76) * | 0.47 (0.34–0.66) * | 0.68 (0.42–1.09) | |||
Binary logistic regression analyses were used to calculate weighted OR values. Reference (Ref.); model 1 adjusted for age and gender; model 2 adjusted for age and gender, race, educational level, marital status, income, body mass index (BMI), season of exam, physical activity level, drinking status, smoking status, hypertension, diabetes, and stroke. Energy was adjusted when we studied the intake of dietary VD. * p-value ≤ 0.05.
3.3. Association between Serum Total 25(OH)D and Cognition
When the serum total 25(OH)D concentration was higher than 86.30 nmol/L, the serum total 25(OH)D was positively correlated with DSST score (0.68 (0.47–0.97)) (Table 5). After adjusting for all covariates, the effect of serum total 25(OH)D concentration on cognitive performance was not significantly different in males and females. When the total 25(OH)D concentration was higher than 61.41 nmol/L, the association between serum total 25(OH)D concentration and Animal Fluency test score was statistically significant in males, but not in females (Model 1). Additionally, the association between the serum total 25(OH)D concentration and CERAD score was only statistically significant in the male when total 25(OH)D concentration was higher than 86.30 nmol/L in Model 1 (Table 6).
Table 6.
Weighted ORs (95%CI) for scores on the Consortium to CERAD test, Animal Fluency test, DSST across quartiles of serum total 25(OH)D, 25(OH)D2 and 25(OH)D3, NHANES 2011–2014 (N = 2735).
| CERAD Test | Animal Fluency Test | Digit Symbol Test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case/Participants | Crude | Model 1 | Model 2 | Case/Participants | Crude | Model 1 | Model 2 | Case/Participants | Crude | Model 1 | Model 2 | |
| Total 25-Hydroxyvitamin D (nmol/L) | ||||||||||||
| ≤61.41 | 274/911 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 326/911 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 307/911 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| ≤86.30 | 252/915 | 0.96 (0.77–1.20) | 0.94 (0.75–1.15) | 1.02 (0.77–1.34) | 259/915 | 0.75 (0.59–0.96) * | 0.75 (0.58–0.97) * | 1.12 (0.83–1.51) | 237/915 | 0.60 (0.44–0.81) * | 0.58 (0.43–0.80) * | 0.93 (0.61–1.42) |
| >86.30 | 194/909 | 0.68 (0.53–0.87) * | 0.67 (0.52–0.86) * | 0.77 (0.55–1.08) | 236/909 | 0.64 (0.47–0.88) * | 0.59 (0.44–0.81) * | 0.95 (0.70–1.28) | 177/909 | 0.44 (0.34–0.55) * | 0.41 (0.32–0.52) * | 0.68 (0.47–0.97) * |
| 25-Hydroxyvitamin D3 (nmol/L) | ||||||||||||
| ≤55.14 | 264/911 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 334/911 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 316/911 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| ≤80.63 | 266/913 | 1.16 (0.85–1.59) | 1.10 (0.81–1.48) | 1.23 (0.86–1.77) | 259/913 | 0.74 (0.56–0.99) * | 0.74 (0.56–0.98) * | 1.10 (0.92–1.46) | 238/913 | 0.61 (0.46–0.81) * | 0.58 (0.43–0.80) * | 0.92 (0.64–1.33) |
| >80.63 | 190/911 | 0.74 (0.53–1.03) | 0.72 (0.51–1.02) | 0.92 (0.58–1.45) | 228/911 | 0.61 (0.47–0.99) * | 0.57 (0.44–0.75) * | 0.98 (0.75–1.29) | 167/911 | 0.36 (0.29–0.46) * | 0.41 (0.32–0.52) * | 0.62 (0.44–0.86) * |
| 25-Hydroxyvitamin D2 (nmol/L) | ||||||||||||
| ≤1.45 | 516/2028 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 594/2028 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 527/2028 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| >1.45 | 204/707 | 1.27 (0.92–1.77) | 1.30 (0.93–1.81) | 1.47 (0.98–2.22) | 227/707 | 0.99 (0.74–1.33) | 0.97 (0.72–1.29) | 1.05 (0.74–1.47) | 194/707 | 0.95 (00.73–1.25) | 0.94 (0.70–1.26) | 1.00 (0.68–1.49) |
Binary logistic regression analyses were used to calculate weighted OR values. Reference (Ref.); model 1 adjusted for age and gender; model 2 adjusted for age and gender, race, educational level, marital status, income, body mass index (BMI), season of exam, physical activity level, drinking status, smoking status, hypertension, diabetes, and stroke. Energy was adjusted when we studied the intake of dietary VD. * p-value ≤ 0.05.
3.4. Association between Serum 25(OH)D2, 25(OH)D3 and Cognition
In model 1, when the 25(OH)D3 concentration was higher than 55.14 nmol/L, the serum 25(OH)D3 concentration was associated with the Animal Fluency test score and DSST score. In the fully adjusted model, the serum 25(OH)D3 concentration was associated with DSST score (0.62 (0.44–0.86)) when the serum 25(OH)D3 concentration was higher than 80.63 nmol/L. However, this association was not found between serum 25(OH)D2 and cognitive test scores (Table 6).
3.5. Dose–Response Relationships
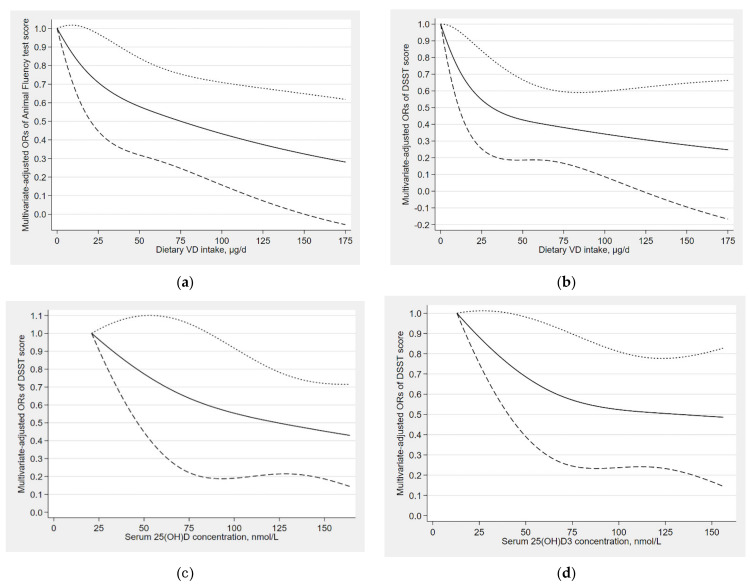
As shown in Figure 2, L-shaped dose–response relationships were found between dietary VD intake and the risk of low cognitive performance (the Animal Fluency test, P for nonlinearity = 0.426; DSST, P for nonlinearity = 0.239). Additionally, L-shaped dose–response relationships were also found in between serum total 25(OH)D and the risk of low cognitive performance (DSST, P for nonlinearity = 0.697), as well as found in between serum 25(OH)D3 and the risk of low cognitive performance (DSST, P for nonlinearity = 0.409). For the Animal Fluency test, when dietary VD intake was higher than 20 μg/d, the risk of low cognitive performance began to decrease (Figure 2a); for the DSST, the risk of low cognitive performance began to decrease when dietary VD intake was higher than 4 μg/d (Figure 2b). When the serum total 25(OH)D concentration was higher than 87 nmol/L, the serum total 25(OH)D was statistically significantly associated with the decreased risk of low cognitive performance in DSST (Figure 2c). The serum 25(OH)D3 was also associated with a decreased risk of low cognitive performance assessed by DSST when 25(OH)D3 concentration was higher than 44 nmol/L (Figure 2d).
Figure 2.
(a) Dose–response relationship between dietary VD intake and the risk of low cognitive performance (Animal Fluency test); (b) Dose–response relationship between dietary VD intake and the risk of low cognitive performance (DSST); (c) Dose–response relationship between serum total 25(OH)D concentration and the risk of low cognitive performance (DSST); (d) Dose–response relationship between serum 25(OH)D3 concentration and the risk of low cognitive performance (DSST). The solid line represents the OR values and dashed lines represent the 95% confidence intervals.
4. Discussion
In this study, we investigated the associations between dietary VD intake and serum 25(OH)D with cognitive performance in older adults. The study found that the dietary VD intake, serum total 25(OH)D, and 25(OH)D3 concentration were negatively associated with low cognitive performance risk, and linear L-shaped dose–response relationships between them were identified. In stratified analysis by gender, the associations between dietary VD intake, serum total 25(OH)D, and cognitive performance were not different between genders.
Recent observational studies have researched the relationship between dietary VD intake and cognitive performance [11,18,38,39,40]. The study of Przybelski et al. showed that elderly adults with higher levels of VD intake had better cognitive performance [40]. Additionally, other studies came to the same conclusion, which was consistent with our study findings [18,38], while the study of Elske et al. showed that the effect of dietary VD intake on cognitive performance was non-significant. [11] Our results were inconsistent with this study and the reason may be the use of different populations. Elske et al. included 127 Dutch older adults over 65 years, but our study included 2524 older adults over 60 years. In addition, in our study, dietary vitamin D intake was grouped by RNIs (5.00 μg/d) provided by FAO/WHO, and the results show that RNIs were also preferable for protecting cognitive performance.
For serum 25(OH)D, some studies supported our view [17,41,42,43]. The study of Littlejohns et al. found a negative correlation between the incidence of Alzheimer’s disease (AD) and serum total 25(OH)D concentration [41]. Additionally, another French cohort study showed that higher level of serum total 25(OH)D could delay the development of cognitive decline and dementia in older adults [42]. The relationship between serum 25(OH)D2, 25(OH)D3 and cognition has not been explored in past studies. In our study, we found that higher 25(OH)D3 concentrations had a protective effect on cognitive performance, while the association between 25(OH)D2 and cognitive performance was not significant. Meanwhile, a study involved patients with AD or mild cognitive impairment (MCI) found that 25(OH)D3 reduced the risk of MCI and AD [44]. It is important to further explore the role of 25(OH)D3 in cognitive performance.
At the same time, in our study, we found that the associations between dietary VD intake, serum total 25(OH)D, and cognitive performance remained unchanged between genders. The research of Morello et al. supported our point [45]. In addition, some studies only focused on women [10,46,47], and few studies explored the effect of serum 25(OH)D on cognition in males; therefore, the relationship between 25(OH)D and cognition in males should not be ignored in future research.
To our knowledge, no studies have explored the dose–response relationships between dietary VD intake, serum 25(OH)D, and cognitive performance. In our study, we found a linear L-shaped dose–response relationship between dietary VD intake, serum total 25(OH)D, serum 25(OH)D3, and cognition. Referable values of serum total 25(OH)D, 25(OH)D3 concentration, and dietary VD intake were proposed to protect cognitive performance, which need to be researched by further studies.
Increasingly, recent studies have focused on the influence of VD on cognitive performance. Several studies have shown that VD affects cognitive performance by affecting cell differentiation, neurotransmitter synthesis, the expression of genes and proteins involved in neural structure, and so on [48]. These action mechanisms emphasize the important role of VD in brain function, so we think that VD may be an important nutrient for maintaining better cognitive performance and preventing cognitive decline in the elderly.
There are several advantages present in the study. First of all, we explored both the dose–response relationships of dietary VD intake, serum total 25(OH)D, 25(OH)D3, and 25(OH)D2 concentrations with cognitive performance, respectively. Referable cutoff values of serum 25(OH)D concentration and dietary VD intake were also provided. In addition, when we explored the relationship between dietary VD intake and cognitive performance, we not only adopted the three-digit grouping, but also referred to RNIs for grouping. Next, we explored the associations between 25(OH)D3, 25(OH)D2 and cognition, respectively, and found that 25(OH)D3 were related to cognitive performance, while 25(OH)D2 was not. Finally, we explored gender differences in dietary VD intake, serum total 25(OH)D concentration, and cognitive performance, respectively.
However, there are some limitations in the study. First of all, the study was a cross-sectional study and could not determine the cause and effect. Secondly, the data of dietary VD intake obtained through 24 h dietary recall could not accurately judge individual dietary intake, and there was recall bias. In the next place, comparing the included with excluded populations, there were no differences in gender (serum: p = 0.199; diet: p = 0.661), but differences in age, race, education level, marital status, and income level. Although we conducted a weighted analysis, extrapolation still needs to be approached with caution. Finally, after adjusting for all covariates, dietary VD intake and serum 25(OH)D were only associated with cognitive performance assessed by DSST, not with all cognitive dimensions.
5. Conclusions
In the study, the associations between dietary VD intake, serum total 25(OH)D, 25(OH)D2, 25(OH)D3, and cognitive performance were investigated separately and positive associations between dietary VD intake, serum total 25(OH)D, 25(OH)D3 with cognitive performance were found. In addition, L-shaped dose–response relationships of them with cognitive performance were found. Future studies should delve into the relationships between them and mechanisms of their impact on cognition, respectively.
Acknowledgments
The authors would like to thank all participants and contributors of NHANES.
Author Contributions
Conceptualization, R.W. and D.Z.; methodology, R.W.; software, R.W.; validation, P.H., R.Z. and X.D.; data curation, R.W.; writing—original draft preparation, R.W..; writing—review and editing, R.Z. and X.D.; visualization, W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (National Science Foundation of China), grant number 82073641.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board Institutional Review Boards of Carle Foundation Hospital and the University of Illinois at Urbana-Champaign (Project ID: 17088; UIUC number: 181933; Original Approval Date: 1 February 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are openly available vis this link: http://www.cdc.gov/nchs/nhanes.htm(accessed on 28 June 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hebert L.E., Weuve J., Scherr P.A., Evans D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afzal S., Bojesen S.E., Nordestgaard B.G. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimers Dement. J. Alzheimer’s Assoc. 2014;10:296–302. doi: 10.1016/j.jalz.2013.05.1765. [DOI] [PubMed] [Google Scholar]
- 3.Jaroudi W., Garami J., Garrido S., Hornberger M., Keri S., Moustafa A.A. Factors underlying cognitive decline in old age and Alzheimer’s disease: The role of the hippocampus. Rev. Neurosci. 2017;28:705–714. doi: 10.1515/revneuro-2016-0086. [DOI] [PubMed] [Google Scholar]
- 4.Dong X., Li S., Sun J., Li Y., Zhang D. Association of Coffee, Decaffeinated Coffee and Caffeine Intake from Coffee with Cognitive Performance in Older Adults: National Health and Nutrition Examination Survey (NHANES) 2011-2014. Nutrients. 2020;12:840. doi: 10.3390/nu12030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travica N., Ried K., Sali A., Scholey A., Hudson I., Pipingas A. Vitamin C Status and Cognitive Function: A Systematic Review. Nutrients. 2017;9:960. doi: 10.3390/nu9090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dusso A.S., Brown A.J., Slatopolsky E. Vitamin D. Am. J. Physiol. Ren. Physiol. 2005;289:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 7.Aspell N., Lawlor B., O’Sullivan M. Is there a role for vitamin D in supporting cognitive function as we age? Proc. Nutr. Soc. 2018;77:124–134. doi: 10.1017/S0029665117004153. [DOI] [PubMed] [Google Scholar]
- 8.Olsson E., Byberg L., Karlström B., Cederholm T., Melhus H., Sjögren P., Kilander L. Vitamin D is not associated with incident dementia or cognitive impairment: An 18-y follow-up study in community-living old men. Am. J. Clin. Nutr. 2017;105:936–943. doi: 10.3945/ajcn.116.141531. [DOI] [PubMed] [Google Scholar]
- 9.Lips P. Worldwide status of vitamin D nutrition. J. Steroid. Biochem. Mol. Biol. 2010;121:297–300. doi: 10.1016/j.jsbmb.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Annweiler C., Schott A.M., Rolland Y., Blain H., Herrmann F.R., Beauchet O. Dietary intake of vitamin D and cognition in older women: A large population-based study. Neurology. 2010;75:1810–1816. doi: 10.1212/WNL.0b013e3181fd6352. [DOI] [PubMed] [Google Scholar]
- 11.Brouwer-Brolsma E.M., van de Rest O., Tieland M., van der Zwaluw N.L., Steegenga W.T., Adam J.J., van Loon L.J., Feskens E.J., de Groot L.C. Serum 25-hydroxyvitamin D is associated with cognitive executive function in Dutch prefrail and frail elderly: A cross-sectional study exploring the associations of 25-hydroxyvitamin D with glucose metabolism, cognitive performance and depression. J. Am. Med. Dir. Assoc. 2013;14:852.e9–852.e17. doi: 10.1016/j.jamda.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Da Rosa M.I., Beck W.O., Colonetti T., Budni J., Falchetti A.C.B., Colonetti L., Coral A.S., Meller F.O. Association of vitamin D and vitamin B(12) with cognitive impairment in elderly aged 80 years or older: A cross-sectional study. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2019;32:518–524. doi: 10.1111/jhn.12636. [DOI] [PubMed] [Google Scholar]
- 13.Navarrete-Reyes A.P., García-Muñoz I., García-Lara J.M., Torres-Carrillo N.M., Amieva H., Avila-Funes J.A. 25-OH-Vitamin D Is Not Associated with Cognitive Performance among Mexican Community-Dwelling Older Persons. J. Frailty Aging. 2015;4:74–79. doi: 10.14283/jfa.2015.44. [DOI] [PubMed] [Google Scholar]
- 14.Lau H., Mat Ludin A.F., Rajab N.F., Shahar S. Identification of Neuroprotective Factors Associated with Successful Ageing and Risk of Cognitive Impairment among Malaysia Older Adults. Curr. Gerontol. Geriatr. Res. 2017;2017:4218756. doi: 10.1155/2017/4218756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodwill A.M., Szoeke C. A Systematic Review and Meta-Analysis of The Effect of Low Vitamin D on Cognition. J. Am. Geriatr. Soc. 2017;65:2161–2168. doi: 10.1111/jgs.15012. [DOI] [PubMed] [Google Scholar]
- 16.Duchaine C.S., Talbot D., Nafti M., Giguère Y., Dodin S., Tourigny A., Carmichael P.H., Laurin D. Vitamin D status, cognitive decline and incident dementia: The Canadian Study of Health and Aging. Can. J. Public Health Rev. Can. De Sante Publique. 2020;111:312–321. doi: 10.17269/s41997-019-00290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chei C.L., Raman P., Yin Z.X., Shi X.M., Zeng Y., Matchar D.B. Vitamin D levels and cognition in elderly adults in China. J. Am. Geriatr. Soc. 2014;62:2125–2129. doi: 10.1111/jgs.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annweiler C., Rolland Y., Schott A.M., Blain H., Vellas B., Herrmann F.R., Beauchet O. Higher vitamin D dietary intake is associated with lower risk of alzheimer’s disease: A 7-year follow-up. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012;67:1205–1211. doi: 10.1093/gerona/gls107. [DOI] [PubMed] [Google Scholar]
- 19.Palacios N., Scott T., Sahasrabudhe N., Gao X., Tucker K.L. Serum vitamin D and cognition in a cohort of Boston-area Puerto Ricans. Nutr. Neurosci. 2020;23:688–695. doi: 10.1080/1028415X.2018.1545291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castle M., Fiedler N., Pop L.C., Schneider S.J., Schlussel Y., Sukumar D., Hao L., Shapses S.A. Three Doses of Vitamin D and Cognitive Outcomes in Older Women: A Double-Blind Randomized Controlled Trial. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020;75:835–842. doi: 10.1093/gerona/glz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NHANES 2011 2012: Vitamin D Data Documentation, Codebook, and Frequencies. [(accessed on 23 August 2021)]; Available online: https://wwwn.cdc.gov/nchs/nhanes/vitamind/analyticalnote.aspx?h=/Nchs/Nhanes/2011-2012/VID_G.htm&t=VID_G%20Doc.
- 22.Swanson C.M., Nielson C.M., Shrestha S., Lee C.G., Barrett-Connor E., Jans I., Cauley J.A., Boonen S., Bouillon R., Vanderschueren D., et al. Higher 25(OH)D2 is associated with lower 25(OH)D3 and 1,25(OH)2D3. J. Clin. Endocrinol. Metab. 2014;99:2736–2744. doi: 10.1210/jc.2014-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romagnoli E., Mascia M.L., Cipriani C., Fassino V., Mazzei F., D’Erasmo E., Carnevale V., Scillitani A., Minisola S. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J. Clin. Endocrinol. Metab. 2008;93:3015–3020. doi: 10.1210/jc.2008-0350. [DOI] [PubMed] [Google Scholar]
- 24.National Health and Nutrition Examination Survey Cognitive Functioning. [(accessed on 23 August 2021)]; Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/CFQ_G.htm.
- 25.Chen S.P., Bhattacharya J., Pershing S. Association of Vision Loss With Cognition in Older Adults. JAMA Ophthalmol. 2017;135:963–970. doi: 10.1001/jamaophthalmol.2017.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S., Sun W., Zhang D. Association of Zinc, Iron, Copper, and Selenium Intakes with Low Cognitive Performance in Older Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES) J. Alzheimer’s Dis. JAD. 2019;72:1145–1157. doi: 10.3233/JAD-190263. [DOI] [PubMed] [Google Scholar]
- 27.National Health and Nutrition Examination Survey Measuring Guides for the Dietary Recall Interview. [(accessed on 28 June 2021)]; Available online: https://www.cdc.gov/nchs/nhanes/measuring_guides_dri/measuringguides.htm.
- 28.Guidelines on Food Fortification with Micronutrients. [(accessed on 28 June 2021)]. Available online: https://www.who.int/publications/i/item/9241594012.
- 29.NHANES 2011–2012 Laboratory Data Overview. [(accessed on 28 June 2021)]; Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory&CycleBeginYear=2011.
- 30.Laboratory Procedure Manual. [(accessed on 28 June 2021)]; Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/labmethods/vid_g_met_vitamin_d.pdf.
- 31.NHANES Analytical Note for 25 Hydroxyvitamin D Data Analysis. [(accessed on 23 August 2021)]; Available online: https://wwwn.cdc.gov/nchs/nhanes/vitamind/analyticalnote.aspx?h=/Nchs/Nhanes/2011.
- 32.Bouillon R., Van Schoor N.M., Gielen E., Boonen S., Mathieu C., Vanderschueren D., Lips P. Optimal vitamin D status: A critical analysis on the basis of evidence-based medicine. J. Clin. Endocrinol. Metab. 2013;98:E1283–E1304. doi: 10.1210/jc.2013-1195. [DOI] [PubMed] [Google Scholar]
- 33.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 34.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G., et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease, Control, and Prevention National Health and Nutrition Examination Survey. Demographic Data and Related Documentation. [(accessed on 28 June 2021)]; Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/DEMO_G.htm.
- 36.Peeri N.C., Egan K.M., Chai W., Tao M.H. Association of magnesium intake and vitamin D status with cognitive function in older adults: An analysis of US National Health and Nutrition Examination Survey (NHANES) 2011 to 2014. Eur. J. Nutr. 2021;60:465–474. doi: 10.1007/s00394-020-02267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NHANES Tutorials Module 3 Weighting. [(accessed on 28 June 2021)]; Available online: https://wwwn.cdc.gov/nchs/nhanes/tutorials/Module3.aspx.
- 38.Beydoun M.A., Hossain S., Fanelli-Kuczmarski M.T., Beydoun H.A., Canas J.A., Evans M.K., Zonderman A.B. Vitamin D Status and Intakes and Their Association With Cognitive Trajectory in a Longitudinal Study of Urban Adults. J. Clin. Endocrinol. Metab. 2018;103:1654–1668. doi: 10.1210/jc.2017-02462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossom R.C., Espeland M.A., Manson J.E., Dysken M.W., Johnson K.C., Lane D.S., LeBlanc E.S., Lederle F.A., Masaki K.H., Margolis K.L. Calcium and vitamin D supplementation and cognitive impairment in the women’s health initiative. J. Am. Geriatr. Soc. 2012;60:2197–2205. doi: 10.1111/jgs.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Przybelski R., Agrawal S., Krueger D., Engelke J.A., Walbrun F., Binkley N. Rapid correction of low vitamin D status in nursing home residents. Osteoporos. Int. A J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA. 2008;19:1621–1628. doi: 10.1007/s00198-008-0619-x. [DOI] [PubMed] [Google Scholar]
- 41.Littlejohns T.J., Henley W.E., Lang I.A., Annweiler C., Beauchet O., Chaves P.H., Fried L., Kestenbaum B.R., Kuller L.H., Langa K.M., et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83:920–928. doi: 10.1212/WNL.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feart C., Helmer C., Merle B., Herrmann F.R., Annweiler C., Dartigues J.F., Delcourt C., Samieri C. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer’s disease in older adults. Alzheimers Dement. J. Alzheimer’s Assoc. 2017;13:1207–1216. doi: 10.1016/j.jalz.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Landel V., Annweiler C., Millet P., Morello M., Féron F. Vitamin D, Cognition and Alzheimer’s Disease: The Therapeutic Benefit is in the D-Tails. J. Alzheimer’s Dis. JAD. 2016;53:419–444. doi: 10.3233/JAD-150943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouma S., Suenaga M., Bölükbaşı Hatip F.F., Hatip-Al-Khatib I., Tsuboi Y., Matsunaga Y. Serum vitamin D in patients with mild cognitive impairment and Alzheimer’s disease. Brain Behav. 2018;8:e00936. doi: 10.1002/brb3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morello M., Landel V., Lacassagne E., Baranger K., Annweiler C., Féron F., Millet P. Vitamin D Improves Neurogenesis and Cognition in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018;55:6463–6479. doi: 10.1007/s12035-017-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Owusu J.E., Islam S., Katumuluwa S.S., Stolberg A.R., Usera G.L., Anwarullah A.A., Shieh A., Dhaliwal R., Ragolia L., Mikhail M.B., et al. Cognition and Vitamin D in Older African-American Women- Physical performance and Osteoporosis prevention with vitamin D in older African Americans Trial and Dementia. J. Am. Geriatr. Soc. 2019;67:81–86. doi: 10.1111/jgs.15607. [DOI] [PubMed] [Google Scholar]
- 47.Annweiler C., Rolland Y., Schott A.M., Blain H., Vellas B., Beauchet O. Serum vitamin D deficiency as a predictor of incident non-Alzheimer dementias: A 7-year longitudinal study. Dement. Geriatr. Cogn. Disord. 2011;32:273–278. doi: 10.1159/000334944. [DOI] [PubMed] [Google Scholar]
- 48.Mayne P.E., Burne T.H.J. Vitamin D in Synaptic Plasticity, Cognitive Function, and Neuropsychiatric Illness. Trends Neurosci. 2019;42:293–306. doi: 10.1016/j.tins.2019.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available vis this link: http://www.cdc.gov/nchs/nhanes.htm(accessed on 28 June 2021).